Abstract
Objective:
Upper airway injury is a recognized complication of prolonged endotracheal intubation, yet little attention has been paid to the consequences of laryngeal injury its functional impact. The purpose of our study was to prospectively define the incidence of acute laryngeal injury and investigate the impact of injury on breathing and voice outcomes.
Design:
Prospective cohort study.
Setting:
Tertiary referral critical care center.
Patients:
Consecutive adult patients intubated greater than 12 hours in the medical intensive care unit from August 2017 through May 2018 who underwent laryngoscopy within 36 hours of extubation.
Interventions:
Laryngoscopy following endotracheal intubation.
Measurements and Main Results:
One hundred consecutive patients (62% male, median age 58.5 years) underwent endoscopic examination after extubation. Acute laryngeal injury (i.e. mucosal ulceration or granulation tissue in the larynx) was present in 57 patients (57%). Patients with laryngeal injury had significantly worse patient-reported breathing (CCQ median 1.05, IQR 0.48–2.10) and vocal symptoms (VHI-10 median 2, IQR 0–6) compared to patients without injury (CCQ median 0.20, IQR 0–0.80; p<0.001; VHI-10 median 0, IQR 0–1; p=0.005). Multivariable logistic regression independently associated diabetes, body habitus, and endotracheal tube size greater than 7.0 with the development of laryngeal injury.
Conclusions:
Acute laryngeal injury occurs in more than half of patients who receive mechanical ventilation and is associated with significantly worse breathing and voicing ten weeks after extubation. An endotracheal tube greater than size 7.0, diabetes, and larger body habitus may predispose to injury. Our results suggest that acute laryngeal injury impacts functional recovery from critical illness.
Keywords: Dyspnea, Laryngostenosis, Laryngoscopy, Intubation, Mechanical Ventilation
Introduction
In the United States, six million people, or 2% of the population, are admitted to an intensive care unit (ICU) each year.(1, 2) Respiratory failure is the most common reason for admission and over a third of patients require mechanical ventilation.(3) Although 50% to 70% of patients survive their acute illness, many develop persistent disabilities that limit return to their pre-illness functioning.(4–6) Little attention has been paid to the laryngeal consequences of endotracheal intubation and how acute laryngeal injury (ALgI) may impair functional recovery from critical illness.(8)
Transient dysphonia, dysphagia, and sore throat are common post-procedural complications after intubation during surgery.(9, 10) In contrast, critically ill patients experience longer durations of mechanical ventilation and have a greater prevalence of conditions thought to predispose to laryngeal complications.(11–18) It has long been recognized that endotracheal tubes (ETT) are forced posteriorly in the larynx by the tongue base and lordosis of the cervical vertebral column. This deforms the tube into an S-like shape with the posterior glottis acting as a fulcrum.(19) Laryngeal injury during mechanical ventilation occurs at the mucosal interface of the ETT and posterior glottis and can progress to fibrosis, restricted glottic mobility, and ventilation impairment that dramatically impacts quality of life.(20–25) While laryngeal injury after prolonged intubation has been recognized for decades, several barriers have limited rigorous investigation into risk factors for disease development or linked injury to clinical outcomes. Because the larynx is rarely examined after extubation in the ICU and laryngeal injury may initially present with minor clinical symptoms, restricted glottic mobility has historically been identified after hospital discharge, leaving critical care specialists who participate in frontline care unaware of the scope of the issue.
We designed a single-institution, prospective cohort study to define the incidence of ALgI following mechanical ventilation and to evaluate potential risk factors for laryngeal injury. We additionally collected patient-reported outcome measures ten weeks after hospital discharge to establish the impact of ALgI on breathing and voice outcomes. With these efforts, we sought to investigate if ALgI may represent an unrecognized component of PICS.
Materials and Methods
We conducted an Institutional Review Board (IRB No. 171066) approved, single-center, prospective, observational study of patients who received mechanical ventilation in the medical ICU at Vanderbilt University Medical Center. In accordance with the Declaration of Helsinki, written informed consent was obtained from the patient or a legal surrogate in the case of mental incapacity.
Study Design
Consecutive adult (≥18 years of age) patients intubated for greater than 12 hours in the medical ICU between August 1, 2017 and May 30, 2018 at a single center were screened for enrollment. Patients with an established tracheostomy prior to mechanical ventilation, known laryngeal or tracheal pathology, or a history of head and neck radiation were excluded. Within 36 hours of extubation, eligible patients were recruited and underwent informed consent prior to enrollment. The study was undertaken for a duration to include 100 patients.
Laryngeal Endoscopy
Enrolled patients underwent endoscopic examination of their larynx with flexible nasolaryngoscopy (Karl Storz Flexible Rhino-Laryngoscope, Tuttlingen, Germany). Video and photographic evaluation was collected using an iPhone five (Cupertino, California, USA) with a Karl Storz Smart Scope, Smartphone adapter. Each exam was uploaded securely via Box Capture (Redwood City, CA, USA) for subsequent adjudication.
Study Population
Baseline characteristics and functional status were recorded for each case and weighted comorbidities as a measure of general health were assessed with the Charlson Comorbidity Index (CCI).(26) The details of intubation, admitting ICU diagnosis, and APACHE II scores, were abstracted from the electronic medical record.(27, 28) Additionally, the presence of delirium, hypotension, vasopressor requirement, acute kidney injury (AKI), pneumonia (PNA), acute respiratory distress syndrome (ARDS), and systemic steroid use during the period of intubation were collected. Delirium and hypotension were defined by standardized criteria.(29, 30) Vasopressor therapy was defined as medical pressor use of at least eight hours. This study did not provide any recommendations during the peri-extubation period and systemic steroids were used at the discretion of the ICU care team. Finally, we initially sought to record ETT cuff pressure greater than 30 mm Hg, however, due to institutional protocol with regular monitoring by respiratory therapists, all patients had minimal cuff pressure <30 mm Hg.
Care of the Intubated Patient
Treating clinicians determine all management prior to extubation, including the approach to sedation, timing of spontaneous breathing and awakening trials, and readiness for extubation. In our ICU ETT tubes are secured in the midline using a Hollister AnchorFast Oral Endotracheal Tube Fastener (Libertyville, IL, USA). The study ICU has established clinical protocols for the care of patients receiving invasive mechanical ventilation including:
Critical Care Pain Observation Tool (CPOT score) [36]
Daily spontaneous awakening trial (SAT) safety screen, SAT performance, spontaneous breathing trial (SBT) safety screen, and SBT performance [2]
Choice of analgesia and sedation
Early Mobility [41]
The clinical protocols used in the study unit can be found in the supplementary appendix.
Outcomes
The primary outcome, ALgI, was defined as one or more of the following on endoscopic evaluation within 36 hours of extubation: glottic mucosal ulceration/granulation or subglottic granulation tissue/stenosis (Figure 1). Laryngotracheal edema and erythema were not considered as evidence of ALgI (due to their subjective assessment and minimal impact on long-term outcomes). Each case was video recorded and the determination of ALgI was made by central, independent review by two otolaryngologists who were blinded to patient characteristics and clinical outcomes. Disagreements were adjudicated by independent review by a third otolaryngologist. All enrolled patients were contacted by phone at ten weeks after extubation and administered validated outcome questionnaires, using the Voice Handicap Index-10 (VHI) and the Clinical COPD Questionnaire (CCQ), by a surveyor who was blinded to the results of the patient’s endoscopic exam.(31, 32) The CCQ is a routinely used instrument to guide clinicians in assessing the airway patency, activity limitation, and emotional impact of COPD. CCQ scores of ≥1 are consistent with mild COPD and the minimal clinically important difference has been established at 0.4.(33, 34) The VHI-10 ranges from zero to 40 and higher scores indicate increased functional, physical, and emotional voice disability with a minimal clinical difference of four.(35) If a patient was unable to be contacted, at least five attempts were made on nonconsecutive days until they were deemed “unreachable.”
Figure 1; Mechanism of intubation-induced laryngeal injury:
The tongue base drives the endotracheal tube into the posterior glottis and direct pressure results in laryngeal mucosal and soft tissue injury at the endotracheal tube interface.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics. Frequencies were used for categorical variables and continuous variables were summarized by the sample median and interquartile range (IQR). Wilcoxon rank sum and Pearson’s chi-squared tests were used to compare the difference between those with and without ALgI for continuous and categorical variables, respectively. A multivariable logistic regression model was fit with the outcome of ALgI and potential causes as independent variables. Independent variables were specified a priori and included ETT size, intubation length, diabetes mellitus type two (DMII), and BMI (height and weight combined) based on literature findings.(36–39) CCI was additionally included to evaluate the relationship between the extent of chronic comorbidities and laryngeal injury.
Prior literature suggests a relationship between laryngotracheal injury and larger ETT size.(36–38) In order to determine if currently proposed ETT size thresholds are sufficient to protect patients from ALgI, we performed additional analysis, determined a priori assessing the probability of ALgI dichotomized by “appropriate” and “inappropriate” ETT size utilizing a published height-based nomogram.(40) Patients were considered inappropriately sized if the tube was one size greater than recommended.
Given multiple analyses implicating larger endotracheal tubes in ALgI development, we performed a post-hoc investigation to assess factors that may determine ETT size selection. We fit a multivariable proportional odds model on gender, BMI, and the type of specialist performing the intubation. Significance was defined as two-sided p ≤0.05. Statistical analyses were conducted using R version 3.5.0 (https://www.R-project.org).
Results
During the study period, 833 patients were mechanically ventilated in the medical ICU, including 166 who were palliatively extubated, 76 who were tracheostomy dependent, and 42 who died of cardiopulmonary arrest prior to extubation. There were 487 patients who survived to extubation (58.5%) and 65 who were intubated for less than 12 hours. Therefore, 100 of 422 patients (23.7%) underwent nasolaryngoscopy within 36 hours of extubation (Figure E1 in the online data supplement).
Baseline Characteristics
ALgI was present in 57 patients at the time of endoscopic examination. Nineteen patients had granulation tissue, 48 had posterior glottic ulceration, and eight had subglottic mucosal ulceration (Figure E2 in the online data supplement). Findings were not mutually exclusive and, ulceration in combination with adjacent granulation tissue was the most common synchronous lesion (N=12). Only one case of subglottic involvement was found in isolation.
Baseline covariates are shown in Table 1. ALgI and non-ALgI patients did not show evidence of significant differences with respect to age, gender, or race. ALgI patients were significantly heavier (median difference 14 kg) and consequently had a larger BMI (median difference 3.8 kg/m2) but were not significantly shorter (median difference 2 cm). Patients with ALgI were more likely to have DMII (46% vs. 21%) and were sicker (median CCI 3.00, IQR 2–5 vs. 2.00, IQR 1–4); however, the two groups did not show evidence of difference in functional status, proportion with COPD, hospital course, ICU admission diagnosis, or APACHE scores. Provider-specific factors including ETT size (median 8.0, IQR 7.5–8.0 vs. median 7.5, IQR 7.0–8.0; p<0.001) and duration of endotracheal intubation (median 3 vs. 2 days; p=0.024) were associated with ALgI.
Table 1:
Patient Characteristics
| ALgI (n=57) | No ALgI (n=43) | Total (n=100) | Significance (p) | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, Median years (IQR) | 59 (47–69) | 57 (41–64) | 58.5 (43–67) | 0.15 | |
| Sex, Male, N (%) | 38 (67) | 24 (56) | 62 (62) | 0.27 | |
| Race, Caucasian, N (%) | 47 (82) | 34 (79) | 81 (81) | 0.67 | |
| Height, cm, Median (IQR) | 173 (165–180) | 171 (165–178) | 172 (165–178) | 0.65 | |
| Weight, kg, Median (IQR) | 91 (77–105) | 77 (69–101.5) | 88.5 (73–105) | 0.043 | |
| Body Mass Index (BMI), kg/m2, Median (IQR) | 31.3 (27.6–36.3) | 27.5 (22.5–33.5) | 30.2 (25.6–35.2) | 0.018 | |
| Health Status / Comorbid Disease | |||||
| Charlson Comorbidity Index (CCI), Median (IQR) | 3.00 (2–5) | 2.00 (1–4) | 3.00 (1–5) | 0.046 | |
| Pre-illness Independent Functional Status, N (% Independent) | 40 (70) | 29 (67) | 69 (69) | 0.25 | |
| Diabetes Mellitus type II (DMII), N (%) | 26 (46) | 9 (21) | 35 (35) | 0.010 | |
| Obstructive Sleep Apnea (OSA), N (%) | 11 (19) | 4 (9) | 15 (15) | 0.17 | |
| Chronic Obstructive Pulmonary Disease (COPD), N (%) | 21 (37) | 12 (28) | 33 (33) | 0.35 | |
| Hospital Course | |||||
| Hypotension, N (%) | 26 (46) | 23 (53) | 49 (49) | 0.44 | |
| Pressor Requirement, N (%) | 21 (37) | 19 (44) | 40 (40) | 0.46 | |
| Acute Kidney Injury (AKI), N (%) | 28 (49) | 21 (49) | 49 (49) | 0.98 | |
| Systemic Steroids, N (%) | 17 (30) | 15 (35) | 32 (32) | 0.59 | |
| Delirium, N (%) | 42 (74) | 26 (60) | 68 (68) | 0.16 | |
| Pneumonia (PNA), N (%) | 22 (39) | 15 (35) | 37 (37) | 0.70 | |
| Acute Respiratory Distress Syndrome (ARDS), N (%) | 5 (9) | 6 (14) | 11 (11) | 0.41 | |
| Intubation Details | |||||
| Length of Intubation, Median Days (IQR) | 3 (3–6) | 2 (2–4) | 3 (2–5) | 0.024 | |
| Endotracheal Tube (ETT) Size, N (%) | 7.0 | 2 (4) | 13 (30) | 15 (15) | <0.001 |
| 7.5 | 23 (40) | 17 (40) | 40 (40) | ||
| 8 | 32 (56) | 13 (30) | 45 (45) | ||
| Laryngeal Exposure, N (%) | 1 | 25 (68) | 32 (89) | 78 (57) | 0.067 |
| 2 | 10 (27) | 4 (11) | 14 (19) | ||
| 3 | 2 (5) | 0 (0) | 2 (3) | ||
| Intubation Attempts, N (%) | 1 | 33 (92) | 30 (81) | 63 (86) | 0.19 |
| 2 | 3 (8) | 7 (19) | 10 (14) | ||
| 3 | 21 (37) | 6 (14) | 27 (27) | ||
| Prior Intubation Attempts, N (%) | 4 (10) | 5 (9) | 9 (9) | 0.94 | |
Breathing and Voice Outcomes
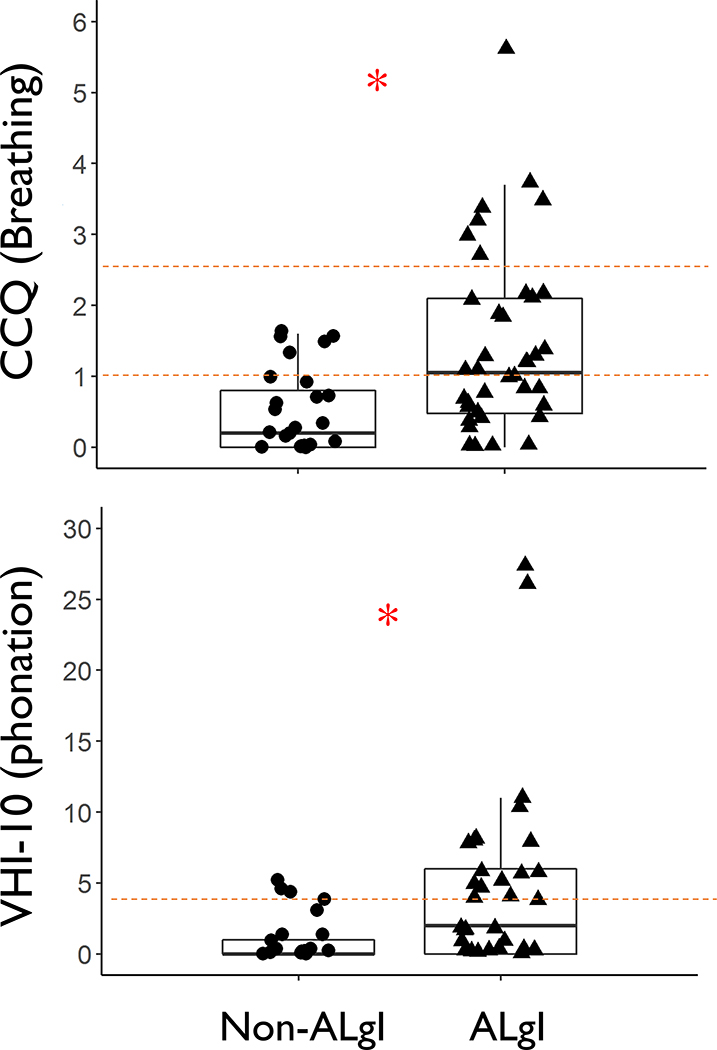
A total of 13 patients died prior to the first follow up phone call at ten weeks following enrollment. Of the remaining 87, ten patients were deemed “unreachable” after five unsuccessful attempts and ten patients had disconnected phone numbers. Thus, 67 patients (77% overall response rate) completed the patient-reported breathing and phonatory function questionnaires, 70% (n=40) with and 63% (n=27) without ALgI. Overall, patients with ALgI reported worse breathing (median CCQ 1.05, IQR 0.48–2.10) than their non-ALgI counterparts (median CCQ 0.20, IQR 0–0.80; p<0.001). Similarly, patients with ALgI reported worse voice outcomes (median VHI 2, IQR 0–6) compared to patients without ALgI (median VHI 0, IQR 0–1; p=0.005) (Figure 2).
Figure 2; Long-term breathing and voice outcomes:
Sixty seven patients (70% with and 63% without acute laryngeal injury) completed outcome questionnaires with both the surveyor and patient blinded to endoscopic findings during hospital admission. Clinical COPD Questionnaire (CCQ): Red dashed lines represents mild COPD (lower line; CCQ ≥ 1) and severe COPD (upper line; CCQ ≥ 2.5). Voice Handicap Index-10 (VHI): Red dashed line represents the minimal clinical significance (VHI ≥ 4). Wilcoxon rank sum test p=0.005 for VHI and <0.001 for CCQ.
ALgI Associated Factors from Multivariable Regression
In a multivariable logistic regression model, DMII, BMI, and ETT size were independently associated with ALgI (Table 2). A 7.0 ETT was associated with a significantly lower probability of ALgI when compared with both a 7.5 tube (adjusted OR=0.04, 95% CI 0.003–0.44, p=0.004) and an 8.0 ETT (OR=0.03, 95% CI 0.003–0.32, p=0.004). No protective effect was afforded a 7.5 vs. 8.0 ETT (OR=0.77, 95% CI 0.28–2.01, p=0.61). DMII modified the effect of BMI (p=0.003 for the formal test of interaction). In patients without DMII, the probability of ALgI increased as BMI increased. However, in patients with DMII, ALgI risk remained elevated across all BMI ranges (Table 2 and Figure E3 in the online data supplement). Receiver operating curve for the multivariable model is shown in Figure E4 in the online data supplement.
Table 2:
Multivariable logistic regression to predict acute laryngeal injury
| Factors | OR | 95% CI | P |
|---|---|---|---|
| Charlson Comorbidity Index (CCI), 0.4 unit increase | 1.13 | 0.42–3.02 | 0.81 |
| Duration of intubation, 3 day increase | 1.49 | 0.79–2.82 | 0.22 |
| Endotracheal Tube (ETT) Size | |||
| 7.0 vs 7.5 | 0.04 | 0.004–0.43 | 0.007 |
| 7.0 vs 8.0 | 0.03 | 0.003–0.31 | 0.003 |
| 7.5 vs 8.0 | 0.76 | 0.28–2.02 | 0.58 |
| Diabetes Mellitus Type II (DMII)a | 3.14 | 0.94–10.48 | 0.002 |
| Body Mass Index (BMI)* | 3.09 | 1.36–7.03 | 0.007 |
Diabetes for those with BMI of 30; BMI controlled for without DM (see Figure E3 for interaction details)
Odds ratios, 95% confidence intervals and significance
Seventeen patients had endotracheal tubes that were ‘inappropriately sized’ based on their height, and the incidence of ALgI was numerically higher in these patients than in patients with ‘appropriately sized’ endotracheal tubes (76.5% vs 53%; p=0.075) (Figure E5 in the online data supplement). In ‘appropriately’ sized patients, in addition to the previously described differences observed in the entire 100 patient cohort (ETT size, DMII, weight/BMI), the proportion of ALgI patients with a longer length of intubation, delirium, and worse grade of laryngeal view on intubation was higher than patients without ALgI (Table E1 in the online data supplement). In multivariable analysis of patients who received an ‘appropriate’ ETT size for their height, BMI and DMII remained significantly associated with ALgI and a 7.5 tube continued to represent a critical threshold for ALgI development (7.0 ETT vs. 7.5: OR=0.05, 95% CI 0.005–0.54, p=0.01) (Table E2 in the online data supplement).
Endotracheal Tube Size Selection
Post hoc multivariable proportional odds model utilizing gender, BMI, and the type of specialist performing the intubation showed that gender (female vs male OR 0.3, 95% CI 0.13–0.78, p=0.01) and intubating physician were significantly associated with selection of ETT size (Figure 3 and Table E3 in the online supplement). Anesthesiologists and Emergency Medical Services placed smaller ETTs, while critical care and emergency department physicians placed larger ETTs.
Figure 3; Association of endotracheal tube size with gender, body mass index, and intubating provider:
Predicted log odds of selecting a larger size endotracheal tube from an ordinal regression model (proportional odds model) investigating the association of endotracheal tube size with gender, body mass index, and intubating provider. The log odds values were adjusted to male, body mass index = 30.2, and emergency department personnel. These results confirm the general practice among doctors—males and larger patients are intubated with larger endotracheal tubes. However, anesthesia and emergency medical service providers used smaller tubes, while critical care specialists and emergency department physicians tended to use larger endotracheal tubes. Y-axis: log odds of larger endotracheal tube size placement (7.5 vs 7.0 or 8.0 vs 7.5 tubes). Ans = Anesthesia; CCT = Critical care team; ED = Emergency department physicians; EMS = Emergency medical service; OSP = Other/Outside personnel. Body Mass Index = kg/m2.
Discussion
Over a third of patients admitted to a critical care unit require endotracheal intubation. Although lifesaving, the laryngeal consequences of endotracheal intubation and long-term recovery of laryngeal injury have been persistent knowledge gaps in modern critical care. With a clear definition of ALgI based on objective exam findings, our results indicate that mechanically ventilated patients develop laryngeal injury at an unexpectedly high rate (57%). Our data suggest there is an elevated risk imparted by an endotracheal tube larger than 7.0. We also show ALgI disproportionally affects particular patient subgroups (type two diabetics and the obese) and may predispose to clinically significant dyspnea after hospital discharge.
While the role of endotracheal intubation and prolonged mechanical ventilation in the development of chronic airway injury is not novel, laryngeal injury after intubation is often initially hidden from both the patient and physician, limiting the ability to develop risk-reduction strategies. Our work provides unambiguous exam findings that can potentially be utilized immediately after extubation to risk stratify patients for long-term functional breathing impairment. Our findings are strongly supported by human reports and animal models that show that direct pressure from an ETT can generate mucosal injury in the posterior larynx (which contains the cricoarytenoid joints responsible for vocal cord mobility), akin to a pressure ulcer.(21) Progressive fibrotic contracture of the mucosal injury overlying the cricoarytenoid joints can reduce the cross-sectional area through the narrowest segment of the adult airway and culminate in symptomatic dyspnea.
This study’s results are consistent with prior reports associating laryngeal and tracheal injury after mechanical ventilation to ETT size, DMII and BMI.(36–39) Subset analysis also suggests that mechanical factors (patient movement associated with delirium, longer duration of intubation, and difficult anatomic exposure of the larynx) may play a role in predisposing patients to ALgI.(36, 41) Although specific patient subgroups (such as the Acute Respiratory Distress Syndrome) can require prolonged mechanical ventilation(42–44), intubation times in this study closely mirror those of published medical ICU populations, which range from 0.5–3.3 days,
Patient-specific factors including DMII and BMI may predispose the larynx to mucosal injury from an ETT or impair mucosal healing once it has occurred. Diabetes is known to inhibit pressure ulcer healing (via a chronic inflammatory state coupled with microvascular disease).(45, 46) Similarly, obesity has been shown to generate systemic inflammation, and obese critical care patients are at much greater risk of systemic inflammatory responses.(47) Alternatively, a larger body habitus may increase the size of the tongue base(48–50), resulting in greater posteriorly-oriented vector forces on the ETT, similar to the forces generated by an anteriorly located larynx.
Consequently, laryngeal injury may be preventable with modifiable procedural factors (ETT size and length of intubation) that could be targeted to potentially reduce the incidence of ALgI. Historically, the recognition that subglottic stenosis developed secondary to elevated ETT cuff pressures led to the utilization of high volume, low-pressure cuffs. This change dramatically lowered the rate of subglottic injury in mechanically ventilation patients. Similarly, future prospective studies could explore personalized strategies to reduce the rate of laryngeal injury (e.g. reduced ETT size, earlier tracheostomy, and increased post-extubation surveillance) in at risk populations (including diabetics and the obese).
Endotracheal Tube Selection
Controversy surrounds ETT size selection for use in mechanical ventilation. Pulmonary physiology in this setting involves a sophisticated interplay of disease pathophysiology, lung compliance, ventilator mode, respiratory flow rate, and circuit resistance.(51–53) Although larger diameter ETTs decrease upper airway resistance, the flow dynamics with medium- (7.0–7.5) and small-sized (6.0–6.5) ETTs adequately allow ventilation and oxygenation of critically ill patients, do not influence peak intrathoracic pressure, and show no significant difference in work of breathing between <8.0 and ≥8.0 ETTs.(51, 54–56) Although the need for bronchoscopy is a frequently cited rationale for the use of large ETTs, only 5% of the patients cared for in our medical ICU between 2016 to 2017 underwent bronchoscopy. Thus, only a fraction of patients intubated with an 8.0 ETT require bronchoscopy while ALgI occurred in more than half of intubated patients. Moreover, though 8.0 ETTs are preferred for bronchoscopy, a 7.0 ETT can accommodate a standard flexible (4–6.3 mm outer diameter) bronchoscope and achieve the appropriate generation of negative pressure while minimizing the decreased effect on ventilation volumes.(57, 58) While there has been a trend to smaller endotracheal tubes over time (online supplement Figure E6 compares the distribution of ETT sizes used in our study to that of Gaynor and colleagues’ 1985 study), it is clear that there is still room for progress.(59) Our data suggest that intensivists should consider using ETT sizes less than 7.5 for patients to limit laryngeal injury and highlights the importance of adhering to recommended guidelines on appropriate endotracheal tube size based on height.(40) Further research is needed to determine whether or not decreasing endotracheal tube size beyond what is currently recommended could further reduce the risk of laryngeal injury.
Strengths and Limitations
The prospective cohort study design allowed us to screen all ventilated patients and reduced the risk of selection bias. We defined ALgI a priori and used a multistep and independent assessment scheme to prevent observer bias. Functional outcomes (via patient-reported outcome measures) were collected on all available patients by interviewers blinded to endoscopic exam results to prevent recall bias. The use of previously validated patient-centered outcomes for breathing and voicing increases the relevance of the results.
The study design also has limitations. Objective measurements of laryngeal function were not collected at ten weeks. Instead, voice and breathing were assessed using patient-reported outcome measure scores. While results show a high rate of ALgI and that ALgI is associated with impairments in breathing and voicing at ten weeks post-intubation, the mechanism for this association and the natural history of laryngeal injury requires further study. Due to the study size, all endoscopic lesions are treated as synonymous and it is possible that different lesions in various locations will produce varying breathing and voice outcomes. Even so, the vast majority of lesions were mucosal ulceration and granulation tissue that occurred in the posterior glottis. In addition to the limitations inherent to observational studies, we did not collect information on other currently unknown factors that may affect the risk of laryngeal injury. Additionally, enrollment of all patients in a single medical center limits generalizability. Finally, we did not evaluate the proportion of patients with simple versus difficult ventilatory weaning; the presence of pre-existing laryngeal lesions; or the reason, degree of urgency, and conditions for intubation or extubation, which could have affected our results.
Future Directions
Given our results, we believe future research efforts in ALgI should concentrate on: 1. Validation of our findings with formal multi-center clinical trials of ETT selection to investigate the laryngeal protection afforded by smaller caliber ETTs. 2. Integration of laryngeal examinations into ICU pathways of care (particularly in those at risk of ALgI) in order to raise awareness among critical care providers. 3. Development of clear and patient-specific ETT size guidelines that trend towards smaller tubes rather than instinctive placement of bigger ETTs in men or “larger” patients.
Conclusions:
Acute laryngeal injury occurred in more than half of patients who received mechanical ventilation in an intensive care unit and may be associated with worse breathing and voice outcomes. Endotracheal tubes greater than size 7.0, diabetes mellitus type two, and larger body habitus may predispose to the development of ALgI. Our results suggest that acute laryngeal injury may impact functional recovery from critical illness and may contribute to the morbidity of post-intensive care syndrome.
Supplementary Material
Figure E1; Study design: Prospective cohort study that was undertaken for a duration to include 100 patients. Eighty one patients consented for themselves, while the remaining 19 had surrogate consent, typically due to delirium (N=9 with and N=10 without laryngeal injury). Fifty seven of 100 patients (57%) were found to have acute laryngeal injury on endoscopic examination. Sixty seven patients completed the patient-reported outcome questionnaires (70% with and 67% without laryngeal injury).
Figure E2; Acute laryngeal injury patterns: Mucosal ulceration (48%) was the most common examination finding, followed by granulation tissue (19%) and subglottic ulceration/granulation (8%). Findings were not mutually exclusive and ulceration in combination with adjacent early granulation tissue formation was the most common simultaneous finding (N=12). Eight patients were found to have subglottic ulceration or subglottic mucosal changes and all but one was associated with glottic ulceration with or without granulation tissue. There was one case of true vocal cord hemorrhage and one case of false vocal fold hemorrhage, both of which were associated with mucosal ulceration.
Figure E3; Diabetes and body mass index interaction: (A) ANOVA with chi-square test statistics and significant for endotracheal tube size, body mass index, and diabetes type two. (B) The effects of body mass index among type two diabetics and non-diabetics. The y-axis values were adjusted to intubation duration=3 days, Charlson Comorbidity Index=3, and endotracheal tube size of 8.0. For every 10 unit increase in body mass, those without diabetes had a significant association of laryngeal injury (OR 3.23, 95% CI 1.37–7.62, p=0.007) compared to those with diabetes (OR 0.63, 95% CI 0.34–1.16, p=0.14).
Figure E4; Receiver operating curve for multivariable logistic regression model for acute laryngeal injury: Harrell’s c-index was 0.83 (95% CI 0.75–0.91) and 0.78 after adjusting for optimism due to over-fitting by internal validation.
Figure E5; ‘Appropriately’ compared to ‘inappropriately’ sized endotracheal tubes: Data fitted to height-based nomogram to help determine if relatively larger endotracheal tubes can help predict the development of acute laryngeal injury and to assist with endotracheal tube size selection for patients.
Figure E6: Endotracheal tube size selection over time: Gaussian distribution of endotracheal tube sizes from the current study conducted in 2018 (dark green) compared to tube sizes from Gaynor et al in 1985 (grey).
Acknowledgements:
JRS, KK, BRC, ASL, and AG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JRS (Vanderbilt University Medical Center, Department of Otolaryngology), LD (Vanderbilt University, Department of Biostatistics), and AG (Vanderbilt University Medical Center, Division of Laryngology) were involved with statistical analysis. All authors provided revisions and approved the final manuscript.
Conflicts of Interest and Source of Funding: ATH consults for Ambu and Olympus. The remaining authors have no conflicts of interest. This study was in part supported by a Vanderbilt Institute for Clinical and Translational Research (VICTR) grant to purchase video laryngoscopy recording capabilities.
Footnotes
Trial Registration: Clinical Trials ID# NCT03250975
References
- 1.Angus DC, Shorr AF, White A, et al. : Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med 2006;34(4):1016–1024. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, et al. : Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29(7):1303–1310. [DOI] [PubMed] [Google Scholar]
- 3.Wunsch H, Wagner J, Herlim M, et al. : ICU occupancy and mechanical ventilator use in the United States. Crit Care Med 2013;41(12):2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashiku SK, Kuzucu A, Grillo HC, et al. : Idiopathic laryngotracheal stenosis: effective definitive treatment with laryngotracheal resection. J Thorac Cardiovasc Surg 2004;127(1):99–107. [DOI] [PubMed] [Google Scholar]
- 5.Needham DM, Davidson J, Cohen H, et al. : Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012;40(2):502–509. [DOI] [PubMed] [Google Scholar]
- 6.Gardner AK, Ghita GL, Wang Z, et al. : The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs. Crit Care Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevin CM, Bloom SL, Jackson JC, et al. : Comprehensive care of ICU survivors: Development and implementation of an ICU recovery center. J Crit Care 2018;46:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtake PJ, Lee AC, Scott JC, et al. : Physical Impairments Associated With Post-Intensive Care Syndrome: Systematic Review Based on the World Health Organization’s International Classification of Functioning, Disability and Health Framework. Phys Ther 2018;98(8):631–645. [DOI] [PubMed] [Google Scholar]
- 9.Pacheco-Lopez PC, Berkow LC, Hillel AT, et al. : Complications of airway management. Respir Care 2014;59(6):1006–1019; discussion 1019–1021. [DOI] [PubMed] [Google Scholar]
- 10.Geraci G, Cupido F, Lo Nigro C, et al. : Postoperative laryngeal symptoms in a general surgery setting. Clinical study. Ann Ital Chir 2013;84(4):377–383. [PubMed] [Google Scholar]
- 11.Donnelly WH: Histopathology of endotracheal intubation. An autopsy study of 99 cases. Arch Pathol 1969;88(5):511–520. [PubMed] [Google Scholar]
- 12.Dubick MN, Wright BD: Comparison of laryngeal pathology following long-term oral and nasal endotracheal intubations. Anesth Analg 1978;57(6):663–668. [PubMed] [Google Scholar]
- 13.Brandwein M, Abramson AL, Shikowitz MJ: Bilateral vocal cord paralysis following endotracheal intubation. Arch Otolaryngol Head Neck Surg 1986;112(8):877–882. [DOI] [PubMed] [Google Scholar]
- 14.Colice GL, Stukel TA, Dain B: Laryngeal complications of prolonged intubation. Chest 1989;96(4):877–884. [DOI] [PubMed] [Google Scholar]
- 15.Santos PM, Afrassiabi A, Weymuller EA Jr.: Risk factors associated with prolonged intubation and laryngeal injury. Otolaryngol Head Neck Surg 1994;111(4):453–459. [DOI] [PubMed] [Google Scholar]
- 16.Massard G, Rouge C, Dabbagh A, et al. : Tracheobronchial lacerations after intubation and tracheostomy. Ann Thorac Surg 1996;61(5):1483–1487. [DOI] [PubMed] [Google Scholar]
- 17.Eckerbom B, Lindholm CE, Alexopoulos C: Airway lesions caused by prolonged intubation with standard and with anatomically shaped tracheal tubes. A post-mortem study. Acta Anaesthesiol Scand 1986;30(5):366–373. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky MB, Gonzalez-Fernandez M, Mendez-Tellez PA, et al. : Factors associated with swallowing assessment after oral endotracheal intubation and mechanical ventilation for acute lung injury. Ann Am Thorac Soc 2014;11(10):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedden M, Ersoz CJ, Donnelly WH, et al. : Laryngotracheal damage after prolonged use of orotracheal tubes in adults. JAMA 1969;207(4):703–708. [PubMed] [Google Scholar]
- 20.Gelbard A, Francis DO, Sandulache VC, et al. : Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2015;125(5):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courey MS, Bryant GL Jr., Ossoff RH: Posterior glottic stenosis: a canine model. Ann Otol Rhinol Laryngol 1998;107(10 Pt 1):839–846. [DOI] [PubMed] [Google Scholar]
- 22.Kastanos N, Estopa Miro R, Marin Perez A, et al. : Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med 1983;11(5):362–367. [DOI] [PubMed] [Google Scholar]
- 23.Howard NS, Shiba TL, Pesce JE, et al. : Photodocumentation of the development of type I posterior glottic stenosis after intubation injury. Case Rep Surg 2015;2015:504791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lano CF Jr., Duncavage JA, Reinisch L, et al. : Laryngotracheal reconstruction in the adult: a ten year experience. Ann Otol Rhinol Laryngol 1998;107(2):92–97. [DOI] [PubMed] [Google Scholar]
- 25.Colton House J, Noordzij JP, Murgia B, et al. : Laryngeal injury from prolonged intubation: a prospective analysis of contributing factors. Laryngoscope 2011;121(3):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 27.Cormack RS, Lehane J: Difficult tracheal intubation in obstetrics. Anaesthesia 1984;39(11):1105–1111. [PubMed] [Google Scholar]
- 28.Mallampati SR, Gatt SP, Gugino LD, et al. : A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985;32(4):429–434. [DOI] [PubMed] [Google Scholar]
- 29.Pandharipande PP, Girard TD, Ely EW: Long-term cognitive impairment after critical illness. N Engl J Med 2014;370(2):185–186. [DOI] [PubMed] [Google Scholar]
- 30.Boone MD, Massa J, Mueller A, et al. : The organizational structure of an intensive care unit influences treatment of hypotension among critically ill patients: A retrospective cohort study. J Crit Care 2016;33:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouraei SA, Randhawa PS, Koury EF, et al. : Validation of the Clinical COPD Questionnaire as a psychophysical outcome measure in adult laryngotracheal stenosis. Clin Otolaryngol 2009;34(4):343–348. [DOI] [PubMed] [Google Scholar]
- 32.Rosen CA, Lee AS, Osborne J, et al. : Development and validation of the voice handicap index-10. Laryngoscope 2004;114(9):1549–1556. [DOI] [PubMed] [Google Scholar]
- 33.Kocks JW, Tuinenga MG, Uil SM, et al. : Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir Res 2006;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Molen T, Willemse BW, Schokker S, et al. : Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes 2003;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young VN, Jeong K, Rothenberger SD, et al. : Minimal clinically important difference of voice handicap index-10 in vocal fold paralysis. Laryngoscope 2018;128(6):1419–1424. [DOI] [PubMed] [Google Scholar]
- 36.Hillel AT, Karatayli-Ozgursoy S, Samad I, et al. : Predictors of Posterior Glottic Stenosis: A Multi-Institutional Case-Control Study. Ann Otol Rhinol Laryngol 2016;125(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsantonis NG, Kabagambe EK, Wootten CT, et al. : Height is an independent risk factor for postintubation laryngeal injury. Laryngoscope 2018. [DOI] [PubMed] [Google Scholar]
- 38.Whited RE: A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope 1984;94(3):367–377. [DOI] [PubMed] [Google Scholar]
- 39.Zias N, Chroneou A, Tabba MK, et al. : Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med 2008;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coordes A, Rademacher G, Knopke S, et al. : Selection and placement of oral ventilation tubes based on tracheal morphometry. Laryngoscope 2011;121(6):1225–1230. [DOI] [PubMed] [Google Scholar]
- 41.Francois B, Bellissant E, Gissot V, et al. : 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet 2007;369(9567):1083–1089. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez G, Vaquero C, Gonzalez P, et al. : Effect of Postextubation High-Flow Nasal Cannula vs Conventional Oxygen Therapy on Reintubation in Low-Risk Patients: A Randomized Clinical Trial. JAMA 2016;315(13):1354–1361. [DOI] [PubMed] [Google Scholar]
- 43.Bellani G, Laffey JG, Pham T, et al. : Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 44.Miltiades AN, Gershengorn HB, Hua M, et al. : Cumulative Probability and Time to Reintubation in U.S. ICUs. Crit Care Med 2017;45(5):835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assar ME, Angulo J, Rodriguez-Manas L: Diabetes and ageing-induced vascular inflammation. J Physiol 2016;594(8):2125–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baltzis D, Eleftheriadou I, Veves A: Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 2014;31(8):817–836. [DOI] [PubMed] [Google Scholar]
- 47.Akinnusi ME, Pineda LA, El Solh AA: Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med 2008;36(1):151–158. [DOI] [PubMed] [Google Scholar]
- 48.Godoy IR, Martinez-Salazar EL, Eajazi A, et al. : Fat accumulation in the tongue is associated with male gender, abnormal upper airway patency and whole-body adiposity. Metabolism 2016;65(11):1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nashi N, Kang S, Barkdull GC, et al. : Lingual fat at autopsy. Laryngoscope 2007;117(8):1467–1473. [DOI] [PubMed] [Google Scholar]
- 50.Kim AM, Keenan BT, Jackson N, et al. : Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014;37(10):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straus C, Louis B, Isabey D, et al. : Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med 1998;157(1):23–30. [DOI] [PubMed] [Google Scholar]
- 52.Shapiro M, Wilson RK, Casar G, et al. : Work of breathing through different sized endotracheal tubes. Crit Care Med 1986;14(12):1028–1031. [DOI] [PubMed] [Google Scholar]
- 53.Haberthur C, Elsasser S, Eberhard L, et al. : Total versus tube-related additional work of breathing in ventilator-dependent patients. Acta Anaesthesiol Scand 2000;44(6):749–757. [DOI] [PubMed] [Google Scholar]
- 54.Bersten AD, Rutten AJ, Vedig AE, et al. : Additional work of breathing imposed by endotracheal tubes, breathing circuits, and intensive care ventilators. Crit Care Med 1989;17(7):671–677. [DOI] [PubMed] [Google Scholar]
- 55.Stenqvist O, Sonander H, Nilsson K: Small endotracheal tubes: ventilator and intratracheal pressures during controlled ventilation. Br J Anaesth 1979;51(4):375–381. [DOI] [PubMed] [Google Scholar]
- 56.Wall MA: Infant endotracheal tube resistance: effects of changing length, diameter, and gas density. Crit Care Med 1980;8(1):38–40. [PubMed] [Google Scholar]
- 57.Baier H, Begin R, Sackner MA: Effect of airway diameter, suction catheters, and the bronchofiberscope on airflow in endotracheal and tracheostomy tubes. Heart Lung 1976;5(2):235–238. [PubMed] [Google Scholar]
- 58.Rosen M, Hillard EK: The effects of negative pressure during tracheal suction. Anesth Analg 1962;41:50–57. [PubMed] [Google Scholar]
- 59.Gaynor EB, Greenberg SB: Untoward sequelae of prolonged intubation. Laryngoscope 1985;95(12):1461–1467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1; Study design: Prospective cohort study that was undertaken for a duration to include 100 patients. Eighty one patients consented for themselves, while the remaining 19 had surrogate consent, typically due to delirium (N=9 with and N=10 without laryngeal injury). Fifty seven of 100 patients (57%) were found to have acute laryngeal injury on endoscopic examination. Sixty seven patients completed the patient-reported outcome questionnaires (70% with and 67% without laryngeal injury).
Figure E2; Acute laryngeal injury patterns: Mucosal ulceration (48%) was the most common examination finding, followed by granulation tissue (19%) and subglottic ulceration/granulation (8%). Findings were not mutually exclusive and ulceration in combination with adjacent early granulation tissue formation was the most common simultaneous finding (N=12). Eight patients were found to have subglottic ulceration or subglottic mucosal changes and all but one was associated with glottic ulceration with or without granulation tissue. There was one case of true vocal cord hemorrhage and one case of false vocal fold hemorrhage, both of which were associated with mucosal ulceration.
Figure E3; Diabetes and body mass index interaction: (A) ANOVA with chi-square test statistics and significant for endotracheal tube size, body mass index, and diabetes type two. (B) The effects of body mass index among type two diabetics and non-diabetics. The y-axis values were adjusted to intubation duration=3 days, Charlson Comorbidity Index=3, and endotracheal tube size of 8.0. For every 10 unit increase in body mass, those without diabetes had a significant association of laryngeal injury (OR 3.23, 95% CI 1.37–7.62, p=0.007) compared to those with diabetes (OR 0.63, 95% CI 0.34–1.16, p=0.14).
Figure E4; Receiver operating curve for multivariable logistic regression model for acute laryngeal injury: Harrell’s c-index was 0.83 (95% CI 0.75–0.91) and 0.78 after adjusting for optimism due to over-fitting by internal validation.
Figure E5; ‘Appropriately’ compared to ‘inappropriately’ sized endotracheal tubes: Data fitted to height-based nomogram to help determine if relatively larger endotracheal tubes can help predict the development of acute laryngeal injury and to assist with endotracheal tube size selection for patients.
Figure E6: Endotracheal tube size selection over time: Gaussian distribution of endotracheal tube sizes from the current study conducted in 2018 (dark green) compared to tube sizes from Gaynor et al in 1985 (grey).