Abstract
Background:
In preclinical models, benfotiamine efficiently ameliorates the clinical and biological pathologies that define Alzheimer’s disease (AD) including impaired cognition, amyloid-β plaques, neurofibrillary tangles, diminished glucose metabolism, oxidative stress, increased advanced glycation end products (AGE), and inflammation.
Objective:
To collect preliminary data on feasibility, safety, and efficacy in individuals with amnestic mild cognitive impairment (aMCI) or mild dementia due to AD in a placebo-controlled trial of benfotiamine.
Methods:
A twelve-month treatment with benfotiamine tested whether clinical decline would be delayed in the benfotiamine group compared to the placebo group. The primary clinical outcome was the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). Secondary outcomes were the clinical dementia rating (CDR) score and fluorodeoxyglucose (FDG) uptake, measured with brain positron emission tomography (PET). Blood AGE were examined as an exploratory outcome.
Results:
Participants were treated with benfotiamine (34) or placebo (36). Benfotiamine treatment was safe. The increase in ADAS-Cog was 43% lower in the benfotiamine group than in the placebo group, indicating less cognitive decline, and this effect was nearly statistically significant (p = 0.125). Worsening in CDR was 77% lower (p = 0.034) in the benfotiamine group compared to the placebo group, and this effect was stronger in the APOE ε4 non-carriers. Benfotiamine significantly reduced increases in AGE (p = 0.044), and this effect was stronger in the APOE ε4 non-carriers. Exploratory analysis derivation of an FDG PET pattern score showed a treatment effect at one year (p = 0.002).
Conclusion:
Oral benfotiamine is safe and potentially efficacious in improving cognitive outcomes among persons with MCI and mild AD.
Keywords: Advanced glycation endproducts, Alzheimer’s disease, benfotiamine, glucose, inflammation, oxidative stress
INTRODUCTION
Alzheimer’s disease (AD) therapies targeting brain amyloid-β (Aβ) have in most cases shown a lack of efficacy, suggesting that AD treatment development should consider alternative targets. In addition to plaques, tangles, and cognitive decline, multiple changes accompany AD including inflammation, oxidative stress, and metabolic dysregulation. Cerebral glucose metabolism as measured by fluorine-18 (18F) fluorodeoxyglucose positron-emission tomography (FDG PET) changes decades before AD is typically diagnosed [1], and in AD patients reductions in glucose utilization correlate highly with cognitive decline [2].
Abnormalities in glucose metabolism, vascular changes, and inflammation are closely linked and common features of AD [3, 4]. Thiamine diphosphate (ThDP)-dependent enzymes regulate key steps in brain glucose metabolism, and the activities of ThDP-dependent enzymes decline in blood and brain of AD patients. The reduction in the activity of these enzymes provide a plausible underlying mechanism for the metabolic abnormalities [5–7]. In pre-clinical models, thiamine deficiency induces inflammation and change in vasculature [8]. Abnormal metabolism often leads to over production of free radicals that damage other molecules. At autopsy, oxidative stress in the brain is as widespread as plaques and tangles [9]. Increases in advanced glycation end products (AGE), toxic protein modifications that are indicative of altered glucose metabolism, and their receptor, RAGE, occur in the brain [10] and periphery [11] of AD patients, in both plaques and tangles [12].
Benfotiamine, a synthetic thiamine precursor, has direct actions on multiple metabolic enzymes and pathways, inflammation, and oxidative stress [13, 14]. Benfotiamine’s activation of the enzyme transketolase [15] accelerates the shunting of the precursors of AGE toward the pentose phosphate pathway thereby reducing the production of AGE [16, 17]. The reduction in AGE decreases metabolic stress, which reduces vascular complications [18–21]. By being more effective in raising blood thiamine concentrations than direct thiamine administration, benfotiamine may overcome the reduction in activity of ThDP dependent enzymes in AD [18, 19]. For example, mice [22] and humans [23] that have genetic defects in the thiamine transporter can be treated with high dose benfotiamine. Benfotiamine is an antioxidant [24–26], modulates arachidonic acid inflammation pathways, nuclear transcription factor κB, protein kinase B, mitogen-activated protein kinases, and vascular endothelial growth factor receptor 2 signaling pathways [14]. Recent studies suggest that restoring cerebral perfusion by preventing neutrophil adhesion may provide another strategy for improving cognition in AD participants [27]. Benfotiamine prevents lipopolysaccharide-induced macrophage death and monocyte adhesion to endothelial cells [28]. Multiple approaches suggest that benfotiamine inhibits inflammatory mediators and enhances anti-inflammatory factor production in activated microglia [28, 29].
Benfotiamine diminishes pathology in multiple pre-clinical models of disease including animal models of AD, which have human gene mutations that cause AD [25]. In a transgenic mouse model of tauopathy, benfotiamine treatment diminishes tangles, activates the Nrf2/ARE pathway, is neuroprotective, and improves behavioral deficits [30]. In animal models of amyloid plaque formation, benfotiamine reduces amyloid plaque numbers and phosphorylated tau levels, elevates the phosphorylation of glycogen synthase kinase-3α and —3β, and improves memory [31]. In other animal models, benfotiamine modulates activation of GSK3-β [32], restores neurogenesis [26, 33], modulates AMPA receptor expression [25], and decreases oxidative stress [26]. Together, these results suggest that benfotiamine may be therapeutically beneficial for AD.
Benfotiamine also diminishes AGE. Measures of AGE in the serum assess peripheral abnormalities and may mirror CNS abnormalities in glucose homeostasis. AGE are a biomarker implicated in aging and the development, or worsening of many degenerative diseases, such as diabetes, atherosclerosis, chronic renal disease, and AD. High concentrations of AGE appear predictive of long-term decline in cognition-related daily living performance in patients with AD as measured by Clinical Dementia Rating (CDR) [11] or Mini-Mental Status Exam (MMSE) [34]. Thus, AGE may be a promising therapeutic target to prevent or delay the progression of AD [35]. Numerous studies in patients with diabetes show that benfotiamine diminishes AGE [21]. A preliminary study of five patients without placebo control that was published after our trial was initiated showed promise [36].
Benfotiamine is safe compound in AD patients as demonstrated in trials conducted for the treatment of peripheral neuropathy in diabetes [13, 20, 37]. The dosage studied most extensively in diabetics is 300 mg in the morning and night, but dosages as high as 900 mg per day show no significant toxicity [20].
The aim of this study was to conduct a double-blind early phase II randomized placebo-controlled trial of benfotiamine with the objective of collecting preliminary data on feasibility, safety, and efficacy. The goal was to test whether benfotiamine treatment could delay clinical decline in amyloid positive patients with amnestic mild cognitive impairment (aMCI) or mild dementia due to AD with MMSE scores of >21. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) served as the primary endpoint. Brain glucose utilization, measured using FDG PET imaging, was assessed as a secondary endpoint. Cerebral glucose metabolism declines in temporoparietal regions with the progression of AD, correlates with clinical decline, and is also a sensitive measure of changes in regional neuronal function associated with disease or treatment effect [1, 2]. AGE levels were used as a peripheral marker of efficacy. Measures of thiamine and its esters thiamine diphosphate (ThDP) and thiamine monophosphate (ThMP) provided blood markers of efficacy of drug delivery.
MATERIALS AND METHODS
This clinical trial was a collaborative study between investigators at the Burke Rehabilitation Center including the Burke Rehabilitation Hospital and the Burke Neurological Institute [an affiliate of Weill Cornell Medicine (WCM)], WCM, and investigators at Columbia University Irving Medical Center (CUMC). The trial was approved by the Institutional Review Boards of the Burke Rehabilitation Hospital, WCM and CUMC.
Patient population
Seventy amyloid positive patients 60 years and older with aMCI (21 <MMSE <26) or mild AD dementia (MMSE ≥26) were included. Table 1 shows the inclusion and exclusion criteria for what we define as AD in this trial. These criteria are especially important because new imaging capabilities will likely redefine AD [38].
Table 1.
Selection criteria for the patients
|
Inclusion criteria. Each patient met the following criteria: • Subjects who are able and willing to provide informed consent. • Male and non-pregnant, non-lactating, postmenopausal, or surgically sterilized female subjects at least 60 years of age or older. • Clinical diagnosis of amnestic MCI by the Peterson criteria or probable AD dementia according to the National Institute of Neurological Disorders and stroke and the Alzheimer’s Disease related Disorders Association (NINCDS/ADRDA). • MMSE score >21, CDR score >0.5 and <1 Cornell Scale for Depression in Dementia (CSDD) score <10. • Ambulatory or ambulatory with aide. • Has a caregiver willing to accompany the patient to each visit, accept responsibility for supervising treatment and provided input to clinical outcome assessments. • Reside at home. • Speak English. • Amyloid positive PET-scan. • Patients taking FDA approved medications for the treatment of Alzheimer’s disease [e.g., donepezil (Aricept), galantamine (Razadyne), rivastigmine (Exelon), or memantine (Namenda)] for three months prior to baseline. Patients not on these medications did not initiate them during the study. Exclusion criteria • Significant neurological disorder other than AD including hypoxia, stroke, traumatic brain injury. • A current psychiatric disorder according the DSM-IV diagnosis of major depression unless successfully treated on a stable dose of an antidepressant for at least 4 weeks and continues on stable dose throughout the study. • Any other DSM-IV Axis l diagnosis including other primary neurodegenerative dementia, schizophrenia or bipolar depression. • A current diagnosis of uncontrolled Type I or Type II diabetes mellitus [Hemoglobin A1 C (Hb A1C<8]. Patients with uncontrolled diabetes (i.e., if glucose values exceed 200 mg/ml. • A current diagnosis of active, uncontrolled seizure disorder. • A current diagnosis of probable or possible vascular dementia according to NINDS-AIREN. • An investigational drug during the previous 4 weeks. • Any previous exposure to Benfotiamine. • A current diagnosis of severe unstable cardiovascular disease. • A current diagnosis of acute severe, or unstable asthmatic condition (e.g., severe chronic obstructive pulmonary disease). • A current diagnosis of cardiac, renal or hepatic disease. • A current diagnosis of cancer including any active treatment. • History of alcoholism, current or within past 5 years. • A disability that may prevent the patient from completing all study requirements (e.g., blindness, deafness, severe language difficulty). |
Study design
Sample size justification
In addition to literature that states a four-point change on the ADAS-Cog is considered clinically significant, several randomized clinical trials have found ADAS-Cog change scores differed by 3–4 points between placebo and treatment groups over a 6-month time period. Moreover, other studies report annual changes in the ADAS-Cog among those who are untreated to average 9.6 points (SD = 8.2) [39, 40]. Power was calculated based on expected difference in change on the ADAS-Cog of 3 points between the treatment and control groups. Estimates based on using a two-sided alpha of 0.05 and a standard deviation of 4, enrolling 29 patients per group, (N = 58) suggest 80% power to detect a mean change of 3 between treatment and placebo.
Assignment of patients
A randomized, placebo-controlled, double-blinded trial of benfotiamine in persons with aMCI or AD dementia with a duration of 12 months was conducted. Using blocked, stratified randomization design, patients were assigned to the treatment or control group. By the inclusion criteria all subjects had MMSE of >21. Within this group, a separate randomization schedule was generated using the proc plan function in SAS statistics program for those with an MMSE greater than or less than or equal to 26 to balance their allocation patients to placebo or treatment groups. Using a block size of four for a total of seventy-six patients, 19 blocks were created to help ensure balanced recruitment into treatment and control groups within strata. The schedule was generated in advance by the statistician and provided to the blinded pharmacist in charge of executing the randomization. Two randomization worksheets stratified by MMSE were provided to the pharmacist, who randomized the patients. One sheet had MMSE scores ≥26 (randomized to Active or Placebo). The other sheet had MMSE scores <26. The patients were enrolled by the clinical study team and randomized by the pharmacist. The assignment to the treatment or placebo group was known only to the pharmacist and kept behind a triple lock. The patients received numbered bottles.
Study procedures
The trial was registered in ClinicalTrials.gov (NCT02292238 (Fig. 1). Participants were prescreened from the database of the Memory Evaluation Treatment Service (METS) at Burke Rehabilitation Center or referrals from the Center for the Aging Brain (CAB) at Montefiore/Einstein Medical College, Alzheimer’s Association, primary care physicians, and private neurologists from the lower Hudson Valley region. aMCI or mild AD dementia were diagnosed according to NIA-AA workgroups criteria [41, 42]. Patients who met the inclusion criteria for aMCI or mild dementia due to AD were invited for a screening initial visit at the METS outpatient department at the Burke Rehabilitation Hospital. After informed consent was obtained from patients and their health care proxies, a physical examination including EKG, laboratory tests (complete blood count, complete metabolic panel, vitamin B12, folate, thyroid function tests), a neurological exam, and the MMSE were administered. If eligible (Table 1), participants were referred to Westchester Imaging Center for an Amyloid PET/CT scan of the brain. Only participants with a positive amyloid scan were sent to CUIMC for a baseline 18F-FDG PET/CT scan of the brain. At the baseline visit, the cognitive tests were performed and blood drawn for measurement of thiamine, ThDP, and ThMP by HPLC [43] and APOE genotyping. Enrolled patients returned to the Burke outpatient clinic at month 3, 6, 9, and 12 for subsequent visits. At month 12, the final FDG PET scan was performed at CUIMC.
Fig. 1.
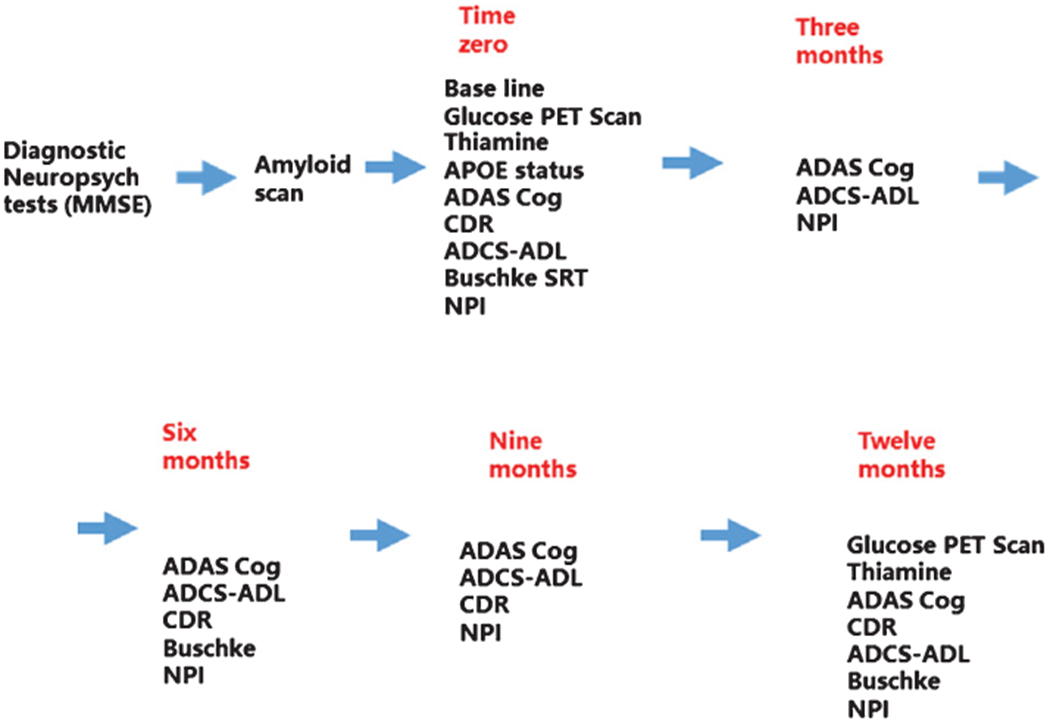
Summary of the treatment protocol for the one-year trial.
The trial duration per participant was twelve months. Participants in the treatment group took one 300 mg capsule of benfotiamine in the morning and one in the evening. The participants in the placebo group took one 300 mg capsule in the morning and evening with microcrystalline cellulose without benfotiamine. At each visit, the patients returned the pill bottles for that period. The number of pills returned was used to assess compliance (the percent of pills consumed).
Characterization procedures
Amyloid scans
Amyloid-β was assessed using PET imaging with 18F-Betapir F18 PET [44] to help confirm the presence of AD pathology in study participants. Positivity was determined by a visual read.
APOE genotyping method
Total nucleic acid was isolated from whole blood samples for APOE genotyping using the Master Pure™ Complete DNA and RNA purification kit (Lucigen) with a starting volume of 150 μl of blood, according to the manufacturer’s instructions. Genotyping of the two human APOE polymorphisms was carried out using the TaqMan® SNP genotyping assays (ThermoFisher Scientific): C_3084793_20 for SNP rs429358 and C_904973_10 for SNP rs7412. An initial 5 min step at 95°C was followed by 40 cycles of 15 s at 95°C and 30 s at 60°C. Genotyping was performed in duplicate with controls for all six possible APOE genotypes and no DNA controls using a QuantStudio™ 12K Flex real-time PCR system (ThermoFisher Scientific).
Treatment
The trial duration per participant was twelve months. Participants in the treatment group took one 300 mg capsule of benfotiamine in the morning and one in the evening. The participants in the placebo group took one 300 mg capsule in the morning and evening with microcrystalline cellulose without benfotiamine. At each visit, the patients returned the pill bottles for that period. The number of pills returned was used to assess compliance (the percent of pills consumed). The benfotiamine and placebo were manufactured and provided by the Advanced Orthomolecular Research, Canada. They prepared the benfotiamine according to an FDA-approved IND, which was prepared by the Cornell Translational Science Center, and issued to the Burke Neurological Institute.
Cognitive measures
The following cognitive tests were conducted at the intervals indicated in Fig. 1:
AD Assessment Scale-Cognitive Subscale (ADAS-Cog) was the primary outcome measure. It indicates the severity of the most important symptoms of AD. It consists of 11 tasks measuring the disturbances of memory, language, praxis, attention, and other cognitive abilities [45, 46].
Clinical dementia rating (CDR) is a 5-point scale used to characterize six domains of cognitive and functional performance applicable to AD and related dementias: Memory, Orientation, Judgment & Problem Solving, Community Affairs, Home & Hobbies, and Personal Care. A higher score indicates greater dementia [47].
The Buschke Selective Reminding Test (SRT) [48] is a standard diagnostic tool in the assessment of verbal memory. Several studies attest to its predictive value for dementia [49, 50].
Neuropsychiatric Inventory (NPI) assesses a wide range of behaviors encountered in dementia patients to provide a means of distinguishing frequency and severity of behavioral changes. Ten behavioral and two neuro-vegetative domains are evaluated through an interview with the caregiver [51–53].
Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) is a caregiver-based ADL scale composed of 19 items developed for use in dementia clinical studies [54]. It assesses the patient’s performance of both basic and instrumental activities of daily living such as those necessary for personal care, communicating and interacting with other people, maintaining a household, conducting hobbies and interests, as well as making judgments and decisions. Higher numbered scores and answers of “yes” reflect a more self-sufficient individual. Therefore, the higher total score correlates with higher cognitive function. The total score is the sum of all items and sub-questions [55].
Biomarker outcomes
AGE are formed during the Maillard reaction where reducing carbohydrates react with lysine side chains and N-terminal amino groups of various macromolecules, particularly proteins. AGE can adversely affect the function of these macromolecules. One of the most prevalent AGE, N-epsilon-(carboxymethyl) lysine, has been implicated in oxidative stress and vascular damage. The quantity of AGE adduct in protein samples is determined by comparison with that of a known AGE-BSA standard curve.
AGE levels were measured on plasma sample with a kit from ABCAM (AB238539), Cambridge, MA., USA
Fluorodeoxyglucose positron emission tomography
Image acquisition, processing, and measurement
FDG PET imaging of glucose metabolism was acquired at baseline and after 12 months of treatment. All scans were acquired on a Siemens MCT 64 PET/CT PET-CT scanner at CUIMC. Study participants were maintained in an awake, at-rest state with eyes and ears open in dim lighting during tracer uptake. Forty minutes after injection of the tracer, the emission image was acquired in four contiguous 5-min frames. Frames were aligned with SPM 12, averaged, and then spatially normalized to the MNI template using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), resulting in one image per participant for each time point. Average voxel values within 90 regions of interest (ROIs) in the Automated Anatomic Labeling (AAL) Atlas [56] were computed. A subset of 16 pre-specified bilateral ROIs were chosen for the group analysis due to their relevance to AD including: posterior cingulate, precuneus, frontal, inferior parietal, mid temporal, hippocampus, paracentral lobule, and cerebellum. The paracentral lobule and cerebellum were included as reference regions given their relative preservation during AD progression.
Derivation of spatial covariance patterns for glucose FDG PET
A multivariate machine learning approach was also applied to evaluate the FDG PET data (Fig. 10). Pattern-based methods have been increasingly applied to the evaluation of neurodegeneration and therapeutic response as they address the issue of complexity in comparing multiple regions and can increase signal to noise for analysis. Feature reduction was performed through use of the scaled subprofile model (SSM) [57–61], a form of principal components analysis (PCA). The resulting components were used in regression modeling that determined spatial patterns of hypometabolism and hypermetabolism (or preservation relative to other regions) associated with the CDR score.
Fig. 10.
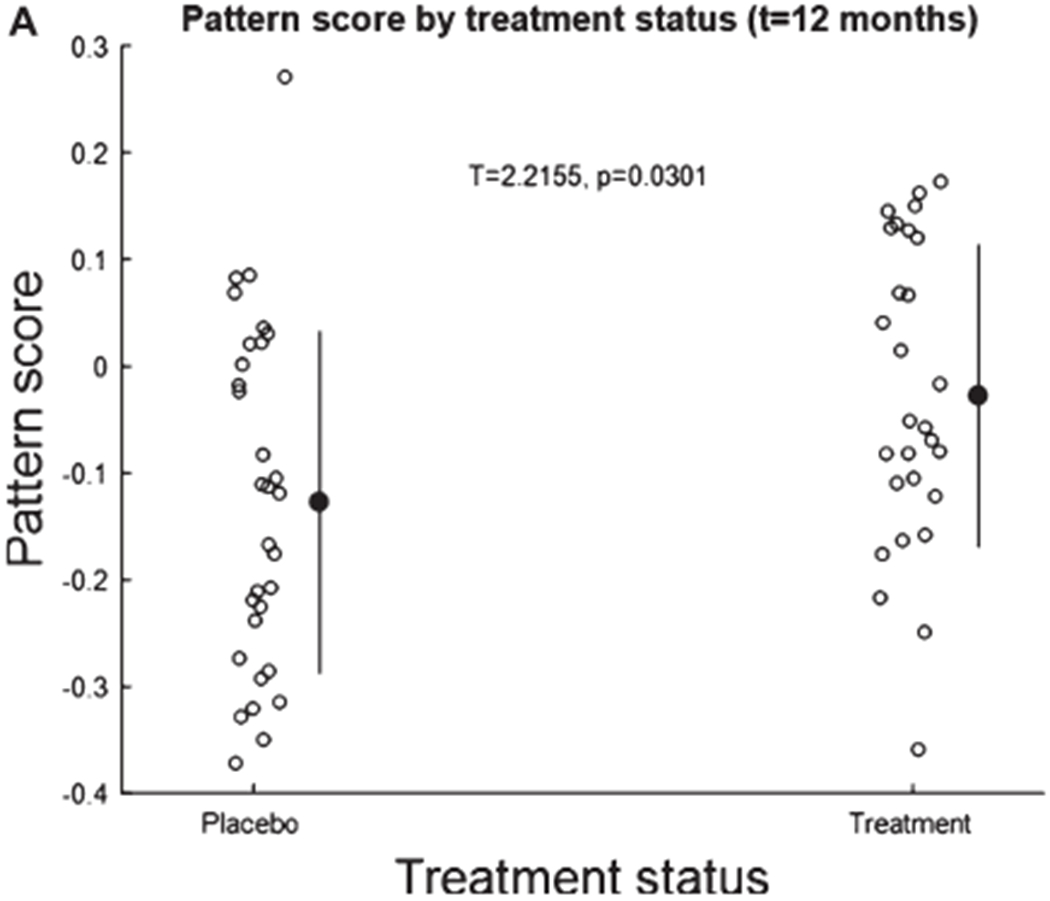
A. Pattern score as function of the 12-month treatment period. The pattern is a linear combination of the first two principal components whose pattern score is slightly but significantly higher for treatment than untreated participants at time point 12 months.
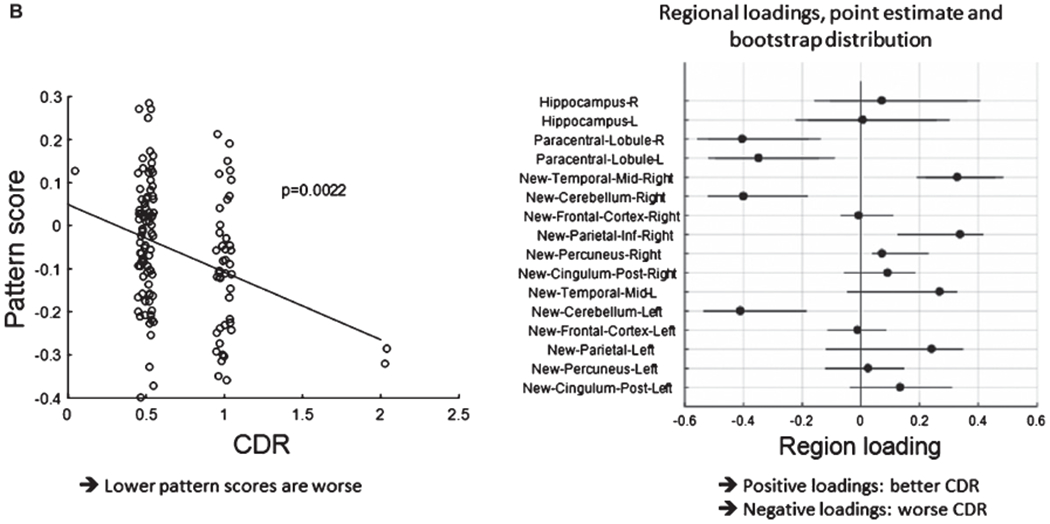
B. The left panel shows the pattern score plotted against CDR status (p-level obtained from whole-model F-test.) A higher pattern score implies lower CDR status. The right panel shows loading distributions from a bootstrap test with 90% coverage intervals. We stress that these loadings sizes and signs are relative since we removed the whole-brain mean from the analysis prior to the pattern derivation. Thus, high positive loadings are found in the right mid temporal and inferior parietal cortex, implying relatively higher signal in participants with lower CDR. Bilateral cerebellum and paracentral lobule on the other hand, had relatively lower signal in participants with lower CDR.
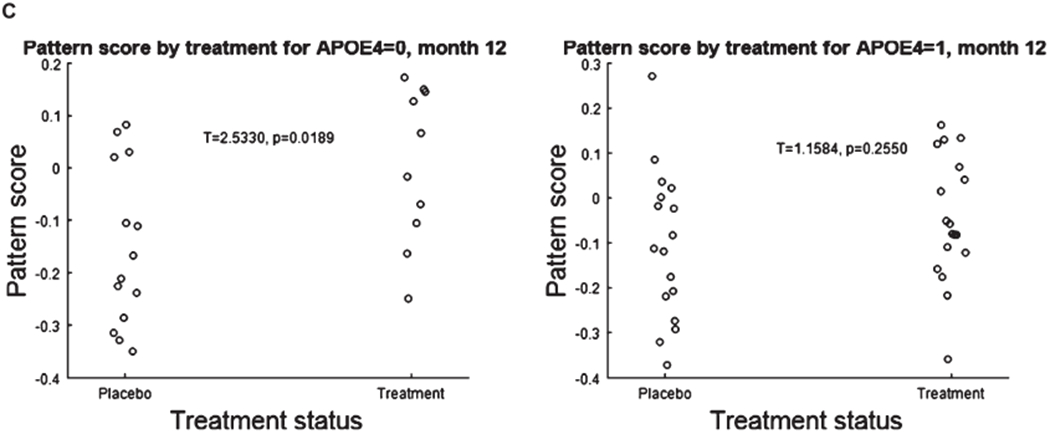
C. Stratification of the pattern score by APOE status reveals that APOE4 negative patients show the greatest response. APOE ε4 = 0 patients show a treatment effect (left panel), APOE ε4 = 1 do not (right panel).
Specifically, SSM by performing PCA on the PET-data array was run, with a subsequent brain-behavioral regression to derive a best-fitting pattern whose pattern scores correlates with the CDR score in a negative direction (i.e., the higher the pattern score, the lower the CDR). The best-fitting set of principal components was obtained via the Akaike criterion [62], and came out as PC1-2.
To help with the imputation of the multivariate analysis, a generic multivariate decomposition was written as: Y(s,x) = w(s) v(x) + ε (s,x), where Y denotes the (log-transformed) data which depends on a participant and time index s and the voxel location x. The pattern score w(s) is a scalar that solely depends on subject and time, but not voxel location, whereas the derived pattern v(x) depends on voxel location, but shows invariance across participants and time, i.e., does not depend on index s; ε(s,x) denotes residual signal that is dependent on participant, time, and voxel location, but which was discarded for our purposes. The pattern score w(s) was chosen to correlate negatively with CDR across the data. The pattern v(x) is normalized to have unity Euclidean norm, i.e., ‖v‖ = 1. This means that the pattern score carries all information about the strength of the signal associated with the spatial pattern. Higher values of w(s) imply higher values of pattern-associated FDG PET signal in direct proportion in all regions.
To estimate the topographic robustness of any patterns of interest, a bootstrap resampling procedure [63, 64] was performed 10,000 times, for which data were resampled with replacement and the complete analytic recipe was executed on the resampled data, generating distribution for pattern loadings. Regional loadings were considered robust if the 95% coverage interval (= [2.5%, 97.5%]) did not overlap with, and lay to one side of, zero. For the correct interpretation, it is important to keep in mind that positive and negative loadings describe only relative, and not absolute differences, in the signal associated with any covariance pattern. Since the residual signal in ε(s,x) was stripped off, there cannot be assurance that there are absolute differences in the total data for the regions with robust loadings.
After deriving and estimating the topographic robustness of the pattern, the pattern score was inspected for an effect of treatment at baseline and follow-up, also broken down by APOE ε4 status.
Statistical methods [65, 66]
Our primary clinical outcome was ADAS-Cog and secondary outcomes were the CDR score and FDG PET imaging of the brain. AGE levels were an exploratory outcome.
Our primary analysis followed Intention-to-Treat (ITT) and the secondary analysis was per-protocol. The per-protocol analysis omitted one placebo participant who took benfotiamine from a commercial vendor. The ITT and per-protocol analysis are presented for the primary outcome ADAS-Cog and the secondary measure CDR. For the other measures only per-protocol analysis are presented.
Spearman correlation coefficient was used to assess the correlation between continuous variables. Student’s t-test was used to compare the continuous variables between placebo and treatment groups; Fisher’s exact test was used to compare categorical variables between placebo and treatment groups. Specifically, two-sample Student’s t-test was used to compare the score changes (ADAS score, normalized PET-related scores, etc.) from baseline between Placebo and Treatment groups when normality was satisfied, otherwise Wilcoxon Rank-sum test was used. ANCOVA was used to test the group difference while adjusting for covariates.
The primary analysis was done on the ITT data. The Last-Observation-Carry-Forward (LOCF) method was used to impute the missing values of ADAS total score and the secondary endpoints such as CDR as well for each time point. The primary analysis was done on ITT data which were imputed with LOCF method. Per-protocol analysis was done as a sensitivity analysis and as observational comparisons [66].
In the time to event analysis, time to ≥3 points of ADAS change was calculated based on whether the ADAS score changed from baseline ≥3 (event) at each time point. When no change ≥3 points was observed at any time point, the observation is censored and the last follow-up time (12 month) was used to calculate the duration. Kaplan-Meier estimator was then used to estimate probability of time-to-event. The difference between groups was tested by log-rank test for statistical significance.
As sensitivity analyses, repeated-measure ANOVA, generalized estimating equation (GEE) and Mixed effect model, and Wilcoxon Rank-sum test were also performed on primary endpoints with and without imputation to compare differences between placebo and treatment groups.
Subgroup analyses in MMSE, APOE, and sex were either in the per-protocol analysis or exploratory. A Student’s t-test was used in each of the subgroup comparisons. An ANCOVA was also used to analyze the treatment difference while adjusting for each of these covariates. Interaction between MMSE and ADAS-Cog responses was assessed by ANCOVA with interaction term. Multiple comparisons were present in our analyses with secondary endpoints, subgroup analyses, or analyses with multiple PET-related scores. Due to exploratory nature of those analyses and early trial of this study, we did not apply correction of p-values for multiple comparisons. All statistical tests were two-sided with an alpha level of 0.05 as the significance cutoff. All analyses were performed in statistical software SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the populations at baseline
The first participant entered the trial on February 12, 2015 and the final participant finished July 9, 2019. This allowed us to exceed our enrollment goal of 58. Pre-screening of 634 patients at the METS at the Burke Rehabilitation Hospital excluded all but 120 participants (Fig. 1). Only 83 of these patients were amyloid positive. Twelve declined to participate. Seventy-one of these participants agreed to be part of the trial, and were randomized to receive either placebo or benfotiamine. Eight subjects were prematurely discontinued from the trial prior to Month 12. Three participants were withdrawn due to non-compliance <80%; three withdrew consent due to unwillingness to complete study procedures; one participant was lost to follow-up and one was withdrawn by PI due to physical limitations. Patients with uncontrolled diabetes were excluded. Eight patients were being successfully managed for diabetes. Patients had to have an HbA1c <8% trial and/or a fasting glucose <200 mg/dl to be enrolled in the trial. None of the participants randomized to the treatment group withdrew due to adverse reactions or adverse effects. Since the ones who withdrew did not have final scores, their dropout did not affect 12-month scores. After the trial completion and after the data were locked, one patient in the placebo group was determined to be on benfotiamine from another source and was excluded from the per-protocol analysis. Thus, 37 (placebo) and 34 (benfotiamine) were included in the ITT analysis, and 36 (placebo) and 34 (benfotiamine) were included in the per-protocol analysis.
Whether the patient took the required medication was referred to as compliance. If the patients who withdrew are included, the percent compliance in the placebo group was 87.7 (3.5%) and in the treatment group was 89.8 (3%). If the patients that withdrew are not included, the percent compliance in the placebo group was 94.1 (1.3%) and in the treatment groups percent compliance was 94.8 (1.4%).
The demographic characteristics of the patients are described in Table 2. The randomization procedure was based on the order of patient entry into the study. There were no statistically significant differences in age, race, MMSE, and demographic or clinical characteristics. The goal to recruit patients with an average MMSE of 26 was met. The percentage of females in the benfotiamine group (67.6%) was higher than in the placebo group (50%). Although the distribution by race was similar, only 2.9% of the population was Non-Hispanic Black. The distribution of APOE ε4 carriers and non-carriers (60% and 40%, respectively) in the whole population was reflected in the benfotiamine (64.7 and 35.3%, respectively) and placebo (55.6% and 44.4%, respectively) groups. Nearly identical proportions were also observed for males (58.6% and 41.4%) and females (61% and 39%). The scores on the neuropsychological tests at baseline did not differ between the two groups, with the exception of NPI, which differed between groups at baseline (p = 0.040) (Table 2B).
Table 2A.
Baseline comparison between benfotiamine (n = 34) and placebo (n = 36). A. Baseline demographic characteristics
| Total | Placebo | Benfotiamine | p | ||
|---|---|---|---|---|---|
| Age | T | ||||
| Mean (SD) | 75.77 (7.01) | 75.81 (7.19) | 75.74 (6.91) | 0.967 | |
| Gender | F | ||||
| Female | 41 (58.6) | 18 (50.0) | 23 (67.6) | 0.153 | |
| Male | 29 (41.4) | 18 (50.0) | 11 (32.4) | ||
| Race | F | ||||
| Black | 2 (2.9) | 1 (2.8) | 1 (2.9) | 1.000 | |
| White | 68 (97.1) | 35 (97.2) | 33 (97.1) | ||
| Ethnicity | F | ||||
| Hispanic/Latino | 4 (5.7) | 4 (11.1) | 0 (0.0) | 0.115 | |
| Not Hispanic/Latino | 66 (94.3) | 32 (88.9) | 34 (100) | ||
| MMSE total | T | ||||
| Mean (SD) | 25.33 (2.63) | 25.33 (2.52) | 25.32 (2.78) | 0.988 | |
| Dichotomized MMSE | F | ||||
| <26 | 34 (48.6) | 18 (50.0) | 16(47.1) | 0.816 | |
| ≥26 | 36 (51.4) | 18 (50.0) | 18 (52.9) | ||
| APOE genotype | F | ||||
| 2/3 | 4 (5.7) | 2 (5.6) | 2 (5.9) | 0.883 | |
| 2/4 | 1 (1.4) | 0 (0.0) | 1 (2.9) | ||
| 3/3 | 24 (34.3) | 14 (38.9) | 10 (29.4) | ||
| 3/4 | 34 (48.6) | 17 (47.2) | 17 (50.0) | ||
| 4/4 | 7 (10.0) | 3 (8.3) | 4 (11.8) |
T, t-test (with equal variances); F, Fisher’s exact t-test.
Table 2B.
Baseline neuropsychological outcome measures
| Total | Placebo | Benfotiamine | p | |
|---|---|---|---|---|
| ADAS total score (ITT) | 15.34 (6.36) | 15.50 (6.61) | 15.19 (6.16) | 0.835 t |
| ADAS total score (Per protocol) | 15.34 (6.40) | 15.48 (6.70) | 15.19 (6.16) | 0.849 t |
| CDR score | 0.50 (0.50–1.00) | 0.50 (0.50–1.00) | 0.50 (0.50–1.00) | 0.334 w |
| Median(range) | ||||
| ADCS-ADL total score | 47.44 (4.29) | 47.42 (4.65) | 47.47 (3.95) | 0.959 t |
| NPI | 13.50 (10.44) | 11.03 (10.15) | 16.12 (10.23) | 0.040 t |
| Buschke score | 27.09 (9.74) | 26.03 (9.01) | 28.21 (10.49) | 0.354t |
Values are Mean (SD). T denotes t-test (with equal variances). W Wilcoxon rank sum test.
Baseline thiamine and ThMP, but not ThDP distributions were similar in the two groups. Blood ThDP was lower (p = 0.038) in the benfotiamine group (Table 2C). In agreement with the literature [67]. ThDP was lower in females than males (p = 0.0003). At baseline, ThDP did not correlate with MMSE (p = 0.644), CDR (p = 0.618), ADAS-Cog (p = 0.883), or whole brain glucose utilization (p = 0.644).
Table 2C.
Baseline Thiamine, ThDP, and ThMP
| Total | Placebo | Benfotiamine | p | |
|---|---|---|---|---|
| Thiamine | ||||
| Mean (SD) | 5.72 (11.31) | 5.26 (4.50) | 6.20 (15.56) | 0.735 |
| Thiamine diphosphate | ||||
| Mean (SD) | 69.71 (19.40) | 74.46 (20.21) | 64.82 (17.50) | 0.038 |
| Thiamine monophosphate | ||||
| Mean (SD) | 3.21 (1.72) | 3.46 (1.83) | 2.97 (1.59) | 0.250 |
Comparisons were by t-test (with equal variances).
Baseline FDG PET measures are presented in Table 2D. In agreement with prior findings, FDG PET in whole brain at baseline correlated with the MMSE (Spearman correlation, r = 0.288, p = 0.015). Brain glucose utilization was 4.4% higher in females than males (p = 0.003). At baseline, FDG PET in the mid-temporal region was significantly higher in the benfotiamine treatment group than placebo group (p = 0.020), and the cingulate was higher in the treatment group at trend level (p = 0.069) (Table 2D).
Table 2D.
Baseline comparison of FDG PET
| Total | Placebo | Benfotiamine | p | |
|---|---|---|---|---|
| Posterior cingulate | ||||
| Left | 0.85 (0.09) | 0.83 (0.10) | 0.87 (0.08) | 0.087 |
| Right | 0.81 (0.07) | 0.79 (0.07) | 0.82 (0.06) | 0.069 |
| Precuneus | ||||
| Left | 1.09 (0.09) | 1.08 (0.10) | 1.10 (0.08) | 0.332 |
| Right | 1.09 (0.10) | 1.08 (0.11) | 1.11 (0.09) | 0.287 |
| Medial temporal | ||||
| Left | 0.97 (0.11) | 0.94 (0.11) | 1.00 (0.10) | 0.022 |
| Right | 1.01 (0.12) | 0.98 (0.12) | 1.04 (0.10) | 0.020 |
| Frontal cortex | ||||
| Left | 0.99 (0.09) | 0.98 (0.09) | 0.99 (0.09) | 0.480 |
| Right | 1.01 (0.09) | 1.00 (0.09) | 1.03 (0.08) | 0.268 |
| Hippocampus | ||||
| Left | 0.74 (0.08) | 0.74 (0.08) | 0.75 (0.08) | 0.938 |
| Right | 0.76 (0.09) | 0.75 (0.10) | 0.76 (0.08) | 0.764 |
| Entorhinal_cortex | ||||
| Left | 0.88 (0.13) | 0.88 (0.14) | 0.87 (0.13) | 0.693 |
| Right | 0.89 (0.18) | 0.87 (0.21) | 0.90 (0.14) | 0.477 |
| Prefrontal_cortex | ||||
| Left | 0.82 (0.09) | 0.81 (0.09) | 0.83 (0.08) | 0.417 |
| Right | 0.87 (0.09) | 0.86 (0.10) | 0.88 (0.08) | 0.293 |
| Whole brain | 0.88 (0.05) | 0.87 (0.06) | 0.89 (0.05) | 0.122 |
All values were normalized to the cerebellum as described in methods. All values are mean (SD). All comparisons were by the t-test (equal variances).
Safety profile
No adverse events related to the 2 × 300 mg benfotiamine per day were observed and patients did not complain about the medication (Table 3A).
Table 3A.
Consequences a 12-month treatment with benfotiamine. A. Benfotiamine did not cause any adverse events
| Symptom | Placebo (n = 36) | Treatment (n = 34) |
|---|---|---|
| Anxiety | 4 (11%) | 5 (14%) |
| Bruise | 5 (14%) | 2 (6%) |
| Cold symptoms | 3 (8%) | 3 (8%) |
| Depression | 2 (6%) | 1 (3%) |
| Dizziness | 3 (8%) | 3 (8%) |
| Fall | 12 (34%) | 6 (17%) |
| Head injury | 2 (6%) | 0 (0%) |
| Heart arrhythmia | 2 (6%) | 1 (3%) |
| Pain | 4 (11%) | 5 (14%) |
| Pneumonia | 3 (8%) | 0 (0%) |
| Sprain | 2 (6%) | 0 (0%) |
| Surgery | 3 (8%) | 1 (3%) |
| Allergy | 2 (2%) | 0 (0%) |
| Gastrointestinal problem | 12 (34%) | 9 (26%) |
| Stroke | 0 (0%) | 2 (6%) |
| Total | 59 | 38 |
Benfotiamine and ADAS-Cog changes (Fig. 2, Table 3B)
Fig. 2.
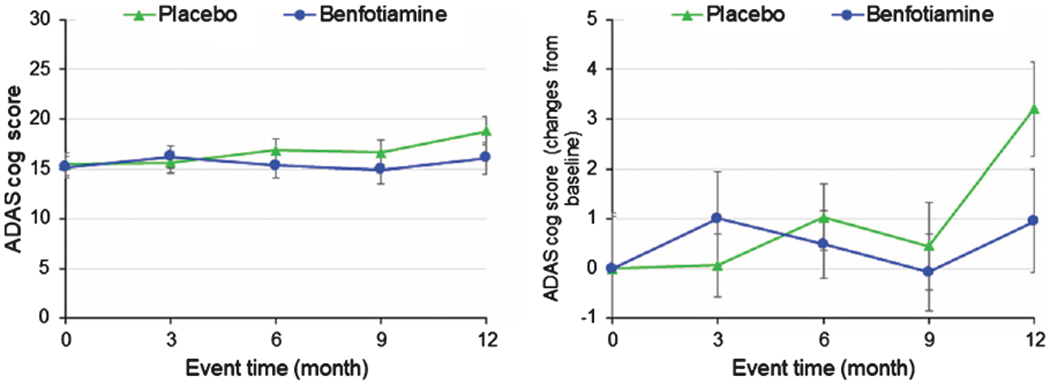
Changes in ADAS-Cog with benfotiamine treatment compared to controls. See Table 7 for statistical comparisons.
Table 3B.
Changes in ADAS-Cog following 12-month treatment with placebo or benfotiamine (ITT and per-protocol analysis)
| Variable | Total | Placebo | Benfotiamine | p1 |
|---|---|---|---|---|
| Unadjusted comparison of the changes from baseline to month 12 in ADAS score between intervention and control (ITT data after LOCF imputation) | ||||
| ADAS score change Mean (SD) | 2.37 (5.61) | 3.26 (5.52) | 1.39 (5.63) | 0.162 [T] |
| Unadjusted comparison of the baseline to month 12 in ADAS score between benfotiamine and control (Per-protocol) | ||||
| ADAS score change Mean (SD) | 2.10 (5.59) | 3.2 (5.66) | 0.96 (5.41) | 0.125 [T] |
Repeated measures ANOVA p-value: 0.5626; Mixed effect model p-value: 0.0708; GEE p-value: 0.1373; Wilcoxon Rank sum p-value: 0.0980.
p-values obtained from the statistical tests: [T] t-test (equal variances). Repeated measures ANOVA p-value: 0.355; Mixed effect model p-value: 0.056; GEE p-value: 0.107; Wilcoxon Rank sum p-value: 0.069. p-values obtained from the statistical tests:[T] t-test (equal variances).
A comparison of unadjusted changes from baseline to 12 months with ITT analysis revealed a difference between the benfotiamine and placebo groups favoring benfotiamine using a mixed effect model (p = 0.071), GEE (p = 0.137), and a non-parametric Wilcoxon rank sum test (p = 0.098) (Fig. 2, Table 3B). At 12 months, the change in the placebo group was 3.26 whereas in the benfotiamine group the change was 1.39. This difference was not apparent at 3, 6, or 9 months. The per-protocol analysis (Table 3B) suggested that the differences were significant when analyzed by a mixed effect model (p = 0.035), GEE (p = 0.069), or Wilcoxon Rank-Sum (p = 0.049). The sub-category exploratory analysis of ADAS-Cog revealed that the changes from baseline in the commands component (p = 0.001) and the word finding difficulty (p = 0.033) were significant at 12 months.
An exploratory analysis of effect modification by sex suggests that males might have been more responsive to benfotiamine, although none of the differences were statistically significant. Furthermore, there was no effect modification by APOE ε4 allele carrier status. Finally, no significant correlation occurred between blood thiamine, ThDP or ThMP values, and ADAS-Cog. No significant interaction was found between MMSE score and ADAS-Cog response (p = 0.122), but a post-hoc analysis suggested that benfotiamine had a stronger response among those with a higher MMSE at baseline (MMSE ≥26 difference in change ADAS-Cog was significant (p = 0.027) whereas this was not the case for MMSE <26 (p = 0.99).
CDR
Mean change in global CDR from baseline to 12 months was significantly different between placebo and benfotiamine groups (p = 0.034), favoring the benfotiamine group (Fig. 3). The difference in the placebo group was 0.22 whereas the change in the benfotiamine group was 0.05, corresponding to a reduction of deterioration by 77%. The mean change in CDR-SB from baseline to 12 months showed a difference at trend level between placebo and benfotiamine groups (p = 0.078). In an analysis of individual CDR subscores, the “home and hobbies score” differed between groups (p = 0.032) whereas other subscores did not differ.
Fig. 3.
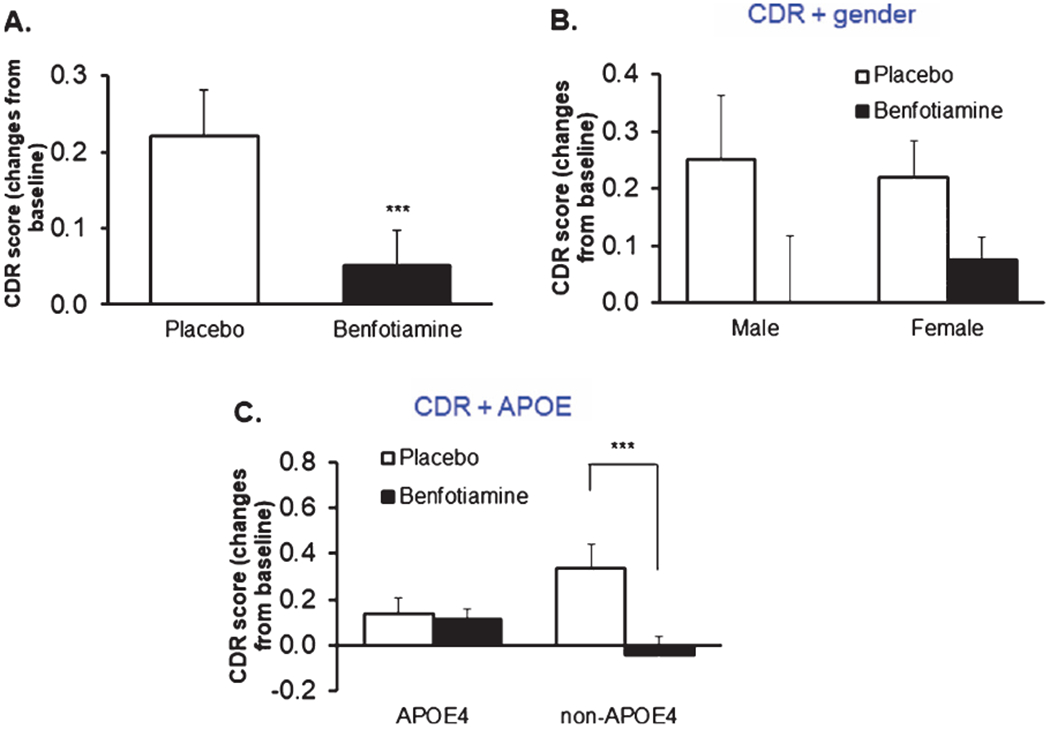
Benfotiamine treatment and the CDR. CDR Placebo = 34, benfotiamine = 29. On the figure *** indicates significantly different (p = 0.034) (A). When the groups are also separated by sex, large but non-significant differences occur (B). When the groups are separated by APOE4 only the non-APOE ε4 allele group differs. In the non-APOE4 group the *** indicates values significantly different (p = 0.013) (C). The APOE4 denotes at least one ε4 allele. p-values here are when there are subgroups are all obtained from subgroup analysis, not interaction from ANOVA (C).
APOE ε4 status (Fig. 3C), but not sex (Fig. 3B), was associated with a differential response to benfotiamine. The performance of males and females was not significantly different (Fig. 3B). The change from baseline in females 0.219 was nearly identical to that in males. However, the non-APOE ε4 group seemed to respond much more than those with the ε4 allele (Fig. 3C). Indeed, the change from baseline was significant in the non-APOE ε4 group (p = 0.013) although only eleven participants were in this category. No significant interaction was found by comparing patients that had MMSE values ≥26 versus <26 (p = 0.878).
The Buschke SRT (Fig. 4)
Fig. 4.
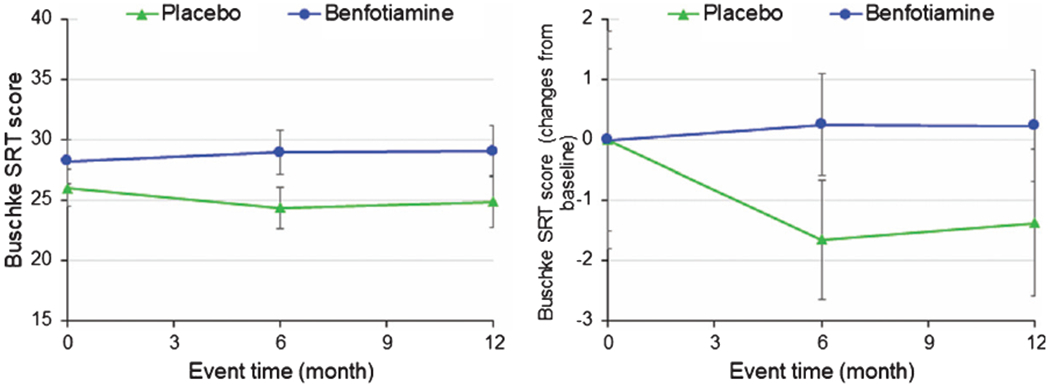
Benfotiamine and the Buschke Selective Reminding Test (SRT).
No significant change in the SRT (p = 0.177) nor the change in score (0.315) (Fig. 4) occurred. Placebo treated participants showed a downward trend while benfotiamine treated participants had stable scores. Trend analysis shows that the non-APOE ε4 are the most responsive at 6 months (compared baseline p = 0.028) and 12 months (compared to baseline p = 0.066).
NPI (Fig. 5)
Fig. 5.
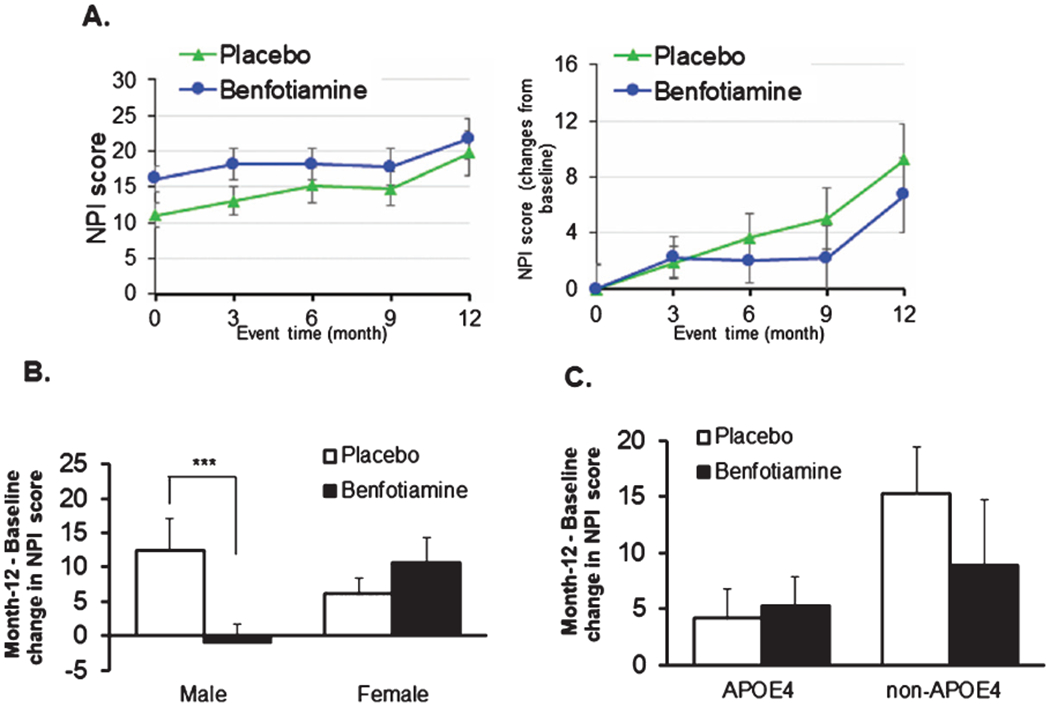
Benfotiamine and the Neuropsychiatric Inventory (NPI). No differences were seen in the overall scores (A). However, separation of the groups by sex revealed a highly significant benefit in males but not females. *** indicates p = 0.035 (B). No significant difference was seen with APOE ε4 alleles (C).
No differences in change in NPI were observed with benfotiamine treatment when the whole population was analyzed (Fig. 5A). However, benfotiamine was associated with significantly reduced scores in males at month 9 (0.014) and month 12 (p = 0.035) (Fig. 5B). The effects of benfotiamine were not altered by APOE4 status (Fig. 5C).
ADCS-ADL (Fig. 6)
Fig. 6.
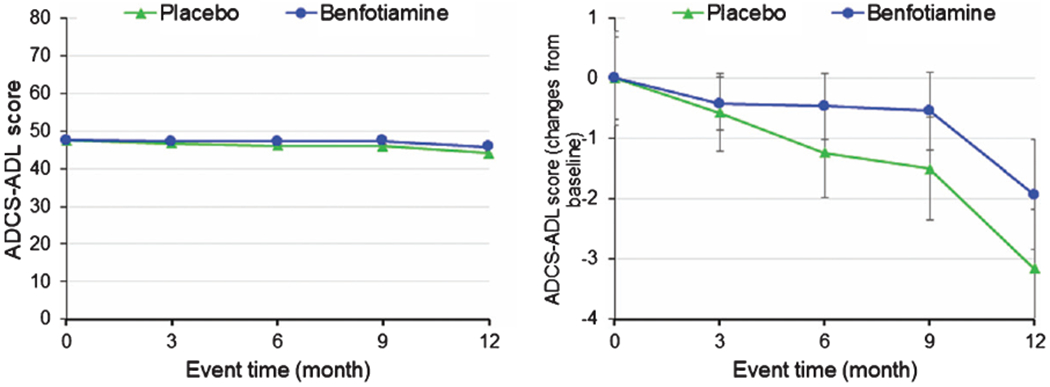
Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL).
No significant differences were observed in ADCS-ADL. In the sub-analysis of sex and APOE, a trend was observed that was consistent with a beneficial effect of benfotiamine (Fig. 6).
Response of thiamine, ThDP, and ThMP to benfotiamine treatment (Table 3C; Figs. 7 and 8)
Table 3C.
Changes in Thiamine, ThDP, and ThMP after 12 months of placebo (n = 36) or benfotiamine (n = 34)
| Baseline | 12 months | p | |
|---|---|---|---|
| Changes in thiamine and its esters after 12 months of placebo | |||
| Thiamine | 5.48 ± 0.77 | 13.64 ± 4.06 | 0.044 |
| Thiamine diphosphate | 74.46 ± 3.42 | 91.70 ± 7.94 | 0.044 |
| Thiamine monophosphate | 3.38 ± 0.31 | 4.05 ± 0.73 | 0.382 |
| Changes in thiamine and its esters after 12 months of benfotiamine | |||
| Thiamine | 6.20 ± 2.67 | 999.51 ± 147.4 | <0.001 |
| Thiamine diphosphate | 64.82 ± 3.00 | 197.39 ± 17.75 | <0.001 |
| Thiamine monophosphate | 2.97 ± 0.27 | 20.73 ± 2.16 | <0.001 |
Comparisons were by t-test (with equal variances).
Fig. 7.
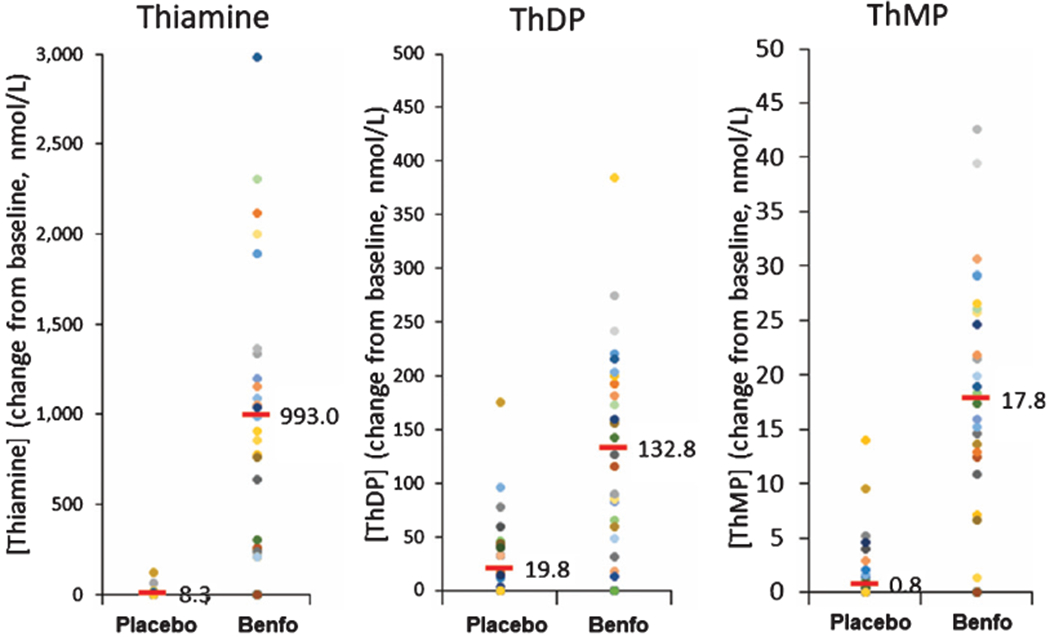
Blood thiamine, ThMP, and ThDP concentrations at baseline and month 12. Each dot represents a different patient. The bar represents the mean value. All values are per protocol after omitting a patient designated as placebo who was taking benfotiamine from another source.
Fig. 8.
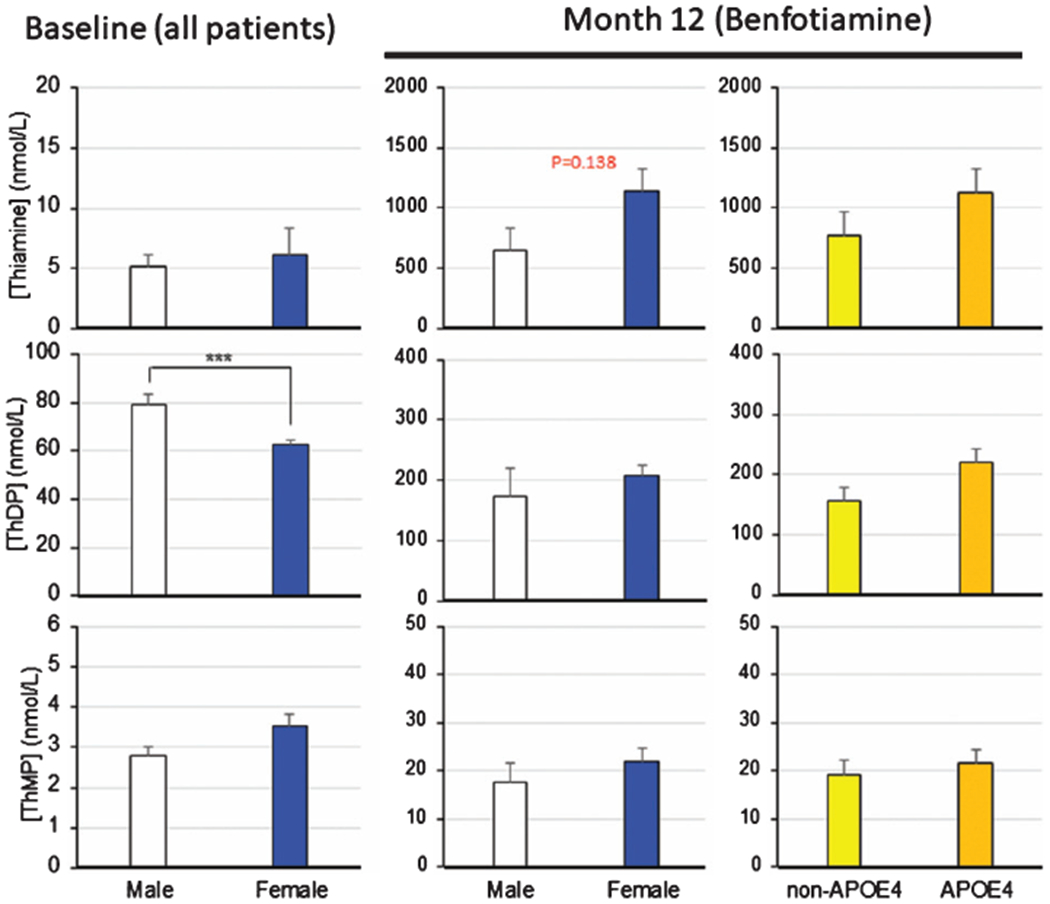
Relation of sex and APOE ε4 genotype to thiamine, ThDP and ThMP. Values are means ± SEM. *** denotes significantly different (p <0.0001) by t-test.
The 161-fold increase in in blood thiamine indicated the administration of the drug was successful. In the placebo group, small increases for the levels of thiamine (5.5 to 13.6; p = 0.044) and ThDP (74.5 to 91.7; p = 0.044) occurred, but not ThMP (3.4 to 4.0; p = 0.382) (Table 3C). After completion of the trial, it was discovered that one patient in the placebo group took commercial benfotiamine during the trial. Consequentially, data from the patient was excluded for all per-protocol analysis. The twelve-month treatment with benfotiamine significantly elevated blood thiamine from 6.2 to 999 (161-fold) above baseline, ThDP (two-fold) and ThMP (five-fold) (Table 3C). Although the differences were significant, the scatter grams revealed large variations (Fig. 7). These changes were apparent even though the timing between the taking the last capsule and taking blood were not standardized. The much larger changes than expected may be related to the duration of the treatment or the purity of the benfotiamine.
There was a trend for APOE ε4 and sex related differences in thiamine response to benfotiamine but the differences were not significant (Fig. 8). Thiamine levels after benfotiamine were about two times higher in females than males. Thiamine values were approximately 50% higher in APOE ε4 carriers than non-APOE ε4 carriers.
The concentrations of blood thiamine, ThDP, ThMP after benfotiamine treatment did not correlate with ADAS-Cog scores (p = 0.736, 0.917, 0.500, respectively) nor CDR (p = 0.762, 0.896, 0.767, respectively).
The response of AGE to benfotiamine treatment
Benfotiamine inhibited the increase in AGE over the course of the disease and the effect was more apparent in non-APOE ε4 patients (Fig. 9).
Fig. 9.
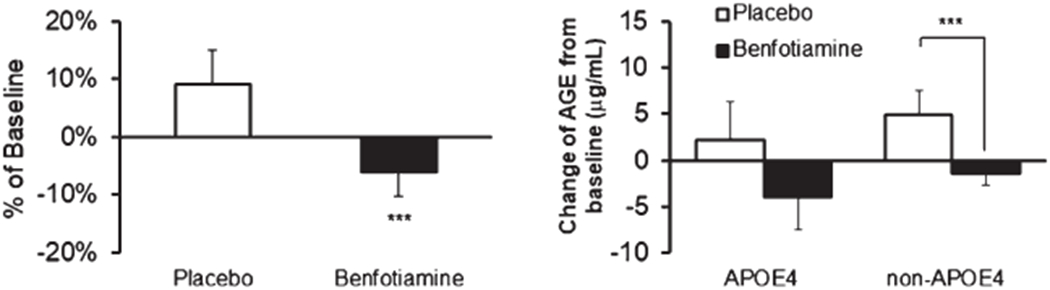
Advanced glycation end products (AGE) after benfotiamine treatment. These were done as an exploratory analysis. They were measured on serum and several samples were contaminated with RBC. In the left panel, the n’s are 12 placebo and 13 benfotiamine patients. The asterisk indicates p = 0.043. In the right panel, in the APOE ε4 group the n = 6. In the non-APOE4 group n = 7. The APOE ε4 denotes at least one ε4 allele.
The response of FDG PET to benfotiamine treatment
The comparison of regions of interest is presented in Table 3D, using the paracentral lobule and cerebellum as the reference region. No significant differences were observed between the benfotiamine and placebo populations in the pre-specified regions of interest.
Table 3D.
Comparison of the Month 12 – Baseline change in FDG PET between benfotiamine (n = 34) and placebo (n = 36)
| Total | Placebo | Benfotiamine | p | |
|---|---|---|---|---|
| Posterior cingulate | ||||
| Left | −0.03 (0.03) | −0.03 (0.04) | −0.02 (0.03) | 0.629 |
| Right | −0.02 (0.03) | −0.02 (0.03) | −0.02 (0.03) | 0.742 |
| Parietal | ||||
| Left | −0.02 (0.04) | −0.03 (0.04) | −0.02 (0.05) | 0.448 |
| Right | −0.03 (0.04) | −0.03 (0.04) | −0.02 (0.04) | 0.323 |
| Precuneus | ||||
| Left | −0.03 (0.04) | −0.02 (0.04) | −0.03 (0.04) | 0.719 |
| Right | −0.03 (0.04) | −0.03 (0.04) | −0.03 (0.04) | 0.722 |
| Medial temporal | ||||
| Left | −0.03 (0.04) | −0.03 (0.04) | −0.03 (0.04) | 0.956 |
| Right | −0.03 (0.05) | −0.03 (0.05) | −0.03 (0.05) | 0.748 |
| Frontal cortex | ||||
| Left | −0.02 (0.04) | −0.02 (0.04) | −0.03 (0.04) | 0.646 |
| Right | −0.02 (0.04) | −0.02 (0.04) | −0.03 (0.04) | 0.616 |
| Hippocampus | ||||
| Left | −0.02 (0.04) | −0.02 (0.05) | −0.01 (0.04) | 0.451 |
| Right | −0.02 (0.04) | −0.02 (0.05) | −0.02 (0.04) | 0.503 |
| Entorhinal_cortex | ||||
| Left | −0.02 (0.11) | −0.01 (0.10) | −0.02 (0.11) | 0.774 |
| Right | −0.02 (0.09) | −0.01 (0.08) | −0.02(0.10) | 0.502 |
| Prefrontal_cortex | ||||
| Left | −0.02 (0.04) | −0.02 (0.04) | −0.02 (0.04) | 0.742 |
| Right | −0.02 (0.04) | −0.02 (0.04) | −0.03 (0.04) | 0.559 |
| Whole_brain | −0.01 (0.02) | −0.02 (0.02) | −0.01 (0.02) | 0.753 |
The multivariate pattern derived through the regression against CDR correlated negatively with CDR (p = 0.001) (Fig. 10B) across all participants and time points. Robust positive loadings, i.e., with more than 97.5% of bootstrap loadings larger than zero, were found in the right precuneus, inferior parietal and mid frontal cortex: higher relative signal in these areas was associated with a better (=lower) CDR score. Robust negative loadings, i.e., with more than 97.5% of bootstrap loadings smaller than zero, were found in the bilateral paracentral lobules and bilateral cerebellum: higher relative signal at these locations was associated with a higher (=worse) CDR score. Pattern scores were higher at 12 months in treated than untreated participants (Fig. 10A). However, a difference was observed between placebo and treatment arm at baseline (T = 2.1582, p = 0.034) when APOE status was not considered. Calculation of differences with sex was complicated by differences in the rates of the two groups at baseline.
Stratification by APOE ε4 revealed that that the CDR-derived FDG PET pattern showed a treatment effect at 12 months in APOE ε4 negative population (p = 0.029) but not in APOE ε4 positive population (p = 0.314) (Fig. 10C). In the APOE positive population there was no difference between treatment groups at baseline (p = 0.164); in the APOE negative population, pattern scores were higher at trend level (p = 0.086) in the benfotiamine group.
For 59 participants who completed follow-up, the longitudinal change in pattern score (follow-up minus baseline) also correlated negatively with the accompanying change in CDR score (R = −0.446, p < 0.001). No difference in longitudinal change was observed between treated and untreated participants (p = 0.638). Additional analyses to adjust for any baseline differences and to explore other baseline heterogeneity effects or comparison patterns were deferred for subsequent evaluation.
DISCUSSION
The results show that benfotiamine administration in patients with aMCI and dementia due to AD is safe and successful in increasing peripheral thiamine levels. The trial provides preliminary evidence of efficacy of benfotiamine on cognitive and functional outcomes. In aggregate, our results provide proof of principle that justify testing the efficacy of benfotiamine in ameliorating cognitive and functional decline among participants with aMCI and dementia due to AD in a trial with a larger sample size and study duration. Measures of blood thiamine (a pharmacokinetic marker of drug delivery), FDG PET patterning (a CNS biomarker of synaptic activity) and serum AGE (a peripheral biomarker of metabolic dysregulation) provided further evidence of the effects of benfotiamine that could benefit cognition.
The results support benfotiamine’s effectiveness which was reported in a preliminary study of five patients without placebo control that was published after our trial was initiated [36]. That study found that 300 mg daily of commercial benfotiamine over 18 months improved MMSE by three points in with greater severity of dementia (i.e., MMSE of 12–25) than our patient population (MMSE >21). Levels of blood thiamine and thiamine esters were not reported in the previous study and too few patients were reported to examine sex or APOE effects.
The large increases in whole blood thiamine, ThDP and ThMP provided a robust indication that oral tablets effectively delivered the treatment. Indeed, the 161-fold increase serum thiamine was more robust than predicted, but the variation was large. The large increase in thiamine with relatively small increases in ThDP (two-fold) and ThMP (five-fold) was also reported following benfotiamine in mouse brain [30]. Appreciable differences in thiamine levels were observed by sex (two-fold) and APOE ε4 carrier status (three-fold) following treatment, but these observations need to be replicated in a larger sample.
The ability of blood thiamine or its esters to predict AD at baseline that was suggested by other trials [67–70] was not evident in our patients. Unlike previous studies which included more severe patients [68, 70], blood ThDP did not correlate significantly with MMSE (0.664), CDR (0.618) or ADAS-Cog (0.883) at baseline or following benfotiamine. Thus, the baseline studies are not supportive of a critical role of blood ThDP in AD. Our studies do support the finding that ThDP is lower in females than males [67, 70]. These results suggest that thiamine, ThDP, and ThMP should be tested in any subsequent study, and additional aspects of thiamine homeostasis such as cellular localization or ThDP effect on transketolase should be tested as well. At minimum, the blood measures provide a measure of drug delivery.
The significant correlation of MMSE and the normalized FDG PET at screening, as well as the correlation between CDR and the derived multivariate pattern, are consistent with the well-documented tight relation of glucose metabolism to AD. Several factors may have contributed to the lack of FDG PET treatment effect findings despite the large measured changes in blood thiamine and observed differences in ADAS-Cog changes. These include baseline heterogeneity in regional hypometabolism, the number of participants having both initial and post-treatment scans, use of CDR as the sole target outcome for the progression pattern, and the very small longitudinal changes that occur in FDG PET over 12 months in this mild population. Next steps include alternate a priori and data driven pattern-based analyses to further understand these relationships. As other potential considerations, the positive effects of benfotiamine/thiamine, including improved cognition, in neurodegeneration occur with minimal change in ThDP [30, 31, 33]. Thus, benfotiamine/thiamine could be acting at steps of glucose metabolism that do not change brain glucose uptake or by one of thiamine/benfotiamine’s actions not directly linked to metabolism. Thiamine also regulates activities of enzyme like malate dehydrogenase and glutamate dehydrogenase [71]. Thiamine can act as an antioxidant [13, 19, 26, 72, 73] and may act directly in cholinergic transmission [74]. Thiamine serves as an allosteric regulator of many proteins [73]. Benfotiamine and thiamin may act as Nrf2 activators [30], which would help the brain deal with many oxidative insults. Finally, benfotiamine/thiamine could be acting on endothelial cells as has been demonstrated in studies of diabetes [20, 21, 37].
The CDR, FDG PET data, and AGE response to benfotiamine suggest that AD patients without APOE ε4 were more responsive to benfotiamine in this study population. The diminished response did not seem to be a difference in drug availability since blood thiamine (+46%), and its esters were all higher in patients with APOE ε4 following benfotiamine (not statistically significant). Patients with APOE ε4 may have a more severe form of the disease since they have more plaques and they occur earlier [75–77]. APOE ε4 carriers have higher levels of the glyoxal, fluorescent AGEs, Nε-carboxymethyllysine, and the receptor for AGE (sRAGE) (p = 0.018) when compared to non-carriers [78].
The role of AGE in AD as a biomarker and progression of disease is not well developed. Recent studies demonstrate that the development of AGE parallels the development of the cognitive deficit [11]. The AGE pentosidine is an indicator of AD [79]. Methylglyoxal and glyoxal levels in serum are higher in MCI patients. Methylglyoxal in serum distinguishes MCI from controls but not from AD. Meanwhile, serum glyoxal levels differentiate MCI from control and AD groups [35]. The levels of carboxymethylysine in serum correlate negatively with the clinical cognitive as measured by MMSE [34]. AGE increase in healthy APOE ε4 and this may provide a link between APOE ε4 and AGE and our responses [78]. Both sex and APOE status alter the AD serum metabolome [80]. In animals, even mild thiamine deficiency leads to increases in AGE [81]. Increased AGE are common in diabetes, which predisposes to the development of AD, and there are many intriguing overlaps between diabetes and AD [18]. Benfotiamine prevents the micro and macro vascular damage in diabetes related to AGE [20, 37, 82]. The mechanisms for the protection have been studied extensively [83].
Our study has several limitations. Our sample size, while appropriate for a pilot study, was relatively small and of short duration, which particularly affected our subgroup analyses. Some significant findings in the secondary endpoints, subgroup analyses and multiple PET-related scores could be due to chance in the context of multiple comparisons without p-value correction. However, we believe that this approach is appropriate in the setting of a pilot study and inform the proposal of a larger confirmatory clinical trial. It is also important to point out that the observed effects for primary and secondary outcomes were consistently in the direction of benefit for benfotiamine. Another potential limitation is our definition of AD. Our study participants had aMCI and dementia that met the criterion for the Alzheimer’s continuum in the NIA/AA research framework [38], which we ascertained through amyloid positivity on PET scans. However, we cannot say with certainty that amyloid was the primary pathology causing cognitive impairment, as other pathologies that we did not ascertain could have caused the cognitive impairment. Lastly, the lack of ethnic and racial diversity is also of concern, and a larger trial must aim to recruit a sample with representation of all ethnic and racial groups.
In summary, benfotiamine is safe and cost effective, and the results of this pilot study are encouraging, providing preliminary evidence of efficacy. Our next step is to propose a larger clinical trial appropriately powered to replicate our findings. We believe that further studies would be very valuable to determine whether benfotiamine may be helpful in delaying onset or treating AD.
ACKNOWLEDGMENTS
We thank the patients and caregivers for their time and commitment. We also thank the following: Alzheimer Drug Discovery Foundation, NIA R01AG043679 (GEG), Burke Rehabilitation Hospital, Burke Neurological Institute, NIA R56AG062 305 (SAF). NIH K24AG045334 (JAL) for funding, Advanced Orthomolecular Medicine for providing the benfotiamine (Dr. Traj Nibber), Dawn C. Matthews, M.S., M.B.A., ADM Diagnostics, Northbrook, IL, USA for help interpreting the imaging, Dr. Michael Reding, Dr. Mary Beth Walsh and Marina Harmon (Burke Rehabilitation Hospital) for their help. Sarah Mink and Tiffany Moy of the Burke Neurological Institute for their help with patient care.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Sonia Sequeir from this center was critical in preparing the IND. Lucien Bettendorff is Research Director of the Fund for Scientific Research (FNRS, Belgium).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0896r1).
REFERENCES
- [1].Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR, Hornbeck RC, Paumier KL, Ances BM, Berman SB, Brickman AM, Cash DM, Chhatwal JP, Correia S, Förster S, Fox NC, Graff-Radford NR, la Fougère C, Levin J, Masters CL, Rossor MN, Salloway S, Saykin AJ, Schofield PR, Thompson PM, Weiner MM, Holtzman DM, Raichle ME, Morris JC, Bateman RJ, Benzinger TLS (2018) Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol 17, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ (2009) FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med 36, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT (2020) Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med 26, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gibson GE, Sheu K-FR, Blass JP, Baker A, Carlson KC, Harding B, Perrino P (1988) Reduced Activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol 45, 836–840. [DOI] [PubMed] [Google Scholar]
- [6].Gibson GE, Haroutunian V, Zhang H, Park LCH, Shi Q, Lesser M, Mohs RC, Sheu RKF, Blass JP (2000) Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol 48, 297–303. [PubMed] [Google Scholar]
- [7].Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE (2005) Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann Neurol 57, 695–703. [DOI] [PubMed] [Google Scholar]
- [8].Karnppagounder S, Gibson GE (2009) Thiamine deficiency: A model of metabolic encephalopathy and of selective neuronal vulnerability In Metabolic Encephalopathy, McCandless DW, ed. Springer New York, New York, NY, pp. 235–260. [Google Scholar]
- [9].Calingasan NY, Uchida K,Gibson GE (1999)Protein-bound acrolein. J Neurochem 72, 751–756. [DOI] [PubMed] [Google Scholar]
- [10].Derk J, MacLean M, Juranek J, Schmidt AM (2018) The receptor for advanced glycation endproducts (RAGE) and mediation of inflammatory neurodegeneration. J Alzheimers Dis Parkinsonism 8, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chou P-S, Wu M-N, Yang C-C, Shen C-T, Yang Y-H (2019) Effect of advanced glycation end products on the progression of Alzheimer’s disease. J Alzheimers Dis 72, 191–197. [DOI] [PubMed] [Google Scholar]
- [12].Ataç ZS, Alaylioğlu M, Dursun E, Gezen-Ak D, Yılmazer S, Gürvit H (2019) G82S polymorphism of receptor for advanced glycation end products gene and serum soluble RAGE levels in mild cognitive impairment and dementia of Alzheimer’s type patients in Turkish population. J Clin Neurosci 59, 197–201. [DOI] [PubMed] [Google Scholar]
- [13].Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A (2010) The multifaceted therapeutic potential of benfotiamine. Pharmacol Res 61, 482–488. [DOI] [PubMed] [Google Scholar]
- [14].Raj V, Ojha S, Howarth FC, Belfur PD, Subramanya SB (2018) Therapeutic potential of benfotiamine and its molecular targets. Eur Rev Medl Pharmacol Sci 22, 3261–3273. [DOI] [PubMed] [Google Scholar]
- [15].Pekovich SR, Martin PR, Singleton CK (1998) Thiamine deficiency decreases steady-state transketolase and pyruvate dehydrogenase but not α-ketoglutarate dehydrogenase mRNA levels in three human cell types. J Nutr 128,683–687. [DOI] [PubMed] [Google Scholar]
- [16].Ahmed N (2005) Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin 67, 3–21. [DOI] [PubMed] [Google Scholar]
- [17].Peyroux J, Sternberg M (2006) Advanced glycation end-products (AGEs): Pharmacological inhibition in diabetes. Pathol Biol 54, 405–419. [DOI] [PubMed] [Google Scholar]
- [18].Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J (2013) Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Mol Cell Neurosci 55, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gibson GE, Hirsch JA, Fonzetti P, Jordan BD, Cirio RT, Elder J (2016) Vitamin B1 (thiamine) and dementia. Ann NY Acad Sci 1367,21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG (2008) Benfotiamine in diabetic polyneuropathy (BENDIP): Results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes 116, 600–605. [DOI] [PubMed] [Google Scholar]
- [21].Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW (2007) High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 50, 2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Suzuki K, Yamada K, Fukuhara Y, Tsuji A, Shibata K, Wakamatsu N (2017) High-dose thiamine prevents brain lesions and prolongs survival of Slc19a3-deficient mice. PLoS One 12, e0180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].El-Hattab AW, Zarante AM, Almannai M, Scaglia F (2017) Therapies for mitochondrial diseases and current clinical trials. Mol Genet Metab 122, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schmid U, Stopper H, Heidland A, Schupp N (2008) Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev 24, 371–377. [DOI] [PubMed] [Google Scholar]
- [25].Gorlova A, Pavlov D, Anthony DC, Ponomarev ED, Sambon M, Proshin A, Shafarevich I, Babaevskaya D, Lesch K-P, Bettendorff L, Strekalova T (2019) Thiamine and benfotiamine counteract ultrasound-induced aggression, normalize AMPA receptor expression and plasticity markers, and reduce oxidative stress in mice. Neuropharmacology 156, 107543. [DOI] [PubMed] [Google Scholar]
- [26].Sambon M, Napp A, Demelenne A, Vignisse J, Wins P, Fillet M, Bettendorff L (2019) Thiamine and benfotiamine protect neuroblastoma cells against paraquat and β-amyloid toxicity by a coenzyme-independent mechanism. Heliyon 5, e01710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, Park L, Vinarcsik LK, Ivasyk I, Rivera DA, Kang Y, Cortes-Canteli M, Peyrounette M, Doyeux V, Smith A, Zhou J, Otte G, Beverly JD, Davenport E, Davit Y, Lin CP, Strickland S, Iadecola C, Lorthois S, Nishimura N, Schaffer CB (2019) Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 22, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shoeb M, Ramana KV (2012) Anti-inflammatory effects of benfotiamine are mediated through the regulation of the arachidonic acid pathway in macrophages. Free Radic Biol Med 52, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bozic I, Savic D, Laketa D, Bjelobaba I, Milenkovic I, Pekovic S, Nedeljkovic N, Lavrnja I (2015) Benfotiamine attenuates inflammatory response in LPS stimulated BV-2 microglia. PLoS One 10, e0118372–e0118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tapias V, Jainuddin S, Ahuja M, Stack C, Elipenahli C, Vignisse J, Gerges M, Starkova N, Xu H, Starkov AA, Bettendorff L, Hushpulian DM, Smirnova NA, Gazaryan IG, Kaidery NA, Wakade S, Calingasan NY, Thomas B, Gibson GE, Dumont M, Beal MF (2018) Benfotiamine treatment activates the Nrf2/ARE pathway and is neuroprotective in a transgenic mouse model of tauopathy. Hum Mol Genet 27, 2874–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, Sun X, Zhao L, Yu M, Xu Z, Dong W, Qin Y, Fei G, Zhong C, Xu TL (2010) Powerful beneficial effects of benfotiamine on cognitive impairment and β-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain 133, 1342–1351. [DOI] [PubMed] [Google Scholar]
- [32].Markova N, Bazhenova N, Anthony DC, Vignisse J, Svistunov A, Lesch K-P, Bettendorff L, Strekalova T (2017) Thiamine and benfotiamine improve cognition and ameliorate GSK-3β-associated stress-induced behaviours in mice. Prog Neuropsychopharmacol 75, 148–156. [DOI] [PubMed] [Google Scholar]
- [33].Vignisse J, Sambon M, Gorlova A, Pavlov D, Caron N, Malgrange B, Shevtsova E, Svistunov A, Anthony DC, Markova N, Bazhenova N, Coumans B, Lakaye B, Wins P, Strekalova T, Bettendorff L (2017) Thiamine and benfotiamine prevent stress-induced suppression of hippocampal neurogenesis in mice exposed to predation without affecting brain thiamine diphosphate levels. Mol Cell Neurosci 82, 126–136. [DOI] [PubMed] [Google Scholar]
- [34].Haddad M, Perrotte M, Landri S, Lepage A, Fiilop T, Ramassamy C (2019) Circulating and extracellular vesicles levels of N-(1-Carboxymethyl)-L-Lysine (CML) differentiate early to moderate Alzheimer’s disease. J Alzheimers Dis 69, 751–762. [DOI] [PubMed] [Google Scholar]
- [35].Haddad M, Perrotte M, Khedher MRB, Demongin C, Lepage A, Fülöp T, Ramassamy C (2019) Methylglyoxal and glyoxal as potential peripheral markers for MCI diagnosis and their effects on the expression of neurotrophic, inflammatory and neurodegenerative factors in neurons and in neuronal derived-extracellular vesicles. Int J Mol Sci 20, 4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pan X, Chen Z, Fei G, Pan S, Bao W, Ren S, Guan Y, Zhong C (2016) Long-term cognitive improvement after benfotiamine administration in patients with Alzheimer’s disease. Neurosi Bull 32, 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D (2006) Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care 29, 2064–2071. [DOI] [PubMed] [Google Scholar]
- [38].Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rockwood K, Fay S, Gorman M, Carver D, Graham JE (2007) The clinical meaningfulness of ADAS-Cog changes in Alzheimer’s disease patients treated with donepezil in an open-label trial. BMC Neurol 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C (2000) A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology 54, 2269. [DOI] [PubMed] [Google Scholar]
- [41].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P, Grisar T, Bettendorff L (2010) Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One 5, e13616–e13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, Grundman M, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Clark CM, Joshi AD, Mintun MA, Skovronsky DM, Pontecorvo MJ (2012) Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline. Neurol 79, 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Balsis S, Unger AA, Benge JF, Geraci L, Doody RS (2012) Gaining precision on the Alzheimer’s Disease Assessment Scale-cognitive: A comparison of item response theory-based scores and total scores. Alzheimers Dement 8, 288–294. [DOI] [PubMed] [Google Scholar]
- [46].Weyer G, Erzigkeit H, Kanowski S, Ihl R, Hadler D (1997) Alzheimer’s Disease Assessment Scale: Reliability and validity in a multicenter clinical trial. Int Psychogeriatr 9, 123–138. [DOI] [PubMed] [Google Scholar]
- [47].Hughes CP, Berg L, Danziger W, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140, 566–572. [DOI] [PubMed] [Google Scholar]
- [48].Buschke H, Fuld PA (1974) Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24, 1019. [DOI] [PubMed] [Google Scholar]
- [49].Fuld PA, Buschke H (1976) Stages of retrieval in verbal learning. J Verb Learning Verb Behav 15, 401–410. [Google Scholar]
- [50].Sliwinski M, Buschke H, Stewart WF, Masur D, Lipton RB (1997) The effect of dementia risk factors on comparative and diagnostic selective reminding norms. J Intl Neuropsychol Soc 3, 317–326. [PubMed] [Google Scholar]
- [51].Cummings JL (1997) The Neuropsychiatric Inventory. Neurology 48, 10S. [DOI] [PubMed] [Google Scholar]
- [52].Cummings J (2020) The Neuropsychiatric Inventory: Development and applications. J Geriatr Psychiatry Neurol 33, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cummings JL, McPherson S (2001) Neuropsychiatric assessment of Alzheimer’s disease and related dementias. Aging Clin Exp Res 13, 240–246. [DOI] [PubMed] [Google Scholar]
- [54].Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S (1997) An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis Assoc Disord 11, S33–S39. [PubMed] [Google Scholar]
- [55].Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD, Alzheimer’s Disease Cooperative Study (2006) ADCS Prevention Instrument Project: Assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord 20, S152–S169. [DOI] [PubMed] [Google Scholar]
- [56].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- [57].Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA (1987) Scaled subprofile model: A statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab 7, 649–658. [DOI] [PubMed] [Google Scholar]
- [58].Strother SC, Anderson JR, Schaper KA, Sidtis JJ, Liow JS, Woods RP, Rottenberg DA (1995) Principal component analysis and the scaled subprofile model compared to intersubject averaging and statistical parametric mapping: I. “Functional connectivity” of the human motor system studied with [15O]water PET. J Cereb Blood Flow Metab 15, 738–753. [DOI] [PubMed] [Google Scholar]
- [59].Habeck C, Foster NL, Perneczky R, Kurz A, Alexopoulos P, Koeppe RA, Drzezga A, Stern Y (2008) Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. Neuroimage 40, 1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Habeck C, Stern Y, Alzheimer’s Disease Neuroimaging Initiative (2010) Multivariate data analysis for neuroimaging data: Overview and application to Alzheimer’s disease. Cell Biochem Biophys 58, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Habeck CG (2010) Basics of multivariate analysis in neuroimaging data. J Vis Exp, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Burnham KP, Anderson DR, Burnham KP (2002) Model selection and multimodel inference a practical information-theoretic approach. Springer, New York. [Google Scholar]
- [63].Efron B (1982) The jackknife, the bootstrap, and other resampling plans. Society for Industrial and Applied Mathematics, Philadelphia. [Google Scholar]
- [64].Efron B, Tibshirani R (1993) An introduction to the bootstrap, Chapman & Hall, New York. [Google Scholar]
- [65].Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, Hager K, Andreasen N, Scarpini E, Liu-Seifert H, Case M, Dean RA, Hake A, Sundell K, Poole Hoffmann V, Carlson C, Khanna R, Mintun M, DeMattos R, Selzler KJ, Siemers E (2018) Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 378, 321–330. [DOI] [PubMed] [Google Scholar]
- [66].Molnar FJ, Hutton B, Fergusson D (2008) Does analysis using “last observation carried forward” introduce bias in dementia research? Can Med Assoc J 179, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pan X, Fei G, Lu J, Jin L, Pan S, Chen Z, Wang C, Sang S, Liu H, Hu W, Zhang H, Wang H, Wang Z, Tan Q, Qin Y, Zhang Q, Xie X, Ji Y, Cui D, Gu X, Xu J, Yu Y, Zhong C (2016) Measurement of blood thiamine metabolites for Alzheimer’s disease diagnosis. EBioMedicine 3, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Molina JA, Jiménez-Jiménez FJ, Hernánz A, Fernández-Vivancos E, Medina S, de Bustos F, Gómez-Escalonilla C, Sayed Y (2002) Cerebrospinal fluid levels of thiamine in patients with Alzheimer’s disease. J Neural Transm 109, 1035–1044. [DOI] [PubMed] [Google Scholar]
- [69].Glasø M, Nordbø G, Diep L, Bøhmer T (2004) Reduced concentrations of several vitamins in normal weight patients with late-onset dementia of the Alzheimer type without vascular disease. J Nutr Health Aging 8, 407–413. [PubMed] [Google Scholar]
- [70].Sang S, Pan X, Chen Z, Zeng F, Pan S, Liu H, Jin L, Fei G, Wang C, Ren S, Jiao F, Bao W, Zhou W, Guan Y, Zhang Y, Shi H, Wang Y, Yu X, Wang Y, Zhong C (2018) Thiamine diphosphate reduction strongly correlates with brain glucose hypometabolism in Alzheimer’s disease, whereas amyloid deposition does not. Alzheimers Res Ther 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mezhenska OA, Aleshin VA, Kaehne T, Artiukhov AV, Bunik VI (2020) Regulation of malate dehydrogenases and glutamate dehydrogenase of mammalian brain by thiamine in vitro and in vivo. Biochemistry (Mosc) 85, 27–39. [DOI] [PubMed] [Google Scholar]
- [72].Gibson GE, Blass JP (2007) Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxid Redox Signal 9, 605–1620. [DOI] [PubMed] [Google Scholar]
- [73].Mkrtchyan G, Aleshin V, Parkhomenko Y, Kaehne T, Luigi Di Salvo M, Parroni A, Contestabile R, Vovk A, Bettendorff L, Bunik V (2015) Molecular mechanisms of the non-coenzyme action of thiamin in brain: Biochemical, structural and pathway analysis. Sci Rep 5, 12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Von Muralt A (1958) The role of thiamine (vitamin B1) in nervous excitation. Exp Cell Res 14, 72–79. [PubMed] [Google Scholar]
- [75].Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, Visser PJ, and the Amyloid Biomarker Study G (2015) Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 313, 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 90, 9649–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rebeck GW, Reiter JS, Strickland DK, Hyman BT (1993) Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 11, 575–580. [DOI] [PubMed] [Google Scholar]
- [78].Deo P, Dhillon VS, Chua A, Thomas P, Fenech M (2019) APOE ε4 carriers have a greater propensity to glycation and sRAGE which is further influenced by RAGE G82S polymorphism. J Gerontol A Biol 75, 1899–1905. [DOI] [PubMed] [Google Scholar]
- [79].Meli M, Perier C, Ferron C, Parssegny F, Denis C, Gonthier R, Laurent B, Reynaud E, Frey J, Chamson A (2002) Serum pentosidine as an indicator of Alzheimer’s disease. J Alzheimers Dis 4, 93–96. [DOI] [PubMed] [Google Scholar]
- [80].Arnold M, Nho K, Kueider-Paisley A, Massaro T, Huynh K, Brauner B, MahmoudianDehkordi S, Louie G, Moseley MA, Thompson JW, John-Williams LS, Tenenbaum JD, Blach C, Chang R, Brinton RD, Baillie R, Han X, Trojanowski JQ, Shaw LM, Martins R, Weiner MW, Trushina E, Toledo JB, Meikle PJ, Bennett DA, Krumsiek J, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R, Kastenmiiller G (2020) Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat Commun 11, 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Depeint F, Shangari N, Furrer R, Bruce WR, O’Brien PJ (2007) Marginal thiamine deficiency increases oxidative markers in the plasma and selected tissues in F344 rats. Nutr Res 27, 698–704. [Google Scholar]
- [82].Goldin A, Beckman Joshua A, Schmidt Ann M, Creager Mark A (2006) Advanced glycation end products. Circulation 114, 597–605. [DOI] [PubMed] [Google Scholar]
- [83].Hammes H-P, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9, 294–299. [DOI] [PubMed] [Google Scholar]


