Structured Abstract
INTRODUCTION:
Clinical differentiation between Alzheimer’s disease (AD) and AD with Lewy Body disease (LBD) is relatively imprecise. The current study examined pathologically-confirmed group differences in neuropsychological functioning, and specific tests’ classification ability.
METHODS:
51 participants with postmortem diagnoses of AD (n=34) and AD+LBD (n=17) were drawn from the Predictors Study. One-way ANOVAs and Chi Square analyses examined group differences in neuropsychological performance. Binary logistic regressions examined predictive utility of specific tests for pathological diagnosis.
RESULTS:
Individuals with AD had better visuoconstruction (p=.006), phonemic fluency, (p = 0.08), and processing speed than AD+LBD, (p = 0.013). No differences were found in memory, naming, semantic fluency or set-switching. Processing speed and visuoconstruction predicted pathologic group, (p=0.03).
DISCUSSION:
Processing speed and visuoconstruction predicted postmortem diagnosis of AD versus AD plus LBD. Current results offer guidance in the selection and interpretation of neuropsychological tests to be used in the differential diagnosis of early dementia.
Keywords: Lewy Body dementia, Alzheimer’s disease, neuropsychological performance, diagnosis, autopsy
1. Background
Alzheimer’s disease (AD) is a progressive, dementing disease pathologically characterized by plaques and neurofibrillary tangles beginning in the temporal lobes, which eventually spread to the networks connecting the frontal, anterior and parietal lobes.[see 1, 2] Lewy Body Disease (LBD), another cause of dementia, is pathologically characterized by abnormal accumulation of alpha synuclein in the brain stem as well as diffusely throughout the cortex, with depleted neurotransmitters such as dopamine and acetylcholine. Additionally, it is common for Lewy bodies to spread to limbic areas.[3] Although AD and LBD have unique pathological profiles, patients commonly present with pathologies characteristic of both diseases.[e.g., 4, 5] In fact, approximately 50% of individuals with LBD have enough AD pathology to be characterized as having a secondary diagnosis of AD, and vice versa.[6, 7]
However, the clinical differentiation between AD and AD plus LBD is relatively imprecise, as there is considerable overlap of cognitive symptoms and neuroanatomic substrates.[8, 9] In fact, although there are current criteria for clinical diagnosis of pure LBD, these criteria do not map on well to AD with LBD, the latter for which new diagnostic criteria remain to be determined.[7] Further characterization of differences, such as specific cognitive profiles, between AD versus AD with LBD may help specify what aspects of the clinical presentation are key for the differentiation of these overlapping disorders.
The study of various cognitive abilities across patients with AD and those diagnosed with LBD has revealed that visual-spatial, attentional, information processing, and executive deficits are often more severe in LBD cases, while memory deficits (particularly recognition memory) are more severe in AD, at least in the beginning stages of disease.[9–11] Individuals with LBD may thus be expected to show a profile of relatively preserved memory storage and differentially impaired visuospatial abilities, phonemic fluency, processing speed, and executive abilities tasks relative to individuals with AD.[10, 12] The majority of the studies that have examined these cognitive differences though, have been based on clinical diagnoses.[13–22] Using clinical diagnosis as the independent grouping variable can be inherently circular, however, because the same data used to predict group classification were utilized for classifying diagnostic group at the outset. Further, the frequencies of pure LBD cases versus mixed AD with Lewy bodies cannot be specified in clinical studies, thus it is not clear if results are driven by pure LBD pathology or by mixed pathology.
In contrast to clinically based diagnoses, studies based on pathologically confirmed diagnoses provide an objective marker against which to examine cognitive symptoms in each group. These studies are scarce, however, likely due to challenges associated with recruiting individuals for autopsy, and the significant length of time required to obtain pathologic specimens.[10, 11, 23] Within pathological studies examining cognitive functioning across AD versus AD with LBD, results suggest that the mixed group is more likely to have better performance in memory (recall and/or recognition),[24, 25] worse visuospatial abilities (pentagons or clock drawing),[26–28] worse verbal fluency,[27–29] worse processing speed,[27, 28] and worse attention.[26] These results though are not consistent across all studies, with several studies observing no cognitive differences across groups.[e.g., 11, 30] Additional studies based on pathological data are thus needed to further examine if cognitive symptoms differ as a function of underlying pathology, to ultimately aid in the clinical differentiation of AD versus AD with LBD. Importantly, identifying the earliest cognitive and clinical predictors of diagnosis is critical as targeted cholinergic therapies might be more effective when delivered earlier [4] as well as identification of targeted treatment and functionally meaningful outcomes based on cognitive functioning that are tailored to each patient.
The current paper examined group differences in performance across a range of neuropsychological tests including measures of visuoconstruction, processing speed, memory, language, attention, and executive functioning. Based on neuroanatomical and clinical evidence to date, it was hypothesized that the AD group would have higher visuoconstruction, phonemic fluency, executive functioning, and processing speed scores than the AD with LBD group. The AD group was also expected to perform worse on memory (e.g., recall, recognition) and semantic processing tasks (i.e., naming, semantic fluency ratio) than those with mixed pathology. Finally, we examined the ability of neuropsychological tests that differed at the group level to classify individuals into pathologic group, while accounting for non-cognitive symptoms (i.e., behavioral and extrapyramidal signs) that are often present in individuals with LBD.
2. Methods
2.1. Participants
The current sample was drawn from the Predictors 2 cohort’s baseline visit, and was comprised of individuals whose autopsy data revealed the presence of AD (n = 34), or mixed AD with LBD (n = 17) with a Clinical Dementia Rating Scale score of 1 or 2. Participants were included in the current study if neuropsychological variables of interest and pathological diagnoses were available. The Predictor’s 2 cohort was initiated in 1997 following the same methods as the Predictors 1 cohort described previously.[31] This cohort primarily consisted of individuals clinically diagnosed with AD, but also included an additional subset of patients with clinically diagnosed dementia with Lewy Bodies (DLB) diagnosed according to the 1996 Consensus Guidelines for DLB. In the current sample, 32 individuals carried clinical diagnoses of AD, and 9 of DLB. Specific details of the general inclusion/exclusion criteria have been described previously.[9] Only individuals from Predictors 2 cohort were included in the current study due to more specific neuropsychological and pathological data collected and thus available for the current study. In all, 211 subjects with probable AD, and 28 with probable LBD, were recruited into the cohort at three sites: Columbia University, Johns Hopkins University, and Massachusetts General Hospital. These individuals were diagnosed in the clinic and referred by their physicians to this study.
2.2. Measures and Procedure
Neuropsychological measures included memory recall and recognition total (Hopkins Verbal learning test), naming (i.e., Boston naming test), verbal fluency (Category fluency, CFL), processing speed (Trail Making Part A, TMT-A), executive functioning (Trail Making Part B, TMT-B), and pentagon copy from the (modified Mini-Mental State Exam (mMMSE,[32]). In addition to examining performance on each test, we also examined the ratio of semantic to phonemic fluency (semantic / (semantic + phonemic), as it has been shown to be particularly specific to AD.[33] It reflects the relative contribution of semantic impairment to fluency deficits independent of defective retrieval that may occur secondary to frontal or subcortical compromise. Lower values represent fewer words generated for semantic versus phonemic fluency. Global cognitive (mMMSE) and functional impairment (Clinical Dementia Rating Scale; CDR,[34]) were measured for the study and used to determine whether groups were at a similar level of disease severity, but these were not primary outcomes of interest. Given the high relevance of non-cognitive symptoms in clinical classification of disease, specifically in LBD, visual hallucinations (coded dichotomously, 1 representing endorsement and 0 representing absence of symptoms), and parkinsonism scores were included in analyses. Parkinsonism was rated on a 5-point scale for each of the following: tremor at rest, rigidity in neck, limbs, and posture, and bradykinesia for a total possible sum score of 0–25. Informants answered questions regarding medications, psychiatric and neurological history. The project was approved by the institutional review board at each of the 3 respective sites. All patients and their proxy decision makers provided written informed consent.[11]
2.3. Procedure of Pathological Diagnoses
Cases were classified as having AD neuropathology if Braak Stage for neurofibrillary tangles was IV, V, or VI and CERAD neuritic plaque score was “moderate” or “frequent”. Coexistent Lewy body disease was classified based on the presence of alpha-syunculein immunohistochemistry positive inclusions consistent with “limbic” or “neocortical” Lewy body pathology.[35, 36]
2.4. Data analysis
All analyses were conducted using SPSS 22. Chi Square analyses and one-way analyses of variance (ANOVA) examined demographic and cognitive differences between the two autopsy-confirmed groups. Binary logistic regressions were used to determine the predictive value of individual neuropsychological performance measures on pathological diagnosis post-mortem, and a single binary regression included multiple neuropsychological predictors established to be significant in the previous models; all models was also conducted while accounting for presence of non-cognitive symptoms (i.e., visual hallucinations and parkinsonism). Pathological groups were coded (AD = 0, mixed AD & LBD = 1). One individual with CDR = 0 was removed from the analyses to ensure similar functional levels across participants. Individuals classified as having only LBD comprised a small sample (n=12), hindering adequate group comparisons (i.e., requiring greater power to detect differences across the three groups) and thus warranting lack of its inclusion in the study.
3. Results
3.1. Demographics
The average global cognitive performance, as measured by the mMMS, was 35.95 (SD = 7.55). The average age ranged from 56 to 90 (M= 74.71, SD= 8.38) and education ranged from 8 years to 20 years (M= 14.59, SD= 3.22). 49.2% were male, and 98.3% were white. One-way ANOVAs and Chi Square analyses revealed no differences in disease severity (CDR, mMMSE) or demographic variables across pathological diagnosis (See Table 1). Overall, clinical diagnoses were accurate in 76.5% of cases. Specifically, clinical diagnoses of AD were accurate in 76.1% cases (32 out of 42). 23.9% were misclassified as DLB. Clinical diagnoses of DLB were accurate in 77.8% of cases (7 out of 9; defined as AD+DLB). 22.2% of mixed AD + LBD were misclassified as AD.
Table 1.
Demographics and clinical characteristics of sample
| Pure AD (n=34) | Mixed AD and LBD (n=17) | F | p-value | |
|---|---|---|---|---|
| Gender (% Female) | 52.9 | 64.7 | X2= 0.65 | 0.31 |
| Ethnicity (% Caucasian) | 100 | 94.1 | X2= 2.20 | 0.34 |
| Age | 74.18(7.97) | 72.65(7.97) | 0.42 | 0.52 |
| Education | 14.62(3.04) | 15.53(2.76) | 1.08 | 0.30 |
| mMMSE Total Score | 21.15(3.58) | 19.07(5.04) | 2.70 | 0.11 |
| CDR | 1.74(0.37) | 1.21(0.47) | 1.20 | 0.08 |
| Time from assessment to death | 1888.33(861.41) | 1906.00(1219.67) | 0.01 | 0.96 |
3.2. Cognitive comparisons
The AD group demonstrated higher phonemic fluency F (1,41) = 5.16, p = 0.029 and faster processing speed F (1,38) = 5.72, p = 0.022 than the AD + LBD group. Pentagon copy was also less frequently impaired in AD (24%; 8 of 33) than AD + LDB (73%; 11 of 15), X2(1) = 10.49, p= .003. All other cognitive measures were comparable across groups. For comparison of performance on the full neuropsychological battery, see Table 2.
Table 2.
Cognitive Scores (Mean and SD)
| AD only (n=34) | Mixed AD+LBD (n=17) | p-value (Difference between AD only vs. mixed) | |
|---|---|---|---|
| Total Recall (out of 36) | 10.93 (3.99) | 9.50 (4.60) | 0.30 |
| % retained | 17.43 (29.75) | 22.36 (29.94) | 0.61 |
| Recognition (# of hits out of 12) | 9.56 (2.38) | 9.14 (2.03) | 0.58 |
| Recognition Discriminability | 5.21 (3.32) | 4.29 (2.97) | 0.38 |
| Naming (out of 30) | 23.10 (6.15) | 19.67 (8.24) | 0.13 |
| CFL (mean) | 8.79 (3.66) | 6.45 (3.67) | 0.03 |
| Animal Fluency | 9.13 (3.10) | 8.54 (5.25) | 0.64 |
| Trails A Time (seconds) | 78.28 (47.4) | 125.64 (74.77) | 0.02 |
| Trails B Time (seconds) | 240.58 (132.67) | 263.13 (136.48) | 0.68 |
| Fluency Ratio | .51 (.16) | .56 (.14) | 0.56 |
| Pentagon copy (frequency impaired) | 8 (24%) | 11 (73%) | <.01 |
Note: Significant values are bolded and defined by p<0.05.
3.3. Prediction of group membership
Three binary logistic regression models examined the ability of each of the significant neuropsychological tests (CFL, Trails A, pentagons) to predict group membership. For model comparison purposes, participants were selected only if they had available data for all three measures, resulting in a sample of 39 patients (28 AD and 11 AD + LBD). Additionally, to enable more direct comparison of the predictors, CFL and TMT-A raw scores were converted to standardized scores adjusted for age and education using the same normative data set.[37, 38] Normative data were not available for pentagon copy.
Standardized CFL score was marginally predictive of group (b = −0.18, SE = 0.11, odds ratio = 0.86, p = 0.09, 95% CI [0.69, 1.03]), X2(1) = 3.10, p = 0.08. The Hosmer-Lemeshow test of homogeneity was not significant X2(7) = 7.92, p = 0.34. CFL correctly classified 76.9% of participants (96.4% of the AD group, 27.3% of mixed AD and LBD), explaining a relatively small proportion of the variance in diagnosis classification, Cox and Snell R2 = .08, Nagelkerke’s R2 = .11. Figure 1 shows the area under the ROC curve = 0.67 (95% CI, 0.48 to 0.86), reflecting poor discrimination according to Hosmer and colleagues.[39] Once the same model was run with inclusion of behavioral and extrapyramidal factors (i.e., visual hallucinations and parkinsonism), the results continued to hold, with CFL score predicting group classification (b= −0.21, SE=0.10, odds ratio= 0.82, p=0.03, 95% CI [0.67, 1.00], X2(3)=7.99, p=0.05). CFL correctly classified 78.9% of participants (92.3% of the AD group, 45.5% of the mixed AD and LBD group), explaining a relatively small proportion of the variance in diagnosis classification, Cox and Snell R2 =0.19.
Figure 1.
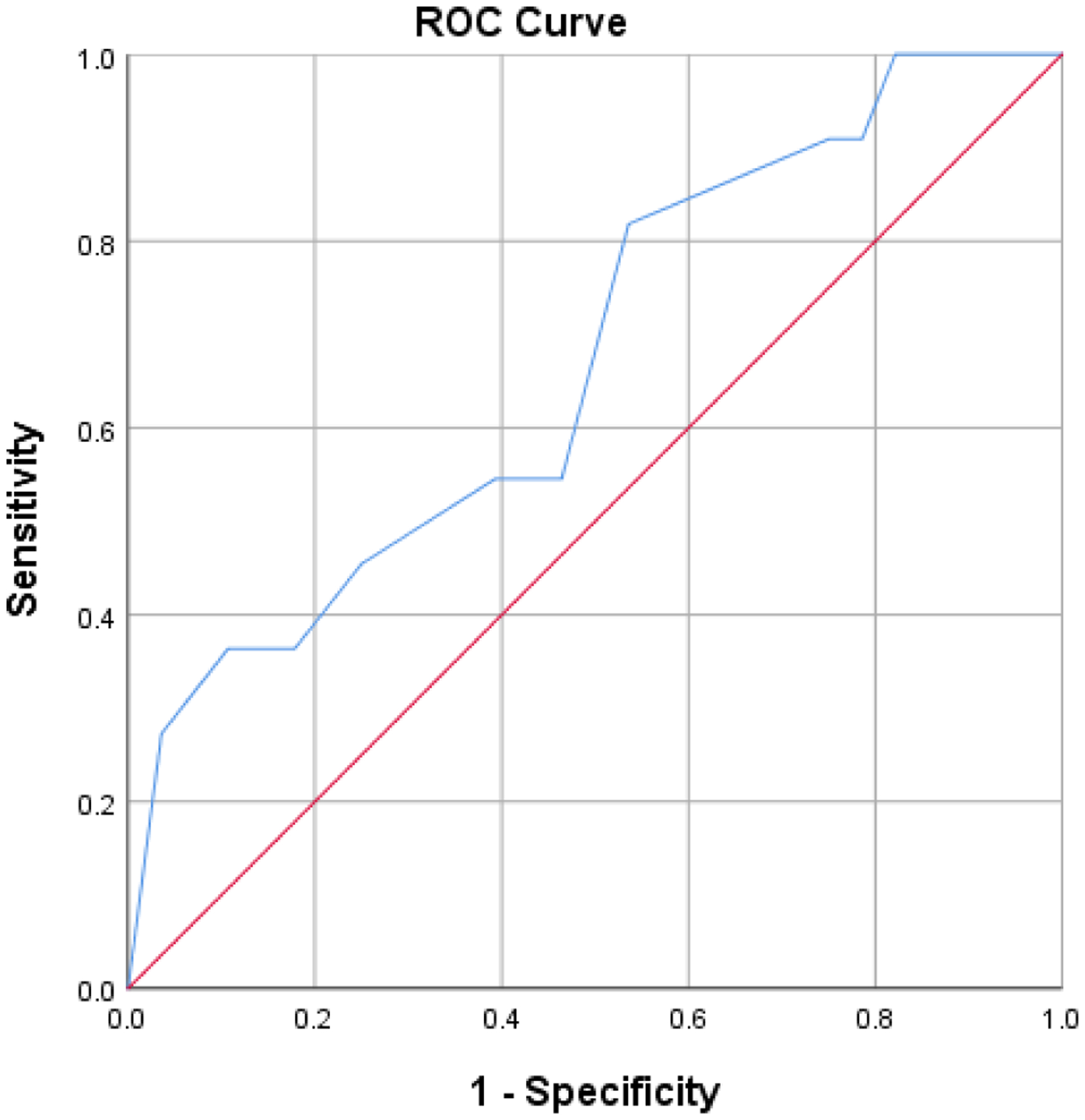
ROC curve of phonemic fluency
Standardized TMT-A score was significantly predictive of group classification with the odds of being classified as mixed pathology increasing as time to complete TMT-A increases (b = −0.30, SE = 0.14, odds ratio = 0.74, p = 0.03, 95% CI [0.57, 0.98]), X2(1) = 6.13, p = 0.013. The Hosmer-Lemeshow test of homogeneity was not significant X2(8) = 7.95, p = 0.44. This second model correctly classified 76.9% of participants (92.9% of AD and 36.4% of AD + LBD). Figure 2 shows the area under the ROC curve as 0.75 (95% CI, 0.57 to 0.92), having acceptable discrimination according to Hosmer and colleagues.[39] Once again, this model was conducted with inclusion of non-cognitive factors (i.e., visual hallucinations and parkinsonism). The findings reflected that TMT-A was significantly predictive of group classification, above and beyond presence of visual hallucinations and extrapyramidal signs (b=0.02, SE=0.01, odds ratio=1.02, p=0.05, 95% CI [1.00, 1.03], X2(3) =6.87, p=0.07).
Figure 2.
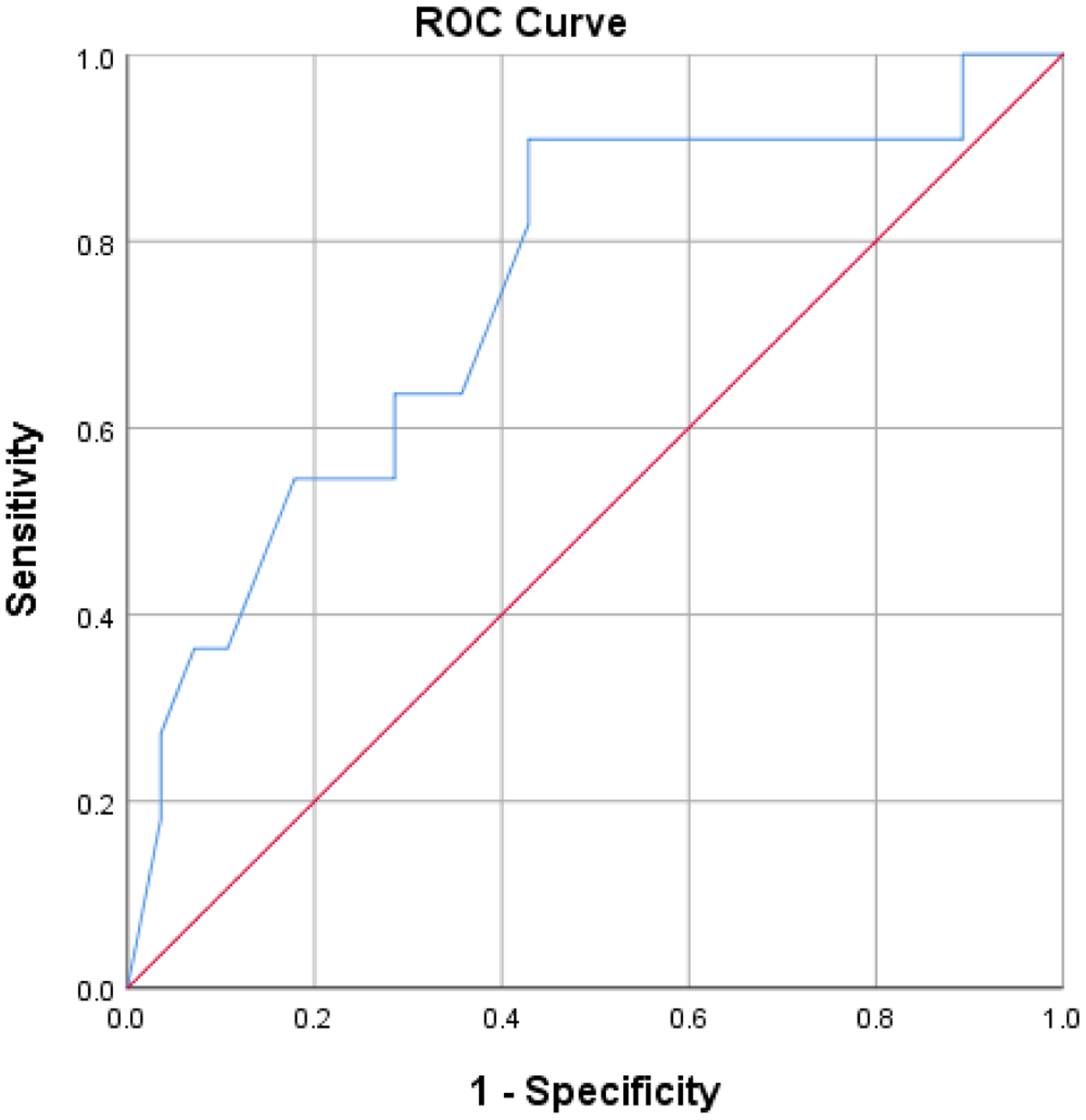
ROC curve of processing speed measure
Pentagon copy significantly contributed to group classification (b = 2.08, SE = 0.81, p = 0.01, odds ratio = 8.00, 95% CI [1.65, 38.79]), X2(1) = 7.59, p = 0.006, and explained approximately 20 % of the variance (Cox and Snell R2 = .18, Nagelkerke’s R2 = .25). The model classified 74.4% of cases correctly (75% pure AD and 72.7% AD + LBD). Figure 3 shows the area under the curve = 0.74 (95% CI, 0.56 to .092), having acceptable discrimination.[39] Once this model was conducted taking into consideration visual hallucinations and parkinsonism symptoms, visuoconstruction (per the pentagon copy score) remained a significant predictor over pathological group classification, (b=−2.60, SE=0.97, odds ratio=0.08, p=0.01, 95% CI [0.01, 0.49], X2(3)=11.20, p=0.01).
Figure 3.
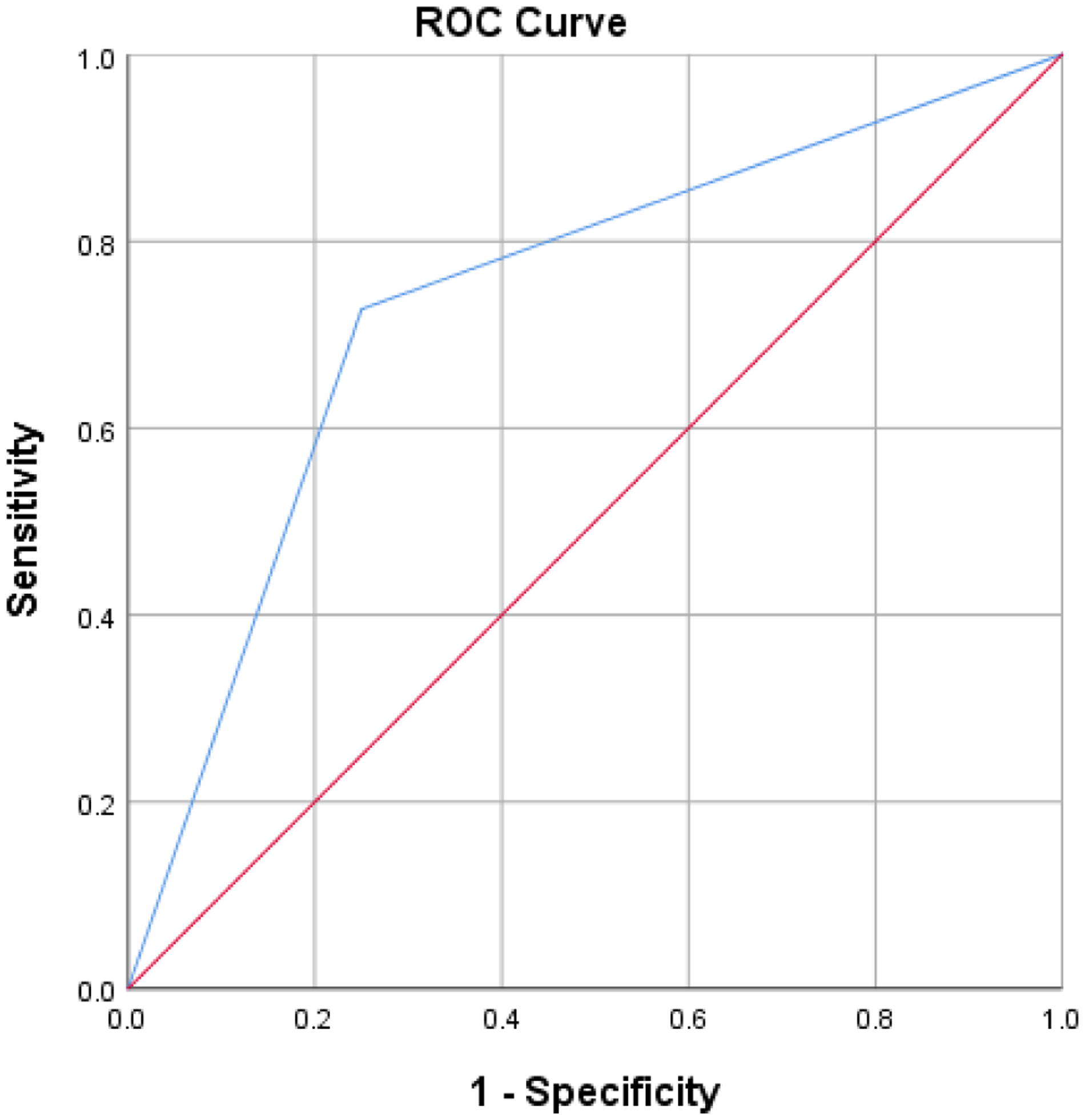
ROC curve of visuoconstruction
A final model included TMT-A and pentagon copy, both deemed to have acceptable discrimination. Although this model significantly predicted diagnosis, X2(3) = 8.95, p=0.03, neither predictor remained an independently significant group classifier (TMTA, b = −0.16, SE = 0.18, p = 0.39, 95% CI [0.60 – 1.22]; pentagons, b = −1.47, SE = 0.93, p = 0.23, 95% CI [0.04 – 1.44]). Further, combining these predictors overall predicted 76.9% of cases (85.7% correctly classified AD and 54.5% correctly classified AD + LBD) and not improve overall classification above that of TMT-A alone (76.9 %) or pentagon copy (74.4%). Figure 4 depicts the area under the curve as 0.78 (95% CI, .61 to .95), reflecting an acceptable discrimination.[39]
Figure 4.

ROC curve of processing speed and visuoconstruction measures
4. Discussion
The current study sought to further the understanding of neuropsychological differences across pathologically confirmed cases of AD versus AD + LBD, and to identify cognitive tests that may aid the clinical differentiation of these overlapping diseases. Previous research has indicated that certain cognitive functions may help discriminate these groups, such that memory storage may be relatively spared in LBD in contrast to greater impairments in visuospatial function, executive function, processing speed and attention, although findings have not been consistent [e.g., 11, 24, 30]. Results from the current study indicated that the AD and AD + LBD groups differed on assessments of phonemic fluency (CFL), processing speed (Trails A), and visuoconstruction (pentagon copy). As will be discussed below, each of these tests had different utility for classifying participants into the correct pathological group.
Findings from the current study are generally in line with previous pathological studies comparing AD versus AD + LBD, particularly those showing that the latter group is more likely to be impaired in phonemic fluency and other measures of executive functioning than pure AD.[e.g., 27, 29] These results are not consistent across all studies, however (Yoshizawa, Vonsattel [11]), potentially reflecting sampling differences. For example, Yoshizawa and colleagues[40] included patients in a somewhat earlier stage of dementia than the current study (e.g., CDR ranging from 0 to 1). It is possible that differences in executive functioning had not emerged yet across groups in that study. However, the ROC curve for fluency in the current study indicated that it was a poor discriminator of diagnosis; it correctly classified over 70% of the sample, but this was mainly driven by correct classification of AD (> 90%) and generally poor classification of AD + LBD (< 30%). Phonemic fluency alone, therefore, may not represent a very reliable determinant of the presence of LBD.
Our results also showed that participants with AD + LBD had slower processing speed than those with AD. This result, to our knowledge, has only been reflected in two other studies.[27, 28] In the current study, the processing speed measure (TMT-A) correctly classified the same percentage of overall patients as the phonemic fluency measure (77%), and its classification of AD + LBD was only slightly more accurate (36%). It is interesting that Trail Making Part B (TMT-B), a measure that relies on both speed and executive function, did not differ across groups. It may be that the slowing associated with DLB differentially impairs performance on TMT-A, a relatively simple measure of attention and speed on which patients with mild AD can perform well. However, on TMT-B, the executive set-shifting component may lower performance in both groups to a similar enough degree that statistically meaningful differences do not emerge across groups. We also considered the possibility that a floor effect on TMT-B could have reduced a potential difference across groups; however, inspection of data (not shown) is not consistent with this idea.
Like phonemic fluency and processing speed, visuoconstruction (pentagon copy) was more likely to be impaired in AD + LBD than AD alone, and this measure was deemed to have acceptable discrimination ability[39], correctly classifying a total of 74% of patients, above and beyond contribution of behavioral and extrapyramidal factors often presenting with LBD symptomatology. This finding is in line with other studies that have reported worse visuospatial functioning (e.g., pentagons, clock drawing) in AD + LBD than AD alone.[26–28, 41] Interestingly, of the three neuropsychological measures that differed across groups, pentagon copy seemed to be most sensitive to the presence of comorbid LBD pathology, correctly classifying 73% of this specific group (versus 27% and 36% for the other two measures). In turn, however, the pentagon copy misclassified more AD cases (25%) than the other measures (4 – 7%). A caveat of this model is the dichotomous nature of the pentagon task which limits its ability capture a broad range of visuoconstruction and visuospatial ability, and hence likely limits its predictive ability. A more fine-grained or qualitative evaluation of such abilities may detect more subtle differences between groups and improve group classification. Indeed, future studies with more comprehensive neuropsychological assessments, a more nuanced visuospatial measure, and attention to the qualitative examination of errors are needed to further elucidate how specific or combined cognitive abilities can contribute to the correct classification of disease.
Current results demonstrate the trade-off between the sensitivity and specificity of each measure for the identification of different pathologies, raising the question of whether combining the measures may yield the highest predictive accuracy. A final regression was thus conducted to determine whether combining the two best predictors of diagnosis (e.g., processing speed and visuoconstruction, both of which were deemed to have acceptable discrimination) improved overall group classification. Interestingly, the final model did not improve classification or area under the curve over that of processing speed or visuoconstruction alone.
Despite the expectation that the pure AD group would show greater depletion in memory and retention given previous work demonstrating that recognition discriminability, are more impaired in AD than in AD + DLB.[4] and that hippocampal volume is relatively better preserved in LBD than AD [42], no significant differences were observed in memory (i.e., retention and recognition) or in semantic processing abilities generally associated with temporal lobe functioning (i.e., naming, semantic fluency, or the fluency ratio calculated to reflect semantic degradation specifically). The reason for similar levels of memory and semantic abilities in the current study, versus studies which compare AD to pure LBD in particular, may well be that both groups have AD pathology and are at similar levels of global cognitive impairment. It is also possible that heterogeneity in the regional distribution of both AD and DLB pathologies contributes to differences seen across studies. Specifically, it is possible that in the current sample, there could have been a relatively low burden of temporal pathology in the AD group, or relatively high burden of temporal pathology in the AD + DLB group, with either scenario leading to comparable rather than dissimilar memory and language abilities. Indeed, AD can present with greater frontal involvement [24] or, conversely, with disproportionate posterior burden as is seen in in Posterior Cortical Atrophy (PCA; 76]. In such cases, memory can be less affected than in the classic amnestic presentation of AD. In order to produce more reliable findings across studies, it will be important not only to compare pathological diagnosis in a dichotomous manner, but with consideration for the degree and distribution of each pathology.
This study supports the idea that cognitive testing can aid in the clinical differentiation between autopsy-confirmed AD and AD + DLB, a common and important differential diagnosis that is not well differentiated when individuals come into the clinic for testing. In particular, processing speed and to a lesser extent, visuoconstruction, predicted pathological diagnosis with acceptable discrimination, and may assist in the clinical differentiation of these groups, above and beyond consideration of behavioral and extrapyramidal symptoms. It is worth noting, however, that although models had acceptable statistical AUC discrimination values, higher AUC (closer to 1) represents the highest degree of both sensitivity and specificity. Results in this study inform the manner in which neuropsychological testing aids diagnostic classification, but should be considered in a broader context when applying to patient care, and in light of risks and benefits associated with the diagnostic process. Examination of classical LBD features such as extrapyramidal and psychiatric features other than parkinsonism and visual hallucinations [see 7 for current diagnostic criteria] should be examined in conjunction with these cognitive abilities to further understand the profile of symptoms which taken together best discriminate between AD versus AD + LBD. Certainly, amyloid imaging studies, DaTScan and SPECT scans (123I-FP-CIT SPECT;[43–45]) can aid in detecting specific pathologies. For example, one previous study showed that cortical PiB retention differentiated patients with pathologically confirmed AD (LBD-AD and AD) from those with pure LBD with 93% accuracy, and the regional pattern of Aβ spared the occipital lobes when LBD was present, regardless of whether AD was also present [46]. However, identifying the mixed presentation of AD + LBD would likely require multiple costly and invasive scans, and may not always be feasible. As such, determining the extent to which non-invasive, routine, and inexpensive neuropsychological measures inform differential diagnosis is an important endeavor.
Regarding the clinical implications of the current findings, it is worth noting that clinicians would benefit from accurate differentiation between diagnosis to provide adequately tailored recommendations and refer patients to specific services that educate and target symptoms common in either diagnosis (i.e., AD, LBD, mixed AD and LBD). Accurate diagnosis is crucial to help in prognosis, medical decision-making, education on treatment options, and treatments to alleviate symptoms. Specifically, this distinction can allow individuals to receive appropriate services and obtain cognitive enhancing medications for the specific diagnosis (i.e., cholinergic therapy), and better educate patients and their families on the expected timeline of progression of and extent of symptoms. Further, tailored clinical recommendations can be directed towards comorbid symptoms that are more commonly present in specific or combined diagnoses (i.e., depression in AD, or hallucinations, sleep difficulty and extrapyramidal features in LBD) and improvement of quality of life (i.e., community resources, support groups). As services recommended on a neuropsychological evaluation often depend on the extent, type, and etiology of deficit, clinical differentiation is essential. As such, clinical differentiation is key for addressing tailored functionally meaningful outcomes for each patient. Visuospatial and processing speed difficulties often observed in LBD may directly influence driving ability and thus would warrant a driving evaluation once these deficits are clinically established. Executive deficits, including attention, working memory, and set-switching may influence decision-making and adherence to recommendations provided by respective medical providers (i.e., medication management). Thus, recommendations to attain specific services and educate caregivers and patients can only occur once these deficits are identified, preferrably early in the disease process. Not only do the current findings point to aspects of the neuropsychological profile that can inform differential diagnosis if subtle or questionable signs of LBD are present along with cognitive impairment, but when comprehensive neuropsychological assessment is not available, brief assessments of processing speed and pentagon copy may inform the presence of LBD.
There are several limitations of the current study. Regarding the clinical characterization of participants, it is possible that individuals developed motor symptoms after the current study visit. Though we cannot account for this in the current study, it lends for future longitudinal research to establish a timeline of cognitive and non-cognitive (i.e., extrapyramidal, behavioral) symptoms that account for changes in clinical profile throughout the respective disease process. Another limitation of the current study included disparate and relatively small sample sizes. Due to this limitation, we were unable to include a pure LBD group in the current study; this group could have informed the extent to which differences in test performance were due solely to the presence of LBD pathology or represented an interaction between mixed pathologies. Nonetheless, the current study sought to focus on those individuals that are often difficult to diagnose (i.e., AD with LBD) from those relatively easier to classify (i.e., pure AD) and thus fill a greater gap in the literature. This limitation reflects the challenges associated with recruiting individuals for autopsy, as previously mentioned, and should be improved in future research. Regarding analyses, this study did not conduct a Bonferroni correction of the initial between group analyses examining neuropsychological measures, which allowed CFL and Trail Making Test A to remain significant and thus be included in subsequent clasification models. However, given that the sample consisted of cases with CDR 1 and 2, subtle cognitive differences were expected between the groups and providing such a stringent correction might have led to increased likelihood of type two error. Additionally, it is possible that more complex analytical methods such as machine learning (i.e., Random forest model) may have allowed for interesting and more nuanced observations of interactions of multivariate data presented in the current study. This represents an interesting area of future research.
Table 3.
Non-Cognitive Scores (Sum of symptoms; Mean and SD)
| AD only (n=34) | Mixed AD+LBD (n=17) | p-value (Difference between AD only vs. mixed) | |
|---|---|---|---|
| Parkinsonism | 2.50(4.05) | 8.53(8.63) | 0.001 |
| Visual Hallucinations | 0.09 (0.38) | 0.71(1.05) | 0.004 |
Research in Context.
1. Systematic review:
The authors reviewed the literature using traditional (i.e., PubMed) sources, including abstracts and presentations.
2. Interpretation:
Our findings extend previous research on neuropsychological differences between pure AD and mixed AD + LBD, noting that the pure AD and AD+LBD group differed in executive, processing speed, and visuoconstructional abilities but not in memory or semantic processing. However, only processing speed and visuoconstruction were adequate discriminants between pathologic groups.
3. Future Directions:
The manuscript highlights that processing speed and visuoconstruction may assist in the clinical differentiation of AD versus AD+LBD above and beyond the presence of visual hallucinations and extrapyramidal signs. In an effort to improve clinical decision-making and intervention earlier in the disease course, future work should examine whether qualitatively characterizing visuoconstructional dysfunction further improves its predictive utility and enhances that of classical LBD features.
Acknowledgments:
The authors would like to thank the National Institute of Aging (NIA) for funding this research (R01 AG007370).
Footnotes
Conflicts of Interest and Source of Funding: None declared.
References
- 1.Serrano-Pozo A, et al. , Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med, 2011. 1(1): p. a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreisl WC, et al. , Distinct patterns of increased translocator protein in posterior cortical atrophy and amnestic Alzheimer’s disease. Neurobiol Aging, 2017. 51: p. 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson DW, Dementia with Lewy bodies: neuropathology. J Geriatr Psychiatry Neurol, 2002. 15(4): p. 210–6. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton JM, et al. , A comparison of episodic memory deficits in neuropathologically-confirmed Dementia with Lewy bodies and Alzheimer’s disease. J Int Neuropsychol Soc, 2004. 10(5): p. 689–97. [DOI] [PubMed] [Google Scholar]
- 5.Hansen LA and Samuel W, Criteria for Alzheimer’s disease and the nosology of dementia with Lewy bodies. Neurology, 1997. 48(1): p. 126–32. [DOI] [PubMed] [Google Scholar]
- 6.Irwin DJ and Hurtig HI, The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders. J Alzheimers Dis Parkinsonism, 2018. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeith IG, et al. , Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology, 2017. 89(1): p. 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiraboschi P, et al. , What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain, 2006. 129(Pt 3): p. 729–35. [DOI] [PubMed] [Google Scholar]
- 9.Stavitsky K, et al. , The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol, 2006. 63(10): p. 1450–6. [DOI] [PubMed] [Google Scholar]
- 10.Metzler-Baddeley C, A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex, 2007. 43(5): p. 583–600. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa H, Vonsattel JP, and Honig LS, Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatry, 2013. 84(12): p. 1326–30. [DOI] [PubMed] [Google Scholar]
- 12.Collerton D, et al. , Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dement Geriatr Cogn Disord, 2003. 16(4): p. 229–37. [DOI] [PubMed] [Google Scholar]
- 13.Gnanalingham KK, et al. , Motor and cognitive function in Lewy body dementia: comparison with Alzheimer’s and Parkinson’s diseases. Journal of neurology, neurosurgery, and psychiatry, 1997. 62(3): p. 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker Z, et al. , Neuropsychological performance in Lewy body dementia and Alzheimer’s disease. Br J Psychiatry, 1997. 170: p. 156–8. [DOI] [PubMed] [Google Scholar]
- 15.Mori E, et al. , Visuoperceptual Impairment in Dementia With Lewy Bodies. Archives of Neurology, 2000. 57(4): p. 489–493. [DOI] [PubMed] [Google Scholar]
- 16.Lambon Ralph MA, et al. , Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer’s type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry, 2001. 70(2): p. 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calderon J, et al. , Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry, 2001. 70(2): p. 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballard C, et al. , Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol, 2001. 58(6): p. 977–82. [DOI] [PubMed] [Google Scholar]
- 19.Doubleday EK, et al. , Qualitative performance characteristics differentiate dementia with Lewy bodies and Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 2002. 72(5): p. 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simard M, van Reekum R, and Myran D, Visuospatial impairment in dementia with Lewy bodies and Alzheimer’s disease: a process analysis approach. International Journal of Geriatric Psychiatry, 2003. 18(5): p. 387–391. [DOI] [PubMed] [Google Scholar]
- 21.Cormack F, et al. , Pentagon drawing and neuropsychological performance in Dementia with Lewy Bodies, Alzheimer’s disease, Parkinson’s disease and Parkinson’s disease with dementia. Int J Geriatr Psychiatry, 2004. 19(4): p. 371–7. [DOI] [PubMed] [Google Scholar]
- 22.Perriol M-P, et al. , Disturbance of sensory filtering in dementia with Lewy bodies: comparison with Parkinson’s disease dementia and Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 2005. 76(1): p. 106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breitve MH, et al. , A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer’s disease. Alzheimers Res Ther, 2014. 6(5–8): p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connor DJ, et al. , Cognitive profiles of autopsy-confirmed Lewy body variant vs pure Alzheimer disease. Arch Neurol, 1998. 55(7): p. 994–1000. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DK, Morris JC, and Galvin JE, Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology, 2005. 65(8): p. 1232–8. [DOI] [PubMed] [Google Scholar]
- 26.Ala TA, et al. , The Mini-Mental State exam may help in the differentiation of dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry, 2002. 17(6): p. 503–9. [DOI] [PubMed] [Google Scholar]
- 27.Hansen L, et al. , The Lewy body variant of Alzheimer’s disease. A clinical and pathologic entity, 1990. 40(1): p. 1–1. [DOI] [PubMed] [Google Scholar]
- 28.Galasko D, et al. , Clinical and Neuropathological Findings in Lewy Body Dementias. Brain and Cognition, 1996. 31(2): p. 166–175. [DOI] [PubMed] [Google Scholar]
- 29.Jicha GA, et al. , Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiology of aging, 2010. 31(10): p. 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forstl H, et al. , The Lewy-body variant of Alzheimer’s disease. Clinical and pathological findings. Br J Psychiatry, 1993. 162: p. 385–92. [DOI] [PubMed] [Google Scholar]
- 31.Stern Y, et al. , Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord, 1993. 7(1): p. 3–21. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, and McHugh PR, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975. 12(3): p. 189–98. [DOI] [PubMed] [Google Scholar]
- 33.Rascovsky K, et al. , Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology, 2007. 21(1): p. 20–30. [DOI] [PubMed] [Google Scholar]
- 34.Hughes CP, et al. , A new clinical scale for the staging of dementia. Br J Psychiatry, 1982. 140: p. 566–72. [DOI] [PubMed] [Google Scholar]
- 35.Pillai JA, et al. , Impact of Alzheimer’s Disease, Lewy Body and Vascular Co-Pathologies on Clinical Transition to Dementia in a National Autopsy Cohort. Dement Geriatr Cogn Disord, 2016. 42(1–2): p. 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulloch J, et al. , APOE DNA methylation is altered in Lewy body dementia. Alzheimers Dement, 2018. 14(7): p. 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivnik RJ, et al. , Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist, 1996. 10(3): p. 262–278. [Google Scholar]
- 38.Strauss E, Sherman EMS, and Spreen O, A Compendium of Neuropsychological Tests: Adiministration, Norms and Commentary. Third ed. 2006, New York: Oxford University Press. [Google Scholar]
- 39.Hosmer DWJ, Lemeshow S, and Sturdivant RX, Applied logistic regression. 3rd ed. 2013, NJ: Wiley. [Google Scholar]
- 40.Yoshizawa H, Vonsattel JPG, and Honig LS, Early neuropsychological discriminants for Lewy body disease: an autopsy series. Journal of Neurology, Neurosurgery & Psychiatry, 2013. 84(12): p. 1326–1330. [DOI] [PubMed] [Google Scholar]
- 41.Ala TA, et al. , Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry, 2001. 70(4): p. 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kantarci K, et al. , Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology, 2016. 87(22): p. 2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rioboo PJ, Varela Lema L Serena Puig A, Ruano-Ravina A, Effectiveness of 123I-ioflupane (DaTSCAN) in the diagnosis of Parkinsonian syndromes. A systematic review. Revista Espanola Medicina Nuclear, 2007. 26(6): p. 375–384. [DOI] [PubMed] [Google Scholar]
- 44.McCleery J, et al. , Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev, 2015. 1: p. CD010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaamonde-Gamo J, Flores-Barragan JM, Ibanez R, Gudin M, Hernandez A, DaT-SCAN SPECT in the differential diagnosis of dementia with Lewy bodies and Alzheimer’s disease. Revista de Neurologia, 2005. 41(11). [PubMed] [Google Scholar]
- 46.Landau SM and Villemagne VL. Can amyloid PET differentiate “pure” LBD from AD with or without LBD copathology. Neurology, 2020. 94(3): p. 103–104. [DOI] [PubMed] [Google Scholar]


