Abstract
A majority of children with fetal alcohol spectrum disorders (FASD) have demonstrated attention and executive function deficits as measured by both parent report measures and performance on tasks requiring sustained levels of attention. However, prior studies have consistently reported a lack of association between parental report-based and task-based performance measures. The current study investigated whether changes in performance over time within-task (i.e., first-half versus second-half) better correspond to parental reports of executive function and temperament in children with FASD. Greater differences in split-half performance during a continuous performance task were found to be associated with higher parent-reported levels of behavioral regulation and inhibitory control. These findings suggest that within-task performance differences may more accurately reflect individual differences in executive function and temperament as measured by parental report and help to further inform the way in which cognitive processes are measured in children with FASD.
Keywords: fetal alcohol spectrum disorders, sustained attention, executive function, task performance, parental report, temperament
Background/Introduction
Fetal alcohol spectrum disorders (FASD) are a group of conditions that occur if an individual is exposed to alcohol prenatally (Astley 2011). These children often experience challenges with memory – in particular, verbal memory – and challenges with visuospatial processing (Pei et al. 2008, Manji et al. 2009). Notably, children with FASD frequently demonstrate executive function and effortful control deficits, with difficulties in attention and reduced inhibition (Mattson and Riley 2011, Mattson et al. 2013, Lange et al. 2019; see Lange et al. 2017, Mattson et al. 2019 for review).
Much attention has been focused on the specific executive function impairments that exist in children with FASD (see Kingdon et al. 2016 for review), specifically due to the prevalence of impairments associated with frontostriatal brain regions involved in inhibition (Fryer et al. 2007) and prefrontal cortex regions involved in working memory (Malisza et al. 2012; see Glass et al. 2014b for review). The Behavior Rating Inventory of Executive Function (BRIEF; Gioia et al. 2000b, Gioia et al. 2015) parent-report questionnaire has been used to assess executive functioning and includes the following subscales: Inhibit, Shift, Emotional Control, Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor. Prior group studies using the BRIEF parent report for children with FASD have demonstrated clinically elevated scores (T-scores >65) for most subscales. These areas of impairment have included Inhibit, Initiate, and Working Memory subscales (Rasmussen et al. 2007), with the relative sparing of Organization of Materials (Rai et al. 2017, Mohamed et al. 2019). As such, the BRIEF has yielded information about the specific executive function challenges encountered in children with FASD, with the potential to link these dimensional aspects of parent-report measures of everyday executive functioning to specific task-based measures of executive function and behavior.
Additionally, one parental report measure in the developmental psychological literature that has been employed in relation to executive function is the use of temperament ratings, specifically those related to effortful control (Rothbart et al. 2007, Morasch and Bell 2011). Effortful control is a construct largely overlapping with executive function, although historically studied from a different ‘tradition’ and representing a trait-level aspect of executive function (Nigg 2017, Zhou et al. 2012). While infants with prenatal alcohol exposure have been shown to demonstrate more difficult temperaments (Alvik et al. 2011) and increased emotional withdrawal (Molteno et al. 2014), in general there has been a relative paucity of studies examining effortful control specifically in children with prenatal alcohol exposure (Garrison et al. 2019). Thus, there is an opportunity to contribute to what is known about specific aspects of parent-reported temperament in children with FASD, and to investigate whether parent-reported temperament measures of effortful control relate to the BRIEF and to direct assessments of executive function and behavioral regulation.
In order to investigate how performance-based neuropsychological measures correspond with everyday functioning in children with FASD, efforts have been made to determine whether parental report measures of executive function in children with FASD correlate with task-based measures of executive function and attention (Gross et al. 2015, Nguyen et al. 2014, Glass et al. 2014a, Rai et al. 2017, Mohamed et al. 2019). These studies have demonstrated little-to-no correlation between task-based and parent report-based measures of executive function. Discrepancies between everyday functioning measures and directly observed performance on cognitive tasks have also been found to extend to older adults with developmental disabilities (Geurts et al. 2020). Various explanations have been proposed for this lack of concordance, such as the theory that a child may perform well on a task during specific experimental conditions yet be ‘unable to deploy the appropriate skills in their daily lives’, which is better captured by parental report (Gross et al. 2015). Task-based and parent-based assessments have also been thought to reflect different aspects of executive functioning (Toplak et al. 2013, Ten Eycke and Dewey 2016). For example, prior studies examining the relationship between sustained attention on continuous performance task and parent-reported BRIEF measures have found no significant relationship between the two, but have demonstrated that parents of children with ADHD report consistently higher levels of executive dysfunction relative to that measured by laboratory task (Bodnar et al. 2007) and that levels of executive dysfunction in children with myelomeningocele form of spina bifida are best predicted by parent report (Brown et al. 2008). Similarly, a recent study in toddlers by Acar et al. (2019) investigating temperament ratings of attention-focusing versus performance on tasks requiring focused attention found no significant relationship between parent-report and task-based measures. The hypothesis that executive function and attention measures based on parental report are uniquely distinct from traditional task-based assessments is further bolstered by the finding that functional activity in specific brain regions seems to correspond to different parent-report versus experiment-based measures (Faridi et al. 2015).
It is unclear, however, whether the failure to demonstrate correspondence between direct assessment of executive function using task-based measures and indirect assessment of executive function using parental report results from the conventional ways in which task performance is measured (e.g., task-wide summary scores that fail to reflect within-task changes in performance). Recent studies, for example, have shown that children with prenatal alcohol exposure demonstrate increased variability in performance on certain executive function tasks (Ali et al. 2018) and increased day-to-day variability in engagement in everyday tasks in classroom settings (Kjellmer and Olswang 2013). Furthermore, one study by Ali et al. (2019) demonstrated that intra-individual variability in reaction time (RT) on a go/no-go task was found to correlate with parental reports of attention. Thus, intra-individual variability may not be adequately captured in an ‘absolute’ measure of task performance.
Given the pattern of deficits in executive function demonstrated in children with prenatal alcohol exposure, one specific task that has been used to systematically measure sustained attention over time is the continuous performance task (CPT), which requires that the participant attend to a series of changing stimuli and respond appropriately to target items while inhibiting responses to distractor items (Klee and Garfinkel 1983,, Riccio et al. 2002, Shalev et al. 2018). The CPT requires actively directed focused attention and continuous inhibitory control and is thought to be foundational for the emergence of higher executive function processes (Fisher 2019). Children with a history of prenatal alcohol exposure have been shown to make more omission and commission errors on CPT than those without a history of prenatal alcohol exposure (see Dolan et al. 2010 for review). Additionally, in children with ADHD, it has been suggested that BRIEF behavioral regulation subscales are correlated with CPT performance measures that more directly measure intra-individual variability (Cak et al. 2017). While slower RTs on CPT in children with FASD have been found to modestly correlate with one BRIEF subscale (Organization of Materials; Rai et al. 2017), intra-individual variability in RT in children with FASD has not yet been explored.
Another measure of intra-individual variation in task performance that may better reflect differences in everyday executive function and attention is split-task performance (i.e., the difference in performance between first and second halves of the CPT). A prior study by Shalev et al. (2019) using the CPT in children with developmental disabilities (Down syndrome and Williams syndrome) demonstrated that split-half performance reveals syndrome-specific profiles. Children with Williams syndrome demonstrated overall poorer CPT performance than typically developing participants (75% accuracy in Williams syndrome versus 90% accuracy in typical development), and uniquely demonstrated a significant decrease in performance during the second half of the task, with a decrease in accuracy from 76% to 72% between task-halves. Meanwhile, those with Down syndrome had overall poorer CPT performance that did not vary across split halves, with overall accuracy of 74%. This innovative, within-task, change-over-time-based approach to measuring CPT performance was shown to correlate with teacher report of inattention in children with Williams syndrome. Compared to children with Williams syndrome, children with FASD also demonstrate executive function deficits, with specific difficulties in attention and visuospatial processing (Rhodes et al. 2010; Atkinson and Braddick 2011; Paolozza et al. 2014). Split-half CPT performance may thus distinctly capture variability in executive function and attention measures for this population and help inform the relation between task-based and parent-rated executive measures for children with FASD.
The current study tested the hypothesis that task-based measures of executive function and attention that represent within-person differences in children with FASD will align with parent-based estimates of executive function. Task performance variability was assessed on a visual CPT requiring continuous inhibition and modulation of attention, with the hypothesis that individual differences in RT variability and split-half performance may more closely correspond with measures of executive function based on parental report.
The following specific research questions were addressed:
Do children with FASD demonstrate within-task changes in performance during a continuous performance task (CPT) assessing sustained attention and inhibition? Specifically, is there intraindividual variability in RT and are there degradations in second-half compared to first-half CPT performance among children with FASD?
Do parental report measures of behavioral regulation (assessed with the BRIEF) and effortful control (assessed with a temperament rating scale) correspond with within-task changes in CPT performance – based on intra-individual variability in RT and split-half performance measures – in children with FASD?
Methods
Participants
Participants with prenatal alcohol exposure were recruited as volunteers from the University of Washington Fetal Alcohol Syndrome Diagnostic and Prevention Network clinical research registry and database, composed of over 3,000 patients. As part of this database, all participants had known in utero alcohol exposure and were diagnostically confirmed to meet criteria for FASD based on interdisciplinary evaluation including neuropsychological testing performed by an interdisciplinary assessment team and medical examination performed by a physician trained on the University of Washington FASD 4-Digit Diagnostic Code (Astley 2004, Astley 2013). The 4 digits of the Code reflect the magnitude of expression of the 4 key diagnostic features of FASD in the following order: 1) growth deficiency, 2) FAS facial phenotype, 3) CNS structural/functional abnormalities, and 4) prenatal alcohol exposure. The magnitude of expression of each feature is ranked on a 4-point Likert scale with 1 reflecting complete absence of the FASD feature and 4 reflecting a strong “classic” presence of the FASD feature. The 4-Digit codes cluster under one of 4 diagnoses under the umbrella of FASD from most severe to least severe: FAS, partial FAS, Static Encephalopathy/Alcohol Exposed (SE/AE) and Neurobehavioral Disorder/Alcohol Exposed (ND/AE). The 12 participants in the current study received the following FASD diagnostic classifications and codes, which fall on the ‘less severe’ end of FASD:
ND/AE (n = 9) – mild to moderate CNS functional impairment (CNS Rank 2)
SE/AE (n = 3) – significant CNS functional impairment or structural abnormality (CNS Rank 3 or 4)
Participants (N = 12; 7 females) were selected on the basis of having attempted and completed the CPT in the context of a larger study. Although 14 participants (8 females; ages 4.9 to 9.3 years; M = 7 years) attempted the CPT, 2 participants were excluded due to task incompletion. Participants spoke English as the primary language in the home and had no uncorrected hearing or vision impairments that would preclude engagement in the lab activities. Of the 12 included participants, medication usage for ADHD symptoms was reported by a caregiver for 7. Participants completed two research visits on the same day for individual assessment by an examiner, during which time parent questionnaires were completed. Due to scheduling preferences, the research visits were on consecutive days for one participant.
Nonverbal IQ
Nonverbal IQ was assessed with the Leiter International Performance Scale, Third Edition (Leiter-3), a standardized assessment for individuals ages 2 through 20 designed to be administered nonverbally (Roid et al. 2013). A nonverbal IQ scaled score was calculated according to the published manual as a composite score of the following scales: Figure Ground Segregation, Form Completion, Sequential Ordering, and Classification & Analogies. Scores on the Leiter-3 are presented descriptively in Table 1; IQ was not significantly correlated with CPT performance or parent report measures.
Table 1.
Mean Participant Age, IQ, BRIEF parent report subscales (included subjects, n=12)
| Age in years (range; SD) | Non-verbal IQ (range; SD) | BRIEF-Inhibit (range; SD) | BRIEF-Shift (range; SD) | BRIEF-Emotional Control (range; SD) | Temperament: Attention Focusing (range; SD) | Temperament: Inhibitory Control (range; SD) |
|---|---|---|---|---|---|---|
| 7.1 (4.9–9.25; 1.5) | 102 (71–120; 12.6) | 73.9 (55–90; 9.9) | 70.2 (53–87; 9.6) | 68.8 (50–81; 10.4) | 5.3 (2.3–7; 1.4) | 3.3 (1.5–5.2; 1.1) |
Parent-Report Questionnaires
Parents of each participant completed the Behavioral Rating Inventory of Executive Function (BRIEF; Gioia et al. 2000b; Gioia et al. 2015). Among the 12 participants, 1 BRIEF-P (preschool version) was completed, 8 BRIEF-1st edition questionnaires were completed, and 3 BRIEF-2nd edition questionnaires were completed. There has been strong internal consistency demonstrated for BRIEF measures on reliability studies, with Cronbach’s alpha coefficient measuring between 0.80 and 0.98 for clinical and normative samples (Gioia et al. 2000a). Of note, a BRIEF T-score of 50 is the normative mean; a score of 65 (i.e., 1.5 SD from that mean) or greater is considered clinically noteworthy. Amongst the 5 BRIEF subscales that overlapped across BRIEF versions (i.e., Inhibit, Shift, Emotional Control, Working Memory, Plan/Organize), those 3 scales that fell in the category of behavioral regulation were used for analysis (see Table 1): Inhibit, Shift, Emotional Control.
Additionally, parents of each participant completed an age-specific temperament questionnaire developed by Rothbart and colleagues: Children’s Behavior Questionnaire (3–7 years; Rothbart et al. 2001), Temperament in Middle Childhood Questionnaire (7–10 years), or Early Adolescent Temperament Questionnaire (>10 years; Capaldi and Rothbart 1992). Given known links between executive function and effortful control – and the view that attention control and inhibitory control are key components for each construct (Kim-Spoon et al. 2019) – two effortful control indices overlapping across the temperament questionnaires were the focus of analysis: attention-focusing and inhibitory control. These indices were adjusted across questionnaire versions to a 1–7 point Likert scale. Again, see Table 1.
Visual Recognition Memory Task
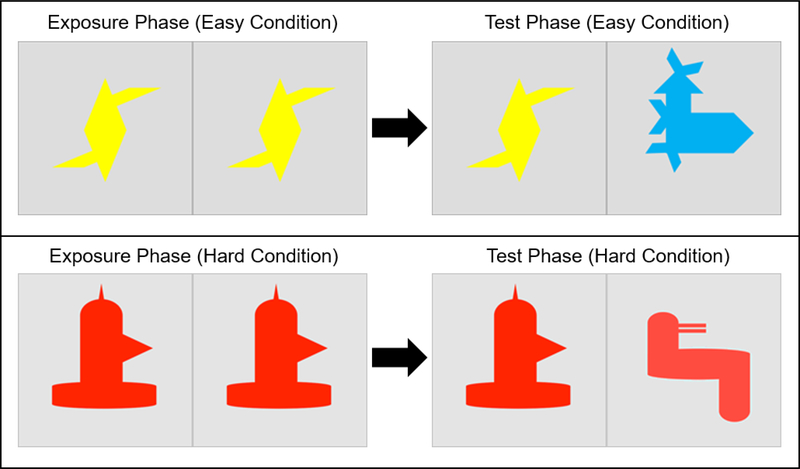
As a measure of foundational visual processing, participants also completed a visual paired comparison (VPC) task based on Rose et al. (2001), presumed to measure explicit visual recognition and memory (Manns et al. 2000). Each task condition (‘easy’, ‘hard’) consisted of 8 trials, with an exposure and a test phase for each trial. Participants were randomized to one of two different stimuli orders for each task version. During each VPC trial, images of geometric figures were presented using E-prime 2.0 on the left and right side of a wall-mounted screen. Participants were seated 1 meter from the screen, with a visual angle of approximately 44 degrees. Test phase stimuli consisted of an ‘old’ item from the preceding exposure phase – randomized to either the left or right side of the screen accompanied by a ‘new’ item on the opposite side of the screen. A black screen was presented during the 500ms inter-stimulus interval. During the exposure and test phases of each trial, visual stimuli were presented for a minimum of 2 seconds with participants required to accumulate a minimum of 2 seconds of looking time as gauged by the examiner via key press in real time (Venker 2013). Average looking times – calculated using eye-gaze coding methods described below – across participants for each trial phase was 2.2 seconds (SD = 0.7) and did not significantly vary across exposure versus test phases or ‘easy’ versus ‘hard’ task versions. Average trial duration was 3.2 seconds (range: 2.5–4.8; SD = 1.2). Task difficulty was manipulated by altering perceptual similarity of stimulus items, with ‘easy’ and ‘hard’ task conditions presented to each participant in a randomized order. During the ‘easy’ condition, old and new items presented during the test phase were different colors and in the ‘hard’ version both old and new items during the test phase were the same color. See Figure 1.
Fig 1.
Example visual paired comparison test stimulus for multi-colored (top panel) and Monochromatic (bottom panel) Conditions, where one image from each test pairing was previously presented to the participant during the exposure slide immediately preceding test
EyeCoder and Datawiz software (Fernald et al. 2008) were used for data analysis to code eye-gaze recordings for each trial, with a frame rate of 30 FPS. Gaze to the left and right (exposure phase) and old and new (test phase) geometric figures was coded frame-by-frame offline by trained coders who completed quarterly reliability exercises, with lab-wide inter-coder agreement maintained at > 98% frame agreement and > 95% shift agreement. Frames in which the participant looked away from the screen were excluded from the analysis. A discrimination ratio (DR) for each test phase was calculated using the following formula (Sivakumaran et al. 2018):
Discrimination ratios were averaged across trials to calculate a mean discrimination ratio for each task condition for each participant.
Amongst the 12 participants, 8 participants completed both ‘easy’ and ‘hard’ task conditions of VPC task. Average DR across the ‘easy’ task condition was 0.68 (range: 0.55–0.87; SD=0.11). Average DR across the ‘hard’ task condition was 0.57 (range: 0.31–0.71; SD=0.12). DR was significantly higher for ‘easy’ compared to hard condition (p = 0.02, 1-tailed). Similar DR values of approximately 0.60 have been reported for pattern-based stimuli in infants as young as 5 months of age (Rose et al. 2001). This demonstrates that participants were able to recognize previously presented images, a foundational visual processing task.
Continuous Performance Task (Sustained Visual Attention)
A standard continuous performance task assessed sustained visual attention in E-prime 2.0 using a Cedrus RB-540 response pad. Participants were seated approximately 1 meter from the wall-mounted screen with the response pad (button box) held in lap. Stimuli comprised an approximate 32 degree visual angle. The task consisted of two practice blocks (one slow, one regular-speed) followed by three stimulus blocks of 15 trials each. Each stimulus block lasted approximately 2 minutes, with each target item presented at pseudo-random intervals (3, 4, or 5 distractor stimuli between each target stimulus). Average time between target stimuli presentation was 4.2 seconds. Presentation duration of target stimulus was 600 ms, with 200 ms inter-stimulus interval. Responses within 2.4 seconds of target onset were counted as correct. Modeled after Scerif et al. (2012) and Cornish et al. (2013), the CPT was presented as a fishing game and participants were instructed to watch the ‘wave’ and press the response button when they saw the ‘big waves’ to ‘catch the fish’ located under the big waves. Stimuli consisted of centrally-presented circular vertical Gabor patches with gray color map, using sinusoidal gradient with phase shift of 0.5π and frequency of 10 Hz. Gaussian standard deviation was 1 wave length for target items and 0.75 wave lengths for distractor items. Gabor images used were demonstrated previously to have supra-threshold intensity discrimination in preschool-to-early school-age boys with other neurodevelopmental disorders (i.e., fragile X syndrome; Scerif et al. 2012, Cornish et al. 2013). Participants were instructed to press a response button when higher contrast ‘big waves’ (i.e., target items; Figure 2, upper left) were presented and refrain from pressing the response button when lower contrast stimuli (i.e., distractor items; Figure 2, upper right) were presented. If participants responded correctly, a reinforcer image (cartoon fish; Figure 2, lower) appeared on the screen during practice; auditory feedback was provided through computer speakers for correct responses during the task.
Fig 2.
Continuous performance task visual attention stimuli
The variables derived from task performance were: (1) Accuracy: extent that the hit rate exceeds the false alarm rate, (2) Sensitivity: ability to distinguish between target and distractor items, and (3) Response Bias: tendency to commit more false alarms versus miss more target items. For data analysis, trials with response times of <200 ms were removed given physiological limitations of reaction time to visual stimuli. For each participant, accuracy for first and second task halves was calculated by subtracting the proportion of false alarms-only trials from the proportion of hits - including trials for which false alarms were made. Additionally, target sensitivity and response bias were calculated for the first and second halves of the task using the following formulas where F = False Alarms and H = Hits (Stanislaw and Todorov 1999, Shalev et al. 2019):
See Table 2. To address the first research question, first-half and second-half performance was compared for each CPT variable using paired-sample t-tests.
Table 2.
Mean Split-Half and Overall Continuous Performance Task Measures (n =12)
| Hits (range; SD) | False Alarms (range; SD) | Accuracy (range; SD) | Sensitivity (range; SD) | Response Bias* (range; SD) | |
|---|---|---|---|---|---|
| 1st half | 0.92 (0.77–1.0; 0.09) | 0.22 (0–0.70; 0.19) | 0.71 (0.24–1.0; 0.22) | 0.91 (0.75–1.0; 0.08) | −0.42 (−1–0.55; 0.53) |
| 2nd half | 0.89 (0.59–1.0; 0.11) | 0.32 (0–0.95; 0.26) | 0.58 (−0.06–1; 0.30) | 0.86 (0.44–1.0; 0.14) | −0.40 (−1–0.29; 0.41) |
| Overall | 0.91 (0.69–1.0; 0.09) | 0.27 (0–0.83; 0.22) | 0.64 (0.14–1.0; 0.24) | 0.89 (0.64–1.0; 0.10) | −0.40 (−1–0.29; 0.36) |
calculations exclude 1 subject who did not have false alarms for response bias calculation
Intra-Individual RT Variability
To further address the second research question, intra-individual variability in CPT reaction times (RT) was calculated by performing a linear regression of trial number x RT for each participant and measuring the standard deviation of the residuals. This yielded an intra-individual standard deviation (ISD) for each participant (Ali et al. 2019). A coefficient of variation (ICV) was calculated by dividing ISD by mean RT. Pearson correlations were performed to measure the strength of association between ICV and BRIEF executive function subscales/effortful control temperament indices, with an α = 0.05 significance level cutoff.
Split-Half Performance
Differences in each measure (dAccuracy, dSensitivity, dResponse Bias) between 1st versus 2nd halves were calculated by subtracting 2nd half performance from 1st half performance, hereafter also referred to as ‘split-half task performance.’ To address the second research question, Pearson correlations were performed to measure the strength of association between split-half task performance measures and BRIEF executive function subscales/temperament effortful control indices.
Results
Continuous Performance Task (Sustained Visual Attention): Research Question 1
Mean reaction time (for first response of each trial) across all trials for all participants was 604ms (range: 436–713; SD=82). Mean ISD was 233ms (range: 96–383; SD=96). Mean ICV was 0.38 (range: 0.22–0.56; SD=0.12). These values are descriptively higher than those demonstrated in prior study by Klein et al. (2006), which utilized the CPT in children with ADHD ages 7 to 14 and demonstrated significant differences in mean reaction time (545ms), mean ISD (145ms), and mean ICV (0.25) as compared to a control group. Average sensitivity, accuracy, and response bias measures across participants for 1st half, 2nd half, and overall CPT performance are summarized in Table 2. Participants demonstrated a significant difference in accuracy (Cohen’s d=−0.49; p=0.01, one-tailed) and sensitivity (Cohen’s d=−0.48; p=0.03, one-tailed) between first and second halves, with higher accuracy and sensitivity in 1st compared to 2nd task halves. Mean accuracy dropped by 14% (from 0.71 to 0.58) between first and second halves, which was descriptively larger than the 4% split-half performance decrement noted by Shalev et al. (2019) in children with Williams syndrome. No significant difference was observed in response bias between first and second halves (p=0.45, one-tailed). Higher false alarm rates in the 2nd half compared to the 1st (Cohen’s d=0.43; p=0.01, one-tailed) were observed. There was no significant difference in hit rates between first and second halves (p=0.14, one-tailed).
Relationship Between Task Performance and Parent Report Measures: Research Question 2
BRIEF Executive Function Behavioral Regulation and Temperament Effortful Control: To examine the correspondence between BRIEF executive function and temperament effortful control (and given the overlapping pattern of findings in relation to task performance, described below), Pearson correlations were calculated between BRIEF-Inhibit and Inhibitory Control from parent-report measures of temperament. As would be expected, there was a significant relationship between these two measures (r=−0.60, p=0.026), with more clinically elevated levels of BRIEF-Inhibit parental report scores correlating with lower levels of parent-reported Inhibitory Control. See Figure 3.
Fig 3.
BRIEF-Inhibit versus Temperament Inhibitory Control Measures
Intra-Individual Variability in CPT Reaction Time and Relationship with Parental Report and Task Performance Measures
ISD and ICV did not significantly correlate with BRIEF behavioral regulation or temperament effortful control parent measures. Although ISD and ICV did not significantly correlate with split-half task performance, there was a significant relationship between ISD/ICV and overall performance measures. ISD and ICV were negatively correlated with overall CPT accuracy (R=−0.75, −0.72 respectively; p=0.0046, 0.0078 respectively) and overall CPT sensitivity (R=−0.69, −0.65 respectively; p=0.013, 0.022 respectively). There was no significant relationship between ISD and ICV with CPT response bias.
Split-Half CPT Performance and Relationship with Parental Report
BRIEF Everyday Executive Function Behavioral Regulation: Of the three behavioral regulation subscales examined in relation to CPT performance, significant associations were observed for Inhibit, but not Shift or Emotional Control. There was a significant negative relationship between BRIEF-Inhibit parent report scores and dAccuracy (r=−0.60, p=0.040) as well as dSensitivity (r=−0.77, p=0.0031) measures on the sustained visual attention CPT, but not dResponse Bias (r=0.24, p=0.46). More clinically elevated BRIEF-Inhibit parental report scores (i.e., decreased inhibition) were associated with smaller differences in split-task performance (Figure 4). No significant relationship was demonstrated between BRIEF-Shift and dAccuracy (r=−0.26, p=0.41), dSensitivity (R=−0.42, p=0.17), or dResponse Bias (R=−0.40,p=0.20. Similarly, no significant relationship was demonstrated between BRIEF-Emotional Control and dAccuracy (r=0.057, p=0.86), dSensitivity (r=−0.35, p=0.27), or dResponse Bias (r=0.53, p=0.07).
Fig 4.
Visual Attention Task Performance versus BRIEF Parent Report Measures
Temperament Effortful Control: Of the two effortful control indices examined in relation to CPT performance, significant associations were observed for Inhibitory Control, but not Attention Focusing. There was a significant positive relationship between Inhibitory Control temperament parent report scores and dAccuracy (r=0.60, p=0.040) but not dSensitivity (r=0.55, p=0.062) for the CPT. High inhibitory control from parent-report measures was associated with greater differences in split-task performance (Figure 5, left). There was a significant negative relationship between Inhibitory Control temperament parent-report scores and dResponse Bias (r=−0.61, p=0.039; Figure 5, right). Higher inhibitory control from parent-report measures was associated with increased levels of shift in response bias away from false alarms as dominant error type between first and second task halves. Participants with lower inhibitory control from parent-report measures demonstrated smaller shifts in response bias – or even increased bias toward false alarms as dominant error type – from first to second task halves. No significant relationship was demonstrated between Attention Focusing and dAccuracy (r=−0.18, p=0.59), dSensitivity (r=0.098, p=0.76), or dResponse Bias (r=−0.33, p=0.29).
Fig 5.
Visual Attention Task Performance versus Temperament Parent Report Measures
Discussion
The current findings reflect the possibility that prior discordance between parental report and task-related measures of executive function – with extensions to effortful control – in children with fetal alcohol exposure may be due to task performance measures which fail to capture the challenges in performance across time that individuals with fetal alcohol spectrum disorders may experience. In particular, these findings are consistent with the notion that parent-report measures are more reflective of ‘typical performance’ in real-word settings as opposed to ‘optimal performance’ situations elicited by many experimental task conditions (Toplak et al. 2013, Ten Eycke and Dewey 2016; see McCoy 2019 for review).
Parent ratings of executive function are uniquely thought to reflect processes that extend beyond the ‘typical’ versus ‘optimal’ performance distinction, and instead may be a more nuanced combination of ‘day-to-day’ versus ‘maximal’ performance estimates (Toplak et al. 2013). Parental reports of executive function via the BRIEF have been proposed to more accurately reflect a wide range of concerns – including behavioral dysregulation and impairments in attention – as opposed to performance-based measures on goal-directed tasks (McAuley 2010). Meanwhile, parent ratings of temperament – and attention-focusing in particular – have been posited to be more reflective of a wide range of focused attention behaviors in young children (Acar et al. 2019). Given that the BRIEF parental report is designed to estimate everyday executive functioning abilities over the past six months and temperament measures are designed to reflect constitutional measures, it follows that task-based measures more sensitive to within-task variation may be a more ecologically valid metric with which to assess executive function. Additionally, the employment of a continuous performance task which largely taxed processes needed for executive functions – requiring sustained attention and continual active inhibition – likely afforded the opportunity to capture performance variability over time, despite a relatively short task duration.
The direction of the parent report versus task-based associations suggests that higher levels of parent-reported inhibitory control are associated with greater split-half performance differences. While somewhat counterintuitive, we postulate that parental report values of inhibitory control aspects of executive functioning are more reflective of parent-perceived capacity in executive functioning rather than absolute measures. Although prior report by Shalev et al. (2019) suggested that greater split-half performance differences were associated with higher levels of inattentive symptoms as assessed by teacher report, there is evidence that teacher and parent reports of attention and other executive function measures have low inter-rater reliability (Schneider et al. 2019) – including in children with FASD (Taylor and Enns 2019) – and may actually demonstrate opposite directional trajectories over time (Murray et al. 2018).
Executive function measures as estimated by parent ratings may very well reflect a combination of ‘typical’ and ‘optimal’ performance measures that are best captured from an experimental standpoint by estimates of variation in task performance rather than conventional measures of task performance. It may be that, when asked about their child’s level of inhibitory control, parents reflect on their own child’s relative ‘day-to-day’ within-person or intraindividual variability in modulating levels of inhibitory control rather than identifying an absolute (i.e. ‘typical’ or ‘optimal’) level of inhibitory control. Parents may, for example, factor in situational, environmental, and motivational factors when assessing their child’s overall executive functioning abilities. Indeed, parent ratings could be influenced by dyad-specific situational factors, such as levels of parental frustration (Gross et al. 2015). Children with more variability in the execution of cognitive control processes may be perceived by parents as having higher levels of inhibitory control. That is, parents may view their child as better able to engage in behavioral self-regulation because parents are assessing their child against the child’s own baseline and in the context of situational factors rather than comparing their child’s level of inhibitory control directly to that of their peers.
In support of this, higher levels of parent-reported inhibitory control were associated with increased shifts away from false alarms as dominant error type between first and second halves. Those with increased response bias shifts between task halves generally demonstrated higher levels of response bias toward false alarms in the first half, whereas those with decreased fluctuations between task halves (and lower levels of inhibitory control on parental report temperament measures) tended not to demonstrate the same level of response bias to begin with. This again suggests that parent report measures are a potential proxy for their own child’s ‘day-to-day’ differential ability to exercise inhibitory control across time as opposed to inherent ‘typical’ level of inhibitory control.
The current study did not replicate prior findings by Ali et al. (2019) of intra-individual variability in CPT reaction time correlating with parental estimates of attention. However, our study did demonstrate a significant negative relationship between intra-individual variability in reaction time and overall task performance, where participants with greater reaction time variability demonstrated lower task accuracy and sensitivity. This finding supports the notion that sustained attention tasks are subject to a speed-accuracy trade-off (Dang et al. 2018) and that individual variations in allocation of cognitive processing resources are important to factor in when comparing differences in CPT performance (Head and Helton 2014).
Overall, these study results suggest that measures of executive function based on parental report – particularly in a population where executive dysfunction is known to occur, as demonstrated by clinically elevated BRIEF measures in our participant group – are more situational (state-based) in nature. Thus, when applying these parent report indices to task-based measures, they are best predictive of intra-task performance variation, which is more state-based in nature and prone to state-based functions such as ‘day-to-day’ fatigue, attention span, and working memory. Conventional task performance measures of ‘overall’ task performance average across time and may reflect more trait-based characteristics of executive function.
The findings that BRIEF-Inhibit behavioral regulation and Inhibitory Control temperament effortful control measures both demonstrate significant relationships with CPT performance – and are significantly predictive of one another – highlights the role of each of these constructs in supporting sustained attention and response inhibition. CPT performance in particular is thought to reflect a convergence of ‘top-down’ and ‘bottom-up’ attention processing, with top-down processing considered a requisite for cortically-mediated executive functioning (see Sarter et al. 2001 for review). The current results support the notion that effortful control is thought to – from a theoretical framework – reflect self-regulation traits required for development of top-down executive function processes and can in some ways be considered a ‘lower-level’ executive function (Tiego et al. 2019; see Nigg 2017 for review). Our study reflects the utility of parent report measures of behavioral regulation and effortful control in predicting task-specific sustained visual attention performance measures and sheds further light on the emerging notion of the specific interplay between child temperament and executive function development (Suor et al. 2019). Further research is needed to investigate the relationship between these constructs.
With respect to the VPC, participants demonstrated an increased discrimination ratio for an ‘easy’ versus ‘hard’ task condition. In the easy condition, a color cue assisted performance; in the hard condition, color did not serve as a cue for task success. While further investigation is needed, the majority of our study participants also demonstrated a novelty preference and significant sensitivity to perceptual difficulty in non-verbal visual processing and memory, the first use of this visual memory task (the VPC) to our knowledge in the FASD population. These findings suggest that children with FASD are able to demonstrate reliable performance on a presumed measure of declarative memory, but this performance – as measured by novelty preference across two conditions – is impacted by altering perceptual similarity. The visual perception deficits that have previously been demonstrated in children with FASD (Pei et al. 2011, Manji et al. 2009, Castillo Castejon et al. 2019) may contribute to the decreased discrimination ability in the ‘hard’ condition where there was an increased demand on detailed visual perception recognition versus more salient color cue differences.
The observed susceptibility to perceptual difficulty demonstrated by significant performance differences in the ‘easy’ versus ‘hard’ conditions during the VPC task may have also played a role in CPT performance, as this task relies on the ability to visually discriminate different spatial frequencies. Although it has been suggested that tasks involving lower-frequency Gabor patches are less taxing on spatial attention processes (Lawrence et al. 2020), the CPT nonetheless requires integration of non-attentional processes known to be affected in children with FASD, such as motion detection (Gummel et al. 2012), oculomotor accuracy (Zhang et al. 2019), and sensorimotor integration (Paolozza et al. 2013). Further studies are needed to determine the contribution of each of these processes to within-task performance variability in the FASD population.
Though the relationships between parent report and task-based measures were robust, our small sample size limits the ability to make inferences about other potential correlates to within-task changes in performance. The current findings may not generalize to all children with FASD, particularly because participants fell on the ‘less severe’ end of the FASD spectrum and demonstrated relatively high non-verbal IQs, with mean non-verbal IQ of 102. While IQ was not significantly correlated with task-based or parent report-based measures in our study, IQ levels in children with FASD are typically low to low-average (see Mattson et al. 2011 for review). It is unclear whether similar profiles of within-task performance differences would be expected in children on the more ‘severe’ end of FASD (i.e., those with fetal alcohol syndrome). However, our findings highlight the notion that even children with history of in utero alcohol exposure who have arguably more ‘mild’ levels of CNS impairment still demonstrate vulnerabilities in task performance and differences in parent-based executive function as well as temperament measures that are predictive of one another. Further studies are additionally needed to determine whether this relationship generalizes to other task types and modalities (Roebuck et al. 2016) or age groups.
Conclusions
These results shed light on the difficulties in quantifying cognitive processing measures in children with FASD. Specifically, the current results show that prior studies investigating the relationship between parental report and task-based measures have not demonstrated a significant correlation perhaps because parental measures of behavioral regulation are more reflective of within-individual capabilities over time as opposed to absolute measures. Our study is, to our knowledge, the first to investigate within-task metrics of CPT performance in children with FASD, such as split-half performance differences. Within-task performance measures essentially normalize each individual’s task performance to themselves, potentially providing a more clinically useful measure with which to assess strengths – and susceptibilities – in capacities for effortful control and behavioral regulation. Moreover, the predictive nature of this intra-individual performance measure to parent report-based assessments of executive function abilities in our study participants may help inform and individualize behavioral assessments and therapies in children with FASD.
Author Acknowledgements
We thank each of the families who participated in this research, Susan Astley Hemingway, director and founder of the FAS-DPN, as well as Beth Gendler, and the NeuDLL Lab members who contributed to data coding in support of these analyses: Emily Fowler, Emi Preston, Laura Cannon, and Claire Kozel.
Funding: This research was supported by a University of Washington Alcohol and Drug Abuse Institute (ADAI) Small Grant, as well as by the National Center for Advancing Translational Sciences of the National Institutes of Health to ITHS under Award Number NIH UL1TR000423.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Declarations
Ethics approval: This study was approved by the University of Washington Institutional Review Board, Human Subjects Division No. 48563.
Consent to participate: Written informed consent was obtained from the parents of all individual participants included in the study. Verbal assent was obtained from each participant.
Consent for publication: Not applicable
Availability of data and material: Not applicable
Code availability: Not applicable
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Acar IH, Frohn S, Prokasky A, Molfese VJ, & Bates JE (2019). Examining the Associations Between Performance Based and Ratings of Focused Attention in Toddlers: Are We Measuring the Same Constructs? Infant and Child Development, 28: pii: e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Kerns KA, Mulligan BP, Olson HC, & Astley S (2018). An investigation of intra-individual variability in children with fetal alcohol spectrum disorder (FASD). Child Neuropsychology, 24: 617–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Macoun SJ, Bedir B, & MacDonald S (2019). Intraindividual variability in children is related to informant ratings of attention and executive function. Journal of Clinical and Experimental Neuropsychology, 41: 740–748. [DOI] [PubMed] [Google Scholar]
- Alvik A, Torgerson AM, Aalen OO, & Lindemann R (2011). Binge alcohol exposure once a week in early pregnancy predicts temperament and sleeping problems in the infant. Early Human Development, 87: 827–833. [DOI] [PubMed] [Google Scholar]
- Astley SJ (2004). Diagnostic guide for fetal alcohol syndrome disorders: The 4-digit diagnostic code (3rd ed.). Seattle: FAS Diagnostic and Prevention Network, University of Washington; Retrieved from http://fasdpn.org. [Google Scholar]
- Astley SJ (2011). Diagnosing Fetal Alcohol Spectrum Disorders (FASD) In Adubato SA, & Cohen DE (Eds.), Prenatal Alcohol Use and FASD: Diagnosis, Assessment and New Directions in Research and Multimodal Treatment (pp 3–29). Bentham Science Publishers. [Google Scholar]
- Astley SJ (2013). Validation of the fetal alcohol spectrum disorder (FASD) 4-digit diagnostic code. Journal of Population Therapeutics and Clinical Pharmacology, 20: e416–e467. [PubMed] [Google Scholar]
- Atkinson J, & Braddick O (2011). From genes to brain development to phenotypic behavior: “Dorsal-stream vulnerability” in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. Progress in Brain Research, 189: 261–83. [DOI] [PubMed] [Google Scholar]
- Bodnar LE, Prahme MC, Cutting LE, Denckla MB, & Mahone EM (2007). Construct validity of parent ratings of inhibitory control. Child Neuropsychology, 13: 345–62. [DOI] [PubMed] [Google Scholar]
- Brown TM, Ris MD, Beebe D, Ammerman RT, Oppenheimer SG, Yeates KO, & Enrile BG (2008). Factors of biological risk and reserve associated with executive behaviors in children and adolescents with spina bifida myelomeningocele. Child Neuropsychology, 14: 118–34. [DOI] [PubMed] [Google Scholar]
- Cak HT, Cengel Kultur SE, Gokler B, Oktem F, & Taskiran C (2017). The Behavior Rating Inventory of Executive Function and Continuous Performance Test in Preschoolers with Attention Deficit Hyperactivity Disorder. Psychiatry Investigation, 14: 260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, & Rothbart MK (1992). Development and Validation of an Early Adolescent Temperament Measure. Journal of Early Adolescence, 12: 153–73. [Google Scholar]
- Castillo Castejon O, Gonzalez I, Prieto E, Perez T, Pablo LE, & Pueyo V (2019). Visual cognitive impairments in children at risk of prenatal alcohol exposure. Acta Paediatrica, 108: 2222–28. [DOI] [PubMed] [Google Scholar]
- Cornish K, Cole V, Longhi E, Karmiloff-Smith A, & Scerif G (2013). Mapping developmental trajectories of attention and working memory in fragile X syndrome: Developmental freeze or developmental change? Development and Psychopathology, 25: 365–76. [DOI] [PubMed] [Google Scholar]
- Dang JS, Figueroa IJ, & Helton WS (2018). You are measuring decision to be fast, not inattention: the Sustained Attention to Response Task does not measure sustained attention. Experimental Brain Research, 236: 2255–62. [DOI] [PubMed] [Google Scholar]
- Dolan GP, Stone DH, & Briggs AH (2010). A systematic review of continuous performance task research in children prenatally exposed to alcohol. Alcohol & Alcoholism, 45: 30–8. [DOI] [PubMed] [Google Scholar]
- Faridi N, Karama S, Burgaleta M, White M, Evans AC, Fonov V, … Waber DP (2015). Neuroanatomical correlates of behavior rating versus performance measures of working memory in typically developing children and adolescents. Neuropsychology, 29: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A, Zangl R, Luz A, Virginia P, & Marchman V (2008). Looking while listening: Using eye movements to monitor spoken language comprehension by infants and young children In Sekerina IA, Fernandez EM, & Clahsen E (Eds.), Developmental psycholinguistics: On-line methods in children’s language processing (pp. 97–135). Amsterdam, Netherlands: John Benjamins Publishing Company. [Google Scholar]
- Fisher AV (2019). Selective sustained attention: a developmental foundation for cognition. Current Opinion in Psychology, 29: 248–253. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, & Riley EP (2007). Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research, 31: 1415–24. [DOI] [PubMed] [Google Scholar]
- Garrison L, Morley S, Chambers CD, & Bakhireva LN (2019). Forty Years of Assessing Neurodevelopmental and Behavioral Effects of Prenatal Alcohol Exposure in Infants: What Have We Learned? Alcoholism: Clinical and Experimental Research, 43: 1632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Pol SE, Lobbestael J, & Simons CJP (2020). Executive Functioning in 60+ Autistic Males: The Discrepancy Between Experienced Challenges and Cognitive Performance. Journal of Autism and Developmental Disorders, 50: 1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, & Kenworthy L (2000a). Behavior Rating Inventory of Executive Function. Child Neuropsychology, 6: 235–238. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, & Kenworthy L (2000b). Behavior Rating Inventory of Executive Function. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, & Kenworthy L (2015). BRIEF2: Behavior Rating Inventory of Executive Function. Lutz, FL: Second Psychological Assessment Resources. [Google Scholar]
- Glass L, Graham DM, Deweese BN, Jones KL, Riley EP, & Mattson SN (2014a). Correspondence of parent report and laboratory measures of inattention and hyperactivity in children with heavy prenatal alcohol exposure. Neurotoxicology and Teratology, 42: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass L, Ware AL, & Mattson SN (2014b). Neurobehavioral, neurologic, and neuroimaging characteristics of fetal alcohol spectrum disorders. Handbook of Clinical Neurology, 125: 435–62. [DOI] [PubMed] [Google Scholar]
- Gross AC, Deling LA, Wozniak JR, Boys CJ (2015). Objective measures of executive functioning are highly discrepant with parental with parent-report in fetal alcohol spectrum disorders. Child Neuropsychology, 21: 531–8. [DOI] [PubMed] [Google Scholar]
- Gummel K, Ygge J, Benassi M, & Bolzani R (2012). Motion perception in children with foetal alcohol syndrome. Acta Paediatrica, 101: e327–32. [DOI] [PubMed] [Google Scholar]
- Head J, & Helton WS (2014). Sustained attention failures are primarily due to sustained cognitive load not task monotony. Acta Psychologica, 153: 87–94. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Calkins SD, King-Casas B, & Bell MA (2019). Commonality between executive functioning and effortful control related to adjustment. Journal of Applied Developmental Psychology, 60: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C, & McGrath JJ (2016). Research review: executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder – a meta-analysis. Journal of Child Psychology and Psychiatry, 57: 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer L, Olswang LB (2013). Variability in classroom social communication: performance of children with fetal alcohol spectrum disorders and typically developing peers. Journal of Speech, Language, and Hearing Research, 56: 982–93. [DOI] [PubMed] [Google Scholar]
- Klee SH, & Garfinkel BD (1983). The computerized continuous performance task: a new measure of inattention. Journal of Abnormal Child Psychology, 11: 487–95. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, & Peper M (2006). Intra-Subject Variability in Attention-Deficit Hyperactivity Disorder. Biological Psychiatry, 60: 1088–97. [DOI] [PubMed] [Google Scholar]
- Lange S, Rovet J, Rehm J, Popova S (2017). Neurodevelopmental profile of fetal alcohol spectrum disorder: a systematic review. BMC Psychology, 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Shield K, Rehm J, Anognostou E, & Popova S (2019). Fetal alcohol spectrum disorder: neurodevelopmentally and behaviorally indistinguishable from other neurodevelopmental disorders. BMC Psychiatry, 19: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RK, Edwards M, & Talipski LA (2020). A critical review of the cognitive and perceptual factors influencing attentional scaling and visual processing. Psychonomic Bulletin & Review. https://doi:10.3758/s13423019–01692-9. [DOI] [PubMed] [Google Scholar]
- Manji S, Pei J, Loomes C, & Rasmussen C (2009). A review of the verbal and visual memory impairments in children with foetal alcohol spectrum disorders. Developmental Neurorehabilitation, 12: 239–47. [DOI] [PubMed] [Google Scholar]
- Manns JR, Stark CE, & Squire LR (2000). The visual paired-comparison task as a measure of declarative memory. Proceedings of the National Academy of Sciences of the United States of America. 97:12375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Buss JL, Bolster RB, de Gervai PD, Woods-Frohlich L, Summers R, … Longstaffe S (2012). Comparison of spatial working memory in children with prenatal alcohol exposure and those diagnosed with ADHD; A functional magnetic resonance imaging study. Journal of Neurodevelopmental Disorders, 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, & Doyle LR. (2019). Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research, 43: 1046–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, & Nguyen TT (2011). Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology Review, 21: 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, & Riley EP (2011). The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Research & Health, 34: 51–5. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, … Riley EP; CIFASD. (2013). Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 37: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley T, Chen S, Goos L, Schachar R, & Crosbie J (2010). Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? Journal of the International Neuropsychological Society, 16: 495–505. [DOI] [PubMed] [Google Scholar]
- McCoy DC (2019). Measuring Young Children’s Executive Function and Self-Regulation in Classrooms and Other Real-World Settings. Clinical Child and Family Psychology Review, 22: 63–74. [DOI] [PubMed] [Google Scholar]
- Mohamed Z, Carlisle AC, Livesey AC, Mukherjee RAS (2019). Comparisons of the BRIEF parental report and neuropsychological clinical tests of executive function in fetal alcohol spectrum disorders: Data from the UK national specialist clinic. Child Neuropsychology, 25: 648–63. [DOI] [PubMed] [Google Scholar]
- Molteno CD, Jacobson JL, Carter RC, Dodge NC, & Jacobson SW (2014). Infant emotional withdrawal: a precursor of affective and cognitive disturbance in Fetal Alcohol Spectrum Disorders. Alcoholism: Clinical and Experimental Research, 38: 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasch KC, & Bell MA (2011). The role of inhibitory control in behavioral and physiological expressions of toddler executive function. Journal of Experimental Child Psychology, 108: 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AL, Booth T, Ribeaud D, & Eisner M (2018). Disagreeing about development: An analysis of parentteacher agreement in ADHD symptom trajectories across the elementary school years. International Journal of Methods in Psychiatric Research, 27: e1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Glass L, Coles CD, Kable JA, May PA, Kalberg WO, … Mattson SN; CIFASD. (2014). The clinical utility and specificity of parent report of executive function among children with prenatal alcohol exposure. Journal of the International Neuropsychological Society, 20: 704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58: 361–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolozza A, Rasmussen C, Pei J, Hanlon-Dearman A, Nikkel SM, Andrew G, … Reynolds JN (2014). Working memory and visuospatial deficits correlate with oculomotor control in children with fetal alcohol spectrum disorder. Behavioural Brain Research, 263: 70–9. [DOI] [PubMed] [Google Scholar]
- Paolozza A, Titman R, Brien D, Munoz DP, & Reynolds J (2013). Altered Accuracy of Saccadic Eye Movements in Children with Fetal Alcohol Spectrum Disorder. Alcoholism: Clinical and Experimental Research, 37: 1491–8. [DOI] [PubMed] [Google Scholar]
- Pei J, Job J, Kully-Martens K, & Rasmussen C (2011). Executive function and memory in children with Fetal Alcohol Spectrum Disorder. Child Neuropsychology, 17: 290–309. [DOI] [PubMed] [Google Scholar]
- Pei JR, Rinaldi CM, Rasmussen C, Massey V, & Massey D (2008). Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. Canadian Journal of Clinical Pharmacology, 15: e44–56. [PubMed] [Google Scholar]
- Rai JK, Abecassis M, Casey JE, Flaro L, Erdodi LA, & Roth RM (2017). Parent rating of executive function in fetal alcohol spectrum disorder: A review of the literature and new data on Aboriginal Canadian children. Child Neuropsychology, 23: 713–32. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, McAuley R, & Andrew G (2007). Parental ratings of children with fetal alcohol spectrum disorder on the Behavior Rating Inventory of Executive Function (BRIEF). Journal of FAS International, 5: e2. [Google Scholar]
- Rhodes SM, Riby DM, Park J, Fraser E, & Campbell LE (2010). Executive neuropsychological functioning in individuals with Williams syndrome. Neuropsychologia, 48: 1216–26. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, & Moore JJ (2002). The continuous performance test: a window on the neural substrates for attention? Archives of Clinical Neuropsychology, 17: 235–72. [PubMed] [Google Scholar]
- Roebuck H, Freigang C, & Barry JG (2016). Continuous Performance Tasks: Not Just About Sustaining Attention. Journal of Speech, Language, and Hearing Research, 59: 501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH, Miller LJ, Pomplun M, & Koch C (2013). Leiter international performance scale – third edition. Wood Dale, IL: Stoelting Company. [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2001). Attention and recognition memory in the 1st year of life: a longitudinal study of preterm and full-term infants. Developmental Psychology, 37: 135–51. [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, & Fisher P (2001). Investigations of temperament at three to seven years: the Children’s Behavior Questionnaire. Child Development, 72: 1394–1408. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE, & Posner MI (2007). Executive attention and effortful control: Linking temperament, brain networks and genes. Child Development Perspectives, 1: 2–7. [Google Scholar]
- Sarter M, Givens B, & Bruno JP (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research Reviews, 35: 146–160. [DOI] [PubMed] [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, & Cornish K (2012). Attention across modalities as a longitudinal predictor of early outcomes: the case of fragile X syndrome. Journal of Child Psychology and Psychiatry, 53: 641–50. [DOI] [PubMed] [Google Scholar]
- Schneider H, Ryan M, & Mahone EM (2020). Parent versus teacher ratings on the BRIEF-preschool version in children with and without ADHD. Child Neuropsychology, 26: 113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev N, Humphreys G, & Demeyere N (2018). Manipulating perceptual parameters in a continuous performance task. Behavior Research Methods, 50: 380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev N, Steele A, Nobre AC, Karmiloff-Smith A, Cornish K, & Scerif G (2019). Dynamic sustained attention markers differentiate atypical development: The case of Williams syndrome and Down syndrome. Neuropsychologia, 132: 107148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran MH, Mackenzie AK, Callan IR, Ainge JA, & O’Connor AR (2018). The discrimination ratio derived from novel object recognition tasks as a measure of recognition memory sensitivity, not bias. Scientific Reports, 8: 11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, & Todorov N (1999). Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers, 31: 137–49. [DOI] [PubMed] [Google Scholar]
- Suor JH, Sturge-Apple ML, Davies PT, Jones-Gordils HR (2019). The interplay between parenting and temperament in associations with children’s executive function. Journal of Family Psychology, 33: 841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NM, & Enns LN (2019). Factors predictive of a fetal alcohol spectrum disorder diagnosis: Parent and teacher ratings. Child Neuropsychology, 25: 507–27. [DOI] [PubMed] [Google Scholar]
- Ten Eycke KD, & Dewey D (2016). Parent-report and performance-based measures of executive function assess different constructs. Child Neuropsychology, 8: 889–906. [DOI] [PubMed] [Google Scholar]
- Tiego J, Bellgrove MA, Whittle S, Pantelis C, & Testa R (2019). Common mechanisms of executive attention underlie executive function and effortful control in children. Developmental Science, 4: e12918. [DOI] [PubMed] [Google Scholar]
- Toplak ME, West RF, & Stanovich KE (2013). Practitioner review: do performance-based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry, 54: 131–43. [DOI] [PubMed] [Google Scholar]
- Venker CE, dissertant. (2013). Statistical word learning and non-social visual attention in children with autism. Ann Arbor, MI: ProQuest LLC. [Google Scholar]
- Zhang C, Paolozza A, Tseng P, Reynolds J, Munoz DP, & Itti L (2019). Detection of Children/Youth with Fetal Alcohol Spectrum Disorder Through Eye Movement, Psychometric, and Neuroimaging Data. Frontiers in Neurology, 10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chen SH, & Main A (2012). Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives, 6: 112–21. [Google Scholar]