Abstract
By the beginning of the global pandemic, SARS-CoV-2 infection has dramatically impacted on oncology daily practice. In the current oncological landscape, where immunotherapy has revolutionized the treatment of several malignancies, distinguishing between COVID-19 and immune-mediated pneumonitis can be hard because of shared clinical, radiological and pathological features. Indeed, their common mechanism of aberrant inflammation could lead to a mutual and amplifying interaction.
We describe the case of a 65–year-old patient affected by metastatic squamous head and neck cancer and candidate to an experimental therapy including an anti-PD-L1 agent. COVID-19 ground-glass opacities under resolution were an incidental finding during screening procedures and worsened after starting immunotherapy. The diagnostic work-up was consistent with ICIs-related pneumonia and it is conceivable that lung injury by SARS-CoV-2 has acted as an inflammatory primer for the development of the immune-related adverse event.
Patients recovered from COVID-19 starting ICIs could be at greater risk of recall immune-mediated pneumonitis. Nasopharyngeal swab and chest CT scan are recommended before starting immunotherapy. The awareness of the phenomenon could allow an easier interpretation of radiological changes under treatment and a faster diagnostic work-up to resume ICIs. In the presence of clinical benefit, for asymptomatic ICIs-related pneumonia a watchful-waiting approach and immunotherapy prosecution are suggested.
Keywords: head and neck neoplasms, immunotherapy, programmed cell death 1 receptor, therapies, investigational, clinical trials as topic
Background
Since December 2019, SARS-Coronavirus-2 (SARS-CoV-2) global pandemic has deeply disrupted health, social and economic aspects of daily life worldwide. Facing this unprecedented outbreak, the cancer community is dealing with many arising and tough challenges. Patients with cancer have historically been considered more susceptible to viral infections because of their own immunosuppression and according to several studies are at major risk of developing severe complications of the Coronavirus Disease 2019 (COVID-19), including interstitial pneumonia.1 2
In the last decade, immunotherapy has dramatically changed the therapeutic landscapes of clinical oncology. With the aim to retrain immune system against cancer cells, several monoclonal antibodies (mAbs) targeting the immune checkpoints, such as programmed death 1 (PD-1), its ligand (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), have now joined the therapeutic armamentarium of several malignancies, including non-small cell lung cancer (NSCLC), melanoma and squamous head and neck cancer (SHNC).3
This novel class of immunomodulating agents called immune checkpoint inhibitors (ICIs) presents a peculiar safety profile, with a large spectrum of immune-related adverse events (ir-AEs) potentially affecting any tissue or organ. Immune-mediated pneumonitis is one of the most frequent, severe and potentially life-threatening ir-AEs. When considering clinical trial population, the frequency of anti-PD-1-related pneumonia across all cancer types is around 2.7%.4 In a real-life setting of patients with NSCLC, the overall incidence appears to be significantly higher (19%) and the development of immune-related pneumonia is more frequently associated with anti-PD-1 agents compared with those targeting PD-L1.5 6 7 Its management differs according to clinical and radiological presentation, from a watchful-waiting approach for asymptomatic cases to high-dose immunosuppressive steroid therapy (1–2 mg/kg of prednisone or equivalent) if respiratory symptoms are present or airway support is needed.7
Given the rapid spread of SARS-CoV-2 across the general population and the continuously growing number of patients with cancer receiving immunotherapy, to clinically distinguish between COVID-19 and ICIs-related pneumonia can be very difficult. Both entities are interstitial lung diseases often sharing several clinical and radiological features. Fever, dyspnea and productive cough represent the most common presenting symptoms, while ground-glass opacities (GGOs) with peripheral distribution are typical radiological findings on chest CT scan.8–12 These two conditions exhibit frequently also the same histopathologic picture, including alveolar damage, interstitial proliferation and fibrosis.8–12 In alveoli SARS-CoV-2 seems to have the capacity to engage and even directly infect immune cells such as neutrophils, macrophages and T lymphocytes, leading to a massive production of inflammatory cytokines such as interleukin 6 (IL-6).8–12 A similar pathogenic mechanism is thought to be responsible also for the development of immune-mediated pneumonitis, with pathological reports showing interstitial inflammation and lymphocytic infiltrate.8–12 Taking together these observations, an aberrant inflammation and reprogrammed immune cells functions seem to sustain both COVID-19 and ICIs-related pneumonia, with the potential risk of a mutual and amplifying interplay.8–12
No specific correlation has been demonstrated so far between ICIs treatment and disease severity or mortality for COVID-19.12–14 However, the potential effects of immunotherapy in patients recovered from SARS-CoV-2 infection are largely unknown and still have to be investigated.
Case presentation
Here, we describe the clinical case of a 65-year-old woman affected by relapsing oral cavity SHNC. In July 2018, the patient underwent left hemiglossectomy and homolateral cervical lymphadenectomy, with a pathological Tumor, Node, Metastasis (pTNM) stage T2N0 and no evidence of residual disease. No adjuvant treatments were indicated and the patient started a clinical and radiological follow-up. In February 2020, due to a left parapharyngeal relapse involving homolateral neurovascular bundle, the patient received cervical radiotherapy (70 Gy in 35 fractions) with concurrent cisplatin chemotherapy (100 mg/m2 every 21 days) for three cycles. In June 2020, the patient experienced a further local disease progression and was referred to our phase I unit for enrollment in an early phase trial exploring safety and activity of a combination regimen including an anti-PD-L1 agent with an oral multi tyrosine kinase inhibitor (TKI) targeting several receptors, such as hepatocyte growth factor receptor (HGFR), rearranged during transfection (RET) and vascular endothelial growth factor receptor types 1–3 (VEGFR 1-3).
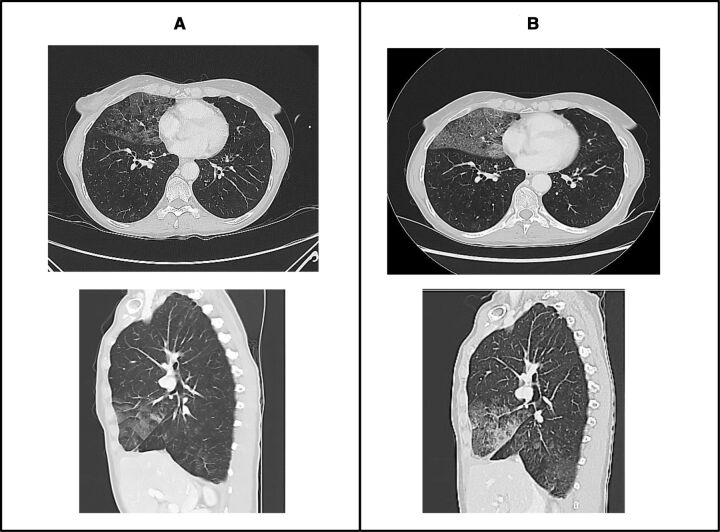
During the screening procedures, chest CT scan showed, as an occasional finding, GGOs in the middle right lobe suggestive for COVID-19, with a score of 4/6 according to COVID-19 Reporting and Data System (CO-RADS) criteria (figure 1A).15 No recent chest CT scans were available for a direct comparison, although the CT scout of a positron emission tomography scan performed at the beginning of May did not display any radiological alteration on the lungs.
Figure 1.
Screening CT scan showing ground-glass opacities (GGOs) in middle lobe (A) and first evaluation CT scan showing a worsening of GGOs even including right lower lobes (B).
The patient was asymptomatic and blood exams, including absolute lymphocyte count, C reactive protein (CRP) and IL-6, were within the normal range. The patient was suddenly admitted to a specific path in emergency room to rule out SARS-CoV-2 infection. Two consecutive nasopharyngeal swabs (NFSs) resulted negative, whereas IgG against SARS-CoV-2 were positive (48.1 AU/mL, with a threshold positivity of 15 AU/mL), clearly indicating a previous exposure to the virus. After a careful multidisciplinary discussion with radiologists, pneumologists and infectivologists, the scenario was interpreted as a recovered COVID-19 with lung radiological alterations still under resolution.
The patient started the experimental treatment with the anti PD-L1 mAb administered intravenously every 3 weeks and a daily oral TKI assumption. As per protocol, the first tumor assessment was performed 6 weeks later, after the first three cycles of immunotherapy. Alongside a stable disease according to response evaluation criteria in solid tumor (RECIST) version 1.1, chest CT scan showed an enlargement of GGOs, even affecting bilateral lower lobes and matching again a score of 4/6 according to CO-RADS criteria (figure 1B).15 An active infection was unlikely considering the good and stable clinical conditions, the absence of respiratory symptoms and results of blood exams (including IL-6) still within the normal range. The patient was hospitalized in an isolated ward to perform further investigations. A new NFS was negative and a bronco-alveolar lavage (BAL) did not detect malignant cells or any pathogens into a broad microbiological panel including SARS-CoV-2, Aspergillus fumigatus, Pneumocystis jirovecii, Mycobacterium tuberculosis and common respiratory viruses (influenza virus, parainfluenza virus, cytomegalovirus, respiratory syncytial virus, rhinovirus, metapneumovirus, adenoviruses, enteroviruses and common coronaviruses). As finding potentially suggestive for immune-related pneumonitis, a particularly high lymphocyte count of 26% was observed in the BAL.
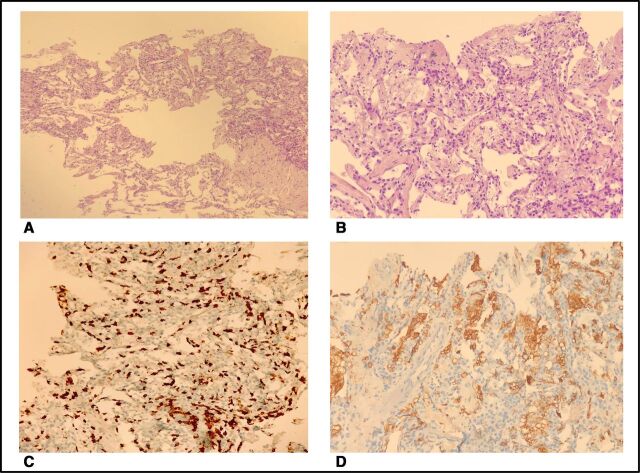
A CT-guided lung biopsy was performed to complete the diagnostic work-up. The microbiological analysis was negative, while pathological examination coupled with immunohistochemical analysis for cluster differentiation 8 (CD8) and 68 (CD68) and the more common cytokeratines showed a mixed picture of interstitial proliferation, CD8+ lymphocytic infiltration and alveolar damage, with no evidence of malignancy (figure 2A–D).
Figure 2.
Pathological issues showing interstitial fibrosis and inflammatory infiltrate (A, B), CD8+ lymphocytic infiltrate (C) and CD68+ alveolar histiocytes (D).
Given the absence of infections and malignant lymphangitis, clinical, radiological and pathological data taken together were consistent with immune-mediated pneumonitis. Our hypothesis is that immunotherapy with PD-L1 blockade led to a worsening in GGOs detecting during screening procedure, with the COVID-19 lung injury acting as an inflammatory primer for the subsequent ICI-related pneumonia development. Due to the complete absence of symptoms, this lung toxicity was classified as grade 1 according to common toxicity criteria for adverse events (CTCAE) version 4, and no further interventions were needed. An involvement of TKI assumption in the worsening of radiological findings was very unlikely. Its well-established safety profile along with its mechanism of action do not suggest any role in the development of this lung damage.
Considering the clinical and radiological benefit on the underlying tumor, the patient restarted experimental immunotherapy after 6 weeks from the last infusion and is currently on-treatment. The last tumor assessment performed after two other infusions of anti-PD-L1 agent showed a further shrinkage of local disease in the neck configuring a partial response (PR), while GGOs appeared unmodified.
Discussion and conclusion
To our knowledge, this is the first report describing a sort of recall immune-mediated pneumonitis in a patient with cancer recovered from COVID-19 and receiving immunotherapy. Several other lung injuries or pre-existing pulmonary comorbidities such as idiopathic pulmonary fibrosis are thought to be risk factors for the development of ICIs-related pneumonia.16 Moreover, immune-related pneumonitis appears to be more frequent in NSCLC compared with other malignancies.5 Several reports described a priming effect of radiation-induced lung damage in patients receiving immunotherapy and subsequently developing immune-mediated pneumonitis in anatomical regions within and around the radiotherapy treatment field.17 18 Similarly, it is conceivable that SARS-CoV-2 inflammatory lung disease could represent a fertile ground for an immune-mediated response sustained by ICI therapy and manifesting radiologically as interstitial pneumonia. SARS-CoV-2 active infection could induce the release of self-antigen and even cross-reactivity between viral and host proteins, enhancing autoimmunity.12 19 As after any infection, the PD-1/PD-L1 axis is likely to be upregulated during recovery phase, switching inflammatory response off and inducing peripheral tolerance.20 21 The PD-L1 blockade induced by ICI could have break self-tolerance, reactivating autoimmune CD8+ T clones and leading to immune-mediated pneumonitis.4 5 7 11
Focusing on clinical management of the present case, we had first to rule out a SARS-CoV-2 reinfection or other forms of active infection potentially leading to interstitial lung alterations. Given the absence of respiratory symptoms and the negative value of CRP, we avoided empirical use of steroids and antibiotics and we moved on the diagnostic work-out performing the BAL, representing the gold standard examination for the differential diagnosis.
Despite not having an immunophenotype study available, we considered the lymphocyte count of 26% found in the BAL suggestive of immune-mediated lung injury, confirming our hypothesis. However, no general consensus exists so far neither for a definite percentage of lymphocyte nor for a specific T cell population pattern in the diagnostic work-up of immune-mediated pneumonitis, which remains primarily a diagnosis of exclusion.22 23
Intriguingly, in this case the blood levels of IL-6 were within the normal range soon after the screening and remained low even when enlargement of GGOs was observed. IL-6 elevation, which often characterizes the acute phase of SARS-CoV-2 infection and is detected occasionally in other form of ir-AEs such as dermatitis and myocarditis, seems not to be a key mediator of ICIs-related pneumonia.24 25
After all microbiological results came negative and malignant lymphangitis was excluded, we deemed appropriate continuing ICI-treatment. Our choice could be debatable, due to the potential further worsening of GGOs. However, in our clinical experience many patients can develop asymptomatic ICIs-related interstitial radiological alterations during the course of treatment, but only very few cases progress and become symptomatic if immunotherapy is pursued. In the presence of a demonstrated disease control or for cancer types that are expected to achieve a robust clinical benefit from ICIs, continuing immunotherapy represents in our opinion an acceptable risk. Our approach is also based on the already established relationship between ir-AEs onset, response rate and survival with ICIs therapy.26 Our patient achieved a PR and GGO is still clinically asymptomatic and radiologically not progressing.
Looking ahead to the close future characterized by the forced coexistence with SARS-CoV-2 and by a concrete risk for a second wave of contagion, mixed scenarios of COVID-19 and ICIs-related pneumonia will become more and more frequent in our daily practice and clinicians should take into account a potential interplay between these two entities. Our case suggests that lung injury induced by SARS-CoV-2 infection could increase the risk of immune-mediated pneumonitis in patients receiving ICIs treatment. Our recommendation is to perform NFS and chest CT scan in all patients before starting ICIs or any other form of experimental immunotherapy.27 For the subgroup of patients recovered from COVID-19 but with radiological alteration still under resolution, physician should keep in mind the possibility of a recall ICIs-related pneumonia. This awareness could make easier the interpretation of dynamic changes in radiological pictures during immunotherapy and faster the work-up to rule out the main differential diagnoses. We believe that all these measures are important to accelerate the resumption of ICIs in patients potentially achieving durable benefit from immunotherapy. Finally, based on our clinical experience with the use of ICIs and the well-recognized correlation between ir-AEs and long-term disease control, in case of asymptomatic ICIs-related interstitial pneumonia we recommend a watchful-waiting approach and the prosecution of immunotherapy.26
Waiting for a mass vaccination or the advent of disease-modifying antiviral agents, we feel that sharing experience still remains the stronger weapon for a better management of patients with cancer during this pandemic.
Acknowledgments
The authors thank the patient and his family for entrusting them with his care.
Footnotes
Twitter: @AngeloDipa_
Correction notice: This paper has been updated to amend author affiliations.
Contributors: AD and MS analyzed and interpreted the patient case, wrote and edited the manuscript. DR provided the imaging figures. PP, EL and AS reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–91. 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218–23. 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801. 10.1038/s41467-020-17670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2:1607–16. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 5. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 2018;13:1930–9. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 6. Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer 2018;124:271–7. 10.1002/cncr.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–80. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 8. Zhu Y, Liu Y-L, Li Z-P, et al. Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19). J Infect 2020:30104–3. 10.1016/j.jinf.2020.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 2020;153:725–33. 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Porcu M, De Silva P, Solinas C, et al. Immunotherapy associated pulmonary toxicity: biology behind clinical and radiological features. Cancers 2019;11:305. 10.3390/cancers11030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russano M, Citarella F, Napolitano A, et al. COVID-19 pneumonia and immune-related pneumonitis: critical issues on differential diagnosis, potential interactions, and management. Expert Opin Biol Ther 2020;20:959–64. 10.1080/14712598.2020.1789097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gambichler T, Reuther J, Scheel CH, et al. On the use of immune checkpoint inhibitors in patients with viral infections including COVID-19. J Immunother Cancer 2020;8:e001145. 10.1136/jitc-2020-001145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26. 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo J, Rizvi H, Egger JV, et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov 2020;10:1121–8. 10.1158/2159-8290.CD-20-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-Definition and evaluation. Radiology 2020;296:E97–104. 10.1148/radiol.2020201473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9:847–55. 10.1111/1759-7714.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoenfeld JD, Nishino M, Severgnini M, et al. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features. J Immunother Cancer 2019;7:112. 10.1186/s40425-019-0583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manapov F, Roengvoraphoj O, Dantes M, et al. Pneumonitis in irradiated lungs after nivolumab: a brief communication and review of the literature. J Immunother 2018;41:96-99. 10.1097/CJI.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 19. Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun 2020;3:100051. 10.1016/j.jtauto.2020.100051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018;18:153–67. 10.1038/nri.2017.108 [DOI] [PubMed] [Google Scholar]
- 21. Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 24. Crisafulli S, Isgrò V, La Corte L, et al. Potential Role of Anti-interleukin (IL)-6 Drugs in the Treatment of COVID-19: Rationale, Clinical Evidence and Risks. BioDrugs 2020;34:415–22. 10.1007/s40259-020-00430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esfahani K, Elkrief A, Calabrese C, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol 2020;17:504–15. 10.1038/s41571-020-0352-8 [DOI] [PubMed] [Google Scholar]
- 26. Petrelli F, Grizzi G, Ghidini M, et al. Immune-Related adverse events and survival in solid tumors treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother 2020;43:1–7. 10.1097/CJI.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 27. Dipasquale A, Persico P, Lorenzi E, et al. Conducting phase I trials during the SARS-Coronavirus-2 outbreak: about science and care. Front Oncol 2020;10:926. 10.3389/fonc.2020.00926 [DOI] [PMC free article] [PubMed] [Google Scholar]