Abstract
Background
The World Health Organization recommends screening for the cryptococcal antigen (CrAg), a predictor of cryptococcal meningitis, among antiretroviral therapy (ART)–naïve people with HIV (PWH) with CD4 <100 cells/mm3. CrAg positivity among ART-experienced PWH with viral load (VL) nonsuppression is not well established, yet high VLs are associated with cryptococcal meningitis independent of CD4 count. We compared the frequency and positivity yield of CrAg screening among ART-experienced PWH with VL nonsuppression and ART-naïve PWH with CD4 <100 cells/mm3 attending rural public health facilities in Uganda.
Methods
We reviewed routinely generated programmatic reports on cryptococcal disease screening from 104 health facilities in 8 rural districts of Uganda from January 2018 to July 2019. A lateral flow assay (IMMY CrAg) was used to screen for cryptococcal disease. PWH were eligible for CrAg screening if they were ART-naïve with CD4 <100 cell/mm3 or ART-experienced with an HIV VL >1000 copies/mL after at least 6 months of ART. We used Pearson’s chi-square test to compare the frequency and yield of CrAg screening.
Results
Of 71 860 ART-experienced PWH, 7210 (10.0%) were eligible for CrAg screening. Among 15 417 ART-naïve PWH, 5719 (37.1%) had a CD4 count measurement, of whom 937 (16.4%) were eligible for CrAg screening. The frequency of CrAg screening was 11.5% (830/7210) among eligible ART-experienced PWH compared with 95.1% (891/937) of eligible ART- naïve PWH (P < .001). The CrAg positivity yield was 10.5% among eligible ART-experienced PWH compared with 13.8% among eligible ART-naïve PWH (P = .035).
Conclusions
The low frequency and high positivity yield of CrAg screening among ART-experienced PWH with VL nonsuppression suggest a need for VL- directed CrAg screening in this population. Studies are needed to evaluate the cost-effectiveness and impact of CrAg screening and fluconazole prophylaxis on the outcomes of ART-experienced PWH with VL nonsuppression.
Keywords: ART-experienced, viral load, nonsuppression, HIV, CrAg, cryptococcal meningitis
Cryptococcal meningitis accounts for 15% of HIV-related deaths globally [1]. In Sub-Saharan Africa, mortality from HIV-associated cryptococcal meningitis is reported to be 44%, although most of the data are among antiretroviral therapy (ART)–naïve individuals [2]. Among ART-naïve people with HIV (PWH), a positive serum cryptococcal antigen (CrAg) predicts development of cryptococcal meningitis and meningitis-related death, which are preventable by preemptive treatment with fluconazole [3, 4]. Modeling studies show that programmatic CrAg screening is cost-effective and averts 40%–43% of would-be deaths from cryptococcal meningitis in advanced HIV disease among ART-naïve PWH [5, 6]. PWH with a CD4 count <100 cells/mm3 have a higher yield of CrAg positivity compared with those with higher CD4 counts owing to impaired cell-mediated immune responses against Cryptococcus spp. [7, 8]. However, CrAg positivity appears to predict cryptococcal meningitis and death even at a cutoff of <200 cells/mm3 among ART-naïve PWH [9]. The World Health Organization (WHO) recommends CrAg screening among newly diagnosed PWH with a CD4 count <100 cells/mm3, and conditionally among those with a CD4 count of 100–200 cells/mm3 [10].
While the prevalence of CrAg positivity among PWH, regardless of CD4 count and ART status, ranges from 1.7% to 33% in low-resource settings [11], the prevalence of CrAg positivity in a homogenous population of ART-experienced PWH is not well established. The prevalence among ART-experienced PWH with low CD4 counts has been reported to be 8.5%–10% in Nigeria [12, 13] and 4.1%–14% in Ethiopia [14, 15]. There is little evidence to support CrAg screening among PWH with VL nonsuppression regardless of the CD4 count. Using reflex laboratory-based testing, Mpoza et al. report a CrAg positivity yield of 3% among leftover plasma samples of PWH with VL nonsuppression (VL >1000 copies/mL) regardless of CD4 count [16]. The CrAg positivity yield among ART-experienced PWH with VL nonsuppression in programmatic settings is not well documented.
Local guidelines in Uganda for CrAg screening among PWH have previously recommended CrAg screening among ART-experienced PWH with VL nonsuppression [17, 18]. However, the frequency of CrAg screening is historically low even among ART-naïve PWH. Only 19% of ART-naïve PWH with CD4 <100 cells/mm3 were screened for CrAg positivity in 2017 [5]. The frequency of CrAg screening among ART-experienced PWH with VL nonsuppression has not been established in programmatic settings.
The objective of this study was to compare the frequency and positivity yield of CrAg screening among ART-experienced PWH with VL nonsuppression and ART-naïve PWH with CD4 <100 cells/mm3 receiving HIV care at rural public health facilities in Uganda. The purpose was to provide preliminary evidence for the need, if any, of VL-directed programmatic CrAg screening among PWH with VL nonsuppression.
METHODS
Study Setting and Population
We reviewed routinely generated programmatic reports on cryptococcal disease screening from 104 health facilities in 8 rural districts of Uganda. The districts were Kassanda, Kiboga, Kyankwanzi, Luwero, Mityana, Mubende, Nakaseke, and Nakasongola, located in the central region of Uganda. The 104 public health facilities are supported by Mildmay Uganda, a large HIV care nongovernmental organization, with funding from the US President’s Emergency Plan for AIDS Relief (PEPFAR), through the US Centers for Disease Control and Prevention (CDC), to implement the country’s operational plan for accelerating HIV epidemic control. The public health facilities consisted of 1 regional referral hospital, 4 district hospitals, 16 health center IVs, 79 health center IIIs, and 4 health center IIs. Health facilities are graded according to infrastructural size, size of population served, and human resources expertise, with referral hospitals as tertiary care facilities, while health center IIs are the lowest unit of care. As such, hospitals have consultant physicians and offer specialized care, health center IVs provide inpatient, outpatient, laboratory, and theater services, health center IIIs offer limited inpatient, outpatient, and laboratory services, and health center IIs are outpatient units that manage mild cases and offer antenatal care [20]. Programmatic reports on cryptococcal disease screening are generated and submitted by health workers at the facilities on a quarterly basis. A consolidated report on AHD is submitted by Mildmay Uganda to the CDC. In this analysis, the inclusion criteria were all registered ART-naïve or ART-experienced PWH with VL nonsuppression receiving routine HIV care from the supported public health facilities that were reported on in the quarterly reports spanning from January 2018 to July 2019.
Study Measurements
Data were extracted from AHD quarterly reports from the health facilities. All newly diagnosed ART-naïve PWH were recommended to have a CD4 count measurement and sequential CrAg screening for those with CD4 <100 cells/mm3 at ART initiation [10]. We defined eligibility for CrAg screening as all PWH who were either ART-naïve with a CD4 <100 cells/mm3 or ART-experienced PWH with VL nonsuppression. VL nonsuppression was defined as having a VL of >1000 copies/mL after at least 6 months of ART [18]. Across public health facilities in Uganda, the CrAg test is performed on plasma, serum, or finger prick blood using a lateral flow assay (IMMY CrAg) as a point-of-care test at the health facility [18]. Health center IIs (and other facilities without a CD4 machine) refer samples of PWH to a higher-level facility with a CD4 machine. Thereafter, CrAg testing is performed on leftover samples at CD4 testing sites if CD4 <100 cells/mm3, but the patient’s result is reported under the referring facility. As of July 2019, there were 37 facilities in the region with a CD4 machine. Routine VL monitoring is centrally performed by the Uganda National Health Laboratory Services on dry blood spot or plasma samples that are delivered through a hub system across the country [21]. It is these routine VL monitoring results that are used to determine VL nonsuppression at 6 months of ART. After receiving VL results from the national central laboratory, clinicians at the health facilities in this region performed CrAg testing on ART-experienced PWH with VL nonsuppression on the next client’s routine clinic visit or proactively followed up with them via a phone call and scheduled a nonroutine clinic visit. In ART-experienced PWH with VL nonsuppression, CrAg testing was performed regardless of the CD4 count or symptoms of AHD in the period under evaluation. However, the national guidelines recommended CrAg testing among ART-experienced PWH with VL nonsuppression between 2016 and September 2018 [18]. In the September 2018 guidelines [19], VL-directed CrAg screening was not explicitly recommended, although this approach was practiced in the region. In May 2019, the AHD management toolkit [17] recommended CrAg screening among ART-experienced PWH with VL nonsuppression if they had symptoms of AHD or CD4 <200 cells/mm3 (if available). Programmatic AHD reports routinely indicate the proportions of ART-naïve PWH for whom a baseline CD4 count was performed, ART-experienced PWH with VL nonsuppression, eligible PWH for whom a CrAg test was performed, and the positivity yield of the CrAg screening. All patients reported upon were, reportedly, being screened for CrAg for the first time.
Data Analysis
Aggregated data from narrative reports were entered in Microsoft Excel and analyzed in Stata 14 (StataCorp, College Station, TX, USA). All data were categorical and were summarized as frequencies. We compared proportions across study variables using the Pearson chi-square test or Fisher exact test. The study variables were district name, level of health facility, sex, and year of CrAg screening eligibility. A P value <.05 was considered statistically significant at the 95% confidence level. The frequency of CrAg screening was calculated as the proportion of eligible PWH who received a CrAg test to the total number of PWH who were eligible for the test. The CrAg positivity yield was calculated as the proportion of eligible PWH who were reported to have a positive CrAg test to the total number of eligible individuals who received a CrAg test.
Patient Consent Statement
The study protocol was approved by the Mildmay Uganda research and ethics committee (REC REF 0804-2018). The project was also reviewed in accordance with the CDC human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. We used aggregated data from publicly available reports. We thus did not seek informed consent because there was no information that could identify individual PWH.
RESULTS
Characteristics of PWH Eligible for CrAg Screening
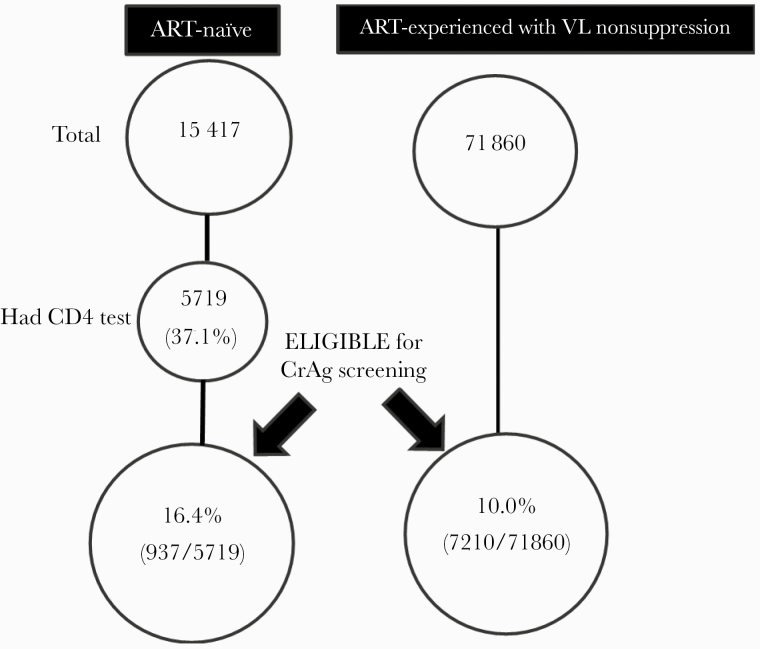
Figure 1 shows the patient flowchart. From January 2018 to July 2019, 15 417 individuals were newly diagnosed with HIV. However, of these, only 5719 (37.1%) had a CD4 test measurement. Among those for whom a CD4 test was performed, 937 (16.4%) had a CD4 <100 cell/mm3 and were thus eligible for CrAg screening. During the same period, there were 71 860 ART-experienced PWH, of whom 7210 (10.0%) had VL nonsuppression and were eligible for CrAg screening. Data regarding the sex of eligible PWH were available for 3077, of whom 1747 (56.8%) were female. While the proportion of eligible ART-experienced PWH was evenly distributed across health center IIs, IIIs, IVs, and hospitals, eligible ART-naïve PWH were mostly at health center IVs (60.3%; P < .001). Characteristics of PWH who were eligible for CrAg screening are shown in Table 1.
Figure 1.
Study flow diagram. Abbreviations: ART, antiretroviral therapy; CrAg, cryptococcal antigen; PWH, people with HIV; VL, viral load.
Table 1.
Characteristics of PWH Eligible for Cryptococcal Antigen Screening
| ART-Naïve (N = 937) | ART-Experienced (n = 7210) | Total (n = 8147) | ||
|---|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) | P Valueb |
| Sex (n = 3077) | .213 | |||
| Female | 209 (59.9) | 1538 (56.4) | 1747 (56.8) | |
| Male | 140 (40.1) | 1190 (43.6) | 1330 (43.2) | |
| Level of health facility | ||||
| Health center II | 0 (0.0) | 33 (0.5) | 33 (0.4) | <.001a |
| Health center III | 245 (26.1) | 2336 (32.4) | 2581 (31.7) | |
| Health center IV | 565 (60.3) | 2505 (34.7) | 3070 (37.7) | |
| Hospital | 127 (13.6) | 2336 (32.4) | 2463 (30.2) | |
| District | ||||
| Kassanda | 91 (9.7) | 658 (9.1) | 749 (9.2) | <.001a |
| Kiboga | 107 (11.4) | 617 (8.6) | 724 (8.9) | |
| Kyankwanzi | 32 (3.4) | 342 (4.7) | 374 (4.6) | |
| Luwero | 351 (37.5) | 1467 (20.3) | 1,818 (22.3) | |
| Mityana | 129 (13.8) | 1401 (19.4) | 1530 (18.8) | |
| Mubende | 59 (6.3) | 1749 (24.3) | 1808 (22.2) | |
| Nakaseke | 92 (9.8) | 598 (8.3) | 690 (8.5) | |
| Nakasongola | 76 (8.1) | 378 (5.2) | 454 (5.6) | |
| Year | ||||
| 2018 | 532 (56.8) | 3976 (55.1) | 4508 (55.3) | .893 |
| 2019 | 405 (43.2) | 3234 (44.9) | 3639 (44.7) |
Abbreviations: ART, antiretroviral therapy; PWH, people with HIV.
aStatistically significant result.
b P value from chi-square test.
Frequency of CrAg Screening Among Eligible PWH
Of 8147 eligible PWH (both ART-experienced and ART-naïve), only 1721 (21.1%) were screened for CrAg. A total of 830 (11.5%) of the 7210 eligible ART-experienced PWH were screened for CrAg compared with 891 (95.1%) of the 937 eligible ART-naïve PWH (P < .001). The frequency of screening significantly differed across health facility levels and districts. The highest frequency of screening was reported at health center IVs (52.2%) and in Luweero district (31.3%; P < .001). There was no CrAg screening at health center IIs. Table 2 compares the proportions of eligible PWH who were screened with a CrAg test with those who were not screened.
Table 2.
Comparison of Eligible PWH Screened With Crag With Those Not Screened
| Screened (n = 1721) | Not Screened (n = 6426) | ||
|---|---|---|---|
| Characteristic | No. (%) | No. (%) | P Valueb |
| Sex (n = 3077) | |||
| Female | 380 (56.8) | 1367 (56.8) | .988 |
| Male | 289 (43.2) | 1041 (43.2) | |
| Level of health facility | |||
| Health center II | 0 (0.0) | 33 (0.5) | <.001a |
| Health center III | 327 (19.0) | 2254 (35.1) | |
| Health center IV | 899 (52.2) | 2171 (33.8) | |
| Hospital | 495 (28.8) | 1968 (30.6) | |
| District | |||
| Kassanda | 125 (7.3) | 624 (9.7) | <.001a |
| Kiboga | 184 (10.7) | 540 (8.4) | |
| Kyankwanzi | 46 (2.7 | 328 (5.1) | |
| Luwero | 538 (31.3) | 1280 (19.9) | |
| Mityana | 394 (22.9) | 1136 (17.7) | |
| Mubende | 208 (12.1) | 1600 (24.9) | |
| Nakaseke | 153 (8.9) | 537 (8.4) | |
| Nakasongola | 73 (4.2) | 381 (5.9) | |
| Year | |||
| 2018 | 967 (56.2) | 3541 (55.1) | .422 |
| 2019 | 754 (43.8) | 2885 (44.9) | |
| Eligibility criteria | |||
| ART-naïve | 891 (51.8) | 46 (0.7) | <.001a |
| ART-experienced | 830 (48.2) | 6380 (99.3) |
Abbreviations: ART, antiretroviral therapy; PWH, people with HIV.
aStatistically significant result.
b P value from chi-square test.
Yield of CrAg Positivity Among Eligible PWH
Of the 1721 eligible PWH who were tested, 210 (12.2%) had a positive CrAg. The CrAg positivity yield was 10.5% (87/830) among eligible ART-experienced compared with 13.8% (123/891) among eligible ART-naïve PWH (P = .035). CrAg positivity among eligible ART-naïve and ART-experienced PWH significantly varied across health facility levels and districts. While most CrAg-positive ART-naïve PWH were reported from health center IVs (67.5%), CrAg-positive ART-experienced individuals were mostly from hospitals (65.5%; P < .001). Most CrAg-positive ART-experienced PWH were from Mityana (32.2%), while ART-naïve individuals were mostly from Luweero district (42.3%; P < .001). Table 3 compares CrAg-positive ART-naïve with ART-experienced PWH. There was no difference by sex, level of facility, district, or year in the CrAg positivity yield among all PWH who were screened (Table 4).
Table 3.
Comparison of CrAg-Positive ART-Naïve and ART-Experienced PWH
| ` | CrAg-Positive ART-Naïve (N = 123) | CrAg-Positive ART-Experienced (n = 87) | Total (n = 210) | |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | P Valueb | |
| Sex (n = 85) | ||||
| Female | 26 (55.3) | 25 (65.8) | 51 (60.0) | .327 |
| Male | 21 (44.7) | 13 (34.2) | 34 (40.0) | |
| Level of health facility | ||||
| Health center III | 24 (19.5) | 10 (11.5) | 34 (16.2) | <.001a |
| Health center IV | 83 (67.5) | 20 (23.0) | 103 (49.0) | |
| Hospital | 16 (13.0) | 57 (65.5) | 73 (34.8) | |
| District | ||||
| Kassanda | 8 (6.5) | 4 (4.6) | 12 (5.7) | <.001a,b |
| Kiboga | 16 (13.0) | 12 (13.8) | 28 (13.3) | |
| Kyankwanzi | 1 (0.8) | 3 (3.4) | 4 (1.9) | |
| Luwero | 52 (42.3) | 10 (11.5) | 62 (29.5) | |
| Mityana | 16 (13.0) | 28 (32.2) | 44 (21.0) | |
| Mubende | 8 (6.5) | 15 (17.2) | 23 (11.0) | |
| Nakaseke | 11 (8.9) | 14 (16.1) | 25 (11.9) | |
| Nakasongola | 11 (8.9) | 1 (1.1) | 12 (5.7) | |
| Year | ||||
| 2018 | 71 (57.7) | 54 (62.1) | 125 (59.5) | .527 |
| 2019 | 52 (42.3) | 33 (37.9) | 85 (40.5) |
Abbreviations: ART, antiretroviral therapy; CrAg, cryptococcal antigen; PWH, people with HIV.
aStatistically significant result.
b P value from the Pearson chi-square test.
c P value from the Fisher exact test.
Table 4.
Comparison Between CrAg-Positive and CrAg-Negative PWH
| Total Screened (n = 1721) | CrAg-Negative (n = 1511) | CrAg-Positive (n = 210) | ||
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | P Valuea | |
| Sex (n = 669) | .523 | |||
| Female | 380 (56.8) | 329 (86.6) | 51 (13.4) | |
| Male | 289 (43.2) | 255 (88.2) | 34 (11.8) | |
| Level of health facility | ||||
| Health center III | 327 (19.0) | 293 (89.6) | 34 (10.4) | .108 |
| Health center IV | 899 (52.2) | 796 (88.5) | 103 (11.5) | |
| Hospital | 495 (28.8) | 422 (85.3) | 73 (14.7) | |
| District | ||||
| Kassanda | 125 (7.3) | 113 (90.4) | 12 (9.6) | .386 |
| Kiboga | 184 (10.7) | 156 (84.8) | 28 (15.2) | |
| Kyankwanzi | 46 (2.7 | 42 (91.3) | 4 (8.7) | |
| Luwero | 538 (31.3) | 476 (88.5) | 62 (11.5) | |
| Mityana | 394 (22.9) | 350 (88.8) | 44 (11.2) | |
| Mubende | 208 (12.1) | 185 (88.9) | 23 (11.1) | |
| Nakaseke | 153 (8.9) | 128 (83.7) | 25 (16.3) | |
| Nakasongola | 73 (4.2) | 61 (83.6) | 12 (16.4) | |
| Year | ||||
| 2018 | 967 (56.2) | 842 (87.1) | 125 (12.9) | .298 |
| 2019 | 754 (43.8) | 669 (88.7) | 85 (11.3) |
Abbreviations: CrAg, cryptococcal antigen; PWH, people with HIV.
a P value from the Pearson chi-square test.
DISCUSSION
In this study, we compared the frequency and yield of CrAg screening among ART-experienced PWH with VL nonsuppression—defined as having a VL of >1000 copies/mL after at least 6 months of ART—and ART-naïve PWH with CD4 <100 cells/mm3 attending rural public health facilities in central Uganda. The overall frequency of screening was low (21%) but was disproportionately lower among ART-experienced (12%) than ART-naïve PWH (95%). The CrAg positivity yield was lower among ART-experienced (11%) than ART-naïve PWH (14%), although we were unable to account for intersite clustering. Moreover, only 37% of ART-naïve PWH had a CD4 count test measurement, indicating that a large proportion of ART-naïve PWH were not assessed for CrAg screening eligibility. The proportions of eligible PWH, frequency of CrAg screening, and CrAg positivity yield (comparing ART-naïve and ART-experienced) significantly varied across districts and levels of health facility. To our knowledge, our study is the first to report CrAg positivity yield among ART-experienced PWH with VL nonsuppression in programmatic settings.
Our findings indicate a low rate of screening among ART-experienced PWH with VL nonsuppression, yet the yield of CrAg positivity was high. Although local guidelines from 2016 to September 2018 [18] and the advanced HIV disease management toolkit of May 2019 recommended CrAg screening in this population [19], CrAg screening in this population appears to be nonuniform across districts and health facilities, yet eligible patients were evenly distributed across the health centers. This reflects a gap in implementation of guideline recommendations for CrAg screening. There are several reasons that there was a low frequency of screening among ART-experienced PWH with VL nonsuppression. First, there were several changes in the recommendations for CrAg screening in this population over a span of 3 years and VL-directed screening was not recommended (although practiced in this region) for a period of 8 months. It is likely that these changes could not be easily rolled out and/or adopted consistently. From our results, CrAg screening occurred even during the period in which VL-directed testing was not recommended among ART-experienced PWH. Second, VL monitoring is performed at a central national laboratory [21]. When results showing VL nonsuppression are relayed to the facility, clinicians must trace the patient in order to screen them for CrAg. This creates a delay and dropout in the screening cascade. Reflexive CrAg screening at the central laboratory on samples showing incident VL nonsuppression could be an alternative approach to mitigate this problem. Lastly, facilities in Uganda experience stock outs of test kits and medicines for opportunistic infections (such as fluconazole preemptive therapy), and this can affect the frequency of screening even among ART-naïve individuals [22].
From our results, it was evident that even among ART-naïve PWH, CD4 count measurement, a prerequisite for CrAg screening, was performed in only 37%. This is possibly because only 37 facilities (out of 104) had a CD4 machine during the period under evaluation. This low coverage of CD4 testing impacts CrAg screening among PWH. All levels of health facilities across districts should be supported to consistently implement guidelines in order to identify PWH at risk of cryptococcal disease. A gap between recommendations and implementation has also been observed in other health care services in Uganda [23]. The WHO recommends CrAg screening for ART-naïve PWH with low CD4 counts, but there is no such international recommendation for ART-experienced PWH with VL nonsuppression due to lack of data to support this recommendation [10]. More studies are needed to evaluate the impact of CrAg screening and subsequent fluconazole preemptive therapy on the outcomes of ART-experienced PWH with VL nonsuppression to inform such guidelines.
There are few studies reporting CrAg screening positivity yield among ART-experienced PWH with VL nonsuppression (VL >1000 copies/mL). Our yield is higher than the prevalence of cryptococcal antigenemia of 3% reported by Mpoza et al. in this population [16]. However, their study population included leftover plasma from PWH with VL nonsuppression drawn from across the entire country. Therefore, a dissimilar estimate should be expected from our programmatic data generated from a single region of the country. Moreover, under programmatic settings, it is likely that symptomatic ART-experienced PWH with VL nonsuppression were preferentially screened over asymptomatic individuals. This would increase the yield of CrAg screening. Additionally, preferential screening of symptomatic individuals in some centers/districts may explain why there were differences in CrAg positivity yield across districts and health facilities when we compared the yield by eligibility criteria. Nevertheless, as our estimate is derived from programmatic data, our finding of CrAg positivity among ART-experienced PWH with VL nonsuppression reflects a “real-life” estimate that could guide allocation of resources for fluconazole preemptive therapy.
High VLs are associated with incident opportunistic infections, including cryptococcal meningitis, independent of CD4 counts [24–26]. The yield of CrAg positivity has been reported to be higher, at a VL cutoff of >5000 copies/mL vs >1000 copies/mL [16]. However, of ART-experienced (>6 months of therapy) PWH presenting with a first episode of cryptococcal meningitis in Uganda, 87% were reported to have a VL of >1000 copies/mL [27]. If VL-directed CrAg screening among ART-experienced PWH is to be adopted, more studies are needed to guide the optimal VL cutoff for CrAg screening. Although the yield of CrAg positivity is high (reportedly 4.8% and 21.9% among outpatients and inpatients in Botswana, respectively [28]), among ART-experienced PWH with CD4 ≤100 cells/mm3, requiring CD4 testing before screening ART-experienced PWH with VL nonsuppression is not cost-effective and is likely to affect the frequency of screening [16, 29]. Requiring CD4 before CrAg screening would create 2 “delays” for lower health facilities: first, the delay in tracing nonsuppressed PWH once VL results are relayed from a central national laboratory and, second, the delay in relaying results from a higher facility with CD4 testing services. Moreover, the long turnaround time for CD4 results experienced in the lower health facilities [30], where >60% of eligible PWH were reported in our study, could be another reason for the low CD4 testing rates among ART-naïve PWH observed in our study. The use of a semiquantitative cheap point-of-care CD4 test (Visitect CD4 lateral flow assay) has been shown to have high diagnostic accuracy and usability in low-resource settings and has the potential to be decentralized across lower health facilities [31]. Its impact on the frequency of CD4-directed CrAg screening is currently unknown.
The yield of CrAg positivity among eligible ART-naïve PWH reported in our study is well within the range of the reported prevalence of cryptococcal antigenemia among ART-naïve patients with CD4 <100 cells/mm3 in Uganda. Oyella et al. reported a prevalence of 19% [32], while Meya et al. reported prevalence rates of 8.2% and 9.3% [4, 33]. An exception is Andama et al., who reported a prevalence rate of 5.7% [34]. However, their study was conducted among patients with presumptive tuberculosis (TB), of whom 60% were determined to have TB. Similar to our findings, ART-experienced HIV patients had lower CrAg positivity yields than newly diagnosed HIV patients even at the same CD4 count in Ethiopia [14]. In Namibia, a cross-sectional study found the prevalence of cryptococcal antigenemia to be 3.3% among ART-experienced and ART-naïve PWH with severe immune suppression (CD4 <200 cells/mm3), although they did not provide a subanalysis to compare the prevalence in both populations [35].
Our study had some limitations. The lack of individual patient data limited our ability to evaluate CD4 counts and absolute HIV VLs. This would otherwise better characterize the relationship between VL and CD4 among CrAg-positive ART-experienced PWH with VL nonsuppression. Additionally, site information was not available, and as such, we were unable to account for facility-level clustering in our analyses. Although programmatic reports specifically capture PWH tested for the first time, we could not ascertain whether there were individuals who had repeat CrAg testing, in which case the yield of CrAg positivity may be overestimated. Additionally, there were no data on patient symptoms and signs. Accordingly, the yield of CrAg screening may be high if patients with symptoms (and therefore established disease) were preferentially screened. Further, there are patient, health worker, and systemic factors that we could not control for that could be influencing the frequency and yield of CrAg screening. The change in the local guidelines between September 2019 and May 2019 (which did not recommend VL-directed testing among ART-experienced PWH) may have affected the frequency (and not yield) of screening. Also, the use of secondary data sources could be affected by low reliability in performing, reading, and reporting of the CrAg test across the many sites and districts. Lastly, we evaluated reports from central Uganda. This could affect the generalizability of our findings. A countrywide evaluation of the CrAg screening yield among ART-experienced PWH with VL nonsuppression is therefore desirable.
CONCLUSIONS
The overall frequency of CrAg screening was only 21%. The CrAg positivity yield among ART-experienced PWH with VL nonsuppression was high (11%), yet the frequency of screening was low (12%). Even among ART-naïve PWH, the CrAg positivity yield was high (14%), but CD4 testing, a prerequisite for CrAg screening, was low (37%). More prospective studies are needed to evaluate the cost-effectiveness and impact of VL-directed CrAg screening on outcomes of ART-experienced PWH. Barriers to CrAg screening need to be identified and addressed to ensure fidelity to recommendations even among ART-naïve PWH.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This research was supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of 1NU2GGH002046-01-00.
Disclaimer. The findings and conclusions are those of the authors and do not necessarily represent the official position of the funding agencies.
Potential conflicts of interest. All authors: no reported conflicts of interest. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data availability. Data sets used in this analysis are available from the corresponding author upon reasonable request.
Author contributions. J.B.B.: conceptualization, data accrual, formal analysis, interpretation of results, drafting manuscript, manuscript revision, manuscript approval. P.M.: formal analysis, interpretation of results, manuscript revision, manuscript approval. S.M.: data accrual, interpretation of results, manuscript revision, manuscript approval. J.N.: interpretation of results, manuscript revision, manuscript approval. C.S.: interpretation of results, manuscript revision, manuscript approval. J.P.O.: interpretation of results, manuscript revision, manuscript approval. B.M.: interpretation of results, manuscript revision, manuscript approval.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tenforde MW, Gertz AM, Lawrence DS, et al. Mortality from HIV‐associated meningitis in Sub‐Saharan Africa: a systematic review and meta‐analysis. J Int AIDS Soc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Letang E, Müller MC, Ntamatungiro AJ, et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2015; 2:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajasingham R, Meya DB, Greene GS, et al. Evaluation of a national cryptococcal antigen screening program for HIV-infected patients in Uganda: a cost-effectiveness modeling analysis. PLoS One 2019; 14:e0210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramachandran A, Manabe Y, Rajasingham R, Shah M. Cost-effectiveness of CRAG-LFA screening for cryptococcal meningitis among people living with HIV in Uganda. BMC Infect Dis 2017; 17:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford N, Shubber Z, Jarvis JN, et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis 2018; 66:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tenforde MW, Scriven JE, Harrison TS, Jarvis JN. Immune correlates of HIV-associated cryptococcal meningitis. PLoS Pathog 2017; 13:e1006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wykowski J, Galagan SR, Govere S, et al. Cryptococcal antigenemia is associated with meningitis or death in HIV-infected adults with CD4 100-200 cells/mm3. BMC Infect Dis 2020; 20:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Guidelines for the Diagnosis, Prevention, and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children, March 2018. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- 11. Derbie A, Mekonnen D, Woldeamanuel Y, Abebe T. Cryptococcal antigenemia and its predictors among HIV infected patients in resource limited settings: a systematic review. BMC Infect Dis 2020; 20:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oladele RO, Akanmu AS, Nwosu AO, et al. Cryptococcal antigenemia in Nigerian patients with advanced human immunodeficiency virus: influence of antiretroviral therapy adherence. Open Forum Infect Dis 2016; 3:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egbe CA, Omoregie R, Alex-Ighodalo O.“Cryptococcus neoformans” infection among human immunodeficiency virus patients on highly active antiretroviral therapy in Benin City, Nigeria. New Zealand J Medical Lab Sci 2015; 69:21–3. [Google Scholar]
- 14. Beyene T, Woldeamanuel Y, Asrat D, et al. Comparison of cryptococcal antigenemia between antiretroviral naïve and antiretroviral experienced HIV positive patients at two hospitals in Ethiopia. PLoS One 2013; 8:e75585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alemu AS, Kempker RR, Tenna A, et al. High prevalence of cryptococcal antigenemia among HIV-infected patients receiving antiretroviral therapy in Ethiopia. PLoS One 2013; 8:e58377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mpoza E, Rajasingham R, Tugume L, et al. Cryptococcal antigenemia in human immunodeficiency virus antiretroviral therapy–experienced Ugandans with virologic failure. Clin Infect Dis 2020; 71:1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ministry of Health. Advance HIV Disease Management Toolkit. Kampala, Uganda: Uganda Ministry of Health; 2019. [Google Scholar]
- 18. Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. Kampala, Uganda: Uganda Ministry of Health; 2016. [Google Scholar]
- 19. Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. Kampala, Uganda: Uganda Ministry of Health; 2018. [Google Scholar]
- 20. Kamwesiga J. Uganda Health Care System. Kampala, Uganda: Makerere University; 2011. [Google Scholar]
- 21. Kiyaga C, Sendagire H, Joseph E, et al. Uganda’s new national laboratory sample transport system: a successful model for improving access to diagnostic services for early infant HIV diagnosis and other programs. PLoS One 2013; 8:e78609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Windisch R, Waiswa P, Neuhann F, et al. Scaling up antiretroviral therapy in Uganda: using supply chain management to appraise health systems strengthening. Global Health 2011; 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nabyonga Orem J, Wavamunno JB, Bakeera SK, Criel B. Do guidelines influence the implementation of health programs? — Uganda’s experience. Implementation Sci 2012; 7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swindells S, Evans S, Zackin R, et al. ; AIDS Clinical Trial Group 722 Study Team Predictive value of HIV-1 viral load on risk for opportunistic infection. J Acquir Immune Defic Syndr 2002; 30:154–8. [DOI] [PubMed] [Google Scholar]
- 25. Weissberg D, Mubiru F, Kambugu A, et al. Ten years of antiretroviral therapy: Incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PLoS One 2018; 13:e0206796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan JE, Hanson DL, Jones JL, Dworkin MS; Adult and Adolescent Spectrum of HIV Disease Project Investigators Viral load as an independent risk factor for opportunistic infections in HIV-infected adults and adolescents. AIDS 2001; 15:1831–6. [DOI] [PubMed] [Google Scholar]
- 27. Rhein J, Hullsiek KH, Evans EE, et al. ; ASTRO-CM study team Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hurt WJ, Tenforde MW, Molefi M, et al. Prevalence and sequelae of cryptococcal antigenemia in antiretroviral therapy–experienced populations: an evaluation of reflex cryptococcal antigen screening in Botswana. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahn JG, Marseille E, Moore D, et al. CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ 2011; 343:d6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lofgren SM, Nalintya E, Meya DB, et al. A qualitative evaluation of an implementation study for cryptococcal antigen screening and treatment in Uganda. Medicine (Baltimore) 2018; 97:e11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ndlovu Z, Massaquoi L, Bangwen NE, et al. Diagnostic performance and usability of the VISITECT CD4 semi-quantitative test for advanced HIV disease screening. PLoS One 2020; 15:e0230453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oyella J, Meya D, Bajunirwe F, Kamya MR. Prevalence and factors associated with cryptococcal antigenemia among severely immunosuppressed HIV-infected adults in Uganda: a cross-sectional study. J Int AIDS Soc 2012; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meya DB, Kiragga AN, Nalintya E, et al. Reflexive laboratory-based cryptococcal antigen screening and preemptive fluconazole therapy for cryptococcal antigenemia in HIV-infected individuals with CD4 <100 cells/µL: a stepped-wedge, cluster-randomized trial. J Acquir Immune Defic Syndr 2019; 80:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andama AO, den Boon S, Meya D, et al. ; International HIV-Associated Opportunistic Pneumonias (IHOP) Study Prevalence and outcomes of cryptococcal antigenemia in HIV-seropositive patients hospitalized for suspected tuberculosis in Uganda. J Acquir Immune Defic Syndr 2013; 63:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sawadogo S, Makumbi B, Purfield A, et al. Estimated prevalence of Cryptococcus antigenemia (CrAg) among HIV-Infected adults with advanced immunosuppression in Namibia justifies routine screening and preemptive treatment. PLoS One 2016; 11:e0161830. [DOI] [PMC free article] [PubMed] [Google Scholar]