Abstract
Purpose of Review:
Opioid-related deaths are a leading cause of mortality during pregnancy through 12 months postpartum. Buprenorphine use during pregnancy is increasing, yet expert opinion on its dosing through the perinatal period is limited. We provide a review of the current clinical literature on buprenorphine dosing during pregnancy through 12 months postpartum. and present data from a retrospective chart review of patients at our institution describing trends in buprenorphine dosing during pregnancy and postpartum. Utilizing this information, we synthesize findings to provide clinical recommendations for providers.
Recent Findings:
Existing literature during pregnancy reflects how many women increase and split total daily buprenorphine doses as gestational age advances.
Summary:
We present data from a retrospective chart review of patients at our institution describing trends in buprenorphine dosing during pregnancy and postpartum. Utilizing this information, we synthesize findings to provide clinical recommendations for providers. Changes in the total daily dose of buprenorphine used across pregnancy and through 12 months postpartum at the individual level do not follow consistent patterns, highlighting substantial individual variability. Altogether, buprenorphine dosing should be individualized through pregnancy and postpartum with frequent evaluations by providers and solicited input from patients.
Keywords: Buprenorphine, women, opioid use disorder, addiction, pregnancy, postpartum
Introduction
The life expectancy in the United States is decreasing, largely due to increasing opioid overdoses during reproductive age years, with women seeing more rapid changes in these poor outcomes than men over the past decade [1]. Pregnant and postpartum women are no exception to this unfortunate statistic. Opioid-related deaths are becoming the leading cause of pregnancy-associated mortality (during pregnancy through 12 months after delivery) [2, 3•,4]. This indicates a need for increased screening, support, treatment and recovery support for people with opioid use disorder during this vulnerable period.
Evidence-based treatment for pregnant and postpartum women with opioid use disorder includes medication, specifically methadone or buprenorphine, concurrent with wrap-around services such as psychiatry, behavioral health, social work and recovery support [5 •]. This comprehensive, integrated approach reduces overdose risk and improves both maternal and child outcomes [5 •]. Medication for opioid use disorder is a key component of this integrated approach, as overdose events among women on medication for opioid use disorder are fewer than among women not on such medication during pregnancy through 12 months postpartum [6].
Likely due to buprenorphine’s lower risk profile for neonatal opioid withdrawal syndrome [7] and office-based administration, it is becoming a predominant medication for opioid use disorder used during pregnancy across regions in the United States [8]. Despite this, evidence to guide buprenorphine dose changes through the perinatal period are based on limited pharmacokinetic data and expert panel recommendations [9•]. With the physiological, medical, and environmental changes that women experience throughout pregnancy and postpartum, careful consideration should be paid to dosing of buprenorphine, with frequent assessment of withdrawal symptoms, cravings, and sedation.
Overall, with the increasing integration of buprenorphine management into primary care [10] and women’s health settings, providers need evidence-based guidelines on how to optimize dosing regimens for this important medication through the perinatal period. This study provides a scoping review of the current clinical literature on buprenorphine dosing during pregnancy through 12 months postpartum, and we present data from our institution describing trends in buprenorphine dosing during this period. Utilizing this information, we synthesize findings into clinical recommendations.
Methods
Scoping review of the literature
We performed a scoping review of the current clinical literature that addressed buprenorphine dosing during pregnancy and/or through the 12-month postpartum period. We searched MEDLINE/PubMED for studies of human subjects during pregnancy and/or through 12 months after delivery on buprenorphine for opioid use disorder MeSH search terms included: [“buprenorphine”] AND [“pregnancy” OR “postpartum period”]. This search yielded 370 citations. Titles followed by abstracts were screened for the following inclusion criteria: (1) human subjects, (2) pregnant or postpartum women comprised the study population, (3) article presented original data, (4) buprenorphine dose information provided. Exclusion criteria included: (1) Non-English language, (2) buprenorphine dosing information only provided at the one time point of delivery. 355 articles were excluded based on these criteria. Full text review was then completed for the remaining 15 articles based on the same criteria, and 14 articles remained. Citations of these articles were also reviewed for any additional eligible articles not included in the original search. An additional 38 articles were searched, resulting in 22 meeting inclusion criteria for full text review, after which 11 remained. In total, 25 articles remained and were reviewed.
Retrospective cohort study of pregnant and postpartum women on buprenorphine for opioid use disorder
a. Clinical population
We conducted a retrospective chart review of pregnant and postpartum patients receiving care at Virginia Commonwealth University (VCU) Health System from January 2017 to March 2020. In 2017, the department of psychiatry opened an outpatient addiction medicine clinic. More than 90% of patients seeking care for opioid use disorder at this clinic are on buprenorphine. A subset of providers are obstetrician-gynecologists and family medicine physicians who treat women during pregnancy and postpartum for substance use disorders across two sites: the outpatient addiction medicine clinic and an integrated addiction program within an obstetrics and gynecology clinic. This integrated Obstetrics/Gynecology--Addiction program is the only one in the region that provides on-site prenatal/postpartum/well-woman care, opioid use disorder treatment with buprenorphine, and behavioral health services. Incarcerated pregnant women on buprenorphine for opioid use disorder from local jails are also cared for by these providers. Lastly, this program collaborates with community opioid-based treatment centers to provide recovery-oriented perinatal care to pregnant and postpartum women with opioid use disorder. Today, the outpatient addiction medicine clinic provides care to almost 500 unique patients, of which about 50 at any given time are pregnant or postpartum. More than half of patients are African American, and almost all have Medicaid as well as at least one co-morbid psychiatric diagnosis (depression, anxiety, etc.).
b. Study population
In order to evaluate the buprenorphine dosing details within our study population of pregnant and postpartum women, we queried the electronic medical records for all women on buprenorphine for opioid use disorder who engaged in care with VCU Health System at least once from January 1, 2017 to March 30, 2020, either during pregnancy or 12 months postpartum on buprenorphine for opioid use disorder. This study was approved by the Virginia Commonwealth University Institutional Review Board. The study population was identified using the following steps. First, billing data was searched for the ICD code Z37 to identify women who delivered at VCU Health System. Next, the medical record was searched for patients whose prenatal records were not included within the study timeframe. Of these patients, those who had at least one outpatient buprenorphine prescription or inpatient order during pregnancy or through 12 months after delivery were included. Patients who were included could be on buprenorphine or buprenorphine-naloxone sublingual tabs or films.
c. Outcomes
Buprenorphine dosing information was abstracted from the medical record for all patients included in our study population. First, all participants were assigned a delivery date in order to extrapolate a corresponding time during pregnancy and postpartum for each buprenorphine dosing data point. For patients who delivered at VCU Health System, the true delivery date was extracted from the billing date. For patients who did not deliver at VCU, the estimated delivery date was abstracted from the medical record (used delivery date entered into prenatal record; if not entered, then the estimated due date for the pregnancy was used as the estimated delivery date). Next, buprenorphine dose information was abstracted from the medical record using both outpatient prescription and inpatient orders. Buprenorphine or buprenorphine-naloxone orders within 280 days before the assigned delivery date or up to 365 days after the delivery date were included. We also abstracted the following information for each buprenorphine order within the dataset: formulation (buprenorphine vs. buprenorphine-naloxone), total daily dose of buprenorphine (1–24 mg), sublingual tab or film, and dosing frequency (once, twice or three times daily dosing). If a patient had more than one buprenorphine order within a 6-day window (e.g., multiple inpatient orders while patient was admitted for buprenorphine induction or delivery), these data points were individually examined to exclude duplicates; the highest total daily dose of duplicate buprenorphine orders was kept while others were deleted.
d. Analysis
All data was exported into an excel document. Data was then de-identified using study ID numbers. In a cross-sectional descriptive analysis, we first report buprenorphine dosing data for all participants who contributed data for defined time periods during pregnancy, at delivery, and postpartum. Delivery buprenorphine doses were categorized as those corresponding to 3 days before through 3 days after delivery. During pregnancy and postpartum, delivery dates were used to generate intervals (e.g., first trimester, second trimester, third trimester of pregnancy; 0–4 months, 4–8 months, and 8–12 months postpartum). If participants had more than one buprenorphine order during an interval, a mean buprenorphine dose was calculated for the individual. All buprenorphine data points within these time intervals were combined to generate descriptive statistics (overall means, standard deviations and ranges) of total daily doses. We also describe the prevalence of formulations, tabs vs films and dosing frequency. Next, a subset of participants who provided buprenorphine dosing data for all trimesters during pregnancy or all postpartum time intervals were selected to provide longitudinal descriptive data of individual-level buprenorphine dose changes through the perinatal period. We used spaghetti plots to describe the individual-level longitudinal data of mean buprenorphine doses during pregnancy and postpartum.
Results
Review of published literature
Table 1 describes the 25 studies included in our literature review of buprenorphine dosing through the perinatal period. Most (n=23) provide buprenorphine dose information for at least one time point during pregnancy, representing a total of 3008 pregnant women. Many of these studies (n=16) reported on buprenorphine dosing at delivery in addition to another time point during pregnancy. Considering the postpartum period, nine studies included dose information after delivery representing 287 postpartum women. Only 1 study [11•] was solely focused on postpartum women. Two studies presented complete dosing information in pregnancy, at delivery, and in the postpartum period (including total daily dose, dosing frequency, formulation).
Table 1. Review of current buprenorphine dosing guidelines in the literature.
Scoping review of existing literature buprenorphine dosing through the perinatal period organized by category of study (randomized controlled trials, cohort, case control, observational, and case studies). For each citation, we discuss the demographics of their participants, formulation of buprenorphine used (including mono vs combination product, tab vs film administration), buprenorphine dosing frequency, dose reported during pregnancy, at delivery, and during postpartum, as well as changes in dosing discussed in each study
| Study citation | Population demographics | Formulation (dosing frequency, route of administration) | Dose during pregnancy (mean, mg/day) | Dose at delivery (mean, mg/day) | Dose postpartum (mean, mg/day) | Changes in dose |
|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||
| Fischer et al. (2006) |
|
|
|
14.0 | Not reported |
|
| Cohort studies | ||||||
| Lo-Ciganic et al. (2019) |
|
|
During first trimester (T1):
|
Not reported | Not reported, though the study did follow women through 12 weeks postpartum |
|
| Brogly et al. (2018) |
|
|
At pregnancy initiation:
|
14.7 | Not reported |
|
| Coker et al. (2018) |
|
|
Overall at last visit: 14.98
|
See other 2 columns | Adherent at last visit: 17 | Overall for adherent: +7 mg/day |
| O’Connor et al. (2018) |
|
|
Not reported prior to delivery |
|
Not reported, though changes to postpartum reported in next column |
S6:
|
| Welle-Strand et al. (2015) |
|
Buprenorphine | At pregnancy determination:
|
|
Not reported | From pregnancy determination to delivery:
|
| Simmat-Durand et al. (2009) |
|
|
Overall during “early pregnancy”: 6.9
|
Overall: 5.4
|
Not reported |
|
| Kahila et al. (2007) |
|
|
At start (<18 wk estimated gestational age, EGA):
|
|
Not reported |
|
| Case control studies | ||||||
| Concheiro et al. (2010) |
|
|
|
18.4 | Not reported |
|
| Observational studies | ||||||
| Bastian et al. (2017) |
|
|
|
Not reported |
|
|
| Caritis et al. (2017) |
|
|
|
Not reported |
|
|
| Concheiro et al. (2011) |
|
|
|
Not reported |
|
|
| Ilett et al. (2011) |
|
|
Not reported | Not reported |
|
Not reported |
| Lacroix et al. (2011) |
|
|
|
Not reported | Not reported |
|
| O’Connor et al. (2011) |
|
Buprenorphine |
|
13.3 | Not reported |
|
| Kacinko et al. (2009) |
|
|
|
18.6 | 17.4 |
|
| Lindelman et al. (2009) |
|
|
|
Not reported |
|
|
| Kacinko et al. (2008) |
|
|
|
18.7 | Not reported |
|
| Goodwin et al. (2007) |
|
|
Initial: 8.2 | 18.7 | Not reported, though does note that participants were maintained “up to 10 wks after delivery” |
|
| Lacroix et al. (2004) |
|
|
|
Not reported | Not reported |
|
| Johnson et al. (2001) |
|
|
Throughout pregnancy: 9.3 | 9.3 | After delivery: women were maintained on buprenorphi ne ×21 days, then tapered off either with 21-day inpatient or 10-wk outpatient schedule | From pregnancy to 13wks postpartum: −9.3 mg/day (due to study design—postpartum taper) |
| Fischer et al. (2000) |
|
|
At induction: 8.4 | 7.4 | Not reported |
|
| Fischer et al. (1998) |
|
|
8.1 | 8.1 | Not reported |
|
| Case report/series | ||||||
| Ross. (2004) |
|
Buprenorphine | During “later stage of pregnancy, up to day of induction”: 34 | 34 | Not reported | Not reported, though paper does insinuate that patient’s dose increased during pregnancy |
| Schindler et al. (2003) |
|
|
9 | 9 | Not reported | From conception to delivery: 0 mg/day |
a. Buprenorphine dosing during pregnancy
During pregnancy, the range of doses provided to women varied widely: first trimester 4.9 to 17.8 mg/day [12–13], second trimester 1.1 to 16 mg/day [9•, 12, 14 •] third trimester 0.1 to 34 mg/day [12, 15]. Doses at delivery ranged from 2.3 to 34 mg/day [13, 16–17]. Of the studies that report longitudinal changes in buprenorphine dosing through pregnancy (n=18), mean changes in doses during pregnancy ranged from - 12.3 to +10.5 mg/day [13, 18]. None of these studies describe associations between buprenorphine dose changes and maternal opioid use disorder treatment outcomes. However, two studies did find higher buprenorphine doses to be associated with higher levels of treatment continuation during pregnancy [19–20 •]. Of the studies that did specify formulation, all used buprenorphine sublingual tablets except for one that had some women who used buprenorphine-naloxone [20 •]. Many studies did not specify dosing frequency, but of those that did, most were administered using daily dosing while a few studies did report on women using twice [9 •, 14 •, 21] or three times [22] daily dosing regimens.
Of the six studies that reported a decrease in total dose during pregnancy [12–13, 23–26], all either included women undergoing a planned taper [12–13, 23–25] or did not provide information on how medication management was handled clinically for women [26].
The majority of studies (n=9) reported increases in buprenorphine total daily doses during pregnancy:
5.9 mg from 1st trimester to delivery [27]
2–4 mg between the second and third trimesters [9•, 14 •, 16].
The remaining three studies reported neither an increase nor decrease in dose during pregnancy. These included a case report of two women that lacked details about medication management [31], and two case series: one of women (n=9) in a program that standardized doses limited to 10 mg or less [32] and the other of 3 women planning to undergo a taper postpartum [33].
b. Buprenorphine dosing during postpartum
Overall, postpartum buprenorphine doses and dose changes varied widely in the literature. One study (n=7) reported a median dose of 7 mg at 3 weeks postpartum, but it is notable that individual doses ranged from 2.4 to 24 mg [21]. At 2 months postpartum, reported mean doses were 16–18.6 mg [9•, 14 •, 16]. Two additional studies described mean doses of 17–17.4 mg at an unknown time after delivery [19, 30]. Most studies did not give formulation and dose frequency information, but O’Connor et al did describe women switching from buprenorphine to buprenorphine-naloxone after delivery [11 •].
Seven studies described dose changes over time with mixed results. From 34 weeks to 2 months postpartum, two studies reported 3 mg dose decreases [9•, 14 •], while another reported a 2 mg increase [16]. Reported dose changes over other timeframes also varied. One study (n=7) reported a median buprenorphine dose decrease of 0.3 mg/kg/day between 11 weeks gestation and 6 days postpartum [22]. Another study (n=9) reported a 4.6 mg increase between 23 weeks and an unknown time postpartum [30]. Lastly, one study reported a 9.7 mg decrease from delivery to 13 weeks postpartum, but this was among women (n=3) undergoing a scheduled taper [33].
Only one study described dosing in detail through 12 months after delivery [11 •]. O’Connor et al. reported retrospectively on postpartum treatment retention of 190 women prescribed buprenorphine at delivery. The average dose at delivery was 15 mg (14 mg for those not maintained in treatment postpartum). Among those maintained in treatment at 6 months (n=151; mean dose 15.1 mg), 51% were on the same dose, 16% a higher dose (mean dose increase 4.94 mg), and 33% a lower dose (mean dose decrease 4.55 mg) than at delivery. Among those maintained in treatment at 12 months (n=135; mean dose 15.3 mg), 36% were on the same dose, 16% were on a higher dose than at delivery (mean dose increase 4.31 mg) and 48% were on a lower dose (mean dose decrease 5.15 mg). Overall, delivery dose was not associated with treatment retention, but the association of postpartum dose changes with treatment retention was not evaluated [11 •].
Retrospective cohort study of pregnant and postpartum women on buprenorphine for opioid use disorder
We identified 240 women during the study timeframe on buprenorphine who engaged in care with VCUHS at least once during pregnancy, at delivery or through 12 months postpartum. A total of 51 women contributed buprenorphine dose data at least once during the first trimester (0–12 weeks gestation), 107 the second trimester (13–24 weeks), and 128 the third trimester (28–40 weeks gestation). At delivery, 131 women contributed buprenorphine dosing data. Postpartum, a total of 104 women contributed buprenorphine dose data during 0–4 months after delivery, 64 during 4–8 months, and 46 during 8–12 months. Ten patients who were on sublingual buprenorphine during pregnancy transitioned to extended release injectable buprenorphine postpartum; these data points were not included in the analyses. Considering the subgroups of patients who contributed longitudinal data during pregnancy or postpartum, 19 women (7.9%) provided buprenorphine dosing data for all pregnancy trimesters (Figure 1), and 32 women (13.3%) provided dosing data for all postpartum time intervals (Figure 2).
Fig. 1.
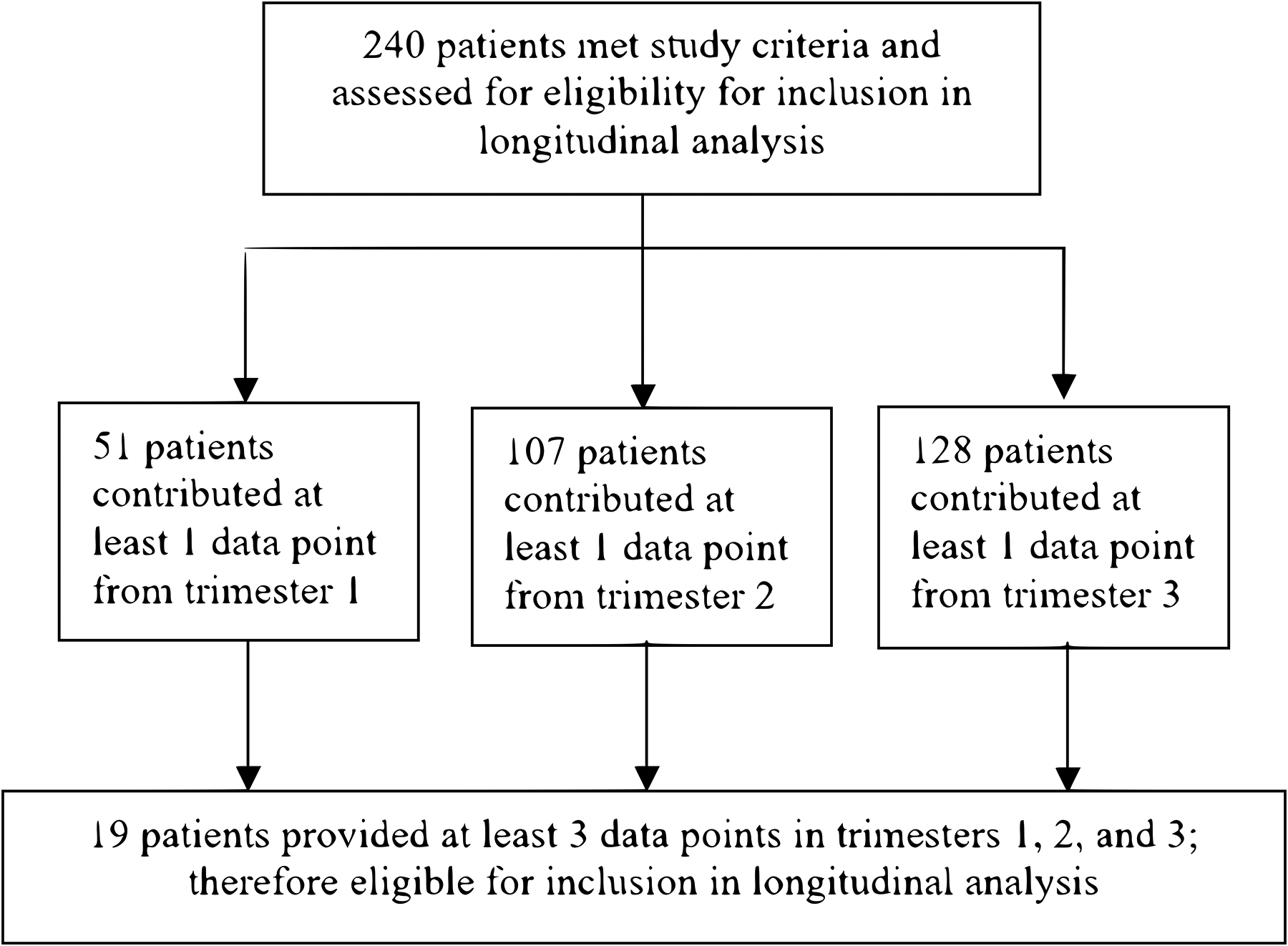
Patient flow diagram for inclusion in longitudinal analysis based on pregnancy data. 240 participants were assessed for eligibility, with 51 contributing at least 1 data point from trimester 1, 107 from trimester 2, and 128 from trimester 3. 19 participants provided at least 3 data points, including 1 from each of the trimesters, and were included in pregnancy longitudinal analysis
Fig. 2.
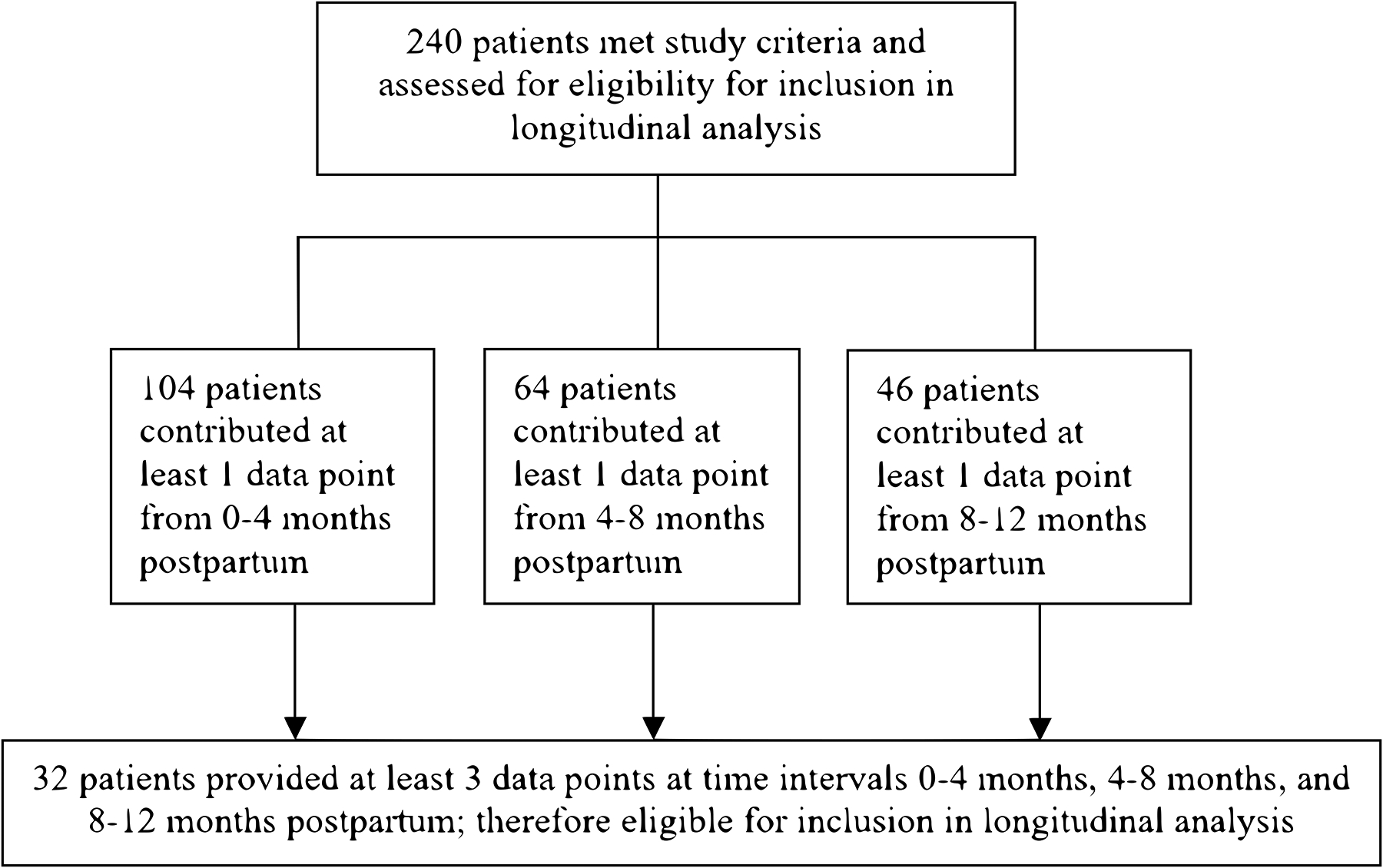
Patient flow diagram for inclusion in longitudinal analysis based on postpartum data. 240 participants were assessed for eligibility, with 104 contributing at least 1 data point from 0–4 months postpartum, 64 from 4–8 months postpartum, and 46 from 8–12 months postpartum. 32 participants provided at least 3 data points, 1 from each of the postpartum time intervals, and were included in postpartum longitudinal analysis
Figure 3 shows the overall mean total daily doses among the study population (N=240) throughout the time intervals of pregnancy, delivery, and postpartum. During pregnancy, mean buprenorphine total daily doses used by the groups providing data for each trimester steadily increased over time: 12.85 mg (n=51; range 2–24 mg) during the first trimester, 13.40 mg (n=107; range 2–24 mg) the second, and 15.01 mg (n=128; range 2–24 mg) the third trimester. At delivery (n=131), total daily doses ranged from 2 mg to 24 mg with a mean dose of 13.98 mg (SD 6.14). Postpartum, mean total daily doses were overall higher than during pregnancy: 16.78 mg (n=104; range 1–24 mg) during 0–4 months after delivery, 18.22 mg (n=64; range 4–24 mg) from 4–8 months postpartum, and 18.06 mg (n=46; range 1–24 mg) from 9–12 months postpartum.
Fig. 3.
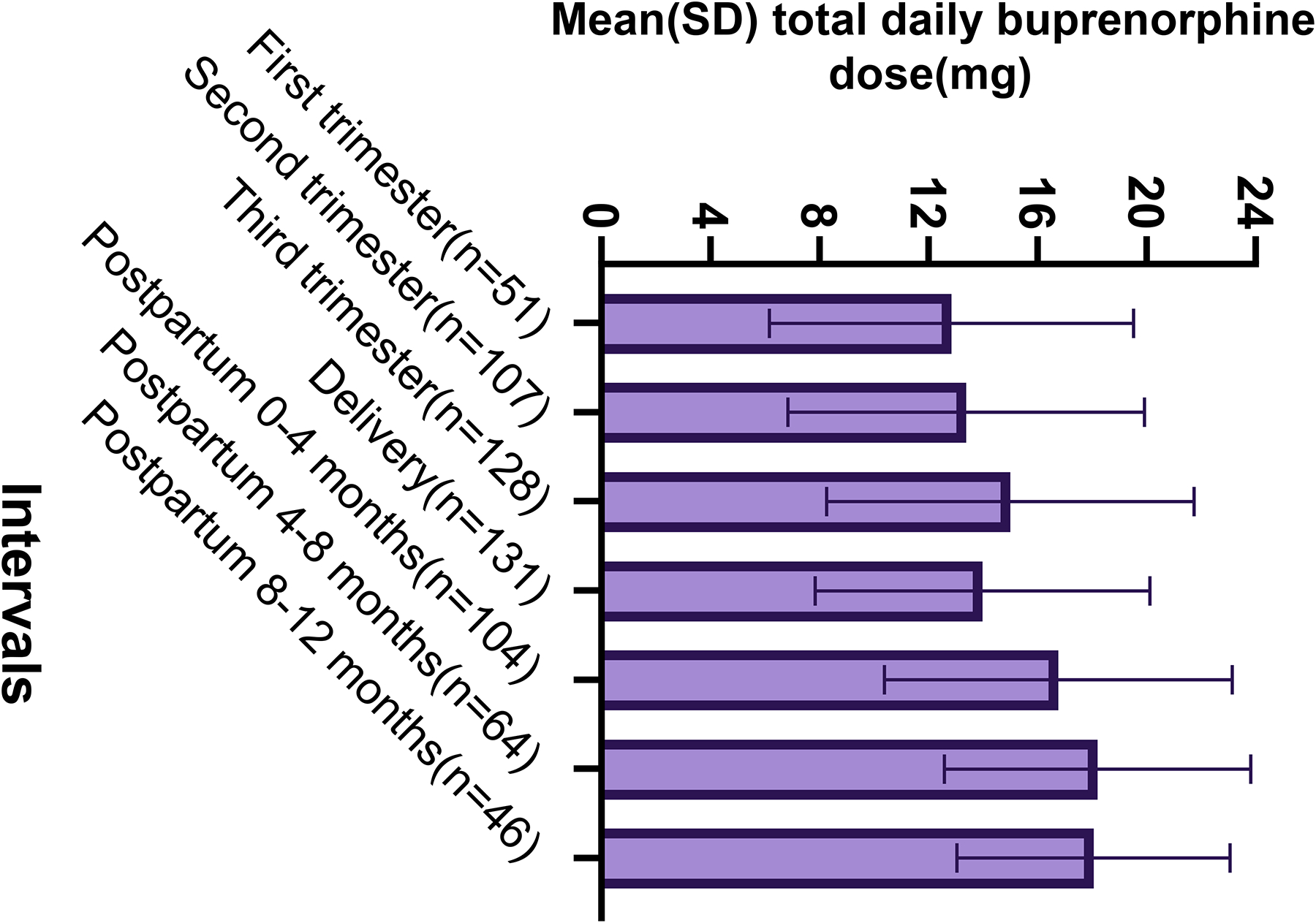
Bar graph of mean (SD; standard deviation) buprenorphine total daily dose through the perinatal period by perinatal time interval among the study population (N=240) demonstrating a steady increase in the mean total daily dose of the groups providing data for each pregnancy trimester, a slight decrease in the group mean dose at the time of delivery, followed by again an increase in the mean total daily dose of the groups providing data for the postpartum time periods.
Considering individual level changes in buprenorphine dose, patients generally underwent increases in their mean total daily dose with increasing gestational age (data not shown). The following dose changes occurred among patients providing data between two consecutive perinatal time intervals during pregnancy: mean 4.5 mg increase (n=17; range 1.5–12 mg) from the first to second trimester, mean 4.9 mg increase (n=37; range 2–12.8 mg) from second to third trimester, and 3.4 mg increase (n=32; range 0.3–10 mg) from third trimester to delivery. Among patients providing data between two consecutive postpartum time intervals, dose changes varied. From delivery through 4 months postpartum (n=85), 43.5% increased their total dose (mean dose increase 6.9 mg), 15.3% decreased (mean dose decrease 4.6 mg) and 41.2% remained at the same dose as at delivery (mean dose 18.2 mg). From 0–4 to 4–8 months postpartum (n=54), 33.3% increased their total dose (mean dose increase 4.1 mg), 13.0% decreased (mean dose decrease 2.6 mg) and 53.7% remained at the same dose as at delivery (mean dose 20.5 mg). From 4–8 to 8–12 months postpartum (n=39), 33.3% increased their total dose (mean dose increase 4.6 mg), 18.0% decreased (mean dose decrease 2.9 mg) and 48.7% remained at the same dose as at delivery (mean dose 18.7 mg).
Mean doses for the patients providing data throughout all of pregnancy (n=19; Figure 4) or the postpartum period (n=32; Figure 5) highlight how dose changes over time do vary between individual patients. For example, three patients decreased their dose during pregnancy (Figure 4). One of these patients desired buprenorphine taper during the 2nd trimester. She was also undergoing a supervised benzodiazepine taper at the same time. She was encouraged to focus on tapering the benzodiazepine dose and maximize her buprenorphine dose; however, she did well on a buprenorphine dose during 3rd trimester that was half what she was taking during the 1st trimester. Another patient was discharged from jail and entered a residential treatment program where her dose was decreased in the 2nd trimester. However, due to manic symptoms in the setting of withdrawal, her buprenorphine dose was increased in 3rd trimester back to what she had started on in 1st trimester. The last of these three patients was undergoing routine buprenorphine treatment but did not disclose to anyone that she was pregnant until she presented late in her third trimester. She attempted to self-decrease her daily buprenorphine dose. Considering outliers in postpartum dosing (Figure 5), two patients underwent tapers with their addiction provider with shared decision making regarding their treatment plans. They both had a mean dose of 1 mg/day during months 8–12 of the postpartum period.
Fig. 4.
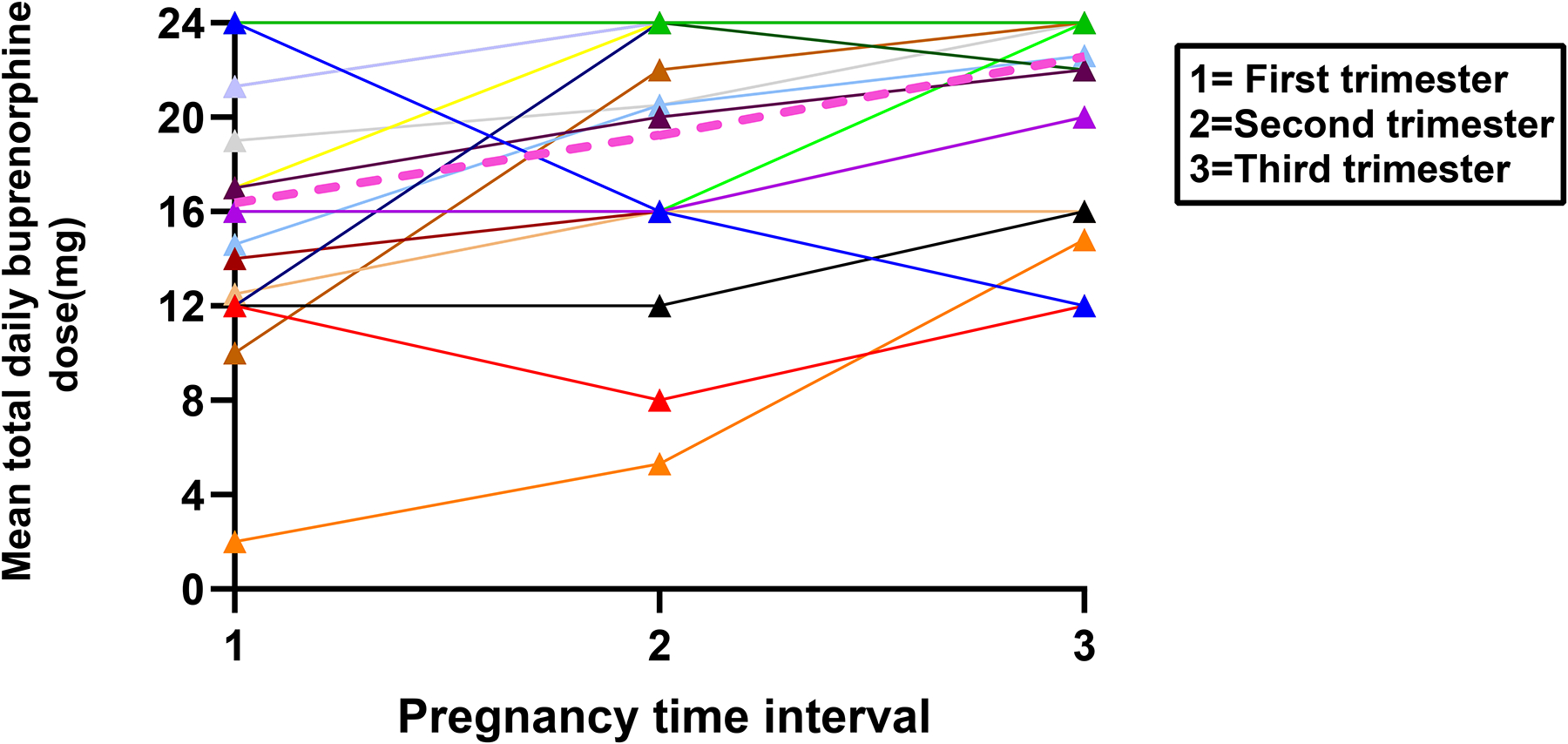
Spaghetti plot of individual trajectories in mean buprenorphine total daily dose for each pregnancy time interval represented in various colors with the mean represented by pink dashed line (N=19)
Fig. 5.
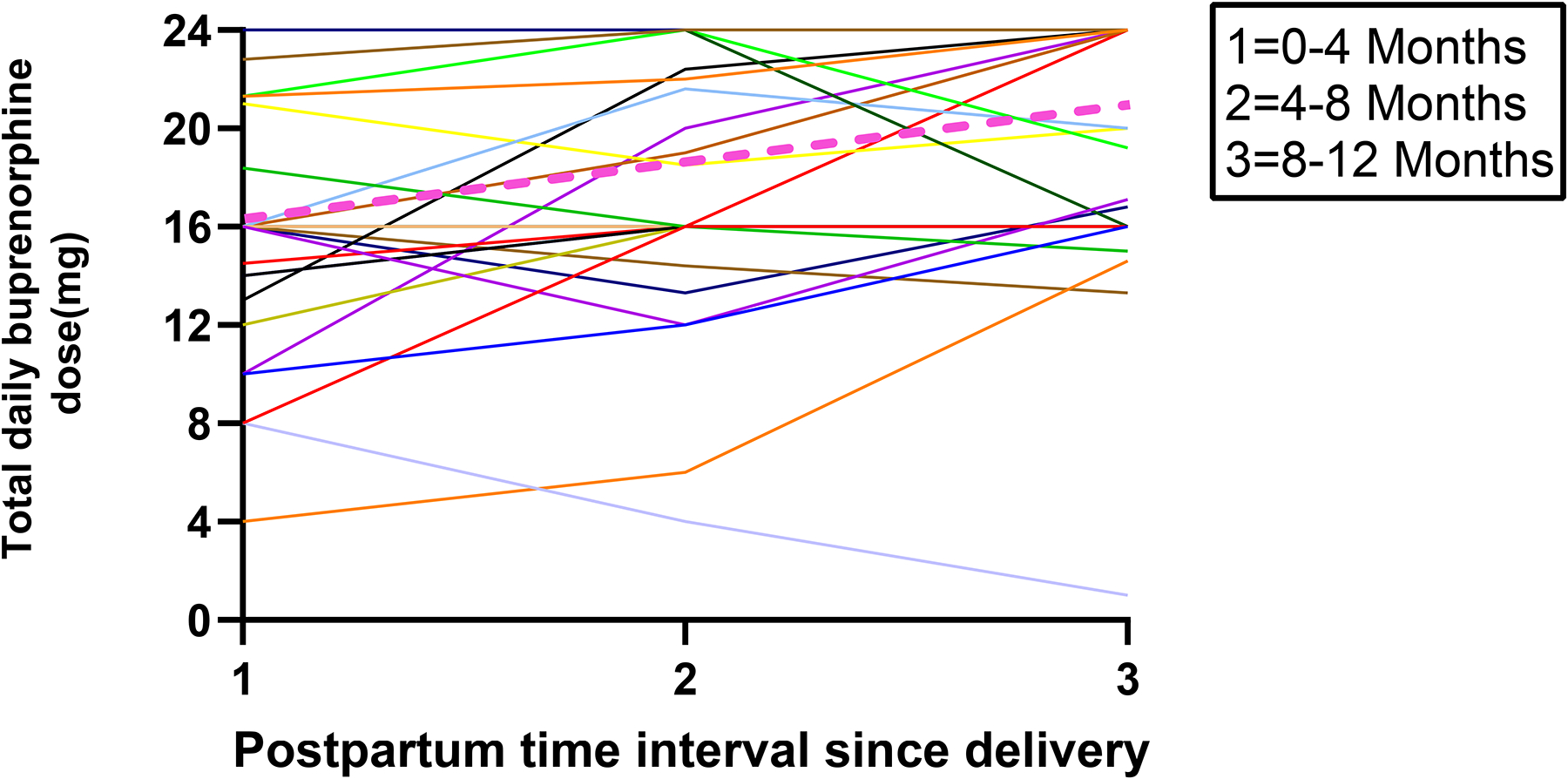
Spaghetti plot of individual trajectories in mean buprenorphine total daily dose for each postpartum time interval represented in various colors with the mean represented by pink dashed line (N=32)
Figure 6 describes the prevalence of buprenorphine mono and combination product used throughout the perinatal period; there were significant differences between the pregnancy and postpartum time intervals. In the 3rd trimester, 65 (51.2%) were using the buprenorphine monoproduct, compared to only 6 (12.5%) in the 8–12 months postpartum. Use of the buprenorphine monoproduct declined sharply immediately after delivery; 78 participants (60.0%) were on the monoproduct at delivery, which decreased to only 17 (16.2%) during months 0–4 postpartum. Conversely, prescription of buprenorphine-naloxone increased after delivery, with the highest number of participants (n=88; 83.8%) on the combination product in the first 0–4 months postpartum.
Fig. 6.
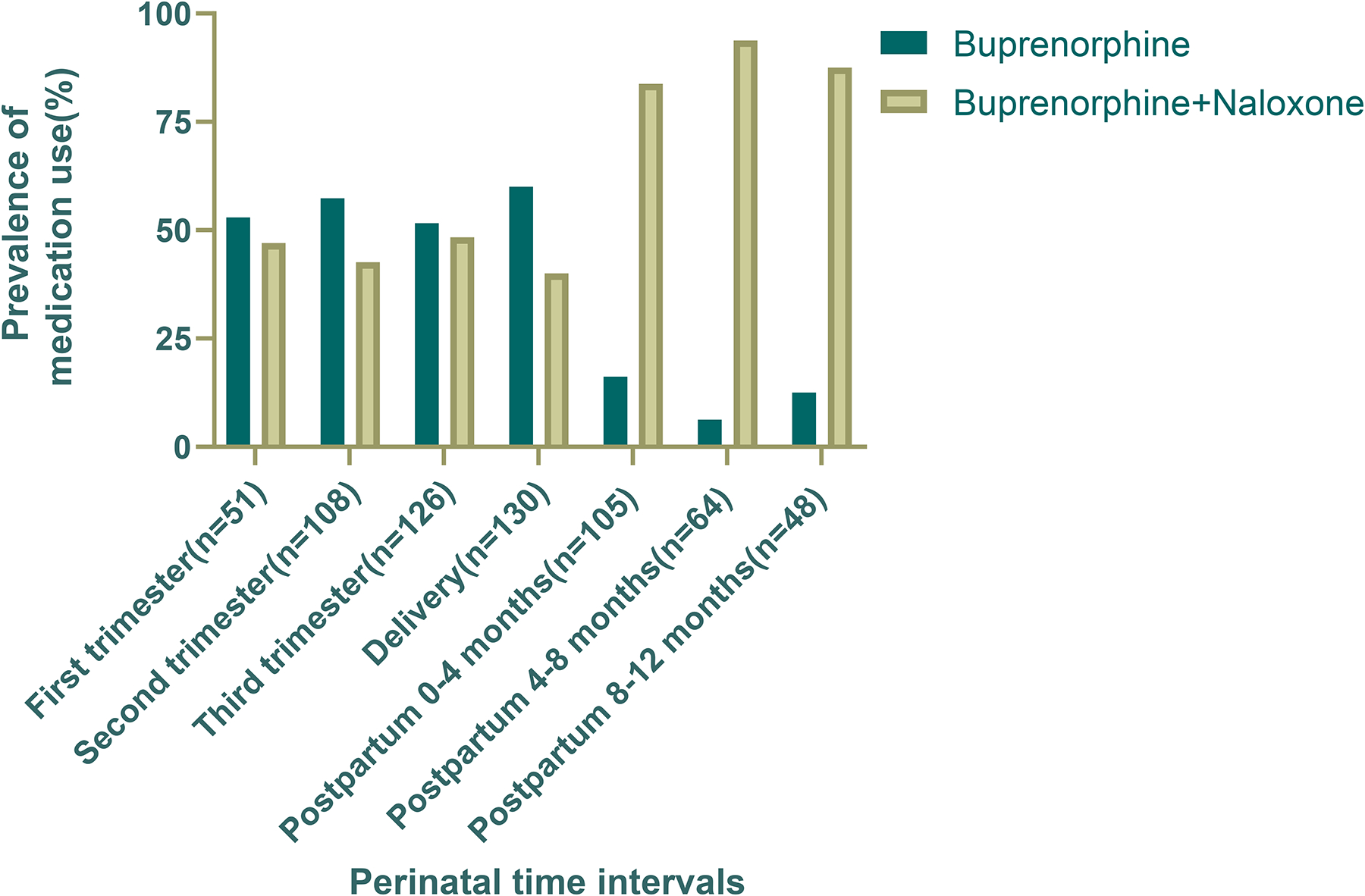
Prevalence of buprenorphine versus buprenorphine-naloxone use through the perinatal period by pregnancy or postpartum time interval (N=240) with teal column representing buprenorphine monoproduct and cream column representing buprenorphine-naloxone combination product. Use of both varied throughout the pregnancy period, and the majority of participants used the combination product throughout postpartum.
Regarding the use of buprenorphine tablet versus film throughout the perinatal period, the differences were not as stark (Figure 7). More women took their medication in tablet form than film throughout pregnancy (62.8–70.4% for tab versus 29.6–37.3% for film). Postpartum, more women used the film formulation than the tablet, with 65 participants (61.9%) taking film in the first 0–4 months postpartum compared to 40 participants (38.1%) taking tab in the same period.
Fig. 7.
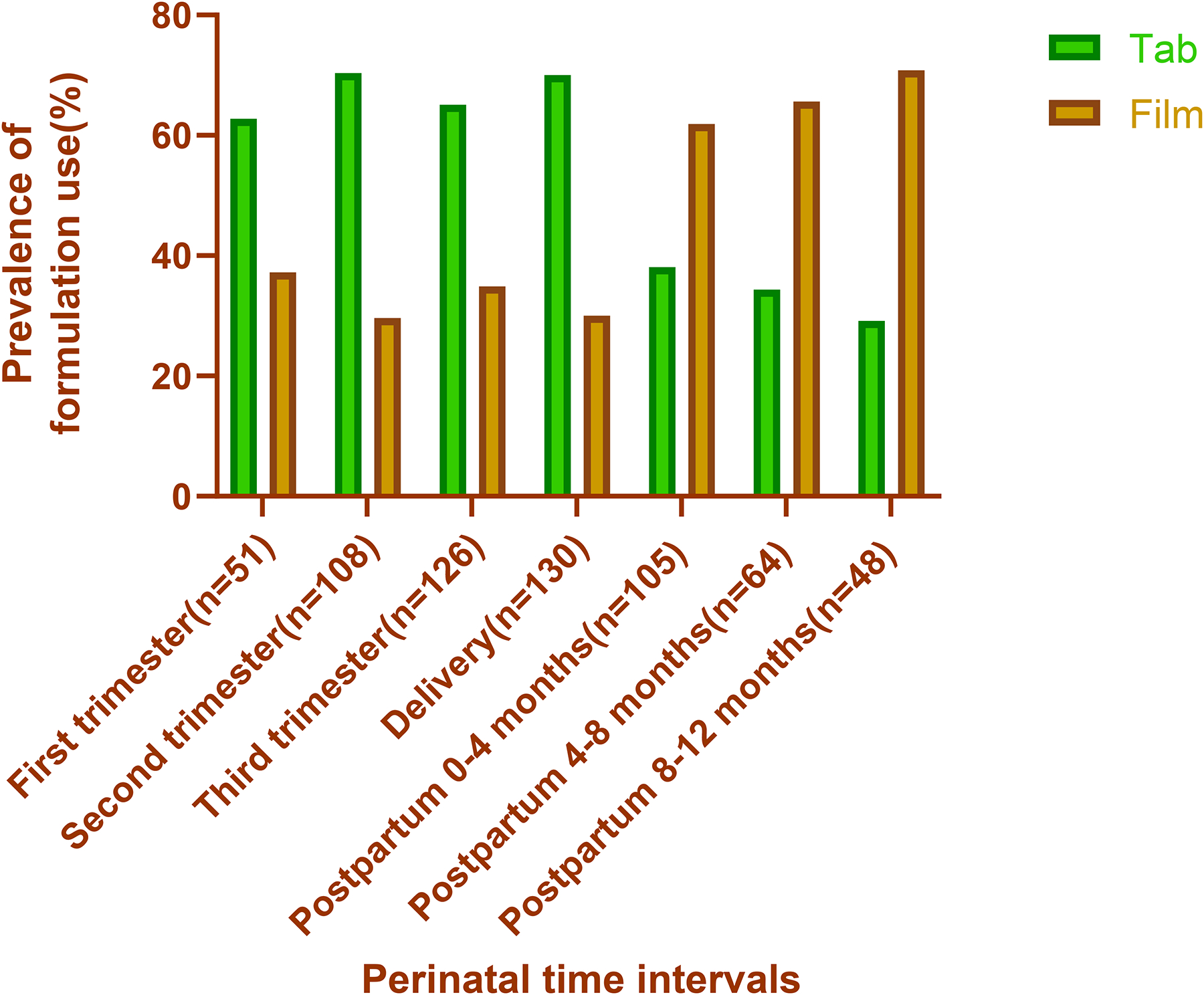
Prevalence of buprenorphine tablet versus film use through the perinatal period by time interval (N=240) with the green column representing tablet and orange representing film. Use of tab varied throughout pregnancy, then steadily decreased in the postpartum period. Use of film varied throughout pregnancy, then steadily increased postpartum and was more frequently used than tablet.
Regarding dosing frequency during pregnancy (Figure 8), more women used once daily dosing during the first trimester (62.8%, compared to 29.4% for twice and 7.84% for thrice daily). This percentage decreased in the second and third trimesters (57.4% and 49.2%, respectively); meanwhile, the percentages of women using twice daily dosing increased in the second and third trimesters (33.3%, 34.1%, respectively), as did the percentages of those using thrice daily dosing (9.3%, 16.7%, respectively). Despite this gradual increase of dosing frequency throughout pregnancy, however, at delivery, most women (77.7%) used once daily dosing, compared to 13.1% at twice daily and 9.2% at thrice.
Fig. 8.
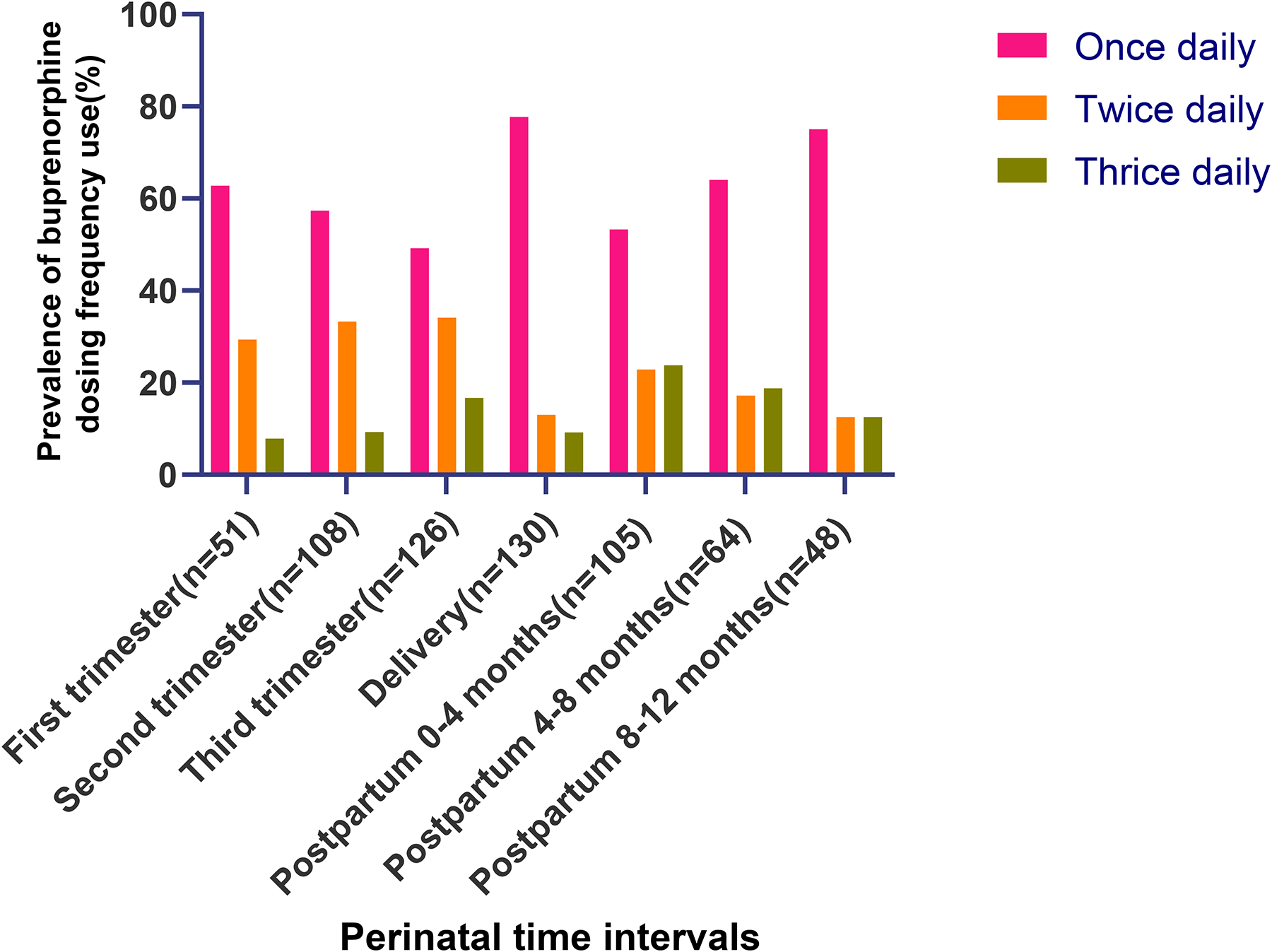
Prevalence of buprenorphine dosing frequencies used through the perinatal period by pregnancy or postpartum time interval (N=240) with the pink column representing once daily dosing, orange twice daily and green thrice daily dosing. Prevalence of split dosing (twice or thrice daily) increased from trimester 1 to 3 then decreased steadily through the postpartum period.
After delivery, frequency of dosing declined steadily overall. During months 0–4 postpartum, 53.3% of the participants used once daily dosing, compared to 22.9% twice daily and 23.8% thrice. Dosing frequency changed to 64.1% once daily, 17.2% twice, and 18.8% thrice, during months 4–8 postpartum. This steady decrease overall of dosing frequency continued into 8–12 months postpartum, with 75.0% of women using once daily dosing, 12.5% twice, and 12.5% thrice, during this time period. Regarding patients who provided frequency data throughout all three postpartum time intervals (data not shown): 27 stayed at the same dosing frequency, 1 increased from once to twice daily, 3 decreased from thrice to once daily, and 2 decreased from twice to once daily. All of the frequency changes that occurred for these participants took place between 0–4 months and 4–8 months postpartum.
Discussion
The existing evidence base to inform clinical guidelines for buprenorphine dosing during pregnancy is limited, and even more so for the postpartum period. Prenatal buprenorphine use is increasing across regions nationally [8], with a substantial contribution by providers in primary care and women’s health responding to the opioid crisis. Medication for opioid use disorder use during pregnancy and postpartum decreases overdose risk [6] and improves both maternal and child outcomes [34 •]. Increased utilization and effectiveness of opioid use disorder treatments for pregnant and postpartum women is urgently needed as opioid overdose has become a leading cause of death for women during pregnancy and through 12 months after delivery [3]. Our findings from the current literature and our institution indicate that dose changes through the perinatal period are prevalent and vary widely but with distinct identified trends, highlighting the need for more research to guide effectively individualizing buprenorphine regimens for this high-risk population.
Our review of the current literature and our retrospective study of our clinical population suggest that generally, most women who are pregnant undergo an increase in total daily buprenorphine dose with split dosing (e.g., twice or three times daily dosing) as pregnancy progresses. However, dose changes need to be individualized as not all patients follow the same trends. This is not surprising given the known changes in maternal physiology of pregnancy, largely in metabolism [9•]. Other contributions to these observed changes during pregnancy include salivary pH affecting sublingual absorption and increased distribution within the bloodstream [9•]. Further, our findings parallel what is described in the literature for methadone. Before the pinnacle MOTHER trial [7], methadone was the only recommended medication for treatment of opioid use disorder in pregnancy. Consequently, more data about dosing in the perinatal period exists for methadone than buprenorphine. A review of the methadone literature similarly demonstrates a need to increase methadone total dose and dose frequency throughout pregnancy. One study of 5 pregnant women prescribed methadone throughout pregnancy found that the average dose increase required between the first and second trimester was 23 mg then between the second and third trimester 19 mg [35]. Further, a higher average daily dose of methadone is associated with better treatment retention during pregnancy [36]. For buprenorphine, higher doses are associated with improved treatment outcomes in non-pregnant populations [37]. Even though this association is likely also true for pregnant and postpartum women, it has not been confirmed [11 •], nor has associations between dose changes and treatment outcomes been investigated, an important gap in research, as dose changes are more common through the perinatal period than other times across the lifecourse.
Buprenorphine providers should engage with patients frequently throughout pregnancy to assess their clinical status, such as increases in withdrawal symptoms or cravings. Patients should be given anticipatory guidance at the beginning of pregnancy and reminded as time goes on to alert their providers if they have signs or symptoms of undertreatment. If this occurs, shared decision making should occur between the patient and provider regarding changes in dosing which may involve increasing the total daily dose and splitting the dosing frequency at the same time whereas providers typically may only proceed with one such dosing change at a time for non-pregnant patients. In these discussions, it is extremely important to inform patients of the clear evidence that higher buprenorphine dose is not correlated with worsened severity of neonatal withdrawal [7]. Some patients may not be aware of this, leading them to not alert providers about their symptoms out of fear for fetal risk, placing them at risk of compromised recovery without a benefit to their fetus.
Given that the postpartum period is particularly high risk for disease recurrence for women with opioid use disorder [4], medication for opioid use disorder is recommended to continue postpartum [5 •]. However, how to optimize buprenorphine dosing through the postpartum period to support recovery is not known. Our findings together with existing literature demonstrate that postpartum dose changes vary widely and need to be individualized, just as for methadone [35]. Importantly, set protocols to decrease buprenorphine dose or switch to once daily from split dosing frequency after delivery should not be routinely implemented. Literature regarding methadone dosing after delivery is more limited than that for pregnancy but indicate that dose changes do need to be individualized, yet dose increases are rare. While many women will need to decrease their methadone dose postpartum, not all will need to do so [38 •]. Also, dose decreases typically are not drastic, and over-sedation events are rare even with these small decreases [39]. We know that a large driver of the variability in methadone dosing stems from genetic factors [40 •]; more research on factors associated with need for buprenorphine dose changes through the pregnancy to postpartum transition is much needed.
While the need for increases in methadone dosing postpartum are rare, this does not appear to be true for buprenorphine. While approximately half of our study population remained at the same buprenorphine dose after delivery, over a third increased their dose with the remainder undergoing a dose decrease. This is an area in great need of investigation. One explanation for this variability in dose changes could be buprenorphine’s role in mood, notably its contribution to alleviating negative affect as a kappa receptor antagonist [41 •]. For example, promising translational work notes how this mechanism could be harnessed as a treatment for chronic pain disorders as it targets the complex emotional aspect of pain (e.g., catastrophizing) seen more commonly in women than men [42]. Postpartum mood lability is very common for all women [43], especially women with pre-existing mood disorders, which are extremely prevalent among women with opioid use disorder [44]. Thus, given its potential antidepressant properties [41 •], it would not be surprising that some women may need increases in buprenorphine after delivery to maintain opioid use disorder stability in the setting of worsening mood. Therefore, providers caring for postpartum women should frequently assess patients not only for signs or symptoms of opioid use disorder over or undertreatment with buprenorphine but also for mood disorders (e.g., using a validated tool such as the Edinburgh Postnatal Depression Scale [45]). For women with co-occurring mood disorders, such as anxiety or depression, postpartum increases in buprenorphine doses could be effective adjuncts to not only achieve mood stability but also reduce substance use recurrence which together can strengthen recovery through this high-risk period. However, buprenorphine alone may not treat increases in depression and adjunctive antidepressant medications may be needed.
The standard of care with non-pregnant patients is to prescribe the combination buprenorphine-naloxone (Suboxone®) film. Historically, clinical practice has been to give buprenorphine monoproduct sublingual tabs during pregnancy due to the concerns regarding the effects of the naloxone on the fetus. A major driver of this practice continuing despite lack of evidence indicating fetal harm is how the few buprenorphine clinical trials during pregnancy have only used the monoproduct tabs [7]. This lack of level 1 evidence is common for many medications during pregnancy, such as psychiatric medications, due to the limited number of pregnant women enrolled in clinical trials and ethical challenges. In these circumstances, robust observational data generally becomes the accepted quality standard by which providers can inform their clinical practices and incorporate into shared decision-making discussions with their patients. Regarding buprenorphine, multiple observational studies indicate the safety and efficacy of buprenorphine-naloxone during pregnancy for both the mother and the fetus [46–50 •]. These findings make pharmacologic sense as the naloxone component has minimal biological activity when taken sublingually. Generally, perinatal addiction specialists now promote pregnant patients using the combination buprenorphine-naloxone. At our institution, our current clinical practice is to not switch patients from buprenorphine-naloxone to buprenorphine during pregnancy, and all patients starting treatment during pregnancy are placed on buprenorphine-naloxone. Exceptions to this clinical practice generally are the same as for non-pregnant patients, such as a documented allergy to naloxone. Further, we have found that for some patients who need to switch to buprenorphine-naloxone postpartum from being on buprenorphine during pregnancy, this change can cause anxiety and stress in an already vulnerable time with regards to maintaining treatment continuation and recovery. Regarding tab versus film formulation, almost all our pregnant, like non-pregnant, patients are on buprenorphine-naloxone films as these are preferred not only by providers (minimize diversion) but also by payers (Medicaid preferred product in Virginia). With the nausea and vomiting of pregnancy, we find that some patients tolerate the tab better than the film, but these changes are made on an individual basis. Overall, based on the available literature, there is evidence to support not transitioning patients between buprenorphine formulations (mono versus combination product, tab versus film) unless indicated for an individual patient’s needs due to the lack of proven fetal harm and the possible risks to maternal stability in recovery through a known high-risk timeframe.
There are some inconsistencies in the literature and clinical recommendations regarding buprenorphine management through the perioperative state. The obstetric literature has been a pioneer among US based studies focused on this issue recommending continuation of buprenorphine through the delivery, including for cesareans [51]. For example, a retrospective cohort study of 185 women on methadone and 88 women on buprenorphine undergoing cesarean delivery found no significant differences in opioid pain requirements through pre, intra and post-operative periods between these two groups [52]. It is not clinical practice to routinely taper patients’ methadone doses before an anticipated painful episode such as surgery; evidence such as this supports managing patients on buprenorphine similarly [52]. There are substantial risks to the mother and fetus with a buprenorphine taper before delivery, including withdrawal and recurrence of substance use, and there is not consistent evidence illustrating compromised pain outcomes with peri-operative buprenorphine continuation. Resultantly, the SAMHSA Clinical Guidance for Treating Pregnant and Parenting Women and their Infants in 2018 included maintaining buprenorphine at the same total daily dose throughout the delivery and immediate postpartum periods in their recommendations [5 •]. Further, recent evidence [53] and clinical guidelines [54 •] support this same practice for non-pregnant patients undergoing surgery except in unique circumstances. Overall, pain management plans should be individualized through collaboration of a multi-disciplinary team that includes the patient. Multi-modal therapies (e.g., regional anesthesia, non-opioid medications) should be maximized, and if opioids are needed for pain control, they should not be withheld. Many patients maintained on buprenorphine may need higher doses and/or more frequent dosing of opioids than people not on opioid agonist therapy. For example, medication regimens used in routine order-sets should be tailored for these patients; instead of oxycodone 5 mg every 6 hours as needed for severe pain, patients on buprenorphine may need oxycodone 10 mg every 4 hours for adequate pain control. At discharge, patients should be provided not only with enough buprenorphine to get them to their next addiction provider appointment but also enough opioid and non-opioid pain medications to provide adequate pain control until follow-up with the surgeon. The amount of opioid prescribed should be consistent with the individual patient’s needs at the time of discharge and be decided upon using shared decision making between provider and patient, similar to recommendations in non-opioid use disorder populations [55].
In addition to these clinical practices, we have all pregnant women on medication for opioid use disorder undergo a prenatal consult with Obstetrics anesthesia and recovery-based pain counseling session with the Obstetrics/Gynecology-Addiction specialist. Pain management plans are documented clearly in the patient’s chart to be reviewed by all providers (and the patient) before delivery. All patients continue buprenorphine at the same total daily dose throughout delivery and the immediate postpartum period. For patients undergoing cesarean delivery, we commonly leave epidurals in place for 12–24 hours post-operatively with a non-opioid anesthetic, provide scheduled acetaminophen and NSAID as well as needed opioids (dose and frequency individualized). For patients who have complex pain histories (e.g., superimposed chronic pain disorder), we discuss with the patient the option of splitting the total daily dose of buprenorphine post-operatively into four times daily dosing (e.g., total daily dose of 24 mg: 6 mg every 6 hours) to take advantage of its potential analgesic effects. In line with recovery-oriented care and harm reduction, follow-up appointments are arranged before discharge for 1 (all cesarean deliveries) to 2 weeks later with both the addiction and Obstetrics/Gynecology providers. If patients have a cesarean section, opioid medication is provided at discharge based on patient needs and preferences. Prescriptions are filled with the hospital pharmacy for buprenorphine, non-opioid and opioid pain medications, and a naloxone kit (if does not already have one) before discharge. Patients are counseled to consider having a family member manage their opioid medications and store them in a lock box. We instruct patients to continue their buprenorphine daily, bring their opioid pill bottles and buprenorphine-naloxone wrappers to follow-up visits for counts where routine urine toxicology is also performed. We have found that patients are highly satisfied with this combination of providing anticipatory guidance through the prenatal and postpartum periods about pain management in a recovery-oriented manner.
There are several limitations to this study. Data from our analysis of buprenorphine dosing was abstracted retrospectively from the medical record. This carries inherent information bias as this data was not collected for research but rather for clinical and billing purposes. For example, the timing pertaining to each buprenorphine dosing data point through pregnancy and postpartum was assigned based on available clinical data. Delivery dates were estimated for patients who did not deliver at VCU which may have skewed the timing of buprenorphine dose changes after delivery. Gestational ages corresponding to buprenorphine dosages during pregnancy were estimated by the assigned delivery dates, not due dates, which could have skewed timing of buprenorphine dose changes before delivery (e.g., patients who delivered preterm). Nonetheless, we used clinical data from multiple sources within our electronic medical record to calculate assigned delivery dates to as much accuracy as possible. In doing so, these limitations likely led to timing inaccuracies by days, or at most weeks, but not months. Similarly, we are only able to provide a clinical context on an institutional level for our results which is not as detailed as would be captured using a database collected for research purposes at an individual level. Observational studies and clinical trials following women prospectively are needed to fill these gaps in knowledge. Our study was conducted at a single institution and may not be generalizable to other regions. However, a strength of our study is that our institution is a large academic medical center with a wide referral base from a diverse patient population socio-demographically. With the goal of informing future research, our study is one of the first to describe buprenorphine dose changes and prevalence of buprenorphine formulation usage over time throughout pregnancy and postpartum.
Conclusions
Providers who care for women through the perinatal period follow accepted recommendations to modify dosing of many common medications through pregnancy and postpartum. These dosing modifications commonly involve increasing total daily dose and splitting dosing through pregnancy then decreasing total daily dose postpartum. Given physiological fluctuations common in the peripartum transition, buprenorphine dose changes are common throughout pregnancy and postpartum, but clinical providers are without data or laboratory markers to inform buprenorphine dosing decisions. We found in our literature review and analysis from our institution’s clinical population that buprenorphine dose changes across individuals do not follow consistent patterns. Although many women increase their buprenorphine total daily dose during pregnancy, not all decrease buprenorphine postpartum. Altogether, there is data to support that buprenorphine dosing should be individualized through pregnancy and postpartum with frequent evaluations by providers and solicited input from patients. Further research in identifying who for and when to change buprenorphine dosing perinatally is urgently needed to equip providers with evidence-based recommendations they can use to better support their patients in opioid use disorder recovery through this critical part of the lifecourse.
Acknowledgement:
The authors wish to thank Dr. Stephen Strakowski for reviewing their manuscript.
Funding: This study was supported by UL1TR002649 and U54DA038999 as well as KL2TR002648.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: Dr. Moeller has research funding from Indivior Pharmaceuticals and Nektar Therapeutics and is a consultant for Astellas and Individor for work unrelated to this study. The authors have no other conflicts of interest.
Availability of data and material: Data was obtained from the VCU medical record, and anonymous level data are available upon request.
Code availability: Database queries were run against customized VCU data and are available upon request.
References:
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.Woolf SH and Schoomaker H (2019). “Life Expectancy and Mortality Rates in the United States, 1959–2017.” Jama 322(20): 1996–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gemmill A, Kiang MV and Alexander MJ (2019). “Trends in pregnancy-associated mortality involving opioids in the United States, 2007–2016.” Am J Obstet Gynecol 220(1): 115–116. [DOI] [PubMed] [Google Scholar]
- 3•.Nielsen T, Bernson D, Terplan M, Wakeman SE, Yule AM, Mehta PK, Bharel M, Diop H, Taveras EM, Wilens TE and Schiff DM (2019). “Maternal and infant characteristics associated with maternal opioid overdose in the year following delivery.” Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]; Well executed study elucidating the postpartum period as a particularly vulnerable time for patients with opioid use disorder.
- 4.Smid MC, Stone NM, Baksh L, Debbink MP, Einerson BD, Varner MW, Gordon AJ and Clark EAS (2019). “Pregnancy-Associated Death in Utah: Contribution of Drug-Induced Deaths.” Obstet Gynecol 133(6): 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SAMHSA (2018). Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants HHS Publication No. (SMA) 18–5054 Rockville, MD, Substance Abuse and Mental Health Services Administration. [Google Scholar]; National recommendations by SAMHSA for management of opioid use disorder during pregnancy, postpartum and infants exposed to opioids in utero.
- 6.Schiff DM, Nielsen T, Terplan M, Hood M, Bernson D, Diop H, Bharel M, Wilens TE, LaRochelle M, Walley AY and Land T (2018). “Fatal and Nonfatal Overdose Among Pregnant and Postpartum Women in Massachusetts.” Obstet Gynecol 132(2): 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR and Fischer G (2010). “Neonatal abstinence syndrome after methadone or buprenorphine exposure.” N Engl J Med 363(24): 2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krans EE, Kim JY, James AE 3rd, Kelley D and Jarlenski MP (2019). “Medication-Assisted Treatment Use Among Pregnant Women With Opioid Use Disorder.” Obstet Gynecol 133(5): 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Bastian JR, Chen H, Zhang H, Rothenberger S, Tarter R, English D, Venkataramanan R and Caritis SN (2017). “Dose-adjusted plasma concentrations of sublingual buprenorphine are lower during than after pregnancy.” Am J Obstet Gynecol 216(1): 64.e61–64.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides important evidence for how buprenorphine is metabolized differently during pregnancy.
- 10.Wakeman SE and Barnett ML (2018). “Primary Care and the Opioid-Overdose Crisis - Buprenorphine Myths and Realities.” N Engl J Med 379(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 11•.O’Connor AB, Uhler B, O’Brien LM and Knuppel K (2018). “Predictors of treatment retention in postpartum women prescribed buprenorphine during pregnancy.” J Subst Abuse Treat 86: 26–29. [DOI] [PubMed] [Google Scholar]; Demonstrates improvement in opioid use treatment retention postpartum when co-morbid depression and anxiety are treated with antidepressants.
- 12.Kahila H, Saisto T, Kivitie-Kallio S, Haukkamaa M and Halmesmaki E (2007). “A prospective study on buprenorphine use during pregnancy: effects on maternal and neonatal outcome.” Acta Obstet Gynecol Scand 86(2): 185–190. [DOI] [PubMed] [Google Scholar]
- 13.Welle-Strand GK, Skurtveit S, Tanum L, Waal H, Bakstad B, Bjarko L and Ravndal E (2015). “Tapering from Methadone or Buprenorphine during Pregnancy: Maternal and Neonatal Outcomes in Norway 1996–2009.” Eur Addict Res 21(5): 253–261. [DOI] [PubMed] [Google Scholar]
- 14•.Caritis SN, Bastian JR, Zhang H, Kalluri H, English D, England M, Bobby S and Venkataramanan R (2017). “An evidence-based recommendation to increase the dosing frequency of buprenorphine during pregnancy.” Am J Obstet Gynecol 217(4): 459.e451–459.e456. [DOI] [PMC free article] [PubMed] [Google Scholar]; Further evidence for buprenorphine concentrations in plasma being lower in pregnancy than in non-pregnancy.
- 15.Ross D (2004). “High dose buprenorphine in pregnancy.” Aust N Z J Obstet Gynaecol 44(1):80. [DOI] [PubMed] [Google Scholar]
- 16.Concheiro M, Jones HE, Johnson RE, Choo R and Huestis MA (2011). “Preliminary buprenorphine sublingual tablet pharmacokinetic data in plasma, oral fluid, and sweat during treatment of opioid-dependent pregnant women.” Ther Drug Monit 33(5): 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Concheiro M, Jones HE, Johnson RE, Choo R, Shakleya DM and Huestis MA (2010). “Maternal buprenorphine dose, placenta buprenorphine, and metabolite concentrations and neonatal outcomes.” Ther Drug Monit 32(2): 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin RS, Wilkins DG, Averin O, Choo RE, Schroeder JR, Jasinski DR, Johnson RE, Jones HE and Huestis MA (2007). “Buprenorphine and norbuprenorphine in hair of pregnant women and their infants after controlled buprenorphine administration.” Clin Chem 53(12): 2136–2143. [DOI] [PubMed] [Google Scholar]
- 19.Coker JL, Catlin D, Ray-Griffith S, Knight B and Stowe ZN (2018). “Buprenorphine medication-assisted treatment during pregnancy: An exploratory factor analysis associated with adherence.” Drug Alcohol Depend 192: 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Lo-Ciganic WH, Donohue JM, Kim JY, Krans EE, Jones BL, Kelley D, James AE and Jarlenski MP (2019). “Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States.” Pharmacoepidemiol Drug Saf 28(1): 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recommends escalation of buprenorphine dose in pregnancy due to apparent increased clearance.
- 21.Ilett KF, Hackett LP, Gower S, Doherty DA, Hamilton D and Bartu AE (2012). “Estimated dose exposure of the neonate to buprenorphine and its metabolite norbuprenorphine via breastmilk during maternal buprenorphine substitution treatment.” Breastfeed Med 7: 269–274. [DOI] [PubMed] [Google Scholar]
- 22.Lindemalm S, Nydert P, Svensson JO, Stahle L and Sarman I (2009). “Transfer of buprenorphine into breast milk and calculation of infant drug dose.” J Hum Lact 25(2): 199–205. [DOI] [PubMed] [Google Scholar]
- 23.Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, Langer M and Aschauer HN (2000). “Treatment of opioid-dependent pregnant women with buprenorphine.” Addiction 95(2): 239–244. [DOI] [PubMed] [Google Scholar]
- 24.Lacroix I, Berrebi A, Chaumerliac C, Lapeyre-Mestre M, Montastruc JL and Damase-Michel C (2004). “Buprenorphine in pregnant opioid-dependent women: first results of a prospective study.” Addiction 99(2): 209–214. [DOI] [PubMed] [Google Scholar]
- 25.Simmat-Durand L, Lejeune C and Gourarier L (2009). “Pregnancy under high-dose buprenorphine.” Eur J Obstet Gynecol Reprod Biol 142(2): 119–123. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix I, Berrebi A, Garipuy D, Schmitt L, Hammou Y, Chaumerliac C, Lapeyre-Mestre M, Montastruc JL and Damase-Michel C (2011). “Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study.” Eur J Clin Pharmacol 67(10): 1053–1059. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor A, Alto W, Musgrave K, Gibbons D, Llanto L, Holden S and Karnes J (2011). “Observational study of buprenorphine treatment of opioid-dependent pregnant women in a family medicine residency: reports on maternal and infant outcomes.” J Am Board Fam Med 24(2): 194–201. [DOI] [PubMed] [Google Scholar]
- 28.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M and Aschauer H (2006). “Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study.” Addiction 101(2): 275–281. [DOI] [PubMed] [Google Scholar]
- 29.Kacinko SL, Jones HE, Johnson RE, Choo RE and Huestis MA (2008). “Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes.” Clin Pharmacol Ther 84(5): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kacinko SL, Jones HE, Johnson RE, Choo RE, Concheiro-Guisan M and Huestis MA (2009). “Urinary excretion of buprenorphine, norbuprenorphine, buprenorphine-glucuronide, and norbuprenorphine-glucuronide in pregnant women receiving buprenorphine maintenance treatment.” Clin Chem 55(6): 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindler SD, Eder H, Ortner R, Rohrmeister K, Langer M and Fischer G (2003). “Neonatal outcome following buprenorphine maintenance during conception and throughout pregnancy.” Addiction 98(1): 103–110. [DOI] [PubMed] [Google Scholar]
- 32.Fischer G, Etzersdorfer P, Eder H, Jagsch R, Langer M and Weninger M (1998). “Buprenorphine maintenance in pregnant opiate addicts.” Eur Addict Res 4 Suppl 1: 32–36. [DOI] [PubMed] [Google Scholar]
- 33.Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, Kissin WB, Alpan G, Lantz ME, Cone EJ, Wilkins DG, Golden AS, Huggins GR and Lester BM (2001). “Buprenorphine treatment of pregnant opioid--dependent women: maternal and neonatal outcomes.” Drug Alcohol Depend 63(1): 97–103. [DOI] [PubMed] [Google Scholar]
- 34•.Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, Campopiano M and Jones HE (2017). “Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance.” J Addict Med 11(3): 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]; Good discussion of the positive impacts of medication for opioid use disorder for both mothers and developing fetuses.
- 35.Bogen DL, Perel JM, Helsel JC, Hanusa BH, Romkes M, Nukui T, Friedman CR and Wisner KL (2013). “Pharmacologic evidence to support clinical decision making for peripartum methadone treatment.” Psychopharmacology (Berl) 225(2): 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilder C, Lewis D, and Winhusen T (2015). “Medication assisted treatment discontinuation in pregnant and postpartum women with opioid use disorder.” Drug Alcohol Depend 149:225–31. [DOI] [PubMed] [Google Scholar]
- 37.Mattick RP, Breen C, Kimber J and Davoli M (2014). “Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence.” Cochrane Database Syst Rev(2): Cd002207. [DOI] [PubMed] [Google Scholar]
- 38•.McCarthy JJ, E. J., Leamon MH, Graas J, Ward C and Fassbender C (2018). “The Use of Serum Methadone/Metabolite Ratios to Monitor Changing Perinatal Pharmacokinetics.” J Addict Med 12(3): 241–246. [DOI] [PubMed] [Google Scholar]; Advocates for increased dosing in methadone throughout pregnancy, which lays the foundation for increased dosing in buprenorphine as well.
- 39.Pace CA, Kaminetzky LB, Winter M, Cheng DM, Saia K, Samet JH and Walley AY (2014). “Postpartum changes in methadone maintenance dose.” J Subst Abuse Treat 47(3): 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Crist RC, Clarke TK and Berrettini WH (2018). “Pharmacogenetics of Opioid Use Disorder Treatment.” CNS Drugs 32(4): 305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]; Good discussion of the genetics of opioid use disorder and how that affects treatment with medication.
- 41•.Serafini G, Adavastro G, Canepa G, De Berardis D, Valchera A, Pompili M, Nasrallah H and Amore M (2018). “The Efficacy of Buprenorphine in Major Depression, Treatment-Resistant Depression and Suicidal Behavior: A Systematic Review.” Int J Mol Sci 19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]; Important discussion of buprenorphine’s adjunctive use in treating depression, so often co-morbid with opioid use disorder.
- 42.Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM and Cahill CM (2019). “Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain.” J Neurosci 39(21): 4162–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G and Swinson T (2005). “Perinatal depression: a systematic review of prevalence and incidence.” Obstet Gynecol 106(5 Pt 1): 1071–1083. [DOI] [PubMed] [Google Scholar]
- 44.Benningfield MM, Dietrich MS, Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Fischer G and Martin PR (2012). “Opioid dependence during pregnancy: relationships of anxiety and depression symptoms to treatment outcomes.” Addiction 107 Suppl 1: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox JL, Holden JM and Sagovsky R (1987). “Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale.” Br J Psychiatry 150: 782–786. [DOI] [PubMed] [Google Scholar]
- 46.Debelak K, Morrone WR, O’Grady KE and Jones HE (2013). “Buprenorphine + naloxone in the treatment of opioid dependence during pregnancy-initial patient care and outcome data.” Am J Addict 22(3): 252–254. [DOI] [PubMed] [Google Scholar]
- 47.Wiegand SL, Stringer EM, Stuebe AM, Jones H, Seashore C and Thorp J (2015). “Buprenorphine and naloxone compared with methadone treatment in pregnancy.” Obstet Gynecol 125(2): 363–368. [DOI] [PubMed] [Google Scholar]
- 48.Jumah NA, Edwards C, Balfour-Boehm J, Loewen K, Dooley J, Gerber Finn L and Kelly L (2016). “Observational study of the safety of buprenorphine+naloxone in pregnancy in a rural and remote population.” BMJ Open 6(10): e011774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen L, Lander LR, O’Grady KE, Marshalek PJ, Schmidt A, Kelly AK and Jones HE (2018). “Treating women with opioid use disorder during pregnancy in Appalachia: Initial neonatal outcomes following buprenorphine + naloxone exposure.” Am J Addict 27(2): 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Mullins N, Galvin SL, Ramage M, Gannon M, Lorenz K, Sager B and Coulson CC (2019). “Buprenorphine and Naloxone Versus Buprenorphine for Opioid Use Disorder in Pregnancy: A Cohort Study.” J Addict Med. Rationale for the differences in monoproduct versus combination product in pregnancy [DOI] [PubMed] [Google Scholar]
- 51.Meyer M, Paranya G, Keefer Norris A and Howard D (2010). “Intrapartum and postpartum analgesia for women maintained on buprenorphine during pregnancy.” Eur J Pain 14(9): 939–943. [DOI] [PubMed] [Google Scholar]
- 52.Vilkins AL, Bagley SM, Hahn KA, Rojas-Miguez F, Wachman EM, Saia K and Alford DP (2017). “Comparison of Post-Cesarean Section Opioid Analgesic Requirements in Women With Opioid Use Disorder Treated With Methadone or Buprenorphine.” J Addict Med 11(5): 397–401. [DOI] [PubMed] [Google Scholar]
- 53.Mehta D, Thomas V, Johnson J, Scott B, Cortina S and Berger L (2020). “Continuation of Buprenorphine to Facilitate Postoperative Pain Management for Patients on Buprenorphine Opioid Agonist Therapy.” Pain Physician 23(2): E163–e174. [PubMed] [Google Scholar]
- 54•.Goel A, Azargive S, Weissman JS, Shanthanna H, Hanlon JG, Samman B, Dominicis M, Ladha KS, Lamba W, Duggan S, Di Renna T, Peng P, Wong C, Sinha A, Eipe N, Martell D, Intrater H, MacDougall P, Kwofie K, St-Jean M, Rashiq S, Van Camp K, Flamer D, Satok-Wolman M and Clarke H (2019). “Perioperative Pain and Addiction Interdisciplinary Network (PAIN) clinical practice advisory for perioperative management of buprenorphine: results of a modified Delphi process.” Br J Anaesth 123(2): e333–e342. [DOI] [PMC free article] [PubMed] [Google Scholar]; Well devised guidelines for peri-operative pain management for patients on buprenorphine.
- 55.Prabhu M, McQuaid-Hanson E, Hopp S, Burns SM, Leffert LR, Landau R, Lauffenburger JC, Choudhry NK, Kaimal A and Bateman BT (2017). “A Shared Decision-Making Intervention to Guide Opioid Prescribing After Cesarean Delivery.” Obstet Gynecol 130(1): 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


