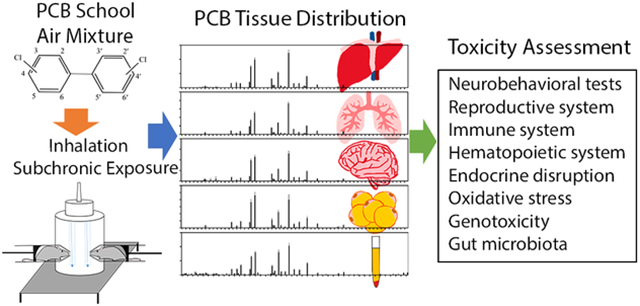
Abstract
Few in vivo inhalation studies have explored the toxicity of environmentally-relevant mixtures of polychlorinated biphenyls (PCBs). The manufacture of industrial PCBs was banned in 1978, but PCBs continue to be formed in industrial and consumer products. Schools represent a significant source of airborne exposures to legacy and non-legacy PCBs, placing children at risk. To evaluate the impact of these exposures, we generated an airborne mixture of PCBs, called the School Air Mixture (SAM), to match the profile of an older school from our adolescent cohort study. Female Sprague-Dawley rats were exposed either to SAM or filtered air in nose-only exposure systems, 4 h/day for 4 weeks. Congener-specific air and tissue PCB profiles were assessed using GC-MS/MS. PCB exposures recapitulated the target school air profile with a similarity coefficient, cos θ of 0.83. PCB inhalation yielded μg/g Σ209 PCB levels in tissues. Neurobehavioral testing demonstrated a modest effect on spatial learning and memory in SAM-exposed rats. PCB exposure induced oxidative stress in liver and lung, affected the maturational stages of hematopoietic stem cells, reduced telomerase activity in bone marrow cells, and altered the gut microbiota. This is the first study to emulate PCB exposures in a school and comprehensively evaluate toxicity.
Keywords: Aroclor 1254, inhalation toxicity, polychlorinated biphenyls, PCBs, school indoor air, subchronic exposure
Graphical Abstract
INTRODUCTION
Polychlorinated biphenyls (PCBs) are manmade mixtures that were used mainly as electrical insulating fluids in transformers, capacitors, and fluorescent light ballasts due to their distinct physicochemical properties. PCBs were also utilized as plasticizers in construction materials, including caulks, sealants, and adhesives. The manufacture of PCBs for industrial and commercial use was banned in the U.S. in 1978 but PCBs continue to be formed inadvertently. They appear as byproducts in pigments, paints and other building materials. Thus, there is both legacy inhalation exposure from Aroclors, Kanechlors and Clophens as well as exposure from non-legacy products. PCB-containing building materials were extensively used from the 1930s to 1970s, which coincided with the massive construction of U.S. schools.1 PCBs were detected in 88% of caulk samples collected in a variety of building types including schools from San Francisco Bay Area in 2010, with 40% exceeding U.S. EPA’s 50 ppm Toxic Substances Control Act criterion for bulk material.2
Slow and continuous volatilization of PCBs from light ballasts, caulks and sealants is the principal source of indoor PCBs. Although there are no national level data about the prevalence of PCB in indoor school air, some schools in the U.S. have been found to exceed EPA’s “exposure levels for evaluating PCBs in school indoor air” of 500 ng/m3 for middle school children.3–5 One school room in New York was found with an airborne ΣPCB concentration of 2,920 ng/m3.3 PCB-contaminated schools in Germany were measured with a peak airborne ΣPCBs level up to 20,800 ng/m3 using only six indicator congeners, which is 4–5 orders of magnitude greater than typical ambient air.5, 6 Schools therefore represent a significant source of airborne PCBs, placing children at particular risk.
The Airborne Exposure to Semi-volatile Organic Pollutants (AESOP) Study has been tracking a cohort of mother-child dyads living in East Chicago, Indiana and Columbus Junction, Iowa, USA since 2008.7 Findings from the AESOP Study indicate that inhalation exposure of lower-chlorinated PCB congeners is often higher than ingestion due to the elevated levels of airborne PCBs in older schools.5 Meanwhile, dietary exposure to PCBs has declined over the last several decades.8 Public concern has been raised for the potential toxicity of chronic inhalation of PCBs in schools, especially for children.9 Animal toxicology studies, usually at high doses, have revealed adverse effects of PCBs on neurodevelopment; immune, endocrine, and reproductive systems; and their demonstrated carcinogenic potency.10–12 Neurological effects of PCBs such as memory, learning, and intellectual impairment have been investigated in human and animal studies focused on prenatal PCB exposure.13–16 Less attention has been paid to the impact of long-term postnatal exposure to PCBs. Moreover, most exposure studies were conducted via ingestion or intraperitoneal injection using specific PCB congeners or commercial products (e.g., Aroclors). Evaluation of the toxicity of an environmentally-relevant school air PCB mixture delivered by inhalation is novel.
We exposed rats to a PCB school air mixture (SAM) resembling the profile of indoor air in an AESOP Study school built in 1968. Adverse outcomes on the nervous, reproductive, immune, hematopoietic, and endocrine system, as well as the gut microbiota and genotoxicity, were evaluated. PCB tissue burdens were quantified, and possible underlying cellular and molecular mechanisms were discussed. Using innovative nose-only inhalation toxicology approaches and novel methods to assess toxicities, we sought to identify the most sensitive outcomes or biomarkers among a wide range of toxicologic endpoints. With these comprehensive risk assessments, we can provide evidence to extrapolate to human risk.
MATERIALS AND METHODS
Generation of SAM Vapor.
Aroclors are formerly commercially-available PCB mixtures and are the sources of PCB contaminants in many older schools. A series of modeling efforts using the ideal gas law and congener vapor pressures, showed that Aroclor 1254 (A1254, Monsanto Lot KC-12–638, St. Louis, MO) would serve well as the base material for the generation of SAM vapor. A1254 was dissolved in dichloromethane and coated onto 3 mm diameter glass beads using a rotary evaporator (Buchi Rotavapor R-200, New Castle, DE) until dichloromethane had evaporated. High-efficiency particulate air (HEPA)-filtered laboratory air was passed through the glass beads at a flow rate of 12 L/min. The resultant PCB vapor-laden air was delivered to a mixing chamber before being supplied to a nose-only inhalation system. A sham exposure group was exposed to HEPA-filtered clean air in an identical nose-only exposure system in an adjacent lab with no supplied PCB. The PCB generating system and mixing chamber were kept at 25.0°C using a precision water bath. A sampling cartridge filled with Amberlite XAD-2 polymeric absorbent resin (Sigma-Aldrich, St. Louis, MO) was used to capture PCB vapor at a flow rate of 2.0 L/min for profile characterization. Each cartridge accumulated three days of exposure atmosphere, providing a time-weighted average PCB concentration. The schematic is illustrated in Supporting Information (SI) Figure S1.
Animals and Exposure.
All animal protocols were approved by our Institutional Animal Care and Use Committee. Animals were housed in a temperature and humidity-controlled room (21°C, 55% relative humidity) with a 12 h light/dark cycle and provided with food and water ad libitum. Considering rats have a very short period of adolescence (< two weeks), and females have a higher susceptibility to PCBs, adult female Sprague-Dawley rats (Envigo, Inc., Indianapolis, IN) were selected as our animal model. Rats weighing 239±8 g at the start were assigned to exposure group using a random number generator and simultaneously exposed to either SAM vapor (n=8) or filtered lab air (n=8) through nose-only inhalation systems. To minimize stress induced by nose-only restraint, we limited the daily exposure duration to 4 h. Positive control rats (n=6) for neurobehavioral testing were administered 1-bromopropane (1-BP, Sigma-Aldrich, St. Louis, MO) daily by gavage at a dose of 800 mg/kg for 12 days. Two sentinel rats were housed in the vivarium for health surveillance and showed no evidence of adverse health. Animal body weight was monitored biweekly. All animals were euthanized with carbon dioxide 22–26 hrs after last exposure. Whole blood was collected by cardiac puncture, and organs were excised and stored at −80°C for later analysis.
PCB Extraction and Quantification from Tissue and XAD.
The liver, lung, brain, white adipose tissue (from visceral adipose), and serum were collected for PCB quantification. PCBs in tissues and XAD from exposure monitoring were extracted using pressurized liquid extraction, as previously described.17 The concentrated extracts were quantified using gas chromatography with tandem mass spectrometry (GC-MS/MS). More details are described in SI.
Quality Assurance and Quality Control.
The quality of data was assured by method blanks, tissue blanks, and extraction recovery. Method blanks were carried out in parallel with each batch of sample extraction. Sample values were corrected based on surrogate recoveries. Method Detection Limit (MDL) was calculated from method blanks via the EPA formula:18
where is the mean of replicates from method blanks, t(n-1, 1-α = 0.99) is the Student’s t-test value for n-1 degree of freedom with 99% confidence level, and SD represents the standard deviation of the replicates. The MDL for each PCB congener or co-eluted congeners are listed in SI Table S1.
Neurobehavioral Testing.
The Morris Water Maze (MWM) and a video capture system (ANY-maze, Stoelting Co., Wood Dale, IL) were used to evaluate spatial learning and memory six days before necropsy with five days spatial acquisition testing and one day probe trial (see SI for more details). In brief, during the spatial acquisition test, rats were released into a tank filled with water until they found the hidden platform. The swimming distance and duration were recorded. On probe trial, the hidden platform was removed, the number of times they crossed where the platform had been located and the time spent in that target quadrant were recorded. The MWM was conducted 2 hours before the exposure and the test was carried out by an analyst blinded with regard to exposure groups.
Toxicity Assessment.
Cytokine expression in plasma was measured using the Bio-Plex Pro assay with a panel of 12 cytokines (Bio-Rad, Hercules, CA). The thyroid hormones, total triiodothyronine (T3), and thyroxine (T4) in serum were analyzed by MSU Veterinary Diagnostic Laboratory (Lansing, MI). Redox status including lipid peroxidation products malondialdehyde (MDA, Cayman Chemical, Ann Arbor, MI) and 4-hydroxynonenal (4-HNE), reactive oxygen species and reactive nitrogen species, ROS/RNS (Cell Biolabs, San Diego, CA) in tissue homogenate were evaluated following the manufacturers’ protocols. Blood redox parameters and redox potentials were quantified by high-performance liquid chromatography (HPLC) at the Division of Gastroenterology, Hepatology and Nutrition (Louisville, KY). Antioxidant enzyme activities in the liver, including glutathione peroxidase 1 (GPx1), glutathione peroxidase 4 (GPx4), and catalase (CAT), were assayed by the Free Radical and Radiation Biology Lab19, 20 (University of Iowa, Iowa City, IA) (see SI for methodologic details). To explore the relationship of selenium-dependent enzymes GPx1 and GPx4 with selenium and other trace metals, four trace metals were quantified using inductively coupled plasma-mass spectrometry (ICP-MS). Protein content was determined by the Bradford assay (Bio-Rad, Hercules, CA) using bovine serum albumin for data normalization.
To observe the genotoxicity of the PCB mixture, whole blood micronuclei (MN) were counted per 1000 reticulocytes on acridine orange-coated slides under fluorescence microscopy. We isolated bone marrow cells and performed a colony-forming cell (CFC) assay to measure the absolute number of viable and functional progenitor cells in the bone marrow as described in the SI. In brief, bone marrow cells were washed with IMDM/2% FBS and added to Methycellulose Complete Media without Epo (R&D Systems, Minneapolis, MN) to set the final cell number at 3×104 cells/ml. Then cells were plated in culture dishes and incubated at 37°C, 5% CO2 for 12 days prior to enumeration. Telomerase activity was determined by a highly sensitive PCR-based telomerase assay: the telomeric repeat amplification protocol (TRAP) assay as previously described.21 The gene expression level of telomerase reverse transcriptase (Tert), telomeric repeat binding factors 1 and 2 (Trf-1 and Trf-2) were measured by quantitative real-time PCR (qRT-PCR) to explore the effects on telomerase in bone marrow hematopoietic stem cells (HSCs) and progenitor cells. (See SI for detailed methods.)
The accessory lobe of right lung, the left lateral lobe of liver, brain, thymus, kidney, and reproductive organs were embedded in paraffin and evaluated for histomorphologic changes via hematoxylin and eosin (H&E) staining by a board-certified veterinary pathologist. In addition, numbers of ovarian follicles in different stages were enumerated. Fecal pellets were collected every week for gut microbiota analysis (see SI for more details). In brief, DNA was extracted from colon fecal samples. PCR amplicon libraries were created using primers specific for the V4 region of the 16S rRNA encoding gene and DNA sequence data were generated using Illumina MiSeq (Illumina, San Diego, CA). The 16S rRNA sequences were categorized into operational taxonomic units (OTUs) using the standard of 97% identity and QiiME2 were used to produce taxonomic profiles and metadata files.
Statistical Methods.
The differences among means were compared using one-way or two-way ANOVA with Bonferroni post hoc testing. The student’s t-test was used if only comparing sham- and SAM-exposed groups. P-values less than 0.05 were considered significant (SPSS 25, IBM Inc., Armonk, NY).
RESULTS AND DISCUSSION
PCB Vapor Profile.
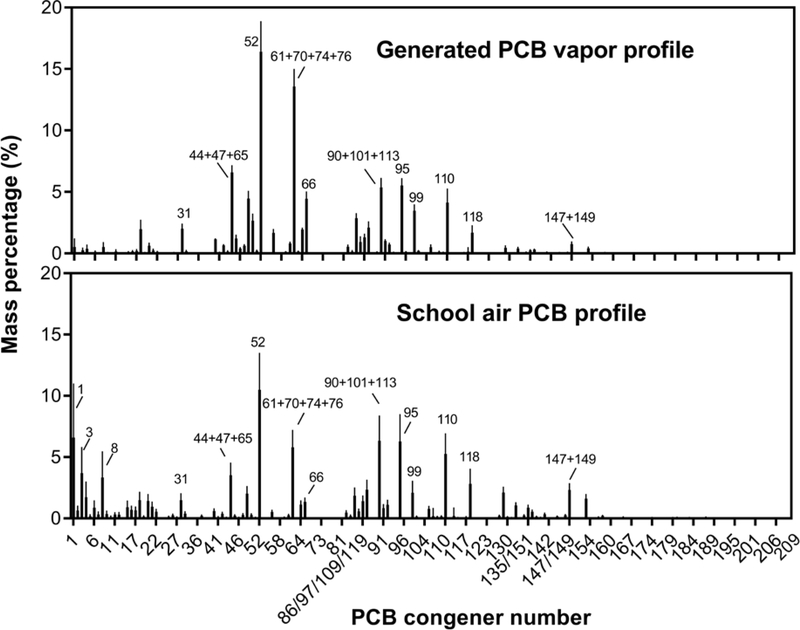
A1254 is frequently detected in caulks and sealants at older schools and many other buildings.2, 22 We generated a PCB vapor from A1254 with a concentration of 62.1±17.7 μg/m3 total PCB, which is comparable with some schools contaminated with PCBs ranging between 0.69 and 20.8 μg/m3.3, 6 The vapor was dominated by tetra- and penta-chlorinated biphenyls. Congeners 52, 66, 95, 99 and 110 and co-eluting PCBs 44+47+65, 61+70+74+76, and 90+101+113 accounted for approximately 90% of total PCBs (Figure 1).
Figure 1.
PCB congener profile of generated PCB vapor and school air in East Chicago. Values were expressed as mean±standard deviation (n=9 for generated PCB vapor, n=12 for school air). Major PCB congeners or co-eluting congeners were labeled with congener numbers.
Congener- and homolog-specific vapor profiles indicated a stable generation profile albeit with some depletion of mono-, di-, and tri-CB over time (Figure S2 and S3). Tetra- and penta-CBs, which comprised 61% of total PCBs, were also the major homologs in indoor school air (Figure 1). However, in our generated vapor, several tetra-CBs were slightly overrepresented including PCB 44+47+65, 52, 61+70+74+76, and 66, while a few lower chlorinated biphenyls (PCB 1, 3 and 8) were underrepresented (Figure 1). Nona- and deca-CBs were not detected either in school air or in generated PCB vapor due to their exceedingly low vapor pressure. Our generated PCB vapor strongly resembled school air with cosθ of 0.83 (cosθ=1 represents identical profiles). We estimated the dose of PCBs as 237 μg/kg based on the exposure concentration (μg/m3); disposition rate of PCB (α=0.998 based on our previous study23); and air volume each rat breathed during the exposure period (95 breaths/min at 1.5 mL/breath for 250 g rats, 4 h/day for 28 days).24
PCB Distribution in Tissues.
Many PCB congeners were retained in tissues after subchronic inhalation exposure to SAM vapor. Total PCB concentration in tissues of SAM-exposed rats was significantly higher than that in shams (p<0.001), ranging from 2-fold (serum) to 100-fold (adipose) higher (Table 1). The most bioaccumulation occurred in adipose tissue as a result of the lipophilicity of PCBs. Sham tissues were comparable to sentinels, which indicated no additional PCBs were introduced other than the unavoidable trace levels received through diet and background PCBs in air.
Table 1.
Total PCB concentration, Toxic Equivalency (WHO-TEQ), and Neurotoxic Equivalency (NEQ)25 (in ng/g lipid weight) in tissues after subchronic inhalation of SAM vapor.
| Sentinel |
Sham |
SAM |
|||
|---|---|---|---|---|---|
| ΣPCB | ΣPCB | ΣPCB | WHO-TEQ | NEQ | |
| Liver | 115 | 145 ± 9 | 1290 ± 77*** | (3.44 ± 0.25) × 10−3 | 330 ± 30 |
| Lung | 308 | 329 ± 21 | 3420 ± 170*** | (9.91 ± 0.75) × 10−3 | 853 ± 51 |
| Brain | 69.2 | 87.7 ± 3.7 | 760 ± 35*** | (2.05 ± 0.15) × 10−3 | 192 ± 22 |
| Adipose | 37.1 | 47.7 ± 5.9 | 5090 ± 581*** | (1.23 ± 0.22) × 10−2 | 1390 ± 175 |
| Serum | 2151 | 1736 ± 86 | 3340 ± 150*** | (1.38 ± 0.15) × 10−2 | 920 ± 62 |
Values are mean ± standard error (n=2 for sentinel, n=8 for sham and SAM).
p < 0.001, compared to sham, Student’s t-test.
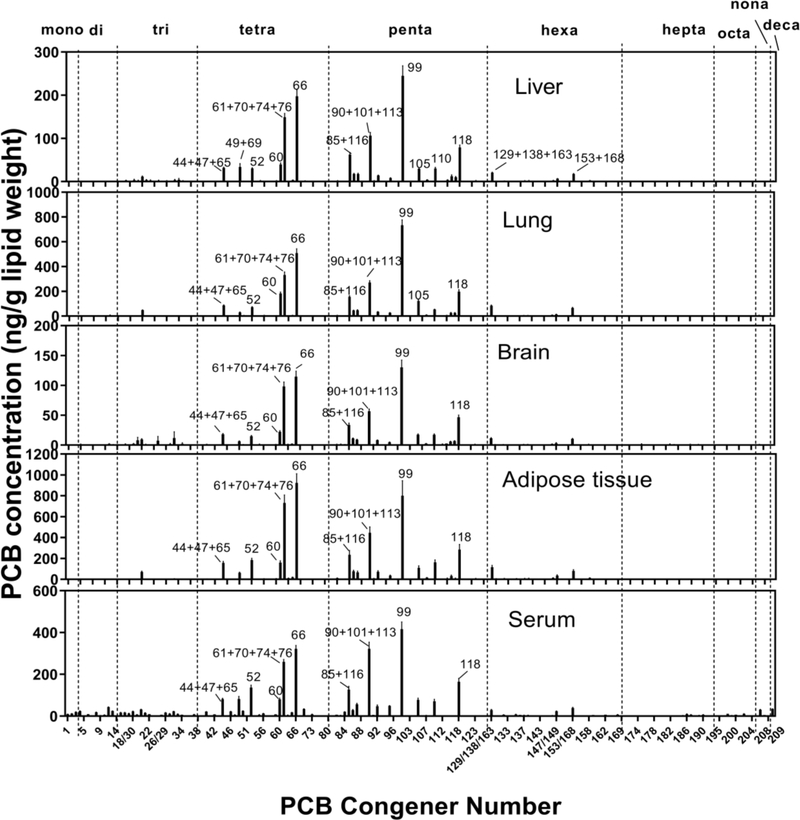
PCB congener-specific profiles were quantified in different tissues (see SI Table S3.1–S3.10 for profiles normalized by wet weight and lipid weight). Generally, the PCB profiles were similar, with leading congeners being PCB 61+70+74+76, 66, 85+116, 90+101+113, 99, and 118 (Figure 2). Minor differences among tissues were observed in terms of PCB composition. For example, PCB 114, 115, and 117 had a larger proportion in serum than in any tissues; several lower CBs were detected only in serum. The PCB homolog profile of tissues is shown in Figure S4 and indicates that tetra- and penta-CBs dominated in all tissues accounting for more than 90% of total PCBs. In comparison with inhaled PCB vapor, higher chlorinated PCBs (penta, hexa, and hepta) were preferentially retained by tissues.
Figure 2.
PCB distribution (ng/g lipid weight) in tissues of rats exposed to SAM. Graphs show mean ± standard error (n=8 for liver, lung, adipose, and serum, n=5 for brain). Major PCB congeners are labeled with congener numbers. PCB homolog groups are indicated on top.
Minor differences between tissues likely resulted from the differential distribution of cytochrome P450 (CYP) enzymes involved in the metabolism of PCBs. The rate and extent of PCB metabolism depends on the number and location of chlorines on the biphenyl molecule.26 Generally, higher-chlorinated congeners are metabolized more slowly. In addition, CYPs most effectively biotransform PCBs with unsubstituted carbons at meta- and para- positions on at least one ring. As a result, PCB31 (2,4’,5-trichlorobiphenyl), PCB52 (2,2’,5,5’-tetrachlorobiphenyl), and PCB95 (2,2’,3,5’,6-pentachlorobiphenyl) are retained less in tissues due to their faster metabolic rate even though they prevail in the vapor. In contrast, PCB66 (2,3’,4,4’-tetrachlorobiphenyl), PCB74 (2,4,4’,5-tetrachlorobiphenyl), PCB99 (2,2’,4,4’,5-pentachlorobiphenyl), and PCB118 (2,3’,4,4’,5-pentachlorobiphenyl) accumulated in tissues as a result of slower metabolism (Figure 2).
To estimate the body burden of dioxin-like PCBs, we calculated the toxic equivalency (WHO-TEQ) using the revised toxic equivalency factors (TEF).27 The TEQ has a descending concentration in serum>adipose tissue>lung>liver>brain (Table 1), and the value was mainly contributed by PCB118 and 105. The Neurotoxic Equivalent (NEQ) values were developed in a manner similar to the better-known TEF values by Simon et al. (2007), which allows a convenient measure to evaluate the neurological effects related to the Ca2+ homeostasis.25 NEQ showed a descending order of adipose tissue>serum>lung> liver>brain with major contributions from PCB 61+70+74+76, 66, 99 and 118.
Cognitive Dysfunction Revealed by Morris Water Maze (MWM) Testing.
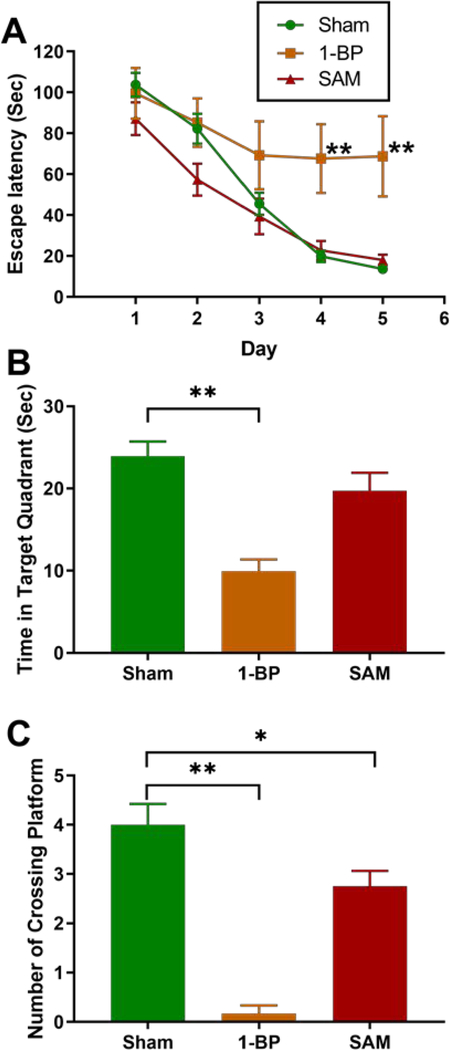
The 1-BP group (as positive control28) exhibited longer escape latency at day 4 (p=0.002) and day 5 (p=0.0002) of the spatial acquisition test, compared to the sham group. However, there were no significant differences between the SAM-exposed group and the sham group (Figure 3A). As shown in Figure 3B, in the probe trial, the swimming time in the target quadrant was lower in the 1-BP group (p=0.0002) compared to the sham group. Moreover, SAM and 1-BP groups had significantly fewer platform crossings than shams (Figure 3C, p=0.043, p<0.0001, respectively) which indicates that reference memory was compromised with SAM or 1-BP exposure.
Figure 3.
Effects of SAM and 1-BP on spatial learning and memory using the Morris Water Maze test. Data expressed as mean ± standard error (n=8 for SAM and 1-BP, n=6 for sham). *p < 0.05, **p < 0.01, vs. sham group. Figure 3A: two-way repeated-measures ANOVA; Figures 3B to 3C: one-way ANOVA with Bonferroni correction for multiple comparisons.
Several studies have investigated the neurodevelopmental toxicity of prenatal exposure to PCBs in the human population and animal studies. Neurological effects of in utero exposure to PCB mixture on infants and young children include impaired short-term memory, reduced IQ score, poor focused attention and comprehensive ability.13–15, 29–32 However, the neurological effects of postnatal PCB exposure are less investigated. In our study, we used MWM to assess neurotoxicity and we observed postnatal exposure to SAM led to modest memory impairment indicated by fewer platform crossings. The hippocampus plays an important role in learning and memory.33, 34 However, sections of the brain stained with H&E showed no gross histopathological changes in the hippocampus of SAM-exposed rats, indicating no severe damage was associated with this dose of PCB. Memory impairment of PCBs could arise from decreased thyroid hormone, which is essential for brain development and function. Thyroid hormone reduction was observed in several PCB exposure studies.35–37 Nevertheless, thyroid hormones (total T4 and T3) were not significantly reduced in our PCB-exposed rats (Figure S7). Another possible mechanism of neurological toxicity is that PCB might disrupt the metabolism of neurotransmitters in the brain, e.g. serotonin, dopamine, and acetylcholine.35, 38–41 Overall, our finding of PCB-induced learning and memory impairment in adult rats is consistent with population42 and animal studies43 while the underlying mechanisms need further study.
Toxicity Assessment.
Body weight (bw) gains in both groups were normal over the 28 days of exposure and no significant changes were observed between SAM- and sham-exposed groups (Figure S5). The relative kidney weight (g kidney/g bw) of SAM was significantly higher than that of shams (p=0.022) (Figure S5). No significant changes were observed in liver or thymus weights.
Cytokines in plasma from the SAM group indicated a small, non-significant elevation (IL-2, IL-5, IL-6, IL-12, and TNF-α) compared to the sham group (Figure S6). No significant differences were observed in thyroid hormones (Figure S7) in serum between sham and SAM groups. Experimental evidence of PCB-related thyroid disruption has been described in cohort studies.44 PCB exposures led to hypothyroidism and thyroid hormone (total T4 and T3) inhibition.45, 46 However, in our study, no significant changes in total T4 or T3 were observed in PCB-treated rats suggesting that thyroid hormone disruption may not be the most sensitive toxicity endpoint for PCBs. Our previously conducted study showed a significant reduction of total T4 using a mixture of Aroclor 1242 and Aroclor 1254 supplemented with PCB 11. However, the PCB exposure concentration was 9-fold higher in that study (533±93 μg/m3, compared to 62.1±17.7 μg/m3 in the present study).47 It is also noteworthy that lower chlorinated congeners were minimal which may explain the lack of changes in thyroid hormone. Heiger-Bernays et al.46 reported thyroid dysfunction risk was associated with exposure of airborne PCBs, especially lower chlorinated congeners. We can conclude that lower exposure concentrations and the school profile of PCBs represented mainly by tetra- (57%) and penta- (31%) chlorinated congeners, as seen in our SAM vapor did not induce endocrine disruption after subchronic exposure.
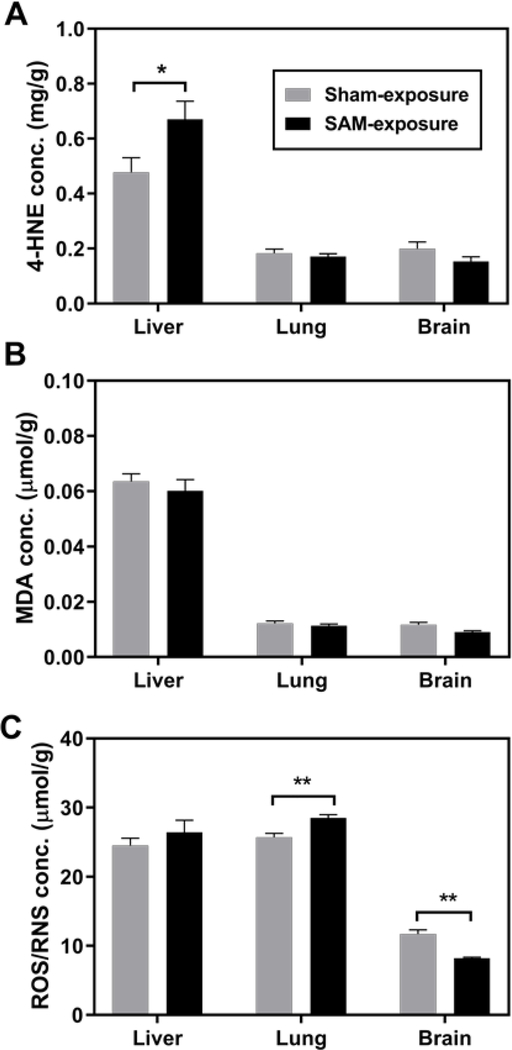
Oxidative stress is a potential mechanism of PCB-induced toxicity. Free radicals or reactive oxygen species (ROS) induced by PCB and metabolites may result in significant damage to cell structure, e.g., DNA, lipid, and protein. MDA and 4-HNE are lipid peroxidation products that may play a cytotoxic role by inhibiting gene expression and inducing cell death.48 Livers from SAM-exposed rats showed signs of oxidative stress indicated by elevated 4-HNE (p=0.039, Figure 4A), whereas another lipid peroxidation product MDA did not significantly differ between SAM and sham (p=0.442, Figure 4B). Our results imply that the production of different lipid peroxidation products may be generated independently. In addition, elevated ROS/RNS concentration was observed in the lungs of SAM-exposed rats (p=0.003, Figure 4C). However, a surprising reduction of ROS/RNS was observed in SAM-exposed rat brains (p=0.0004), suggesting a unique redox environment in the brain. The lung with more PCB deposition gave rise to a higher load of ROS/RNS. In the liver, activities of antioxidant enzymes including catalase, GPx1, and GPx4 (Figure S8), trace metal content (Figure S9), and the whole blood redox parameters (Table S4) showed no difference between SAM- and sham-exposed groups (p>0.05).
Figure 4.
Biomarkers of oxidative stress including 4-HNE (Figure 4A), MDA (Figure 4B), and ROS/RNS (Figure 4C) concentration in tissue homogenate after subchronic exposure to SAM vapor. Values are normalized by protein content and show mean ± standard error (n=8 for liver and lung, and n=5 for brain). *p < 0.05, **p < 0.01, Student’s t-test, compared to sham exposure.
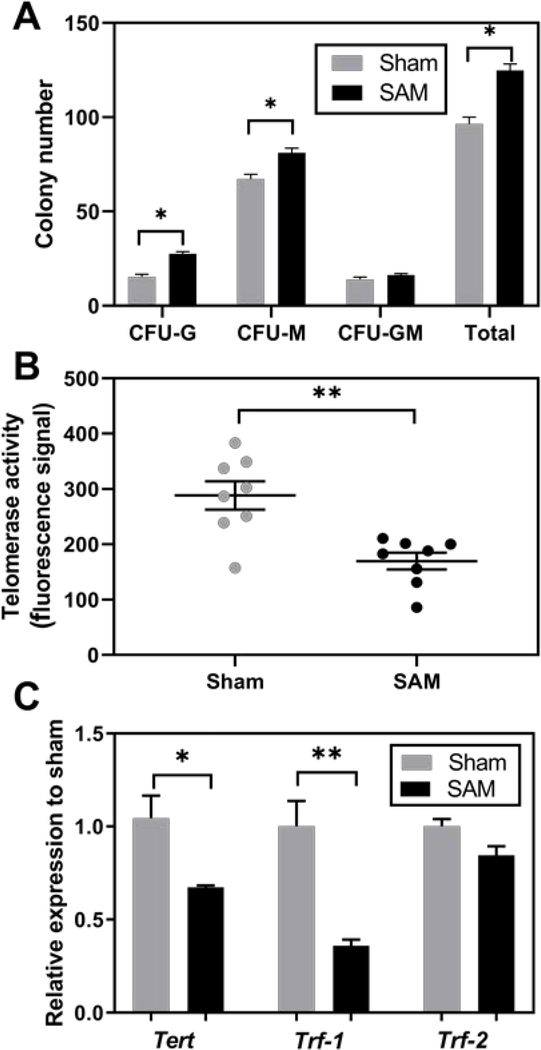
No significant differences were observed in whole blood micronuclei between SAM-exposed rats and shams (p=0.60, Figure S10) and there was a low count (less than 1 per 1000) of reticulocytes in every sample. Hematopoietic stem cells undergo various intermediate maturational stages generating multi-potential and lineage-committed progenitor cells before reaching maturity. Inhaled PCBs as compared to sham-exposed control rats have the potential to affect the maturational stages and induce modification of the differentiation pattern towards the myelocytic pathway. SAM exposure significantly increased the total colony-forming units (CFU) numbers compared to the sham group (p<0.0001), increasing the number of granulocyte CFU (CFU-G) and macrophage CFU (CFU-M) (p<0.0001, p=0.0006, Figure 5A), whereas, granulocyte-macrophage CFU (CFU-GM) was not significantly changed (p=0.129).
Figure 5.
Colony-forming cell counts in bone marrow (Figure 5A) with each bar mean ± standard error (n=16). Figure 5B shows telomerase activity described in fluorescence signal per 200μg protein. Figure shows mean ± standard error (n=8) with each dot representing an individual value. Figure 5C shows expression of Tert, Trf-1, and Trf-2 in bone marrow relative to sham-exposed rats as mean ± standard error (n=4). *p < 0.05, **p < 0.01, Student’s t-test, compared to sham exposure.
Telomerase is necessary to keep telomeres at the proper length and retention of telomerase activity is an important characteristic of stem cells or progenitor cells. Telomerase activity is typically reduced when cells stop proliferating or differentiate into mature tissue cells. Interference of telomere maintenance may lead to premature aging, immune suppression and different types of cancers such as leukemia and non-Hodgkin lymphoma.49 SAM exposure significantly suppressed telomerase activity in bone marrow cells (p=0.0013, Figure 5B). Our previous study has demonstrated PCB28, PCB52 and a synthetic Chicago Air Mixture (CAM) significantly reduced telomerase activity.21 TERT is the limiting factor responsible for catalyzing the addition of nucleotides TTAGGG to the ends of a chromosome. TRF-1 and TRF-2 are members of the sheltering complex that protect the chromosome ends. The ultimate function of TRF-1 is inhibition of telomerase, suppression of telomere elongation, and stabilization of telomere length. TRF-2 is a down-regulator of telomere length, playing a key role in blocking the ATM signaling pathway to prevent chromosome fusion. Inhaled PCBs may exhibit hematotoxicity by negatively affecting the immortality of the telomerase in hematopoietic stem cells. Our results showed a significant downregulation of Tert and Trf-1 gene expression in bone marrow cells after SAM exposure (p=0.022, and p=0.004, respectively, Figure 5C).
Brain histopathology evaluation showed no morphologic features of necrosis or apoptosis for neurons in any of the examined areas. No overt group-specific lesions were observed in the liver, lung, thymus and kidney in either SAM- or sham-exposed rats. There were no noticeable pathological changes in ovary and uterus in SAM-exposed rats, yet primary follicle numbers in rats exposed to SAM were significantly reduced in comparison to sham-exposed rats (p=0.046, Figure S11).
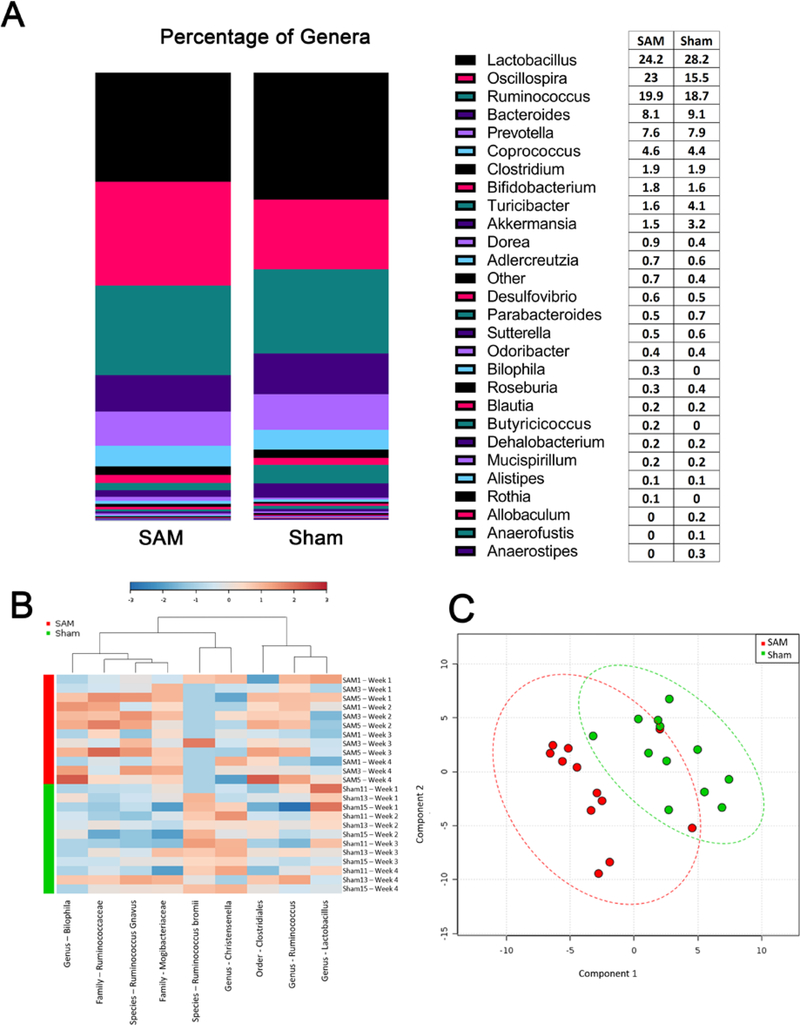
The gut microbiome plays an essential role in maintaining energy balance, immune function, neurological conditions, and overall health.50–52 Fecal microbiota were evaluated weekly and are presented in Figure 6. These data were analyzed through partial least squares discriminant analysis (PLS-DA) score plot, relative abundance, and heat map. The heat map was graphed by the top features throughout all taxonomic ranks (Figure 6B). We saw differences between treatment groups by different abundances. For instance, Ruminococus bromii were decreased in SAM-treated rats, while conversely, Mogibacteriaceae were increased in SAM-treated rats as compared to sham rats. The PLSDA score plot, which shows the diversity between treatment groups (Figure 6C), indicated a separation of the two groups. Conversely, the relative abundance of genera plot shows that on average, SAM and sham treatments contained the same relative ratio of each genus of bacteria. However, SAM and sham groups do have bacterial genera that are absent in the opposite treatment group (Figure 6A). Thus, we find evidence that PCB exposure leads to fecal microbiota dysbiosis. Studies have shown PCB exposure via ingestion disrupted the gut microbiota, manifested by imbalanced composition and reduced diversity.53–55 However, the mechanism of how inhalation exposure of PCBs induces gut microbiota dysbiosis is unknown.
Figure 6.
Gut microbiota analysis in SAM and sham-exposed rat feces. Relative abundance of genera (n=3) is shown in Figure 6A. Figure 6B illustrates heat map of fecal microbiome bacteria collected weekly (n=3). Data were analyzed based on statistically significant taxa determined through ANOVA on all of the taxonomic ranks. Blue = low abundance while Red = high abundance. Figure 6C exhibited Partial Least Squares Discriminant Analysis (PLS-DA) of fecal microbiome. Component 1 accounts for 25.8% variance while component 2 explains 24.1%.
Outside our laboratory, few researchers have investigated the inhalation toxicity of PCB congeners or mixtures. The dosimetry and adverse effects from prior studies are summarized in Table 2 – those conducted in our lab are indicated in bold typeface. In a 1956 publication, Treon et al.56 reported that inhaling a massive dose of 59,000 μg/kg bw of Aroclor 1254 vapor led to degenerative lesions in liver. Hu et al.57 found inhalation exposure to A1242 vapor at 6600 μg/kg bw diminished the rats’ weight gain. Hu et al.47 also compared the toxicity of a Chicago air mixture (vapor from A1242, A1254 and PCB11) by nose-only and whole-body inhalation regimens at doses of 1320 and 1980 μg/kg bw, respectively. Diminished weight gain and decreased thyroid hormone T4 were observed in both regimens. Increased liver lipid peroxidation was only shown with the higher dose delivered nose-only. At a lower dose of 446 μg/kg bw, the effects on weight gain and T4 were no longer present, but we still observed minor changes in blood GSH/GSSG.17 Casey et al.58 observed adverse outcomes of A1242 at a much lower stated dose of 19.2 μg/kg bw, including histopathological changes in the thyroid and thymus, increases in serum T3 and T4, decrease in exploratory behavior, and diminished weight gain. However, as discussed previously,47 the Casey et al.58 study had significant shortcomings in its experimental design. Lombardo et al.59 reported hyperactivity in male rats after whole-body inhalation of A1248 at ∑PCB dose of 8.72 μg. No overt toxicity was found in our low dose studies exposing rats to individual PCB congeners, e.g., PCB 11 (7.2 μg/kg bw) and PCB 3 (150–180 μg/kg bw).60, 61
Table 2.
Summary of studies assessing the inhalation toxicity of PCB on rats. Studies in bold typeface were performed in our laboratory.
| Exposure Regimen | Animals | PCB |
Dose |
Observed effects | References | ||
|---|---|---|---|---|---|---|---|
| Vapor source | Conc. (μg/m3) | μg | μg/(kg b.w.) | ||||
| whole-body | rats | Aroclor 1254 | 1500a | 13000a | 59000a | Histopathologic lesions in liver | Treon et al. (1956)56 |
| whole-body | adolescent male rats | Aroclor 1242 | 0.9 | 3.8b | 19.2b | Histopathological changes in the thyroid and thymus, increases in serum T3 and T4, decrease in exploratory behavior, diminished weight gainc. | Casey et al. (1999)58 |
| nose-only | male rats | Aroclor 1242 | 8200 | 985 | 6600 | Diminished weight gain | Hu et al. (2010)57 |
| nose-only | female rats | Aroclor 1242 & 1254 | 520 | 134 | 446 | No effects in weight gain or thyroid hormone, only minor change in blood GSH/GSSG. | Hu et al. (2012)17 |
| nose-onlyd | male rats | PCB 11 | 106 | 1.8 | 7.2 | No overt toxicity | Hu et al. (2013)60 |
| nose-onlyd | female rats | PCB 3 | 2060 | 35 | 150–180 | No effects in serum chemistry parameters including T4, or 8-oxo-dG levels in urine. | Dhakal et al. (2014)61 |
| whole-body | female rats | Aroclor 1242, 1254 & PCB11 | 533 | 307 | 1320 | Diminished weight gain, decrease in T4 | Hu et al. (2015)47 |
| nose-only | 464 | 1980 | Diminished weight gain, decrease in T4, and increase in hepatic lipid peroxidation. | ||||
| whole-body | rats | Aroclor 1248 | 0.56 | 8.72e | -- | Hyperactivity in male rats | Lombardo et al. (2015)59 |
| nose-only | female rats | Aroclor 1254 | 62.1 | 59.3 | 237 | Modest impairment in spatial learning and memory, oxidative stress in liver and lung, reduced telomerase activity in bone marrow cells, altered the gut microbiota | This study |
Estimated by measuring hydrochloric acid formation after thermal decomposition of Aroclor vapor.
Estimated using a minute ventilation volume that is lower than reported by others in literature.
No statistical analysis was presented.
Acute exposure that lasted 2h.
Estimated using the same minute ventilation volume with our current study.
Our study has revealed that subchronic exposure to an environmentally-relevant PCB mixture at ΣPCB dose of 237 μg/kg bw induced subtle spatial learning and memory impairment, led to oxidative stress in liver and lung, reduced telomerase activity and downregulated Tert and Trf-1 gene expression in bone marrow cells, and altered gut microbiota dysbiosis. Nevertheless, no significant differences were observed in plasma cytokine expression, thyroid hormone levels in serum, redox potentials in plasma, antioxidant enzyme activities in liver, and micronuclei in blood between SAM- and sham-exposed rats. With many assessed outcomes, some differences could have arisen by chance. This was mitigated by the use of Bonferroni post hoc testing.
To date, this is the first study conducted using environmentally-relevant PCB mixtures and concentrations to mimic indoor air from a representative older school. This approach provides insight into the potential impact on school children from inhalation of airborne PCBs. The toxicity information obtained in this study suggests that 237 μg/kg bw by subchronic inhalation of a school air mixture approximates the lowest observed adverse effect level (LOAEL). Data generated in this research are available at https://doi.org/10.25820/data.006120.62
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Benjamin R. Steines for assistance in carrying out the study, Ezazul Haque for performing the ICP-MS analysis, Rachel F. Marek and Xueshu Li for laboratory training, and David K. Meyerholz for histopathological evaluation. This research was supported by grant NIH P42 ES013661 and conducted in laboratory facilities supported by the Environmental Health Sciences Research Center, grant NIH P30 ES005605.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
Abbreviations; methods of PCB extraction and quantification from tissues and XAD; methods of Morris Water Maze test; table of Method Detection Limit (MDL) for each PCB congener or co-eluted congeners; table of recovery rate (%) of surrogate standards in tissues and SAM vapor extraction; tables of PCB congener-specific concentrations in tissues in wet tissue weight and lipid weight; table of redox parameters and redox potentials in plasma; schematic of PCB generation and nose-only exposure system; figures of PCB congener and homologue composition in XAD; figure of PCB homolog composition in tissues of SAM-exposed rats and in generated SAM vapor; figure of body weight and relative tissue weight of rats; figures of cytokines, thyroid hormones concentration in serum; figures of antioxidant enzyme activities and trace elements content in liver; figures of micronuclei (MN) number in blood and ovarian follicle counts.
REFERENCES
- 1.Lewis L; Snow K; Farris E; Smerdon B; Cronen S; Kaplan J, Condition of America’s Public School Facilities: 1999. In US Department of Education, Office of Educational Research and Improvement, National Center for Education Statistics, 2000. [Google Scholar]
- 2.Klosterhaus S; McKee LJ; Yee D; Kass JM; Wong A, Polychlorinated biphenyls in the exterior caulk of San Francisco Bay Area buildings, California, USA. Environ Int 2014, 66, 38–43. [DOI] [PubMed] [Google Scholar]
- 3.Thomas K, Polychlorinated biphenyls (PCBs) in school buildings: sources, environmental levels, and exposures. In US Environmental Protection Agency, National Exposure Research Laboratory, Office of Research and Development: 2012. [Google Scholar]
- 4.MacIntosh DL; Minegishi T; Fragala MA; Allen JG; Coghlan KM; Stewart JH; McCarthy JF, Mitigation of building-related polychlorinated biphenyls in indoor air of a school. Environ Health 2012, 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marek RF; Thorne PS; Herkert NJ; Awad AM; Hornbuckle KC, Airborne PCBs and OH-PCBs Inside and Outside Urban and Rural U.S. Schools. Environ Sci Technol 2017, 51, (14), 7853–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liebl B; Schettgen T; Kerscher G; Broding HC; Otto A; Angerer J; Drexler H, Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int J Hyg Environ Health 2004, 207, (4), 315–24. [DOI] [PubMed] [Google Scholar]
- 7.Ampleman MD; Martinez A; DeWall J; Rawn DF; Hornbuckle KC; Thorne PS, Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol 2015, 49, (2), 1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann GM; Christensen K; Maddaloni M; Phillips LJ, Evaluating health risks from inhaled polychlorinated biphenyls: research needs for addressing uncertainty. Environ Health Perspect 2015, 123, (2), 109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osterberg D; Scammell MK, PCBs in schools--where communities and science come together. Environ Sci Pollut Res Int 2016, 23, (3), 1998–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seegal RF, Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit Rev Toxicol 1996, 26, (6), 709–37. [DOI] [PubMed] [Google Scholar]
- 11.Brown JF Jr.; Mayes BA; Silkworth JB; Hamilton SB, Polychlorinated biphenyls modulated tumorigenesis in Sprague Dawley rats: correlation with mixed function oxidase activities and superoxide (O2*) formation potentials and implied mode of action. Toxicol Sci 2007, 98, (2), 375–94. [DOI] [PubMed] [Google Scholar]
- 12.NTP, NTP toxicology and carcinogenesis studies of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) in female Harlan Sprague-Dawley rats (Gavage Studies). Natl Toxicol Program Tech Rep Ser 2006, (520), 4–246. [PubMed] [Google Scholar]
- 13.Jacobson SW; Fein GG; Jacobson JL; Schwartz PM; Dowler JK, The effect of intrauterine PCB exposure on visual recognition memory. Child Development 1985, 56, (4), 853–860. [PubMed] [Google Scholar]
- 14.Jacobson JL; Jacobson SW, Intellectual impairment in children exposed to polychlorinated biphenyls in utero. New England Journal of Medicine 1996, 335, (11), 783–789. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson JL; Jacobson SW; Humphrey HE, Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. The Journal of pediatrics 1990, 116, (1), 38–45. [DOI] [PubMed] [Google Scholar]
- 16.Yang D; Kim KH; Phimister A; Bachstetter AD; Ward TR; Stackman RW; Mervis RF; Wisniewski AB; Klein SL; Kodavanti PR; Anderson KA; Wayman G; Pessah IN; Lein PJ, Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect 2009, 117, (3), 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X; Adamcakova-Dodd A; Lehmler HJ; Hu D; Hornbuckle K; Thorne PS, Subchronic inhalation exposure study of an airborne polychlorinated biphenyl mixture resembling the Chicago ambient air congener profile. Environ Sci Technol 2012, 46, (17), 9653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.USEPA, Definition and procedure for the determination of the method detection limit, revision 2. In United States Environmental Protection Agency: Washington, DC: 2016. [Google Scholar]
- 19.Stolwijk JM; Falls-Hubert KC; Searby CC; Wagner BA; Buettner GR, Simultaneous detection of the enzyme activities of GPx1 and GPx4 guide optimization of selenium in cell biological experiments. Redox Biol 2020, 32, 101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson AR; O’Leary BR; Du J; Sarsour EH; Kalen AL; Wagner BA; Stolwijk JM; Falls-Hubert KC; Alexander MS; Carroll RS; Spitz DR; Buettner GR; Goswami PC; Cullen JJ, Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity. Cancer Res 2020, 80, (7), 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senthilkumar PK; Klingelhutz AJ; Jacobus JA; Lehmler H; Robertson LW; Ludewig G, Airborne polychlorinated biphenyls (PCBs) reduce telomerase activity and shorten telomere length in immortal human skin keratinocytes (HaCat). Toxicol Lett 2011, 204, (1), 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler M; Tremp J; Zennegg M; Seiler C; Minder-Kohler S; Beck M; Lienemann P; Wegmann L; Schmid P, Joint sealants: an overlooked diffuse source of polychlorinated biphenyls in buildings. Environ Sci Technol 2005, 39, (7), 1967–73. [DOI] [PubMed] [Google Scholar]
- 23.Hu X; Adamcakova-Dodd A; Thorne PS, The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ Int 2014, 63, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauderly JL, Respiration of F344 rats in nose-only inhalation exposure tubes. J Appl Toxicol 1986, 6, (1), 25–30. [DOI] [PubMed] [Google Scholar]
- 25.Simon T; Britt JK; James RC, Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regul Toxicol Pharmacol 2007, 48, (2), 148–70. [DOI] [PubMed] [Google Scholar]
- 26.Bandiera SM, Cytochrome P450 enzymes as biomarkers of PCB exposure and modulators of toxicity PCB: Recent Advances in Environmental Toxicology Health Effects. Lexington, KY: The University Press of Kentucky; 2001, 185–192. [Google Scholar]
- 27.Van den Berg M; Birnbaum LS; Denison M; De Vito M; Farland W; Feeley M; Fiedler H; Hakansson H; Hanberg A; Haws L; Rose M; Safe S; Schrenk D; Tohyama C; Tritscher A; Tuomisto J; Tysklind M; Walker N; Peterson RE, The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006, 93, (2), 223–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Z; Zeng T; Xie K; Zhang C; Chen J; Bi Y; Zhao X, Elevation of 4-hydroxynonenal and malondialdehyde modified protein levels in cerebral cortex with cognitive dysfunction in rats exposed to 1-bromopropane. Toxicology 2013, 306, 16–23. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y-CJ; Guo Y-L; Hsu C-C; Rogan WJ, Cognitive development of Yu-Cheng (‘Oil Disease’) children prenatally exposed to heat-degraded PCBs. Jama 1992, 268, (22), 3213–3218. [PubMed] [Google Scholar]
- 30.Stewart PW; Lonky E; Reihman J; Pagano J; Gump BB; Darvill T, The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect 2008, 116, (10), 1416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson JL; Jacobson SW; Padgett RJ; Brumitt GA; Billings RL, Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Developmental Psychology 1992, 28, (2), 297. [Google Scholar]
- 32.Patandin S; Lanting CI; Mulder PG; Boersma ER; Sauer PJ; Weisglas-Kuperus N, Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. The Journal of pediatrics 1999, 134, (1), 33–41. [DOI] [PubMed] [Google Scholar]
- 33.Squire LR, Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review 1992, 99, (2), 195. [DOI] [PubMed] [Google Scholar]
- 34.Tulving E; Markowitsch HJ, Episodic and declarative memory: role of the hippocampus. Hippocampus 1998, 8, (3), 198–204. [DOI] [PubMed] [Google Scholar]
- 35.Donahue DA; Dougherty EJ; Meserve LA, Influence of a combination of two tetrachlorobiphenyl congeners (PCB 47; PCB 77) on thyroid status, choline acetyltransferase (ChAT) activity, and short- and long-term memory in 30-day-old Sprague-Dawley rats. Toxicology 2004, 203, (1–3), 99–107. [DOI] [PubMed] [Google Scholar]
- 36.Morse DC; Wehler EK; Wesseling W; Koeman JH; Brouwer A, Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254). Toxicol Appl Pharmacol 1996, 136, (2), 269–79. [DOI] [PubMed] [Google Scholar]
- 37.Ness DK; Schantz SL; Moshtaghian J; Hansen LG, Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett 1993, 68, (3), 311–23. [DOI] [PubMed] [Google Scholar]
- 38.Enayah SH; Vanle BC; Fuortes LJ; Doorn JA; Ludewig G, PCB95 and PCB153 change dopamine levels and turn-over in PC12 cells. Toxicology 2018, 394, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fingerman SW; Short EC Jr., Changes in neurotransmitter levels in channel catfish after exposure to benzo(a)pyrene, naphthalene, and Aroclor 1254. Bull Environ Contam Toxicol 1983, 30, (2), 147–51. [DOI] [PubMed] [Google Scholar]
- 40.Seegal RF; Brosch KO; Bush B, Polychlorinated biphenyls produce regional alterations of dopamine metabolism in rat brain. Toxicol Lett 1986, 30, (2), 197–202. [DOI] [PubMed] [Google Scholar]
- 41.Mariussen E; Fonnum F, The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology 2001, 159, (1–2), 11–21. [DOI] [PubMed] [Google Scholar]
- 42.Schantz SL; Gasior DM; Polverejan E; McCaffrey RJ; Sweeney AM; Humphrey H; Gardiner JC, Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environmental Health Perspectives 2001, 109, (6), 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvakumar K; Bavithra S; Ganesh L; Krishnamoorthy G; Venkataraman P; Arunakaran J, Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: anxiolytic-like effects of quercetin. Toxicol Lett 2013, 222, (1), 45–54. [DOI] [PubMed] [Google Scholar]
- 44.Brouwer A; Longnecker MP; Birnbaum LS; Cogliano J; Kostyniak P; Moore J; Schantz S; Winneke G, Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ Health Perspect 1999, 107 Suppl 4, 639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winneke G; Walkowiak J; Lilienthal H, PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology 2002, 181–182, 161–5. [DOI] [PubMed] [Google Scholar]
- 46.Heiger-Bernays WJ; Tomsho KS; Basra K; Petropoulos ZE; Crawford K; Martinez A; Hornbuckle KC; Scammell MK, Human health risks due to airborne polychlorinated biphenyls are highest in New Bedford Harbor communities living closest to the harbor. Sci Total Environ 2020, 710, 135576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu X; Adamcakova-Dodd A; Lehmler HJ; Gibson-Corley K; Thorne PS, Toxicity Evaluation of Exposure to an Atmospheric Mixture of Polychlorinated Biphenyls by Nose-Only and Whole-Body Inhalation Regimens. Environ Sci Technol 2015, 49, (19), 11875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayala A; Munoz MF; Arguelles S, Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014, 2014, 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campisi J; Kim SH; Lim CS; Rubio M, Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol 2001, 36, (10), 1619–37. [DOI] [PubMed] [Google Scholar]
- 50.Turnbaugh PJ; Gordon JI, The core gut microbiome, energy balance and obesity. J Physiol 2009, 587, (Pt 17), 4153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tremlett H; Bauer KC; Appel-Cresswell S; Finlay BB; Waubant E, The gut microbiome in human neurological disease: A review. Ann Neurol 2017, 81, (3), 369–382. [DOI] [PubMed] [Google Scholar]
- 52.Kau AL; Ahern PP; Griffin NW; Goodman AL; Gordon JI, Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, (7351), 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petriello MC; Hoffman JB; Vsevolozhskaya O; Morris AJ; Hennig B, Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environ Pollut 2018, 242, (Pt A), 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chi Y; Lin Y; Zhu H; Huang Q; Ye G; Dong S, PCBs-high-fat diet interactions as mediators of gut microbiota dysbiosis and abdominal fat accumulation in female mice. Environ Pollut 2018, 239, 332–341. [DOI] [PubMed] [Google Scholar]
- 55.Cheng SL; Li X; Lehmler HJ; Phillips B; Shen D; Cui JY, Gut Microbiota Modulates Interactions Between Polychlorinated Biphenyls and Bile Acid Homeostasis. Toxicol Sci 2018, 166, (2), 269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treon JF; Cleveland FP; Cappel JW; Atchley RW, The toxicity of the vapors of aroclor 1242 and aroclor 1254. Am Ind Hyg Assoc Q 1956, 17, (2), 204–13. [DOI] [PubMed] [Google Scholar]
- 57.Hu X; Adamcakova-Dodd A; Lehmler HJ; Hu D; Kania-Korwel I; Hornbuckle KC; Thorne PS, Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ Sci Technol 2010, 44, (17), 6893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey AC; Berger DF; Lombardo JP; Hunt A; Quimby F, Aroclor 1242 inhalation and ingestion by Sprague-Dawley rats. J Toxicol Environ Health A 1999, 56, (5), 311–42. [DOI] [PubMed] [Google Scholar]
- 59.Lombardo JP; Berger DF; Hunt A; Carpenter DO, Inhalation of Polychlorinated Biphenyls (PCB) Produces Hyperactivity in Rats. J Toxicol Environ Health A 2015, 78, (18), 1142–53. [DOI] [PubMed] [Google Scholar]
- 60.Hu X; Lehmler HJ; Adamcakova-Dodd A; Thorne PS, Elimination of inhaled 3,3’-dichlorobiphenyl and the formation of the 4-hydroxylated metabolite. Environ Sci Technol 2013, 47, (9), 4743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhakal K; Uwimana E; Adamcakova-Dodd A; Thorne PS; Lehmler HJ; Robertson LW, Disposition of phenolic and sulfated metabolites after inhalation exposure to 4-chlorobiphenyl (PCB3) in female rats. Chem Res Toxicol 2014, 27, (8), 1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H; Flor S; Gosse LE; Klenov VE; and Stolwijk JM, Toxicity assessment data from subchronic inhalation exposure to a school air mixture (SAM) of PCBs. (dataset). University of Iowa. 2020. 10.25820/data.006120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.