Abstract
Background
During pneumonia, normal alveolar areas coexist adjacently with consolidated areas, and high inspiratory efforts may predispose to lung damage. To date, no study has evaluated different degrees of effort during Biphasic positive airway pressure (BIVENT) on lung and diaphragm damage in experimental pneumonia, though largely used in clinical setting. We aimed to evaluate lung damage, genes associated with ventilator-induced lung injury (VILI) and diaphragmatic injury, and blood bacteria in pressure-support ventilation (PSV), BIVENT with low and high inspiratory efforts in experimental pneumonia.
Material and methods
Twenty-eight male Wistar rats (mean ± SD weight, 333±78g) were submitted Pseudomonas aeruginosa-induced pneumonia. After 24-h, animals were ventilated for 1h in: 1) PSV; 2) BIVENT with low (BIVENTLow-Effort); and 3) BIVENT with high inspiratory effort (BIVENTHigh-Effort). BIVENT was set at Phigh to achieve VT = 6 ml/kg and Plow at 5 cmH2O (n = 7/group). High- and low-effort conditions were obtained through anaesthetic infusion modulation based on neuromuscular drive (P0.1). Lung mechanics, histological damage score, blood bacteria, and expression of genes related to VILI in lung tissue, and inflammation in diaphragm tissue.
Results
Transpulmonary peak pressure and histological damage score were higher in BIVENTHigh-Effort compared to BIVENTLow-Effort and PSV [16.1 ± 1.9cmH2O vs 12.8 ± 1.5cmH2O and 12.5 ± 1.6cmH2O, p = 0.015, and p = 0.010; median (interquartile range) 11 (9–13) vs 7 (6–9) and 7 (6–9), p = 0.021, and p = 0.029, respectively]. BIVENTHigh-Effort increased interleukin-6 expression compared to BIVENTLow-Effort (p = 0.035) as well as expressions of cytokine-induced neutrophil chemoattractant-1, amphiregulin, and type III procollagen compared to PSV (p = 0.001, p = 0.001, p = 0.004, respectively). Tumour necrosis factor-α expression in diaphragm tissue and blood bacteria were higher in BIVENTHigh-Effort than BIVENTLow-Effort (p = 0.002, p = 0.009, respectively).
Conclusion
BIVENT requires careful control of inspiratory effort to avoid lung and diaphragm damage, as well as blood bacteria. P0.1 might be considered a helpful parameter to optimize inspiratory effort.
Introduction
Pneumonia caused by Pseudomonas aeruginosa is frequent in intensive care unit patients [1] and can disrupt upper and lower airway homeostasis by damaging the epithelium and evading innate and adaptive immune responses [2]. Consequently, there is a widespread focal consolidation, which may lead to heterogeneous distortion of lung parenchyma during mechanical ventilation. Partial ventilatory support allows spontaneous breathing during mechanical ventilation, leading to a reduction in the need for sedation [3], improvement in hemodynamic [4], and maintenance of respiratory muscle activity [5]. Biphasic positive airway pressure (BIVENT) is a partial ventilatory support mode that employs pressure-controlled, time-cycled ventilation set at two levels of continuous positive airway pressure (CPAP) with unrestricted spontaneous breathing during the ventilatory cycle. In experimental acute respiratory distress syndrome (ARDS), BIVENT has shown advantages over controlled mechanical ventilation, reducing the risk of ventilator-induced lung injury (VILI) [6–8]. Additionally, lung damage and worse outcomes might occur during airway pressure release ventilation (APRV) [9] when high inspiratory effort is present [10–12]. On the other hand, recent preclinical [13] and clinical studies [14] have shown that increased effort during partial ventilatory support is not associated with worse outcomes in early ARDS. Nevertheless, in pneumonia, where normal alveolar areas coexist adjacently with consolidated areas, high inspiratory efforts may predispose to lung damage. To date, no study has evaluated the impact of different degrees of effort during BIVENT on lung and diaphragm damage in experimental pneumonia. We hypothesized that BIVENT may promote beneficial effects only with low inspiratory effort. For this purpose, lung damage, biological markers associated with both ventilator-induced lung injury (VILI) and diaphragmatic injury, and bacterial translocation were evaluated in three ventilation strategies—BIVENT with low inspiratory effort, BIVENT with high inspiratory effort, and pressure-support ventilation (PSV)—in a model of Pseudomonas aeruginosa-induced pneumonia.
Methods
Study approval
This study was approved by the Ethics Committee of the Health Science Centre (CEUA 116/16), Federal University of Rio de Janeiro, Rio de Janeiro, Brazil. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences, USA. The present study followed the ARRIVE guidelines for reporting of animal research [15]. Animals were housed at a controlled temperature (23°C) and controlled light–dark cycle (12–12 h), with free access to water and food.
Bacterial preparation
Pseudomonas aeruginosa (ATCC27853), supplied by the FIOCRUZ Bacterial Culture Collection, was cultured overnight in Luria Broth Base (Invitrogen™ by Life Technologies, Carlsbad, CA, USA) at 37°C to obtain stationary-phase microorganisms. Subsequently, the culture medium containing the bacteria was centrifuged at 6,500 g for 15 minutes at 4°C and the pellet was washed and resuspended in sterile saline. The sample was analysed by spectrophotometry and adjusted to the desired dose of 5×107 colony-forming units (CFUs) [16]. In previous studies, this level of blood bacteria resulted in pneumonia with no mortality [16].
Pneumonia induction
In the early morning (8:00 a.m.), 28 male Wistar rats (mean body weight ± SD, 333±78g) were anesthetized under spontaneous breathing with 1.5–2.0% isoflurane (Isoforine®; Cristália, Itapira, SP, Brazil) and underwent intratracheal instillation of the above-mentioned bacterial cultures. This bacterial strain is deposited in the Culture Collection of Hospital-Acquired Bacteria (CCBH) located at the Hospital Infection Research Laboratory at Oswaldo Cruz Institute, FIOCRUZ, Brazil.
Animal preparation and experimental protocol
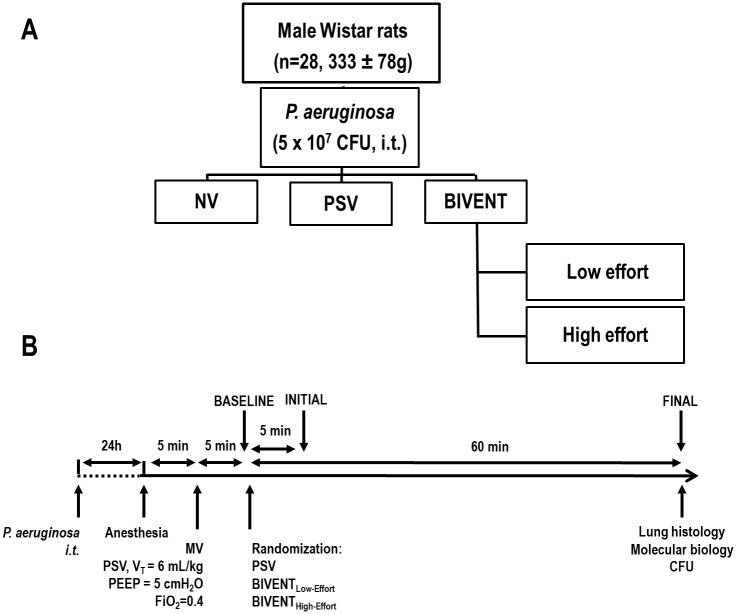
After 24 h, animals were premedicated intraperitoneally (i.p.) with midazolam (1–2 mg/kg) and anesthetized with ketamine (100 mg/kg, i.p.). An intravenous (i.v.) catheter (Jelco 24G, Becton, Dickinson and Company, New Jersey, NJ, USA) was inserted into the tail vein, and anaesthesia induced and maintained with midazolam (2 mg/kg/h) and ketamine (50 mg/kg/h). The adequacy of anaesthesia was assessed by response to a nociceptive stimulus before surgery. Body temperature was maintained at 37.5 ± 1°C with a heating bed (EFF 421, INSIGHT®, Brazil). The neck area was anesthetized by subcutaneous injection of 0.4 ml lidocaine (2%), a tracheostomy was performed, and a polyethylene cannula (internal diameter 1.8 mm, length 7.5 cm; PE 240, Intramedic®, Clay-Adams Inc., New York, USA) was advanced into the trachea. A second catheter (18G; Arrow International, USA) was then placed in the right internal carotid artery for blood sampling and gas analysis (Radiometer ABL80 FLEX, Copenhagen NV, Denmark), as well as monitoring of mean arterial pressure (MAP) (Networked Multiparameter Veterinary Monitor LifeWindow 6000 V; Digicare Animal Health, Boynton Beach, FL, USA). Animals were connected to an airway pressure transducer (UT-PDP-70; SCIREQ, Canada) and a two-sidearm pneumotachograph (internal diameter 2.7 mm, length 25.7 mm, internal volume 0.147 ml, airflow resistance 0.0057 cm H2O·ml-1·s-1) [17] connected to a differential pressure transducer (UT-PDP-02, SCIREQ, Montreal, QC, Canada), for airflow (V′) measurement. A 30-cm-long water-filled catheter (PE-205; Becton, Dickinson and Company) with side holes at the tip, connected to a differential pressure transducer (UT-PL-400; SCIREQ, Canada), was used to measure the oesophageal pressure, and proper positioning was assessed using the “occlusion test” as described elsewhere [18]. Seven of the 28 rats were subjected to P. aeruginosa instillation, but not ventilated. This non-ventilated (NV) group was used for molecular biology analysis. Twenty-one rats were mechanically ventilated (SERVO-i; MAQUET, Solna, Sweden) in PSV with ΔP set to achieve a tidal volume (VT) of 6 ml/kg, PEEP of 5 cmH2O, and FiO2 equal to 0.4. Flow trigger sensitivity was adjusted at BASELINE for adequate inspiratory effort, according to esophageal pressure (Pes) decay. No additional changes to flow trigger sensitivity were done at any point in the experiment. Shortly after, animals were randomly assigned to: 1) PSV according to previous ventilatory settings; 2) BIVENTLow-Effort; or 3) BIVENTHigh-Effort (n = 7/group) (Fig 1A). In PSV and BIVENTLow-Effort, anaesthesia was titrated to obtain low effort according to Pes decay, while in BIVENTHigh-Effort the anaesthesia infusion was reduced to reach high effort according to Pes decay. The criteria to observe Pes decay was based on neuromuscular drive (P0.1 level). Anaesthesia titration lasted few minutes, without affecting the total time under mechanical ventilation among groups. For BIVENT, the following parameters were adjusted: airway pressure spent during inspiratory time (Phigh) to reach VT = 6 ml/kg, Plow = 5 cmH2O, and inspiratory time (Thigh) = 0.3 seconds. Blood gas analysis (Radiometer, Copenhagen, Denmark) and mechanical data were obtained at INITIAL and at the end of 1 h of mechanical ventilation (FINAL) under FiO2 = 0.4 (Fig 1B). At FINAL, blood samples were taken for measurement of bacterial load (CFU count). Animals were euthanized by intravenous overdose of sodium thiopental (60 mg/kg; Cristália, Brazil). The left lung was removed for quantification of heterogeneous airspace enlargement and pneumonia score, and the right lung and diaphragm were harvested for gene expression analysis by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR).
Fig 1.
A. Experimental design. i.t.: intratracheal; NV: non-ventilated; PSV: pressure-support ventilation; BIVENT: biphasic positive airway pressure ventilation; Low effort: anaesthesia infusion was maintained to keep inspiratory effort low, according to oesophageal pressure decay; High effort: anaesthesia infusion was reduced to achieve high inspiratory effort according to oesophageal pressure decay (n = 7/group). B. Timeline of the experiments. i.t.: intratracheal; CFU: colony-forming unit; MV: mechanical ventilation; VT: tidal volume; FiO2: inspiratory fraction of oxygen; PEEP: positive end-expiratory pressure; CFU: colony-forming unit.
Data acquisition and respiratory system mechanics
Airflow, airway pressure (Paw), and Pes were recorded continuously throughout the experiments by a computer running customer-made software written in LabVIEW (National Instruments, USA). VT was calculated by digital integration of the airflow signal. The total respiratory rate (RR) was calculated from the Pes swings as the frequency per minute of each type of breathing cycle. Peak transpulmonary pressure (Ppeak,L) was calculated as the difference between tracheal and esophageal pressure. P0.1 is the esophageal pressure measured 100 ms after the onset of inspiratory effort, and it reflects the neuromuscular drive. All signals were amplified in a three-channel signal conditioner (TAM-DHSE Plugsys Transducers Amplifiers, Module Type 705/2, Harvard Apparatus, Holliston, Massachusetts, USA) and sampled at 200 Hz with a 12-bit analog-to-digital converter (National Instruments; Austin, Texas, USA) [19]. All mechanical data were computed offline by a routine written in MATLAB (Version R2007a; The Mathworks Inc., USA).
Histology
Pneumonia score
The left lung was removed, fixed, and embedded in paraffin. The slides containing the lung sections were stained with haematoxylin and eosin and analysed according to their qualitative and quantitative aspects. For the descriptive analysis, the entire lamina surface was observed, with all pulmonary structures represented, under incremental magnification (25×, 100×, and 400×). Pseudomonas aeruginosa-induced lung damage was quantified by a pulmonary pathology specialist (V.L.C.) blinded to group assignment using a weighted scoring system as previously described [20, 21]. Assessment of perivascular oedema/haemorrhage, septal neutrophil and vasculitis features were based on a previous study that highlighted the histological characteristics most commonly found in models of pneumonia [22]. Values from 0 to 4 were used to represent the severity of a given characteristic, with 0 for no effect and 4 for maximum severity. The extent of involvement in each field of view was also scored on a scale from 0 to 4, with 0 for no appearance and 4 for full involvement. Results were calculated as the product of severity and extent of each feature, thus ranging from 0 to 16. Considering that the total lung damage score was the sum of three features, the pneumonia score ranged from 0 to 48.
Immunohistochemistry analysis
For the immunohistochemistry analysis, the lung sections were subjected to a high temperature (121°C) for 1 minute for antigen retrieval. After blocking of nonspecific sites with 3% hydrogen peroxide for 5 minutes, the specimens were stained with rabbit polyclonal CINC-1 antibody (Millipore; 1:100 dilution, Temecula, California, USA). The reactions were stained with Vectastain ABC (Vector Laboratories). The colour was developed with 3,3-diaminobenzidinetetrahydrochloride (Vector Laboratories) and counterstained with H&E.
Immunofluorescence and confocal analysis
To perform immunostaining for neutrophils and occludin, lung slides were mounted on 3-aminopropyltriethoxysilane (Sigma Chemical Co., St. Louis, MO, USA), dewaxed in xylene, and hydrated in graded ethanol. Antigen retrieval was accomplished using enzymatic treatment with pepsin from porcine gastric mucosa (10,000 dry units/mL) (Sigma Chemical Co. St. Louis, MO, USA) in acetic acid buffer at 0.5 N for 30 minutes at 37°C. Nonspecific sites were blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 30 minutes, at room temperature. The lung specimens were incubated overnight at 4°C with rabbit monoclonal antibody to neutrophil elastase [clone EPR7479; 1:100; ABCAM; USA] and rabbit monoclonal antibody to occludin [clone EPR20992; 1:300; ABCAM, USA]. The lung sections were subsequently washed in PBS with Tween 20 0.05% and incubated for 60 minutes at room temperature. Bound antibodies were visualized with Alexa 488-conjugated anti-mouse IgG and Alexa 546-conjugated anti-rabbit IgG (Invitrogen, Eugene, OR, USA). Specimens were visualized in an immunofluorescence microscope (OLYMPUS BX51). For three-dimensional reconstruction by confocal microscopy, the same procedure described above was followed, but slides were viewed under a laser-scanning microscope (Zeiss LSM 510 META/UV, Germany).
Immunohistochemistry and immunofluorescence staining markers were scored using a membrane pattern to analyze occludin and a cytoplasmic pattern to analyze CINC-1. The intensity and extent of staining were scored as 0 (no staining), 1 (weak staining and focal extension), 2+ (moderate staining and focal extension), 3+ (moderate staining and diffuse extension), and 4+ (strong expression and diffuse extension). The total score was obtained by multiplying extension and intensity, yielding a final score of up to 16.
Morphometric analysis
Quantification of heterogeneous airspace enlargement
Airspace enlargement was assessed by measuring the mean linear intercept between alveolar walls at a magnification of × 400, as described elsewhere [19, 23, 24]. To characterize the heterogeneity of airspace enlargement, the central moment of the mean linear intercept (D2 of mean linear intercept between alveolar walls) was computed from 20 airspace measurements [25], according to Eq 1:
| (1) |
where μ is the mean, σ is the variance of airspace diameters, and γ is the skewness of the diameter distribution. After D2 calculation, the heterogeneity index (β) was derived from D2 and mean linear intercept between alveolar wall values by their ratio [19, 23, 24]. Quantification of heterogeneous airspace enlargement was performed to properly show heterogeneous airspace enlargement, lung slices were scanned (3DHISTECH®, Budapest, Hungary). All analyses were performed by one of the authors (M.C.S.), who was blinded to group assignment.
Blood bacterial load
Blood samples (20 μL) were seeded in Petri dishes with Tryptic Soy Agar growth medium (Fluka Analytical, St Louis, MO, USA). Manual CFU counts were performed after 24 h of incubation at 37°C.
Enzyme-linked immunosorbent assay of lung tissue
Protein levels of CINC-1 (1009127, Murine KC, Preprotech, United States) in lung tissue were measured by enzyme-linked immunosorbent assay (ELISA). The sample was homogenized in lysis buffer (1 M HEPEs, 0.5 M EDTA, 1 M sucrose, 200 mM NaF, Roche protease inhibitor cocktail) and total protein was quantified by Bradford’s assay (5000205, Quick Start™ Bradford 1× Dye Reagent). ELISA was performed as per manufacturer instructions. The result was expressed as pg/mL of CINC-1 normalized by total protein content (μg/mL), and given as pg/μg.
Molecular biology analysis of lung and diaphragm tissue
RT-PCR was performed to measure biological markers associated with inflammation (interleukin [IL]-6, cytokine-induced neutrophil chemoattractant [CINC-1]), alveolar stretch (amphiregulin), and fibrogenesis (type III procollagen [PC-III]) in lung tissue, as well as a marker of inflammation (tumour necrosis factor [TNF]-α) in diaphragm tissue. The primer sequences are listed in S1 Table. Central slices of the right lung or diaphragm were cut, collected in cryotubes, flash-frozen by immersion in liquid nitrogen, and stored at −80°C. Total RNA was extracted from frozen lung tissues using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s recommendations. The RNA concentration was measured by spectrophotometry in a Nanodrop ND-2000 system. First-strand cDNA was synthesized from total RNA using a Quantitec reverse transcription kit (Qiagen, Hilden, Germany). Relative mRNA concentrations were measured with a SYBR green detection system using ABI 7500 real-time polymerase chain reaction (Applied Biosystems, Foster City, CA, USA). Samples were measured in triplicate. For each sample, the expression of each gene was normalized to that of the housekeeping gene 36B4 (acidic ribosomal phosphoprotein P0) [26] and expressed as fold change relative to NV, using the 2-ΔΔCt method, where ΔCt = Ct (reference gene)–Ct (target gene). All analyses were performed by one of the authors (C.L.B.), who was blinded to group assignment.
Statistical analysis
The sample size was judiciously calculated to minimize the use of animals. A sample of 7 animals per group would provide the appropriate power (1-β = 0.8) to identify significant (α = 0.05) differences in volume fractions of the lung occupied by collapsed pulmonary areas between BIVENT-100 and BIVENT-50, since those groups showed distinct inspiratory efforts in previous studies by our group [7], taking into account an effect size d = 1.82, a two-sided test, and a sample size ratio of 1 (G*Power 3.1.9.2, University of Düsseldorf, Germany). The primary outcome was the difference in pneumonia score, whereas the secondary outcomes were respiratory system mechanics, lung heterogeneity, blood CFU count, and expression of genes related to inflammation, alveolar stretch, and fibrogenesis. The Kolmogorov–Smirnov test with Lilliefors’ correction was used to assess normality of data, while the Levene median test was used to evaluate the homogeneity of variances. To compare P0.1 level between High-Effort and Low-Effort groups, Student’s t-test was used (p<0.05). To compare functional parameters over time, a mixed linear model based on a random intercept for each animal followed by Bonferroni’s test was used (p<0.05). For lung damage, airspace enlargement, blood CFU count, and molecular biology assays in lung tissue, a Kruskal–Wallis test followed by Dunn’s multiple comparisons was performed (p<0.05). The mixed linear models were run in IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., USA). All other tests were performed in GraphPad Prism v8.1.1 (GraphPad Software, La Jolla, CA, USA).
Results
No significant differences among groups were observed in the volume of fluids required to keep mean arterial pressure at or above 70 mmHg. Oxygenation improved over time only in the PSV group, whereas PaCO2 increased and pHa decreased in all groups. At FINAL, no significant differences were observed in oxygenation, PaCO2, pHa, or HCO3- among groups (Table 1).
Table 1. Blood gas analysis during mechanical ventilation.
| Groups | INITIAL | FINAL | Time effect | Group effect | Interaction | |
|---|---|---|---|---|---|---|
| PaO2/FiO2 (mmHg) | p = 0.003 | p = 0.949 | p = 0.328 | |||
| PSV | 370 ± 35 | 442 ± 70† | ||||
| BIVENTLow-Effort | 386 ± 71 | 422 ± 86 | ||||
| BIVENTHigh-Effort | 382 ± 49 | 408 ± 52 | ||||
| pHa | p = 0.011 | p = 0.439 | p = 0.543 | |||
| PSV | 7.37 ± 0.04 | 7.34 ± 0.03 | ||||
| BIVENTLow-Effort | 7.39 ± 0.02 | 7.35 ± 0.09 | ||||
| BIVENTHigh-Effort | 7.42 ± 0.03 | 7.35 ± 0.06 | ||||
| PaCO2 (mmHg) | p = 0.048 | p = 0.787 | p = 0.674 | |||
| PSV | 39 ± 8 | 46 ± 7 | ||||
| BIVENTLow-Effort | 39 ± 5 | 42 ± 8 | ||||
| BIVENTHigh-Effort | 37 ± 3 | 43 ± 6 | ||||
| HCO3 (mmol/L) | p = 0.734 | p = 0.831 | p = 0.189 | |||
| PSV | 21.9 ± 3.8 | 24.0 ± 2.5 | ||||
| BIVENTLow-Effort | 23.9 ± 3.1 | 22.7 ± 2.1 | ||||
| BIVENTHigh-Effort | 24.5 ± 1.4 | 22.7 ± 1.9 |
Blood gas analysis at INITIAL and FINAL. PSV: pressure-support ventilation with ΔP set to achieve a VT of 6 mL/kg (n = 7); BIVENTLow-Effort: Biphasic positive airway pressure at 50 controlled breaths/min. Animals were allowed to breath either at high and low positive airway pressures, and anesthesia was modulated to keep low inspiratory effort (n = 7). BIVENTHigh-Effort: Biphasic positive airway pressure at 50 controlled breaths/min. Animals were allowed to breath either at high and low positive airway pressures, and anesthesia was modulated to keep high inspiratory effort (n = 7). Values represent mean ± standard deviation (SD). Comparisons were done using a mixed linear model based on a random intercept for each animal followed by Bonferroni’s test was used (p<0.05).
† vs INITIAL (p<0.05).
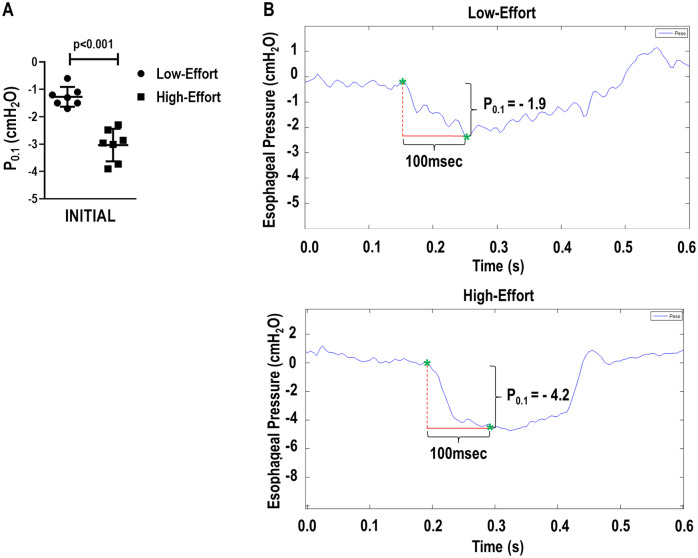
P0.1 was higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV (p = 0.002, and p<0.001, respectively) at INITIAL, and remained unaltered until the end of the experiments (Table 2). The representative P0.1 curves were different between BIVENTHigh-Effort and BIVENTLow-Effort (Fig 2). VT did not differ among groups. At FINAL, Ppeak,L was higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV (p = 0.015, and p = 0.011, respectively). RR and V′E did not differ along time and between groups (Table 2).
Table 2. Respiratory variables during mechanical ventilation.
| Groups | INITIAL | FINAL | Time effect | Group effect | Interaction | |
|---|---|---|---|---|---|---|
| VT (mL/kg) | p = 0.289 | p = 0.194 | p = 0.621 | |||
| PSV | 6.2 ± 0.3 | 6.2 ± 0.6 | ||||
| BIVENTLow-Effort | 5.7 ± 0.4 | 6.0 ± 0.6 | ||||
| BIVENTHigh-Effort | 5.8 ± 0.4 | 6.0 ± 0.4 | ||||
| RR (bpm) | p = 0.455 | p = 0.654 | p = 0.739 | |||
| PSV | 70 ± 11 | 70 ± 14 | ||||
| BIVENTLow-Effort | 64 ± 3 | 70 ± 15 | ||||
| BIVENTHigh-Effort | 72 ± 17 | 74 ± 16 | ||||
| V′E (mL/min) | p = 0.131 | p = 0.601 | p = 0.571 | |||
| PSV | 140 ± 4 | 150 ± 5 | ||||
| BIVENTLow-Effort | 143± 48 | 154 ± 52 | ||||
| BIVENTHigh-Effort | 158 ± 24 | 189 ± 81 | ||||
| Ppeak,L (cmH2O) | p = 0.158 | p = 0.013 | p = 0.158 | |||
| PSV | 13.0 ± 0.9 | 12.5 ± 1.6 | ||||
| BIVENTLow-Effort | 12.0 ± 2.5 | 12.8 ± 1.5 | ||||
| BIVENTHigh-Effort | 14.3 ± 1.9 | 16.1 ± 1.9*# | ||||
| P0.1 (cmH2O) | p = 0.027 | p<0.001 | p = 0.168 | |||
| PSV | -1.1 ± 0.7 | -0.9 ± 0.8 | ||||
| BIVENTLow-Effort | -1.7 ± 0.6 | -1.9 ± 0.8 | ||||
| BIVENTHigh-Effort | -3.0 ± 0.7*# | -4.2 ± 2.5*# |
Respiratory variables at INITIAL and FINAL. PSV: pressure-support ventilation with ΔP set to achieve a VT of 6 mL/kg (n = 7); BIVENTLow-Effort: Biphasic positive airway pressure at 50 controlled breaths/min. Animals were allowed to breath either at high and low positive airway pressures, and anesthesia was modulated to keep low inspiratory effort (n = 7). BIVENTHigh-Effort: Biphasic positive airway pressure at 50 controlled breaths/min. Animals were allowed to breath either at high and low positive airway pressures, and anesthesia was modulated to keep high inspiratory effort (n = 7). VT: tidal volume; RR: respiratory rate; V′E: minute ventilation; Ppeak,L: transpulmonary peak pressure; P01: esophageal pressure measured after 100ms the beginning of inspiratory effort. Values represent mean ± standard deviation (SD). Comparisons were done using a mixed linear model based on a random intercept for each animal followed by Bonferroni’s test was used (p<0.05).
*, vs PSV;
#, vs BIVENTLow-Effort.
Fig 2.
A. P0.1 values obtained at INITIAL in Low-Effort and High-Effort. B. Representative oesophageal pressure signals at INITIAL in Low-Effort and High-Effort. Low-Effort yielded a P0.1 value of -1.9 cmH2O, while High-effort yielded a P0.1 of -4.2 cmH2O. p<0.05 (Student’s t-test).
Pneumonia score was higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV, mainly due to increased oedema/haemorrhage (Fig 3, S2 Table). Accordingly, heterogeneity index was higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV (Fig 3). E-cadherin tissue expression did not differ among groups (median [interquartile range]: PSV, 27% [22% to 56%]; BIVENTLow-Effort, 39% [27% to 55%], BIVENTHigh-Effort: 32% [16% to 42%]; p = 0.547). CINC-1 protein expression in lung tissue was higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV (Fig 4). In addition, neutrophil cell counts were higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV (Fig 5D), while occludin expression was lower in BIVENTHigh-Effort than PSV (Fig 5E). Occludin expression did not differ BIVENTLow-Effort and BIVENTHigh-Effort.
Fig 3.
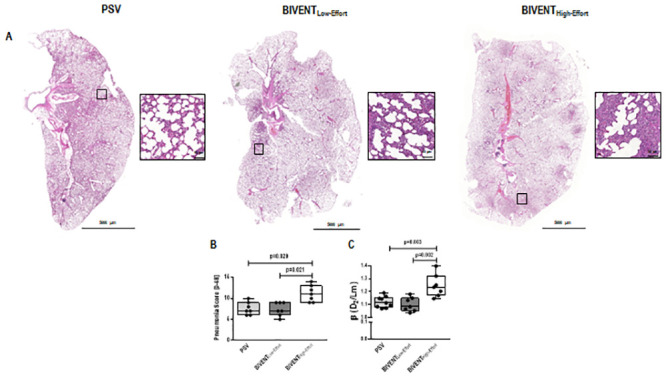
A. Representative scanned lung slices from PSV, BIVENTLow-Effort, and BIVENTHigh-Effort groups. Inset: respective area selected from the whole lung. Bars at 50 μm. B. Pneumonia score [0–32] from PSV, BIVENTLow-Effort, and BIVENTHigh-Effort groups. No difference was observed between PSV and BIVENTLow-Effort (p = 0.999). C. Heterogeneity score (β) from PSV, BIVENTLow-Effort, and BIVENTHigh-Effort groups. No difference was observed between PSV vs BIVENTLow-Effort (p = 0.528). Box plots represent the median and interquartile range. Comparisons were done by Kruskal–Wallis test followed by Dunn’s multiple comparisons (p<0.05).
Fig 4. Representative photographs of immunohistochemistry of serial lung sections for CINC-1 (brown staining).
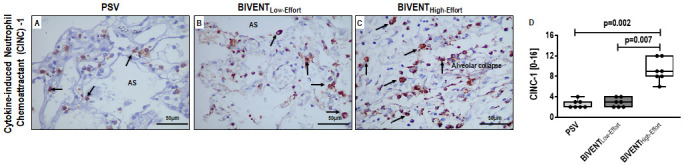
In BIVENTHigh-Effort, intense CINC-1 expression was detected within a dense area of inflammation (panel C; arrows). Light staining for CINC-1 was observed in PSV group lung tissue (panel A; arrows). The middle panel shows moderate staining for CINC-1 in BIVENTLow-Effort (panel B; arrows). AS: alveolar space. No difference in CINC-1 expression in lung tissue was observed between PSV and BIVENTLow-Effort (p = 0.999). Box plots represent the median and interquartile range (panel D). Comparisons were done by the Kruskal–Wallis test followed by Dunn’s multiple comparisons (p<0.05).
Fig 5. Double immunostaining of lung tissue for green-fluorescent neutrophils (GFN) and red-fluorescent occludin (RFO) from PSV, BIVENTLow-Effort, and BIVENTHigh-Effort animals, visualized under confocal microscopy.
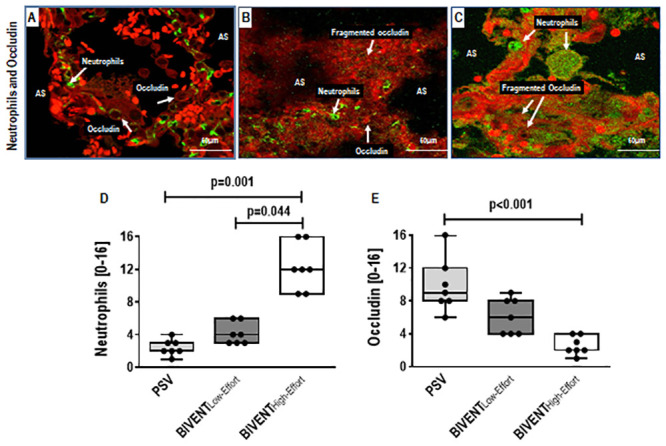
Increased GFN cell counts (panel C; arrows) was seen in BIVENTHigh-Effort compared with PSV (panel A; arrows) and BIVENTLow-Effort (panel B; arrows) lungs. In contrast, increased RFO cell counts (panel A; arrows) were noted in PSV compared to both BIVENTLow-Effort and BIVENTHigh-Effort. Note the fragmented occludin in both BIVENT groups. AS: alveolar space. No difference in neutrophil counts was observed between PSV and BIVENTLow-Effort (p = 0.382). No differences in occludin were observed between PSV vs BIVENTLow-Effort and between BIVENTLow-Effort vs BIVENTHigh-Effort (p = 0.350 and p = 0.078, respectively). Box plots represent the median and interquartile range (panels D and E). Comparisons were done by the Kruskal–Wallis test followed by Dunn’s multiple comparisons (p<0.05).
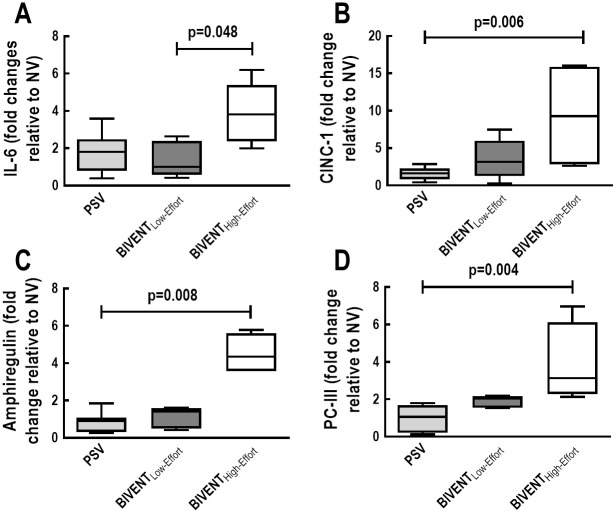
IL-6 and amphiregulin mRNA expression were higher in BIVENTHigh-Effort than BIVENTLow-Effort (Fig 6A and 6C). In addition, CINC-1, amphiregulin and PC-III gene expressions were higher in BIVENTHigh-Effort compared to PSV (Fig 6B–6D). IL-6, CINC-1, amphiregulin, and PC-III expressions did not differ between BIVENTLow-Effort and PSV (Fig 6A–6D). CINC-1 protein levels were higher in BIVENTHigh-Effort than BIVENTLow-Effort and PSV (Fig 7), but did not differ between BIVENTLow-Effort and PSV.
Fig 6. Real-time polymerase chain reaction analysis of biological markers measured in lung tissue for inflammation (A: interleukin [IL]-6; B: cytokine-induced neutrophil chemoattractant [CINC]-1), alveolar stretch (C: amphiregulin), and fibrogenesis (D: procollagen [PC]-III).
Box plots represent the median and interquartile range. Relative gene expression was calculated as a ratio of the average gene expression levels compared with the reference gene (36B4) and expressed as fold change relative to respective NV. No difference was observed between PSV vs BIVENTLow-Effort regarding IL-6, CINC-1, amphiregulin, or PC-III expressions (p = 0.996, p = 0.221, p = 0.999, p = 0.255, respectively). Comparisons were done by the Kruskal–Wallis test followed by Dunn’s multiple comparisons (p<0.05).
Fig 7. Protein levels of cytokine-induced neutrophil chemoattractant (CINC)-1 in lung tissue.
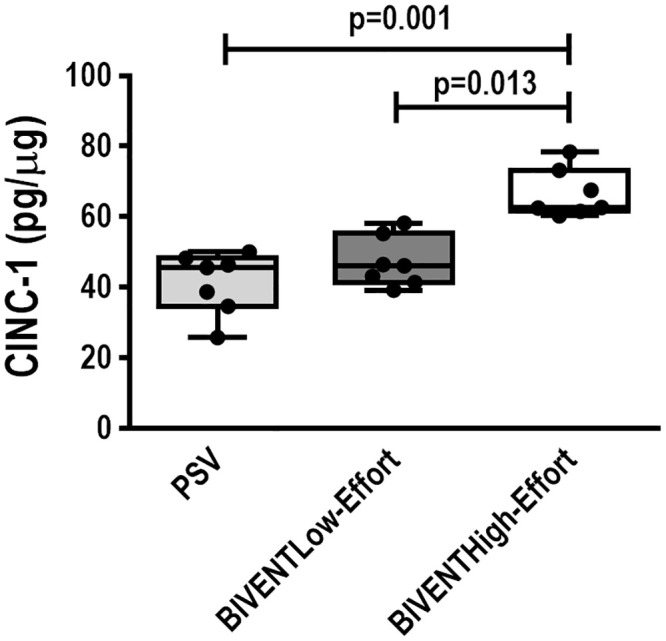
Box plots represent the median and interquartile range in 7 animals/group. No difference in CINC-1 protein levels was observed between PSV and BIVENTLow-Effort (p = 0.999). Comparisons among all groups were done by the Kruskal–Wallis test followed by Dunn’s test (p<0.05).
Blood CFU counts were higher in BIVENTHigh-Effort, but not in BIVENTLow-Effort, compared to PSV (Fig 8).
Fig 8. Blood bacterial counts.
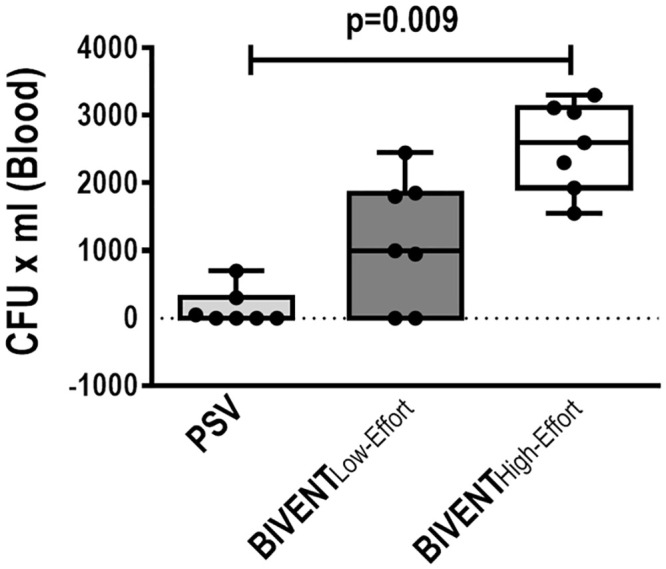
Lines represent the median and interquartile range of 7 animals in each group. The limit for detection was 50 CFU/ml. No difference was observed between PSV vs BIVENTLow-Effort (p = 0.415) and BIVENTLow-Effort vs BIVENTHigh-Effort (p = 0.098). Comparisons were done by the Kruskal–Wallis test followed by Dunn’s multiple comparisons (p<0.05).
Diaphragm TNF-α mRNA expression was higher in BIVENTHigh-Effort compared to BIVENTLow-Effort and PSV (Fig 9); however, no difference was observed between BIVENTLow-Effort and PSV.
Fig 9. Real-time polymerase chain reaction analysis of tumour necrosis factor (TNF)-α in diaphragm tissue.
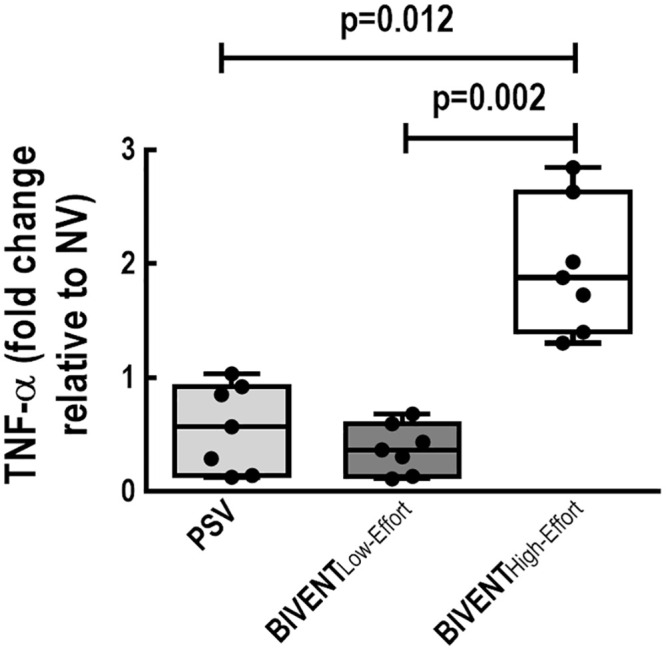
Box plots represent the median and interquartile range. Relative gene expression was calculated as a ratio of the average gene expression levels compared with the reference gene (36B4) and expressed as fold change relative to respective NV. No difference was observed between PSV vs BIVENTLow-Effort (p = 0.999) Comparisons were done by the Kruskal–Wallis test followed by Dunn’s multiple comparisons (p<0.05).
Discussion
In a model of Pseudomonas aeruginosa-induced pneumonia, rats mechanically ventilated with the same protective VT, we found that: 1) BIVENTHigh-Effort was associated with higher transpulmonary peak pressure and histological damage score compared to BIVENTLow-Effort and PSV; 2) BIVENTHigh-Effort was associated with increased CINC-1 protein expression both in lung tissue and homogenate, while reduced occludin expression was detected in immunofluorescence analysis; 3) BIVENTHigh-Effort increased IL-6 gene expression compared to BIVENTLow-Effort, as well as CINC-1, amphiregulin, and PC-III gene expressions in lung tissue compared to PSV; and 4) TNF-α gene expression in diaphragm tissue as well as blood bacterial counts were higher in BIVENTHigh-Effort than BIVENTLow-Effort. BIVENTLow-Effort and PSV, which also exhibited low inspiratory effort, were associated with similar mechanical, histological, and molecular behaviour. This suggests that, once a similar low degree of inspiratory effort is achieved, the mode of partial support ventilation does not affect VILI differently. On the contrary, BIVENT with high effort led to VILI in this model of pneumonia. In addition, P0.1 may be a feasible and easy tool to monitor and optimize the degree of inspiratory effort during assisted ventilation and thus minimize VILI.
Pseudomonas aeruginosa is a common cause of hospital-acquired pneumonia [1]. The present study used a rat model of Pseudomonas aeruginosa-induced pneumonia in which the CFU dosage was adjusted to result in blood bacteria with no mortality [16]. To date, there has been few research evaluating ventilatory strategies in pneumonia; current strategies are based on ARDS studies [27]. In preclinical studies in pneumonia, protective controlled mechanical ventilation (VT = 6 ml/kg and PEEP = 10 cmH2O) was shown to reduce pulmonary bacterial burden in lung tissue [28], whereas different patterns of recruitment manoeuvres were responsible for moving mucus toward distal airways [29]. Both of these studies were done under controlled mechanical ventilation without muscle activity. The present study is the first to evaluate the impact of partial ventilatory support modes, at different inspiratory efforts, on lung and diaphragm damage in a pneumonia model. In this line, BIVENT was used since it is a partial support mode that employs pressure-controlled, time-cycled ventilation set at two levels of continuous positive airway pressure with unrestricted spontaneous breathing at any time of respiratory cycling [7]. In order to test the hypothesis regarding the role of different inspiratory efforts (low and high), anaesthesia was titrated by modulating ketamine and midazolam infusion rates, and P0.1 was kept constant throughout the experiment. To compare the effects of BIVENT with low and high effort, PSV, a method of spontaneous ventilation employed widely in clinical practice, was used [30]. During assisted ventilatory modes, protective VT = 6 ml/kg was always applied.
BIVENTHigh-Effort vs BIVENTLow-Effort
As shown in Table 2, transpulmonary pressure was higher in BIVENTHigh-Effort than BIVENTLow-Effort. Since a similar VT was achieved between groups, we may infer that lung compliance was reduced in BIVENTHigh-Effort. This mechanical change was associated with a higher histological pneumonia score, mainly due to increased oedema/haemorrhage. In addition, biological markers associated with VILI were higher in BIVENTHigh-Effort than BIVENTLow-Effort. Increased inspiratory effort can result in increased Pes swings and lung perfusion [31], thus increasing capillary leakage and oedema [32]. In lungs with bacterial pneumonia, normal alveolar areas coexist with adjacent consolidated areas (alveolar heterogeneity), which may lead to further lung damage when inspiratory effort is increased. Indeed, the heterogeneity score was higher with increased inspiratory effort. High inspiratory effort can cause a significant negative intrapleural pressure, leading to marked tensile stress [33]. Depending on the degree of tensile stress, alveolar unit overdistension and damage may occur even at the same VT [11, 31, 34]. In the present study, the expression and protein level of CINC-1 in lung tissue homogenate were higher in BIVENTHigh-Effort compared to BIVENTLow-Effort, which may result in increased neutrophil chemoattraction after P. aeruginosa infection [35]. Unfortunately, some ventilators do not detect this marked intrapleural pressure. As pointed out by Brochard et al [31], under partial ventilatory assistance, even though modest levels of positive airway pressure and VT during inspiration are adjusted, one should not consider these patients to be protected. On the other hand, P0.1, which is a reasonable index of neuromuscular drive, can be measured in some ventilators and should be used much more widely at the bedside [31, 36]. The occurrence of spontaneous effort during the Phigh period in BIVENTHigh-Effort may increase transpulmonary pressure to dangerous levels.
BIVENT vs PSV
BIVENT and PSV are different forms of partial support ventilation. PSV is a partial support mode which demands a inspiratory effort target for a given airway pressure range. The transition from inspiration to expiration occurs when the inspiratory flow has decayed to less than 25% of the peak inspiratory value (flow-cycled). On the other hand, BIVENT employs pressure control under partial ventilation (time-cycled), set at two levels of CPAP with unrestricted spontaneous breathing. Although there are fundamental differences regarding how BIVENT and PSV work, in the present study, we did not detect any difference between BIVENTLow-effort and PSV, which denotes that the main cause of lung damage was not linked to type of partial ventilatory support, but rather was associated with the level of inspiratory effort. In this line, P0.1 was lower in PSV and BIVENTLow-effort than in BIVENTHigh-Effort, in accordance with PSV values previously observed by our group in experimental ARDS [37, 38]. BIVENTHigh-Effort was associated with higher pneumonia and heterogeneity scores compared to PSV. In addition, CINC-1 gene expression and protein levels, a marker of neutrophil migration into the lungs during pneumonia [39], was higher in BIVENTHigh-Effort compared to PSV. The translocation of neutrophils may increase the gaps among endothelial and epithelial cells and, combined with overdistension, may predispose to fluid shift between capillaries towards the alveolar space. One way to infer the gaps among epithelial cells is to measure the expression of tight-junction proteins that promote epithelial integrity, such as occludin. Previous research has shown that the fragmentation of occludin followed by its reduction suggests epithelial integrity degradation by injurious mechanical ventilation [40]. Amphiregulin, a marker of alveolar stretch, has been found elevated in experimental influenza pneumonia [41], and was higher in BIVENTHigh-Effort compared to PSV and BIVENTLow-Effort. Amphiregulin gene expression is mostly influenced by overdistension, and less so by inflammatory stimuli [42]. We hypothesize that the worse outcomes observed during partial ventilator support in pneumonia are related to the high tensile stress caused by high inspiratory effort, thus exceeding the plasto-elastic threshold of lung tissue [43] and contributing to bacterial translocation and distal organ damage [44].
Furthermore, high inspiratory effort resulted in increased gene expression of markers associated with diaphragm inflammation (TNF-α). The inappropriate use of partial ventilator support has been shown to injure not only the lung, but also the diaphragm [45]. One likely mechanism would be excessive concentric loading, during which vigorous contractions provoke high muscle tension, resulting in muscle inflammation, proteolysis, myofibrillar damage, and derangement of the sarcolemma [46]. Since the total time of mechanical ventilation was set to 1 hour, we chose to analyse the marker of inflammation which rises the fastest, TNF-α. We may further conclude that whether the mode of ventilation was flow- (PSV) or time-cycled (BIVENT) made no difference in terms of diaphragm injury, at least during low inspiratory effort.
Possible clinical implications of study findings
In a prospective, observational, international multicentre cohort study of 13,751 ventilated ARDS patients, which had pneumonia as the major risk factor, spontaneous breathing was not associated with worse outcomes, and appeared to allow earlier weaning from the ventilator [14]. Nevertheless, it is well recognized that increased inspiratory effort has a negative impact on clinical outcome [11]. In the present study, we used BIVENT, which allows spontaneous ventilation at two levels of airway pressure. We also found that P0.1 may be a feasible parameter to monitor and optimize respiratory effort under partial ventilatory support modes [36].
Limitations
Some limitations of this study must be noted. First, pneumonia was induced by intratracheal instillation of Pseudomonas aeruginosa; thus, our findings cannot be extrapolated to other types of pneumonia. Second, we only measured blood bacterial counts, not the distal organ contamination. Third, male animals were used to avoid the effects of female hormones on the expression of pro-inflammatory mediators [47], acknowledging the importance of sex differences in preclinical and basic studies [48]. Fourth, the ventilation period was limited to 1 h, since a longer period of time might result in haemodynamic instability and thus affect the expression of biological markers. One hour of mechanical ventilation was enough to observe molecular changes in key biological markers related to VILI and bacterial translocation from airspaces to the bloodstream. Further studies are required to evaluate whether the level of blood bacteria (CFU/mL) is associated with survival in mechanically ventilated rats during a long-term experiment. Fifth, PSV was done only at low inspiratory effort. Thus, we cannot ensure that, under high inspiratory effort, BIVENT and PSV would behave differently way from how they do under low inspiratory effort. A previous preclinical study found that PSV under intense work of breathing was not associated with worse outcomes [13].
Conclusion
In the experimental model of Pseudomonas aeruginosa-induced pneumonia used herein, at the same protective VT, BIVENT under high inspiratory effort was associated with higher lung damage score and increased expression of genes associated with VILI and diaphragm inflammation, as well as increased translocation of bacteria into the bloodstream, compared to BIVENT and PSV (both with low inspiratory effort). Therefore, regardless of the mode of partial support ventilation (BIVENT or PSV), when low inspiratory effort was used, the lung and diaphragm remained protected and bacterial translocation was reduced. We also found that P0.1 might be a helpful parameter to optimize inspiratory effort during assisted ventilation.
Supporting information
(RTF)
(DOCX)
Acknowledgments
We thank Mr. Andre Benedito da Silva for animal care, Ms. Arlete Fernandes for her help with microscopy, and Mrs. Moira Elizabeth Schottler and Mr. Filippe Vasconcellos for their assistance in editing the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq, 405686/2018-8). http://www.cnpq.br/. PLS: Rio de Janeiro State Research Foundation (FAPERJ, E-26/202.651/2018), Coordination for the Improvement of Higher Education Personnel (CAPES) https://www.capes.gov.br/, and the Department of Science and Technology – Brazilian Ministry of Health (DECIT/MS) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Restrepo MI, Babu BL, Reyes LF, Chalmers JD, Soni NJ, et al. (2018) Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. The European respiratory journal 52 10.1183/13993003.01190-2017 [DOI] [PubMed] [Google Scholar]
- 2.Curran CS, Bolig T, Torabi-Parizi P (2018) Mechanisms and Targeted Therapies for Pseudomonas aeruginosa Lung Infection. American journal of respiratory and critical care medicine 197: 708–727. 10.1164/rccm.201705-1043SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiumello D, Brochard L, Marini JJ, Slutsky AS, Mancebo J, et al. (2017) Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Critical care 21: 240 10.1186/s13054-017-1820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMullen SM, Meade M, Rose L, Burns K, Mehta S, et al. (2012) Partial ventilatory support modalities in acute lung injury and acute respiratory distress syndrome-a systematic review. PloS one 7: e40190 10.1371/journal.pone.0040190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doorduin J, Nollet JL, Roesthuis LH, van Hees HW, Brochard LJ, et al. (2017) Partial Neuromuscular Blockade during Partial Ventilatory Support in Sedated Patients with High Tidal Volumes. American journal of respiratory and critical care medicine 195: 1033–1042. 10.1164/rccm.201605-1016OC [DOI] [PubMed] [Google Scholar]
- 6.Saddy F, Oliveira GP, Garcia CS, Nardelli LM, Rzezinski AF, et al. (2010) Assisted ventilation modes reduce the expression of lung inflammatory and fibrogenic mediators in a model of mild acute lung injury. Intensive care medicine 36: 1417–1426. 10.1007/s00134-010-1808-6 [DOI] [PubMed] [Google Scholar]
- 7.Saddy F, Moraes L, Santos CL, Oliveira GP, Cruz FF, et al. (2013) Biphasic positive airway pressure minimizes biological impact on lung tissue in mild acute lung injury independent of etiology. Critical care 17: R228 10.1186/cc13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieman GF, Gatto LA, Andrews P, Satalin J, Camporota L, et al. (2020) Prevention and treatment of acute lung injury with time-controlled adaptive ventilation: physiologically informed modification of airway pressure release ventilation. Annals of intensive care 10: 3 10.1186/s13613-019-0619-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalgudi Ganesan S, Jayashree M, Chandra Singhi S, Bansal A (2018) Airway Pressure Release Ventilation in Pediatric Acute Respiratory Distress Syndrome. A Randomized Controlled Trial. American journal of respiratory and critical care medicine 198: 1199–1207. 10.1164/rccm.201705-0989OC [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Grieco DL, Brochard L, Fujino Y (2020) Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Current opinion in critical care 26: 59–65. 10.1097/MCC.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, et al. (2013) Spontaneous effort causes occult pendelluft during mechanical ventilation. American journal of respiratory and critical care medicine 188: 1420–1427. 10.1164/rccm.201303-0539OC [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y (2012) Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Critical care medicine 40: 1578–1585. 10.1097/CCM.0b013e3182451c40 [DOI] [PubMed] [Google Scholar]
- 13.Henzler D, Schmidt A, Xu Z, Ismaiel N, Zhang H, et al. (2019) Increased effort during partial ventilatory support is not associated with lung damage in experimental acute lung injury. Intensive care medicine experimental 7: 60 10.1186/s40635-019-0272-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Haren F, Pham T, Brochard L, Bellani G, Laffey J, et al. (2019) Spontaneous Breathing in Early Acute Respiratory Distress Syndrome: Insights From the Large Observational Study to UNderstand the Global Impact of Severe Acute Respiratory FailurE Study. Critical care medicine 47: 229–238. 10.1097/CCM.0000000000003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Journal of pharmacology & pharmacotherapeutics 1: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Magalhaes RF, Samary CS, Santos RS, de Oliveira MV, Rocha NN, et al. (2016) Variable ventilation improves pulmonary function and reduces lung damage without increasing bacterial translocation in a rat model of experimental pneumonia. Respiratory research 17: 158 10.1186/s12931-016-0476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortola JP, Noworaj A (1983) Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. Journal of applied physiology: respiratory, environmental and exercise physiology 55: 250–253. 10.1152/jappl.1983.55.1.250 [DOI] [PubMed] [Google Scholar]
- 18.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. The American review of respiratory disease 126: 788–791. 10.1164/arrd.1982.126.5.788 [DOI] [PubMed] [Google Scholar]
- 19.Pinto EF, Santos RS, Antunes MA, Maia LA, Padilha GA, et al. (2020) Static and Dynamic Transpulmonary Driving Pressures Affect Lung and Diaphragm Injury during Pressure-controlled versus Pressure-support Ventilation in Experimental Mild Lung Injury in Rats. Anesthesiology 132: 307–320. 10.1097/ALN.0000000000003060 [DOI] [PubMed] [Google Scholar]
- 20.Uhlig C, Silva PL, Ornellas D, Santos RS, Miranda PJ, et al. (2014) The effects of salbutamol on epithelial ion channels depend on the etiology of acute respiratory distress syndrome but not the route of administration. Respiratory research 15: 56 10.1186/1465-9921-15-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva PL, Guldner A, Uhlig C, Carvalho N, Beda A, et al. (2013) Effects of intravascular volume replacement on lung and kidney function and damage in nonseptic experimental lung injury. Anesthesiology 118: 395–408. 10.1097/ALN.0b013e31827e554c [DOI] [PubMed] [Google Scholar]
- 22.Mizgerd JP, Skerrett SJ (2008) Animal models of human pneumonia. American journal of physiology Lung cellular and molecular physiology 294: L387–398. 10.1152/ajplung.00330.2007 [DOI] [PubMed] [Google Scholar]
- 23.Wierzchon C, Padilha G, Rocha NN, Huhle R, Coelho MS, et al. (2017) Variability in Tidal Volume Affects Lung and Cardiovascular Function Differentially in a Rat Model of Experimental Emphysema. Frontiers in physiology 8: 1071 10.3389/fphys.2017.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felix NS, Samary CS, Cruz FF, Rocha NN, Fernandes MVS, et al. (2019) Gradually Increasing Tidal Volume May Mitigate Experimental Lung Injury in Rats. Anesthesiology 130: 767–777. 10.1097/ALN.0000000000002630 [DOI] [PubMed] [Google Scholar]
- 25.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B (2006) Quantitative characterization of airspace enlargement in emphysema. Journal of applied physiology 100: 186–193. 10.1152/japplphysiol.00424.2005 [DOI] [PubMed] [Google Scholar]
- 26.Akamine R, Yamamoto T, Watanabe M, Yamazaki N, Kataoka M, et al. (2007) Usefulness of the 5’ region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. Journal of biochemical and biophysical methods 70: 481–486. 10.1016/j.jbbm.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, et al. (2016) Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 63: e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperber J, Nyberg A, Lipcsey M, Melhus A, Larsson A, et al. (2017) Protective ventilation reduces Pseudomonas aeruginosa growth in lung tissue in a porcine pneumonia model. Intensive care medicine experimental 5: 40 10.1186/s40635-017-0152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Bassi G, Comaru T, Marti D, Xiol EA, Chiurazzi C, et al. (2019) Recruitment manoeuvres dislodge mucus towards the distal airways in an experimental model of severe pneumonia. British journal of anaesthesia 122: 269–276. 10.1016/j.bja.2018.07.039 [DOI] [PubMed] [Google Scholar]
- 30.Penuelas O, Muriel A, Abraira V, Frutos-Vivar F, Mancebo J, et al. (2020) Inter-country variability over time in the mortality of mechanically ventilated patients. Intensive care medicine 46: 444–453. 10.1007/s00134-019-05867-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brochard L (2017) Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure: Yes. Intensive care medicine 43: 250–252. 10.1007/s00134-016-4645-4 [DOI] [PubMed] [Google Scholar]
- 32.Kallet RH, Alonso JA, Luce JM, Matthay MA (1999) Exacerbation of acute pulmonary edema during assisted mechanical ventilation using a low-tidal volume, lung-protective ventilator strategy. Chest 116: 1826–1832. 10.1378/chest.116.6.1826 [DOI] [PubMed] [Google Scholar]
- 33.Saddy F, Sutherasan Y, Rocco PR, Pelosi P (2014) Ventilator-associated lung injury during assisted mechanical ventilation. Seminars in respiratory and critical care medicine 35: 409–417. 10.1055/s-0034-1382153 [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Roldan R, Beraldo MA, Torsani V, Gomes S, et al. (2016) Spontaneous Effort During Mechanical Ventilation: Maximal Injury With Less Positive End-Expiratory Pressure. Critical care medicine 44: e678–688. 10.1097/CCM.0000000000001649 [DOI] [PubMed] [Google Scholar]
- 35.Denning GM, Wollenweber LA, Railsback MA, Cox CD, Stoll LL, et al. (1998) Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infection and immunity 66: 5777–5784. 10.1128/IAI.66.12.5777-5784.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, et al. (2020) Airway Occlusion Pressure as an Estimate of Respiratory Drive and Inspiratory Effort During Assisted Ventilation. American journal of respiratory and critical care medicine. 10.1164/rccm.201907-1425OC [DOI] [PubMed] [Google Scholar]
- 37.Moraes L, Santos CL, Santos RS, Cruz FF, Saddy F, et al. (2014) Effects of sigh during pressure control and pressure support ventilation in pulmonary and extrapulmonary mild acute lung injury. Critical care 18: 474 10.1186/s13054-014-0474-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magalhaes PAF, Padilha GA, Moraes L, Santos CL, Maia LA, et al. (2018) Effects of pressure support ventilation on ventilator-induced lung injury in mild acute respiratory distress syndrome depend on level of positive end-expiratory pressure: A randomised animal study. European journal of anaesthesiology 35: 298–306. 10.1097/EJA.0000000000000763 [DOI] [PubMed] [Google Scholar]
- 39.Wen J, Li CM, Gu L, Yin SJ, Li W, et al. (2014) Aging reduces the expression of lung CINC and MCP-1 mRNA in a P. aeruginosa rat model of infection. Inflammation 37: 933–941. 10.1007/s10753-014-9813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao T, Liu M, Gu C, Wang X, Wang Y (2014) Activation of c-Src tyrosine kinase mediated the degradation of occludin in ventilator-induced lung injury. Respiratory research 15: 158 10.1186/s12931-014-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Vermillion MS, Ursin RL, Kuok DIT, Vom Steeg LG, Wohlgemuth N, et al. (2018) Production of amphiregulin and recovery from influenza is greater in males than females. Biology of sex differences 9: 24 10.1186/s13293-018-0184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolinay T, Kaminski N, Felgendreher M, Kim HP, Reynolds P, et al. (2006) Gene expression profiling of target genes in ventilator-induced lung injury. Physiological genomics 26: 68–75. 10.1152/physiolgenomics.00110.2005 [DOI] [PubMed] [Google Scholar]
- 43.Suki B, Bates JH (2011) Lung tissue mechanics as an emergent phenomenon. Journal of applied physiology 110: 1111–1118. 10.1152/japplphysiol.01244.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller-Redetzky HC, Will D, Hellwig K, Kummer W, Tschernig T, et al. (2014) Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Critical care 18: R73 10.1186/cc13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertoni M, Spadaro S, Goligher EC (2020) Monitoring Patient Respiratory Effort During Mechanical Ventilation: Lung and Diaphragm-Protective Ventilation. Critical care 24: 106 10.1186/s13054-020-2777-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, et al. (2001) Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 164: 1734–1739. 10.1164/ajrccm.164.9.2011150 [DOI] [PubMed] [Google Scholar]
- 47.Khramtsova EA, Davis LK, Stranger BE (2019) The role of sex in the genomics of human complex traits. Nature reviews Genetics 20: 173–190. 10.1038/s41576-018-0083-1 [DOI] [PubMed] [Google Scholar]
- 48.Lee SK (2018) Sex as an important biological variable in biomedical research. BMB reports 51: 167–173. 10.5483/bmbrep.2018.51.4.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RTF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.