Abstract
The hypothalamus and pituitary have been identified to play essential roles in maintaining homeostasis. Various diseases can disrupt the functions of these systems, which can often result in serious lifelong symptoms. The current treatment for hypopituitarism involves hormone replacement therapy. However, exogenous drug administration cannot mimic the physiological changes that are a result of hormone requirements. Therefore, patients are at a high risk of severe hormone deficiency, including adrenal crisis.
Pluripotent stem cells (PSCs) self-proliferate and differentiate into all types of cells. The generation of endocrine tissues from PSCs has been considered as another new treatment for hypopituitarism. Our colleagues established a 3-dimensional (3D) culture method for embryonic stem cells (ESCs). In this culture, the ESC-derived aggregates exhibit self-organization and spontaneous formation of highly ordered patterning. Recent results have shown that strict removal of exogenous patterning factors during early differentiation efficiently induces rostral hypothalamic progenitors from mouse ESCs. These hypothalamic progenitors generate vasopressinergic neurons, which release neuropeptides upon exogenous stimulation.
Subsequently, we reported adenohypophysis tissue self-formation in 3D cultures of mouse ESCs. The ESCs were found to differentiate into both nonneural oral ectoderm and hypothalamic neuroectoderm in adjacent layers. Interactions between the 2 tissues appear to be critically important for in vitro induction of a Rathke’s pouch-like developing embryo. Various endocrine cells were differentiated from nonneural ectoderm. The induced corticotrophs efficiently secreted adrenocorticotropic hormone when engrafted in vivo, which rescued hypopituitary hosts.
For future regenerative medicine, generation of hypothalamic and pituitary tissues from human PSCs is necessary. We and other groups succeeded in establishing a differentiation method with the use of human PSCs. Researchers could use these methods for models of human diseases to elucidate disease pathology or screen potential therapeutics.
Keywords: hypothalamus, pituitary, embryonic stem cells, induced pluripotent stem cells, differentiation, regenerative medicine
The hypothalamus and pituitary gland have been considered as essential for controlling the somatic endocrine system. They work by close interactions between hormone secretion and feedback from the periphery. For example, the hypothalamus-pituitary-adrenal axis is a powerful regulator of physiological systems, which can respond to both psychological and physical stressors. The anterior pituitary (adenohypophysis) works by producing 6 important hormones, including adrenocorticotropic hormone (ACTH), growth hormone (GH), thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, and prolactin. The posterior pituitary gland (neurohypophysis) consists of axons and terminals of hypothalamic neurons, ie, vasopressin and oxytocin neurons. Hypothalamic and pituitary functions can be impaired under various conditions, including genetic disorders, tumors, inflammation, and surgery. However, their regeneration remains largely unclear.
Recently, studies have focused on embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). These pluripotent stem cells (PSCs) can self-proliferate and differentiate into all cell types and tissues. Therefore, regenerative medicine, such as the regeneration of cells or tissues from these PSCs, could provide new alternative treatments for intractable diseases.
The Need for Adenohypophysis Regenerative Medicine
Hypopituitarism is one of the most serious pituitary gland dysfunctions, which can cause life-threatening complications such as adrenal crisis due to insufficient glucocorticoid hormone secretion. Hypopituitarism can develop from both congenital and acquired diseases. Postoperative hypopituitarism is one of the most frequent dysfunctions of acquired hypopituitarism. Patients require hormone replacement therapy throughout their lives because the dysfunction of hormone-secreting cells rarely seen to improve. Currently, hormone replacement therapy is the sole effective treatment; however, its therapeutic benefits are sometimes limited. For example, exogenous hormone treatment cannot mimic the drastic physiologic changes that occur with the secretion of endogenous hormones in response to circadian rhythms or various physical and psychological stressors. One clinical research study showed that even in educated patients with chronic adrenal insufficiency who received glucocorticoid replacement therapy, there is still a high incidence of adrenal crisis. Furthermore, in this study, mortality associated with adrenal crisis occurred in approximately 6% of the patients [1].
Therefore, endocrine cells or tissue generated from human PSCs that can respond to both releasing and feedback signals could provide better treatment alternatives to current hormone replacement therapy.
Pituitary Gland Development
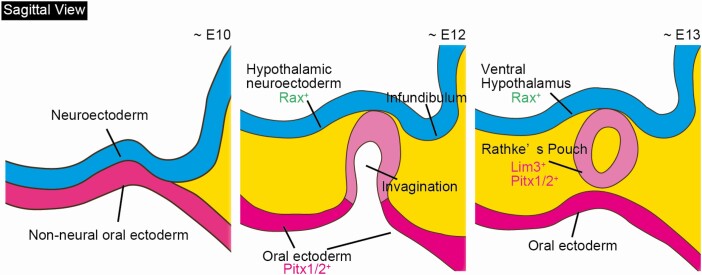
During early mammalian development, the pituitary anlage (Rathke’s pouch) originates as a placode in the nonneural oral ectoderm located immediately anterior to the rostral neural plate. The first step in Rathke’s pouch formation is when the thickened placode epithelium invaginates toward the ventral diencephalon, which finally differentiates into the ventral hypothalamus (Fig. 1). Subsequently, the invaginated placode detaches from the oral ectoderm and then forms a hollowed vesicle called Rathke’s pouch. The invagination of the oral ectoderm has been determined to depend on bone morphogenetic protein (BMP) 4, which is expressed in the overlying ventral diencephalon at E8.5 and later in the infundibulum [2]. Genetic deletion of BMP4 in some genetic backgrounds results in the failure of ectodermal thickening and invagination during E9.5–9.75 [3].
Figure 1.
Schematic of mouse pituitary development. Sagittal view of pituitary embryogenesis. Abbreviation: E, embryonic day.
The close interaction between the nonneural oral ectoderm and the ventral diencephalon is essential for Rathke’s pouch development. Several signaling molecules from both sides are involved in pituitary organogenesis. Sonic hedgehog (Shh) is expressed in the ventral diencephalon and oral ectoderm but is excluded from the invaginating Rathke’s pouch as soon as it becomes morphologically visible [2, 4, 5]. Shh signaling affects both proliferation and cell type determination during pituitary gland development [5].
The opposing signaling molecules BMP4, fibroblast growth factor (FGF) members, and Wnt5 are expressed in the ventral diencephalon. FGF8 and FGF10 have been found in the ventral diencephalon and subsequently in the posterior lobe of the pituitary and play important roles in pituitary development [2, 5, 6].These opposing interactions between the ventralizing and dorsalizing gradients convey the proliferative and positional cues of Rathke’s pouch [2].
Three-Dimensional Embryonic Stem Cell Culture
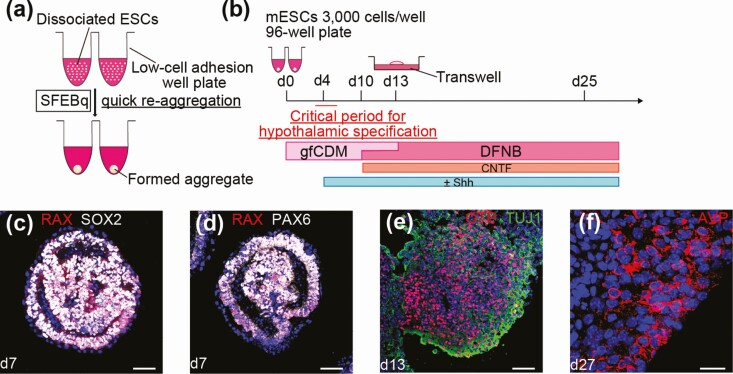
Currently, studies that focus on the induction of multiple types of cells or tissues from mouse and human pluripotent cells are actively ongoing. Our colleagues established a 3D culture method for ESCs, which is now termed as serum-free culture of embryoid body-like aggregates with quick reaggregation (SFEBq) [7, 8]. In brief, maintained pluripotent ESCs are dissociated into single cells by trypsin treatment and pipetting. Then, the dissociated single ESCs are autonomically and quickly aggregated when they are disseminated into low-cell adhesion well plates in differentiation medium (Fig. 2A). Our colleagues initially developed the SFEBq culture to generate telencephalic anlage from mouse [7] and human ESCs [8]. Subsequently, this culture method is considered appropriate for the induction of various ectodermal derivatives from ESCs. In SEFBq cultures, aggregates formed from ESCs exhibit self-organization and spontaneous formation into a highly ordered structure or pattern [9]. This floating culture has revealed intrinsic programs that drive locally autonomous modes of organogenesis and homeostasis. Using the SFEBq method, mesencephalic dopamine neurons [10, 11], cortical neurons [8, 12, 13], the optic cup [14-16], cerebellar neurons [17], and hippocampal neurons [18] have been generated from mouse and human ESCs.
Figure 2.
In vitro hypothalamic differentiation from mouse ESCs. A, Diagram of serum-free culture of embryoid body-like aggregates with quick reaggregation (SFEBq). B, Schematic of the culture protocol for hypothalamic differentiation from mouse ESCs. C, D, Immunostaining of early hypothalamic progenitor cells. RAX (c and d, red), SOX2 (c, white), PAX6 (d, white). E, Immunostaining of intermediate precursor cells. OTP (red), TUJ1 (green). F, In the mature phase of hypothalamic differentiation, hypothalamic neurons were observed. AVP (red). For all relevant panels, nuclear counterstaining was with DAPI (blue). Scale bars: 50µm (c-e); 20µm (f).
Induction of Hypothalamic Neurons from Mouse Pluripotent Stem Cells
Wataya et al proposed that mouse ESCs differentiate into rostral hypothalamic tissues when cultured as floating aggregates in a chemically defined medium lacking growth factors such as insulin [19] (Fig. 2B). They noted that the absence of high insulin signals at the early differentiation period (during days 4 and 5) is a key condition for the retina and anterior neural fold homeobox (Rax) expression, which is considered an early hypothalamic progenitor marker in SFEBq-cultured ES cells and generation of rostral hypothalamic neurons in vitro (Fig. 2C and 2D). In mice, Rax is specifically expressed in the rostral hypothalamic neuroepithelia and retinal neuroepithelia [20].
Shh is actively expressed in the ventral region of the embryonic hypothalamus [21]. Without Shh treatment, mouse ESCs differentiate into hypothalamic progenitors that have the characteristics of dorsal hypothalamic progenitors (Rax+/Pax6+/Nkx2.1-) (Fig. 2D), whereas Shh treatment promotes ventral hypothalamic differentiation (Rax+/Pax6-/Nkx2.1+). This indicates that Shh signaling promotes the specification of rostral–ventral hypothalamic progenitors at the cost of rostral–dorsal progenitors in vitro. Around day 25, mouse ESC-derived dorsal hypothalamic progenitors can generate arginine-vasopressin (AVP) producing neurons, presumably via OTP+/Brn2+ intermediate precursors [22] (Fig. 2E and 2F). Using AVP-release analysis upon high K+ stimulation, these induced AVP+ neurons secrete a substantial amount of neuropeptide AVP. Conversely, in the presence of exogenous Shh, Rax+ hypothalamic progenitor cells differentiate into SF1+/VGLUT2+ ventral hypothalamus neurons, TH+/Nkx2.1+ dopaminergic neurons, and AgRP+/NPY+ neurons which are typically found in the arcuate nucleus of the hypothalamus.
Currently, we have improved the methods for the differentiation of hypothalamic vasopressin neurons using mouse iPSCs [23]. As in the case of mouse ESCs, the previous 3D floating culture was also effective for hypothalamic differentiation from mouse iPSCs. Fgf8 is reported to be necessary to increase Otp expression in the supraoptic nucleus, and both are precursors of AVP [24]. Furthermore, Fgf8-hypomorphic mice showed a reduction in AVP [25]. Therefore, in our system, we integrated Fgf8b and heparin treatment and optimized the timing and concentration, which resulted in the increased numbers of Otp-positive precursors and subsequently AVP-positive neurons. Moreover, K+ stimulation tests showed that AVP secretion of Fgf8b-treated aggregates has greatly increased compared to aggregates in the conventional method.
The Need for Hypothalamus Regenerative Medicine
Diabetes insipidus has been defined as a disorder characterized by the excretion of abnormally large volumes of diluted urine, resulting in excessive urination (polyuria) and thirst (polydipsia) [26]. Deficiency of the antidiuretic nonapeptide hormone AVP in the blood leads to a disorder called central diabetes insipidus (CDI). CDI is the result of a number of somatic and central disorders, such as central nerve tumors, metastases, inflammation, neurosurgical interventions, and congenital disease. Desmopressin (DDAVP) is an AVP analogue used in the treatment of CDI. Deficiency or excess of DDAVP could potentially lead to hypernatremia or hyponatremia, which in turn can result in life-threatening conditions. Moreover, some CDI patients are adipsic because not only the AVP neurons but also hypothalamic osmoreceptors are dysfunctional, and these patients easily become dehydrated unless properly treated [27, 28]. Adipsic CDI patients reportedly cannot feel thirst and do not consume appropriate amounts of water even when they have severe hypernatremia and dehydration. Although most adipsic CDI patients are educated to measure body weight every day and to determine the amount of daily water intake based on their body weight with a fixed amount of DDAVP, they are still at high risk of death compared to those without adipsia [29]. To overcome these limitations of current DDAVP therapy, regenerative medicine involving hypothalamic AVP neurons is warranted.
Induction of Hypothalamic Neurons from Human Pluripotent Stem Cells
Both Wang et al and Merkle et al developed 2-dimensional (2D) differentiation protocols to generate human hypothalamic neurons [30, 31]. A combination of early activation of Shh signaling followed by timed NOTCH inhibition in human ESCs or iPSCs has been determined to induce efficient conversion into ventral hypothalamic NKX2.1+ precursors [30]. The application of a NOTCH inhibitor and brain-derived neurotrophic factor further directed the cells into arcuate nucleus hypothalamic-like neurons that express hypothalamic neuron markers proopiomelanocortin (POMC), neuropeptide Y (NPY), agouti-related peptide (AGRP), somatostatin, and dopamine. Importantly, these hypothalamic neural cells produce and secrete POMC neuropeptides and increase p-AKT and p-STAT3 in response to insulin and leptin.
Merkle et al. differentiated human PSCs in the presence of inhibitors of the BMP and TGFβ/NODAL/activin signaling pathways to promote neural differentiation and used an inhibitor of the posteriorizing WNT pathway combined with Shh signaling to induce ventral neural characteristics [31]. They also adapted a previously described a 3D differentiation protocol for human PSCs. Unlike mouse ESCs, human PSCs died after several days even in the presence of the Rho kinase (ROCK) inhibitor Y27632 [32]. To overcome this cell death, they supplemented gfCDM with insulin and Akt inhibitor since Akt signaling inhibited hypothalamic differentiation under similar conditions [19]. The self-patterned cell aggregates adopted a hypothalamic identity expressing Rax, Nkx2.1, OTP, and single-minded homolog 1 [33]. On day 90, these hypothalamic progenitors have given rise to physiologically important populations of hypothalamic neurons, including αMSH/POMC, AGRP, oxytocin, and AVP. Although human PSC-derived cells display neuronal morphology with extensive arbors of neurites and are immunopositive for hypothalamic neuropeptides, the capacity for neuropeptide secretion in this culture method was not evaluated.
Currently, we have improved the efficiency of hypothalamic neuron induction from human ESCs using a 3D culture [34]. When we added a small amount of knockout serum replacement into gfCDM, human ESCs successfully aggregated. Subsequently, when both smoothened agonist (SAG) and BMP4 were combined, these human ESC-derived aggregates differentiated into RAX-positive hypothalamic progenitor cells. Then, floating aggregates were dissociated between days 30 and 90. As a result, few AVP neurons were detected on days 80 to 150. To promote a more rostral positional identity, we used an Akt inhibitor from day 6 together with BMP4 and SAG. This Akt inhibitor treatment increased the number of human ESC-derived AVP+ neurons. The concentration of secreted AVP was further increased when the neurons were stimulated with KCl, suggesting that they were able to secrete AVP and react to stimulation.
Induction of Pituitary Tissue from Mouse Pluripotent Stem Cells
We previously reported self-formation of functional pituitary tissues from mouse ESCs when we applied the method described above [35]. Mouse ESCs treated with N-methyl-N9-(3-pyridinylbenzyl)-N9-(3-chlorobenzo[b]thiophene-2-carbonyl)-1,4-diaminocyclohexane (smoothened agonist, or SAG), a hedgehog agonist, were stimulated to differentiate into 2 different layers: an outer nonneural ectoderm (oral ectoderm) layer and an inner hypothalamic neuroectoderm layer within the same aggregate. The outer layer expressed LHX3 (also called LIM3) [36, 37], an early Rathke’s pouch marker in SFEBq culture. LHX3 has been determined to be essential for these hormone-producing lineages. Knockdown of LHX3 inhibited subsequent differentiation into hormone-producing cells, which supports altered pituitary development in LHX3 knockout mice [37]. In these SAG-treated SFEBq aggregates, LHX3+ tissues appear as thickened placode epithelium on the surface around day 8, invaginate on days 9 to 10, and form hollow epithelial vesicles on days 10 to 11, as in embryonic Rathke’s pouch development. They differentiated further into various endocrine cell types including corticotrophs and somatotrophs (GH-producing cells). The corticotrophs efficiently produced ACTH in response to corticotrophin-releasing hormone (CRH), a hypothalamic hormone that stimulates ACTH secretion of pituitary corticotrophs. ACTH secretion from the pituitary is negatively regulated by the downstream hormone glucocorticoid. Consistent with this in vivo regulation, the in vitro release of ACTH by CRH was greatly suppressed by glucocorticoid pretreatment. These results indicate that the mouse ESC-derived corticotrophs can respond normally to both positive and negative regulators that function in the hypothalamus-pituitary-adrenal axis in vivo. When these cells were grafted, they rescued the phenotype of the hypopituitary model mice by producing ACTH. The grafted mice can then survive without corticosterone replacement.
Induction of Pituitary Tissue From Human Pluripotent Stem Cells
The application of this mESC culture method to human ESCs has been considered to be necessary for therapeutic application and disease modeling. We recently developed the self-organizing 3D culture of human ESCs to generate regulator-responsive anterior pituitary tissue in vitro [38]. As in mouse ESCs, we found that adding SAG robustly induced RAX- and NKX2.1-positive ventral hypothalamic tissue. In contrast to observations in mouse ESCs, SAG plus BMP4 has led to the generation of pan-Cytokeratin+ Ecad+ nonneural oral ectoderm. Thus, with the combined application of hedgehog and BMP4 signals, human ESCs differentiate into both oral ectoderm and hypothalamic neuroepithelia in adjacent layers within the aggregate. By days 26 to 28, parts of the oral ectoderm were thickened and expressed LHX3. These pituitary placodes subsequently differentiated into pituitary hormone–producing cells. We identified all types of pituitary hormone–producing cells. Among them, we confirmed that the human ESC-derived ACTH+ corticotrophs and GH+ somatotrophs were able to respond normally to releasing and feedback signals in vitro. Grafted human ESC-derived ACTH+ cells rescued physical activity levels and survival of the hosts with hypopituitarism for 14 weeks. Notably, these grafted cells exhibited a substantial induction of ACTH release in response to CRH-loading despite ectopic transplantation. Other groups have reported methods using 2D adherent culture to differentiate human PSCs into the anterior pituitary [39, 40]. Zimmer et al demonstrated that 4 types of pituitary cells producing ACTH, GH, prolactin, and FSH can be induced from human PSCs. They also showed a part of these cells respond to releasing signals in vitro [40]. These human PSC-derived anterior pituitary cells produce hormones at basal levels when grafted into hypophysectomized rats. We do not believe that either 3D or 2D culture is necessarily better than the other. The most important point is how to make completely mature pituitary cells. Each way has its own strengths; therefore, we will perfect development of differentiating technologies by taking advantage of each other’s strengths in the future.
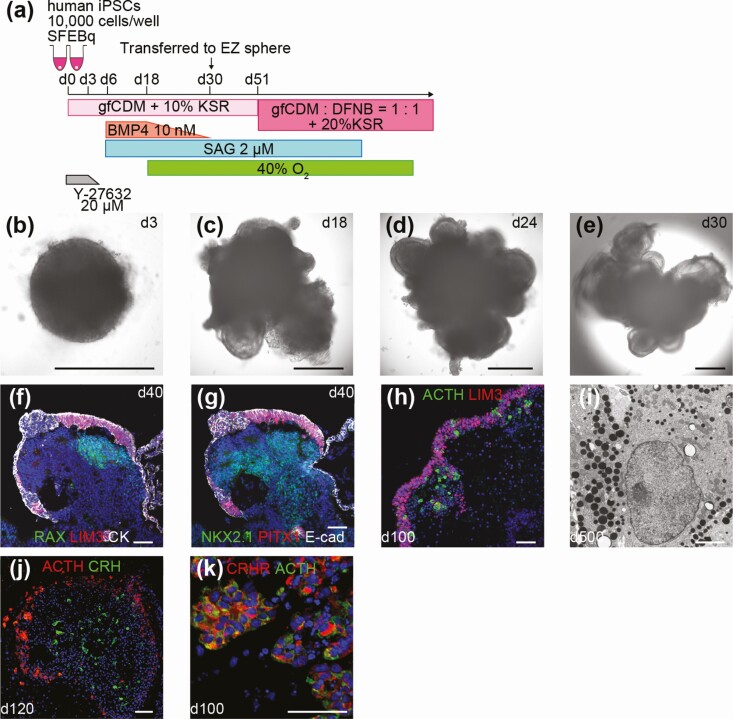
In the next step, we attempted to improve the efficiency of differentiation using human induced PSCs (iPSCs) [41]. The optimized culture conditions (ie, the initial number of cells per well, the concentration of knockout serum replacement added to gfCDM, BMP4 concentration, SAG concentration, and the timing) enabled us to increase the proportion of ACTH+ cells and final ACTH concentration in the culture medium (Fig. 3A-3E). We observed the maturation of hypothalamic tissues adjacent to the anterior pituitary tissue in human iPSC aggregates (Fig. 3F and 3G). As a result of the juxtaposition of the pituitary and hypothalamus during the maturation stage, abundant LHX3+ cells and ACTH+ cells were determined (Fig. 3H). These induced corticotrophs had numerous cytoplasmic storage secretory granules (Fig. 3I). Moreover, our aggregates acquired the ability to secrete ACTH in response to CRH in a self-regulating manner. In vivo, ACTH cells were able to function under various types of regulation. One of the most important stimulants is hypothalamic CRH, whereas the inhibitory system is mediated by glucocorticoids [42, 43]. In addition, ACTH is secreted from the anterior pituitary through hypothalamic neurons such as CRH under hypoglycemic stimulation [44]. In our in vitro aggregates, inner hypothalamic tissues expressed CRH, whereas outer ACTH+ cells expressed CRH receptor (Fig. 3J and 3K). Both the CRH-loading test and low-glucose test efficiently induced ACTH secretion in our aggregates. ACTH levels decreased in response to CRH receptor 1 inhibitor treatment and dexamethasone. These results show that human iPSC-derived aggregates respond to both positive and negative regulators. Therefore, we proposed that a functional hypothalamic-pituitary axis existed within those hybrid organoids as in vitro.
Figure 3.
In vitro hypothalamic-pituitary unit induced from human induced pluripotent stem cells (iPSCs). A, Modified condition for the human iPSC line. B-E, Self-formation of hypothalamic and pituitary tissues from human iPSCs. Double-layered structure was observed in aggregates. F, G, Both anterior pituitary and hypothalamic tissues were developed. LIM3 (f, red), PITX1 (g, red), RAX (f, green), NKX2.1 (g, green), pan-cytokeratin (f, white), E-cadherin (g, white). H, LHX3+ cells and ACTH+ cells were found to be abundant on day 100. LIM3 (red), ACTH (green). I, Electron micrograph of human iPSC-derived corticotrophs on day 500. J, ACTH+ cells and CRH+ cells coexisted in the same aggregates. ACTH (red), CRH (green). K, ACTH+ cells expressed CRHR. CRHR (red), ACTH (green). For all relevant panels, nuclear counterstaining was with DAPI (blue). Scale bars: 500µm (b-e), 50µm (f-h, j, k), 2µm (i).
Hypothalamic Neural Stem/Progenitor Cells
Recently, the existence of neural stem/progenitor cells has been reported to be present in the floor and lateral walls of the hypothalamic third ventricle [45-50]. These cells are called tanycytes, and they play an important role in adult neurogenesis in the hypothalamus. Tanycytes also regulate feeding, weight, and energy balance. Disruption of tanycytes can lead to impaired neuronal differentiation and ultimately the development of obesity and prediabetes [45]. These hypothalamic neural stem/progenitor cells exist abundantly at the neonatal stage, but they gradually diminish with increasing age [50].
In the early development period, Rax is broadly expressed in hypothalamic progenitors. By the mature stage of hypothalamic development, Rax expression is limited in tanycytes and is absent from all other hypothalamic regions [51]. Therefore, tanycytes are considered the only area in the adult hypothalamus that expresses Rax. Based on this idea, we isolated Rax-EGFP+ cells from mouse ESCs in vitro using cell sorting at the mature phase of hypothalamic differentiation. These cells self-renewed and formed neurospheres when cultured with exogenous FGF2. Additionally, these Rax+ neurospheres differentiated into all 3 neuronal lineages (neurons, astrocytes, and oligodendrocytes) including hypothalamic NPY neurons. Thus, Rax+ residual cells in the late stage of hypothalamic differentiation culture were multipotent neural stem/progenitor cells.
These tanycyte-like neural stem/progenitor cells from PSCs could represent a new treatment approach for dysfunction of the hypothalamic homeostatic mechanism or energy regulation caused by disease and aging. As a next step, transplantation experiments will be necessary in order to determine whether these cells can proliferate and differentiate in vivo.
Conclusion and Future Perspectives
Clinically applicable pituitary and hypothalamic tissue generated from human ESCs or iPSCs is crucial for regenerative medicine that uses PSCs in treating intractable endocrinological diseases. Currently, functional corticotrophs can be efficiently induced from human ESCs and iPSCs. When grafted into the kidney subcapsular, they can rescue adrenal insufficiency of hosts and allow long-term survival in rodents. Importantly, these grafted corticotrophs do not function autonomously, but they can reportedly respond to both positive and negative regulators. This is crucial, because endocrine hormone–producing cells have to respond to circadian rhythms or various stress-induced changes, which cannot be perfectly achieved by current oral hormone replacement therapy. For clinical applications, there are still many challenges remaining. It is necessary to eliminate undifferentiated cells or immature precursor cells in pituitary differentiation to avoid tumorigenesis. We have to use fully safe materials and components which can be used clinically [40]. Therefore, our future aim is to establish new culture protocol for efficient pituitary induction using clinically applicable materials. Furthermore, location of tissue transplantation has to be well considered. Ectopic transplantation (eg, subcutaneous transplantation) is easy and safe approach because we can eliminate the grafts if serious side effect or rejection occurs. However, ectopic transplantation is not perfect because physiological CRH released from the hypothalamus does not directly affect these grafted ACTH+ cells. Orthotopic transplantation, which means transplantation of pituitary cells into the sella turcica, might be one of the future candidates for complete therapy. In the future, it will be necessary to perform transplantation into large mammals like nonhuman primates to confirm the efficacy and safety of the generated pituitary tissues.
Another important use for human pluripotent cells is the induction of tissues or organs from a patient’s own iPSCs in vitro, specifically from patients who have congenital diseases due to gene mutations. Our colleagues recently established an iPSC-based congenital pituitary hypoplasia model [52]. They established iPSCs from a patient with pan-hypopituitarism with a heterozygous mutation in OTX2 that plays an important role in the development of the forebrain, eye, and pituitary [53–55]. The patient’s iPSCs showed increased apoptosis in pituitary progenitor cells, and the differentiation into pituitary hormone–producing cells was severely impaired. This method has uncovered underlying mechanisms of congenital pituitary hypoplasia involving an OTX2 mutation that remains largely unknown. These disease-specific iPSCs can be used to elucidate intractable disease pathology or for use in therapy screenings as an in vitro human disease model.
Acknowledgments
Financial Support: This work was supported by grants from the Project for Technological Development (H.S.) of the Research Center Network for Realization of Regenerative Medicine (RCNRRM) funded by the Japan Agency for Medical Research and Development (AMED); the Acceleration Program for Intractable Diseases Research utilizing disease-specific iPSCs (H.S.) of RCNRRM funded by AMED; Grants-in-Aid for Scientific Research (H.S.) from The Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); and Nagoya University Hospital Funding for Clinical Research (H.S.).
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- AGRP
agouti-related peptide
- AVP
arginine-vasopressin
- BMP
bone morphogenetic protein
- CDI
central diabetes insipidus
- CRH
corticotropin-releasing hormone
- DDAVP
desmopressin
- ESC
embryonic stem cell
- FGF
fibroblast growth factor
- GH
growth hormone
- HPA
hypothalamic-pituitary-adrenal
- iPSC
induced pluripotent stem cells
- NPY
neuropeptide Y
- POMC
proopiomelanocortin
- PRL
prolactin
- PSC
pluripotent stem cell
- RAX
retina and anterior neural fold homeobox
- SAG
smoothened agonist (N-methyl-N9-(3-pyridinylbenzyl)-N9-(3-chlorobenzo[b]thiophene-2-carbonyl)-1,4-diaminocyclohexane)
- SFEBq
serum-free culture of embryoid body-like aggregates with quick reaggregation
- Shh
sonic hedgehog
Additional Information
Disclosure summary: The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hahner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. 2015;100(2):407-416. [DOI] [PubMed] [Google Scholar]
- 2. Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87(3):933-963. [DOI] [PubMed] [Google Scholar]
- 3. Takuma N, Sheng HZ, Furuta Y, et al. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125(23):4835-4840. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol. 2010;348(2):199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Treier M, O’Connell S, Gleiberman A, et al. Hedgehog signaling is required for pituitary gland development. Development. 2001;128(3):377-386. [DOI] [PubMed] [Google Scholar]
- 6. Treier M, Gleiberman AS, O’Connell SM, et al. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12(11):1691-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8(3):288-296. [DOI] [PubMed] [Google Scholar]
- 8. Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3(5):519-532. [DOI] [PubMed] [Google Scholar]
- 9. Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: self-organising stem cells. Development. 2012;139(22):4111-4121. [DOI] [PubMed] [Google Scholar]
- 10. Kawasaki H, Suemori H, Mizuseki K, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci U S A. 2002;99(3):1580-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morizane A, Takahashi J, Shinoyama M, et al. Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J Neurosci Res. 2006;83(6):1015-1027. [DOI] [PubMed] [Google Scholar]
- 12. Kadoshima T, Sakaguchi H, Nakano T, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013;110(50):20284-20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danjo T, Eiraku M, Muguruma K, et al. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci. 2011;31(5):1919-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51-56. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102(32):11331-11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215-224. [DOI] [PubMed] [Google Scholar]
- 17. Muguruma K, Nishiyama A, Ono Y, et al. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat Neurosci. 2010;13(10):1171-1180. [DOI] [PubMed] [Google Scholar]
- 18. Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun. 2015;6:8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wataya T, Ando S, Muguruma K, et al. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci U S A. 2008;105(33):11796-11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U S A. 1997;94(7):3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Markakis EA. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol. 2002;23(3):257-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Simeone A. The role of Otx and Otp genes in brain development. Int J Dev Biol. 2000;44(6):669-677. [PubMed] [Google Scholar]
- 23. Mitsumoto K, Suga H, Sakakibara M, et al. Improved methods for the differentiation of hypothalamic vasopressin neurons using mouse induced pluripotent stem cells. Stem Cell Res. 2019;40:101572. [DOI] [PubMed] [Google Scholar]
- 24. Tsai PS, Brooks LR, Rochester JR, Kavanaugh SI, Chung WC. Fibroblast growth factor signaling in the developing neuroendocrine hypothalamus. Front Neuroendocrinol. 2011;32(1):95-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCabe MJ, Gaston-Massuet C, Tziaferi V, et al. Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo-pituitary dysfunction. J Clin Endocrinol Metab. 2011;96(10):E1709-E1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol. 2005;16(10):2836-2846. [DOI] [PubMed] [Google Scholar]
- 27. Di Iorgi N, Napoli F, Allegri AE, et al. Diabetes insipidus–diagnosis and management. Horm Res Paediatr. 2012;77(2):69-84. [DOI] [PubMed] [Google Scholar]
- 28. Fenske W, Allolio B. Clinical review: Current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab. 2012;97(10):3426-3437. [DOI] [PubMed] [Google Scholar]
- 29. Arima H, Wakabayashi T, Nagatani T, et al. Adipsia increases risk of death in patients with central diabetes insipidus. Endocr J. 2014;61(2):143-148. [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Meece K, Williams DJ, et al. Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J Clin Invest. 2015;125(2):796-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merkle FT, Maroof A, Wataya T, et al. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development. 2015;142(4):633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681-686. [DOI] [PubMed] [Google Scholar]
- 33. Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12(20):3264-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogawa K, Suga H, Ozone C, et al. Vasopressin-secreting neurons derived from human embryonic stem cells through specific induction of dorsal hypothalamic progenitors. Sci Rep. 2018;8(1):3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suga H, Kadoshima T, Minaguchi M, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480(7375):57-62. [DOI] [PubMed] [Google Scholar]
- 36. Ellsworth BS, Butts DL, Camper SA. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol. 2008;313(1):118-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sheng HZ, Zhadanov AB, Mosinger B Jr, et al. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272(5264):1004-1007. [DOI] [PubMed] [Google Scholar]
- 38. Ozone C, Suga H, Eiraku M, et al. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7:10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dincer Z, Piao J, Niu L, et al. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep. 2013;5(5):1387-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zimmer B, Piao J, Ramnarine K, Tomishima MJ, Tabar V, Studer L. Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Reports. 2016;6(6):858-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kasai T, Suga H, Sakakibara M, et al. Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3D-cultured human iPS cells. Cell Rep. 2020;30(1):18-24.e5. [DOI] [PubMed] [Google Scholar]
- 42. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394-1397. [DOI] [PubMed] [Google Scholar]
- 43. Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1-24. [DOI] [PubMed] [Google Scholar]
- 44. Plotsky PM, Bruhn TO, Vale W. Hypophysiotropic regulation of adrenocorticotropin secretion in response to insulin-induced hypoglycemia. Endocrinology. 1985;117(1):323-329. [DOI] [PubMed] [Google Scholar]
- 45. Li J, Tang Y, Cai D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14(10):999-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee DA, Blackshaw S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int J Dev Neurosci. 2012;30(8):615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee DA, Bedont JL, Pak T, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15(5):700-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robins SC, Stewart I, McNay DE, et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. [DOI] [PubMed] [Google Scholar]
- 49. Haan N, Goodman T, Najdi-Samiei A, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33(14):6170-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Kim MS, Jia B, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548(7665):52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shimogori T, Lee DA, Miranda-Angulo A, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13(6):767-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsumoto R, Suga H, Aoi T, et al. Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. J Clin Invest. 2020;130(2):641-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chatelain G, Fossat N, Brun G, Lamonerie T. Molecular dissection reveals decreased activity and not dominant negative effect in human OTX2 mutants. J Mol Med (Berl). 2006;84(7):604-615. [DOI] [PubMed] [Google Scholar]
- 54. Acampora D, Mazan S, Lallemand Y, et al. Forebrain and midbrain regions are deleted in Otx2-/- mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121(10):3279-3290. [DOI] [PubMed] [Google Scholar]
- 55. Elliott J, Maltby EL, Reynolds B. A case of deletion 14(q22.1–>q22.3) associated with anophthalmia and pituitary abnormalities. J Med Genet. 1993;30(3):251-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.