Abstract
Perineuronal nets (PNNs) are an extracellular matrix structure rich in chondroitin sulfate proteoglycans (CSPGs), which preferentially encase parvalbumin-containing (PV+) interneurons. PNNs restrict cortical network plasticity but the molecular mechanisms involved are unclear. We found that reactivation of ocular dominance plasticity in the adult visual cortex induced by chondroitinase ABC (chABC)-mediated PNN removal requires intact signaling by the neurotrophin receptor TRKB in PV+ neurons. Additionally, we demonstrate that chABC increases TRKB phosphorylation (pTRKB), while PNN component aggrecan attenuates brain-derived neurotrophic factor (BDNF)-induced pTRKB in cortical neurons in culture. We further found that protein tyrosine phosphatase σ (PTPσ, PTPRS), receptor for CSPGs, interacts with TRKB and restricts TRKB phosphorylation. PTPσ deletion increases phosphorylation of TRKB in vitro and in vivo in male and female mice, and juvenile-like plasticity is retained in the visual cortex of adult PTPσ-deficient mice (PTPσ+/−). The antidepressant drug fluoxetine, which is known to promote TRKB phosphorylation and reopen critical period-like plasticity in the adult brain, disrupts the interaction between TRKB and PTPσ by binding to the transmembrane domain of TRKB. We propose that both chABC and fluoxetine reopen critical period-like plasticity in the adult visual cortex by promoting TRKB signaling in PV+ neurons through inhibition of TRKB dephosphorylation by the PTPσ-CSPG complex.
SIGNIFICANCE STATEMENT Critical period-like plasticity can be reactivated in the adult visual cortex through disruption of perineuronal nets (PNNs) by chondroitinase treatment, or by chronic antidepressant treatment. We now show that the effects of both chondroitinase and fluoxetine are mediated by the neurotrophin receptor TRKB in parvalbumin-containing (PV+) interneurons. We found that chondroitinase-induced visual cortical plasticity is dependent on TRKB in PV+ neurons. Protein tyrosine phosphatase σ (PTPσ, PTPRS), a receptor for PNNs, interacts with TRKB and inhibits its phosphorylation, and chondroitinase treatment or deletion of PTPσ increases TRKB phosphorylation. Antidepressant fluoxetine disrupts the interaction between TRKB and PTPσ, thereby increasing TRKB phosphorylation. Thus, juvenile-like plasticity induced by both chondroitinase and antidepressant treatment is mediated by TRKB activation in PV+ interneurons.
Keywords: BDNF, chABC, CSPG, perineuronal nets, PTPRS, RPTPσ
Introduction
Plasticity is the ability of the brain to change itself through establishing new neuronal connections and rewiring existing ones. Plasticity is prominent in early life and it peaks during so-called “critical periods” when the ability of the brain to adapt is at its highest (Wiesel, 1982). After the end of the critical period, plasticity persists but at significantly diminished levels (Hübener and Bonhoeffer, 2014).
Closure of the critical periods is mediated by changes in cortical excitatory/inhibitory (E/I) balance that take place because of maturation of cortical inhibitory interneurons. Fast-spiking interneurons expressing parvalbumin (PV+) orchestrate synchronous neuronal oscillations and play a particularly important role in this process (Hensch, 2005). Closure of the critical period coincides with the functional maturation of PV+ cells and establishment of perineuronal nets (PNNs) around them (Wang and Fawcett, 2012; Umemori et al., 2018; Fawcett et al., 2019). PNNs are mesh-like structures of extracellular matrix that surround the somata and proximal dendrites of PV+ interneurons in particular (Kwok et al., 2011). Chondroitin sulfate proteoglycans (CSPGs), such as aggrecan and brevican, are major components of PNNs.
After the closure of a critical period, neuronal plasticity can still be modulated, and critical period-like plasticity can be induced in the adult brain by a number of different methods (Bavelier et al., 2010; Sale et al., 2014). Digestion of PNNs by chondroitinase ABC (chABC) treatment has been demonstrated to induce ocular dominance plasticity in the adult visual cortex (Pizzorusso et al., 2002). Local chondroitinase injections into different brain areas have also been shown to promote recovery of spinal cord injury (Bradbury et al., 2002) and extinction of fear memories in adult rodents (Gogolla et al., 2009; Hylin et al., 2013; Shi et al., 2019). However, the mechanisms through which chABC influences plasticity in the CNS remain unclear.
Antidepressant drugs also induce critical period-like plasticity in the adult brain (Castreń, 2013). Antidepressants activate neurotrophic receptor tyrosine kinase 2 (TRKB), the receptor for brain-derived neurotrophic factor (BDNF), and promote plasticity through its signaling pathways (Saarelainen et al., 2003; Duman and Monteggia, 2006; Castrén and Antila, 2017; Umemori et al., 2018). Like ChABC, fluoxetine, a widely prescribed antidepressant, induces ocular dominance plasticity in the rodent visual cortex (Maya Vetencourt et al., 2008) and makes fear-related memories in mice susceptible to erasure (Karpova et al., 2011). We have recently found that the activation of TRKB in PV+ interneurons is both necessary and sufficient for antidepressant-induced plasticity in the mature CNS (Winkel et al., 2020).
ChABC and antidepressant treatment exert similar plasticity-promoting effects in the adult brain; however, it is not known whether they recruit similar molecular mechanisms. We hypothesize that receptor-like protein tyrosine phosphatase σ (PTPσ) might be a nexus that mediates plasticity processes induced by both methods. PTPσ is a receptor for CSPGs (Shen et al., 2009), and it has been demonstrated to be essential for the inhibitory effects of CSPGs on neurite outgrowth (Shen et al., 2009; Duan and Giger, 2010; Coles et al., 2011). PTPσ interacts with and modulates the activity of TRK receptors (Faux et al., 2007; Takahashi et al., 2011), inhibiting TRKB through dephosphorylation (Kurihara and Yamashita, 2012).
We now demonstrate that TRKB activity in PV+ interneurons is essential for plasticity induced by chABC. While CSPGs removal by chABC injection into the visual cortex promotes ocular dominance shift, this effect is abolished in heterozygous mice with reduced TRKB in PV+ neurons (PV-TRKB+/−). We have confirmed that PTPσ interacts with TRKB, and genetic deficiency of PTPσ promotes TRKB phosphorylation (pTRKB) in vitro and in vivo. We further show that PTPσ deficiency promotes plasticity at network levels, as PTPσ+/− mice display critical period-like plasticity in the visual cortex in adulthood. Finally, we have observed that the antidepressant fluoxetine disrupts TRKB:PTPσ interaction in vitro and in vivo.
Materials and Methods
Animals
BALB/c and C57BL/6J mice heterozygous for PTPRS gene (PTPσ+/− mice) and their wild-type (WT) littermates, C57BL/6J mice heterozygous for TRKB gene in PV+ interneurons (PV-TRKB+/−) and their PV-Cre littermates (PV-TRKB+/+), WT C57BL/6J mice were used in the experiments. BALB/c PTPσ+/− mice were originally developed by Michel Tremblay's lab (McGill University, Canada; Elchebly et al., 1999) and kindly donated to us by Heikki Rauvala (University of Helsinki). BALB/c PTPσ+/− mice 10 weeks old were used for brain sample collection and pTRKB level assessment by ELISA. For optical imaging experiments, BALB/c mouse line was rederived to C57BL/6J background, and N4 generation of the offspring was used for testing. The mice were two months old at the beginning of the experiments. PV-TRKB+/− (TRKBflx/wt, PVcre/wt) mice were generated by mating heterozygous floxed TRKB mice (TRKBflx/wt; Minichiello et al., 1999) and homozygous PV-specific Cre line (PVcre/cre; Pvalb-IRES-Cre, JAX: 008069, The Jackson Laboratory; Hippenmeyer et al., 2005). PV heterozygous (TRKBw/w, PVcre/wt) littermates with intact TRKB in PV+ neurons were used as a control group for PV-TRKB+/− mice. The mice were four months old at the beginning of the experiments. WT C57BL/6J mice five months old were used to assess fluoxetine effect on TRKB:PTPσ interaction in the visual cortex in vivo. The mice were kept under standard laboratory conditions with 12/12 h light/dark cycle (lights on at 6 A.M.) and access to food and water ad libitum. All the procedures involving animals were done in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines and were approved by the Experimental Animal Ethical Committee of Southern Finland (ESAVI/10 300/04.10.07/2016).
Brain sample collection and processing
Mice were killed with CO2. The death was confirmed by ascertaining cardiac and respiratory arrest. The animals were decapitated, the visual cortices were dissected and stored at −80°C. Samples from the primary visual cortex were sonicated in NP lysis buffer (137 mm NaCl, 20 mm Tris, 1% NP-40, 10% glycerol, and 48 mm NaF) containing a protease and phosphatase inhibitor mix (#P2714 and #P0044, Sigma-Aldrich) and 2 mm Na2VO3. The homogenate was centrifuged for 15 min at 15,000 × g, 4°C. The supernatant was collected and used for further analysis. Protein levels were measured using DC Protein Assay kit (Bio-Rad, #5000116) by colorimetric Lowry method in Varioskan Flash (Thermo Fisher Scientific).
Cell culture
Cerebral cortical cell cultures were prepared from Wistar rat (Harlan Labs) embryos extracted on embryonic day (E)18 (Sahu et al., 2019). The cells were cultured in a serum-free neurobasal medium with supplements (1% penicillin, 1% L-glutamine, 2% B-27) and collected after 7–9 d in vitro (DIV). For the experiments assessing the effect of PTPσ genetic deficiency on pTRKB in vitro, cortical cell cultures were prepared from BALB/c mouse embryos (PTPσ−/−, PTPσ+/− and their WT littermates) extracted on E18 using the same protocol as described above for the rat cells. Mouse fibroblast cells stably expressing full-length TRKB (MG87.TRKB) or TRKA (MG87.TRKA) were cultured in DMEM supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin (PS), 1% L-glutamine, and 400 mg/ml G418. Human-derived HEK293T cells were cultured in DMEM supplemented with 10% FCS, 1% PS, and 1% L-glutamine.
Transfection
We transfected 70% confluent MG87.TRKA, MG87.TRKB, and HEK293T cell lines using Lipofectamine 2000 (Thermo Fischer Scientific). MG87.TRKA and MG87.TRKB cells were transfected with PTPσ (RefSeq number NM_019140) Myc-DDK-tagged open-reading frame (ORF) plasmid purchased from OriGene (#RR209636), pCMV6-Entry vector with C-terminal Myc-DDK Tag (#PS100001) was used to transfect control cells. HEK293T were transfected with GFP-tagged full-length TRKB plasmid (HP220GFP-TRKB; Haapasalo et al., 2001) or TRKB carrying Y433F/R427A mutation (Cannarozzo et al., 2020).
Antibodies and reagents
Aggrecan was purchased from Sigma-Aldrich (A1960). Anti-phospho-TRKA (Tyr490)/TRKB (Tyr516; C35G9) rabbit monoclonal antibody (mAb) was purchased from Cell Signaling Technology (#4619, #4621, and #4168). Anti-TRKB goat polyclonal antibody (pAb) was bought from R&D Systems, #AF1494. Anti-phosphotyrosine mouse monoclonal antibody (clone PY20) was purchased from Bio-Rad (#MCA2472). Anti-PTPσ (SS-8) mAb was purchased from Santa Cruz Biotechnology (SC-100419). Secondary horseradish peroxidase (HRP)-conjugated antibodies were purchased from Bio-Rad (goat anti-rabbit #1705046 and goat anti-mouse #1705047) and from Invitrogen (rabbit anti-goat #611620). Enhanced chemiluminescent (ECL) substrate WesternBright Quantum HRP substrate (Advansta) was used for Western blotting (#K-12042). Pierce HRP ECL substrate was used to detect luminescence in ELISA (Thermo Fisher Scientific, #32209). Tris-buffered saline with Tween 20 (TBST) was used for Western blotting (20 mm Tris-HCl, 150 mm NaCl, and 0.1% Tween 20; pH 7.6), PBS with Tween 20 (PBST) was used for ELISA (137 mm NaCl, 10 mm phosphate, 2.7 mm KCl, and 0.1% Tween 20; pH 7.4).
Drug treatment
We added 10 µg/ml aggrecan to cortical neurons cultured 6 DIV, and the cells were collected 1 d after the aggrecan treatment (7 DIV); 20 ng/ml BDNF was added to cortical cells for 10 min; 0.1 and 1 μm fluoxetine was added to cortical cells cultured 7 DIV, and the cells were collected after 30 min of treatment. HEK293T cells were treated with 10 μm fluoxetine for 30 min. BALB/c PTPσ+/− and WT mice were injected with 30 mg/kg fluoxetine intraperitoneally and killed with CO2. The brain samples were collected and stored at −80°C until further processing.
Immunoprecipitation and Western blotting
The cells were lysed using NP lysis buffer containing 2 mm sodium orthovanadate and protease inhibitor mix. The homogenized suspension was centrifuged (15,000 × g, 10 min, +4°C), and the resulting supernatant was used for analysis. For immunoprecipitation, TRKB was captured using anti-TRKB antibodies (R&D Systems). The samples were incubated with Sepharose, washed with NP lysis buffer twice and the proteins were separated by heating in 2× Laemmli buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.02% bromophenol blue, and 125 mm Tris HCl; pH 6.8) for 5 min at 95°C. The samples were loaded to NuPAGE 4–12% Bis-Tris Protein polyacrylamide gels (Invitrogen, #NP0323BOX), and the proteins were separated according to their molecular weight using electrophoresis. The samples were transferred to polyvinylidene difluoride (PVDF) membrane, incubated in 1:1000 primary antibody dilution in 3% bovine serum albumin (BSA) in tris-phosphate buffer containing 0.1% Tween 20 (TBST) overnight at 4°C and subsequently incubated in HRP-conjugated secondary antibodies (1:10,000) for 1 h at room temperature (RT). The bands were visualized using chemiluminescent western blotting substrate in Fuji LAS3000 camera (Tamro Medlabs).
ELISA
Levels of pTRKB were evaluated using ELISA for pTRKB developed in our lab (Antila et al., 2014). On day 1, the high-binding 96-well OptiPlate (PerkinElmer) was incubated overnight at 4°C with 1:500 anti-TRKB antibody diluted in a homemade carbonate buffer (57.4 mm sodium bicarbonate and 42.6 mm sodium carbonate, pH 9.8). On day 2, the plate was incubated for 2 h at RT in the blocking buffer (3% BSA in PBST) to block non-specific binding. Homogenized and centrifuged brain samples or lysed cell samples were added to the plate and incubated overnight at 4°C. On day 3, the plate was washed four times with PBST using an automated plate washer (Thermo Fisher Scientific Wellwash Versa), and the samples were incubated in anti-phosphotyrosine or anti-pTRKB antibodies diluted 1:1000 in the blocking buffer overnight at 4°C. On day 4, the samples were washed four times in PBST and incubated with tertiary HRP-conjugated antibodies in the blocking buffer (1:5000) at RT for 1 h. Finally, ECL was added to the plate and luminescence was measured with 1-s integration time using Varioskan Flash plate reader (Thermo Fisher Scientific). Levels of TRKB:PTPσ interaction were evaluated by ELISA using a similar protocol. TRKB was captured using anti-TRKB antibodies, and anti-PTPσ were applied afterward to assess levels of PTPσ bound to TRKB.
Cell-surface ELISA
Cell-surface ELISA was conducted to assess PTPσ levels found on the cell surface as previously described (Zheng et al., 2008; Fred et al., 2019). Briefly, cortical cells were cultivated in clear-bottom 96-well plates (ViewPlate 96, PerkinElmer). On 7 DIV, the cells' medium was removed, washed with cold PBS, and fixed with 100 µl of 4% paraformaldehyde (PFA) per well for 20 min. Then the cells were washed with PBS three times, and non-specific binding was blocked with PBS containing 5% non-fat dry milk and 5% BSA for 1 h at RT. After that, the samples were incubated in primary anti-PTPσ antibody (1:500 in the blocking buffer) overnight at 4°C. On the following day, the cells were washed with PBS once and incubated in HRP-conjugated anti-mouse antibody (1:5000 in the blocking buffer) for 1 h at RT. The cells were washed four times with PBS, ECL was added and chemiluminescence was measured with 1-s integration time using Varioskan Flash (Thermo Fisher Scientific).
Transparent skull surgery
Transparent skull surgery was conducted as described previously (Steinzeig et al., 2017). Animals were anesthetized either with a mixture of 0.05 mg/kg fentanyl (Hameln), 5 mg/kg midazolam (Hameln), and 0.5 mg/kg medetomidine (Orion Pharma) administered intraperitoneally or isoflurane combined with 0.05 mg/kg buprenorphine analgesia administered subcutaneously, 5 mg/kg carprofen (ScanVet) was administered subcutaneously for postoperative analgesia. Under anesthesia, the scalp and periosteum of the animals were removed, the skull was polished, and two layers of transparent acryl powder (EUBECOS) mixed with methyl methacrylate liquid (Dentsply) were applied on the surface. Metal holders (Neurotar) were installed on the top of the head and fixed with a mixture of acryl polymer powder (Dentsply) and cyanoacrylate glue (Steinzeig et al., 2017).
Monocular deprivation (MD)
MD was conducted by suturing the eye contralateral to the imaged hemisphere (left eye) with perma-hand silk thread (Ethicon). The length of the MD was 3.5 d for the PTPσ+/− versus WT experiment and 7 d for the PV-TRKB+/− versus WT experiment. The integrity of the suture was checked on a daily basis before the lights were on.
Optical imaging
Optical imaging of intrinsic signals was conducted as previously described (Steinzeig et al., 2017). Visual cortex of the right hemisphere of each animal was imaged. Continuous-periodic stimulation with continuous synchronized data acquisition was used for the processing of the intrinsic signals. А drifting thin horizontal bar 2° wide moving upwards with a temporal frequency of 1 cycle/8.3 s (0.125 Hz) and a spatial frequency of 1/80° was used to alternatively stimulate the left and right eye, while the other eye was patched. The drifting bars were displayed −15° to +5° from the center to optimally stimulate the binocular area of the right visual cortex. The imaging was done under 1.2% isoflurane anesthesia in a 1:2 mixture of O2:air.
Optical imaging of PV-TRKB+/− mice
Four-month-old mice were used for the experiments. During week 1, the animals underwent transparent skull surgery. After 7 d, during week 2, the animals underwent the first session of the optical imaging under isoflurane anesthesia (IOS1). During week 3, the animals were injected with 50 mU of chABC in PBS or PBS into the binocular area of the visual cortex and were subjected to MD for 7 d. During week 4, the eyes were opened, and IOS2 immediately took place.
Optical imaging of PTPσ+/− mice
Two-month-old mice were used for the experiments. During week 1, the animals underwent transparent skull surgery. After 7 d, the animals underwent the first session of the optical imaging (IOS1) followed by MD for 3.5 d. We have switched to the shorter MD length as compared with the previously described experiment since it has been recently demonstrated to be sufficient for the induction of the critical period-like plasticity (Baho et al., 2019). On day 4 of the MD, the eyes were opened, and IOS2 took place.
Stereotaxic surgeries
The mice were anesthetized with isoflurane combined with 0.05 mg/kg buprenorphine analgesia. Images acquired during the first session of the optical imaging were used to identify the binocular area of the visual cortex. A hole in the skull was made with a drill, and 50 mU of chABC in 1 µl of PBS or 1 µl of PBS was injected in the center of the binocular area using a microsyringe pump; 10-µl Nanofil syringe (WPI Nanofil) with drilled 1.1-mm outlet bore to accommodate 1.0-mm glass needle and custom-made bevelled borosilicate glass needles with a 50-µm tip diameter were used for the infusions. Infusions were done at the speed of 2 nl/s. After the surgery, the animals were left to recover in the home cage, and the second imaging session took place 7 d later.
Experimental design and statistical analysis
The experiments were designed taking into account good laboratory practices and the 3Rs principle of animal research. Both sexes of mice were used in all the experiments involving animal testing, except for the in vivo fluoxetine experiment (samples from only male mice were used because of a low number of female mice available in the cohort). Parametric tests were preferentially used to gain statistical power. Exceptions were made whenever the data presented lacked homoscedasticity or when variables were discrete, in which cases non-parametric tests were chosen. The statistical tests used in each particular experiment and statistical values are described in the legend of figures, and statistical values are provided in Table 1. Differences were considered statistically significant when p < 0.05. Statistical analysis and plots were made in GraphPad Prism 6 software. Data are presented as mean ± SEM. Detailed information on the statistical analysis of each experiment, and number of samples/animals used is given in Table 1.
Table 1.
Statistical analysis and the number of animals/samples used in the experiments
| Graph | Pre hoc | Post hoc | N, number of samples/animals per group |
|---|---|---|---|
| 1A | Three-way ANOVA (time × genotype × drug): General effect: Time (IOS): F(1,34) = 4.139, p = 0.0498* Genotype: F(1,34) = 0.7739, p = 0.3852 chABC: F(1,34) = 5.164, p = 0.0295* Interaction: Time × genotype: F(1,34) = 4.861, p = 0.0343* Time × chABC: F(1,34) = 6.483, p = 0.0156* Genotype × chABC: F(1,34) = 7.244, p = 0.0110* Time × genotype × chABC: F(1,34) = 5.899, p = 0.0206* |
Tukey's multiple comparison test: WT-chABC (IOS1) × WT-chABC (IOS2): p = 0.0002* |
WT-vehicle: 3 (we have used the lowest number of animals possible in the control group to reduce the total number of animals used in the experiments; there is abundant evidence of no shift in ocular dominance in adult WT mice both from our lab and in the literature) WT-chABC: 6 PV-TRKB+/−-vehicle: 5 PV-TRKB+/−-chABC: 7 |
| 1B | Mann–Whitney: U = 0, p = 0.0286* | Vehicle: 4 chABC: 4 |
|
| 1C | Two-way ANOVA: Interaction: F(1,20) = 3.921, p = 0.0616 BDNF: F(1,20) = 155.2, p < 0.0001* Aggrecan: F(1,20) = 5.117, p = 0.035* |
Tukey's multiple comparison test: BDNF-vehicle × BDNF-aggrecan: p = 0.0329* |
Vehicle-vehicle: 6 Vehicle-aggrecan: 6 Vehicle-BDNF: 6 BDNF-aggrecan: 6 |
| 2B | Unpaired t test: T22 = 4384, p = 0.0002* | WT: 12 PTPσ+/−: 12 |
|
| 2C | One-way ANOVA: F(2,14) = 91.44, p < 0.0001* |
Tukey's multiple comparison test: WT × PTPσ+/−: p = 0.0007* WT × PTPσ-/-: p < 0.0001* PTPσ+/− × PTPσ-/-: p < 0.0001* |
WT: 5 PTPσ+/−: 6 PTPσ-/-: 6 |
| 2D | Mann–Whitney: U = 0, p = 0.0002* | WT: 7 PTPσ+/−: 9 |
|
| 2E | Mann–Whitney: U = 9, p = 0.0164* | WT: 7 PTPσ+/−: 9 |
|
| 2F | Two-way ANOVA: Interaction: F(1,8) = 33,13, p = 0.0004* Time: F(1,8) = 40.81, p = 0.0002* Genotype: F(1,8) = 36.70, p = 0.0003* |
Bonferroni's multiple comparison test: IOS1-PTPσ+/− × IOS2- PTPσ+/−: p = 0.0001* |
WT: 6 PTPσ+/−: 4 |
| 3A | One-way ANOVA: F(2,15) = 6.734, p = 0.0082* |
Bonferroni's multiple comparison test: vehicle × fluoxetine 1 μm: p = 0.0046* |
Vehicle: 6 Fluoxetine 0.1 μm: 6 Fluoxetine 1 μm: 6 |
| 3B | Unpaired t test: t(6) = 4.025, p = 0.0069* | Vehicle: 4 Fluoxetine: 4 |
|
| 3C | Unpaired t test: t(28) = 0.9872, p = 0.3320 | Vehicle: 15 Fluoxetine: 15 |
|
| 3D | Two-way ANOVA: Interaction: F(1,19) = 0.8761, p = 0.3610 Fluoxetine: F(1,19) = 2.627, p = 0.1216 TRKB mutation: F(1,19) = 5.131, p = 0.0354* |
WT-TRKB-vehicle: 6 WT-TRKB-fluoxetine: 6 Mutant TRKB-vehicle: 5 Mutant TRKB-fluoxetine: 6 |
*Statistically significant.
Results
TRKB signaling in PV+ neurons is essential for chABC-induced plasticity
Digestion of PNNs by chABC is a well-known activator of plasticity (Fawcett et al., 2019), and it has been shown to induce critical period-like plasticity in the adult rodent visual cortex through degradation of CSPGs in the extracellular matrix (Pizzorusso et al., 2002). However, precise molecular mechanisms responsible for the plasticity-promoting effect of chABC remain largely unknown.
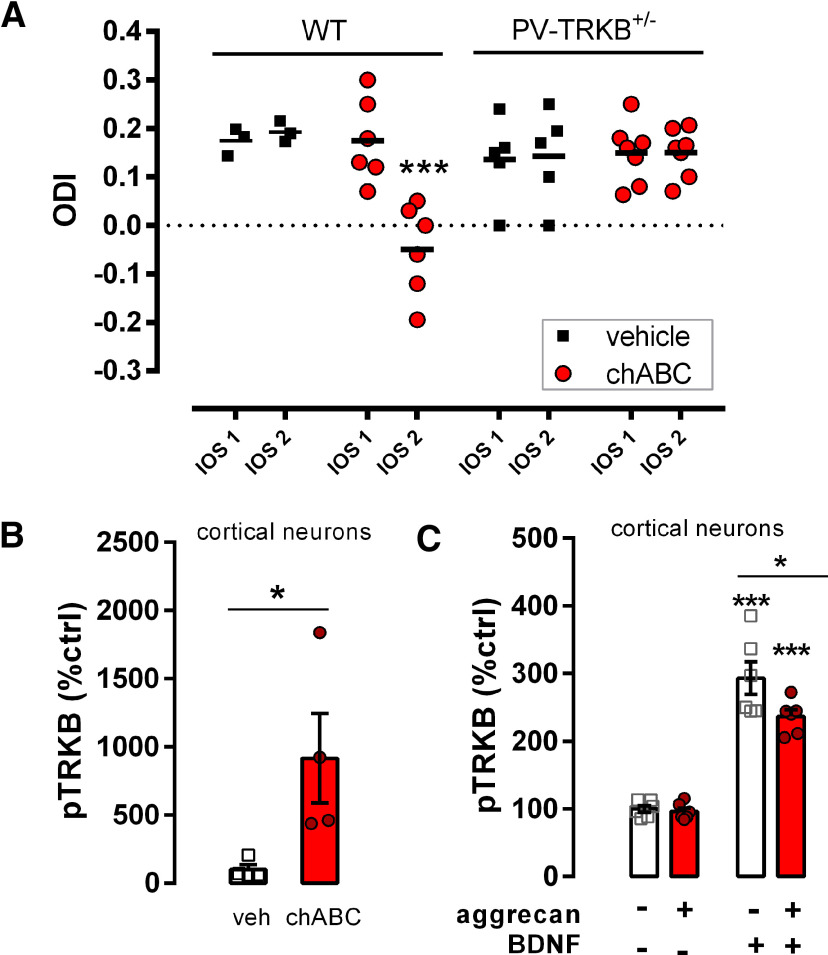
We tested the hypothesis that TRKB functioning in PV+ interneurons is involved in chABC-induced plastic changes. For that purpose, we generated mice heterozygous for full-length TRKB allele in PV+ interneurons (PV-TRKB+/−; Minichiello et al., 1999). We assessed the ability of chABC to promote ocular dominance plasticity in their visual cortex. Four-month-old mice underwent assessment of their ocular dominance by optical imaging of the intrinsic signal (IOS1; Cang et al., 2005; Steinzeig et al., 2017). After the first imaging session, the mice were injected with chABC or vehicle into the binocular area of the visual cortex followed by MD for 7 d. As expected, vehicle-treated WT mice failed to show any OD plasticity in the second imaging session (IOS2) right after the MD, whereas WT mice treated with chABC showed a clear shift in the ocular dominance toward the non-deprived eye (Fig. 1A), as previously shown (Pizzorusso et al., 2002). However, PV-TRKB+/− mice failed to show any shift in ocular dominance regardless of whether they were treated with chABC or not (Fig. 1A). These data demonstrate that chABC-mediated reactivation of visual cortical plasticity is dependent on intact TRKB signaling in the PV+ interneurons.
Figure 1.
ChABC-induced plasticity requires intact TRKB signaling in PV+ neurons. A, PNN removal by local chABC injection reopens critical period-like plasticity in the visual cortex of WT but not in mice heterozygous for TRKB deletion in PV interneurons (PV-TRKB+/−), as measured by optical imaging of the intrinsic signal before (IOS1) and after 7 d of MD (IOS2). ODI: ocular dominance index. B, ChABC treatment (30 min) of cortical neurons (7 DIV) increases pTRKB. C, The CSPG aggrecan, added to cortical neurons (6 DIV) for 24 h, attenuates BDNF-induce pTRKB in cortical neurons. Columns and bars represent mean ± SEM, respectively, and scattered points represent individual values. Data were analyzed by three-way ANOVA and Tukeys's post hoc (A), Mann–Whitney (B), or two-way ANOVA followed by Tukey's multiple comparison test (C); *p < 0.05, **p < 0.005, ***p < 0.0005.
Next, we assessed whether PNN digestion with chABC induces TRKB activation in primary cerebral cortical neurons. We treated rat cortical neurons grown in vitro (DIV) for 7 d with 2 U/ml chABC for 30 min and checked the activation of TRKB by assessing its phosphorylation levels (pTRKB; Antila et al., 2014). We observed that reduction of PNNs by chABC treatment robustly increased pTRKB in vitro (Fig. 1B).
After observing that PNN digestion with chABC treatment positively affects pTRKB, we investigated whether treatment with aggrecan, a major PNN component in the adult CNS, might have an opposing effect on pTRKB and render BDNF-induced TRKB activation less effective. We added 10 µg/ml aggrecan to the primary cortical neurons cultured for 6 DIV. After 24 h, we challenged the cells with 20 ng/ml BDNF for 10 min. Aggrecan treatment did not alter basal pTRKB levels (that are normally very low in vitro); however, it significantly decreased BDNF-induced pTRKB (Fig. 1C). Taken together, these data demonstrate that PNNs regulate plasticity in a TRKB-dependent manner in vivo and that PNNs exert negative effects on TRKB activation in vitro.
Deletion of CSPG receptor PTPσ extends the critical period and facilitates TRKB activation
PTPσ is a recognized inhibitor of neuronal plasticity and a receptor for CSPGs (Shen et al., 2009; Duan and Giger, 2010; Fawcett et al., 2019), and previous data indicate that PTPσ interacts with TRKB (Kurihara and Yamashita, 2012). We therefore hypothesized that PTPσ might restrict TRKB signaling through dephosphorylation in PNN-bearing PV+ interneurons and thereby inhibit TRKB-promoted plasticity. To confirm TRKB:PTPσ interaction, NIH3T3 cell line stably expressing either TRKA or TRKB (MG.TRKA and MG.TRKB, respectively), were transfected with myc-PTPσ, immunoprecipitated with anti-TRKB antibody and blotted for PTPσ. In the transfected samples, we observed a band below 250 kDa (Fig. 2A), which is consistent with the predicted molecular weight of the plasmid's product. Additionally, a 165-kDa band was observed in non-transfected cells, which corresponds to the endogenous mature full-length PTPσ (Faux et al., 2007). When the membrane was stripped and reblotted for TRKB, we observed a band of 140 kDa corresponding to the full-length TRKB. This band was seen in TRKB expressing cells only, which rules out potential unspecific binding of TRKB antibodies to TRKA receptors. These data indicate that PTPσ interacts with TRKB. Furthermore, our previous proteomic study found PTPσ among the proteins that were immunoprecipitated with TRKB in hippocampal samples of the adult mouse brain (Fred et al., 2019).
Figure 2.
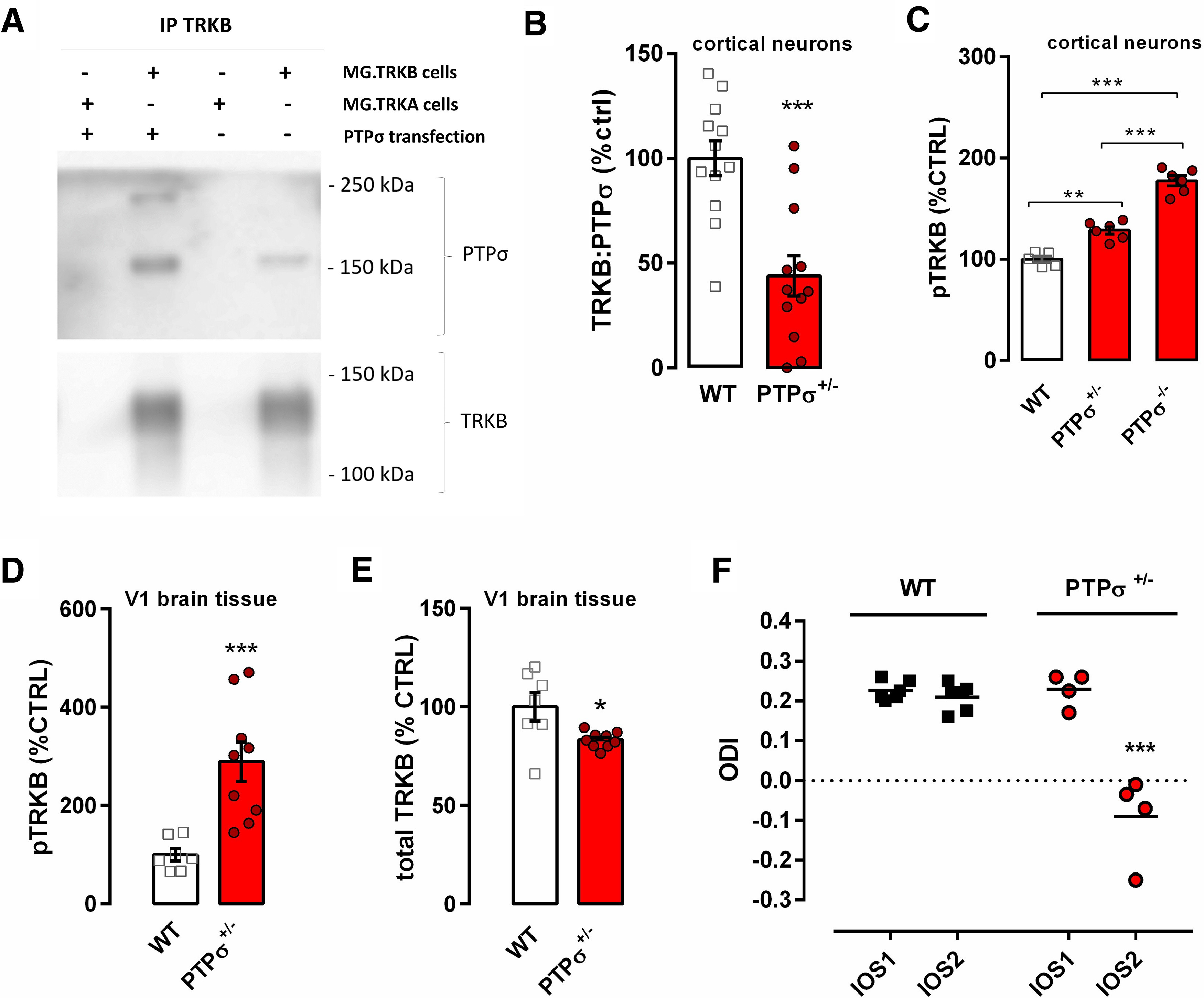
Deletion of CSPG receptor PTPσ facilitates pTRKB and delays closure of the critical period in the visual cortex of adult mice. A, PTPσ can be immunoprecipitated with anti-TRKB antibody in samples from a TRKB-expressing, but not from a TRKA-expressing, cell line. B, Interaction of TRKB and PTPσ is reduced in embryonic cortical cultures from PTPσ+/− mice when compared with those from WT mice. C, pTRKB is increased in cortical cultures from PTPσ+/− and PTPσ−/− mice. D, Adult PTPσ+/− mice have increased pTRKB and (E) a slight decrease in total TRKB levels in the visual cortex. F, Critical period-like plasticity is present in the visual cortex of two-month-old PTPσ+/− mice (red circles) but not in WT littermates (black squares), as measured by optical imaging of the intrinsic signal before (IOS1) and after 3.5 d of MD (IOS2). ODI: ocular dominance index. Columns and bars represent mean ± SEM, respectively, and scattered points represent individual values. Data were analyzed by two-way (F) or one-way ANOVA (C) followed by Bonferroni's or Tukey's post hoc, respectively; unpaired t test (B) or Mann–Whitney test (D, E); *p < 0.05, **p < 0.005, ***p < 0.0005.
Next, we set out to investigate the role of PTPσ in pTRKB by using mice heterozygous for PTPσ (PTPσ+/−). Since transgenic mice quite often develop compensatory mechanisms to counteract genetic deficiency, we wanted to confirm that neurons lacking PTPRS allele would present comparable reduction in PTPσ:TRKB protein interaction. We prepared E18 neuronal cultures from cortex of WT or PTPσ+/− littermates, and compared them after 7 DIV using co-immunoprecipitation. We observed a reduction of ∼50% in the interaction between TRKB and PTPσ in samples from PTPσ+/− mice as compared with WT (Fig. 2B). Then, we further investigated whether PTPσ influences pTRKB. We compared cortical pTRKB levels in embryonic cultures from PTPσ−/− and PTPσ+/− mice with those in cultures prepared from their WT littermates. Notably, PTPσ had a clear gene dosage-dependent suppressive effect on the basal pTRKB. PTPσ+/− cortical cultures demonstrate increased pTRKB as compared with cultures from their WT littermates, and PTPσ−/− samples demonstrate yet further increased pTRKB significantly differing from both PTPσ+/− and WT samples (Fig. 2C). We also checked the levels of pTRKB in samples from the adult visual cortex of mice heterozygous for PTPRS gene (PTPσ+/−). In line with our in vitro data, adult PTPσ+/− mice exhibit increased basal TRKB autophosphorylation in the visual cortex (Fig. 2D), despite having slightly reduced levels of total TRKB (Fig. 2E), which may represent a compensatory downregulation because of a long-term increase in pTRKB.
Finally, we investigated whether increased TRKB signaling produced by genetic deficiency of PTPσ might render the cortical structures of PTPσ+/− mice susceptible to plastic changes. We tested adult PTPσ+/− mice and their WT littermates in an ocular dominance plasticity paradigm using optical imaging. As expected, MD did not induce an ocular dominance shift in adult WT animals; however, it did induce a shift in ocular dominance in PTPσ+/− mice (Fig. 2F), indicating that closure of the critical period was delayed or prevented in the PTPσ+/− mice.
Antidepressant treatment disrupts TRKB:PTPσ interaction
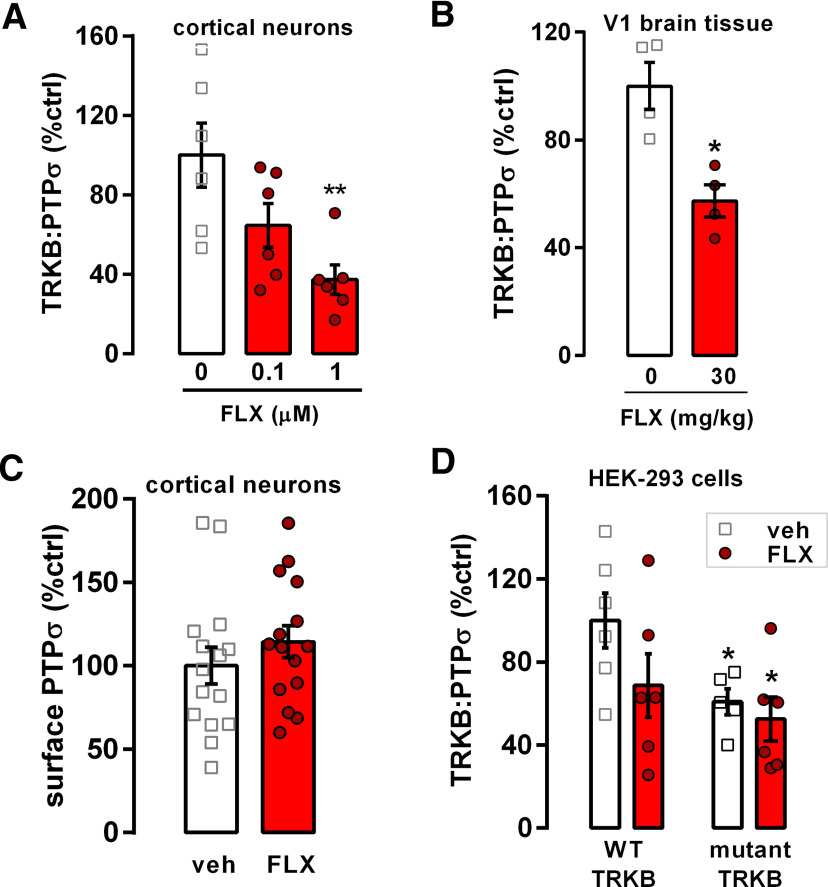
Antidepressants have been demonstrated to induce activation of TRKB receptors in the brain (Saarelainen et al., 2003; Rantamäki et al., 2007; Castrén and Rantamäki, 2010). Moreover, fluoxetine, a widely used antidepressant, induces ocular dominance plasticity in the adult visual cortex through BDNF-TRKB signaling (Maya Vetencourt et al., 2008) and reduces percentage of PNNs enwrapping PV+ interneurons in the amygdala and hippocampus, shifting PV+ interneurons toward an immature state and reopening brain plasticity (Karpova et al., 2011). Therefore, we asked whether fluoxetine treatment might have an effect on TRKB:PTPσ interaction, which could potentially establish a link between antidepressant-induced pTRKB and PNNs. We cultured rat primary cortical neurons for 7 DIV and challenged them with two different doses of fluoxetine (0.1 and 1 μm) for 30 min. Fluoxetine dose-dependently reduced the interaction between TRKB and PTPσ in vitro as measured by ELISA (Fig. 3A). Since fluoxetine has been shown to increase pTRKB as fast as 30 min after systemic injection (Saarelainen et al., 2003), we checked whether fluoxetine would be able to disrupt TRKB:PTPσ interaction in vivo within a similar timeframe. We treated animals with 30 mg/kg fluoxetine (intraperitoneally) and killed the animals 30 min after the injection. Acute fluoxetine treatment significantly reduced TRKB:PTPσ interaction in vivo (Fig. 3B).
Figure 3.
Fluoxetine disrupts TRKB and PTPσ interaction in vitro and in vivo. A, Fluoxetine (FLX) disrupts the interaction between TRKB and PTPσ in 7 DIV cortical neurons, and (B) in the visual cortex (V1) of mice systemically treated (30 mg/kg, i.p.) 30 min before tissue dissection. C, Fluoxetine does not affect the detection of PTPσ extracellular domain on the cell surface, indicating no effect on PTPσ surface exposure or PTPσ ectodomain shedding. D, Mutation of the TRKB transmembrane domain (R427A/Y433F) partially disrupts the TRKB:PTPσ interaction and abolishes the effects of fluoxetine on it. Columns and bars represent mean ± SEM, respectively. Data were analyzed by one-way ANOVA followed by Bonferroni's (A), two-way ANOVA (D), or unpaired t test (B, C); *p < 0.05, **p < 0.005.
PTPσ is proteolytically processed by cleavage in the juxtamembrane region, leading to shedding of the extracellular domain (Faux et al., 2007). To rule out the possibility that TRKB:PTPσ interaction experiments could have been influenced by a potential effect of the treatment on PTPσ shedding, we investigated the presence of PTPσ extracellular domain in the cell surface after fluoxetine treatment in cortical cells using cell-surface ELISA (Zheng et al., 2008; Fred et al., 2019). We found no effect of the drug (Fig. 3C). These data indicate that fluoxetine does not affect PTPσ shedding and positioning on cell surface, and supports the idea that the decrease in PTPσ levels in co-IP experiments is a result of decreased interaction with TRKB.
Antidepressants have recently been shown to directly interact with TRKB through the transmembrane region (Casarotto et al., 2019). Moreover, it has been previously suggested that PTPσ interacts with TRKA receptors through its transmembrane region (Faux et al., 2007). Therefore, we asked whether disruption in TRKB:PTPσ interaction after fluoxetine treatment is potentially mediated by the antidepressant's binding to the same region of TRKB where interaction with PTPσ takes place. We transfected HEK293T cells with either a WT TRKB plasmid or a TRKB plasmid carrying two point mutations in the transmembrane region of TRKB (arginine R427 mutated to alanine, R427A; and tyrosine Y433 mutated to phenylalanine, Y433F); these point mutations disrupt a cholesterol interaction site in the TRKB transmembrane region critical for antidepressant interaction (Cannarozzo et al., 2020). R427A/Y433F mutation caused a dramatic decrease in TRKB:PTPσ interaction levels, however, the interaction was not completely lost, suggesting that multiple sites of interaction may exist. Nevertheless, fluoxetine (10 μm) failed to influence TRKB:PTPσ interaction in the cells carrying R427A/Y433F mutated TRKB (Fig. 3D), providing evidence that the site of interaction of PTPσ and fluoxetine with TRKB lies within the transmembrane region of TRKB.
Discussion
In the current study, we have investigated a possible convergence of the two plasticity-inducing methods, chABC and antidepressant treatment, on the same molecular pathway involving reduced dephosphorylation of TRKB by PTPσ within PV+ interneurons. PNNs, extracellular structures rich in CSPGs, are associated with a reduction of plasticity in the adult brain. Maturation of PNNs coincides with the closure of the critical period (Berardi et al., 2004; Fawcett et al., 2019), and digestion of PNNs has been shown to promote plasticity and re-open critical periods for certain functions in the adult nervous system (Fawcett et al., 2019). Local injections of chABC promote axonal regeneration and functional recovery after spinal cord injury (Bradbury et al., 2002), restore ocular dominance plasticity in the visual cortex (Pizzorusso et al., 2002), and promote extinction of fear memories in rodents (Gogolla et al., 2009). PNNs preferentially enwrap PV+ interneurons that synchronize oscillatory activity of brain networks and mediate neuronal plasticity and learning. Our experiments demonstrate that the effects of chABC depend on TRKB signaling in PV+ interneurons, since genetic deficiency of TRKB in PV+ cells abrogates the ability of chABC to induce plasticity in the visual cortex of adult mice. Moreover, we have shown that chABC treatment increases pTRKB, which promotes TRKB signaling, while treatment with CSPG aggrecan decreases phosphorylation of TRKB induced by BDNF.
CSPGs, major components of PNNs, have been shown to exert inhibitory action on plasticity through protein tyrosine phosphatase receptor type S (Shen et al., 2009). PTPσ has been demonstrated to be critical for the inhibitory effects of CSPGs on plasticity and regeneration since CSPGs exert little inhibitory effect on axonal outgrowth of neurons prepared from PTPσ−/− mice as compared with WT (Shen et al., 2009). Interestingly, PTPσ has been shown to interact with and dephosphorylate all three TRK receptors and inhibit their activation even in the presence of their cognate neurotrophins (Faux et al., 2007). Faux et al. (2007) provided evidence that PTPσ forms stable complexes with TRKA and TRKC and only weakly interacts with TRKB. However, subsequent research has shown that PTPσ co-immunoprecipitates with TRKB in samples from cortical neurons and mediates abrogation of BDNF-induced dendritic spine formation by CSPGs (Kurihara and Yamashita, 2012). We have now demonstrated that PTPσ dephosphorylates TRKB in vitro and in vivo based on increased phosphorylation of TRKB in cultures prepared from PTPσ KO homozygous or heterozygous mice (PTPσ−/− and PTPσ+/−) and in samples from the visual cortex of PTPσ+/− mice. Moreover, we observed that genetic deficiency of PTPσ delays the closure of critical period-like plasticity in the adult visual cortex, an effect that we demonstrated to be dependent on TRKB in PV+ neurons.
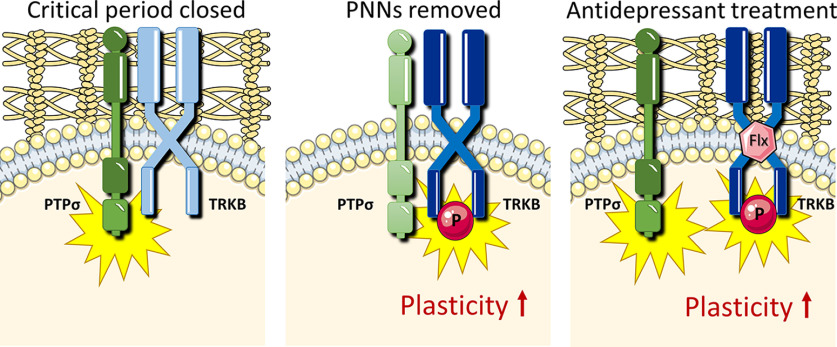
TRKB signaling is known to be critical for activation of plasticity by antidepressant drug treatment and for the behavioral consequences of it (Saarelainen et al., 2003; Duman and Monteggia, 2006; Autry and Monteggia, 2012; Castrén and Antila, 2017). Moreover, our group has recently demonstrated that TRKB activation in PV+ interneurons is sufficient for the induction of juvenile-like plasticity in the adult brain and necessary for the plasticity-inducing effects of fluoxetine (Winkel et al., 2020). We now demonstrate that the antidepressant fluoxetine induces disruption of interaction between TRKB and PTPσ, releasing TRKB from the suppressive activity of the phosphatase and promoting its activation (Fig. 4). These data suggest that reduced dephosphorylation of TRKB by PTPσ is at least one mechanism through which antidepressant treatment promotes plasticity.
Figure 4.
Schematic model of how chondroitinase and antidepressant treatments promote plasticity by releasing TRKB from dephosphorylating control of PTPσ. Left, In the presence of PNNs, active PTPσ dephosphorylates TRKB and suppresses its signaling. Middle, In the absence of PNNs, PTPσ is inactive and pTRKB is facilitated. Right, Fluoxetine (FLX) disrupts TRKB:PTPσ interaction, promoting TRKB signaling.
Antidepressants have recently been shown to directly interact with TRKB through its transmembrane domain (Casarotto et al., 2021). Our data now show that the mutation that inhibits antidepressant binding to the transmembrane region of TRKB partially disrupts its interaction with PTPσ, and that fluoxetine treatment has no further additive effect on TRKB:PTPσ interaction in TRKB mutant cells. These data suggest that TRKB and PTPσ interact in the transmembrane region of TRKB, and that binding of fluoxetine to TRKB disrupts TRKB:PTPσ interaction.
Our findings suggest a general mechanism responsible for the opening of critical period-like plasticity in the adult brain by chABC and antidepressant treatment (Fig. 4). We propose that both methods converge on the same pathway involving reduced inhibitory interaction between TRKB and PTPσ in PV+ neurons, which releases TRKB from the PTPσ-mediated dephosphorylation and promotes its autophosphorylation. Chondroitinase treatment downregulates CSPG-mediated activation of PTPσ, which allows for enhanced signaling of TRKB (Fig. 4). Antidepressants, on the other hand, disrupt the interaction between TRKB and PTPσ in the membrane, promoting TRKB activation (Fig. 4).
Taken together, our data reveal that interaction between TRKB and PTPσ in PV+ interneurons is a critical regulator of chABC-induced and antidepressant-induced plasticity in the adult cortex. The similarity in the plasticity-promoting effects of chABC and antidepressants prompted us to focus on these two treatments, but there are a number of other methods known to reactivate juvenile-like plasticity in the adult cortex (Bavelier et al., 2010), such as enriched environment (Nithianantharajah and Hannan, 2006; Sale et al., 2007, 2014) and cross-modal manipulation of sensory functions (Rodríguez et al., 2018). It will be interesting in future studies to investigate whether induction of plasticity by these means recruits the same molecular pathways involving enhanced TRKB activation in PV+ interneurons.
Footnotes
This work was supported by the Centre for International Mobility (CIMO) Grant TM-17-10472 (to A.L.), the ERC Grant #322742-iPLASTICITY, EU Joint Program — Neurodegenerative Disease Research (JPND) CircProt Projects #301225 and #643417, Doctoral Program Brain & Mind, Sigrid Jusélius Foundation, Jane and Aatos Erkko Foundation, and the Academy of Finland Grants #294710 and #307416 (to E.C.). We thank Sulo Kolehmainen and Seija Lågas for technical help, Dr. Heikki Rauvala for the donation of the PTPσ knockout mice and for his expert comments on the manuscript, and Katja Kaurinkoski for language revision.
The authors declare no competing financial interests.
References
- Antila H, Autio H, Turunen L, Harju K, Tammela P, Wennerberg K, Yli-Kauhaluoma J, Huttunen HJ, Castrén E, Rantamäki T (2014) Utilization of in situ ELISA method for examining Trk receptor phosphorylation in cultured cells. J Neurosci Methods 222:142–146. 10.1016/j.jneumeth.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258. 10.1124/pr.111.005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baho E, Chattopadhyaya B, Lavertu-Jolin M, Mazziotti R, Awad PN, Chehrazi P, Groleau M, Jahannault-Talignani C, Vaucher E, Ango F, Pizzorusso T, Baroncelli L, Di Cristo G (2019) p75 neurotrophin receptor activation regulates the timing of the maturation of cortical parvalbumin interneuron connectivity and promotes juvenile-like plasticity in adult visual cortex. J Neurosci 39:4489–4510. 10.1523/JNEUROSCI.2881-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK (2010) Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30:14964–14971. 10.1523/JNEUROSCI.4812-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L (2004) Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron 44:905–908. 10.1016/j.neuron.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640. 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- Cang J, Kalatsky VA, Löwel S, Stryker MP (2005) Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci 22:685–691. 10.1017/S0952523805225178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannarozzo C, Fred SM, Girych M, Biojone C, Enkavi G, Róg T, Vattulainen I, Casarotto PC, Castrén E (2020) Cholesterol recognition motifs in the transmembrane domain of the tyrosine kinase receptor family: the case for TRKB. bioRxiv 734012. [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz CRAF, Laukkanen L, Cardon I, Antila H, Rog T, Piepponen TP, Bramham CR, Normann C, Lauri SE, Saarma M, Vattulainen I, Castrén E (2021) Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castreń E (2013) Neuronal network plasticity and recovery from depression. JAMA Psychiatry 70:983–989. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70:289–297. 10.1002/dneu.20758 [DOI] [PubMed] [Google Scholar]
- Castrén E, Antila H (2017) Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry 22:1085–1095. 10.1038/mp.2017.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR (2011) Proteoglycan-specific molecular switch for RPTP clustering and neuronal extension. Science 332:484–488. 10.1126/science.1200840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Giger RJ (2010) A new role for RPTPσ in spinal cord injury: signaling chondroitin sulfate proteoglycan inhibition. Sci Signal 3:pe6. 10.1126/scisignal.3110pe6 [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. 10.1016/j.biopsych.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Elchebly M, Wagner J, Kennedy TE, Lanctôt C, Michaliszyn E, Itié A, Drouin J, Tremblay ML (1999) Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase σ. Nat Genet 21:330–333. 10.1038/6859 [DOI] [PubMed] [Google Scholar]
- Faux C, Hawadle M, Nixon J, Wallace A, Lee S, Murray S, Stoker A (2007) PTPσ binds and dephosphorylates neurotrophin receptors and can suppress NGF-dependent neurite outgrowth from sensory neurons. Biochim Biophys Acta 1773:1689–1700. 10.1016/j.bbamcr.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Oohashi T, Pizzorusso T (2019) The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 20:451–465. 10.1038/s41583-019-0196-3 [DOI] [PubMed] [Google Scholar]
- Fred SM, Laukkanen L, Brunello CA, Vesa L, Göös H, Cardon I, Moliner R, Maritzen T, Varjosalo M, Casarotto PC, Castrén E (2019) Pharmacologically diverse antidepressants facilitate TRKB receptor activation by disrupting its interaction with the endocytic adaptor complex AP-2. J Biol Chem 294:18150–18161. 10.1074/jbc.RA119.008837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261. 10.1126/science.1174146 [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Koponen E, Hoppe E, Wong G, Castrén E (2001) Truncated trkB.T1 is dominant negative inhibitor of trkB.TK+-mediated cell survival. Biochem Biophys Res Commun 280:1352–1358. 10.1006/bbrc.2001.4296 [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888. 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S (2005) A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3:e159 10.1371/journal.pbio.0030159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübener M, Bonhoeffer T (2014) Neuronal plasticity: beyond the critical period. Cell 159:727–737. 10.1016/j.cell.2014.10.035 [DOI] [PubMed] [Google Scholar]
- Hylin MJ, Orsi SA, Moore AN, Dash PK (2013) Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn Mem 20:267–273. 10.1101/lm.030197.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agústsdóttir A, Antila H, Popova D, Akamine Y, Bahi A, Sullivan R, Hen R, Drew LJ, Castrén E (2011) Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 334:1731–1734. 10.1126/science.1214592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara D, Yamashita T (2012) Chondroitin sulfate proteoglycans down-regulate spine formation in cortical neurons by targeting tropomyosin-related kinase B (TrkB) protein. J Biol Chem 287:13822–13828. 10.1074/jbc.M111.314070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JCF, Dick G, Wang D, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71:1073–1089. 10.1002/dneu.20974 [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L (2008) The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320:385–388. 10.1126/science.1150516 [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R (1999) Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24:401–414. 10.1016/s0896-6273(00)80853-3 [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7:697–709. 10.1038/nrn1970 [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251. 10.1126/science.1072699 [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Männistö PT, Castrén E (2007) Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 32:2152–2162. 10.1038/sj.npp.1301345 [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Chakraborty D, Schrode KM, Saha R, Uribe I, Lauer AM, Lee HK (2018) Cross-modal reinstatement of thalamocortical plasticity accelerates ocular dominance plasticity in adult mice. Cell Rep 24:3433–3440.e4. 10.1016/j.celrep.2018.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castrén E (2003) Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu MP, Nikkilä O, Lågas S, Kolehmainen S, Castrén E (2019) Culturing primary neurons from rat hippocampus and cortex. Neuronal Signal 3:NS20180207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L (2007) Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci 10:679–681. 10.1038/nn1899 [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L (2014) Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol Rev 94:189–234. 10.1152/physrev.00036.2012 [DOI] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG (2009) PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596. 10.1126/science.1178310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Wei X, Wang X, Du S, Liu W, Song J, Wang Y (2019) Perineuronal nets protect long-term memory by limiting activity-dependent inhibition from parvalbumin interneurons. Proc Natl Acad Sci USA 116:27063–27073. 10.1073/pnas.1902680116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinzeig A, Molotkov D, Castrén E (2017) Chronic imaging through “transparent skull” in mice. PLoS One 12:e0181788. 10.1371/journal.pone.0181788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, Craig AM (2011) Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron 69:287–303. 10.1016/j.neuron.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori J, Winkel F, Didio G, Llach Pou M, Castrén E (2018) iPlasticity: induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin Neurosci 72:633–653. 10.1111/pcn.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fawcett J (2012) The perineuronal net and the control of CNS plasticity. Cell Tissue Res 349:147–160. 10.1007/s00441-012-1375-y [DOI] [PubMed] [Google Scholar]
- Wiesel TN (1982) Postnatal development of the visual cortex and the influence of environment. Nature 299:583–591. 10.1038/299583a0 [DOI] [PubMed] [Google Scholar]
- Winkel F, Voigt MB, Didio G, Mateo S, Jetsonen EL, Pou M, Steinzeig A, Ryazantseva M, Harkki J, Englund J, Khirug S, Rivera C, Palva S, Taira T, Lauri S, Umemori J, Castren E (2020) TrkB activation in Parvalbumin interneurons orchestrates cortical plasticity. bioRxiv. doi: 10.1101/2020.04.27.063503 [DOI] [Google Scholar]
- Zheng J, Shen WH, Lu TJ, Zhou Y, Chen Q, Wang Z, Xiang T, Zhu YC, Zhang C, Duan S, Xiong ZQ (2008) Clathrin-dependent endocytosis is required for TrkB-dependent Akt-mediated neuronal protection and dendritic growth. J Biol Chem 283:13280–13288. 10.1074/jbc.M709930200 [DOI] [PubMed] [Google Scholar]