Abstract
The rapid spread of COVID-19 across the world has raised concerns about the responsiveness of cities and healthcare systems during pandemics. Recent studies try to model how the number of COVID-19 infections will likely grow and impact the demand for hospitalization services at national and regional levels. However, less attention has been paid to the geographic access to COVID-19 healthcare services and to hospitals’ response capacity at the local level, particularly in urban areas in the Global South. This paper shows how transport accessibility analysis can provide actionable information to help improve healthcare coverage and responsiveness. It analyzes accessibility to COVID-19 healthcare at high spatial resolution in the 20 largest cities of Brazil. Using network-distance metrics, we estimate the vulnerable population living in areas with poor access to healthcare facilities that could either screen or hospitalize COVID-19 patients. We then use a new balanced floating catchment area (BFCA) indicator to estimate spatial, income, and racial inequalities in access to hospitals with intensive care unit (ICU) beds and mechanical ventilators while taking into account congestion effects. Based on this analysis, we identify substantial social and spatial inequalities in access to health services during the pandemic. The availability of ICU equipment varies considerably between cities, and it is substantially lower among black and poor communities. The study maps territorial inequalities in healthcare access and reflects on different policy lessons that can be learned for other countries based on the Brazilian case.
Keywords: Accessibility, Floating catchment area, ICU, Ventilators, Brazil, COVID-19, Equity, Race
1. Introduction
The global outbreak of the new coronavirus (SARS-CoV-2) has raised serious concerns about the responsiveness of healthcare systems and particularly about how vulnerable population groups might be affected (Lancet, 2020; WHO, 2020, Almeida et al., 2020). A rapidly growing body of research has emerged to model how the number of COVID-19 infections will likely grow and impact the demand for hospitalization services globally (Petropoulos and Makridakis, 2020, Walter, 2020) and at the national level (Arenas et al., 2020; Moghadas et al., 2020; Paez, 2020; Paez et al., n.d.; Wu et al., 2020) as well as at the regional and local levels (Barrozo et al., 2020a; Castro et al., 2020). However, less attention has been paid to the geographic access to COVID-19 healthcare services and to hospitals’ response capacity at the local level within urban areas, despite the potential relationships between accessibility to healthcare resources and mortality (Ji et al., 2020). Early work by Ji et al. (2020) and Rader et al. (2020), for example, considered resources at the provincial level in China and at the county level in the USA. Still, we are not aware of studies that investigate the issue of resource allocation at higher spatial resolutions, particularly in the context of Latin America, where the epicenter of the pandemic shifted in June 2020.
This study aims to present estimates of geographic accessibility to COVID-19 healthcare at a high spatial resolution within Brazil's 20 largest cities. Healthcare services in Brazil are known to be unevenly distributed across the country and also within cities (Amaral et al., 2017). In this context, it is crucial to map where vulnerable social groups confront poor accessibility to health services. Similarly, it becomes paramount to identify which healthcare facilities are likely to face surges in demand due to the need to hospitalize severely ill patients. In this paper, we combine traditional and novel accessibility metrics to address these questions. Using network-distance metrics, we first estimate the number of vulnerable people living in areas with poor access to inpatient or outpatient facilities able to provide care for patients with suspected or confirmed cases of COVID-19. Next, we use a new balanced floating catchment area method (BFCA) proposed by (Paez et al., 2019) to analyze levels of access to hospitals that could treat patients with severe symptoms of COVID-19, taking into account healthcare system capacity and competition effects for Intensive Care Unit (ICU) beds with mechanical ventilators. This new BFCA indicator is used to estimate income and racial inequalities in access to COVID-19 health services while accounting for congestion effects.
The remainder of this paper is as follows. The next section provides relevant background information regarding the evolution of the COVID-19 pandemic in Brazil and accessibility analysis. Sections 3, 4 present the data and methods used in this research. Then, the results of the empirical analysis are presented and discussed in section 5. Finally, we offer some concluding remarks, including policy implications and directions for future research.
2. Background
2.1. COVID-19 in Brazil
The first confirmed case of COVID-19 in Latin America was in late February 2020, in Brazil. The person was a man from São Paulo who had traveled that month to Italy (Souza et al., 2020). This case was typical of the beginning of the epidemic in Brazil, with other early disease cases imported via international flights coming mostly from Italy and the United States (Candido et al., 2020). By early June, Brazil had become, after the United States, the country with the highest number of cases of COVID-19 in the world. In early July, less than five months after the first case of COVID-19 in Brazil, over 1.8 million cases have been confirmed, and over 70 thousand deaths have been attributed to the disease, 45% of which concentrated in the 20 largest cities of the country.
The earliest cases of COVID-19 in Brazil were concentrated among middle and upper-class people (Souza et al., 2020; Li et al., 2020), who typically can afford to pay for private healthcare or use health services intermediated by private health insurance. This is not the case for lower-income groups, who are largely dependent on Brazil's public health system (Castro et al., 2019), and among whom community transmission rapidly increased the number of infections. This development is particularly worrisome as low-income groups also typically face poor transport services and poor access to health, education, and employment opportunities (Pereira et al., 2019). In fact, previous research has identified critical spatial gaps in accessibility to emergency services in Brazil (Rocha et al., 2017). To further complicate matters, other research has linked poor accessibility to higher pneumonia mortality (Zaman et al., 2014).
Given the rapid growth of COVID-19 in Brazil, it is essential to map the potential stress on the country's healthcare system (Almeida et al., 2020; Barrozo et al., 2020a). Previous studies show an unusual increase in the numbers of deaths (Barrozo et al., 2020a) and admissions to hospitals due to COVID-19 (InfoGripe, 2020). These studies raise concerns about the overload the pandemic can generate to the public Unified Health System (Sistema Único de Saúde - SUS), which already started showing signs of collapse similar to those observed in Italy and Spain (Grasselli et al., 2020; Legido-Quigley et al., 2020). The work of Noronha et al. (2020) found that even in an optimistic scenario, of an infection level of 0.1% in the first month, roughly half of the nation's health regions would face a grave deficit of ICU beds to meet the demand for admission of COVID-19 patients. Research by Barrozo et al. (2020a) and regional modeling studies conducted by Coelho et al. (2020) and Castro et al. (2020) suggest the pressure on the health system is more likely to reach critical levels in large urban centers, where the number of confirmed cases is higher.
These previous studies in Brazil analyze the dynamics of COVID-19 across different regions, states, and municipalities. Nonetheless, there is still a lack of studies that look at the COVID-19 healthcare provision within urban areas, and at what actionable insights can be drawn from the analysis of vulnerable groups and their access to health services at a higher spatial resolution. The looming crisis faced by the health system due to COVID-19 requires many emergency actions. For this purpose, it is essential for healthcare planners to have an understanding of the neighborhoods with less access to health services and equipment, and to identify the hospitals that might suffer overloaded demand for admissions.
2.2. Healthcare accessibility
A commonly used indicator to measure geographic access to healthcare is the shortest distance/travel time to the closest facility (Geurs and van Wee, 2004; Neutens, 2015). This indicator is widely used in part because it is relatively simple to calculate and straightforward to interpret, and thus easily communicated to policy-makers. However, a well-known limitation of this indicator is that it overlooks congestion effects since it does not account for potential population demand nor for levels of service supply.
Another popular approach to measure access to healthcare is the family of Floating Catchment Area (FCA) methods (Matthews et al., 2019). A key advantage of this family of indicators is that it accounts for capacity restrictions, local congestion effects, as well as cross-border healthcare-seeking behavior (Neutens, 2015). The common rationale underlying FCA methods is to calculate accessibility levels in sequential steps. The first step is to calculate the provider-to-population ratio (PPR) of each health facility as a ratio between its service supply (e.g., number of ICU beds) and its potential service demand given by the population that falls within some catchment area. The second step is to calculate accessibility levels of each population center by aggregating the PPR of every healthcare provider accessible from each population center.
The first indicator of this sort is the two-step floating catchment area (2SFCA), proposed in the early 2000s (Luo and Wang, 2003; Radke and Mu, 2000). Since then, multiple authors have proposed incremental improvements to the basic model to incorporate more sophisticated impedance functions (Dai, 2010; Luo and Qi, 2009), to consider suboptimal configurations of health systems (Delamater, 2013) and to account for spatially adaptive floating catchments (Matthews et al., 2019; Matthew R. McGrail and Humphreys, 2009) and trip-chaining behavior (Fransen et al., 2015).
A fundamental limitation of FCA methods is that they overestimate both service demand and supply, which can generate misleading accessibility estimates (Delamater, 2013; Paez et al., 2019; Wan et al., 2012). Demand inflation occurs when populations that fall within the overlap of catchment areas are counted more than once as potential demand for multiple facilities. Supply inflation, on the other hand, happens when the level of service of a healthcare unit is simultaneously allocated to multiple population centers (Paez et al., 2019). Until recently, two approaches had been proposed to address this issue. Wan et al. (2012) introduced the Three-Step Float Catchment Area (3SFCA), which deflates demand by introducing an initial step that splits a population center's potential demand over multiple health facilities proportional to transport costs/distances. Meanwhile, Delamater (2013) proposed the Modified Two-Step Floating Catchment Area (M2SFCA), which deflates the supply side by increasing the friction of distance in a way that allocates levels of service more locally. Nonetheless, both methods only partially fix the inflation problem, as they compound the effects of impedance functions to address either demand inflation (3SFCA) or supply inflation (M2SFCA).
To overcome this limitation, Paez et al. (2019) recently introduced a new indicator to the FCA family, which we term here as the Balanced Float Catchment Area (BFCA). The new BFCA uses a standardized impedance matrix to generate a proportional allocation of demand and service level, fixing both demand and supply inflation issues in FCA calculations. The result is a more intuitive measure of accessibility that 1) accounts for competition effects, 2) provides a local version of the provider-to-population ratio (PPR) that is interpreted similarly to a regional PPR; and 3) preserves system-wide population and level of service, overcoming inflation issues of both demand and service levels. Estimating accessibility during a pandemic is a suitable application of this method because congestion over the short term is one of the fundamental issues to address. Therefore, the analysis must account accurately for competition for scarce resources if policy interventions are to have any hope of addressing shortfalls effectively. The BFCA approach is described in more detail in the following section.
3. Data
Data for Brazil's 20 largest cities (Appendix Figure A) are drawn from the Access to Opportunities Project (Pereira et al., 2019).1 The method combines data from national household surveys, administrative records of the federal and municipal governments, satellite images and collaborative mapping data to estimate accessibility at a high spatial resolution. The analysis is based on a hexagonal grid that corresponds to the global H3 index at resolution 8, with a size of 357 m (short diagonal) and an area of 0.74 km2; this is approximately the size of a typical city block (https://h3geo.org/docs/core-library/restable).
Original sociodemographic data comes from the 2010 population census conducted by the Brazilian Institute of Geography and Statistics (IBGE). These data are aggregated to the hexagonal grid using dasymetric interpolation in two steps, as follows. Data on population count, income, race, and age distribution are gathered at the census tract level. Population counts are then updated based on municipal-level demographic projections for 2020, published by Freire et al. (2020). The total projected population of each city in 2020 was distributed across census tracts assuming that the population's relative distribution by district and age cohort of each sector remained constant between 2010 and 2020. We then used dasymetric interpolation to pass information from census tracts to a finer regular grid of 200 m with population count data considering aerial intersection and population sizes. Finally, these data were reaggregated from the regular grid to the hexagonal grid.
Data on healthcare facilities associated with the SUS, as registered in the National Registry of Health Facilities (CNES), at the end of 2019 were geocoded and made publicly available by Pereira et al. (2019). For this paper, these data were complemented with updated information from the CNES for February 2020 about the number of adult ICU beds and ventilators in each healthcare facility. We also included geolocated data on 30 field hospitals and reactivated hospitals in 15 cities up to April 2, 2020. These hospitals have eased demand on other hospitals and expanded the health system's capillarity by adding a total of 868 beds in ICUs and semi-intensive or semi-critical care units.
Finally, we used street network data from OpenStreetMap and topography data from satellite images generated by the Japanese Spatial Agency (JAXA, 2011). These data were processed with OpenTripPlanner to generate the door-to-door travel times and distances between the centroids of hexagons with vulnerable populations and healthcare facilities in each city. OpenTripPlanner is an open routing algorithm for multimodal transport networks that takes street network characteristics and terrain elevation into account to model pedestrian routing.
4. Methods
The analysis presented in the paper is divided into two parts, as described next.
4.1. Maximum distance to the nearest facility by vulnerable population
As noted in the background, a commonly used measure of accessibility is distance/travel time to the nearest facility. In the first part of the analysis, we estimate for each city the number and residential locations of vulnerable populations who: (a) cannot access within 30 min on foot an establishment associated with the SUS that can perform triage and refer patients suspected of COVID-19 infection for hospitalization; and (b) live further than 5 km from a hospital with capacity to admit COVID-19 patients with adult ICU beds and ventilators. Vulnerable population groups were defined as people above 50 years old with lower income (in the bottom half of the income distribution). These criteria were chosen based on evidence found by previous studies regarding the vulnerability of people older than 50 years in Brazil to COVID-19 (Souza et al., 2020). As well, low-income individuals tend to depend more on the public health system (Castro et al., 2019) and to face more significant difficulties of urban mobility and access to health services (Pereira et al., 2019).2 That said, the method is applicable to any population group of interest.
All time and distance thresholds were chosen as a first exploratory analysis following the official recommendation from the Health Ministry and local officials (state and municipal), who recommend that people with suspected COVID-19 infection stay at home if the symptoms are mild or go to the nearest health unit for an initial interview (triage) and notification of the surveillance team (Brasil, 2020b). Patients with severe symptoms should be referred for admission to hospitals in general wards or ICUs (Brasil, 2020b). In Brazil, the typical pattern is for people to use hospitals as entry points for daily health services and emergency care (Brasil, 2020b). Following clinical management and fast track service protocols (Brasil, 2020a, 2020b), this analysis included those primary health service facilities with capabilities to screen patients suspected of COVID-19, refer patients to specialized services, as well as facilities with more advanced service levels, such as emergency care centers, first aid posts, and hospitals.3
Accessibility levels by public transportation were not considered because collective transportation is not recommended for people with symptoms of COVID-19. Besides this, various cities saw a drop of over 70% in the supply of transit services due to isolation measures (NTU, 2020). This reduction makes public transport services less reliable and more hazardous for contagion due to the agglomeration of people at stops/stations and aboard vehicles (Costa et al., 2020).
4.2. Balanced Floating Catchment Areas
In the second part of the analysis, we consider accessibility to health services while taking into account healthcare system capacity and congestion effects by means of Balanced Floating Catchment Areas. In this part, we focus on access to those facilities that could provide hospitalization in ICUs to support patients with COVID-19. This analysis estimates provider-to-population ratios from both origin and destination perspectives. To do so, we use (1) the balanced float catchment area (BFCA) to calculate for each hexagonal cell the number of accessible ICU beds with ventilators, and (2) a partial version of BFCA to calculate for each hospital the ratio between the number of ICU beds with ventilators available and the population of the corresponding catchment area.
Floating Catchment Area approaches are generally defined in two key steps. In the first stage, the population to be serviced is allocated to each service point (in this case, a health care facility) as follows:
where is the population at population center () and is a weight for location pair (). This weight typically depends on the cost of transportation between locations (which are population centers, for example grid cells), and locations (which are service points, e.g., clinics). In this way, is the weighted sum of the population serviced by location . The level of service at each service point is the capacity at the location divided by its estimated serviced population:
where is the service capacity at (e.g., number of beds) and is the level of service at (for instance, beds per 1000 people).
In the second step of the approach, the accessibility of population center is calculated as the weighted sum of the level of service of all clinics reachable from there according to the weights:
Weights are usually obtained from a distance-decay function that depend on the cost of transportation between locations and , as follows:
There are numerous modifications of this basic framework that use binary, stepwise, or smooth functions for the distance-decay function (e.g., McGrail and Humphreys, 2009; Luo and Qi, 2009; Dai, 2010; Bauer and Groneberg, 2016). Other researchers have identified a certain bias in the calculations (termed inflation) that results from allocating the same population to multiple clinics, and then allocating the level of service of each clinic to multiple population centers (see Wan et al., 2012; Delamater, 2013), in built-in double counting effects that seldom cancel each other. A detailed discussion of this issue can be found in Páez et al. (2019), where the BFCA approach is introduced.
The BFCA differs from previous indicators by simultaneously correcting for inflation of demand and service levels. To do this, this approach replaces the weights in the calculations above, and uses instead a set of suitably normalized weights as follows:
and:
These weights satisfy the following properties:
and:
So that accessibility is calculated as:
The intuition of the normalized weights is that population is allocated proportionally to service points, and the sum of the allocated population is the total of the population. Likewise, the level of service is allocated proportionally to population centers, and the total sum of the level of service is the total level of service. In this way, double counting is eliminated at both stages, and the resulting indicator can be interpreted as an exact provider-to-population ratio (PPR).
4.3. Practical issues for implementation
Accessibility measures can be implemented using positive and normative approaches (Páez et al., 2012). Positive approaches consider the willingness of people to travel, whereas the latter captures a norm to be satisfied. In practice, the difference between positive and normative accessibility is the definition of the impedance function. In this study we consider both approaches. In the first part of the empirical analysis, we consider a normative implementation with a threshold of 30 min travel time based on research by McGrail et al. (2015). This threshold was chosen with a policy assumption that tries to minimize the distance that patients travel as a way to reduce contagion risks for others. In the second part of the analysis, we calculated accessibility considering as a threshold the maximum distance to the nearest hospital in each city. This threshold can be interpreted as the minimum necessary distance that guarantees that every person can reach at least one hospital and is normatively in line with the recommendation from the Ministry of Health (Brasil, 2020b) that suspected cases should visit their nearest hospital.
Because this treatment of patients with COVID-19 often requires combined availability of ICU beds and mechanical ventilators, we only considered the joint availability of bed/ventilator. Thus, in the case of a hospital with 30 adult ICU beds but only 20 ventilators, we considered only 20 beds (one ventilator per bed). As a rule, however, there are more ventilators than ICU beds. For ICU beds in field hospitals, we assumed there was at least one ventilator available per bed.
A limitation of this method is that it considered the ICU beds and ventilators that are in use, but these might not be the appropriate models for prolonged use as in COVID-19 cases. Another limitation is that we analyzed the public healthcare system's attendance capacity focusing only on the number of adult ICU beds and ventilators. Other studies should also consider restrictions imposed by the availability of healthcare professionals to staff ICUs when the data is available.
Another limitation of the method is that our analyses are restricted to the population and supply of services within cities. This generates two effects. The first is the tendency to underestimate the level of access to services by people who live near the border between two cities since they could possibly access hospitals in the neighboring city. The second effect is the underestimation of the demand from people from other neighboring cities who can seek admission to hospitals in the 20 cities analyzed. To minimize this second problem, we applied an adjustment factor for hospital admissions by non-residents (Brasil, 2005) according to the size of the population living in each hexagon, as suggested by Paez et al. (2019). A factor of 1.5, for example, would simulate that the size of the demand for admission to each hospital is 50% higher due to patients living in other cities. In a recent study, Servo et al. (2019) demonstrated that in 2015 on average 30% of the hospitalizations for medium complexity treatments in cities were from patients living in other cities. Based on data from the SUS Hospital Information System for 2019, we calculated the value of the correction factors for each city (see Appendix Table A).
5. Results
5.1. Access to health services
We find that the 20 largest Brazilian cities comprise nearly 228 thousand people above 50 years of age who are in low income brackets, and reside more than 30 min away (by walking) from a health unit that provides triage and referral services to patients with suspected infection (Table 1 , column A). These health units, particularly primary health and emergency care units play a fundamental role as entry points to the system. As such, the spatial capillarity and longitudinality of these units are very important to facilitate the population's access to health services and to avoid hazardous agglomeration of patients with suspected coronavirus infection.
Table 1.
Low-income population above 50 years old with access to healthcare in Brazil's 20 largest cities, 2020.
| City | Total population | Vulnerable population** | (A) Vulnerable Pop. with poor access to basic health services |
(B) Vulnerable Pop. with poor access to ICU hospitalization |
(B)/Vulnerable Pop. (%) |
|---|---|---|---|---|---|
| Rio de Janeiro | 6592.2 | 692.5 | 51.9 | 384.3 | 55.5 |
| São Paulo | 12142.6 | 1053.6 | 33.2 | 263.1 | 25 |
| Brasília | 3052.5 | 180.3 | 21.1 | 121 | 67.1 |
| Curitiba | 1927 | 172.9 | 5.1 | 116.4 | 67.3 |
| Belo Horizonte | 2469.9 | 244 | 7.2 | 92.3 | 37.8 |
| Fortaleza | 2651.8 | 193.5 | 6.5 | 77.7 | 40.2 |
| São Gonçalo | 1075.4 | 112.9 | 8.8 | 72.6 | 64.3 |
| Duque de Caxias | 905.1 | 81.3 | 13.5 | 67 | 82.4 |
| Porto Alegre | 1480.5 | 159.6 | 8.6 | 60.3 | 37.8 |
| Goiânia | 1509.4 | 118.3 | 11.4 | 59.4 | 50.2 |
| Campinas | 1208.9 | 115.1 | 12.2 | 58.1 | 50.5 |
| Guarulhos | 1389.9 | 98.7 | 4.3 | 48 | 48.6 |
| Recife | 1607 | 147.1 | 0.6 | 42.9 | 29.2 |
| Campo Grande | 895.6 | 69.7 | 5 | 42.9 | 61.5 |
| Maceió | 1042 | 74.2 | 8.4 | 38.2 | 51.5 |
| Salvador | 2831.6 | 217.4 | 7.8 | 35.3 | 16.2 |
| Belém | 1360.1 | 97.2 | 8.7 | 32.9 | 33.8 |
| Manaus | 2216.1 | 111.6 | 2.3 | 24.8 | 22.2 |
| São Luís | 1080.4 | 69.3 | 10.2 | 18.6 | 26.8 |
| Natal |
867.9 |
63 |
1.6 |
10.2 |
16.2 |
| Total | 48305.9 | 4072.2 | 228.4 | 1666 | 40.9 |
Obs.
- Population in thousands.
- ** Population above 50 years old and in the bottom half of the income distribution.
- (A) Low-income people above 50 years old who cannot access a healthcare facility in less than 30 min walking.
- (B) Low-income people above 50 years old who live more than 15 km away from the nearest hospital with an ICU bed and mechanical ventilator.
Source: authors' own elaboration using data from Pereira et al. (2019), healthcare facilities data from CNES on February 2020, and population projections for 2020 from Freire et al. (2020).
The highest proportion of people in this situation were found in the cities of Rio de Janeiro, São Paulo, Brasília, Duque de Caxias, Campinas, and Goiânia. These six cities concentrate more than 60% of the vulnerable population who live more than 30 min on foot from a health service point. However, analysis of the proportion in each city reveals that Duque de Caxias, São Luís, Brasília, Maceió, and Campinas stand out with more than 10% of their vulnerable population living more than 30 min from a health facility qualified for this triage.
Furthermore, Table 1 also shows there are some 1.6 million vulnerable people who live farther than 5 km from a health unit able to admit patients in serious condition due to COVID-19. This total represents 41% of the vulnerable population in the 20 cities. These numbers vary widely across cities due to the different patterns of urban occupation and the spatial distribution of healthcare facilities in each area. Cities like Rio de Janeiro, São Paulo, Brasília, and Curitiba stand out for having more than 100,000 inhabitants in potentially vulnerable conditions and with poor access to hospitals with ICU beds and mechanical ventilators. It is also noteworthy that half of the 20 cities have more than 50% of their vulnerable population living farther than 5 km from inpatient facilities.
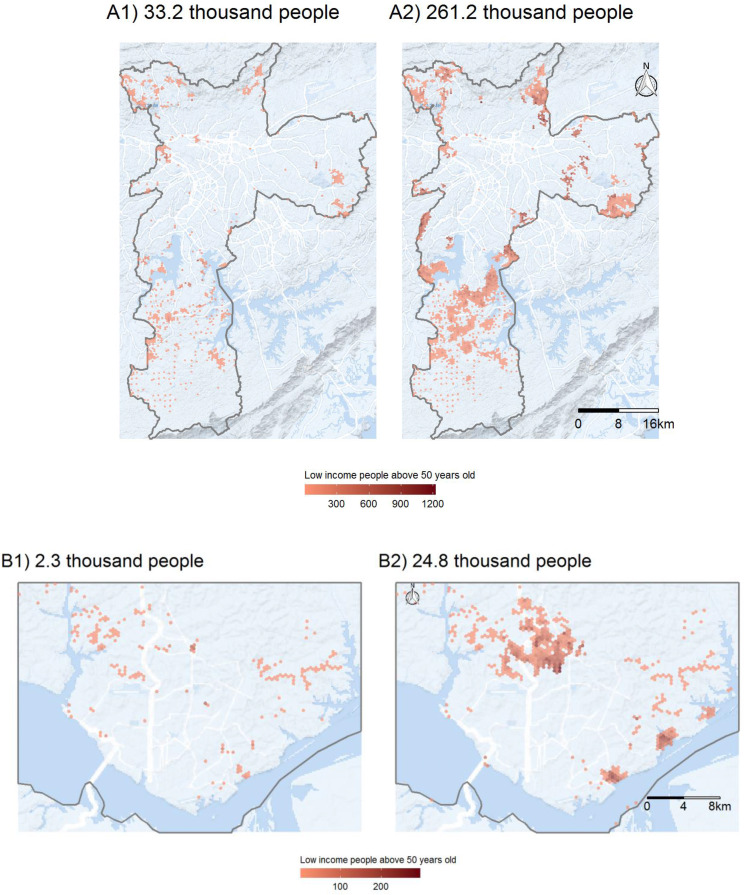
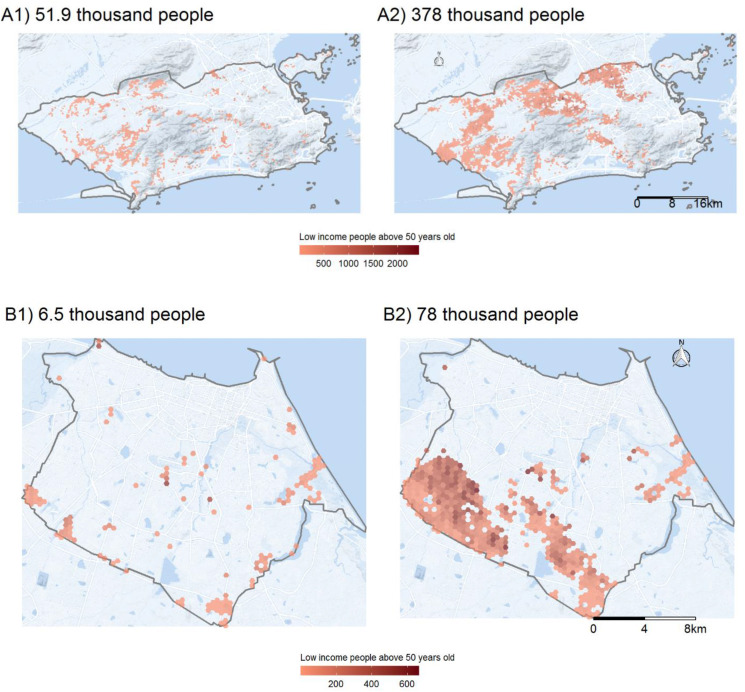
As crucial as estimating how many vulnerable people have poor access to healthcare is mapping where this population lives. For four selected cities, Fig. 1, Fig. 2 present the size and residential locations of the low-income population older than 50 years who (1) cannot access any primary healthcare establishment in less than 30 min by walking; and (2) live more than 5 km from the nearest hospital with at least one ICU bed and one ventilator. For the sake of brevity, we only present the maps for Sao Paulo, Rio de Janeiro, Fortaleza, and Manaus, the four cities most affected by COVID-19 in Brazil. As of the end of June, these four cities alone concentrated 18% of COVID-19 confirmed cases and 32% of deaths in the country. These maps show that vulnerable populations with poor access to health services are mostly located in urban peripheries, indicating those areas which would be good candidates for local policy interventions, such as setting up field hospitals or engaging pre-hospital mobile units (such as the Urgent Mobile Response Service - SAMU) or community health agents.
Fig. 1.
Access to COVID-19 healthcare in São Paulo (A) and Manaus (B), 2020. (Panel 1) Vulnerable populations that cannot access a primary healthcare facility in less than 30 min walking. (Panel 2) Vulnerable populations that live farther than 5 km to the nearest hospital with ICU bed and mechanical ventilator.
Fig. 2.
Access to COVID-19 healthcare in Rio de Janeiro (A) and Fortaleza (B), 2020. (Panel 1) Vulnerable populations that cannot access a primary healthcare facility in less than 30 min walking. (Panel 2) Vulnerable populations that live farther than 5 km to the nearest hospital with ICU bed and mechanical ventilator.
5.2. Health system capacity
A primary indicator to measure a health system's capacity is the number of hospital beds per inhabitant in a given area. According to the parameters defined by the Brazilian Ministry of Health (Brasil, 2015) the minimum standard provision should be 1 adult ICU bed for each 10,000 people.4 The average number of adult ICU beds with ventilators in hospitals in the public health system in the 20 largest Brazilian cities is 1.06 per 10 thousand people (Table 2 ). This value is only slightly higher than the minimum recommended by the Ministry of Health under normal circumstances. The value of 1.06 can be considered insufficient in an epidemic situation, posing a risk of overload in scenarios for COVID-19 contagion indicated in previous studies (Castro et al., 2020; Noronha et al., 2020). The ratio of beds per population also varies significantly across cities (Table 2). Thirteen out of the 20 cities analyzed are below the recommended level of service and by July 1st most of these cities had ICU bed occupancy rates above 80% (Folha de S.Paulo, 2020).
Table 2.
Number of adult ICU beds and mechanical ventilators in SUS per 10 thousand people in Brazil's 20 greatest municipalities, 2020.
| Municipality | ICU beds* | Population (in thousands)** | ICU beds per 10 thousand people |
|---|---|---|---|
| São Gonçalo | 149 | 760.5 | 2 |
| Goiânia | 596 | 2965.9 | 2 |
| Belo Horizonte | 792 | 4348.2 | 1.8 |
| Rio de Janeiro | 1419 | 8259.4 | 1.7 |
| Porto Alegre | 327 | 2602.8 | 1.3 |
| Salvador | 565 | 4363.9 | 1.3 |
| São Paulo | 1211 | 13852.3 | 0.9 |
| Campo Grande | 102 | 1180.3 | 0.9 |
| Curitiba | 211 | 2550.1 | 0.8 |
| Campinas | 141 | 1701.9 | 0.8 |
| Guarulhos | 93 | 1197.7 | 0.8 |
| Recife | 373 | 4477 | 0.8 |
| São Luís | 159 | 1886.4 | 0.8 |
| Belém | 139 | 2111 | 0.7 |
| Manaus | 170 | 2419.3 | 0.7 |
| Natal | 123 | 1784.9 | 0.7 |
| Fortaleza | 241 | 4003 | 0.6 |
| Brasília | 181 | 3860.3 | 0.5 |
| Maceió | 82 | 1893.2 | 0.4 |
| Duque de Caxias | 30 | 946.3 | 0.3 |
| Total | 7104 | 67,164.4 | 1.06 |
Source: authors' own elaboration using data from Pereira et al. (2019), healthcare facilities data from CNES on February 2020, and population projections for 2020 from Freire et al. (2020). Obs.: * Number of ICU beds with mechanical ventilators in the public healthcare system. ** City population corrected by the adjustment factor from Appendix Table A.
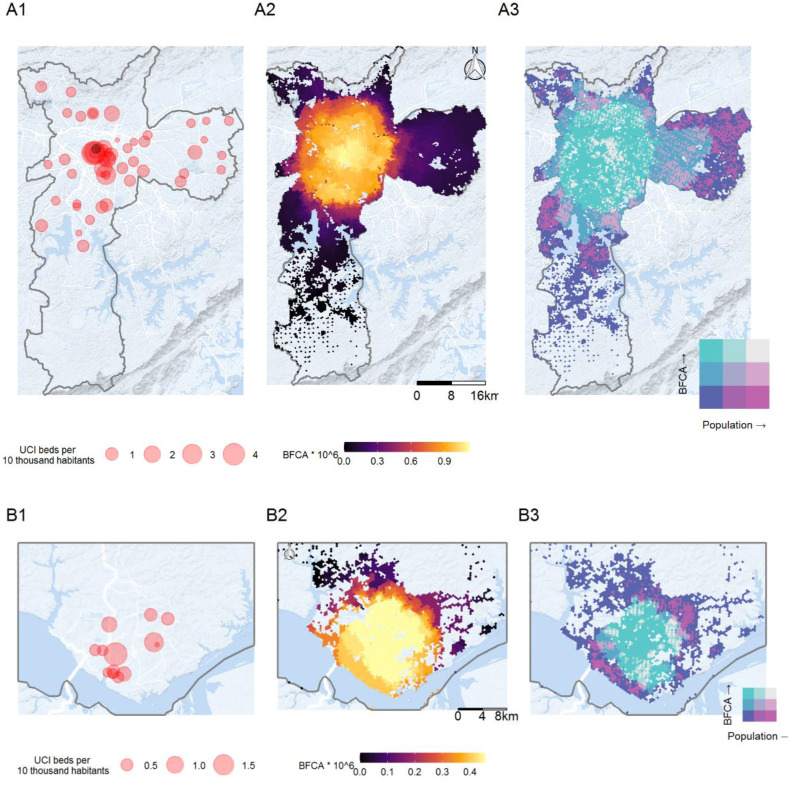
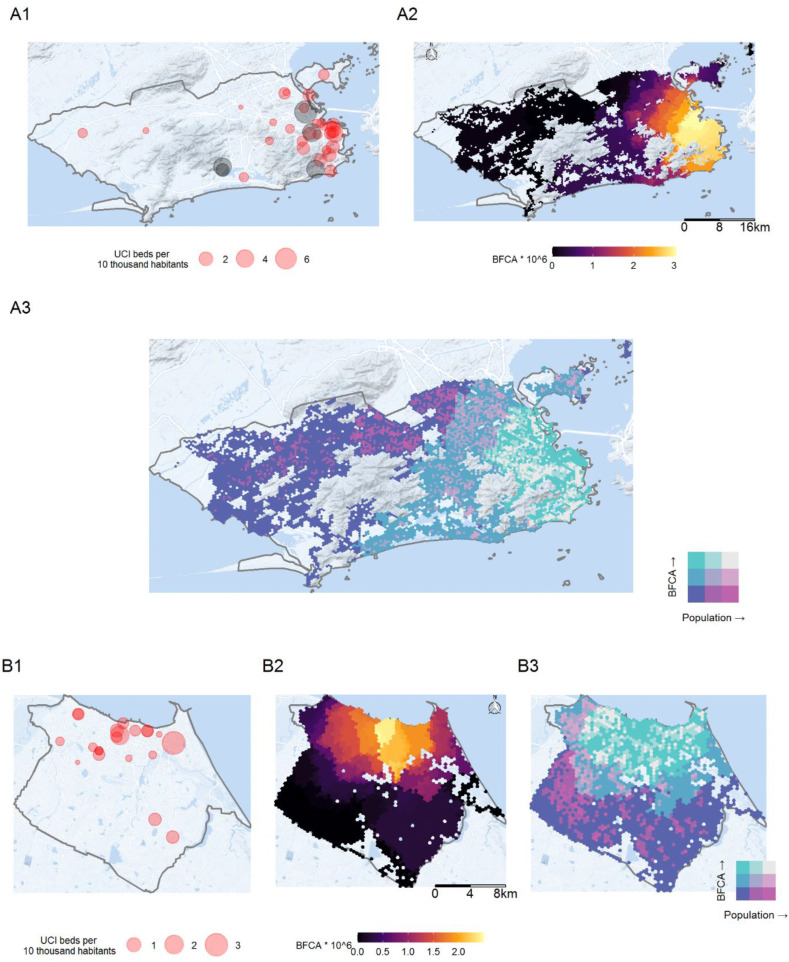
The most significant variations in the availability of health services, however, occur within cities. In Fig. 3, Fig. 4 below we present a set of maps that provide a detailed view of the spatial distribution of hospitals with adult ICU beds and ventilators and the level of access to these services considering competition effects. The maps on Panel 1 show the spatial distribution of hospitals. Each hospital is represented by a circle whose size reflects the ratio between the number of beds/ventilators of that hospital and the population of its catchment area. Although the situations vary across cities, as a rule, downtown areas generally concentrate the greatest number of hospitals, especially the ones with more beds per inhabitant. This is the case, for example, in Fortaleza, Manaus, and Rio de Janeiro. The availability of ICU beds and ventilators to serve patients with severe COVID-19 tends to be considerably lower in the peripheral regions of these cities. In these regions, it is common to observe ratios of ICU beds per 10 thousand inhabitants between 0.5 and 1.0. These ratios can be considered critical against a backdrop of an epidemic with growing numbers of patients needing hospitalization for respiratory complications. The example of cities like Rio de Janeiro also illustrates how setting up field hospitals in farther areas can increase the capillarity of health services in epidemic situations like the COVID-19 outbreak.
Fig. 3.
Spatial distribution of hospitals with adult ICU beds and ventilators (1), the level of access to these services considering competition effects (2), and population distribution (3) in São Paulo (A) and Manaus (B), 2020.
Fig. 4.
Spatial distribution of hospitals with adult ICU beds and ventilators (Panel 1), the level of access to these services considering competition effects (Panel 2) and population distribution (Panel 3) in Rio de Janeiro (A) and Fortaleza (B), 2020.
Meanwhile, the maps on Panel 2 show the number of ICU beds and ventilators accessible from each location, considering at the same time the level of service availability and the potential overlap of demand and supply competition effects estimated with the balanced float catchment area (BFCA). Compared to the results in Panel 1, these maps present in more detail how the geographic access to COVID-19 healthcare is particularly higher in central urban areas. This is perhaps more clearly seen in the city of São Paulo, where accessibility to equipped beds steadily declines from the central parts of the city to the periphery. Considering the average population per tile of the hexagonal grid in São Paulo, this indicates that in the regions with the highest accessibility, there are approximately 0.000012 beds per 10,000 people serving on average 1240 persons. This translates into 9.76e-6 beds per person, compared to 8.74e-5 beds per person average for São Paulo. This shows how the regional PPR can be misleading, by assuming that every person in the region has equal access to medical facilities. Analysis using the BFCA indicator also illustrates how living close to a hospital does not necessarily translate into high levels of accessibility once congestion effects are taken into account. This is the case in cities like Fortaleza, Rio de Janeiro, and São Paulo, where some peripheral neighborhoods, despite being close to a hospital, face poor access to health services due to the limited capacity of the healthcare system to support the expected demand of much larger areas.
Finally, the maps on Panel 3 present bivariate choropleth maps with the combined spatial distribution of population densities and accessibility levels by automobile using city-specific thresholds so that every person could reach at least one hospital. This panel complements previous figures by highlighting those areas with large populations underserved by healthcare services (bright pink = larger population and lower accessibility) and those areas which face higher service levels for a comparable lower demand (bright green = smaller population but high accessibility). It is possible to see that even in those places with low accessibility, there are pockets where the situation is made worse by afflicting larger populations. The figures in Panel 3 are useful to differentiate low-accessibility areas with large and small population numbers, which can provide actionable information for policy-makers to choose which low-accessibility areas should be prioritized in emergency situations.
Obs. Gray circles represent field hospitals or temporarily reactivated facilities.
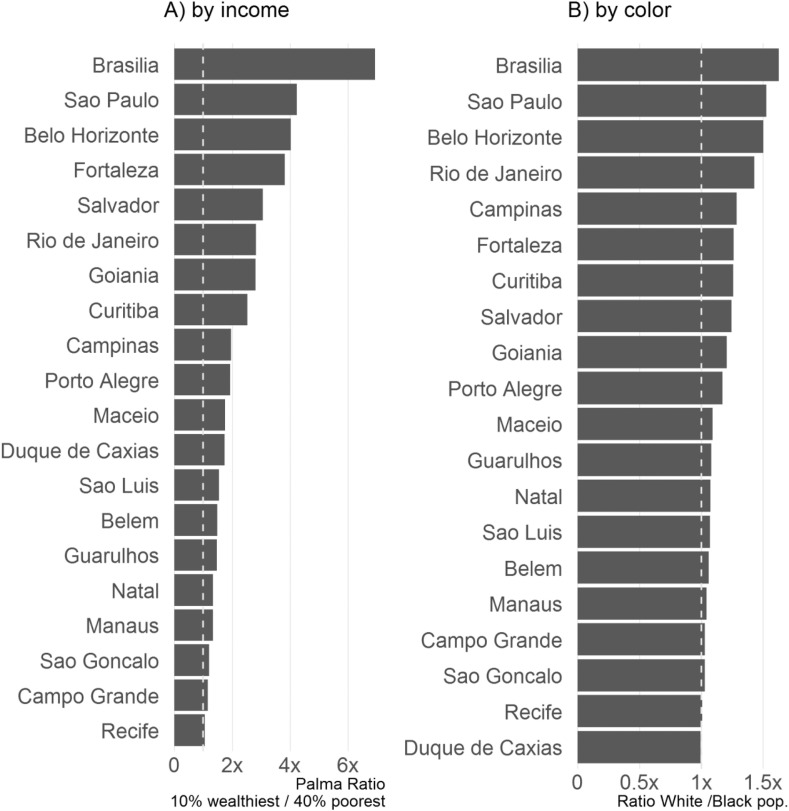
The geographic access to COVID-19 healthcare in Brazil presents not only spatial but also marked social inequalities. Fig. 5 shows the magnitude of the racial and income inequalities in access to ICU beds and ventilators considering competition effects. One of the most extreme cases is the capital of the country Brasília, where the number of ICU beds with ventilators accessible by the wealthiest population is more than six times larger than for the poor. While racial inequalities are relatively lower compared to inequalities by income, they are still present in most cities. This is particularly true in Brasília, São Paulo, and Belo Horizonte, where black communities can only access half as many health resources as the white population.
Fig. 5.
Income and racial inequalities in access to ICU beds and ventilators considering competition effects. Brazil's 20 largest cities, 2020.
In summary, our findings point to a worrying pattern. Across Brazil's 20 largest cities, we find substantially lower healthcare system capacity in peripheral urban areas and among low-income and black communities. In particular, urban peripheries with high population density coupled with low incomes and poor sanitation services create a worrying scenario with strong potential for propagation of the COVID-19 precisely among communities that are most vulnerable to the disease and with the lowest access to healthcare. Combined, the analyses presented provide valuable information that can help local authorities map the areas which should receive more immediate response from local healthcare community agents and perhaps the construction of new field hospitals to address short-term needs induced by the COVID-19 pandemic.
6. Final remarks
This paper examined the geographical access to COVID-19 healthcare in the 20 largest Brazilian cities. The study looked at access to both health facilities with capacity for triage and referral of patients with suspected COVID-19 to hospitals, as well as those able to hospitalize patients with the support of ICU beds and mechanical ventilators. We mapped approximately 228 thousand vulnerable people (low-income and above 50 years old) living more than 30 min walking from primary and emergency care units. We also found some 1.6 million vulnerable people living farther than 5 km from a hospital with capacity for ICU admission. Because patients suspected of COVID-19 might face mobility constraints due to grave conditions, it becomes crucial to develop strategies to provide transport and health services to these people. This is particularly true of low-income communities on the outskirts of cities, where there are fewer mobility options and where health services are scarce.
The study also analyzed the support capacity of the public health system in Brazil's largest cities, looking at the number of ICU beds/ventilators per person in the catchment area of each hospital. We find that thirteen out of the 20 cities analyzed have fewer ICU beds/ventilators than the minimum level recommended by national authorities. This number could be considered insufficient to cope with the growth of demand for hospital admissions given the rapid propagation of COVID-19 in Brazil. Accessibility analysis using the new balanced float catchment area (BFCA) shows this scenario is particularly aggravated when accounting for competition effects on both supply and demand for health services. The BFCA estimates show large spatial inequalities with substantially lower access to health services in low-income and black communities in urban peripheries, which could more easily be overwhelmed by the near-future hospitalization demands.
As a whole, the study illustrates how transport accessibility analyses can provide actionable information to help local governments improve access to healthcare during pandemic outbreaks. Our analyses put disadvantaged communities with poor access to health services on the map, indicating in which neighborhoods local authorities could prioritize building makeshift hospitals or engage mobile units and health community agents. These analyses may also help identify which hospitals might face greater admission overload and hence would need supplementary funding to expand capacity. The application of the novel BFCA in this paper illustrates how considering competition effects in access to healthcare can have important but often overlooked implications for policy planning.
Future research could potentially indicate the areas where the construction of makeshift hospitals would be more effective to improve healthcare accessibility, particularly for vulnerable groups. New studies are also necessary to examine the potential role of community health agents in improving healthcare accessibility in more remote areas. More research is still needed to investigate how the availability of health professionals could hinder the availability of services to the population. Future studies could also benefit from the work developed by Barrozo et al. (2020b) to further examine the relationship between socioeconomic and health conditions, including those associated with COVID-19.
Contributions
Conception and design of study: RHMP, LMS, AP. Acquisition of data: RHMP, CKVB, LMS, NG. Analysis and/or interpretation of data: RHMP, CKVB, BS, PA, AP. Drafting the manuscript: RHMP, LMS, BS. All authors read and revised the final manuscript
Footnotes
More information about the Access to Opportunities Project and its databases are available at: https://www.ipea.gov.br/acessooportunidades/en/.
Ideally, it would be important also to consider the population with comorbidities, such as hypertension, diabetes and cardiovascular or respiratory diseases, because people with these profiles are at greater risk of COVID-19 infection, with greater severity and lethality (Guan et al., 2020; Yang et al., 2020). However, data with this level of detail are not yet available.
The following types of healthcare units for first-response services and triage were considered: community health posts, basic health centers/units, polyclinics, general hospitals, specialized hospitals, mixed care units, general first aid posts, specialized first aid posts, and units for indigenous health response. As established by the Management Protocol for the New Coronavirus (Protocolo de Manejo para o Novo Coronavirus), “All patients who seek health services (Primary Health Response Units, Emergency Care Units, First Aid Posts, Mobile Pre-Hospital Service Units and Hospitals), must be submitted to clinical triage that includes early recognition of suspected cases, and if necessary, immediate referral of the patient to an area separated from those that contain respiratory and hand hygiene supplies” (free translation from Ministério da Saúde, 2020b).
Edict 1101 of June 12, 2002, and Resolution 7 of February 24, 2010.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.socscimed.2021.113773.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- Almeida D., Pinheiro L.B., Moschini L.E., Bogaert J. Brazil's vulnerability to COVID-19 quantified by a spatial metric. Public Health Practice. 2020;1 doi: 10.1016/j.puhip.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral P.V., Rocha T.A.H., Barbosa A.C.Q., Vissoci J.R.N. Spatially balanced provision of health equipment: a cross-sectional study oriented to the identification of challenges to access promotion. Int. J. Equity Health. 2017;16(1):209. doi: 10.1186/s12939-017-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas A., Cota W., Gómez-Gardeñes J., Gómez S., Granell C., Matamalas J.T., Soriano-Paños D., Steinegger B. Modeling the spatiotemporal epidemic spreading of COVID-19 and the impact of mobility and social distancing interventions. Phys. Rev. X. 2020;10(4) doi: 10.1103/PhysRevX.10.041055. [DOI] [Google Scholar]
- Barrozo L.V., Serafim M.B., de Moraes S.L., Mansur G. Hygeia-Revista Brasileira de Geografia Médica e da Saúde; 2020. Monitoramento espaço-temporal das áreas de alto risco de COVID-19 nos municípios do Brasil; pp. 417–425. [DOI] [Google Scholar]
- Barrozo L.V., Fornaciali M., de André C.D.S., Morais G.A.Z., Mansur G., Cabral-Miranda W., et al. GeoSES: a socioeconomic index for health and social research in Brazil. PloS One. 2020;15(4) doi: 10.1371/journal.pone.0232074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Groneberg D.A. Measuring spatial accessibility of health care providers - introduction of a variable distance decay function within the floating catchment area 834 (fca) method. PloS One. 2016;11 doi: 10.1371/journal.pone.0159148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil . Secretaria de Atenção à Saúde. Departamento de Regulação, Avaliação e Controle de Sistemas; 2015. Critérios e parâmetros para o planejamento e programação de ações e serviços de saúde no âmbito do Sistema Único de Saúde. Brasília: MS: Ministério da Saúde. [Google Scholar]
- Brasil . Secretaria de Atenção Primária à Saúde; 2020. Protocolo de Manejo Clínico do Coronavírus (Covid-19) na Atenção Primária à Saúde. Brasília: MS: Ministério da Saúde. [Google Scholar]
- Brasil . MS: Ministério da Saúde. Secretaria de Atenção Primária à Saúde; Brasília: 2020. Protocolo de Manejo Clínico para o Novo Coronavírus (2019-nCoV) [Google Scholar]
- Brasil . Secretaria de Atenção Primária à Saúde; 2020. Protocolo de Manejo Clínico para o Novo Coronavírus (2019-nCoV). Brasília: MS: Ministério da Saúde. [Google Scholar]
- Candido D.D.S., Watts A., Abade L., Kraemer M.U.G., Pybus O.G., Croda J., de Oliveira W., Khan K., Sabino E.C., Faria N.R. Routes for COVID-19 importation in Brazil. J. Trav. Med. 2020;27(taaa042) doi: 10.1093/jtm/taaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M.C., Massuda A., Almeida G., Menezes-Filho N.A., Andrade M.V., de Souza Noronha K.V.M., et al. Brazil's unified health system: the first 30 years and prospects for the future. Lancet. 2019;394:345–356. doi: 10.1016/S0140-6736(19)31243-7. 10195. [DOI] [PubMed] [Google Scholar]
- Castro M.C., Carvalho L. R. de, Chin T., Oliveira W. K. de. Demand for hospitalization services for COVID-19 patients in Brazil. MedRxiv. 2020 doi: 10.1101/2020.03.30.20047662. 2020.03.30.20047662. [DOI] [Google Scholar]
- Coelho F.C., Lana R.M., Cruz O.G., Gomes M.F.C. MedRxiv; 2020. Assessing the Potential Impact of COVID-19 in Brazil: Mobility, Morbidity and the Burden on the Health Care System. 2020.03.19.20039131. [DOI] [Google Scholar]
- Costa S.F., Giavina-Bianchi P., Buss L., Mesquita Peres C.H., Rafael M.M., dos Santos L.G.N., Bedin A.A., Francisco M.C.P.B., Satakie F.M., Jesus Menezes M.A., Dal Secco L.M., Rodrigues Caron D.M., de Oliveira A.B., de Faria M.F.L., de Aurélio Penteado A.S., de Souza I.O.M., de Fatima Pereira G., Pereira R., Matos Porto A.P., Levin A.S. Clinical Infectious Diseases; 2020. SARS-CoV-2 Seroprevalence and Risk Factors Among Oligo/asymptomatic Healthcare workers(HCW): Estimating the Impact of Community Transmission. ciaa1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D. Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health Place. 2010;16(5):1038–1052. doi: 10.1016/j.healthplace.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Delamater P.L. Spatial accessibility in suboptimally configured health care systems: a modified two-step floating catchment area (M2SFCA) metric. Health Place. 2013;24:30–43. doi: 10.1016/j.healthplace.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Fransen K., Neutens T., De Maeyer P., Deruyter G. A commuter-based two-step floating catchment area method for measuring spatial accessibility of daycare centers. Health Place. 2015;32:65–73. doi: 10.1016/j.healthplace.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Freire F.H.M.A., Gonzaga M.R., Gomes M.M.F. Projeções populacionais por sexo e idade para pequenas áreas no Brasil. Rev. Latinoamericana de Poblac. 2020;14(26):124–149. doi: 10.31406/relap2020.v14.i1.n26.6. [DOI] [Google Scholar]
- Geurs K., van Wee B. Accessibility evaluation of land-use and transport strategies: review and research directions. J. Transport Geogr. 2004;12(2):127–140. doi: 10.1016/j.jtrangeo.2003.10.005. [DOI] [Google Scholar]
- Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in lombardy, Italy: early experience and forecast during an emergency response. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Guan W., Liang W., Zhao Y., He J. Comorbidity and its impact on 1590 patients with covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- InfoGripe . 2020, March 4. Monitoramento de casos reportados de síndrome respiratória aguda grave (SRAG) hospitalizados.http://info.gripe.fiocruz.br/ Retrieved. [Google Scholar]
- JAXA . Japan Aerospace Exploration Agency (JAXA); 2011. ALOS PALSAR Digital Elevation Model Data.https://search.asf.alaska.edu Retrieved from ASF DAAC. [Google Scholar]
- Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health. 2020;8(4) doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet T. COVID-19: learning from experience. Lancet. 2020;395:1011. doi: 10.1016/S0140-6736(20)30686-3. 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legido-Quigley H., Mateos-García J.T., Campos V.R., McKee M. The resilience of the Spanish health system against the COVID-19 pandemic. Lancet Pub. Health. 2020 doi: 10.1016/S2468-2667(20)30060-8. 0(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.L., Pereira R.H., Prete C.A., Zarebski A.E., Emanuel L., Alves P.J., et al. Social and racial inequalities in COVID-19 risk of hospitalisation and death across São Paulo state. Brazil. medRxiv. 2020 doi: 10.1101/2020.12.09.20246207. [DOI] [Google Scholar]
- Luo W., Qi Y. An enhanced two-step floating catchment area (E2SFCA) method for measuring spatial accessibility to primary care physicians. Health Place. 2009;15(4):1100–1107. doi: 10.1016/j.healthplace.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Luo W., Wang F. Measures of spatial accessibility to health care in a GIS environment: synthesis and a case study in the chicago region. Environ. Plann. Plann. Des. 2003;30(6):865–884. doi: 10.1068/b29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K.A., Gaglioti A.H., Holt J.B., Croft J.B. Using spatially adaptive floating catchments to measure the geographic availability of a health care service: pulmonary rehabilitation in the southeastern United States. Health Place. 2019;56:165–173. doi: 10.1016/j.healthplace.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail Matthew R., Humphreys J.S. Measuring spatial accessibility to primary care in rural areas: improving the effectiveness of the two-step floating catchment area method. Appl. Geogr. 2009;29(4):533–541. doi: 10.1016/j.apgeog.2008.12.003. [DOI] [Google Scholar]
- McGrail Matthew Richard, Humphreys J.S., Ward B. Accessing doctors at times of need–measuring the distance tolerance of rural residents for health-related travel. BMC Health Serv. Res. 2015;15(1):212. doi: 10.1186/s12913-015-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas S.M., Shoukat A., Fitzpatrick M.C., Galvani A.P. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(16):9122–9126. doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutens T. Accessibility, equity and health care: review and research directions for transport geographers. J. Transport Geogr. 2015;43:14–27. doi: 10.1016/j.jtrangeo.2014.12.006. [DOI] [Google Scholar]
- Noronha K., et al. Belo Horizonte: Cedepar-Face-UFMG; 2020. Análise de demanda e oferta de leitos hospitalares gerais, UTI e equipamentos de ventilação assistida no Brasil em função da pandemia do COVID-19: Impactos microrregionais ponderados pelos diferenciais de estrutura etária, perfil etário de infecção e risco etário de internação (Nota Técnica) [Google Scholar]
- NTU . Associação Nacional das Empresas de Transportes Urbanos - NTU; Brasilia: 2020. COVID-19 e o transporte público por ônibus: Impactos no setor e ações realizadas. [Google Scholar]
- Paez A. Using google community mobility reports to investigate the incidence of COVID-19 in the United States. Transp. Findings. 2020:12976. doi: 10.32866/001c.12976. [DOI] [Google Scholar]
- Paez, A., Lopez, F. A., Menezes, T., … Pitta, M. G. da R. (n.d.). A spatio-temporal analysis of the environmental correlates of COVID-19 incidence in Spain. Geogr. Anal., n/a(n/a). doi:10.1111/gean.12241. [DOI] [PMC free article] [PubMed]
- Páez A., Scott D.M., Morency C. Measuring accessibility: positive and normative implementations of various accessibility indicators. J. Transport Geogr. 2012;25:141–153. doi: 10.1016/j.jtrangeo.2012.03.016. [DOI] [Google Scholar]
- Paez A., Higgins C.D., Vivona S.F. Demand and level of service inflation in Floating Catchment Area (FCA) methods. PloS One. 2019;14(6) doi: 10.1371/journal.pone.0218773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo Folha de S. Folha de S.Paulo; 2020, July 1. Ocupação de UTI volta a subir e supera 80% em 13 capitais.https://www1.folha.uol.com.br/cotidiano/2020/07/ocupacao-de-uti-volta-a-subir-e-supera-80-em-13-capitais.shtml Retrieved from. [Google Scholar]
- Pereira R.H.M., Braga C.K.V., Serra Bernardo, Nadalin V. Texto para Discussão IPEA; 2019. Desigualdades socioespaciais de acesso a oportunidades nas cidades brasileiras, 2019; p. 2535.http://www.ipea.gov.br/portal/images/stories/PDFs/TDs/td_2535.pdf Retrieved from. [Google Scholar]
- Petropoulos F., Makridakis S. Forecasting the novel coronavirus COVID-19. PloS One. 2020;15(3) doi: 10.1371/journal.pone.0231236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader B., Astley C.M., Sy K.T.L., Kraemer M.U.G. Geographic access to United States SARS-CoV-2 testing sites highlights healthcare disparities and may bias transmission estimates. J. Trav. Med. 2020 doi: 10.1093/jtm/taaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke J., Mu L. Spatial decompositions, modeling and mapping service regions to predict access to social programs. Geograph. Inform. Sci. 2000;6(2):105–112. doi: 10.1080/10824000009480538. [DOI] [Google Scholar]
- Rocha T.A.H., da Silva N.C., Amaral P.V., Facchini L.A. Access to emergency care services: a transversal ecological study about Brazilian emergency health care network. Publ. Health. 2017;153:9–15. doi: 10.1016/j.puhe.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Servo L., Andrade M., do Amaral P. Anais do XXI Encontro da Encontro da Associação Brasileira de Estudos Populacionais (ABEP) 2019. Análise das regiões de saúde no Brasil a partir do Pacto pela Saúde: adequação da regionalização e acesso geográfico. [Google Scholar]
- Souza W. M. de, Buss L.F., Candido D. da S., Carrera J.-P., Li S., Zarebski A.E., Pereira R.H.M., Prete C.A., de Souza-Santos A.A., Parag K.V., Belotti M.C.T.D., Vincenti-Gonzalez M.F., Messina J., da Silva Sales F.C., Andrade P., dos S., Nascimento V.H., Ghilardi F., Abade L., Gutierrez B., Faria N.R. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020;4(8):856–865. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- Wan N., Zou B., Sternberg T. A three-step floating catchment area method for analyzing spatial access to health services. Int. J. Geogr. Inf. Sci. 2012;26(6):1073–1089. doi: 10.1080/13658816.2011.624987. [DOI] [Google Scholar]
- Walter P.G., et al. Imperial College London; London: 2020. Report 12: The Global Impact of COVID-19 and Strategies for Mitigation and Suppression | CRUB.http://www.crub.org.br/blog/covid-19-reports-faculty-of-medicine-imperial-college-london/ Retrieved from. [Google Scholar]
- Who . World Health Organization; 2020. Addressing Human Rights As Key To the COVID-19 Response (No. WHO/2019-nCoV/SRH/Rights/2020.1)https://www.who.int/publications-detail/addressing-human-rights-as-key-to-the-covid-19-response Retrieved from. [Google Scholar]
- Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. 10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S.M.A., Cox J., Enwere G.C., Cutts F.T. The effect of distance on observed mortality, childhood pneumonia and vaccine efficacy in rural Gambia. Epidemiol. Infect. 2014;142(12):2491–2500. doi: 10.1017/S0950268814000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.