Abstract
Hydroxychloroquine, initially used as an antimalarial, is used as an immunomodulatory and anti-inflammatory agent for the management of autoimmune and rheumatic diseases such as systemic lupus erythematosus. Lately, there has been interest in its potential efficacy against severe acute respiratory syndrome coronavirus 2, with several speculated mechanisms. The purpose of this review is to elaborate on the mechanisms surrounding hydroxychloroquine. The review is an in-depth analysis of the antimalarial, immunomodulatory, and antiviral mechanisms of hydroxychloroquine, with detailed and novel pictorial explanations. The mechanisms of hydroxychloroquine are related to potential cardiotoxic manifestations and demonstrate potential adverse effects when used for coronavirus disease 2019 (COVID-19). Finally, current literature associated with hydroxychloroquine and COVID-19 has been analyzed to interrelate the mechanisms, adverse effects, and use of hydroxychloroquine in the current pandemic. Currently, there is insufficient evidence about the efficacy and safety of hydroxychloroquine in COVID-19.
KEY MESSAGES
HCQ, initially an antimalarial agent, is used as an immunomodulatory agent for managing several autoimmune diseases, for which its efficacy is linked to inhibiting lysosomal antigen processing, MHC-II antigen presentation, and TLR functions.
HCQ is generally well-tolerated although severe life-threatening adverse effects including cardiomyopathy and conduction defects have been reported.
HCQ use in COVID-19 should be discouraged outside clinical trials under strict medical supervision.
Keywords: Hydroxychloroquine, COVID-19, cardiotoxicity, mechanism of action
Introduction
Initially used to treat malaria, hydroxychloroquine (HCQ) is an important therapeutic option for several autoimmune diseases, especially systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). The efficacy of HCQ in rheumatic illnesses stems from its anti-inflammatory and immunomodulatory effects, the mechanisms of which are unclear. Although initially thought to exert its immunomodulatory effects by interfering with lysosomal enzymatic actions and major histocompatibility complex class-II (MHC-II)-mediated antigen presentation, emerging evidence suggests interference with Toll-like receptor (TLR) functions as an additional pathway [1].
HCQ is one of the safest immunomodulatory agents for rheumatic illness. However, rare but serious adverse effects have been reported, mostly with long-term use. HCQ-induced acquired lysosomal storage disease causes some of these adverse effects, including myopathy and cardiomyopathy [1]. Corrected QT (QTc) interval prolongation is associated with HCQ owing to human ether-à-go-go-related gene (hERG) voltage-gated potassium channel inhibition [2].
In vitro studies [3–7] have revealed the antiviral properties of HCQ, raising interest in its potential therapeutic role in coronavirus disease 2019 (COVID-19). As of 30 August 2020, 24,854,140 cases of COVID-19 were reported with 838,924 deaths globally according to the World Health Organization (WHO) [8] and currently there is no effective treatment for this novel disease. Although HCQ was among the first drugs evaluated for COVID-19 treatment, clinical trials [9] reported so far have largely been inadequate to confirm its efficacy owing to poor methodology and small sample sizes. Furthermore, recent studies [10–15] have raised concerns about the safety of HCQ, especially in combination with other drugs.
This review illustrates the mechanisms of action underlying the antimalarial, immunomodulatory, and potentially antiviral properties of HCQ, and the pathophysiological aspects of HCQ-mediated cardiotoxicity, with novel pictorial explanations. Additionally, the controversial role of HCQ for COVID-19 treatment is summarized with currently available clinical trials.
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE, PubMed, EMBASE, SCOPUS, Google Scholar, Science Citation Index and references from relevant articles using the search terms “hydroxychloroquine,” “severe acute respiratory syndrome coronavirus 2,” “SARS-CoV-2,” “COVID-19,” “2019-nCoV,” “Wuhan,” and “coronavirus.” Only articles published in English from inception to 31st August 2020, restricted to humans, and directly related to this review were included.
Indications of HCQ
Antimalarials have been used for the treatment of RA since the 1950s. HCQ is one of the mildest and safestdisease-modifying antirheumatic drugs [16]. Although initially used predominantly for RA, HCQ may be most efficacious in SLE, for which besides treating skin and joint disease, HCQ prevents disease flares, promotes long-term survival, and improves overall prognosis [17]. Furthermore, the antithrombotic effects of HCQ are beneficial in patients with SLE and anti-phospholipid syndrome [18,19]. HCQ may have utility in several infectious disease processes. Although the overall efficacy of HCQ in infectious diseases, besides malaria, is unknown, HCQ is being explored in human immunodeficiency viruses, Coxiella burnetii, Zika virus, chikungunya, and Whipple’s disease [18].
Mechanisms of action
HCQ and chloroquine (CQ) are 4-aminoquinolines with similar chemical structures, except for an ethyl group substitution by a hydroxyethyl group on the tertiary amino acid side chain in HCQ. HCQ, 2-[[4-[(7-chloro-4-quinolyl)amino]pentyl]ethylamino]ethanol sulphate [20], has antimalarial and immunomodulatory properties. HCQ is absorbed rapidly after oral administration and has a long half-life of 30–60 days, reaching steady plasma levels up to 6 months after therapy initiation [21]. HCQ can be detected in plasma and tissues several months to years after discontinuation [22]. HCQ is metabolized in the liver by CYP450 and undergoes renal excretion.
Antimalarial action
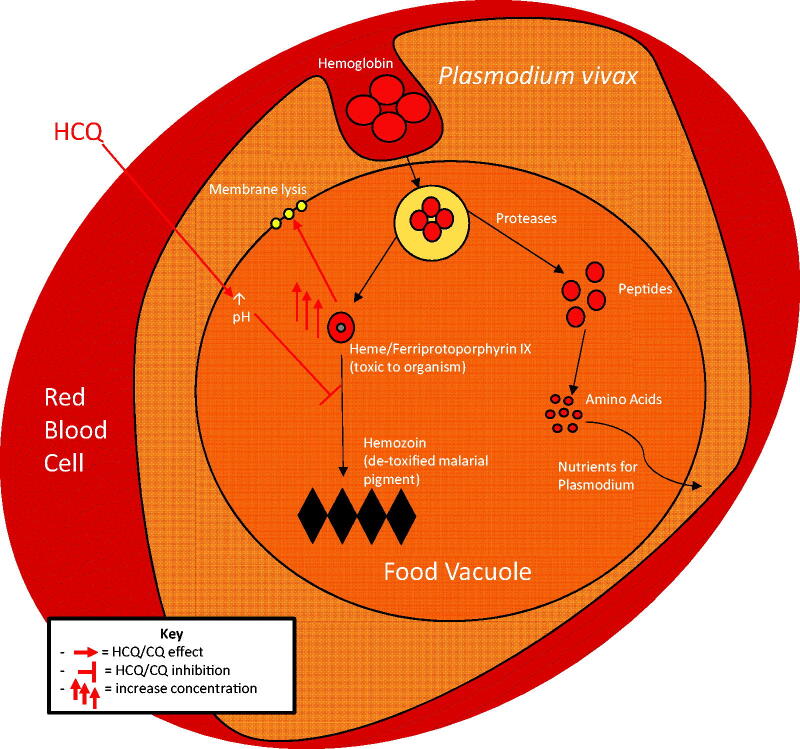
HCQ was widely used as an antimalarial agent before the rapid development of drug-resistance. In malaria, HCQ acts as a blood schizonticide against trophozoites in red blood cells (RBCs). In RBCs, a trophozoite obtains the amino acids required for growth by haemoglobin breakdown in its food vacuole. A by-product of this breakdown is haem (ferriprotoporphyrin IX), which is toxic to the parasite as it lyses cell membranes. In the food vacuole, this toxic haem is converted to non-toxic crystallized hemozoin [23].
The antimalarial action of HCQ is dependent on its lipophilicity to permeate and accumulate in intracellular structures, including lysosomes and food vacuoles of the malaria parasite. Once inside an intracellular vesicle, HCQ increases the pH as a weak base [24]. By increasing food vacuole pH, HCQ interferes with the conversion of haem to hemozoin, thereby increasing the toxic haem level, which lyses the parasite (Figure 1) [18].
Figure 1.
Antimalarial actions of hydroxychloroquine (HCQ). Being lipophilic, HCQ easily permeates the red blood cell that contains the malaria parasite and enters the food vacuole of the parasite. Being weakly alkaline, HCQ increases the pH of the food vacuole, which inhibits the conversion of toxic haem to non-toxic hemozoin. Accumulation of toxic haem leads to membrane lysis and parasite death.
Immunomodulatory and anti-inflammatory action
Although HCQ is efficacious in several autoimmune and inflammatory disorders, including SLE and RA, the exact mechanism underlying the anti-inflammatory and immunomodulatory actions of HCQ is unclear.
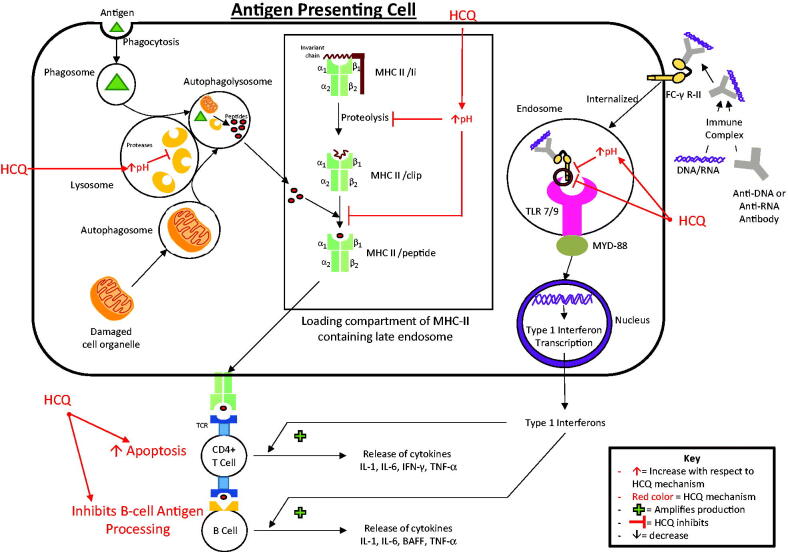
Being lipophilic, HCQ easily permeates cell membranes and accumulates in intracellular vesicles, including lysosomes, endosomes, and autophagosomes. In these acidic vesicles, it interferes with vesicular enzyme functionality (such as proteases) by increasing the pH [25]. In antigen-presenting cells (APCs), HCQ interferes with the processing of antigens to peptides, thereby preventing peptide presentation for MHC-II [1,26]. Furthermore, in the loading compartment of MHC-II-containing acidic endosomes, HCQ possibly interferes with the interaction of peptides with MHC-II. A crucial step in this interaction is the clipping of the MHC-II invariant chain and replacement by antigen peptides, which forms the MHC-II/peptide complex. An increase in pH caused by HCQ inhibits invariant chain clipping by proteases. This selectively inhibits the binding of low-affinity self-antigen peptides to the MHC-II binding site but not of high-affinity foreign-antigen peptides (such as bacterial peptides), possibly explaining why HCQ is not associated with an increased infection risk [1,21] (Figure 2).
Figure 2.
Immunomodulatory actions of hydroxychloroquine (HCQ). In antigen-presenting cells, HCQ increases the pH of lysosomes and inhibits lysosomal proteases, thereby inhibiting antigen processing and presentation to major histocompatibility complex class-II proteins (MHC-II). HCQ increases the pH of the late endosome loading compartment that contains MHC-II, which inhibits the clipping and replacement of the invariant chain (Ii) by antigenic peptides and prevents the formation of the MHC-II/peptide complex, thereby inhibiting MHC-II-mediated antigen presentation to CD4+ T-cells. In plasmacytoid dendritic cells, HCQ inhibits immune complex-mediated Toll-like receptor (TLR) 7 and 9 in the endosome by increasing the pH of the endosome and directly inhibiting the binding of the immune complex to the TLR 7 and 9, thereby preventing downstream type-1 interferon transcription. HCQ promotes T-cell apoptosis and inhibits B-cell antigen processing, thereby decreasing T-cell- and B-cell-mediated cytokine release.
Thus, by interfering with MHC-II-related autoantigen presentation to cluster of differentiation (CD) 4+ T-cells via APCs, HCQ interferes with cytokine release. This action also interferes with B-cell activation by CD4+ T-cells. Additionally, HCQ induces apoptosis of autoreactive T-cells and interferes with antigen processing by B-cells, thereby interfering with their functions and cytokine production (interleukin [IL]-1, IL-6, interferon-gamma, tumour necrosis factor [TNF], and B-cell activating factor) [21,27].
A recently highlighted immunomodulatory mechanism associated with HCQ is the inhibition of TLR signalling pathways [28]. Immune complexes that contain DNA or RNA bind Fc-gamma receptor-II on plasmacytoid dendritic cells and are internalized to endosomes that contain intracellular TLR7 and TLR9, which recognizes single-stranded RNA and DNA respectively. The binding of immune complexes to TLR7 and TLR9 leads to the downstream induction of type-1 interferon transcription through the myeloid differentiation primary response protein 88. The pathogenic role of type-1 interferons in various rheumatic diseases, such as SLE, has been well described [29]. Type-1 interferons activate T-cells, B-cells, natural killer cells, myeloid dendritic cells, and monocytes, leading to further cytokine production [1,21]. HCQ accumulates in TLR7 and TLR9-containing endosomes and directly inhibits the binding of TLR7 and TLR9 to the immune complexes. By increasing the pH of the endosome, HCQ can also interfere with TLR processing [30]. Thus, by interfering with TLR7 and TLR9 signalling, HCQ inhibits the transcription of type-1 interferons, which results in immunomodulatory and anti-inflammatory effects [1,21] (Figure 2).
Antiviral action
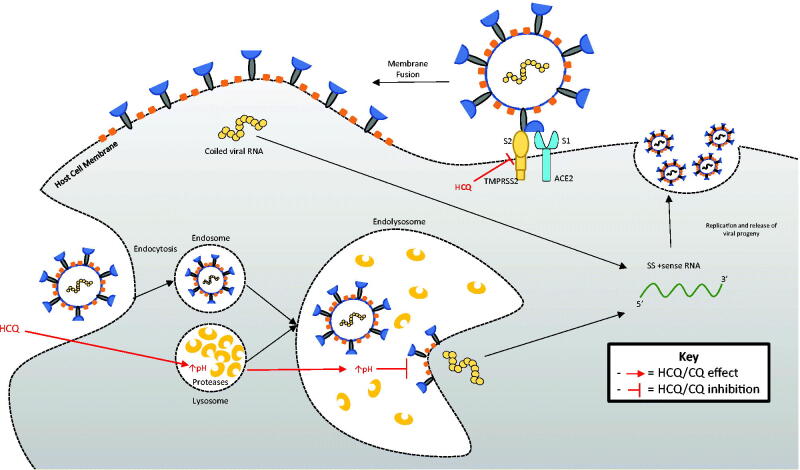
Owing to the current COVID-19 pandemic, several therapies are under investigation for potential efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using current and historical data. In vitro studies have shown potential antiviral properties associated with HCQ and CQ, raising interest in their role as potential therapies against SARS-CoV-2. The anti-inflammatory action of HCQ is dependent on immunomodulation and the downstream production of cytokines. The attenuation of inflammation results in a successful response in a rheumatic setting and possibly SARS-CoV-2 infection [31]. Furthermore, successful SARS-CoV-2 entry into host cells is strongly dependent on angiotensin-converting enzyme-2 (ACE-2) interaction with the viral spike protein [32]. CQ reduces the glycosylation of ACE-2, which inhibits the binding of the SARS-CoV-2 spike protein to the cell surface and cell integration [33,34]. Recent investigation also suggests that by binding the gangliosides, HCQ inhibits communication between the spike protein and the cell membrane, thus inhibiting viral entry into the cell [33]. Additionally, HCQ and CQ accumulate in lysosomes and, by increasing the pH of lysosomes, prevent viral particle release by disrupting vital cellular pathways [34]. Moreover, the inhibition of glycosyl-transferases, post-translational viral modification, quinone reductase-2 and sialic acid synthesis, and viral replicative mechanisms is implicated in the antiviral effect of HCQ (Figure 3) [31]. However, to date, no in vivo studies have confirmed the potential antiviral action of HCQ in humans.
Figure 3.
Proposed theoretical antiviral actions of hydroxychloroquine (HCQ). By increasing the pH of the lysosome, HCQ may inhibit endosomal acidification to prevent viral RNA shedding into the cytoplasm, thereby interfering with downstream viral replication. HCQ may bind the gangliosides and inhibit the communication between the spike protein and the cell membrane, thus inhibiting viral entry into the cell.
Adverse effects
HCQ is widely used in rheumatology and is generally safe and well-tolerated; however, several adverse effects have been reported, some irreversible and life-threatening [35].
Cardiotoxicity
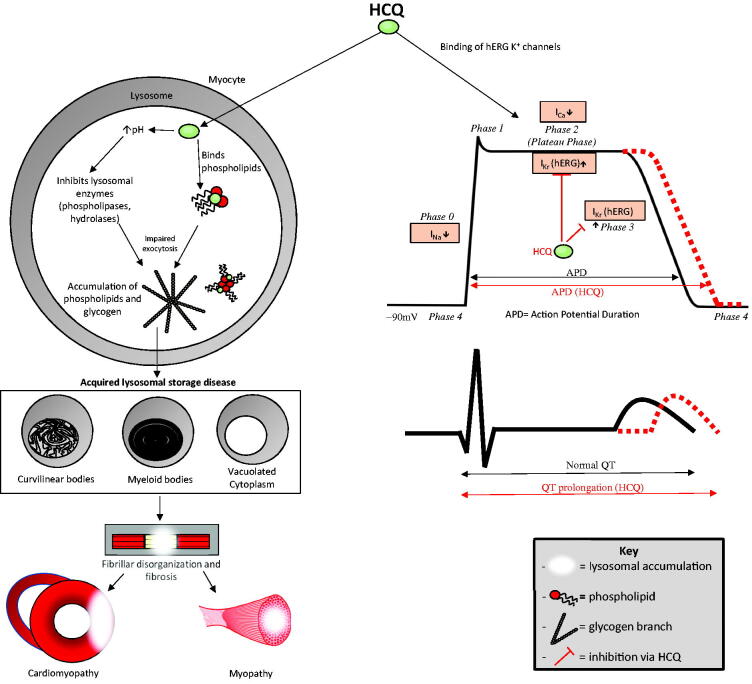
Cardiomyopathy and conduction abnormalities with HCQ have been described and recently highlighted with its use for COVID-19. Unlike cardiomyopathy, which is rare and occurs after prolonged exposure to HCQ, conduction abnormalities are common and acute (Figure 4) [36].
Figure 4.
Hydroxychloroquine (HCQ)-induced cardiomyopathy and myopathy. HCQ permeates the lysosomes of myocytes and causes glycogen and phospholipid accumulation by binding phospholipids and increasing the pH, thereby inhibiting phospholipases and hydrolases. This leads to the formation of curvilinear and myeloid bodies and cytoplasmic vacuoles causing an acquired lysosomal storage disease, which causes fibrillar disorganization, atrophy, and fibrosis. These changes lead to cardiomyopathy and proximal myopathy in skeletal muscles. HCQ-induced conduction abnormalities. HCQ binds Ikr (hERG) potassium channels, slowing potassium efflux in phase 2 and especially phase 3, thereby prolonging the action potential duration that leads to QTc prolongation (depicted in red). The action potential begins with sodium influx, phase 0; rapid potassium efflux, phase 1; calcium influx balanced by potassium efflux, phase 2; potassium efflux, phase 3; and subsequent restoration of resting membrane potential, phase 4.
Acquired lysosomal storage disease induced by HCQ is the pathogenetic pathway for the development of cardiomyopathy, mostly with long-term use [37]. Most cases are caused by accumulation, which can be augmented by CYP450 2C8 mutation [38]. Being lipophilic, HCQ easily permeates myocytes, in which it binds lysosomal phospholipids, leading to lysosomal accumulation of phospholipids. Furthermore, by increasing the pH of the lysosome, HCQ inhibits lysosomal enzymes, such as hydrolases and phospholipases, which interferes with lysosomal function and exocytosis, leading to the accumulation of glycogen and phospholipids [39]. The abnormal accumulation of metabolic products and lysosomal inclusions in cardiac myocytes induces an acquired lysosomal storage disease, leading to myofibrillar disorganization, atrophy, and fibrosis, which may lead to cardiomyopathy [40]. Acquired lysosomal storage disease can be visualized by electron microscopy as vacuoles, myeloid bodies, and curvilinear bodies. Although vacuoles are more commonly detected, curvilinear bodies are pathognomonic of HCQ-induced lysosomal storage disease. Histopathologically, HCQ-induced lysosomal storage disease appears identical to inherited lysosomal storage diseases, including Anderson-Fabry disease, except for the presence of curvilinear bodies [41]. The most frequent clinical presentation is acute exacerbation of right, left, or biventricular heart failure. Risk factors for the development of HCQ-induced cardiomyopathy include prolonged exposure to the drug (several years), elderly age, renal insufficiency, and chronic liver disease. Diffusely thickened ventricular walls on a transthoracic echocardiogram are hallmarks of this cardiomyopathy, although this is not specific to HCQ-induced cardiomyopathy [42,43]. A cardiac magnetic resonance image (MRI) that shows late gadolinium enhancement is a marker for fibrosis, especially in hypertrophic cardiomyopathy, and prognostic marker for cardiac death. MRI can also be used to guide biopsy sampling; hence, MRI plays an important role in cardiac evaluation and risk stratification [44]. Endomyocardial biopsy with electron microscopy is the most specific diagnostic test [45]. Fortunately, most patients report symptom resolution after drug cessation [46].
HCQ-induced conduction disorders are usually acute, owing to cardiac channel blockage. Several structurally-related medications, such as quinolones, CQ, and HCQ, that affect myocardial depolarization and repolarization mainly via cardiac K+ channel blockage cause QT/QTc prolongation, which is an indicator of an increased risk of drug-induced torsade de pointes (TdP). TdP is usually self-limiting but can degenerate into lethal ventricular fibrillation and cause sudden cardiac death [38]. The main mechanism of HCQ-induced QT prolongation is blockage of hERG K+ channels [47]. hERG, located on chromosome 7 q35-36, encodes the pore-forming subunits of hERG K+ channels, which mediate rapid delayed rectifier potassium currents (Ikr), resulting in phase 2 and phase 3 of repolarization in the cardiac cycle. The blockage of hERG K+ Ikr channels increases the duration of phase 2 and especially phase 3 repolarization, leading to a prolonged QT interval (class III antiarrhythmic effect) [2]. Additionally, HCQ can cause hypotension owing to alpha blockade, leading to arteriolar and venular dilation, sodium (class 1 antiarrhythmic effect) and calcium channel blockage, and a negative inotropic effect at low micromolar concentrations [2]. These effects explain the reduction in the maximum velocity of cardiac action potential and conduction disturbances, such as atrioventricular block, bundle branch block, and a QT prolongation effect [38]. HCQ blocks cardiac channels in a dose-dependent manner. At the currently recommended dose of less than 5 mg/kg/day, HCQ is usually safe, although prolongation of the QT/QRS is rarely observed on a surface electrocardiogram [48]. Ventricular ectopy and lethal ventricular arrhythmias have been reported, mostly with supra-therapeutic doses. Risk factors for the development of lethal ventricular arrhythmias include underlying structural heart disease, electrolyte abnormalities (e.g. hypokalaemia and hypomagnesemia), female sex, elderly age, genetic defects of cardiac ion channels (inherited long QT syndrome owing to hERG mutation), renal insufficiency, chronic liver disease, and, concomitant use of other drug classes that cause QT prolongation, such as azithromycin (AZ) [2]. A QT/QTc interval of over 500 ms is associated with a higher risk of TdP and sudden cardiac death. The risk of cardiotoxicity secondary to HCQ is theoretically greater in critically ill patients with COVID-19 owing to the potential for viral myocarditis, cardiac injury owing to cytokine storm, and multiorgan failure [47].
Other adverse effects
HCQ-induced ocular toxicity has been recognized, especially Bilateral bull’s eye maculopathy with central macular involvement sparing the parafovea, which is observed rarely (< 1%) in the first 5 years of therapy, in < 2% of cases after 10 years, and in up to 20% of cases after 20 years [49,50]. HCQ binds melanin in the retinal pigment epithelium (RPE) and accumulation results in macular damage. Furthermore, by increasing RPE lysosome pH and inhibiting lysosomal enzymes and phagocytosis, HCQ inhibits the clearance of shed outer photoreceptor segments, leading to accumulation. This leads to the migration of pigment-containing RPE cells to outer retinal layers, and in the loss of photoreceptors and RPE atrophy. Damage to the outer retinal photoreceptor layer precedes RPE damage and atrophy according to optical coherence tomography data. HCQ-induced retinopathy is irreversible and may continue for several months after drug discontinuation owing to its long half-life [49,51]. HCQ can also bind cellular lipids in the cornea and deposit in the corneal basal epithelial layer leading to corneal deposition (vortex keratopathy), which is reversible after drug discontinuation. Other ocular adverse effects include ciliary body deposition leading to disturbances in accommodation and blurred vision, which are also reversible [52].
Proximal myopathy, possibly associated with neuropathy, owing to HCQ has been reported, with a similar pathogenesis to that of HCQ-induced cardiomyopathy, i.e. acquired lysosomal storage disease (Figure 4). Risk factors include higher cumulative dose, elderly age, and renal disease. HCQ-induced myopathy presents with proximal weakness with normal creatine phosphokinase levels but abnormal electromyogram and muscle biopsy results that reveal vacuoles, myeloid bodies, or curvilinear bodies, the latter being the most specific to this disease [53]. HCQ-induced myopathy is usually reversible, with rapid clinical improvement after drug discontinuation [54].
Other adverse effects of HCQ include gastrointestinal distress (e.g. nausea, vomiting, diarrhoea, abdominal pain, and anorexia) and skin rash, which are common and observed in 5–10% of patients administered with HCQ [16,55]. Rare adverse effects include skin hyperpigmentation, alopecia, agranulocytosis, aplastic anaemia, leukopoenia, thrombocytopenia, haemolytic anaemia in glucose-6-phosphate dehydrogenase deficiency, irritability, nervousness, headaches, dizziness, vertigo, tinnitus, and transaminitis [56].
COVID-19 and HCQ literature review
In December 2019, China reported a novel viral illness caused by SARS-CoV-2, later defined by the WHO as COVID-19. HCQ gained interest as a potential therapeutic option for COVID-19 based on in vitro studies suggesting efficacy of HCQ and CQ against SARS-COv and SARS-Cov-2 [3–7] (Table 1). Although the initial studies showed potential efficacy, these had several flaws and the risk of bias and several further trials failed to confirm the efficacy of HCQ for COVID-19.
Table 1.
Summary of in vitro studies with hydroxychloroquine and chloroquine in coronaviruses.
| Date | Authors | Results |
|---|---|---|
| August 2004 | Keyaerts et al. [3] | CQ inhibits SARS-CoV |
| August 2005 | Vincent et al. [4] | CQ inhibits SARS-CoV |
| February 2020 | Wang et al. [5] | CQ inhibits SARS-CoV-2 |
| March 2020 | Yao et al. [6] | HCQ > CQ against SARS-CoV-2, dosage recommendations |
| April 2020 | Andreani et al. [7] | HCQ and AZ show synergistic effect against SARS-CoV-2 |
Studies suggesting HCQ efficacy in COVID-19
HCQ efficacy investigation began with a French investigation of 36 patients [57]. The investigation claimed efficacy of HCQ ± AZ in COVID-19 as significantly more patients administered HCQ ± AZ had negative polymerase chain reaction (PCR) results on Day 6 than those not administered HCQ. However, this study had several major limitations, including small sample size, lack of randomization and blinding, heterogeneous patient recruitment, and poorly selected endpoints. Furthermore, the six patients lost to follow-up were all in the treatment arm, some with adverse outcomes. Despite several significant concerns with the methodology, this study gained widespread attention that led to the use of HCQ in patients with COVID-19. Another study by the same authors evaluated the efficacy of HCQ with AZ in 80 patients with COVID-19 and showed efficacy in 65 patients [58]. However, a significant pitfall of this study was the lack of a control arm. Furthermore, the viral PCR threshold value, which determined patient discharge from the hospital, was changed multiple times during the study.
The first randomized controlled trial (RCT) suggesting efficacy of HCQ in COVID-19 was reported by Chen et al., who reported a shorter duration of symptoms (fever and cough) and radiographic improvements in patients with mild COVID-19 treated with HCQ for 5 days compared with those with standard of care treatments [59]. However, this study was also limited by sample size (31 patients in each arm), strict inclusion criteria excluding severe cases, which raises concerns of selection bias. Additionally, clinical improvement was assessed by only fever and cough, excluding other important outcomes, such as oxygen saturation. Another open-label RCT by Chen et al. suggested shorter time to clinical recovery which was 5.50 days in CQ arm (n = 18), 6.00 days in HCQ arm (n = 18) and 7.50 days in control arm (n = 12). Besides the small sample size, this study was nonblinded. Further, the study was terminated early and was underpowered [60].
The recent evidence about the efficacy of HCQ in COVID-19 has been through retrospective case series or retrospective non-randomized, non-blinded observational studies. Ahmad et al. reported clinical recovery defined as improvement in fever and dyspnoea in 85% of the patients hospitalized with COVID-19 when treated with HCQ and doxycycline. This case series had a small sample size (n = 54) with no control arm. Further, 14.8% (8) patients in this study clinically deteriorated or died and radiographic improvement was observed only in 11% of the patients [61]. Million et al. reported “good clinical outcome” and virological clearance in 91·7% out of 1,061 patients with COVID-19 treated with HCQ + AZ [62]. However, drawbacks of this retrospective case series included no control arm, poorly defined clinical outcomes, unsupervised treatment, and incomplete data with computed tomography scans and serum drug levels unavailable in some cases. Yu et al. reported decreased mortality (18·88% vs 45·8%) and reduced IL-6 levels with the use of HCQ in 48 critically ill patients with COVID-19 [63]. Antivirals were used in several patients and significantly more patients receiving interferon and antibiotics in the non-HCQ group than in the HCQ group. Additionally, with the cause of mortality not specified, drug interactions and comorbidities could have influenced the results. Novales et al. reported decreased mortality in patients treated with HCQ (27 out of 123, 22%) compared to the control arm (21 out of 43, 48.8%) in a retrospective analysis in patients admitted with COVID-19. Besides small sample size, lack of blinding and retrospective study method, other limitations of this study included use of other antiviral and anti-inflammatory medications, younger patients in the HCQ arm (61.5 years vs 68.7 years), and cause of mortality not specified [64]. A large retrospective population wide analysis of patients with confirmed/suspected COVID-19 from Portugal compared the incidence of PCR positivity in those who were already on HCQ to those who were not [65]. About, 0.29% of all patients who tested positive for COVID-19 were on HCQ while 0.36% of all patients who tested negative were on HCQ. No data was available about patient comorbidities, and drug compliance in this retrospective analysis. A direct causation effect could be deduced based on this retrospective observational analysis [65]. A recent multicenter retrospective analysis of HCQ ± AZ in 2,541 inpatients with COVID-19 reported significantly lower mortality in patients treated with HCQ alone (13.5%), or in combination with AZ (20.1%) than in patients in the control arm (26.4%) [66]. However, significantly more patients received corticosteroids (78·9% and 74·3% versus 35·7%) and tocilizumab (3·4% and 9·2% versus 1·2%) in the HCQ and HCQ + AZ arms than in the control arm. Furthermore, significantly more patients were more than 65 years old (61·4% versus 48·9% and 45·5%) in the control arm than in the HCQ and HCQ + AZ arms. Although propensity score matching suggested a 51% decline in the mortality hazard ratio in patients who received HCQ, unmeasured biases may exist, in addition to the limitations of a non-randomized, non-blinded observational trial.
Studies suggesting no efficacy of HCQ for COVID-19 treatment
Several retrospective cohort analyses have failed to show efficacy of HCQ in virological clearance of COVID-19. Mallat et al. reported a delay in virological clearance in patients with COVID-19 treated with HCQ [67]. No improvement in lymphopenia or inflammatory markers was observed. This study was limited by small sample size (21 in HCQ and 13 in control arm) and exclusion of severe illness. A small case series of 11 patients with COVID-19 treated with HCQ + AZ revealed no virological clearance in eight patients 5–6 days after treatment [68]. The small sample size, lack of a control arm, and short follow-up were the limitations of this study. A prospective study measuring consecutive viral loads in 66 patients admitted with moderately severe COVID-19 did not find any difference in viral load clearance over time in vivo with use of HCQ compared to those not treated with HCQ [69]. Again, small sample size and variation in time for serial sampling were some limitations with this study. Similarly, another observational study evaluated the effect of HCQ on seroconversion in 43 patients with mild COVID-19 by PCR on Days 0.3 and 8 [70] and did not find any association of HCQ use with increase or decrease in viral RNA copy number. Small sample size, short follow up and exclusion of more severe disease were some of the limitations of this study. A retrospective observational study (n = 85) actually reported that use of HCQ + AZ was associated with decreased and delayed virological clearance, with median time to negative PCR being 23 days in HCQ + AZ arm and 19 days in control arm, and 77% patients in HCQ + AZ arm compared to 100% patients in control arm being PCR negative at Day 28 [71]. Again, small sample size in addition to a younger patient population, unequal use of other medications, and more symptomatic patients in HCQ + AZ arm were some limitations of this study.
Lack of efficacy of HCQ in clinical outcomes of COVID-19 has been observed in many retrospective cohort analyses. An observational study in patients with COVID-19, who required oxygen but not in an intensive care unit, from France did not observe any difference in clinical outcomes (survival or transfer to intensive care unit) in 84 patients who received HCQ and 89 patients who did not [72]. Furthermore, more patients had QTc prolongation in the HCQ arm than in the control arm. A retrospective observational study in 80 patients admitted to the ICU with severe COVID-19 did not find any difference in clinical outcomes (need for treatment escalation), ventilator free days, and mortality between the HCQ and control arm [73]. Another retrospective observational study (n = 84) in patients admitted with COVID-19 did not find any difference in risk of unfavourable clinical outcomes (death or transfer to ICU) in patients treated with HCQ and control arm [74]. Another observational analysis of 1,376 patients from New York determined that HCQ has no significant impact on intubation or death [75]. Although this analysis used a large sample size ensuring power, the confounding bias of unmeasured variables, such as older age and more comorbidities in the HCQ arm, must be considered. A retrospective cohort analysis of 4642 patients from France did not find any mortality benefit of HCQ ± AZ in patients hospitalized with COVID-19 [76]. Limitations of this study included more comorbidities in HCQ and HCQ + AZ arms, and lack of direct information on drug doses and study variables such as oxygen requirement. In another retrospective observational study of 2512 patients hospitalized with COVID-19, no difference in mortality rate was seen in patients prescribed HCQ ± AZ [77]. Significantly more patients who received HCQ were younger and less likely to be nursing home residents, although were more symptomatic. Further, there were variations in dosing, duration, and prescribing patterns of HCQ. Another large retrospective cohort analysis of inpatients with COVID-19 did not find any improvement in mortality or need for mechanical ventilation in those treated with HCQ ± AZ [78]. There were significant differences in baseline characteristics of the patients in either arm in this study. An investigation of 368 males in a Veterans Affairs hospital suggested no mortality benefit from HCQ ± AZ. The observers also suggested an increase in all-cause mortality with HCQ use [79]. Although a large sample was evaluated, the study population was all-male with more severe cases receiving HCQ ± AZ, possibly skewing the observed mortality increase with HCQ. A retrospective analysis of 1,438 New York hospital patients observed no significant difference in in-hospital mortality in patients receiving HCQ, AZ, and HCQ + AZ compared to no treatment [80]. As this was observational within a specific setting, the analysis of other hospital visits after discharge was limited. Only in-hospital deaths were measured, leaving the possibility for unmeasured deaths in another setting or hospital. A large multicenter retrospective analysis from the Netherlands (n = 1893) showed no difference in 21-day mortality in patients treated at hospitals that routinely used HCQ or CQ in patients admitted with COVID-19, compared to hospitals that did not [81]. Another study that suggested no survival benefit and increased risk of ventricular arrhythmias in patients with COVID-19 owing to HCQ or CQ was retracted owing to significant concerns with the accuracy of data acquisition and analysis [82,83]. Although retrospective analyses as mentioned above have the benefit of rapidly evaluating a hypothesis, several limitations exist including lack of randomization, risk of selection bias and confounding bias of unmeasured variables.
The first RCT evaluating the role of HCQ in COVID-19 by Chen et al. reported no benefit of HCQ in virological clearance, as 86·7% of patients in the HCQ arm and 90% in the conventional arm were nasopharyngeal swab PCR negative for SARS-CoV-2 by Day 7 [84]. No significant differences were noted in the resolution of fever or radiographic progression between groups. However, this study had several limitations, including the small sample size (15 patients in each arm), lack of intervention uniformity, and use of antiviral agents. Tang et al. also did not find any significant difference in clinical improvement time or PCR negativity in 70 patients treated with HCQ compared with 80 treated with the standard of care [85]. This study was open-label and antiviral treatments were used in both arms. Furthermore, the initial intention to treat protocol was not followed as several patients were switched to the other arm after the initial randomization, raising concerns of bias. Chen et al. reported no efficacy of HCQ in virological clearance in a multicenter open-label RCT (n = 33) with 81% patients in the HCQ arm and 75% patients in the standard of care arm being RT-PCR negative, with median time to negative PCR being 5 and 11 days respectively, none of these measures reaching statistical significance [86]. This study had small sample size, younger patients with only mild-moderate disease, and antivirals and antibacterials were used in the study. Another multicenter open-label RCT (n = 293) reported no difference in virological clearance at days 3 and 7 in patients treated with HCQ compared to patients in the control arm [87]. Unequal use of antivirals (more in HCQ arm), short follow up, lack of placebo masking, lack of blinding and overrepresentations of younger patients and healthcare workers (>80%) were some of the limitations of this study.
Use of HCQ ± AZ in 667 patients admitted with mild to moderate COVID-19 was evaluated by Cavalcanti et al. in a multicenter, randomized, non-blinded, open-label, three-group, controlled trial using a 7-level ordinal scale to evaluate the clinical status at day 15 [88]. Use of HCQ ± AZ was not associated with improvement in clinical status, need for mechanical ventilation, mortality rates, acute kidney injury and thromboembolic complications. QTc prolongation was observed in more patients treated with HCQ + AZ than those with HCQ alone or neither drug. This study was unblinded. Patients requiring oxygen 4 l/min or more were excluded from the trial. Protocol deviation was noted, and many patients had previously received HCQ and/or AZ 24 h prior to enrolment. Another single centre open label RCT evaluated the efficacy of HCQ in 500 patients admitted with mild COVID-19 [89]. No difference was noted in PCR negativity at Days 7 and/or 14, or the likelihood for disease progression between HCQ + standard of care arm and standard of care only arm. The study was unblinded and patients were mostly younger (35.96 ± 11.2 years), males (93.2), and all had mild infection with only 7.6% having comorbid conditions, limiting the ability of this trial to judge efficacy of HCQ in more severe cases.
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial was established to evaluate the efficacy of several drugs, including HCQ, for COVID-19 [90]. On 5 June 2020, enrolment into the HCQ arm of the trial was stopped as preliminary data did not show any beneficial effect of HCQ in hospitalized patients with COVID-19. The preliminary results of this large (1561 HCQ arm, 3155 usual care arm) randomized, controlled clinical trial show no difference in the 28-day mortality rate between the HCQ (26·8%) and control (25%) arms [90]. Furthermore, patients in the HCQ arm had a lower probability of discharge, with a longer time to discharge, than patients in the control arm, with a higher probability of needing mechanical ventilation and death if mechanical ventilation was not used. No beneficial effect of HCQ on hospital stay duration was observed. As this trial included only hospitalized patients, with a mean duration of symptoms of 9 days and more than 75% needing some form of oxygen supplementation, the effects of HCQ earlier in the course of infection in patients with less severe illness could not be assessed. In another randomized, double-blind, placebo-controlled clinical trial evaluating the efficacy of HCQ among 423 outpatients with early COVID-19, the use of HCQ was not associated with reductions in the severity or duration of symptoms [91]. There was no statistically significant difference in hospitalizations or deaths between arms. This study was limited by the lack of cases with confirmed SARS-CoV-2 infection, with a PCR test performed on only 58% of the participants and 16% participants with a negative PCR test contributing to the data. The participants in this study were mostly “low-risk” with a median age of 40 years, 68% with no comorbidities, and only 3% being African-Americans, which limit the ability of this trial to inform the efficacy of HCQ for severe COVID-19 infection in higher-risk populations.
On 20 June 2020, The National Institutes of Health stopped the clinical trial to evaluate safety and efficacy of HCQ in patients hospitalized with COVID-19 after interim results did not show any benefit of HCQ compared to placebo [92]. On 4 July 2020, WHO discontinued the HCQ arm of the SOLIDARITY trial after reviewing the interim results, which showed no mortality benefit of HCQ in hospitalized patients with COVID-19 compared with the standard of care [93,94].
Studies suggesting no prophylactic efficacy of HCQ for COVID-19
In a randomized, placebo-controlled, double-blinded trial of HCQ as a postexposure prophylactic initiated within 4 days after moderate- to high-risk exposure, HCQ was not associated with the prevention of illness compatible with COVID-19 [95]. This trial was limited by case definition (PCR confirmed or clinically compatible) and a lack of uniform PCR testing. The risk of asymptomatic infections could not be assessed owing to the lack of testing. Although patients in the HCQ arm experienced more adverse effects than those in the non-HCQ arm, most adverse effects were mild with no reports of arrhythmias, although an asymptomatic increase in QTc was not assessed.
The data available suggest that patients with rheumatic diseases (e.g. SLE) currently undergoing HCQ therapy remain at risk for COVID-19 and are not protected by HCQ use [96]. A group of 17 patients with SLE on long-term HCQ therapy who contracted COVID-19 progressed to severe disease despite baseline treatment with HCQ [97]. Data from the COVID-19 Global Rheumatology Alliance Global Registry show COVID-19 in 874 individuals with primary rheumatic disease, 27·4% of whom were on HCQ or CQ before COVID-19 diagnosis. There was no association between the use of HCQ and risk of hospitalization or serious infection in these patients, including those with SLE [98,99].
Studies evaluating adverse effects of HCQ in COVID-19
The effect of HCQ with or without AZ on QTc prolongation has been investigated in several observational and case studies [10–15]. Significant QTc prolongation with HCQ with or without AZ was reported in 90% of patients in intensive care with COVID-19 (n = 40) by Bessière et al. [10]. Chorin et al. reported severe QTc prolongation (>500 ms) in 11% of patients (n = 84) treated with HCQ + AZ [11]. In anotherstudy, which included the previous 84 patients, severe QTc prolongation in 23% of patients (n = 251) was reported; eight patients discontinued therapy owing to severe QTc prolongation and one developed polymorphic ventricular tachycardia, suspected as TdP, needing cardioversion [12]. Ramireddy et al. reported critical QTc prolongation in 12% of patients (n = 490), with greater prolongation with the combination of HCQ and AZ than with either drug alone [13]. Saleh et al. also reported greater QTc prolongation with the combination of HCQ and AZ than with either drug alone (n = 210) [14]. Although these observational studies indicate critical QTc prolongation secondary to HCQ use in COVID-19, especially in combination with AZ, they have several limitations, including lack of a control arm and effects of confounding factors such as underlying comorbidities, disease severity, and other medications. A retrospective cohort analysis of 90 patients with COVID-19 treated with HCQ reported a significant increase in QTc interval, with a greater increase in those treated with AZ (53/90) [15]. Furthermore, this study reported one patient administered HCQ + AZ who developed TdP and other ventricular arrhythmias needing lidocaine. The limitations of this study included the lack of a control arm, short follow-up, small sample size, and effects of confounding factors such as underlying comorbidities, disease severity, and other medications. A transversal study evaluating effect of HCQ in ambulatory and admitted patients with COVID (n = 219) reported a significant but not clinically relevant increase in QTc from baseline of 416 ms to 423 ms [100] 48 h after treatment initiation with none of the participants showing an increase of more than 25% in QTc. The limitations of this study included the lack of a control arm, short follow-up, and exclusion of more severe disease. Finally, an analysis from 3 RCTs evaluating HCQ as pre-exposure prophylaxis, post-exposure prophylaxis, and early treatment in COVID-19 reported safety data on HCQ in this population (n = 2795) [101]. Patients treated with HCQ experienced more adverse effects, mostly gastrointestinal and mild. Only one patient in the HCQ arm developed supraventricular tachycardia and no cases of sudden cardiac death or ventricular arrhythmias were noted, although specific effects of HCQ on QTc were not evaluated. Limitations of this analysis included inclusion of only outpatient and mostly younger, healthcare worker participants with less underlying comorbidities, thus excluding those with more severe disease and more comorbidities.
Summary of available literature
This review of the available literature suggests a scarcity of well-conducted clinical trials on HCQ for COVID-19 (Table 2). While the initial observational data suggested possible efficacy of HCQ in COVID-19, recent clinical trial data has largely been unable to reproduce these results. However, several methodological drawbacks have been noted in the available literature so far. Further, the currently available data is insufficient to definitively confirm or rule out cardiotoxicity from HCQ when used in COVID-19. However, this concern retains significance as most critical patients infected with SARS-CoV-2 have underlying cardiac comorbidities [9]. Owing to the paucity of research in COVID-19, recommendations for or against therapy cannot be suggested in earnest [102]. It is appropriate to remain mindful of potential cardiovascular risk when prescribing HCQ, especially to those with comorbidities.
Table 2.
Summary of COVID-19 studies involving hydroxychloroquine.
| Date | Authors | Study type | N | Study results (HCQ associated with:) | Study limitations |
|---|---|---|---|---|---|
| March 2020 | Gautret et al. [57] | Cohort | 36 | Improved viral clearance | Obs, SS, no randomization, unblinded, LTFU all in HCQ group |
| March 2020 | Chen J. et al. [84] | RCT | 30 | No improvement in viral clearance, mortality | SS, no intervention unifromity, antivirals used |
| March 2020 | Chen Z. et al.[59] | RCT | 62 | Improved time to clinical recovery | SS, severe cases excluded, inadequate primary end points |
| March 2020 | Molina et al. [68] | Case series | 11 | No improvement in viral clearance | Obs, SS, no control arm, short follow up |
| April 2020 | Gautret et al. [58] | Case series | 80 | Improved clinical course and viral clearance | Obs, SS, no control arm, changes to viral PCR threshold |
| April 2020 | Mathian et al.[97] | Case series | 17 | No impact on clinical course in patients with SLE | Obs, SS, obs., Rheumatic population |
| April 2020 | Chorin et al. [11] | Case series | 84 | QTc prolongation | SS, no control arm, effect of other medications, severity of illness, CM |
| April 2020 | Saleh et al. [14] | Case series | 201 | QTc prolongation | Obs, no control arm |
| April 2020 | Magagnoli et al. [79] | Cohort | 368 | No improvement in mortality, intubation. Increased all-cause mortality | Obs, all male, more severe cases received HCQ +/- AZ |
| April 2020 | Ramireddy et al. [13] | Case series | 98 | QTc prolongation | Obs, no control arm |
| May 2020 | Ahmad et al. [61] | Case series | 54 | Improved clinical recovery | Obs, no control arm, clinically worsened patients not included in final analysis |
| May 2020 | Yu et al. [63] | Cohort | 568 | Improved mortality and decreased IL-6 levels in critically ill | Obs, use of antivirals. Unequal use of other medications |
| May 2020 | Bessière et al. [10] | Case series | 40 | QTc prolongation | Obs, Early discontinuation, use of other cardiotoxic drugs, ICU patients, CM |
| May 2020 | Chorin et al. [12] | Case series | 251 | QTc prolongation | Obs, No control arm, effect of other medications, severity of illness, CM |
| May 2020 | Mercuro et al. [15] | Cohort | 90 | QTc prolongation | Obs, SS, no control arm |
| May 2020 | Mallat et al. [67] | Cohort | 34 | Dealy in viral clearance, no improvement in lab-markers | Obs, SS, exclusion of severe illness |
| May 2020 | Gianfrancesco et al. [98] | Case series | 600 | No reduction in hospitalization in patients with rheumatic diseases | Obs, Retrospective analysis, focus beyond HCQ in rheumatic populations |
| May 2020 | Novales et al. [64] | Cohort | 166 | Improved mortality | Obs, SS, use of other medications, potential baseline confounders |
| May 2020 | Million et al. [62] | Case series | 1061 | Improved viral clearance, mortality, clinical outcomes | Obs, No control arm, therapy unsupervised, incomplete data |
| May 2020 | Geleris et al. [75] | Cohort | 1376 | No improvement in mortality, intubation | Obs, HCQ arm with older age, more comorbidities |
| May 2020 | Rosenberg et al. [80] | Cohort | 1438 | No improvement in clinical outcomes or mortality in inpatients | Obs, only in-hospital deaths measured |
| May 2020 | Tang et al. [85] | RCT | 150 | No improvement in viral clearance, time to clinical recovery | Open label, antivirals in both arms, patients switched arms |
| May 2020 | Mahévas et al. [72] | Cohort | 181 | No improvement in ICU transfer, mortality | Obs, no randomization, potential baseline confounders |
| May 2020 | Singh et al. [78] | Cohort | 3372 | No improvement in mortality and need for mechanical ventillation | Obs, potential baseline confounders |
| May 2020 | Ip et al. [77] | Cohort | 2512 | No improvement in mortality | Obs, no randomization, variation in HCQ prescribing patterns |
| June 2020 | Ferreira et al. [65] | Cohort | 360304 | Less odds of PCR positivity | Obs, missing information on comorbidities, compliance |
| June 2020 | Paccoud et al. [74] | Cohort | 84 | No improvement in clinical outcomes | Obs, SS, no randomization, |
| June 2020 | Faico-Filho et al. [69] | Cohort | 66 | No improvement in viral clearance | Obs, SS, variable time for serial sampling |
| June 2020 | Boulware et al. [95] | RCT | 821 | No efficacy as post-exposure prophylaxis | Case definiation limitation, lack of uniform PCR testing |
| June 2020 | Sbidian et al. [76] | Cohort | 4642 | No improvement in mortality | Obs, lack of direct information on study variables |
| June 2020 | Chen L et al. [60] | RCT | 48 | Improved time to clinical recovery | SS, unblinded, terminated early, underpowered |
| June 2020 | Arshad et al. [66] | Cohort | 2541 | Improved mortality | Obs, effect of other medications |
| July 2020 | Lecronier et al. [73] | Cohort | 80 | No improvement in clinical outcomes or mortality in ICU patients | Obs, SS, unblinded, more severe illness |
| July 2020 | Horby et al. [90] | RCT | 4716 | No improvement in mortality, more death and ventillation in non-ventillated patients | More severe illness, therapy initiated after prolonged illness |
| July 2020 | Cavalcanti et al. [88] | RCT | 667 | No improvement in clinical status | Unblinded, protocol deviation |
| July 2020 | Mitja et al. [87] | RCT | 293 | No improvement in viral clearance | Unblinded, unequal use of other medications, younger patients |
| July 2020 | Chen CP et al. [86] | RCT | 32 | No improvement in viral clearance | SS, exclusion of severe illness, antivirals used |
| July 2020 | Skipper et al. [78] | RCT | 432 | No improvement in symptom duration or severity in outpatients | Lack of uniform PCR testing, healthier and lower-risk participants |
| July 2020 | Komissarov et al. [70] | Cohort | 43 | No improvement in viral clearance | Obs, SS, short follow up, exclusion of severe illness |
| August 2020 | Peters et al. [81] | Cohort | 1893 | No improvement in mortality | Obs, variability in standard of care, unequal use of other medications |
| August 2020 | Saleemi et al. [71] | Cohort | 85 | Delayed and decreased chances of virological clearance | Obs, SS, unequal use of other medications, potential baseline confounders |
| August 2020 | Lofgren et al. [101] | RCT | 2719 | More GI adverse effects (mild), no ventricular arrythmias or sudden cardiac death | Younger healthier participants, exclusion of severe illness |
| August 2020 | Jaimez et al. [100] | Case series | 219 | Significant but not clinically relevant QTc prolongation | Obs., No control arm, exclusion of severe illness |
| August 2020 | Kamran et al. [89] | RCT | 500 | No improvement in disease progression and PCR conversion | Unblinded, younger, healthier, male patients with mild illness. |
SS: small sample; obs.: observational limitations; CM: co-morbidities; LTFU: Lost to follow up; N-PCR: Nasopharyngeal Polymerase Chain Reaction; Green: Showing efficacy of HCQ; Red: Showing no efficacy of HCQ; Yellow: Studying adverse effects of HCQ.
As early literature suggested the efficacy of HCQ against COVID-19, several organizations [103,104] supported the cautionary use of this medication. For example, the Food and Drug Administration (FDA) approved HCQ for emergency use [103]. Although these early studies were crucial steps, recent trials suggest that the purported benefits of this medication may not outweigh the potentially life-threatening adverse effects. Owing to the evolving literature, several agencies have now advised against HCQ administration. The Centre for Disease Control, FDA, European Medical Agency, American College of Physicians, Infectious Disease Society of America, and National Institutes of Health have publicly stated the need for caution when prescribing HCQ for COVID-19 outside hospital and clinical trial settings [105–110].
Conclusion
HCQ, initially an antimalarial agent, is used as an immunomodulatory agent for managing several autoimmune diseases, for which its efficacy is linked to inhibiting lysosomal antigen processing, MHC-II antigen presentation, and TLR functions. It is generally well-tolerated although severe life-threatening adverse effects have been reported. HCQ gained popularity as a potential therapy for COVID-19, owing to in vitro data suggesting its antiviral activities by interfering with lysosomal functions. However, data on its efficacy and safety in COVID-19 are still insufficient, with several methodological difficulties and small sample sizes. Recent clinical trials suggest no role of HCQ in COVID-19 treatment or prevention, and there are unanswered questions about its cardiac safety in patients with COVID-19. Until further randomized controlled trials eliciting the efficacy and safety are available, HCQ use in COVID-19 should be discouraged outside clinical trials under strict medical supervision. Although rapid publication of small trials and observational analyses are necessary during a global pandemic, well-performed clinical trials with better methodology will best present reliable and valid data moving forward. Further low-powered investigation will only continue to cloud the overall information on this topic. Although it may not be possible to perform flawless clinical trials, researchers should plan future trials by assessing the limitations of published studies to achieve high-quality research with minimal bias and few methodological errors.
Acknowledgments
There was no funding for the work associated with this publication. None of the authors have been paid by any agency or pharmaceutical company to write this article. All authors have full access to the manuscript and all the data in the study, and the corresponding author has the final responsibility for the decision to submit for publication.
Disclosure statement
JMD has research grants from Pfizer and has served on advisory boards sponsored by Abbvie and Sanofi-Genzyme, outside of the submitted work. The other authors declare no competing interests.
Author contributions
PB and AG designed the study concept. PB, AG and AC wrote the original draft and designed the schematic figures and tables. SL, DMD, PB(2), PBB, FA, HSD, TD, DS, BS, LC and SR edited the figure legends, and revised the manuscript. AG and PB performed the final revisions and approved the final version of the article after reviewing feedback from all other authors and reviewers. All authors contributed to study design, critically reviewed the first draft, approved the final version and agreed to be accountable for the work.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- 1.Schrezenmeier E, Dörner T.. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. [DOI] [PubMed] [Google Scholar]
- 2.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–558. [DOI] [PubMed] [Google Scholar]
- 3.Keyaerts E, Vijgen L, Maes P, et al. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [16115318] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreani J, Le Bideau M, Duflot I, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Novel Coronavirus ; [cited 2020 August 31]. Available from: 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 9.Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. Faseb J. 2020;34(5):6027–6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with Coronavirus Disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chorin E, Dai M, Shulman E, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26(6):808–809. [DOI] [PubMed] [Google Scholar]
- 12.Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17(9):1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramireddy A, Chugh H, Reinier K, et al. Experience with hydroxychloroquine and azithromycin in the Coronavirus Disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9(12):e017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh M, Gabriels J, Chang D, et al. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13(6):e008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, Anderson JJ, Meenan RF.. The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis. Results of two metaanalyses. Arthritis Rheum. 1990;33(10):1449–1461. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007;66(9):1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plantone D, Koudriavtseva T.. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological Diseases: a mini-review. Clin Drug Investig. 2018;38(8):653–671. [DOI] [PubMed] [Google Scholar]
- 19.Erkan D, Yazici Y, Peterson MG, et al. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatol Oxf Engl. 2002;41(8):924–929.1093/rheumatology/41.8.924 [DOI] [PubMed] [Google Scholar]
- 20.National Center for Biotechnology Information. PubChem Database; [cited 2020. Jun 7]. Available from: CID = 123133626, https://pubchem.ncbi.nlm.nih.gov/compound/.
- 21.Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(2 Suppl 1):82–91. [DOI] [PubMed] [Google Scholar]
- 22.Rainsford KD, Parke AL, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. [DOI] [PubMed] [Google Scholar]
- 23.Slater AFG, Cerami A.. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992;355(6356):167–169. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie AH. Pharmacologic actions of 4-aminoquinoline compounds. Am J Med. 1983;75(1A):5–10. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Noriega A, Grubb JH, Talkad V, et al. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980;85(3):839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz SJ, Russell AS.. Re-evaluation of antimalarials in treating rheumatic diseases: re-appreciation and insights into new mechanisms of action. Curr Opin Rheumatol. 2011;23(3):278–281. [DOI] [PubMed] [Google Scholar]
- 27.Wozniacka A, Carter A, Mccauliffe DP.. Antimalarials in cutaneous lupus erythematosus: mechanisms of therapeutic benefit. Lupus. 2002;11(2):71–81. [DOI] [PubMed] [Google Scholar]
- 28.Lafyatis R, York M, Marshak-Rothstein A.. Antimalarial agents: closing the gate on toll-like receptors? Arthritis Rheum. 2006;54(10):3068–3070. [DOI] [PubMed] [Google Scholar]
- 29.Kirou KA, Lee C, George S, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. [DOI] [PubMed] [Google Scholar]
- 30.Macfarlane DE, Manzel L.. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol Baltim Md. 1950;160(3):1122–1131. [PubMed] [Google Scholar]
- 31.Sinha N, Balayla G.. Hydroxychloroquine and COVID-19. Postgrad Med J. 2020;96(1139):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79(23):14614–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fantini J, Di Scala C, Chahinian H, et al. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gbinigie K, Frie K.. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open. 2020;4(2):bjgpopen20X101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. [DOI] [PubMed] [Google Scholar]
- 36.Singh AP, Tousif S, Umbarkar P, et al. A pharmacovigilance study of hydroxychloroquine cardiac safety profile: potential implication in COVID-19 Mitigation. J Clin Med. 2020;9(6):1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumpter M, Tatro L, Stoecker W, et al. Evidence for risk of cardiomyopathy with hydroxychloroquine. Lupus. 2012;21(14):1594–1596. [DOI] [PubMed] [Google Scholar]
- 38.Chatre C, Roubille F, Vernhet H, et al. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41(10):919–931. [DOI] [PubMed] [Google Scholar]
- 39.Frustaci A, Morgante E, Antuzzi D, et al. Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int J Cardiol. 2012;157(1):117–119. [DOI] [PubMed] [Google Scholar]
- 40.Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 2014;30(12):1706–1715. [DOI] [PubMed] [Google Scholar]
- 41.Roos JM, Aubry M-C, Edwards WD.. Chloroquine cardiotoxicity. Cardiovasc Pathol. 2002;11(5):277–283. [DOI] [PubMed] [Google Scholar]
- 42.Joyce E, Fabre A, Mahon N.. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013;2(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cervera A. Cardiac toxicity secondary to long term treatment with chloroquine. Ann Rheum Dis. 2001;60(3):301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buggey J, ElAmm CA.. Myocarditis and cardiomyopathy. Curr Opin Cardiol. 2018;33(3):341–346. [DOI] [PubMed] [Google Scholar]
- 45.Ratliff NB, Estes ML, Myles JL, et al. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med. 1987;316(4):191–193. [DOI] [PubMed] [Google Scholar]
- 46.Cotroneo J, Sleik KM, Rene Rodriguez E, et al. Hydroxychloroquine-induced restrictive cardiomyopathy. Eur J Echocardiogr. 2007;8(4):247–251. [DOI] [PubMed] [Google Scholar]
- 47.Kamp TJ, Hamdan MH, January CT.. Chloroquine or Hydroxychloroquine for COVID-19: is cardiotoxicity a concern? J Am Heart Assoc. 2020;9(12):e016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HCQ dosing information ; [cited 2020 Jun 19]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf.
- 49.Melles RB, Marmor MF.. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453–1460. [DOI] [PubMed] [Google Scholar]
- 50.Block JA. Hydroxychloroquine and retinal safety. Lancet Lond Engl. 1998;351(9105):771. [DOI] [PubMed] [Google Scholar]
- 51.Martín-Iglesias D, Artaraz J, Fonollosa A, et al. Evolution of retinal changes measured by optical coherence tomography in the assessment of hydroxychloroquine ocular safety in patients with systemic lupus erythematosus. Lupus. 2019;28(4):555–559. [DOI] [PubMed] [Google Scholar]
- 52.Rynes RI, Krohel G, Falbo A, et al. Ophthalmologic safety of long-term hydroxychloroquine treatment. Arthritis Rheum. 1979;22(8):832–836. [DOI] [PubMed] [Google Scholar]
- 53.Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65(3):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein M, Bell MJ, Ang LC.. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27(12):2927–2931. [PubMed] [Google Scholar]
- 55.Avina-Zubieta JA, Galindo-Rodriguez G, Newman S, et al. Long term effectiveness of antimalarial drugs in rheumatic diseases. Ann Rheum Dis. 1998;57(10):582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plaquenil Package Insert ; [cited 2020. Jun 9]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf.
- 57.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Gautret P, Lagier J-C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. Epidemiology. 2020;369:m1844. [Google Scholar]
- 60.Chen L, Zhang Z, Fu J, et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study. MedRxiv. 2020. [Google Scholar]
- 61.Ahmad I, Alam M, Saadi R, et al. Doxycycline and hydroxychloroquine as treatment for high-risk COVID-19 patients: experience from case series of 54 patients in long-term care facilities. MedRxiv. 2020. [Google Scholar]
- 62.Million M, Lagier J-C, Gautret P, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35:101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu B, Wang DW, Li C.. Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19. Emer Med. 2020. DOI: 10.1101/2020.04.27.20073379 [DOI] [Google Scholar]
- 64.de Novales FJM, Ramírez-Olivencia G, Estébanez M, et al. Early hydroxychloroquine is associated with an increase of survival in COVID-19 patients: an observational study. 2020. DOI: 10.20944/preprints202005.0057.v1 [DOI]
- 65.Ferreira A, Oliveira E, Silva A, Bettencourt P.. Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection. J Med Virol. 2020. DOI: 10.1002/jmv.26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallat J, Hamed F, Balkis M, et al. Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: a retrospective study. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faico-Filho KS, Conte DD, Souza Luna LK, et al. Effect of hydroxychloroquine on SARS-CoV-2 viral load in patients with COVID-19. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komissarov A, Molodtsov I, Ivanova O, et al. Hydroxychloroquine has no effect on SARS-CoV-2 load in nasopharynx of patients with mild form of COVID-19. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saleemi SA, Alrajhi A, Alhajji M, et al. Time to negative PCR from symptom onset in COVID-19 patients on hydroxychloroquine and azithromycin – a real world experience. MedRxiv. 2020. [Google Scholar]
- 72.Mahévas M, Tran V-T, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;2020:m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lecronier M, Beurton A, Burrel S, et al. Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis. Crit Care. 2020;24(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paccoud O, Tubach F, Baptiste A, et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe COVID-19 in a French university hospital. Clin Infect Dis off Publ Infect Dis Soc Am. 2020:ciaa791. DOI: 10.1093/cid/ciaa791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382(25):2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sbidian E, Josse J, Lemaitre G, et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. MedRxiv. 2020. [Google Scholar]
- 77.Ip A, Berry DA, Hansen E, et al. Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients – an observational study. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh S, Khan A, Chowdhry M, et al. Outcomes of hydroxychloroquine treatment among hospitalized COVID-19 patients in the United States- real-world evidence from a federated electronic medical record network. MedRxiv. 2020. [Google Scholar]
- 79.Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United states veterans hospitalized with COVID-19. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters EJ, Collard D, van Assen S, et al. Outcomes of persons with COVID-19 in hospitals with and without standard treatment with (Hydroxy) chloroquine. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehra MR, Desai SS, Ruschitzka F, et al. Retracted: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet Lond Engl. 2020;22:PMC7255293. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Mehra MR, Ruschitzka F, Patel AN.. Retraction—hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020;395(10240):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J, Liu D, Liu L, et al. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Zhejiang Xue Xue Bao Yi Xue Ban J Zhejiang Univ Med Sci. 2020;49(2):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen C-P, Lin Y-C, Chen T-C, et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus Disease 2019 (COVID-19). MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 87.Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial. Clin Infect Dis off Publ Infect Dis Soc Am. 2020:ciaa1009. DOI: 10.1093/cid/ciaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020;2020:NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehmood K. s, Mirza Z e H, Naseem A, et al. Clearing the fog: is hcq effective in reducing COVID-19 progression: a randomized controlled trial. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020. 2020.07.15.20151852. [Google Scholar]
- 91.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020:M20–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.NIH halts clinical trial of hydroxychloroquine ; [cited 2020. Jul 18]. Available from: https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine.
- 93.“Solidarity” clinical trial for COVID-19 treatments discontinued; [cited 2020. Jul 18]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-COVID-19-treatments.
- 94.WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 ; [cited 2020. Oct 18]. Available from: www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-COVID-19.
- 95.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383(6):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing ; [cited 2020. Oct 19]. Available from: https://www.whitehouse.gov/briefingsstatements/remarks-president-trump-vice-president-pence-members-coronavirus-task-forcepress-briefing-19/.
- 97.Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79(6):837–839. [DOI] [PubMed] [Google Scholar]
- 98.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Konig MF, Kim AH, Scheetz MH, et al. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. 2020;79(10):1386–1388. annrheumdis-2020-217690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaimez JJ, Macias R, Bermudez F, et al. Absence of relevant QT interval prolongation in not critically ill COVID-19 patients. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lofgren SMM, Nicol MR, Bangdiwala AS, et al. Safety of hydroxychloroquine among outpatient clinical trial participants for COVID-19. MedRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Healio Cardiology Today ; [cited 2020. May 6]. Available from: https://www.healio.com/cardiology/arrhythmia-disorders/news/online/%7B7c68a265-6c04-4f7d-ace5-2c7f7f8182bb%7D/fda-improper-use-of-hydroxychloroquine-chloroquine-for-COVID-19-may-increase-risk-for-arrhythmias-death.
- 103.On March 28 , 2020. the FDA issued an Emergency Use Authorization (EUA) to allow hydroxychloroquine sulfate and chloroquine phosphate products donated to the Strategic National Stockpile(SNS) to be distributed and used for certain hospitalized patients with COVID-19; [cited 2020 Jul 18]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-daily-roundup-march-30-2020.
- 104.The AEMPS reports the controlled distribution of the entire hydroxychloroquine/chloroquine stock ; [cited 2020. Jul 18]. Available from: https://www.aemps.gob.es/informa/notasinformativas/laaemps/2020-laaemps/la-aemps-informa-de-la-distribucion-controlada-de-todo-el-stock-de-hidroxicloroquina-cloroquina/?lang=en%20(accessed%20June%2020,%202020).
- 105.Among patients with COVID-19, the IDSA guideline panel recommends hydroxychloroquine/chloroquine only in the context of a clinical trial; [cited 2020. Jul 18]. Available from: https://www.idsociety.org/practice-guideline/COVID-19-guideline-treatment-and-management/#toc-3.
- 106.EMA statement of COVID-19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine; [cited 2020. Jul 18]. Available from: https://www.ema.europa.eu/en/news/COVID-19-reminder-risk-serious-side-effects-chloroquine-hydroxychloroquine.
- 107.April, 24 2020. Hydroxychloroquine or Chloroquine for COVID-19: Drug Safety Communication – FDA Cautions Against Use Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems; [cited 2020 Jul 18]. Available from: https://www.fda.gov/safety/medical-product-safety-information/hydroxychloroquine-or-chloroquine-COVID-19-drug-safety-communication-fda-cautions-against-use.
- 108.Based on ongoing analysis and emerging scientific data , FDA has revoked the emergency use authorization (EUA) to use hydroxychloroquine and chloroquine to treat COVID-19 in certain hospitalized patients when a clinical trial is unavailable or participation is not feasible; [cited 2020. Jul 18]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-COVID-19-outside-hospital-setting-or.
- 109.NIH: The COVID-19 Treatment Guidelines Panel (the Panel) recommends against the use of chloroquine or hydroxychloroquine for the treatment of COVID-19 , except in a clinical trial (AII); [cited 2020. May 17]. Available from: https://www.COVID19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine/.
- 110.ACP: evidence does not support chloroquine or HCQ use alone or in combination with azithromycin as prophylaxis for COVID-19 ; [cited 2020 Jul 18]. Available from: Accessed July 18, 2020. https://www.acponline.org/acp-newsroom/acp-evidence-does-not-support-chloroquine-or-hcq-use-alone-or-in-combination-with-azithromycin-as
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.