Abstract
With the growing number of transgender and gender-nonbinary individuals who are becoming visible, it is clear that there is a need to develop a rigorous evidence base to inform care practice. Transgender health research is often limited to HIV/AIDS or mental health research and is typically subsumed in larger studies with general LGBTQ focus. Although the number of knowledgeable health care providers remains modest, the model for the medical approach to transgender health is shifting owing to growing social awareness and an appreciation of a biological component. Gender-affirming medicine facilitates aligning the body of the transgender person with the gender identity; typical treatment regimens include hormone therapy and/or surgical interventions. While broadly safe, hormone treatments require some monitoring for safety. Exogenous estrogens are associated with a dose-dependent increase in venous thromboembolic risk, and androgens stimulate erythropoiesis. The degree to which progressing gender-affirming hormone treatment changes cancer risk, cardiac heart disease risk, and/or bone health remains unknown. Guidelines referencing the potential exacerbation of cancer, heart disease, or other disease risk often rely on physiology models, because conclusive clinical data do not exist. Dedicated research infrastructure and funding are needed to address the knowledge gap in the field.
Introduction
Transgender people face barriers in care that stem from gaps in medical research, provider comfort, and health care coverage. Adopting language specific to transgender health care that includes definitions with consensus is essential for engaging in meaningful dialogue with patients and communicating within the medical community (refs. 1, 2; and Table 1). Importantly, “gender identity” is the term used to reference a person’s internal sense of their own sex, that is, of being male, female, neither, or some combination. Transgender persons are broadly defined as having gender identity that is not aligned with sex recorded at birth, the latter typically reflecting visible external genitalia at the time (1, 2). Adjectives used to label the incongruence between gender identity and sex recorded at birth include “transgender,” “trans,” “transsexual,” “gender nonbinary,” “gender incongruent,” and “genderqueer.” The adjective used to label individuals who are not transgender is “cisgender.” “Gender expression” references how a person signals gender identity both internally and to others, while “gender dysphoria” is the mental health diagnosis that describes the discomfort felt by some gender-incongruent individuals. Although dysphoria is a mental health diagnosis, it is the only diagnosis in the current International Classification of Diseases (ICD) for transgender people. Even though the need for medical treatment for transgender individuals does not depend on the individual suffering from dysphoria, many payors require the label for reimbursement of transgender medical and surgical interventions (3).
Table 1. Terms and definitions.
Since the number of transgender and gender-nonbinary individuals becoming visible is increasing, there is a pressing need for clinicians to conduct rigorous research and provide evidence-informed care. The most recent broad national survey in the United States estimated that 0.6% of US adults, or 1.4 million people, were transgender in 2016 (4). In addition, from 0.6% to 2.7% of children report gender incongruence (5), although not all such children seek medical intervention as they age (6). Most other population-based surveys fail to accurately measure gender identity and may underestimate the number of transgender people. Similarly, researchers often include transgender populations in larger studies that focus generally on lesbian, gay, bisexual, and transgender (LGBT) health, a practice that may misguide interpretation and may misguide and bias interpretation of health problems (7). In fact, research on transgender health has focused often on HIV/AIDS, neglecting other priorities in transgender medicine. This Review focuses on the gaps in gender-affirming medical research and care, emphasizing the principles and potential risks of hormone therapy.
Health care disparities and negative health outcomes
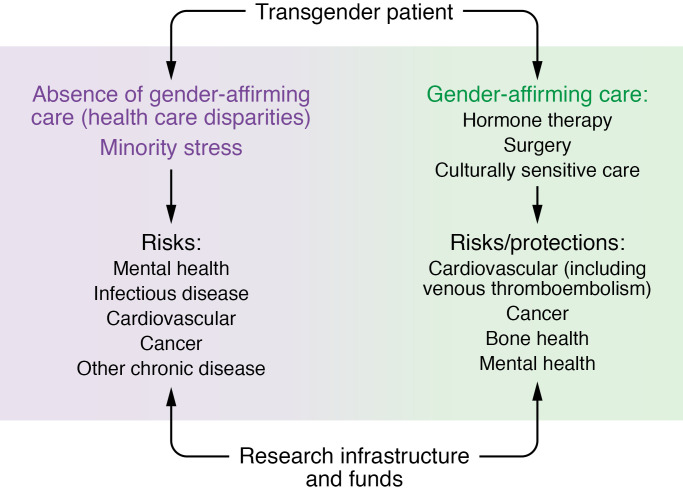
The most rigorous studies of transgender people have focused on general disparities in health access and outcomes among transgender people independent of treatment considerations (8). For example, cross-sectional studies report disproportionate barriers to health care services including frank medical mistreatment (9–12). Such barriers to access likely play a substantial role in the health outcome disparities among transgender people, which include increased rates of some cancers, increased substance abuse, greater numbers of people reporting mental health conditions, increased infections, and higher rates of chronic diseases (refs. 10, 11, 13–21; and Figure 1).
Figure 1. Potential research priorities in transgender health care to address with a dedicated research infrastructure and funding.
Although there is a modest literature, gaps remain in determining the degree of morbidity and mortality associated with the limited access to health care suffered by transgender people, along with minority stress exacerbated by an unwelcoming health care environment. Areas of increased risk of harm include mental health morbidity, cardiac disease, infectious disease, and chronic disease in general. Gaps in research regarding provision of gender-affirming care are even greater than gaps in defining the harms from lack of care. While gender-affirming medical care, including hormone treatment, may associate with decreased mental health morbidity, the degree and direction of impact of hormone treatment on numerous other physiological and health processes remain to be studied robustly. Areas to study include cardiovascular disease risk, cancer risk, bone health, and athleticism. To address the gaps in research and evidence-based care, dedicated research infrastructure and funding are needed.
Work from the Veterans Health Administration has identified health disparities among transgender veterans (16, 22–25). A retrospective study involving 5117 veterans found that the top two causes of mortality among the general US population, cardiovascular disease and cancer, were not increased among transgender people (16). However, it was noted that mortality among transgender people related to suicide was increased relative to the general population (16, 25). The data were not stratified according to gender-affirming hormone treatment or surgery.
As one may predict in the setting of decreased access to care, mental health morbidity is greater among transgender people relative to cisgender people. Several studies show increased rates of suicide, suicide attempts, depression, and anxiety among transgender individuals (22, 24, 26, 27). In the European Network for the Investigation of Gender Incongruence (ENIGI) study, transgender people had a 30% rate of suicide ideation or attempt (28). Gender-affirming care, including hormone therapy, appears to have beneficial psychosocial effects, at least in part (29–31).
Although health disparities research may be more robust than some other research areas for transgender people, there are still enormous gaps. Despite an abundant literature (32), there are major voids in understanding the degree to which stigma and minority stress influence the reported disparities and even health outcomes independent of disparities. Further, existing literature fails to adequately consider ethnicity, affluence, and other demographics (8). Such failures may mask areas of even greater morbidity than is recognized on the basis of results from the existing heterogeneous cohorts.
Gender identity biology training reduces barriers to care
To date, most data regarding gender identity are limited to cross-sectional analyses of convenience samples with occasional passive evaluation of data collected for clinical purposes. Still, the approach to both the study of gender identity and the development of best practices has shifted substantially over the past two decades (1, 2).
Transgender people historically report that the largest barrier to care is a lack of knowledgeable providers (8) resulting in an inability to access appropriate and culturally sensitive care. Data suggest that a deficit in formal instruction regarding transgender care in establishment medical education, beyond cultural sensitivity (33–37), results in a lack of providers. While efforts to increase cultural sensitivity training among medical providers are useful, they have failed to close the gap in provider comfort with delivering gender-affirming care to transgender people (37). Indeed, physicians attribute their discomfort when treating transgender patients to insufficient training (38–40). Additional benefit has been reported when training includes data on gender identity biology and data on gender-affirming treatment strategy (37).
Some of the neglect in conventional medical education may relate to the failure to recognize the substantial organic component underlying gender identity. The biological basis for gender identity has become more apparent in the recent past (41), shifting perspectives away from a malleable gender identity model (42). Notably, data suggest a durable, biological underpinning to gender identity that is present at birth for cisgender people, transgender people, and nonbinary people (41, 43). For example, for intersex/differences of sex development (DSD) persons, attempts to manipulate gender identity by external means have failed as a strategy (44, 45). In addition, identical twins have greater concordance with regard to transgender identity than do fraternal twins (46). Further, there are data connecting gender identity to brain anatomy (47).
The only mechanistic data on the biology underlying gender identity come from observational studies relating to in utero androgen exposure. Increased rates of male gender identity are noted among people with female chromosomes (XX) with congenital adrenal hyperplasia who were exposed to excess androgen in utero (48). Those with complete androgen insensitivity syndrome, by contrast, have female gender identity among other traits associated with the lack of androgen action in their bodies (49).
Gender-affirming treatment approaches for transgender people
Understanding of gender-affirming treatment approaches can guide studies of best practices in the care of transgender people. In addition, gender-affirming treatment interventions can serve as an opportunity to learn about hormone mechanisms in general human biology.
Hormone treatment for transfeminine individuals
Many transgender women seek hormone treatment to better align their bodies with their gender identities (1, 2). The current approach relies on reference ranges for testosterone levels as the main treatment target to decrease testosterone levels from the male range (300–1000 ng/dL) to the female range (<50 ng/dL), although such levels are not always achieved (50, 51). While orchiectomy is the most effective means to decrease testosterone levels, many transgender women opt only for medical treatment, especially initially. Transgender women with intact testes may require higher estrogen doses to suppress testosterone into the female range (50); the typical formulations are oral, transdermal, and parenteral estradiol. Oral conjugated estrogens are sometimes used, and oral ethinyl estradiol is avoided.
Supraphysiological doses of estrogens suppress androgen production (via central feedback) but may associate with increased risk of thrombosis. Thus, typical regimens may also include other testosterone-lowering agents so that lower doses of estrogens can be administered. The three most common adjunctive androgen-lowering/-inhibiting agents are spironolactone, which blocks androgen action at its receptor and may decrease testosterone levels (1, 2, 52, 53); cyproterone acetate, a progestin popular in Europe; and gonadotropin-releasing hormone (GnRH) agonist therapy. Estrogen and adjunct antiandrogen therapies are started together.
Progestins like medroxyprogesterone acetate and micronized progesterone can suppress gonadotropins and, therefore, suppress testosterone secretion via central feedback. Progestins are not recommended by the Endocrine Society because medroxyprogesterone associates with both cardiovascular and breast cancer risk in older postmenopausal women when used in combination with conjugated estrogens (54, 55). Finasteride inhibits conversion of testosterone to the more potent dihydrotestosterone, which targets some tissues, including prostate and scalp. Finasteride is a treatment option for individuals with higher testosterone levels who are experiencing male pattern hair loss.
Hormone treatment for transmasculine individuals
For transgender men, testosterone can be administered in a dose appropriate to achieve hormone levels in the normal physiological range for men (300–1000 ng/dL). The most widely used testosterone formulations include testosterone esters, testosterone gels, and testosterone patches. Injectable testosterone esters may be administered subcutaneously rather than intramuscularly (56). Other formulations include a long-acting testosterone (testosterone undecanoate) and a buccal testosterone patch.
Note that maximum dosing is unnecessary for transmasculine and transfeminine individuals interested in modified regimens, for reasons that include nonbinary gender identity. The chief clinical concern among those prescribing gender-affirming hormones is to avoid risk to bone health that is associated with hypogonadism.
Risks associated with gender-affirming medical interventions
Transfeminine regimens and thromboembolism
Reports suggest that transgender women undergoing hormone therapy have an increased risk of venous thromboembolism (VTE) (refs. 57–61; and Figure 1). Indeed, VTE is highlighted at the top of the list of concerns referenced by the Endocrine Society transgender care guideline (62). It remains unestablished whether and to what degree such risks might relate to hormone dose, route of administration, duration of hormone therapy, or some other risk factor unique to transgender individuals.
The VTE risk was noted historically in studies that used the more thrombogenic estrogen ethinyl estradiol (63, 64). By contrast, conjugated equine estrogens and 17β-estradiol appear much safer (65), and there are indications that 17β-estradiol is the safer of the two (66). Subsequent studies less consistently demonstrated VTE risk when the estrogen of choice was 17β-estradiol (21, 67). Further limiting data interpretation, hypercoagulable risk factors accompanied many of the VTE cases (58).
More recent large cross-sectional analyses continue to reveal increased VTE risk, with some contribution from duration of therapy, although the impact of estrogen type and other aspects of transgender health care (including neglect of care) remains unaddressed (57).
In a large meta-analysis of cisgender women, increased thrombogenicity associated with increased estrogen doses and with the addition of progestins to estrogen regimens across numerous estrogen products used (68). Studies also report a substantially lower risk of VTE in transgender women with the use of transdermal estradiol (61, 65, 69), although there are no head-to-head studies. Transgender women taking oral conjugated estrogens displayed elevated proinflammatory cytokine levels (IL-1, IL-6, IL-8, and TNF-α) along with increased white blood cell count (58, 70). Transdermal estrogens have not been reported to increase proinflammatory cytokines and procoagulant factors in either cisgender women or transgender women. However, studies comparing transdermal estrogen delivery with oral estrogen delivery to date have failed to consider serum estradiol levels or doses of estrogens used as potential contributing risk factors for VTE. Further, patient demographics and comorbidities may also alter VTE risk profile. Many of the studies analyzed report coexisting conditions that increase study participants’ risk for VTE, including smoking, HIV disease, malignancy, high cholesterol, clotting disorder, and hypertension. Thus, whether the benefit ascribed to transdermal estrogen preparations relates to decreased dose delivered, the avoidance of first-pass proinflammatory cytokine metabolism in the liver, or some other mechanism, is unknown.
Transmasculine regimens and erythropoiesis
Although exogenous testosterone treatment stimulates erythropoiesis, no increase in rates of thrombogenicity has been reported in cross-sectional studies of transgender men (57, 60, 71–73). There are moderate-quality data suggesting a rise in hematocrit associated with testosterone treatment in transgender persons. However, data are insufficient to associate testosterone therapy in transgender persons with polycythemia per se. When polycythemia is observed, health care providers must seek additional causes. Data are also insufficient to stratify the rise in hematocrit by route of testosterone administration or by testosterone formulation.
Hormone treatment and cardiovascular disease risk
The mechanisms of cardiovascular disease risk among transgender people are likely complex. Hormone therapy might increase risk or protect individuals depending on the circumstance (74). Further, historical studies vary in regard to the controls used to assess risk, including both cisgender men and cisgender women (57). In addition, risk might reasonably relate both to current sex steroid use and to historical sex steroid levels.
Historical studies with cardiovascular endpoints observed increased rates among transgender women relative to cisgender women (72, 74). Unfortunately, there was no ability to control for important factors like greater tobacco use among transgender women relative to cisgender people. Further, such studies did not observe an actual increase in anticipated risk for a given individual beginning hormone therapy. When cisgender men were used as controls, the risk for transgender women was comparable or improved (72).
Remarkably, transgender men did not exhibit any difference in the rate of stroke, transient ischemic attack, or myocardial infarction compared with cisgender men used as reference controls in these studies.
Diabetes mellitus.
Conditions like diabetes mellitus might serve as flags for increased incidence of morbidity that worsens cardiac risk. There are no broad population data on the prevalence or incidence of diabetes among transgender people overall relative to cisgender people.
With hormone therapy, transgender women show increased insulin resistance, possible increased fasting glucose, and increased subcutaneous fat (75, 76). Transgender men are reported to have increased insulin resistance, possible decreased fasting glucose, and increased fat (75, 76). The fact that data suggest increased insulin resistance over time in both transgender men and transgender women (despite opposite treatment regimens) leaves open the possibility that the reports describe a secular trend with aging rather than a hormone-dependent change.
A prospective study of transgender men and women treated with hormone therapy showed an increase in metabolic syndrome above that predicted for the general population; however, the risks related to some other morbidities were not addressed (77). For example, the highest risk observed was among the patients with coexisting psychiatric morbidity.
Hypertension.
Whether sex steroid therapy like the gender-affirming hormone regimens for transgender people results in increased hypertension is not known. A 2012 European study reported increased rates of hypertension among hormone-treated transgender people after 10 years (60). Like the diabetes mellitus studies that noted similar risk for people with opposite hormone regimens, increased hypertension rates could simply reflect secular change over time. Indeed, other groups have been unable to demonstrate significant blood pressure changes with hormone therapy among transgender people (74, 78, 79).
Lipids.
Studies of lipids in transgender people consistently show that estrogen-treated transgender women have higher HDL-cholesterol levels and testosterone-treated transgender men have lower HDL-cholesterol levels (60, 74, 78–83). Such changes occurred within months. It is unclear whether testosterone therapy demonstrably increases LDL-cholesterol. Although some studies report increases in triglyceride levels in both transgender men and transgender women, the change is inconsistent (73, 74, 76) and, when present, has been criticized as reflecting secular trends.
Hormone treatment and cancer
For both transgender men and transgender women, the overall incidence of malignancy has not been found to differ relative to cisgender controls (72).
Breast cancer.
Transgender women have lower rates of breast cancer than cisgender women (84, 85), although studies have been small. A Dutch study indicated that the breast cancer rate of transgender women was 25% that of cisgender women (84). However, the study lacked sufficient power to be conclusive, because the finding was based on 18 breast cancer diagnoses among 2260 transgender women. Similarly, a study of transgender US veterans reported a low rate of breast cancer but also lacked sufficient power to be conclusive, with only three cases of breast cancer among transgender women and seven cases among transgender men among a total of 5135 persons (24).
There are several proposed explanations for the relatively low breast cancer risk observed to date in transgender women. First, low reported cancer rates may be due both to underreporting among people with poor access to care and to failure to correctly classify transgender people in registries (86). Second, transgender women may have shorter lifetime exposure to estrogen if hormone treatment is initiated later in life (87). Finally, other elements may provide protection. For example, typical transfeminine hormone regimens do not contain progesterone, while among cisgender women, exogenous estrogen/progesterone combination regimens associate with increased breast cancer incidence relative to regimens containing estrogen alone (88).
Preliminary attempts to investigate the relative aggressiveness of breast cancer among transgender women include a study of ten breast cancers in transgender women in which the median age of diagnosis was 48 years. The majority of tumors were estrogen receptor negative, and the background lobular development in noncancerous tissue was similar to that observed in adolescent girls (89). There has also been one reported case of ductal carcinoma in situ (89).
For transgender men, a question is whether androgen exposure is of consequence to breast tissue, with worry expressed that higher levels of androgens might increase breast cancer risk (90). Alternatively, some suggest that testosterone exposure may have antiproliferative and apoptotic effects (91), with in vitro studies demonstrating that some androgens (testosterone and dihydrotestosterone) can inhibit the growth of cancer cells (91). In addition, a single study that examined the histology of mammary tissue from transgender men who had taken testosterone before mastectomy showed a reduction in glandular tissue and an increase in fibrous connective tissue (55, 85).
Prostate cancer.
Decreased androgen in transgender women might decrease the risk of prostate cancer. However, no rigorous data are available. There have been multiple case reports of prostate cancer among transgender women (92, 93). A 2014 Dutch retrospective chart review of 2306 orchiectomized transgender women reported an overall incidence of prostate cancer of 0.04% versus 8%–25% for cisgender men depending on the population studied. Conclusions from the data are limited owing both to limited screening and to the young average age (29 years) among subjects at the start of hormone therapy (94).
Other reproductive organ cancers.
Although transgender men have presented with ovarian, uterine, and cervical cancers (95), there is no current evidence to suggest that the rates of these conditions among transgender men are higher than the background rate in cisgender women (16, 72).
Confounding analysis, most transgender men in the European studies have undergone hysterectomy within 5 years of starting hormone therapy, limiting prolonged testosterone exposure to nonreproductive tissue.
Hormone treatment and other conditions
The literature contains case reports of conditions that are observed in people undergoing feminizing hormone therapy even when there is not much underlying expectation of a causal relationship. Reports include transgender individuals with meningiomas (96, 97), prolactinomas (98), and other pituitary tumors.
While the human pituitary cells (lactotrophs) are estrogen sensitive, there are no studies associating prolactin rise with treatment with estrogen alone in transgender people (51). There are modest data supporting an association between development of hyperprolactinemia and combination estrogen and cyproterone acetate treatment (99). However, prolactin levels return toward baseline when cyproterone acetate is discontinued, suggesting that the progestin is the operative stimulus for the prolactin rather than the estrogen (99). Notably, when estrogen is combined with either spironolactone or a GnRH agonist, prolactin levels are maintained (100, 101). Although the collected data are quite heterogeneous, no studies to date demonstrate prolactinoma development even when estrogens are combined with cyproterone acetate.
There are also reports of the occurrence of autoimmune conditions with a female predominance in transgender women receiving estrogen therapy, such as systemic lupus erythematosus (102). While there is underlying logic based on understanding of physiology, no causality has been demonstrated.
Hormone treatment and osteoporosis
Two main observations have been made regarding bone density in transgender individuals. The first observation is that transfeminine individuals might have lower bone density than their cisgender peers prior to any medical intervention. Proposed explanations to investigate include reduced rates of physical activity prior to treatment and increased rates of vitamin D deficiency (103). The second observation is that hormone-treated transgender people have higher bone density relative to cisgender people. Among transgender men, uninterrupted testosterone therapy may maintain or increase bone density (104), while prospective studies in transgender men with oophorectomy suggest that bone mineral density may decrease if testosterone use is interrupted or the dose is inadequate. Studies on feminizing hormone therapy are mixed because of heterogeneity in methodology, with cisgender men rather than cisgender women sometimes being used as a reference group. Despite the previously noted studies that used bone density as an endpoint, no long-term studies have examined specific hormone regimen or fracture risk (60).
Hormone treatment and athletic performance
A nascent area of research relates to the impact of androgens on athletic performance and the implications of such impact on transgender athletes. Although most transgender people may not be elite athletes, further understanding of physiology changes with sex hormones may be of broader use.
Based on research comparing cisgender men with cisgender women (105), there is evidence that testosterone level markedly drives many differences in athletic performance between elite male athletes and elite female athletes (105).
Although transgender women are not the only female athletes with XY chromosomes and they are not the only female athletes with potentially higher levels of testosterone than what is typically considered the female range (105), more public participation of transgender women in sports has resulted in interest in the subtleties of androgen impact on aspects of athletic performance (106). Despite a virtual absence of data, the androgen impact tends to be divided into two categories. The first is the impact of existing total testosterone levels on specific athletic skills (107). The second is the impact of puberty on athletic skills, specifically the puberty changes, such as bone mass, that persist despite later hormone manipulation (108).
Current regulations of the international track and field oversight organization World Athletics (formerly called the International Association of Athletics Federations) include a rule that women must have serum testosterone levels below 5 nmol/L to participate in the women’s category in some elite events. The concept is derived from data showing improved performance in some events among women with higher testosterone levels (107). Although not binding on athletic federations, the International Olympic Committee (IOC) has published guidance with similar logic except that the IOC uses a total testosterone level of 10 nmol/L (equivalent to 288 ng/dL) as a potential cut point.
The only specific data to date regarding comparative athletic performance of transgender women are from a study of eight long-distance runners who began gender-affirming interventions as adults (109). The investigation reported that, after medical treatment, the transgender women’s race placement in the women’s category was the same as their race placement had been in the men’s category prior to treatment.
The degree to which typical male puberty serves as an advantage or a disadvantage for transgender women later in life with regard to specific athletic skills remains uninvestigated (108). For example, transgender women who go through typically male puberty may have larger bones than cisgender women. Absence of typical male levels of testosterone and muscle mass to propel increased bone mass could diminish athletic capacity. Further, in sports in which athletes are segregated by weight classes, the fact that a transgender woman may have increased bone mass may pose an athletic disadvantage due to a lower muscle/bone ratio in comparison with women in the same weight class who have smaller bones.
Interest within the athletic community regarding the participation of transgender men in sports has been limited. Further, the impact of androgens on athletic skills of people who have undergone typically female puberty before androgen treatment remains unstudied. As well, there are no studies on transgender individuals who began treatment at puberty to determine whether there are components of athletic performance that are independent of hormone levels.
Gaps in patient-centered and comparative efficacy studies
There are virtually no data regarding patient-derived study priorities (110, 111). There are small-scale studies on the topic of gender-affirming hormone outcomes, including a series that examined the degree of breast development and time to cessation of menses. Studies on satisfaction regarding surgery outcome are limited to series with ill-matched controls or, in some cases, controls adopted from historical publications (112–114). There are also small-scale studies that evaluate people who have had both hormone treatment and surgical treatment but that fail to investigate the individual treatment entities in isolation.
Conclusion
Despite the need to inform care for transgender and gender-nonbinary patients and the potential opportunity for data applicable to all people, rigorous medical intervention outcomes studies among transgender people remain rare. Cross-sectional studies are compromised by heterogeneity in access to care among regions of the world. Where data do exist, study subjects are usually young, the studies themselves are not well controlled, and demographic stratification is lacking. Larger European studies are confounded by the fact that the research participants have had mastectomy, gonadectomy, and/or genital surgeries. By contrast, surgery is far rarer in the United States. In addition, the demographics of US, European, and other populations differ substantially.
Although the gaps in transgender medical research are large, the body of literature is increasing. Observations include increased morbidity and mortality in transgender individuals not receiving optimal care, along with modest increases in thromboembolic risk associated with feminizing hormone therapy.
Challenges to transgender medical research include an insufficient patient base at any given center, which hampers the statistical rigor for studying heterogeneous populations. A dedicated infrastructure and funding are needed to advance transgender health research (Figure 1).
Version 1. 02/15/2021
Electronic publication
Footnotes
Conflict of interest: JDS served on an advisory panel for Endo Pharmaceuticals.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(4):e142029.https://doi.org/10.1172/JCI142029.
References
- 1.Safer JD, Tangpricha V. Care of the transgender patient. Ann Intern Med. 2019;171(1):ITC1–ITC16. doi: 10.7326/AITC201907020. [DOI] [PubMed] [Google Scholar]
- 2.Safer JD, Tangpricha V. Care of transgender persons. N Engl J Med. 2019;381(25):2451–2460. doi: 10.1056/NEJMcp1903650. [DOI] [PubMed] [Google Scholar]
- 3. Lambda Legal. Accessing Coverage for Transition-Related Health Care. https://www.lambdalegal.org/know-your-rights/article/trans-health-care Updated July 7, 2020. Accessed July 7, 2020.
- 4. UCLA. How Many Adults Identify as Transgender in the United States? https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/ Accessed January 11, 2021.
- 5.Rider GN, et al. Health and care utilization of transgender and gender nonconforming youth: a population-based study. Pediatrics. 2018;141(3):e20171683. doi: 10.1542/peds.2017-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steensma TD, et al. Desisting and persisting gender dysphoria after childhood: a qualitative follow-up study. Clin Child Psychol Psychiatry. 2011;16(4):499–516. doi: 10.1177/1359104510378303. [DOI] [PubMed] [Google Scholar]
- 7. Institute of Medicine. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 8.Safer JD, et al. Barriers to healthcare for transgender individuals. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):168–171. doi: 10.1097/MED.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan KJ, et al. Estrogen levels do not rise with testosterone treatment for transgender men. Endocr Pract. 2018;24(4):329–333. doi: 10.4158/EP-2017-0203. [DOI] [PubMed] [Google Scholar]
- 10.Reisner SL, et al. Global health burden and needs of transgender populations: a review. Lancet. 2016;388(10042):412–436. doi: 10.1016/S0140-6736(16)00684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffee KD, et al. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016. doi: 10.1097/MLR.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 12.Meyer IH, et al. Demographic characteristics and health status of transgender adults in select US regions: behavioral risk factor surveillance system, 2014. Am J Public Health. 2017;107(4):582–589. doi: 10.2105/AJPH.2016.303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuttbrock L, et al. Lifetime risk factors for HIV/STI infections among male-to-female transgender persons. J Acquir Immune Defic Syndr 1999. 2009;52(3):417–421. doi: 10.1097/QAI.0b013e3181ab6ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baral SD, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 15.Feldman J, et al. HIV risk behaviors in the U.S. transgender population: prevalence and predictors in a large internet sample. J Homosex. 2014;61(11):1558–1588. doi: 10.1080/00918369.2014.944048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blosnich JR, et al. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269–276. doi: 10.1089/lgbt.2014.0050. [DOI] [PubMed] [Google Scholar]
- 17.Coulter RWS, et al. Differences in alcohol use and alcohol-related problems between transgender- and nontransgender-identified young adults. Drug Alcohol Depend. 2015;154:251–259. doi: 10.1016/j.drugalcdep.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Pedro KT, et al. Substance use among transgender students in California public middle and high schools. J Sch Health. 2017;87(5):303–309. doi: 10.1111/josh.12499. [DOI] [PubMed] [Google Scholar]
- 19.Guss CE, et al. Disordered weight management behaviors, nonprescription steroid use, and weight perception in transgender youth. J Adolesc Health. 2017;60(1):17–22. doi: 10.1016/j.jadohealth.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhejne C, et al. Long-term follow-up of transsexual persons undergoing sex reassignment surgery: cohort study in Sweden. PLoS One. 2011;6(2):e16885. doi: 10.1371/journal.pone.0016885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asscheman H, et al. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635–642. doi: 10.1530/EJE-10-1038. [DOI] [PubMed] [Google Scholar]
- 22.Brown GR, Jones KT. Racial health disparities in a cohort of 5,135 transgender veterans. J Racial Ethn Health Disparities. 2014;1(4):257–266. doi: 10.1007/s40615-014-0032-4. [DOI] [Google Scholar]
- 23.Brown GR, Jones KT. Mental health and medical health disparities in 5,135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122–131. doi: 10.1089/lgbt.2015.0058. [DOI] [PubMed] [Google Scholar]
- 24.Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191–198. doi: 10.1007/s10549-014-3213-2. [DOI] [PubMed] [Google Scholar]
- 25.Blosnich JR, et al. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27–e32. doi: 10.2105/AJPH.2013.301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotondi NK, et al. Depression in male-to-female transgender Ontarians: results from the trans PULSE project. Can J Commun Ment Health. 2011;30(2):113–133. doi: 10.7870/cjcmh-2011-0020. [DOI] [Google Scholar]
- 27.Bockting WO, et al. Stigma, mental health, and resilience in an online sample of the US transgender population. Am J Public Health. 2013;103(5):943–951. doi: 10.2105/AJPH.2013.301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heylens G, et al. Psychiatric characteristics in transsexual individuals: multicentre study in four European countries. Br J Psychiatry. 2014;204(2):151–156. doi: 10.1192/bjp.bp.112.121954. [DOI] [PubMed] [Google Scholar]
- 29.Colizzi M, et al. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65–73. doi: 10.1016/j.psyneuen.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Gil E, et al. Hormone-treated transsexuals report less social distress, anxiety and depression. Psychoneuroendocrinology. 2012;37(5):662–670. doi: 10.1016/j.psyneuen.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Murad MH, et al. Hormonal therapy and sex reassignment: a systematic review and meta-analysis of quality of life and psychosocial outcomes. Clin Endocrinol (Oxf) 2010;72(2):214–231. doi: 10.1111/j.1365-2265.2009.03625.x. [DOI] [PubMed] [Google Scholar]
- 32.Meyer IH. Does an improved social environment for sexual and gender minorities have implications for a new minority stress research agenda? Psychol Sex Rev. 2016;7(1):81–90. [PMC free article] [PubMed] [Google Scholar]
- 33.Safer JD, Pearce EN. A simple curriculum content change increased medical student comfort with transgender medicine. Endocr Pract. 2013;19(4):633–637. doi: 10.4158/EP13014.OR. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DD, Safer JD. A simple intervention raised resident-physician willingness to assist transgender patients seeking hormone therapy. Endocr Pract. 2015;21(10):1134–1142. doi: 10.4158/EP15777.OR. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson SES, Safer JD. Evidence-based curricular content improves student knowledge and changes attitudes towards transgender medincine. Endocr Pract. 2016;22(7):837–841. doi: 10.4158/EP151141.OR. [DOI] [PubMed] [Google Scholar]
- 36.Chan B, et al. Gaps in transgender medicine content identified among Canadian medical school curricula. Transgend Health. 2016;1(1):142–150. doi: 10.1089/trgh.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JA, Safer JD. Clinical exposure to transgender medicine improves students’ preparedness above levels seen with didactic teaching alone: a key addition to the Boston University model for teaching transgender healthcare. Transgend Health. 2018;3(1):10–16. doi: 10.1089/trgh.2017.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidge-Pitts C, et al. Transgender health in endocrinology: current status of endocrinology fellowship programs and practicing clinicians. J Clin Endocrinol Metab. 2017;102(4):1286–1290. doi: 10.1210/jc.2016-3007. [DOI] [PubMed] [Google Scholar]
- 39.Davidge-Pitts CJ, et al. Endocrinolgy fellows’ perception of their confidence and skill level in providing transgender healthcare. Endocr Pract. 2018;24(12):1038–1042. doi: 10.4158/EP-2018-0307. [DOI] [PubMed] [Google Scholar]
- 40.Irwig MS. Transgender care by endocrinologists in the United States. Endocr Pract. 2016;22(7):832–836. doi: 10.4158/EP151185.OR. [DOI] [PubMed] [Google Scholar]
- 41.Saraswat A, et al. Evidence supporting the biologic nature of gender identity. Endocr Pract. 2015;21(2):199–204. doi: 10.4158/EP14351.RA. [DOI] [PubMed] [Google Scholar]
- 42.Diamond M. Sex, gender, and identity over the years: a changing perspective. Child Adolesc Psychiatr Clin N Am. 2004;13(3):591–607. doi: 10.1016/j.chc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Safer JD, Tangpricha V. Out of the shadows: it is time to mainstream treatment for transgender patients. Endocr Pract. 2008;14(2):248–250. doi: 10.4158/EP.14.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer-Bahlburg HFL. Gender identity outcome in female-raised 46,XY persons with penile agenesis, cloacal exstrophy of the bladder, or penile ablation. Arch Sex Behav. 2005;34(4):423–438. doi: 10.1007/s10508-005-4342-9. [DOI] [PubMed] [Google Scholar]
- 45.Reiner WG, Gearhart JP. Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N Engl J Med. 2004;350(4):333–341. doi: 10.1056/NEJMoa022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heylens G, et al. Gender identity disorder in twins: a review of the case report literature. J Sex Med. 2012;9(3):751–757. doi: 10.1111/j.1743-6109.2011.02567.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhou JN, et al. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378(6552):68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- 48.Dessens AB, et al. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch Sex Behav. 2005;34(4):389–397. doi: 10.1007/s10508-005-4338-5. [DOI] [PubMed] [Google Scholar]
- 49.Mazur T. Gender dysphoria and gender change in androgen insensitivity or micropenis. Arch Sex Behav. 2005;34(4):411–421. doi: 10.1007/s10508-005-4341-x. [DOI] [PubMed] [Google Scholar]
- 50.Liang JJ, et al. Testosterone levels achieved by medically treated transgender women in a United States endocrinology clinic cohort. Endocr Pract. 2017;24(2):135–142. doi: 10.4158/EP-2017-0116. [DOI] [PubMed] [Google Scholar]
- 51.Greene DN, et al. Reproductive endocrinology reference intervals for transgender men on stable hormone therapy. J Appl Lab Med. doi: 10.1093/jalm/jfaa169. [published online November 26, 2020]. [DOI] [PubMed] [Google Scholar]
- 52.Moore E, et al. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88(8):3467–3473. doi: 10.1210/jc.2002-021967. [DOI] [PubMed] [Google Scholar]
- 53.Tangpricha V, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9(1):12–21. doi: 10.4158/EP.9.1.12. [DOI] [PubMed] [Google Scholar]
- 54.Manson JE, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chlebowski RT, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324(4):369–380. doi: 10.1001/jama.2020.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spratt DI, et al. Subcutaneous injection of testosterone is an effective and preferred alternative to intramuscular injection: demonstration in female-to-male transgender patients. J Clin Endocrinol Metab. 2017;102(7):2349–2355. doi: 10.1210/jc.2017-00359. [DOI] [PubMed] [Google Scholar]
- 57.Getahun D, et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med. 2018;169(4):205–213. doi: 10.7326/M17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldstein Z, et al. Managing the risk of venous thromboembolism in transgender adults undergoing hormone therapy. J Blood Med. 2019;10:209–216. doi: 10.2147/JBM.S166780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinand JD, Safer JD. Hormone therapy in transgender adults is safe with provider supervision; a review of hormone therapy sequelae for transgender individuals. J Clin Transl Endocrinol. 2015;2(2):55–60. doi: 10.1016/j.jcte.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wierckx K, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641–2651. doi: 10.1111/j.1743-6109.2012.02876.x. [DOI] [PubMed] [Google Scholar]
- 61.Asscheman H, et al. Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review. Andrologia. 2014;46(7):791–795. doi: 10.1111/and.12150. [DOI] [PubMed] [Google Scholar]
- 62.Hembree WC, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 63.Khan J, et al. Venous thrombotic risk in transgender women undergoing estrogen therapy: a systematic review and metaanalysis. Clin Chem. 2019;65(1):57–66. doi: 10.1373/clinchem.2018.288316. [DOI] [PubMed] [Google Scholar]
- 64.Defreyne J, et al. Effects of gender-affirming hormones on lipid, metabolic, and cardiac surrogate blood markers in transgender persons. Clin Chem. 2019;65(1):119–134. doi: 10.1373/clinchem.2018.288241. [DOI] [PubMed] [Google Scholar]
- 65.Laliberté F, et al. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059. doi: 10.1097/gme.0b013e3182175e5c. [DOI] [PubMed] [Google Scholar]
- 66.Seal LJ, et al. Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens. J Clin Endocrinol Metab. 2012;97(12):4422–4428. doi: 10.1210/jc.2012-2030. [DOI] [PubMed] [Google Scholar]
- 67.Gooren LJ, et al. Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience. J Clin Endocrinol Metab. 2008;93(1):19–25. doi: 10.1210/jc.2007-1809. [DOI] [PubMed] [Google Scholar]
- 68.Vinogradova Y, et al. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canonico M, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115(7):840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 70.Greene DN, et al. Hematology reference intervals for transgender adults on stable hormone therapy. Clin Chim Acta. 2019;492:84–90. doi: 10.1016/j.cca.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Mueller A, et al. Long-term administration of testosterone undecanoate every 3 months for testosterone supplementation in female-to-male transsexuals. J Clin Endocrinol Metab. 2007;92(9):3470–3475. doi: 10.1210/jc.2007-0746. [DOI] [PubMed] [Google Scholar]
- 72.Wierckx K, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169(4):471–478. doi: 10.1530/EJE-13-0493. [DOI] [PubMed] [Google Scholar]
- 73.Nota NM, et al. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation. 2019;139(11):1461–1462. doi: 10.1161/CIRCULATIONAHA.118.038584. [DOI] [PubMed] [Google Scholar]
- 74.Elamin MB, et al. Effect of sex steroid use on cardiovascular risk in transsexual individuals: a systematic review and meta-analyses. Clin Endocrinol (Oxf) 2010;72(1):1–10. doi: 10.1111/j.1365-2265.2009.03632.x. [DOI] [PubMed] [Google Scholar]
- 75.Elbers JMH, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003;58(5):562–571. doi: 10.1046/j.1365-2265.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- 76.Gooren LJ, et al. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809–819. doi: 10.1530/EJE-14-0011. [DOI] [PubMed] [Google Scholar]
- 77.Colizzi M, et al. Concomitant psychiatric problems and hormonal treatment induced metabolic syndrome in gender dysphoria individuals: a 2 year follow-up study. J Psychosom Res. 2015;78(4):399–406. doi: 10.1016/j.jpsychores.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Deutsch MB, et al. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol. 2015;125(3):605–610. doi: 10.1097/AOG.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wierckx K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11(8):1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 80.Ott J, et al. Cross-sex hormone therapy alters the serum lipid profile: a retrospective cohort study in 169 transsexuals. J Sex Med. 2011;8(8):2361–2369. doi: 10.1111/j.1743-6109.2011.02311.x. [DOI] [PubMed] [Google Scholar]
- 81.Pelusi C, et al. Effects of three different testosterone formulations in female-to-male transsexual persons. J Sex Med. 2014;11(12):3002–3011. doi: 10.1111/jsm.12698. [DOI] [PubMed] [Google Scholar]
- 82.Chandra P, et al. Alterations in lipids and adipocyte hormones in female-to-male transsexuals. Int J Endocrinol. 2010;2010:945053. doi: 10.1155/2010/945053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goodrum BA. The effects of long-term testosterone use on lipid-related cardiovascular risk factors among FtM patients. Int J Transgenderism. 2014;15(3–4):164–172. doi: 10.1080/15532739.2014.995261. [DOI] [Google Scholar]
- 84.de Blok CJM, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. The BMJ. 2019;365:l1652. doi: 10.1136/bmj.l1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reisman T, et al. A review of breast development in cisgender women and implications for transgender women. Endocr Pract. 2019;25(12):1338–1345. doi: 10.4158/EP-2019-0183. [DOI] [PubMed] [Google Scholar]
- 86.Sherman RL, et al. Misclassification of sex in central cancer registries. J Registry Manag. 2014;41(3):120–124. [PubMed] [Google Scholar]
- 87.Pike MC, et al. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 88.Chlebowski RT, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 89.Maglione KD, et al. Breast cancer in male-to-female transsexuals: use of breast imaging for detection. AJR Am J Roentgenol. 2014;203(6):W735–W740. doi: 10.2214/AJR.14.12723. [DOI] [PubMed] [Google Scholar]
- 90.Key T, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 91.Somboonporn W, et al. Testosterone effects on the breast: implications for testosterone therapy for women. Endocr Rev. 2004;25(3):374–388. doi: 10.1210/er.2003-0016. [DOI] [PubMed] [Google Scholar]
- 92.Molokwu CN, et al. Detection of prostate cancer following gender reassignment. BJU Int. 2008;101(2):259–260. doi: 10.1111/j.1464-410X.2007.07394_4.x. [DOI] [PubMed] [Google Scholar]
- 93.Turo R, et al. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7–8):E544–E546. doi: 10.5489/cuaj.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gooren L, Morgentaler A. Prostate cancer incidence in orchidectomised male-to-female transsexual persons treated with oestrogens. Andrologia. 2014;46(10):1156–1160. doi: 10.1111/and.12208. [DOI] [PubMed] [Google Scholar]
- 95.Urban RR, et al. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12. doi: 10.1016/j.ajog.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 96.Bergoglio MT, et al. Symptomatic meningioma induced by cross-sex hormone treatment in a male-to-female transsexual. Endocrinol Nutr. 2013;60(5):264–267. doi: 10.1016/j.endonu.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Razavi HB. Meningioma: the unusual growth in a transsexual patient after estrogen-progesterone therapy. SOJ Neurol. 2014;1(2):1–3. [Google Scholar]
- 98.Cunha FS, et al. Diagnosis of prolactinoma in two male-to-female transsexual subjects following high-dose cross-sex hormone therapy. Andrologia. 2015;47(6):680–684. doi: 10.1111/and.12317. [DOI] [PubMed] [Google Scholar]
- 99.Defreyne J, et al. Transient elevated serum prolactin in trans women is caused by cyproterone acetate treatment. LGBT Health. 2017;4(5):328–336. doi: 10.1089/lgbt.2016.0190. [DOI] [PubMed] [Google Scholar]
- 100.Bisson JR, et al. Prolactin levels do not rise among transgender women treated with estradiol and spironolactone. Endocr Pract. 2018;24(7):646–651. doi: 10.4158/EP-2018-0101. [DOI] [PubMed] [Google Scholar]
- 101.Dittrich R, et al. Endocrine treatment of male-to-female transsexuals using gonadotropin-releasing hormone agonist. Exp Clin Endocrinol Diabetes. 2005;113(10):586–592. doi: 10.1055/s-2005-865900. [DOI] [PubMed] [Google Scholar]
- 102.Chan K, Mok C. Development of systemic lupus erythematosus in a male-to-female transsexual: the role of sex hormones revisited. Lupus. 2013;22(13):1399–1402. doi: 10.1177/0961203313500550. [DOI] [PubMed] [Google Scholar]
- 103.Van Caenegem E, et al. Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone. 2013;54(1):92–97. doi: 10.1016/j.bone.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 104.Van Caenegem E, et al. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (ENIGI) Eur J Endocrinol. 2015;172(2):163–171. doi: 10.1530/EJE-14-0586. [DOI] [PubMed] [Google Scholar]
- 105.Handelsman DJ, et al. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803–829. doi: 10.1210/er.2018-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rogol AD, Pieper LP. The interconnected histories of endocrinology and eligibility in women’s sport. Horm Res Paediatr. 2018;90(4):213–220. doi: 10.1159/000493646. [DOI] [PubMed] [Google Scholar]
- 107.Bermon S, Garnier P-Y. Serum androgen levels and their relation to performance in track and field: mass spectrometry results from 2127 observations in male and female elite athletes. Br J Sports Med. 2017;51(17):1309–1314. doi: 10.1136/bjsports-2017-097792. [DOI] [PubMed] [Google Scholar]
- 108.Knox T, et al. Transwomen in elite sport: scientific and ethical considerations. J Med Ethics. 2019;45(6):395–403. doi: 10.1136/medethics-2018-105208. [DOI] [PubMed] [Google Scholar]
- 109.Harper J. Race times for transgender athletes. J Sport Cult Identities. 2015;6(1):1–9. doi: 10.18848/2381-6678/CGP/v06i01/54079. [DOI] [Google Scholar]
- 110.Horbach SER, et al. Outcome of vaginoplasty in male-to-female transgenders: a systematic review of surgical techniques. J Sex Med. 2015;12(6):1499–1512. doi: 10.1111/jsm.12868. [DOI] [PubMed] [Google Scholar]
- 111.Rossi NR, et al. Gender reassignment surgery — a 13 year review of surgical outcomes. Eur Urol Suppl. 2013;12(1):e559. doi: 10.1016/S1569-9056(13)61042-8. [DOI] [PubMed] [Google Scholar]
- 112.Costantino A, et al. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321–335. doi: 10.1080/0092623X.2012.736920. [DOI] [PubMed] [Google Scholar]
- 113.Bouman M-B, et al. Intestinal vaginoplasty revisited: a review of surgical techniques, complications, and sexual function. J Sex Med. 2014;11(7):1835–1847. doi: 10.1111/jsm.12538. [DOI] [PubMed] [Google Scholar]
- 114.Davis SA, Meier SC. Effects of testosterone treatment and chest reconstruction surgery on mental health and sexuality in female-to-male transgender people. Int J Sex Health. 2014;26(2):113–128. doi: 10.1080/19317611.2013.833152. [DOI] [Google Scholar]