Abstract
Objectives:
Pancreatic neuroendocrine neoplasms include well differentiated tumors (PanNETs) and poorly differentiated carcinomas (PanNECs). Previous reports suggested a role for platinum-based therapy largely in PanNEC. We sought to investigate the role of platinum-based therapy in pancreatic neuroendocrine neoplasms regardless of tumor grade and differentiation.
Methods:
Patients with pancreatic neuroendocrine neoplasms treated with platinum-based therapy at Memorial Sloan Kettering (1994–2016) and Verona University Hospital (2008–2016) were retrospectively identified. Response to treatment by RECISTv1.1, overall survival and progression-free survival were defined. Among patients with available tissue, DAXX, ATRX, Rb, and p53 expression was evaluated to support the histologic grade of differentiation.
Results:
Fifty PanNETs, 29 PanNECs, and 22 high grade tumors with undeterminable differentiation were included. No patients achieved complete response. Overall rate of partial response was 31%, 41% for PanNEC and 20% for PanNETs. Among PanNETs, PR was achieved in 33% of G1 (2/6), 10% of G2 (2/19), and 24% of G3 (6/25) tumors. Median overall survival was 29.3 months for PanNETs and 10.9 months for PanNEC (P <0.001). There was no significant difference in median progression-free survival (P = 0.2).
Conclusions:
Platinum-based therapies demonstrated increased activity in PanNEC, however promising efficacy was also observed in PanNETs, irrespective of grade.
Keywords: pancreatic neuroendocrine tumor, poorly differentiated neuroendocrine tumor, platinum-based treatment, PanNEC, PanNET
Introduction
Pancreatic neuroendocrine neoplasms include the well differentiated neuroendocrine tumors (PanNETs) and the poorly differentiated neuroendocrine carcinomas (PanNECs). Poorly differentiated neuroendocrine carcinomas is universally aggressive, whereas PanNETs include a heterogeneous group of neoplasms with varying degrees of aggressiveness and is defined by grade according to World Health Organization (WHO) classification.1,2
The treatment paradigms are generally based on the differentiation of the neoplasm (well- or poorly-differentiated), and, for well differentiated PanNETs, by the grade. Management of PanNETs includes several therapeutic options, such as somatostatin analogs, peptide receptor radionuclide therapy and targeted therapies (everolimus, sunitinib) that are generally considered as first- and second-line treatments.3 Cytotoxic chemotherapy with alkylating agents is used across the spectrum of PanNETs and is reserved for patients with heavy tumor burden, rapidly progressive disease, and high-grade neoplasms.4–6 For PanNEC, treatment options are more limited and cytotoxic chemotherapy is used first-line with the exclusive use of platinum-based regimens.4,6 However, high-grade PanNETs have only been recently recognized as a distinct entity from PanNEC and therefore many patients with this disease have previously received platinum-based chemotherapy.1,7 This treatment strategy was investigated in the NORDIC study, in which patients with neuroendocrine neoplasms with a Ki-67 >20% received platinum-based chemotherapy, without controlling for tumor differentiation; in this study, a higher response rate was seen in neuroendocrine neoplasms with a Ki-67 >55%, despite a longer overall survival (OS) in tumors with Ki-67 <55%.5 Although high-grade PanNETs exhibit lower average Ki-67 compared to PanNEC, the ranges of Ki-67 indices do overlap and there is no clear cutoff that sharply distinguishes these two entities.8 Without an accurate classification of the tumor differentiation, it is difficult to determine from prior investigation the efficacy of platinum-based treatments in high-grade neuroendocrine neoplasms. Additionally, other features can differentiate high grade PanNET from PanNEC.7,9,10 The first share similar mutations with low and intermediate grade PanNETs, including inactivation of MEN1 and DAXX or ATRX. In contrast, PanNEC universally exhibit alterations in RB1, and often have alterations in TP53. Therefore, Rb, p53, DAXX, and ATRX immunoexpression can be used in the clinical practice as a surrogate for the genetic alteration to help differentiate highgrade PanNET from PanNEC.7,9–13
An additional limitation of current recommendations for use of platinum-based treatment in neuroendocrine neoplasms is that the current body of knowledge comes from small and nonrandomized series, largely focused on treatment with platinum/etoposide regimens, with data lacking for other platinum-based regimens.5,14,15 Also, little data has been reported on the efficacy of platinum-based regimens for patients with low or intermediate grade disease.16–18
In the present study, we sought to evaluate the response of pancreatic neuroendocrine neoplasms to platinum-based therapy regardless of the tumor grade and differentiation in order to better identify predictors of response. We retrospectively reviewed clinical, pathological, and treatment data of patients diagnosed with pancreatic neuroendocrine neoplasms undergoing platinum-based chemotherapy. To distinguish PanNETs from PanNEC, we evaluated the protein expression of DAXX, ATRX, Rb, and p53 in a subgroup of cases with material available for immunohistochemistry (IHC).
MATERIALS AND METHODS
Study Population and Data Collection
This study was approved by a waiver authorization from the Memorial Sloan Kettering (MSK) and Verona University Hospital (VUH) institutional review boards. Electronic pharmacy records from institutional databases were queried for patients with pancreatic neuroendocrine neoplasms who underwent platinum-based treatment. The time of the study, depending on data availability, was 1994–2016 for MSK and 2008–2016 for VUH. Inclusion criteria included a histopathologically confirmed diagnosis of pancreatic neuroendocrine neoplasm and WHO grade. Morphologically, the tumor was classified as either well differentiated or poorly differentiated. Neoplasms with mixed pathologic differentiation (ie, with ductal adenocarcinoma or acinar cell carcinoma components) were excluded. Medical records were retrospectively reviewed for patient demographics and characteristics, pathology reports, systemic treatments, and outcomes. Patients who received at least one cycle of platinum-based treatment were included. Patients who received platinum-based chemotherapy for the treatment of a second nonpancreatic primary tumor, those who received a platinum-based treatment in association with an alkylating agent, and those with unknown tumor grade or with no clinical records available for review, were excluded.
In both institutions, the pathological assessment was performed by a dedicated gastrointestinal pathologist experienced in the diagnosis and classification of pancreatic neuroendocrine neoplasms. When the tissue was available, the Ki-67 proliferation index was repeated; otherwise, the grade was obtained from the pathology reports. Tumors were graded according to the 2019 WHO classification system.1 When Ki-67 was assessed multiple times, the highest value was used for the analysis. The classification of differentiation status was based upon the morphologic features of the neoplasm as previously described.7,8 When representative tissue was available, immunohistochemistry (IHC) stains for DAXX, ATRX, Rb, and p53 were performed as surrogate markers for genomic alterations, with the results supplementing the morphologic assessment to define differentiation status. In selected cases, next-generation sequencing of tumor tissue was performed using the MSK-IMPACT™ assay19, and alterations in the four genes listed above were similarly used to establish differentiation.
Evaluation of Response
The overall response rate was assessed according to the RECISTv.1.1 and classified as: complete response (CR), partial response (PR), stable disease (SD), and progression of disease (POD).20 Platinum-based chemotherapy regimens were categorized as follows: (1) platinum/etoposide (cisplatin/etoposide, carboplatin/etoposide), (2) platinum/irinotecan (cisplatin/irinotecan, carboplatin/irinotecan), (3) platinum/gemcitabine (cisplatin/gemcitabine, oxaliplatin/gemcitabine), (4) oxaliplatin/Fluorouracil (5-FU) (FOLFIRINOX [a comibation of leucovorin, 5-Fu, irinotecan, oxaliplatin], FOLFOX [leucovorin, 5-FU, oxaliplatin], XELOX [capecitabine and oxaliplatin]) and (5) platinum (carboplatin). For patients who received more than one platinum-based regimen, the regimen with the best overall response was reported.
Immunohistochemistry Analysis
When available, paraffin-embedded tissues were used for IHC analysis for Ki-67, DAXX, ATRX, Rb, and p53. Additional details are provided in the Supplemental Methods. Neoplasms with abnormal expression (ie, loss) of DAXX or ATRX were regarded to be well differentiated. Those with abnormal expression (ie, loss) of Rb were considered poorly differentiated. Those with abnormal overexpression of p53 were also considered poorly differentiated, unless there was a concurrent abnormality in DAXX or ATRX, in which case a well differentiated categorization was established.8 In cases with ambiguous morphologic results and no further data from IHC (due to insufficient tissue or normal expression of all 4 markers), the differentiation was considered “unknown.”
Statistical Analysis
Clinicopathologic characteristics of patients were summarized by frequency and percentages for categorical variables, and median and interquantile range for continuous variables. Fisher’s exact test and Wilcoxon rank-sum test for categorical and continuous variables, respectively, were used to comparing characteristics between best response status. Overall survival was calculated from the date of first platinum-based treatment to date of death or last follow-up. Progression-free survival (PFS) was calculated from the date of first platinum-based treatment until the first disease progression, or death, whichever occurred first. Overall survival and PFS were estimated using Kaplan-Meier methods and compared between subgroups using log-rank test. The duration of response was calculated among patients who achieved the best response (CR + PR) until the date of the first progression and estimated using Kaplan-Meier methods. All P-values were based on 2-tailed statistical analysis, and P < 0.05 was considered statistically significant. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.1 (R Core Team, Vienna, Austria).
RESULTS
A total of 133 patients with a diagnosis of pancreatic neuroendocrine neoplasms who received platinum-based chemotherapy were identified and 32 patients were excluded from the study; 9 patients had unknown tumor grade and no tissue available for testing, 6 patients received platinum-based therapies for a second primary tumor, 12 patients had a combination of platinum plus alkylating agents and 1 patient had platinum-based therapy in the adjuvant setting; 4 patients had no medical records available for review. Final cohort included 101 patients, 69 from MSK, and 32 from VUH. The clinicopathologic characteristics of the study population are presented in Table 1. All patients with low and intermediate grade PanNETs had progressive disease before initiating platinum-based therapies.
TABLE 1.
Clinical and Pathological Characteristics of Study Population
| Variable | Overall (n = 101) |
|---|---|
| Age, median (IQR), y | 60 (49–68) |
| Sex, male, n | 58 |
| Performance status, n | |
| 0 | 32 |
| 1 | 50 |
| 2 | 19 |
| Functional, n | 5 |
| Somatostatin receptor imaging, n | |
| Negative | 17 |
| Positive | 47 |
| Unknown | 37 |
| Ki-67, median (IQR), % | 50 (20–80) |
| Ki-67, n | |
| <55% | 52 |
| ≥55% | 35 |
| Unknown | 14 |
| Differentiation, n | |
| Well-differentiated (PanNET) | 50 |
| Poorly differentiated (PanNEC) | 29 |
| Unknown differentiation | 22 |
| Grade (PanNET only), n | |
| Grade 1 | 6 |
| Grade 2 | 19 |
| Grade 3 | 25 |
| Stage at presentation, n | |
| III | 5 |
| IV | 96 |
| Best overall response scheme, n (%) | |
| Platinum/Etoposide | 57 |
| Platinum/Irinotecan | 9 |
| Platinum/Gemcitabine | 17 |
| Oxaliplatin/5-FU | 15 |
| Platinum | 3 |
PanNET indicates pancreatic neuroendocrine tumor; PanNEC, pancreatic neuroendocrine carcinoma.
Response to Treatment
In the entire treated cohort, CR was 0%, PR 31%, SD 41%, and POD 28%. No clinicopathologic characteristics included in the analysis were significantly associated with response to treatment (Table 2). Details on the clinicopathologic characteristics of each patient who had PR or SD to platinum-based chemotherapy are reported in Supplemental Tables 1 and 2.
TABLE 2.
Clinical and Pathological Characteristics Associated With Response to Platinum-based Chemotherapy
| Variable | PR | SD | POD | P |
|---|---|---|---|---|
| All Cases (n = 101) | (n = 31) | (n = 42) | (n = 28) | |
| Age, median (IQR), y | 59 (49–69) | 61 (50–68) | 60 (50–68) | 0.97 |
| Sex, male, n (%) | 20 (35) | 21 (36) | 17 (29) | 0.45 |
| Functional, n (%) | 1 (20) | 3 (60) | 1 (20) | 0.73 |
| Somatostatin receptor imaging, n (%) | 0.14 | |||
| Negative | 2 (12) | 8 (47) | 7 (41) | |
| Positive | 15 (32) | 22 (47) | 10 (21) | |
| Not Available | 14 (38) | 12 (32) | 11 (30) | |
| Differentiation, n (%) | 0.11 | |||
| Well-differentiated (PanNET) | 10 (20) | 27 (54) | 13 (26) | |
| Poorly differentiated (PanNEC) | 12 (41) | 10 (36) | 7 (24) | |
| Unknown differentiation | 9 (41) | 5 (23) | 8 (36) | |
| Grade (PanNET only), n (%) | 0.73 | |||
| Grade 1 | 2 (33) | 3 (50) | 1 (17) | |
| Grade 2 | 2 (10) | 11 (58) | 6 (32) | |
| Grade 3 | 6 (24) | 13 (52) | 6 (24) | |
| Ki-67, median (IQR), % | 65 (33–80) | 40 (15–60) | 40 (15–70) | 0.10 |
| Ki-67, n (%) | 0.08 | |||
| <55% | 11 (21) | 27 (52) | 14 (27) | |
| ≥55% | 15 (43) | 11 (31) | 9 (26) | |
| Not available | 5 (36) | 4 (28) | 5 (36) | |
| G3 and PD PanNEC cases (n = 76) | (n = 27) | (n = 28) | (n = 21) | |
| Age, median (IQR), y | 59 (4–69) | 61 (47–68) | 60 (50–67) | 0.97 |
| Sex, male, n (%) | 19 (44) | 13 (30) | 11 (26) | 0.06 |
| Somatostatin receptor imaging, n (%) | 0.12 | |||
| Negative | 2 (13) | 7 (47) | 6 (40) | |
| Positive | 11 (42) | 10 (39) | 5 (19) | |
| Not available | 14 (40) | 11 (31) | 10 (29) | |
| Differentiation, n (%) | 0.34 | |||
| Well-differentiated (PanNET) | 6 (24) | 13 (52) | 6 (24) | |
| Poorly differentiated (PanNEC) | 12 (41) | 10 (35) | 7 (24) | |
| Unknown differentiation | 9 (41) | 5 (23) | 8 (36) | |
| Ki-67, median (IQR), % | 70 (50–83) | 50 (40–80) | 55 (33–80) | 0.39 |
| Ki-67 cut-off, n (%) | 0.31 | |||
| <55% | 7 (26) | 13 (48) | 7 (26) | |
| ≥55% | 15 (43) | 11 (31) | 9 (26) |
PanNET indicates pancreatic neuroendocrine tumor; PanNEC, pancreatic neuroendocrine cancer; PD, poorly differentiated; PR, partial response; SD, stability of disease; POD, progression of disease.
In patients that achieved a PR, the time elapsed from diagnosis (defined by the time of biopsy) and initiation of platinum-based treatment was significantly longer for PanNETs (median, 8 months [interquartile range, 1–39]) than for PanNEC (median, 0.7 months [interquartile range, 0.4–1.7]), P = 0.004. Indeed, whereas 97% of patients (n = 28) with PanNEC received platinum-based chemotherapy as first-line treatment, patients having PanNETs were previously treated with somatostatin analogs (74%, n = 37), with alkylating agents (46%, n = 23), targeted therapies (44%, n = 22) and with PRRT (10%, n = 5).
No significant differences in response rate were observed by type of platinum-based therapy (P = 0.41). Best overall response to platinum-based therapies according to regimen, grade, and differentiation is shown in Table 3.
TABLE 3.
Best Overall Response to Platinum-based Therapies According to the Scheme, Tumor Differentiation, and for Well-Differentiated PanNET, Grade
| Pancreatic Neuroendocrine Neoplasms (n = 101) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platinum/Etoposide | Platinum/Irinotecan | Platinum/Gemcitabine | Oxaliplatin/5-FU | Platinum | |||||||||||
| Characteristic | PR (n = 16) |
SD (n = 20) |
POD (n = 21) |
PR (n = 3) |
SD (n = 4) |
POD (n = 2) |
PR (n = 6) |
SD (n = 8) |
POD (n = 3) |
PR (n = 5) |
SD (n = 9) |
POD (n = 1) |
PR (n = 1) |
SD (n = 1) |
POD (n = 1) |
| Well-differentiated (PanNET), | 2/17 (12) |
8/17 (47) |
7/17 (41) |
0/5 (0) |
3/5 (60) |
2/5 (40) |
4/13 (31) |
6/13 (46) |
3/13 (23) |
4/13 (31) |
9/13 (69) |
0/13 (0/0) | 0/2 (0) |
1/2 (50) |
1/2 (50) |
| Grade 1 | 0/2 (0) |
1/2 (50) |
1/2 (50) |
0/0 (0) |
0/0 (0) |
0/0 (0) |
1/2 (50) |
1/2 (50) |
0/2 (0) |
1/2 (50) |
1/2 (50) |
0/2 (0) |
0 /0 (0) |
0/0 (0) |
0/0 (0) |
| Grade 2 | 1/6 (17) |
4/6 (67) |
1 /6 (17) |
0/2 (0) |
1/2 (50) |
1/2 (50) |
1/6 (17) |
2/6 (33) |
3/6 (50) |
0/4 (0) |
4/4 (83) |
0/4 (17) |
0/1 (0) |
0/1 (0) |
1/1 (100) |
| Grade 3 | 1/9 (11) |
3/9 (33) |
5/9 (56) |
0/3 (0) |
2/3 (67) |
1/3 (33) |
2/4 (50) |
2/4 (50) |
0/4 (0) |
3/7 (38) |
4/7 (62) |
0/7 (0) |
0/1 (0) |
1/1 (100) |
0/1 (0) |
| Poorly differentiated (PanNEC) | 7/22 (32) |
8/22 (36) |
7/22 (32) |
3/4 (75) |
1/4 (25) |
0/4 (0) |
1/2 (50) |
1/2 (50) |
0/2 (0) |
0/0 (0) |
0/0 (0) |
0/0 (0) |
1/1 (100) |
0/1 (0) |
0/1 (0) |
| Unknown differentiation | 7/18 (39) |
4/18 (22) |
7/18 (39) |
0/0 (0) |
0/0 (0) |
0/0 (0) |
1/2 (50) |
1/2 (50) |
0/2 (0) |
1/2 (50) |
0/2 (0) |
1/2 (50) |
0/0 (0) |
0/0 (0) |
0/0 (0) |
Proportions and perentages are expressed in row.
PanNEN indicates pancreatic neuroendocrine neoplasm; PanNET, pancreatic neuroendocrine tumor; PanNEC, pancreatic neuroendocrine cancer; PR, partial response; SD, stability of disease; POD, progression of disease.
Among patients who achieved a PR, the median duration of response was 5.4 months (95% confidence interval (CI), 3.5–8.5 months) and this was not significantly different between PanNETs and PanNEC (PanNETs: 13.5 months [95% CI, 9.1–not available]; PanNEC: 8.6 months [95% CI, 6.2–not available]; P = 0.64, Fig. 1).
FIGURE 1.
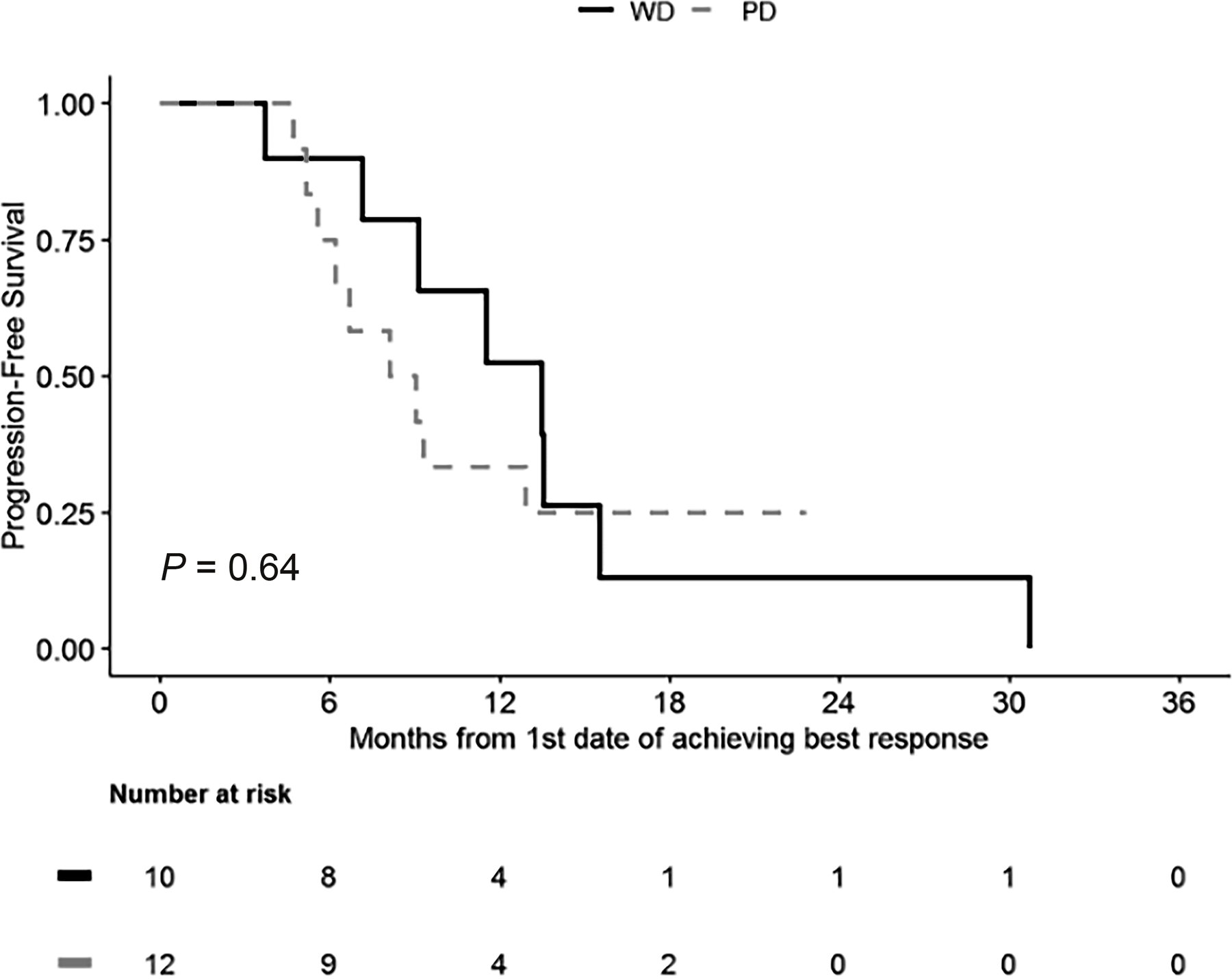
Duration of response according to the differentiation status among patients who achieved partial response with the platinum-based treatment. WD, well-differentiated; PD, poorly differentiated.
In 26 cases, archival tissue was available for IHC labeling for ATRX, DAXX, p53, and Rb and was used to support the histologic determination of differentiation between PanNETs and PanNEC. The clinicopathologic features of these patients are shown in Supplemental Table 3. The IHC analysis confirmed that 4 of 6 cases with PR to platinum-based treatment were PanNETs with a Ki-67 ≤20%. DAXX expression was lost in 7/17 (41%) PanNETs and among those 1 had a PR (14%), 4 (57%) had SD and 2 (29%) had POD. ATRX was expressed in all except 1 PanNET with POD. p53 expression was abnormal (positive IHC) in 3 PanNEC; no relationship was seen with regards to therapy response (1/3 PR, 1/3 SD, and 1/3 POD). Finally, abnormal expression of Rb (negative IHC) was found in 2/2 patients achieving a PR. For one patient (Fig. 2, Supplemental Table 3, case #3), multiple tissue samples taken at different time points of the course of the disease, were available for IHC and next generation sequencing (NGS) analysis. Figure 2 illustrates the mutational and IHC status over the course of time and disease treatment.
FIGURE 2.
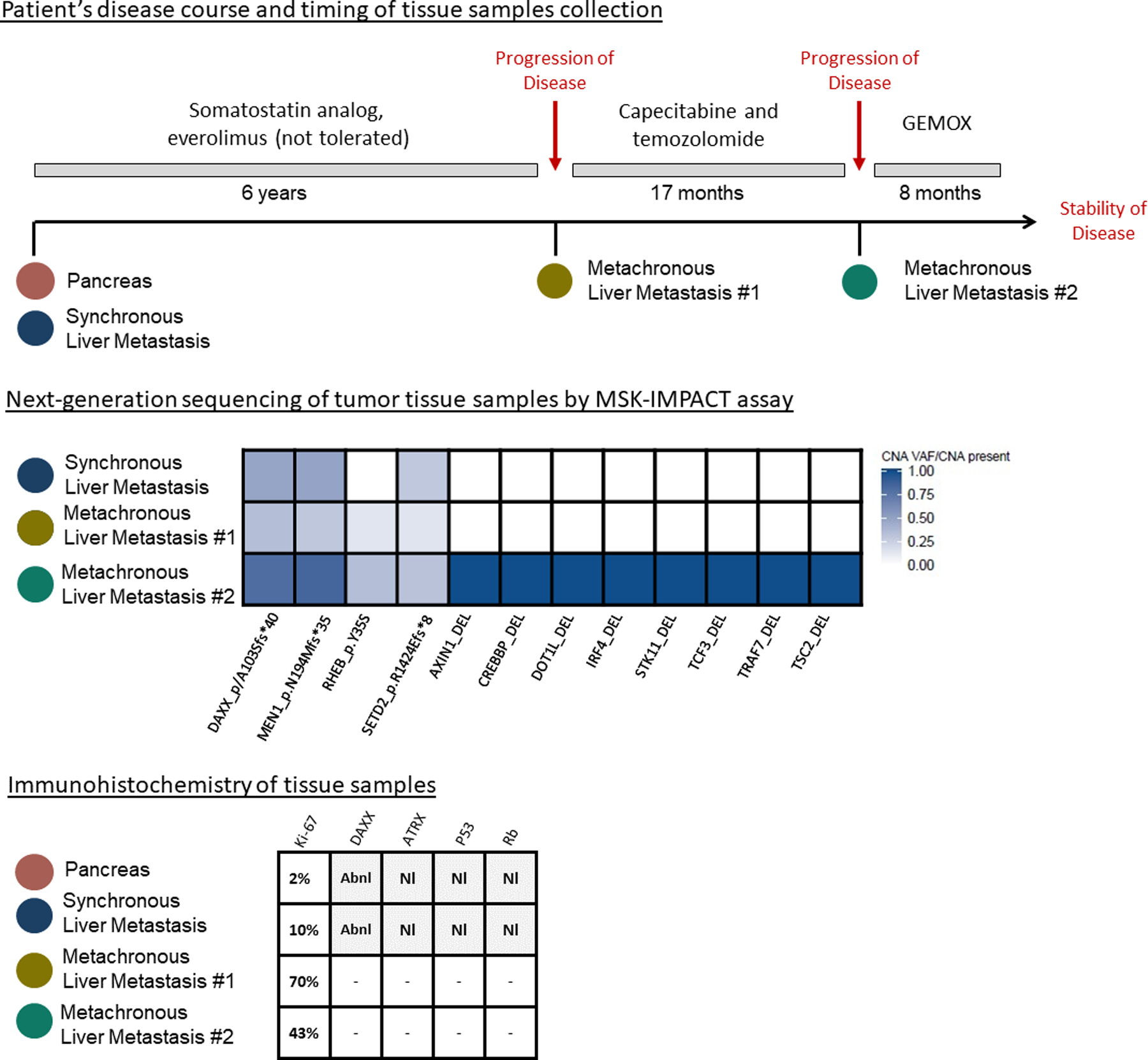
Changes in mutational profile in one patient having a grade 2 PanNET during grade progression and after treatment with a somatostatin analog, mTOR pathway inhibitor, and capecitabine/temozolomide. Single nucleotide variations were annotated with gene name and their respective amino acid change with shades of color denoting detected variant allele frequency. Copy number alterations were annotated with gene name and their respective change (amplification or amplification/deletion or deletion). SNV, single nucleotide variations; CAN, copy number alterations; SV, structural variations; DEL, deletion; Abn, abnormal expression; Nl, normal expression.
Survival
Median OS in the entire cohort was 17 months (95% CI, 13.5–24.4) and was significantly shorter in the PanNEC group compared to the PanNET group [PanNEC: 10.9 months (95% CI, 7.2–19.0; PanNET: 29.2 months (95% CI, 22.2–41.5; P < 0.001, Fig. 3A]. Median PFS from the date of first platinum-based treatment was 5.6 months (95% CI, 4.57–7.1), and specifically, 7.1 months (95% CI, 4.4–13.54) for PanNET and 5.2 months (95% CI, 4.1–8.1) for PanNEC (P = 0.2, Fig. 3B).
FIGURE 3.
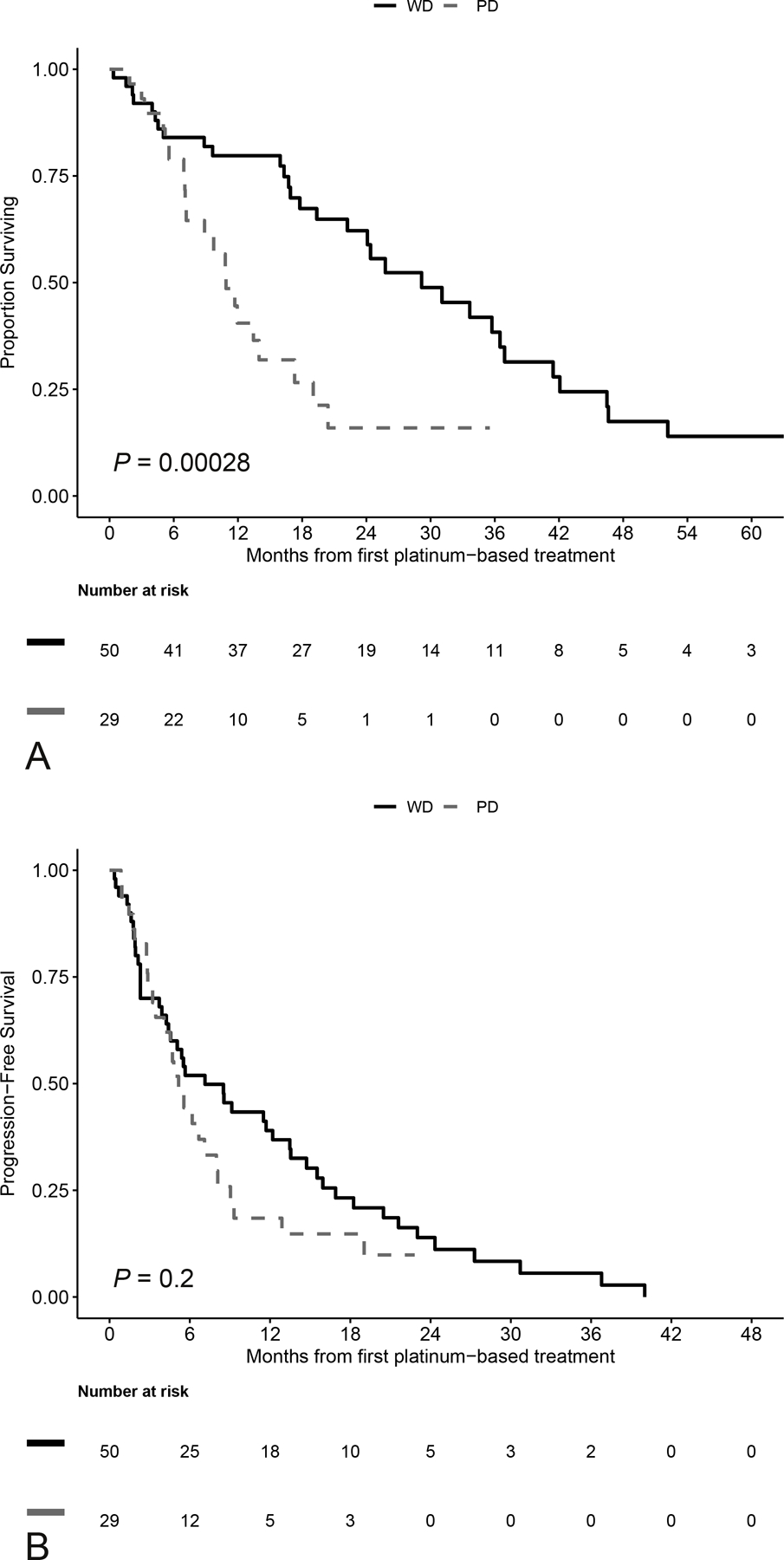
A, Overall Survival from first platinum treatment by differentiation. B, Progression free survival: calculated from date of first platinum until date of first progression, or date of death, whichever occurred first. WD, well-differentiated; PD, poorly differentiated.
DISCUSSION
Systemic chemotherapy with platinum-based combinations is the frontline treatment for the poorly differentiated PanNEC.5,21 However, the role and efficacy of platinum-based therapy for the well differentiated PanNET has not been previously explored in detail.
In this study, we reviewed the largest series of pancreatic neuroendocrine neoplasms that have been treated with platinum-based chemotherapy. In contrast with previous investigations suggesting that only patients with PanNEC benefit from platinum-based chemotherapy, we have documented that these therapies might also offer meaningful activity and efficacy in patients with PanNETs irrespective of grade, with similar PFS between PanNETs and PanNEC.
In this series, we observed that 20% of patients with PanNETs achieved a PR whereas 54% experienced SD. Patients with PanNEC did have a higher PR rate of 41%, with 32% of patients experiencing SD. However, it should be noted that in patients who experienced a PR, irrespective of the tumor grade, those with PanNETs not only had comparable PFS but also had a similar duration of response to those with PanNEC (13.4 vs 8.5 months). In addition, within the PanNET group, there was no difference in terms of response among different grades. Although our analysis is limited by the small numbers, this finding is still clinically relevant. In this study, patients with low and intermediate grade PanNETs were recommended to receive platinum-based treatments after experiencing disease progression and following other therapeutic approaches and obtained disease control (either PR or SD) in 83% and 68% of cases, respectively. However, it is important to acknowledge that, in most patients, the grade of the tumor was defined at the time of the diagnosis on small samples. Therefore, it is possible that some of the low and intermediate grade PanNETs had higher grade components not sampled by the biopsies, or had progressed to a higher grade neoplasm, at the time of treatment with platinum-based regimens.7 This phenomenon of grade progression with time and treatment is illustrated in Figure 2, in one patient undergoing surgery and several biopsies of recurrent lesions. In this patient with an intermediate grade PanNET at initial diagnosis, high-grade progression was documented six years following initial diagnosis in a biopsied liver metastasis; NGS of this high grade liver metastasis revealed a rising tumor mutation burden compared to that originally identified in the synchronous metastasis, supporting the concept of a possible association between genetic progression and grade progression, and indicating that an accumulation of genetic alterations can be identified as a PanNET progresses from low to high grade disease, consistent with previous reports.7,10,22–25 To date, no data support that genetic changes in PanNETs are predictive of response to platinum-based treatments. Therefore, although patients received platinum-based regimens later in their treatment course, further investigations are needed to clarify their efficacy at an earlier point.
In the NORDIC study, Sorbye et al, evaluaved high-grade PanNETs and PanNEC together5, and identified a lower response rate of 15% for neoplasms with a Ki-67% <55% treated with a cisplatin-carboplatin/etoposide combination, with POD observed in 38% of the cases.24 Similarly, Raj et al26 evaluated response to therapy in 45 high grade pancreatic neuroendocrine neoplasms according to cellular morphology, observed a response rate of 10% for PanNETs and 37% for PanNEC treated with platinum/etoposide or platinum/oxaliplatin regimens. A multicentric study from Hijioka et al27 observed no response to platinum in patients with high grade PanNETs, in comparison to a 61% response rate in patients with PanNEC.
The different results of our study can be explained by several observations. First, most previous investigations on platinum-based regimens focused on platinum-etoposide or platinum-irinotecan combinations and were limited to first-line treatments.5,27 In the present series, these two regimens were adopted to treat almost all PanNECs, with a response rate of 32% and 75% respectively. PR rates for PanNETs were minimal with these regimens (12% and 0%, respectively). Despite the small numbers, we observed better responses in the PanNET cohort with GEMOX (gemcitabine and oxaliplatin), and oxaliplatin/5-FU (FOLFIRINOX, FOLFOX, XELOX) regimens (both 31% response rate) and a disease control in 77–100% of cases, suggesting that oxaliplatin-based therapies might have meaningful efficacy in those tumors, although also agents other than oxaliplatin might have played a role. Other studies have reported similar rates of response for oxaliplatin-based regimens: Dussol et al17 documented a 38% response rate to GEMOX in a population of 38 metastatic PanNETs. Similarly, a study by Spada et al16 included 36 PanNETs treated with oxaliplatin-based chemotherapy, and reported a 33% response rate. Finally, combination treatment with bevacizumab and either FOLFOX or XELOX has been associated with response rates of 42% and 19%, respectively, in metastatic PanNETs.18
We further validated our clinical results by using immunohistochemical staining to clarify tumor differentiation status. In a subset of 26 cases, we performed IHC using a selected panel of immunostains. We were unable to identify an IHC profile statistically predictive of response to platinum-based therapy, as expected given that the immunoprofile correlated with differentiation status, which did not predict response. However, although our numbers were too small to draw any definitive conclusions, we observed in this cohort that Rb loss, which is a well-recognized marker of PanNEC, was associated with response to treatment when present in the tumor. This is consistent with the prior study supporting the concept that Rb is a marker of platinum-sensitivity for PanNEC and worthy of further investigations.27
This study has some limitations. First, because of its retrospective nature, there was a selection bias for platinum treatment for patients having low and intermediate grade PanNETs, who received these schemes as salvage therapy after progressing with multiple previous treatments. Second, most patients had metastases at the time of diagnosis and pathological data were assessed on biopsy or fine-needle aspiration specimens. For many of these patients, tissue was therefore not available for the additional IHC characterizations we pursued, and our sample size for this correlative analysis was small; in these cases, the pathologic characteristics of many PanNETs were assessed at the time of diagnosis and not at the time of therapy with platinum; therefore, an underestimation of Ki-67 might have occurred, given our understanding of tumor grade heterogeneity and progression in PanNETs with time and through therapy.25
In conclusion, our data showed that, although platinum-based therapies are more effective in patients with the poorly differentiated PanNEC, some platinum-based regimens provide promising activity and efficacy in patients with the well differentiated PanNETs, irrespective of grade. These therapies should be considered as an alternative option in PanNETs after failure of prior systemic therapies in the setting of moderate to severe disease progression.
Supplementary Material
Footnotes
Disclosure: D.S.K. is consultant and equity holder for Paige.AI. The rest of the authors declare no conflicts of interest.
REFERENCES
- 1.Klimstra DS, Klöppel G, La Rosa S, et al. Classification of neuroendocrine neoplasms of the digestive system In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2019:16. [Google Scholar]
- 2.Pulvirenti A, Javed AA, Landoni L, et al. Multi-institutional development and external validation of a nomogram to predict recurrence after curative resection of pancreatic neuroendocrine tumors. Ann Surg. 2019. September 24 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 3.Raj N, Reidy-Lagunes D. Systemic Therapies for Advanced Pancreatic Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103:186–194. [DOI] [PubMed] [Google Scholar]
- 5.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152–160. [DOI] [PubMed] [Google Scholar]
- 6.Pavel M, O’’Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 7.Tang LH, Untch BR, Reidy DL, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016;22:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang LH, Basturk O, Sue JJ, et al. A practical approach to the classification of WHO Grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol. 2016;40:1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas. Am J Surg Pathol. 2014;38:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from. Am J Surg Pathol. 2012;36:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pea A, Yu J, Marchionni L, et al. Genetic analysis of small well-differentiated pancreatic neuroendocrine tumors identifies subgroups with differing risks of liver metastases. Ann Surg. 2020;271:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pea A, Hruban RH, Wood LD. Genetics of pancreatic neuroendocrine tumors: Implications for the clinic. Expert Review of Gastroenterology and Hepatology. 2015;9:1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heetfeld M, Chougnet CN, Olsen IH, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657–664. [DOI] [PubMed] [Google Scholar]
- 15.Vélayoudom-Céphise FL, Duvillard P, Foucan L, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–657. [DOI] [PubMed] [Google Scholar]
- 16.Spada F, Antonuzzo L, Marconcini R, et al. Oxaliplatin-based chemotherapy in advanced neuroendocrine tumors: clinical outcomes and preliminary correlation with biological factors. Neuroendocrinology. 2016;103:806–814. [DOI] [PubMed] [Google Scholar]
- 17.Dussol AS, Joly MO, Vercherat C, et al. Gemcitabine and oxaliplatin or alkylating agents for neuroendocrine tumors: Comparison of efficacy and search for predictive factors guiding treatment choice. Cancer. 2015;121:3428–3434. [DOI] [PubMed] [Google Scholar]
- 18.Kunz PL, Balise RR, Fehrenbacher L, et al. Oxaliplatin-fluoropyrimidine chemotherapy plus bevacizumab in advanced neuroendocrine tumors. Pancreas. 2016;45:1394–1400. [DOI] [PubMed] [Google Scholar]
- 19.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT). J Mol Diagnostics. 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 21.Pulvirenti A, Rao D, Mcintyre CA, et al. Limited role of chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB (Oxford). 2019;21:612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohmoto A, Rokutan H, Yachida S. Pancreatic neuroendocrine neoplasms: Basic biology, current treatment strategies and prospects for the future. Int J Mol Sci. 2017;18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klimstra DS. Reassessing the grade of gastroenteropancreatic neuroendocrine neoplasms. Endocrine. 2016;53:4–6. [DOI] [PubMed] [Google Scholar]
- 24.Basturk O, Yang Z, Tang LH, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj N, Shah R, Stadler Z, et al. Real-time genomic characterization of metastatic pancreatic neuroendocrine tumors has prognostic implications and identifies potential germline actionability. JCO Precis Oncol. 2018;1–18. [DOI] [PMC free article] [PubMed]
- 26.Raj N, Valentino E, Capanu M, et al. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology. Pancreas. 2016;00:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijioka S, Hosoda W, Matsuo K, et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: A Japanese multicenter pancreatic NEN-G3 study. Clin Cancer Res. 2017;23:4625–4632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


