Abstract
Objective
To determine the prevalence, predictors and case fatality risk of hypothermia among neonates in Lira district, Northern Uganda.
Setting
Three subcounties of Lira district in Northern Uganda.
Design
This was a community-based cross-sectional study nested in a cluster randomised controlled trial.
Participants
Mother–baby pairs enrolled in a cluster randomised controlled trial. An axillary temperature was taken during a home visit using a lithium battery-operated digital thermometer.
Primary and secondary outcomes
The primary outcome measure was the prevalence of hypothermia. Hypothermia was defined as mild if the axillary temperature was 36.0°C to <36.5°C, moderate if the temperature was 32.0°C to <36.0°C and severe hypothermia if the temperature was <32.0°C. The secondary outcome measure was the case fatality risk of neonatal hypothermia. Predictors of moderate to severe hypothermia were determined using a generalised estimating equation model for the Poisson family.
Results
We recruited 1330 neonates. The prevalence of hypothermia (<36.5°C) was 678/1330 (51.0%, 95% CI 46.9 to 55.1). Overall, 32% (429/1330), 95% CI 29.5 to 35.2 had mild hypothermia, whereas 18.7% (249/1330), 95% CI 15.8 to 22.0 had moderate hypothermia. None had severe hypothermia. At multivariable analysis, predictors of neonatal hypothermia included: home birth (adjusted prevalence ratio, aPR, 1.9, 95% CI 1.4 to 2.6); low birth weight (aPR 1.7, 95% CI 1.3 to 2.3) and delayed breastfeeding initiation (aPR 1.2, 95% CI 1.0 to 1.5). The case fatality risk ratio of hypothermic compared with normothermic neonates was 2.0 (95% CI 0.60 to 6.9).
Conclusion
The prevalence of neonatal hypothermia was very high, demonstrating that communities in tropical climates should not ignore neonatal hypothermia. Interventions designed to address neonatal hypothermia should consider ways of reaching neonates born at home and those with low birth weight. The promotion of early breastfeeding initiation and skin-to-skin care could reduce the risk of neonatal hypothermia.
Trial registration number
ClinicalTrial.gov as NCT02605369.
Keywords: epidemiology, paediatrics, public health
Strengths and limitations of this study.
This is the first purely community-based assessment of neonatal hypothermia in sub-Saharan Africa.
Estimates obtained are generalisable to settings with a significant proportion of home births unlike previous estimates from health facility-based studies.
We included a large number of neonates (1330), which increased the precision of our estimates.
The choice of a digital thermometer placed in the axilla could have underestimated hypothermia, but this was the most socially acceptable option.
We did not measure some predictors, such as delivery room temperature and maternal body temperature.
Introduction
Neonatal mortality (death of neonates less than 28 days) in Uganda is unacceptably high, at 22.3 deaths per 1000 live births compared with 1.6 deaths per 1000 live births in high-income countries.1 In order to attain the global target of reducing neonatal mortality to under 12 deaths per 1000 live births by 2030,2 there is a need to identify and quantify the predictors of neonatal mortality; especially those that are preventable by available low-cost interventions.3 4 One of the predictors of neonatal mortality that can easily be solved by available low-cost interventions is neonatal hypothermia.5
Neonatal hypothermia, defined as an axillary temperature less than 36.5°C,6 7 is associated with increased neonatal morbidity and mortality.8–10 Countries with high neonatal mortality have high rates of neonatal hypothermia.11 Hypothermia mainly contributes to mortality by worsening outcomes of severe neonatal infections, preterm birth and birth asphyxia.5 6 11 It is estimated that 20% of deaths due to prematurity and 10% of deaths in term babies could be prevented by improved thermal care.12 In addition, neonatal hypothermia results in reduced growth and development.13
Neonates are unable to maintain their body temperature without thermal protection.14 They are susceptible to hypothermia due to physical and environmental factors. Physical factors that predispose neonates to hypothermia include a large surface area to volume ratio, thin skin and low amounts of insulating fat.5 11 14 15 Environmental factors that predispose neonates to hypothermia include poor thermal practices around the time of birth, such as keeping the neonate away from the mother and bathing the newborn within 24 hours of birth,16 which are common practices in sub-Saharan Africa.17 18 WHO recommends a 10-step warm chain to prevent neonatal hypothermia: a warm delivery room, immediate drying, delayed bathing, skin-to-skin contact, early and exclusive breast feeding, appropriate clothing/bedding, keeping the baby with the mother, warm transportation and resuscitation, and training/raising awareness on the dangers of hypothermia.6 However, these actions are often suboptimal in most communities in sub-Saharan Africa,19 and disregarded with the misguided assumption that a warm climate guarantees thermal protection to the neonates.20 21 Neonates are at greatest risk of hypothermia on the first day of life and this is mainly a result of evaporation of amniotic fluid and the neonate’s limited ability to generate heat.15 22
Despite a significant proportion of births and deaths taking place at home in sub-Saharan Africa, there is little to no data on hypothermia obtained from community studies.5 23 Previous estimates of hypothermia in sub-Saharan Africa have mostly been obtained from health facility studies9 10 20 21 24 25 and may, therefore, not be representative of populations with poor health-seeking behaviours. Researchers conducting community-based studies have been encouraged to incorporate axillary temperatures with standard inexpensive digital thermometers in their study protocols in order to enrich the literature on community estimates of neonatal hypothermia.5 This information is necessary when advocating for the scale-up of existing interventions known to reduce hypothermia.23 Therefore, in this study, we determined the prevalence, predictors and case fatality risk of hypothermia among neonates in Lira district, Northern Uganda.
Materials and Methods
Study setting
This study was conducted in Lira district, located in Lango region a postconflict area in Northern Uganda, in the subcounties of Aromo, Agweng and Ogur between January 2018 and March 2019. About 400 000 people live in Lira; the majority live in rural areas and practice subsistence farming.26 In Lango region, 97% of pregnant women attend at least one antenatal care visit from a skilled provider, only 66% of births take place in a health facility, and approximately 29 out of every 1000 neonates died in the first 28 days of life.27 During the period of this study, the average monthly temperatures ranged from 27.8°C to 35.0°C (Ngeta weather station, Lira district). Women who give birth vaginally are discharged from health facilities within 24 hours and those who give birth by caesarean section are discharged within 72 hours, unless complications occur.
Study design
This was a cross-sectional study conducted between January 2018 and March 2019. The study was nested in a cluster randomised controlled trial designed to promote health facility birth, newborn care practices (early and exclusive breast feeding, skin-to-skin care), and timely postnatal health facility visits (Survival Pluss study registered on ClinicalTrial.gov as NCT02605369).
Study participants
All neonates born to mothers participating in the cluster randomised controlled trial were eligible for this study. We excluded neonates whose mothers were too sick to participate in the interview, and neonates that died before we visited.
Power and sample size
A total of 1330 neonates participated in our study. The participants were initially enrolled in a cluster randomised controlled study, which had a neonatal hypothermia intracluster correlation coefficient of 0.044, and average cluster sample size of 65, giving us a design effect of 3.8, and effective sample size of 350, resulting in absolute precision of 1.5%–5.2%, that is, the difference between the point estimate and the 95% CI for prevalence values ranging from 2% to 50%. Since we were studying a very common outcome, we deemed this precision adequate.
Main variables
Outcome variable
The outcome variable in this study was hypothermia, which was defined as mild hypothermia if the axillary temperature was between 36.0°C and less than 36.5°C, moderate if the temperature was between 32.0°C and less than 36.0°C, and severe hypothermia if the temperature was less than 32.0°C. We also graded hypothermia according to a classification proposed by Mullany.23 Briefly, Mullany classified hypothermia as follows: grade 1 (36.0°C−36.5°C), grade 2 (35.0°C to <36.0°C), grade 3 (34.0°C to <35.0°C) and grade 4 (<34.0°C).
Exposure variables
Data were collected on several predictors during pregnancy and immediately after birth. These included: maternal age, parity, maternal education, paternal education, wealth, singleton or multiple birth, sex of the newborn, place of birth, birth weight, early breastfeeding initiation, bathing of the newborn, and whether the baby was placed on the mother’s chest or abdomen immediately after birth. We classified the season as wet if the average monthly precipitation was 60 mm or more (Koppen-Geiger climate classification).28 The average monthly precipitation and temperature for the study period were obtained from the Ngeta weather station in Lira district. Wealth quintiles were calculated from an asset-based index using principal component analysis. The following assets and house characteristics were considered: cupboard, bicycle, radio, mobile phone, motorcycle, cement floor, iron sheets, burnt bricks and land ownership. We defined early breastfeeding initiation as the initiation of breast feeding within 1 hour of birth. Education level was categorised into primary, secondary and tertiary. The primary level corresponds to 1–7 years of education, the secondary level to 8–13 years of education and the tertiary level to more than 13 years of education.
Data collection
As part of the trial in which this study was nested, a team of 42 research assistants collected data and conducted measurements on the first day of birth, or as soon as possible after birth at the mother’s home. A temperature was taken high in the axilla during the study visit. We used a lithium battery-operated digital thermometer: Model TM01 (manufactured by Cotronic Manufacturing, Shenzhen). The research assistants were trained on how to measure temperature and supervised by a team consisting of three paediatricians, one obstetrician, two general practitioners, two nurses and one data analyst. Temperature measurements were mostly conducted before taking the baby’s anthropometric measurements, with emphasis placed on minimising the time the babies were exposed to the cold. Measurements involved putting the tip of the thermometer high up in the apex of the axilla, halfway between the anterior and posterior margins, and holding the arm in place until an automatic audible beep was heard. Two measurement readings in degrees Celsius were taken and the average of these used. Thermometers were cleaned with cotton wool soaked in 70% alcohol after the examination.
Recruitment and follow-up
All villages had a recruiter who was elected during the community sensitisation meetings of the trial. The recruiter was a female resident in the cluster. Recruiters identified pregnant women and accompanied research assistants to the home of the women during the recruitment. They were trained during a 1-day workshop, which emphasised ethics, confidentiality and good record keeping. Recruiters were also given a cell phone to contact the team (site supervisor/research assistants) whenever they identified a pregnant woman or whenever a pregnant woman had given birth. They were paid Uganda Shillings 5000 (US$1.4) whenever they identified an eligible participant and whenever they informed the team within 24 hours of a mother giving birth. Approximately 250 recruiters were trained. After a recruiter informed the team of an eligible participant, a research assistant visited the mother to ascertain eligibility, to obtain informed consent and to conduct the interview. To ensure that recruiters were reporting all pregnant women, we employed community health workers (village health team members) to conduct a census of all pregnant women in the area. Pregnant mothers and their relatives were encouraged to contact the study team immediately after giving birth. Research assistants also obtained phone numbers of pregnant women and their relatives and periodically conducted follow-up phone calls and visits to ensure that mothers were visited immediately after birth. The process of notification was similar between health facility and home births. Data collectors conducted the follow-up visits to assess whether the neonates were alive at 7 days and at 28 days.
Patient and public involvement
The public was not involved in the design and conceptualisation of the study but they were involved in the recruitment of participants. We held community meetings in each village during which a recruiter was elected from among the village members. The recruiter was responsible for recruitment in their village. The results of this study will be disseminated to the wider community through community dialogue meetings at parish level in each participating village.
Statistical analysis
Data were analysed using Stata V.14.0 (StataCorp). Study characteristics were compared across the exposure status and summarised as proportions for categorical data and means for continuous data. Hypothermia was categorised using both the WHO classification,6 and a classification suggested by Mullany23, and presented as proportions with corresponding 95% CIs adjusted for clustering. Factors associated with moderate to severe hypothermia were determined using a generalised estimating equation model for the Poisson family, with a log link, allowing for the clustering and assuming an exchangeable correlation. We used robust variance estimation in our model. Predictors of hypothermia included in our multivariable model were determined a priori during a review of the literature on the subject. Factors included as predictors in our model were: mother’s age, mother’s education, mode of birth, place of birth, low birth weight, wealth, parity, season, baby placed on mother’s chest or abdomen immediately after birth, cleaning/drying the baby immediately after birth, bathing the baby, delayed initiation of breastfeeding.5 6 11 16 20–23 All variables included in the model were assessed for collinearity and considered collinear if they had a variance inflation factor greater than 10. In the case of collinearity, we retained the variable with greater biological plausibility and/or measure of association. The multivariable analyses were based on a complete case analysis. However, we conducted sensitivity analyses of best case, worst-case and most realistic scenarios to assess the potential effect of the missing data. We also conducted subgroup analysis of the prevalence of hypothermia by date of neonate on examination and by place of birth. Since this study was nested in a cluster randomised controlled trial, the trial arm was added as a fixed effect in all the models.
Results
Participant characteristics
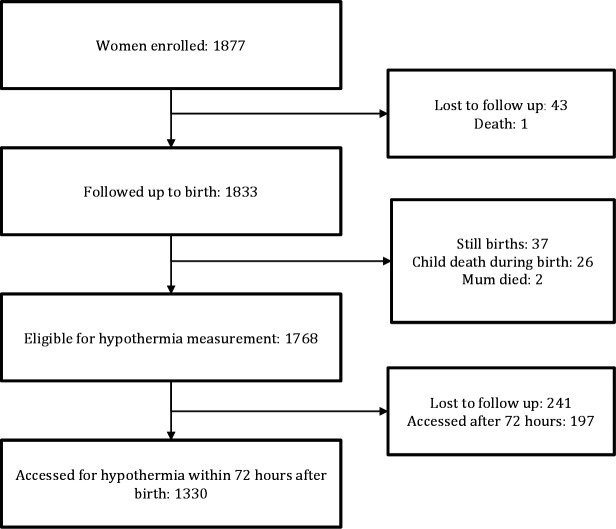
On our visits to the mothers, we were able to take the temperature measurements of 1527 neonates; of these we used the data of 1330 for whom temperatures were taken within the first 72 hours after birth (figure 1). The mean age of mothers was 24.6 years (SD 6.8) and their median education was 5 years (IQR 3–6). The mean weight of neonates was 3.2 kg (SD 0.5) (table 1).
Figure 1.
Study profile of neonates assessed for hypothermia in Lira district, Northern Uganda.
Table 1.
Participant characteristics of neonates assessed for hypothermia in northern Uganda
| All participants | Late participants* | Missed participants† | |||
| No hypothermia | Hypothermia | No hypothermia | Hypothermia | Unknown | |
| N=652 | N=678 | N=88 | N=109 | N=241 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age of mother | |||||
| ≤19 | 148 (22.7) | 201 (29.7) | 28 (31.8) | 33 (30.3) | 66 (27.4) |
| 20–30 | 367 (56.3) | 347 (51.2) | 48 (54.6) | 56 (51.4) | 121 (50.2) |
| >30 | 137 (21.0) | 130 (19.2) | 12 (13.6) | 20 (18.4) | 54 (22.4) |
| Mother’s education | |||||
| None | 74 (11.4) | 105 (15.5) | 6 (06.8) | 12 (11.0) | 34 (14.1) |
| Primary | 513 (78.7) | 519 (76.6) | 73 (83.0) | 85 (78.0) | 190 (78.8) |
| Secondary | 51 (7.8) | 47 (6.9) | 7 (8.0) | 9 (8.3) | 17 (7.1) |
| Tertiary | 14 (02.2) | 07 (01.0) | 2 (02.3) | 3 (02.8) | – |
| Father’s education | |||||
| None | 14 (2.2) | 11 (1.6) | 1 (1.1) | 1 (0.92) | 6 (2.5) |
| Primary | 377 (57.8) | 416 (61.4) | 52 (59.1) | 55 (50.5) | 151 (62.7) |
| Secondary | 177 (27.2) | 147 (21.7) | 23 (26.1) | 28 (25.7) | 51 (21.2) |
| Tertiary | 41 (6.3) | 38 (5.6) | 4 (4.6) | 8 (7.3) | 12 (5.0) |
| Missing | 43 (6.6) | 66 (9.7) | 8 (9.1) | 17 (15.6) | 21 (8.7) |
| Parity | |||||
| ≤1 | 286 (43.9) | 325 (47.9) | 44 (50.0) | 55 (50.5) | 101 (41.9) |
| 2–4 | 219 (33.6) | 218 (32.2) | 36 (40.9) | 28 (25.7) | 86 (35.7) |
| >4 | 147 (22.6) | 135 (19.9) | 8 (9.1) | 26 (23.9) | 54 (22.4) |
| Place of birth | |||||
| Home | 157 (24.1) | 254 (37.5) | 26 (29.6) | 40 (36.7) | 100 (41.5) |
| Health facility | 495 (75.9) | 424 (62.5) | 62 (70.5) | 69 (63.3) | 141 (58.5) |
| Caesarean section | |||||
| No | 641 (98.3) | 670 (98.8) | 79 (89.8) | 94 (86.2) | 232 (96.3) |
| Yes | 11 (1.7) | 8 (1.2) | 9 (10.2) | 15 (13.8) | 9 (3.7) |
| Marital status | |||||
| Married | 609 (93.4) | 612 (90.3) | 80 (90.9) | 92 (84.4) | 220 (91.3) |
| Single | 43 (6.6) | 66 (9.7) | 8 (9.1) | 17 (15.6) | 21 (8.7) |
| Electricity | |||||
| Yes | 71 (10.9) | 86 (12.7) | 4 (4.6) | 6 (5.5) | 24 (10.0) |
| No | 581 (89.1) | 592 (87.3) | 84 (95.5) | 103 (94.5) | 217 (90.0) |
| Presence of mobile phone in the household | |||||
| Yes | 346 (53.1) | 363 (53.5) | 42 (47.7) | 53 (48.6) | 159 (66.0) |
| No | 306 (46.9) | 315 (46.5) | 46 (52.3) | 56 (51.4) | 82 (34.0) |
| Source of drinking water | |||||
| Borehole | 319 (48.9) | 340 (50.2) | 54 (61.4) | 58 (53.2) | 138 (57.3) |
| Tap/piped water | 88 (13.5) | 84 (12.4) | 9 (10.2) | 10 (09.2) | 20 (8.3) |
| Protected natural spring | 131 (20.1) | 150 (22.1) | 13 (14.8) | 20 (18.4) | 43 (17.8) |
| Unprotected water source | 114 (17.5) | 104 (15.3) | 12 (13.6) | 21 (19.3) | 40 (16.6) |
| Twin | |||||
| No | 648 (99.4) | 668 (98.5) | 87 (98.9) | 107 (98.2) | 237 (98.3) |
| Yes | 4 (0.61) | 10 (1.5) | 1 (1.1) | 2 (1.8) | 4 (1.7) |
| Low birth weight | |||||
| No | 613 (94.0) | 622 (91.7) | 83 (94.3) | 101 (92.7) | 15 (6.2) |
| Yes | 35 (5.4) | 45 (6.6) | 4 (4.6) | 6 (5.5) | 1 (0.41) |
| Missing | 4 (0.6) | 11 (01.6) | 1 (1.1) | 2 (1.8) | 225 (93.4) |
| Wealth quintiles | |||||
| 1 (poorest) | 146 (22.4) | 140 (20.7) | 19 (21.6) | 23 (21.1) | 35 (14.5) |
| 2 | 143 (21.9) | 185 (27.3) | 20 (22.7) | 22 (20.2) | 63 (26.1) |
| 3 | 123 (18.9) | 121 (17.9) | 19 (21.6) | 18 (16.5) | 45 (18.7) |
| 4 | 105 (16.1) | 114 (16.8) | 10 (11.4) | 20 (18.4) | 49 (20.3) |
| 5 (richest) | 135 (20.7) | 118 (17.4) | 20 (22.7) | 26 (23.9) | 49 (20.3) |
| Season | |||||
| Wet | 589 (90.3) | 579 (85.4) | 74 (84.1) | 87 (79.8) | 47 (19.5) |
| Dry | 63 (9.7) | 99 (14.6) | 14 (15.9) | 22 (20.2) | 194 (80.5) |
| Baby placed on mother’s chest or abdomen immediately after birth | |||||
| Yes | 547 (83.9) | 548 (80.8) | 68 (77.3) | 76 (69.7) | 163 (67.6) |
| No | 105 (16.1) | 130 (19.2) | 20 (22.7) | 33 (30.3) | 78 (32.4) |
| Clean and dry baby immediately | |||||
| No | 68 (10.4) | 104 (15.3) | 10 (11.4) | 21 (19.3) | 41 (17.0) |
| Yes | 584 (89.6) | 574 (84.7) | 78 (88.6) | 88 (80.7) | 200 (83.0) |
| Bathed baby before visit | |||||
| No | 326 (50.0) | 274 (40.4) | 1 (1.1) | 2 (1.8) | 81 (34.3) |
| Yes | 326 (50.0) | 404 (59.6) | 87 (98.9) | 107 (98.2) | 155 (65.7) |
| Died in first month | |||||
| No | 643 (98.6) | 675 (99.6) | 88 (100.0) | 108 (99.1) | 227 (94.2) |
| Yes | 9 (1.4) | 3 (0.44) | 0 (0.0) | 1 (0.92) | 14 (5.8) |
| Early breastfeeding initiation | |||||
| No | 208 (31.9) | 257 (37.9) | 35 (39.8) | 58 (53.2) | 110 (48.0) |
| Yes | 444 (68.1) | 421 (62.1) | 53 (60.2) | 51 (46.8) | 119 (52.0) |
*Participants whose temperature was measured after 3 days.
†Missed participants: eligible participants whose temperature was not measured.
Hypothermia
The mean temperature was 36.4°C (SD 0.7), and the median temperature was 36.4°C (IQR 36.1°C−36.8°C). The minimum temperature recorded was 32.0°C and the maximum temperature recorded was 39.4°C. The prevalence of hypothermia (temperature less than 36.5°C) was 678/1330 (51.0%: 95% CI 46.9 to 55.1). Overall, 32% (429/1330), 95% CI 29.5 to 35.2) had mild hypothermia (temperature 36.0°C to <36.5°C), whereas 18.7% (249/1330), 95% CI 15.8 to 22.0 had moderate hypothermia (temperature 32.0°C to <36.0°C). No neonate had severe hypothermia (temperature less than 32.0°C) (table 2A). We also graded hypothermia according to a classification proposed by Mullany23 and present the results in table 2B. Sensitivity analyses conducted suggested that we might have underestimated the burden (online supplemental tables 1 and 2). Hypothermia was more common among home births and on the first day of birth. Results of the third day of life were very imprecise (online supplemental tables 3 and 4).
Table 2.
(A) Prevalence of hypothermia (defined by the WHO classification) in Lira district, Northern Uganda
| Hypothermia | n/N (all) | % (95% CI) |
| (A) | ||
| Mild (36.0–36.5) | 429/1330 | 32.3 (29.5 to 35.2) |
| Moderate (32.0–35.9) | 249/1330 | 18.7 (15.8 to 22.0) |
| Severe (<32.0) | 0/1330 | 0 |
| Any | 678/1330 | 51.0 (46.9 to 55.1) |
| (B) | ||
| Grade 1 (36.0–36.5) | 429/1330 | 32.3 (29.5 to 35.2) |
| Grade 2 (35.0–35.99) | 218/1330 | 16.4 (14.0 to 19.1) |
| Grade 3 (34.0–34.99) | 26/1330 | 2.0 (1.2 to 3.1) |
| Grade 4 (less than 34.0) | 5/1330 | 0.38 (0.16 to 0.90) |
(B) Prevalence of hypothermia (defined by the Mullany classification) in Lira district, Northern Uganda.
bmjopen-2020-041723supp001.pdf (27.6KB, pdf)
bmjopen-2020-041723supp002.pdf (26KB, pdf)
bmjopen-2020-041723supp003.pdf (30.5KB, pdf)
bmjopen-2020-041723supp004.pdf (28.3KB, pdf)
Factors associated with hypothermia
Using multivariable analysis, the factors associated with neonatal hypothermia included: home birth (adjusted prevalence ratio, aPR, 1.9, 95% CI 1.4 to 2.6), low birth weight (aPR 1.7, 95% CI 1.3 to 2.3), and delayed breastfeeding initiation (aPR 1.2, 95% CI 1.0 to 1.5) (table 3).
Table 3.
Factors associated with moderate to severe hypothermia among neonates in Lira district Northern Uganda
| Bivariable N=1330 |
Multivariable RR N=1315 |
|
| Crude prevalence ratio (95% CI) | Adjusted prevalence ratio (95% CI) | |
| Trial arm | ||
| Control | 1 | 1 |
| Intervention | 0.85 (0.62 to 1.2) | 1.0 (0.79 to 1.4) |
| Age of mother | ||
| ≤19 | 1 | 1 |
| 20–30 | 0.71 (0.58 to 0.88) | 0.81 (0.59 to 1.1) |
| >30 | 0.70 (0.50 to 0.96) | 0.75 (0.43 to 1.3) |
| Mother’s education | ||
| None | 1 | 1 |
| Primary | 0.93 (0.69 to 1.2) | 0.94 (0.70 to 1.3) |
| ≥Secondary | 0.53 (0.31 to 0.88) | 0.63 (0.39 to 1.0) |
| Father’s education | ||
| None | 1 | |
| Primary | 1.2 (0.58 to 2.6) | |
| Secondary | 0.81 (0.35 to 1.9) | – |
| Tertiary | 0.73 (0.27 to 2.0) | |
| Parity | ||
| ≤1 | 1 | 1 |
| 2–4 | 0.75 (0.57 to 0.99) | 0.85 (0.57 to 1.3) |
| >4 | 0.77 (0.55 to 1.1) | 0.84 (0.50 to 1.4) |
| Place of birth | ||
| Health Facility | 1 | 1 |
| Home | 2.0 (1.5 to 2.6) | 1.9 (1.4 to 2.6) |
| Caesarean section | ||
| No | 1 | 1 |
| Yes | 0.94 (0.44 to 2.0) | 0.82 (0.31 to 2.1) |
| Marital status | ||
| Single | 1 | – |
| Married | 0.77 (0.55 to 1.1) | |
| Low birth weight* (less than 2.5) | ||
| No | 1 | 1 |
| Yes | 1.9 (1.4 to 2.6) | 1.7 (1.3 to 2.3) |
| Wealth quintiles | ||
| 1 (poorest) | 1 | 1 |
| 2 | 1.1 (0.82 to 1.6) | 1.3 (0.91 to 1.7) |
| 3 | 0.81 (0.57 to 1.1) | 0.93 (0.67 to 1.3) |
| 4 | 0.71 (0.46 to 1.1) | 0.87 (0.59 to 1.3) |
| 5 (richest) | 0.59 (0.40 to 0.87) | 0.79 (0.53 to 1.2) |
| Season | ||
| Wet | 1 | 1 |
| Dry | 1.3 (0.92 to 1.8) | 1.4 (1.0 to 1.9) |
| Baby placed on mother’s chest or abdomen immediately after birth | ||
| No | 1 | 1 |
| Yes | 0.78 (0.61 to 0.99) | 0.98 (0.76 to 1.3) |
| Clean and dry baby immediately | ||
| No | 1 | 1 |
| Yes | 0.87 (0.59 to 1.3) | 0.96 (0.65 to 1.4) |
| Bathed baby before visit | ||
| No | 1 | 1 |
| Yes | 1.2 (0.98 to 1.5) | 1.0 (0.81 to 1.2) |
| Breastfeeding initiation | ||
| Early | 1 | 1 |
| Late | 1.4 (1.1 to 1.8) | 1.2 (1.0 to 1.5) |
| Child’s sex | ||
| Male | 1 | |
| Female | 1.1 (0.95 to 1.3) | – |
Case fatality risk
The risk of death among neonates with moderate hypothermia was 3/249 (1.2%, 95% CI 0.38% to 3.7%), compared with 6/1023 (0.59%, 95% CI 0.28% to 1.2%) among neonates with normal temperature, resulting in a case fatality risk ratio of 2.0 (95% CI 0.60 to 6.9).
Discussion
The prevalence of hypothermia in this study was high. Half of the neonates developed hypothermia; 33% developed mild hypothermia; 19% developed moderate hypothermia. Similar findings were observed in a community-based study in Nepal, where 59% of neonates developed hypothermia on the first day,29 and in another community-based study in India where the prevalence of hypothermia was 45%.30 However, the prevalence of hypothermia observed in our study was much higher than that observed in two other studies in India, which observed a prevalence of 11%31 and 17%.32 The difference could be explained by the different definitions of hypothermia used in the studies. We defined hypothermia as a temperature less than 36.5°C in accordance with recommendations from WHO,6 whereas Kumar et al defined hypothermia as a temperature less than 35.6°C, and Bang et al defined hypothermia as a temperature less than 35°C.
Neonates who had low birth weight were more likely to be hypothermic compared with neonates with normal birth weight. This finding is not surprising. Similar findings were observed in a community-based study conducted in Nepal33 and in many other hospital-based studies in Uganda, Ethiopia24 25 and other countries.20 34 Low birthweight neonates have less capability to conserve and generate heat. This is mainly because of physiological factors such as the reduced amount of brown fat and a poor shivering reflex.15 35 These thermoprotective mechanisms are needed to maintain a normal temperature in neonates who are exposed to hypothermic situations.
Neonates born at home were more likely to be hypothermic compared with neonates born in health facilities. This finding has also been reported in other settings.35 A study in Uganda found that mothers who gave birth at home were more likely to practice suboptimal thermal care practices.36 Mothers who give birth at home are more likely to bathe their neonates soon after birth,37 38 which could explain the increased risk of hypothermia observed in neonates born at home. The main reason for bathing neonates early is the belief that neonates are dirty, having come into contact with maternal fluids and the vernix caseosa.17 39–41 Bathing neonates is also perceived as a prerequisite to good rest and sleep.39 However, early bathing has been shown to result in a substantial drop in the neonate’s temperatures.16 We recommend that neonates are not bathed within the first 24 hours after birth14 and that bathing be done with warm water, after which the neonate should be placed on the skin of the caregiver or placed in adequate warm clothing if available.
Despite the generally impoverished nature of the study area, belonging to a relatively lower socialeconomic status was also a predictor of hypothermia in this population. Mothers with low socioeconomic status often lack resources to buy materials that can keep the neonate warm42 and may have limited access to health information.43 This should not be a big problem if the mother practices skin-to-skin care. Unfortunately, many mothers in Uganda,41 Ethiopia,44 Ghana,44 Tanzania44 and Mali44 do not practice skin-to-skin care. Reasons for not practising adequate neonatal care include beliefs that skin-to-skin care could result in the transmission of diseases to the neonate and could hurt the umbilical cord of the neonate.41 45 46
Mothers who delayed putting their neonates to the breast were more likely to have hypothermic babies. This finding was also observed in the community-based study in Nepal.33 Neonates who are breastfed early receive warmth from their mothers and this explains the reduction in hypothermia.23 47 Mothers who had higher education were less likely to have hypothermic babies, although this finding was imprecise. There was also no difference between mothers in the intervention group and the control group, meaning the intervention did not prevent the neonate from becoming hypothermic.
Methodological considerations
We did not measure some predictors such as delivery room temperature and maternal temperature. We could also have underestimated hypothermia by using a digital thermometer, placed in the axilla. Digital thermometers might slightly over or underestimate temperature readings as compared with mercury thermometers.48–50 We used these because they are inexpensive, locally available and easy to use by community workers.29 In addition, digital thermometers are easier to use in poorly lit rural homes.29 We used axillary measurements because they were easier to do, safer and more acceptable than rectal measurements.29 In a systematic review studying differences between rectal and axillary temperatures, the pooled mean difference of rectal minus axillary temperature was estimated to be 0.17°C, ranging from −0.15°C to 0.5°C.51 Our study could have suffered from a selection bias since only 75% of eligible participants were recruited. From our sensitivity analysis, we believe hypothermia is still a big challenge, and that this selection bias might have slightly underestimated the burden since it was possibly the very sick who were not visited within 72 hours of birth. We believe this selection bias also greatly underestimated the mortality attributed to hypothermia since many more neonates died in the unmeasured group. This is understandable since the majority of newborn deaths in the study, as would be expected, occurred in the first few hours after birth before our teams were able to reach the scene. The lack of gestational age data is another limitation in our study. We believe that our findings are generalisable to rural areas in tropical low-income countries with similar newborn care practices.
Conclusion
The prevalence of neonatal hypothermia was very high, demonstrating that communities in tropical climates should not ignore neonatal hypothermia. Interventions designed to address neonatal hypothermia should consider ways of reaching neonates born at home, as these are at greater risk of hypothermia. Low birthweight neonates, and neonates born to mothers in the poorest socioeconomic status, should also be prioritised. We recommend promotion of low-cost interventions such as skin-to-skin care for all neonates born in similar settings to prevent neonatal hypothermia.
Supplementary Material
Acknowledgments
In a special way, we acknowledge the District Health Office of Lira district, and the various district, sub-county, parish, and village leaders for their assistance in this study. We thank the study participants for accepting to be part of the study and research assistants for working tirelessly to make this work a reality. In a special way, we acknowledge the excellent work performed by our recruiters in making this study possible. Finally, we extend heartfelt appreciation to Ms. Jo Weeks for the excellent English editing.
Footnotes
Contributors: DM, JKT, VN, GN and TT conceived, designed, supervised the study, analysed the data, and wrote the first draft of manuscript. MWM, JKT, JBT, AN, VZ, VA, BO and AAA were instrumental in the design and supervision of the study, and in drafting of the manuscript. All authors read and approved the final version to be published.
Funding: Funding was obtained from the Survival Pluss project; grant number UGA-13-0030 at Makerere University. Survival Pluss project is funded by The Norwegian Programme for Capacity Development in Higher Education and Research for Development (NORHED) under The Norwegian Agency for Development Cooperation (NORAD).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval and consent to participate: Ethical approval to conduct the study was obtained from the following bodies: (1) Research and Ethics committee School of Medicine, Makerere University (SOMREC: REF 2015-121); (2) Uganda National Council of Science and Technology (UNCST: SS 3954); (3) Regional Committees for Medical and Health Research Ethics (REKVEST 2017/2079). We also obtained permission from the Ministry of Health and Lira Local Government. Written informed consent was obtained from the respondents in the study. Research assistants were trained in confidentiality and the right of the respondent to withdraw their participation at any time during the study. At the community level, we obtained permission to include clusters during community sensitisation meetings, after which the community members democratically elected recruiters, and peer buddies when applicable, from among themselves.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.GBD 2016 Mortality Collaborators Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1084–150. 10.1016/S0140-6736(17)31833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Sustainable development goals. secondary sustainable development goals, 2015. Available: http://www.un.org/sustainabledevelopment/summit/
- 3.Darmstadt GL, Bhutta ZA, Cousens S, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 2005;365:977–88. 10.1016/S0140-6736(05)71088-6 [DOI] [PubMed] [Google Scholar]
- 4.Knippenberg R, Lawn JE, Darmstadt GL, et al. Systematic scaling up of neonatal care in countries. Lancet 2005;365:1087–98. 10.1016/S0140-6736(05)71145-4 [DOI] [PubMed] [Google Scholar]
- 5.Lunze K, Bloom DE, Jamison DT, et al. The global burden of neonatal hypothermia: systematic review of a major challenge for newborn survival. BMC Med 2013;11:24. 10.1186/1741-7015-11-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Thermal protection of the newborn: a practical guide. Geneva: World Health Organization, 1997. [Google Scholar]
- 7.Lunze K, Yeboah-Antwi K, Marsh DR, et al. Prevention and management of neonatal hypothermia in rural Zambia. PLoS One 2014;9:e92006. 10.1371/journal.pone.0092006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensson K, Bhat GJ, Eriksson B, et al. The effect of routine hospital care on the health of hypothermic newborn infants in Zambia. J Trop Pediatr 1995;41:210–4. 10.1093/tropej/41.4.210 [DOI] [PubMed] [Google Scholar]
- 9.Kambarami R, Chidede O. Neonatal hypothermia levels and risk factors for mortality in a tropical country. Cent Afr J Med 2003;49:103–6. [PubMed] [Google Scholar]
- 10.Sodemann M, Nielsen J, Veirum J, et al. Hypothermia of newborns is associated with excess mortality in the first 2 months of life in Guinea-Bissau, West Africa. Trop Med Int Health 2008;13:980–6. 10.1111/j.1365-3156.2008.02113.x [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Shearer JC, Kumar A, et al. Neonatal hypothermia in low resource settings: a review. J Perinatol 2009;29:401–12. 10.1038/jp.2008.233 [DOI] [PubMed] [Google Scholar]
- 12.Bhutta ZA, Das JK, Bahl R, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet 2014;384:347–70. 10.1016/S0140-6736(14)60792-3 [DOI] [PubMed] [Google Scholar]
- 13.Glass L, Silverman WA, Sinclair JC. Relationship of thermal environment and caloric intake to growth and resting metabolism in the late neonatal period. Biol Neonat 1969;14:324–40. 10.1159/000240198 [DOI] [PubMed] [Google Scholar]
- 14.Lunze K, Hamer DH. Thermal protection of the newborn in resource-limited environments. J Perinatol 2012;32:317–24. 10.1038/jp.2012.11 [DOI] [PubMed] [Google Scholar]
- 15.ADAMSON SK, Towell ME. Thermal homeostasis in the fetus and newborn. Anesthesiology 1965;26:531–48. 10.1097/00000542-196507000-00017 [DOI] [PubMed] [Google Scholar]
- 16.Bergström A, Byaruhanga R, Okong P. The impact of newborn bathing on the prevalence of neonatal hypothermia in Uganda: a randomized, controlled trial. Acta Paediatr 2005;94:1462–7. 10.1080/080352505100366750 [DOI] [PubMed] [Google Scholar]
- 17.Waiswa P, Kemigisa M, Kiguli J, et al. Acceptability of evidence-based neonatal care practices in rural Uganda - implications for programming. BMC Pregnancy Childbirth 2008;8:21. 10.1186/1471-2393-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill Z, Tawiah-Agyemang C, Manu A, et al. Keeping newborns warm: beliefs, practices and potential for behaviour change in rural Ghana. Trop Med Int Health 2010;15:1118–24. 10.1111/j.1365-3156.2010.02593.x [DOI] [PubMed] [Google Scholar]
- 19.Coalter WS, Patterson SL. Sociocultural factors affecting uptake of home-based neonatal thermal care practices in Africa: a qualitative review. J Child Health Care 2017;21:132–41. 10.1177/1367493516686201 [DOI] [PubMed] [Google Scholar]
- 20.Manji KP, Kisenge R. Neonatal hypothermia on admission to a special care unit in Dar-es-Salaam, Tanzania: a cause for concern. Cent Afr J Med 2003;49:23–7. [PubMed] [Google Scholar]
- 21.Byaruhanga R, Bergstrom A, Okong P. Neonatal hypothermia in Uganda: prevalence and risk factors. J Trop Pediatr 2005;51:212–5. 10.1093/tropej/fmh098 [DOI] [PubMed] [Google Scholar]
- 22.Smales OR, Kime R. Thermoregulation in babies immediately after birth. Arch Dis Child 1978;53:58–61. 10.1136/adc.53.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullany LC Neonatal hypothermia in low-resource settings. Semin Perinatol 2010;34:426–33. 10.1053/j.semperi.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasew H, Gebrekristos K, Kidanu K, et al. Determinants of hypothermia on neonates admitted to the intensive care unit of public hospitals of central zone, Tigray, Ethiopia 2017: unmatched case-control study. BMC Res Notes 2018;11:576. 10.1186/s13104-018-3691-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demissie BW, Abera BB, Chichiabellu TY, et al. Neonatal hypothermia and associated factors among neonates admitted to neonatal intensive care unit of public hospitals in Addis Ababa, Ethiopia. BMC Pediatr 2018;18:263. 10.1186/s12887-018-1238-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uganda Bureau of statistics The National population and housing census 2014 – area specific profile series, Kampala, Uganda secondary the National population and housing census 2014 – area specific profile series, Kampala, Uganda, 2017. Available: http://www.ubos.org/onlinefiles/uploads/ubos/2014CensusProfiles/MUKONO.pdf
- 27.Uganda Bureau of Statistics (UBOS) and ICF Uganda demographic and health survey 2016. Kampala, Uganda and Rockville, Maryland, USA: UBOS and ICF, 2018. [Google Scholar]
- 28.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 2007;11:1633–44. 10.5194/hess-11-1633-2007 [DOI] [Google Scholar]
- 29.Mullany LC, Katz J, Khatry SK, et al. Incidence and seasonality of hypothermia among newborns in southern Nepal. Arch Pediatr Adolesc Med 2010;164:71–7. 10.1001/archpediatrics.2009.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darmstadt GL, Kumar V, Yadav R, et al. Introduction of community-based skin-to-skin care in rural Uttar Pradesh, India. J Perinatol 2006;26:597–604. 10.1038/sj.jp.7211569 [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, Aggarwal AK. Body temperatures of home delivered newborns in North India. Trop Doct 1998;28:134–6. 10.1177/004947559802800304 [DOI] [PubMed] [Google Scholar]
- 32.Bang AT, Reddy HM, Baitule SB, et al. The incidence of morbidities in a cohort of neonates in rural Gadchiroli, India: seasonal and temporal variation and a hypothesis about prevention. J Perinatol 2005;25 Suppl 1:S18–28. 10.1038/sj.jp.7211271 [DOI] [PubMed] [Google Scholar]
- 33.Mullany LC, Katz J, Khatry SK, et al. Neonatal hypothermia and associated risk factors among newborns of southern Nepal. BMC Med 2010;8:43. 10.1186/1741-7015-8-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zayeri F, Kazemnejad A, Ganjali M, et al. Hypothermia in Iranian newborns. incidence, risk factors and related complications. Saudi Med J 2005;26:1367–71. [PubMed] [Google Scholar]
- 35.Onalo R Neonatal hypothermia in sub-Saharan Africa: a review. Niger J Clin Pract 2013;16:129–38. 10.4103/1119-3077.110120 [DOI] [PubMed] [Google Scholar]
- 36.Kabwijamu L, Waiswa P, Kawooya V, et al. Newborn care practices among adolescent mothers in Hoima district, Western Uganda. PLoS One 2016;11:e0166405. 10.1371/journal.pone.0166405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mrisho M, Schellenberg JA, Mushi AK, et al. Understanding home-based neonatal care practice in rural southern Tanzania. Trans R Soc Trop Med Hyg 2008;102:669–78. 10.1016/j.trstmh.2008.04.029 [DOI] [PubMed] [Google Scholar]
- 38.Salasibew MM, Filteau S, Marchant T. A qualitative study exploring newborn care behaviours after home births in rural Ethiopia: implications for adoption of essential interventions for saving newborn lives. BMC Pregnancy Childbirth 2014;14:412. 10.1186/s12884-014-0412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adejuyigbe EA, Bee MH, Amare Y, et al. "Why not bathe the baby today?": A qualitative study of thermal care beliefs and practices in four African sites. BMC Pediatr 2015;15:156. 10.1186/s12887-015-0470-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shamba D, Schellenberg J, Hildon ZJ-L, et al. Thermal care for newborn babies in rural southern Tanzania: a mixed-method study of barriers, facilitators and potential for behaviour change. BMC Pregnancy Childbirth 2014;14:267. 10.1186/1471-2393-14-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byaruhanga RN, Nsungwa-Sabiiti J, Kiguli J, et al. Hurdles and opportunities for newborn care in rural Uganda. Midwifery 2011;27:775–80. 10.1016/j.midw.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 42.Lunze K, Dawkins R, Tapia A, et al. Market mechanisms for newborn health in Nepal. BMC Pregnancy Childbirth 2017;17:428. 10.1186/s12884-017-1599-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owor MO, Matovu JKB, Murokora D, et al. Factors associated with adoption of beneficial newborn care practices in rural eastern Uganda: a cross-sectional study. BMC Pregnancy Childbirth 2016;16:83. 10.1186/s12884-016-0874-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bee M, Shiroor A, Hill Z. Neonatal care practices in sub-Saharan Africa: a systematic review of quantitative and qualitative data. J Health Popul Nutr 2018;37:9. 10.1186/s41043-018-0141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byaruhanga RN, Bergström A, Tibemanya J, et al. Perceptions among post-delivery mothers of skin-to-skin contact and newborn baby care in a periurban hospital in Uganda. Midwifery 2008;24:183–9. 10.1016/j.midw.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 46.Waiswa P, Peterson S, Tomson G, et al. Poor newborn care practices - a population based survey in eastern Uganda. BMC Pregnancy Childbirth 2010;10:9. 10.1186/1471-2393-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huffman SL, Zehner ER, Victora C. Can improvements in breast-feeding practices reduce neonatal mortality in developing countries? Midwifery 2001;17:80–92. 10.1054/midw.2001.0253 [DOI] [PubMed] [Google Scholar]
- 48.Smith J Are electronic thermometry techniques suitable alternatives to traditional mercury in glass thermometry techniques in the paediatric setting? J Adv Nurs 1998;28:1030–9. 10.1046/j.1365-2648.1998.00745.x [DOI] [PubMed] [Google Scholar]
- 49.Jones HL, Kleber CB, Eckert GJ, et al. Comparison of rectal temperature measured by digital vs. mercury glass thermometer in infants under two months old. Clin Pediatr 2003;42:357–9. 10.1177/000992280304200409 [DOI] [PubMed] [Google Scholar]
- 50.Latman NS, Hans P, Nicholson L, et al. Evaluation of clinical thermometers for accuracy and reliability. Biomed Instrum Technol 2001;35:259–65. [PubMed] [Google Scholar]
- 51.Craig JV, Lancaster GA, Williamson PR, et al. Temperature measured at the axilla compared with rectum in children and young people: systematic review. BMJ 2000;320:1174–8. 10.1136/bmj.320.7243.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041723supp001.pdf (27.6KB, pdf)
bmjopen-2020-041723supp002.pdf (26KB, pdf)
bmjopen-2020-041723supp003.pdf (30.5KB, pdf)
bmjopen-2020-041723supp004.pdf (28.3KB, pdf)