Abstract
The supply of safe drinking and clean water is becoming increasingly challenging proposition throughout the world. The deployment of environmentally sustainable nanomaterials with unique advantages namely high efficiency and selectivity, earth-abundance, recyclability, low-cost of production processes, and stability, has been a priority although several important challenges and constraints still remained unresolved. Carbon nanomaterials namely activated carbon, multi-walled- and single-walled carbon nanotubes, have been developed and applied as adsorbents for wastewater treatment and purification; graphene and graphene oxide-based nanomaterials as well as carbon and graphene quantum dots-derived nanomaterials have shown significant promise for water and wastewater treatment and purification, especially, for industrial- and pharmaceutical-laden wastes. This review encompasses advanced carbonaceous nanomaterials and methodologies that are deployed for the elimination of contaminants and ionic metals in aqueous media, and as novel nanosorbents for wastewater, drinking and ground water treatment. Additionally, recent trends and challenges pertaining to the sustainable carbon and graphene quantum dots-derived nanomaterials and their appliances for treating and purifying wastewater are highlighted.
Keywords: Sustainable nanomaterials, Carbon nanotubes, Graphene, Carbon dots, Quantum dots, Wastewater treatment
GRAPHICAL ABSTRACT
Advanced nanomaterials for water treatment comprising carbon nanotubes, carbon- and graphene quantum dots and graphene-based nanomaterials, are highlighted.
1. Introduction
Water is one of the foremost environmental stress issues as resources of water are limited, and the population which depends on these constrained supplies is predictably growing. Conventional technologies for treating wastewater include ion-exchange, reverse osmosis, oxidation, adsorption, flocculation, ultra-filtration, sedimentation, membrane, and advanced oxidation processes (AOPs). Wastewater treatment approaches, including bio-processes, UV photolysis/photocatalysis, activated carbon adsorption, and ozonation, have been deployed for removing organic contaminants and pollutants from waters (Cai et al., 2018; Colmenares et al., 2016; Khan et al., 2020a; Nasrollahzadeh et al., 2020a; Padhye et al., 2014; Pakzad et al., 2019).
Nanomaterials can be employed for removing organic pollutants and pharmaceutically active compounds via processes such as adsorption, photocatalysis, AOPs, filtration, etc. (Cai et al., 2018; Nasrollahzadeh et al., 2020b, 2020c). There are numerous emerging contaminants in wastewater discharge which have adverse health effects namely pesticides, textile dyes, plasticizers, disinfection byproducts, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), various endocrine disrupting materials, pharmaceutical and personal care products (Bousselmi et al., 2004; Mozia et al., 2007; Rizzo et al., 2009). Per- and polyfluoroalkyl substances (PFASs) contaminants can be removed from aqueous environments by carbonaceous nanomaterials as adsorbents; electrostatic and hydrophobic interactions, hydrogen bonding and ligand exchange being the main mechanisms for the adsorption of PFOS and PFOA (Liu et al., 2019; Saleh et al., 2019). In this regard, innovative engineered nanomaterials are very promising for removing these hazardous contaminants, as they have high surface areas and remarkable reactivities (Zhang et al., 2019).
The development of “greener” and environmentally sustainable techniques namely using less hazardous and toxic solvents, the deployment of less chemical reagents, precursors and catalysts with fewer steps in the production pathways have been the core emphasis lately. Green chemistry and nanotechnology can potentially be applied to address emerging and critical challenges posed by various contaminants and microorganisms; novel and suitable approaches for safer destruction of hazardous contaminants and pollutants is imperative. Green nanotechnology may enhance the environmental sustainability of underlying procedures producing negative externalities, as attested by the application of renewable energy and hydrogen for water treatment. It has a significant potential for decreasing the costs and enhancing the efficiency in prevention and treatment of wastewater contaminants as exemplified by nanoscale filtration techniques, adsorption of pollutants on nanoparticles (NPs), and breakdown of contaminants by nanocatalysts (Carpenter et al., 2015; Chorawalaa and Mehta, 2015; Cloete et al., 2010; Ma et al., 2011; Meng et al., 2011; Nasrollahzadeh et al., 2020a; Sajjadi et al., 2020; Shatkin et al., 2013; Xie et al., 2005; Zhang et al., 2014, 2016). The need for potable fresh water is significantly increasing in both, developing and industrialized world and assorted micropollutants are entering in the water resources (Ali et al., 2017; Das et al., 2017, 2018; Khin et al., 2012; Simeonidis et al., 2016). Contemporarily, the conventional decontamination methods (e.g., chlorination and ozonation) utilize excessive amounts of chemicals, and can generate hazardous and toxic by-products (Gehrke et al., 2015; Sajjadi et al., 2020; Varma, 2016; Westerhoff et al., 2016).
Nanomaterials with their high surface area, significant chemical reactivity, mechanical properties, cost-effectiveness, and low power consumption, can meaningfully be applied for water treatment and remediation. These materials, endowed with well-defined and controllable morphologies of proper size and porosity, can be potential good adsorbents (Perez-Page et al., 2016; Yan et al., 2020).
Indeed, advanced nanomaterials are being applied in the development of economical and high-performance water treatment systems for ‘real-time’ and continuous monitoring of water quality; nanomaterials have been extensively utilized for the remediation, prevention and treatment of effluence. These recyclable materials can preserve the long-term water quality, accessibility and feasibility of water supply while constantly detecting the biological and chemical contamination from man-made, municipal or industrial waste. The next section discusses nanomaterials comprising graphene-based nanomaterials, carbon-based nanotubes, carbon- and graphene-derived quantum dots for the treatment of wastewater and removal of pharmaceutical contaminants (Fig. 1).
Fig. 1.
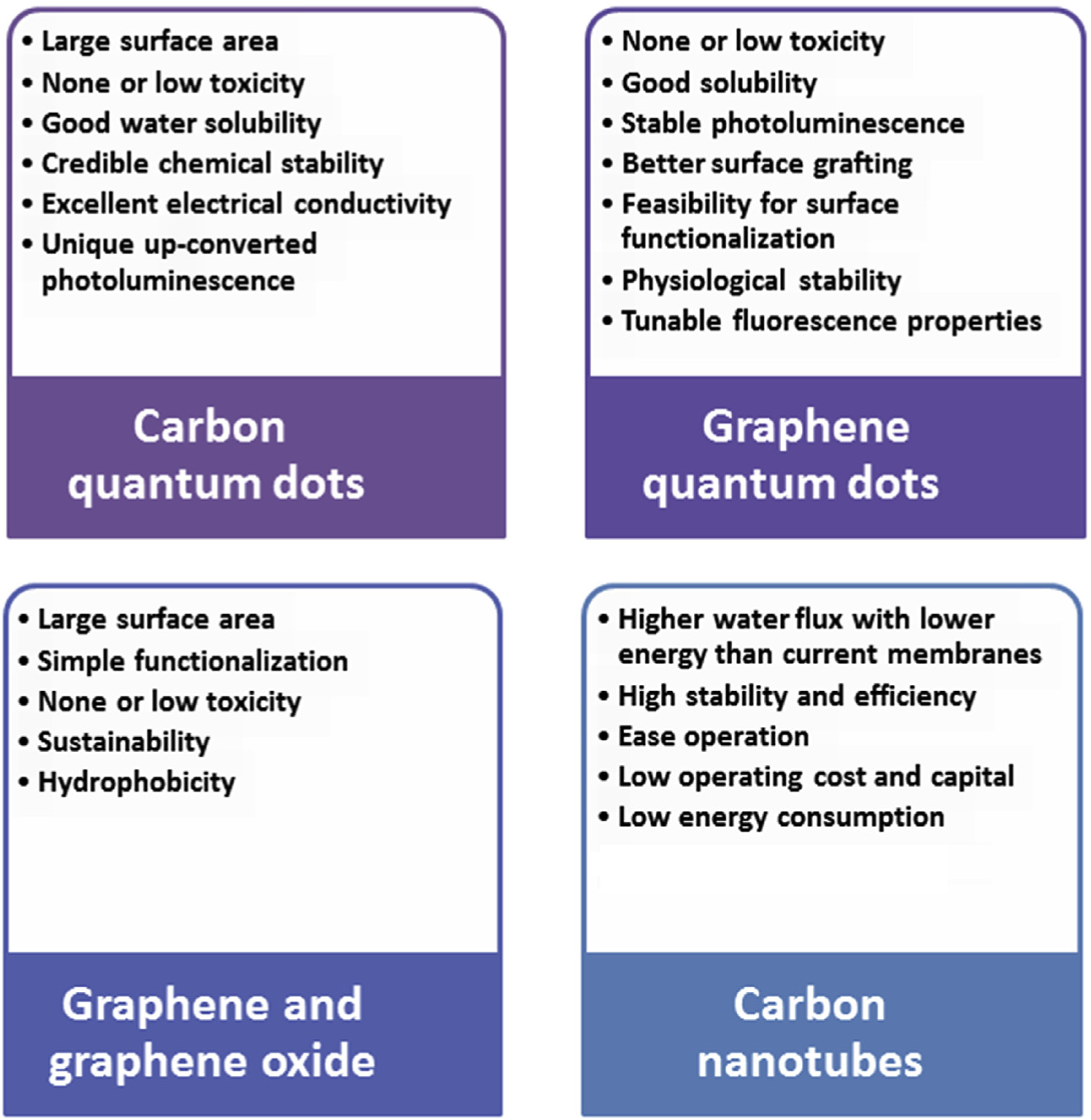
Specific advantages of diverse carbon-based nanomaterials.
It appears that these nanomaterials with their unique advantages can be considered as promising alternatives for wastewater treatment, although some significant challenges still persist namely potential health risks, higher cost of production, specific selectivity, sustainability, and recyclability (Lu and Astruc, 2020). Moreover, additional detailed studies should be focused on standardized analytical methodologies, removal kinetics, simulation models, and evaluation of environmental behaviors of hazardous contaminants, toxicity issues and the environmental effects of applied nanomaterials. Consequently, researchers have focused on the production of highly efficient and environmentally sustainable nanomaterials at an affordable cost for eco-friendly treatment of waters and wastewaters, especially comprising pharmaceuticals, endocrine disrupting chemical materials, common industrial wastes, pesticides, hazardous organic dyes, personal care products, detergents, and other emerging and recalcitrant pollutants (Lu and Astruc, 2020; Mukherjee et al., 2020).
This review underscores the advanced sustainable nanomaterials for the elimination of aqueous pollutants and contaminants using carbon-based nanomaterials, namely carbon nanotubes, graphene and its oxide, including carbon- and graphene quantum dots.
2. Carbon nanotubes (CNTs)
2.1. Chemistry and properties
High aqueous solubility of drugs can lead to their adsorption in target tissues/cells in humans and animals or plants; often they are extremely resistant to biodegradability and are stable under normal conditions. Each year, more than thousand tons of active drug and pharmaceutical components enter the environment, a large proportion being the unconsumed pharmaceuticals. Further, they are converted to active components anew during metabolism processes (Carballa et al., 2004) and urban sewage is especially contaminated by diverse pharmaceutical contaminants. Carbon-based (nano)materials have interestingly large surface-to-area ratio, superior chemical stability, low cost, and reduced chemical impact on the environment, and these properties render them favorable for the treatment (Hu et al., 2011; Konicki et al., 2012; Wei et al., 2013; Yang et al., 2011). Carbon nanotubes (CNTs), and carbon allotropes with a cylindrical nanostructure, can be applied for eliminating the pharmaceutical-derived contaminants (Cong et al., 2013; Das et al., 2014a; Ji et al., 2009; Qu et al., 2013) or their metabolites (Table S1); they are superior nanosorbents for the removal of organic/inorganic pollutants in comparison to common sorbents e.g. zeolite, clay, activated carbon and diatomite (Upadhyayula et al., 2009). CNTs as good adsorbents have well-defined cylindrical hollow structures, stronger physicochemical interactions, high aspect ratios, large surface area, high sorbent capacity, hydrophobic side and easily modifiable exteriors (Das et al., 2014b).
2.2. Applications for water treatment
CNTs and CNT-based composites exhibit the capacity for dye adsorption from aqueous systems which include multi-walled nanotubes (MWCNTs) and single-walled nanotubes (SWCNTs); later demonstrated superior adsorption capacity than MWCNTs (Gupta et al., 2013; Liu et al., 2013a, 2013b). Though, CNTs have interesting edge over activated carbon, their large-scale applications for sizeable wastewater treatment is not favorable due to the excessive manufacturing costs (De Volder et al., 2013). In addition to high surface area, CNTs have modifiable surfaces and are endowed with significantly measurable adsorption sites located on their outer and/or inner layer surfaces; theoretically a favorable third generation of carbon-based adsorbents. CNT-based composites exhibit remarkable wastewater remediation capabilities for multiple organic/inorganic and biological water contaminants (Upadhyayula et al., 2009); CNTs function as scaffolds in water purification as illustrated in Fig. 2. Because of their hydrophobic surface, however, they should be stabilized in aqueous suspensions for avoiding aggregation which can reduce their active surface area (De Volder et al., 2013).
Fig. 2.
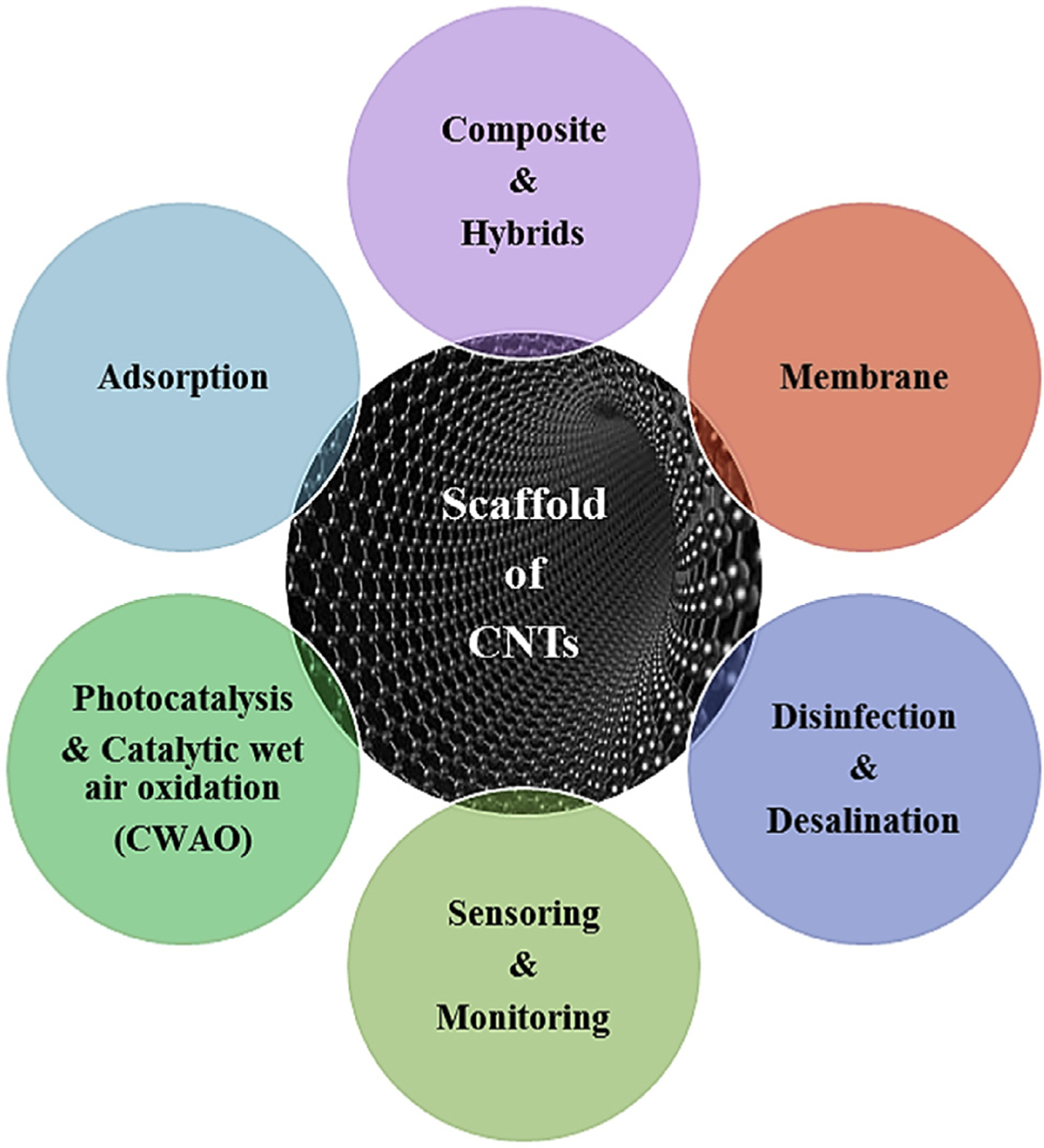
Functions of CNTs as scaffolds in wastewater remediation technologies.
Fugetsu et al. (2004) reported the first application of the encapsulated MWCNTs for removing ionic dyes (Fig. S1), wherein Ba2+-alginate background constituting an enclosure that preserves the entombed MWCNTs. Apparently, cages carry negative charge on their surface which restricts the capture of the large molecular weight anions e.g. humic acids, due to electrostatic repulsion. Thus, Ba2+-ALG/MWCNT composite could be used for the elimination of eosin bluish, acridine orange, orange G, and ethidium bromide with the adsorption efficiency of 0.44, 0.31, 0.33 and 0.43 μmol mg−1, respectively. Ghaedi et al. (2011) studied the adsorption efficiency of activated carbon and MWCNTs for Eriochrome Cyanine R at diverse pH, electrolyte, temperature, initial dye concentration and adsorbent amounts, where pH is a significant factor controlling adsorption. Higher adsorption capacities are attainable expeditiously by MWCNTs (95.2 mg g−1) with respect to activated carbon (40.6 mg g−1). In another study, alkali-activated high surface area CNTs, with large number of mesoporous, were applied for the elimination of methyl blue and MO with high adsorption capacities of 399 and 149 mg g−1, respectively (Ma et al., 2012); several studies have ensued for the removal of dyes using CNTs from the textile industries wastewaters (Mishra et al., 2010). Qu et al. (2008) reported a wet chemical process for combination of MWCNTs with Fe2O3 NPs for decontamination of neutral red and MB from polluted water (Qu et al., 2008); adsorption capacities being 77.5 and 42.3 mg g−1, respectively. Similarly, Gong et al. (2009) researched the elimination of neutral red, MB and brilliant cresyl blue deploying magnetic MWCNT nanocomposite which could effectively degrade cationic dye pollutants in water. Gao et al. (2013) offered a magnetic polymer MWCNT nanocomposite for remediation of anionic azo dyes. Several CNT-based nanosorbents have been reported for the adsorption of dyes e.g. CNTs-cellulose, CNTs-graphene, CNTs-Fe3O4, CNT-chitosan and CNT-activated carbon fiber, among others (Ai and Jiang, 2012; Gupta et al., 2013; Rajabi et al., 2017).
CNT sheets can be utilized to remove toxic heavy metals e.g. cadmium (AlSaadi et al., 2016), lead (Kabbashi et al., 2009), copper (Jeon et al., 2010; Ouni et al., 2019), zinc (Pyrzynska and Stafiej, 2012; Zhao et al., 2015), arsenic (Naghizadeh et al., 2012) and cobalt (Ouni et al., 2019; Wang et al., 2011); the efficiencies achieved were 94 for Cd2+, 85 for Pb2+ and more than 50 percent for Co2+ from contaminated water besides being effective for the removal of Cu and As. The low adsorption capacities for toxic heavy metals using raw CNTs could be considerably increased up on oxidation by KMnO4, HNO3 and NaOCl as shown by Kuo and Lin (2009) for the efficient adsorption of aqueous Cd2+ pollutants onto modified MWCNTs; microwave (MW)-assisted oxidation by oxidants and/or acids demonstrated that the Cd2+ adsorption capacity on MW/KMnO4/H2SO4-modified MWCNTs was increased in comparison with MW/H2SO4-modified CNTs. In another study with CNT sheets, exclusion of various heavy divalent metals from aqueous solutions was reported (Tofighy and Mohammadi, 2011); sheets were prepared by chemical vapor deposition of ferrocene and cyclohexanol under N2 environment at 750 °C followed by room temperature oxidation with conc. nitric acid. The adsorption of Cr(VI) pollutants from drinking water could be achieved by immobilizing ceria NPs on aligned CNTs (CeO2/ACNTs) as a novel adsorbent (Di et al., 2006). The chemical transformation of CeCl3, with aqueous NaOH in aligned CNT tailed by thermal treatment, afforded the adsorbent whose maximum adsorption capacity, as investigated by Langmuir model at pH 7.0, was found to be 30.2 mg g−1. Furthermore, oxidized MWCNTs and SWCNTs using a NaClO solution were deployed for Ni2+ adsorption from aqueous solutions (Lu and Liu, 2006) including HNO3 oxidized MWCNTs (Chen and Wang, 2006). A detailed investigation on the Pb2+ adsorption was undertaken using MWCNTs (Yu et al., 2013); apparently, MWCNTs with a higher O2 content and smaller diameter were shown to have greater lead removal ability. In contrast to conventional materials, CNT-based nanosorbents have grown as excellent materials for the remediation of diverse pollutants as shown in Table S2 (Kumar et al., 2014); an array of contaminants or heavy metals has been reported with diverse surface chemistry and physical structures.
Metal ions are adsorbed by CNTs via chemical bonding or electrostatic attraction (Rao et al., 2007) and additionally, they show antimicrobial characteristics by triggering oxidative stress in bacteria and abolishing the cell membranes. Interestingly, oxidative chemistry occurs with no generation of hazardous or toxic byproducts, which is a salient advantageous feature over established disinfection approaches besides easy renewal of CNTs via suitable regulations of conditions (e.g., pH) (Li et al., 2013b). Indeed, conventionally applied desalination approaches are high energy-demanding and technically challenging, while adsorption-centered approaches can find appliances at point-of-use water decontamination gadgets, although their competence for removing salts is slightly restricted. Plasma-altered ultra-long CNTs have been produced with very high adsorption capacity (>400% by weight) specifically for salt (Xing et al., 2013) relative to conventional carbon-based water treatment systems; they can be applied in multimodal membranes for removing salt, organics, and metal contaminants thus comprising an integral part of next-generation potable water purification strategies for desalination, disinfection, and filtration (Hashim et al., 2012).
Due to exceptional electrical, optical and mechanical properties, graphitic carbon-based nanocomposites (particularly CNTs) can serve as a promising building block for hybrid nanocatalysts thus increasing their performances in wastewater treatment. Semiconductors or metallic NPs has been discussed for the photoreduction of diverse organic/inorganic contaminants (Alwash et al., 2018; Gan and Qiao, 2012; Hoffmann et al., 1995); although several semiconductors e.g. TiO2, CdS and ZnO, possess low quantum efficiency, large band gap and slow ultraviolet photoresponse. CNTs have high electron-storage capacity (Kongkanand and Kamat, 2007) and can extend the light sorption region and increase the quantum ability of hybrid nanocatalysts, due to their notable chemical stability, hollow and high specific surface area, unique electronic structure and high absorbability (Liu et al., 2018; Woan et al., 2009; Xu et al., 2010, 2015; Zhang et al., 2018). Thus, novel CNT-based photo (nano)catalysts can be effectively deployed as ideal catalyst supports in wastewater remediation. Lee et al. (2011) fabricated the N-doped CNTs/TiO2 nanowires of core-shell configuration for light-mediated biomineralization of MB by TiO2 nanoshells (~5 nm) at a surface of graphitic carbon; direct contact of TiO2 nanoshell and NCNTs surface with no adhesive interlayer offered a novel carbon energy level for the band gap of TiO2, initiating an effective visible light photocatalytic degradation of MB. Besides, this ideal NCNTs/TiO2 core-shell hybrid nanostructures can facilitate a diversity of pragmatic applications in (nano)catalysts, sensors, energy conversion and/or storage. Zhang et al. (2010) described the fabrication of CNT-pillared rGO (reduced graphene oxide) platelets by chemical vapor deposition processes for photocatalytical degradation of RhB (Fig. S2). Li et al. (2011) described the synthesis of Au NPs@POM (polyoxometalate)-CNT nanohybrids for the degradation of RhB where POMs serve as encapsulating, bridging molecules and reducing agents; high electron-conducting capability of MWCNTs render these nanohybrids as efficient photocatalysts with a selective overview for their use in water remediation being summarized in Table S3.
3. Graphene and graphene oxide (GO)
3.1. Chemistry and properties
Graphene nanomaterials have been applied for treating wastewater (Perreault et al., 2015) as they show significant relevant physicochemical characteristics that include high surface area, simple functionalization, and hydrophobicity. Graphene nanocomposites can be utilized as nanosorbents for eliminating hazardous and toxic mix of multiple pollutants, and for their subsequent catalytic degradation using catalytic, photocatalytic, electrocatalytic and photo-electrocatalytic oxidation, and reduction processes (Al-Wafi et al., 2020; Gandhi et al., 2016; Khan et al., 2020b; Nasrollahzadeh et al., 2019a, 2020b; Wani et al., 2020) via their commercial production, and use is still a formidable challenge.
Uniform nanoporous graphene sheets can be applied for filtrating and desalinating water by varying their pore size and hence the pressure (Aghigh et al., 2015), the main drawback being their mechanical stability after increasing the pore number. Yan et al. (2018), produced N-doped sandwich-type graphene composites, and applied them as capacitive deionization electrodes via a self-assembly method. This sandwich composite offered substantial accessible surface area and lower electronic resistivity and displayed considerable salt adsorption capability with superior modification ability coupled with recycling operations. It has been shown that the GO nanosheets could be applied as suitable selective barriers for water permeability (Goh et al., 2015); super-hydrophobic and superoleophilic porous graphene has been generated as absorbent for various materials, demonstrating extreme selectivity, suitable recyclability, and significant absorption capacities of more than 90% (Tabish et al., 2018). Additionally, GO-based nanofiltration membrane with an enormously porous polyacrylonitrile nanofibrous mat has been fabricated for treating water; graphene oxide could generate a barricade on a polyacrylonitrile nanofibrous mat with well-regulated thickness (Wang et al., 2016a).
Unlike CNT-based nanomaterials, the aqueous removal of toxic dyes by graphene-based nanoadsorbents have varied advantages wherein single-layered graphene or GO nanosheets have two basal planes accessible for contaminant adsorption (Nasrollahzadeh et al., 2019a; Zhao et al., 2014); CNTs inner walls are not available to adsorbates (Sitko et al., 2013b). Moreover, graphene and its analogues could be simply fabricated via chemical/mechanical exfoliation of graphite without employing metallic catalysts (e.g. nickel) and complex apparatus (Nasrollahzadeh et al., 2019a; Sitko et al., 2013b). Scheme 1 illustrates the accepted common mechanisms for adsorption and pros and cons of utilizing graphene-based nanosorbents to eliminate metallic pollutants (Perreault et al., 2015) Notably, the resulting graphene-based nanomaterials are completely devoid of catalytic residues, thus not necessitating additional purification steps. Concerning GO, the nanosorbents contain a vast quantity of O2-bearing functionalities that precludes additional acid treatments to enhance reactivity and hydrophilicity character to graphene oxide (Zhao et al., 2011). It could positively interact with a diversity of organic/inorganic contaminants in ionic and/or molecular forms through the mechanisms, commonly known as the electrostatic π-π and hydrophobic interactions, among others. Thus, GO has drawn considerable attention as an effective nanosorbent for contaminated water/wastewater (Nasrollahzadeh et al., 2019a; Perreault et al., 2015; Wang et al.,2019).
Scheme 1.
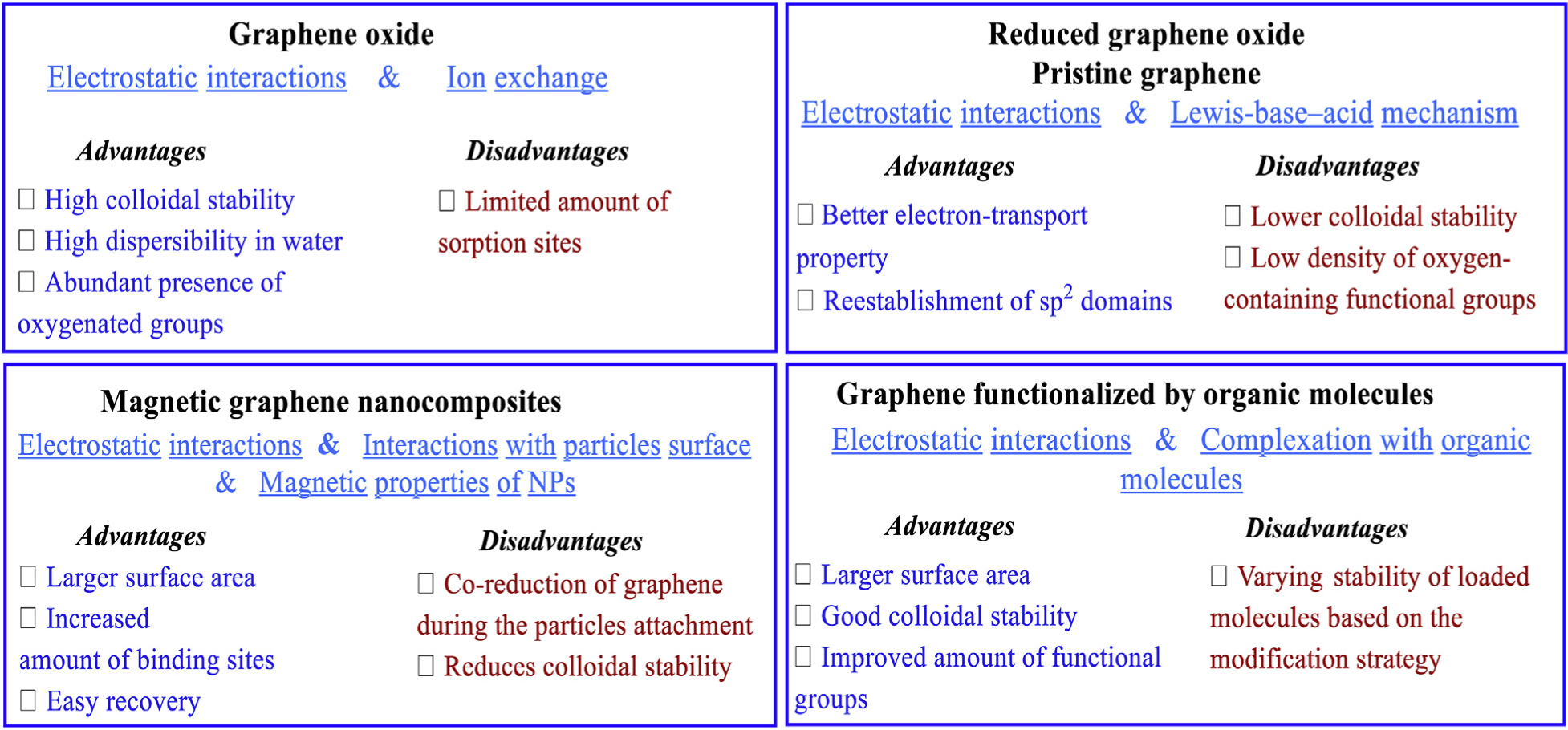
Specific interaction mechanisms and probable advantages/disadvantages for using graphene-based nanosorbents for aqueous degradation of pollutants.
3.2. Applications for water treatment
Graphene-based objects are deployed as efficient adsorbents for treating pharmaceuticals-laden wastewaters; their adsorption capacity can be varied by changing the experimental conditions (Carmalin Sophia et al., 2016). Additionally, they can be harnessed for removing radionuclides and heavy metals from wastewater; various key parameters, embracing the initial dosage and characteristics of graphene materials and metal ions pH, temperature, contact time, acidic organic ligands, and coexisting ions, can affect their adsorption capacity (Xu and Wang, 2017) as is evident from the recent data on remediation of toxic dyes and heavy metals in water (Tables S4 and S5) (Huang et al., 2011; Sitko et al., 2013a; Zhao et al., 2011). Significance of O2-bearing functional groups has been illustrated (Huang et al., 2011); GNS-500 and GNS-700 (heated at 500 or 700 °C after exfoliation) exhibited a greater adsorption capabilities for Pb2+ compared with pristine graphene thus signifying the importance of carboxyl/hydroxyl groups in the Pb2+ adsorption. Ghaedi et al. (2014) reported an efficient removal technology for mixed adsorption of brilliant green and MB as cationic toxic dyes by GO fabricated from modified Hummers process as calculated by Langmuir model at pH 7.0 were 416.67 and 476.19 mg g−1, respectively; removal was apparently via a pseudo-second-order (PSO) kinetic process based on a monolayer adsorption manner, mechanism attributed to π-π interactions or electrostatic attraction (Jiang and Fan, 2014; Shen et al., 2014; Sui et al., 2013; Wu et al., 2013) between positively charged dyes and negatively charged GO. Sabzevari et al. (2018) researched the fabrication of GO-chitosan without sonication for the MB removal to find that high adsorption ability for MB (402.6 mg g−1) could be obtained in comparison with other GO from original Hummers means and/or via sonication.
Graphene can be applied as a suitable separation membrane, by forming nanoscale pores in its layer (Song et al., 2018; Surwade et al., 2015); it has exclusive characteristics such as flexibility, mechanical and chemical stability, with essentially thickness of one-atom. Nanometer-sized pores in monolayer of graphene have been prepared by applying oxygen plasma engraving procedure, enabling the fine tuning of the pore size; ensuing membranes showed a salt refusal rate of nearly quantitative and speedy water transference. Particularly, water fluidities of up to 106 gm−2s−1 were attained by using powerful force via pressure alteration, while water fluxes evaluated at osmotic pressure did not surpass 70 gm−2s−1atm−1 (Surwade et al., 2015). It has been reported that single-layered self-supporting graphene with nanometer-scale pores could efficiently sieve NaCl salt from water (Cohen-Tanugi and Grossman, 2012) which varied significantly by changing pore diameter. Additionally, by analyzing the function of chemical groups attached to the graphene pore’s edges, it was revealed that commonly present hydroxyl groups could nearly double the water fluidity owing to their hydrophilic nature (Cohen-Tanugi and Grossman, 2012). A recyclable nanocomposite was prepared by growing both iron oxide and silver NPs on the GO’s surface; relative to silver NPs, the produced nanocomposite showed much superior antibacterial efficiency towards the Gram-positive and -negative bacteria. Moreover, magnetic iron oxide NPs facilitated repeated applications by enabling easy recycling of the nanocomposite via magnetic separation (Tian et al., 2014). Blue luminescent sulfur-doped graphene quantum dots (~1–5 nm) have been synthesized via a single-step pyrolysis method in presence of 3-mercaptosuccinic and citric acid (Anh et al., 2019) where 4-NP can function as quencher of fluorescence by π-π interaction with sulfur-doped graphene quantum dots, as shown by the linear reduction in fluorescence intensity after adding varying amounts of 4-NP (10–200 μM). Additionally, the generated quantum dots served as sensors to accelerate the analytic implementation of 4-NP detection, the limiting values being 0.7 and 3.5 nM in deionized water and wastewater, respectively; sulfur-doped graphene quantum dots-based paper sensor could quickly monitor 4-NP in wastewater within a minute (Anh et al., 2019).
Assorted (nano)catalysts have been fabricated via physicochemical and/or biological methods comprising a series of metals such as Pd, Co, Au, Ni, Cu, etc. (Nasrollahzadeh et al., 2019b, 2019c). Graphene-based (photo)nanocatalysts (G, GO or rGO-based) has been recently explored and employed for the alteration of photocatalysts or nanocomposites because of their exceptional features, namely, prominent surface area (~2630 m2 g−1), high electron conductivity (~7200 Sm−1), high thermal/chemical stability, and excellent capacity for the immobilization of metals (Ak and Novoselov, 2007; Nasrollahzadeh et al., 2019a, 2019c; Neto et al., 2009; Qiu et al., 2015) or via their decoration with numerous NPs e.g. metal oxides, noble metals and magnetic NPs (Julkapli and Bagheri, 2015; Naghdi et al., 2018; Nasrollahzadeh et al., 2019a).
GO decorated with highly stable lanthanum-substituted manganese ferrites (LMF) have been synthesized for the removal of PFOA from aqueous environments (Fig. S3) (Elanchezhiyan et al., 2020) After the substitution of lanthanum on MF, the surface charge density and the crystalline nature changed remarkably; electrostatic interaction and hydrogen bonding had significant roles in PFOA adsorption. Additionally, it has been revealed that the coexisting pollutants, including anions, cations, and other organic compounds had no significant effects on the adsorption of PFOA deploying these nanohybrids (Elanchezhiyan et al., 2020).
Multifunctional chitosan-attached plasmonic Au NPs conjugated GO architecture-based 3D porous membrane, chitosan-Au NPs-GO (Fig. 3A), was designed and fabricated by Jones et al. (2017) for the supply of clean and safe drinking water. The 3D porous membrane was used as a novel nanocatalyst in efficient separation and label-free Surface Enhanced Raman spectroscopy (SERS) documentation of pharmaceutical pollutants and killing of methicillin-resistant Staphylococcus aureus (MRSA) in polluted water. The HRSEM image in Fig. 3B illustrates the morphology of chitosan-attached Au NPs on the GO sheet and Fig. 3C depicts MRSA bacteria being easily trapped using highly porous chitosan-Au NPs-GO membrane. Besides, the amount of colonies of live MRSA on the extremely porous 3D membrane was determined (Fig. 3D and E), affirming that ~100% of MRSA superbug was killed using as-prepared porous membrane.
Fig. 3.
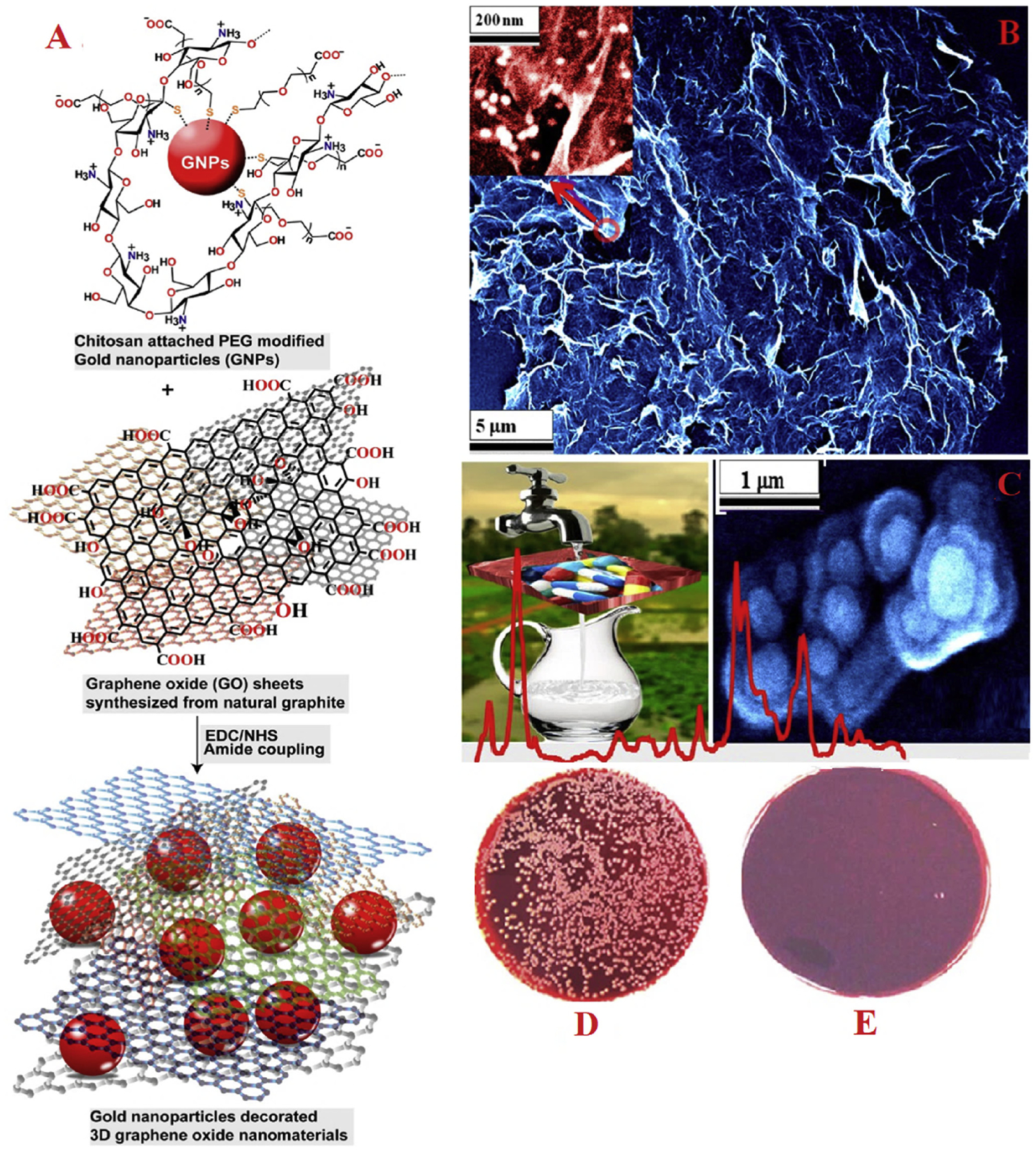
Fabrication of (A) chitosan-attached Au NPs conjugated GO architecture-based multifunctional 3D porous membrane. (B) SEM image of chitosan-attached plasmonic Au NPs conjugated with hybrid 3D GO membrane. (C) SEM image showing the MRSA being captured using the 3D membrane. (D & E) Colonies of bacteria displaying the amount of live MRSA (D) after filtration by only a GO-based 3D membrane, (E) after filtration by using the chitosan-attached plasmonic Au NPs-based 3D GO membrane. Reproduced with permission from Ref. (Jones et al., 2017).
4. Carbon and graphene quantum dots
4.1. Chemistry and properties
The research community is optimistic about the pervasive potential utilization of carbon nanostructured materials to ameliorate sustainable energy conversion and wastewater treatment. Among the nanocarbon materials, carbon- and graphene quantum dots are a fascinating category of the nanomaterials that can find widespread applications. Quantum-sized carbon dots (or carbon NPs) were initially discovered by Xu’s group in 2004 (Xu et al., 2004), (i.e., a few months before graphene analogues), and the common name “CQDs” was assigned by Sun’s group in 2006 (Sun et al., 2006), although their utilization as a heterogeneous photocatalysts only started recently. Carbon quantum dots refer to carbon-based sub-ten-nanometer NPs (at least one dimension below 10 nm), and flat or quasi-spherical shape and comprise sp3 and/or sp2 hybridized carbon atoms (i.e., diamond-like and/or graphene/GO sheets), with composition-dependent fluorescence and possess diverse surface functional groups (Lim et al., 2015; Yu et al., 2016a). Graphene quantum dots comprise graphene of single and/or a few layers (<2 nm thick) with lateral dimensions (<10 nm) being considerably larger than their height. Carbon nanodots (CNDs), CQDs, GQDs, N-doped CDs/GQDs, carbon nitride dots, and CDs/GQDs (nano)composites and (nano)hybrids represent the same (nano)carbon family (Garg and Bisht, 2016; Mahmoud et al., 2020; Zhu et al., 2015).
These carbon-based nanostructured materials have garnered significant interest as they could be applied in non-biomedical areas, such as optical devices (Jiang et al., 2015) and in photocatalysis (Ke et al., 2017; Su et al., 2020), because of their unique features e.g. strongly size-dependent electronic, electrochemical, and optical, and quantum size effects (Cai et al., 2019; Cao et al., 2012), easy surface functionalization, adjustable composition, ability to harness long-wavelength light, and easy synthesis (bottom-up and/or top-down approaches) (Cao et al., 2012; Fernando et al., 2015; Hisatomi et al., 2014; Zhao et al., 2016). Besides, CQDs/GQDs are promising candidates for biomedical appliances, such as drug delivery (Tang et al., 2013; Zheng et al., 2014), biosensing (Dai et al., 2014; Ding et al., 2013; Tajik et al., 2020), gene delivery (Hu et al., 2014; Zemmouri et al., 2013), and bioimaging (Ding et al., 2013; Luo et al., 2013; Zhang and Ding, 2018), because of their distinctive attributes e.g. nontoxicity, high photostability, excellent mechanical/thermal stability in vivo, and high biocompatibility, electrochemiluminescence (Dong et al., 2013), nonlinear optical response (Bourlinos et al., 2013), and intense and tunable photoluminescence (PL) by using surface functional groups and dot size (Bourlinos et al., 2008, 2011; Hola et al., 2014; Li et al., 2010). Based on DFT (density functional theory) and time-dependent DFT calculations, Chen et al. (Sk et al., 2014) determined that the GQDs properties can be adjusted from deep UV-to-NIR (near infrared) via alteration of morphology, particle size, surface functional groups, surface defects, edge configuration and heteroatom doping.
Nanocomposites with carbon-based nanomaterials are known mostly for graphene (i.e., 0D CQDs onto 2D graphene sheets), and to a lesser extent for nanodiamonds and CNTs. Moreover, GQDs could self-assemble to fabricate honeycomb nanostructures (Fan et al., 2012) and/or hollow microspheres (Ding et al., 2012). Compared to their 2D counterpart, GQDs and their functionalized analogues can offer novel application opportunities and unique merits, namely, better tunability in physicochemical features, high dispersibility, bandgap opening owing to quantum confinement, comparable sizes to biomolecules, and also more abundant active sites (edges, and functional moieties) (Anastopoulos et al., 2019; Li et al., 2013; Wang et al., 2016b). Because of these intriguing properties and their possible fabrication from biomass, CQDs and/or GQDs have potential to substitute conventional semiconductor/quantum which have severe disadvantages of high cost and toxicity; they could ameliorate the performance of several nano/photocatalysts via band gap alignment, morphology change, enhancement of light adsorption, synergic cooperation, and/or the charge transferal promotion. Thus, exploiting good light absorption, fully available surface, excellent photostability, high dispersibility, tunable bandgap structure, and their capability to fabricate heterojunction or hybrid with other nanostructures, CQDs/GQDs have been often applied as efficient nano/photocatalysts for (photo)catalytic energy generation (Chen et al., 2016a; Yeh et al., 2014; Yu et al., 2016b) and (photo)degradation of pollutants/contaminants (Chen and Bai, 2020; Ebrahimi et al., 2016; Pan et al., 2015; Yan et al., 2017).
4.2. Applications for water treatment
CQDs (1.2–3.8 nm) with high up-conversion PL properties were first prepared by Li et al. via a single step alkali-assisted electrochemical process (Li et al., 2010); the preparation of TiO2/CQDs or SiO2/CQDs semiconductor nanocomposites was reported with a strong and stable visible-light response for the decomposition of an organic dye, MB. The maximum photodegradation abilities of SiO2/CQDs (TiO2/CQDs) nanocomposites towards aqueous MB pollutants were found to be ~100% after 25 min irradiation with halogen lamp (300 W), whereas control experiments with pure CQDs and/or without either the metal oxide component (SiO2 or TiO2) demonstrated a minimal MB (0%) photodegradation. A CQDs/ZnO nanocomposite (~20–30 nm) was fabricated via a hydrothermal one-step route (Yu et al., 2012), which was then utilized for the photodegradation of toxic methanol and benzene (>80% degradation efficiency after 24 h) in air under visible-light conditions at room temperature.
The fabrication of nanocomplex-based photocatalysts (e.g. WO3/CQDs, N-ZnO/CQDs, CQDs/BiOI) has been revealed with substantial proficiency towards the photodegradation of a variety of organic contaminants (Chen et al., 2016b; Muthulingam et al., 2016; Yan et al., 2016a). Zhao et al. (Qian et al., 2016) established the preparation and photocatalytic prowess of CQDs decorated nanocomposites of Bi2WO6 for the photocatalytic removal of gaseous VOCs under both, the UV irradiation and visible-light; it could broaden the absorption visible-light and improve the photoexcited charge recombination/separation thus revealing an enhanced photo-oxidation performances and stabilities towards toluene and acetone. Similarly, Mendes et al. reported on the performance of N-CQDs/TiO2 (P25) nanocomposite for NO pollutant’s photo-oxidation under both, UV and visible-light irradiation (Martins et al., 2016) wherein the NO conversion augmented from ~10% (for P25) to ~27% (for N-CQDs/TiO2) and from 58.4% to 79.6% under both, UV-Vis light, respectively.
One of the candidates for the improvement of CQDs-based photocatalysts is the utilization of metal sulfides (e.g. CdS) with their superior properties, namely narrow band gap, good transport, and high chemical/thermal stability. In this context, CQDs/CdS photocatalysts were prepared by Zhang and co-workers via a hydrothermal route and their application towards the photodegradation of RhB was evaluated (Li et al., 2013b) where CQDs could efficiently trap electrons and slow down the recombination of the photoexcited carriers (holes or electrons); ~50% RhB degradation efficiency was attained by flower-shaped CdS while 1% CQDs/CdS could enhance the degradation efficiency to 90%. The photodegradation of Alizarin red S (ARS) dye was accomplished using CQDs/ZnS photo/nanocatalyst by Kansal et al. (Kaur et al., 2016), where it demonstrated the superior photocatalytic activity for ARS degradation (~89% after 250 min), 1.4 times better than pristine ZnS (~63%) under comparable visible-light irradiation conditions.
Polymer dots (PDs) have been fabricated from polymers such as polyvinyl alcohol with core comprising CDs (Zhu et al., 2015) as exemplified by hydrothermal treatment of PVA precursor (Li et al., 2018). CDs/PDs-TiO2 nanocomposites with Ti-O-C bands were made by grafting CDs/PDs with TiO2 NPs via facile hydrothermal thermal treatment, and the activities of ensuing semiconductor/CD nanohybrids were evaluated for degradation of MO dye degradation in aqueous solutions (Fig. 4A). Synergy between CDs/PDs and TiO2 could effectively improve the removal of toxic MO, and the nano/photocatalytic rate constant of PDs/TiO2 is 9.5 and 3.6 times more than commercially available pure P25 and TiO2, respectively (Fig. 4B and C).
Fig. 4.
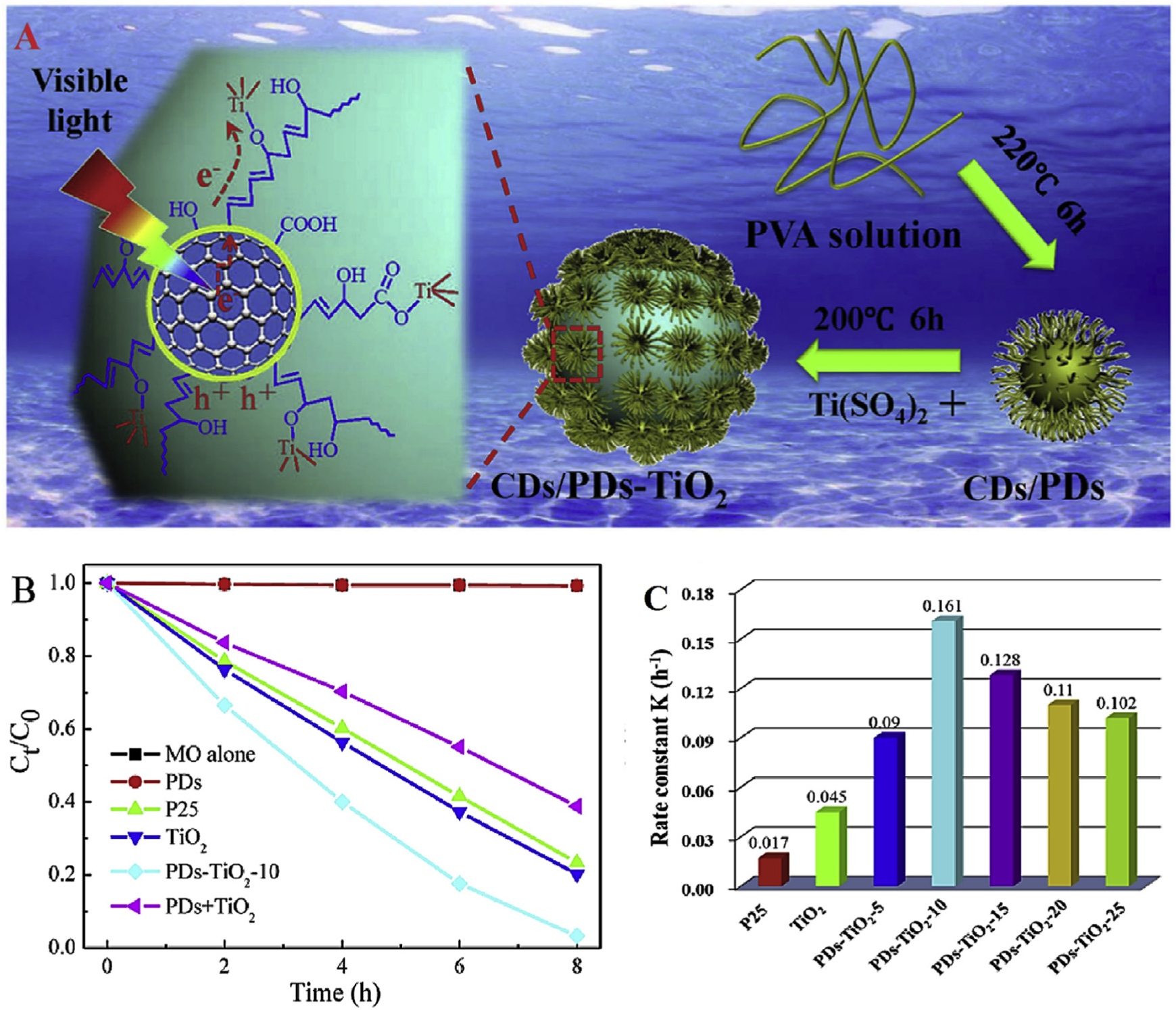
(A) Mechanistic photocatalytic illustration for CDs/PDs-TiO2 under vis-light irradiation. (B) MO degradation under UV/Visible-light irradiation (220–800 nm), (C) rate constant k of CDs/PDs-TiO2 nanohybrids, pure TiO2, and P25 under vis-light treatment. Reproduced with permission from Ref. (Li et al., 2018).
The fabrication of new hybrid nanocomposites comprising CQDs and inorganic NPs namely zinc oxide, iron oxide, titania, and silica have been accomplished. Zhang et al. (2011) synthesized, via hydrothermal process, Fe2O3/CQDs nanocomposites as effective photocatalysts for the degradation of noxious gases namely benzene or methanol in vis-light conditions; pathway for Fe2O3/CQDs nanohybrid with cubic morphology of magnetic architecture and their photochemical application, are shown in Fig. 5. Owing to the CQDs nature, magnetic nanohybrids can combine magnetic response with fluorescence on a single platform. CQD has high-electron storage where photon-excited electron from Fe2O3 NPs can easily be transported in the CQD conducting system, as exhibited in Fig. 6. The adsorbed reductants/oxidants (such as O2 and/or OH) interact with the electron-hole pairs, forming an enhanced quantity of active oxygen radicals with a strong oxidation prowess for removing toxic gases. Besides, the resulting nanohybrids could effectively combine the fluorescence features of CQDs with magnetic, mechanical or optical properties/features of the oxide’s core culminating in a core-shell fluorescent/magnetic photocatalyst (Li et al., 2010; Markova et al., 2012; Ruan et al., 2009; Yu et al., 2012) where an iron oxide core is attached to CQDs either through a covalent bond (by utilization of surface functional groups present on the nanohybrid components) and/or electrostatic interactions (via an electrostatically charged shells of the nanohybrid elements). In this context, a series of core-shell fluorescent photocatalysts using shells-derived from CQDs and a variety of NP-cores (biogenic magnetite, CNTs and silver) were prepared by Markova et al. (2012) by a novel and effectual protocol for the modification of negatively charged NP-cores using CQDs and betaine hydrochloride via electrostatic interactions (Fig. S4) which illustrates a core-shell architecture attained by coating magnetite and CNTs using CQDs.
Fig. 5.

Up: Fabrication of Fe2O3/CQDs nanocomposites via the hydrothermal process, which photographs illustrate; (i) aqueous CQDs dispersion, (ii) solution comprising CQDs, (NH2)2CO, and, FeCl3, and (iii) final product. Down: (a) SEM image of Fe2O3 NPs, (b) (HR) TEM images of CQDs, (c & d) SEM and TEM images of Fe2O3/CQDs nanocomposites. Reproduced with permission from Ref. (Zhang et al., 2011).
Fig. 6.
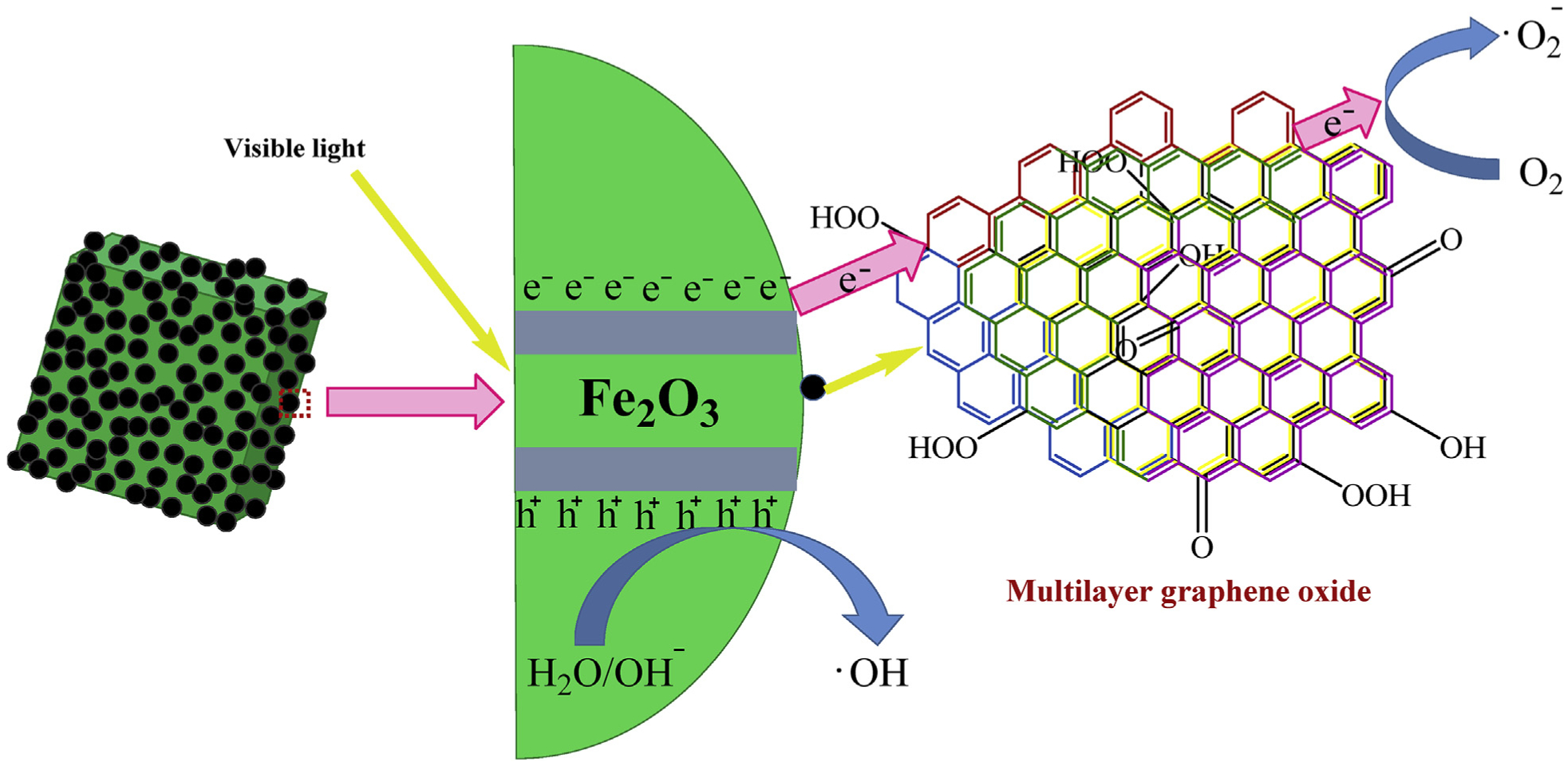
Schematic model of the mutual interactions between CQDs and Fe2O3 NPs describing the enhanced photocatalytic ability of nanocomposite under vis-light (Zhang et al., 2011).
GQDs have been similarly deployed in photocatalytic environmental remediation by elimination of organic pollutants such as azo dyes, and VOCs. Several procedures have emerged to ameliorate photocatalytic efficiency of semiconductor GQDs-based photocatalysts, including loading of noble metal and/or metal oxides, structure design, and the preparation of a semiconductor nanocomposites/hybrids (Kaur et al., 2018; Kemp et al., 2013; Putri et al., 2015; Yan et al., 2019). A series of newly designed GQDs-based photo/nanocatalysts, including GQDs coupled with TiO2 (Bian et al., 2017; Qu et al., 2013), Fe3O4 (Wu et al., 2014), zinc porphyrin (Lu et al., 2015), MnNb2O6 (Yan et al., 2017), ZnO (Ebrahimi et al.,2016), g-C3N4 (graphitic carbon nitrides) (Bian et al., 2017; Liu et al., 2017), and/or N-Bi2O2CO3 (Yu et al., 2017), have been designed for the visible-light- and/or NIR-driven photodegradation. In this context, GQDs/modified mesoporous-C3N4 heterojunction can be produced via an electrostatic interaction (Yu et al., 2017) wherein the nanocomposite can form more superoxide radical species and small holes towards an efficient photodegradation of RhB and tetracycline (TC), as displayed in Fig. S5. Similarly, Liu et al. (Lu et al., 2015) have reported GQD/zinc porphyrin heterojunction, and evaluated its performance for the faster decomposition of aqueous MB visible-light-driven photodegradation reactions.
GQDs have been known for a more intact aryl-basal flat structure and rich periphery carboxylic groups than the micro-sized GO layers (Zhang et al., 2013; Zhou et al., 2012). More attractively, GQDs demonstrated much better inherent peroxidase-like prowess compared to micro-sized GO, thereby improving efficiency and stability in detection of H2O2. Zhang et al. (2013) reported that Au NPs/GQDs nanohybrids exhibited a synergetic and switchable peroxidase-like ability for H2O2 decomposition and/or H2O2 detection in physiology or pathology. Similarly, nanocomposites comprising GQDs and Fe3O4 NPs, as encouraging peroxidase-mimic nanocatalytic systems, have been reported (Wu et al., 2014), and they could be fabricated via a single-step co-precipitation route to remove phenolic compounds. The as-prepared GQDs/Fe3O4 nanocomposite bearing a weight ratio of 1:1 for H2O2- facilitated the oxidation of 3,3′,5,5′-tetramethylbenzidine demonstrated comparable or superior catalytic performances, compared to individual Fe3O4 NPs (~22 fold higher), GQDs (~25 fold higher), and also GO/Fe3O4 NPs (~9 fold higher). In fact, the combination of Fe3O4 NPs via Fe-O chemical links led to electron transfer from electron-rich GQDs to magnetic NPs, preserving a greater number of Fe(II) ions which play a main role in the peroxidase-like nanocatalytic activities of Fe3O4 NPs.
The doping of nitrogen is one of the novel strategies to modify and modulate the structural functionalities, chemical and electronic properties/features of graphene and GQDs (Li et al., 2012; Subramanian et al., 2017) as shown for the preparation of N-GQDs/TiO2 nanocomposite via a hydrothermal procedure and its deployment as a photo/nanocatalyst for the photodegradation of aqueous MB (Safardoust-Hojaghan and Salavati-Niasari, 2017); N-GQDs displayed an eminent effect on the photocatalytic efficiency of TiO2 NPs from 40% to 85% for MB degradation under UV-light. A recent study (Yan et al., 2016b) revealed that photocatalytic activity of N-GQDs-BiVO4@C3N4-Z scheme heterojunctions to (photo) degrade the antibiotics pollutants such as oxytetracycline, tetracycline, and ciprofloxacin was higher than BiVO4@C3N4; as-prepared heterojunction exhibited superior photocatalytic efficiency of 91.5% tetracycline degradation in 30 min. Besides, the combined S, N co-doped GQDs with g-C3N4 was fabricated as an ideal photo/nanocatalyst for pollutant degradations (Cai et al., 2017); as-prepared S, N-GQD/g-C3N4 (6–10 nm) composites (Fig. S6) revealed higher photocatalytic activities in remediation of RhB dye in vis-light condition than by pure g-C3N4 under identical conditions. Indeed, enhancement in degradation was because of the appropriate separation of photogenerated holes and electrons in S,N-GQD/g-C3N4 nanocomposite.
A variety of CQDs- and GQDs-based photocatalysts, deployed in eliminating highly toxic pollutants namely dyes and heavy metals, are shown in Table S6, where diverse preparations of CQDs- and GQDs-based photocatalysts via hydrothermal-, solvothermal-, ultrasonic treatment and microwave-assisted routes are documented. Among these methods, the hydrothermal route is a facile, greener and an effective approach (Chu et al., 2019; Pirsaheb et al., 2018; Yu et al., 2016a; Zeng et al., 2018). Natural bio-wastes/biosources have also been used as environmentally-friendly, low-cost and abundant carbon sources for the synthesis of these photocatalysts.
5. Conclusion and future prospects
Treatment of water and wastewater including industrial water is very critical and essential for safeguarding the environment and human health, and it is a major apprehension for the public health. As is obvious from this review, tremendous progresses have been achieved in recent years pertaining to the environmental pollution abatement. Among advanced technologies, greener nanotechnology and applications of sustainable nanomaterials can be exploited for the treatment of wastewater, and especially in the handling of previously untreatable hazardous contaminants. However, there are still several hurdles to endure, mainly the lack of infrastructure and resource utilization that need to be addressed besides the obvious concern about the potential toxicity of the nanomaterials themselves where carbon-based materials may have an edge. The consequence of releasing nanomaterials into the aquatic system is more nuanced, especially if the nanomaterials are part of a composite material.
Nanotechnology has the potential to make industrial water treatment more efficient where the contaminants can be removed by using relatively greener nanotechnology, but this field needs extensive explorations. Eventually, carbon nanotubes, carbon and graphene quantum dots and graphene-based nanomaterials may emerge as effectual, cost-effective, and environmentally-friendly substitutes for the prevailing treatment supplies, from the viewpoints of both environmental cleanup and resource conservation. For instance, the modification of CNTs such as, oxidation, and applying electrochemical assistance can meaningfully improve their adsorption rate and capacities for the removal of contaminants, and may classify as an innovative strategy in environmental remediation, but the toxicity issues need to be evaluated, comprehensively.
CNTs and some earth-abundant nanometals are naturally green and extraordinarily applicable nanoadsorbents for removing the heavy metals and hazardous contaminants; major benefit for CNTs is their strong adsorption capacity for polar organic compounds due to their assorted interactions with pollutants. Unfortunately, CNTs are not cost-competitive relative to adsorbents like activated carbon. The manufacture of CNTs is rather an expensive proposition, and supplementary technical devices, for example, plants equipped with membrane filtration, need to be incorporated to ensure that no NPs are emitted into the aqueous streams or air. Impending appliances may emphasize very particular adsorption activities, where only miniscule amounts of CNTs are needed or unique situations where CNTs are the exclusive adsorbent. Commercialization of natural nano-engineered materials for wastewater technology is greatly contingent on their effect on the aquatic ecosystem (Dwivedi et al., 2015). Several investigations embracing toxicity analyses, life cycle assessments, strategy evaluations, and dispersion of NPs in water systems have to be conducted to assess the risks of nanomaterials to human health (Bakand and Hayes, 2016; Borm et al., 2006; Nasrollahzadeh et al., 2018). These informative findings would lead to better awareness of the conduct of NPs (like CNTs) in aqueous systems (Asghari et al., 2012; Clemente et al., 2012; Jackson et al., 2013; Petersen et al., 2011). Besides CNTs-based nanoadsorbents, other allotropes of nanocarbon, such as graphene, GO, CDs or GQDs, have shown promise to enhance the (photo)catalytic activities of conventional semiconductors, rendering them widely applicable for the (photo) catalytic degradation of contaminants.
In spite of the remarkable advances in fabrication and the demonstrated catalytic prowess of sustainable nanomaterials such as carbon nanotubes, carbon and graphene quantum dots and graphene-based nanomaterials, more attention need to be focused on the following issues in future investigations:
Novel and innovative synthetic methods to reduce the cost of production of nanomaterials and improve their catalytic prowess.
Improvement and reuse of the magnetic nanomaterials for water treatment.
Advancement and use of mild, water soluble and low-cost reductants for the degradation of various pollutants.
Utilization of biowaste-derived nanomaterials for wastewater treatment.
Exploitation of natural materials such as clays, montmorillonite, bentonite, zeolites as nontoxic and cost-effective and abundant supports for the synthesis of nanomaterials and application in water treatment.
Manipulation of animal residues such as bone, eggshell and bristles for the synthesis of nanomaterials and application in water treatment.
Toxicity evaluations should be accomplished for all the aforementioned proposals; comprehensive assessments of the risks and the potential impact of nanomaterials to human health and ecosystems, mainly potential cellular toxicities, must be evaluated and documented.
Supplementary Material
Acknowledgement
The support of the Iranian Nano Council, the University of Qom and Isfahan University of Medical Sciences for this work is greatly appreciated.
Footnotes
Publisher's Disclaimer: Disclaimer
The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the U.S. Environmental Protection Agency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2020.128005.
References
- Aghigh A, Alizadeh V, Wong HY, Islam MS, Amin N, Zaman M, 2015. Recent advances in utilization of graphene for filtration and desalination of water: a review. Desalination 365, 389–397. [Google Scholar]
- Ai L, Jiang J, 2012. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene-carbon nanotube hybrid. Chem. Eng. J 192, 156–163. [Google Scholar]
- Ak G, Novoselov K, 2007. The rise of graphene. Nat. Mater 6,183–191. [DOI] [PubMed] [Google Scholar]
- Al-Wafi R, Ahmed MK, Mansour SF, 2020. Tuning the synthetic conditions of graphene oxide/magnetite/hydroxyapatite/cellulose acetate nanofibrous membranes for removing Cr(VI), Se(IV) and methylene blue from aqueous solutions. Journal of Water Process Engineering 38, 101543. [Google Scholar]
- Ali I, Peng C, Naz I, Khan ZM, Sultan M, Islam T, Abbasi IA, 2017. Phytogenic magnetic nanoparticles for wastewater treatment: a review. RSC Adv. 7, 40158–40178. [Google Scholar]
- AlSaadi MA, Al Mamun A, Alam MZ, Amosa MK, Atieh MA, 2016. Removal of cadmium from water by CNT-PAC composite: effect of functionalization. Nano 11,1650011. [Google Scholar]
- Alwash A, Adil H, Hussain Z, Yousif E, 2018. Potential of carbon nanotubes in enhance of photocatalyst activity. Arch Nano Op Acc J 1, 65–70. [Google Scholar]
- Anastopoulos I, Pashalidis I, Hosseini-Bandegharaei A, Giannakoudakis DA, Robalds A, Usman M, Escudero LB, Zhou Y, Colmenares JC, Núñez-Delgado A, 2019. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq 295, 111684. [Google Scholar]
- Anh NTN, Chang P-Y, Doong R-A, 2019. Sulfur-doped graphene quantum dot-based paper sensor for highly sensitive and selective detection of 4-nitrophenol in contaminated water and wastewater. RSC Adv. 9, 26588–26597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghari S, Johari SA, Lee JH, Kim YS, Jeon YB, Choi HJ, Moon MC, Yu IJ, 2012. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J. Nanobiotechnol 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakand S, Hayes A, 2016. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci 17, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Zhou C, Li P, Liu J, Dong X, Xi F, 2017. Graphene quantum dots decorated titania nanosheets heterojunction: efficient charge separation and enhanced visible-light photocatalytic performance. ChemCatChem 9, 3349–3357. [Google Scholar]
- Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J, 2006. The potential risks of nanomaterials: a review carried out for ECETOC. Part. Fibre Toxicol 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlinos AB, Karakassides MA, Kouloumpis A, Gournis D, Bakandritsos A, Papagiannouli I, Aloukos P, Couris S, Hola K, Zboril R, 2013. Synthesis, characterization and non-linear optical response of organophilic carbon dots. Carbon 61, 640–643. [Google Scholar]
- Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Georgakilas V, Giannelis EP, 2008. Photoluminescent carbogenic dots. Chem. Mater 20, 4539–4541. [Google Scholar]
- Bourlinos AB, Zboril R, Petr J, Bakandritsos A, Krysmann M, Giannelis EP, 2011. Luminescent surface quaternized carbon dots. Chem. Mater 24, 6–8. [Google Scholar]
- Bousselmi L, Geissen S-U, Schroeder H, 2004. Textile wastewater treatment and reuse by solar catalysis: results from a pilot plant in Tunisia. Water Sci. Technol 49, 331–337. [PubMed] [Google Scholar]
- Cai A, Wang Q, Chang Y, Wang X, 2017. Graphitic carbon nitride decorated with S, N co-doped graphene quantum dots for enhanced visible-light-driven photocatalysis. J. Alloys Compd 692, 183–189. [Google Scholar]
- Cai Y, Wei Z, Song C, Tang C, Han W, Dong X, 2019. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev 48, 22–37. [DOI] [PubMed] [Google Scholar]
- Cai Z, Dwivedi AD, Lee W-N, Zhao X, Liu W, Sillanpää M, Zhao D, Huang C-H, Fu J, 2018. Application of nanotechnologies for removing pharmaceutically active compounds from water: development and future trends. Environ. Sci. J. Integr. Environ. Res.: Nano 5, 27–47. [Google Scholar]
- Cao L, Meziani MJ, Sahu S, Sun Y-P, 2012. Photoluminescence properties of graphene versus other carbon nanomaterials. Acc. Chem. Res 46, 171–180. [DOI] [PubMed] [Google Scholar]
- Carballa M, Omil F, Lema JM, Llompart M a, Garcıa-Jares C, Rodrıguez I, Gomez M, Ternes T, 2004. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 38, 2918–2926. [DOI] [PubMed] [Google Scholar]
- Carmalin Sophia A, Lima EC, Allaudeen N, Rajan S, 2016. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater-a review. Desalination and Water Treatment 57, 27573–27586. [Google Scholar]
- Carpenter AW, de Lannoy C-F, Wiesner MR, 2015. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol 49, 5277–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wang X, 2006. Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind. Eng. Chem. Res 45, 9144–9149. [Google Scholar]
- Chen LC, Teng CY, Lin CY, Chang HY, Chen SJ, Teng H, 2016a. Architecting nitrogen functionalities on graphene oxide photocatalysts for boosting hydrogen production in water decomposition process. Advanced Energy Materials 6, 1600719. [Google Scholar]
- Chen Y, Bai X, 2020. A review on quantum dots modified g-C3N4-based photocatalysts with improved photocatalytic activity. Catalysts 10, 142. [Google Scholar]
- Chen Y, Lu Q, Yan X, Mo Q, Chen Y, Liu B, Teng L, Xiao W, Ge L, Wang Q, 2016b. Enhanced photocatalytic activity of the carbon quantum dot-modified BiOI microsphere. Nanoscale research letters 11, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorawalaa KK, Mehta M, 2015. Applications of nanotechnology in wastewater treatment. Int J Innov Emerg Res Eng 2, 21–26. [Google Scholar]
- Chu K-W, Lee SL, Chang C-J, Liu L, 2019. Recent progress of carbon dot precursors and photocatalysis applications. Polymers 11, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente Z, Castro V, Jonsson C, Fraceto L, 2012. Ecotoxicology of nano-TiO2-an evaluation of its toxicity to organisms of aquatic ecosystems. Int. J. Environ. Res 6, 33–50. [Google Scholar]
- Cloete TE, De Kwaadsteniet M, Botes M, 2010. Nanotechnology in Water Treatment Applications. Horizon Scientific Press. [Google Scholar]
- Cohen-Tanugi D, Grossman JC, 2012. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608. [DOI] [PubMed] [Google Scholar]
- Colmenares JC, Varma RS, Lisowski P, 2016. Sustainable hybrid photocatalysts: titania immobilized on carbon materials derived from renewable and biodegradable resources. Green Chem. 18, 5736–5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Q, Yuan X, Qu J, 2013. A review on the removal of antibiotics by carbon nanotubes. Water Sci. Technol 68, 1679–1687. [DOI] [PubMed] [Google Scholar]
- Dai H, Shi Y, Wang Y, Sun Y, Hu J, Ni P, Li Z, 2014. A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sensors and Actuators B: Chemicals 202, 201–208. [Google Scholar]
- Das R, Ali ME, Hamid SBA, Ramakrishna S, Chowdhury ZZ, 2014a. Carbon nanotube membranes for water purification: a bright future in water desalination. Desalination 336, 97–109. [Google Scholar]
- Das R, Hamid SBA, Ali ME, Ismail AF, Annuar M, Ramakrishna S, 2014b. Multifunctional carbon nanotubes in water treatment: the present, past and future. Desalination 354, 160–179. [Google Scholar]
- Das R, Vecitis CD, Schulze A, Cao B, Ismail AF, Lu X, Chen J, Ramakrishna S, 2017. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev 46, 6946–7020. [DOI] [PubMed] [Google Scholar]
- Das S, Chakraborty J, Chatterjee S, Kumar H, 2018. Prospects of biosynthesized nanomaterials for the remediation of organic and inorganic environmental contaminants. Environ. Sci. J. Integr. Environ. Res.: Nano 5, 2784–2808. [Google Scholar]
- De Volder MF, Tawfick SH, Baughman RH, Hart AJ, 2013. Carbon nanotubes: present and future commercial applications. Science 339, 535–539. [DOI] [PubMed] [Google Scholar]
- Di Z-C, Ding J, Peng X-J, Li Y-H, Luan Z-K, Liang J, 2006. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 62, 861–865. [DOI] [PubMed] [Google Scholar]
- Ding C, Zhu A, Tian Y, 2013. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Accounts Chem. Res 47, 20–30. [DOI] [PubMed] [Google Scholar]
- Ding Y, Cheng H, Zhou C, Fan Y, Zhu J, Shao H, Qu L, 2012. Functional microspheres of graphene quantum dots. Nanotechnology 23, 255605. [DOI] [PubMed] [Google Scholar]
- Dong Y, Chen C, Lin J, Zhou N, Chi Y, Chen G, 2013. Electrochemiluminescence emission from carbon quantum dot-sulfite coreactant system. Carbon 56, 12–17. [Google Scholar]
- Dwivedi AD, Dubey SP, Sillanpää M, Kwon Y-N, Lee C, Varma RS, 2015. Fate of engineered nanoparticles: implications in the environment. Coord. Chem. Rev 287, 64–78. [Google Scholar]
- Ebrahimi M, Samadi M, Yousefzadeh S, Soltani M, Rahimi A, Chou T.-c., Chen L-C, Chen K-H, Moshfegh AZ, 2016. Improved solar-driven photocatalytic activity of hybrid graphene quantum dots/ZnO nanowires: a direct Z-scheme mechanism. ACS Sustain. Chem. Eng 5, 367–375. [Google Scholar]
- Elanchezhiyan SS, Prabhu SM, Kim Y, Park CM, 2020. Lanthanum-substituted bimetallic magnetic materials assembled carboxylate-rich graphene oxide nanohybrids as highly efficient adsorbent for perfluorooctanoic acid adsorption from aqueous solutions. Appl. Surf. Sci 509, 144716. [Google Scholar]
- Fan Y, Cheng H, Zhou C, Xie X, Liu Y, Dai L, Zhang J, Qu L, 2012. Honeycomb architecture of carbon quantum dots: a new efficient substrate to support gold for stronger SERS. Nanoscale 4, 1776–1781. [DOI] [PubMed] [Google Scholar]
- Fernando KS, Sahu S, Liu Y, Lewis WK, Guliants EA, Jafariyan A, Wang P, Bunker CE, Sun Y-P, 2015. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 7, 8363–8376. [DOI] [PubMed] [Google Scholar]
- Fugetsu B, Satoh S, Shiba T, Mizutani T, Lin Y-B, Terui N, Nodasaka Y, Sasa K, Shimizu K, Akasaka T, 2004. Caged multiwalled carbon nanotubes as the adsorbents for affinity-based elimination of ionic dyes. Environ. Sci. Technol 38, 6890–6896. [DOI] [PubMed] [Google Scholar]
- Gan Y, Qiao L, 2012. Optical properties and radiation-enhanced evaporation of nanofluid fuels containing carbon-based nanostructures. Energy Fuels 26, 4224–4230. [Google Scholar]
- Gandhi MR, Vasudevan S, Shibayama A, Yamada M, 2016. Graphene and graphene-based composites: a rising star in water purification-a comprehensive overview. Chemistry 1, 4358–4385. [Google Scholar]
- Gao H, Zhao S, Cheng X, Wang X, Zheng L, 2013. Removal of anionic azo dyes from aqueous solution using magnetic polymer multi-wall carbon nanotube nanocomposite as adsorbent. Chem. Eng. J 223, 84–90. [Google Scholar]
- Garg B, Bisht T, 2016. Carbon nanodots as peroxidase nanozymes for biosensing. Molecules 21, 1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke I, Geiser A, Somborn-Schulz A, 2015. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, Shokrollahi A, Hossainian H, Kokhdan SN, 2011. Comparison of activated carbon and multiwalled carbon nanotubes for efficient removal of eriochrome cyanine R (ECR): kinetic, isotherm, and thermodynamic study of the removal process. J. Chem. Eng. Data 56, 3227–3235. [Google Scholar]
- Ghaedi M, Zeinali N, Ghaedi A, Teimuori M, Tashkhourian J, 2014. Artificial neural network-genetic algorithm based optimization for the adsorption of methylene blue and brilliant green from aqueous solution by graphite oxide nanoparticle. Spectrochim. Acta Mol. Biomol. Spectrosc 125, 264–277. [DOI] [PubMed] [Google Scholar]
- Goh K, Setiawan L, Wei L, Si R, Fane AG, Wang R, Chen Y, 2015. Graphene oxide as effective selective barriers on a hollow fiber membrane for water treatment process. J. Membr. Sci 474, 244–253. [Google Scholar]
- Gong J-L, Wang B, Zeng G-M, Yang C-P, Niu C-G, Niu Q-Y, Zhou W-J, Liang Y, 2009. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard Mater 164, 1517–1522. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat M, 2013. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci 193, 24–34. [DOI] [PubMed] [Google Scholar]
- Hashim DP, Narayanan NT, Romo-Herrera JM, Cullen DA, Hahm MG, Lezzi P, Suttle JR, Kelkhoff D, Munoz-Sandoval E, Ganguli S, 2012. Covalently bonded three-dimensional carbon nanotube solids via boron induced nano-junctions. Sci. Rep 2, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi T, Kubota J, Domen K, 2014. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev 43, 7520–7535. [DOI] [PubMed] [Google Scholar]
- Hoffmann MR, Martin ST, Choi W, Bahnemann DW, 1995. Environmental applications of semiconductor photocatalysis. Chem. Rev 95, 69–96. [Google Scholar]
- Hola K, Bourlinos AB, Kozak O, Berka K, Siskova KM, Havrdova M, Tucek J, Safarova K, Otyepka M, Giannelis EP, 2014. Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO- induced red-shift emission. Carbon 70, 279–286. [Google Scholar]
- Hu J, Shao D, Chen C, Sheng G, Ren X, Wang X, 2011. Removal of 1-naphthylamine from aqueous solution by multiwall carbon nanotubes/iron oxides/cyclodextrin composite. J. Hazard Mater 185, 463–471. [DOI] [PubMed] [Google Scholar]
- Hu L, Sun Y, Li S, Wang X, Hu K, Wang L, Liang X.-j., Wu Y, 2014. Multifunctional carbon dots with high quantum yield for imaging and gene delivery. Carbon 67, 508–513. [Google Scholar]
- Huang Z-H, Zheng X, Lv W, Wang M, Yang Q-H, Kang F, 2011. Adsorption of lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27, 7558–7562. [DOI] [PubMed] [Google Scholar]
- Jackson P, Jacobsen NR, Baun A, Birkedal R, Kühnel D, Jensen KA, Vogel U, Wallin H, 2013. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J 7, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S-Y, Yun J-M, Lee Y-S, Kim H-I, 2010. Removal of Cu(II) ions by alginate/carbon nanotube/maghemite composite magnetic beads. Carbon Letters (Carbon Lett.) 11, 117–121. [Google Scholar]
- Ji L, Chen W, Duan L, Zhu D, 2009. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol 43, 2322–2327. [DOI] [PubMed] [Google Scholar]
- Jiang K, Sun S, Zhang L, Lu Y, Wu A, Cai C, Lin H, 2015. Red, green, and blue luminescence by carbon dots: full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed 54, 5360–5363. [DOI] [PubMed] [Google Scholar]
- Jiang L, Fan Z, 2014. Design of advanced porous graphene materials: from graphene nanomesh to 3D architectures. Nanoscale 6,1922–1945. [DOI] [PubMed] [Google Scholar]
- Jones S, Pramanik A, Kanchanapally R, Nellore B, Begum S, Sweet C, Ray P, 2017. ACS sustainable chem. Eng. Times 5, 7175–7187. [Google Scholar]
- Julkapli NM, Bagheri S, 2015. Graphene supported heterogeneous catalysts: an overview. Int. J. Hydrogen Energy 40, 948–979. [Google Scholar]
- Kabbashi NA, Atieh MA, Al-Mamun A, Mirghami ME, Alam M, Yahya N, 2009. Kinetic adsorption of application of carbon nanotubes for Pb(II) removal from aqueous solution. J. Environ. Sci 21, 539–544. [DOI] [PubMed] [Google Scholar]
- Kaur M, Kaur M, Sharma VK, 2018. Nitrogen-doped graphene and graphene quantum dots: a review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci 259, 44–64. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sharma S, Kansal SK, 2016. Synthesis of ZnS/CQDs nanocomposite and its application as a photocatalyst for the degradation of an anionic dye, ARS. Superlattice. Microst 98, 86–95. [Google Scholar]
- Ke J, Li X, Zhao Q, Liu B, Liu S, Wang S, 2017. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci 496, 425–433. [DOI] [PubMed] [Google Scholar]
- Kemp KC, Seema H, Saleh M, Le NH, Mahesh K, Chandra V, Kim KS, 2013. Environmental applications using graphene composites: water remediation and gas adsorption. Nanoscale 5, 3149–3171. [DOI] [PubMed] [Google Scholar]
- Khan A, Colmenares JC, Gläser R, 2020a. Lignin-based composite materials for photocatalysis and photovoltaics Lignin Chemistry. Springer, pp. 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Goepel M, Colmenares JC, Gläser R, 2020b. Chitosan-based N-doped carbon materials for electrocatalytic and photocatalytic applications. ACS Sustain. Chem. Eng 8, 4708–4727. [Google Scholar]
- Khin MM, Nair AS, Babu VJ, Murugan R, Ramakrishna S, 2012. A review on nanomaterials for environmental remediation. Energy Environ. Sci 5, 8075–8109. [Google Scholar]
- Kongkanand A, Kamat PV, 2007. Electron storage in single wall carbon nanotubes. Fermi level equilibration in semiconductor-SWCNT suspensions. ACS Nano 1, 13–21. [DOI] [PubMed] [Google Scholar]
- Konicki W, Pełech I, Mijowska E, Jasińska I, 2012. Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbon nanotubes-Fe3C nanocomposite: kinetics, equilibrium and thermodynamics. Chem. Eng. J 210, 87–95. [Google Scholar]
- Kumar R, Khan MA, Haq N, 2014. Application of carbon nanotubes in heavy metals remediation. Crit. Rev. Environ. Sci. Technol 44, 1000–1035. [Google Scholar]
- Kuo C-Y, Lin H-Y, 2009. Adsorption of aqueous cadmium(II) onto modified multi-walled carbon nanotubes following microwave/chemical treatment. Desalination 249, 792–796. [Google Scholar]
- Lee WJ, Lee JM, Kochuveedu ST, Han TH, Jeong HY, Park M, Yun JM, Kwon J, No K, Kim DH, 2011. Biomineralized N-doped CNT/TiO2 core/shell nanowires for visible light photocatalysis. ACS Nano 6, 935–943. [DOI] [PubMed] [Google Scholar]
- Li G, Wang F, Liu P, Chen Z, Lei P, Xu Z, Li Z, Ding Y, Zhang S, Yang M, 2018. Polymer dots grafted TiO2 nanohybrids as high performance visible light photocatalysts. Chemosphere 197, 526–534. [DOI] [PubMed] [Google Scholar]
- Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee ST, 2010. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed 49, 4430–4434. [DOI] [PubMed] [Google Scholar]
- Li L, Wu G, Yang G, Peng J, Zhao J, Zhu J-J, 2013. Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale 5, 4015–4039. [DOI] [PubMed] [Google Scholar]
- Li LL, Ji J, Fei R, Wang CZ, Lu Q, Zhang JR, Jiang LP, Zhu JJ, 2012. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv. Funct. Mater 22, 2971–2979. [Google Scholar]
- Li S, Yu X, Zhang G, Ma Y, Yao J, Keita B, Louis N, Zhao H, 2011. Green chemical decoration of multiwalled carbon nanotubes with polyoxometalate-encapsulated gold nanoparticles: visible light photocatalytic activities. J. Mater. Chem 21, 2282–2287. [Google Scholar]
- Lim SY, Shen W, Gao Z, 2015. Carbon quantum dots and their applications. Chem. Soc. Rev 44, 362–381. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu H, Xu Y, Song Y, Lian J, Zhao Y, Wang L, Huang L, Ji H, Li H, 2017. Graphene quantum dots modified mesoporous graphite carbon nitride with significant enhancement of photocatalytic activity. Appl. Catal. B Environ 207, 429–437. [Google Scholar]
- Liu L, Liu Y, Gao B, Ji R, Li C, Wang S, 2019. Removal of perfluorooctanoic acid (PFOA) andperfluorooctane sulfonate (PFOS) from water bycarbonaceous nanomaterials: a review. Critical Reviews in Environmental Science and Technology 1–34. 10.1080/10643389.10642019.11700751. [DOI] [Google Scholar]
- Liu S, Yan Z, Zhang Y, Wang R, Luo S-Z, Jing F, Chu W, 2018. Carbon nanotubes supported nickel as the highly efficient catalyst for hydrogen production through glycerol steam reforming. ACS Sustain. Chem. Eng 6, 14403–14413. [Google Scholar]
- Liu Y, Yu Y-X, Zhang W-D, 2013a. Carbon quantum dots-doped CdS microspheres with enhanced photocatalytic performance. J. Alloys Compd 569, 102–110. [Google Scholar]
- Liu X, Wang M, Zhang S, Pan B, 2013b. Application potential of carbon nanotubes in water treatment: a review. J. Environ. Sci 25, 1263–1280. [DOI] [PubMed] [Google Scholar]
- Lu C, Liu C, 2006. Removal of nickel (II) from aqueous solution by carbon nanotubes. Journal of chemical technology & biotechnology: international research in process. Environmental & Clean Technology 81,1932–1940. [Google Scholar]
- Lu F, Astruc D, 2020. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev 408, 213180. [Google Scholar]
- Lu Q, Zhang Y, Liu S, 2015. Graphene quantum dots enhanced photocatalytic activity of zinc porphyrin toward the degradation of methylene blue under visible-light irradiation. J. Mater. Chem 3, 8552–8558. [Google Scholar]
- Luo PG, Sahu S, Yang S-T, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L, Sun Y-P, 2013. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 1, 2116–2127. [DOI] [PubMed] [Google Scholar]
- Ma H, Hsiao BS, Chu B, 2011. Ultrafine cellulose nanofibers as efficient adsorbents for removal of in water. ACS Macro Lett. 1, 213–216. [DOI] [PubMed] [Google Scholar]
- Ma J, Yu F, Zhou L, Jin L, Yang M, Luan J, Tang Y, Fan H, Yuan Z, Chen J, 2012. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 4, 5749–5760. [DOI] [PubMed] [Google Scholar]
- Mahmoud ME, Fekry NA, Abdelfattah AM, 2020. Removal of uranium(VI) from water by the action of microwave-rapid green synthesized carbon quantum dots from starch-water system and supported onto polymeric matrix. Journal of Hazardous Materials, 122770. [DOI] [PubMed] [Google Scholar]
- Markova Z, Bourlinos AB, Safarova K, Polakova K, Tucek J, Medrik I, Siskova K, Petr J, Krysmann M, Giannelis EP, 2012. Synthesis and properties of core-shell fluorescent hybrids with distinct morphologies based on carbon dots. J. Mater. Chem 22, 16219–16223. [Google Scholar]
- Martins NC, Ângelo J, Girão AV, Trindade T, Andrade L, Mendes A, 2016. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B Environ 193, 67–74. [Google Scholar]
- Meng Z-D, Zhu L, Choi J-G, Park C-Y, Oh W-C, 2011. Preparation, characterization and photocatalytic behavior of WO3-fullerene/TiO2 catalysts under visible light. Nanoscale research letters 6, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Arockiadoss T, Ramaprabhu S, 2010. Study of removal of azo dye by functionalized multi walled carbon nanotubes. Chem. Eng. J 162, 1026–1034. [Google Scholar]
- Mozia S, Tomaszewska M, Morawski AW, 2007. Photocatalytic membrane reactor (PMR) coupling photocatalysis and membrane distillation—effectiveness of removal of three azo dyes from water. Catal. Today 129, 3–8. [Google Scholar]
- Mukherjee S, Philip L, Pradeep T, 2020. Sustainable Materials for Affordable Point-of-Use Water Purification In: Naddeo V, Balakrishnan M, Choo KH (Eds.), Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development). Springer, Cham. [Google Scholar]
- Muthulingam S, Bae KB, Khan R, Lee I-H, Uthirakumar P, 2016. Carbon quantum dots decorated N-doped ZnO: synthesis and enhanced photocatalytic activity on UV, visible and daylight sources with suppressed photocorrosion. Journal of Environmental Chemical Engineering 4,1148–1155. [Google Scholar]
- Naghdi S, Sajjadi M, Nasrollahzadeh M, Rhee KY, Sajadi SM, Jaleh B, 2018. Cuscuta reflexa leaf extract mediated green synthesis of the Cu nanoparticles on graphene oxide/manganese dioxide nanocomposite and its catalytic activity toward reduction of nitroarenes and organic dyes. Journal of the Taiwan Institute of Chemical Engineers 86, 158–173. [Google Scholar]
- Naghizadeh A, Yari AR, Tashauoei HR, Mahdavi M, Derakhshani E, Rahimi R, Bahmani P, Daraei H, Ghahremani E, 2012. Carbon nanotubes technology for removal of arsenic from water. Archives of Hygiene Sciences 1, 6–11. [Google Scholar]
- Nasrollahzadeh M, Nezafat Z, Gorab MG, Sajjadi M, 2020a. Recent progresses in graphene-based (photo)catalysts for reduction of nitro compounds. Molecular Catalysis 484, 110758. [Google Scholar]
- Nasrollahzadeh M, Baran T, Baran NY, Sajjadi M, Tahsili MR, Shokouhimehr M, 2020b. Pd nanocatalyst stabilized on amine-modified zeolite: antibacterial and catalytic activities for environmental pollution remediation in aqueous medium. Separ. Purif. Technol 239, 116542. [Google Scholar]
- Nasrollahzadeh M, Sajadi MS, Atarod M, Sajjadi M, Isaabadi Z, 2019c. An Introduction to Green Nanotechnology. Academic Press. [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS, 2020. Green-synthesized nanocatalysts and nanomaterials for water treatment: current challenges and future perspectives. Journal of Hazardous Materials, 123401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Komber H, Khonakdar HA, Sajadi SM, 2019b. In situ green synthesis of Cu-Ni bimetallic nanoparticles supported on reduced graphene oxide as an effective and recyclable catalyst for the synthesis of N-benzyl-N-aryl-5-amino-1H-tetrazoles. Appl. Organomet. Chem 33, e4938. [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Maham M, Sajadi SM, Barzinjy AA, 2018. Biosynthesis of the palladium/sodium borosilicate nanocomposite using Euphorbia milii extract and evaluation of its catalytic activity in the reduction of chromium (VI), nitro compounds and organic dyes. Mater. Res. Bull 102, 24–35. [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Sajadi SM, 2019. Functionalized-graphene and graphene oxide: fabrication and application in catalysis. Photoenergy and thin film materials, 661–727. [Google Scholar]
- Neto AC, Guinea F, Peres NM, Novoselov KS, Geim AK, 2009. The electronic properties of graphene. Rev. Mod. Phys 81, 109. [Google Scholar]
- Ouni L, Ramazani A, Fardood ST, 2019. An overview of carbon nanotubes role in heavy metals removal from wastewater. Front. Chem. Sci. Eng 1–22. [Google Scholar]
- Padhye LP, Yao H, Kung’u FT, Huang C-H, 2014. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 51, 266–276. [DOI] [PubMed] [Google Scholar]
- Pakzad K, Alinezhad H, Nasrollahzadeh M, 2019. Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram. Int 45, 17173–17182. [Google Scholar]
- Pan D, Jiao J, Li Z, Guo Y, Feng C, Liu Y, Wang L, Wu M, 2015. Efficient separation of electron-hole pairs in graphene quantum dots by TiO2 heterojunctions for dye degradation. ACS Sustain. Chem. Eng 3, 2405–2413. [Google Scholar]
- Perez-Page M, Yu E, Li J, Rahman M, Dryden DM, Vidu R, Stroeve P, 2016. Template-based syntheses for shape controlled nanostructures. Adv. Colloid Interface Sci 234, 51–79. [DOI] [PubMed] [Google Scholar]
- Perreault F, De Faria AF, Elimelech M, 2015. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev 44, 5861–5896. [DOI] [PubMed] [Google Scholar]
- Petersen EJ, Zhang L, Mattison NT, O’Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang Q, Henry TB, Holbrook RD, 2011. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol 45, 9837–9856. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M, Asadi A, Sillanpää M, Farhadian N, 2018. Application of carbon quantum dots to increase the activity of conventional photocatalysts: a systematic review. J. Mol. Liq 271, 857–871. [Google Scholar]
- Putri LK, Ong W-J, Chang WS, Chai S-P, 2015. Heteroatom doped graphene in photocatalysis: a review. Appl. Surf. Sci 358, 2–14. [Google Scholar]
- Pyrzynska K, Stafiej A, 2012. Sorption behavior of Cu(II), Pb(II), and Zn(II) onto carbon nanotubes. Solvent Extr. Ion Exch 30, 41–53. [Google Scholar]
- Qian X, Yue D, Tian Z, Reng M, Zhu Y, Kan M, Zhang T, Zhao Y, 2016. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Appl. Catal. B Environ 193, 16–21. [Google Scholar]
- Qiu B, Zhou Y, Ma Y, Yang X, Sheng W, Xing M, Zhang J, 2015. Facile synthesis of the Ti 3+ self-doped TiO 2-graphene nanosheet composites with enhanced photocatalysis. Sci. Rep 5, 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Zheng M, Du P, Zhou Y, Zhang L, Li D, Tan H, Zhao Z, Xie Z, Sun Z, 2013. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 5, 12272–12277. [DOI] [PubMed] [Google Scholar]
- Qu S, Huang F, Yu S, Chen G, Kong J, 2008. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. Hazard Mater 160, 643–647. [DOI] [PubMed] [Google Scholar]
- Rajabi M, Mahanpoor K, Moradi O, 2017. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: critical review. RSC Adv. 7, 47083–47090. [Google Scholar]
- Rao GP, Lu C, Su F, 2007. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Separ. Purif. Technol 58, 224–231. [Google Scholar]
- Rizzo L, Meric S, Guida M, Kassinos D, Belgiorno V, 2009. Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res. 43, 4070–4078. [DOI] [PubMed] [Google Scholar]
- Ruan G, Thakur D, Deng S, Hawkins S, Winter J, 2009. Fluorescent-magnetic nanoparticles for imaging and cell manipulation. Proc. Inst. Mech. Eng. - Part N J. Nanoeng. Nanosyst 223, 81–86. [Google Scholar]
- Sabzevari M, Cree DE, Wilson LD, 2018. Graphene oxide-chitosan composite material for treatment of a model dye effluent. ACS Omega 3, 13045–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safardoust-Hojaghan H, Salavati-Niasari M, 2017. Degradation of methylene blue as a pollutant with N-doped graphene quantum dot/titanium dioxide nanocomposite. J. Clean. Prod 148, 31–36. [Google Scholar]
- Sajjadi M, Baran NY, Baran T, Nasrollahzadeh M, Tahsili MR, Shokouhimehr M, 2020. Palladium nanoparticles stabilized on a novel Schiff base modified Unye bentonite: highly stable, reusable and efficient nanocatalyst for treating wastewater contaminants and inactivating pathogenic microbes.Separ. Purif. Technol 237, 116383. [Google Scholar]
- Saleh NB, Khalid A, Tian Y, Ayres C, Sabaraya IV, Pietari J, Hanigan D, Chowdhury I, Apul OG, 2019. Removal of poly- and per-fluoroalkyl substances from aqueous systems by nano-enabled water treatment strategies. Environ. Sci.: Water Res. Technol 5, 198–208. [Google Scholar]
- Shatkin J, Wegner T, Neih W, 2013. Incorporating life-cycle thinking into risk assessment for nanoscale materials: case study of nanocellulose, 2013 TAPPI International Conference on Nanotechnology for Renewable Materials. [Google Scholar]
- Shen Y, Fang Q, Chen B, 2014. Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection. Environ. Sci. Technol 49, 67–84. [DOI] [PubMed] [Google Scholar]
- Simeonidis K, Mourdikoudis S, Kaprara E, Mitrakas M, Polavarapu L, 2016. Inorganic engineered nanoparticles in drinking water treatment: a critical review. Environ. Sci. J. Integr. Environ. Res.: Water Research & Technology 2, 43–70. [Google Scholar]
- Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, Gagor A, Feist B, Wrzalik R, 2013a. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 42, 5682–5689. [DOI] [PubMed] [Google Scholar]
- Sitko R, Zawisza B, Malicka E, 2013b. Graphene as a new sorbent in analytical chemistry. Trac. Trends Anal. Chem 51, 33–43. [Google Scholar]
- Sk MA, Ananthanarayanan A, Huang L, Lim KH, Chen P, 2014. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem C 2, 6954–6960. [Google Scholar]
- Song N, Gao X, Ma Z, Wang X, Wei Y, Gao C, 2018. A review of graphene-based separation membrane: materials, characteristics, preparation and applications. Desalination 437, 59–72. [Google Scholar]
- Su Y, Liu G, Zeng C, Lu Y, Luo H, Zhang R, 2020. Carbon quantum dots-decorated TiO2/g-C3N4 film electrode as a photoanode with improved photo-electrocatalytic performance for 1,4-dioxane degradation. Chemosphere, 12638. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Pan Z, Rong G, Li H, Zhou L, Li W, Qiu Y, Xu Y, Hou Y, Zheng Z, 2017. Graphene quantum dot antennas for high efficiency Förster resonance energy transfer based dye-sensitized solar cells. J. Power Sources 343, 39–46. [Google Scholar]
- Sui Z-Y, Cui Y, Zhu J-H, Han B-H, 2013. Preparation of three-dimensional graphene oxide-polyethylenimine porous materials as dye and gas adsorbents. ACS Appl. Mater. Interfaces 5, 9172–9179. [DOI] [PubMed] [Google Scholar]
- Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, 2006. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc 128, 7756–7757. [DOI] [PubMed] [Google Scholar]
- Surwade SP, Smirnov SN, Vlassiouk IV, Unocic RR, Veith GM, Dai S, Mahurin SM, 2015. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol 10, 459–464. [DOI] [PubMed] [Google Scholar]
- Tabish TA, Memon FA, Gomez DE, Horsell DW, Zhang S, 2018. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep 8, 1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajik S, Dourandish Z, Zhang K, Beitollahi H, Van Le Q, Jang HW, Shokouhimehr M, 2020. Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 10, 15406–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Kong B, Wu H, Xu M, Wang Y, Wang Y, Zhao D, Zheng G, 2013. Carbon nanodots featuring efficient FRET for real-time monitoring of drug delivery and two-photon imaging. Adv. Mater 25, 6569–6574. [DOI] [PubMed] [Google Scholar]
- Tian T, Shi X, Cheng L, Luo Y, Dong Z, Gong H, Xu L, Zhong Z, Peng R, Liu Z, 2014. Graphene-based nanocomposite as an effective, multifunctional, and recyclable antibacterial agent. ACS Appl. Mater. Interfaces 6, 8542–8548. [DOI] [PubMed] [Google Scholar]
- Tofighy MA, Mohammadi T, 2011. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard Mater 185, 140–147. [DOI] [PubMed] [Google Scholar]
- Upadhyayula VK, Deng S, Mitchell MC, Smith GB, 2009. Application of carbon nanotube technology for removal of contaminants in drinking water: a review. Sci. Total Environ 408, 1–13. [DOI] [PubMed] [Google Scholar]
- Varma RS, 2016. Greener and sustainable trends in synthesis of organics and nanomaterials. ACS Sustain. Chem. Eng 4, 5866–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang P, Liang B, Liu Y, Xu T, Wang L, Cao B, Pan K, 2016a. Graphene oxide as an effective barrier on a porous nanofibrous membrane for water treatment. ACS Appl. Mater. Interfaces 8, 6211–6218. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li J, Chen C, Ren X, Hu J, Wang X, 2011. Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem. Eng. J 174, 126–133. [Google Scholar]
- Wang X, Sun G, Li N, Chen P, 2016b. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev 45, 2239–2262. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pan C, Chu W, Vipin AK, Sun L, 2019. Environmental remediation applications of carbon nanotubes and graphene oxide: adsorption and catalysis. Nanomaterials 9, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani AA, Khan AM, Manea YK, Shahadat M, Shaikh ZA, Ali SW, 2020. Graphene-supported organic-inorganic layered double hydroxides and their environmental applications: a review. Journal of cleaner production, 122980. [Google Scholar]
- Wei H, Deng S, Huang Q, Nie Y, Wang B, Huang J, Yu G, 2013. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution. Water Res. 47, 4139–4147. [DOI] [PubMed] [Google Scholar]
- Westerhoff P, Alvarez P, Li Q, Gardea-Torresdey J, Zimmerman J, 2016. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. J. Integr. Environ. Res.: Nano 3, 1241–1253. [Google Scholar]
- Woan K, Pyrgiotakis G, Sigmund W, 2009. Photocatalytic carbon-nanotube-TiO2 composites. Adv. Mater 21, 2233–2239. [Google Scholar]
- Wu T, Chen M, Zhang L, Xu X, Liu Y, Yan J, Wang W, Gao J, 2013. Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J. Mater. Chem 1, 7612–7621. [Google Scholar]
- Wu X, Zhang Y, Han T, Wu H, Guo S, Zhang J, 2014. Composite of graphene quantum dots and Fe3O4 nanoparticles: peroxidase activity and application in phenolic compound removal. RSC Adv. 4, 3299–3305. [Google Scholar]
- Xie X-L, Mai Y-W, Zhou X-P, 2005. Dispersion and alignment of carbon nanotubes in polymer matrix: a review. Mater. Sci. Eng. R Rep 49, 89–112. [Google Scholar]
- Xing Z, Ju Z, Yang J, Xu H, Qian Y, 2013. One-step solid state reaction to selectively fabricate cubic and tetragonal CuFe2O4 anode material for high power lithium ion batteries. Electrochim. Acta 102, 51–57. [Google Scholar]
- Xu C, Wang J, Yang T, Chen X, Liu X, Ding X, 2015. Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydr. Polym 121, 79–85. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang J, 2017. The application of graphene-based materials for the removal of heavy metals and radionuclides from water and wastewater. Crit. Rev. Environ. Sci. Technol 47, 1042–1105. [Google Scholar]
- Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA, 2004. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc 126, 12736–12737. [DOI] [PubMed] [Google Scholar]
- Xu Y-J, Zhuang Y, Fu X, 2010. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: a case study on degradation of benzene and methyl orange. J. Phys. Chem. C 114, 2669–2676. [Google Scholar]
- Yan F, Kong D, Fu Y, Ye Q, Wang Y, Chen L, 2016a. Construction of carbon nanodots/tungsten trioxide and their visible-light sensitive photocatalytic activity. J. Colloid Interface Sci 466, 268–274. [DOI] [PubMed] [Google Scholar]
- Yan M, Hua Y, Zhu F, Gu W, Jiang J, Shen H, Shi W, 2017. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 pn junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B Environ 202, 518–527. [Google Scholar]
- Yan M, Zhu F, Gu W, Sun L, Shi W, Hua Y, 2016b. Construction of nitrogen-doped graphene quantum dots-BiVO4/gC3N4 Z-scheme photocatalyst and enhanced photocatalytic degradation of antibiotics under visible light. RSC Adv. 6, 61162–61174. [Google Scholar]
- Yan P, Zhang X, Wang X, Zhang X, 2020. Controllable preparation of mono-disperse mesoporous silica from microspheres to microcapsules and catalytic loading of Au nanoparticles. Langmuir 36, 5271–5279. [DOI] [PubMed] [Google Scholar]
- Yan T, Liu J, Lei H, Shi L, An Z, Park HS, Zhang D, 2018. Capacitive deionization of saline water using sandwich-like nitrogen-doped graphene composites via a self-assembling strategy. Environ. Sci. J. Integr. Environ. Res.: Nano 5, 2722–2730. [Google Scholar]
- Yan Y, Gong J, Chen J, Zeng Z, Huang W, Pu K, Liu J, Chen P, 2019. Recent advances on graphene quantum dots: from chemistry and physics to applications. Adv. Mater 31, 1808283. [DOI] [PubMed] [Google Scholar]
- Yang S, Hu J, Chen C, Shao D, Wang X, 2011. Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol 45, 3621–3627. [DOI] [PubMed] [Google Scholar]
- Yeh TF, Teng CY, Chen SJ, Teng H, 2014. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light Illumination. Adv. Mater 26, 3297–3303. [DOI] [PubMed] [Google Scholar]
- Yu F, Wu Y, Ma J, Zhang C, 2013. Adsorption of lead on multi-walled carbon nanotubes with different outer diameters and oxygen contents: kinetics, isotherms and thermodynamics. J. Environ. Sci 25, 195–203. [DOI] [PubMed] [Google Scholar]
- Yu H, Shi R, Zhao Y, Waterhouse GI, Wu LZ, Tung CH, Zhang T, 2016a. Smart utilization of carbon dots in semiconductor photocatalysis. Adv. Mater 28, 9454–9477. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhang H, Huang H, Liu Y, Li H, Ming H, Kang Z, 2012. ZnO/carbon quantum dots nanocomposites: one-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature. New J. Chem 36, 1031–1035. [Google Scholar]
- Yu S, Zhong Y-Q, Yu B-Q, Cai S-Y, Wu L-Z, Zhou Y, 2016b. Graphene quantum dots to enhance the photocatalytic hydrogen evolution efficiency of anatase TiO2 with exposed {001} facet. Phys. Chem. Chem. Phys 18, 20338–20344. [DOI] [PubMed] [Google Scholar]
- Yu Z, Dang Q, Liu C, Cha D, Zhang H, Zhu W, Zhang Q, Fan B, 2017. Preparation and characterization of poly (maleic acid)-grafted cross-linked chitosan microspheres for Cd(II) adsorption. Carbohydr. Polym 172, 28–39. [DOI] [PubMed] [Google Scholar]
- Zemmouri H, Drouiche M, Sayeh A, Lounici H, Mameri N, 2013. Chitosan application for treatment of Beni-Amrane’s water dam. Energy Procedia 36, 558–564. [Google Scholar]
- Zeng Z, Chen S, Tan TTY, Xiao F-X, 2018. Graphene quantum dots (GQDs) and its derivatives for multifarious photocatalysis and photoelectrocatalysis. Catal. Today 315, 171–183. [Google Scholar]
- Zhang H, Gong Q, Ren S, Arshid MA, Chu W, Chen C, 2018. Implication of iron nitride species to enhance the catalytic activity and stability of carbon nanotubes supported Fe catalysts for carbon-free hydrogen production via low-temperature ammonia decomposition. Catalysis Science & Technology 8, 907–915. [Google Scholar]
- Zhang H, Ming H, Lian S, Huang H, Li H, Zhang L, Liu Y, Kang Z, Lee S-T, 2011. Fe2O3/carbon quantum dots complex photocatalysts and their enhanced photocatalytic activity under visible light. Dalton Trans. 40, 10822–10825. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Xiong Z, Zhao X, 2010. Pillaring chemically exfoliated graphene oxide with carbon nanotubes for photocatalytic degradation of dyes under visible light irradiation. ACS Nano 4, 7030–7036. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, Xu C, Merlin D, 2016. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther 24, 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Ding Z, 2018. Recent advances in graphene quantum dots as bioimaging probes. Journal of Analysis and Testing 2, 45–60. [Google Scholar]
- Zhang W, Zhang D, Liang Y, 2019. Nanotechnology in remediation of water contaminated by poly- and perfluoroalkyl substances: a review. Environ. Pollut 247, 266–276. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu C, Zhou X, Wu X, Yang Y, Wu H, Guo S, Zhang J, 2013. Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 5, 1816–1819. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sèbe G, Rentsch D, Zimmermann T, Tingaut P, 2014. Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem. Mater 26, 2659–2668. [Google Scholar]
- Zhao G, Li J, Ren X, Chen C, Wang X, 2011. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol 45, 10454–10462. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang Z, White JC, Xing B, 2014. Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol 48, 9995–10009. [DOI] [PubMed] [Google Scholar]
- Zhao X-H, Jiao F-P, Yu J-G, Xi Y, Jiang X-Y, Chen X-Q, 2015. Removal of Cu(II) from aqueous solutions by tartaric acid modified multi-walled carbon nanotubes. Colloid. Surface. Physicochem. Eng. Aspect 476, 35–41. [Google Scholar]
- Zhao Y, Zhao B, Liu J, Chen G, Gao R, Yao S, Li M, Zhang Q, Gu L, Xie J, 2016. Oxide-modified nickel photocatalysts for the production of hydrocarbons in visible light. Angew. Chem. Int. Ed 55, 4215–4219. [DOI] [PubMed] [Google Scholar]
- Zheng M, Liu S, Li J, Qu D, Zhao H, Guan X, Hu X, Xie Z, Jing X, Sun Z, 2014. Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv. Mater 26, 3554–3560. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang Y, Wang C, Wu X, Yang Y, Zheng B, Wu H, Guo S, Zhang J, 2012. Photo-Fenton reaction of graphene oxide: a new strategy to prepare graphene quantum dots for DNA cleavage. ACS Nano 6, 6592–6599. [DOI] [PubMed] [Google Scholar]
- Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B, 2015. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano research 8, 355–381. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


