Abstract
Introduction:
Reactive carbonyl species including methylglyoxal (MGO) are oxidation metabolites of glucose and precursors of advanced glycation end products (AGEs). They are important mediators of cellular oxidative stress and exacerbate skin complications. Published data supports that certain phenolic compounds can exert cellular protective effects by their antioxidant activity. A phenolic-enriched maple syrup extract (MSX) was previously reported to show protective effects against AGEs- and MGO-induced cytotoxicity in human colon cells but its skin protective effects remain unknown.
Objective:
The protective effects of MSX were evaluated against hydrogen peroxide (H2O2)- and MGO-induced cytotoxicity in human keratinocytes (HaCaT cells).
Methods:
Cellular viability and antioxidant activity were evaluated by the luminescent cell viability CellTiter-Glo® assay and the reactive oxygen species (ROS) assay, respectively. A single-cell gel electrophoresis (Comet assay) was used to measure the strand breaks in the DNA of HaCaT cells.
Results:
MSX (at 50 μg/mL) ameliorated H2O2- and MGO-induced cytotoxicity by increasing cell viability by 21.5 and 25.9%, respectively. MSX reduced H2O2- and MGO-induced ROS production by 69.4 and 56.6%, respectively. MSX also reduced MGO-induced DNA damage by 47.5%.
Conclusion:
MSX showed protective effects against H2O2- and MGO-induced cytotoxicity in HaCaT cells supporting its potential for dermatological and/or cosmeceutical applications.
Keywords: Maple syrup extract, phenolics, methylglyoxal, keratinocytes, antioxidant, DNA integrity
1. INTRODUCTION
Skin aging and skin complications associated with many systemic disorders such as diabetes are linked to the accumulation of a group of proteins known as advanced glycation endproducts (AGEs) (Ahmed, 2005; Gkogkolou & Böhm, 2012). The formation of AGEs is a non-enzymatic process in which reducing sugar molecules e.g. glucose, interact with free amino acids of biological macromolecules including protein, DNA, and lipids to form covalent-bond Maillard reaction products. This Maillard reaction leads to the generation of a group of unstable compounds known as Schiff bases (aldehyde- or ketone-like chemicals in which the carbonyl moiety is replaced by an imine or azomethine group), which can further undergo a series of chemical rearrangements to form stable keto-amine adducts known as Amadori products. Both Schiff bases and Amadori products react with amino acids of proteins to form cross-linked protein complexes and undergo multi-step reactions including oxidation, dehydration, and polymerization to form late stage AGEs. During the formation of AGEs, glucose and Amadori products are oxidized to generate highly reactive dicarbonyl chemicals including 3-deoxyglucosone (3-DG), glyoxal (GO), and methylglyoxal (MGO) (Desai & Wu, 2007). These reactive dicarbonyl species (RCS) are regarded as metabolites of sugar molecules and are known to exacerbate the formation of AGEs. As the precursors of AGEs, RCS can induce intracellular oxidative stress and cause damage to human dermal cells including fibroblasts and keratinocytes, which can further lead to accelerated skin aging (Roberts, Wondrak, Laurean, Jacobson, & Jacobson, 2003). Therefore, AGEs inhibitors have immense potential for the management of skin complications including those associated with diabetes mellitus (Ahmed, 2005). Considerable research efforts have been directed towards the development of AGEs inhibitors for the treatment of AGE-mediated skin complications associated with diabetes mellitus (Rahbar, Kumar Yernini, Scott, Gonzales, & Lalezari, 1999). Several synthetic chemicals, including aminoguanidine, are promising AGEs inhibitors but many have failed drug approval due to their adverse effect profile (Abdel-Rahman & Kline Bolton, 2002; Nilsson, 1999). Conversely, natural products, including several food-derived phenolic compounds (e.g. curcumin, epigallocatechin gallate, and flavonoids including kaempferol, luteolin, quercetin, naringenin, and rutin) are generally regarded as safe and have been reported to inhibit AGEs formation (Wu, Huang, Lin, & Yen, 2011; Wu & Yen, 2005). In addition, a growing body of data suggests that these phenolic AGE-inhibitors can exert protective effects on keratinocytes by reducing cellular oxidative stress (Babu, Sabitha, & Shyamaladevi, 2006; Huang et al., 2007).
Our laboratory has initiated a research program focused on the identification of AGE inhibitors from several medicinal plants and functional foods (foods that provide health benefits in addition to macro- and micronutrients) (Liu et al., 2014, 2017; Liu et al., 2016; Ma et al., 2016; Ma et al., 2018, 2015; Sun et al., 2016; Zhang, Ma, Liu, Yuan, & Seeram, 2015). Among these natural products, we reported that phenolic-enriched extracts of pomegranate fruit (Punica granatum) and red maple leaves (Acer rubrum) showed potent inhibitory effects against AGEs formation and protected human keratinocytes against oxidative stress induced cytotoxicity (Liu et al., 2014; Ma et al., 2016; Liu et al., 2019). In addition, we also reported that a polyphenol-enriched maple syrup extract (MSX) showed inhibitory activity against AGEs formation and protective effects against AGEs- and MGO-induced cytotoxicity in normal human colon cells (Liu et al., 2017). MSX has also been reported to show a diverse range of biological activities including antioxidant, anti-diabetic, anti-inflammatory and anti-neuroinflammatory effects (Liu et al., 2017; Ma et al., 2016; Nahar, Driscoll, Li, Slitt, & Seeram, 2014; Zhang et al., 2014) but its skin protective effects in human keratinocytes remain unknown. Herein, we evaluated the protective effects of MSX against hydrogen peroxide (H2O2)- and MGO-induced cytotoxicity, as well as its antioxidant and cellular DNA protection activities in human keratinocytes (HaCaT cells).
2. MATERIALS AND METHODS
2.1. Materials
A standardized food grade phenolic-enriched maple syrup extract (MSX), which contains over 90% of phenolic compounds (determined by High-performance liquid chromatography, liquid chromatography-mass spectrometry, and nuclear magnetic resonance methods), was prepared in our laboratory as previously reported (Li & Seeram, 2010, 2011; Liu, Ma, & Seeram, 2016; Zhang et al., 2014). Our previous phytochemical characterization studies of MSX led to the isolation and identification of several phenolic sub-classes including lignans, gallic acid derivatives and other phenolic acids, coumarins, and stilbenes (Zhang et al., 2014). However, lignans are the major type of phenolics present in MSX. Other minor constituents in MSX include ash (ca. 2.21%), fiber (ca. 11%), minerals (ca. 788.6 mg/100g), amino acids (ca. 31.7 mg/100g), organic acids (ca. 796.9 mg/100g), and vitamins (ca. 16670.7 mg/100g) (Zhang et al., 2014). Methylglyoxal (MGO), hydrogen peroxide (H2O2), crystal violet staining agent, trypsin solution, dimethyl sulfoxide (DMSO), and 2’,7’-dichlorofluorescin diacetate (DCFDA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Ground Island, NY, USA). A luminescent cell viability CellTiter-Glo® (CTG 2.0 assay) kit was purchased from Promega (Fitchburg, WI, USA). Comet assay kit was purchased from TREVIGEN (Gaithersburg, MD, USA).
2.2. Cell culture and cell viability
Human keratinocytes (HaCaT cells) were obtained from American Type Culture Collection (Manassas, VA, USA). HaCaT cells were maintained in DMEM supplemented with 5% FBS at 37 °C under an atmosphere of 5% CO2. Cellular viability was assessed using the luminescent cell viability CellTiter-Glo® (CTG 2.0 assay). Briefly, HaCaT cells were seeded at 5×104 cells/mL to 50–60% confluency in a 96-well microplate. HaCaT cells were exposed to the test compounds at different concentrations (6.25 – 100 μg/mL) for 24 h. Then the CTG 2.0 agent was added in a 1:1 ratio with existing media and mixed for 5 min on an orbital shaker prior to luminescence measurement using a plate reader (Spectramax M2, Molecular Devices, Sunnyvale, CA, USA).
2.3. Effect of MSX on H2O2 or MGO induced cell toxicity
HaCaT cells were seeded at 5×104 cells/mL to 60% confluency in a 96-well microplate. After 12 h incubation, cells were pre-treated with different concentrations of MSX (12.5, 25, and 50 μg/mL) for 2 h. Cells were washed with PBS twice and treated with H2O2 (400 μM) or MGO (400 μM) and then cell viability was determined using the CTG 2.0 assay.
2.5. Crystal violet staining
Crystal violet staining solution was prepared by dissolving 5 g of crystal violet powder into 100 mL 20% aqueous ethanol. HaCaT cells seeded at 25×104 cells/mL to 80% confluency in 6-well plates. Cells were fixed with 75% ethanol for 15 min at room temperature and incubated with staining solution for 10 min, followed by washing with PBS for 5 times. Images were captured with a fluorescence microscope cells imaging system (EVOS; Invitrogen, Waltham, MA, USA).
2.6. Reactive oxygen species (ROS) assay
HaCaT cells were seeded at at 5×104 cells/mL to 50–60% confluency in a 96-well microplate. After 12 h incubation, cells were pre-treated with MSX (12.5, 25, and 50 μg/mL) for 2 h and medium was replaced with fresh medium containing DCFDA (20 μM). Then cells were treated with H2O2 (400 μM) or MGO (400 μM) for 24 h. The fluorescence signals were read at excitation and emission wavelengths of 485 and 525 nm, respectively, using a plate reader (Spectra Max M2 spectrometer, Molecular Devices, Sunnyvale, CA, USA).
2.7. Comet assay
Comet assay, a single-cell gel electrophoresis that is used to measure the strand breaks in the DNA of HaCaT cells, was performed according to the instructions of the manufacturers (Trevigen, Gaithersburg, MD, USA). Briefly, HaCaT cells were seeded at about 5×104 cells/mL to yield 70–90% confluence in a 6-well plate. Cells were pre-treated with MSX (50 μg/mL) for 2 h and then washed with PBS twice followed by treatment of H2O2 (400 μM) or MGO (400 μM) for 24h. Cells were then collected and combined with melted LMAgarose (a 1% low melting agarose in PBS that is designed for the Comet assay) at a ratio of 1:10 (v/v). Then the respective suspensions were transferred onto comet slides and incubated with cell lysis at 4 °C for 12 h. The slides were immersed into alkaline unwinding solution for 20 min and subjected to electrophoresis in an alkaline electrophoresis solution at 21 volts for 30 min. Slides were fixed in 75% ethanol and stained in diluted SYBR GOLD solution (1:3000). SYBR GOLD is a cyanine dye that exhibits higher binding affinity to nucleic acids including double- or single-stranded DNA. Comet images were captured with EVOS fluorescence microscope and the percentage of tailed cell DNA were analyzed with CASP software program (Końca et al., 1981).
2.8. Statistical analysis
All data was expressed as the mean ± the standard error of the mean (S.E.M.). The significance of differences was determined using a two-way analysis of variance (ANOVA) followed by a post hoc Student-Newman–Keuls multiple comparison test (SNK). A threshold of p value < 0.05 was considered the cut-off for statistical significance of results.
3. RESULTS AND DISCUSSION
3.1. MSX ameliorates H2O2- and MGO-induced cytotoxicity
Reactive carbonyl species exacerbate cellular oxidative stress and suppress cell viability (Desai & Wu, 2007). Therefore, we first evaluated whether MSX can reduce H2O2- and MGO-induced toxicity in HaCaT cells. As shown in Figure 1 A, MSX at concentrations ranging from 6.25 – 100 μg/mL did not affect cell viability of HaCaT cells (viability > 98.0%), and concentrations of 12.5, 25, and 50 μg/mL were selected for further evaluations. Both H2O2 and MGO (at 400 μM) significantly induced cytotoxicity by reducing cell viability by 59.2 and 61.7%, respectively. Treatment of MSX (12.5, 25, and 50 μg/mL) increased the viability of cells exposed to H2O2 by 11.0, 14.1, and 21.5%, respectively, as compared to the model (H2O2-treated) group (Figure 1 B). MSX also reduced MGO-induced cytotoxicity by increasing cell viability by 25.9% at concentration of 50 μg/mL (Figure 1 C). This protective effect was further supported by data obtained from crystal violet staining assay. Treatment of MSX maintained the normal shape of cell nuclei, as compared to H2O2 and MGO challenged cells, which had irregular shaped nuclei (Figure 1 D). This finding is in agreement with our previously reported study showing that phenolics from a commercially available standardized pomegranate fruit extract (Pomella®) attenuated H2O2-induced cytotoxicity in HaCaT cells (Liu et al., 2019).
FIGURE 1.
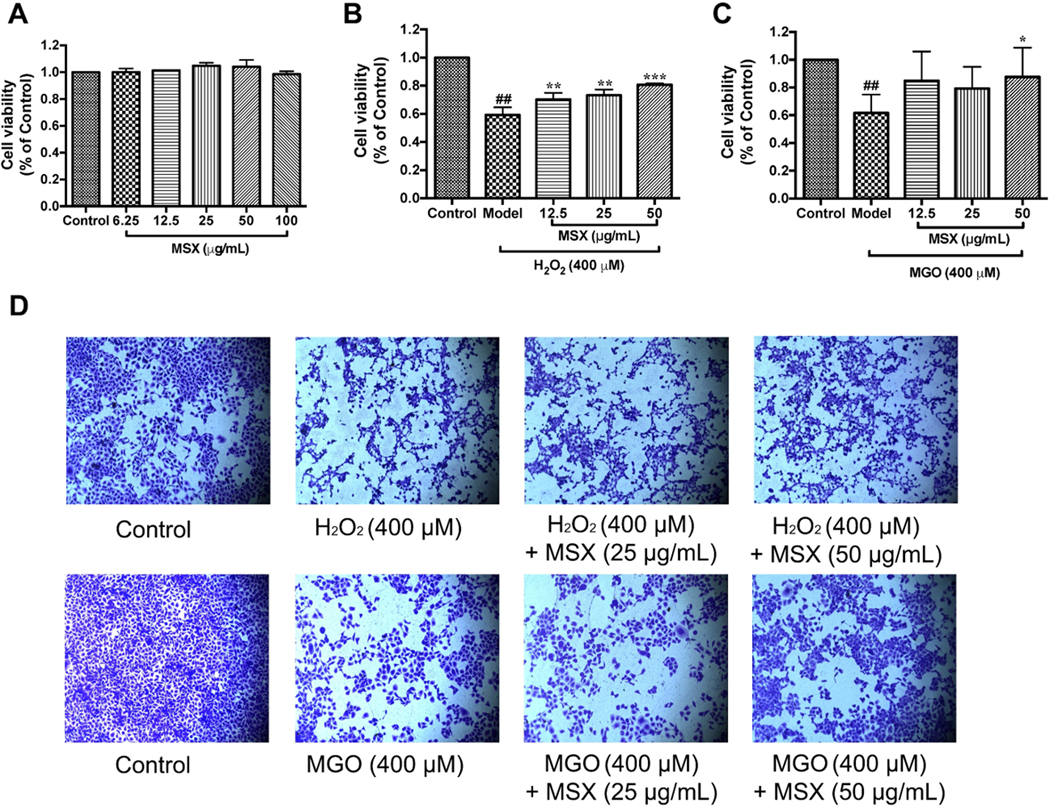
Effects of MSX on the cell viability of H2O2 and MGO challenged HaCaT cells. MSX (6.25-100 μg/mL) were nontoxic to HaCaT cells, A. HaCaT cells were pretreated with MSX (12.5, 25, and 50 μg/mL) for 2 hours, then treated with H2O2 (400 μM; B), or MGO (400 μM, C). Representative images of cells stained with crystal violet reagent. HaCaT cells were pretreated with MSX and then exposed to H2O2 or MGO, D. ##Compared to control P < .01; *compared to model P < .05, **Compared to model P < .01, ***compared to model P < .001
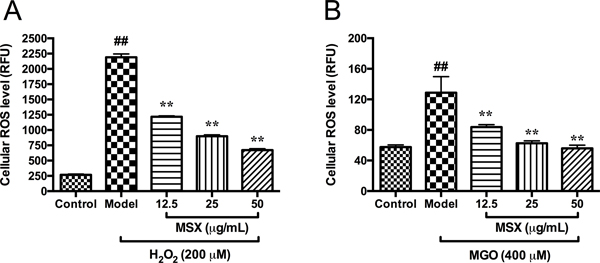
3.2. MSX reduces H2O2- and MGO-induced cellular reactive oxygen species (ROS)
Hydrogen peroxide (H2O2) and MGO induce cytotoxicity in HaCaT cells by mediating the production of cellular reactive oxygen species (ROS) (Roberts et al., 2003). Levels of ROS in HaCaT cells were elevated by 8.18- and 2.24-fold when cells were stimulated by H2O2 (200 μM) and MGO (400 μM), respectively, as compared to the control group. Treatment of MSX reduced the production of ROS in cells exposed to H2O2 and MGO in a concentration dependent manner. MSX (12.5, 25, and 50 μg/mL) reduced H2O2- and MGO-induced ROS production by 44.4, 59.0, and 69.4%, and 34.9, 51.3, and 56.6%, respectively, as compared to the model group (Figure 2). This is in agreement with our previously reported studies showing that MSX exerts cytoprotective effects by attenuating cellular ROS in normal human colon CCD-18Co cells (Liu et al., 2017) and murine microglial BV-2 cells (Ma, et al., 2016).
FIGURE 2.
Effects of MSX on the production of ROS in HaCaT cells in the DCFDA assay. HaCaT cells were pretreated with MSX (12.5, 25, and 50 μg/mL) for 2 hours and then treated with DCFDA reagent (20 μM) followed by incubation with H2O2 (200 μM) for 1 hour, A; or with MGO (400 μM) for 24 hours, B. Levels of cellular ROS were determined by measuring the fluorescent intensity of cells. ##Compared to control P < .01; **compared to model P < .01
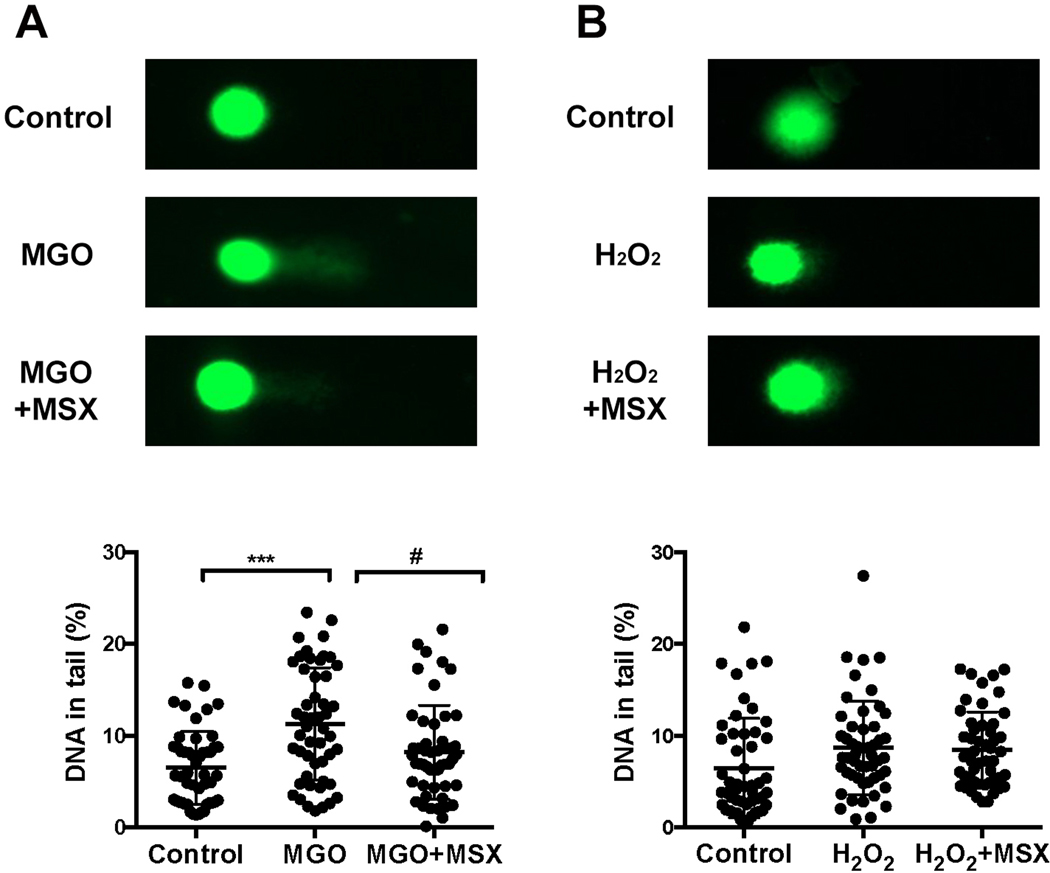
3.3. MSX maintains MGO-induced cellular DNA damage
Reported studies suggested that MGO can bind to nucleotides and induce DNA chain fracture in human skin cells (Roberts et al., 2003). The protective effects of MSX against H2O2- and MGO-induced DNA damage were evaluated in the Comet assay. As shown in Figure 3 A, MGO (400 μM) significantly increased the levels of fractured DNA chain in HaCaT cells (by 73.9%) and the treatment of MSX (50 μM) maintained the integrity of DNA structures in HaCaT cells with a lower percentage of tailed DNA (47.5%; Figure 3 A lower panel). Treatment of H2O2 (400 μM) did not result in DNA damage in HaCaT cells as shown in the comet assay (Figure 3 B), suggesting that different mechanism(s) are involved in the oxidative stress induced cell damage. This difference was in agreement with our previously reported observation where H2O2 induced cell apoptosis (Liu et al., 2019) while MGO did not show apoptotic effects in HaCaT cells (data not shown). Although several synthetic small molecules including penicillamine (Roberts et al., 2003) and N-acetylcysteine (Yang et al., 2014) are reported to show protective effects, to the best of our knowledge, this is the first report showing that a polyphenol-enriched natural product extract protects the integrity of DNA in HaCaT cells from MGO induced damage.
FIGURE 3.
Effects of MSX (50 μM) on the integrity of DNA in MGO- and H2O2-challenged HaCaT cells, A and B. Representative fluorescent images of SYBR GOLD stained comet slides captured by EVOS microscope system. The percentages of tailed DNA were measured from at least fifty randomly selected cells and analyzed with CASP software. ***Compared to control P < .001; #compared to model P < .05
TRANSLATIONAL THERAPEUTIC IMPLICATIONS OF OUR RESEARCH
A growing body of basic pharmacological research support the utilization of several natural polyphenols to combat AGEs-mediated dermatological dysfunctions and skin disorders induced by oxidative stress (Ho & Wang, 2013; Jeanmaire, Danoux, & Pauly, 2001). Several of these polyphenols are found in plant based-diets including in fruits, vegetables, grains, and plant-derived beverages (Khan, Liu, Wang, & Sun, 2020). Therefore, because of their inherent lack of toxicity and natural origin, these polyphenols show great promise for preventive and therapeutic effects against skin aging, skin damage and cutaneous toxicity, including ulcer, burns, and wounds (Działo et al., 2016). Moreover, several clinical studies support the skin protective effects of polyphenols and their dermatological applications (Gianeti, Mercurio, & Maia Campos, 2013; Mirnezami, Jafarimanesh, Rezagholizamenjany, Alimoradian, & Ranjbaran, 2020; Palmer & Kitchin, 2010). The skin protective effects of dietary polyphenols as well as their therapeutic potential for the treatment of skin disorders have been reviewed in several articles (Daniyal et al., 2019; Działo et al., 2016; Svobodová, Psotová, & Walterová, 2003; Zillich, Schweiggert-Weisz, Eisner, & Kerscher, 2015). For instance, the skin protective effects of honey, a natural sweetener, has been supported by empirical evidence and modern scientific studies (Bogdanov, Jurendic, Sieber, & Gallmann, 2008; Saranraj & Sivasakthi, 2018). Published data suggest that honey is suitable as a remedy for skin conditions including wounds and burns (Burlando & Cornara, 2013; McLoone, Warnock, & Fyfe, 2016). Apart from bioactives including amino acids, vitamins, minerals, and phenolics, some honey, for e.g. Manuka honey, has been reported to contain MGO, which partially contributes to its antimicrobial activity (Alvarez-Suarez, Gasparrini, Forbes-Hernández, Mazzoni, & Giampieri, 2014). To date, the skin protective effects of maple syrup, a plant derived natural sweetener, are unknown. Therefore, we evaluated the cytoprotective effects of a phenolic-enriched maple syrup extract (MSX) on human keratinocytes HaCaT cells against oxidative and glycative stress induced cytotoxicity. MSX at non-toxic concentrations (ranging from 6.25 – 100 μg/mL) reduced H2O2- and MGO-induced cytotoxicity and production of ROS in HaCaT cells. Results from the Comet assay showed that MSX can protect the integrity of DNA of HaCaT cells from MGO-induced DNA damage. It is possible that the protective effects of MSX in skin cells are involved with several molecular pathways, such as activation of extracellular signal-regulated kinases (ERK) 1/2 phosphorylation, which was observed in our previously reported study (Liu et al., 2017). Further studies on the underlying mechanism(s) of the skin protective effects of MSX are warranted. In summary, the findings from the current study support the protective effects of MSX in keratinocytes and its potential for dermatological and/or cosmeceutical applications.
ACKNOWLEDGMENTS
Data were acquired from instruments located in the RI-INBRE core facility (at the University of Rhode) supported by Grant P20GM103430 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Abbreviation
- MGO
methylglyoxal
- AGEs
advanced glycation end products
- MSX
maple syrup extract
- ROS
reactive oxygen species
- RCS
reactive dicarbonyl species
- FBS
fetal bovine serum
- DCFDA
2’,7’-dichlorofluorescin diacetate
- DMEM
Dulbecco’s modified Eagle’s medium
- CTG
CellTiter-Glo®
Footnotes
Conflict of interest
The authors declare no potential conflict of interest.
References
- Abdel-Rahman E, & Kline Bolton W. (2002). Pimagedine: A novel therapy for diabetic nephropathy. Expert Opinion on Investigational Drugs. 10.1517/13543784.11.4.565 [DOI] [PubMed] [Google Scholar]
- Ahmed N. (2005). Advanced glycation endproducts - Role in pathology of diabetic complications. Diabetes Research and Clinical Practice. 10.1016/j.diabres.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Alvarez-Suarez J, Gasparrini M, Forbes-Hernández T, Mazzoni L, & Giampieri F. (2014). The composition and biological activity of honey: A focus on Manuka honey. Foods. 10.3390/foods3030420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu PVA, Sabitha KE, & Shyamaladevi CS (2006). Therapeutic effect of green tea extract on advanced glycation and cross-linking of collagen in the aorta of streptozotocin diabetic rats. Clinical and Experimental Pharmacology and Physiology. 10.1111/j.1440-1681.2006.04374.x [DOI] [PubMed] [Google Scholar]
- Bogdanov S, Jurendic T, Sieber R, & Gallmann P. (2008). Honey for nutrition and health: A review. Journal of the American College of Nutrition. 10.1080/07315724.2008.10719745 [DOI] [PubMed] [Google Scholar]
- Burlando B, & Cornara L. (2013). Honey in dermatology and skin care: A review. Journal of Cosmetic Dermatology. 10.1111/jocd.12058 [DOI] [PubMed] [Google Scholar]
- Daniyal M, Akram M, Zainab R, Munir N, Shah SMA, Liu B, … Jabeen F. (2019). Progress and prospects in the management of psoriasis and developments in phyto-therapeutic modalities. Dermatologic Therapy. 10.1111/dth.12866 [DOI] [PubMed] [Google Scholar]
- Desai K, & Wu L. (2007). Methylglyoxal and advanced glycation endproducts: New therapeutic horizons? Recent Patents on Cardiovascular Drug Discovery. 10.2174/157489007780832498 [DOI] [PubMed] [Google Scholar]
- Działo M, Mierziak J, Korzun U, Preisner M, Szopa J, & Kulma A. (2016). The potential of plant phenolics in prevention and therapy of skin disorders. International Journal of Molecular Sciences. 10.3390/ijms17020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianeti MD, Mercurio DG, & Maia Campos PMBG (2013). The use of green tea extract in cosmetic formulations: Not only an antioxidant active ingredient. Dermatologic Therapy. 10.1111/j.1529-8019.2013.01552.x [DOI] [PubMed] [Google Scholar]
- Gkogkolou P, & Böhm M. (2012). Advanced glycation end products: Keyplayers in skin aging? Dermato-Endocrinology. 10.4161/derm.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CT, & Wang M. (2013). Dietary phenolics as reactive carbonyl scavengers: Potential impact on human health and mechanism of action. Journal of Traditional and Complementary Medicine. 10.4103/2225-4110.114892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Wu W. Bin, Fang JY, Chiang HS, Chen SK, Chen BH, … Hung CF (2007). (−)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules. 10.3390/12081845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmaire C, Danoux L, & Pauly G. (2001). Glycation during human dermal intrinsic and actinic ageing: An in vivo and in vitro model study. British Journal of Dermatology. 10.1046/j.1365-2133.2001.04275.x [DOI] [PubMed] [Google Scholar]
- Khan M, Liu H, Wang J, & Sun B. (2020). Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Research International. 10.1016/j.foodres.2019.108933 [DOI] [PubMed] [Google Scholar]
- Końca Krzysztof, Lankoff Anna, Banasik Anna, Lisowska Halina, Kuszewski Tomasz, Góźdź Stanisław, Koza Zbigniew, and A. W. (1981). A cross-platform public domain PC image-analysis program for the comet assay. Profesional Psychology. 10.1163/_q3_SIM_00374 [DOI] [PubMed] [Google Scholar]
- Li L, & Seeram NP (2010). Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. Journal of Agricultural and Food Chemistry. 10.1021/jf1033398 [DOI] [PubMed] [Google Scholar]
- Li L, & Seeram NP (2011). Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals. In Journal of Agricultural and Food Chemistry. 10.1021/jf2011613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Guo H, DaSilva NA, Li D, Zhang K, Wan Y, … Ma H. (2019). Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. Journal of Functional Foods. 10.1016/j.jff.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ma H, Frost L, Yuan T, Dain JA, & Seeram NP (2014). Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food and Function, 5(11). 10.1039/c4fo00538d [DOI] [PubMed] [Google Scholar]
- Liu W, Wei Z, Ma H, Cai A, Liu Y, Sun J, … Seeram NP (2017). Anti-glycation and anti-oxidative effects of a phenolic-enriched maple syrup extract and its protective effects on normal human colon cells. Food and Function, 8(2). 10.1039/c6fo01360k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ma H, DaSilva NA, Rose KN, Johnson SL, Zhang L, … Seeram NP (2016). Development of a neuroprotective potential algorithm for medicinal plants. Neurochemistry International. 10.1016/j.neuint.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wei Z, Ma H, Cai A, Liu Y, Sun J, … Seeram NP (2017). Anti-glycation and anti-oxidative effects of a phenolic-enriched maple syrup extract and its protective effects on normal human colon cells. Food and Function. 10.1039/c6fo01360k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ma H, & Seeram NP (2016). Development and UFLC-MS/MS characterization of a product-specific standard for phenolic quantification of maple-derived foods. Journal of Agricultural and Food Chemistry. 10.1021/acs.jafc.6b01381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, DaSilva NA, Liu W, Nahar PP, Wei Z, Liu Y, … Seeram NP (2016). Effects of a standardized phenolic-enriched maple syrup extract on β-amyloid aggregation, neuroinflammation in microglial and neuronal cells, and β-amyloid induced neurotoxicity in Caenorhabditis elegans. Neurochemical Research, 41(11). 10.1007/s11064-016-1998-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Liu W, Frost L, Kirschenbaum LJ, Dain JA, & Seeram NP (2016). Glucitol-core containing gallotannins inhibit the formation of advanced glycation end-products mediated by their antioxidant potential. Food and Function, 7(5). 10.1039/c6fo00169f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Johnson SL, Liu W, Dasilva NA, Meschwitz S, Dain JA, & Seeram NP (2018). Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and mic. International Journal of Molecular Sciences. 10.3390/ijms19020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Liu W, Frost L, Wang L, Kong L, Dain JA, & Seeram NP (2015). The hydrolyzable gallotannin, penta-O-galloyl-beta-D-glucopyranoside, inhibits the formation of advanced glycation endproducts by protecting protein structure. Molecular Biosystems. 10.1039/c4mb00722k [DOI] [PubMed] [Google Scholar]
- McLoone P, Warnock M, & Fyfe L. (2016). Honey: an immunomodulatory agent for disorders of the skin. Food and Agricultural Immunology. 10.1080/09540105.2015.1104653 [DOI] [Google Scholar]
- Mirnezami M, Jafarimanesh H, Rezagholizamenjany M, Alimoradian A, & Ranjbaran M. (2020). The effect of silymarin on liver enzymes in patients taking isotretinoin: A randomized clinical trial. Dermatologic Therapy. 10.1111/dth.13236 [DOI] [PubMed] [Google Scholar]
- Nahar PP, Driscoll MV, Li L, Slitt AL, & Seeram NP (2014). Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. Journal of Functional Foods. 10.1016/j.jff.2013.09.026 [DOI] [Google Scholar]
- Nilsson BO (1999). Biological effects of aminoguanidine: An update. Inflammation Research. 10.1007/s000110050495 [DOI] [PubMed] [Google Scholar]
- Palmer DM, & Kitchin JS (2010). A double-blind, randomized, controlled clinical trial evaluating the efficacy and tolerance of a novel phenolic antioxidant skin care system containing Coffea arabica and concentrated fruit and vegetable extracts. Journal of Drugs in Dermatology. 9(12):1480–1487 [PubMed] [Google Scholar]
- Rahbar S, Kumar Yernini K, Scott S, Gonzales N, & Lalezari I. (1999). Novel inhibitors of advanced glycation endproducts. Biochemical and Biophysical Research Communications. 10.1006/bbrc.1999.1275 [DOI] [PubMed] [Google Scholar]
- Roberts MJ, Wondrak GT, Laurean DC, Jacobson MK, & Jacobson EL (2003). DNA damage by carbonyl stress in human skin cells. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis. 10.1016/S0027-5107(02)00232-4 [DOI] [PubMed] [Google Scholar]
- Saranraj P, & Sivasakthi S. (2018). Comprehensive review on honey: Biochemical and medicinal properties. Journal of Academia and Industrial Research. [Google Scholar]
- Sun J, Liu W, Ma H, Marais JPJ, Khoo C, Dain JA, … Seeram NP (2016). Effect of cranberry (Vaccinium macrocarpon) oligosaccharides on the formation of advanced glycation end-products. Journal of Berry Research. 10.3233/JBR-160126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svobodová A, Psotová J, & Walterová D. (2003). Natural phenolics in the prevention of UV-induced skin damage. A review. Biomedical Papers. 10.5507/bp.2003.019 [DOI] [PubMed] [Google Scholar]
- Wu CH, Huang SM, Lin JA, & Yen GC (2011). Inhibition of advanced glycation endproduct formation by foodstuffs. Food and Function. 10.1039/c1fo10026b [DOI] [PubMed] [Google Scholar]
- Wu CH, & Yen GC (2005). Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. Journal of Agricultural and Food Chemistry. 10.1021/jf048550u [DOI] [PubMed] [Google Scholar]
- Yang CT, Meng FH, Chen L, Li X, Cen LJ, Wen YH, Li CC and Zhang H. (2014). Inhibition of Methylglyoxal-Induced AGEs/ RAGE Expression Contributes to Dermal Protection by N-Acetyl-L-Cysteine. 10.1163/_q3_SIM_00374 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma H, Liu W, Yuan T, & Seeram NP (2015). New antiglycative compounds from cumin (Cuminum cyminum) spice. Journal of Agricultural and Food Chemistry, 63(46). 10.1021/acs.jafc.5b04796 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yuan T, Li L, Nahar P, Slitt A, & Seeram NP (2014). Chemical compositional, biological, and safety studies of a novel maple syrup derived extract for nutraceutical applications. Journal of Agricultural and Food Chemistry. 10.1021/jf501924y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillich OV, Schweiggert-Weisz U, Eisner P, & Kerscher M. (2015). Polyphenols as active ingredients for cosmetic products. International Journal of Cosmetic Science. 10.1111/ics.12218 [DOI] [PubMed] [Google Scholar]