Abstract
Dyslexia is a neurodevelopmental disorder mainly defined by reading difficulties. During reading, individuals with dyslexia exhibit hypoactivity in left-lateralized language systems. Lower activity in one brain circuit can be accompanied by greater activity in another, and, here, we examined whether right-hemisphere-based emotional reactivity may be elevated in dyslexia. We measured emotional reactivity (i.e., facial behavior, physiological activity, and subjective experience) in 54 children ages 7–12 with (n = 32) and without (n = 22) dyslexia while they viewed emotion-inducing film clips. Participants also underwent task-free functional magnetic resonance imaging. Parents of children with dyslexia completed the Behavior Assessment System for Children, which assesses real-world behavior. During film viewing, children with dyslexia exhibited significantly greater reactivity in emotional facial behavior, skin conductance level, and respiration rate than those without dyslexia. Across the sample, greater emotional facial behavior correlated with stronger connectivity between right ventral anterior insula and right pregenual anterior cingulate cortex (pFWE<.05), key salience network hubs. In children with dyslexia, greater emotional facial behavior related to better real-world social skills and higher anxiety and depression. Our findings suggest there is heightened visceromotor emotional reactivity in dyslexia, which may lead to interpersonal strengths as well as affective vulnerabilities.
Keywords: reading, development, emotion generation, autonomic nervous system, laterality
1. Introduction
Dyslexia is a common neurodevelopmental disorder characterized by prominent reading difficulties, and approximately 5–17% of children and adults have significant trouble learning to read despite adequate intelligence, effort, and education (S. E. Shaywitz, 1998; Silani et al., 2005). Reading is a complex process during which meaning is extracted from written words via visual and language systems (Gaillard, Balsamo, Ibrahim, Sachs, & Xu, 2003; Wandell & Le, 2017). Dyslexia is a heterogeneous disorder, but a problem with phonological processing—the ability to break words down into smaller sound units and then to associate these sound units with the written word (S. E. Shaywitz, 1998)—is the most common underlying mechanism (Bradley & Bryant, 1983; Frith, 1999; Lyon, Shaywitz, & Shaywitz, 2003; O’Brien, Wolf, & Lovett, 2012).
Neuroanatomical studies of classic phonological dyslexia have revealed altered brain structure and function in predominantly left-lateralized language systems (Goswami, 2008; Norton, Beach, & Gabrieli, 2015; Richlan, 2012; Silani et al., 2005). While post-mortem studies have shown reduced leftward asymmetry of the planum temporale in dyslexia (Galaburda, 1994; Vanderauwera et al., 2016), neuroimaging studies have found smaller gray matter volume in the left fusiform gyrus and left inferior temporal gyrus (Kronbichler et al., 2008; Linkersdorfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012), smaller gray matter volume and reduced cortical thickness in left occipitotemporal cortex (Krafnick, Flowers, Luetje, Napoliello, & Eden, 2014; Williams, Juranek, Cirino, & Fletcher, 2018), lower fractional anisotropy in white matter tracts (Vandermosten, Boets, Wouters, & Ghesquière, 2012), and enhanced gyrification of left lateral temporal and middle frontal gyri (Caverzasi et al., 2018) in dyslexia. Functional neuroimaging studies that measure brain activity during reading and phonological decision-making tasks have found that individuals with dyslexia exhibit hypoactivation of bilateral temporoparietal and left occipitotemporal structures, regions that support reading (Hoeft et al., 2007; Paulesu, Danelli, & Berlingeri, 2014; Richlan, Kronbichler, & Wimmer, 2011, 2013). Similar patterns have been found in pre-reading children with a family history of dyslexia, who also exhibit smaller gray matter volume (Brambati et al., 2004; Raschle et al., 2017; Raschle, Chang, & Gaab, 2011), atypical sulcal patterns (Im, Raschle, Smith, Ellen Grant, & Gaab, 2016), lower functional and structural connectivity (Kuhl et al., 2020; Skeide et al., 2015), white matter alterations (Langer et al., 2017; Vanderauwera, Wouters, Vandermosten, & Ghesquière, 2017; Vandermosten et al., 2015), and lower functional activity during phonological processing (Raschle, Zuk, & Gaab, 2012) in language networks. Taken together, these studies offer convergent evidence that dyslexia is characterized by predominant neural alterations in the left hemisphere. There is some variability across studies, however, with others suggesting the anatomical underpinnings of dyslexia are more diffuse and involve the right hemisphere as well (Beelen, Vanderauwera, Wouters, Vandermosten, & Ghesquière, 2019; Raschle et al., 2011).
In various clinical disorders, lateralized dysfunction in one hemisphere may facilitate function in the other, an imbalance that can lead to strengths as well as vulnerabilities (Kapur, 1996; B. L. Miller, Ponton, Benson, Cummings, & Mena, 1996; Seeley, Matthews, et al., 2008; Zhou et al., 2010). Language and emotions have long been associated with opposing hemispheres of the brain: while the left hemisphere is crucial for language, the right hemisphere plays a dominant role in emotion generation and recognition (Blonder, Bowers, & Heilman, 1991; Borod et al., 1998; Demaree, Everhart, Youngstrom, & Harrison, 2005; Gainotti, 1972; Tucker, 1981). Emotions are adaptive, multisystem responses that are accompanied by coordinated changes in autonomic nervous system activity and facial expression (i.e., herein, “visceromotor” activities), rapid bursts of activity that sweep across the body and move an individual from rest to action (Levenson, 2003). In clinical studies, individuals with predominant right hemisphere damage have diminished emotional expression and impaired recognition of emotional faces, prosody, and gestures (Blonder et al., 1991; Borod et al., 1998; Sturm, Ascher, Miller, & Levenson, 2008). In dyslexia, there is some indication that diminished functioning in language systems in the left hemisphere is accompanied by accentuated functioning in emotion systems in the right. In addition to hypoactivity in left hemisphere language systems during phonological processing tasks, for example, individuals with dyslexia exhibit hyperactivity in right hemisphere regions that promote emotions including the anterior insula and thalamus (Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; Richlan, Kronbichler, & Wimmer, 2009).
The salience network, an intrinsic connectivity network anchored by structures in the right hemisphere, plays a central role in emotion generation and sensation (Seeley et al., 2007; Seeley, Zhou, & Kim, 2012). Intrinsic connectivity networks are comprised of spatially distributed brain regions that exhibit synchronous blood oxygen level-dependent (BOLD) fluctuations in task-free settings and support various cognitive, motor, sensory, social, and affective processes (Beckmann, DeLuca, Devlin, & Smith, 2005; Fox et al., 2005). The salience network has primary hubs in the right ventral anterior insula (vAI) and right anterior cingulate cortex (ACC), regions that typically coactivate during a wide range of functional neuroimaging studies including those that elicit emotions, empathy, pain, and reward (Craig, 2009, 2011). Through projections to subcortical structures (i.e., hypothalamus, central nucleus of the amygdala, periaqueductal gray, and brainstem nuclei), the ACC and vAI play critical roles in visceromotor emotion generation and interoception, triggering and sensing the physiological and motor changes that arise during emotions (Craig, 2002; Critchley & Harrison, 2013; Levenson, 1994; Ongur & Price, 2000; Saper, 2002; Seeley et al., 2012; Vogt, 2005). Salience network connectivity, which is reliable over time and considered to be trait-like, varies in strength across people (C. C. Guo et al., 2012) and relates to variability in socioemotional sensitivity (Toller et al., 2018) such that individuals with stronger salience network connectivity are inclined to have more intense physiological and experiential reactions to affectively charged contexts than those with lower salience network connectivity (Hermans et al., 2011; Seeley et al., 2007; Xia, Touroutoglou, Quigley, Feldman Barrett, & Dickerson, 2017). Although the salience network is detectable in infancy and has a spatial topography that resembles adults (Gao, Alcauter, Smith, Gilmore, & Lin, 2015), its connections may expand and get stronger during childhood and adolescence (Uddin, Supekar, Ryali, & Menon, 2011; Zielinski, Gennatas, Zhou, & Seeley, 2010).
By guiding behavior and coloring subjective experience, emotions play an important role in everyday life and are critical for physical survival and social harmony (Levenson, 1994). Emotions not only help people to stay safe from physical threats but also encourage them to form and maintain close interpersonal bonds (Griskevicius, Shiota, & Neufeld, 2010; Lerner & Keltner, 2000). Individuals who manage their emotions with ease are better equipped to navigate complex interpersonal situations and to develop meaningful social connections (Eisenberg, Spinrad, & Eggum, 2010; Lopes, Mestre, Guil, Kremenitzer, & Salovey, 2012; Lopes, Salovey, Côté, Beers, & Petty, 2005). In children and adolescents, those who express their emotions in adaptive ways are more socially adept, more likable, and less anxious in everyday life (Denham, McKinley, Couchoud, & Holt, 1990; Lopes et al., 2012; A. L. Miller et al., 2006), whereas those who tend to express high-intensity emotions have poorer mental health, lower social skills, and less robust relationships with teachers, parents, and peers (Eisenberg et al., 1993; Eisenberg et al., 2010; Frick & Morris, 2004). While some prior research suggests children with dyslexia have poorer social skills than those without dyslexia (Parhiala et al., 2015), this finding is not consistent, and other studies have found that children and adults with dyslexia are rated as socially competent (Frederickson & Jacobs, 2001; Hellendoorn & Ruijssenaars, 2000). Although experiencing emotions is often advantageous, emotions that are too frequent or too intense can be problematic and lead to affective symptoms (Cole, Michel, & Teti, 1994; Kring & Sloan, 2009). Affective symptoms, which are associated with alterations in gray matter volume and functional connectivity in salience network structures (Davis, Margolis, Thomas, Huo, & Marsh, 2018; Goodkind et al., 2015; Sha, Wager, Mechelli, & He, 2019), are common in dyslexia (Carroll & Iles, 2006; Haft, Duong, Ho, Hendren, & Hoeft, 2019; Hendren, Haft, Black, White, & Hoeft, 2018; Novita, 2016). Children with dyslexia and reading disorders often report feelings of anxiety and depression (Mugnaini, Lassi, La Malfa, & Albertini, 2009; Willcutt & Pennington, 2000), and even mild reading deficits in children ages 8 – 12 are associated with lower mood and self-esteem (Casey, Levy, Brown, & Brooks-Gunn, 1992).
In the present study, we investigated whether children with phonological dyslexia have enhanced emotional reactivity. Children with and without dyslexia underwent a laboratory-based assessment of emotion and “resting state,” task-free functional magnetic resonance imaging (tf-fMRI). Parents of children with dyslexia also reported on their child’s real-world social behavior, mood, and anxiety. To measure emotional reactivity, participants viewed five film clips that elicited specific positive and negative emotions while facial behavior and physiological activity were recorded continuously. Subjective experience was also assessed after each film clip by asking participants to rate how much they felt various specific emotions. We hypothesized that children with dyslexia would show accentuated emotional reactivity while viewing the film clips and that greater emotional reactivity would relate to stronger intrinsic connectivity between right vAI and right ACC, key salience network hubs. Given that emotional reactivity has been associated with social advantages (Lopes et al., 2005) as well as affective vulnerabilities (Cole et al., 1994; Kring & Sloan, 2009), we expected that higher emotional reactivity in dyslexia may be associated with greater interpersonal strengths as well as greater symptoms of anxiety and depression.
2. Materials and Methods
We report how we determined our sample size, all data exclusions, all inclusion and exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures included in the present study.
2.1. Participants
Fifty-four participants, including 32 children with dyslexia and 22 controls without dyslexia, were included in the present study. All participants were fluent English speakers between the ages of 7 and 12 years of age. Guardians of participants were invited to report on their child’s ethnicity. Guardians were also asked to report on their household income on a 16-point scale, ranging from “less than $10,000” to “over $500,000,” which provided an indication of socioeconomic status, and on the type of school their child attended (public or private). The study was approved by the University of California, San Francisco (UCSF) Committee on Human Research. Guardians of the participants provided informed written consent, and participants provided verbal assent. No part of the study procedures was pre-registered prior to the research being conducted.
Children with a formal diagnosis of dyslexia made by a licensed psychologist were recruited from the UCSF Pediatric Brain Clinic, local schools, or a specialized school for students with dyslexia. Children with dyslexia were included in the present study if they had notable difficulties in reading and phonological processing and were of at least average intelligence compared to same-aged peers at the time of the UCSF evaluation (see the “Cognitive Assessment” section for details).
Controls without dyslexia were recruited through local schools and underwent limited neuropsychological and reading testing at UCSF to ensure they did not currently meet diagnostic criteria for dyslexia. Exclusion criteria included acquired brain injury, known genetic conditions that impact cognition or brain development, psychiatric disorders including autism spectrum disorders and sensory processing disorders, history of academic difficulties, or prior diagnoses of developmental disorders. Children without dyslexia were included as controls in the present study if they were at least of average intelligence and showed no notable signs of reading or phonological impairment (see Table 1 for demographic information). Inclusion and exclusion criteria were established prior to data analysis.
Table 1 –
Participant demographics and cognitive test scores.
| Dyslexia M(SD) | Controls M(SD) | p | |
|---|---|---|---|
| N | 32 | 22 | |
| Age (years) | 10.3 (1.4) | 10.5 (1.5) | .50 |
| Sex (male/female) | 17/15 | 11/11 | .82 |
| Handedness (right/non-right) | 28/4 | 22/0 | .08 |
| Ethnicity (%) | |||
| White | 63 | 64 | .93 |
| Asian or Pacific Islander | 6 | 4 | .79 |
| Mixed Race | 9 | 9 | .97 |
| Declined to State | 22 | 23 | .94 |
| Annual Household Income (%) | |||
| $60,000 – $249,000 | 28 | 50 | .10 |
| $250,000+ | 44 | 27 | .22 |
| Declined to State | 28 | 23 | .66 |
| Schooling (%) | |||
| Public | 6 | 46 | <.001 |
| Private | 91 | 36 | <.001 |
| Declined to State | 3 | 18 | .06 |
| WASI: Matrix Reasoning (raw score) | 24.1 (3.8) | 24.7 (4.9) | .64 |
| WASI: Matrix Reasoning (percentile) | 71.3 (21.7) | 74.4 (22.5) | .64 |
| TOWRE-2: Sight Word Efficiency Subscale (raw score) | 48.1 (17.5) | 71.4 (11.1) | <.001 |
| TOWRE-2: Sight Word Efficiency Subscale (percentile) | 15.7 (18.7) | 53.4 (26.5) | <.001 |
| TOWRE-2: Phonemic Decoding Efficiency Subscale (raw score) | 21.5 (11.6) | 38.8 (9.4) | <.001 |
| TOWRE-2: Phonemic Decoding Efficiency Subscale (percentile) | 12.7 (12.4) | 54.6 (23.3) | <.001 |
| Woodcock Johnson IV: Letter-Word Identification (raw score) | 50.7 (9.8) | N/A | N/A |
| Woodcock Johnson IV: Letter-Word Identification (percentile) | 29.4 (23.3) | N/A | N/A |
| Woodcock Johnson IV: Word Attack (raw score) | 18.8 (3.7) | N/A | N/A |
| Woodcock Johnson IV: Word Attack (percentile) | 32.6 (23.3) | ||
| GORT-5 Rate (raw score) | 20.8 (9.7) | N/A | N/A |
| GORT-5 Rate (percentile) | 21.2 (19.0) | ||
| GORT-5 Accuracy (raw score) | 15.3 (6.7) | N/A | N/A |
| GORT-5 Accuracy (percentile) | 11.6 (12.9) | ||
| GORT-5 Fluency (raw score) | 36.1 (15.1) | N/A | N/A |
| GORT-5 Fluency (percentile) | 14.2 (12.6) | ||
| GORT-5 Comprehension (raw score) | 23.8 (7.9) | N/A | N/A |
| GORT-5 Comprehension (percentile) | 25.7 (17.6) | N/A | N/A |
| BASC-2: Social Skills subscale (raw score) | 16.4(4.1) | N/A | N/A |
| BASC-2: Social Skills subscale (T-score) | 53.1 (8.7) | N/A | N/A |
| BASC-2: Anxiety subscale (raw score) | 10.0 (4.8) | N/A | N/A |
| BASC-2: Anxiety subscale (T-score) | 46.1 (8.3) | N/A | N/A |
| BASC-2: Depression subscale (raw score) | 4.1 (3.5) | N/A | N/A |
| BASC-2: Depression subscale (T-score) | 44.4 (9.4) | N/A | N/A |
T-tests and chi-square tests were used to determine whether there were significant differences between the groups. Cognitive scores are reported in percentiles; means (M) and standard deviations (SD) are presented unless otherwise noted. N/A = not applicable. Behavior Assessment System for Children, Second Edition (BASC-2), Gray Oral Reading Test – Fifth Edition (GORT-5), Test of Word Reading Efficiency – Version 2 (TOWRE-2), and Wechsler Abbreviated Scale of Intelligence (WASI). Clinical measures including the BASC-2 (which was used here to assess social skills, anxiety, and depression) were not administered to parents of children in the control group. Additional measures of reading, beyond the TOWRE-2, were also not administered in the control sample in the interest of brevity and retention of participants.
2.2. Cognitive Assessment
At UCSF, children with dyslexia underwent a clinical interview, neurological examination, and neuropsychological testing. We have reported all clinical data that were analyzed as a part of the present study. Matrix Reasoning from the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) was used to assess non-verbal reasoning, and the Woodcock-Johnson IV (Schrank, Mather, & McGrew, 2014) was used to evaluate academic skills (Table 1). Reading was assessed with untimed single-word reading measures from the Woodcock-Johnson IV (i.e., Letter-Word Identification and Word Attack) as well as timed measures from the Test of Word Reading Efficiency – Version 2 (TOWRE-2) (Torgesen, Wagner, & Rashotte, 2012) and the Gray Oral Reading Test – Fifth Edition (GORT-5) (Wiederholt & Bryant, 2012), which measure paragraph reading. Five children with dyslexia did not complete all of the tests due to time constraints.
The UCSF evaluation confirmed that all of the children with dyslexia had difficulty reading (at least one reading score < 25th percentile, to account for the extensive reading remediation in this group) and were of at least average intelligence (global cognitive estimates ≥ 16th percentile) compared to same-aged peers. Controls underwent a limited cognitive assessment, which included tests of non-verbal reasoning (WASI Matrix Reasoning) and single-word reading (TOWRE-2: Sight Word Efficiency Subscale and Phonemic Decoding Efficiency Subscale), and had scores ≥ 16th percentile compared to same-aged peers at the time of their UCSF evaluation.
2.3. Parent-Reported Real-World Behavior
Parents of children with dyslexia completed the Behavior Assessment System for Children, Second Edition (BASC-2) child and adolescent parent rating scale forms (Reynolds & Kamphaus, 2004). The BASC-2 is a standardized, well-validated, multi-dimensional rating system that assesses a broad range of skills and personality traits as well as adaptive and problem behaviors. The child form (ages 6–11) consists of 160 items; the adolescent form (ages 12–21) consists of 150 items. The BASC-2 scoring algorithm standardizes participants’ scores within their age group, making scores on the child and adolescent forms equivalent. The parent is asked to rate each item according to the frequency of the behavior on a four-point scale, ranging from N (never), S (sometimes), O (often), to A (almost always). Item raw scores are summed to obtain subscale scores for 14 behavioral domains. Here, we focused on one adaptive subscale (i.e., Social Skills) and two clinical subscales (i.e., Anxiety and Depression). Item raw scores were summed, and subscale scores were converted into standardized T scores (mean = 50; standard deviation = 10) for interpretation. For the adaptive scales, lower scores represent deficits, with T scores between 31 and 40 falling in the at-risk range, and scores ≤ 30 considered clinically significant. For the clinical scales, on which high scores represent more problematic behaviors, T scores between 60 and 69 are considered at-risk, and scores ≥ 70 are considered clinically significant. Legal copyright restrictions prevent public archiving of the BASC-2 used in this study; the BASC-2 is available from the copyright holder in the cited references.
2.4. Laboratory Assessment of Emotion
2.4.1. Procedure
Participants underwent a laboratory assessment of emotion at the UCSF Center for Psychophysiology and Behavior. This assessment included other emotion-relevant tasks and measures, but these were outside the scope of the present study and, thus, were not analyzed here. Participants were seated in a comfortable chair in a well-lit experiment room. Sensors were applied, and participants were videotaped throughout the testing session with a semi-obscured, remotely controlled video camera. Participants were informed they would be videotaped prior to the start of the testing session. All stimuli were presented on a 21.5-inch computer monitor placed 4.25 feet in front of them. Instructions were presented visually and via audio recordings.
2.4.2. Emotion Word Knowledge
At the beginning of the testing session, participants completed a task that assessed whether they understood the meaning of each of the emotion terms that would be used throughout the laboratory assessment. Participants were asked, “For each question, you will see an emotion word at the top of the screen. Pick the situation where you’d feel the emotion.” For each emotion term, they were presented with three choices depicting different emotional situations. All emotion terms and situations were presented visually as words and via audio. The three scenarios were also represented pictorially, with a representative image, to limit the potentially confounding influence of reading ability. After completion of the task, the experimenter reviewed any incorrect responses with the participant and explained the correct answers. This step was taken to ensure that participants understood all of the emotion terms that would be used throughout the testing session. If participants asked for clarification about the meaning of any word later in the session, the experimenter reminded them of the meaning as often as needed using standardized prompts.
2.4.3. Emotional Reactivity
During the emotional reactivity task, participants watched five film clips that each elicited a specific emotion (Figure 1). At the beginning of the task, participants were presented with the following instructions, “Now you will watch some movies. After each movie, we will ask you some questions. We want to know how YOU feel while watching the movie. If you find the videos too upsetting, please close your eyes. Before each movie, you will see an ‘X’ on the screen. Please relax and try to clear your mind when you see an ‘X’ on the screen. Let’s begin. Watch the ‘X,’ please.”
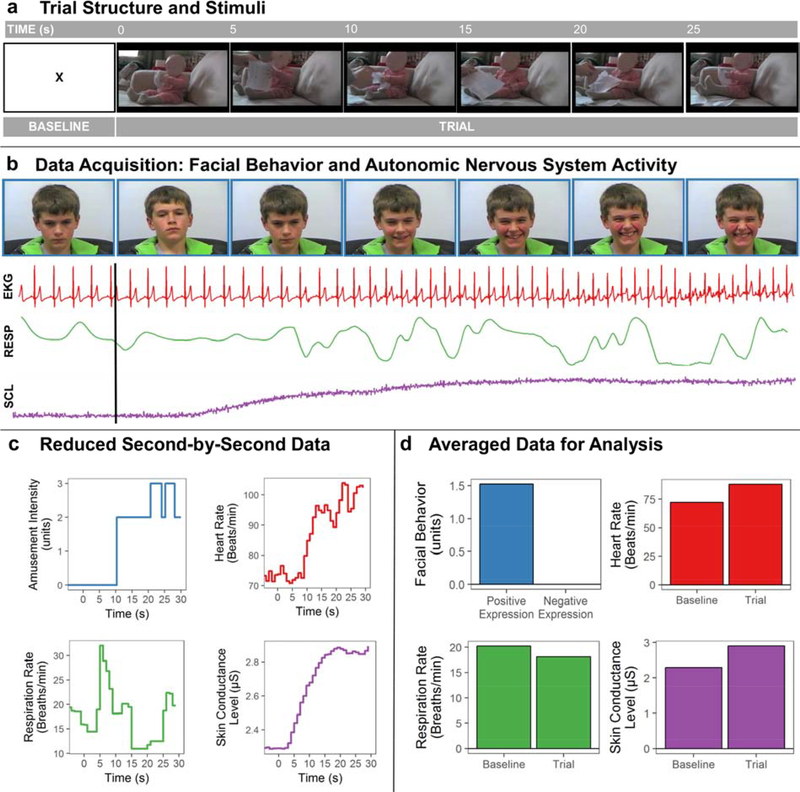
Figure 1. Laboratory-based assessment of emotional reactivity.
(A) Participants viewed emotion-eliciting film clips after a 60-second resting baseline period in which they viewed an “X” on the computer monitor. Screenshots of the first 30 seconds of the amusement film (captured at 5-second intervals) are shown for illustrative purposes. (B) Participants were videotaped throughout the testing session. The screenshots of one participant (he and his surrogate gave informed consent to publish his image), which are time-locked with the stimuli shown in (A), are provided to illustrate the happiness/amusement behavior that he expressed while watching the film clip. Physiological activity was recorded continuously, and the raw electrocardiogram (EKG), skin conductance level (SCL), and respiration (RESP) data during the baseline and trial are shown. Subjective emotional experience was assessed via self-report questions at the end of each trial (not shown). (C) Emotional facial behavior was later coded using an objective system that quantifies facial muscle movement; the second-by-second happiness/amusement intensity codes are plotted here. After the raw physiological signals were processed, they were reduced and exported as second-by-second averages. (D) Total emotional facial behavior scores were computed by summing the intensity scores of all emotional facial expressions displayed by the participant during each trial. To measure physiological reactivity, mean activity during the baseline and trial were computed for each channel; reactivity scores were computed for each channel by subtracting the mean activity level during the 60-second baseline from the mean level during the most intense 30 seconds of the trial. The participant and his guardian gave consent to use his image in this publication.
Each trial began with a 60-second resting baseline period in which participants watched a black “X” on a white computer screen. They then viewed an approximately 90-second film clip that elicited a specific positive (i.e., awe, nurturant love, or amusement) or negative (i.e., sadness or disgust) emotion. Each participant viewed the film clips in the same order (i.e., awe, sadness, amusement, disgust, and nurturant love). The awe film clip was from either Lord of the Rings or Planet Earth and showed landscapes and vistas; the sad film clip was from 21 Grams and showed a mother finding out her family died in a car accident; the nurturant love film clip was from Babies and showed babies crawling and playing with animals; the disgust film clip showed an ear being cleaned; and the amusement film clip showed a baby laughing while watching someone ripping up paper. Pilot testing in an independent sample of healthy children indicated that these film clips elicited the target emotions. Legal copyright restrictions prevent public archiving of the film clips used in this study; the film clips will be shared unconditionally upon request to the corresponding author.
After viewing each film clip, participants were asked a series of questions. First, they were asked a question about the content of each film clip to ensure they had paid attention during the trial. They were provided with three choices and were asked to identify the correct response. Second, participants rated their subjective experience of numerous positive and negative emotions (i.e., afraid, amused or happy, angry, awe or amazement, disgusted, embarrassed, excited or enthusiastic, love or affection, proud, sad, and surprised) while watching each film clip. They were asked, “Did you feel ______ while watching the movie?” and were given the following choices: “no”, “a little,” or “a lot”. Third, they were asked if they had seen the film clip before and were given the following choices: “yes,” “no,” or “not sure.”
2.4.4. Measures
Emotion Word Knowledge.
Participants’ emotion word knowledge was computed by summing their total correct responses during the test of emotion word knowledge. Higher scores indicated greater knowledge of emotion terms (maximum score = 15).
Emotional Facial Behavior.
Videotaped recordings of the laboratory testing session were coded with Noldus version 13.0 software (Noldus Technologies, Leesburg, VA). Participants’ emotional facial expressions during the most intense 30 seconds of each film clip were coded on a second-by-second basis using a modified version of the Emotional Expressive Behavior coding scale (Gross & Levenson, 1995). Twenty percent of the videos were rated by multiple coders; interrater reliability was excellent (Cohen’s kappa = .79). We computed a total emotional facial behavior score for each trial by summing the intensity scores of the anger, sadness, contempt, fear, disgust, surprise, concentration, interest, happiness/amusement, and embarrassment codes.
Physiological Recordings.
Emotions are accompanied by dynamic changes in autonomic nervous system activity, as well as other bodily systems, that moderately cohere over time (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005). To capture a broad array of activity in the cardiovascular, electrodermal, and respiratory systems, we obtained continuous recordings of autonomic nervous system activity using Biopac MP150 bioamplifiers and a computer equipped with data acquisition software: (1) heart rate: Electrodes were placed in a bipolar configuration on opposite sides of the participant’s chest; heart rate was calculated as the number of R waves per minute from the electrocardiogram; (2) skin conductance level: A constant-voltage device was used to pass a small voltage between Ag/Acl Silver 8mm electrodes (using an electrolyte of sodium chloride) attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand; and (3) respiration rate: A pneumatic bellows or respiration transducer was stretched around the thoracic region, and respiration rate was measured as the number of inspirations per minute.
Physiological data were processed using a custom pipeline scripted in AcqKnowledge software (v5.0, www.biopac.com). Briefly, algorithms identified and marked the signature components of each waveform, and these marks were then visually inspected for errors and noise. Outliers in the raw data were considered to be +/− 3 standard deviations from the mean level during the trial; these periods were interpolated if their duration was three seconds or less and deleted if their duration was greater than three seconds. We computed reactivity scores for each channel by subtracting the mean level during the 60-second pre-trial baseline from the mean level during the 30-second portion of the trial that had been coded for emotional facial behavior.
Subjective Emotional Experience.
We coded response to the questions regarding subjective emotional experience as 0 (“no”), 1 (“a little”), or 2 (“a lot”). We summed participants’ total subjective experience (i.e., afraid, angry, disgusted, sad, amused/happy, awe/amazement, excited/enthusiastic, love/affection, embarrassed, surprised, and proud) during each trial to capture their overall emotional experience while watching each film clip.
Film Clip Content.
Correct responses to the questions regarding the content of the film clips were given scores of 1; incorrect responses were given scores of 0.
Film Clip Familiarity.
After viewing each film clip, participants responded “yes,” “no,” or “not sure” to the question, “Have you seen this film before?” We coded “yes” responses as 1, and “no” and “not sure” responses as 0.
2.5. Neuroimaging
2.5.1. Image Acquisition
Fifty-three participants underwent research-quality magnetic resonance imaging (MRI). One participant in the control group declined an MRI. Participants were scanned at the UCSF Neuroscience Imaging Center within four months of the emotion assessment, with the majority (70%) within 90 days. Images were obtained on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil (n = 28, 53% children with dyslexia) or 3.0 Tesla Siemens Prisma scanner equipped with a 64-channel head coil (n = 25, 47% children with dyslexia). Head movements were minimized by stabilizing the participant’s head with cushions. Structural whole-brain images were acquired using a volumetric 3D T1-weighted sagittal Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence (160 sagittal slices; slice thickness = 1.0 mm; field-of-view [FOV] = 256 × 240 mm2; matrix 256 × 240; voxel size 1.0 × 1.0 × 1.0 mm3; repetition time [TR] = 2300 ms; echo-time [TE] = 2.98 ms; flip angle = 9°).
Tf-fMRI were collected with a single-shot echo planar imaging sequence on the Trio scanner (TR = 2000 ms; TE = 31 ms; flip angle = 80°; in plane resolution = 3 × 3 mm; slice thickness = 3.5 mm; number of slices = 31; ascending; FOV = 220 mm) or Prisma scanner (TR = 1290 ms; TE = 32.4 ms; flip angle = 45°; in plane resolution = 2.2 × 2.2 mm; slice thickness = 2.2 mm; number of slices = 68; ascending; FOV = 211 mm). Before entering the MRI scanner and immediately prior to the tf-fMRI acquisition, participants were instructed to rest with their eyes closed in the scanner without falling asleep; following the tf-fMRI acquisition, the operator also asked participants whether they had followed those instructions. Since head motion represents a challenge in fMRI studies, especially in pediatric populations, we allowed participants to take a break every 20 minutes. It has been shown that this approach is useful in reducing head motion during functional MRI (Meissner, Walbrin, Nordt, Koldewyn, & Weigelt, 2020). The task-free acquisition was performed after one of the breaks, and its total duration was six minutes.
2.6. Statistical Analyses
Behavioral analyses were carried out in R Project (R Core Team, 2017). All statistical analyses were two-tailed with an alpha level of .05. No part of the study analyses was pre-registered prior to the research being conducted. All analysis code is publicly available (https://osf.io/gk57j/?view_only=21da1e90c1a9498b8731876a42d9517a).
2.6.1. Power Analysis
We conducted a post hoc power analysis using GLIMMPSE (Kreidler et al., 2013), a program well-suited for repeated measures power analyses (Y. Guo, Logan, Glueck, & Muller, 2013). We entered values from the linear mixed effects model to determine the power our study had to detect a significant main effect of diagnosis on facial behavior (α = .05). These estimates included the standard deviation of our dependent variable (aggregated across participants, the standard deviation was 10.18), the magnitude of the difference between the diagnostic groups (which was 5.4), and the variability across the repeated measures (the correlation coefficients for each pair of trials were entered into a matrix). We specified a total sample size of 54 and a categorical repeated measure (i.e., trial) with five levels. From these parameters, the power of our study was estimated to be .87, which is greater than standard power of .80. Although we may have been underpowered to examine group by trial interactions, we did include an interaction term in our models to explore potential differences between the groups during specific trials.
2.6.2. Emotional Reactivity
We ran separate linear mixed-effects models for emotional facial behavior, heart rate reactivity, skin conductance level reactivity, respiration rate reactivity, and subjective emotional experience to examine whether there were main effects of diagnosis or diagnosis by trial interactions on any measure. Random intercepts were specified for each participant. We controlled for age, sex, and film clip familiarity in all analyses. Visual inspection of model residuals via histogram and partial probability plots showed normal distributions. To compute effect sizes (Cohen’s D), we conducted an additional set of regression analyses but omitted the diagnosis by trial interaction term to isolate the main effect of group on each emotion measure. Analyses of covariance (controlling for age and sex) were used to determine if there were group differences in emotion word knowledge; emotion word knowledge was included as an additional covariate in a follow-up analysis of subjective emotional experience. Two participants (one with dyslexia and one control) failed to answer one film content question correctly; these two trials were removed from all analyses. One participant (a child with dyslexia) did not have physiological data due to technical problems during testing and, thus, was not included in the physiological analyses. Three participants (all in the control group) were not included in the skin conductance level analyses because of a faulty sensor.
2.6.3. Task-Free Functional Neuroimaging Analyses
Processing of the tf-fMRI data was performed using the Statistical Parametric Mapping 12 package (SPM12; http://www.fil.ion.ucl.ac.uk/spm) and the Conn Toolbox (version 17f) (Whitfield-Gabrieli & Nieto-Castanon, 2012) in the MATLAB computing environment (The MathWorks, Natick, MA). Functional data were corrected for interleaved slice acquisition order, realigned to the first volume of the series using a rigid transformation, and analyzed for the presence of motion. Images were excluded if relative motion exceeded 2 mm (11 children with dyslexia and five controls were excluded from the neuroimaging analyses according to these criteria, leaving a final sample for the tf-fMRI analysis of n = 37). To further reduce the effect of head movement on functional connectivity, volumes with < 2 mm/TR frame-wise displacement were detected as outliers using the Art toolbox (https://www.nitrc.org/projects/artifact_detect) and later included as nuisance regressors during the denoising step. Two-sample t-tests found low levels of movement on average and no significant group differences in mean relative displacement (children with dyslexia = 0.09 ± 0.04, children without dyslexia = 0.10 ± 0.04, t = 0.85, p = .39) or maximum relative displacement (children with dyslexia = 0.47 ± 0.31, children without dyslexia = 0.52 ± 0.35, t = 0.43, p = .68).
Data were then spatially normalized to Montreal Neurological Institute (MNI) space using a non-linear registration algorithm and resultant images were resampled to 2 × 2 × 2 mm3 voxels in MNI space. Spatial smoothing was done with an isotropic Gaussian kernel (full-width at half maximum = 8 mm). Finally, the functional data were denoised using the CompCor technique implemented in the CONN toolbox (Behzadi, Restom, Liau, & Liu, 2007). The gray matter signal was bandpass filtered (0.01 – 0.1 Hz) and detrended. Then, sixteen principal components were extracted from white matter and CSF regions and, in addition to the six motion parameters and their first-order temporal derivatives, regressed out from the gray matter signal.
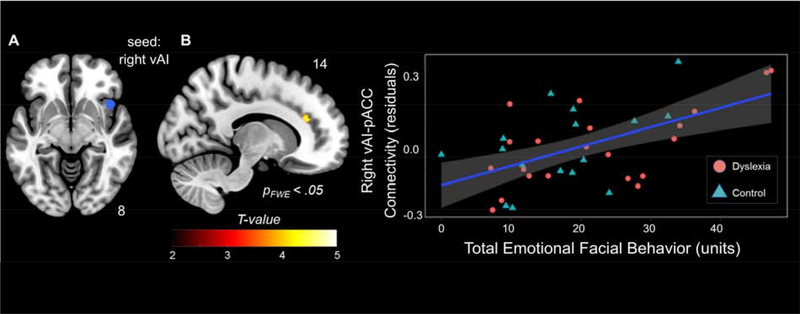
Single-subject correlation maps of the salience network (Seeley et al., 2007) were generated using a seed-based approach by calculating the correlation between the BOLD signal time series in the seed region of interest (ROI), and the time series in each voxel in the rest of the brain. The salience network has been previously identified with both seed-based and independent component analysis techniques on task-free data as well as during task performance (Hermans et al., 2011; Lee et al., 2017; Seeley et al., 2007). The seed ROI was defined as a 5 mm-radius sphere in right vAI (Figure 3A), centered at MNI coordinates x = 42, y = 17, z = −10 as previously described (Lee et al., 2014; Seeley, Crawford, et al., 2008). Correlation maps, which represent the temporal correlation of the average time series in right vAI with all of the other voxels in the brain, were converted to z-score maps by Fisher’s r-to-z transformations to enable parametric statistical comparisons. Next, we conducted a one-sample t-test (with age, sex, scanner, and diagnosis as nuisance covariates) to investigate the group-level spatial organization of the salience network. Family Wise Error (FWE) correction on the resultant connectivity maps was set to pFWE<.001 (k > 40 for cluster extent).
Figure 3. Emotional facial behavior reflects variability in intrinsic connectivity between right vAI and right ACC.
(A) We seeded the right ventral anterior insula (vAI) to test whether stronger intrinsic connectivity between vAI and right pregenual anterior cingulate cortex (pACC) was related to greater emotional reactivity across the sample (n = 37). (B) Results indicated that stronger vAI – pACC connectivity was associated with greater emotional facial behavior when controlling for age, sex, scanner type, diagnosis, and time interval between the MRI and emotion assessment (pFWE < .05). Color bars represent T-scores. The shaded area represents the 95% confidence interval.
We then conducted voxel-wise multiple regressions to investigate whether the emotional reactivity measures that significantly differed between the groups were associated with intrinsic salience network connectivity. Although the groups did not differ in the proportion of children who were scanned on each scanner, χ2(1) = 0.12, p = .73, we included age, sex, scanner type (i.e., Prisma or Trio scanner), diagnosis (i.e., with dyslexia or without dyslexia), and the time interval (in months) between the MRI scan and the emotion assessment as nuisance covariates in the regression models. Given that the ACC is a crucial node in the salience network and has a critical role in visceromotor emotion generation, we restricted our regression analyses to the bilateral ACC, as defined by the AAL atlas (Tzourio-Mazoyer et al., 2002). We performed this analysis to identify the specific location within the ACC that correlated with the emotional reactivity measures. Results were considered significant at pFWE < .05 after correcting for multiple comparisons within the bilateral ACC mask. We also report unmasked whole-brain results at p < .001, uncorrected. Images were overlaid using MRIcron (http://mccauslandcenter.sc.edu/CRNL) on the MNI template for visualization purposes.
2.6.4. Associations Between Emotional Reactivity and Real-World Behavior
We used linear regressions (controlling for age and sex) to examine whether higher emotional reactivity predicted higher scores on the BASC-2 Social Skills, Anxiety, and Depression subscales in children with dyslexia.
2.6.5. Emotional Reactivity and Potential Associations with Reading and Age
We used linear regressions (controlling for age and sex) to examine whether reading fluency (as measured with the GORT-5) or phonemic decoding ability (as measured with the TOWRE-2) predicted emotional reactivity in children with dyslexia. We selected these tests because they are particularly sensitive to reading difficulties in dyslexia, and we conducted these analyses to confirm that reading challenges alone did not account for emotional reactivity in the children with dyslexia. To ensure that any potential results were not accounted for by variability in age, we also conducted linear regressions (controlling for sex) to examine whether age predicted emotional reactivity in the children with dyslexia.
2.7. Data Availability
Data generated by the UCSF Dyslexia Center are available upon request; data requests can be submitted through the UCSF Memory and Aging Center Resource Request form: http://memory.ucsf.edu/resources/data. Academic, not-for-profit investigators with Institutional Review Board approval from the UCSF Human Research Protection Program (HRPP) can request data for research studies. The UCSF HRPP will not review the application until the UCSF Memory and Aging Center Executive Committee has signed off on the proposal and consent form. Data are not publicly available because they contain information that could compromise the privacy of the participants.
3. Results
3.1. Participant Demographics and Clinical Information
The groups of children with and without dyslexia included approximately equal numbers of girls and boys and had a similar mean age, which was 10 years old (see Table 1). The groups did not differ in handedness, and both were comprised of children of comparable ethnic backgrounds and socioemotional statuses (as measured by the mean annual income of the families). In general, both groups were predominantly white and had annual family income levels that ranged from the low average to above average range for the surrounding area. Given that many of the children with dyslexia attended private schools that specialize in learning differences, a greater proportion of the children with dyslexia attended private schools than those without dyslexia. As expected, the children with dyslexia had low scores on tests of reading; 70% had at least one score in the impaired range, 23% were in the low average range, and 7% were in the average range. On the BASC-2, the majority of children with dyslexia had social skills, anxiety, and depression scores that were in the average range. Two children’s scores indicated they were in the at-risk range for clinically significant anxiety, and one child was in the at-risk range for clinically significant depression (see Table 1).
3.2. Visceromotor Emotional Reactivity is Elevated in Dyslexia
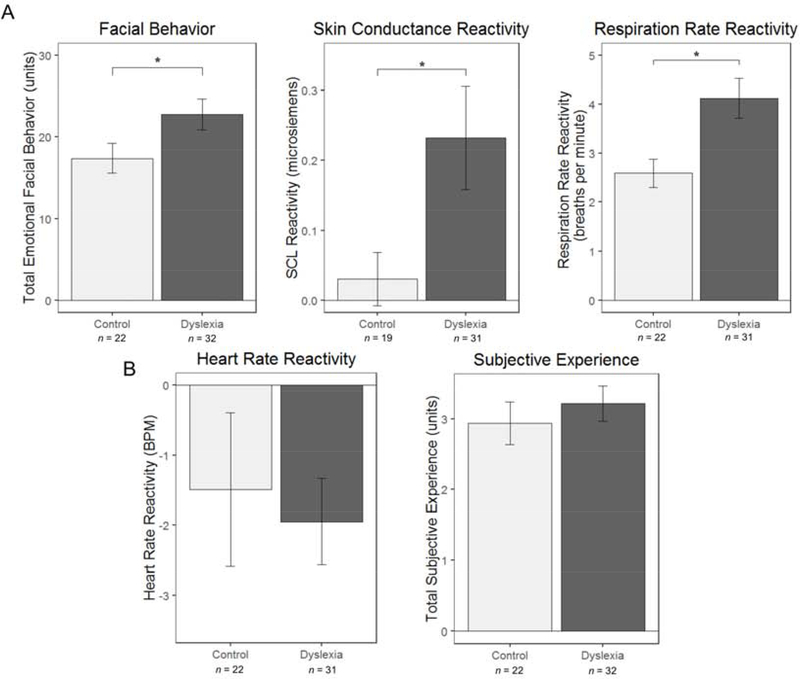
The linear mixed-effects models (controlling for age, sex, and film clip familiarity) revealed a main effect of diagnosis on emotional facial behavior, F(1,50) = 4.50, p = .04; skin conductance level reactivity, F(1,46) = 4.47, p = .04; and respiration rate reactivity, F(1,49) = 7.69, p = .01; but not on heart rate reactivity, F(1,49) = 0.14, p = .71 (Figure 2). These results indicated that children with dyslexia displayed greater emotional facial behavior and were more physiologically reactive than children without dyslexia while watching the film clips. There was no main effect of diagnosis on subjective emotional experience, F(1,50) = 0.37, p = .55 (Figure 2), even when accounting for emotion word knowledge, F(1,49) = 0.01, p = .91, which was lower in dyslexia compared to controls, F(1,50) = 13.67, p < .001. No significant diagnosis by trial interactions emerged for any of the behavioral, autonomic, or experiential measures (Table 2), which suggests that the heightened visceromotor reactivity in children with dyslexia was not specific to any particular emotion trial. Regression models that included the same covariates but omitted the diagnosis by trial interaction terms showed a medium effect size for the main effects of group on facial behavior (Cohen’s D = .60) and skin conductance reactivity (Cohen’s D = .62) and a large effect size for respiration rate reactivity (Cohen’s D = .80), according to established criteria (Cohen, 1992). See Supplemental Table 1.
Figure 2. Visceromotor emotional reactivity during film-viewing is elevated in dyslexia.
Linear mixed-effects models found a significant main effect of diagnosis for (A) emotional facial behavior, skin conductance level reactivity, and respiration rate reactivity but not for (B) heart rate reactivity or subjective emotional experience, suggesting that children with dyslexia were more reactive to emotionally evocative stimuli than control children without dyslexia. For each measure, averaged reactivity levels across the trials are shown to illustrate this result. Error bars are the standard error of the mean computed across the trials. * indicates p< .05.
Table 2 –
Emotional reactivity during film viewing.
| Dyslexia M(SD) | Controls M(SD) | |
|---|---|---|
| Awe Film | ||
| Emotional Facial Behavior (units) | 7.4 (10.5) | 7.6 (9.5) |
| Heart Rate Reactivity (beats per minute) | −0.3 (3.6) | 0.8 (4.6) |
| Skin Conductance Level Reactivity (microsiemens) | 0.2 (0.5) | 0 (0.3) |
| Respiration Rate Reactivity (breaths per minute) | 3.2 (3.1) | 0.8 (2.6) |
| Subjective Emotional Experience (units) | 3.1 (2.1) | 3.0 (2.0) |
| Sadness Film | ||
| Emotional Facial Behavior (units) | 17.1 (14.9) | 10.5 (11.6) |
| Heart Rate Reactivity (beats per minute) | −2 (5.7) | −1.1 (8.6) |
| Skin Conductance Level Reactivity (microsiemens) | 0.6 (1) | 0.2 (0.3) |
| Respiration Rate Reactivity (breaths per minute) | 4.2 (3.5) | 3.5 (3.3) |
| Subjective Emotional Experience (units) | 3.0 (1.9) | 2.5 (1.5) |
| Amusement Film | ||
| Emotional Facial Behavior (units) | 41.8 (22.0) | 42.4 (22.7) |
| Heart Rate Reactivity (beats per minute) | 0.2 (6.2) | −0.5 (7.2) |
| Skin Conductance Level Reactivity (microsiemens) | 0.2 (0.6) | 0.1 (0.2) |
| Respiration Rate Reactivity (breaths per minute) | 3.2 (4.8) | 2.4 (3.3) |
| Subjective Emotional Experience (units) | 4.1 (2.5) | 3.6 (1.8) |
| Disgust Film | ||
| Emotional Facial Behavior (units) | 30.5 (23.9) | 21.2 (26.5) |
| Heart Rate Reactivity (beats per minute) | −2.6 (7) | −1.6 (6.6) |
| Skin Conductance Level Reactivity (microsiemens) | 0.2 (0.8) | 0.1 (0.2) |
| Respiration Rate Reactivity (breaths per minute) | 5.2 (3.7) | 3.4 (3.3) |
| Subjective Emotional Experience (units) | 2.9 (1.3) | 3.0 (1.5) |
| Nurturant Love Film | ||
| Emotional Facial Behavior (units) | 18.8 (18.5) | 19.6 (18.2) |
| Heart Rate Reactivity (beats per minute) | −4.9 (5.5) | −4.9 (7) |
| Skin Conductance Level Reactivity (microsiemens) | 0 (0.5) | −0.2 (0.3) |
| Respiration Rate Reactivity (breaths per minute) | 4.5 (3.5) | 2.6 (2.9) |
| Subjective Emotional Experience (units) | 3.1 (2.3) | 3.4 (2.4) |
Means (M) and standard deviations (SD) for emotional facial behavior, physiological reactivity (trial minus baseline), and subjective emotional experience are presented for each group.
3.3. Greater Emotional Facial Behavior Reflects Stronger Connectivity Between Right vAI and Right ACC
Given that we found main effects of diagnosis on emotional facial behavior, skin conductance level reactivity, and respiration rate reactivity, we focused our neuroimaging analyses on those variables. Here, we averaged the reactivity scores across the trials to obtain a single overall reactivity metric for each measure for each participant.
As expected, the salience network maps were consistent with prior studies and showed that across the sample of children with and without dyslexia, the right vAI had strong functional connections with the ACC as well as the anterior midcingulate cortex, amygdala, thalamus, hypothalamus, and brainstem (see Supplemental Figure 1).
Next, we correlated the emotional reactivity measures of interest with salience network connectivity. Across the sample (controlling for age, sex, scanner type, diagnosis, and time interval between the MRI and emotion assessment), greater emotional facial behavior was associated with stronger intrinsic connectivity between the right vAI and the right ACC in a cluster that bordered the pregenual ACC and anterior midcingulate cortex (pFWE< .05; cluster size k = 58; cluster peak: 14, 30, 20; T = 4.1456; Figure 3). When we removed the ACC mask to examine connectivity between the right vAI and the whole brain, this cluster survived at uncorrected levels (p< .001). In the whole-brain analysis, only one other cluster in the right frontal pole emerged as being correlated with emotional facial behavior (p< .001 cluster size k = 78; cluster peak: 26, 58, −16; T = 4.44). No significant associations emerged with skin conductance level reactivity or respiration rate reactivity at this threshold.
3.4. Emotional Facial Behavior Relates to Real-World Behavior in Dyslexia
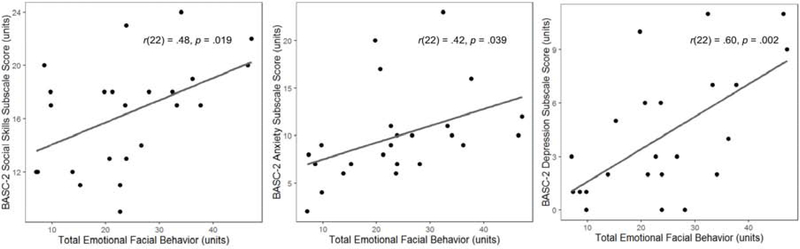
Linear regressions (controlling for age and sex) revealed that children with dyslexia who displayed greater total emotional facial behavior had higher scores on the Social Skills, b = 0.17, t(20) = 2.37, p = .03; Anxiety, b = 0.19, t(20) = 2.20, p = .04; and Depression, b = 0.21, t(20) = 4.04, p < .001, BASC-2 subscales (Figure 4). Skin conductance level reactivity and respiration rate reactivity were not significant predictors of any of these subscale scores.
Figure 4. Associations between emotional facial behavior and real-world behavior.
In dyslexia, greater emotional facial behavior during film-viewing was associated with greater social skills, anxiety, and depression, per parent reports on the BASC-2.
3.5. Visceromotor Emotional Reactivity Is Not Associated with Reading or Age
Linear regression analyses (controlling for age and sex) in the children with dyslexia revealed no significant associations between reading fluency (as measured with the GORT-5) and emotional facial behavior, b = 0.25, t(25) = 1.61, p = .12; skin conductance level reactivity, b = 0.002, t(24) = 0.35, p = .73; or respiration rate reactivity, b = 0.02, t(24) = 0.76, p = .46. Likewise, there were no significant associations between phonemic decoding ability (as measured with the TOWRE-2) and emotional facial behavior, b = 0.07, t(26) = 0.36, p = .72; skin conductance level reactivity, b = 0.01, t(25) = 1.87, p = .07; or respiration rate reactivity, b = 0.05, t(25) = 1.25, p = .22. These results suggest elevated emotional reactivity in dyslexia was not accounted for by reading difficulties. Linear regression analyses (controlling for sex) in the children with dyslexia also found age had no significant associations with emotional facial behavior, b = 1.19, t(29) = 0.88, p = .39; skin conductance level reactivity, b = −0.01, t(28) = −0.13, p = .89; or respiration rate reactivity, b = −0.10, t(28) = −0.34, p = .74.
4. Discussion
We found evidence for elevated visceromotor emotional reactivity in dyslexia. While viewing emotion-eliciting film clips, children with dyslexia exhibited greater reactivity in emotional facial behavior, skin conductance level, and respiration rate than children without dyslexia. The groups did not differ in heart rate reactivity during the film-viewing task. There was no significant difference between the groups in subjective emotional experience, even when accounting for lower emotion word knowledge in children with dyslexia. Across the sample, greater emotional facial behavior was associated with stronger intrinsic connectivity between the right vAI and right pregenual ACC, key salience network hubs (Seeley et al., 2007). Enhanced visceromotor emotional reactivity in dyslexia had real-world implications: children with dyslexia who displayed greater emotional facial behavior had better social skills as well greater symptoms of anxiety and depression. These findings suggest that accentuated visceromotor emotional reactivity in dyslexia may have both positive and negative impacts on social functioning, leading to interpersonal benefits as well as affective vulnerabilities.
Our results are consistent with longstanding models of brain asymmetry, which would predict heightened emotional reactivity in dyslexia, a disorder typically characterized by alterations in gray matter volume, white matter connectivity, gyrification, and task-based activity in left-lateralized language networks (Caverzasi et al., 2018; Hoeft et al., 2007; Krafnick et al., 2014; Langer et al., 2017; Paulesu et al., 2014; Richlan et al., 2013; Vandermosten et al., 2015). Emotional facial behavior and autonomic nervous system activity are direct readouts of the salience network, a distributed neural network critical for emotion generation and sensation (Levenson et al., 2008; Seeley et al., 2012). The salience network is anchored by the right vAI (Seeley et al., 2007), a final waystation in interoceptive pathways that represent contextually embedded internal cues that color subjective experience and guide behavior (Craig, 2011; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). The vAI has tight reciprocal connections with the ACC (Craig, 2009), a salience network hub that is critical for triggering the coordinated visceromotor cascades that accompany emotions (Critchley et al., 2003; Sturm et al., 2013; Vogt, 2005). Previous studies have shown that tighter intrinsic connectivity between the vAI and ACC is associated with more intense emotional experience, greater autonomic nervous system responding, and higher socioemotional sensitivity (Hermans et al., 2011; Seeley et al., 2007; Toller et al., 2018; Xia et al., 2017). One previous study of children with reading disorders found they had elevated connectivity between the amygdala and medial prefrontal cortex compared to children without reading disorders and that greater connectivity between these structures related to higher anxiety symptoms (Davis et al., 2018). These findings, like ours, suggest stronger connectivity between emotion-relevant structures may relate to elevated emotionality in dyslexia. At an uncorrected statistical threshold, greater connectivity between the right vAI and the right frontal pole, a region critical for self-awareness and self-monitoring (Seeley & Sturm, 2006), was also related to greater facial expressivity.
Heightened visceromotor emotional reactivity in dyslexia may result from alterations in any number of underlying emotion systems, but further studies will be required to elucidate the precise mechanisms underlying this enhancement. Emotional reactivity refers to the generation of visceromotor outflow and subjective experience that unfold during emotions, products of the salience network (Seeley, 2019). The salience network, though present from the early days of life (Gao et al., 2015), undergoes structural and functional refinement across development (Uddin et al., 2011; Zielinski et al., 2010). Although the salience network shows a largely adult organization in middle childhood, connectivity between certain nodes, including between right vAI and ACC, continues to strengthen until early adulthood (Uddin et al., 2011). The developmental trajectory of the salience network in dyslexia is unknown, but children with dyslexia may exhibit different patterns of within- or between-network structural and functional connections that encourage visceromotor emotional reactivity. Emotions unfold after an appraisal process (Ellsworth, 2013), and it is also possible that elevated visceromotor emotional reactivity in dyslexia reflects an underlying hypersensitivity or hyperreactivity to non-verbal (e.g., visual) cues that convey affect (Diehl et al., 2014) or to difficulties with emotion regulation, the ability to modulate our emotions to meet prevailing goals and demands (Gross, 2013). Emotional reactivity is inextricably linked with emotion regulation (Campos, Frankel, & Camras, 2004; Mauss, Bunge, & Gross, 2007), a process that often occurs automatically and is critical for adaptive social functioning (Eisenberg et al., 2010; Mauss et al., 2007). Emotion regulation develops throughout childhood and adolescence and engages a lateral frontoparietal network that supports cognitive, behavioral, and motor control (Ochsner & Gross, 2005). Children ages 8 – 12 engage emotion regulation systems in a different manner than adults and are less effective at cognitive control (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002), but how this system functions in children with dyslexia is not well understood. Prior studies of dyslexia have found both lower functional connectivity at rest (Margolis et al., 2019) and greater activity during phonological tasks (B. A. Shaywitz et al., 2002) in lateral inferior frontal regions that support cognitive, emotional, and behavioral inhibition, making it possible that greater visceromotor emotional reactivity in dyslexia may result from less efficient engagement of emotion regulation systems. Additional studies are needed to examine the different neural contributions to elevated visceromotor emotional reactivity in dyslexia and to investigate whether our results differ across a wider range of developmental stages. When emotions run high due to atypical functioning in any of these underlying biological mechanisms, however, affective symptoms may emerge and impact everyday life and well-being (Kring & Sloan, 2009).
There are limitations to the present study that should be considered. First, dyslexia is a heterogeneous disorder, and debate continues to surround its etiology, diagnosis, and treatment (S. E. Shaywitz, 1998). Like other complex clinical disorders, dyslexia likely includes multiple subtypes (O’Brien et al., 2012), and it is likely that not all children with dyslexia will exhibit the accentuated visceromotor emotional reactivity that we detected on average at the group level. Here, we focused on children with dyslexia who have prominent phonological processing deficits, but other subtypes of dyslexia may exhibit different patterns of visceromotor emotional reactivity. The demographic characteristics of our sample were also fairly homogenous, and many of the children were white and of middle to high socioeconomic status, which may further limit the generalizability of our results. In future studies of dyslexia, it will be important to identify subgroups of children with higher visceromotor emotional reactivity who may benefit from early interventions that enhance this strength and that teach strategies for handling strong emotions (Haft, Myers, & Hoeft, 2016). Music therapy may represent one promising avenue. The majority of work on music therapy in dyslexia has focused on stimulating perception of rhythm and musical syntax, which is mediated by the left hemisphere, including language areas (Forgeard, Winner, Norton, & Schlaug, 2008; Habib et al., 2016; Overy, 2003). Such efforts have proven successful in improving phonological awareness (Overy, 2003) and reading ability (Habib et al., 2016). As music perception involves the right hemisphere (Bever & Chiarello, 1974; Halpern & Zatorre, 1999; Pallesen et al., 2005), elicits changes in autonomic activity (Bernardi, Porta, & Sleight, 2006), and reduces anxiety symptoms (Goldbeck & Ellerkamp, 2012), future studies should investigate whether music can also be used to promote rewarding positive emotional experiences and to reduce affective vulnerability in dyslexia. Second, it is not possible to determine the causal relationship between reading difficulties and accentuated visceromotor emotional reactivity in dyslexia. While one possibility is that elevated emotional reactivity in dyslexia develops in response to chronic reading difficulties and academic challenges, another possibility is that heightened emotional reactivity in people with dyslexia is present prior to reading instruction. Previous research has found that prereaders who later received a diagnosis of dyslexia (Clark et al., 2014) had smaller gray matter volume in brain regions that are critical for emotion and social regulation (e.g., right orbitofrontal cortex), which suggests structures outside of the reading network may also be involved early in dyslexia (Wang et al., 2019), even before reading problems are evident (Beelen et al., 2019). Although it is possible that heightened visceromotor emotional reactivity is directly associated with reading difficulties, our results did not suggest this was the case given that the domains in which we detected emotional reactivity enhancement in dyslexia were non-verbal (i.e., facial behavior and physiological activity) and were not related to reading fluency or phonemic decoding scores. Many questions remain regarding the association between reading and emotions, however, and additional research in this area will help to clarify these important issues. Third, it is plausible that certain emotions are more affected than others in dyslexia. Alternative models of brain asymmetry propose that the left and right hemispheres not only differ in their functional specialization for language and emotion but also in their dominance in affective valence (Davidson & Fox, 1982; Sackeim et al., 1982). Future studies that further elucidate each hemisphere’s unique and shared roles in negative and positive emotion generation will improve our understanding of how emotion alterations may manifest in lateralized clinical disorders such as dyslexia.
Elevated visceromotor emotional reactivity is an important aspect of dyslexia that has previously gone overlooked. Our results suggest that although individuals with dyslexia may have reading difficulties, they may also exhibit strengths as well as vulnerabilities secondary to enhanced visceromotor emotional reactivity. Being highly attuned and sensitive to the world around us can be an asset as well as a liability, making people with dyslexia keen observers of salient cues in the environment yet potentially at risk for too many powerful feelings. A more detailed conceptualization of language and non-language functioning in dyslexia will be essential for improving treatment planning, prognosis, and well-being in children and adults who struggle with reading.
Supplementary Material
Highlights.
Autonomic reactivity and facial behavior during emotions are elevated in dyslexia
Emotional reactivity relates to stronger salience network hubs connectivity
Emotional reactivity correlates with greater social skills, anxiety, and depression
Acknowledgements
We are grateful to the children and their families for participating in this research.
Funding
This work was supported by the University of California, San Francisco Dyslexia Center; the Charles and Helen Schwab Foundation; the National Institute of Neurological Disorders and Stroke (R01NS050915); and the National Institutes of Health (K24DC015544).
Abbreviations:
- ACC
anterior cingulate cortex
- vAI
ventral anterior insula
Footnotes
Competing Interests
The authors report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckmann CF, DeLuca M, Devlin JT, & Smith SM (2005). Investigations into restingstate connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1457), 1001–1013. doi: 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen C, Vanderauwera J, Wouters J, Vandermosten M, & Ghesquière P (2019). Atypical gray matter in children with dyslexia before the onset of reading instruction. Cortex, 121, 399–413. doi: 10.1016/j.cortex.2019.09.010 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Porta C, & Sleight P (2006). Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: the importance of silence. Heart, 92(4), 445–452. doi: 10.1136/hrt.2005.064600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever TG, & Chiarello RJ (1974). Cerebral dominance in musicians and nonmusicians. Science, 185(4150), 537–539. doi: 10.1126/science.185.4150.537 [DOI] [PubMed] [Google Scholar]
- Blonder LX, Bowers D, & Heilman KM (1991). The role of the right hemisphere in emotional communication. Brain, 114 ( Pt 3), 1115–1127. [DOI] [PubMed] [Google Scholar]
- Borod JC, Cicero BA, Obler LK, Welkowitz J, Erhan HM, Santschi C, … Whalen JR. (1998). Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology, 12(3), 446–458. [DOI] [PubMed] [Google Scholar]
- Bradley L, & Bryant PE (1983). Categorizing sounds and learning to read—a causal connection. Nature, 301(5899), 419. [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, & Perani D (2004). Regional reductions of gray matter volume in familial dyslexia. Neurology, 63(4), 742–745. doi: 10.1212/01.WNL.0000134673.95020.EE [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, & Gabrieli JD (2002). Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron, 33(2), 301–311. doi: 10.1016/S0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JJ, Frankel CB, & Camras L (2004). On the nature of emotion regulation. Child development, 75(2), 377–394. doi: 10.1111/j.1467-8624.2004.00681.x [DOI] [PubMed] [Google Scholar]
- Carroll JM, & Iles JE (2006). An assessment of anxiety levels in dyslexic students in higher education. Br J Educ Psychol, 76(Pt 3), 651–662. doi: [DOI] [PubMed] [Google Scholar]
- Casey R, Levy SE, Brown K, & Brooks-Gunn J (1992). Impaired emotional health in children with mild reading disability. J Dev Behav Pediatr, 13(4), 256–260. doi: 10.1097/00004703-199208000-00003 [DOI] [PubMed] [Google Scholar]
- Caverzasi E, Mandelli ML, Hoeft F, Watson C, Meyer M, Allen IE, … Gorno-Tempini ML. (2018). Abnormal age-related cortical folding and neurite morphology in children with developmental dyslexia. Neuroimage Clin, 18, 814–821. doi: 10.1016/j.nicl.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, & Hugdahl K (2014). Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain, 137(Pt 12), 3136–3141. doi: 10.1093/brain/awu229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological bulletin, 112(1), 155–159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Cole PM, Michel MK, & Teti LOD (1994). The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the society for research in child development, 73–100. doi: 10.1111/j.1540-5834.1994.tb01278.x [DOI] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature reviews neuroscience, 3, 655–666. doi: 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci, 10(1), 59–70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig AD (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. doi: 10.1111/j.1749-6632.2011.05990.x [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. doi: 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, … Dolan RJ. (2003). Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain, 126(Pt 10), 2139–2152. doi: 10.1093/brain/awg216 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nature neuroscience, 7(2), 189–195. doi: 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & Fox NA (1982). Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science, 218(4578), 1235–1237. doi:: 10.1126/science.7146906 [DOI] [PubMed] [Google Scholar]
- Davis K, Margolis AE, Thomas L, Huo Z, & Marsh R (2018). Amygdala sub-regional functional connectivity predicts anxiety in children with reading disorder. Developmental science, 21(5), e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, & Harrison DW (2005). Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev, 4(1), 3–20. doi: 10.1177/1534582305276837 [DOI] [PubMed] [Google Scholar]
- Denham SA, McKinley M, Couchoud EA, & Holt R (1990). Emotional and behavioral predictors of preschool peer ratings. Child development, 61(4), 1145–1152. doi: 10.1111/j.1467-8624.1990.tb02848.x [DOI] [PubMed] [Google Scholar]
- Diehl JJ, Frost SJ, Sherman G, Mencl WE, Kurian A, Molfese P, … Pugh KR. (2014). Neural correlates of language and non-language visuospatial processing in adolescents with reading disability. NeuroImage, 101, 653–666. doi: 10.1016/j.neuroimage.2014.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Bernzweig J, Karbon M, Poulin R, & Hanish L (1993). The relations of emotionality and regulation to preschoolers’ social skills and sociometric status. Child development, 64(5), 1418–1438. doi: 10.1111/j.1467-8624.1993.tb02961.x [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, & Eggum ND (2010). Emotion-related self-regulation and its relation to children’s maladjustment. Annual review of clinical psychology, 6, 495–525. doi: 10.1146/annurev.clinpsy.121208.131208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PC (2013). Appraisal theory: Old and new questions. Emotion Review, 5(2), 125–131. doi: 10.1177/1754073912463617 [DOI] [Google Scholar]
- Forgeard M, Winner E, Norton A, & Schlaug G (2008). Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS One, 3(10), e3566. doi: 10.1371/journal.pone.0003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson N, & Jacobs S (2001). Controllability attributions for academic performance and the perceived scholastic competence, global self-worth and achievement of children with dyslexia. School Psychology International, 22(4), 401–416. doi:. 10.1177/0143034301224002 [DOI] [Google Scholar]
- Frick PJ, & Morris AS (2004). Temperament and developmental pathways to conduct problems. Journal of clinical child and adolescent psychology, 33(1), 54–68. doi:. 10.1207/S15374424JCCP3301_6 [DOI] [PubMed] [Google Scholar]
- Frith U (1999). Paradoxes in the definition of dyslexia. Dyslexia, 5(4), 192–214. doi:. [DOI] [Google Scholar]
- Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, & Xu B (2003). fMRI identifies regional specialization of neural networks for reading in young children. Neurology, 60(1), 94–100. [DOI] [PubMed] [Google Scholar]
- Gainotti G (1972). Emotional behavior and hemispheric side of the lesion. Cortex, 8(1), 41–55. doi: 10.1016/S0010-9452(72)80026-1 [DOI] [PubMed] [Google Scholar]
- Galaburda AM (1994). Developmental dyslexia and animal studies: at the interface between cognition and neurology. Cognition, 50(1–3), 133–149. [DOI] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, & Lin W (2015). Development of human brain cortical network architecture during infancy. Brain Structure and Function, 220(2), 1173–1186. doi: 10.1007/s00429-014-0710-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeck L, & Ellerkamp T (2012). A randomized controlled trial of multimodal music therapy for children with anxiety disorders. J Music Ther, 49(4), 395–413. doi: 10.1093/jmt/49.4.395 [DOI] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, … Korgaonkar MS. (2015). Identification of a common neurobiological substrate for mental illness. JAMA psychiatry, 72(4), 305–315. doi: 10.1001/jamapsychiatry.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U (2008). Reading, dyslexia and the brain. Educational Research, 50(2), 135–148. doi: 10.1080/00131880802082625 [DOI] [Google Scholar]
- Griskevicius V, Shiota MN, & Neufeld SL (2010). Influence of different positive emotions on persuasion processing: a functional evolutionary approach. Emotion, 10(2), 190–206. doi: 10.1037/a0018421 [DOI] [PubMed] [Google Scholar]
- Gross JJ (2013). Emotion regulation: taking stock and moving forward. Emotion, 13(3), 359–365. doi: 10.1037/a0032135 [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1995). Emotion elicitation using films. Cognition and Emotion, 9, 87–108. doi: 10.1080/02699939508408966 [DOI] [Google Scholar]
- Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH, & Seeley WW (2012). One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage, 61(4), 1471–1483. doi: 10.1016/j.neuroimage.2012.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Logan HL, Glueck DH, & Muller KE (2013). Selecting a sample size for studies with repeated measures. BMC Med Res Methodol, 13, 100. doi: 10.1186/1471-2288-13-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M, Lardy C, Desiles T, Commeiras C, Chobert J, & Besson M (2016). Music and Dyslexia: A New Musical Training Method to Improve Reading and Related Disorders. Front Psychol, 7, 26. doi: 10.3389/fpsyg.2016.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft SL, Duong PH, Ho TC, Hendren RL, & Hoeft F (2019). Anxiety and Attentional Bias in Children with Specific Learning Disorders. Journal of Abnormal Child Psychology, 47(3), 487–497. doi: 10.1007/s10802-018-0458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft SL, Myers CA, & Hoeft F (2016). Socio-Emotional and Cognitive Resilience in Children with Reading Disabilities. Curr Opin Behav Sci, 10, 133–141. doi: 10.1016/j.cobeha.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern AR, & Zatorre RJ (1999). When that tune runs through your head: a PET investigation of auditory imagery for familiar melodies. Cereb Cortex, 9(7), 697–704. doi: 10.1093/cercor/9.7.697 [DOI] [PubMed] [Google Scholar]
- Hellendoorn J, & Ruijssenaars W (2000). Personal experiences and adjustment of Dutch adults with dyslexia. Remedial and special education, 21(4), 227–239. doi: 10.1177/074193250002100405 [DOI] [Google Scholar]
- Hendren RL, Haft SL, Black JM, White NC, & Hoeft F (2018). Recognizing Psychiatric Comorbidity With Reading Disorders. Front Psychiatry, 9, 101. doi: 10.3389/fpsyt.2018.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, … Fernandez G. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science, 334(6059), 1151–1153. doi: 10.1126/science.1209603 [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, … Gabrieli JD. (2007). Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci U S A, 104(10), 4234–4239. doi: 10.1073/pnas.0609399104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Ellen Grant P, & Gaab N (2016). Atypical Sulcal Pattern in Children with Developmental Dyslexia and At-Risk Kindergarteners. Cerebral Cortex, 26(3), 1138–1148. doi: 10.1093/cercor/bhu305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N (1996). Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain, 119 (Pt 5), 1775–1790. [DOI] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, & Eden GF (2014). An investigation into the origin of anatomical differences in dyslexia. Journal of Neuroscience, 34(3), 901–908. doi: 10.1523/jneurosci.2092-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidler SM, Muller KE, Grunwald GK, Ringham BM, Coker-Dukowitz ZT, Sakhadeo UR, … Glueck DH. (2013). GLIMMPSE: Online Power Computation for Linear Models with and without a Baseline Covariate. J Stat Softw, 54(10). doi: 10.18637/jss.v054.i10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, & Sloan DM (2009). Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment: Guilford Press. [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, & Ladurner G (2008). Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Human Brain Mapping, 29(5), 613–625. doi: 10.1002/hbm.20425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl U, Neef NE, Kraft I, Schaadt G, Dörr L, Brauer J, … Kirsten H. (2020). The emergence of dyslexia in the developing brain. Neuroimage, 211, 116633. doi: 10.1016/j.neuroimage.2020.116633 [DOI] [PubMed] [Google Scholar]
- Langer N, Peysakhovich B, Zuk J, Drottar M, Sliva DD, Smith S, … Gaab N. (2017). White matter alterations in infants at risk for developmental dyslexia. Cerebral Cortex, 27(2), 1027–1036. doi: 10.1093/cercor/bhv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, … Seeley WW. (2014). Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain, 137(Pt 11), 3047–3060. doi: 10.1093/brain/awu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Sias AC, Mandelli ML, Brown JA, Brown AB, Khazenzon AM, … Seeley WW. (2017). Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin, 14, 286–297. doi: 10.1016/j.nicl.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner JS, & Keltner D (2000). Beyond valence: Toward a model of emotion-specific influences on judgement and choice. Cognition and Emotion, 14(4), 473–493. doi: 10.1080/026999300402763 [DOI] [Google Scholar]
- Levenson RW (1994). Human emotion: A functional view In Ekman P & Davidson RJ (Eds.), The nature of emotion: Fundamental questions (pp. 123–126). New York: Oxford University Press. [Google Scholar]
- Levenson RW (2003). Blood, sweat, and fears: The autonomic architecture of emotion. Annals of the New York Academy of Sciences, 1000, 348–366. doi: 10.1196/annals.1280.016 [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ascher E, Goodkind M, McCarthy M, Sturm V, & Werner K (2008). Chapter 25 Laboratory testing of emotion and frontal cortex. Handb Clin Neurol, 88, 489–498. doi: 10.1016/s0072-9752(07)88025-0 [DOI] [PubMed] [Google Scholar]
- Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, & Fiebach CJ (2012). Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS ONE, 7(8), e43122. doi: 10.1371/journal.pone.0043122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes PN, Mestre JM, Guil R, Kremenitzer JP, & Salovey P (2012). The role of knowledge and skills for managing emotions in adaptation to school: Social behavior and misconduct in the classroom. American Educational Research Journal, 49(4), 710–742. doi: 10.3102/0002831212443077 [DOI] [Google Scholar]
- Lopes PN, Salovey P, Côté S, Beers M, & Petty RE (2005). Emotion regulation abilities and the quality of social interaction. Emotion, 5(1), 113. doi: 10.1037/1528-3542.5.1.113 [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, & Shaywitz BA (2003). A definition of dyslexia. Ann Dyslexia, 53(1), 1–14. doi: 10.1007/s11881-003-0001-9 [DOI] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, & Eden GF (2008). A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci, 1145, 237–259. doi: 10.1196/annals.1416.024 [DOI] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Davis KS, Thomas L, Banker SM, Cyr M, & Marsh R (2019). Neural correlates of cognitive control deficits in children with reading disorder. Brain imaging and behavior, 1–12. doi: 10.1007/s11682-019-00083-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, & Gross JJ (2007). Automatic emotion regulation. Social and Personality Psychology Compass, 1(1), 146–167. doi: 10.1111/j.1751-9004.2007.00005.x [DOI] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175–190. doi: 10.1037/1528-3542.5.2.175 [DOI] [PubMed] [Google Scholar]
- Meissner TW, Walbrin J, Nordt M, Koldewyn K, & Weigelt S (2020). Head motion during fMRI tasks is reduced in children and adults if participants take breaks. Developmental Cognitive Neuroscience, 100803. doi: 10.1016/j.dcn.2020.100803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Fine SE, Kiely Gouley K, Seifer R, Dickstein S, & Shields A (2006). Showing and telling about emotions: Interrelations between facets of emotional competence and associations with classroom adjustment in Head Start preschoolers. Cognition and Emotion, 20(8), 1170–1192. doi: 10.1080/02699930500405691 [DOI] [Google Scholar]
- Miller BL, Ponton M, Benson DF, Cummings JL, & Mena I (1996). Enhanced artistic creativity with temporal lobe degeneration. Lancet, 348(9043), 1744–1745. [DOI] [PubMed] [Google Scholar]
- Mugnaini D, Lassi S, La Malfa G, & Albertini G (2009). Internalizing correlates of dyslexia. World J Pediatr, 5(4), 255–264. doi: 10.1007/s12519-009-0049-7 [DOI] [PubMed] [Google Scholar]
- Norton ES, Beach SD, & Gabrieli JD (2015). Neurobiology of dyslexia. Current opinion in neurobiology, 30, 73–78. doi: 10.1016/j.conb.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novita S (2016). Secondary symptoms of dyslexia: a comparison of self-esteem and anxiety profiles of children with and without dyslexia. European journal of special needs education, 31(2), 279–288. doi: 10.1080/08856257.2015.1125694 [DOI] [Google Scholar]
- O’Brien BA, Wolf M, & Lovett MW (2012). A taxometric investigation of developmental dyslexia subtypes. Dyslexia, 18(1), 16–39. doi: 10.1002/dys.1431 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Science, 9(5), 242–249. doi: 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ongur D, & Price JL (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex, 10, 206–219. doi: 10.1093/cercor/10.3.206 [DOI] [PubMed] [Google Scholar]
- Overy K (2003). Dyslexia and music. From timing deficits to musical intervention. Ann N Y Acad Sci, 999, 497–505. doi: 10.1196/annals.1284.060 [DOI] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey C, Korvenoja A, Koivisto J, Gjedde A, & Carlson S (2005). Emotion processing of major, minor, and dissonant chords: a functional magnetic resonance imaging study. Ann N Y Acad Sci, 1060, 450–453. doi: 10.1196/annals.1360.047 [DOI] [PubMed] [Google Scholar]
- Parhiala P, Torppa M, Eklund K, Aro T, Poikkeus AM, Heikkilä R, & Ahonen T (2015). Psychosocial Functioning of Children with and without Dyslexia: A Follow-up Study from Ages Four to Nine. Dyslexia, 21(3), 197–211. doi: 10.1002/dys.1486 [DOI] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, & Berlingeri M (2014). Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front Hum Neurosci, 8, 830. doi: 10.3389/fnhum.2014.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Becker BLC, Smith S, Fehlbaum LV, Wang Y, & Gaab N (2017). Investigating the influences of language delay and/or familial risk for dyslexia on brain structure in 5-year-olds. Cerebral Cortex, 27(1), 764–776. doi: 10.1093/cercor/bhv267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Chang M, & Gaab N (2011). Structural brain alterations associated with dyslexia predate reading onset. Neuroimage, 57(3), 742–749. doi: 10.1016/j.neuroimage.2010.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, & Gaab N (2012). Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proceedings of the National Academy of Sciences of the United States of America, 109(6), 2156–2161. doi: 10.1073/pnas.1107721109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2004). BASC-2 Behavior Assessment for Children Manual. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Richlan F (2012). Developmental dyslexia: dysfunction of a left hemisphere reading network. Front Hum Neurosci, 6, 120. doi: 10.3389/fnhum.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2009). Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp, 30(10), 3299–3308. doi: 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2011). Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage, 56(3), 1735–1742. doi: 10.1016/j.neuroimage.2011.02.040 [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2013). Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Hum Brain Mapp, 34(11), 3055–3065. doi: 10.1002/hbm.22127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, & Geschwind N (1982). Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Archives of neurology, 39(4), 210–218. doi: 10.1001/archneur.1982.00510160016003 [DOI] [PubMed] [Google Scholar]
- Saper CB (2002). The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience, 25, 433–469. doi: 10.1146/annurev.neuro.25.032502.111311 [DOI] [PubMed] [Google Scholar]
- Schrank FA, Mather N, & McGrew KS (2014). Woodcock-Johnson IV tests of achievement. Rolling Meadows, IL: Riverside. [Google Scholar]
- Seeley WW (2019). The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J Neurosci, 39(50), 9878–9882. doi: 10.1523/jneurosci.1138-17.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, & Gorno-Tempini ML (2008). Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of neurology, 65(2), 249–255. doi: 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, & Miller BL (2008). Unravelling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain, 131(Pt 1), 39–49. doi: 10.1093/brain/awm270 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, & Sturm VE (2006). Self-representation and the frontal lobes In Miller BL & Cummings JL (Eds.), The human frontal lobes: Functions and disorders (2nd ed., pp. 317–334). New York: The Guilford Press. [Google Scholar]
- Seeley WW, Zhou J, & Kim EJ (2012). Frontotemporal dementia: What can the behavioral variant teach us about human brain organization? Neuroscientist, 18(4), 373–385. doi: 10.1177/1073858411410354 [DOI] [PubMed] [Google Scholar]
- Sha Z, Wager TD, Mechelli A, & He Y (2019). Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biological psychiatry, 85(5), 379–388. doi: 10.1016/j.biopsych.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, … Lyon GR (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological psychiatry, 52(2), 101–110. doi: 10.1016/S0006-3223(02)01365-3 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE (1998). Dyslexia. New England Journal of Medicine, 338(5), 307–312. doi: 10.1056/nejm199801293380507 [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, … Paulesu E (2005). Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain, 128(Pt 10), 2453–2461. doi: 10.1093/brain/awh579 [DOI] [PubMed] [Google Scholar]
- Skeide MA, Kirsten H, Kraft I, Schaadt G, Muller B, Neef N, … Friederici AD. (2015). Genetic dyslexia risk variant is related to neural connectivity patterns underlying phonological awareness in children. NeuroImage, 118, 414–421. doi: 10.1016/j.neuroimage.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, & Levenson RW (2008). Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion, 8(6), 861–869. doi: 10.1037/a0013765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, … Levenson RW. (2013). Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci, 8(4), 468–474. doi: 10.1093/scan/nss023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller G, Brown J, Sollberger M, Shdo SM, Bouvet L, Sukhanov P, … Rankin KP. (2018). Individual differences in socioemotional sensitivity are an index of salience network function. Cortex, 103, 211–223. doi: 10.1016/j.cortex.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen J, Wagner R, & Rashotte C (2012). TOWRE-2: Test of Word Recognition Efficiency–Examiner Manual. Austin, TX: Pro-Ed. [Google Scholar]
- Tucker DM (1981). Lateral brain function, emotion, and conceptualization. Psychological bulletin, 89(1), 19–46. doi: 10.1037/0033-2909.89.1.19 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, & Menon V (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience, 31(50), 18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera J, Altarelli I, Vandermosten M, De Vos A, Wouters J, & Ghesquière P (2016). Atypical Structural Asymmetry of the Planum Temporale is Related to Family History of Dyslexia. Cerebral Cortex, 28(1), 63–72. doi: 10.1093/cercor/bhw348 [DOI] [PubMed] [Google Scholar]
- Vanderauwera J, Wouters J, Vandermosten M, & Ghesquière P (2017). Early dynamics of white matter deficits in children developing dyslexia. Developmental Cognitive Neuroscience, 27, 69–77. doi: 10.1016/j.dcn.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, & Ghesquière P (2012). A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience & Biobehavioral Reviews, 36(6), 1532–1552. doi: 10.1016/j.neubiorev.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, … Ghesquière P (2015). A DTI tractography study in pre-readers at risk for dyslexia. Developmental Cognitive Neuroscience, 14, 8–15. doi: 10.1016/j.dcn.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci, 6(7), 533–544. doi: 10.1038/nrn1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, & Le RK (2017). Diagnosing the Neural Circuitry of Reading. Neuron, 96(2), 298–311. doi: 10.1016/j.neuron.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yan X, Liu Y, Spray GJ, Deng Y, & Cao F (2019). Structural and functional abnormality of the putamen in children with developmental dyslexia. Neuropsychologia, 130, 26–37. doi: 10.1016/j.neuropsychologia.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler, D. (1999) Manual for the Wechsler abbreviated intelligence scale (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2(3), 125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wiederholt J, & Bryant B (2012). Gray Oral Reading Test–Fifth Edition (GORT-5): Examiner’s manual. Austin, TX: Pro-Ed. [Google Scholar]
- Willcutt EG, & Pennington BF (2000). Psychiatric comorbidity in children and adolescents with reading disability. J Child Psychol Psychiatry, 41(8), 1039–1048. doi: 10.1111/1469-7610.00691 [DOI] [PubMed] [Google Scholar]
- Williams VJ, Juranek J, Cirino P, & Fletcher JM (2018). Cortical Thickness and Local Gyrification in Children with Developmental Dyslexia. Cerebral Cortex, 28(3), 963–973. doi: 10.1093/cercor/bhx001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Touroutoglou A, Quigley KS, Feldman Barrett L, & Dickerson BC (2017). Salience Network Connectivity Modulates Skin Conductance Responses in Predicting Arousal Experience. Journal of Cognitive Neuroscience, 29(5), 827–836. doi: 10.1162/jocn_a_01087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, … Seeley WW. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(Pt 5), 1352–1367. doi: 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, & Seeley WW (2010). Network-level structural covariance in the developing brain. Proceedings of the National Academy of Sciences, 107(42), 18191–18196. doi: 10.1073/pnas.1003109107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated by the UCSF Dyslexia Center are available upon request; data requests can be submitted through the UCSF Memory and Aging Center Resource Request form: http://memory.ucsf.edu/resources/data. Academic, not-for-profit investigators with Institutional Review Board approval from the UCSF Human Research Protection Program (HRPP) can request data for research studies. The UCSF HRPP will not review the application until the UCSF Memory and Aging Center Executive Committee has signed off on the proposal and consent form. Data are not publicly available because they contain information that could compromise the privacy of the participants.