Abstract
Aim: We investigated the outcomes of transcatheter (TAVR) and surgical aortic valve replacement (SAVR) in Finland during the last decade.
Methods: The nationwide FinnValve registry included data from 6463 patients who underwent TAVR or SAVR with a bioprosthesis for aortic stenosis from 2008 to 2017.
Results: The annual number of treated patients increased three-fold during the study period. Thirty-day mortality declined from 4.8% to 1.2% for TAVR (p = .011) and from 4.1% to 1.8% for SAVR (p = .048). Two-year survival improved from 71.4% to 83.9% for TAVR (p < .001) and from 87.2% to 91.6% for SAVR (p = .006). During the study period, a significant reduction in moderate-to-severe paravalvular regurgitation was observed among TAVR patients and a reduction of the rate of acute kidney injury was observed among both SAVR and TAVR patients. Similarly, the rate of red blood cell transfusion and severe bleeding decreased significantly among SAVR and TAVR patients. Hospital stay declined from 10.4 ± 8.4 to 3.7 ± 3.4 days after TAVR (p < .001) and from 9.0 ± 5.9 to 7.8 ± 5.1 days after SAVR (p < .001).
Conclusions: In Finland, the introduction of TAVR has led to an increase in the invasive treatment of severe aortic stenosis, which was accompanied by improved early outcomes after both SAVR and TAVR.
Trial registration: NCT03385915
Key Messages
This study demonstrated that the introduction of transcatheter aortic valve replacement has led to its widespread use as an invasive treatment for severe aortic stenosis.
Early and 2-year survival after transcatheter and surgical aortic valve replacement has improved during past decade.
Transcatheter aortic valve replacement has fulfilled its previously unmet clinical needs and has surpassed surgical aortic valve replacement as the most common invasive treatment for aortic stenosis.
Keywords: Transcatheter aortic valve implantation (TAVI), surgical aortic valve replacement (SAVR), aortic valve stenosis (AS)
Introduction
In the field of structural heart disease, transcatheter aortic valve replacement (TAVR) occupies currently a significant position as a valid invasive treatment option for aortic valve stenosis (AS). Since the first TAVR procedure in 2002 [1], it has rapidly grown as a treatment of choice for a large proportion of AS patients. It is currently recommended and FDA approved treatment option for AS among inoperable patients and high-risk patients as well as patients with intermediate operative risk [2–8]. Furthermore, preliminary data suggest that catheter approaches may be comparable to surgery even in low-risk patients [9]. To date, TAVR has been widely adopted across the world and the number of patients undergoing this procedure has increased rapidly during last few years in the whole western hemisphere [10].
Since TAVR has dramatically transformed the treatment of symptomatic AS worldwide, we created a national database to investigate the outcomes of TAVR and SAVR treatments in Finland during past decade. The purpose of this national database is to detect possible trends and changes in survival as well as in numbers of untoward complications. Also changes in patient clinical characteristics during the years were of particular interest. We anticipated that although this therapy was initially used to treat inoperable and high-risk patients, improved procedural techniques as well as dedicated pre-evaluation of the patient has led to excellent consisted results with low morbidity and mortality among TAVR patients with intermediate risk as well as among SAVR patients.
Materials and methods
Study population
The FinnValve registry is a nationwide registry NCT03385915, which retrospectively collects data from consecutive and unselected patients who underwent TAVR or SAVR with a bioprosthesis for severe AS at all five Finnish university hospitals (Helsinki, Kuopio, Oulu, Tampere and Turku) from January 2008 to November 2017. During the study period, only these five university hospitals performed TAVR and SAVR procedures. A small number of TAVR procedures have been performed in three central hospitals during a short period of time during which this procedure was temporarily allowed by the national authorities. Similarly, a small number of SAVR procedures were performed in a central hospital not performing TAVR procedures. Data from patients treated in central hospitals were not collected in to this registry, because these procedures were performed outside a heart team environment and this might have introduced bias in to the results.
This study was approved by and a waiver for the requirement of informed consent was obtained from the Institutional Review Board of each participating centre. The inclusion criteria for this registry were as follows: (1) AS with or without aortic valve regurgitation; (2) patients aged >18 years; and (3) primary TAVR or SAVR with a bioprosthesis with or without concomitant coronary revascularization. The exclusion criteria of this study were as follows: (1) any prior TAVR or surgical intervention on the aortic valve; (2) concomitant major cardiac procedure on the ascending aorta and/or other heart valves or structures; (3) transcatheter or surgical procedure for isolated aortic valve regurgitation; (4) acute endocarditis; and (5) SAVR with a mechanical valve prosthesis.
This nationwide registry was planned during several meetings of the main investigators, who developed an electronic case report form which was filled by clinicians and research nurses with specific experience in TAVR and SAVR procedures and their related outcomes. No data from prior institutional datasets contributed to this registry. This registry includes a consecutive series of patients, whose operating code referring to TAVR and/or SAVR with a bioprosthesis were retrieved from Institutional administrative registries. The definition criteria of baseline, operative and postoperative variables were prespecified and listed in a table with a similar output of the electronic case report for rapid consultation by the researchers, who were instructed before starting the data collection to comply with these criteria. Data underwent robust checking of its completeness and quality. Data on mortality was retrieved from the national registry Statistics Finland, which is based on death certificates reviewed by local authorities. Based on this, follow-up was considered complete for all patients, but for two patients who were not residing in Finland and whose follow-up was truncated at hospital discharge.
Definition criteria of baseline risk factors
Baseline variables were defined according to the EuroSCORE II criteria [11]. The operative risk of these patients was stratified according to the EuroSCORE II and STS [12] risk scores. Severe frailty was defined according to the Geriatric Status Scale (GSS) and herein is defined as GSS grades 2–3 as proposed by Rockwood et al. [13]. Coronary artery disease was defined as any stenosis ≥50% of the main coronary branches.
Outcome measures
The primary outcome of this study was 30-day mortality. The secondary outcomes were stroke, postoperative use of intra-aortic balloon pump or extracorporeal membrane oxygenation, conversion to cardiac surgery, coronary artery occlusion, aortic annulus rupture, aortic dissection/rupture, major vascular complication, red blood cell transfusion, severe bleeding, resternotomy for bleeding, moderate-to-severe paravalvular regurgitation, implantation of permanent pace-maker, severe acute kidney injury, atrial fibrillation, postoperative length of stay in the hospital where the index procedure was performed and 2-year survival (including only patients operated from 2008 and 2015, i.e. those with at least 2 years of follow-up). The length of stay in the intensive care unit was not considered in this analysis as an end-point because of inter-institutional differences in the organizational program of postoperative care of TAVR patients. All the aforementioned adverse events, but mortality were recorded only during the index hospitalization. The postprocedural echocardiographic findings of paravalvular regurgitation were not adjudicated by a core lab and its severity was graded before discharge by the treating physicians. Stroke was defined according to the Valve Academic Research Consortium-2 (VARC-2) definition criteria [14] as any focal or global neurological deficit lasting 24 h or longer with a new brain infarct or haemorrhage detected at neuroimaging, or a neurological deficit resulting in death during the index hospitalization. Severe bleeding was defined as transfusion of more than 4 units of red blood cells and/or any operation for excessive bleeding. Severity of bleeding and major vascular complications were defined also according to the VARC-2 definition criteria [14]. Stage-3 acute kidney injury was defined as according to the KDIGO classification criteria as any increase in serum creatinine ≥3.0 times the baseline level or serum creatinine increase ≥353.65 μmol/l during the hospital stay and/or de novo renal replacement therapy during the hospital stay [14,15].
Statistical analysis
Statistical analysis was performed using SPSS v. 25.0 statistical software (IBM Corporation, New York, USA). Comparative analysis of the TAVR and SAVR cohorts were performed using the Mann–Whitney, the Chi-square and the Fisher exact tests. Trends over time were plotted and analyzed across the 10 years intervals using the Mantel–Haenszel linear-by-linear (LLA) association chi-squared test for trend and linear regression with year categories regressed as an ordinal variable. Differences in the long-term survival were evaluated by the Kaplan-Meier method with the log-rank test. A p < .05 was set for statistical significance.
Results
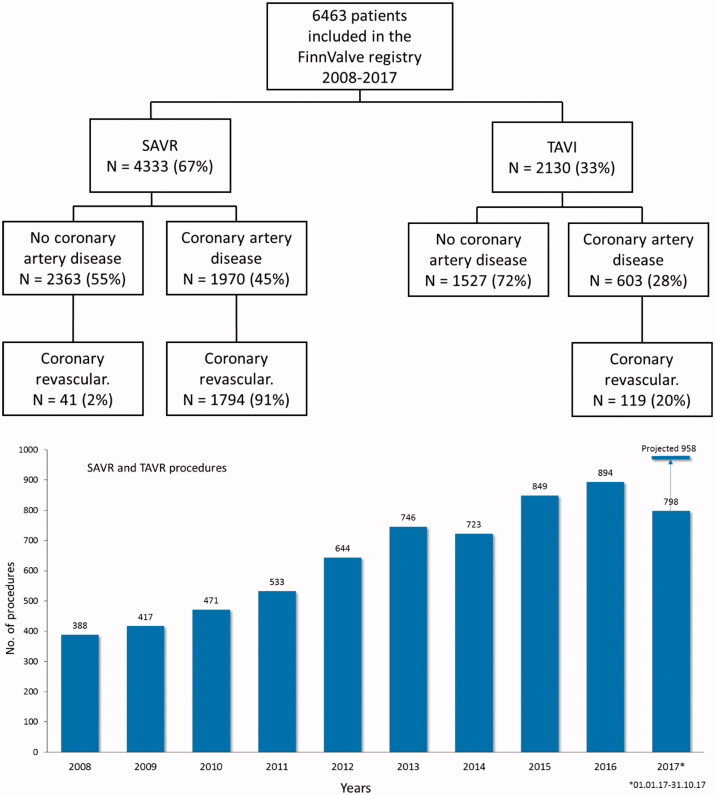
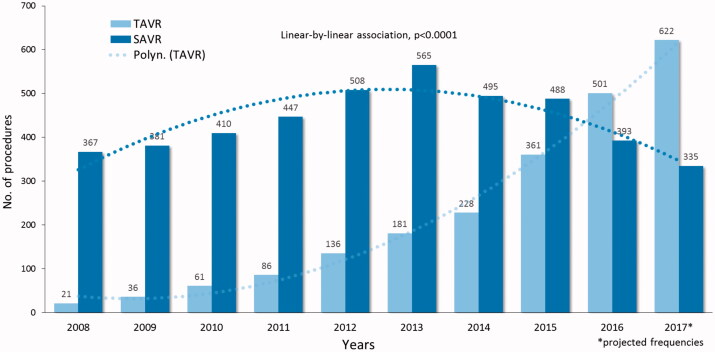
The FinnValve registry includes data from 6 463 patients who underwent primary TAVR (2 130 patients) and SAVR (4 333 patients) with a bioprothesis for severe AS (Figure 1). Among the TAVR prostheses, 1689 were mechanically expanding valves (79.4%) and 439 were self-expandable valves (20.6%, data on TAVR prosthesis was missing in two patients). Third-generation TAVR prosthesis were implanted in 1451 patients (68.2%). Among the SAVR prostheses, 126 were sutureless prostheses (0.3%) and 4308 (99.5%) were stented valve prostheses (data on SAVR prosthesis was missing in two patients). Clinical characteristics of patients who underwent TAVR or SAVR are presented in Table 1. Nationwide progressive increase in number of invasively treated AS patients during whole study period was evident (Figure 1). In Finland TAVR surpassed SAVR as most prevalent treatment option in 2016 (Figure 2).
Figure 1.
Study flowchart and annual number of transcatheter (TAVR) and surgical aortic valve replacement (SAVR) along the study period.
Table 1.
Characteristics of patients who underwent transcatheter or surgical aortic valve replacement.
| Characteristics | TAVR 2130 patients |
SAVR 4333 patients |
p-Value |
|---|---|---|---|
| Age, mean (years) | 81.2 ± 6.6 | 75.1 ± 6.5 | <.0001 |
| Female, n (%) | 1172 (55.0) | 2026 (46.8) | <.0001 |
| Body mass index, mean (kg/m2) | 27.1 ± 4.8 | 27.7 ± 4.8 | <.0001 |
| Hemoglobin, mean (mg/L) | 125 ± 16 | 132 ± 15 | <.0001 |
| eGFR, mean (mL/min/1.73 m2) | 65 ± 23 | 76 ± 22 | <.0001 |
| Active malignancy, n (%) | 84 (3.9) | 60 (1.4) | <.0001 |
| Diabetes, n (%) | 605 (28.4) | 1154 (26.6) | .133 |
| Stroke, n (%) | 247 (11.6) | 299 (6.9) | <.0001 |
| Pulmonary disease, n (%) | 456 (21.4) | 642 (14.8) | <.0001 |
| Oxygen therapy, n (%) | 14 (0.7) | 16 (0.4) | .109 |
| Frailty GSS grades 2–3, n (%) | 318 (14.9) | 107 (2.5) | <.0001 |
| Extracardiac arteriopathy, n (%) | 412 (19.3) | 539 (12.4) | <.0001 |
| LVEF ≤50%, n (%) | 596 (28.0) | 909 (21.0) | <.0001 |
| Atrial fibrillation, n (%) | 932 (43.8) | 955 (22.0) | <.0001 |
| NYHA class 4, n (%) | 244 (11.5) | 453 (10.5) | .223 |
| SPAP, n (%) | <.0001 | ||
| 31–55 mmHg | 853 (40.0) | 1526 (35.2) | |
| >55 mmHg | 286 (13.4) | 305 (7.0) | |
| Moderate-severe mitral valve regurgitation, n (%) | 288 (14.3) | 256 (6.5) | <.0001 |
| Porcelain aorta, n (%) | 124 (5.8) | 15 (0.3) | <.0001 |
| Recent myocardial infarction, n (%) | 49 (2.3) | 312 (7.2) | <.0001 |
| Acute heart failure ≤60 days/critical preop. state, n (%) | 253 (11.9) | 501 (11.6) | .710 |
| Coronary artery disease, n (%) | 603 (28.3) | 1970 (45.5) | <.0001 |
| Prior PCI, n (%) | 467 (21.9) | 405 (9.3) | <.0001 |
| Prior cardiac surgery | 431 (20.2) | 97 (2.2) | <.0001 |
| Permanent pace-maker, n (%) | 208 (9.8) | 174 (4.0) | <.0001 |
| Emergency procedure, n (%) | 6 (0.3) | 59 (1.4) | <.0001 |
| Bicuspid aortic valve, n (%) | 114 (5.4) | 920 (21.0) | <.0001 |
| Concomitant PCI/CABG, n (%) | 119 (5.6) | 1835 (42.3) | <.0001 |
| EuroSCORE II, mean (%) | 7.2 ± 7.4 | 4.2 ± 5.5 | <.0001 |
| STS Score, mean (%) | 4.6 ± 3.3 | 3.0 ± 2.9 | <.0001 |
Continuous variables are reported as means ± standard deviation. Categorical variables as counts and percentages. Clinical variables are according to the EuroSCORE II definition criteria. SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement; eGFR: glomerular filtration estimated according to the MDRD equation; LVEF: left ventricular ejection fraction; Frailty, GSS grades 2–3; SPAP: systolic pulmonary artery pressure; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting.
Figure 2.
Frequencies of transcatheter (TAVR) and surgical aortic valve replacement (SAVR) along the study period.
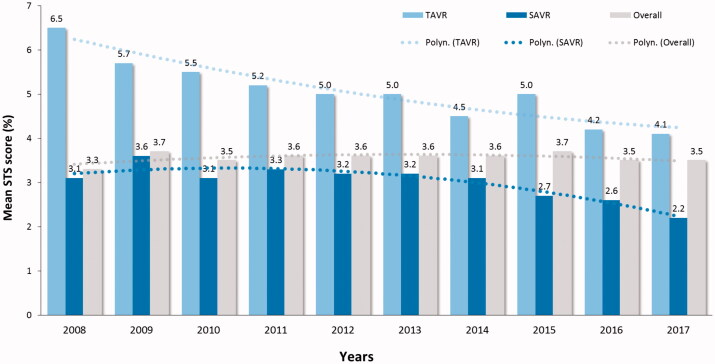
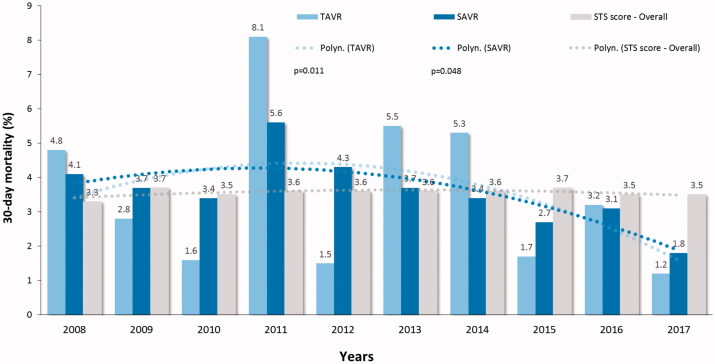
During 10-year study period, the mean STS preoperative risk score declined from 6.5 ± 5.7% to 4.1 ± 3.1% level for TAVR (p < .001) and from 3.1 ± 2.3 to 2.2 ± 1.5% for SAVR (p < .001) (Figure 3). The 30-day mortality of decreased from 4.8% to 1.2% (75% decrease, p = .011) for TAVR and from 4.1% to 1.8% (56% decrease, p = .048) for SAVR (Figure 4). This trend was evident during second half of the study period and led to a significant decrease of the overall 30-day mortality (p = .001) (Figure 4). Two-year survival improved from 71.4% to 83.9% for TAVR patients and from 87.2% to 91.6% for SAVR patients (Figure 5). Thirty-day, 1-, 3, and 5-year survival rates after TAVI were 97.1%, 90.6%, 73.0% and 51.9%, and after SAVR 96.4%, 92.9%, 86.9% and 78.7%, respectively (log-rank test, p < .0001).
Figure 3.
Operative risk stratified by the Society of Thoracic Surgeons (STS) score in patients who underwent transcatheter (TAVR) or surgical aortic valve replacement (SAVR) along the study period.
Figure 4.
Observed and predicted 30-day mortality by the Society of Thoracic Surgeons (STS) score in patients who underwent transcatheter (TAVR) or surgical aortic valve replacement (SAVR) along the study period. P-values are from the linear-by-linear association test.
Figure 5.
Two years survival in patients who underwent transcatheter (TAVR) or surgical aortic valve replacement (SAVR) from 2018 to 2015. p-Values are from the linear-by-linear association test.
Early adverse events are summarized in Table 2. Conversion to open surgery was required in 13 patients (0.6%) who underwent TAVR and the related 30-day and 1-year mortality were 38.5% and 48.7%, respectively (p < .0001 compared to patients not requiring conversion to cardiac surgery). The reasons for conversion to cardiac surgery were as follows: aortic root rupture in four patients, wire perforation of the left ventricle in in four patients, TAVR prosthesis migration in three patients, bleeding from pace-maker lead perforation of the innominate vein in one patient and severe bleeding from unspecified site in one patient.
Table 2.
Crude rates of adverse events occurred during the index hospitalization in patients undergoing transcatheter or surgical aortic valve replacement.
| Outcomes | TAVR 2130 patients |
SAVR 4333 patients |
p-Value |
|---|---|---|---|
| 30-day death, (%) | 62 (2.9) | 158 (3.6) | .125 |
| Stroke, n (%) | 53 (2.5) | 165 (3.8) | .006 |
| Postop. IABP/ECMO, n (%) | 4 (0.2) | 80 (1.8) | <.0001 |
| Conversion to cardiac surgery, n (%) | 13 (0.6) | – | – |
| Coronary ostium occlusion, n (%) | 8 (0.4) | 10 (0.2) | .320 |
| Aortic annulus rupture, n (%) | 10 (0.5) | 8 (0.2) | .047 |
| Aortic dissection/rupture | 17 (0.8) | 31 (0.7) | .716 |
| Major vascular complication, n (%) | 191 (9.0) | 69 (1.6) | <.0001 |
| RBC trasfusion, n (%) | 403 (19.2) | 3010 (70.4) | <.0001 |
| RBC transfusion, mean (units) | 0.6 ± 1.7 | 3.0 ± 3.8 | <.0001 |
| RBC transfusion, >4 units | 76 (3.6) | 926 (21.7) | <.0001 |
| Resternotomy for bleeding, n (%) | 30 (1.4) | 351 (8.1) | <.0001 |
| Severe bleeding,an (%) | 116 (5.5) | 1033 (24.1) | <.0001 |
| VARC-2 bleeding events | <.0001 | ||
| Major, n (%) | 567 (26.7) | 1564 (36.2) | |
| Life-threatening, n (%) | 167 (7.9) | 2597 (60.0) | |
| KDIGO acute kidney injury grades 3,bn (%) | 21 (1.0) | 127 (3.0) | <.0001 |
| Renal replacement therapy, n (%) | 30 (1.4) | 113 (2.6) | .002 |
| Moderate-severe paravalvular regurgitation, n (%) | 79 (3.7) | 29 (0.7) | <.0001 |
| Atrial fibrillation,cn (%) | 160 (13.4) | 1645 (48.7)) | <.0001 |
| Permanent pacemaker, n (%) | 185 (8.7) | 170 (3.9) | <.0001 |
| Hospital stay, mean (days) | 5.5 ± 5.0 | 8.3 ± 6.4 | <.0001 |
Continuous variables are reported as means ± standard deviation. Categorical variables as counts and percentages. a, it refers to transfusion of more than 4 units of red blood cells and/or operation for excessive bleeding; b, it excludes patients with estimated glomerular filtration rate <15 mL/min/1.73 m2 or dialysis; c, it excludes patients with preoperative atrial fibrillation. TAVR: transcatheter aortic valve replacement; SAVR: surgical aortic valve replacement; IABP: intraaortic balloon pump; ECMO: extracorporeal membrane oxygenation; RBC: red blood cells; VARC-2: Valve Academic Research Consortium-2; KDIGO: Kidney Disease: Improving Global Outcomes.
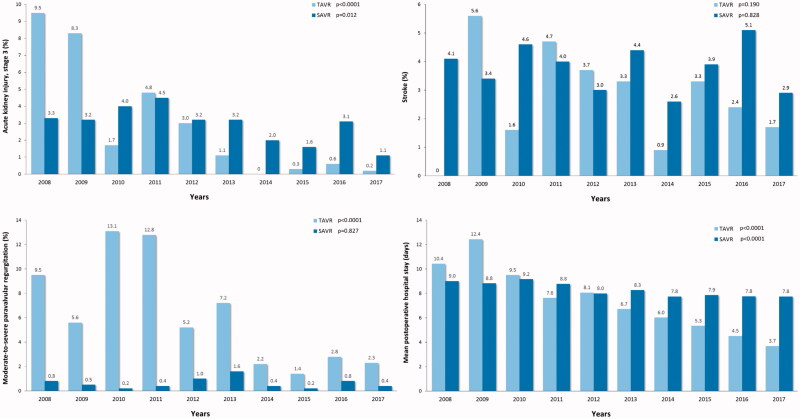
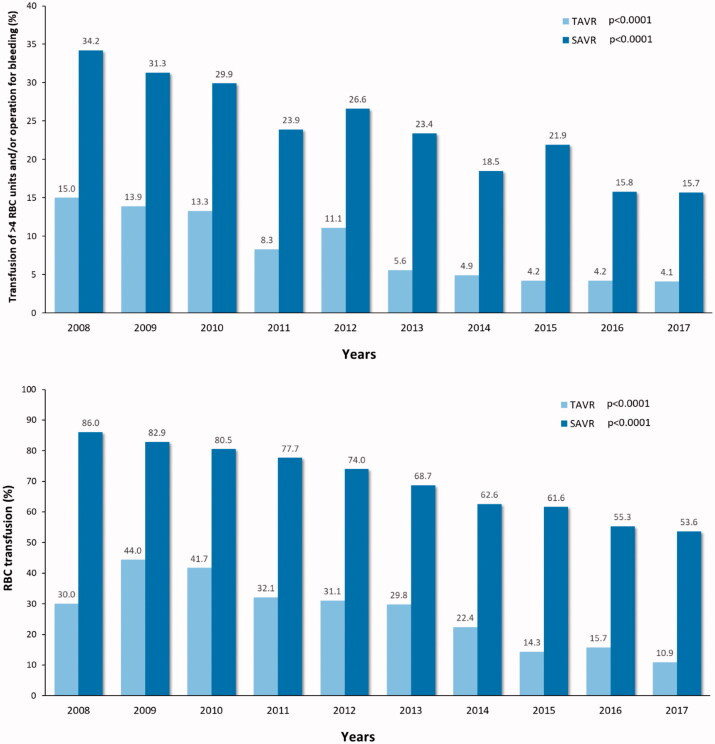
The number of complications declined during past decade among both operation modalities. A significant reduction in moderate-to-severe paravalvular regurgitation was observed among TAVR patients and a reduction of the rate of stage 3 acute kidney injury was observed among both SAVR and TAVR patients (Figure 6). Similarly, the rate of red blood cell transfusion and severe bleeding (transfusion of >4 units of red blood cells and/or operation for bleeding) decreased significantly among SAVR and TAVR patients (Figure 7). A trend toward reduction in stroke rate was observed among TAVR patients during the study period. The mean hospital stay declined from 10.4 ± 8.4 to 3.7 ± 3.4 days after TAVR and from 9.0 ± 5.9 to 7.8 ± 5.1 days after SAVR (Figure 6).
Figure 6.
Incidence of stroke, moderate-to-severe paravalvular regurgitation, acute kidney injury stage 3 and length of hospital stay after transcatheter (TAVR) and surgical aortic valve replacement (SAVR) along the study period. P-values are from the linear-by-linear association test.
Figure 7.
Rates of red blood cell (RBC) transfusion and of transfusion of >4 units of RBC and/or operation for excessive bleeding in patients who underwent transcatheter (TAVR) or surgical aortic valve replacement (SAVR). P-values are from the linear-by-linear association test.
Discussion
The main findings of the present study are that: [1] the early and intermediate mortality in patients with AS has decreased during past 5-year period among TAVR treated patients as well as among SAVR treated patients despite relatively stable overall preoperative risk among this patient group; [2] in Finland, TAVR is now extensively used for patients at high and intermediate risk and it has surpassed SAVR as the most common treatment strategy for severe AS; [3] advanced technology of TAVR has led to reduced rates of perioperative bleeding, stroke, severe acute kidney injury and other procedural complications placing TAVR now as an effective standardized procedure with the safe and feasible treatment results for most patients with symptomatic severe AS.
With over 500,000 TAVR patients treated to date worldwide, indications for TAVR continue to widen. Current estimates predict 200,000 annual candidates for TAVR in 2020 [16]. TAVR is currently recommended for inoperable and high-risk patients as well as for patients with intermediate operative risk [17]. This retrospective study confirms that also in Finland this trend is evident and likely to continue. Improved survival of current TAVR operations nationwide, reduction of major and minor complications as well as decreased demand for postoperative care will likely cause higher TAVR implantation rate in the future among patients with severe AS. TAVR treatment surpassed SAVR as the most common treatment for severe AS in 2016 in Finland. The same shift has occurred in 2014 in Germany [18]. Adoption rates of this therapy vary from country to country as a result of interpretation differences of the results of scientific clinical trials as well as heterogeneous reimbursement programs [19–21].
Complication rates of TAVR operations has rapidly declined and are currently comparable or even better (kidney injury, severe bleeding) than SAVR, despite the older age and higher comorbidities of TAVR patients relative to SAVR patients. Severe TAVR specific complications, like annular rupture, are becoming very rare due to improved implantation and imaging techniques and development of TAVR devices. However, a word of caution is in place while reaching this therapy to low risk patients. Several issues will play a key role in expanding TAVR indication in healthier individuals. Particularly issues like valve durability, need for permanent pacemaker, valve performance as well as optimal antithrombotic or anticoagulation medication are questions needed to be answered when expanding TAVR treatment to low-risk patient populations [22].
Since the short-term TAVR results in procedure mortality, stroke, valve performance and vascular complications has been excellent [2–8], the main attention is directed now to long-term durability of TAVR valves. Most SAVR bio-prosthesis has a 10-year freedom from reoperation that is above 97% [23]. Long-term survival free of structural valve degeneration is a well-known fact for SAVR while it still is somewhat unclear for TAVR. A report from 5-year follow-up from PARTNER I trial revealed excellent durability without significant structural valve degeneration up to 5 years [24]. A meta-analysis confirmed the good long-term durability of TAVR devices [25]. However, the long-term durability is of pivotal importance in younger patient with a life-expectance greater than 10 years when TAVR or SAVR options are considered. Another major practical issue which needs to be solved is whether TAVR is to replace SAVR among younger patient population because of a relatively high permanent pacemaker implantation rate for some TAVR devices [26]. Heart conduction disturbances are the most common complication of TAVR. Need for permanent pacemaker varies in relation with the valve type used. Even if mortality has not been increased in patients undergoing TAVR or SAVR and requiring permanent pacemaker implantation, the long-term effects of having permanent pacemaker particularly in younger patient populations may be significant. In the present study, implantation of permanent pacemaker was more frequent among TAVR patients than among SAVR patients. However, with modern devices very low permanent pacemaker implantation rates have been reported [27]. Ongoing clinical trials currently in progress for low-risk patients as well as results from long-term follow-ups of previous trials and large registries will clarify the role of TAVR and SAVR operations among low risk younger patients in not too distant future [28–29].
Despite the excellent results in this nationwide database and other large registries as well as several randomized clinical trials with positive results [2–9], resistance from surgeons [10] and the abovementioned lack of randomized long-term data to provide solid information concerning valve durability will currently curb TAVR adoption rate. Although extensive laboratory testing of used devices has been conducted, long-term durability of TAVR devices in vivo remains somewhat unclear and reliable long-term outcome data over 10 years concerning durability is not currently available. Therefore, routine use of TAVR in younger patients may not be feasible beyond the limits of randomized controlled clinical trials.
Although it has been postulated that TAVR might become the standard of care for most patients with severe AS in near future, accounting for even 90% of all aortic valve replacement procedures [30], SAVR will remain the treatment of choice for various patient groups, such as patients with coexistent severe multivessel coronary artery disease, diseases of the ascending aorta, and infective endocarditis. Therefore, development of SAVR procedural techniques is also of pivotal importance. This scenario may become valid, if ongoing clinical trials with low-risk patients will provide positive results for TAVR treatment.
Limitations
The present study has limitations inherent to all retrospective analyses. First, the reasons for treatment allocation cannot be fully resolved and likely changed along the study period. In this context, the direction and magnitude of bias cannot be determined. However, this nationwide registry is mainly descriptive in nature offering an excellent view to evaluate TAVR and SAVR operations and their results independently.
Conclusions
This nationwide registry demonstrated that the introduction of TAVR has led to a widespread use of invasive treatment for severe AS, which was accompanied by improved early outcomes after both SAVR and TAVR. During the past decade, TAVR has fulfilled its previously unmet clinical needs and has surpassed SAVR as the most common invasive treatment for AS.
Disclosure statement
T.M., none; M.P.J., none; T.L, none; M.V., none; M.N., none; T.A, none; T.T, none; P.M., none; A.H., none; E.M.K., none; S.D. received a grant from the Finnish Society of Angiology; J.J., none; J.A., none; V.A., none; S.R, none; P.D.E, none; M.S., is proctor for Medtronic, relationship is significant; M.L., is proctor for Boston Scientific, relationship is significant; T.M., none; A.V., none; P.R. none; M.E, none; F.B., none.
References
- 1.Cribier A, Eltchaninoff H, Bash A, et al. . Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, et al. . Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ, et al. . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 4.Gilard M, Eltchaninoff H, Iung B, et al. . Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. [DOI] [PubMed] [Google Scholar]
- 5.Wenaweser P, Stortecky S, Schwander S, et al. . Clinical outcomes of patients with estimated low or intermediate surgical risk undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:1894–1905. [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack MJ, et al. . Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 7.Reardon MJ, Van Mieghem NM, Popma JJ, et al. . Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 8.Thourani VH, Kodali S, Makkar RR, et al. . Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 9.Thyregod HG, Steinbruchel DA, Ihlemann N, et al. . Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 10.Cribier A Commemorating the 15-year anniversary of TAVI: insights into the early stages of development, from concept to human application, and perspectives. EuroIntervention. 2017;13:29–37. [DOI] [PubMed] [Google Scholar]
- 11.Nashef SA, Roques F, Sharples LD, et al. . EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744. [DOI] [PubMed] [Google Scholar]
- 12.The Society of Thoracic Surgeons. Online STS adult cardiac surgery risk calculator 2017. http://riskcalc.sts.org/stswebriskcalc/#/. Accessed October 7, 2018.
- 13.Rockwood K, Stadnyk K, MacKnight C, et al. . A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. [DOI] [PubMed] [Google Scholar]
- 14.Kappetein AP, Head SJ, Généreux P, et al. . Valve Academic Research Consortium-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 15.Machado MN, Nakazone MA, Maia LN. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durko AP, Osnabrugge RL, Van Mieghem NM, et al. . Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635–2642. [DOI] [PubMed] [Google Scholar]
- 17.Falk V, Baumgartner H, Bax JJ, et al. . 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017;52:616–664. [DOI] [PubMed] [Google Scholar]
- 18.Eggebrecht H, Mehta RH. Transcatheter aortic valve implantation (TAVI) in Germany 2008-2014: on its way to standard therapy for aortic valve stenosis in the elderly?. EuroIntervention. 2016;11:1029–1033. [DOI] [PubMed] [Google Scholar]
- 19.Benamer H, Auffret V, Cayla G, et al. . Position paper of French Interventional Group (GACI) for TAVI in France in 2018. Ann Cardiol Angeiol. 2018;67:455–465. [DOI] [PubMed] [Google Scholar]
- 20.Cerrato E, Nombela-Franco L, Nazif TM, et al. . Evaluation of current practices in transcatheter aortic valve implantation: The WRITTEN (WoRldwIde TAVI ExperieNce) survey. Int J Cardiol. 2017;228:640–647. [DOI] [PubMed] [Google Scholar]
- 21.Wenaweser P, Stortecky S, Heg D, et al. . Short-term clinical outcomes among patients undergoing transcatheter aortic valve implantation in Switzerland: the Swiss TAVI registry. EuroIntervention. 2014;10:982–989. [DOI] [PubMed] [Google Scholar]
- 22.Terré JA, George I, Smith CR. Pros and cons of transcatheter aortic valve implantation (TAVI). Ann Cardiothorac Surg. 2017;6:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahimtoola SH Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55:2413–2426. [DOI] [PubMed] [Google Scholar]
- 24.Kapadia SR, Leon MB, Makkar RR, et al. . 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. [DOI] [PubMed] [Google Scholar]
- 25.Chakos A, Wilson-Smith A, Arora S, et al. . Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5-year survival and beyond. Ann Cardiothorac Surg. 2017;6:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. Eur Heart J. 2018;39:2003–2013. [DOI] [PubMed] [Google Scholar]
- 27.Toggweiler S, Nissen H, Mogensen B, et al. . Very low pacemaker rate following ACURATE neo transcatheter heart valve implantation. EuroIntervention. 2017;13:1273–1280. [DOI] [PubMed] [Google Scholar]
- 28.Rogers T, Torguson R, Bastian R, et al. . Feasibility of transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis: rationale and design of the Low Risk TAVR (LRT) study. Am Heart J. 2017;189:103–109. [DOI] [PubMed] [Google Scholar]
- 29.Junquera L, Ferreira-Neto A, Guimaraes L, et al. . Transcatheter aortic valve replacement in low risk patients. Minerva Cardioangiol. 2019;67:19–38. [DOI] [PubMed] [Google Scholar]
- 30.O’Sullivan CJ, Wenaweser P. A glimpse into the future: in 2020, which patients will undergo TAVI or SAVR? Interv Cardiol. 2017;12:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]