Abstract
A new schema proposes that the bone-derived osteocalcin (Ocn) peptide hormone activates the G-protein–coupled receptor GPRC6A to directly regulate glucose and fat metabolism in liver, muscle, and fat, and to stimulate the release of metabolism-regulating hormones, including insulin, fibroblast growth factor 21, glucagon-like peptide 1, testosterone, and interleukin 6. Ocn/GPRC6A activation has also been implicated in cancer progression. GPRC6A is activated by cations, amino acids, and testosterone. The multiligand specificity, the regulation of energy metabolism in diverse tissues, and the coordinated release of metabolically active hormones make the GPRC6A endocrine networks unique. Recently, the significance of Ocn/GPRCA has been questioned. There is a lack of metabolic abnormalities in newly created genetically engineered Ocn- and Gprc6a-deficient mouse models. There are also paradoxical observations that GPRC6A may function as a tumor suppressor. In addition, discordant published studies have cast doubt on the function of the most prevalent uniquely human GPRC6A-KGKY polymorphism. Explanations for these divergent findings are elusive. We provide evidence that the metabolic susceptibility of genetically engineered Ocn- and Gprc6a-deficient mice is influenced by environmental challenges and genetic differences in mouse strains. In addition, the GPRC6A-KGKY polymorphism appears to be a gain-of-function variant. Finally, alternatively spliced isoforms of GPRC6A may alter ligand specificity and signaling that modulate oncogenic effects. Thus, genetic, post-translational and environmental factors likely account for the variable results regarding the functions of GPRC6A in animal models. Pending additional information, GPRC6A should remain a potential therapeutic target for regulating energy and fat metabolism, hormone production, and cancer progression.
Keywords: amino acid, GPRC6A, glucose and fat metabolism, insulin, osteocalcin, testosterone
There is an ongoing controversy regarding the roles of the nutrient-sensing G-protein–coupled receptor GPRC6A and its putative bone-derived peptide ligand, osteocalcin (Ocn), in regulating glucose and fat metabolism (1-5). Because of the potential unique role of the Ocn/GPRC6A endocrine network in animal and human health and disease, we believe that an attempt to explain these discrepancies regarding the functions of Ocn and GPRC6A are warranted.
Original Premise
Osteocalcin is a Ligand for GPRC6A that Regulates Energy Metabolism
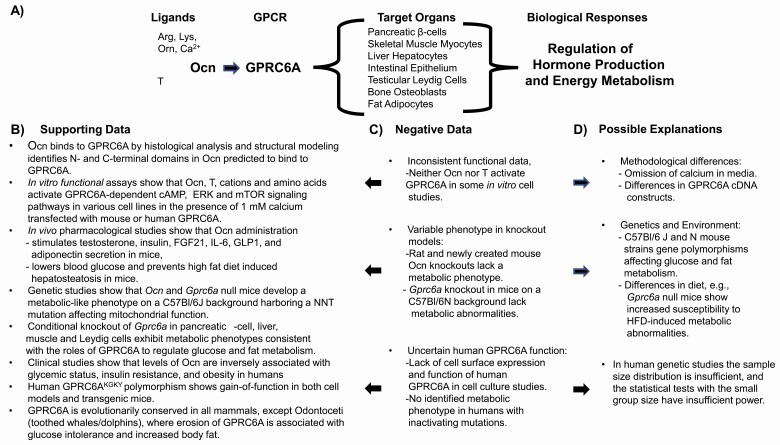
A new schema of regulating energy metabolism and fertility proposes the release into the circulation of the peptide hormone Ocn from bone (3, 6, 7) and its activation of GPRC6A, a member of the Family C G-protein–coupled receptors (8, 9) that is located in various organs (Fig. 1A).
Figure 1.
(A) Schema showing the bone derived peptide hormone osteocalcin (Ocn) activation of GPRC6A in various organs leading to regulation of glucose and fat metabolism through direct tissue effects and release of an ensemble of metabolically active hormones. (B) Evidence supporting the physiological and clinical importance of the Ocn/GPRC6A endocrine network. Structural data support Ocn binding to GPRC6A; in vitro functional assays demonstrate Ocn activation of transfected and endogenous GPRC6A in various cell lines; pharmacological and genetic studies demonstrate the hormonal function of Ocn in mice; global and conditional knockout of Gprc6a in mice support the organ specific function of this GPCR to regulate glucose and fat metabolism and the release of metabolically active hormones; evolutionary analysis, “humanized” KGKY transgenic mouse models, and in vitro assessment of human GPRC6A cDNA constructs support a functional role of GPRC6A in humans. (C) Discordant data. In vitro studies of transfected GPRC6A cDNA constructs in heterologous cell systems failed to confirm activation by Ocn or testosterone in several studies; recently created genetic knockout models for Ocn and Gprc6a failed to identify metabolic phenotypes in Ocn and Gprc6a deficient animals; studies of human GPRC6A, characterized by the KGKY polymorphism in the third intracellular loop, is reported to be located intracellularly and to lack function in in vitro assays; the function of human GPRC6A is questioned by the failure to observe an association between inactivating mutations of GPRC6A and clinical phenotypes in human population studies. (D) Possible explanations for variable findings. Negative in vitro functional studies omitted calcium from the culture media, an essential cofactor for Ocn activation of GPRC6A; negative Ocn and Gprc6a knockout studies may be explained by genetic and environmental factors; studies investigating the association between GPRC6A inactivating mutations and metabolic phenotypes in humans did not assess patients with inactivation of both alleles and were inadequately powered. See text for references.
The concept of an Ocn/GPRC6A endocrine network was originally published in 2007 by Gerard Karsenty and collaborators (6), and was based, in part, on the observations that Ocn null mice, in addition to having increased bone formation (10), had an unexpected metabolic syndrome–like phenotype (6). These studies also confirmed our prior finding from 2005 that GPRC6A is an Ocn-sensing receptor (8, 9). Structural models of Ocn/GPRC6A interactions, binding studies, and functional assays showed that Ocn is a ligand for GPRC6A (9, 11, 12). The discovery in 2008 that Gprc6a null mice have abnormalities in glucose and fat metabolism resembling Ocn null mice (13), and the subsequent observation of genetic interactions between Ocn and GPRC6A in compound mutant mice (14), solidified the hypothesis that Ocn and GPRC6A function in a common pathway regulating energy metabolism (Fig. 1B).
The hormone function of Ocn is thought to reside in an uncarboxylated form. The structure of GPRC6A has not been determined, but models have been created based on homologies to other Family C receptors for which the structures have been solved (9). The exact binding sites for Ocn interactions with GPRC6A are uncertain. Binding to GPRC6A recently has been proposed to localize to a 15 amino acid fragment of Ocn present in the blood with the sequence NH2-YLGASVPSPDPLEP-COOH (12). This region of Ocn is flanked by protease cleavage sites suggesting that Ocn fragments may have bioactivity. The C-terminus of Ocn has also been proposed to bind to GPRC6A (9). Additional structural and target engagement studies are needed to define sites of interactions between Ocn and GPRC6A.
Both in vitro and in vivo genetic and pharmacological studies confirm that Ocn acts as a hormone through activation of GPRC6A. GPRC6A is located in multiple tissues, including liver hepatocytes (15), skeletal muscle (16, 17), pancreatic β-cells (9, 11, 18, 19), and testicular Leydig cells (14), as well as others tissues, such as adipocytes (20-22), gastrointestinal epithelia (23, 24), skin keratinocytes (25), vascular endothelial cells (26), osteoblasts (27), and prostate (28, 29), to create complex networks. Indeed, Ocn activation of GPRC6A directly regulates signaling pathways and metabolic processes controlling glucose and fat metabolism in these tissues, as well as stimulates the release of an ensemble of metabolically active hormones, including insulin (18), testosterone (14, 15), fibroblast growth factor 21 (FGF-21) (15), glucagon-like peptide 1 (GLP-1) (30, 31), and interleukin 6 (IL-6) (17) in mice. These direct and indirect actions create multiple positive feedback loops that have net anabolic effects (29).
Systemic administration of the Ocn full-length peptide induces metabolic effects in all species tested (as reviewed in references (3) and (29)). Of particular note are the pharmacological studies in mice consistently showing that Ocn and GPRC6A regulate glucose and fat metabolism and activation of the Ocn/GPRC6A, which pathway can prevent development of type 2 diabetes (T2D) and nonalcoholic fatty liver disease induced by high-fat diets and glucocorticoid exposure (15, 32-37).
Epidemiological and clinical association studies in humans also show that levels of the GPRC6A ligand, Ocn, are inversely associated with glycemic status and insulin (38), body mass index, fasting glucose and insulin, triglycerides, and leptin, and are positively correlated with adiponectin (39, 40). Human association studies also support a metabolic role of Ocn, showing that polymorphisms in Ocn’s target, GPRC6A, correlate with insulin and fertility abnormalities (41, 42). The clinical association studies are consistent with a role for Ocn in regulating energy metabolism, but human interventional studies are needed to confirm causality between Ocn and the measures of regulatory hormones and energy status in patients. The importance of the hormonal functions has been reviewed in a recent article in PLOS Genetics (3).
There is also evidence that GPRC6A is involved in regulating energy metabolism in certain cancers. Ocn polymorphisms have also been linked to human prostate cancer (43-45). GPRC6A is increased in certain forms of human prostate cancer, where it regulates prostate cancer progression through Ocn stimulation of mammalian target of rapamycin (mTOR) pathways (28, 46-48).
In addition, GPRC6A has been shown to mediate the nongenomic effects of testosterone (49). Testosterone is a ligand for both mouse and human GPRC6A as shown in transfected HEK-293 and in endogenously expressing prostate cancer cell lines, hepatocytes, β-cells, and skin fibroblasts that has similar effects to Ocn to activate downstream effectors (25, 27, 46, 47). Computational modeling has also defined putative testosterone binding domains in GPRC6A (49). Since Ocn stimulates testosterone production by Leydig cells (14, 15), GPRC6A is 1 receptor with 2 ligands with potential different responses derived from testosterone activating both GPRC6A and androgen receptors. Indeed, testosterone binding by GPRC6A modulates the genomic effects of androgen receptor (AR) (27). In HEK-293 cells coexpressing both GPRC6A and androgen response element (ARE), R1881-mediated activation of ARE-luciferase activity was significantly attenuated, suggesting potential cross-talk between GPRC6A and AR pathways (27).
Finally, there is at least 1 other receptor for Ocn, Gpr158, which is located in the brain (50). The role of this receptor is less well documented than GPRC6A, and it could mediate other responses to Ocn.
Divergent Observations and Possible Explanations
Disparate Results Regarding GPRC6A’s Endocrine Networks
In contrast to the above findings, others have questioned the premise that Ocn/GPRC6A form an endocrine network (Fig. 1C). Several studies show that Ocn is neither a hormone or ligand for GPRC6A, and that GPRC6A has minimal or no functions, especially in humans (4, 51, 52).
Additional doubts about the proposed Ocn and GPRC6A functions to regulate energy metabolism is mainly based on the lack of metabolic phenotypes in newly generated Ocn (1, 2, 53) and Gprc6a (54-56) knockout animal models.
In the mouse, osteocalcin is encoded by 3 genes Bglap1, Bglap2, and Bglap3. Bglap 1 and 2 are expressed in bone, whereas Bglap3, which differs by 4 amino acids, is expressed in nonosseous tissues, and does not contribute to circulating Ocn levels (2). Unlike mice, but similar to humans, the rat osteocalcin gene locus consists of a single copy of Ocn (53).
Originally Karsenty (10) and more recently Moriishi et al. (1) used homologous recombination to delete Bglap1 and Bglap2 in mice, leaving the Bglap3 intact. Unlike robust metabolic phenotype in the mice derived by Karsenty’s group, the Ocn knockout mouse made by Komori and Moriishi’s group failed to show a role for Ocn in exercise-induced bone formation, glucose metabolism, exercise-induced enhancement of glucose uptake, testosterone synthesis, spermatogenesis, or muscle mass (1). CRISPR-Cas9 gene editing was also used to delete the single Ocn gene in rats (53) and both Bglap1 and Bglap2 in mice (2). CRISPR-Cas9 deletion of Ocn gene failed to produce a metabolic phenotype in either mice (2) or rat (53) models.
Various strategies were also used to generate the three existing Gprc6a-deficient mouse models, including homologous recombination to target exon 2 in the extracellular domain (13), exon 6 encoding the 7TM domain of Gprc6a (56, 57), and the entire Gprc6a locus (55). Three different Gprc6a floxed mice have also been created (9, 11, 14-17, 58). In contrast, to metabolic abnormalities in the original global Gprc6a knockout mouse (13) and the organ-specific Gprc6a knockouts in skeletal muscle myocytes, pancreatic β-cells, liver hepatocytes (9, 11, 14, 15, 19, 58), neither glucose intolerance, insulin resistance, nor alterations in fat metabolism were reported in the newly created Gprc6a knockout mouse models (54-56).
Environmental and Genetic Factors May Account for the Phenotypic Differences in Glucose and Fat Metabolism
It is estimated that 70% of researchers fail to confirm another scientist’s experiments (59, 60). Experience and logic dictate, however, that multiple positive findings cannot be disproven by the failure to reproduce the results in other laboratories. The failure to reproduce research results may be due to many factors, including variation in methodologies, the rigor and depth of phenotyping, environmental and dietary exposure, differences in genetic background, species and model systems, dissimilarities in gene targeting approaches, and compensation by genetic modifying genes affecting complex metabolic traits, to name a few (Fig. 1C). Of these genetic and environmental factors are high on the list to explain the discrepancies in the Ocn and GPRC6A data, given the known importance of genetic modifiers and dietary factors in the pathogenesis of metabolic syndrome and T2D (61).
An in-depth metabolic phenotyping was not uniformly performed in these knockout animals. Investigators initially reporting no metabolic abnormalities in Gprc6a null mice, later reported a greater propensity of their Gprc6a knockout mice to develop an abnormal metabolic phenotype when fed a high-fat diet (62). There were other perturbations that appear to be related to energy metabolism, as there was enhanced voluntary wheel running in Gprc6a null mice (63). In addition, L-arginine-induced GLP-1 secretion was attenuated (24). GLP-1 is an incretin hormone regulator of energy metabolism. Finally, circulating and bone Ocn, the GPRC6A ligand, were decreased in the full Gprc6a gene deletion model (54).
The fact that some studies do not find a metabolic-like phenotype in Gprc6a deficient mice (55, 56, 64), raises the possibility that GPRC6A effects are modified by dietary factors, particularly dietary fat. However, unlike the impact of dietary fat on the Gprc6a null mouse model, exposure of Ocn null mice to a high-fat diet did not lead to greater metabolic derangements compared with control mice (1). None of these studies have examined the effects of a Western style diet of excess carbohydrate and fat content, which may be needed to uncover metabolic abnormalities in Ocn and Gprc6a deficient mouse models.
Furthermore, genetically engineered mouse models investigating Ocn and GPRC6A are not ideal for dissecting polygenic networks or genetic and environmental interactions that regulate glucose and fat metabolism, because they have been optimized to study actions of a single gene on single genetic background. Energy metabolism is a complex trait and influenced by hundreds of genes that interact with each other within the context of an integrated biological network. Coregulated genes in the network may be responsible for the variance of metabolic phenotypes in the knockout and response to high-fat diets. Indeed, studies in inbreed BXD mouse strains show that the susceptibility to abnormalities in glucose and fat metabolism differ between different mouse strains (65-68).
Negative studies regarding the function of Ocn as a hormone and GPRC6A as its biologically relevant receptor are having an unexpected impact to stifle publication of new findings in this area, casting doubt as to whether additional investigations of GPRC6A should be funded, and discouraging interest by pharmaceutical companies in Ocn and GPRC6A as therapeutic targets.
Additional observations point to a more specific genetic modifier of Ocn and GPRC6A metabolic functions in mice. For the Ocn and GPRC6A deficient models generated in the United States, the mice were backcrossed for multiple generations to the inbred line C57BL/6J. In contrast, in the Ocn knockout mice generated in Japan (69) and Europe (2) and the 2 Gprc6a knockout mouse models generated in Europe (54-56, 63) were backcrossed for multiple generation on to the C57BL/6N mouse strain.
C57BL/6J and C57BL/6N coding sequences differ in 34 single-nucleotide polymorphisms, as well as have structural variants in 15 genes, including Nnt (which has an in-frame 5-exon deletion in C57BL/6J mice (70). Of these, Nnt was identified as a trait locus accounting for glucose intolerance in mice (71). Nnt encodes an integral protein of the inner mitochondrial membrane that functions to couple proton flow down the electrochemical proton gradient. NNT deletion can have an overriding effect on glucose regulation by elevating the mitochondrial NADH/NAD+ ratio and leading to changes in activities of the tricarboxylic acid cycle and decreases in glycolysis (72). Indeed, C57BL/6 J mice have defective insulin secretion in response to intravenous glucose, decreased ambulation and increased lean mass but lower fat mass, O2 consumption, and heat production than C57BL/6N mice (73-75). Thus, Nnt or other genetic modifiers could account for the differences in glucose and fat metabolism of Ocn and GPRC6A loss of function in C57BL/6J and C57BL/6N mice, a possibility that is now being experimentally tested.
Given the essential role of mitochondria in energy metabolism, the possible effect of mitochondrial dysfunction in C57BL/6J mice to exacerbate the metabolic effects of Ocn and Gprc6a deficiency in mice and susceptibility to developing metabolic complications in response to high-fat diet, raises the question of whether mitochondria are central targets of Ocn/GPRC6A signaling. The effects of Ocn activation of GPRC6A to activate mTOR pathways controlling mitochondrial functions and effects of GPRC6A in muscle to regulate ATP production support mitochondria as a cellular target for Ocn/GPRC6A. It is intriguing to speculate that L-arginine sensing by GPRC6A in endosomes and cell surface membranes evolved to provide alternative molecular pathways to the ancestral lysosomal L-arginine sensing pathway present in yeast and all other cells in lysosomes that links mTOR signaling and mitochondrial functions (76). If so, Ocn and GPRC6A may have important effects on autophagy and endoplasmic reticulum stress, which is a feature of metabolic syndrome and metabolic dysregulation in certain cancers (77).
Disparate Results on Ocn and T Activation of GPRC6A
Amino acids and cations were initially identified as the ligands for GPRC6A (51, 52, 78) (Fig. 1B). Publications also showed that Ocn and T do not activate GPRC6A (51, 52, 64, 79) (Fig. 1C).
A cause for this discrepancy may be the calcium concentration during ligand activation (Fig. 1D). Media calcium is an obligate requirement for Ocn and T activation of GPRC6A in cultured cells (64, 80), negative in vitro studies may be due to the omission of calcium from the culture media. Negative findings may also be due to different methods used for GPRC6A receptor transfection into cells (64).
GPRC6A Function in Humans, an Unresolved Question
The GPRC6A gene, which emerged during vertebrate evolution, is highly conserved across 56 mammalian genomes that have been evaluated to date, including humans, mice, goat, sheep, and cows as well as other species (78). The conservation of the GPRC6A sequence across multiple species supports a physiological role for this receptor.
All species, except for humans, are characterized by an RKLP sequence in the third intracellular loop that regulates membrane trafficking (64). The function of this ancestral GPRC6A has been confirmed by multiple groups that show it is localized to the cell surface and undergoes β-arrestin–dependent internalization (8, 79, 80) (Fig. 1B).
A unique KGKY polymorphism is present in most humans that replaced the ancestral RKLP sequence in the third intracellular loop of GPRC6A (42, 81). Some published results maintain that the KGKY variant lacks function based on in vitro studies showing that a transfected GPRC6A_KGKY cDNA is retained intracellularly and is not activated by either Ocn or T in heterologous cell models (64, 81) (Fig. 1C). The same studies showed that the human KGKY variant is constitutively active intracellularly due to omnipresent amino acid ligands and cation agonists, and that the variant is recycled through the plasma membrane through a slow recycling pathway (64, 82). Supporting evidence for lack of function stems from the lack of hereditary monogenic disorders caused by inactivating mutation in GPRC6A in humans.
The conclusion that there is a lack of GPRC6A function due to its intracellular localization, however, may not be correct. Instead of a lack of function with Ocn and constitutive function due to amino acid and cations in the intracellular compartment, a recent publication proves that the human GPRC6A KGKY variant is a gain-of-function polymorphism. The human GPRC6A KGKY polymorphism allows sustained signals via Ocn and T ligand binding through activation of mTOR in early endosomes (47). Thus, GPRC6A is analogous to other GPCRs capable sustained signaling from internal compartments (83, 84). Regarding the purported lack of a phenotype associated with inactivating GPRC6A mutations in humans, the diversity of human physiology and inadequate assessments of environmental conditions may limit identification of clinical disorders associated with GPRC6A inactivation (Fig. 1D).
Additional evidence for the function of human GPRC6A comes from the study of prostate cancer. The endogenous hGPRC6A in PC-3 prostate cancer cells is functional and deletion of endogenous GPRC6A by Crispr/CAS9 resulted in loss of T-induced signaling (46). Gelman and Withers reported that GPRC6A drives a cell type specific migration phenotype in prostate cancer cell lines (85). Knockdown of GPRC6A in the androgen sensitive LNCaP cell line resulted in enhanced chemotactic migration and Matrigel invasion with little to no effect on proliferation or general motility (86). Finally, we have “humanized” the mouse GPRC6A gene by replacing the IC3_KGRKLP sequence in the mouse with the IC3_KGKY in humans. We found that Gprc6a_KGKY-knockin mice had significantly lower fasting blood glucose levels, improved glucose tolerance test, and increased FGF-21 compared with wild-type Gprc6a_KGRKLP, consistent with GPRC6A_KGKY being a gain-of-function variant (82).
Interestingly, evolutionary erosion of GPRC6A is found in odontocete cetaceans (dolphins and other toothed whales), marine mammals that have elevated blood glucose and excess body fat in the form of blubber (83), which resembles the elevated glucose levels and increased body fat observed in Gprc6a null mice (13).
Conclusions
Maintenance of energy metabolism is complex and redundant, with multiple genes having small effects on the metabolic phenotype that is further influenced by a multitude of environmental factors (61). We review how a combination of methodological differences as well as genetic and environmental factors may account for the variable results regarding the metabolic functions of Ocn and GPRC6A. Existing genetically engineered mouse models should be studied on both the C57BL/6J and C57BL/6N background and under various dietary conditions to more fully understand the genetic and environmental factors modulating the GPRC6A endocrine network. In addition, the effects of Ocn and GPRC6A on glucose and energy metabolism studies in in-bred mouse strains that more closely replicate the complexity of human diseases may identify other modulating genes relevant to the pathogenesis of metabolic syndrome. More comprehensive studies are also needed in humans to determine if polymorphisms of GPRC6A are associated with diseases. Even if the overall effects of Ocn and GPRC6A are small, the preponderance of evidence suggest that Ocn and GPRC6A provide a new schema for understanding the regulation energy and fat metabolism, hormone production, and prostate cancer progression.
In summary, negative data regarding Ocn/GPRC6A has cast doubt about the relevance of this hormone network. It is premature, however, to dismiss the physiological and pathological roles of Ocn and GPRC6A. Positive outcomes of future studies that consider the influence of genetic and environmental factors will determine where Ocn and GPRC6A fit within the systems biology of metabolic control of energy metabolism, particularly in humans. If the schema is substantiated, agonists for the Ocn/GPRC6A endocrine axis could be a therapeutic target for treating metabolic syndrome and T2D, and antagonists might modulate oncogenic metabolic pathways in cancer that express GPRC6A.
Acknowledgments
Financial Support: This work was supported by 1R01DK120567-01 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health.
Author Contributions: L.D.Q., S.K.N., and M.P. conceived and wrote the paper.
Glossary
Abbreviations
- AR
androgen receptor
- Bglap1
bone gamma-carboxyglutamic acid-containing protein 1
- Crspr/CAS9
clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9
- FGF-21
fibroblast growth factor 21
- GLP-1
glucagon-like peptide 1
- GPRC6A
G protein–coupled receptor class C group 6 member A
- IL-6
interleukin 6
- mTOR
mammalian target of rapamycin
- NNT
nicotinamide nucleotide transhydrogenase
- Ocn
osteocalcin
- T
testosterone
- T2D
type 2 diabetes
Additional Information
Disclosures: There are no financial interests of the authors that could be perceived as being a conflict of interest.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed for this review.
References
- 1. Moriishi T, Ozasa R, Ishimoto T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020;16(5):e1008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diegel CR, Hann S, Ayturk UM, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020;16(5):e1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karsenty G. The facts of the matter: what is a hormone? PLoS Genet. 2020;16(6):e1008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manolagas SC. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020;16(6):e1008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diegel CR, Hann S, Ayturk UM, et al. ; VAI Vivarium and Transgenics Core . Independent validation of experimental results requires timely and unrestricted access to animal models and reagents. PLoS Genet. 2020;16(6):e1008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Komori T. What is the function of osteocalcin? J Oral Biosci. 2020;62(3):223-227. [DOI] [PubMed] [Google Scholar]
- 8. Pi M, Faber P, Ekema G, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280(48):40201-40209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pi M, Kapoor K, Ye R, et al. Evidence for osteocalcin binding and activation of GPRC6A in β-cells. Endocrinology. 2016;157(5):1866-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ducy P, Desbois C, Boyce B, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448-452. [DOI] [PubMed] [Google Scholar]
- 11. Oury F, Ferron M, Huizhen W, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123(6):2421-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teng B, Huang C, Cheng CL, et al. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor GPRC6A. J Hepatol. 2020;73(2):383-393. [DOI] [PubMed] [Google Scholar]
- 13. Pi M, Chen L, Huang MZ, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3(12):e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oury F, Sumara G, Sumara O, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5): 796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pi M, Xu F, Ye R, et al. Role of GPRC6A in regulating hepatic energy metabolism in mice. Sci Rep. 2020;10(1): 7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2017;25(1):218. [DOI] [PubMed] [Google Scholar]
- 17. Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016;5(10):1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26(7):1680-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sabek OM, Nishimoto SK, Fraga D, Tejpal N, Ricordi C, Gaber AO. Osteocalcin effect on human β-cells mass and function. Endocrinology. 2015;156(9):3137-3146. [DOI] [PubMed] [Google Scholar]
- 20. Li X, Li P, Wang L, Zhang M, Gao X. Lysine enhances the stimulation of fatty acids on milk fat synthesis via the GPRC6A-PI3K-FABP5 signaling in bovine mammary epithelial cells. J Agric Food Chem. 2019;67(25):7005-7015. [DOI] [PubMed] [Google Scholar]
- 21. Otani T, Mizokami A, Hayashi Y, et al. Signaling pathway for adiponectin expression in adipocytes by osteocalcin. Cell Signal. 2015;27(3):532-544. [DOI] [PubMed] [Google Scholar]
- 22. Guedes JAC, Esteves JV, Morais MR, Zorn TM, Furuya DT. Osteocalcin improves insulin resistance and inflammation in obese mice: Participation of white adipose tissue and bone. Bone. 2018;115(10):68-82. [DOI] [PubMed] [Google Scholar]
- 23. Namai F, Shigemori S, Sudo K, et al. Recombinant mouse osteocalcin secreted by lactococcus lactis promotes glucagon-like peptide-1 induction in STC-1 cells. Curr Microbiol. 2018;75(1):92-98. [DOI] [PubMed] [Google Scholar]
- 24. Clemmensen C, Jørgensen CV, Smajilovic S, Bräuner-Osborne H. Robust GLP-1 secretion by basic L-amino acids does not require the GPRC6A receptor. Diabetes Obes Metab. 2017;19(4):599-603. [DOI] [PubMed] [Google Scholar]
- 25. Ko E, Choi H, Kim B, et al. Testosterone stimulates Duox1 activity through GPRC6A in skin keratinocytes. J Biol Chem. 2014;289(42):28835-28845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qaradakhi T, Gadanec LK, Tacey AB, et al. The effect of recombinant undercarboxylated osteocalcin on endothelial dysfunction. Calcif Tissue Int. 2019;105(5):546-556. [DOI] [PubMed] [Google Scholar]
- 27. Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010;285(51):39953-39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pi M, Quarles LD. GPRC6A regulates prostate cancer progression. Prostate. 2012;72(4):399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pi M, Nishimoto SK, Quarles LD. GPRC6A: jack of all metabolism (or master of none). Mol Metab. 2017;6(2):185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizokami A, Mukai S, Gao J, et al. GLP-1 signaling is required for improvement of glucose tolerance by osteocalcin. J Endocrinol. 2020;244(2):285-296. [DOI] [PubMed] [Google Scholar]
- 31. Mizokami A, Yasutake Y, Higashi S, et al. Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone. 2014;69(12):68-79. [DOI] [PubMed] [Google Scholar]
- 32. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50(2):568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pi M, Kapoor K, Ye R, et al. Computationally identified novel agonists for GPRC6A. PLoS One. 2018;13(4): e0195980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupte AA, Sabek OM, Fraga D, et al. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology. 2014;155(12):4697-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin X, Parker L, McLennan E, et al. Undercarboxylated osteocalcin improves insulin-stimulated glucose uptake in muscles of corticosterone-treated mice. J Bone Miner Res. 2019;34(8):1517-1530. [DOI] [PubMed] [Google Scholar]
- 36. Du J, Zhang M, Lu J, et al. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine. 2016;53(3):701-709. [DOI] [PubMed] [Google Scholar]
- 37. Yasutake Y, Mizokami A, Kawakubo-Yasukochi T, et al. Long-term oral administration of osteocalcin induces insulin resistance in male mice fed a high-fat, high-sucrose diet. Am J Physiol Endocrinol Metab. 2016;310(8):E662-E675. [DOI] [PubMed] [Google Scholar]
- 38. Iki M, Tamaki J, Fujita Y, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo osteoporosis risk in men (FORMEN) Study. Osteoporos Int. 2011;23(2):761-770. [DOI] [PubMed] [Google Scholar]
- 39. Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94(3):827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Foresta C, Strapazzon G, De Toni L, et al. Androgens modulate osteocalcin release by human visceral adipose tissue. Clin Endocrinol (Oxf). 2011;75(1):64-69. [DOI] [PubMed] [Google Scholar]
- 41. De Toni L, Di Nisio A, Speltra E, et al. Polymorphism rs2274911 of GPRC6A as a novel risk factor for testis failure. J Clin Endocrinol Metab. 2016;101(3):953-961. [DOI] [PubMed] [Google Scholar]
- 42. Di Nisio A, Rocca MS, Fadini GP, et al. The rs2274911 polymorphism in GPRC6A gene is associated with insulin resistance in normal weight and obese subjects. Clin Endocrinol (Oxf). 2017;86(2):185-191. [DOI] [PubMed] [Google Scholar]
- 43. Takata R, Akamatsu S, Kubo M, et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42(9):751-754. [DOI] [PubMed] [Google Scholar]
- 44. Long QZ, Du YF, Ding XY, et al. Replication and fine mapping for association of the C2orf43, FOXP4, GPRC6A and RFX6 genes with prostate cancer in the Chinese population. PLoS One. 2012;7(5):e37866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haiman CA, Han Y, Feng Y, et al. Genome-wide testing of putative functional exonic variants in relationship with breast and prostate cancer risk in a multiethnic population. PLoS Genet. 2013;9(3):e1003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ye R, Pi M, Cox JV, Nishimoto SK, Quarles LD. CRISPR/Cas9 targeting of GPRC6A suppresses prostate cancer tumorigenesis in a human xenograft model. J Exp Clin Cancer Res. 2017;36(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ye R, Pi M, Nooh MM, Bahout SW, Quarles LD. Human GPRC6A mediates testosterone-induced mitogen-activated protein kinases and mTORC1 signaling in prostate cancer cells. Mol Pharmacol. 2019;95(5):563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hayashi Y, Kawakubo-Yasukochi T, Mizokami A, Takeuchi H, Nakamura S, Hirata M. Differential roles of carboxylated and uncarboxylated osteocalcin in prostate cancer growth. J Cancer. 2016;7(12):1605-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pi M, Kapoor K, Wu Y, et al. Structural and functional evidence for testosterone activation of GPRC6A in peripheral tissues. Mol Endocrinol. 2015;29(12):1759-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khrimian L, Obri A, Ramos-Brossier M, et al. Gpr158 mediates osteocalcin’s regulation of cognition. J Exp Med. 2017;214(10):2859-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clemmensen C, Smajilovic S, Wellendorph P, Bräuner-Osborne H. The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol. 2014;171(5):1129-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jørgensen CV, Bräuner-Osborne H. Pharmacology and physiological function of the orphan GPRC6A receptor. Basic Clin Pharmacol Toxicol. 2020;126(Suppl 6):77-87. [DOI] [PubMed] [Google Scholar]
- 53. Lambert LJ, Challa AK, Niu A, et al. Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology. Dis Model Mech. 2016;9(10):1169-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jørgensen CV, Gasparini SJ, Tu J, Zhou H, Seibel MJ, Bräuner-Osborne H. Metabolic and skeletal homeostasis are maintained in full locus GPRC6A knockout mice. Sci Rep. 2019;9(1):5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinsey-Jones JS, Alamshah A, McGavigan AK, et al. GPRC6a is not required for the effects of a high-protein diet on body weight in mice. Obesity (Silver Spring). 2015;23(6):1194-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wellendorph P, Johansen LD, Jensen AA, et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol. 2009;42(3):215-223. [DOI] [PubMed] [Google Scholar]
- 57. Smajilovic S, Clemmensen C, Johansen LD, et al. The L-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in L-arginine-induced insulin release. Amino Acids. 2013;44(2):383-390. [DOI] [PubMed] [Google Scholar]
- 58. Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. GPRC6A mediates the effects of L-arginine on insulin secretion in mouse pancreatic islets. Endocrinology. 2012;153(10):4608-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baker M. 1500 scientists lift the lid on reproducibility. Nature. 2016;533(7604):452-454. [DOI] [PubMed] [Google Scholar]
- 60. Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature. 2012;483(7391):531-533. [DOI] [PubMed] [Google Scholar]
- 61. Barroso I, McCarthy MI. The genetic basis of metabolic disease. Cell. 2019;177(1):146-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clemmensen C, Smajilovic S, Madsen AN, Klein AB, Holst B, Bräuner-Osborne H. Increased susceptibility to diet-induced obesity in GPRC6A receptor knockout mice. J Endocrinol. 2013;217(2):151-160. [DOI] [PubMed] [Google Scholar]
- 63. Clemmensen C, Pehmøller C, Klein AB, Ratner C, Wojtaszewski JF, Bräuner-Osborne H. Enhanced voluntary wheel running in GPRC6A receptor knockout mice. Physiol Behav. 2013;118(6):144-151. [DOI] [PubMed] [Google Scholar]
- 64. Jacobsen SE, Ammendrup-Johnsen I, Jansen AM, Gether U, Madsen KL, Bräuner-Osborne H. The GPRC6A receptor displays constitutive internalization and sorting to the slow recycling pathway. J Biol Chem. 2017;292(17):6910-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barthel A, Okino ST, Liao J, et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274(29):20281-20286. [DOI] [PubMed] [Google Scholar]
- 66. Williams EG, Wu Y, Jha P, et al. Systems proteomics of liver mitochondria function. Science. 2016;352(6291):aad0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu Y, Williams EG, Dubuis S, et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell. 2014;158(6):1415-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang X, Pandey AK, Mulligan MK, et al. Joint mouse-human phenome-wide association to test gene function and disease risk. Nat Commun. 2016;7(2):10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moriishi T, Komori T. Lack of reproducibility in osteocalcin-deficient mice. PLoS Genet. 2020;16(6):e1008939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simon MM, Greenaway S, White JK, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14(7):R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55(7):2153-2156. [DOI] [PubMed] [Google Scholar]
- 72. Ho HY, Lin YT, Lin G, Wu PR, Cheng ML. Nicotinamide nucleotide transhydrogenase (NNT) deficiency dysregulates mitochondrial retrograde signaling and impedes proliferation. Redox Biol. 2017;12(8):916-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48(4):675-686. [DOI] [PubMed] [Google Scholar]
- 74. Fergusson G, Ethier M, Guévremont M, et al. Defective insulin secretory response to intravenous glucose in C57Bl/6J compared to C57Bl/6N mice. Mol Metab. 2014;3(9):848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rendina-Ruedy E, Hembree KD, Sasaki A, et al. A comparative study of the metabolic and skeletal response of C57BL/6J and C57BL/6N mice in a diet-induced model of type 2 diabetes. J Nutr Metab. 2015;2015(6):758080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin X, Parker L, Mclennan E, et al. Uncarboxylated osteocalcin enhances glucose uptake ex vivo in insulin-stimulated mouse oxidative but not glycolytic muscle. Calcif Tissue Int. 2018;103(2):198-205. [DOI] [PubMed] [Google Scholar]
- 77. Zhou B, Li H, Liu J, et al. Intermittent injections of osteocalcin reverse autophagic dysfunction and endoplasmic reticulum stress resulting from diet-induced obesity in the vascular tissue via the NFκB-p65-dependent mechanism. Cell Cycle. 2013;12(12):1901-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Turakhia Y, Chen H, Marcovitz A, Bejerano G. Loss of critical developmental and human disease-causing genes in 58 mammals. BioRxiv. 2019. https://t.co/dJw7p3JxyE [Google Scholar]
- 79. Jacobsen SE, Nørskov-Lauritsen L, Thomsen AR, et al. Delineation of the GPRC6A receptor signaling pathways using a mammalian cell line stably expressing the receptor. J Pharmacol Exp Ther. 2013;347(2):298-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rueda P, Harley E, Lu Y, et al. Murine GPRC6A mediates cellular responses to L-amino acids, but not osteocalcin variants. PLoS One. 2016;11(1):e0146846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jorgensen S, Have CT, Underwood CR, et al. Genetic variations in the human GPRC6A receptor control cell surface expression and function. J Biol Chem. 2017;292(4):1524-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pi M, Xu F, Ye R, et al. Humanized GPRC6AKGKY is a gain-of-function polymorphism in mice. Sci Rep. 2020;10(1):11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Venn-Watson SK, Ridgway SH. Big brains and blood glucose: common ground for diabetes mellitus in humans and healthy dolphins. Comp Med. 2007;57(4):390-395. [PubMed] [Google Scholar]
- 84.Heissel S, Masoudi A, Ben-Hail D, et al. Structure of an endosomal signaling GPCR-G protein-beta-arrestin megacomplex. Nat Struct Mol Biol. 2019;26(12):1123-1131. [DOI] [PMC free article] [PubMed]
- 85.Thomsen AR, Plouffe B, Cahill TJ, et al. GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell. 2016;166(4):907-919. [DOI] [PMC free article] [PubMed]
- 86. Withers HG, Gelman IH. Characterization of an off-target RNAi genomic screen hit identifies GPRC6A as a novel suppressor of metastatic chemotaxis and invasiveness. AACR; 2017;77(13 Suppl):Abstract nr LB-148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed for this review.