Abstract
COVID-19 mainly causes pulmonary disease. Involvement of gastrointestinal and hepatobiliary systems, among other systems, has been reported. We report a case of acute pancreatitis in a patient with resolving COVID-19 pneumonia. History taking and investigations excluded other causes of pancreatitis. This case demonstrates the possibility of pancreatic injury in patients with COVID-19, in line with previously reported similar cases. We believe that it is imperative to screen patients presenting with acute pancreatitis for SARS-CoV-2. It is also important to take into consideration that patients with a complicated course who require an invasive procedure such as drainage might pose a risk of transmission to the operating surgeon or interventionist.
Keywords: pancreatitis, general surgery, pneumonia (infectious disease)
Background
COVID-19, caused by SARS-CoV-2, mainly causes pulmonary disease. It emerged and was first identified in Wuhan, China in hospitalised patients with pneumonia of unknown origin between December 2019 and January 2020. However, extrapulmonary manifestations affecting gastrointestinal and hepatobiliary systems, among other systems, have been reported. Patients can present with symptoms including anorexia, nausea and/or vomiting, diarrhoea and abdominal pain.1 2 We report a case of acute pancreatitis in a patient with COVID-19 pneumonia without any other known causes.
Case presentation
This patient is a 52-year-old woman with a medical history of type 2 diabetes mellitus, hypertension, hypothyroidism and morbid obesity. The patient developed symptoms of fever, dry cough and shortness of breath over a duration of 1 week and tested positive for SARS-CoV-2 through a nasopharyngeal swab, detected by real-time reverse transcription PCR, with a cycle threshold value of 24. The patient denied abdominal pain on initial presentation. Contact with a positive case was reported. The patient was admitted to an isolation facility as per national protocols for mildly symptomatic patients who are not eligible for home isolation.3
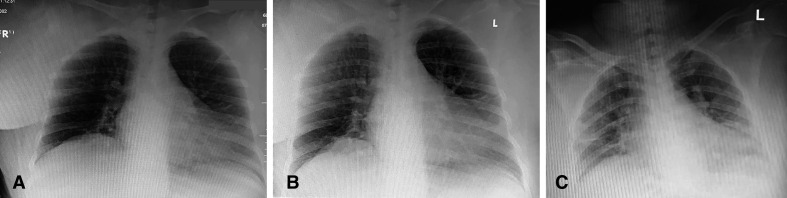
On the sixth day, she developed worsening of cough and shortness of breath with hypoxia and was hence transferred to a treatment facility designated for cases with moderate pneumonia. Chest radiograph showed bilateral infiltrates and so she was treated as COVID-19 pneumonia as per protocol (figure 1A–C).3 The patient was started on a steroid course for a total of 15 days (including dexamethasone and methylprednisolone), ceftriaxone, doxycycline, azithromycin, enoxaparin, vitamin D, zinc, fluticasone furoate/vilanterol, salbutamol, ipratropium bromide, pantoprazole, omeprazole, metoclopramide and paracetamol, in addition to her regular home medications (table 1). Proning and incentive spirometry were employed as well. The patient required oxygen through nasal cannula and face mask.
Figure 1.
(A–C) Chest radiographs of the patient on days 1, 3 and 6 of the disease.
Table 1.
Summary of received medications
| Medication | Dose and route of administration | Duration |
| Dexamethasone | 6 mg intravenously once daily | 2 days |
| Methylprednisolone | 40–60 mg intravenously every 8 hours | 8 days |
| Dexamethasone (following discontinuation of methylprednisolone) | 6 mg per oral once daily | 5 days |
| Ceftriaxone | 2000 mg intravenously once daily | 8 days |
| Doxycycline | 100 mg per oral two times per day | 2 days |
| Azithromycin | 500 mg per oral once daily | 5 days |
| Enoxaparin | 4000–12 000 IU subcutaneously two times per day | Throughout hospital stay |
| Cholecalciferol | 50 000 IU per oral once weekly | |
| Zinc gluconate | 50 mg per oral once daily | |
| Fluticasone furoate/vilanterol | 1 puff once daily | From second day of admission onwards |
| Nebulised salbutamol | 5 mg every 8 hours | |
| Nebulised ipratropium bromide | 250 μg every 8 hours | |
| Pantoprazole | 20 mg per oral once daily | According to permit of oral intake and drug availability |
| Omeprazole | 20 mg intravenously two times per day/20 mg per oral two times per day | |
| Metoclopramide | 10 mg intravenously every 4 hours as needed | Limited use |
| Paracetamol | 1000 mg per oral or intravenously every 6 hours as needed |
μg, micrograms; IU, international units; mg, milligrams.
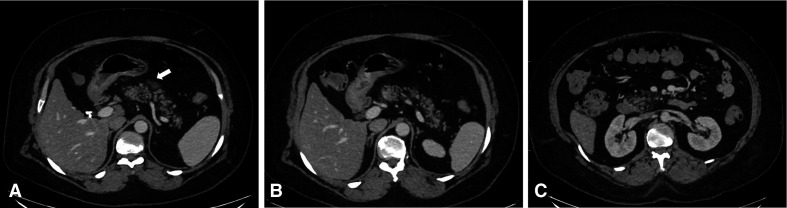
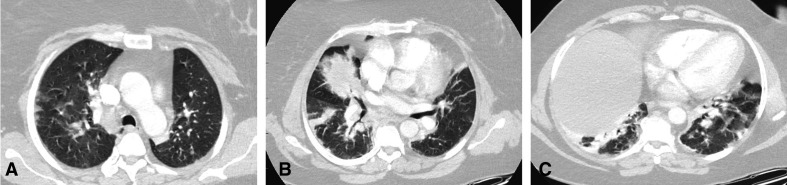
As the pneumonia was resolving and the patient was being prepared to be discharged, she developed abdominal pain. At that time, she had already tested negative for SARS-CoV-2 on two nasopharyngeal swabs obtained as exit swabs on days 17 and 18 from the initial swab. CT scan of the abdomen was done and showed an atrophic pancreas with diffuse fatty infiltration, minimal peripancreatic fat stranding and fluid, and reactive lymph nodes. The imaging findings were suggestive of early acute pancreatitis without necrosis, pseudocyst nor gas formation (figure 2). COVID-19 pneumonia changes were evident in the lower cuts of the chest in the form of bilateral ground-glass consolidation, mainly peripherally (figure 3A–C).
Figure 2.
Axial cuts of intravenous contrast-enhanced CT of the abdomen at the level of the pancreas. Mild fatty infiltration of the pancreatic parenchyma is noted with mild diffuse peripancreatic fat stranding and focal single small volume fluid collection anterior to the neck of the pancreas (arrow). No necrosis, calcification or haemorrhage. According to Balthazar score, the CT severity index is 3. This score is consistent with mild acute pancreatitis.
Figure 3.
(A) Chest CT scan (lung window) showing bilateral peripheral areas of ground-glass opacity, changes during the peak stage of known COVID-19 infection. (B, C) Chest CT scan (lung window) showing massive consolidation in the posterior parts of the lower lung lobes, changes of peak stage of known COVID-19 infection.
General surgery consultation was sought. On obtaining further history, the patient reported that the pain has been present for at least 1 week, treated initially as gastritis. However, it was gradually increasing in severity. The pain was mainly epigastric and affecting the right upper quadrant, burning in nature, often radiating to the back and was aggravated by oral intake. It was accompanied by nausea and vomiting. The patient denied any previous similar episodes. She also denied alcohol intake. Her surgical history was significant for a laparoscopic cholecystectomy performed 10 years earlier for gallstone disease and a hysterectomy 5 months earlier for uterine fibroids.
Investigations
Laboratory investigations done on consulting general surgery are summarised in table 2 including those done to rule out the causes of acute pancreatitis. Liver function tests revealed a mild increase in gamma-glutamyl transferase (GGT) and alanine aminotransferase and were otherwise within normal limits. Lipase is not available in our laboratory and hence was not done. Inflammatory markers were generally improving.
Table 2.
Summary of laboratory investigations
| Laboratory investigation | Results | Laboratory reference range |
| White cell count, ×109/L | 19 | 3.9–9.6 |
| Serum amylase, U/L | 47 | 30–118 |
| Urinary amylase, U/L | 219 | <650 |
| Arterial blood gas on room air | ||
| pH | 7.472 | 7.35–7.45 |
| Partial pressure of carbon dioxide, mm Hg | 34.9 | 35–45 |
| Partial pressure of oxygen, mm Hg | 70.8 | 80–100 |
| Oxygen saturation, % | 95.3 | 95–100 |
| Bicarbonate (HCO3−), mmol/L | 26.3 | 22–28 |
| Lactic acid, mmol/L | 2.9 | 0.2–1.8 |
| Calcium, mmol/L | 2.38 | 2.15–2.50 |
| Total bilirubin, μmol/L | 4 | 5–21 |
| Gamma-glutamyl transferase, U/L | 102 | 5–55 |
| Alanine aminotransferase, U/L | 37 | <33 |
| Triglyceride (initial), mmol/L | 3.4 | 0.2–1.8 |
| Triglyceride (1 day later), mmol/L | 1.9 | |
| Parathyroid hormone, pmol/L | 7.5 | 1.96–9.33 |
| IgG, g/L | 9.27 | 5.4–16.5 |
| C reactive protein, mg/L | 1.09 | 0–3 |
Differential diagnosis
Initially, we thought that pancreatitis was caused by hypertriglyceridaemia; however, lipid profile was repeated and triglyceride level was almost normal. The patient did not undergo any invasive procedure, namely endoscopic retrograde cholangiopancreatography nor recent surgery. No previous similar episodes to suggest chronic nature or genetic predisposition were reported. No recent trauma, exposure to envenomation nor ischaemic insult was reported. Anatomical pancreatic anomalies were not evident on imaging studies. Personal history was negative for pancreatic cancer. Moreover, during her hospital course, our patient received multiple drugs that have been associated with pancreatitis, so drug-induced acute pancreatitis is possible (table 1). Nevertheless, with a multitude of cases published of patients with COVID-19 developing pancreatitis, an association is possible.
Treatment
The patient was transferred to a regular surgical ward for further management. From a surgical point of view, she was started on conservative management including intravenous fluids and bowel rest. Oral intake was resumed gradually as tolerated. The patient was maintaining adequate oxygen saturation on room air. However, her pain was not improving and a repeated abdominal CT was done around a week later. It showed a localised fluid density at the level of the body of the pancreas anteriorly, measuring 3.1×1.5 cm, which was faintly present on the prior scan. On improvement of pain, the patient was discharged home.
Outcome and follow-up
The patient stayed for a total of 23 days in the hospital, of which 9 days were under the care of general surgery. The patient was seen in the general surgery outpatient clinic 10 days following discharge on a follow-up visit and was doing well.
Discussion
Acute pancreatitis is an inflammatory condition of the exocrine pancreas, caused most commonly by gallstones and alcohol consumption. Acinar cell injury and impaired zymogen secretion leading to intrapancreatic protease activation underlie this disease. Diagnosis requires two out of three criteria: typical history, elevated serum amylase or lipase more than three times the upper limit of normal for the laboratory reference range and suggestive imaging findings.4
Several viruses have been implicated in the aetiology of acute pancreatitis. These include cytomegalovirus, Epstein-Barr virus, hepatitis A–E viruses, herpes simplex virus, varicella zoster virus, mumps, measles and coxsackie virus, among others. The exact mechanism by which viruses cause pancreatitis is unknown and each virus might cause pancreatitis through a different mechanism. These mechanisms include viral replication in pancreatic acinar cells resulting in protease leakage and activation, in addition to cholangiopathy and ampullary oedema.5
COVID-19 is caused by SARS-CoV-2. SARS-CoV-2 enters the host cells via its spike (S) protein which binds to angiotensin-converting enzyme 2 (ACE2). In the initial phase of the disease, infection of the upper respiratory tract ACE2-expressing nasal epithelial cells occurs in asymptomatic individuals. Later in the disease course, infection of lower respiratory tract ACE2-expressing cells causes pneumonitis.6 The virus mainly causes pulmonary disease; however, extrapulmonary manifestations affecting gastrointestinal and hepatobiliary systems, among other systems, have been reported. Pathophysiology is likely multifactorial. Mechanisms suggested include direct tissue damage, inflammation-mediated damage and microvascular injury as observed in the small bowel.1 Moreover, in a study by Liu et al,7 expression of ACE2 in normal pancreases was found to be slightly higher than that of the lungs and it was expressed in both the exocrine glands and islets of the pancreas. The study shed light on the possibility of pancreatic injury in patients with COVID-19, based on the above findings and on a cohort of patients with non-severe and severe disease who exhibited features of pancreatic involvement.7 Additionally, Schepis et al8 reported the detection of SARS-CoV-2 RNA in a fluid sample obtained from a pancreatic pseudocyst in a patient with COVID-19 pneumonia and acute pancreatitis.
Several cases of pancreatitis in patients with COVID-19 were reported. Gonzalo-Voltas et al9 reported a case of pancreatitis from Spain in the absence of the most common risk factors: alcohol and gallstones.9 Lakshmanan and Malik10 also reported a case of a nursing home resident who tested positive for SARS-CoV-2 following an outbreak at his residence and developed pancreatitis a few days later. Moreover, Brikman et al11 reported a case of a patient with COVID-19 pneumonia who developed acute pancreatitis 14 days following admission. The patient did not have any history of pancreatitis, gallstones nor alcohol intake.11 Further supportive data are provided by Meireles et al12 in a report of a patient with COVID-19 pneumonia who developed acalculous pancreatitis on the 11th day of the disease in whom other viral causes have been ruled out by laboratory investigations.12 Additional similar cases were reported by Aloysius et al,13 Kataria et al,14 Mazrouei et al,15 Karimzadeh et al,16 Hadi et al,17 Kumaran et al18 and Wang et al.19
Reports from Wuhan, China provide great insight into this possible association. Among 52 patients with COVID-19 pneumonia, 9 (17%) were found to have pancreatic injury. Patients with pancreatic injury were noted to have a more severe illness on admission. They were more likely to have loss of appetite and diarrhoea. With regard to laboratory values, they were noted to have higher levels of aspartate aminotransferase, GGT, creatinine, lactate dehydrogenase and erythrocyte sedimentation rate.20 Interesting findings are provided by Szatmary et al21 on the characteristics of patients with COVID-19 and pancreatitis. They suggest that a combination of male sex, abdominal pain, pancreaticoduodenal inflammation with steatosis on imaging and metabolic stress (indicated by elevation of glucose and triglyceride) represents a distinct subset of patients in whom the endocrine pancreas is particularly vulnerable.21 Moreover, a paediatric case has also been reported. The child presented with multisystem inflammatory syndrome, with acute pancreatitis being the initial manifestation of this syndrome.22
During her hospital course, our patient received multiple drugs that have been associated with pancreatitis (table 1). These include dexamethasone, omeprazole, paracetamol, ceftriaxone and doxycycline. Accordingly, drug-induced pancreatitis can still be considered.23
Linking acute pancreatitis to COVID-19 requires further investigation to establish an association. Acute pancreatitis is a common disease and can often be idiopathic with no identifiable aetiology. Besides, many patients with COVID-19 who are considered contagious might have not been investigated thoroughly with tests such as endoscopic ultrasonography so some causes might have been missed. It should also be taken into consideration that many reported cases have had heterogenous results, making it difficult to draw conclusions. Nevertheless, pancreatitis seems to be an uncommon complication of COVID-19.24 A study from the USA revealed a point prevalence of 0.27% of acute pancreatitis in hospitalised patients with COVID-19.25 Another study from Spain reported a frequency of 0.07%.26 Further studies are needed to evaluate the incidence compared with that of pre-COVID-19 times.24
What does this mean to us as surgeons? The pancreas can be involved in COVID-19 infection. However, acute pancreatitis should be diagnosed based on the standard criteria as elevation of pancreatic enzymes is also seen in COVID-19 infection without pancreatitis. Patients with a complicated course who require an invasive procedure such as drainage might pose a risk of transmission to the operating surgeon or interventionist so precautionary measures must be taken.27
To sum up, in our patient, routine investigations were carried out to determine the cause of pancreatitis and many causes were ruled out. Although causality cannot be established through this case report only, we believe that COVID-19-induced pancreatitis is still to be considered, in line with the above case reports. Further data are required to determine the impact of this virus on the pancreas and the underlying pathophysiology.
Learning points.
Several viruses have been implicated in the aetiology of acute pancreatitis.
The exact mechanism by which viruses cause pancreatitis is unknown.
The pancreas can be involved in COVID-19 infection.
It is imperative to screen patients presenting with acute pancreatitis for SARS-CoV-2.
Further studies are required to determine the association between pancreatitis and COVID-19.
Footnotes
Contributors: RARA: manuscript preparation. TF: revision of manuscript. JSA: preparation of images and captions. KA: primary consultant and revision of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahrain COVID-19 national protocols, 2020. Available: https://www.nhra.bh/Media/Announcement/CovidAlert.aspx [Accessed 30 Sep 2020].
- 4.Goodchild G, Chouhan M, Johnson GJ. Practical guide to the management of acute pancreatitis. Frontline Gastroenterol 2019;10:292–9. 10.1136/flgastro-2018-101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons-Linares CR, Imam Z, Chahal P. Viral-Attributed acute pancreatitis: a systematic review. Dig Dis Sci 2020;143. 10.1007/s10620-020-06531-9. [Epub ahead of print: 12 Aug 2020]. [DOI] [PubMed] [Google Scholar]
- 6.Matheson NJ, Lehner PJ. How does SARS-CoV-2 cause COVID-19? Science 2020;369:510–1. 10.1126/science.abc6156 [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Long X, Zhang B, et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020;18:2128–30. 10.1016/j.cgh.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schepis T, Larghi A, Papa A, et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology 2020;20:1011–2. 10.1016/j.pan.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalo-Voltas A, Fernández-Pérez-Torres CU, Baena-Díez JM. [Acute pancreatitis in a patient with COVID-19 infection]. Med Clin 2020;155:183–4. 10.1016/j.medcli.2020.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshmanan S, Malik A. Acute pancreatitis in mild COVID-19 infection. Cureus 2020;12:e9886. 10.7759/cureus.9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brikman S, Denysova V, Menzal H, et al. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ 2020;192:E858–9. 10.1503/cmaj.201029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meireles PA, Bessa F, Gaspar P, et al. Acalculous acute pancreatitis in a COVID-19 patient. Eur J Case Rep Intern Med 2020;7:001710. 10.12890/2020_001710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aloysius MM, Thatti A, Gupta A, et al. COVID-19 presenting as acute pancreatitis. Pancreatology 2020;20:1026–7. 10.1016/j.pan.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kataria S, Sharif A, Ur Rehman A, et al. COVID-19 induced acute pancreatitis: a case report and literature review. Cureus 2020;12:e9169. 10.7759/cureus.9169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazrouei SSA, Saeed GA, Al Helali AA. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep 2020;15:1601–3. 10.1016/j.radcr.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karimzadeh S, Manzuri A, Ebrahimi M, et al. COVID-19 presenting as acute pancreatitis: lessons from a patient in Iran. Pancreatology 2020;20:1024–5. 10.1016/j.pan.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadi A, Werge M, Kristiansen KT, et al. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology 2020;20:665–7. 10.1016/j.pan.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep 2020;13:e237903. 10.1136/bcr-2020-237903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Luo J, Tan F, et al. Acute pancreatitis as the initial manifestation in 2 cases of COVID-19 in Wuhan, China. Open Forum Infect Dis 2020;7:ofaa324. 10.1093/ofid/ofaa324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Wang H, Fan J, et al. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology 2020;159:367–70. 10.1053/j.gastro.2020.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szatmary P, Arora A, Thomas Raraty MG, et al. Emerging phenotype of severe acute respiratory syndrome-coronavirus 2-associated pancreatitis. Gastroenterology 2020;159:1551–4. 10.1053/j.gastro.2020.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens JP, Brownell JN, Freeman AJ, et al. COVID-19-Associated multisystem inflammatory syndrome in children presenting as acute pancreatitis. J Pediatr Gastroenterol Nutr 2020;71:669–71. 10.1097/MPG.0000000000002860 [DOI] [PubMed] [Google Scholar]
- 23.Simons-Linares CR, Elkhouly MA, Salazar MJ. Drug-Induced acute pancreatitis in adults: an update. Pancreas 2019;48:1263–73. 10.1097/MPA.0000000000001428 [DOI] [PubMed] [Google Scholar]
- 24.de-Madaria E, Capurso G. COVID-19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol 2021;18:3–4. 10.1038/s41575-020-00389-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inamdar S, Benias PC, Liu Y, et al. Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology 2020;159:2226–8. 10.1053/j.gastro.2020.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miró Òscar, Llorens P, Jiménez S, et al. Frequency of five unusual presentations in patients with COVID-19: results of the UMC-19-S1. Epidemiol Infect 2020;148:e189. 10.1017/S0950268820001910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta V COVID-19 and acute pancreatitis: what do surgeons need to know? Indian J Surg 2020:301–4. 10.1007/s12262-020-02447-w [DOI] [PMC free article] [PubMed] [Google Scholar]