Abstract
Aims/hypothesis
Psychological stress has long been considered a possible trigger of type 1 diabetes, although prospective studies examining the link between psychological stress or life events during pregnancy and the child’s type 1 diabetes risk are rare. The objective of this study was to examine the association between life events during pregnancy and first-appearing islet autoantibodies (IA) in young children, conditioned by the child’s type 1 diabetes-related genetic risk.
Methods
The IA status of 7317 genetically at-risk The Environmental Determinants of Diabetes in the Young (TEDDY) participants was assessed every 3 months from 3 months to 4 years, and bi-annually thereafter. Reports of major life events during pregnancy were collected at study inception when the child was 3 months of age and placed into one of six categories. Life events during pregnancy were examined for association with first-appearing insulin (IAA) (N=222) or GAD (GADA) (N=209) autoantibodies in the child until 6 years of age using proportional hazard models. Relative excess risk due to interaction (RERI) by the child’s HLA-DR and SNP profile was estimated.
Results
Overall, 65% of mothers reported a life event during pregnancy; disease/injury (25%), serious interpersonal (28%) and job-related (25%) life events were most common. The association of life events during pregnancy differed between IAA and GADA as the first-appearing autoantibody. Serious interpersonal life events correlated with increased risk of GADA-first only in HLA-DR3 children with the BACH2-T allele (HR 2.28, p<0.0001), an additive interaction (RERI 1.87, p=0.0004). Job-related life events were also associated with increased risk of GADA-first among HLA-DR3/4 children (HR 1.53, p=0.04) independent of serious interpersonal life events (HR 1.90, p=0.002), an additive interaction (RERI 1.19, p=0.004). Job-related life events correlated with reduced risk of IAA-first (HR 0.55, p=0.004). particularly in children with the BTNL2-GG allele (HR 0.48; 95% CI 0.31, 0.76).
Conclusions/interpretation
Specific life events during pregnancy relate differently with age-related IAA vs GADA as first-appearing IA and interact with different HLA and non-HLA genetic factors, supporting the concept of different endotypes underlying type 1 diabetes. However, the mechanisms underlying these associations remain to be discovered. Life events may be markers for other yet-to-be-identified factors important to the development of first-appearing IA.
Keywords: BACH2 single nucleotide polymorphism, BTNL2 single nucleotide polymorphism, GAD autoantibodies, HLA-DR-DQ haplogenotype, Insulin autoantibodies, Islet autoimmunity, Prenatal life events, Psychosocial stress, Type 1 diabetes
Graphical Abstract

Introduction
The Environmental Determinants of Diabetes in the Young (TEDDY) study seeks to identify environmental triggers of type 1 diabetes in genetically at-risk children followed from birth to age 15 at three centres in the USA (Colorado, Georgia/Florida and Washington) and three centres in Europe (Germany, Finland and Sweden). We previously confirmed that diabetes-related insulin autoantibodies (IAA) first appear at an earlier age than GAD autoantibodies (GADA) and that the order of appearance is related to the child’s HLA-DR-DQ haplogenotype (1), suggesting different pathways to the endotypes of type 1 diabetes (2).
Psychological stress has long been considered a possible trigger of type 1 diabetes; literature reviews and prospective studies provide evidence for such a linkage (3–8). However, the mechanism by which psychological stress might lead to type 1 diabetes is unknown. Psychological stress could have a direct effect on the development of diabetes-related autoimmunity or it could have an indirect effect by increasing the likelihood of some other exposures associated with the aetiology of the disease (9). Any link between stress and diabetes-related autoimmunity may also depend on the child’s HLA and non-HLA genetic risk. No study has examined the association between psychological stress and first-appearing IAA and GADA separately nor has the impact of the child’s HLA and non-HLA genetic risk on the possible relationship between stress and IA been explored. Although there is prospective literature documenting a link between life events (LEs) during pregnancy and subsequent infections or illnesses in the child (10–13), only two prospective studies examined the relationship between psychological stress or LEs during pregnancy and the child’s subsequent risk of type 1 diabetes: death of the child’s father or sibling during pregnancy was associated with increased risk (7) and interpersonal events during pregnancy (e.g., divorce, family conflict) were associated with increased risk in HLA-DR3/4 children (8). Neither study assessed the possible link between LEs during pregnancy and first-appearing IA in the child.
In TEDDY, all children were recruited based on their high-risk HLA-DR-DQ haplogenotype. We have reported that non-HLA SNPs are strongly associated with the development of islet autoantibodies (IA) up to 6 years of age: rs2476601 (PTPN22), rs2292239 (ERBB3), rs1004446 (INS), rs3184504 (SH2B3) and rs3763305 (BTNL2). Several SNPs were found to be related differently with IAA compared with GADA: rs231775 (CTLA4), rs689 (INS) and rs3757247 (BACH2) (1,14–16). No study has examined whether the association between psychological stress and first-appearing IAA and GADA is dependent on these SNPs.
Methods
Participants
The TEDDY study design and methods are published elsewhere (17), as well as the characteristics of those who enrolled and those who declined (18,19). Written, informed consents were obtained from parents of all participants and the study was approved by each site’s institutional review or ethics board. All participants joined the TEDDY study before 4.5 months of age.
The current analysis focused on the TEDDY cohort as of March 2019. Of the 8676 children who entered TEDDY, the following were excluded from the analysis: twin or triplet (n=252); child determined not to be HLA eligible (n=120); someone other than the mother was interviewed at 3 months about the mother’s LEs during the pregnancy (n=168) or no one was interviewed (n=5); mother had gestational, type 1 or type 2 diabetes (n=791); or the child’s antibody status was indeterminant (n=23). Hence, we included 7317 TEDDY children followed for the development of IA until 6 years (<84 months) of age. During this interval, 532 (7.3%) of these children developed autoantibodies (222 IAA-first; 209 GADA-first; ten insulinoma antigen-2 (IA-2A)-first; 91 multiple IA at first detection). This paper focuses on the IAA-first and GADA-first samples since the first-appearing IA could not be determined in the multiple-IA cases and the IA-2A sample was too small.
Genotyping
More than 400,000 newborns were HLA genotyped and those with DR3/4, DR4/4, DR4/8 and DR3/3 HLA haplogenotypes were eligible for TEDDY participation. For first-degree relatives of someone with type 1 diabetes, several additional haplogenotypes were eligible for inclusion (electronic supplementary material [ESM] Table 1). Of the 21,589 HLA eligible children, 8676 joined the TEDDY study. When the TEDDY participant was 9-12 months of age, the child’s HLA status was confirmed and diabetes-related SNPs from the Illumina Immuno BeadChip (manifest file: Immuno_BeadChip_11419691.bpm from Illumina, San Diego, CA, USA) were assessed. The methods for genotyping have been published previously (1). For the current analysis, we focused on the high-risk HLA groups and SNPs previously found to be associated with IAA, GADA or both in TEDDY (rs2476601 in PTPN22; rs2292239 in ERBB3; rs1004446 in INS; rs3757247 in BACH2; rs3184504 in SH2B3; rs231775 in CTLA4; and rs3763305 in BTNL2) (1,11,12).
Islet autoantibodies
Blood draws for IA assay were done every 3 months for the first 4 years of participation and then bi-annually unless the child was positive for IA, in which case quarterly visits were maintained. A child was considered to have developed IA if the child had persistent confirmed autoimmunity defined as the presence of confirmed IAA, GADA or IA-2A at each of the two TEDDY reference laboratories on two or more consecutive visits. The assay methods have been published elsewhere (1).
LEs during pregnancy
The most common method for assessing LEs is a self-reported checklist (e.g., Social Readjustment and Rating Scale, Life Experiences Survey) (20,21). To improve the quality of the data obtained, we modified this approach based on expert recommendations from the literature (22). Instead of using an LE checklist, we interviewed the mother at the time of the child’s enrolment in TEDDY (during the child’s first 3-4 months of life) about any major LE that occurred during her pregnancy. As part of the interview process, the mother was provided with a list of 20 LEs commonly reported (23) and was invited to report LEs not listed (ESM Table 2). To enhance accurate recall, the focus was on a specific, relatively short time-frame: her pregnancy. Our approach considered both overall LEs and type of LE grouped into six categories: disease/injury (self or others); significant loss (death of a family member or friend); serious interpersonal (marriage, separation, divorce, conflicts with spouse/relative/friend, moved or had a change in family composition); job-related (self or spouse quit/lost a job or started work/school); financial difficulties (self or spouse/partner); and other. To avoid concerns about quantifying the total number of LEs, and given the short time-frame of interest (pregnancy), we treated LEs as a dichotomous (yes/no) variable.
Statistical methods
LEs during pregnancy and their association with IA overall as well as with IAA or GADA as the first-appearing autoantibodies were evaluated by proportional hazard models. Children negative for autoantibodies were right-censored on the day of the last negative autoantibody test result or on the day before the child’s seventh birthday. The strength of associations was described by HRs and 95% CIs. Factors known to be associated with the development of autoimmunity (country, sex, having a father or sibling with type 1 diabetes, HLA haplogenotype and diabetes-related SNPs) were statistically controlled. Further testing of whether risk factors for IAA-first and GADA-first differed was performed by multivariate logistic regression, modelling factors significantly associated with the ratio of IAA-first to GADA-first. All factors included in the proportional hazard models were also included in the logistic models in addition to age of seroconversion. To account for correlation among the non-mutually exclusive LE categories, all categories were included in the multivariate models and a p value less than 0.05 was considered statistically significant. LEs significantly associated with IA overall or IAA-first vs GADA-first were further examined. Confounding and selection biases were considered by adjusting parsimoniously for maternal factors associated with maternal reports of LEs during pregnancy using an LE propensity score. An inverse probability of treatment (LE) weighting analysis, as described elsewhere (24), was performed to reduce selection bias by weighting children in the proportional hazard models by a stabilised weight created from the LE propensity score (25). Since the propensity score was first an estimate and then a known quantity, standard errors were calculated from 1000 bootstrap samples. Maternal factors were also included in the models if they showed a significant association with outcome. Finally, maternal LEs showing an association with development of IA in the offspring were tested for effect modification by the child’s genetic risk factors. Interactions were examined on the ratio scale (multiplicative interaction), by including a cross-product term in the proportional hazard model, or on the difference scale (additive interaction) by estimating the relative excess risk due to interaction (RERI). The RERI was estimated by including in the model a four-category variable describing the presence (1) and absence (0) of LEs and genetic factors and estimating the RERI as HR11 − HR10 - HR01 + 1 (26). The RERI and 95% CI estimate the additional risk due to interaction, with RERI>0 suggesting synergistic interaction. The strongest interactions are considered to exist on both the additive and multiplicative scales, with an RERI>1 indicating possible sufficient cause interaction between LE and gene. To account for multiple genetic×LE comparisons, a false discovery rate was calculated to account for the number (HLA-DR haplogenotypes and SNPs) and the type (additive or multiplicative) of genetic interaction tests (n=22), and a false discovery rate <0.05 was considered statistically significant. A sensitivity analysis was performed to determine whether results may have been influenced by knowing the first autoantibody for children who developed both IAA and GADA between visits (n=72 children). These children were censored at the time of seroconversion in a competing risk analysis along with 29 children who had developed IA-2A. The model discriminating IAA-first from GADA-first was fitted to children with both IAA and GADA to predict which autoantibody might have come first. Associations examining LEs with first-appearing autoantibodies were repeated to include any additional first-appearing autoantibody cases that had a predicted ratio of 2:1 to have developed one autoantibody over the other (51/72). The sensitivity analysis was extended to examine the influence of attrition bias by including any first-time dropout or loss to follow-up (>1 year since last visit) as a competing risk.
Results
Nearly two-thirds (65%) of all participating mothers reported at least one LE during pregnancy (US mothers: 69%; European mothers: 62%). The most common categories of events reported were: disease/injury (25%); serious interpersonal (28%); and job-related (25%). Financial difficulties were reported by 19% of US mothers but only 5% of European mothers (Table 1). Controlling for all demographic and genetic factors associated with any IA, IAA-first or GADA-first (ESM Table 3), having one or more LE of any kind during pregnancy was not associated with IA overall (ESM Table 4). However, it was associated differently for children developing IAA-first as compared with GADA-first (p=0.04, ESM Table 4). The associations between specific LEs and any IA, IAA-first and GADA-first are provided in Table 2. Having a serious interpersonal LE, independent of other LE categories, was associated with an increased risk of IA overall (HR 1.25; 95% CI 1.03, 1.52; p=0.02). This was largely explained by an increased risk of GADA-first (HR 1.58; 95% CI 1.18, 2.12; p=0.002). In contrast, a job-related LE correlated with a lower risk of IAA-first (HR 0.55; 95% CI 0.40, 0.84; p=0.004). The association of job-related LE with IAA-first differed significantly compared with GADA-first (p=0.005) and there was no association with IA overall. No other LE category showed a correlation with IA, IAA-first or GADA-first.
Table 1.
LEs during pregnancy reported by US and European TEDDY mothers
| LE | All mothers (N=7317), n (%) reporting the event | US mothers (N=3103), n (%) reporting the event | European mothers (N=4214), n (%) reporting the event |
|---|---|---|---|
| Any LE | 4742 (65) | 2132 (69) | 2610 (62) |
| Disease/injury (self or others) | 1842 (25) | 847 (27) | 996 (24) |
| Significant loss (death of family member or friend) | 767 (11) | 380 (12) | 387 (9) |
| Serious interpersonal (separation, divorce, conflicts with spouse/relative/friend) | 2010 (28) | 982 (32) | 1028 (24) |
| Job-related (self or spouse quit/lost job, started work/school) | 1828 (25) | 853 (28) | 975 (23) |
| Financial difficulties (self or spouse) | 807 (11) | 588 (19) | 219 (5) |
| Other | 995 (14) | 412 (13) | 583 (14) |
Table 2.
Maternal LEs reported during pregnancy in relation to the risk of IA in the child until 6 years of age
| Specific LE | Outcome=IA multivariate and adjusted | Outcome=IAA-first multivariate and adjusted | Outcome=GADA-first multivariate and adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LE among cases (% of n=532) | HRa (95% CI) | p value | LE among cases (% of n=222) | HRa (95% CI) | p value | LE among cases (% of n=209) | HRa (95% CI) | p value | |
| Disease/injury (self or others) | 24% | 0.94 (0.77, 1.15) | 0.56 | 28% | 1.27 (0.94, 1.70) | 0.12 | 22% | 0.82 (0.59, 1.15) | 0.25 |
| Significant loss (death of family member or friend) | 9% | 0.89 (0.66, 1.20) | 0.44 | 9% | 0.84 (0.52, 1.35) | 0.48 | 10% | 0.97 (0.61, 1.54) | 0.90 |
| Serious interpersonal (marriage, separation, divorce, conflicts with spouse/relative/friend, move or change in family composition) | 30% | 1.25 (1.03, 1.52) | 0.02 | 26% | 1.02 (0.75, 1.39) | 0.90 | 35% | 1.58 (1.18, 2.12) | 0.002 |
| Job-related (self or spouse quit/lost job, started work/school) | 23% | 0.89 (0.72, 1.10) | 0.26 | 16% | 0.55 (0.40, 0.84)b | 0.004 | 28% | 1.07 (0.78, 1.47)b | 0.68 |
| Financial difficulties (self or spouse) | 8% | 0.95 (0.68, 1.32) | 0.76 | 9% | 1.24 (0.76, 2.04) | 0.39 | 8% | 0.78 (0.46, 1.32) | 0.35 |
| Other | 9% | 0.91 (0.68, 1.22) | 0.53 | 10% | 1.03 (0.66, 1.61) | 0.85 | 10% | 0.88 (0.55, 1.39) | 0.57 |
Multivariable proportional hazard model of specific reported maternal LEs examined together in relation to IA overall as well to IAA-first and GADA-first among 6364 children followed until 6 years of age
Models adjusted for sex, country of residence, family history of type 1 diabetes and genetic risk factors for IA as shown in ESM Table 3.
LE is associated differently with hazard of IAA-first as compared with GADA-first (p<0.05)
The serious interpersonal LE association with GADA and the job-related LE association with IAA remained significant (p values ≤0.01) when sensitivity analysis was performed to address possible bias due to other factors associated with maternal reports of LE (ESM Table 5). Attrition or interval censoring due to lack of determination of the first-appearing IA did not affect the associations. Since we previously reported that respiratory infections during pregnancy exhibited a protective association with IAA for certain genetic subgroups (15) and stressful LEs are known to increase risk of illness (9), we reasoned that maternal illness during pregnancy might explain our job-related LE–IAA association. However, multivariate modelling, controlling for respiratory illness during pregnancy, did not reduce the protective association between job-related LE during pregnancy and IAA-first in the child (ESM Table 6).
We next examined whether the associations of job-related LE with IAA-first and serious interpersonal LE with GADA-first were dependent on the child’s type 1 diabetes genetic risk. Additive and multiplicative interactions were tested between maternal job-related LE and the ten genetic components of the child (HLA-DR3, HLA-DR4, HLA-DR8 and SNPs in PTPN22, INS, ERRB3, SH2B3, BACH2, CTLA4 and BTNL2) (ESM Table 7). Adjusting for multiple comparisons, there were no statistically significant interactions between job-related LE and the genetic components. However, an interaction was observed between job-related LE and the BTNL2 SNP on both the risk difference (additive interaction, RERI 0.56; 95% CI 0.05, 1.08; p=0.03) and ratio scales (multiplicative interaction, HR 2.19; 95% CI 1.01, 4.77; p=0.048). Job-related LE was associated with a reduced risk of IAA among children with the BTNL2-GG genotype (HR 0.48; 95% CI 0.31, 0.76), but no correlation was seen among children with the BTLN2-A allele (HR 1.03; 95% CI 0.54, 1.95). An examination of the overall absolute incidence of IAA-first at age 6 years by job-related LE and the child’s HLA-DR and BTNL2 genotypes showed that job-related LE reduced incidence of IAA-first consistently across HLA-DR haplogenotypes, although only in children with the BTNL2-GG genotype (Fig. 1a). The association was not consistent for children with the BTNL2-A allele (Fig. 1b). In this cohort, 99.4% of HLA-DR3/3 children have the BTNL2-GG genotype and the incidence of IAA-first among HLA-DR3/3 children was low. Thus, we examined the children with at least one HLA-DR4 haplogenotype. The reduced risk of IAA-first by a job-related LE during pregnancy was primarily observed before 3 years of age and only in children with the BTNL2-GG genotype (Fig. 1c,d).
Fig. 1.
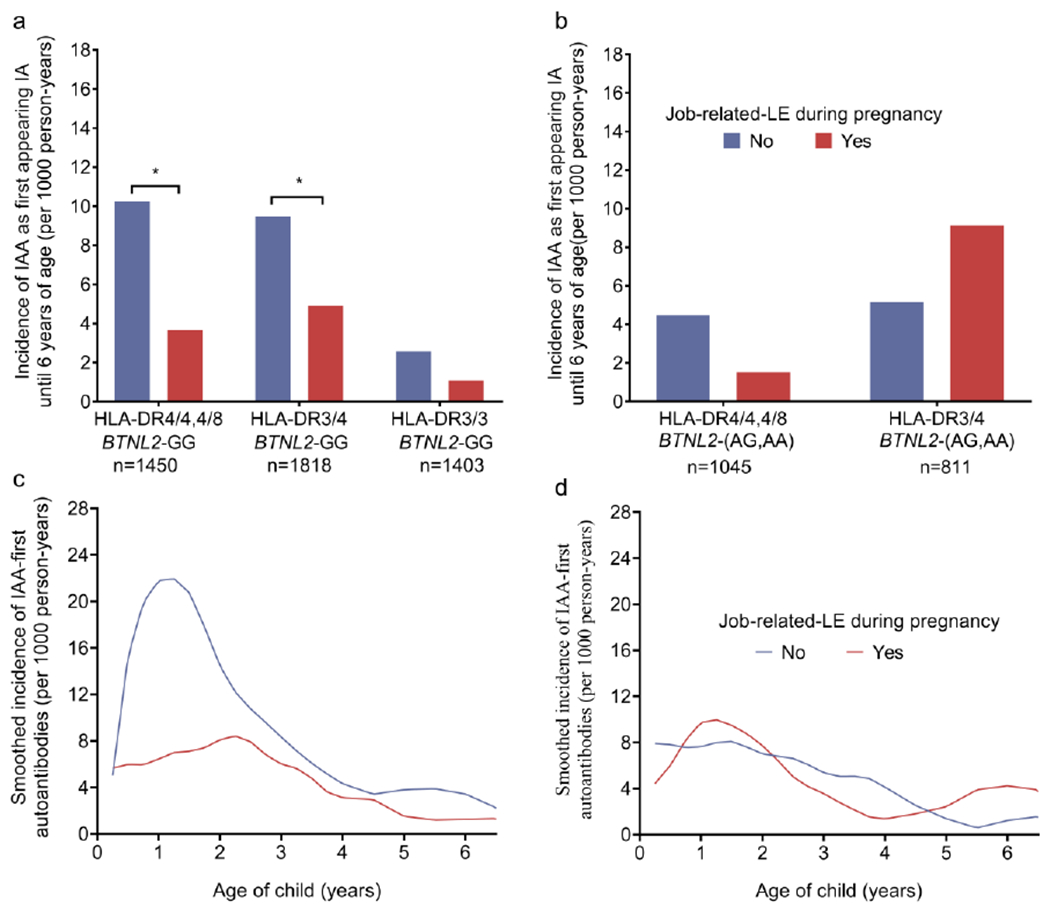
(a) HLA-DR4/4 or 4/8 children and HLA-DR3/4 children with the BTNL2-GG genotype were less likely to develop IAA as the first-appearing IA if the mother experienced a job-related LE during pregnancy (*p<0.05). A job-related LE during pregnancy was unrelated to IAA as the first-appearing IA in HLA-DR3/3 children (p=0.26) with the BTNL2-GG genotype. (b) There was no association between job-related LE in pregnancy and IAA as the first-appearing antibody in HLA-DR4/4 or 4/8 children (p=0.14) or DR3/4 children (p=0.16) with the BTNL2-AA/AG genotype. (c) Age-specific incidence of IAA as the first-appearing IA by job-related LE in pregnancy for HLA-DR4 children with the BTNL2-GG genotype and (d) for HLA-DR4 children with the BTNL2-AA/AG genotype
The additive and multiplicative interactions between a serious interpersonal LE during pregnancy and the ten genetic components of the child on risk of GADA-first are summarised in ESM Table 8. An additive interaction was discovered between the BACH2 SNP and a serious interpersonal LE (RERI 1.25; 95% CI 0.50, 2.00; p=0.001; false discovery rate=0.02). Taking BACH2-CC genotype and no serious interpersonal LE as a reference group, only children with a BACH2-CT or TT genotype and a serious interpersonal LE showed an increased risk of developing GADA-first (HR 2.22; 95% CI 1.48, 3.33), with no increase seen for children with a BACH2-CC genotype and a serious interpersonal LE (HR 0.77; 95% CI 0.40, 1.47) or for BACH2-CT and TT genotypes and no serious interpersonal LE (HR 1.21; 95% CI 0.83, 1.77). The child’s HLA haplogenotype also showed evidence of modifying the serious interpersonal LE effect (ESM Table 8). The absolute risk of GADA-first at 6 years of age stratifying on serious interpersonal LE during pregnancy as well as both HLA-DR and BACH2 genotypes showed that the increased risk of GADA-first by serious interpersonal LE in pregnancy and BACH2-T allele only occurred if children had the HLA-DR3 haplogenotype (HR 2.28, p<0.0001), an additive interaction (RERI 1.87, p=0.0004). (Fig. 2a,b). Among HLA-DR3 children, the impact of the BACH2-T allele on the serious interpersonal LE correlation with GADA-first increased with the age of the child (Fig. 2c,d).
Fig. 2.

(a) There was no association between a serious interpersonal LE in pregnancy and GADA as the first-appearing autoantibody for HLA-DR4/4 or 4/8 (p=0.79), HLA-DR3/4 (p=0.65) and HLA-DR3/3 (p=0.08) children with the BACH2-CC genotype. (b) A serious interpersonal LE was associated with increased risk of GADA as the first-appearing antibody in HLA-DR3/4 children with the BACH2-CT/TT genotype (***p<0.0005) and HLA-DR3/3 children with the BACH2-CT/TT genotype (*p<0.05), but not in HLA-DR4/4 or 4/8 children with the BACH2-CT/TT genotype (p=0.91). (c) Age-specific incidence of GADA as the first-appearing autoantibody by serious interpersonal LE during pregnancy for HLA-DR3 children with the BACH2-CC genotype and for (d) HLA-DR3 children with the BACH2-CT/TT genotype
Since GADA-first generally occurs later than IAA-first, we also examined whether job-related LE interacted with the child’s HLA-DR haplogenotype on risk of GADA-first. Job-related LE showed strong multiplicative (HR 2.44; 95% CI 1.29, 4.61; p=0.006) and additive interactions (RERI 1.19; 95% CI 0.38, 2.00; p=0.004) with the HLA-DR3/4 haplogenotype. Job-related LE correlated with increased risk of GADA-first (HR 1.75; 95% CI 1.17, 2.61; p=0.006) among children with HLA-DR3/4, while no increase was seen in children without both the HLA-DR3 and the HLA-DR4 haplogenotypes (HR 0.68; 95% CI 0.41, 1.12; p=0.12). The interaction was not dependent on the BTNL2 genotype or age (ESM Fig. 1a–d). For children with HLA-DR3/4, serious interpersonal LE (HR 1.90; 95% CI 1.26, 2.84; p=0.002) and job-related LE (HR 1.53; 95% CI 1.01, 2.30; p=0.04) independently correlated with an increased risk of GADA-first after adjusting for all other factors. The incidence of GADA-first by 6 years of age among HLA-DR3/4 children was 13.7/1000 person-years if mothers reported both serious interpersonal LE and job-related LE (n=308), 9.2/1000 person-years if mothers reported only one of these events (n=979) and 5.1/1000 person-years if mothers reporting neither during pregnancy (n=1602).
Discussion
Type 1 diabetes is a complex autoimmune disease, with age and HLA and non-HLA SNPs associated with the first-appearing IA (1). Although the incidence of type 1 diabetes increases until early adolescence, the appearance of IAA as the first IA peaks in the first year of life, predominately among HLA-DR4 children, while GADA-first appears consistently throughout early childhood. Our findings add further clarification of these relationships by identifying specific LEs during pregnancy that correlated differently with IAA-first and GADA-first in genetically at-risk young children. For IAA-first, we found that a job-related LE reported during pregnancy was associated with lower risk, specifically among children having the BTNL2-GG genotype. For GADA-first, a serious interpersonal LE during pregnancy, interacting with HLA-DR3 and BACH2-G alleles, correlated with a significant excess risk. Taken together, these findings show that children born with the highest HLA risk for type 1 diabetes (HLA-DR3/4) had a greater risk of developing GADA-first over IAA-first, and thus IA at a later age, if the mother reported a job-related LE or serious interpersonal LE during pregnancy.
Although these findings are intriguing, they raise many questions about the mechanisms underlying these associations. For example, a job-related LE could be a proxy for other events that occur during pregnancy or after the birth of the child (e.g., exclusivity/duration of breast feeding, age placed in daycare) that could prove to be associated with IAA at a young age. Of interest is our finding that among HLA-DR4 children, the BTNL2-A allele had a protective association with IAA; a job-related LE during pregnancy was not associated with IAA in these children. In contrast, the protective association of a job-related LE occurred in those HLA-DR4 children with the higher-risk BTNL2-GG genotype. Although this interaction was no longer significant when controlling for multiple comparisons, this preliminary finding may warrant further exploration as the BTNL2-G allele, among HLA-DR3/4 children in the TEDDY cohort, was in nearly complete linkage disequilibrium with HLA-DRB1*04 subtypes (*04:01, *04:02, *04:05), while the BTNL2-A allele was associated with HLA-DRB1*04:04 and *04:07 (15). Previously, the BTNL2-GG genotype was associated with increased risk for IA and type 1 diabetes, although its specific association with IAA and GADA as the first-appearing autoantibody was not explored (15). BTNL2 is a butyrophilin family member and mutations in this gene have been associated with several autoimmune diseases (15, 27). There are few studies examining the association of BTNL2 with type 1 diabetes (28, 29), although BTNL2 is thought to have a regulatory function on T cell generation and function (15, 30–32) (ESM Table 9).
Whatever the mechanism, evidence of protection against IAA is important because IAA is seen in very young children who tend to go on to develop type 1 diabetes very rapidly (33); reducing risk of IAA would likely delay type 1 diabetes onset even in those who later develop GADA. While HLA-DR4 is associated with IAA-first, HLA-DR3 is associated with GADA-first. We found that a serious interpersonal LE during pregnancy further increased the risk of GADA in HLA-DR3 children but not in HLA-DR4 children. Further, serious interpersonal LEs were associated with increased risk of GADA in children with the BACH2-T allele. A number of studies have documented an association between the BACH2 SNP and increased risk for type 1 diabetes (34–38), and one study reported BACH2 to be associated with increased risk for GADA as the first-appearing antibody (1). Several studies have suggested that the BACH2 SNP plays a key role in B cell differentiation (39) as well as T cell regulation (40–42). While only 10% of the participants had all three risk factors (DR3, BACH2-T allele, serious interpersonal LE during pregnancy), 24% of the GADA participants did.
However, the mechanism by which a serious interpersonal LE during pregnancy might lead to GADA in DR3, BACH2-T allele children is unknown. Serious interpersonal LEs may be rather chronic in nature, resulting in a maternal stress response affecting the mother’s own immune system as well as the development of the immune system in the child. It is also possible that a serious interpersonal LE during pregnancy is a proxy for other exposures or maternal behaviours (e.g. diet, sleep, prenatal care) that could affect the developing fetus, or mothers with serious interpersonal LEs during their pregnancy may continue to experience such events after the child’s birth, influencing mother–child bonding, breastfeeding behaviour (43) and other parenting practices or environmental exposures that could affect the child.
The HLA-DR3/4 haplogenotype is the most common high-risk subgroup in TEDDY. Children in this subgroup are equally likely to get IAA as GADA as the first-appearing autoantibody. Like children with other HLA-DR haplogenotypes, a job-related LE during pregnancy was protective of IAA, particularly in those with the BTNL2-GG genotype. However, the same job-related LE during pregnancy actually increased risk of GADA in these children, suggesting that in these children the risk for IA was not reduced but simply shifted in time. In fact, these children were at exceedingly high risk for GADA if their mothers experienced both a job-related LE and a serious interpersonal LE during pregnancy and they had the BACH2-T allele.
Although gene–environment interactions are often cited as a causal influence for type 1 diabetes, there are few clear examples in the published literature. Here we report two gene–environment interactions relevant to GADA as the first-appearing antibody: a serious interpersonal LE in pregnancy increased a child’s risk for GADA in HLA-DR3 but not HLA-DR4 children, and increased risk for GADA in children with the BACH2-T allele but not in children with the BACH2-CC genotype. We also report some preliminary evidence that a job-related LE during pregnancy may have a protective association with IAA in those HLA-DR4 children with the BTNL2-GG genotype, but may increase risk for GADA in HLA-DR3/4 children.
By focusing separately on IAA and GADA as the first-appearing autoantibody, we add to our understanding of the different pathways by which a child may develop this disease. By including diabetes-related genetic information in our analyses we have advanced our knowledge of the complex interplay of genes and environment in its early development. Although our study findings highlight important associations between different types of LEs during pregnancy and the child’s subsequent likelihood of developing type 1 diabetes-related autoimmunity, we have yet to understand the mechanisms underlying these associations. Nevertheless, our study findings clearly support the concept of different endotypes underlying type 1 diabetes (2).
The study limitations include a rather crude measure of environmental stress exposure during pregnancy. Unfortunately, there is no gold standard for measuring human environmental stress exposure. We took a number of steps to promote accurate recall of LE data; our prospective study design eliminated the possibility that associations were a product of recall bias. Further, our findings are consistent with a previously published report linking interpersonal events during pregnancy with type 1 diabetes in HLA-DR3/4 children (8). However, certain types of LEs (significant loss and financial difficulties) were infrequently reported, limiting our ability to detect any association between these types of events and the development of IA in the child. Given the previously published study documenting a link between death of a father or a sibling during the prenatal period and the development of type 1 diabetes (7), we conducted an exploratory post hoc analysis examining onset of any IA by HLA-DR haplogenotype and specific LEs. This analysis suggested that a significant loss LE during pregnancy may indeed impact onset of IA in the child, but the effect is dependent on the child’s HLA-DR haplogenotype (ESM Table 10).
Our focus on environmental stress exposure during pregnancy and not the mother’s reaction to that exposure is an additional study limitation. Our purpose was to first test the possible association of environmental stress exposure during pregnancy with the development of diabetes-related autoimmunity in the child. Resilience, coping or adaptation in response to LEs are certainly additional factors important to consider in future studies (44).
Additional study limitations include its narrow focus on IAA-first and GADA-first in children of non-diabetic mothers until 6 years of age. We conducted post hoc analyses of 774 TEDDY children with diabetic mothers of whom 65 developed IA (29 IAA-first, 24 GADA-first). In this small sample, children of mothers reporting a job-related LE during pregnancy were more likely to develop GADA-first than IAA-first (HR 3.16; 95% CI 1.25, 9.02; p=0.02), consistent with the findings we report here. However, we were unable to document an association between serious interpersonal LEs and any IA, possibly due to the small sample size. Future research will need to expand this work to the unique situation experienced by pregnant women with diabetes. Also important will be studies of prenatal LEs and IA in older children, as well as their possible role in a child’s progression to multiple autoantibody status or type 1 diabetes. Because TEDDY study visits occur every 3 to 6 months, we were unable determine the first IA for 20% of children who exhibited multiple autoantibodies in their first positive test results; this was another study limitation. However, sensitivity analysis showed little evidence that determination of first-appearing IA for these multiple-IA children would change the findings.
In an effort to address some of these limitations, TEDDY investigators plan to explore the role of LEs after the child’s birth in both the development of IA and progression toward type 1 diabetes. Plans also include exploring possible associations with stress response-related genotypes in the child using genome-wide SNP coverage, epigenetic and transcriptome profiling, child gut microbiota, metabolomics and proteomics, and the child’s immunological response. Although stress has long been considered a possible trigger of type 1 diabetes, this work will advance the field beyond the simple association studies that have characterised the literature to date. Elucidating the gene–environment interactions underlying the pathogenesis of this disease is critical to our ultimate goal of type 1 diabetes prevention.
Supplementary Material
Research in context.
What is already known about this subject?
Prospective studies have suggested a link between psychological stress and type 1 diabetes
Two prospective studies have documented an association between psychological stress or life events during pregnancy and risk of type 1 diabetes in the child
What is the key question?
Are maternal life events experienced during pregnancy associated with first-appearing insulin autoantibodies (IAA) or GAD autoantibodies (GADA) in the young child and is any association conditioned by the child’s type 1 diabetes genetic risk?
What are the new findings?
The association of maternal life events during pregnancy differed between IAA and GADA as the first-appearing autoantibody and was often conditioned by the child’s type 1 diabetes genetic risk
A job-related life event during pregnancy was associated with reduced risk of IAA as the first-appearing autoantibody in all children except HLA-DR3/4 children with the BTNL2-A allele
In contrast, a serious interpersonal life event during pregnancy was associated with increased risk for GADA as the first-appearing autoantibody in HLA-DR3 children with the BACH2-T allele; a job-related life event during pregnancy was also associated with an increased risk of GADA in HLA-DR3/4 children
How might this impact on clinical practice in the foreseeable future?
The findings add together our understanding of the different pathways by which a child may develop type 1 diabetes and support the need for additional investigation of the role of life events during pregnancy in the child’s development of type 1 diabetes-related autoimmunity
Acknowledgements
We thank the TEDDY families for making this work possible and the many additional members of the TEDDY Study Group (available online in the ESM).
Funding The TEDDY study is funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483 and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Environmental Health Sciences (NIEHS), the Centers for Disease Control and Prevention (CDC) and JDRF. This work was supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR002535). The sponsors of this study were represented on the Steering Committee and played a role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication. The corresponding authors had the final decision to submit the manuscript for publication.
Abbreviations
- BACH2
rs3757247 SNP in BACH2
- BTNL2
rs3763305 SNP in BTNL2
- CTLA4
rs231775 SNP in CTLA-4
- ERBB3
rs2292239 SNP in ERBB3
- GADA
GAD autoantibodies
- IA
Islet autoantibodies
- IAA
Insulin autoantibodies
- IA-2A
Insulinoma-associated protein 2 autoantibodies
- INS
rs1004446 SNP in INS
- LE
Life event
- PTPN22
rs2476601 SNP in PTPN22
- RERI
Relative excess risk due to interaction
- SH2B3
rs3184504 SNP in SH2B3
- TEDDY
The Environmental Determinants of Diabetes in the Young
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability The datasets generated and analysed during the current study will be made available in the NIDDK Central Repository at https://www.niddkrepository.org/studies/teddy. The TEDDY ImmunoChip (SNP) data that support the findings of this study have been deposited in NCBI’s database of Genotypes and Phenotypes (dbGaP) with the primary accession code phs001037.v2.p1.
References
- 1.Krischer JP, Lynch KF, Lernmark A, et al. (2017) Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: The TEDDY study. Diabetes Care 40(9):1194–1202. doi: 10.2337/dcl7-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battaglia M, Ahmed S, Anderson MS, et al. (2020) Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43(1):5–12. doi: 10.2337/dc19-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sepa A, Ludvigsson J (2006). Psychological stress and the risk of diabetes-related autoimmunity: a review article. Neuroimmunomodulation 13(5-6):301–308. doi: 10.1159/000104858. [DOI] [PubMed] [Google Scholar]
- 4.Sharif K, Watad A, Coplan L, Amital H, Shoenfeld Y, Afek A (2018) Psychological stress and type 1 diabetes mellitus: what is the link? Expert Rev Clin Immun 14(12):1081–1088. doi: 10.1080/1744666X.2018.1538787. [DOI] [PubMed] [Google Scholar]
- 5.Sepa A, Frodi A, Ludvigsson J (2005) Mothers’ experiences of serious life events increase the risk of diabetes-related autoimmunity in their children. Diabetes Care 28(10):2394–2399. doi: 10.2337/diacare.28.10.2394. [DOI] [PubMed] [Google Scholar]
- 6.Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A (2015) Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia 58(6):1188–1197. doi: 10.1007/s00125-015-3555-2. [DOI] [PubMed] [Google Scholar]
- 7.Virk J, Li J, Vestergaard M, et al. (2010) Early life disease programming during the preconception and prenatal period: Making the link between stressful life events and type-1 diabetes. PLoS One 5(7):e11523. doi: 10.1371/journal.pone.0011523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren M, Ellstrom K, Larsson H, for the DiPiS study group (2018) Influence of early-life parental severe life events on the risk of type 1 diabetes in children: the DiPiS study. Acta Diabetologia 55(8):797–804. doi: 10.1007/s00592-018-1150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth R, Lynch K, Hyöty H, Lönnrot M, Driscoll KA, Johnson SB, TEDDY Study Group (2019) The association between stressful life events and respiratory infections during the first 4 years of life: The Environmental Determinants of Diabetes in the Young study. Stress Health 35(3):289–303. doi: 10.1002/smi.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tegethoff M, Greene N, Olsen J, Schaffner E, Meinlschmidt G (2011) Stress during pregnancy and offspring pediatric disease: A national cohort study. Environ Health Perspect 119(11):1647–1652. doi: 10.1289/ehp.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stepanikova I, Baker E, Oates G, et al. (2019) Perinatal maternal stress and susceptibility to infectious diseases in later childhood: An early life programming perspective. J Psychol 153(1);67–88. doi: 10.1080/00223980.2018.1483311. [DOI] [PubMed] [Google Scholar]
- 12.Smejda K, Polanska K, Merecz-Kot D, et al. (2018) Maternal stress during pregnancy and allergic diseases in children during the first year of life. Respir Care 63(1):70–76. doi: 10.4187/respcare.05692. [DOI] [PubMed] [Google Scholar]
- 13.Flanigan C, Sheikh A, DunnGalvin A, Brew BK, Almqvist C, Nwaru BI (2018) Prenatal maternal psychosocial stress and offspring’s asthma and allergic disease: A systematic review and meta-analysis. Clin Exp Allergy 48(4):403–414. doi: 10.1111/cea.13091 [DOI] [PubMed] [Google Scholar]
- 14.Torn C, Hadley D, Lee H-S et al. (2015) Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 64(5):1818–1829. doi: 10.2337/db14-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hippich M, Beyerlin A, Hagopian WA et al. (2019) Genetic contribution to the divergence to type 1 diabetes risk beween children from the general population and children from affected families. Diabetes 68(4):847–857. doi: 10.2337/db18-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch KF, Hye-Seung L, Torn C, et al. (2018) Gestational respiratory infections interacting with offspring HLA and CTLA-4 modifies incident B-cell autoantibodies. J Autoimmun 86:93–103. doi: 10.1016/j.jaut.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.TEDDY Study Group (2008) The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci 1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lernmark B, Johnson SB, Vehik K, et al. (2011) Enrollment experiences in a pediatric longitudinal observational study: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Contemp Clin Trials 32(4):517–523. doi: 10.1016/j.cct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter J, Vehik K, Johnson SB, et al. (2012) Differences in recruitment and early retention among ethnic minority participants in a large pediatric cohort: the TEDDY Study. Contemp Clin Trials 33(4):633–640. doi: 10.1016/j.cct.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes TH, Rahe RH (1967) The social readjustment rating scale. J Psychosom Res 11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 21.Sarason IG, Johnson JH, Siegel JM (1978) Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol 46(5):932–46. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 22.Harkness KL, Monroe SM (2016) The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. J Abnorm Psychol 125(5):727–745. doi: 10.1037/abn0000178. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn PM, Rohde P, Gau JM (2003) Comparability of self-report checklist and interview data in the assessment of stressful life events in adults. Psychol Rep 93(2):459–471. doi: 10.2466/pr0.2003.93.2.459. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D (2010) Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 13(2):273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knol M, VanderWeele T (2012) Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 41(2):514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsunaga S, Hosomichi K, Okudaira Y, et al. (2013) Exome sequencing identifies novel rheumatoid arthritis-susceptible variants in the BTNL2. J Hum Genet 58(4):210–215. doi: 10.1038/jhg.2013.2. [DOI] [PubMed] [Google Scholar]
- 28.Orozco G, Eerligh P, Sanchez E et al. (2005) Analysis of a functional BTNL2 polymorphism in type diabetes, rheumatoid arthritis, and systemic lupus erythermatosus. Hum Immunol 66(12):1235–1241. doi: 10.1016/j.humimm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.He C, Hamon S, Li D, Barral-Rodriguez S, Ott B and the Type 1 Diabetes Genetics Consortium (2009) MHC fine mapping of human type 1 diabetes using T1DGC data. Diabetes Obes Metab 11(Suppl 1):53–59. doi: 10.1111/j.1463-1326.2008.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnett HA1, Escobar SS, Viney JL (2009) Regulation of costimulation in the era of butyrophilins. Cytokine 46(3):370–375. doi: 10.1016/j.cyto.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Swanson RM, Gavin MA, Escobar SS, et al. (2013) Butyrophilin-like 2 modulates B7 costimulation to induce Foxp3 expression and regulatory T cell development in mature T cells. J Immunol 190(5):2027–2035. doi: 10.4049/jimmunol.1201760. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen T, Liu XK, Zhang Y, Dong C (2006) BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol 176(12):7354–7360. doi: 10.4049/jimmunol.176.12.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steck AK, Vehik K, Bonifacio E et al. (2015) Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 38(5):808–813. doi: 10.2337/dc14-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper JD, Smyth DJ, Smiles AM et al. (2008) Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet 40(12):1399–1401. doi: 10.1038/ng.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant SFA, Qu HQ, Bradfield JP et al. (2009) Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes 58(1):290–295. doi: 10.2337/db08-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler C, Krumsiek J, Buettner F et al. (2014) Feature ranking of type 1 diabetes susceptibility genes improves prediction of type 1 diabetes. Diabetologia 57(12):2521–2529. doi: 10.1007/s00125-014-3362-1. [DOI] [PubMed] [Google Scholar]
- 37.Onuma H, Kawamura R, Tabara Y et al. (2019) Variant of BACH2 and CLEC16A gene might be associated with susceptibility to insulin-triggered type 1 diabetes. J Diabetes Investig 10(6):1447–1453. doi: 10.1111/jdi.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frohnert BI, Laimighofer M, Krumsiek J et al. (2018) Prediction of type 1 diabetes using a genetic risk model in the Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes 19(2):277–283. doi: 10.1111/pedi.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidwell T, Kallies A (2016) Bach2 is required for B cell and T cell memory differentiation. Nat Immunol 17(7):744–5. doi: 10.1038/ni.3493. [DOI] [PubMed] [Google Scholar]
- 40.Marrroqui L, Santin I, Santos R, Marselli L, Marchetti P, Eizirik D (2014) BACH2, a candidate risk gene for type 1 diabetes, regulates apoptosis in pancreatic B-cells via JNK1 modulation and crosstalk with the candidate gene PTPN2. Diabetes 63(7):251627. doi: 10.2337/db13-1443. [DOI] [PubMed] [Google Scholar]
- 41.Roychoudhuri R, Hirahara K, Mousavi K et al. (2013) BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature 498(7455):506–512. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richer MJ, Lang ML, Butler NS (2016) T cell fates zipped up: How the Bach2 basic leucine zipper transcriptional repressor directs T cell differentiation and function. J Immunol 197(4):1009–1015. doi: 10.4049/jimmunol.1600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buck CO, Gjelsvik A, Vivier PM, Monteiro K, Amanullah S (2018) Prenatale exposure to stressful life events and infant breastfeeding. Breastfeed Med 13(6):426–432. doi: 10.1089/bfm.2017.0200. [DOI] [PubMed] [Google Scholar]
- 44.Dantzer R, Cohen S, Russo SJ, Dinan TG (2018) Resilience and immunity. Brain Behav Immun 74:28–42. doi: 10.1016/j.bbi.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


