Abstract
This is an overview of the metabolic activation of drugs, natural products, physiological compounds, and general chemicals by catalytic activity of cytochrome P450 enzymes belonging to Families 1–4. The data were collected from > 5,152 references. The total number of data entries of reactions catalyzed by P450s Families 1–4 was 7,696 of which 1,121 (~15%) were defined as bioactivation reactions of different degrees. The data were divided into groups of General Chemicals, Drugs, Natural Products, and Physiological Compounds, presented in tabular form. The metabolism and bioactivation of selected examples of each group are discussed. In most of the cases the metabolites are directly toxic chemicals reacting with cell macromolecules, but in some cases the metabolites formed are not direct toxicants but participate as substrates in succeeding metabolic reactions (e.g., conjugation reactions), the products of which are final toxicants. We identified a high level of activation for three groups of compounds (General Chemicals, Drugs, and Natural Products) yielding activated metabolites and the generally low participation of Physiological Compounds in bioactivation reactions. In the group of General Chemicals, P450 enzymes 1A1, 1A2, and 1B1 dominate in the formation of activated metabolites; Drugs are mostly activated by the enzyme P450 3A4, and Natural Products by P450s 1A2, 2E1, and 3A4. Physiological Compounds showed no clearly dominant enzyme, but the highest numbers of activations are attributed to P450 1A, 1B1, and 3A enzymes. The results thus show, perhaps not surprisingly, that Physiological Compounds are infrequent substrates in bioactivation reactions catalyzed by P450 enzyme Families 1–4, with the exception of estrogens and arachidonic acid. The results thus provide information on the enzymes that activate specific groups of chemicals to toxic metabolites.
Keywords: Cytochrome P450, P450, CYP Families 1-4, xenobiotics, natural products, bioactivation
Introduction
Human cytochrome P450 (P450, CYP) enzymes catalyze a great number of metabolic reactions that have important effects on the biological activities (physiologic, therapeutic, and/or toxic) of xenobiotics such as drugs, natural products, general chemicals (e.g., environmental chemicals such as pesticides, pro-carcinogens), and physiological compounds. Their general role and significance for metabolism in humans has been discussed and reviewed previously. In addition, in previous publications efforts were made to estimate the participation of the activity of different groups of enzymes, e.g. oxidoreductase enzymes (FMO (microsomal flavin-containing monooxygenase), AKR (aldo-keto reductase), MAO (monoamine oxidase), and P450 enzymes), in the metabolism of natural products and physiological chemicals and general chemicals in humans. When the groups of chemicals were analyzed, the results showed the highest values for participation of P450 enzymes in the metabolism of drugs and general chemicals as substrates. For P450 enzymes the calculations also showed that, regarding drug metabolism, more than three-fourths of the human P450 reactions can be accounted for by a set of five P450s: 1A2, 2C9, 2C19, 2D6, and 3A4, with the largest fraction of the P450 reactions being catalyzed by P450 3A enzymes. Compared to other oxidoreductase enzymes and taking into consideration chemicals that are classified as carcinogens, our calculations showed that metabolic activations of the compounds to toxic metabolites are dominantly catalyzed by P450 enzymes (66% of bioactivations) and that, within this group, six P450s (1A1, 1A2, 1B1, 2A6, 2E1, and 3A4) accounted for 77% of the P450 activation reactions. In the present review we have updated and extended our calculations to general activation reactions forming potentially toxic metabolites as a consequence of metabolic activation of drugs, natural products, physiological compounds, and general chemicals (Rendic 2002; Rendic and Di Carlo 1997; Rendic and Guengerich 2012; Rendic and Guengerich 2015). We recently reviewed the properties (mechanisms, induction, inhibition, toxic effects, and benefits) of human P450s belonging to the P450 Families 5–51 (i.e., 22 of the total 57 P450s) that are responsible for metabolism and biosynthesis of physiological compounds, including their substrate selectivity, information, and references (Rendic and Guengerich 2018). In the present paper we update and discuss important aspects of many of the P450s belonging to Families 1–4, including the reactions and the roles in metabolic activation of xenobiotics (drugs, natural products, general chemicals) and physiological compounds.
Results and discussion
A synopsis of of the data used for analysis of catalytic activity of P450 Families 1–4 is presented in Table 1. Data were collected from more than 5,152 references. The total number of data entries for enzymatic reactions catalyzed by P450s belonging to 1–4 Families was 7,686 of which 1,114 (~15%) were defined as bioactivation reactions of different degrees. When considering activation of all compounds the results show predominant participation of P450s 3A4, 1A2, and 1A1, followed by P450s 2E1 and 1B1. P450s 2C9, 2D6, 2A6, 2C19, and 2B6 also have significant participation in bioactivation reactions (Fig. 1).
Table 1.
Number of data entries related to metabolic activation of drugs, general chemicals, natural products, and physiological compounds catalyzed by human cytochrome P450 Families 1–4
| Number of data entries | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All compounds | General chemicals | Drugs | Natural products | Physiological compounds | |||||
| Total | Activations | Total | Activations | Total | Activations | Total | Activations | Total | Activations |
| 7686 | 1114 | 2165 | 618 | 4039 | 235 | 952 | 186 | 530 | 75 |
Fig 1.
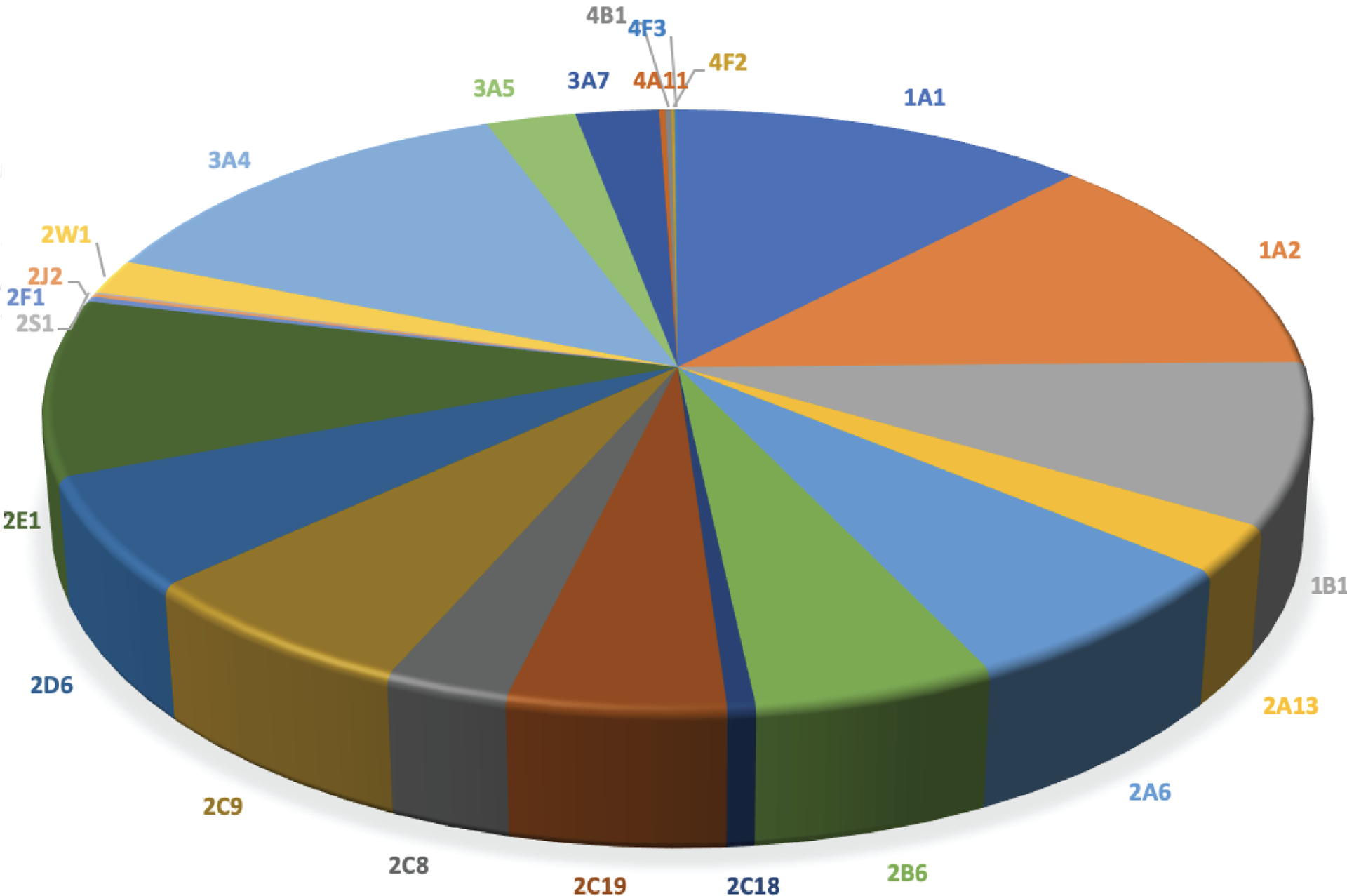
Participation of human P450 Families 1–4 in activation of all compounds to potentially toxic metabolites (7686 reactions, 1114 activation reactions)
Data analyzed were divided into four groups of compounds: General Chemicals, Drugs, Natural Products, and Physiological Compounds. Of the 2,165 reactions for General Chemicals, 618 (29%) were classified as activations; for 4,032 Drugs entries, 237 (6%) were classified as activations; for the 952 reactions under Natural Products, 186 (20%) were classified as activations; for the 530 Physiological Compounds, 75 reactions (14%) were classified as activations (Table 1).
General chemicals
We reported previously that metabolism of General Chemicals catalyzed by human enzymes is predominately catalyzed by P450 enzymes in humans (~92%) (Rendic and Guengerich 2015). Other enzymes, besides P450s, that participate in a greater extent include those in the AKR, FMO, and MAO families (Rendic and Guengerich 2015). P450 enzymes dominate in bioactivation of carcinogens (66%) over other xenobiotic metabolizing enzymes (Rendic and Guengerich 2012). The present data show that among P450 enzymes, Family 1 enzymes (P450s 1A1, 1A2, B1) dominate in activations of General Chemicals, followed by P450s 2E1, 3A4, and 2A6 (Fig. 2).
Fig 2.

Participation of human P450 Families 1–4 in activation of general chemicals to potentially toxic metabolites (2165 reactions, 618 activation reactions)
The following examples illustrate the participation of P450 enzymes in the bioactivation of selected General Chemicals substrates.
Polycyclic aromatic hydrocarbons (PAHs)
Examples (213 data entries) of the metabolic activation of group of general chemicals (e.g., polycyclic aromatic hydrocarbons (PAHs), heterocyclic and aromatic amines, insecticides, organic solvents) are presented in Table 2. The majority of the data presented (75 data entries) involve PAHs and their metabolites. Of the 76 entries presented in Table 2, 24 are attributed as “high activity” or “high activation” and are catalyzed by P450 1A1, 1A2, 1B1, 2A13, and 2A6 enzymes. These data correlate well with experimental findings on the activation of PAHs by P450 enzymes (Shimada et al. 2013). The parent PAH compounds are not toxic per se but their products formed by hydroxylation and epoxidation reactions, catalyzed by P450 enzymes, are reactive and interact with cellular macromolecules. Consequently, the literature data on activation of PAHs are predominately focused on activation of the PAH metabolites (e.g., dihydrodiols possessing different stereochemical structures) to ultimate toxic dihydrodiol epoxides, as exemplified by the classic activation of benzo[a]pyrene (B[a]P) (Fig. 3).
Table 2.
Examples of the metabolic activation of groups of general chemicals (polycyclic aromatic hydrocarbons, heterocyclic and aromatic amines, insecticides, organic solvents) by human cytochrome P450 enzymes
| General chemical | P450 | Category | Reaction | PMID numbers | References |
|---|---|---|---|---|---|
| N-Acetylaminofluorene (2-acetamidofluorene, 2-AAF) | 1A2 | Acyl arylamine | Hydroxylation, N- (major enzyme, activation) | 8095200, 8313839, 1576936, 2813353, 9705755, 8200083, 10503887, 10517985, 11377247, 11013410, 11473383, 15279838, 11375903, 23432465 | (Aryal et al. 2000; Butler et al. 1989; Edwards et al. 1994; Guengerich 1993; Guengerich et al. 1999; Ioannides and Parke 1993; Josephy et al. 2001; Juchau et al. 1992; Oda et al. 2001; Shimada et al. 2013; Turesky et al. 1999; Turesky et al. 1998; Yamazaki et al. 2004; Yueh et al. 2001) |
| 2-AAF | 1A1 | Acetyl arylamine | Hydroxylation, N- (activation) | 8095200, 8313839, 1576936, 11502724, 15279838, 7955101, 23432465 | (Guengerich 1993; Ioannides and Parke 1993; Juchau et al. 1992; Shimada et al. 1994; Shimada et al. 2013; Shimada et al. 2001a; Yamazaki et al. 2004) |
| 2-Aminoanthracene (2-AA) | 2W1 | Arylamine | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| 2-AA | 1A2 | Arylamine | Hydroxylation, N- (activation) | 23432465 | (Shimada et al. 2013) |
| 2-AA | 2A13 | Arylamine | Hydroxylation, N- (activation) | 23432465 | (Shimada et al. 2013) |
| 2-AA | 2A6 | Arylamine | Hydroxylation, N- (high activity, activation) | 23432465 | (Shimada et al. 2013) |
| 2-AA | 1A1 | Arylamine | Hydroxylation, N- (high activity, activation), major enzyme | 7955101, 11502724, 9705755, 11377247, 10964100, 23432465 | (Oda et al. 2001; Shimada et al. 1994; Shimada et al. 2013; Shimada et al. 2001a; Turesky et al. 1998; Williams et al. 2000) |
| 2-AA | 1B1 | Arylamine | Hydroxylation, N- (high activity, activation) | 8674051, 10964100, 11377247, 11473383, 23432465, 27123158 | (Chun and Kim 2016; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1996; Shimada et al. 2013; Williams et al. 2000) |
| 6-Aminochrysene | 3A4 | Arylamine | N-Oxidation (high activity, activation) | 2271712, 8330339 | (Brian et al. 1990; Yamazaki et al. 1993) |
| 6-Aminochrysene | 1A1 | Arylamine | Oxidation (high activity and activation) | 7955101, 8961944, 11502724, 9685642 | (Guengerich and Shimada 1998; Shimada et al. 1994; Shimada et al. 2001a; Shou et al. 1996a) |
| 2-Amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ) | 2A13 | Heterocyclic amine | Activation | 23432465 | (Shimada et al. 2013) |
| MeIQ | 2A6 | Heterocyclic amine | Activation | 23432465 | (Shimada et al. 2013) |
| MeIQ | 2W1 | Heterocyclic amine | Activation | 24278521 | (Eun et al. 2010) |
| MeIQ | 1A1 | Heterocyclic amine | Hydroxylation, N- (activation) | 7955101, 9152602, 11502724, 9705755, 8200083, 11377247, 11473383 | (Edwards et al. 1994; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1994; Shimada et al. 1997b; Shimada et al. 2001a; Turesky et al. 1998) |
| MeIQ | 1B1 | Heterocyclic amine | Hydroxylation, N- (activation) | 8674051, 10964100, 9152602, 10426814, 11377247, 11473383, 9721189, 11502724, 11719446, 23432465, 27123158 | (Chun and Kim 2016; Chun et al. 2001; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1994; Shimada et al. 1997b; Shimada et al. 2013; Shimada et al. 2001a; Shimada et al. 1999; Shimada et al. 1998; Williams et al. 2000) |
| 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) | 2A13 | Heterocyclic amine | Activation | 23432465 | (Shimada et al. 2013) |
| MeIQx | 2A6 | Heterocyclic amine | Activation (weaker activation) | 23432465 | (Shimada et al. 2013) |
| MeIQx | 1B1 | Heterocyclic amine | Activation (weaker activation) | 23432465, 27123158 | (Shimada et al. 2013) |
| MeIQx | 1A1 | Heterocyclic amine | Hydroxylation, N- (activation) | 7955101, 9705755, 8200083, 11377247, 11473383, 11502724, 17627018 | (Bendaly et al. 2007; Edwards et al. 1994; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1994; Shimada et al. 2001a; Turesky et al. 1998) |
| MeIQx | 1A2 | Heterocyclic amine | Hydroxylation, N- (major enzyme, high activity and activation) | 7955101, 9705755, 8200083, 11377247, 11473383, 9111224, 10220313, 11258970, 11453738, 12351158, 14744142, 14725854, 28879062 | (Delannée et al. 2017; Edwards et al. 1994; Hammons et al. 1997; Josephy et al. 2001; Kim and Guengerich 2004; Langouët et al. 2001; Oda et al. 2001; Parikh et al. 1999; Shimada et al. 1994; Turesky et al. 1998; Turesky et al. 2002; Turesky et al. 2001; Zhou et al. 2004) |
| 3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) | 2D6 | Heterocyclic amine | Hydroxylation, N- (activation) | 11377247 | (Oda et al. 2001) |
| Trp-P-1 | 2E1 | Heterocyclic amine | Hydroxylation, N- (activation) | 11377247 | (Oda et al. 2001) |
| Trp-P-1 | 3A4 | Heterocyclic amine | Hydroxylation, N- (high activation) | 11377247 | (Oda et al. 2001) |
| Trp-P-1 | 2C9 | Heterocyclic amine | Oxidation (activation) | 11377247 | (Oda et al. 2001) |
| Trp-P-1 | 2W1 | Heterocyclic amine | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| Trp-P-1 | 2A13 | Heterocyclic amine | Activation (weaker activation) | 23432465 | (Shimada et al. 2013) |
| Trp-P-1 | 2A6 | Heterocyclic amine | Activation (weaker activation) | 23432465 | (Shimada et al. 2013) |
| Trp-P-1 | 2W1 | Heterocyclic amine | Oxidation (activation) | 24278521 | (Eun et al. 2010) |
| Trp-P-1 | 1A2 | Heterocyclic amine | Hydroxylation, N- (major enzyme, activation) | 11377247, 7955101, 9705755, 11473383, 8961944 | (Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1994; Shou et al. 1996a; Turesky et al. 1998) |
| Trp-P-1 | 1A1 | Heterocyclic amine | Hydroxylation, N- (high activation) | 7955101, 8200083, 11377247, 11502724, 11473383, 9705755 | (Edwards et al. 1994; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1994; Shimada et al. 2001a; Turesky et al. 1998) |
| 2-Aminofluorene (2-AF) | 2W1 | Arylamine | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| 2-AF | 2A6 | Arylamine | Hydroxylation, N- (activation) | 23432465 | (Shimada et al. 2013) |
| 2-AF | 2A13 | Arylamine | Hydroxylation, N- (major enzyme, high activity, activation) | 23432465 | (Shimada et al. 2013) |
| 2-AF | 1A2 | Arylamine | Hydroxylation, N- (major enzyme, high activity and activation) | 2334931, 7955101, 8095200, 8313839, 1576936, 9705755, 8200083, 10503887, 10964100, 11377247, 11013410, 2803520, 10815771, 23432465 | (Aoyama et al. 1989; Aryal et al. 2000; Edwards et al. 1994; Guengerich 1993; Ioannides and Parke 1993; Juchau et al. 1992; Lozano et al. 2000; McManus et al. 1990; Oda et al. 2001; Shimada et al. 1994; Shimada et al. 2013; Turesky et al. 1999; Turesky et al. 1998; Williams et al. 2000) |
| 2-AF | 1B1 | Arylamine | Hydroxylation, N- (weaker activation) | 23432465, 27123158 | (Chun and Kim 2016; Shimada et al. 2013) |
| 2-Amino-6-methyldipyrido[1,2-a,3,2’-d]-imidazole (Glu-P-1) | 1A2 | Heterocyclic amine | Hydroxylation, N- (high activity, major enzyme, activation) | 7955101, 9705755, 10503887, 10517985, 11377247, 2803520, 14744142, 14725854 | (Aoyama et al. 1989; Guengerich et al. 1999; Kim and Guengerich 2004; Oda et al. 2001; Shimada et al. 1994; Turesky et al. 1999; Turesky et al. 1998; Zhou et al. 2004) |
| 2-Amino-3-methylimidazo[4,5-f]quinoline (IQ) | 2A13 | Heterocyclic amine | Activation | 23432465 | (Shimada et al. 2013) |
| IQ | 2A6 | Heterocyclic amine | Activation (weaker activation) | 23432465 | (Shimada et al. 2013) |
| IQ | 2W1 | Heterocyclic amine | Activation | 24278521 | (Eun et al. 2010) |
| IQ | 1B1 | Heterocyclic amine | Activation | 23432465, 27123158 | (Chun and Kim 2016; Shimada et al. 2013) |
| IQ | 1A1 | Heterocyclic amine | Hydroxylation, N- (activation) | 7955101, 9705755, 8200083, 11377247, 11473383, 9918136, 8095200 | (Edwards et al. 1994; Guengerich 1993; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 1994; Turesky et al. 1998; Williams et al. 1998) |
| IQ | 1A2 | Heterocyclic amine | Hydroxylation, N- (high activity, major enzyme, activation) | 9675256, 7955101, 8200083, 11377247, 9111224, 9918136, 14744142, 14725854, 11038156 | (Barceló et al. 1998; Edwards et al. 1994; Hammons et al. 1997; Kim and Guengerich 2004; Miranda et al. 2000; Oda et al. 2001; Shimada et al. 1994; Williams et al. 1998; Zhou et al. 2004) |
| 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, PhIP | 2C9 | Heterocyclic amine | Hydroxylation, N2- (activation) | 11377247 | (Oda et al. 2001) |
| PhIP | 2D6 | Heterocyclic amine | Hydroxylation, N2- (activation) | 11377247 | (Oda et al. 2001) |
| PhIP | 2E1 | Heterocyclic amine | Hydroxylation, N2- (low or no activation) | 11377247 | (Oda et al. 2001) |
| PhIP | 1A1 | Heterocyclic amine | Hydroxylation, N2- (activation) | 11502724, 8961944, 9705755, 9855011, 8200083, 11377247, 11473383, 15279838, 9111224, 8095200 | (Crofts et al. 1998; Edwards et al. 1994; Guengerich 1993; Hammons et al. 1997; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 2001a; Shou et al. 1996a; Turesky et al. 1998; Yamazaki et al. 2004) |
| PhIP | 1A2 | Heterocyclic amine | Hydroxylation, N2- (high activity, major enzyme, major reaction, activation) | 1913651, 7955101, 9705755, 8200083, 11377247, 11473383, 10220313, 11258970, 11453738, 12351158, 14725854, 15279838, 16167840 | (Cheung et al. 2005a; Edwards et al. 1994; Josephy et al. 2001; Langouët et al. 2001; Oda et al. 2001; Parikh et al. 1999; Shimada et al. 1994; Shimada and Guengerich 1991; Turesky et al. 1998; Turesky et al. 2002; Turesky et al. 2001; Yamazaki et al. 2004; Zhou et al. 2004) |
| PhIP | 1B1 | Heterocyclic amine | Hydroxylation, N2- (activation) | 8961944, 11502724, 9855011, 10964100, 11377247, 11473383, 9328177 | (Crofts et al. 1997; Crofts et al. 1998; Josephy et al. 2001; Oda et al. 2001; Shimada et al. 2001a; Shou et al. 1996a; Williams et al. 2000) |
| PhIP | 1B1 | Heterocyclic amine | Hydroxylation, N2- and deamination (activation) | 9855011, 9328177, 27123158 | (Chun and Kim 2016; Crofts et al. 1997; Crofts et al. 1998) |
| Aniline reaction with norharman | 1A1 | Arylamine | Aminophenylnorharman formation (activation), high activity | 15279827 | (Nishigaki et al. 2004) |
| Aniline reaction with norharman | 1A2 | Arylamine | Aminophenylnorharman formation, activation, major enzyme | 15279827 | (Nishigaki et al. 2004) |
| Aniline reaction with norharman | 1B1 | Arylamine | Aminophenylnorharman formation, activation, very low activity | 15279827 | (Nishigaki et al. 2004) |
| Aniline reaction with norharman | 2B6 | Arylamine | Aminophenylnorharman formation, activation, very low activity | 15279827 | (Nishigaki et al. 2004) |
| Aniline reaction with norharman | 2D6 | Arylamine | Aminophenylnorharman formation, activation | 15279827 | (Nishigaki et al. 2004) |
| Aniline reaction with norharman | 2E1 | Arylamine | Aminophenylnorharman formation, activation, very low activity | 15279827 | (Nishigaki et al. 2004) |
| Aniline reaction with norharman | 3A4 | Arylamine | Aminophenylnorharman formation, activation, major enzyme | 15279827 | (Nishigaki et al. 2004) |
| Azinphos-methyl | 2C19 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, medium Km, high activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 1A1 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, medium Km, medium activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 1A2 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, medium Km, medium activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 2B6 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, medium Km, medium activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 2C19 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, medium Km, high activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 2C8 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, very low activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 2C9 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, very low activity | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Azinphos-methyl | 3A4 | Insecticide, organophosphate, benzotriazine organothiophosphate | Desulfuration (oxon formation), activation, very low activity, at higher concentrations | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Benzo[a]anthracene-3,4-diol | 1A1 | Polycyclic aromatic hydrocarbon (PAH) metabolite | Activation (weaker) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[a]anthracene-3,4-diol | 1A2 | PAH metabolite | Activation (weaker) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| trans-Benz[a]anthracene-3,4-diol | 2W1 | PAH metabolite | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| Benzo[g]chrysene-11,12-diol | 1A1 | PAH metabolite | Activation | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[g]chrysene-11,12-diol | 1B1 | PAH metabolite | Activation | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[g]chrysene-11,12-diol | 1A2 | PAH metabolite | Activation (weaker) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[b]fluoranthene-9,10-diol | 1A2 | PAH metabolite | Oxidation (activation) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[b]fluoranthene-9,10-diol | 2B6 | PAH metabolite | Oxidation (activation) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[b]fluoranthene-9,10-diol | 2C19 | PAH metabolite | Oxidation (activation) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Benzo[c]phenanthrene (B[c]P) | 2A13 | PAH | Activation (weaker) | 23432465 | (Shimada et al. 2013) |
| B[c]P | 2A6 | PAH | Activation (weaker) | 23432465 | (Shimada et al. 2013) |
| B[c]P | 1A1 | PAH | 3,4-Dihydrodiol-1,2-epoxide formation (weaker activation) | 9168260, 21781864, 11409939 | (Baum et al. 2001; Einolf et al. 1997; Seidel et al. 1998) |
| B[c]P | 1A2 | PAH | 3,4-Dihydrodiol-1,2-epoxide formation (major enzyme, activation) | 9168260, 21781864, 11409939 | (Baum et al. 2001; Einolf et al. 1997; Seidel et al. 1998) |
| B[c]P | 1B1 | PAH | 3,4-Dihydrodiol-1,2-epoxide formation (major enzyme, activation) | 9168260, 21781864, 11409939, 23432465 | (Baum et al. 2001; Einolf et al. 1997; Seidel et al. 1998; Shimada et al. 2013) |
| Benzo[c]phenanthrene 3,4-dihydrodiol (B[c]P-3,4-diol) | 1A2 | PAH metabolite | Activation (weak) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| B[c]P-3,4-diol | 1A1 | PAH metabolite | Activation (weaker) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| B[c]P-3,4-diol | 1B1 | PAH metabolite | Activation (weaker) | 14720319, 23432465, 27123158 | (Chun and Kim 2016; Shimada and Fujii-Kuriyama 2004; Shimada et al. 2013) |
| B[c]P-3,4-diol | 2A13 | PAH metabolite | Activation (weak) | 23432465 | (Shimada et al. 2013) |
| B[c]P-3,4-diol | 2A6 | PAH metabolite | Activation (weak) | 23432465 | (Shimada et al. 2013) |
| Benzo[a]pyrene (B[a]P) | 1A1 | PAH | trans-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (activation) | 17640999, 14720319, 19330882 | (Kabler et al. 2009; Kim et al. 2007; Shimada and Fujii-Kuriyama 2004) |
| B[a]P | 1B1 | PAH | trans-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation. (medium Km, high activity, high efficiency, activation) | 8674051, 9721189, 11502724, 12584184, 9806168, 12628515, 23432465, 14720319, 27123158 | (Buters et al. 2003; Chun and Kim 2016; Guengerich et al. 2003; Kim et al. 1998; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1996; Shimada et al. 2013; Shimada et al. 2001a; Shimada et al. 1998) |
| (±)-Benzo[a]pyrene (B[a]P)-7,8-dihydrodiol | 2W1 | PAH metabolite | Oxidation (activation) | 16551781 | (Wu et al. 2006) |
| (±)-B[a]P-7,8-dihydrodiol | 1A1 | PAH metabolite | Oxidation, diol-epoxide formation (high activation) | 11952781, 21028851, 11502724, 14720319, 29219051, 18402469, 15720144, 16411658, 17295519 | (Gelhaus et al. 2011; Jiang et al. 2005; Jiang et al. 2006; Kisselev et al. 2002; Quinn and Penning 2008; Rendic and Guengerich 2018; Ruan et al. 2007; Shimada and Fujii-Kuriyama 2004; Shimada et al. 2001a) |
| (±)-B[a]P-7,8-dihydrodiol | 1B1 | PAH metabolite | Oxidation, diol-epoxide formation (high activation) | 16551781, 21028851, 11502724, 14720319, 29219051, 18402469, 15720144, 16411658, 17295519 | (Gelhaus et al. 2011; Jiang et al. 2005; Jiang et al. 2006; Quinn and Penning 2008; Rendic and Guengerich 2018; Ruan et al. 2007; Shimada and Fujii-Kuriyama 2004; Shimada et al. 2001a; Wu et al. 2006) |
| cis-(−)-B[a]P-7,8-dihydrodiol | 1A1 | PAH metabolite | cis-(syn)-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (trans-diol epoxide formation), high activation | 14720319, 29219051 | (Rendic and Guengerich 2018; Shimada and Fujii-Kuriyama 2004) |
| cis-(−)-B[a]P-7,8-dihydrodiol | 1B1 | PAH metabolite | cis-(syn)-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (trans-diol epoxide formation), high activation | 14720319, 29219051 | (Rendic and Guengerich 2018; Shimada and Fujii-Kuriyama 2004) |
| trans-(−)-B[a]P-7,8-dihydrodiol | 3A7 | PAH metabolite | Oxidation (activation) | 9328287 | (Gillam et al. 1997) |
| trans-(−)-B[a]P-7,8-dihydrodiol | 1A1 | PAH metabolite | trans-(anti)-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (trans-diol epoxide formation), medium Km, high activity | 7955101, 9152602, 8961944, 11502724, 15720144, 17295519, 9014198, 8043197, 1551116, 11238186, 7581497, 11952781, 12670496, 14633740, 19330882 | (Doehmer et al. 1995; Gautier et al. 1996; Jiang et al. 2005; Kabler et al. 2009; Kisselev et al. 2002; Ruan et al. 2007; Schwarz et al. 2001; Schwarz et al. 2003; Schwarz and Roots 2003; Shimada et al. 1994; Shimada et al. 1997b; Shimada et al. 2001a; Shou et al. 1996a; Shou et al. 1994; Yun et al. 1992) |
| trans-(−)-B[a]P-7,8-dihydrodiol | 1B1 | PAH metabolite | trans-(anti)-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (trans-diol epoxide formation), low Km, high activity | 9152602, 10426814, 12807732, 15720144, 15861043, 16411658, 17295519 | (Jiang et al. 2005; Jiang et al. 2006; Mammen et al. 2005; Mammen et al. 2003; Ruan et al. 2007; Shimada et al. 1997b; Shimada et al. 1999) |
| trans-(+)-B[a]P-7,8-dihydrodiol | 3A7 | PAH metabolite | Oxidation (activation) | 9328287 | (Gillam et al. 1997) |
| trans-(+)-B[a]P-7,8-dihydrodiol | 2A13 | PAH metabolite | Oxidation (activation) | 23432465 | (Shimada et al. 2013) |
| trans-(+)-B[a]P-7,8-dihydrodiol | 2A6 | PAH metabolite | Oxidation (weak activation) | 23432465 | (Shimada et al. 2013) |
| trans-(+)-B[a]P-7,8-dihydrodiol | 1B1 | PAH metabolite | Oxidation, trans-(anti)-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (trans-diol epoxide formation), high efficiency activation | 23432465, 14720319, 29219051 | (Rendic and Guengerich 2018; Shimada and Fujii-Kuriyama 2004; Shimada et al. 2013) |
| trans-(+)-B[a]P-7,8-dihydrodiol | 1A1 | PAH metabolite | trans-(anti)-7,8-Dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro formation (trans-diol epoxide formation), medium Km, high activity, high efficiency activation | 7955101, 9152602, 8961944, 11502724, 15720144, 17295519, 9014198, 8043197, 1551116, 11238186, 7581497, 11952781, 12670496, 14633740, 14720319, 29219051, 19330882 | (Doehmer et al. 1995; Gautier et al. 1996; Jiang et al. 2005; Kabler et al. 2009; Kisselev et al. 2002; Rendic and Guengerich 2018; Ruan et al. 2007; Schwarz et al. 2001; Schwarz et al. 2003; Schwarz and Roots 2003; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1994; Shimada et al. 1997b; Shimada et al. 2001a; Shou et al. 1996a; Shou et al. 1994; Yun et al. 1992) |
| 1,3-Butadiene | 2A6 | Olefin | Butadiene monoxide (epoxybutene) formation (high activity, activation) | 8203896, 9016811, 8901879, 11397415, | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997) |
| 1,3-Butadiene | 1A2 | Olefin | Butadiene monoxide (epoxybutene) formation (activation) | 8203896, 9016811, 8901879, 11397415, 7586124 | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| 1,3-Butadiene | 2B6 | Olefin | Butadiene monoxide (epoxybutene) formation (activation) | 8203896, 9016811, 8901879, 11397415, 7586124 | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| 1,3-Butadiene | 2D6 | Olefin | Butadiene monoxide (epoxybutene) formation (activation) | 8203896, 9016811, 8901879, 11397415, 7586124 | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| 1,3-Butadiene | 2E1 | Olefin | Butadiene monoxide (epoxybutene), (S)- and (R)- formation (high activity, activation, major enzyme) | 8203896, 9635416, 17298833 | (Boysen et al. 2007; Duescher and Elfarra 1994; Nieusma et al. 1998) |
| Butadiene monoxide (1,2-epoxy-3-butene) | 3A4 | Olefin, butadiene metabolite | Diepoxybutane (at high concentrations, activation) | 8203896, 9016811, 8901879, 11397415, 7586124 | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| Butadiene monoxide (1,2-epoxy-3-butene) | 2A6 | Olefin, butadiene metabolite | Diepoxybutane, meso- (major) and (±)- formation (activation) | 8203896, 9016811, 8901879, 11397415, 7586124 | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| Butadiene monoxide (1,2-epoxy-3-butene) | 2C9 | Olefin, butadiene metabolite | Diepoxybutane, meso- (major) and (±)- formation (activation) | 8203896, 9016811, 8901879, 11397415, 7586124 | (Bond and Medinsky 2001; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| Butadiene monoxide (1,2-epoxy-3-butene) | 2E1 | Olefin, butadiene metabolite | Diepoxybutane, meso- (major) and (±)- formation (activation) | 8203896, 9016811, 8901879, 11397415, 7586124, 17298833 | (Bond and Medinsky 2001; Boysen et al. 2007; Duescher and Elfarra 1994; Elfarra et al. 1996; Krause and Elfarra 1997; Seaton et al. 1995) |
| Chloromethylindolines | 2W1 | Indoline | Oxidation (activation) | 27257736, 23589180 | (Guo et al. 2016; Travica et al. 2013) |
| Chlorpyrifos | 2C9 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, low activity | 11502728, 15764407 | (Sams et al. 2004; Tang et al. 2001) |
| Chlorpyrifos | 3A4 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, major at high concentration (100 μM) | 11502728, 21782601, 12620367, 11714865, 15764407, 16757081, 17079358, 17110060, 10996483, 20709133 | (Buratti et al. 2006; Buratti et al. 2002; Buratti et al. 2003; Croom et al. 2010; Dai et al. 2001; Foxenberg et al. 2007; Mutch and Williams 2006; Sams et al. 2004; Sams et al. 2000; Tang et al. 2001) |
| Chlorpyrifos | 1A2 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation, major enzyme), activation, low Km, medium activity | 11502728, 21782601, 12620367, 16757081, 17079358 | (Buratti et al. 2002; Buratti et al. 2003; Foxenberg et al. 2007; Mutch and Williams 2006; Tang et al. 2001) |
| Chlorpyrifos | 2B6 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), major enzyme, activation, medium activity, low Km, high activity and major reaction at low concentrations | 11502728, 21782601, 12620367, 15764407, 16757081, 17079358, 29463407, 20709133, 22281205 | (Buratti et al. 2002; Buratti et al. 2003; Foxenberg et al. 2007; Mutch and Williams 2006; Tang et al. 2001) |
| Chlorpyrifos | 2C19 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, medium to low activity | 11502728, 21782601, 15764407, 16757081, 17079358, 20709133 | (Buratti et al. 2002; Croom et al. 2010; Dai et al. 2001; Mutch and Williams 2006; Sams et al. 2004; Tang et al. 2001) |
| Chlorpyrifos | 2D6 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, low activity | 15764407, 16757081, 10996483 | (Buratti et al. 2002; Croom et al. 2010; Dai et al. 2001; Mutch and Williams 2006; Sams et al. 2004; Tang et al. 2001) |
| Chlorpyrifos | 3A5 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, medium activity | 16757081, 17079358, 17110060, 17079358 | (Buratti et al. 2006; Foxenberg et al. 2007; Mutch and Williams 2006; Sams et al. 2004) |
| Chlorpyrifos | 2C8 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation | 16757081, 21782601 | (Buratti et al. 2002; Sams et al. 2004) |
| Chlorpyrifos | 3A7 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, low activity | 17079358, 17110060 | (Buratti et al. 2006; Mutch and Williams 2006) |
| trans-Chrysene-1,2-diol | 1A2 | PAH, chrysene metabolite | Oxidation (weaker activation) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| trans-Chrysene-1,2-diol | 2W1 | PAH, chrysene metabolite | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| trans-Chrysene-1,2-diol | 1A1 | PAH, chrysene metabolite | Oxidation (activation) | 11502724, 8961944, 9152602, 14720319 | (Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997; Shimada et al. 2001a; Shou et al. 1996a) |
| trans-Chrysene-1,2-diol | 1B1 | PAH, chrysene metabolite | Oxidation (activation) | 8674051, 9721189, 11502724, 12584184, 9152602, 10426814, 9685642, 14720319 | (Buters et al. 2003; Guengerich and Shimada 1998; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shimada et al. 1996; Shimada et al. 2001a; Shimada et al. 1999; Shimada et al. 1998) |
| Diazinon | 3A5 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, high activity | 16757081 | (Mutch and Williams 2006) |
| Diazinon | 1A1 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, medium Km, medium activity | 21969518 | (Ellison et al. 2012) |
| Diazinon | 2E1 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, low Km, medium activity, at lower concentration | 21782601, 12620367 | (Buratti et al. 2002; Buratti et al. 2003) |
| Diazinon | 2B6 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, medium Km, medium to high activity | 21782601, 12620367, 11708902, 15764407, 16757081, 21969518 | (Buratti et al. 2002; Buratti et al. 2003; Dai et al. 2001a; Ellison et al. 2012; Kappers et al. 2001; Sams et al. 2004) |
| Diazinon | 2C19 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, medium to high Km, medium to high activity, major enzyme | 21782601, 12620367, 16757081, 11708902, 21969518 | (Buratti et al. 2002; Buratti et al. 2003; Ellison et al. 2012; Kappers et al. 2001; Sams et al. 2004) |
| Diazinon | 1A2 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, high Km, medium activity | 21782601, 12620367, 21969518 | (Buratti et al. 2002; Buratti et al. 2003; Ellison et al. 2012) |
| Diazinon | 3A4 | Insecticide, organophosphate, phosphorothioate | Desulfuration (oxon formation), activation, medium to high Km, high activity | 21782601, 12620367, 21969518 | (Buratti et al. 2002; Buratti et al. 2003; Ellison et al. 2012) |
| Dibenzo[b,k]fluoranthene | 1A1 | PAH | Oxidation (activation) | 10613181 | (Durant et al. 1999) |
| Dibenzo[a,l]pyrene (DB[a,l]P) (11R,12R)-dihydrodiol | 1A2 | PAH metabolite | Activation (weaker) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| Dibenzo[a,l]pyrene (DB[a,l]P) | 1A2 | PAH | (−)-syn- and (−)-anti-11,12-dihydrodiol-13,14-epoxide form. (medium Km, high activity, high efficiency, activation) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| DB[a,l]P | 2W1 | PAH | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| DB[a,l]P | 1B1 | PAH | (−)-syn- and (−)-anti-11,12-dihydrodiol-13,14-epoxide formation (medium Km, high activity, high efficiency, activation) | 8674051, 9721189, 11502724, 12584184, 9152602, 9625737, 10506751, 8968059, 9354437, 17623886, 12628515, 14720319, 27123158 | (Buters et al. 2003; Chun and Kim 2016; Guengerich et al. 2003; King et al. 1999; Luch et al. 1998; Mahadevan et al. 2007; Shimada et al. 1997a; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shimada et al. 1996; Shimada et al. 2001a; Shimada et al. 1998; Shou et al. 1996c) |
| DB[a,l]P | 1A1 | PAH | (−)-syn- and (−)-anti-11,12-dihydrodiol-13,14-epoxide formation (medium Km, high activity, high efficiency, activation) | 8961944, 9152602, 10207125, 10493514, 10506751, 8968059, 10613181, 14720319, 19330882 | (Durant et al. 1999; Kabler et al. 2009; King et al. 1999; Luch et al. 1999a; Luch et al. 1999b; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shou et al. 1996a; Shou et al. 1996c) |
| trans-DB[a,l]P-11,12-diol | 2W1 | PAH metabolite | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| trans-(−)-DB[a,l]P-(11R,12R)-diol | 1A1 | PAH metabolite | (−)-anti- and (+)-syn-11,12-dihydrodiol-13,14-epoxide formation (medium Km, high activity, activation) | 7955101, 8961944, 11502724, 10207125, 10493514, 14720319 | (Luch et al. 1999a; Luch et al. 1999b; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1994; Shimada et al. 2001a; Shou et al. 1996a) |
| trans-(−)-DB[a,l]P-(11R,12R)-diol | 1B1 | PAH metabolite | (−)-anti-11,12-Dihydrodiol-13,14-epoxide formation (medium Km, high activity, high efficiency, activation) | 8674051, 9721189, 11502724, 12584184, 9625737, 10207125, 10493514, 10506751, 10739169, 11465393, 9354437, 10426814, 12628515, 14720319 | (Buters et al. 2003; Guengerich et al. 2003; King et al. 1999; Luch et al. 1998; Luch et al. 1999a; Luch et al. 1999b; Shimada et al. 1997a; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1996; Shimada et al. 2001a; Shimada et al. 2001b; Shimada et al. 1999; Shimada et al. 1998; Watanabe et al. 2000) |
| trans-2,3-Dihydroxy-2,3-dihydrofluoranthene | 1B1 | PAH metabolite | Oxidation (activation) | 8674051, 9721189, 11502724, 12584184, 9685642 | (Buters et al. 2003; Guengerich and Shimada 1998; Shimada et al. 1996; Shimada et al. 2001a; Shimada et al. 1998) |
| 7,12-Dimethylbenz[a]anthracene (7,12-DMBA) | 2W1 | PAH | Oxidation (activation) | 16551781 | (Wu et al. 2006) |
| 7,12-DMBA | 1B1 | PAH | Oxidation (low Km, high activity and efficiency, activation) | 11502724, 8674051, 9152602, 27123158 | (Chun and Kim 2016; Shimada et al. 1997b; Shimada et al. 1996; Shimada et al. 2001a) |
| 7,12-DMBA | 1B1 | PAH | Oxidation (activation) | 23432465, 27123158 | (Chun and Kim 2016; Shimada et al. 2013) |
| 7,12-DMBA | 1A1 | PAH | Oxidation (low Km, high activity and efficiency, activation) | 7955101, 8961944, 11502724, 10575002 | (Ciolino and Yeh 1999; Shimada et al. 1994; Shimada et al. 2001a; Shou et al. 1996a) |
| 7,12-DMBA | 1A1 | PAH | Oxidation (low Km, high activity and efficiency, activation) | 7955101, 8961944, 11502724, 9152602, 23432465 | (Shimada et al. 1994; Shimada et al. 1997b; Shimada et al. 2013; Shimada et al. 2001a; Shou et al. 1996a) |
| 7,12-DMBA | 1B1 | PAH | Oxidation (activation) | 8674051, 11502724 | (Shimada et al. 1996; Shimada et al. 2001a) |
| trans-7,12-DMBA-3,4-diol | 1A2 | PAH metabolite | Oxidation (weaker activation) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| trans-7,12-DMBA-3,4-diol | 2W1 | PAH metabolite | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| trans-7,12-DMBA-3,4-diol | 2A13 | PAH metabolite | Activation | 23432465 | (Shimada et al. 2013) |
| trans-7,12-DMBA-3,4-diol | 1A1 | PAH metabolite | 3,4-Dihydrodiol-1,2-epoxide formation (medium Km, high activity, high efficiency activation) | 11502724, 9152602, 11360624, 8989918, 9685642, 14720319 | (Guengerich and Shimada 1998; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shimada et al. 2001a; Shou et al. 1996b; Wu et al. 1997) |
| trans-7,12-DMBA-3,4-diol | 1B1 | PAH metabolite | 3,4-Dihydrodiol-1,2-epoxide formation (medium Km, high activity, high efficiency activation) | 8674051, 9721189, 11502724, 12584184, 9152602, 10426814, 23432465, 14720319 | (Buters et al. 2003; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shimada et al. 1996; Shimada et al. 2013; Shimada et al. 2001a; Shimada et al. 1999; Shimada et al. 1998) |
| 5,6-Dimethylchrysene-1,2-diol | 1A1 | PAH metabolite | Oxidation (activation) | 7955101, 8961944, 11502724, 9152602, 16946553, 14720319 | (Shimada 2006; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1994; Shimada et al. 1997b; Shimada et al. 2001a; Shou et al. 1996a) |
| trans-5,6-Dimethylchrysene-1,2-diol | 2W1 | PAH metabolite | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| trans-5,6-Dimethylchrysene-1,2-diol | 1A2 | PAH metabolite | Oxidation (activation) | 7955101, 8961944, 16946553, 14720319 | (Shimada 2006; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1994; Shou et al. 1996a) |
| trans-5,6-Dimethylchrysene-1,2-diol | 1B1 | PAH metabolite | Oxidation (activation) | 8674051, 9721189, 11502724, 12584184, 9152602, 10426814, 9685642, 14720319 | (Buters et al. 2003; Guengerich and Shimada 1998; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shimada et al. 1996; Shimada et al. 2001a; Shimada et al. 1999; Shimada et al. 1998) |
| N,N-Dimethylformamide (DMF) | 2E1 | Organic solvent | N-Demethylation (high activity, activation) | 1538706, 8477011, 11684354 | (Amato et al. 2001; Hyland et al. 1992; Mráz et al. 1993) |
| N-Hydroxymethyl, N-methylformamide, | 2E1 | Organic solvent, dimethylformamide metabolite | Demethylation, N- (high activity, activation) | 1538706, 8477011, 11684354 | (Amato et al. 2001; Hyland et al. 1992; Mráz et al. 1993) |
| 3-Methoxy-4-aminoazobenzene | 2W1 | Azoarylamine | Oxidation (activation) | 16379042 | (Turesky et al. 1998) |
| 3-Methylcholanthrene | 1A1 | PAH | Oxidation (activation) | 11360624, 14720319 | (Shimada and Fujii-Kuriyama 2004; Wu et al. 1997) |
| 5-Methylchrysene | 1A1 | PAH | Oxidation (medium Km, high activity, high efficiency, activation) | 7955101, 8961944, 11502724, 27123158 | (Chun and Kim 2016; Shimada et al. 1994; Shimada et al. 2001a; Shou et al. 1996a) |
| 3-Methylchrysene-1,2-diol | 1A1 | PAH metabolite | Activation | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| 3-Methylchrysene-1,2-diol | 1B1 | PAH metabolite | Activation | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| 3-Methylchrysene-1,2-diol | 1A2 | PAH metabolite | Activation (weaker) | 14720319 | (Shimada and Fujii-Kuriyama 2004) |
| trans-5-Methylchrysene-1,2-diol | 2W1 | PAH metabolite | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
| trans-5-Methylchrysene-1,2-diol | 2A6 | PAH metabolite | Oxidation (medium Km, high activity, high efficiency activation) | 23432465, 14720319 | (Shimada and Fujii-Kuriyama 2004; Shimada et al. 2013) |
| trans-5-Methylchrysene-1,2-diol | 2A13 | PAH metabolite | Oxidation (medium Km, high activity, high efficiency activation) | 23432465, 14720319 | (Shimada and Fujii-Kuriyama 2004; Shimada et al. 2013) |
| trans-5-Methylchrysene-1,2-diol | 1A1 | PAH metabolite | Oxidation (medium Km, high activity, high efficiency activation) | 7955101, 11502724, 9152602, 16485905, 16946553 | (Shimada 2006; Shimada et al. 1994; Shimada et al. 1997b; Shimada and Guengerich 2006; Shimada et al. 2001a) |
| trans-5-Methylchrysene-1,2-diol | 1B1 | PAH metabolite | Oxidation (medium Km, high activity, high efficiency activation) | 8674051, 9721189, 11502724, 12584184, 10426814, 9152602, 16485905, 9685642, 23432465, 14720319 | (Buters et al. 2003; Guengerich and Shimada 1998; Shimada and Fujii-Kuriyama 2004; Shimada et al. 1997b; Shimada and Guengerich 2006; Shimada et al. 1996; Shimada et al. 2013; Shimada et al. 2001a; Shimada et al. 1999; Shimada et al. 1998) |
| N-Nitrosodi-n-propylamine | 3A4 | Nitrosamine | Depropylation, N- (medium Km, high activity, medium efficiency, activation) | 10910959 | (Teiber and Hollenberg 2000) |
| Parathion | 2C9 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, medium activity | 17079358 | (Foxenberg et al. 2007) |
| Parathion | 1A1 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, low activity | 10414794, 21969518 | (Ellison et al. 2012; Mutch et al. 1999) |
| Parathion | 2A6 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation | 10794390, 12669189 | (Eaton 2000; Mutch et al. 2003) |
| Parathion | 3A5 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, medium Km, medium activity | 10794390, 12669189, 10414794, 17079358, 17110060 | (Buratti et al. 2006; Eaton 2000; Foxenberg et al. 2007; Mutch et al. 1999; Mutch et al. 2003) |
| Parathion | 2B6 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, low Km, medium to high activity | 10794390, 12669189, 10414794, 21782601, 12620367, 16757081, 17079358, 21969518 | (Buratti et al. 2002; Buratti et al. 2003; Eaton 2000; Ellison et al. 2012; Foxenberg et al. 2007; Mutch et al. 1999; Mutch et al. 2003; Mutch and Williams 2006) |
| Parathion | 1A2 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, low or medium Km, high activity | 10794390, 12669189, 21782601, 12620367, 16757081, 17079358, 21969518 | (Buratti et al. 2002; Buratti et al. 2003; Eaton 2000; Ellison et al. 2012; Foxenberg et al. 2007; Mutch et al. 2003; Mutch and Williams 2006) |
| Parathion | 2C19 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, low Km, medium activity | 10794390, 12669189, 21782601, 16757081, 17079358, 21969518 | (Buratti et al. 2002; Eaton 2000; Ellison et al. 2012; Foxenberg et al. 2007; Mutch et al. 2003; Mutch and Williams 2006) |
| Parathion | 2E1 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, very low activity | 10794390, 12669189,16757081 | (Eaton 2000; Mutch et al. 2003) |
| Parathion | 2D6 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation and 4-nitrophenol (p-nitrophenol) formation | 10794390, 12669189, 16757081, 10996483 | (Eaton 2000; Mutch et al. 2003; Mutch and Williams 2006; Sams et al. 2000) |
| Parathion | 3A7 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation | 17110060, 17079358 | (Buratti et al. 2006; Foxenberg et al. 2007) |
| Parathion | 2C8 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation | 21782601, 10794390, 12669189, 10414794, 16757081 | (Buratti et al. 2002; Eaton 2000; Mutch et al. 1999; Mutch et al. 2003; Mutch and Williams 2006) |
| Parathion | 3A4 | Insecticide, organophosphate and acaricide, dicarboximide | Desulfuration (oxon formation), activation, major enzyme, high Km, high activity | 9023313, 10794390, 12669189, 10414794, 21782601, 12620367, 17079358, 17110060, 10996483 | (Buratti et al. 2006; Buratti et al. 2002; Buratti et al. 2003; Butler and Murray 1997; Eaton 2000; Foxenberg et al. 2007; Mutch et al. 1999; Mutch et al. 2003; Sams et al. 2000) |
| 3-(N-Phenylamino)propane-1,2-diol (PAP) | 2C18 | Toxic oil compound | Hydroxylation (aromatic), high Km, high activity, activation | 17672514 | (Martínez-Cabot et al. 2007) |
| PAP | 2C8 | Toxic oil compound | Hydroxylation (aromatic), high Km, high activity, activation | 17672514 | (Martínez-Cabot et al. 2007) |
| PAP | 2C9 | Toxic oil compound | Hydroxylation (aromatic), high Km, high activity, activation | 17672514 | (Martínez-Cabot et al. 2007) |
| PAP | 2D6 | Toxic oil compound | Hydroxylation (aromatic), high Km, high activity, activation | 17672514 | (Martínez-Cabot et al. 2007) |
| 2,4,3´,5´-Tetramethoxystilbene | 1B1 | Cancer chemopreventive, trans-hydroxystilbene derivative | Oxidation (major enzyme, activation) | 16120791, 11719446 | (Chun et al. 2001; Chun et al. 2005) |
Fig 3.

Metabolic activation of benzo[a]pyrene to toxic trans-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro product
The following examples taken from Table 2 illustrate PAH compounds for which metabolic activation is attributed as “high activity reaction” and/or “high activation” (for references see Table 2):
P450 1A1: 5-Methylchrysene, trans-5-methylchrysene-1,2-diol, 7,12-dimethylbenz[a]anthracene (7,12-DMBA), trans-7,12-DMBA-3,4-diol, (±)-benzo[a]pyrene (B[a]P) −7,8-dihydrodiol, cis-(−)-B[a]P-7,8-dihydrodiol, trans-(+)-B[a]P-7,8-dihydrodiol, trans-(−)-B[a]P-7,8-dihydrodiol, dibenzo[a,l]pyrene (DB[a,l]P), trans-(−)-DB[a,l]P-(11R,12R)-diol
P4501A2: Dibenzo[a,l]pyrene (DB[a,l]P)
P4501B1: B[a]P, (+,−)-B[a]P-7,8-dihydrodiol, cis-(−)-B[a]P-7,8-dihydrodiol, trans-(+)-B[a]P-7,8-dihydrodiol, trans-5-Methylchrysene-1,2-diol, trans-7,12-DMBA-3,4-diol), dibenzo[a,l]pyrene (DB[a,l]P), trans-(−)-DB[a,l]P-(11R,12R)-diol
P450 2A13: trans-5-Methylchrysene-1,2-diol
P450 2A6: trans-5-Methylchrysene-1,2-diol
Heterocyclic and aromatic amines
Activation of heterocyclic, aromatic, and azoaromatic amines is represented by 58 cadsentries (Table 2) of which 15 are attributed as “high activity” and/or “high activation” catalyzed by P450 1A1, 1A2, 1B1, 2A13, 2A6, and 3A4. The reactions of activation or aromatic and heterocyclic amines are presented in Fig. 4 and Fig. 5 as illustrated by activation of 2-aminofluorene and MeIQx, respectively.
Fig 4.
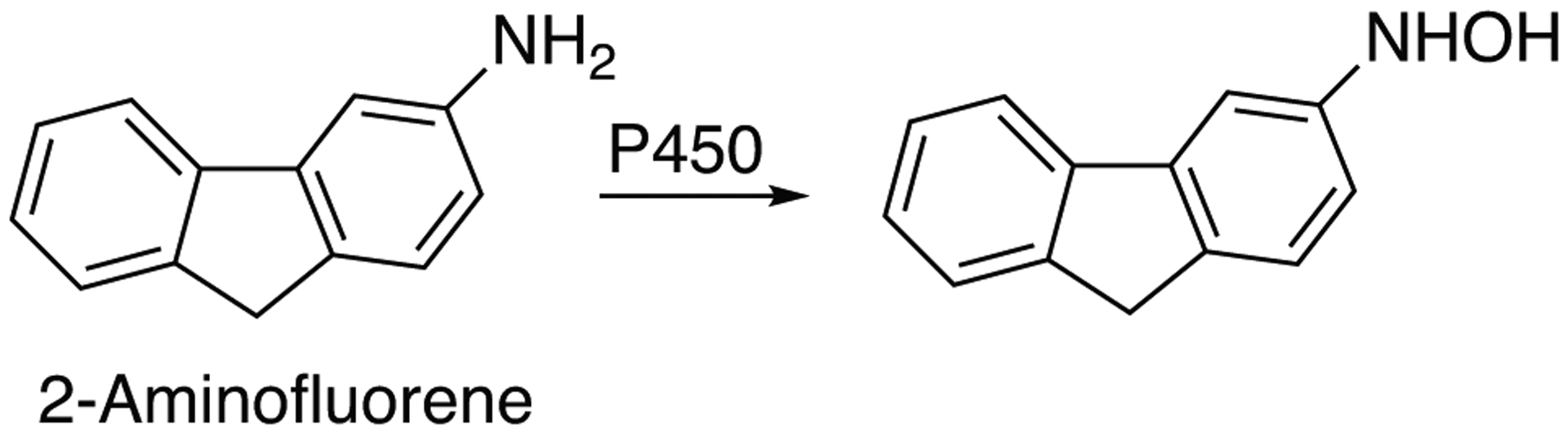
Activation of 2-amimnofluorene to toxic N-hydroxy product by P450 enzymes
Fig 5.
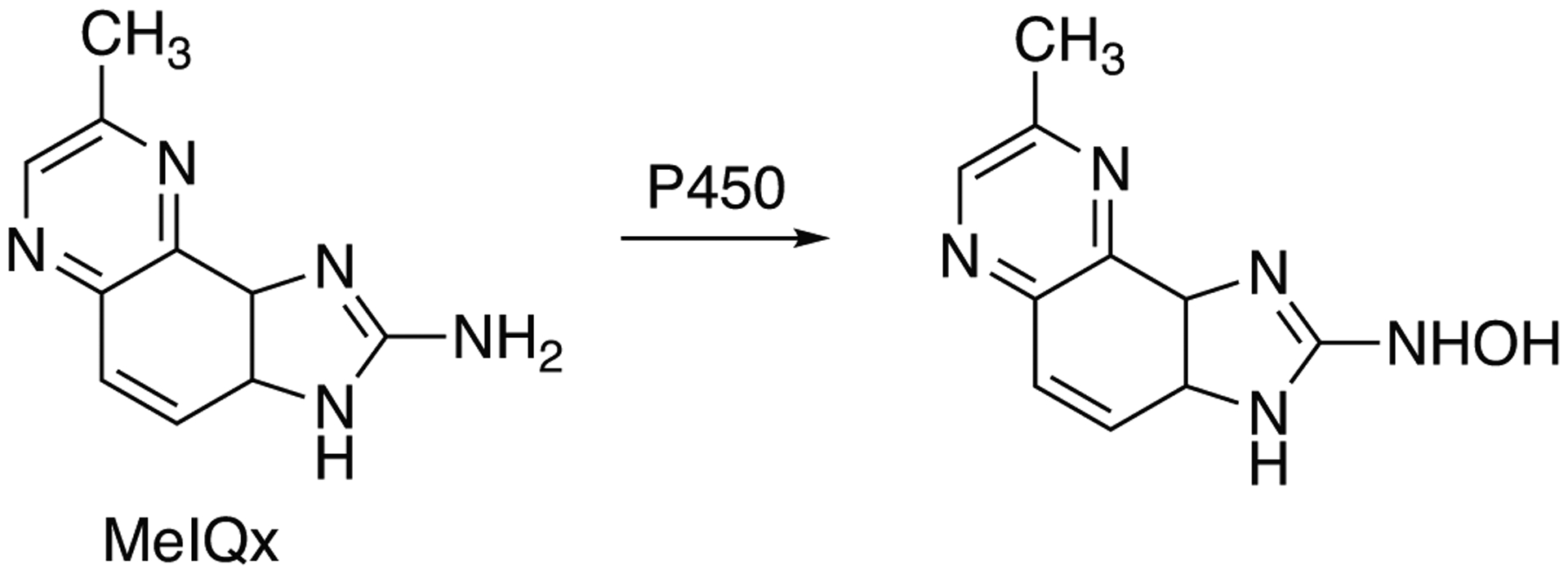
Activation of 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQx) to toxic N-hydroxy product by P450 enzymes
The following examples illustrate metabolic activation of heterocyclic compounds by specific P450s (Table 2 and references therein):
P450 1A2: 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-6-methyldipyrido[1,2-a,3,2’-d]-imidazole (Glu-P-1)
P450 1A1: 2-Aminoanthracene (2-AA), 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1), 6-aminochrysene
P450 1A2: 2-Aminofluorene (2-AF)
P450 1B1: 2-AA
P450 2A6: 2-AA
P450 2A13: 2-AF
P450 3A4: 3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1), 6-aminochrysene
Insecticides
Activation of organophosphate insecticides is represented by chlorpyrifos, diazinon, parathion, and azinphos-methyl (Table 2 and references therein). The compounds are metabolically activated to neurotoxic metabolites (i.e. oxon derivatives) by desulfuration reactions catalyzed by P450 enzymes. Chlorpyrifos (Fig. 7) and parathion (Fig. 8) are activated by P450 1A2, 2B6, 2D6, 2C8, 2C19, 3A4, and 3A5 enzymes, of which P450 2B6 is the most prominent at lower concentrations (20 μM) and having the highest kcat/Km value. In addition to the oxon derivative, chloropyrifos is also metabolized to the less toxic 3,5,6-trichloro-2-pyridinol by P450 3A4 (Jan et al. 2016; Crane et al. 2012a; Crane et al. 2012b; Croom et al. 2010; Mutch and Williams 2006).
Fig 7.

Activation of parathion to a toxic oxon product by P450 enzymes
Fig 8.
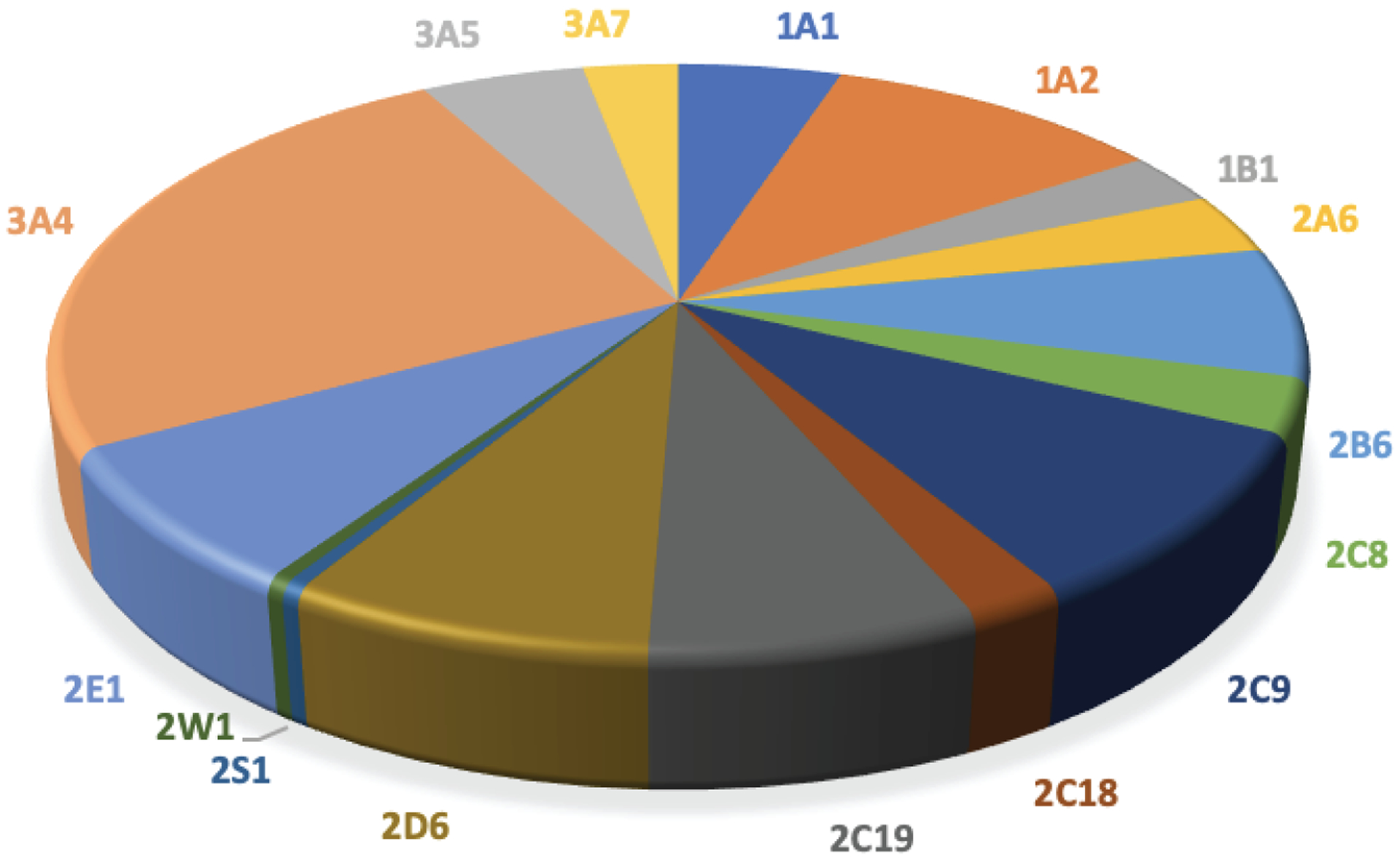
Participation of human P450 Families 1–4 in activation of drugs to potentially toxic products (4039 reactions, 235 activation reactions)
Azinphos-methyl is activated primarily by P450 1A2 (at low concentrations), and 2B6 and 3A4 (at higher concentrations) (Table 2) (Buratti et al. 2002; Buratti et al. 2003). The entries in Table 2 show that at lower concentrations organophosphates are activated predominately by P450 1A1, 1A2, 2B6, and 2C19 and at high concentrations by P450 3A4.
Drugs
A present and historical view of the activation of drugs and their conversion to reactive metabolites as substrates of P450 enzymes has been reviewed recently by one of the authors of the present paper. It has been pointed out that P450 metabolic activity often prevents drug toxicity (for instance making drug elimination faster), but on the opposite side it can, in some cases, result in conversion of drugs to reactive metabolites that cause toxicity (Guengerich 2020). The final properties of the products of drug-P450 enzyme reactions can also be significantly affected by factors such as: a) variations in the activity caused by genetic polymorphism and thus primarily on the level of single nucleotide variations (SNVs), or b) by enzyme induction and/or inhibition of activity by environmental chemicals or by co-administered drug(s) (Guengerich and Rendic 2010; Guengerich 2020). Examples of drugs that are converted to toxic metabolites, due to activity of P450 enzymes, are listed in Table 3. It must be emphasized that most drugs, used in recommended doses, are not or are only slightly toxic per se due to extensive testing in preclinical and clinical testing of drugs. However, as mentioned before, toxic metabolites might be formed under circumstances of enhanced dose, when applied with other drugs/chemicals that might redirect metabolism pathway to formation of toxic metabolites, or when genetic polymorphism of the particular enzyme was not tested or observed in early drug testing. It is prudent to remember the words of Paracelsus, paraphrased, “the dose makes the poison (only the dose distinguishes a medicine from a poison)” (Borzelleca 2000). Selected examples of drugs taken from Table 3 are discussed, for which toxicity is related to metabolic conversion to toxic products and is known to occur during clinical use. In addition, therapeutic compounds are presented that are used as pro-drugs. Such pro-drugs are therapeutically inactive until activated by P450 enzymes but can became also cytotoxic in healthy cells/tissues when used in therapy (e.g., the anticancer drugs cyclophosphamide, ifosfamide, and AQ4N (banoxantrone; 1,4-bis{[2-(dimethylamino)ethyl]amino}−5,8-hydroxy-anthracene-9,10-dione bis-N-oxide)). Also included is the natural product drug ellipticine, which is used in cancer therapy and activated to a cytotoxic metabolite (Table 3). However, while the inherent toxicity of a drug might be lowered, the metabolites formed might be also less toxic and less therapeutically active. An example of such a drug is trabectedin (ecteinascidin 743), an anti-cancer drug of marine origin for which the side effects include myelosuppression, hepatotoxicity, and nausea and vomiting (Held-Warmkessel 2003). Trabectedin is metabolized by P450 3A4 (major enzyme) and in addition by P450s 2C9, 2C19, 2D6, and 2E1. Metabolic and inhibition studies revealed that the metabolites formed are less cytotoxic and less therapeutically active than the parent drug. Inhibitors of P450 enzymes significantly increased cytotoxicity of the drug in a human cell line model system (Reid et al. 2002; Brandon et al. 2005; Brandon et al. 2006).
Table 3.
Examples of the metabolic activation of drugs by human cytochrome P450 enzymes
| Drug | P450 | Category | Reaction | PMIDs | References |
|---|---|---|---|---|---|
| Acetaminophen (paracetamol) | 3A4 | Analgesic, antipyretic | Oxidation to N-acetyl-p-benzoquinone imine (NABQI), activation, major at toxic concentrations | 9152386, 7956731, 8374050, 2729995, 8380689, 10741631, 10872641, 9633991, 9246016, 15576447, 17894464, 19219744 | (Cameron et al. 2007; Cheung et al. 2005b; Laine et al. 2009; Li et al. 1994; Manyike et al. 2000; Patten et al. 1993; Raucy et al. 1989; Roe et al. 1993; Sarich et al. 1997; Sinclair et al. 1998; Thummel et al. 2000; Zhou et al. 1997) |
| Acetaminophen (paracetamol) | 2E1 | Analgesic, antipyretic | Oxidation to NABQI, high Km, high activity, major in vivo enzyme, activation | 9152386, 8374050, 2729995, 8380689, 10741631, 10872641, 9633991, 9246016, 15576447, 9056059, 8354023, 15532721, 31024054, 19219744, 9548799, 11095574, 11866476 | (Bai and Cederbaum 2004; Chen et al. 1998; Cheung et al. 2005b; Dong et al. 2000; Hazai et al. 2002; Laine et al. 2009; Manyike et al. 2000; O’Shea et al. 1997; Patten et al. 1993; Rahman et al. 2019; Raucy et al. 1989; Roe et al. 1993; Sarich et al. 1997; Sinclair et al. 1998; Thummel et al. 2000; Zand et al. 1993; Zhou et al. 1997) |
| Acetaminophen (paracetamol) | 2D6 | Analgesic, antipyretic | Oxidation to NABQI, high Km, low to medium activity, activation | 9152386, 9548799, 11095574, 11866476, 19219744 | (Chen et al. 1998; Dong et al. 2000; Hazai et al. 2002; Laine et al. 2009; Zhou et al. 1997) |
| Acetaminophen (paracetamol) | 1A2 | Analgesic, antipyretic | Oxidation to NABQI, low to medium activity, activation | 9152386, 7956731, 8374050, 2729995, 8380689, 10741631, 10872641, 9633991, 9246016, 15576447, 19219744 | (Cheung et al. 2005b; Laine et al. 2009; Li et al. 1994; Manyike et al. 2000; Patten et al. 1993; Raucy et al. 1989; Roe et al. 1993; Sarich et al. 1997; Sinclair et al. 1998; Thummel et al. 2000; Zhou et al. 1997) |
| Acetaminophen (paracetamol) | 2A6 | Analgesic, antipyretic | Oxidation to NABQI, minor reaction, activation | 9548799, 11095574, 11866476, 19219744 | (Chen et al. 1998; Dong et al. 2000; Hazai et al. 2002; Laine et al. 2009) |
| Aminoflavone, NSC 68628 | 1A2 | Anticancer, antiproliferative, flavone derivative | Hydroxylation, N4’-, N-5, activation | 16775196, 12065765 | (Chen et al. 2006a; Kuffel et al. 2002) |
| Aminoflavone, NSC 68628 | 1A1 | Anticancer, antiproliferative, flavone derivative | Hydroxylation, N4’-, N5-, activation | 12065765, 15210858, 16775196, 12065765 | (Chen et al. 2006a; Kuffel et al. 2002; Loaiza-Pérez et al. 2004) |
| Aminoflavone, NSC 68628 | 2C9 | Anticancer, antiproliferative, flavone derivative | Hydroxylation, N5-, activation, | 16775196 | (Chen et al. 2006a) |
| Aminoflavone, NSC 68628 | 2C19 | Anticancer, antiproliferative, flavone derivative | Hydroxylation, N5- (activation, major enzyme at high conc.) | 16775196 | (Chen et al. 2006a) |
| Banoxantrone; 1,4-bis([2-(dimethylamino)ethyl]amino)-5,8-hydroxy-anthracene-9,10-dione bis-N-oxide (AQ4N) | 1A1 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 12214668, 16410820 | (Patterson 2002; Yakkundi et al. 2006) |
| AQ4N | 1A2 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 12214668 | (Patterson 2002) |
| AQ4N | 1B1 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 12214668 | (Patterson 2002) |
| AQ4N | 2B6 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 15712360, 16410820 | (McErlane et al. 2005; Yakkundi et al. 2006) |
| AQ4N | 3A4 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 9845092, 10834269, 12214668, 12489027 | (McCarthy et al. 2003; Patterson 2002; Patterson et al. 1999; Raleigh et al. 1998) |
| AQ4N | 2S1 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 20566689, 21430234 | (Nishida et al. 2010; Xiao et al. 2011) |
| AQ4N | 2W1 | Anticancer, anthraquinone prodrug | Reduction to AQ4, activation | 20566689 | (Nishida et al. 2010) |
| Carbamazepine | 1A2 | Antiepileptic | Activation | 11760814, 9630846 | (Masubuchi et al. 2001; Wolkenstein et al. 1998) |
| Carbamazepine | 3A4 | Antiepileptic | Activation | 9630846 | (Wolkenstein et al. 1998) |
| Carbamazepine, C2-hydroxy | 3A4 | Antiepileptic, carbamazepine metabolite | Iminostilbene formation (activation, major enzyme) | 16135660 | (Pearce et al. 2005) |
| Carbamazepine, C2-hydroxy | 2C19 | Antiepileptic, carbamazepine metabolite | Iminostilbene formation (activation, minor enzyme) | 16135660, 18463198 | (Pearce et al. 2008; Pearce et al. 2005) |
| Carbamazepine, C2-hydroxy | 2D6 | Antiepileptic, carbamazepine metabolite | Iminostilbene formation (activation, minor enzyme), at high concentration | 16135660 | (Pearce et al. 2005) |
| Carbamazepine, C3-hydroxy | 3A5 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation | 18463198 | (Pearce et al. 2008) |
| Carbamazepine, C3-hydroxy | 3A7 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation | 18463198 | (Pearce et al. 2008) |
| Carbamazepine, C3-hydroxy | 2C19 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation, high activity | 18463198 | (Pearce et al. 2008) |
| Carbamazepine, C3-hydroxy | 3A4 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation, major enzyme | 18463198 | (Pearce et al. 2008) |
| Carbamazepine, C3-hydroxy | 1A1 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation, minor enzyme | 18463198 | (Pearce et al. 2008) |
| Carbamazepine, C3-hydroxy | 1A2 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation, minor enzyme | 18463198 | (Pearce et al. 2008) |
| Carbamazepine, C3-hydroxy | 2C18 | Antiepileptic, carbamazepine metabolite | Hydroxylation, C2- (aromatic), activation, minor enzyme | 18463198 | (Pearce et al. 2008) |
| Clopidogrel | 2C9 | Antiplatelet, P2Y12 antagonist (thiophene) | Activation | 19812348 | (Kazui et al. 2010) |
| Clopidogrel | 2B6 | Antiplatelet, P2Y12 antagonist (thiophene) | Oxidation (2-oxo formation), low activity, or no activity, activation | 12485953, 19812348, 26654298 | (Clarke and Waskell 2003; Kazui et al. 2010; Zhai et al. 2016) |
| Clopidogrel | 3A4 | Antiplatelet, P2Y12 antagonist (thiophene) | Oxidation (2-oxo formation), major enzyme, activation | 12485953, 10331074, 23770199, 19812348 | (Clarke and Waskell 2003; Guengerich 1999; Kazui et al. 2010; Zahno et al. 2013) |
| Clopidogrel | 2C19 | Antiplatelet, P2Y12 antagonist (thiophene) | Oxidation (2-oxo formation), medium activity, activation | 17682072, 19812348, 26654298 | (Kazui et al. 2010; Walsky and Obach 2007; Zhai et al. 2016) |
| Clopidogrel | 1A2 | Antiplatelet, P2Y12 antagonist (thiophene) | Oxidation (2-oxo formation), very low to medium activity, activation | 12485953, 19812348 | (Clarke and Waskell 2003; Kazui et al. 2010) |
| Cyclophosphamide | 3A7 | Anticancer, alkylating, oxazaphosporine | N-Dechloroethylation (high Km, medium activity), activation to neuro- and nephrotoxic metabolite | 15919850 | (Chen et al. 2005) |
| Cyclophosphamide | 3A4 | Anticancer, alkylating, oxazaphosporine | N-Dechloroethylation (major enzyme, major reaction, high Km, high activity), activation to neuro- and nephrotoxic metabolite | 8242617, 9010702, 9331082, 10692561, 15919850 | (Bohnenstengel et al. 1996; Chang et al. 1993; Chen et al. 2005; Huang et al. 2000; Ren et al. 1997) |
| Cyclophosphamide | 3A4 | Anticancer, alkylating, oxazaphosporine | C4-Hydroxylation (high Km, high activity), activation to cytotoxic metabolite, major enzyme at high concentration | 8242617, 9010702, 9331082, 9157990, 10348794, 10991840, 10692561, 9923542 | (Bohnenstengel et al. 1996; Chang et al. 1993; Chang et al. 1997; Huang et al. 2000; Philip et al. 1999; Ren et al. 1997; Roy et al. 1999b; Zhou et al. 2000) |
| Cyclophosphamide | 3A7 | Anticancer, alkylating, oxazaphosporine | C4- Hydroxylation (high Km, low to high activity, major reaction), activation to cytotoxic metabolite | 10348794, 15919850;9157990 | (Chen et al. 2005; Roy et al. 1999b; Chang et al. 1997) |
| Cyclophosphamide | 2C18 | Anticancer, alkylating, oxazaphosporine | C4-Hydroxylation (low Km, activation to cytotoxic metabolite) | 8242617, 9010702, 9331082, 10348794, , 10692561, 9157990 | (Bohnenstengel et al. 1996; Chang et al. 1993; Chang et al. 1997; Huang et al. 2000; Ren et al. 1997; Roy 199b) |
| Cyclophosphamide | 2B6 | Anticancer, alkylating, oxazaphosporine | C4-Hydroxylation (major enzyme, major reaction, high Km, high activity, activation to cytotoxic metabolite), genetic polymorphism influence | 8242617, 9010702, 9331082, 9157990, 10348794, 10991840, 10692561, 15919850, 10471061, 17502835, 11360624 | (Bohnenstengel et al. 1996; Chang et al. 1993; Chang et al. 1997; Chen et al. 2005; Gervot et al. 1999; Huang et al. 2000; Nakajima et al. 2007; Ren et al. 1997; Roy et al. 1999b; Wu et al. 1997) |
| Dacarbazine | 1A1 | Anticancer, alkylating, imidazole carboxamide | N-Demethylation (major enzyme, activation) | 10473105, 27428168 | (Lewis et al. 2016; Reid et al. 1999) |
| Dacarbazine | 1A2 | Anticancer, alkylating, imidazole carboxamide | N-Demethylation (major enzyme, activation) | 10473105, 27428168 | (Lewis et al. 2016; Reid et al. 1999) |
| Dacarbazine | 2E1 | Anticancer, alkylating, imidazole carboxamide | N-Demethylation (activation, at higher concentration) | 10473105 | (Reid et al. 1999) |
| Dapsone | 2B6 | Antileprotic | Activation, minor contribution | 19998329 | (Ganesan et al. 2010) |
| Dapsone | 2D6 | Antileprotic | Activation, minor contribution | 19998329 | (Ganesan et al. 2010) |
| Dapsone | 3A4 | Antileprotic | N-Hydroxylation, (high Km, activation or no activity) | 7586950, 8703658, 8181193, 8742227, 1588928, 19998329 | (Fleming et al. 1992; Ganesan et al. 2010; Gill et al. 1995; Irshaid et al. 1996; May et al. 1994; Mitra et al. 1995) |
| Dapsone | 2C8 | Antileprotic | N-Hydroxylation, (high Km, minor enzyme), activation | 10901692, 19998329 | (Ganesan et al. 2010; Winter et al. 2000) |
| Dapsone | 2C19 | Antileprotic | N-Hydroxylation, (high Km, minor reaction), major enzyme for activation | 10901692 | (Winter et al. 2000) |
| Dapsone | 2E1 | Antileprotic | N-Hydroxylation, (low Km, activation), or no activity | 7586950, 8703658, 8181193, 8742227, 1588928, 10901692 | (Fleming et al. 1992; Gill et al. 1995; Irshaid et al. 1996; May et al. 1994; Mitra et al. 1995; Winter et al. 2000) |
| Dapsone | 2C18 | Antileprotic | N-Hydroxylation, (medium Km, minor reaction), activation | 10901692 | (Winter et al. 2000) |
| Dapsone | 2C9 | Antileprotic | N-Hydroxylation (medium Km, activation) | 7586950, 8703658, 10901692, 9521735, 12920490 | (Gill et al. 1995; Korzekwa et al. 1998; Li et al. 2003; Mitra et al. 1995; Winter et al. 2000) |
| Desogestrol | 3A4 | Estrogen, contraceptive | Oxygenation, mechanism-based inhibition | 2133086, 2256525 | {Guengerich, 1990, 12562; Guengerich, 1990, 13522} |
| Diclofenac | 2C9 | NSAID, cyclooxygenase-2 (COX-2) inhibitor | C4’-Hydroxylation, formation of 1´,4´-benzoquinoneimine (low or medium Km, medium or high activity, high efficiency, major enzyme, major reaction), activation | 9698079, 8417277, 10027801, 10027798, 10950847, 10572000, 10449188, 12464247, 27130197, 19022234 | (Bort et al. 1999; den Braver et al. 2016; Leemann et al. 1993; Mancy et al. 1999; Melet et al. 2003; Ngui et al. 2000; Shen et al. 1999; Tang et al. 1999; Yamazaki et al. 1998a) |
| Diclofenac | 3A4 | NSAID, COX-2 inhibitor | C5-Hydroxylation (major enzyme, high Km), reaction at high concentration (>100 μM), formation of proposed reactive intermediate | 8417277, 10027801, 10027798, 10950847, 10572000, 12438516, 12871048, 17584015, 19022234 | (Kalgutkar et al. 2007; Kumar et al. 2002; Lauer et al. 2009; Leemann et al. 1993; Mancy et al. 1999; Ngui et al. 2000; Shen et al. 1999; Tang 2003; Tang et al. 1999) |
| Diclofenac | 3A4 | NSAID, COX-2 inhibitor | C5-Hydroxylation, formation of 2,5-quinoneimine, activation | 27130197 | (den Braver et al. 2016) |
| Diclofenac 5-hydroxy | 2C9 | NSAID, COX-2 inhibitor | Activation, formation of 2,5-quinoneimine, activation | 27130197 | (den Braver et al. 2016) |
| Ellipticine | 2B6 | Anticancer, topoisomerase II inhibitor | C12-Hydroxylation, weak activation | 11755121, 12123750, 22917556 | (Frei et al. 2002; Stiborová et al. 2001a; Stiborová et al. 2012b) |
| Ellipticine | 3A4 | Anticancer, topoisomerase II inhibitor | C13- and C12-hydroxylation, activation (major enzyme) | 11755121, 12123750, 15548707, 20027146, 22917556 | (Frei et al. 2002; Martinkova et al. 2009:Stiborová, 2012b; Stiborová et al. 2001a; Stiborová et al. 2004) |
| Ellipticine | 2C9 | Anticancer, topoisomerase II inhibitor | C13- and C12-hydroxylation, activation, low activity | 11755121, 12123750, 15548707, 22917556 | (Frei et al. 2002; Stiborová et al. 2001a; Stiborová et al. 2012b; Stiborová et al. 2004) |
| Ellipticine | 2D6 | Anticancer, topoisomerase II inhibitor | C13- and C12-hydroxylation, activation, low activity | 11755121, 12123750, 15548707, 22917556 | (Frei et al. 2002; Stiborová et al. 2001a; Stiborová et al. 2012b; Stiborová et al. 2004) |
| Ellipticine | 2E1 | Anticancer, topoisomerase II inhibitor | C13- and C12-hydroxylation, activation, low activity | 11755121, 12123750, 22917556 | (Frei et al. 2002; Stiborová et al. 2001a; Stiborová et al. 2012b) |
| Ellipticine | 2C19 | Anticancer, topoisomerase II inhibitor | C13- and C12-hydroxylation, activation, low activity | 11755121, 12123750, 15548707, 22917556 | (Frei et al. 2002; Stiborová et al. 2001a; Stiborová et al. 2012b; Stiborová et al. 2004) |
| Ellipticine | 1A1 | Anticancer, topoisomerase II inhibitor | C13-Hydroxylation, activation | 11755121, 12123750, 15548707, 22917556, 20027146 | (Frei et al. 2002; Martinkova et al. 2009; Stiborová et al. 2001a; Stiborová et al. 2012b; Stiborová et al. 2004) |
| Ellipticine | 1A2 | Anticancer, topoisomerase II inhibitor | C13-Hydroxylation, activation, low activity | 11755121, 12123750, 15548707, 22917556 | (Frei et al. 2002; Stiborová et al. 2001a; Stiborová et al. 2012b; Stiborová et al. 2004) |
| Ellipticine | 1B1 | Anticancer, topoisomerase II inhibitor | C13-Hydroxylation, activation, low activity | 11755121, 12123750, 22917556, 20027146 | (Frei et al. 2002; Martinkova et al. 2009; Stiborová et al. 2001a; Stiborová et al. 2012b; Stiborová et al. 2004) |
| 17α-Ethynylestradiol | 3A4 | Estrogen, contraceptive | Epoxidation (activation) | 16251255 | (Chen et al. 2006b) |
| 17α-Ethynylestradiol | 3A4 | Estrogen, contraceptive | Oxygenation, mechanism-based inhibition | 17251390, 17584015, 11907170, 3285175, 2133086, 2256525 | (Guengerich 1988; Guengerich 1990a; Guengerich 1990b; Kalgutkar et al. 2007; Lin and Hollenberg 2007; Lin et al. 2002) |
| 17α-Ethynylestradiol | 3A5 | Estrogen, Contraceptive | Oxygenation, mechanism-based inhibition | 17251390, 17584015 | (Kalgutkar et al. 2007; Lin and Hollenberg 2007) |
| Flutamide | 2C9 | Anticancer, antiandrogen, nonsteroidal, nitroaromatic | C2-Hydroxylation, activation | 16507648 | (Goda et al. 2006) |
| Flutamide | 1B1 | Anticancer, antiandrogen, nonsteroidal, nitroaromatic | C2-Hydroxylation (activation, major enzyme in cancer cells) | 11160641 | (Rochat et al. 2001) |
| Flutamide | 1A2 | Anticancer, antiandrogen, nonsteroidal, nitroaromatic | C2-Hydroxylation (activation, major enzyme) | 8386241, 16507648, 9351907, 12052211, 18411402 | (Berson et al. 1993; Goda et al. 2006; Kang et al. 2008; Patterson and Murray 2002; Shet et al. 1997) |
| Flutamide | 1A2 | Anticancer, antiandrogen, nonsteroidal, nitroaromatic | Amide oxidation, N-hydroxylation following amide cleavage | 17403914, 18411402, 16507648 | (Kang et al. 2007; Kang et al. 2008; Goda et al. 2006) |
| Flutamide | 2C19 | Anticancer, antiandrogen, nonsteroidal, nitroaromatic | Amide oxidation, N-hydroxylation following amide cleavage | 17403914, 18411402, 16507648 | (Kang et al. 2007; Kang et al. 2008; Goda et al. 2006) |
| Flutamide | 3A4 | Anticancer, antiandrogen, nonsteroidal, nitroaromatic | Amide oxidation, N-hydroxylation following amide cleavage | 16507648 | (Kang et al. 2007) |
| Gestodene | 3A4 | Estrogen, contraceptive | Oxygenation, mechanism-based inhibition, hydroxylation | 2133086, 2256525, 8664172 | (Guengerich 1990a; Guengerich 1990b; Ward and Back 1993) |
| Haloperidol | 1A1 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 11717183, 16841959 | (Avent et al. 2006; Fang et al. 2001) |
| Haloperidol | 1B1 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 16841959 | (Avent et al. 2006) |
| Haloperidol | 2D6 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 9140699, 9844810 | (Fang et al. 1997; Usuki et al. 1998) |
| Haloperidol | 3A5 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 9140699, 9844810, 9431831, 10628896, 12584149, 11167668, 16841959 | (Avent et al. 2006; Fang et al. 1997; Kalgutkar et al. 2003; Kudo and Ishizaki 1999; Pan et al. 1997; Shin et al. 2001; Usuki et al. 1998) |
| Haloperidol | 3A7 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 16841959 | (Avent et al. 2006) |
| Haloperidol | 3A4 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 9140699, 9844810, 9431831, 10628896, 12584149, 11167668, 11717183, 24913773 | (Fang et al. 1997; Fang et al. 2001; Kalgutkar et al. 2003; Kudo and Ishizaki 1999; Kurth et al. 2014; Pan et al. 1997; Shin et al. 2001; Usuki et al. 1998) |
| Haloperidol | 3A4 | Antipsychotic, butyrophenone | Oxidation, pyridinium metabolite formation, activation | 9431831, 10628896, 12584149, 11167668, 11717183, 24913773 | (Fang et al. 2001; Kalgutkar et al. 2003; Kudo and Ishizaki 1999; Kurth et al. 2014; Pan et al. 1997; Shin et al. 2001) |
| Halothane | 2E1 | General inhalation anesthetic, haloalkane | Dehalogenation, oxidative, activation (major reaction, major enzyme in vivo, low Km) | 9616199, 9103523, 8886607, 10805064, 11506127, 11684364, 17584015, 24913773 | (Kalgutkar et al. 2007; Kharasch et al. 2000; Kurth et al. 2014; Minoda and Kharasch 2001; Spracklin et al. 1997; Spracklin and Kharasch 1998; Spracklin et al. 1996; White and De Matteis 2001) |
| Halothane | 2A6 | General inhalation anesthetic, haloalkane | Dehalogenation, oxidative, activation (minor reaction, high Km) | 9616199, 9103523, 8886607, 10805064, 11506127, 24913773 | (Kalgutkar et al. 2007; Kharasch et al. 2000; Kurth et al. 2014; Spracklin et al. 1997; Spracklin and Kharasch 1998; Spracklin et al. 1996) |
| Halothane | 3A4 | General inhalation anesthetic, haloalkane | Dehalogenation, reductive, activation (high Km) | 9616199, 9103523, 8886607, 10805064, 11506127, 24913773 | (Kalgutkar et al. 2007; Kharasch et al. 2000; Kurth et al. 2014; Spracklin et al. 1997; Spracklin and Kharasch 1998; Spracklin et al. 1996) |
| Halothane | 2A6 | General inhalation anesthetic, haloalkane | Dehalogenation, reductive, activation (low Km) | 9616199, 9103523, 8886607, 10805064, 11506127, 24913773 | (Driscoll et al. 2007; Kharasch et al. 2000; Kurth et al. 2014; Spracklin et al. 1997; Spracklin and Kharasch 1998; Spracklin et al. 1996) |
| Halothane | 2B6 | General inhalation anesthetic, haloalkane | Oxidation, activation | 11684364, 24913773 | (Kalgutkar et al. 2007; White and De Matteis 2001) |
| Halothane | 2E1 | General inhalation anesthetic, haloalkane | Oxidation, activation | 11684364, 24913773 | (Kalgutkar et al. 2007; White and De Matteis 2001) |
| Ifosfamide, (R)- | 3A5 | Anticancer, alkylating, oxazaphosporine | Hydroxylation, C4, activation to cytotoxic product | 10348794, 15821045, 10534317, 16854777 | (Lu et al. 2006; McCune et al. 2005; Roy et al. 1999a) |
| Ifosfamide, (R)-, (S)- | 3A4 | Anticancer, alkylating, oxazaphosporine | Dechloroethylation, N2-, N3- (high Km, high activity, major enzyme, major reaction for (S)-), activation to neuro- and nephrotoxic metabolites | 8242617, 10692561, 15919850, 15821045, 16854777, 10534317, 8161344, 8071856, 10101149, 9923542, 17464949 | (Chang et al. 1993; Chen et al. 2005; Chugh et al. 2007; Granvil et al. 1999; Huang et al. 2000; Lu et al. 2006; McCune et al. 2005; Murray et al. 1994; Philip et al. 1999; Roy et al. 1999a; Walker et al. 1994) |
| Ifosfamide, (R)-, (S)- | 2B6 | Anticancer, alkylating, oxazaphosporine | Dechloroethylation, N2-, N3- (high Km, high activity, major enzyme, major reaction), activation to neuro- and nephrotoxic metabolites. | 10692561, 15919850, 15821045, 16854777, 10534317 | (Chen et al. 2005; Huang et al. 2000; Lu et al. 2006; McCune et al. 2005; Roy et al. 1999a) |
| Ifosfamide, (R)-, (S)- | 2C9 | Anticancer, alkylating, oxazaphosporine | Hydroxylation, C4- (low Km), activation to cytotoxic metabolites | 8242617, 9157990 | (Chang et al. 1993; Chang et al. 1997) |
| Ifosfamide, (S)- | 2B6 | Anticancer, alkylating, oxazaphosporine | Hydroxylation, C4- (high Km, medium (R-) and high (S-) activity, major enzyme), activation to cytotoxic metabolites | 8242617, 10348794, 10692561, 9157990, 15919850, 25934575 | (Calinski et al. 2015; Chang et al. 1993; Chang et al. 1997; Huang et al. 2000; Roy et al. 1999a) |
| Isoniazid | 2E1 | Antituberculotic, pyridine | Oxidation, activation, major enzyme | 12668988, 18071298 | (Huang et al. 2003; Shen et al. 2008) |
| 3-Ketodesogestrol | 3A4 | Estrogen, contraceptive | Oxygenation, mechanism-based inhibition | 2133086, 2256525 | (Guengerich 1990a; Guengerich 1990b) |
| Levonorgestrol | 3A4 | Estrogen, contraceptive | Oxygenation, mechanism-based inhibition | 2133086, 2256525 | (Guengerich 1990a; Guengerich 1990b) |
| Norethisterone | 3A4 | Estrogen, contraceptive | Oxygenation, mechanism-based inhibition | 2133086, 2256525 | (Guengerich 1990a; Guengerich 1990b) |
| Phencyclidine | 2B6 | Hallucinogenic (angel dust, angel hair, angel mist), analgesic and anesthetic | C-Hydroxylation, (p-hydroxy and quinone methide formation), activation | 17892269, 16326815, 16782764 | (Driscoll et al. 2007; Jushchyshyn et al. 2006; Shebley et al. 2006) |
| Phencyclidine | 2C19 | Hallucinogenic (angel dust, angel hair, angel mist), analgesic and anesthetic | C-Hydroxylation (p-hydroxy and quinone methide formation), activation | 17892269, 16326815, 16782764 | (Driscoll et al. 2007; Jushchyshyn et al. 2006; Shebley et al. 2006) |
| Raloxifene | 2D6 | Anticancer and prevention of osteoporosis, antiestrogen, estrogen receptor modulator, benzothiophene | Oxidation, activation, minor enzyme | 12119000 | (Chen et al. 2002) |
| Raloxifene | 3A4 | Anticancer and prevention of osteoporosis, antiestrogen, estrogen receptor modulator, benzothiophene | 3´-Hydroxylation, oxidation, glutathione (GSH) adduct formation, activation | 12119000, 18052110, 17497897, 17584015, 20405834 | (Baer et al. 2007; Chen et al. 2002; Hollenberg et al. 2008; Kalgutkar et al. 2007; Moore et al. 2010a) |
| Raloxifene | 3A4 | Anticancer and prevention of osteoporosis, antiestrogen, estrogen receptor modulator, benzothiophene | Dehydrogenation, activation, reactive diquinonemethide and 7-hydroxyraloxifene formation | 20405834, 20812728, 17867646, 12119000 | (Chen et al. 2002; Moore et al. 2010a; Moore et al. 2010b; Yukinaga et al. 2007) |
| Tamoxifen | 1A1 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | Hydroxylation, C3-, C4- (catechol formation, activation) | 10348797, 8293548, 12018981, 12124303, 12419838, 12623757 | (Boocock et al. 2002; Crewe et al. 2002; Dehal and Kupfer 1999; Hu et al. 2003; Notley et al. 2002; Styles et al. 1994) |
| Tamoxifen | 3A5 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | Hydroxylation, C3-, C4- (catechol formation, activation) | 10348797, 8293548, 12018981, 12124303, 12419838, 12623757 | (Boocock et al. 2002; Crewe et al. 2002; Dehal and Kupfer 1999; Hu et al. 2003; Notley et al. 2002; Styles et al. 1994) |
| Tamoxifen | 3A5 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | Cα-Hydroxylation, activation, at high concentration (250 μM) | 15159443, 16533026 | (Desta et al. 2004; Notley et al. 2005) |
| Tamoxifen | 3A4 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | Cα-Hydroxylation (major enzyme, activation), at low and high concentrations (18 and 250 μM) | 10348797, 8293548, 12018981, 12124303, 12419838, 12623757, 16533026, 15159443, 14678348, 12971802 | (Boocock et al. 2002; Coller et al. 2004; Crewe et al. 2002; Dehal and Kupfer 1999; Desta et al. 2004; Hu et al. 2003; Kim et al. 2003; Notley et al. 2005; Notley et al. 2002; Styles et al. 1994) |
| Tamoxifen | 2B6 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | Oxidation, activation | 12061800, 7748182 | (Stiborová et al. 2002; White et al. 1995) |
| Tamoxifen | 3A4 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | Oxidation, activation | 7748182 | (White et al. 1995) |
| Tamoxifen, 3-hydroxy (droloxifene) | 2D6 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | C4-Hydroxylation, (catechol form., low extent, activation) | 10348797 | (Dehal and Kupfer 1999) |
| Tamoxifen 3-hydroxy, (droloxifene) | 3A4 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine | C4-Hydroxylation, (catechol formation, major reaction, activation) | 10348797 | (Dehal and Kupfer 1999) |
| Tamoxifen, 4-hydroxy | 3A4 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine, tamoxifen metabolite | N-Demethylation, activation, formation of major antiestrogenic metabolite | 15159443 | (Desta et al. 2004) |
| Tamoxifen, 4-hydroxy | 2D6 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine, tamoxifen metabolite | C3-Hydroxylation (catechol formation, low extent, activation) | 10348797 | (Dehal and Kupfer 1999) |
| Tamoxifen, 4-hydroxy | 3A4 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine, tamoxifen metabolite | C3-Hydroxylation (catechol formation, major reaction, activation) | 10348797, 15159443 | (Dehal and Kupfer 1999; Desta et al. 2004) |
| Tamoxifen, N-desmethyl | 2D6 | Anticancer, antiestrogen, estrogen receptor modulator, triphenylethyleneamine, tamoxifen metabolite | C4-Hydroxylation (major enzyme, activation), formation of major antiestrogenic metabolite, genetic polymorphism influences clinical response, proposed clinically significant drug-drug interaction potential when inhibited | 14652237, 15159443, 15111773, 16815318, 17518364, 23711794, 30536272, 29637493, 26423799 | (Beverage et al. 2007; Bezerra et al. 2018; Borges et al. 2006; Desta et al. 2004; Hansten 2018; Johnson et al. 2004; Stearns et al. 2003; Watanabe et al. 2015; Yang et al. 2013) |
| Thiabendazole | 1A1 | Antifungal, benzimidazole | C5-Hydroxylation, activation, | 12920490 | (Li et al. 2003) |
| Thiabendazole | 1A2 | Antifungal, benzimidazole | C5-Hydroxylation (activation, major enzyme) | 10936227, 9565779, 12920490 | (Coulet et al. 1998; Coulet et al. 2000; Li et al. 2003) |
| Thiabendazole | 1B1 | Antifungal, benzimidazole | C5-Hydroxylation, activation, | 12920490 | (Li et al. 2003) |
| Tienilic acid | 2C9 | Diuretic and uricosuric, 2-aroylthiophene | Epoxidation (thiophene ring), activation, proposed reactive intermediate formation in mechanism-based inhibition process | 8286335, 8075377, 17584015, 8477697 | (Kalgutkar et al. 2007; Lecoeur et al. 1994; Lopez Garcia et al. 1993; López-Garcia et al. 1994) |
| Tienilic acid | 2C9 | Diuretic and uricosuric, 2-aroylthiophene | Oxidation, S-oxygenation (thiophene ring), activation, proposed reactive intermediate formation in mechanism-based inhibition process | 17584015 | (Kalgutkar et al. 2007) |
| Tolcapone, acetylamine | 1A2 | Dopaminergic, catechol-O-methyltransferase (COMT) inhibitor, tolcapone metabolite | Oxidation, activation, major enzyme | 10806608, 12588182 | (Jorga et al. 2000; Smith et al. 2003b) |
| Tolcapone, amine | 1A2 | Dopaminergic, COMT inhibitor, tolcapone metabolite | Oxidation, activation, major enzyme | 10806608, 12588182 | (Jorga et al. 2000; Smith et al. 2003b) |
| Tolmetin | 3A4 | Analgesic, anti-inflammatory | Epoxidation, activation | 16251255 | (Chen et al. 2006b) |
| Toremifene | 3A4 | Antiestrogen, estrogen receptor modulator, triphenylethylene | Cα-Hydroxylation, activation | 12971802, 26423799 | (Kim et al. 2003; Watanabe et al. 2015) |
| Toremifene, N-desmethyl | 2C9 | Antiestrogen, estrogen receptor modulator, triphenylethylene | 4-Hydroxylation, endoxifen formation, activation | 26423799 | (Watanabe et al. 2015) |
| Toremifene, N-desmethyl | 2D6 | Antiestrogen, estrogen receptor modulator, triphenylethylene | 4-Hydroxylation, activation | 26423799 | (Watanabe et al. 2015) |
| N,Ń,N´´-Triethylene thiophosphoramide (ThioTEPA) | 3A4 | Anticancer, alkylating agent, aziridine | Desulfuration, N,Ń,N´´-triethylene phosphoramide (TEPA) formation (major enzyme, activation) | 12107550, 19076156 | (Ekhart et al. 2009; Jacobson et al. 2002) |
| ThioTEPA | 2B6 | Anticancer, alkylating agent, aziridine | Desulfuration, TEPA formation (minor enzyme, activation) | 12107550, 15121764, 17584015, 19076156 | (Ekhart et al. 2009; Harleton et al. 2004; Jacobson et al. 2002; Kalgutkar et al. 2007) |
| ThioTEPA | 1B1 | Anticancer, alkylating agent, aziridine | Desulfuration, TEPA formation (minor enzyme, activation) | 15121764 | (Harleton et al. 2004) |
| Troglitazone | 1A2 | Peroxisome proliferation activation receptor (PPAR)-γ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 15155556 | (He et al. 2004a) |
| Troglitazone | 2C8 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 10534310 | (Yamazaki et al. 1999) |
| Troglitazone | 2C19 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 15155556 | (He et al. 2004a) |
| Troglitazone | 2D6 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 15155556 | (He et al. 2004a) |
| Troglitazone | 2E1 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 15155556 | (He et al. 2004a) |
| Troglitazone | 3A4 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 15155556, 11511170, 11389877, 10534310, 11170509 | (He et al. 2004a; He et al. 2001; Kassahun et al. 2001; Tettey et al. 2001; Yamazaki et al. 1999) |
| Troglitazone | 3A5 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring, activation | 15155556 | (He et al. 2004a) |
| Troglitazone | 2C8 | PPARγ agonist, thiazolidinedione, glitazone | Oxidation of thiazolidinedione ring (activation, major enzyme) | 15155556 | (He et al. 2004a) |
| Valproic acid | 2A6 | Antiepileptic, histone deacetylase (HDAC) inhibitor | Dehydrogenation (desaturation, 4-ene formation, activation), minor enzyme | 16945988, 10461547, 9353388 | (Ekins and Wrighton 1999; Kiang et al. 2006; Sadeque et al. 1997) |
| Valproic acid | 2C9 | Antiepileptic, HDAC inhibitor | Dehydrogenation (desaturation, 4-ene formation, activation), major enzyme | 16945988, 10461547, 9353388, 14597963 | (Ekins and Wrighton 1999; Ho et al. 2003; Kiang et al. 2006; Sadeque et al. 1997) |
| Valproic acid | 2B6 | Antiepileptic, HDAC inhibitor | Dehydrogenation (desaturation, 4-ene formation, activation), minor enzyme | 16945988, 10461547 | (Ekins and Wrighton 1999; Kiang et al. 2006) |
The numbers of activation reactions of drugs as substrates of human P450 enzymes are presented in Fig. 8, calculated from our records. Of the total of 4,039 reactions, 235 (~ 6%) involve activation and formation of potentially toxic intermediates or metabolites. P450 3A4 clearly dominated in the formation of toxic metabolites compared with other P450s, catalyzing ~25% of the bioactivation reactions.
The following examples illustrate the participation of P450 enzymes in the bioactivation of selected drugs (Table 3).
AQ4N
AQ4N, an aliphatic amine di-N-oxide, is a potent topoisomerase II inhibitor and in clinical trials as a potential anticancer drug. It is inactive until enzymatically bioactivated to an active amine under the reductive conditions present in hypoxic tumor cells (Fig. 9) (Patterson 1993; Lalani et al. 2007; O’Rourke et al. 2008). Because the amine AQ4 is very toxic to normal cells, it is not inherently suitable for delivery as an anticancer drug. AQ4N is reported to be substrate of several P450s (i.e. 1A1, 1A2, 1B1, 2B6, 2W1, 2S1, and 3A4) (Table 3 and references therein), but most efficiently by P450s 1A1 and 2B6 (Yakkundi et al. 2006)). Under reducing oxygen conditions (hypoxia) AQ4N is reduced to the cytotoxic AQ4-mono-N-oxide (AQ4M) and amine (AQ4) (Patterson et al. 1999). Of the enzymes involved in the metabolism of AQN4, P450 2W1 is highly expressed in some human colon and adrenal tumors and was suggested as tumor-specific enzyme. In addition, strong expression of P450 2S1 has been reported in tumors of epithelial origin and hypoxic tumors and the gene was found to be overexpressed under hypoxic conditions (Karlgren et al. 2005; Saarikoski et al. 2005; Rivera et al. 2007; Nishida et al. 2010; Xiao et al. 2011).
Fig 9.

Activation of AQ4N by P450 enzymes
Cyclophosphamide and ifosfamide
Cyclophosphamide and ifosfamide are widely used anticancer agents that require metabolic activation by P450 enzymes (Figs. 10 and 11, respectively). While 4-hydroxylation yields DNA-alkylating and cytotoxic metabolites, N-dechloroethylation results in the generation of neuro- and nephrotoxic products. Cyclophosphamide and ifosfamide undergo extensive P450-catalyzed metabolism to yield both active (4-hydroxylated) and therapeutically inactive but neurotoxic N-dechloroethyl metabolites, and ovarian toxicity is a major concern with cyclophosphamide therapy. The human liver microsomal P450 metabolism of cyclophosphamide and ifosfamide 4-hydroxylation is well characterized (Table 3 and references therein). Of all P450 enzymes, P450 3A4 exhibited the highest N-dechloroethylation activity (bioactivation) toward both cyclophosphamide and ifosfamide, whereas P450 2B6 and P450 3A4 displayed high N-dechloroethylation activity toward ifosfamide but not cyclophosphamide. With cyclophosphamide as substrate, P450 3A4 was shown to catalyze ≥ 95% of liver microsomal N-dechloroethylation, whereas with ifosfamide as substrate, P450 3A4 catalyzed an average of ~70% of liver microsomal N-dechloroethylation (range 40–90%), with the balance of this activity catalyzed by P450 2B6 (range 10–70%, depending on the P450 2B6 content of the liver) (Huang and Waxman 2000). In the case of cyclophosphamide treatment, determination of selected P450 enzyme genotypes (such as frequencies of the variant alleles CYP2B6*5, CYP2C19*2, CYP2C9*2, and CYP3A5*3) has been proposed for predicting the risk of premature ovarian failure in lupus nephritis patients (Takada et al. 2004).
Fig 10.
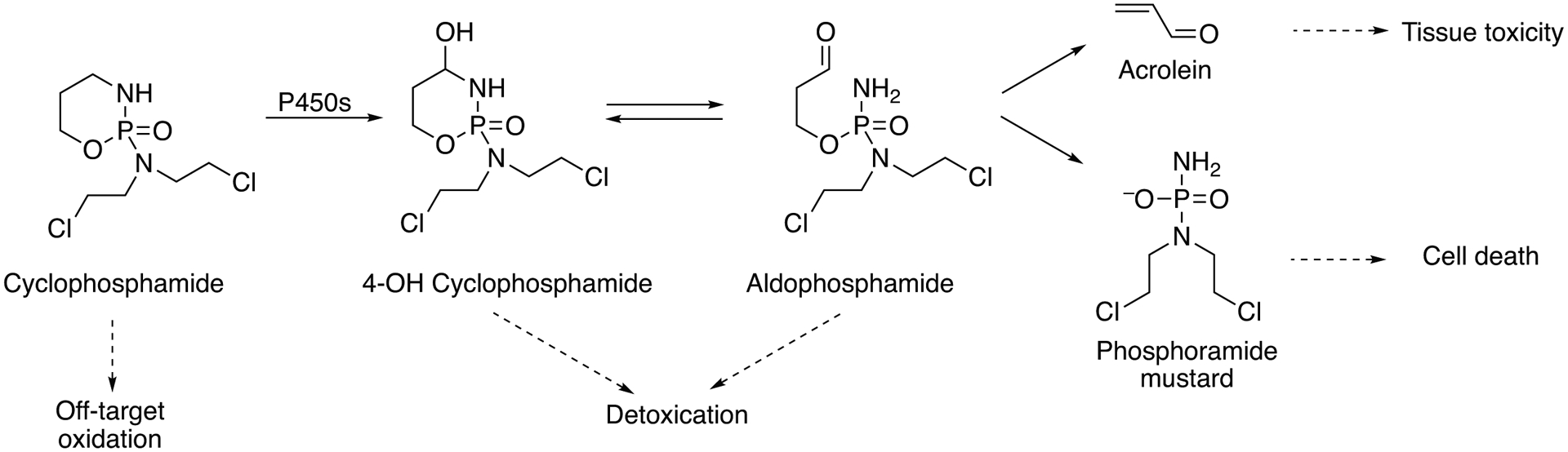
Metabolic activation of CPA
Fig 11.
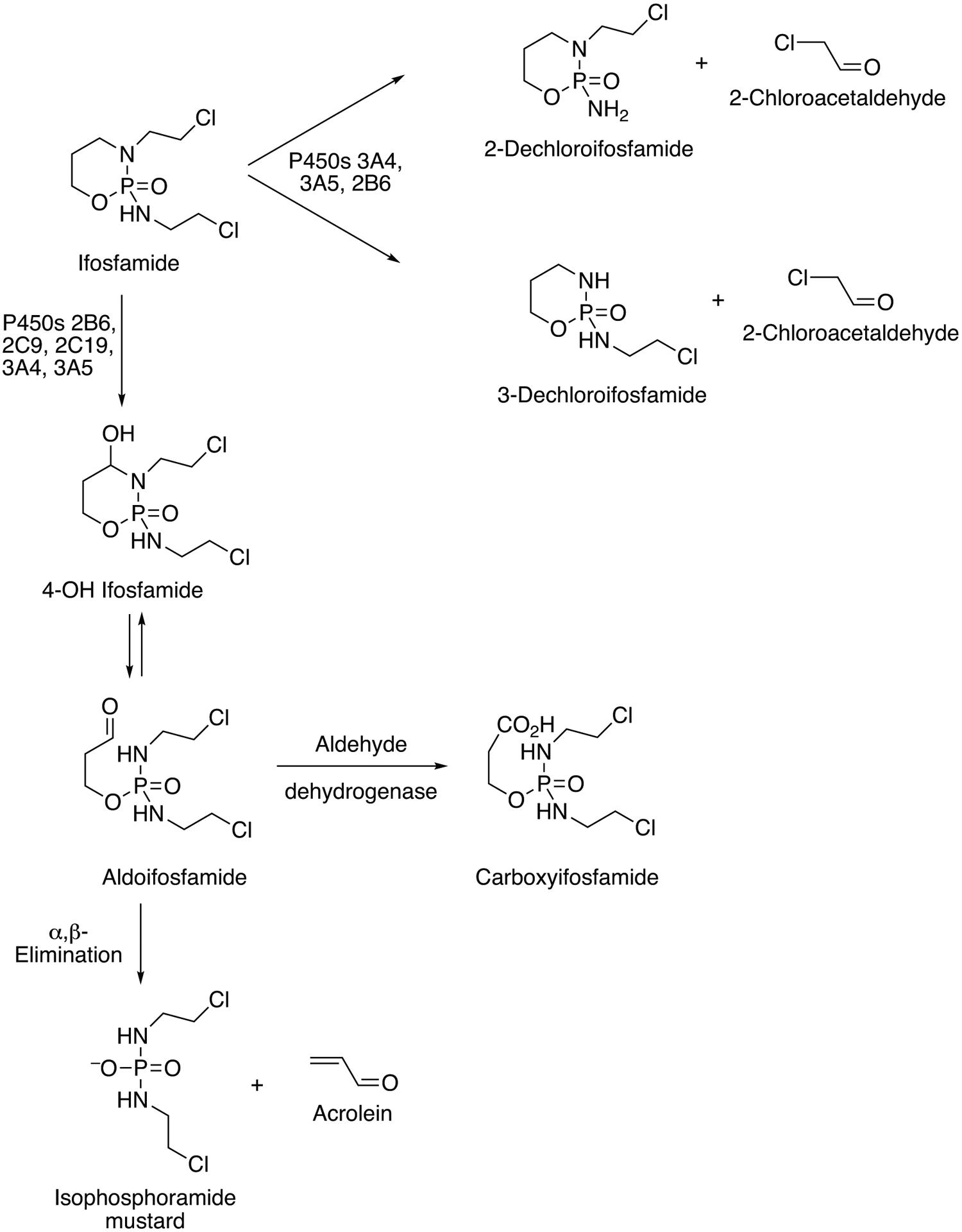
Metabolic activation of IFA
Acetaminophen
Acetaminophen (paracetamol, Tylenol®) is one of the most widely used drugs in the world. It is very safe when used at therapeutic doses. However, it is also involved in ~ ½ of the cases of drug-induced liver failure and well exemplifies the axiom of Paracelsus about the “the dose makes the poison” (Larson et al. 2005). When overdosed, the drug causes mitochondrial dysfunction and centrilobular necrosis in the liver in humans and experimental animals (Yoon et al. 2016). Normally most of the ingested drug is eliminated by glucuronidation and sulfation, catalyzed by UDP-glucuronosyltransferases (UGT1A1 and 1A6) and sulfotransferases (SULT1A1, 1A3/4, and 1E1), respectively, producing nontoxic conjugates (McGill and Jaeschke 2013).
The key mechanism in the hepatotoxicity is P450-catalyzed formation of the reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which depletes hepatic glutathione and accumulates to cause hepatocellular liver damage, including oxidative stress (Fig. 13). Approximately 5% to 9% of orally administered acetaminophen is metabolized by P450 2E1, 1A2, and 3A4 catalyzed oxidation reactions (Table 3 and references therein). At a toxic concentration the formation of the NAPQI-glutathione conjugate was highest with P450 3A4, having the lowest Km value of 130 μM (for comparison therapeutic concentrations of paracetamol are ~50 μM and toxic concentrations are ~1 mM) followed by P450 2E1 and P450 1A2 (Patten et al. 1993)). It has been proposed that human P450 3A4 is the major enzyme catalyzing acetaminophen oxidation to NAPQI (Laine et al. 2009). Other studies using inhibition studies with human recombinant enzymes indicated that P450 1A2 is the enzyme responsible for acetaminophen metabolic activation (Tan et al. 2008). In mice, the deletion of P450 2e1 or 1a2 blocks acetaminophen toxicity (Lee et al. 1996; Zaher et al. 1998).
Fig 13.

Metabolic activation of halothane
Halothane
Halothane was previously the most widely used anesthetic agent and in 1963 was reported to cause liver necrosis in humans. It was shown that halothane liver necrosis was induced following pretreatment of rats with polychlorinated biphenyls, known inducers of P450 enzymes. The liver necrosis caused by halothane anesthesia could be prevented by administration of metyrapone, a rather non-selective inhibitor of P450 enzymes, prior to anesthesia. These findings indicated that halothane toxicity resulted from metabolic activation of halothane by P450 enzymes (Nastainczyk et al. 1982). In addition, halothane hepatotoxicity could be potentiated in rats by chronic administration of ethanol, an inducer of P450 2E1 (Takagi et al. 1983). Human P450s activate halothane by both reductive and oxidative metabolism (Table 3, Fig. 14), and oxidative dehalogenation by P450 2E1 is a major in vivo reaction with low Km values (and also results in lipid peroxidation). Limited participation of P450 2A6 activation has been indicated in vivo. Reductive activation of halothane is catalyzed by P450 2A6 and 3A4 enzymes (Table 3 and references therein). Halothane oxidation, the major metabolic pathway, leads to the production of the reactive electrophile trifluoroacetyl chloride, and subsequent acylation of liver proteins results in the formation of trifluoroacetylated protein neoantigens. Metabolic halothane reduction leads to the formation of the 2-chloro-1,1,1-trifluoroethyl radical by hemolytic cleavage of the C-Br bond (Fig. 13) (Kurth et al. 2014). Halothane has been replaced in most of countries by other, less toxic, inhalation anesthetics due to its induced hepatitis, but there is still the possibility that it is in use in some developing countries.
Fig 14.
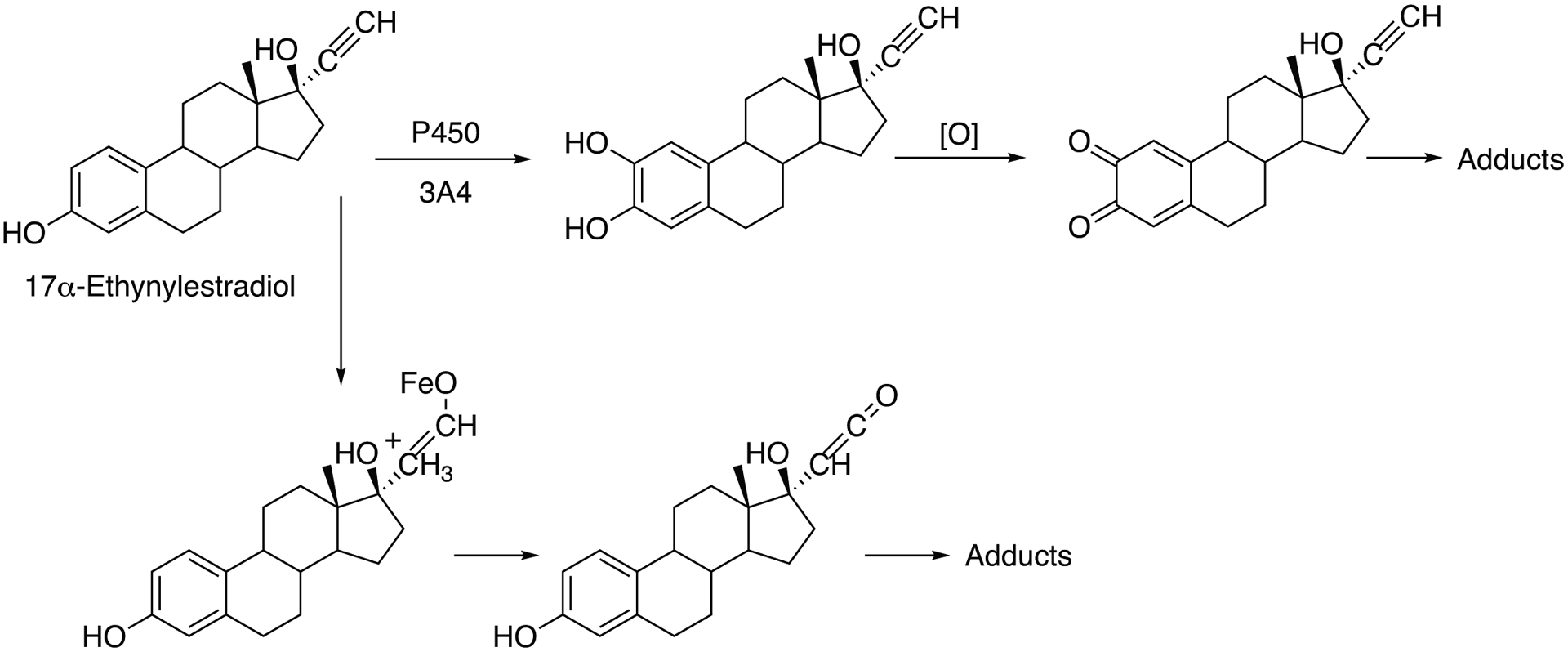
Metabolic activation of 17α-ethynylestradiol
17α-Ethynylestradiol
17α-Ethynylestradiol is in use as the estrogenic component of oral contraceptives (Bolt 1979). Similar to natural estrogens (see the Physiological Compounds section, vide infra) (Lacassagne 1932), 17α-ethynylestradiol is a weak carcinogen in rats, and carcinogenic activity has been associated with the formation of catechol metabolites (Fig. 14) (Zhu et al. 1993). C2-hydroxylation catalyzed by P450 enzymes (mainly P450 3A4) was found to be the main metabolic pathway of 17α-ethynylestradiol in individuals using oral contraceptives (Guengerich 1988)). This metabolite is excreted as 2-methoxyethynylestradiol after O-methylation (Back et al. 1984). Induction of P450 3A4 by rifampicin, barbiturates, or herbal remedies such as St. John’s wort can lead to increased clearance and unplanned pregnancy (Bolt et al. 1977; Guengerich 1988).
Natural products
Natural products, including herbal supplements, can have multiple effects on activity of P450 enzymes, for instance inhibition or induction of activity and/or their expression (St. John’s wort, vide supra). By changing activity and/or expression of the enzymes and applied concomitantly with drugs, natural chemicals can provoke drug-phytochemical interactions. Such activity might result in altered therapeutic and or toxic properties of drugs (Guengerich and Rendic 2010; Rendic and Guengerich 2010). Examples of different classes of natural compounds that can be activated to toxic metabolites by cytochrome P450s (e.g., alkaloids, monoterpenes, mycotoxins, N-nitrosamines) are presented in Table 4. Natural products, as substrates of P450 enzymes, can be both activated to toxic and detoxicated to nontoxic products by P450 enzymes in different ways. For instance, aflatoxin B1 (AFB1) is activated to toxic and detoxicated to nontoxic metabolites by oxidative reactions, while aristolochic acid is activated by nitro reduction under (partially anaerobic conditions), and oxidative metabolism results in the formation of a nontoxic O-demethylated product. Estragole and safrole are examples in which metabolism by P450 enzymes to nontoxic metabolites can be followed by activation to toxic metabolites by conjugation to form a sulfate ester (Table 4 and references therein).
Table 4.
Examples of the metabolic activation of natural products by human cytochrome P450 enzymes
| Natural product | P450 | Category | Reaction | Reference PMED IDs | Reference No. |
|---|---|---|---|---|---|
| Aflatoxin B1 (AFB1) | 2A13 | Mycotoxin, Aspergillus species | Hydroxylation (AFM1 formation) and epoxidation 8,9- (low activity, activation) | 16385575, 30454686, 22743290 | (Deng et al. 2018; He et al. 2006; Yang et al. 2012) |
| AFB1 | 2W1 | Mycotoxin, Aspergillus species | Oxidation, activation | 16379042 | (Brandon et al. 2006) |
| AFB1 | 2B6 | Mycotoxin, Aspergillus species | Epoxidation 8,9- (low activity, activation) | 8597154, 9280407, 11360624 | (Code et al. 1997; Neal 1995; Wu et al. 1997) |
| AFB1 | 2A6 | Mycotoxin, Aspergillus species | Epoxidation, exo-8,9-, activation | 8082563, 1944238, 16385575, 11189750 | (Gonzalez and Gelboin 1994; He et al. 2006; Lewis et al. 1999; Yun et al. 1991) |
| AFB1 | 3A5 | Mycotoxin, Aspergillus species | Epoxidation, exo-C8,9- (activation, a major enzyme at higher concentration), medium Km, medium activity | 9385444, 9730826, 7893152, 16608170, 30454686, 11189750 | (Deng et al. 2018; Gillam et al. 1995; Kamdem et al. 2006; Kim et al. 1997; Lewis et al. 1999; Wang et al. 1998) |
| AFB1 | 2E1 | Mycotoxin, Aspergillus species | Oxidation (weak activation) | 11189750 | (Lewis et al. 1999) |
| AFB1 | 3A4 | Mycotoxin, Aspergillus species | Epoxidation, exo-8,9- (activation), major enzyme, medium Km and activity | 8261428, 7766804, 12079611, 1902334, 9385444, 11782366, 9730826, 11368545, 16608170, 9003190, 30454686, 8975785, 11497333, 1643250, 11189750 | (Deng et al. 2018; Gallagher et al. 1996; Gallagher et al. 1994; Kamdem et al. 2006; Kim et al. 1997; Lewis et al. 1999; Ramsdell et al. 1991; Raney et al. 1992; Shimada and Guengerich 1989; Ueng et al. 1997; Ueng et al. 1995; Van Vleet et al. 2001; Van Vleet et al. 2002a; Van Vleet et al. 2002b; Wang et al. 1998; Xue et al. 2001) |
| AFB1 | 1A2 | Mycotoxin, of Aspergillus species | Epoxidation, exo-8,9- (activation), and endo-8,9- (detoxication), medium Km and activity | 8261428, 7766804, 12079611, 1902334, 11782366, 16385575, 16608170, 30454686, 8975785, 11497333, 11189750 | (Deng et al. 2018; Gallagher et al. 1996; Gallagher et al. 1994; He et al. 2006; Kamdem et al. 2006; Lewis et al. 1999; Ramsdell et al. 1991; Shimada and Guengerich 1989; Ueng et al. 1995; Van Vleet et al. 2001; Van Vleet et al. 2002a; Van Vleet et al. 2002b) |
| AFB1 | 3A7 | Mycotoxin, Aspergillus species | Epoxidation, exo-8,9- (activation, major enzyme at higher concentration), medium Km, medium activity | 30454686 | (Deng et al. 2018) |
| Aflatoxin G1 (AFG1) | 2A13 | Mycotoxin, Aspergillus species | Oxidation, activation | 23907605 | (Zhang et al. 2013) |
| AFG1 | 1A2 | Mycotoxin, Aspergillus species | Oxidation, activation | 11189750 | (Lewis et al. 1999) |
| AFG1 | 3A4 | Mycotoxin, Aspergillus species | Oxidation, activation | 8082563, 8261428, 7766804, 12079611, 1902334, 352361, 12849689, 11189750 | (Buening et al. 1978; Gallagher et al. 1994; Gonzalez and Gelboin 1994; Lewis et al. 1999; Ramsdell et al. 1991; Sabater Vilar et al. 2003; Ueng et al. 1995; Van Vleet et al. 2002a) |
| Aristolochic acid I | 1A1 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | Nitroreduction, reductive activation, N-hydroxyaristolactam formation | 11511187, 15386410, 23701164, 26593908, 24152141, 22086975, 23353840, 26861298 | (Jerabek et al. 2012; Milichovský et al. 2016; Stiborová et al. 2015; Stiborová et al. 2014; Stiborová et al. 2005; Stiborová et al. 2001b; Stiborová et al. 2012a; Stiborová et al. 2013) |
| Aristolochic acid I | 1A2 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | Nitroreduction, reductive activation, N-hydroxyaristolactam (high activity) | 11511187, 15386410, 23701164, 26593908, 24152141, 22086975, 23353840, 26861298 | (Jerabek et al. 2012; Milichovský et al. 2016; Stiborová et al. 2015; Stiborová et al. 2014; Stiborová et al. 2005; Stiborová et al. 2001b; Stiborová et al. 2012a; Stiborová et al. 2013) |
| Aristolochic acid I | 1A1 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | O-Demethylation, detoxication | 26593908, 22086975 | (Stiborová et al. 2015; Stiborová et al. 2012a) |
| Aristolochic acid I | 1A2 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | O-Demethylation, detoxication | 26593908, 22086975 | (Stiborová et al. 2015; Stiborová et al. 2012a) |
| Aristolochic acid I | 2C9 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | O-Demethylation, detoxication | 26593908 | (Stiborová et al. 2015) |
| Aristolochic acid I | 3A4 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | O-Demethylation, detoxication | 26593908 | (Stiborová et al. 2015) |
| Aristolochic acid II | 1A1 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | Nitroreduction, reductive activation | 11511187, 15386410 | (Stiborová et al. 2005; Stiborová et al. 2001b) |
| Aristolochic acid II | 1A2 | Nephrotoxin and carcinogen, from the plant Aristolochia fangchi and other species and several Asarum species | Nitroreduction, reductive activation (high activity) | 11511187, 15386410 | (Stiborová et al. 2005; Stiborová et al. 2001b) |
| Benzophenone | 1A1 | Muscat grape and mango compound, flavoring substance, ultraviolet protection compound | Benzhydrol and 4-hydroxybezophenone formation, activation | 12160905 | (Takemoto et al. 2002) |
| Benzophenone | 1A2 | Muscat grape and mango compound, flavoring substance, ultraviolet protection compound | Benzhydrol and 4-hydroxybezophenone formation, activation | 12160905 | (Takemoto et al. 2002) |
| Benzophenone | 1B1 | Muscat grape and mango compound, flavoring substance, ultraviolet protection compound | Benzhydrol and 4-hydroxybezophenone formation, activation | 12160905 | (Takemoto et al. 2002) |
| Benzophenone | 2A6 | Muscat grape and mango compound, flavoring substance, ultraviolet protection compound | Benzhydrol and 4-hydroxybezophenone formation, activation | 12160905 | (Takemoto et al. 2002) |
| Curcumin | 2D6 | Coloring agent, yellow pigment from Curcuma longa, chemopreventive | O-Demethylation, activation | 12220536 | (Sakano and Kawanishi 2002) |
| Curcumin | 1A1 | Coloring agent, yellow pigment from Curcuma longa, chemopreventive | O-Demethylation, activation | 12220536 | (Sakano and Kawanishi 2002) |
| Curcumin | 1A2 | Coloring agent, yellow pigment from Curcuma longa, chemopreventive | O-Demethylation, activation | 12220536 | (Sakano and Kawanishi 2002) |
| Δ3-Carene | 1A2 | Bicyclic monoterpene | Epoxidation (high Km, medium activity, activation) | 16379671 | (Duisken et al. 2005) |
| Diallyl sulfone | 2E1 | Garlic oil compound, organosulfur | Oxidation (diallyl sulfoxide and diallyl sulfone formation, activation) | 11062148, 16510538, 11238812 | (Black et al. 2006; Forkert et al. 2000; Yang et al. 2001) |
| Ecteinascidin 743, trabectedin (ET-743) | 3A4 | Marine compound, tetrahydroisoquinoline | Oxidation, low Km, major enzyme, activation | 12231541,16162970, 16379042 | (Reid et al. 2002; Brandon et al. 2005; Brandon et al. 2006) |
| Ecteinascidin 743, trabectedin (ET-743) | 2C9 | Marine compound, tetrahydroisoquinoline | Oxidation, activation | 12231541,16162970, 16379042 | (Reid et al. 2002; Brandon et al. 2005; Brandon et al. 2006) |
| Ecteinascidin 743, trabectedin (ET-743) | 2C19 | Marine compound, tetrahydroisoquinoline | Oxidation, activation | 16162970, 16379042 | (Brandon et al. 2005; Brandon et al. 2006) |
| Ecteinascidin 743, trabectedin (ET-743) | 2E1 | Marine compound, tetrahydroisoquinoline | Oxidation, activation | 12231541,16162970, 16379042 | (Reid et al. 2002; Brandon et al. 2005; Brandon et al. 2006) |
| Ecteinascidin 743, trabectedin (ET-743) | 2D6 | Marine compound, tetrahydroisoquinoline | Oxidation, activation | 12231541,16162970, 16379042 | (Reid et al. 2002;Brandon et al. 2005; Brandon et al. 2006) |
| Estragole | 2A6 | Alkenylbenzene | C1´-Hydroxylation (major enzyme, medium Km, medium activity), activation after sulfation | 17407329 | (Jeurissen et al. 2007) |
| Estragole | 2C19 | Alkenylbenzene | C1´-Hydroxylation (minor enzyme, medium Km, medium activity), activation after sulfation | 17407329 | (Jeurissen et al. 2007) |
| Estragole | 1A2 | Alkenylbenzene | C1´-Hydroxylation, activation after sulfation, high Km, medium activity) | 17407329 | (Jeurissen et al. 2007) |
| Estragole | 2D6 | Alkenylbenzene | C1´-Hydroxylation, activation after sulfation, at high concentrations | 17407329 | (Jeurissen et al. 2007) |
| Estragole | 2E1 | Alkenylbenzene | C1´-Hydroxylation, activation after sulfation, at high concentrations | 17407329 | (Jeurissen et al. 2007) |
| Ethanol | 2C19 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km, high activity | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 1A1 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 1B1 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 2B6 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 2D6 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 2C8 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 2C9 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 2J2 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 4A11 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997 | (Hamitouche et al. 2006) |
| Ethanol | 1A2 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997, 10446146, 9368031, 8627510, 9884161 | (Asai et al. 1996; Bell and Guengerich 1997; Bell-Parikh and Guengerich 1999; Hamitouche et al. 2006) |
| Ethanol | 2E1 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997, 8313838, 10446146, 9368031, 8627510, 7687464, 17084997, 9143349, 30362088 | (Asai et al. 1996; Dai et al. 1993; Hamitouche et al. 2006; Guengerich and Avadhani 2018; Yang and Cederbaum 1997) |
| Ethanol | 3A4 | Organic solvent | Oxidation (acetaldehyde formation), activation, high Km (~10 mM), high activity; in vivo after long-term treatment or at high doses | 17084997, 8571359, 10976571, 10446146, 9368031, 8627510, 9884161 | (Asai et al. 1996; Bell and Guengerich 1997; Bell-Parikh and Guengerich 1999; Hamitouche et al. 2006; Novak and Woodcroft 2000; Raucy 1995; Salmela et al. 1998) |
| Ethyl carbamate (urethane) | 2E1 | Carbamic acid derivative, fermentation byproduct | Oxidation to vinyl carbamate epoxide, activation | 1664256, 1912327 | (Guengerich and Kim 1991; Guengerich et al. 1991) |
| 4-Ipomeanol | 1A2 | Pulmonary toxin, alkylating, from Fusarium solani | Oxidation, activation, major enzyme | 1651809, 15892579 | (Baer et al. 2005; Czerwinski et al. 1991) |
| 4-Ipomeanol | 2C19 | Pulmonary toxin, alkylating, from F. solani | Oxidation, activation, major enzyme | 15892579 | (Baer et al. 2005) |
| 4-Ipomeanol | 2D6 | Pulmonary toxin, alkylating, from F. solani | Oxidation, activation | 15892579 | (Baer et al. 2005) |
| 4-Ipomeanol | 2E1 | Pulmonary toxin, alkylating, from F. solani | Oxidation, activation | 15892579 | (Baer et al. 2005) |
| 4-Ipomeanol | 2F1 | Pulmonary toxin, alkylating, from F. solani | Oxidation, activation | 1651809 | (Czerwinski et al. 1991) |
| 4-Ipomeanol | 3A4 | Pulmonary toxin, alkylating, from F. solani | Epoxidation, activation | 14967002, 17584015 | (Alvarez-Diez and Zheng 2004; Kalgutkar et al. 2007) |
| 4-Ipomeanol | 3A4 | Pulmonary toxin, alkylating, from F. solani | Oxidation, activation, minor enzyme | 1651809, 15892579 | (Baer et al. 2005; Czerwinski et al. 1991) |
| 4-Ipomeanol | 4B1 | Pulmonary toxin, alkylating, from F. solani | Oxidation, activation | 1651809, 23748241, 27092941, 30409834 | (Czerwinski et al. 1991; Roellecke et al. 2016; Teitelbaum et al. 2019) |
| 3-Methylindole (skatole) | 1A2 | Pulmonary toxin | Dehydrogenation (3-methyleneindolenine formation, low Km, high activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 2A6 | Pulmonary toxin | Epoxidation (3-methyloxindole formation, at high concentration, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 1A2 | Pulmonary toxin | Epoxidation (3-methyloxindole formation, low Km, high activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 1B1 | Pulmonary toxin | Epoxidation (3-methyloxindole form., low Km, high activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 1A1 | Pulmonary toxin | Epoxidation (3-methyloxindole form., low Km, medium activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 2E1 | Pulmonary toxin | Epoxidation (3-methyloxindole form., low Km, medium activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 1A2 | Pulmonary toxin | Hydroxylation, C- (indole-3-carbinol formation, low Km, high activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 1A1 | Pulmonary toxin | Hydroxylation, C- (indole-3-carbinol formation, low Km, medium activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 1B1 | Pulmonary toxin | Hydroxylation, C- (indole-3-carbinol formation, low Km, medium activity, high efficiency, activation) | 8558432, 11408359 | (Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 2F1 | Pulmonary toxin | Dehydrogenation (desaturation, 3-methyleneindolenine formation, low Km, medium activity, high efficiency and activation) | 8558432, 11408359, 10383923, 17962375 | (Kartha and Yost 2008; Lanza et al. 1999; Lanza and Yost 2001; Thornton-Manning et al. 1996) |
| 3-Methylindole (skatole) | 2F1 | Pulmonary toxin | Dehydrogenation (desaturation, 3-methyleneindolenine formation, high activation) | 8558432, 11408359, 10383923, 17962375, 20795680, 20187624 | (Kartha and Yost 2008; Lanza et al. 1999; Lanza and Yost 2001; Thornton-Manning et al. 1996; Weems et al. 2010; Weems and Yost 2010) |
| 3-Methylindole (skatole) | 1A1 | Pulmonary toxin | Dehydrogenation (desaturation, 3-methyleneindolenine form., low Km, medium activity, high efficiency, activation) | 8558432, 11408359, 20795680, 20187624 | (Lanza and Yost 2001; Thornton-Manning et al. 1996; Weems et al. 2010; Weems and Yost 2010) |
| 3-Methylindole (skatole) | 2A13 | Pulmonary toxin | Dehydrogenation (desaturation, 3-methyleneindolenine formation, activation) | 8558432, 11408359, 20795680, 20187624 | (Lanza and Yost 2001; Thornton-Manning et al. 1996; Weems et al. 2010; Weems and Yost 2010) |
| 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) | 2E1 | Tobacco-specific nitrosamine | Activation | 19156262 | (Krishnan et al. 2009) |
| NNK | 1B1 | Tobacco-specific nitrosamine | Activation (low activation) | 19156262 | (Krishnan et al. 2009) |
| NNK | 2A13 | Tobacco-specific nitrosamine | Hydroxylation, Cα-methylene (keto aldehyde formation), activation, medium Km, medium activity, or high activity | 11016631, 12975327, 15333516, 15528319, 15962925, 12130698 | (Bao et al. 2005; He et al. 2004b; Jalas et al. 2003; Su et al. 2000; Wong et al. 2005b; Zhang et al. 2002) |
| NNK | 2A13 | Tobacco-specific nitrosamine | Hydroxylation, Cα-methyl (keto alcohol), major enzyme for activation, medium Km, medium activity, or high activity | 11016631, 12975327, 15333516, 15528319, 15962925, 12130698, 17671098, 21473878, 23917075 | (Bao et al. 2005; Chiang et al. 2011; He et al. 2004b; Jalas et al. 2003; Megaraj et al. 2014; Su et al. 2000; Wong et al. 2005b; Zhang et al. 2007; Zhang et al. 2002) |
| NNK | 2B6 | Tobacco-specific nitrosamine | Hydroxylation, α-methyl (keto alcohol formation), activation, major reaction | 11360624, 12920169, 16174803, 9280407, 1312898, 8806763, 9106248, 8485585 | (Code et al. 1997; Crespi et al. 1997; Dicke et al. 2005; Patten et al. 1996; Penman et al. 1993; Smith et al. 2003a; Smith et al. 1992; Wu et al. 1997) |
| NNK | 2F1 | Tobacco-specific nitrosamine | Hydroxylation, α-methyl (keto alcohol formation), activation | 1312898, 8806763 | (Patten et al. 1996; Smith et al. 1992) |
| NNK | 2A6 | Tobacco-specific nitrosamine | Hydroxylation, Cα-methyl (keto alcohol and ketoaldehyde formation), high Km, low activity, minor reaction, weak activation | 1312898, 8806763, 1423839, 9106248, 9280407, 8485585, 10837014, 11600130, 12920169, 11016631, 11080669, 14668073, 15333516, 16364922, 21473878 | (Chiang et al. 2011; Code et al. 1997; Crespi et al. 1997; Fujita and Kamataki 2001b; He et al. 2004b; Kushida et al. 2000; Patten et al. 1996; Penman et al. 1993; Sellers et al. 2003; Smith et al. 2003a; Smith et al. 1992; Su et al. 2000; von Weymarn et al. 2006; Yamazaki et al. 1992) |
| NNK | 2E1 | Tobacco-specific nitrosamine | Hydroxylation, Cα-methylene (keto aldehyde formation), high Km, low activity, minor reaction, activation | 1312898, 8806763, 1423839, 9106248, 9280407, 8485585, 10837014, 11600130, 12920169 | (Code et al. 1997; Crespi et al. 1997; Fujita and Kamataki 2001b; Kushida et al. 2000; Patten et al. 1996; Penman et al. 1993; Smith et al. 2003a; Smith et al. 1992; Yamazaki et al. 1992) |
| NNK | 2E1 | Tobacco-specific nitrosamine | Hydroxylation, Cα-methyl (keto alcohol), high Km, medium activity, major reaction, activation | 1312898, 8806763, 1423839, 9106248, 9280407, 8485585, 10837014, 11600130 | (Code et al. 1997; Crespi et al. 1997; Fujita and Kamataki 2001b; Kushida et al. 2000; Patten et al. 1996; Penman et al. 1993; Smith et al. 1992; Yamazaki et al. 1992) |
| NNK | 2D6 | Tobacco-specific nitrosamine | Hydroxylation, Cα-methyl (keto alcohol), high Km, medium activity, or high activity, major reaction, activation | 1312898, 8806763, 9106248, 9280407, 8485585 | (Code et al. 1997; Crespi et al. 1997; Patten et al. 1996; Penman et al. 1993; Smith et al. 1992) |
| NNK | 1A2 | Tobacco-specific nitrosamine | Hydroxylation, α-methyl (keto alcohol formation), high Km, medium activity, activation | 1312898, 8806763, 9106248, 9280407, 8485585, 11774366, 12214673, 16174803, 21473878, 19156262 | (Chiang et al. 2011; Code et al. 1997; Crespi et al. 1997; Dicke et al. 2005; Fujita and Kamataki 2001a; Kamataki et al. 2002; Krishnan et al. 2009; Patten et al. 1996; Penman et al. 1993; Smith et al. 1992) |
| 4-Methylphenol (p-cresol) | 1A1 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 1A2 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (activation, high activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2C19 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2D6 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2C9 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 3A4 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2E1 | Antiseptic, disinfectant | Hydroxylation, C-aromatic, formation of 4-methyl-o-hydroquinone (activation, high activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2C19 | Antiseptic, disinfectant | Hydroxylation, C-aromatic, formation of 4-methyl-o-hydroquinone (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2C9 | Antiseptic, disinfectant | Hydroxylation, C-aromatic, formation of 4-methyl-o-hydroquinone (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 1A1 | Antiseptic, disinfectant | Hydroxylation, C-aromatic, formation of 4-methyl-o-hydroquinone (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 1A2 | Antiseptic, disinfectant | Hydroxylation, C-aromatic, formation of 4-methyl-o-hydroquinone (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2D6 | Antiseptic, disinfectant | Hydroxylation, C-aromatic, formation of 4-methyl-o-hydroquinone (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 3A4 | Antiseptic, disinfectant | Hydroxylation, C-aromatic (activation, low activity) | 16174805 | (Yan et al. 2005) |
| 4-Methylphenol (p-cresol) | 2E1 | Antiseptic, disinfectant | Hydroxylation, C-methyl, 4-hydroxybenzaldehyde formation (low activity) | 16174805 | (Yan et al. 2005) |
| 3-N-Nitrosoguvacine (NGC) | 1A1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 23983642 | (Lin et al. 2013) |
| NGC | 2A6 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 23983642 | (Lin et al. 2013) |
| NGC | 2E1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 23983642 | (Lin et al. 2013) |
| 3-N-Nitrosoguvacoline (NGL) | 2A13 | Nitrosamine, betel quid, Areca nut compound | Oxidation, major enzyme, activation | 15725615 | (Miyazaki et al. 2005) |
| NGL | 2A6 | Nitrosamine, betel quid, Areca nut compound | Oxidation, major enzyme, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| NGL | 2E1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 23983642 | (Lin et al. 2013) |
| 3-(N-Nitrosomethylamino)propionaldehyde (NMPA) | 1A1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| NMPA | 1B1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 15725615 | (Miyazaki et al. 2005) |
| NMPA | 2A13 | Nitrosamine, betel quid, Areca nut compound | Oxidation, major enzyme, activation | 15725615 | (Miyazaki et al. 2005) |
| NMPA | 2A6 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| NMPA | 2E1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| 3-(N-Nitrosomethylamino)propionitrile (NMPN) | 1B1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 15725615 | (Miyazaki et al. 2005) |
| NMPN | 2A13 | Nitrosamine, betel quid, Areca nut compound | Oxidation, activation | 15725615 | (Miyazaki et al. 2005) |
| NMPN | 2A6 | Nitrosamine, betel quid, Areca nut compound | Oxidation, major enzyme, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| NMPN | 2E1 | Nitrosamine, Betel quid, Areca nut compound | Oxidation, major enzyme, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| NMPN | 1A1 | Nitrosamine, betel quid, Areca nut compound | Oxidation, major enzyme, activation | 15725615, 23983642 | (Lin et al. 2013; Miyazaki et al. 2005) |
| Menthofuran | 1A2 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | Activation | 26969934 | (Lassila et al. 2016) |
| Menthofuran | 3A4 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | Activation | 26969934 | (Lassila et al. 2016) |
| Menthofuran | 2B6 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | Activation | 26969934 | (Lassila et al. 2016) |
| Menthofuran (R)-(+)- | 2A6 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | C2-Hydroxylation, (2-hydroxymethofuran formation), activation | 10220485 | (Khojasteh-Bakht et al. 1999) |
| Menthofuran (R)-(+)- | 1A2 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | C2-Hydroxylation, (2-hydroxymethofuran formation), high Km, low activity, activation | 10220485, 26969934 | (Khojasteh-Bakht et al. 1999; Lassila et al. 2016) |
| Menthofuran (R)-(+)- | 2C19 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | C2-Hydroxylation, (2-hydroxymethofuran formation), high Km, low activity, activation | 10220485 | (Khojasteh-Bakht et al. 1999) |
| Menthofuran (R)-(+)- | 2E1 | Monoterpene, pennyroyal herb and oil compound, pulegone metabolite | C2-Hydroxylation, (2-hydroxymethofuran formation), medium Km, low activity, activation | 10220485 | (Khojasteh-Bakht et al. 1999) |
| Methyleugenol | 2C19 | Phenylpropene, from Rhizoma acorigraminei | C1´-Hydroxylation, medium activity, high Km, activation, followed by formation of 1´-sulfooxymethyleugenol | 16411663 | (Jeurissen et al. 2006) |
| Methyleugenol | 2D6 | Phenylpropene, from Rhizoma acorigraminei | C1´-Hydroxylation, medium activity, high Km, activation, followed by formation of 1´-sulfooxymethyleugenol | 16411663 | (Jeurissen et al. 2006) |
| Methyleugenol | 2E1 | Phenylpropene, from Rhizoma acorigraminei | C1´-Hydroxylation, medium activity, high Km, activation, followed by formation of 1´-sulfooxymethyleugenol | 16411663, 9328175 | (Gardner et al. 1997; Jeurissen et al. 2006) |
| Methyleugenol | 1A2 | Phenylpropene, from Rhizoma acorigraminei | C1´-Hydroxylation, major enzyme, activation, followed by formation of 1´-sulfooxymethyleugenol | 16411663, 25549870 | (Al-Subeihi et al. 2015; Jeurissen et al. 2006) |
| Methyleugenol | 2C9 | Phenylpropene, from Rhizoma acorigraminei | C1´-Hydroxylation, major enzyme, activation, followed by formation of 1´-sulfooxymethyleugenol | 16411663 | (Jeurissen et al. 2006) |
| Methyleugenol | 2B6 | Phenylpropene, from Rhizoma acorigraminei | Epoxidation, major enzyme, activation, followed by formation of 1-sulfooxymethyleugenol | 25549870 | (Al-Subeihi et al. 2015) |
| Monocrotaline | 3A4 | Pyrrolizidine alkaloid, genotoxic | Pyrrole formation, dehydrogenation, activation | 15649625 | (Wang et al. 2005) |
| N´-Nitrosonornicotine (NNN) | 1A1 | Tobacco-specific nitrosamine | Activation | 11774366 | (Fujita and Kamataki 2001a) |
| NNN | 1A2 | Tobacco-specific nitrosamine | Activation | 11774366 | (Fujita and Kamataki 2001a) |
| NNN | 1B1 | Tobacco-specific nitrosamine | Activation | 11774366 | (Fujita and Kamataki 2001a) |
| NNN | 2A6 | Tobacco-specific nitrosamine | 5´-Hydroxylation (activation, major enzyme) | 11774366, 12214673, 9029045, 9276639, 15651850 | (Fujita and Kamataki 2001a; Kamataki et al. 2002; Patten et al. 1997; Staretz et al. 1997; Wong et al. 2005b) |
| NNN | 2C19 | Tobacco-specific nitrosamine | Activation | 11774366 | (Fujita and Kamataki 2001a) |
| NNN | 3A4 | Tobacco-specific nitrosamine | 2´-Hydroxylation (activation) | 11774366, 9029045, 9276639 | (Fujita and Kamataki 2001a; Patten et al. 1997; Staretz et al. 1997) |
| NNN | 3A5 | Tobacco-specific nitrosamine | Activation | 11774366 | (Fujita and Kamataki 2001a) |
| NNN | 2A13 | Tobacco-specific nitrosamine | 2´-Hydroxylation (activation, major enzyme) | 15651850 | (Wong et al. 2005b) |
| NNN | 2E1 | Tobacco-specific nitrosamine | 5´-Hydroxylation (activation) | 9276639 | (Patten et al. 1997) |
| NNN | 2D6 | Tobacco-specific nitrosamine | 5´-Hydroxylation (activation, major enzyme) | 9276639 | (Patten et al. 1997) |
| Ochratoxin A | 2C9 | Mycotoxin, from Aspergillus ochraceus and Penicillium verrucosum | Oxidation, activation | 10712746 | (El Adlouni et al. 2000) |
| Ochratoxin A | 1A1 | Mycotoxin, from Aspergillus ochraceus and Penicillium verrucosum | Oxidation, activation | 8542584 | (de Groene et al. 1996) |
| Ochratoxin A | 1A2 | Mycotoxin, from Aspergillus ochraceus and Penicillium verrucosum | Oxidation, activation | 8542584, 11189750 | (de Groene et al. 1996; Lewis et al. 1999) |
| Ochratoxin A | 3A4 | Mycotoxin, from Aspergillus ochraceus and Penicillium verrucosum | Oxidation, activation | 8542584, 16139406 | (de Groene et al. 1996; Simarro Doorten et al. 2006) |
| Pulegone (R)-(+)- | 1A2 | Monoterpene, pennyroyal herb and oil compound | Oxidation, menthofuran formation (high Km, medium activity, medium efficiency, activation) | 10220485 | (Khojasteh-Bakht et al. 1999) |
| Pulegone (R)-(+)- | 2C19 | Monoterpene, pennyroyal herb and oil compound | Oxidation, menthofuran formation (medium Km, medium activity, medium efficiency, activation) | 10220485 | (Khojasteh-Bakht et al. 1999) |
| Pulegone (R)-(+)- | 2E1 | Monoterpene, pennyroyal herb and oil compound | Oxidation, menthofuran formation (major enzyme, medium Km, high activity, medium efficiency, activation) | 10220485 | (Khojasteh-Bakht et al. 1999) |
| Retrorsine | 3A4 | Pyrrolizidine alkaloid, genotoxic | Pyrrole formation, activation | 15649625, 25651456, 24799337, 32469285, 19818743 | (Dai et al. 2010; Fashe et al. 2015; Lu et al. 2020; Tu et al. 2014; Wang et al. 2005) |
| Retrorsine | 2C19 | Pyrrolizidine alkaloid, genotoxic | Pyrrole formation, activation | 19818743 | (Dai et al. 2010) |
| Riddelliine | 3A4 | Pyrrolizidine alkaloid, genotoxic | Pyrrole formation, activation dehydrogenation | 15649625, 32798647 | (Li et al. 2020; Wang et al. 2005) |
| Riddelliine | 3A5 | Pyrrolizidine alkaloid, genotoxic | Pyrrolic metabolite formation, dehydrogenation, activation | 32798647 | (Li et al. 2020) |
| Riddelliine | 3A7 | Pyrrolizidine alkaloid, genotoxic | Pyrrolic metabolite formation, dehydrogenation, activation, low activity | 32798647 | (Li et al. 2020) |
| Safrole | 1B1 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, activation | 15310247 | (Ueng et al. 2004) |
| Safrole | 2A6 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, major enzyme at low concentration, medium Km, medium activity, activation | 15377158, 15310247, 17407329, 23112005 | (Jeurissen et al. 2004; Jeurissen et al. 2007; Ueng et al. 2004; Uno et al. 2013) |
| Safrole | 2C19 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, high Km, medium activity, activation | 15377158, 17407329 | (Jeurissen et al. 2004; Jeurissen et al. 2007) |
| Safrole | 2C9 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, major enzyme at higher conc., high Km, medium activity, activation | 15377158, 15310247, 17407329 | (Jeurissen et al. 2004; Jeurissen et al. 2007; Ueng et al. 2004) |
| Safrole | 3A4 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, activation | 15310247 | (Ueng et al. 2004) |
| Safrole | 2E1 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, major enzyme, activation | 15310247, 15377158 | (Jeurissen et al. 2004; Ueng et al. 2004) |
| Safrole | 2D6 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, activation, high Km, low activity | 15377158 | (Jeurissen et al. 2004) |
| Safrole | 1A2 | Methylenedioxyphenyl, sassafras oil and betel quid component | o-Quinone formation, activation | 30865484, 29082813 | (Hu et al. 2019; Yang et al. 2018) |
| Safrole | 1A1 | Methylenedioxyphenyl, sassafras oil and betel quid component | C1´-Hydroxylation, activation | 15310247 | (Ueng et al. 2004) |
| Senecionine | 3A4 | Pyrrolizidine alkaloid, genotoxic | Pyrrole formation, N-oxygenation, dehydrogenation, activation | 2009596, 8095200 | (Guengerich 1993; Miranda et al. 1991) |
| Sterigmatocystin | 1A1 | Mycotoxin, fungal product | Epoxidation, activation (minor reaction) | 7955101, 20929267, 21913247 | (Cabaret et al. 2011; Cabaret et al. 2010; Shimada et al. 1994) |
| Sterigmatocystin | 1A2 | Mycotoxin, fungal product, xanthon | Epoxidation, activation (minor reaction) | 7955101, 20929267, 21913247 | (Cabaret et al. 2011; Cabaret et al. 2010; Shimada et al. 1994) |
| Sterigmatocystin | 2W1 | Mycotoxin, fungal product | Oxidation, activation | 16551781 | (Wu et al. 2006) |
| Sterigmatocystin | 3A4 | Mycotoxin, fungal product | Epoxidation, activation (minor reaction) | 9328287, 20929267, 21913247, 7554070 | (Cabaret et al. 2011; Cabaret et al. 2010; Gillam et al. 1997; Yamazaki et al. 1995) |
| Sterigmatocystin | 3A7 | Mycotoxin, fungal product | Oxidation, activation | 9328287 | (Gillam et al. 1997) |
| Sterigmatocystin | 1B1 | Mycotoxin, fungal product | Oxidation (weak activation) | 16551781 | (Wu et al. 2006) |
| Sterigmatocystin | 2W1 | Fungal product | Oxidation (activation) | 16379042 | (Brandon et al. 2006) |
3-Methylindole (skatole) is formed in nature by microbial degradation of tryptophan and tyrosine (Carlson and Breeze 1984), but is also present humans where it is formed by the decarboxylation of tryptophan in the large intestine. 3-Methylindole is selective pulmonary toxicant and, in addition to intestinal formation and absorption, cigarette smoke is additional source of 3-methylindole in smokers. 3-Methylindole may provoke pneumotoxicity and lung cancer by activity of P450 1A1 and P450 2F1 (Weems et al. 2010). Toxicity of 3-methylindole depends on bioactivation by several reactions: epoxidation (3-methyloxindole formation, P450 1A1, 1A2, 1B1, 2E1, 2A6), C-hydroxylation (indole-3-carbinol formation, P450 1A1, 1A2, 1B1), and dehydrogenation (3-methyleneindolenine formation, P450 1A1, 1A2, 2A13, 2F1 (Table 4 and references therein).
The numbers of activation reactions catalyzed by human P450 enzymes reacting with natural products as substrates are presented in Fig. 15. Of the total of 952 reactions identified in our records, 152 (~16%) involve bioactivation and the formation of potentially toxic products. The major P450s involved in the activations are P450s 1A2 (~12%), P450s 2E1 and P450 3A4 (~11% each), followed by P450 1A1 and 2A6 (~10%).
Fig 15.
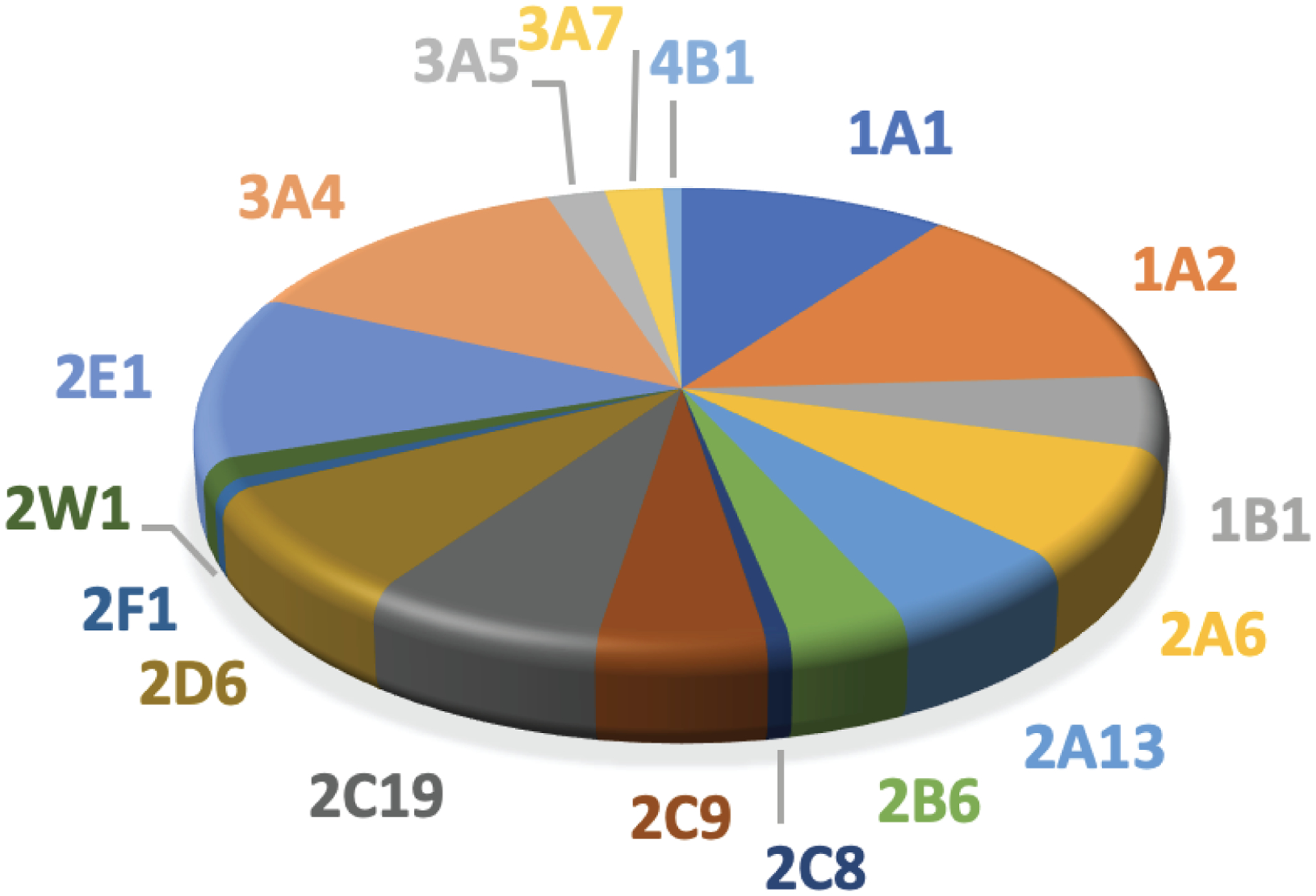
Participation of human P450 Families 1–4 in activation of natural products to potentially toxic products (952 reactions, 186 activation reactions)
The following examples illustrate the participation of P450 enzymes in the bioactivation of selected natural compounds (Table 4).
Aflatoxins
AFB1 is a potent hepatocarcinogen in animal models and also classified as a hepatocarcinogen in humans. AFB1 is metabolically activated by P450 enzymes to form cytotoxic and DNA-reactive intermediates (Fig. 16). AFB1 is activated to the toxic exo-8,9 epoxide most prominently by P450 Subfamily 3A enzymes in liver and P450 2A13 in lung (Shimada and Guengerich 1989; Deng et al. 2018). In addition to its hepatotoxicity, AFB1 can be toxic in lungs (at least in animal models) due to the activity of P450 2A enzymes. P450 3A enzymes (3A4 and 3A5) oxidize AFB1 to the highly mutagenic exo-8,9-epoxide (Fig. 16), while P450 1A2 oxidizes it to a roughly equimolar mixture of toxic exo- plus the endo-epoxide, the latter of which is essentially non-mutagenic (Iyer et al. 1994). Both P450 3A4 and 1A2 enzymes also catalyze AFB1 detoxication reactions, i.e. 3α-hydroxylation in the case of P450 3A4 (aflatoxin Q1 formation) and 9a-hydroxylation in the case of P450 1A2 (aflatoxin M1 formation) (Rendic and Guengerich 2012)). This example illustrates that P450 enzymes can catalyze both activation and detoxication reactions acting on the same substrate. The toxic AFB1-exo-8,9-epoxide is detoxicated by glutathione (GSH) transferases by conjugation of GSH to the epoxide (Johnson et al. 1997; Deng et al. 2018; Yang et al. 2012). In addition to a being substrate of P450 enzymes, AFB1 is an inducer of P450 1A1, 1B1, and 3A4 in monocytes (Bahari et al. 2014), and the compound might enhance its own metabolism or metabolism of another substrate of the enzyme.
Fig 16.

Activation (formation of 8,9-exo-epoxide) and detoxication (formation of AFQ1) of AFB1 by P450 3A4
Artistocholic acid
Aristolochic acids constitute a group of compounds found naturally in many types of plants known as Aristolochiaceae, including Aristolochia and Asarum (wild ginger) grown worldwide. Aristolochic acid I and II are the predominant chemical toxins in the plants. Aristolochic acid compounds were shown to be the cause of a kidney disease called Chinese herb nephropathy, now renamed aristolochic acid nephropathy (Arlt et al. 2002; Schmeiser et al. 2009; Gökmen et al. 2013). Aristocholic acid is classified by the International Agency for Research on Cancer as a Group I carcinogen. This natural product has also been implicated in the development of another kidney disease, Balkan endemic nephropathy, and its associated urothelial malignancy. The disease is endemic in certain rural areas of Balkan countries located closed to the tributaries of the Danube river basin (Arlt et al. 2007; Grollman et al. 2007; Han et al. 2019). As already mentioned, aristolochic acid I is activated by reduction of the nitro group (under partially anaerobic conditions), and oxidative metabolism results in formation of nontoxic O-demethylated metabolites. Nitro reduction of aristolochic acid I, considered as the major factor causing its toxicity, is required to exert its carcinogenic properties. The reaction catalyzed by P450s 1A1 and 1A2 results in the generation of N-hydroxyaristolactam I, which leads to the formation of a cyclic acyl nitrenium ion, the intermediate that either forms DNA adducts or rearranges to 7-hydroxyaristolactam I (Fig. 17). Aristolochic acid I oxidation to a nontoxic metabolite by O-demethylation of the methoxy group is catalyzed by the same enzymes, i.e. P450 1A1 and 1A2, with contribution from P450 2C9 and 3A4 (Table 4). The product of the reactions is 8-hydroxyaristolochic acid I, a detoxication product. The O-demethylated metabolite is excreted either in its free or conjugated form (Chan et al. 2006; Shibutani et al. 2010; Arlt et al. 2011; Stiborová et al. 2012a).
Fig 17.

Activation of aristolochic acid I and II by P450 enzymes
Estragole
Estragole is a common component of herbs and spices and is a natural constituent of basil oil. It is also a genotoxic hepatocarcinogen in rodents, and its potential toxic effect in humans is still under consideration. One of the major sources of human exposure to this phytochemical is Foeniculum vulgare Mill. (fennel) (Levorato et al. 2018). Toxicity is ascribed to its hydroxylation in position C1´, catalyzed by P450s 1A2, 2A6, 2C19, 2D6, and 2E1, of which P450s 1A2 and 2A6 are the major enzymes (Table 4 and references therein). Other enzymes can also contribute but at relatively high concentrations of estragole. The metabolite of P450 oxidation is not inherently toxic; however, C1´-hydroxylation of estragole is the first step in activation followed by sulfate conjugation by a sulfotransferase to produce genotoxic 3´-sulfoxyestragole (Fig. 18) (Monien et al. 2019).
Fig 18.
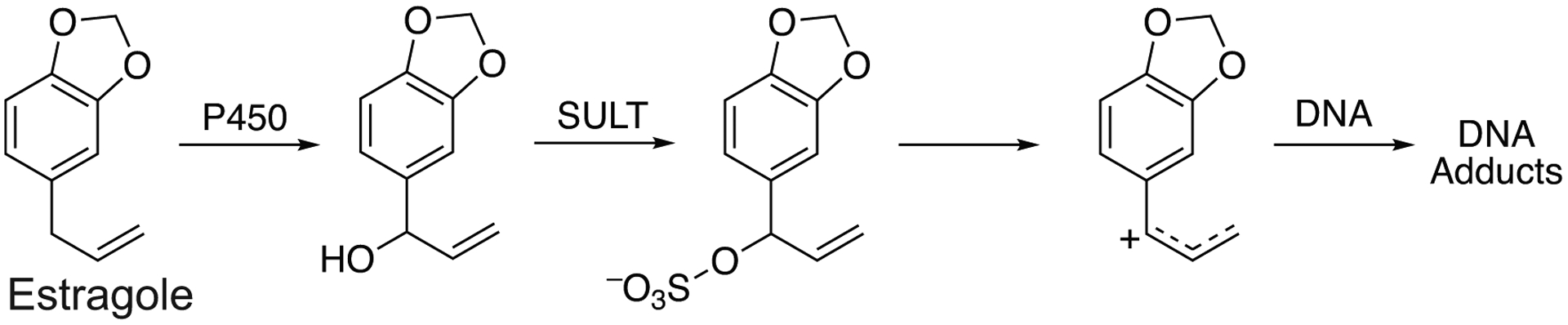
Activation of estragole by P450 enzymes and sulfotransferases
Ethanol
Ethanol is widely consumed and is metabolically activated to toxic acetaldehyde (Fig. 19). The metabolism and activation of ethanol is primarily catalyzed by alcohol dehydrogenase and, to a lesser extent, catalase but under certain circumstances (e.g., high doses) P450 enzymes can also be involved. Many P450 enzymes in Families 1–4 oxidize ethanol to acetaldehyde at high concentrations, namely 1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 (Table 3), but P450 2E1 has the highest catalytic activity (i.e., specificity constant, kcat/Km).
Fig 19.
Activation of ethanol to toxic acetaldehyde by P450 enzymes
The role of P450 2E1 in ethanol metabolism has been reviewed recently. P450 2E1, 3A4, and 1A2 were reported as P450s that are significantly involved in oxidation of ethanol under conditions of high concentration (Km ~10 mM) and chronic use (Hamitouche et al. 2006; Guengerich and Avadhani 2018; Guengerich 2020).
Safrole
Safrole is a natural compound categorized as an IARC Group 2B carcinogen. It is extracted from sassafras oil or certain other essential oils and also from betel quid. Safrole was reported to be a rodent hepatocarcinogen, and DNA adducts were identified in liver samples of patients having a history of betel quid chewing (Bolton et al. 1994; Chung et al. 2008). In addition, betel quid chewing is associated with oral and hypopharynx cancers (Shield et al. 2017; Chen et al. 2017). The metabolism of safrole was reported to be predominantly catalyzed by P450 1A2, with minor contributions by P450 2E1. It was suggested that the ortho-quinone metabolite may mediate safrole hepatotoxicity (Fig. 20, Table 4 and references therein). Safrole can also, as in the case of estragole, undergo bioactivation by sequential 1´-hydroxylation and sulfation, resulting in reactive intermediates capable of forming DNA adducts (Jeurissen et al. 2004). In addition, it has been reported that safrole is a mechanism-based inhibitor of P450 1A2 (Hu et al. 2019; Yang et al. 2018). It has been also reported that safrole induced P450 2A6 activity and tobacco specific 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolic activation, resulting in higher NNK-induced genotoxicity (Tsou et al. 2019).
Fig 20.

Activation of safrole by P450 enzymes and sulfotransferases
N´-Nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)
The tobacco specific nitrosamines NNN and NNK are potent carcinogens in animal models and are believed to be causative agents for esophageal cancer in smokers and those using chewing tobacco and snuff. Metabolic activation of NNN is required to exert carcinogenic potential (Fig. 21) and occurs through P450 catalyzed 2´- and 5´-hydroxylation, which generates unstable metabolites that decompose to 4-hydroxy-1-(3-pyridyl)-1-butanone (‘keto alcohol’) and 4-hydroxy-4-(3-pyridyl)butanal, respectively. The latter cyclizes to 5-(3-pyridyl)-2-hydroxytetrahydrofuran (‘lactol’). P450s 2E1, 2A6, and 3A4 were identified as major catalysts for NNN 5´-hydroxylation in human liver microsomes (Yamazaki et al. 1992; Hecht 1998; Wong et al. 2005a; Patten et al. 1996; Patten et al. 1997; Carlson et al. 2016; Fan et al. 2019; Staretz et al. 1997; Fujita and Kamataki 2001a).
Fig 21.

Activation of NNN by P450 enzymes
NNK, a potent tobacco-specific carcinogen, has been demonstrated to induce lung tumors in animals and is suspected to be a human carcinogen. P450s are the major enzymes responsible for the activation of NNK in lung and liver microsomes of rats and mice, as well as in human liver. Human P450s 2A6 and 3A4 are involved in the activation of NNK (Smith et al. 1995; Staretz et al. 1997). In addition, it was demonstrated that P450s 1A2, 2A6, and 3A4 may be important for the activation of NNK to a DNA-methylating agent (‘keto aldehyde’) via the α-methylene hydroxylation pathway (Fig. 22). P450s 1A2, 2E1, and 2D6 are selective for α-methyl hydroxylation of NNK, leading to keto alcohol and a DNA-pyridyloxobutylating agent. P450 1A2 exhibits at least twice the specificity toward NNK bioactivation compared to P450 2E1 and catalyzed the formation of both, keto alcohol and 4-oxo-1-(3-pyridyl)-1-butanone (keto aldehyde) with the keto alcohol being the major product (Patten et al. 1996; Patten et al. 1997; Krishnan et al. 2009; Smith et al. 1996) (Table 4 and references therein).
Fig 22.

Activation of NNK by P450 enzymes
Physiological compounds
Physiological substrates of P450 Family 1–4 enzymes include eicosanoids, estrogens (e.g., estradiol), fatty acids (e.g., arachidonic acid), cholesterol, fat-soluble vitamins (e.g., vitamins A, D3, E, and K), neurotransmitters (serotonin, tryptamine), leukotrienes, prostaglandins, fatty acids (e.g. arachidonic acid), bile acids (e.g. lithocholic, deoxycholic, cholic acid), corticosteroids, androgens (e.g., androstenedione, testosterone, dihydrotestosterone), and progesterone. In addition to being substrates of P450s Families 1–4, these compounds are predominately substrates of the enzymes belonging to Families 5–51 (with 22 enzymes) (Rendic and Di Carlo 1997; Rendic and Guengerich 2018). The data presented in Fig. 23 show the participation of Family 1–4 P450s in the activation of physiological compounds to some potentially toxic products. Of the total 530 metabolic reactions (data from our records), 75 (14 %) involve bioactivation. The highest involvement is with P450s 1A1, 1A2, 1B1, 3A4, and 3A5 (~9% each), followed closely by P450 2C9 (8%). Physiological substrates in activation reactions include estrogenic hormones (17β-estradiol and estrone) and fatty acids (Table 5). In addition to being activated to toxic products, fatty acids (exemplified by arachidonic acid) can both down-regulate (Palacharla et al. 2017) or induce P450 activity by changing their expression (Finn et al. 2009). In some cases, the reaction products are not inherently reactive but may have deleterious signaling properties (e.g., 20-HETE, EETs (Sausville et al. 2018)).
Fig 23.
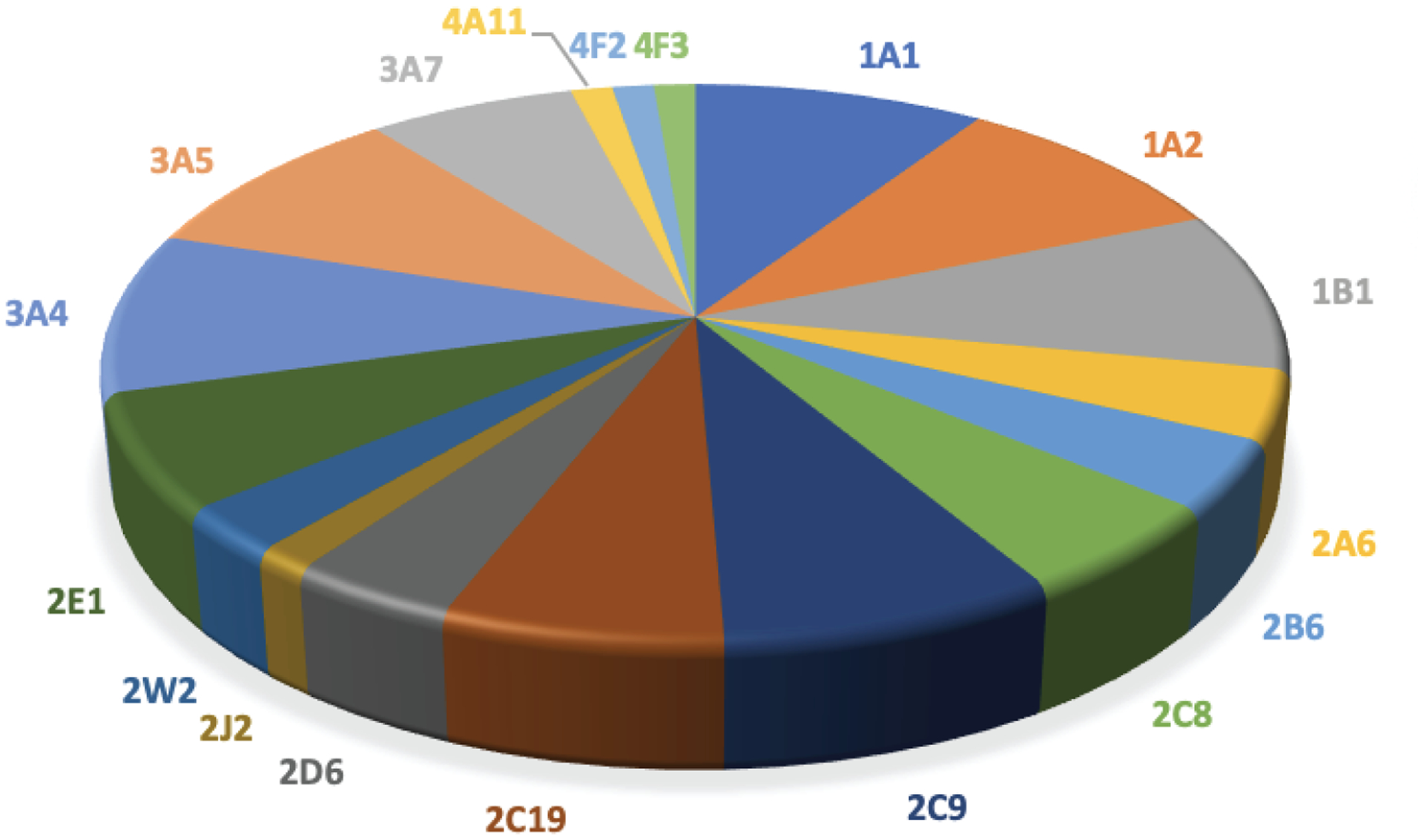
Participation of human P450 Families 1–4 in metabolic activation of physiological compounds to potentially toxic metabolites (530 reactions, 75 activation reactions)
Table 5.
Examples of the metabolic activation of physiological compounds by human cytochrome P450 enzymes.
| Compound | Enzyme | Subcategory | Reaction | PMIDs | References |
|---|---|---|---|---|---|
| Arachidonic acid | 2E1 | Fatty acid, polyunsaturated | Oxidation | 9169410, 14744237 | (Caro and Cederbaum 2004; Chen et al. 1997) |
| Arachidonic acid | 2W1 | Fatty acid, polyunsaturated | Epoxidation, epoxyeicosatrienoic acid (EET) formation, very low activity, if any, under physiological conditions | 26936974 | (Zhao et al. 2016) |
| Arachidonic acid | 2C8 | Fatty acid, polyunsaturated | Epoxidation, EET formation | 28701518, 7996455, 7625847, 15652503, 8651708, 7574697, 15766564, 11668219 | (Barbosa-Sicard et al. 2005; Dai et al. 2001b; Daikh et al. 1994; Fan and Roman 2017; Lundblad et al. 2005; Rifkind et al. 1995; Zeldin et al. 1995; Zeldin et al. 1996) |
| Arachidonic acid | 2C9 | Fatty acid, polyunsaturated | Epoxidation, EET formation, 14,15-, 11,12- and 8,9-EETs | 28701518, 7996455, 7625847, 8651708, 7574697, 15766564, 15652503 | (Barbosa-Sicard et al. 2005; Daikh et al. 1994; Fan and Roman 2017; Lundblad et al. 2005; Rifkind et al. 1995; Zeldin et al. 1995) |
| Arachidonic acid | 2J2 | Fatty acid, polyunsaturated | Epoxidation, EET formation | 28701518 | (Fan and Roman 2017) |
| Arachidonic acid | 4F3 | Fatty acid, polyunsaturated | Hydroxylation, 20-hydroxy eicosatetraenoic acid (HETE) formation, activation to pro-hypertensive activity | 28701518, 12709424 | (Christmas et al. 2003; Fan and Roman 2017) |
| Arachidonic acid | 4F2 | Fatty acid, polyunsaturated | Hydroxylation, 20-HETE formation, low activity, activation to pro-hypertensive activity | 9618440, 10660572, 28701518, 18662666 | (Fan and Roman 2017; Hirani et al. 2008; Lasker et al. 2000; Powell et al. 1998) |
| Arachidonic acid | 4A11 | Fatty acid, polyunsaturated | Hydroxylation, 20-HETE formation, activation to pro-hypertensive activity | 9618440, 10660572, 28701518 | (Fan and Roman 2017; Lasker et al. 2000; Powell et al. 1998) |
| Arachidonic acid | 1A2 | Fatty acid, polyunsaturated | Epoxidation, EET formation | 7625847, 15258110, 8651708, 7574697 | (Choudhary et al. 2004; Rifkind et al. 1995; Zeldin et al. 1995; Zeldin et al. 1996) |
| Arachidonic acid | 2E1 | Fatty acid, polyunsaturated | Hydroxylation, 20-HETE formation, activation to pro-hypertensive activity | 7625847 | (Rifkind, 1995) |
| Arachidonic acid | 2B6 | Fatty acid, polyunsaturated | Epoxidation, EET formation, activation, low activity | 7625847, 8651708, 7574697 | (Rifkind, 1995;Zeldin et al. 1996; Zeldin et al. 1995) |
| Arachidonic acid | 1A1 | Fatty acid, polyunsaturated | Epoxidation, EET formation | 15041462 | (Schwarz et al. 2004) |
| Arachidonic acid | 1B1 | Fatty acid, polyunsaturated | Epoxidation, EET formation | 15258110 | (Choudhary et al. 2004) |
| Arachidonic acid | 2C19 | Fatty acid, polyunsaturated | Epoxidation, EET formation | 9866708, 9435160 | (Bylund et al. 1998a; Bylund et al. 1998b) |
| 17β-Estradiol | 2D6 | Estrogen | C16α-Hydroxylation, minor reaction | 9667077, 9625734 | (Niwa et al. 1998; Yamazaki et al. 1998b) |
| 17β-Estradiol | 3A5 | Estrogen | C4-Hydroxylation, activation | 11454902, 15784278, 12865317, 12124305 | (Lee et al. 2003a; Lee et al. 2001; Williams et al. 2002; Zhu and Lee 2005) |
| 17β-Estradiol | 1B1 | Estrogen | C4-Hydroxylation, major extrahepatic enzyme, medium to low Km, medium activity, medium to low efficiency, activation | 8790407, 9498279, 9152602, 15784278, 12865317, 12902195, 10426814, 14703066, 10739169, 11465393, 10403516, 16112414, 12423652, 10862525, 10963622, 15142886, 11555828, 10910054, 11854143 11854439, 11719446, 25678418 | (Aklillu et al. 2002; Badawi et al. 2001; Chen et al. 2004; Chun et al. 2001; Hanna et al. 2000; Hayes et al. 1996; Jefcoate et al. 2000; Lee et al. 2003a; Li et al. 2000; Modugno et al. 2003; Niwa et al. 2015; Pang et al. 1999; Shimada et al. 1997b; Shimada et al. 2001b; Shimada et al. 1999; Spink et al. 2002a; Spink et al. 2002b; Spink et al. 1998; Tsuchiya et al. 2005; van Duursen et al. 2003; Watanabe et al. 2000; Zhu and Lee 2005) |
| 17β-Estradiol | 1A2 | Estrogen | C4-Hydroxylation, minor reaction, activation | 1449532, 15784278, 9625734, 9054608, 9667077, 9152602, 11555828, 12865317 | (Badawi et al. 2001; Kerlan et al. 1992; Lee et al. 2003a; Niwa et al. 1998; Shimada et al. 1997b; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| 17β-Estradiol | 3A4 | Estrogen | C4-Hydroxylation, minor reaction, medium Km, medium activity, activation | 1449532, 11454902, 15784278, 9625734, 9054608, 9667077, 12865317, 12124305, 11555828, 9003190 | (Badawi et al. 2001; Kerlan et al. 1992; Lee et al. 2003a; Lee et al. 2001; Niwa et al. 1998; Shou et al. 1997; Ueng et al. 1997; Williams et al. 2002; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| 17β-Estradiol | 1A2 | Estrogen | C16α-Hydroxylation (major enzyme, high Km, low activity) | 9625734, 9054608, 12865317, 9667077, 11555828 | (Badawi et al. 2001; Lee et al. 2003a; Niwa et al. 1998; Shou et al. 1997; Yamazaki et al. 1998b) |
| 17β-Estradiol | 1B1 | Estrogen | C16α-Hydroxylation,(minor enzyme, medium and high Km, low activity) | 9667077, 11555828, 11854439, 10910054 | (Aklillu et al. 2002; Badawi et al. 2001; Hanna et al. 2000; Niwa et al. 1998) |
| 17β-Estradiol | 1A1 | Estrogen | C16α-Hydroxylation (high Km, low activity) | 9667077, 11555828, 12865317, 15784278, 10403516 | (Badawi et al. 2001; Lee et al. 2003a; Niwa et al. 1998; Pang et al. 1999; Zhu and Lee 2005) |
| 17β-Estradiol | 1A1 | Estrogen | C4-Hydroxylation (minor reaction, medium Km, medium efficiency, low activity, activation) | 8790407, 11555828, 12865317, 15784278, 10403516, 25678418 | (Badawi et al. 2001; Hayes et al. 1996; Lee et al. 2003a; Niwa et al. 2015; Pang et al. 1999; Zhu and Lee 2005) |
| 17β-Estradiol | 2A6 | Estrogen | C4-Hydroxylation (low activity, activation) | 12865317, 15784278 | (Lee et al. 2003a; Zhu and Lee 2005) |
| 17β-Estradiol | 2C19 | Estrogen | C4-Hydroxylation (low activity, activation) | 11067738 | (Satoh et al. 2000) |
| 17β-Estradiol | 2C19 | Estrogen | C16α-Hydroxylation (low activity) | 9667077, 9625734, 15784278 | (Niwa et al. 1998; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| 17β-Estradiol | 2C8 | Estrogen | C4-Hydroxylation (low activity, activation) | 12865317, 11067738 | (Lee et al. 2003a; Satoh et al. 2000) |
| 17β-Estradiol | 2C9 | Estrogen | C4-Hydroxylation (very low activity, activation) | 9625734, 12865317, 15784278 | (Lee et al. 2003a; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| 17β-Estradiol | 2C9 | Estrogen | C16α-Hydroxylation (minor reaction, low activity) | 9667077, 9625734, 15784278 | (Niwa et al. 1998; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| 17β-Estradiol | 2D6 | Estrogen | C4-Hydroxylation (very low activity, activation) | 11067738 | (Satoh et al. 2000) |
| 17β-Estradiol | 3A4 | Estrogen | C16α-Hydroxylation, high Km, low activity | 9625734, 11454902, 12865317, 14559847, 9667077, 14703066, 11555828 | (Badawi et al. 2001; Lee et al. 2003a; Lee et al. 2003b; Lee et al. 2001; Modugno et al. 2003; Niwa et al. 1998; Yamazaki et al. 1998b) |
| 17β-Estradiol | 3A5 | Estrogen | C16α-Hydroxylation, high Km, low activity | 12865317, 14559847 | (Lee et al. 2003a; Lee et al. 2003b) |
| 17β-Estradiol | 3A7 | Estrogen | C4-Hydroxylation, high Km, low activity, activation | 14559847 | (Lee et al. 2003b) |
| 17β-Estradiol | 3A4 | Estrogen | C2-Hydroxylation, activation, major enzyme in liver | 11454902, 11067738, 14703066, 12865317, 11555828, 16112414 | (Badawi et al. 2001; Lee et al. 2003a; Lee et al. 2001; Modugno et al. 2003; Satoh et al. 2000; Tsuchiya et al. 2005) |
| 17β-Estradiol | 3A5 | Estrogen | C2-Hydroxylation, activation | 11454902, 12865317 | (Lee et al. 2003a; Lee et al. 2001) |
| 17β-Estradiol | 2D6 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 1A1 | Estrogen | C15α-Hydroxylation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 1A1 | Estrogen | C6α-Hydroxylation, | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 1A1 | Estrogen | C7α-Hydroxylation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 1B1 | Estrogen | C2-Hydroxylation, low Km and low activity, activation | 8790407, 12865317, 11555828, 25678418 | (Badawi et al. 2001; Hayes et al. 1996; Lee et al. 2003a; Niwa et al. 2015) |
| 17β-Estradiol | 3A4 | Estrogen | C16β-Hydroxylation, low activity | 12865317, 14703066 | (Lee et al. 2003a; Modugno et al. 2003) |
| 17β-Estradiol | 1A2 | Estrogen | C2-Hydroxylation, high activity, activation, major enzyme in liver | 12865317, 11555828, 16112414 | (Badawi et al. 2001; Lee et al. 2003a; Tsuchiya et al. 2005) |
| 17β-Estradiol | 1A1 | Estrogen | C2-Hydroxylation, high activity, major extrahepatic enzyme, activation | 8790407, 9498279, 11854143, 12902195, 14703066, 16112414, 15142886, 25678418 | (Chen et al. 2004; Hayes et al. 1996; Modugno et al. 2003; Niwa et al. 2015; Spink et al. 2002b; Spink et al. 1998; Tsuchiya et al. 2005; van Duursen et al. 2003) |
| 17β-Estradiol | 2W1 | Estrogen | C2-Hydroxylation, very low activity under physiological conditions, activation | 26936974 | (Zhao et al. 2016) |
| 17β-Estradiol | 2A6 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 2B6 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 2C8 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 2C9 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | 2C19 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| 17β-Estradiol | P450 2E1 | Estrogen | C4-Hydroxylation (low activity, activation) | 11067738, 15784278 | (Satoh et al. 2000; Zhu and Lee 2005) |
| 17β-Estradiol | 3A7 | Estrogen | C16α-Hydroxylation, very low activity | 12865317, 14559847 | (Lee et al. 2003a; Lee et al. 2003b) |
| Estrone | 1A1 | Estrogen | C15α-Hydroxylation, high activity | 12865317, 15784278 | (Lee et al. 2003a; Zhu and Lee 2005) |
| Estrone | 2A6 | Estrogen | C16α-Hydroxylation, at higher concentration | 9635876 | (Huang et al. 1998) |
| Estrone | 3A7 | Estrogen | C16α-Hydroxylation, minor reaction | 12865317, 14559847 | (Lee et al. 2003a; Lee et al. 2003b) |
| Estrone | 1A1 | Estrogen | C16α-Hydroxylation, minor reaction | 9635876, 15784278, 16537715 | (Cribb et al. 2006; Huang et al. 1998; Zhu and Lee 2005) |
| Estrone | 1B1 | Estrogen | C4-Hydroxylation, low Km, major reaction, activation | 9152602, 15784278, 12865317, 16537715, 10426814, 10739169, 11465393, 16207128, 25678418 | (Cribb et al. 2006; Lee et al. 2003a; Niwa et al. 2015; Paracchini et al. 2005; Shimada et al. 1997b; Shimada et al. 2001b; Shimada et al. 1999; Watanabe et al. 2000; Zhu and Lee 2005) |
| Estrone | 1A1 | Estrogen | C4-Hydroxylation, medium activity, activation | 12865317, 15784278, 16537715, 25678418 | (Cribb et al. 2006; Lee et al. 2003a; Niwa et al. 2015; Zhu and Lee 2005) |
| Estrone | 1A1 | Estrogen | C7α-Hydroxylation, minor reaction, activation | 12865317, 15784278 | (Lee et al. 2003a; Zhu and Lee 2005) |
| Estrone | 1A2 | Estrogen | C4-Hydroxylation (medium Km, very low to medium activity, activation) | 9625734, 9054608, 12865317, 15784278, 16537715 | (Cribb et al. 2006; Lee et al. 2003a; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 1A2 | Estrogen | C16α-Hydroxylation (minor reaction, very low activity, activation) | 9625734, 9054608, 12865317, 25678418 | (Lee et al. 2003a; Niwa et al. 2015; Shou et al. 1997; Yamazaki et al. 1998b) |
| Estrone | 3A7 | Estrogen | C4-Hydroxylation (low activity, medium Km, activation) | 12865317, 14559847 | (Lee et al. 2003a; Lee et al. 2003b) |
| Estrone | 3A7 | Estrogen | C2-Hydroxylation (low activity, activation) | 14559847 | (Lee et al. 2003b) |
| Estrone | 3A5 | Estrogen | C16α-Hydroxylation (high Km, low activity) | 9635876, 9625734, 9054608, 12865317, 9667077, 15784278, 16537715, 14559847 | (Cribb et al. 2006; Huang et al. 1998; Lee et al. 2003a; Lee et al. 2003b; Niwa et al. 1998; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2C9 | Estrogen | C16α-Hydroxylation (low activity, activation) | 9635876, 9625734, 9054608, 14703066, 9667077, 15784278 | (Huang et al. 1998; Modugno et al. 2003; Niwa et al. 1998; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 3A4 | Estrogen | C16α-Hydroxylation (high Km, low activity, major enzyme) | 9625734, 9054608, 9667077, 9635876, 12865317, 15784278, 14559847, 25678418 | (Huang et al. 1998; Lee et al. 2003a; Lee et al. 2003b; Niwa et al. 2015; Niwa et al. 1998; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 3A4 | Estrogen | C4-Hydroxylation (high Km, low activity, major enzyme, activation) | 9635876, 9625734, 9054608, 9667077, 12865317, 15784278 | (Huang et al. 1998; Lee et al. 2003a; Niwa et al. 1998; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2C9 | Estrogen | C4-Hydroxylation, (very low activity, activation) | 9625734, 12865317, 15784278 | (Lee et al. 2003a; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2C19 | Estrogen | C16α-Hydroxylation (low activity) | 9054608, 9625734, 15784278, 16537715 | (Cribb et al. 2006; Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2E1 | Estrogen | C16α-Hydroxylation (very low activity) | 9054608, 9625734, 15784278 | (Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2B6 | Estrogen | C4-Hydroxylation (very low activity, activation) | 9054608, 9625734, 15784278 | (Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2E1 | Estrogen | C4-Hydroxylation (very low activity, activation) | 9054608, 9625734, 15784278 | (Shou et al. 1997; Yamazaki et al. 1998b; Zhu and Lee 2005) |
| Estrone | 2C8 | Estrogen | C-4 Hydroxylation (very low activity, activation) | 9054608, 9625734 | (Shou et al. 1997; Yamazaki et al. 1998b) |
| Estrone | 1A1 | Estrogen | C6α-Hydroxylation, low activity | 12865317 | (Lee et al. 2003a) |
| Estrone | 1A1 | Estrogen | C2-Hydroxylation, major metabolite, activation | 12865317, 25678418 | (Lee et al. 2003a; Niwa et al. 2015) |
| Estrone | 1A2 | Estrogen | C2-Hydroxylation, high activity, activation | 12865317 | (Lee et al. 2003a) |
| Estrone | 1B1 | Estrogen | C2-Hydroxylation, low activity, activation | 12865317, 25678418 | (Lee et al. 2003a; Niwa et al. 2015) |
| Estrone | 3A4 | Estrogen | C2-Hydroxylation, activation | 12865317, 25678418 | (Lee et al. 2003a; Niwa et al. 2015) |
| Estrone | 3A5 | Estrogen | C2-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
| Estrone | 3A5 | Estrogen | C4-Hydroxylation, activation | 12865317 | (Lee et al. 2003a) |
Although a relatively low number of activations are ascribed to P450 enzymes interacting with physiological compounds, some of them are important because they can possibly cause either cancer (e.g., estrogenic hormones) or have important impact on physiological processes related to high blood pressure (arachidonic acid).
17β-Estradiol and estrone
Estrogenic hormones (e.g., 17β-estradiol and estrone) can induce tumors in various organs of experimental animals (Lacassagne 1932). In humans, elevated circulating estrogen levels increase the risk of breast and uterine cancer. Estrogens can act as hormone stimulating cell proliferators and also as procarcinogens, inducing genetic damage (Yager 2000; Liehr 2000). 17β-Estradiol and estrone are eliminated from the body by metabolic conversion to inactive metabolites that are excreted in the urine and/or feces following oxidations and conjugation reactions. The first step in the metabolism of estrogens is hydroxylation catalyzed by P450 enzymes (Fishman et al. 1970; Zhu and Lee 2005). A large number of hydroxylated metabolites are formed and catalyzed by P450 Family 1–4 (Table 5); however, we focus here on reactions leading to formation of activated and toxic metabolites. Activations of 17β-estradiol and estrone by hydroxylation at positions C2 and C4 have been suggested to be major reactions involved in mammary carcinogenesis and other cancers (Cavalieri and Rogan 2006; Cavalieri et al. 2006). The data (Table 5 and references therein) also show that formation of the major metabolite of 17β-estradiol, 2-hydroxyestradiol, is mainly catalyzed by P450s 1A2 and 3A4, and by P450 1A1 in extrahepatic tissues. P450 1B1, which is highly expressed in estrogen target tissues including mammary, ovary, and uterus, selectively catalyzes the 4-hydroxylation of 17β-estradiol (Guengerich et al. 2003; Chun and Kim 2016; Wen et al. 2007) Formation of catechols of estrone and estradiol is considered as a part of the carcinogenic process, in that these compounds can readily be further oxidized to reactive quinones, semiquinones, and reactive oxygen species are formed (Bolton and Thatcher 2008). 4-Hydroxyestradiol can generate free radicals from redox cycling, with formation of corresponding semiquinone and quinone forms causing cellular damage. Local formation of 4-hydroxyestradiol in breast and endometrial cancers has been reported (Tsuchiya et al. 2005; Hayes et al. 1996; Spink et al. 1997; Liehr 2000; Shimada et al. 1999; Bolton 2002; Bolton and Thatcher 2008; Fussell et al. 2011). Estradiol-3,4-quinone is more reactive with DNA than estradiol-2,3-quinone, and the relative reactivities of estradiol-3,4-quinone and estradiol-2,3-quinone to form depurinating adducts have been correlated with the carcinogenicity, mutagenicity, and cell-transforming activity of their precursors, the catechol estrogens 4-hydroxyestradiol and 2-hydroxyestradiol (Zahid et al. 2006).
Numerous P450s have been detected in breast tumor or adjacent tissue, including P450s 1A1, 1B1, 2A5, 2B6, 2C9, 2D6, 2E1, 2J2, 2S1, 2U1, 3A4, 3A5, 3A43, 4A11, 4V2, 4X1, 4Z1, 26A1, and of course 19A1 (Hellmold et al. 1998; Huang et al. 1996; Iscan et al. 2001; Schmidt et al. 2004). Of these, three enzymes are involved to major extent in estradiol hydroxylation (i.e. P450s 1A1, 1B1, and 3A4) (Fig. 24). P450 2C9 is also involved in the conversion of both estradiol and estrone, with low activity in forming C4- and C16-hydroxylated products (Table 5). P450 enzymes involved in estrogen metabolism are expressed in both tumor and non-tumor breast tissue; however, higher levels of P450 1B1 and 3A4 were found more often in non-tumor tissue than in tumor tissue. It has been suggested that local activation of estrogen to potentially reactive metabolites by the P450s in breast tissue may play a role in initiating and promoting the carcinogenic process (Modugno et al. 2003).
Fig 24.
Metabolism and activation of 17β-estradiol and estrone by P450 enzymes
In breast tumor cells, P450 1A1 and 1B1 mRNA levels and rates of both estradiol 2- and 4-hydroxylation were elevated following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Spink et al. 1998). In addition, the inhibitory effects of ketoconazole, cyclosporin A, and cimetidine (inhibitors of P450 enzymes) toward P450 3A4-catalyzed estradiol 2-hydroxylation were reported, and the IC50 values were 7 nM, 64 nM, and 290 μM, respectively (Satoh et al. 2000). It was also reported that non-ortho-substituted polychlorinated biphenyl congeners can, depending on the structure, induce or inhibit P450 1B1 and 1A1 activity and consequently that they might affect the formation of 2- and 4- hydroxylated metabolites of estradiol and the potential for mammary tumorigenesis (Spink et al. 2002a; Pang et al. 1999). Resveratrol was reported to strongly inhibit the TCDD-induced aryl hydrocarbon receptor DNA binding activity, the expression of P450 1A1 and 1B1, and P450 1A1 and 1B1 catalytic activities in MCF-10A breast cancer cells. Resveratrol also reduced the formation of 2- and 4-hydroxyestradiol from 17β-estradiol by recombinant human P450s 1A1 and 1B1, respectively. Furthermore, resveratrol significantly attenuated intracellular reactive oxygen species formation and oxidative DNA damage, and the cytotoxicity induced by the catechol estrogens (Chen et al. 2004). In addition to chemicals that induce or inhibit activity of P450 enzymes, genetic variation of the enzymes (e.g., P450 1B1) can also affect the metabolic activation and carcinogenesis of 17β-estradiol and estrone, although the effects have not been shown to be large (Shimada et al. 1999; Watanabe et al. 2000). Changes in the expression levels of estrogen-metabolizing P450s not only alter the activity of substrates but may also have physiological effects in liver and target tissues (Chun and Kim 2016).
Arachidonic acid
Arachidonic acid metabolites are key mediators involved in the pathogenesis of numerous cardiovascular, pulmonary, inflammatory, and thromboembolic diseases. Thromboxane A2 is produced by the action of thromboxane synthase (P450 5A1) on the prostaglandin endoperoxide H2 (PGH2), a product of the enzymatic transformation of arachidonic acid by the cyclooxygenases (Rendic and Guengerich 2018). Arachidonic acid is metabolized in a number of tissues (liver, kidney, lung, brain, and the vasculature) by P450 enzymes that form hydroxyeicosatetraenoic acids (HETEs) or epoxides (epoxyeicosatrienoic acids, EETs) (Fig. 25). The reactions occur in different organs (brain, kidney, lung, vasculature, liver). EETs and HETEs have different biological properties, based on sites of production, and can be stored in tissue lipids and released in response to hormonal stimuli.
Fig 25.

Metabolism and activation of arachidonic acid to 20-HETE and EETs by P450 enzymes
20-HETE has both pro- and anti-hypertensive actions that result from modulation of vascular and kidney function. 20-HETE is a potent vasoconstrictor, and upregulation of the production of this compound can contributes to elevation of endothelial dysfunction and the increase in peripheral vascular resistance associated with some forms of hypertension. In kidney, 20-HETE exerts anti-hypertensive action by inhibiting sodium reabsorption by the kidney in both the proximal tubule and thick ascending limb of Henle (Williams et al. 2010; Garcia et al. 2017; Zhang et al. 2018; Roman 2002). Formation of 20-HETE is catalyzed by human P450s 4A11, 4F2, and F3B and the epoxygenation of arachidonic acid to EETs is catalyzed by P450s 2C8, 2C9, 2C19, and 2J2 and (to a much lesser extent) by P450 2W1 (Table 5 and references therein). The arachidonic acid products 20-HETE and EETs compose a group of compounds that participate in the regulation of liver metabolic activity and hemodynamics, may be involved in abnormalities related to liver diseases (e.g., cirrhosis), and play a key role in the pathophysiology of portal hypertension and renal failure (Sacerdoti et al. 2003). Arachidonic acid, as a model for metabolic activation of polyunsaturated fatty acids, produced a concentration- and time-dependent toxicity to Hep G2-MV2E1–9 cells, which express P450 2E1, proposed to be related to reactive oxygen intermediates and lipid peroxidation (Chen et al. 1997).
Concluding remarks
The data on activation of xenobiotics and endobiotics catalyzed by P450 enzymes in Families 1–4 are divided into groups of General Chemicals, Drugs, Natural Products, and Physiological Compounds. The metabolites formed are direct toxicants reacting with cell macromolecules in many cases. However, in selected cases the metabolites are not direct toxicants but participate as substrates in additional metabolic reactions (e.g., conjugation reactions) and the resulting products are final toxicants (e.g., estragole). In other cases, the product elicits physiological responses through indirect biological activities (e.g., 20-HETE, EETs). We have emphasized the observed higher number of activations of three groups of compounds (General Chemicals, Drugs, and Natural Products) yielding activated metabolites and the lower fraction of Physiological Compounds involved as substrates in activation reactions catalyzed by P450 enzymes belonging to Families 1–4, exemplified by estrogen hormones and arachidonic acid. In the group of General Chemicals, P450s 1A1, 1A2, and 1B1 are dominant in the formation of activated metabolites, followed by P450s 3A4 and 2E1 (Fig. 2); in the group Drugs (Fig. 9) P450 3A4 dominates in the formation of activated metabolites. In the group of Natural Products, P450s 1A2, 3A4, and 2E1 dominate in the formation of activated metabolites, followed by P450s 1A1 and 2A6 (Fig. 16); in the group of Physiological Compounds there was no clearly dominant P450 but the highest number of activations is attributed to P450s 1A, 1B1 and 3A (Fig. 23). The results show that Physiological Compounds are substrates infrequently in bioactivation reactions catalyzed by P450 enzymes belonging to Families 1–4, with the gexception of estrogens and arachidonic acid.
The results presented give information on the enzymes that dominate in bioactivation of specific group of chemicals and might be used as guide on which enzymes to direct research when testing their bioactivation to toxic metabolites.
Fig 6.

Activation of chlorpyrifos to a toxic oxon product by P450 enzymes
Fig 12.

Metabolic activation of acetaminophen (paracetamol)
Acknowledgements
We thank K. Trisler for assistance in preparation of the manuscript.
Funding F.P.G. acknowledges current support from the United States National Institutes of Health Grant R01 GM118122. The Alexander von Humbolt Fundation, Bon Bad Godesberg, FR Germany, supported research of S.R. in the field of P450 enzymes on several occasions from 1978-2001 at the Institute for Physiological Chemistry, University of Saarland, Homburg, Saar, Germany, Faculty of Biology, University of Konstanz, Germany, and Institute of Biochemistry of the German Sport University Cologne, Cologne, Germany, and his collaboration with Prof. Dr. Volker Ullrich and Prof. Dr. Manfred Donike.
Footnotes
Availability of data and materials (data transparency). All the data are available in the text and tables of the review.
Conflict of interest The authors declare no conflict of interest, financial or otherwise.
References
- Aklillu E, Oscarson M, Hidestrand M, Leidvik B, Otter C, Ingelman-Sundberg M (2002) Functional analysis of six different polymorphic CYP1B1 enzyme variants found in an Ethiopian population. Mol Pharmacol 61(3):586–594 doi: 10.1124/mol.61.3.586 [DOI] [PubMed] [Google Scholar]
- Al-Subeihi AA, Alhusainy W, Kiwamoto R, et al. (2015) Evaluation of the interindividual human variation in bioactivation of methyleugenol using physiologically based kinetic modeling and Monte Carlo simulations. Toxicol Appl Pharmacol 283(2):117–126 doi: 10.1016/j.taap.2014.12.009 [DOI] [PubMed] [Google Scholar]
- Alvarez-Diez TM, Zheng J (2004) Mechanism-based inactivation of cytochrome P450 3A4 by 4-ipomeanol. Chem Res Toxicol 17(2):150–157 doi: 10.1021/tx034143l [DOI] [PubMed] [Google Scholar]
- Amato G, Grasso E, Longo V, Gervasi PG (2001) Oxidation of N,N-dimethylformamide and N,N-diethylformamide by human liver microsomes and human recombinant P450s. Toxicol Lett 124(1–3):11–19 doi: 10.1016/s0378-4274(01)00324-1 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Gonzalez FJ, Gelboin HV (1989) Human cDNA-expressed cytochrome P450 IA2: mutagen activation and substrate specificity. Mol Carcinog 2(4):192–198 doi: 10.1002/mc.2940020405 [DOI] [PubMed] [Google Scholar]
- Arlt VM, Levová K, Bárta F, et al. (2011) Role of P450 1A1 and P450 1A2 in bioactivation versus detoxication of the renal carcinogen aristolochic acid I: studies in Cyp1a1−/−, Cyp1a2−/−, and Cyp1a1/1a2−/− mice. Chem Res Toxicol 24(10):1710–1719 doi: 10.1021/tx200259y [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborova M, Schmeiser HH (2002) Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 17(4):265–277 doi: 10.1093/mutage/17.4.265 [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborová M, vom Brocke J, et al. (2007) Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis 28(11):2253–2261 doi: 10.1093/carcin/bgm082 [DOI] [PubMed] [Google Scholar]
- Aryal P, Terashita T, Guengerich FP, Shimada T, Oda Y (2000) Use of genetically engineered Salmonella typhimurium OY1002/1A2 strain coexpressing human cytochrome P450 1A2 and NADPH-cytochrome P450 reductase and bacterial O-acetyltransferase in SOS/umu assay. Environ Mol Mutagen 36(2):121–126 doi: [DOI] [PubMed] [Google Scholar]
- Asai H, Imaoka S, Kuroki T, Monna T, Funae Y (1996) Microsomal ethanol oxidizing system activity by human hepatic cytochrome P450s. J Pharmacol Exp Ther 277(2):1004–1009 [PubMed] [Google Scholar]
- Avent KM, DeVoss JJ, Gillam EMJ (2006) Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites. Chem Res Toxicol 19(7):914–920 doi: 10.1021/tx0600090 [DOI] [PubMed] [Google Scholar]
- Back DJ, Maggs JL, Purba HS, Newby S, Park BK (1984) 2-Hydroxylation of ethinyloestradiol in relation to the oxidation of sparteine and antipyrine. Br J Clin Pharmacol 18(4):603–607 doi: 10.1111/j.1365-2125.1984.tb02511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi AF, Cavalieri EL, Rogan EG (2001) Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16a-hydroxylation of 17b-estradiol. Metabolism 50(9):1001–1003 doi: 10.1053/meta.2001.25592 [DOI] [PubMed] [Google Scholar]
- Baer BR, Rettie AE, Henne KR (2005) Bioactivation of 4-ipomeanol by CYP4B1: adduct characterization and evidence for an enedial intermediate. Chem Res Toxicol 18(5):855–864 doi: 10.1021/tx0496993 [DOI] [PubMed] [Google Scholar]
- Baer BR, Wienkers LC, Rock DA (2007) Time-dependent inactivation of P450 3A4 by raloxifene: identification of Cys239 as the site of apoprotein alkylation. Chem Res Toxicol 20(6):954–964 doi: 10.1021/tx700037e [DOI] [PubMed] [Google Scholar]
- Bahari A, Mehrzad J, Mahmoudi M, Bassami MR, Dehghani H (2014) Cytochrome P450 isoforms are differently up-regulated in aflatoxin B₁-exposed human lymphocytes and monocytes. Immunopharmacol Immunotoxicol 36(1):1–10 doi: 10.3109/08923973.2013.850506 [DOI] [PubMed] [Google Scholar]
- Bai J, Cederbaum AI (2004) Adenovirus mediated overexpression of CYP2E1 increases sensitivity of HepG2 cells to acetaminophen induced cytotoxicity. Mol Cell Biochem 262(1–2):165–176 doi: 10.1023/b:mcbi.0000038232.61760.9e [DOI] [PubMed] [Google Scholar]
- Bao Z, He XY, Ding X, Prabhu S, Hong JY (2005) Metabolism of nicotine and cotinine by human cytochrome P450 2A13. Drug Metab Dispos 33(2):258–261 doi: 10.1124/dmd.104.002105 [DOI] [PubMed] [Google Scholar]
- Barbosa-Sicard E, Markovic M, Honeck H, Christ B, Muller DN, Schunck WH (2005) Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem Biophys Res Commun 329(4):1275–1281 doi: 10.1016/j.bbrc.2005.02.103 [DOI] [PubMed] [Google Scholar]
- Barceló S, Macé K, Pfeifer AM, Chipman JK (1998) Production of DNA strand breaks by N-nitrosodimethylamine and 2-amino-3-methylimidazo[4,5-f]quinoline in THLE cells expressing human CYP isoenzymes and inhibition by sulforaphane. Mutat Res 402(1–2):111–120 doi: 10.1016/s0027-5107(97)00288-1 [DOI] [PubMed] [Google Scholar]
- Baum M, Amin S, Guengerich FP, Hecht SS, Köhl W, Eisenbrand G (2001) Metabolic activation of benzo[c]phenanthrene by cytochrome P450 enzymes in human liver and lung. Chem Res Toxicol 14(6):686–693 doi: 10.1021/tx000240s [DOI] [PubMed] [Google Scholar]
- Bell LC, Guengerich FP (1997) Oxidation kinetics of ethanol by human cytochrome P450 2E1. Rate-limiting product release accounts for effects of isotopic hydrogen substitution and cytochrome b5 on steady-state kinetics. J Biol Chem 272(47):29643–29651 doi: 10.1074/jbc.272.47.29643 [DOI] [PubMed] [Google Scholar]
- Bell-Parikh LC, Guengerich FP (1999) Kinetics of cytochrome P450 2E1-catalyzed oxidation of ethanol to acetic acid via acetaldehyde. J Biol Chem 274(34):23833–23840 doi: 10.1074/jbc.274.34.23833 [DOI] [PubMed] [Google Scholar]
- Bendaly J, Zhao S, Neale JR, et al. (2007) 2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline-induced DNA adduct formation and mutagenesis in DNA repair-deficient Chinese hamster ovary cells expressing human cytochrome P450 1A1 and rapid or slow acetylator N-acetyltransferase 2. Cancer Epidemiol Biomarkers Prev 16(7):1503–1509 doi: 10.1158/1055-9965.Epi-07-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson A, Wolf C, Chachaty C, et al. (1993) Metabolic activation of the nitroaromatic antiandrogen flutamide by rat and human cytochromes P-450, including forms belonging to the 3A and 1A subfamilies. J Pharmacol Exp Ther 265(1):366–372 [PubMed] [Google Scholar]
- Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD (2007) CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci 96(9):2224–2231 doi: 10.1002/jps.20892 [DOI] [PubMed] [Google Scholar]
- Bezerra LS, Santos-Veloso MAO, Bezerra Junior NDS, Fonseca LCD, Sales WLA (2018) Impacts of cytochrome P450 2D6 (CYP2D6) genetic polymorphism in tamoxifen therapy for breast cancer. Rev Bras Ginecol Obstet 40(12):794–799 doi: 10.1055/s-0038-1676303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black GP, Collins KS, Blacquiere DP, Forkert PG (2006) Formation of N-alkylprotoporphyrin IX from metabolism of diallyl sulfone in lung and liver. Drug Metab Dispos 34(6):895–900 doi: 10.1124/dmd.106.009928 [DOI] [PubMed] [Google Scholar]
- Bohnenstengel F, Hofmann U, Eichelbaum M, Kroemer HK (1996) Characterization of the cytochrome P450 involved in side-chain oxidation of cyclophosphamide in humans. Eur J Clin Pharmacol 51(3–4):297–301 doi: 10.1007/s002280050201 [DOI] [PubMed] [Google Scholar]
- Bolt HM (1979) Metabolism of estrogens–natural and synthetic. Pharmacol Ther 4(1):155–181 doi: 10.1016/0163-7258(79)90018-4 [DOI] [PubMed] [Google Scholar]
- Bolt HM, Bolt M, Kappus H (1977) Interaction of rifampicin treatment with pharmacokinetics and metabolism of ethinyloestradiol in man. Acta Endocrinol (Copenhagen) 85(1):189–197 doi: 10.1530/acta.0.0850189 [DOI] [PubMed] [Google Scholar]
- Bolton JL (2002) Quinoids, quinoid radicals, and phenoxyl radicals formed from estrogens and antiestrogens. Toxicology 177(1):55–65 doi: 10.1016/s0300-483x(02)00195-6 [DOI] [PubMed] [Google Scholar]
- Bolton JL, Acay NM, Vukomanovic V (1994) Evidence that 4-allyl-o-quinones spontaneously rearrange to their more electrophilic quinone methides: potential bioactivation mechanism for the hepatocarcinogen safrole. Chem Res Toxicol 7(3):443–450 doi: 10.1021/tx00039a024 [DOI] [PubMed] [Google Scholar]
- Bolton JL, Thatcher GR (2008) Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol 21(1):93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JA, Medinsky MA (2001) Insights into the toxicokinetics and toxicodynamics of 1,3-butadiene. Chem-Biol Interact 135–136:599–614 doi: 10.1016/s0009-2797(01)00199-5 [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Brown K, Gibbs AH, Sanchez E, Turteltaub KW, White IN (2002) Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis 23(11):1897–1901 doi: 10.1093/carcin/23.11.1897 [DOI] [PubMed] [Google Scholar]
- Borges S, Desta Z, Li L, et al. (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80(1):61–74 doi: 10.1016/j.clpt.2006.03.013 [DOI] [PubMed] [Google Scholar]
- Bort R, Macé K, Boobis A, Gómez-Lechón MJ, Pfeifer A, Castell J (1999) Hepatic metabolism of diclofenac: role of human CYP in the minor oxidative pathways. Biochem Pharmacol 58(5):787–796 doi: 10.1016/s0006-2952(99)00167-7 [DOI] [PubMed] [Google Scholar]
- Borzelleca JF (2000) Profiles in Toxicology–Paracelsus: herald of modern toxicology. Toxicol Sci 53:2–4 [DOI] [PubMed] [Google Scholar]
- Boysen G, Scarlett CO, Temple B, et al. (2007) Identification of covalent modifications in P450 2E1 by 1,2-epoxy-3-butene in vitro. Chem–Biol Interact 166(1–3):170–175 doi: 10.1016/j.cbi.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Brandon EF, Meijerman I, Klijn JS, et al. (2005) In-vitro cytotoxicity of ET-743 (trabectedin, yondelis), a marine anti-cancer drug, in the Hep G2 cell line: influence of cytochrome P450 and phase II inhibition, and cytochrome P450 induction. Anticancer Drugs 16(9):935–943 doi: 10.1097/01.cad.0000180121.16407.38 [DOI] [PubMed] [Google Scholar]
- Brandon EF, Sparidans RW, Guijt KJ, et al. (2006) In vitro characterization of the human biotransformation and CYP reaction phenotype of ET-743 (yondelis, trabectidin), a novel marine anti-cancer drug. Invest New Drugs 24(1):3–14 doi: 10.1007/s10637-005-4538-9 [DOI] [PubMed] [Google Scholar]
- Brian WR, Sari M-A, Iwasaki M, Shimada T, Kaminsky LS, Guengerich FP (1990) Catalytic activities of human liver cytochrome P-450 IIIA4 expressed in Saccharomyces cerevisiae. Biochemistry 29(51):11280–11292 doi: 10.1021/bi00503a018 [DOI] [PubMed] [Google Scholar]
- Buening MK, Fortner JG, Kappas A, Corney AH (1978) 7,8-Benzoflavone stimulates the metabolic activation of aflatoxin B1 to mutagens by human liver. Biochem Biophys Res Commun 82(1):348–355 doi: 10.1016/0006-291x(78)90616-2 [DOI] [PubMed] [Google Scholar]
- Buratti FM, Leoni C, Testai E (2006) Foetal and adult human CYP3A isoforms in the bioactivation of organophosphorothionate insecticides. Toxicol Lett 167(3):245–255 doi: 10.1016/j.toxlet.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Buratti FM, Volpe MT, Fabrizi L, Meneguz A, Vittozzi L, Testai E (2002) Kinetic parameters of OPT pesticide desulfuration by c-DNA expressed human CYPs. Environ Toxicol Pharmacol 11(3–4):181–190 doi: 10.1016/s1382-6689(02)00010-8 [DOI] [PubMed] [Google Scholar]
- Buratti FM, Volpe MT, Meneguz A, Vittozzi L, Testai E (2003) CYP-specific bioactivation of four organophosphorothioate pesticides by human liver microsomes. Toxicol Appl Pharmacol 186(3):143–154 doi: 10.1016/s0041-008x(02)00027-3 [DOI] [PubMed] [Google Scholar]
- Buters J, Quintanilla-Martinez L, Schober W, et al. (2003) CYP1B1 determines susceptibility to low doses of 7,12-dimethylbenz[a]anthracene-induced ovarian cancers in mice: correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis 24(2):327–334 doi: 10.1093/carcin/24.2.327 [DOI] [PubMed] [Google Scholar]
- Butler AM, Murray M (1997) Biotransformation of parathion in human liver: participation of CYP3A4 and its inactivation during microsomal parathion oxidation. J Pharmacol Exp Ther 280(2):966–973 [PubMed] [Google Scholar]
- Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF (1989) Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A 86(20):7696–7700 doi: 10.1073/pnas.86.20.7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund J, Ericsson J, Oliw EH (1998a) Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS. Anal Biochem 265(1):55–68 doi: 10.1006/abio.1998.2897 [DOI] [PubMed] [Google Scholar]
- Bylund J, Kunz T, Valmsen K, Oliw EH (1998b) Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J Pharmacol Exp Ther 284(1):51–60 [PubMed] [Google Scholar]
- Cabaret O, Puel O, Botterel F, Pean M, Bretagne S, Delaforge M (2011) Contribution of uniformly 13C-enriched sterigmatocystin to the study of its pulmonary metabolism. Rapid Commun Mass Spectrom 25(19):2704–2710 doi: 10.1002/rcm.5068 [DOI] [PubMed] [Google Scholar]
- Cabaret O, Puel O, Botterel F, et al. (2010) Metabolic detoxication pathways for sterigmatocystin in primary tracheal epithelial cells. Chem Res Toxicol 23(11):1673–1681 doi: 10.1021/tx100127b [DOI] [PubMed] [Google Scholar]
- Calinski DM, Zhang H, Ludeman S, Dolan ME, Hollenberg PF (2015) Hydroxylation and N-dechloroethylation of ifosfamide and deuterated ifosfamide by the human cytochrome P450s and their commonly occurring polymorphisms. Drug Metab Dispos 43(7):1084–1090 doi: 10.1124/dmd.115.063628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MD, Wen B, Roberts AG, Atkins WM, Campbell AP, Nelson SD (2007) Cooperative binding of acetaminophen and caffeine within the P450 3A4 active site. Chem Res Toxicol 20(10):1434–1441 doi: 10.1021/tx7000702 [DOI] [PubMed] [Google Scholar]
- Carlson JR, Breeze RG (1984) Ruminal metabolism of plant toxins with emphasis on indolic compounds. J Anim Sci 58(4):1040–1049 doi: 10.2527/jas1984.5841040x [DOI] [PubMed] [Google Scholar]
- Carlson ES, Upadhyaya P, Hecht SS (2016) Evaluation of nitrosamide formation in the cytochrome P450-mediated metabolism of tobacco-specific nitrosamines. Chem Res Toxicol 29(12):2194–2205 doi: 10.1021/acs.chemrestox.6b00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI (2004) Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol 44:27–42 doi: 10.1146/annurev.pharmtox.44.101802.121704 [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Chakravarti D, Guttenplan J, et al. (2006) Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta 1766(1):63–78 doi: 10.1016/j.bbcan.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Rogan E (2006) Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann N Y Acad Sci 1089:286–301 doi: 10.1196/annals.1386.042 [DOI] [PubMed] [Google Scholar]
- Chan W, Cui L, Xu G, Cai Z (2006) Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20(11):1755–1760 doi: 10.1002/rcm.2513 [DOI] [PubMed] [Google Scholar]
- Chang TK, Weber GF, Crespi CL, Waxman DJ (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53(23):5629–5637 [PubMed] [Google Scholar]
- Chang TK, Yu L, Maurel P, Waxman DJ (1997) Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res 57(10):1946–1954 [PubMed] [Google Scholar]
- Chen C, Meng L, Ma X, et al. (2006a) Urinary metabolite profiling reveals CYP1A2-mediated metabolism of NSC686288 (aminoflavone). J Pharmacol Exp Ther 318(3):1330–1342 doi: 10.1124/jpet.106.105213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Jounaidi Y, Waxman DJ (2005) Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab Dispos 33(9):1261–1267 doi: 10.1124/dmd.105.004788 [DOI] [PubMed] [Google Scholar]
- Chen PH, Mahmood Q, Mariottini GL, Chiang TA, Lee KW (2017) Adverse health effects of betel quid and the risk of oral and pharyngeal cancers. Biomed Res Int 2017:3904098 doi: 10.1155/2017/3904098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Doss GA, Tung EC, et al. (2006b) Evidence for the bioactivation of zomepirac and tolmetin by an oxidative pathway: identification of glutathione adducts in vitro in human liver microsomes and in vivo in rats. Drug Metab Dispos 34(1):145–151 doi: 10.1124/dmd.105.004341 [DOI] [PubMed] [Google Scholar]
- Chen Q, Galleano M, Cederbaum AI (1997) Cytotoxicity and apoptosis produced by arachidonic acid in HepG2 cells overexpressing human cytochrome P4502E1. J Biol Chem 272(23):14532–14541 doi: 10.1074/jbc.272.23.14532 [DOI] [PubMed] [Google Scholar]
- Chen Q, Ngui JS, Doss GA, et al. (2002) Cytochrome P450 3A4-mediated bioactivation of raloxifene: irreversible enzyme inhibition and thiol adduct formation. Chem Res Toxicol 15(7):907–914 doi: 10.1021/tx0200109 [DOI] [PubMed] [Google Scholar]
- Chen W, Koenigs LL, Thompson SJ, et al. (1998) Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 11(4):295–301 doi: 10.1021/tx9701687 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Hurh YJ, Na HK, et al. (2004) Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 25(10):2005–2013 doi: 10.1093/carcin/bgh183 [DOI] [PubMed] [Google Scholar]
- Cheung C, Ma X, Krausz KW, et al. (2005a) Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol 18(9):1471–1478 doi: 10.1021/tx050136g [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu AM, Ward JM, et al. (2005b) The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos 33(3):449–457 doi: 10.1124/dmd.104.002402 [DOI] [PubMed] [Google Scholar]
- Chiang HC, Wang CY, Lee HL, Tsou TC (2011) Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced gene mutation–a mammalian cell-based mutagenesis approach. Toxicol Appl Pharmacol 253(2):145–152 doi: 10.1016/j.taap.2011.03.022 [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB (2004) Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos 32(8):840–847 doi: 10.1124/dmd.32.8.840 [DOI] [PubMed] [Google Scholar]
- Christmas P, Carlesso N, Shang H, et al. (2003) Myeloid expression of cytochrome P450 4F3 is determined by a lineage-specific alternative promoter. J Biol Chem 278(27):25133–25142 doi: 10.1074/jbc.M302106200 [DOI] [PubMed] [Google Scholar]
- Chugh R, Wagner T, Griffith KA, et al. (2007) Assessment of ifosfamide pharmacokinetics, toxicity, and relation to CYP3A4 activity as measured by the erythromycin breath test in patients with sarcoma. Cancer 109(11):2315–2322 doi: 10.1002/cncr.22669 [DOI] [PubMed] [Google Scholar]
- Chun Y-J, Kim D (2016) Cancer activation and polymorphisms of human cytochrome P450 1B1. Toxicol Res 32(2):89–93 doi: 10.5487/tr.2016.32.2.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Y-J, Kim S, Kim D, Lee SK, Guengerich FP (2001) A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res 61(22):8164–8170 [PubMed] [Google Scholar]
- Chun Y-J, Lee SK, Kim MY (2005) Modulation of human cytochrome P450 1B1 expression by 2,4,3´,5´-tetramethoxystilbene. Drug Metab Dispos 33(12):1771–1776 doi: 10.1124/dmd.105.006502 [DOI] [PubMed] [Google Scholar]
- Chung YT, Chen CL, Wu CC, Chan SA, Chi CW, Liu TY (2008) Safrole-DNA adduct in hepatocellular carcinoma associated with betel quid chewing. Toxicol Lett 183(1–3):21–27 doi: 10.1016/j.toxlet.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Yeh GC (1999) The steroid hormone dehydroepiandrosterone inhibits CYP1A1 expression in vitro by a post-transcriptional mechanism. J Biol Chem 274(49):35186–35190 doi: 10.1074/jbc.274.49.35186 [DOI] [PubMed] [Google Scholar]
- Clarke TA, Waskell LA (2003) The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos 31(1):53–59 doi: 10.1124/dmd.31.1.53 [DOI] [PubMed] [Google Scholar]
- Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TK, Waxman DJ (1997) Human cytochrome P450 2B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos 25(8):985–993 [PubMed] [Google Scholar]
- Coller JK, Krebsfaenger N, Klein K, et al. (2004) Large interindividual variability in the in vitro formation of tamoxifen metabolites related to the development of genotoxicity. Br J Clin Pharmacol 57(1):105–111 doi: 10.1046/j.1365-2125.2003.01970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulet M, Dacasto M, Eeckhoutte C, et al. (1998) Identification of human and rabbit cytochromes P450 1A2 as major isoforms involved in thiabendazole 5-hydroxylation. Fundam Clin Pharmacol 12(2):225–235 doi: 10.1111/j.1472-8206.1998.tb00946.x [DOI] [PubMed] [Google Scholar]
- Coulet M, Eeckhoutte C, Larrieu G, et al. (2000) Evidence for cytochrome P450 1A2-mediated protein covalent binding of thiabendazole and for its passive intestinal transport: use of human and rabbit derived cells. Chem-Biol Interact 127(2):109–124 doi: 10.1016/s0009-2797(00)00167-8 [DOI] [PubMed] [Google Scholar]
- Crane AL, Klein K, Olson JR (2012a) Bioactivation of chlorpyrifos by CYP2B6 variants. Xenobiotica 42(12):1255–1262 doi: 10.3109/00498254.2012.702246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane AL, Klein K, Zanger UM, Olson JR (2012b) Effect of CYP2B6*6 and CYP2C19*2 genotype on chlorpyrifos metabolism. Toxicology 293(1–3):115–122 doi: 10.1016/j.tox.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS, Sutter TR (1997) Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis 12(2):83–89 doi: 10.1093/mutage/12.2.83 [DOI] [PubMed] [Google Scholar]
- Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM (2002) Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4´-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30(8):869–874 doi: 10.1124/dmd.30.8.869 [DOI] [PubMed] [Google Scholar]
- Cribb AE, Knight MJ, Dryer D, et al. (2006) Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol Biomarkers Prev 15(3):551–558 doi: 10.1158/1055-9965.Epi-05-0801 [DOI] [PubMed] [Google Scholar]
- Crofts FG, Strickland PT, Hayes CL, Sutter TR (1997) Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human cytochrome P4501B1. Carcinogenesis 18(9):1793–1798 doi: 10.1093/carcin/18.9.1793 [DOI] [PubMed] [Google Scholar]
- Crofts FG, Sutter TR, Strickland PT (1998) Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P450 1A1, P450 1A2 and P450 1B1. Carcinogenesis 19(11):1969–1973 doi: 10.1093/carcin/19.11.1969 [DOI] [PubMed] [Google Scholar]
- Croom EL, Wallace AD, Hodgson E (2010) Human variation in CYP-specific chlorpyrifos metabolism. Toxicology 276(3):184–191 doi: 10.1016/j.tox.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Czerwinski M, McLemore TL, Philpot RM, et al. (1991) Metabolic activation of 4-ipomeanol by complementary DNA-expressed human cytochromes P-450: evidence for species-specific metabolism. Cancer Res 51(17):4636–4638 [PubMed] [Google Scholar]
- Dai D, Tang J, Rose R, et al. (2001a) Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther 299(3):825–831 [PubMed] [Google Scholar]
- Dai D, Zeldin DC, Blaisdell JA, et al. (2001b) Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 11(7):597–607 doi: 10.1097/00008571-200110000-00006 [DOI] [PubMed] [Google Scholar]
- Dai J, Zhang F, Zheng J (2010) Retrorsine, but not monocrotaline, is a mechanism-based inactivator of P450 3A4. Chem-Biol Interact 183(1):49–56 doi: 10.1016/j.cbi.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Dai Y, Rashba-Step J, Cederbaum AI (1993) Stable expression of human cytochrome P450 2E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry 32(27):6928–6937 doi: 10.1021/bi00078a017 [DOI] [PubMed] [Google Scholar]
- Daikh BE, Lasker JM, Raucy JL, Koop DR (1994) Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J Pharmacol Exp Ther 271(3):1427–1433 [PubMed] [Google Scholar]
- de Groene EM, Hassing IG, Blom MJ, Seinen W, Fink-Gremmels J, Horbach GJ (1996) Development of human cytochrome P450-expressing cell lines: application in mutagenicity testing of ochratoxin A. Cancer Res 56(2):299–304 [PubMed] [Google Scholar]
- Dehal SS, Kupfer D (1999) Cytochrome P-450 3A and 2D6 catalyze ortho-hydroxylation of 4-hydroxytamoxifen and 3-hydroxytamoxifen (droloxifene) yielding tamoxifen catechol: involvement of catechols in covalent binding to hepatic proteins. Drug Metab Dispos 27(6):681–688 [PubMed] [Google Scholar]
- Delannée V, Langouët S, Théret N, Siegel A (2017) A modeling approach to evaluate the balance between bioactivation and detoxification of MeIQx in human hepatocytes. PeerJ 5:e3703 doi: 10.7717/peerj.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braver MW, den Braver-Sewradj SP, Vermeulen NP, Commandeur JN (2016) Characterization of cytochrome P450 isoforms involved in sequential two-step bioactivation of diclofenac to reactive p-benzoquinone imines. Toxicol Lett 253:46–54 doi: 10.1016/j.toxlet.2016.04.022 [DOI] [PubMed] [Google Scholar]
- Deng J, Zhao L, Zhang NY, et al. (2018) Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res 778:79–89 doi: 10.1016/j.mrrev.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310(3):1062–1075 doi: 10.1124/jpet.104.065607 [DOI] [PubMed] [Google Scholar]
- Dicke KE, Skrlin SM, Murphy SE (2005) Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-butanone metabolism by cytochrome P450 2B6. Drug Metab Dispos 33(12):1760–1764 doi: 10.1124/dmd.105.006718 [DOI] [PubMed] [Google Scholar]
- Doehmer J, Holtkamp D, Soballa V, et al. (1995) Cytochrome P450 mediated reactions studied in genetically engineered V79 Chinese hamster cells. Pharmacogenetics 5 Spec No:S91–96 doi: 10.1097/00008571-199512001-00008 [DOI] [PubMed] [Google Scholar]
- Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD (2000) Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab Dispos 28(12):1397–1400 [PubMed] [Google Scholar]
- Driscoll JP, Kornecki K, Wolkowski JP, Chupak L, Kalgutkar AS, O’Donnell JP (2007) Bioactivation of phencyclidine in rat and human liver microsomes and recombinant P450 2B enzymes: evidence for the formation of a novel quinone methide intermediate. Chem Res Toxicol 20(10):1488–1497 doi: 10.1021/tx700145k [DOI] [PubMed] [Google Scholar]
- Duescher RJ, Elfarra AA (1994) Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch Biochem Biophys 311(2):342–349 doi: 10.1006/abbi.1994.1246 [DOI] [PubMed] [Google Scholar]
- Duisken M, Benz D, Peiffer TH, Blömeke B, Hollender J (2005) Metabolism of Δ3-carene by human cytochrome P450 enzymes: identification and characterization of two new metabolites. Curr Drug Metab 6(6):593–601 doi: 10.2174/138920005774832614 [DOI] [PubMed] [Google Scholar]
- Durant JL, Lafleur AL, Busby WF Jr., Donhoffner LL, Penman BW, Crespi CL (1999) Mutagenicity of C24H14 PAH in human cells expressing CYP1A1. Mutat Res 446(1):1–14 doi: 10.1016/s1383-5718(99)00135-7 [DOI] [PubMed] [Google Scholar]
- Eaton DL (2000) Biotransformation enzyme polymorphism and pesticide susceptibility. Neurotoxicology 21(1–2):101–111 [PubMed] [Google Scholar]
- Edwards RJ, Murray BP, Murray S, et al. (1994) Contribution of CYP1A1 and CYP1A2 to the activation of heterocyclic amines in monkeys and human. Carcinogenesis 15(5):829–836 doi: 10.1093/carcin/15.5.829 [DOI] [PubMed] [Google Scholar]
- Einolf HJ, Story WT, Marcus CB, et al. (1997) Role of cytochrome P450 enzyme induction in the metabolic activation of benzo[c]phenanthrene in human cell lines and mouse epidermis. Chem Res Toxicol 10(5):609–617 doi: 10.1021/tx960174n [DOI] [PubMed] [Google Scholar]
- Ekhart C, Doodeman VD, Rodenhuis S, Smits PH, Beijnen JH, Huitema AD (2009) Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa. Br J Clin Pharmacol 67(1):50–60 doi: 10.1111/j.1365-2125.2008.03321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Wrighton SA (1999) The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev 31(3):719–754 doi: 10.1081/dmr-100101942 [DOI] [PubMed] [Google Scholar]
- El Adlouni C, Pinelli E, Azémar B, Zaoui D, Beaune P, Pfohl-Leszkowicz A (2000) Phenobarbital increases DNA adduct and metabolites formed by ochratoxin A: role of CYP 2C9 and microsomal glutathione-S-transferase. Environ Mol Mutagen 35(2):123–131 doi: [DOI] [PubMed] [Google Scholar]
- Elfarra AA, Krause RJ, Selzer RR (1996) Biochemistry of 1,3-butadiene metabolism and its relevance to 1,3-butadiene-induced carcinogenicity. Toxicology 113(1–3):23–30 doi: 10.1016/0300-483x(96)03423-3 [DOI] [PubMed] [Google Scholar]
- Ellison CA, Tian Y, Knaak JB, Kostyniak PJ, Olson JR (2012) Human hepatic cytochrome P450-specific metabolism of the organophosphorus pesticides methyl parathion and diazinon. Drug Metab Dispos 40(1):1–5 doi: 10.1124/dmd.111.042572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun CY, Han S, Lim YR, et al. (2010) Bioactivation of aromatic amines by human CYP2W1, an orphan cytochrome P450 enzyme. Toxicol Res 26(3):171–175 doi: 10.5487/tr.2010.26.3.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Roman RJ (2017) Effect of cytochrome P450 metabolites of arachidonic acid in nephrology. J Am Soc Nephrol 28(10):2845–2855 doi: 10.1681/asn.2017030252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Sun G, Zhao L, Cui X, Zhong R (2019) Metabolic activation and carcinogenesis of tobacco-specific nitrosamine Ń-nitrosonornicotine (NNN): a density function theory and molecular docking study. Int J Environ Res Public Health 16(2) doi: 10.3390/ijerph16020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Baker GB, Silverstone PH, Coutts RT (1997) Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol 17(2):227–233 doi: 10.1023/a:1026317929335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, McKay G, Song J, Remillrd A, Li X, Midha K (2001) In vitro characterization of the metabolism of haloperidol using recombinant cytochrome P450 enzymes and human liver microsomes. Drug Metab Dispos 29(12):1638–1643 [PubMed] [Google Scholar]
- Fashe MM, Juvonen RO, Petsalo A, Vepsäläinen J, Pasanen M, Rahnasto-Rilla M (2015) In silico prediction of the site of oxidation by cytochrome P450 3A4 that leads to the formation of the toxic metabolites of pyrrolizidine alkaloids. Chem Res Toxicol 28(4):702–710 doi: 10.1021/tx500478q [DOI] [PubMed] [Google Scholar]
- Finn RD, Henderson CJ, Scott CL, Wolf CR (2009) Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J 417(1):43–54 doi: 10.1042/bj20080740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J, Guzik H, Hellman L (1970) Aromatic ring hydroxylation of estradiol in man. Biochemistry 9(7):1593–1598 doi: 10.1021/bi00809a018 [DOI] [PubMed] [Google Scholar]
- Fleming CM, Branch RA, Wilkinson GR, Guengerich FP (1992) Human liver microsomal N-hydroxylation of dapsone by cytochrome P-450 3A4. Mol Pharmacol 41(5):975–980 [PubMed] [Google Scholar]
- Forkert PG, Premdas PD, Bowers RJ (2000) Epoxide formation from diallyl sulfone is associated with CYP2E1 inactivation in murine and human lungs. Am J Respir Cell Mol Biol 23(5):687–695 doi: 10.1165/ajrcmb.23.5.4149 [DOI] [PubMed] [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR (2007) Human hepatic cytochrome P450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos 35(2):189–193 doi: 10.1124/dmd.106.012427 [DOI] [PubMed] [Google Scholar]
- Frei E, Bieler CA, Arlt VM, Wiessler M, Stiborová M (2002) Covalent binding of the anticancer drug ellipticine to DNA in V79 cells transfected with human cytochrome P450 enzymes. Biochem Pharmacol 64(2):289–295 doi: 10.1016/s0006-2952(02)01072-9 [DOI] [PubMed] [Google Scholar]
- Fujita K, Kamataki T (2001a) Predicting the mutagenicity of tobacco-related N-nitrosamines in humans using 11 strains of Salmonella typhimurium YG7108, each coexpressing a form of human cytochrome P450 along with NADPH-cytochrome P450 reductase. Environ Mol Mutagen 38(4):339–346 doi: 10.1002/em.10036 [DOI] [PubMed] [Google Scholar]
- Fujita K, Kamataki T (2001b) Role of human cytochrome P450 (CYP) in the metabolic activation of N-alkylnitrosamines: application of genetically engineered Salmonella typhimurium YG7108 expressing each form of CYP together with human NADPH-cytochrome P450 reductase. Mutat Res 483(1–2):35–41 doi: 10.1016/s0027-5107(01)00223-8 [DOI] [PubMed] [Google Scholar]
- Fussell KC, Udasin RG, Smith PJ, Gallo MA, Laskin JD (2011) Catechol metabolites of endogenous estrogens induce redox cycling and generate reactive oxygen species in breast epithelial cells. Carcinogenesis 32(8):1285–1293 doi: 10.1093/carcin/bgr109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher EP, Kunze KL, Stapleton PL, Eaton DL (1996) The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol Appl Pharmacol 141(2):595–606 doi: 10.1006/taap.1996.0326 [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Wienkers LC, Stapleton PL, Kunze KL, Eaton DL (1994) Role of human microsomal and human complementary DNA-expressed cytochromes P450 1A2 and P450 3A4 in the bioactivation of aflatoxin B1. Cancer Res 54(1):101–108 [PubMed] [Google Scholar]
- Ganesan S, Sahu R, Walker LA, Tekwani BL (2010) Cytochrome P450-dependent toxicity of dapsone in human erythrocytes. J Appl Toxicol 30(3):271–275 doi: 10.1002/jat.1493 [DOI] [PubMed] [Google Scholar]
- Garcia V, Gilani A, Shkolnik B, et al. (2017) 20-HETE signals through G-protein-coupled receptor GPR75 (G(q)) to affect vascular function and trigger hypertension. Circ Res 120(11):1776–1788 doi: 10.1161/circresaha.116.310525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner I, Wakazono H, Bergin P, et al. (1997) Cytochrome P450 mediated bioactivation of methyleugenol to 1´-hydroxymethyleugenol in Fischer 344 rat and human liver microsomes. Carcinogenesis 18(9):1775–1783 doi: 10.1093/carcin/18.9.1775 [DOI] [PubMed] [Google Scholar]
- Gautier JC, Lecoeur S, Cosme J, et al. (1996) Contribution of human cytochrome P450 to benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol metabolism, as predicted from heterologous expression in yeast. Pharmacogenetics 6(6):489–499 doi: 10.1097/00008571-199612000-00002 [DOI] [PubMed] [Google Scholar]
- Gelhaus SL, Harvey RG, Penning TM, Blair IA (2011) Regulation of benzo[a]pyrene-mediated DNA- and glutathione-adduct formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human lung cells. Chem Res Toxicol 24(1):89–98 doi: 10.1021/tx100297z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervot L, Rochat B, Gautier JC, et al. (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9(3):295–306 [PubMed] [Google Scholar]
- Gill HJ, Tingle MD, Park BK (1995) N-Hydroxylation of dapsone by multiple enzymes of cytochrome P450: implications for inhibition of haemotoxicity. Br J Clin Pharmacol 40(6):531–538 doi: 10.1111/j.1365-2125.1995.tb05797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam EMJ, Guo Z, Ueng Y-F, et al. (1995) Expression of cytochrome P450 3A5 in Escherichia coli: effects of 5´ modification, purification, spectral characterization, reconstitution conditions, and catalytic activities. Arch Biochem Biophys 317(2):374–384 doi: 10.1006/abbi.1995.1177 [DOI] [PubMed] [Google Scholar]
- Gillam EMJ, Wunsch RM, Ueng Y-F, et al. (1997) Expression of cytochrome P450 3A7 in Escherichia coli: effects of 5´ modification and catalytic characterization of recombinant enzyme expressed in bicistronic format with NADPH-cytochrome P450 reductase. Arch Biochem Biophys 346(1):81–90 doi: 10.1006/abbi.1997.0286 [DOI] [PubMed] [Google Scholar]
- Goda R, Nagai D, Akiyama Y, et al. (2006) Detection of a new N-oxidized metabolite of flutamide, N-[4-nitro-3-(trifluoromethyl)phenyl]hydroxylamine, in human liver microsomes and urine of prostate cancer patients. Drug Metab Dispos 34(5):828–835 doi: 10.1124/dmd.105.008623 [DOI] [PubMed] [Google Scholar]
- Gökmen MR, Cosyns JP, Arlt VM, et al. (2013) The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann Intern Med 158(6):469–477 doi: 10.7326/0003-4819-158-6-201303190-00006 [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Gelboin HV (1994) Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev 26(1–2):165–183 doi: 10.3109/03602539409029789 [DOI] [PubMed] [Google Scholar]
- Granvil CP, Madan A, Sharkawi M, Parkinson A, Wainer IW (1999) Role of CYP2B6 and CYP3A4 in the in vitro N-dechloroethylation of (R)- and (S)-ifosfamide in human liver microsomes. Drug Metab Dispos 27(4):533–541 [PubMed] [Google Scholar]
- Grollman AP, Shibutani S, Moriya M, et al. (2007) Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A 104(29):12129–12134 doi: 10.1073/pnas.0701248104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP (1988) Oxidation of 17a-ethynylestradiol by human liver cytochrome P-450. Mol Pharmacol 33(5):500–508 [PubMed] [Google Scholar]
- Guengerich FP (1990a) Inhibition of oral contraceptive steroid-metabolizing enzymes by steroids and drugs. Am J Obstetrics Gynecol 163(6 Pt 2):2159–2163 [DOI] [PubMed] [Google Scholar]
- Guengerich FP (1990b) Mechanism-based inactivation of human liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem Res Toxicol 3(4):363–371 [DOI] [PubMed] [Google Scholar]
- Guengerich FP (1993) The 1992 Bernard B. Brodie Award Lecture. Bioactivation and detoxication of toxic and carcinogenic chemicals. Drug Metab Dispos 21(1):1–6 [PubMed] [Google Scholar]
- Guengerich FP (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17 doi: 10.1146/annurev.pharmtox.39.1.1 [DOI] [PubMed] [Google Scholar]
- Guengerich FP (2020) A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol Res:1–23 doi: 10.1007/s43188-020-00056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Avadhani NG (2018) Roles of cytochrome P450 in metabolism of ethanol and carcinogens. Adv Exp Med Biol 1032:15–35 doi: 10.1007/978-3-319-98788-0_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Chun Y-J, Kim D, Gillam EMJ, Shimada T (2003) Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat Res 523–524:173–182 doi: 10.1016/s0027-5107(02)00333-0 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Kim D-H (1991) Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1,N6-ethenoadenosine. Chem Res Toxicol 4(4):413–421 doi: 10.1021/tx00022a003 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M (1991) Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 4(2):168–179 doi: 10.1021/tx00020a008 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Parikh A, Turesky RJ, Josephy PD (1999) Inter-individual differences in the metabolism of environmental toxicants: cytochrome P450 1A2 as a prototype. Mutat Res 428(1–2):115–124 doi: 10.1016/s1383-5742(99)00039-3 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Rendic S (2010) Update information on drug metabolism systems–2009, part I. Curr Drug Metab 11(1):1–3 doi: 10.2174/138920010791110908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Shimada T (1998) Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res 400(1–2):201–213 doi: 10.1016/s0027-5107(98)00037-2 [DOI] [PubMed] [Google Scholar]
- Guo J, Johansson I, Mkrtchian S, Ingelman-Sundberg M (2016) The CYP2W1 enzyme: regulation, properties and activation of prodrugs. Drug Metab Rev 48(3):369–378 doi: 10.1080/03602532.2016.1188939 [DOI] [PubMed] [Google Scholar]
- Hamitouche S, Poupon J, Dreano Y, Amet Y, Lucas D (2006) Ethanol oxidation into acetaldehyde by 16 recombinant human cytochrome P450 isoforms: role of CYP2C isoforms in human liver microsomes. Toxicol Lett 167(3):221–230 doi: 10.1016/j.toxlet.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Hammons GJ, Milton D, Stepps K, Guengerich FP, Tukey RH, Kadlubar FF (1997) Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis 18(4):851–854 doi: 10.1093/carcin/18.4.851 [DOI] [PubMed] [Google Scholar]
- Han J, Xian Z, Zhang Y, Liu J, Liang A (2019) Systematic overview of aristolochic acids: nephrotoxicity, carcinogenicity, and underlying mechanisms. Front Pharmacol 10:648 doi: 10.3389/fphar.2019.00648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF (2000) Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res 60(13):3440–3444 [PubMed] [Google Scholar]
- Hansten PD (2018) The underrated risks of tamoxifen drug interactions. Eur J Drug Metab Pharmacokinet 43(5):495–508 doi: 10.1007/s13318-018-0475-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harleton E, Webster M, Bumpus NN, Kent UM, Rae JM, Hollenberg PF (2004) Metabolism of N,Ń,Ń´-triethylenethiophosphoramide by CYP2B1 and CYP2B6 results in the inactivation of both isoforms by two distinct mechanisms. J Pharmacol Exp Ther 310(3):1011–1019 doi: 10.1124/jpet.104.069112 [DOI] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR (1996) 17b-Estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A 93(18):9776–9781 doi: 10.1073/pnas.93.18.9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazai E, Vereczkey L, Monostory K (2002) Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun 291(4):1089–1094 doi: 10.1006/bbrc.2002.6541 [DOI] [PubMed] [Google Scholar]
- He K, Talaat RE, Pool WF, et al. (2004a) Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab Dispos 32(6):639–646 doi: 10.1124/dmd.32.6.639 [DOI] [PubMed] [Google Scholar]
- He K, Woolf TF, Kindt EK, Fielder AE, Talaat RE (2001) Troglitazone quinone formation catalyzed by human and rat CYP3A: an atypical CYP oxidation reaction. Biochem Pharmacol 62(2):191–198 doi: 10.1016/s0006-2952(01)00653-0 [DOI] [PubMed] [Google Scholar]
- He XY, Shen J, Ding X, Lu AYH, Hong JY (2004b) Identification of critical amino acid residues of human CYP2A13 for the metabolic activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific carcinogen. Drug Metab Dispos 32(12):1516–1521 doi: 10.1124/dmd.104.001370 [DOI] [PubMed] [Google Scholar]
- He XY, Tang L, Wang SL, Cai QS, Wang JS, Hong JY (2006) Efficient activation of aflatoxin B1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. Int J Cancer 118(11):2665–2671 doi: 10.1002/ijc.21665 [DOI] [PubMed] [Google Scholar]
- Hecht SS (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 11(6):559–603 doi: 10.1021/tx980005y [DOI] [PubMed] [Google Scholar]
- Held-Warmkessel J (2003) Ecteinascidin-743. Clin J Oncol Nurs 7(3):313–319 doi: 10.1188/03.Cjon.313-319 [DOI] [PubMed] [Google Scholar]
- Hellmold H, Rylander T, Magnusson M, Reihner E, Warner M, Gustafsson J-Å (1998) Characterization of cytochrome P450 enzymes in human breast tissue from reduction mammaplasties. J Clin Endocrinol Metab 83(3):886–895 doi: 10.1210/jcem.83.3.4647 [DOI] [PubMed] [Google Scholar]
- Hirani V, Yarovoy A, Kozeska A, Magnusson RP, Lasker JM (2008) Expression of CYP4F2 in human liver and kidney: assessment using targeted peptide antibodies. Arch Biochem Biophys 478(1):59–68 doi: 10.1016/j.abb.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PC, Abbott FS, Zanger UM, Chang TK (2003) Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenomics J 3(6):335–342 doi: 10.1038/sj.tpj.6500210 [DOI] [PubMed] [Google Scholar]
- Hollenberg PF, Kent UM, Bumpus NN (2008) Mechanism-based inactivation of human cytochromes P450s: experimental characterization, reactive intermediates, and clinical implications. Chem Res Toxicol 21(1):189–205 doi: 10.1021/tx7002504 [DOI] [PubMed] [Google Scholar]
- Hu L, Wu F, He J, Zhong L, Song Y, Shao H (2019) Cytotoxicity of safrole in HepaRG cells: studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica 49(12):1504–1515 doi: 10.1080/00498254.2019.1590882 [DOI] [PubMed] [Google Scholar]
- Hu Y, Dehal SS, Hynd G, Jones GB, Kupfer D (2003) CYP2D6-mediated catalysis of tamoxifen aromatic hydroxylation with an NIH shift: similar hydroxylation mechanism in chicken, rat and human liver microsomes. Xenobiotica 33(2):141–151 doi: 10.1080/0049825021000042733 [DOI] [PubMed] [Google Scholar]
- Huang YS, Chern HD, Su WJ, et al. (2003) Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 37(4):924–930 doi: 10.1053/jhep.2003.50144 [DOI] [PubMed] [Google Scholar]
- Huang Z, Fasco MJ, Figge HL, Keyomarsi K, Kaminsky LS (1996) Expression of cytochromes P450 in human breast tissue and tumors. Drug Metab Dispos 24(8):899–905 [PubMed] [Google Scholar]
- Huang Z, Guengerich FP, Kaminsky LS (1998) 16a-Hydroxylation of estrone by human cytochrome P4503A4/5. Carcinogenesis 19(5):867–872 doi: 10.1093/carcin/19.5.867 [DOI] [PubMed] [Google Scholar]
- Huang Z, Roy P, Waxman DJ (2000) Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol 59(8):961–972 doi: 10.1016/s0006-2952(99)00410-4 [DOI] [PubMed] [Google Scholar]
- Hyland R, Gescher A, Thummel K, et al. (1992) Metabolic oxidation and toxification of N-methylformamide catalyzed by the cytochrome P450 isoenzyme CYP2E1. Mol Pharmacol 41(2):259–266 [PubMed] [Google Scholar]
- Ioannides C, Parke DV (1993) Induction of cytochrome P450 1 as an indicator of potential chemical carcinogenesis. Drug Metab Rev 25(4):485–501 doi: 10.3109/03602539308993983 [DOI] [PubMed] [Google Scholar]
- Irshaid Y, Branch RA, Adedoyin A (1996) Metabolic interactions of putative cytochrome P450 3A substrates with alternative pathways of dapsone metabolism in human liver microsomes. Drug Metab Dispos 24(2):164–171 [PubMed] [Google Scholar]
- Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H (2001) The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast Cancer Res Treat 70(1):47–54 doi: 10.1023/a:1012526406741 [DOI] [PubMed] [Google Scholar]
- Iyer R, Coles B, Raney KD, Thier R, Guengerich FP, Harris TM (1994) DNA adduction by the potent carcinogen aflatoxin B1: mechanistic studies. J Am Chem Soc 116:1603–1609 [Google Scholar]
- Jacobson PA, Green K, Birnbaum A, Remmel RP (2002) Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thioTEPA to TEPA. Cancer Chemother Pharmacol 49(6):461–467 doi: 10.1007/s00280-002-0453-3 [DOI] [PubMed] [Google Scholar]
- Jalas JR, Ding X, Murphy SE (2003) Comparative metabolism of the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol by rat cytochrome P450 2A3 and human cytochrome P450 2A13. Drug Metab Dispos 31(10):1199–1202 doi: 10.1124/dmd.31.10.1199 [DOI] [PubMed] [Google Scholar]
- Jan YH, Richardson JR, Baker AA, et al. (2016) Novel approaches to mitigating parathion toxicity: targeting cytochrome P450-mediated metabolism with menadione. Ann N Y Acad Sci 1378(1):80–86 doi: 10.1111/nyas.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate CR, Liehr JG, Santen RJ, et al. (2000) Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr(27):95–112 doi: 10.1093/oxfordjournals.jncimonographs.a024248 [DOI] [PubMed] [Google Scholar]
- Jerabek P, Martinek V, Stiborova M (2012) Theoretical investigation of differences in nitroreduction of aristolochic acid I by cytochromes P450 1A1, 1A2 and 1B1. Neuro Endocrinol Lett 33 Suppl 3:25–32 [PubMed] [Google Scholar]
- Jeurissen SM, Bogaards JJ, Awad HM, et al. (2004) Human cytochrome P450 enzyme specificity for bioactivation of safrole to the proximate carcinogen 1´-hydroxysafrole. Chem Res Toxicol 17(9):1245–1250 doi: 10.1021/tx040001v [DOI] [PubMed] [Google Scholar]
- Jeurissen SM, Bogaards JJ, Boersma MG, et al. (2006) Human cytochrome P450 enzymes of importance for the bioactivation of methyleugenol to the proximate carcinogen 1´-hydroxymethyleugenol. Chem Res Toxicol 19(1):111–116 doi: 10.1021/tx050267h [DOI] [PubMed] [Google Scholar]
- Jeurissen SM, Punt A, Boersma MG, et al. (2007) Human cytochrome P450 enzyme specificity for the bioactivation of estragole and related alkenylbenzenes. Chem Res Toxicol 20(5):798–806 doi: 10.1021/tx700012d [DOI] [PubMed] [Google Scholar]
- Jiang H, Shen YM, Quinn AM, Penning TM (2005) Competing roles of cytochrome P450 1A1/1B1 and aldo-keto reductase 1A1 in the metabolic activation of (±)-7,8-dihydroxy-7,8-dihydro-benzo[a]pyrene in human bronchoalveolar cell extracts. Chem Res Toxicol 18(2):365–374 doi: 10.1021/tx0497245 [DOI] [PubMed] [Google Scholar]
- Jiang H, Vudathala DK, Blair IA, Penning TM (2006) Competing roles of aldo-keto reductase 1A1 and cytochrome P4501B1 in benzo[a]pyrene-7,8-diol activation in human bronchoalveolar H358 cells: role of AKRs in P450 1B1 induction. Chem Res Toxicol 19(1):68–78 doi: 10.1021/tx0502488 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Zuo H, Lee KH, et al. (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85(2):151–159 doi: 10.1023/B:BREA.0000025406.31193.e8 [DOI] [PubMed] [Google Scholar]
- Johnson WW, Ueng Y-F, Widersten M, et al. (1997) Conjugation of highly reactive aflatoxin B1 exo-8,9-epoxide catalyzed by rat and human glutathione transferases: estimation of kinetic parameters. Biochemistry 36(11):3056–3060 doi: 10.1021/bi962537o [DOI] [PubMed] [Google Scholar]
- Jorga KM, Fotteler B, Gasser R, Banken L, Birnboeck H (2000) Lack of interaction between tolcapone and tolbutamide in healthy volunteers. J Clin Pharmacol 40(5):544–551 doi: 10.1177/00912700022009161 [DOI] [PubMed] [Google Scholar]
- Josephy PD, Batty SM, Boverhof DR (2001) Recombinant human P450 forms 1A1, 1A2, and 1B1 catalyze the bioactivation of heterocyclic amine mutagens in Escherichia coli lacZ strains. Environ Mol Mutagen 38(1):12–18 doi: 10.1002/em.1045 [DOI] [PubMed] [Google Scholar]
- Juchau MR, Lee QP, Fantel AG (1992) Xenobiotic biotransformation/bioactivation in organogenesis-stage conceptual tissues: implications for embryotoxicity and teratogenesis. Drug Metab Rev 24(2):195–238 doi: 10.3109/03602539208996293 [DOI] [PubMed] [Google Scholar]
- Jushchyshyn MI, Wahlstrom JL, Hollenberg PF, Wienkers LC (2006) Mechanism of inactivation of human cytochrome P450 2B6 by phencyclidine. Drug Metab Dispos 34(9):1523–1529 doi: 10.1124/dmd.106.010579 [DOI] [PubMed] [Google Scholar]
- Kabler SL, Seidel A, Jacob J, Doehmer J, Morrow CS, Townsend AJ (2009) Differential protection by human glutathione S-transferase P1 against cytotoxicity of benzo[a]pyrene, dibenzo[a,l]pyrene, or their dihydrodiol metabolites, in bi-transgenic cell lines that co-express rat versus human cytochrome P450 1A1. Chem-Biol Interact 179(2–3):240–246 doi: 10.1016/j.cbi.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalgutkar AS, Obach RS, Maurer TS (2007) Mechanism-based inactivation of cytochrome P450 enzymes: chemical mechanisms, structure-activity relationships and relationship to clinical drug-drug interactions and idiosyncratic adverse drug reactions. Curr Drug Metab 8(5):407–447 doi: 10.2174/138920007780866807 [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, Taylor TJ, Venkatakrishnan K, Isin EM (2003) Assessment of the contributions of CYP3A4 and CYP3A5 in the metabolism of the antipsychotic agent haloperidol to its potentially neurotoxic pyridinium metabolite and effect of antidepressants on the bioactivation pathway. Drug Metab Dispos 31(3):243–249 doi: 10.1124/dmd.31.3.243 [DOI] [PubMed] [Google Scholar]
- Kamataki T, Fujita K, Nakayama K, Yamazaki Y, Miyamoto M, Ariyoshi N (2002) Role of human cytochrome P450 (CYP) in the metabolic activation of nitrosamine derivatives: application of genetically engineered Salmonella expressing human CYP. Drug Metab Rev 34(3):667–676 doi: 10.1081/dmr-120005668 [DOI] [PubMed] [Google Scholar]
- Kamdem LK, Meineke I, Gödtel-Armbrust U, Brockmöller J, Wojnowski L (2006) Dominant contribution of P450 3A4 to the hepatic carcinogenic activation of aflatoxin B1. Chem Res Toxicol 19(4):577–586 doi: 10.1021/tx050358e [DOI] [PubMed] [Google Scholar]
- Kang P, Dalvie D, Smith E, Zhou S, Deese A (2007) Identification of a novel glutathione conjugate of flutamide in incubations with human liver microsomes. Drug Metab Dispos 35(7):1081–1088 doi: 10.1124/dmd.107.014860 [DOI] [PubMed] [Google Scholar]
- Kang P, Dalvie D, Smith E, Zhou S, Deese A, Nieman JA (2008) Bioactivation of flutamide metabolites by human liver microsomes. Drug Metab Dispos 36(7):1425–1437 doi: 10.1124/dmd.108.020370 [DOI] [PubMed] [Google Scholar]
- Kappers WA, Edwards RJ, Murray S, Boobis AR (2001) Diazinon is activated by CYP2C19 in human liver. Toxicol Appl Pharmacol 177(1):68–76 doi: 10.1006/taap.2001.9294 [DOI] [PubMed] [Google Scholar]
- Karlgren M, Miura S, Ingelman-Sundberg M (2005) Novel extrahepatic cytochrome P450s. Toxicol Appl Pharmacol 207(2 Suppl):57–61 doi: 10.1016/j.taap.2004.12.022 [DOI] [PubMed] [Google Scholar]
- Kartha JS, Yost GS (2008) Mechanism-based inactivation of lung-selective cytochrome P450 CYP2F enzymes. Drug Metab Dispos 36(1):155–162 doi: 10.1124/dmd.107.017897 [DOI] [PubMed] [Google Scholar]
- Kassahun K, Pearson PG, Tang W, et al. (2001) Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem Res Toxicol 14(1):62–70 doi: 10.1021/tx000180q [DOI] [PubMed] [Google Scholar]
- Kazui M, Nishiya Y, Ishizuka T, et al. (2010) Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 38(1):92–99 doi: 10.1124/dmd.109.029132 [DOI] [PubMed] [Google Scholar]
- Kerlan V, Dreano Y, Bercovici JP, Beaune PH, Floch HH, Berthou F (1992) Nature of cytochromes P450 involved in the 2-/4-hydroxylations of estradiol in human liver microsomes. Biochem Pharmacol 44(9):1745–1756 doi: 10.1016/0006-2952(92)90068-t [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Hankins DC, Fenstamaker K, Cox K (2000) Human halothane metabolism, lipid peroxidation, and cytochromes P450 2A6 and P450 3A4. Eur J Clin Pharmacol 55(11–12):853–859 doi: 10.1007/s002280050707 [DOI] [PubMed] [Google Scholar]
- Khojasteh-Bakht SC, Chen W, Koenigs LL, Peter RM, Nelson SD (1999) Metabolism of (R)-(+)-pulegone and (R)-(+)-menthofuran by human liver cytochrome P-450s: evidence for formation of a furan epoxide. Drug Metab Dispos 27(5):574–580 [PubMed] [Google Scholar]
- Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK (2006) Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci 94(2):261–271 doi: 10.1093/toxsci/kfl096 [DOI] [PubMed] [Google Scholar]
- Kim B-R, Oh HS, Kim D-H (1997) Differential inhibition of aflatoxin B1 oxidation by gestodene action on human liver microsomes. Biochem Mol Biol Int 43(4):839–846 doi: 10.1080/15216549700204651 [DOI] [PubMed] [Google Scholar]
- Kim D, Guengerich FP (2004) Selection of human cytochrome P450 1A2 mutants with enhanced catalytic activity for heterocyclic amine N-hydroxylation. Biochemistry 43(4):981–988 doi: 10.1021/bi035593f [DOI] [PubMed] [Google Scholar]
- Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR (1998) Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis 19(10):1847–1853 doi: 10.1093/carcin/19.10.1847 [DOI] [PubMed] [Google Scholar]
- Kim JY, Chung JY, Park JE, et al. (2007) Benzo[a]pyrene induces apoptosis in RL95–2 human endometrial cancer cells by cytochrome P450 1A1 activation. Endocrinology 148(10):5112–5122 doi: 10.1210/en.2007-0096 [DOI] [PubMed] [Google Scholar]
- Kim SY, Suzuki N, Santosh Laxmi YR, Rieger R, Shibutani S (2003) a-Hydroxylation of tamoxifen and toremifene by human and rat cytochrome P450 3A subfamily enzymes. Chem Res Toxicol 16(9):1138–1144 doi: 10.1021/tx0300131 [DOI] [PubMed] [Google Scholar]
- King LC, Adams L, Allison J, et al. (1999) A quantitative comparison of dibenzo[a,l]pyrene-DNA adduct formation by recombinant human cytochrome P450 microsomes. Mol Carcinog 26(2):74–82 [PubMed] [Google Scholar]
- Kisselev P, Schwarz D, Platt KL, Schunck WH, Roots I (2002) Epoxidation of benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1 in reconstituted membranes. Effects of charge and nonbilayer phase propensity of the membrane. Eur J Biochem 269(7):1799–1805 doi: 10.1046/j.1432-1033.2002.02848.x [DOI] [PubMed] [Google Scholar]
- Korzekwa KR, Krishnamachary N, Shou M, et al. (1998) Evaluation of atypical cytochrome P450 kinetics with two-substrate models: evidence that multiple substrates can simultaneously bind to cytochrome P450 active sites. Biochemistry 37(12):4137–4147 doi: 10.1021/bi9715627 [DOI] [PubMed] [Google Scholar]
- Krause RJ, Elfarra AA (1997) Oxidation of butadiene monoxide to meso- and (±)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch Biochem Biophys 337(2):176–184 doi: 10.1006/abbi.1996.9781 [DOI] [PubMed] [Google Scholar]
- Krishnan S, Hvastkovs EG, Bajrami B, Schenkman JB, Rusling JF (2009) Human cyt P450 mediated metabolic toxicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) evaluated using electrochemiluminescent arrays. Mol Biosyst 5(2):163–169 doi: 10.1039/b815910f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo S, Ishizaki T (1999) Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet 37(6):435–456 doi: 10.2165/00003088-199937060-00001 [DOI] [PubMed] [Google Scholar]
- Kuffel MJ, Schroeder JC, Pobst LJ, et al. (2002) Activation of the antitumor agent aminoflavone (NSC 686288) is mediated by induction of tumor cell cytochrome P450 1A1/1A2. Mol Pharmacol 62(1):143–153 doi: 10.1124/mol.62.1.143 [DOI] [PubMed] [Google Scholar]
- Kumar S, Samuel K, Subramanian R, et al. (2002) Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: role of acyl glucuronidation and sequential oxidative metabolism of the acyl glucuronide. J Pharmacol Exp Ther 303(3):969–978 doi: 10.1124/jpet.102.038992 [DOI] [PubMed] [Google Scholar]
- Kurth MJ, Yokoi T, Gershwin ME (2014) Halothane-induced hepatitis: paradigm or paradox for drug-induced liver injury. Hepatology 60(5):1473–1475 doi: 10.1002/hep.27253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida H, Fujita K, Suzuki A, et al. (2000) Metabolic activation of N-alkylnitrosamines in genetically engineered Salmonella typhimurium expressing CYP2E1 or CYP2A6 together with human NADPH-cytochrome P450 reductase. Carcinogenesis 21(6):1227–1232 [PubMed] [Google Scholar]
- Lacassagne A (1932) Results of the treatment of cancer of the cervix uteri. Br Med J 2(3750):912–913 doi: 10.1136/bmj.2.3750.912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JE, Auriola S, Pasanen M, Juvonen RO (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39(1):11–21 doi: 10.1080/00498250802512830 [DOI] [PubMed] [Google Scholar]
- Lalani AS, Alters SE, Wong A, Albertella MR, Cleland JL, Henner WD (2007) Selective tumor targeting by the hypoxia-activated prodrug AQ4N blocks tumor growth and metastasis in preclinical models of pancreatic cancer. Clin Cancer Res 13(7):2216–2225 doi: 10.1158/1078-0432.Ccr-06-2427 [DOI] [PubMed] [Google Scholar]
- Langouët S, Welti DH, Kerriguy N, et al. (2001) Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem Res Toxicol 14(2):211–221 doi: 10.1021/tx000176e [DOI] [PubMed] [Google Scholar]
- Lanza DL, Code E, Crespi CL, Gonzalez FJ, Yost GS (1999) Specific dehydrogenation of 3-methylindole and epoxidation of naphthalene by recombinant human CYP2F1 expressed in lymphoblastoid cells. Drug Metab Dispos 27(7):798–803 [PubMed] [Google Scholar]
- Lanza DL, Yost GS (2001) Selective dehydrogenation/oxygenation of 3-methylindole by cytochrome P450 enzymes. Drug Metab Dispos 29(7):950–953 [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, et al. (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42(6):1364–1372 [DOI] [PubMed] [Google Scholar]
- Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK (2000) Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem 275(6):4118–4126 doi: 10.1074/jbc.275.6.4118 [DOI] [PubMed] [Google Scholar]
- Lassila T, Mattila S, Turpeinen M, Pelkonen O, Tolonen A (2016) Tandem mass spectrometric analysis of S- and N-linked glutathione conjugates of pulegone and menthofuran and identification of P450 enzymes mediating their formation. Rapid Commun Mass Spectrom 30(7):917–926 doi: 10.1002/rcm.7518 [DOI] [PubMed] [Google Scholar]
- Lauer B, Tuschl G, Kling M, Mueller SO (2009) Species-specific toxicity of diclofenac and troglitazone in primary human and rat hepatocytes. Chem-Biol Interact 179(1):17–24 doi: 10.1016/j.cbi.2008.10.031 [DOI] [PubMed] [Google Scholar]
- Lecoeur S, Bonierbale E, Challine D, et al. (1994) Specificity of in vitro covalent binding of tienilic acid metabolites to human liver microsomes in relationship to the type of hepatotoxicity: comparison with two directly hepatotoxic drugs. Chem Res Toxicol 7(3):434–442 doi: 10.1021/tx00039a023 [DOI] [PubMed] [Google Scholar]
- Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT (2003a) Characterization of the oxidative metabolites of 17b-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology 144(8):3382–3398 doi: 10.1210/en.2003-0192 [DOI] [PubMed] [Google Scholar]
- Lee AJ, Conney AH, Zhu BT (2003b) Human cytochrome P450 3A7 has a distinct high catalytic activity for the 16a-hydroxylation of estrone but not 17b-estradiol. Cancer Res 63(19):6532–6536 [PubMed] [Google Scholar]
- Lee AJ, Kosh JW, Conney AH, Zhu BT (2001) Characterization of the NADPH-dependent metabolism of 17b-estradiol to multiple metabolites by human liver microsomes and selectively expressed human cytochrome P450 3A4 and 3A5. J Pharmacol Exp Ther 298(2):420–432 [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ (1996) Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem 271(20):12063–12067 doi: 10.1074/jbc.271.20.12063 [DOI] [PubMed] [Google Scholar]
- Leemann T, Transon C, Dayer P (1993) Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4´-hydroxylation in human liver. Life Sci 52(1):29–34 doi: 10.1016/0024-3205(93)90285-b [DOI] [PubMed] [Google Scholar]
- Levorato S, Dominici L, Fatigoni C, et al. (2018) In vitro toxicity evaluation of estragole-containing preparations derived from Foeniculum vulgare Mill. (fennel) on HepG2 cells. Food Chem Toxicol 111:616–622 doi: 10.1016/j.fct.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Lewis BC, Korprasertthaworn P, Miners JO (2016) Impaired dacarbazine activation and 7-ethoxyresorufin deethylation in vitro by polymorphic variants of CYP1A1 and CYP1A2: implications for cancer therapy. Pharmacogenet Genomics 26(10):453–461 doi: 10.1097/fpc.0000000000000236 [DOI] [PubMed] [Google Scholar]
- Lewis CW, Smith JE, Anderson JG, Freshney RI (1999) Increased cytotoxicity of food-borne mycotoxins toward human cell lines in vitro via enhanced cytochrome P450 expression using the MTT bioassay. Mycopathologia 148(2):97–102 doi: 10.1023/a:1007130923558 [DOI] [PubMed] [Google Scholar]
- Li DN, Seidel A, Pritchard MP, Wolf CR, Friedberg T (2000) Polymorphisms in P450 CYP1B1 affect the conversion of estradiol to the potentially carcinogenic metabolite 4-hydroxyestradiol. Pharmacogenetics 10(4):343–353 doi: 10.1097/00008571-200006000-00008 [DOI] [PubMed] [Google Scholar]
- Li X, He X, Chen S, et al. (2020) Evaluation of pyrrolizidine alkaloid-induced genotoxicity using metabolically competent TK6 cell lines. Food Chem Toxicol 145:111662 doi: 10.1016/j.fct.2020.111662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Björkman A, Andersson TB, Gustafsson LL, Masimirembwa CM (2003) Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol 59(5–6):429–442 doi: 10.1007/s00228-003-0636-9 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang E, Patten CJ, Chen L, Yang CS (1994) Effects of flavonoids on cytochrome P450-dependent acetaminophen metabolism in rats and human liver microsomes. Drug Metab Dispos 22(4):566–571 [PubMed] [Google Scholar]
- Liehr JG (2000) Is estradiol a genotoxic mutagenic carcinogen? Endocrinol Rev 21(1):40–54 doi: 10.1210/edrv.21.1.0386 [DOI] [PubMed] [Google Scholar]
- Lin CY, Pan TS, Ting CC, et al. (2013) Cytochrome P450 metabolism of betel quid-derived compounds: implications for the development of prevention strategies for oral and pharyngeal cancers. Scientific World J 2013:618032 doi: 10.1155/2013/618032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Hollenberg PF (2007) The inactivation of cytochrome P450 3A5 by 17a-ethynylestradiol is cytochrome b5-dependent: metabolic activation of the ethynyl moiety leads to the formation of glutathione conjugates, a heme adduct, and covalent binding to the apoprotein. J Pharmacol Exp Ther 321(1):276–287 doi: 10.1124/jpet.106.117861 [DOI] [PubMed] [Google Scholar]
- Lin HL, Kent UM, Hollenberg PF (2002) Mechanism-based inactivation of cytochrome P450 3A4 by 17a-ethynylestradiol: evidence for heme destruction and covalent binding to protein. J Pharmacol Exp Ther 301(1):160–167 doi: 10.1124/jpet.301.1.160 [DOI] [PubMed] [Google Scholar]
- Loaiza-Pérez AI, Kenney S, Boswell J, et al. (2004) Aryl hydrocarbon receptor activation of an antitumor aminoflavone: basis of selective toxicity for MCF-7 breast tumor cells. Mol Cancer Ther 3(6):715–725 [PubMed] [Google Scholar]
- López-Garcia MP, Dansette PM, Valadon P, et al. (1993) Human-liver cytochromes P-450 expressed in yeast as tools for reactive-metabolite formation studies. Oxidative activation of tienilic acid by cytochromes P-450 2C9 and 2C10. Eur J Biochem 213(1):223–232 doi: 10.1111/j.1432-1033.1993.tb17752.x [DOI] [PubMed] [Google Scholar]
- López-Garcia MP, Dansette PM, Mansuy D (1994) Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry 33(1):166–175 doi: 10.1021/bi00167a022 [DOI] [PubMed] [Google Scholar]
- Lozano JJ, Pastor M, Cruciani G, et al. (2000) 3D-QSAR methods on the basis of ligand-receptor complexes. Application of COMBINE and GRID/GOLPE methodologies to a series of CYP1A2 ligands. J Comput Aided Mol Des 14(4):341–353 doi: 10.1023/a:1008164621650 [DOI] [PubMed] [Google Scholar]
- Lu H, Wang JJ, Chan KK, Philip PA (2006) Stereoselectivity in metabolism of ifosfamide by CYP3A4 and CYP2B6. Xenobiotica 36(5):367–385 doi: 10.1080/00498250600598486 [DOI] [PubMed] [Google Scholar]
- Lu Y, Wong KY, Tan C, Ma J, Feng B, Lin G (2020) Establishment of a novel CYP3A4-transduced human hepatic sinusoidal endothelial cell model and its application in screening hepatotoxicity of pyrrolizidine alkaloids. J Environ Sci Health C Toxicol Carcinog 38(2):169–185 doi: 10.1080/26896583.2020.1769409 [DOI] [PubMed] [Google Scholar]
- Luch A, Coffing SL, Tang YM, et al. (1998) Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chem Res Toxicol 11(6):686–695 doi: 10.1021/tx970236p [DOI] [PubMed] [Google Scholar]
- Luch A, Kishiyama S, Seidel A, Doehmer J, Greim H, Baird WM (1999a) The K-region trans-8,9-diol does not significantly contribute as an intermediate in the metabolic activation of dibenzo[a,l]pyrene to DNA-binding metabolites by human cytochrome P450 1A1 or 1B1. Cancer Res 59(18):4603–4609 [PubMed] [Google Scholar]
- Luch A, Schober W, Soballa VJ, et al. (1999b) Metabolic activation of dibenzo[a,l]pyrene by human cytochrome P450 1A1 and P450 1B1 expressed in V79 Chinese hamster cells. Chem Res Toxicol 12(4):353–364 doi: 10.1021/tx980240g [DOI] [PubMed] [Google Scholar]
- Lundblad MS, Stark K, Eliasson E, Oliw E, Rane A (2005) Biosynthesis of epoxyeicosatrienoic acids varies between polymorphic CYP2C enzymes. Biochem Biophys Res Commun 327(4):1052–1057 doi: 10.1016/j.bbrc.2004.12.116 [DOI] [PubMed] [Google Scholar]
- Mahadevan B, Luch A, Atkin J, Haynes M, Nguyen T, Baird WM (2007) Inhibition of human cytochrome P450 1B1 further clarifies its role in the activation of dibenzo[a,l]pyrene in cells in culture. J Biochem Mol Toxicol 21(3):101–109 doi: 10.1002/jbt.20168 [DOI] [PubMed] [Google Scholar]
- Mammen JS, Kleiner HE, DiGiovanni J, Sutter TR, Strickland PT (2005) Coumarins are competitive inhibitors of cytochrome P450 1B1, with equal potency for allelic variants. Pharmacogenet Genomics 15(3):183–188 doi: 10.1097/01213011-200503000-00007 [DOI] [PubMed] [Google Scholar]
- Mammen JS, Pittman GS, Li Y, et al. (2003) Single amino acid mutations, but not common polymorphisms, decrease the activity of CYP1B1 against (−)benzo[a]pyrene-7R-trans-7,8-dihydrodiol. Carcinogenesis 24(7):1247–1255 doi: 10.1093/carcin/bgg088 [DOI] [PubMed] [Google Scholar]
- Mancy A, Antignac M, Minoletti C, et al. (1999) Diclofenac and its derivatives as tools for studying human cytochromes P450 active sites: particular efficiency and regioselectivity of P450 2Cs. Biochemistry 38(43):14264–14270 doi: 10.1021/bi991195u [DOI] [PubMed] [Google Scholar]
- Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT (2000) Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther 67(3):275–282 doi: 10.1067/mcp.2000.104736 [DOI] [PubMed] [Google Scholar]
- Martínez-Cabot A, Morató A, Commandeur JN, Vermeulen NP, Messeguer A (2007) In vitro bioactivation of 3-(N-phenylamino)propane-1,2-diol by human and rat liver microsomes and recombinant P450 enzymes. Implications for toxic oil syndrome. Chem Res Toxicol 20(8):1218–1224 doi: 10.1021/tx700209p [DOI] [PubMed] [Google Scholar]
- Martinkova E, Dontenwill M, Frei E, Stiborova M (2009) Cytotoxicity of and DNA adduct formation by ellipticine in human U87MG glioblastoma cancer cells. Neuro Endocrinol Lett 30 Suppl 1:60–66 [PubMed] [Google Scholar]
- Masubuchi Y, Nakano T, Ose A, Horie T (2001) Differential selectivity in carbamazepine-induced inactivation of cytochrome P450 enzymes in rat and human liver. Arch Toxicol 75(9):538–543 doi: 10.1007/s002040100270 [DOI] [PubMed] [Google Scholar]
- May DG, Porter J, Wilkinson GR, Branch RA (1994) Frequency distribution of dapsone N-hydroxylase, a putative probe for P450 3A4 activity, in a white population. Clin Pharmacol Ther 55(5):492–500 doi: 10.1038/clpt.1994.62 [DOI] [PubMed] [Google Scholar]
- McCarthy HO, Yakkundi A, McErlane V, et al. (2003) Bioreductive GDEPT using cytochrome P450 3A4 in combination with AQ4N. Cancer Gene Ther 10(1):40–48 doi: 10.1038/sj.cgt.7700522 [DOI] [PubMed] [Google Scholar]
- McCune JS, Risler LJ, Phillips BR, Thummel KE, Blough D, Shen DD (2005) Contribution of CYP3A5 to hepatic and renal ifosfamide N-dechloroethylation. Drug Metab Dispos 33(7):1074–1081 doi: 10.1124/dmd.104.002279 [DOI] [PubMed] [Google Scholar]
- McErlane V, Yakkundi A, McCarthy HO, et al. (2005) A cytochrome P450 2B6 meditated gene therapy strategy to enhance the effects of radiation or cyclophosphamide when combined with the bioreductive drug AQ4N. J Gene Med 7(7):851–859 doi: 10.1002/jgm.728 [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30(9):2174–2187 doi: 10.1007/s11095-013-1007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus ME, Burgess WM, Veronese ME, Huggett A, Quattrochi LC, Tukey RH (1990) Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res 50(11):3367–3376 [PubMed] [Google Scholar]
- Megaraj V, Zhou X, Xie F, Liu Z, Yang W, Ding X (2014) Role of CYP2A13 in the bioactivation and lung tumorigenicity of the tobacco-specific lung procarcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: in vivo studies using a CYP2A13-humanized mouse model. Carcinogenesis 35(1):131–137 doi: 10.1093/carcin/bgt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melet A, Assrir N, Jean P, et al. (2003) Substrate selectivity of human cytochrome P450 2C9: importance of residues 476, 365, and 114 in recognition of diclofenac and sulfaphenazole and in mechanism-based inactivation by tienilic acid. Arch Biochem Biophys 409(1):80–91 doi: 10.1016/s0003-9861(02)00548-9 [DOI] [PubMed] [Google Scholar]
- Milichovský J, Bárta F, Schmeiser HH, et al. (2016) Active site mutations as a suitable tool contributing to explain a mechanism of aristolochic acid I nitroreduction by cytochromes P450 1A1, 1A2 and 1B1. Int J Mol Sci 17(2):213 doi: 10.3390/ijms17020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoda Y, Kharasch ED (2001) Halothane-dependent lipid peroxidation in human liver microsomes is catalyzed by cytochrome P450 2A6 (CYP2A6). Anesthesiology 95(2):509–514 doi: 10.1097/00000542-200108000-00037 [DOI] [PubMed] [Google Scholar]
- Miranda CL, Reed RL, Guengerich FP, Buhler DR (1991) Role of cytochrome P450 IIIA4 in the metabolism of the pyrrolizidine alkaloid senecionine in human liver. Carcinogenesis 12(3):515–519 doi: 10.1093/carcin/12.3.515 [DOI] [PubMed] [Google Scholar]
- Miranda CL, Yang YH, Henderson MC, et al. (2000) Prenylflavonoids from hops inhibit the metabolic activation of the carcinogenic heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline, mediated by cDNA-expressed human CYP1A2. Drug Metab Dispos 28(11):1297–1302 [PubMed] [Google Scholar]
- Mitra AK, Thummel KE, Kalhorn TF, Kharasch ED, Unadkat JD, Slattery JT (1995) Metabolism of dapsone to its hydroxylamine by CYP2E1 in vitro and in vivo. Clin Pharmacol Ther 58(5):556–566 doi: 10.1016/0009-9236(95)90176-0 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Sugawara E, Yoshimura T, Yamazaki H, Kamataki T (2005) Mutagenic activation of betel quid-specific N-nitrosamines catalyzed by human cytochrome P450 coexpressed with NADPH-cytochrome P450 reductase in Salmonella typhimurium YG7108. Mutat Res 581(1–2):165–171 doi: 10.1016/j.mrgentox.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Modugno F, Knoll C, Kanbour-Shakir A, Romkes M (2003) A potential role for the estrogen-metabolizing cytochrome P450 enzymes in human breast carcinogenesis. Breast Cancer Res Treat 82(3):191–197 doi: 10.1023/b:Brea.0000004376.21491.44 [DOI] [PubMed] [Google Scholar]
- Monien BH, Sachse B, Niederwieser B, Abraham K (2019) Detection of N-acetyl-S-[3´-(4-methoxyphenyl)allyl]-l-Cys (AMPAC) in human urine samples after controlled exposure to fennel tea: a new metabolite of estragole and trans-anethole. Chem Res Toxicol 32(11):2260–2267 doi: 10.1021/acs.chemrestox.9b00287 [DOI] [PubMed] [Google Scholar]
- Moore CD, Reilly CA, Yost GS (2010a) CYP3A4-Mediated oxygenation versus dehydrogenation of raloxifene. Biochemistry 49(21):4466–4475 doi: 10.1021/bi902213r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CD, Shahrokh K, Sontum SF, Cheatham TE 3rd, Yost GS (2010b) Improved cytochrome P450 3A4 molecular models accurately predict the Phe215 requirement for raloxifene dehydrogenation selectivity. Biochemistry 49(41):9011–9019 doi: 10.1021/bi101139q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mráz J, Jheeta P, Gescher A, Hyland R, Thummel K, Threadgill MD (1993) Investigation of the mechanistic basis of N,N-dimethylformamide toxicity. Metabolism of N,N-dimethylformamide and its deuterated isotopomers by cytochrome P450 2E1. Chem Res Toxicol 6(2):197–207 doi: 10.1021/tx00032a009 [DOI] [PubMed] [Google Scholar]
- Murray M, Butler AM, Stupans I (1994) Competitive inhibition of human liver microsomal cytochrome P450 3A-dependent steroid 6b-hydroxylation activity by cyclophosphamide and ifosfamide in vitro. J Pharmacol Exp Ther 270(2):645–649 [PubMed] [Google Scholar]
- Mutch E, Blain PG, Williams FM (1999) The role of metabolism in determining susceptibility to parathion toxicity in man. Toxicol Lett 107(1–3):177–187 doi: 10.1016/s0378-4274(99)00044-2 [DOI] [PubMed] [Google Scholar]
- Mutch E, Daly AK, Leathart JB, Blain PG, Williams FM (2003) Do multiple cytochrome P450 isoforms contribute to parathion metabolism in man? Arch Toxicol 77(6):313–320 doi: 10.1007/s00204-003-0452-0 [DOI] [PubMed] [Google Scholar]
- Mutch E, Williams FM (2006) Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology 224(1–2):22–32 doi: 10.1016/j.tox.2006.04.024 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Komagata S, Fujiki Y, et al. (2007) Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genomics 17(6):431–445 doi: 10.1097/FPC.0b013e328045c4fb [DOI] [PubMed] [Google Scholar]
- Nastainczyk W, Rendic S, Ullrich V, Krüger R (1982) [Prevention by metyrapone of halothane induced liver necrosis in rats (author’s transl)]. Anaesthesist 31(4):181–184 [PubMed] [Google Scholar]
- Neal GE (1995) Genetic implications in the metabolism and toxicity of mycotoxins. Toxicol Lett 82–83:861–867 doi: 10.1016/0378-4274(95)03600-8 [DOI] [PubMed] [Google Scholar]
- Ngui JS, Tang W, Stearns RA, et al. (2000) Cytochrome P450 3A4-mediated interaction of diclofenac and quinidine. Drug Metab Dispos 28(9):1043–1050 [PubMed] [Google Scholar]
- Nieusma JL, Claffey DJ, Koop DR, et al. (1998) Oxidation of 1,3-butadiene to (R)- and (S)-butadiene monoxide by purified recombinant cytochrome P450 2E1 from rabbit, rat and human. Toxicol Lett 95(2):123–129 doi: 10.1016/s0378-4274(98)00026-5 [DOI] [PubMed] [Google Scholar]
- Nishida CR, Lee M, Ortiz de Montellano PR (2010) Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol Pharmacol 78(3):497–502 doi: 10.1124/mol.110.065045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki R, Totsuka Y, Takamura-Enya T, Sugimura T, Wakabayashi K (2004) Identification of cytochrome P-450s involved in the formation of APNH from norharman with aniline. Mutat Res 562(1–2):19–25 doi: 10.1016/j.mrgentox.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Niwa T, Murayama N, Imagawa Y, Yamazaki H (2015) Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev 47(2):89–110 doi: 10.3109/03602532.2015.1011658 [DOI] [PubMed] [Google Scholar]
- Niwa T, Yabusaki Y, Honma K, et al. (1998) Contribution of human hepatic cytochrome P450 isoforms to regioselective hydroxylation of steroid hormones. Xenobiotica 28(6):539–547 doi: 10.1080/004982598239290 [DOI] [PubMed] [Google Scholar]
- Notley LM, Crewe KH, Taylor PJ, Lennard MS, Gillam EMJ (2005) Characterization of the human cytochrome P450 forms involved in metabolism of tamoxifen to its a-hydroxy and a,4-dihydroxy derivatives. Chem Res Toxicol 18(10):1611–1618 doi: 10.1021/tx050140s [DOI] [PubMed] [Google Scholar]
- Notley LM, De Wolf CJ, Wunsch RM, Lancaster RG, Gillam EMJ (2002) Bioactivation of tamoxifen by recombinant human cytochrome P450 enzymes. Chem Res Toxicol 15(5):614–622 doi: 10.1021/tx0100439 [DOI] [PubMed] [Google Scholar]
- Novak RF, Woodcroft KJ (2000) The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch Pharm Res 23(4):267–282 doi: 10.1007/bf02975435 [DOI] [PubMed] [Google Scholar]
- O’Rourke M, Ward C, Worthington J, et al. (2008) Evaluation of the antiangiogenic potential of AQ4N. Clin Cancer Res 14(5):1502–1509 doi: 10.1158/1078-0432.Ccr-07-1262 [DOI] [PubMed] [Google Scholar]
- O’Shea D, Kim RB, Wilkinson GR (1997) Modulation of CYP2E1 activity by isoniazid in rapid and slow N-acetylators. Br J Clin Pharmacol 43(1):99–103 doi: 10.1111/j.1365-2125.1997.tb00039.x [DOI] [PubMed] [Google Scholar]
- Oda Y, Aryal P, Terashita T, Gillam EMJ, Guengerich FP, Shimada T (2001) Metabolic activation of heterocyclic amines and other procarcinogens in Salmonella typhimurium umu tester strains expressing human cytochrome P4501A1, 1A2, 1B1, 2C9, 2D6, 2E1, and 3A4 and human NADPH-P450 reductase and bacterial O-acetyltransferase. Mutat Res 492(1–2):81–90 doi: 10.1016/s1383-5718(01)00154-1 [DOI] [PubMed] [Google Scholar]
- Palacharla RC, Uthukam V, Manoharan A, et al. (2017) Inhibition of cytochrome P450 enzymes by saturated and unsaturated fatty acids in human liver microsomes, characterization of enzyme kinetics in the presence of bovine serum albumin (0.1 and 1.0% w/v) and in vitro–in vivo extrapolation of hepatic clearance. Eur J Pharm Sci 101:80–89 doi: 10.1016/j.ejps.2017.01.027 [DOI] [PubMed] [Google Scholar]
- Pan LP, Wijnant P, De Vriendt C, Rosseel MT, Belpaire FM (1997) Characterization of the cytochrome P450 isoenzymes involved in the in vitro N-dealkylation of haloperidol. Br J Clin Pharmacol 44(6):557–564 doi: 10.1046/j.1365-2125.1997.t01-1-00629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Cao JQ, Katz BH, Hayes CL, Sutter TR, Spink DC (1999) Inductive and inhibitory effects of non-ortho-substituted polychlorinated biphenyls on estrogen metabolism and human cytochromes P450 1A1 and 1B1. Biochem Pharmacol 58(1):29–38 doi: 10.1016/s0006-2952(99)00070-2 [DOI] [PubMed] [Google Scholar]
- Paracchini V, Pedotti P, Raimondi S, et al. (2005) A common CYP1B1 polymorphism is associated with 2-OH E1/16-OH E1 urinary estrone ratio. Clin Chem Lab Med 43(7):702–706 doi: 10.1515/cclm.2005.119 [DOI] [PubMed] [Google Scholar]
- Parikh A, Josephy PD, Guengerich FP (1999) Selection and characterization of human cytochrome P450 1A2 mutants with altered catalytic properties. Biochemistry 38(17):5283–5289 doi: 10.1021/bi990142+ [DOI] [PubMed] [Google Scholar]
- Parkinson OT, Liggitt HD, Rettie AE, Kelly EJ (2013) Generation and characterization of a Cyp4b1 null mouse and the role of CYP4B1 in the activation and toxicity of ipomeanol. Toxicol Sci 134(2):243–250 doi: 10.1093/toxsci/kft123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CJ, Smith TJ, Friesen MJ, Tynes RE, Yang CS, Murphy SE (1997) Evidence for cytochrome P450 2A6 and 3A4 as major catalysts for Ń-nitrosonornicotine a-hydroxylation by human liver microsomes. Carcinogenesis 18(8):1623–1630 doi: 10.1093/carcin/18.8.1623 [DOI] [PubMed] [Google Scholar]
- Patten CJ, Smith TJ, Murphy SE, et al. (1996) Kinetic analysis of the activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by heterologously expressed human P450 enzymes and the effect of P450-specific chemical inhibitors on this activation in human liver microsomes. Arch Biochem Biophys 333(1):127–138 doi: 10.1006/abbi.1996.0373 [DOI] [PubMed] [Google Scholar]
- Patten CJ, Thomas PE, Guy RL, et al. (1993) Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 6(4):511–518 doi: 10.1021/tx00034a019 [DOI] [PubMed] [Google Scholar]
- Patterson LH (1993) Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev 12(2):119–134 doi: 10.1007/bf00689805 [DOI] [PubMed] [Google Scholar]
- Patterson LH (2002) Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab Rev 34(3):581–592 doi: 10.1081/dmr-120005659 [DOI] [PubMed] [Google Scholar]
- Patterson LH, McKeown SR, Robson T, Gallagher R, Raleigh SM, Orr S (1999) Antitumour prodrug development using cytochrome P450 (CYP) mediated activation. Anticancer Drug Des 14(6):473–486 [PubMed] [Google Scholar]
- Patterson LH, Murray GI (2002) Tumour cytochrome P450 and drug activation. Curr Pharm Des 8(15):1335–1347 doi: 10.2174/1381612023394502 [DOI] [PubMed] [Google Scholar]
- Pearce RE, Lu W, Wang Y, Uetrecht JP, Correia MA, Leeder JS (2008) Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab Dispos 36(8):1637–1649 doi: 10.1124/dmd.107.019562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RE, Uetrecht JP, Leeder JS (2005) Pathways of carbamazepine bioactivation in vitro: II. The role of human cytochrome P450 enzymes in the formation of 2-hydroxyiminostilbene. Drug Metab Dispos 33(12):1819–1826 doi: 10.1124/dmd.105.004861 [DOI] [PubMed] [Google Scholar]
- Penman BW, Reece J, Smith T, et al. (1993) Characterization of a human cell line expressing high levels of cDNA-derived CYP2D6. Pharmacogenetics 3(1):28–39 doi: 10.1097/00008571-199302000-00003 [DOI] [PubMed] [Google Scholar]
- Philip PA, Ali-Sadat S, Doehmer J, et al. (1999) Use of V79 cells with stably transfected cytochrome P450 cDNAs in studying the metabolism and effects of cytotoxic drugs. Cancer Chemother Pharmacol 43(1):59–67 doi: 10.1007/s002800050863 [DOI] [PubMed] [Google Scholar]
- Powell PK, Wolf I, Jin R, Lasker JM (1998) Metabolism of arachidonic acid to 20-hydroxy-5,8,11,14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther 285(3):1327–1336 [PubMed] [Google Scholar]
- Quinn AM, Penning TM (2008) Comparisons of (±)-benzo[a]pyrene-trans-7,8-dihydrodiol activation by human cytochrome P450 and aldo-keto reductase enzymes: effect of redox state and expression levels. Chem Res Toxicol 21(5):1086–1094 doi: 10.1021/tx700345v [DOI] [PubMed] [Google Scholar]
- Rahman MA, Kodidela S, Sinha N, et al. (2019) Plasma exosomes exacerbate alcohol- and acetaminophen-induced toxicity via CYP2E1 pathway. Sci Rep 9(1):6571 doi: 10.1038/s41598-019-43064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh SM, Wanogho E, Burke MD, McKeown SR, Patterson LH (1998) Involvement of human cytochromes P450 (CYP) in the reductive metabolism of AQ4N, a hypoxia activated anthraquinone di-N-oxide prodrug. Int J Radiat Oncol Biol Phys 42(4):763–767 doi: 10.1016/s0360-3016(98)00308-3 [DOI] [PubMed] [Google Scholar]
- Ramsdell HS, Parkinson A, Eddy AC, Eaton DL (1991) Bioactivation of aflatoxin B1 by human liver microsomes: role of cytochrome P450 IIIA enzymes. Toxicol Appl Pharmacol 108(3):436–447 doi: 10.1016/0041-008x(91)90090-2 [DOI] [PubMed] [Google Scholar]
- Raney KD, Shimada T, Kim D-H, Groopman JD, Harris TM, Guengerich FP (1992) Oxidation of aflatoxins and sterigmatocystin by human liver microsomes: significance of aflatoxin Q1 as a detoxication product of aflatoxin B1. Chem Res Toxicol 5(2):202–210 doi: 10.1021/tx00026a009 [DOI] [PubMed] [Google Scholar]
- Raucy JL (1995) Risk assessment: toxicity from chemical exposure resulting from enhanced expression of CYP2E1. Toxicology 105(2–3):217–224 doi: 10.1016/0300-483x(95)03216-3 [DOI] [PubMed] [Google Scholar]
- Raucy JL, Lasker JM, Lieber CS, Black M (1989) Acetaminophen activation by human liver cytochromes P450 IIE1 and P450 IA2. Arch Biochem Biophys 271(2):270–283 doi: 10.1016/0003-9861(89)90278-6 [DOI] [PubMed] [Google Scholar]
- Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM (1999) Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2, and CYP2E1. Clin Cancer Res 5(8):2192–2197 [PubMed] [Google Scholar]
- Reid JM, Kuffel MJ, Ruben SL, et al. (2002) Rat and human liver cytochrome P-450 isoform metabolism of ecteinascidin 743 does not predict gender-dependent toxicity in humans. Clin Cancer Res 8(9):2952–2962 [PubMed] [Google Scholar]
- Ren S, Yang JS, Kalhorn TF, Slattery JT (1997) Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 57(19):4229–4235 [PubMed] [Google Scholar]
- Rendic S (2002) Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev 34(1–2):83–448 doi: 10.1081/dmr-120001392 [DOI] [PubMed] [Google Scholar]
- Rendic S, DiCarlo FJ (1997) Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev 29(1–2):413–580 doi: 10.3109/03602539709037591 [DOI] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP (2010) Update information on drug metabolism systems–2009, part II: summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr Drug Metab 11(1):4–84 doi: 10.2174/138920010791110917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP (2012) Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol 25(7):1316–1383 doi: 10.1021/tx300132k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP (2015) Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol 28(1):38–42 doi: 10.1021/tx500444e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic SP, Guengerich FP (2018) Development and uses of offline and web-searchable metabolism databases–the case of benzo[a]pyrene. Curr Drug Metab 19(1):3–46 doi: 10.2174/1389200219666171207123939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind AB, Lee C, Chang TK, Waxman DJ (1995) Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch Biochem Biophys 320(2):380–389 doi: 10.1016/0003-9861(95)90023-3 [DOI] [PubMed] [Google Scholar]
- Rivera SP, Wang F, Saarikoski ST, et al. (2007) A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of cytochrome P450 2S1 by both dioxin and hypoxia. J Biol Chem 282(15):10881–10893 doi: 10.1074/jbc.M609617200 [DOI] [PubMed] [Google Scholar]
- Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL (2001) Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther 296(2):537–541 [PubMed] [Google Scholar]
- Roe AL, Snawder JE, Benson RW, Roberts DW, Casciano DA (1993) HepG2 cells: an in vitro model for P450-dependent metabolism of acetaminophen. Biochem Biophys Res Commun 190(1):15–19 doi: 10.1006/bbrc.1993.1003 [DOI] [PubMed] [Google Scholar]
- Roellecke K, Virts EL, Einholz R, et al. (2016) Optimized human CYP4B1 in combination with the alkylator prodrug 4-ipomeanol serves as a novel suicide gene system for adoptive T-cell therapies. Gene Ther 23(7):615–626 doi: 10.1038/gt.2016.38 [DOI] [PubMed] [Google Scholar]
- Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82(1):131–185 doi: 10.1152/physrev.00021.2001 [DOI] [PubMed] [Google Scholar]
- Roy P, Tretyakov O, Wright J, Waxman DJ (1999a) Stereoselective metabolism of ifosfamide by human P-450s 3A4 and 2B6. Favorable metabolic properties of R-enantiomer. Drug Metab Dispos 27(11):1309–1318 [PubMed] [Google Scholar]
- Roy P, Yu LJ, Crespi CL, Waxman DJ (1999b) Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 27(6):655–666 [PubMed] [Google Scholar]
- Ruan Q, Gelhaus SL, Penning TM, Harvey RG, Blair IA (2007) Aldo-keto reductase- and cytochrome P450-dependent formation of benzo[a]pyrene-derived DNA adducts in human bronchoalveolar cells. Chem Res Toxicol 20(3):424–431 doi: 10.1021/tx060180b [DOI] [PubMed] [Google Scholar]
- Saarikoski ST, Rivera SP, Hankinson O, Husgafvel-Pursiainen K (2005) CYP2S1: a short review. Toxicol Appl Pharmacol 207(2 Suppl):62–69 doi: 10.1016/j.taap.2004.12.027 [DOI] [PubMed] [Google Scholar]
- Sabater Vilar M, Kuilman-Wahls ME, Fink-Gremmels J (2003) Inhibition of aflatoxin B1 mutagenicity by cyclopiazonic acid in the presence of human liver preparations. Toxicol Lett 143(3):291–299 doi: 10.1016/s0378-4274(03)00196-6 [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Gatta A, McGiff JC (2003) Role of cytochrome P450-dependent arachidonic acid metabolites in liver physiology and pathophysiology. Prostaglandins Other Lipid Mediat 72(1–2):51–71 doi: 10.1016/s1098-8823(03)00077-7 [DOI] [PubMed] [Google Scholar]
- Sadeque AJ, Fisher MB, Korzekwa KR, Gonzalez FJ, Rettie AE (1997) Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J Pharmacol Exp Ther 283(2):698–703 [PubMed] [Google Scholar]
- Sakano K, Kawanishi S (2002) Metal-mediated DNA damage induced by curcumin in the presence of human cytochrome P450 isozymes. Arch Biochem Biophys 405(2):223–230 doi: 10.1016/s0003-9861(02)00302-8 [DOI] [PubMed] [Google Scholar]
- Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS (1998) Respective roles of human cytochrome P-450 2E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res 22(9):2125–2132 [PubMed] [Google Scholar]
- Sams C, Cocker J, Lennard MS (2004) Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP). Xenobiotica 34(10):861–873 doi: 10.1080/00498250400017273 [DOI] [PubMed] [Google Scholar]
- Sams C, Mason HJ, Rawbone R (2000) Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes. Toxicol Lett 116(3):217–221 doi: 10.1016/s0378-4274(00)00221-6 [DOI] [PubMed] [Google Scholar]
- Sarich T, Kalhorn T, Magee S, et al. (1997) The effect of omeprazole pretreatment on acetaminophen metabolism in rapid and slow metabolizers of S-mephenytoin. Clin Pharmacol Ther 62(1):21–28 doi: 10.1016/s0009-9236(97)90148-x [DOI] [PubMed] [Google Scholar]
- Satoh T, Fujita KI, Munakata H, et al. (2000) Studies on the interactions between drugs and estrogen: analytical method for prediction system of gynecomastia induced by drugs on the inhibitory metabolism of estradiol using Escherichia coli coexpressing human CYP3A4 with human NADPH-cytochrome P450 reductase. Anal Biochem 286(2):179–186 doi: 10.1006/abio.1999.4775 [DOI] [PubMed] [Google Scholar]
- Sausville LN, Gangadhariah MH, Chiusa M, et al. (2018) The cytochrome P450 slow metabolizers CYP2C9*2 and CYP2C9*3 directly regulate tumorigenesis via reduced epoxyeicosatrienoic acid production. Cancer Res 78(17):4865–4877 doi: 10.1158/0008-5472.Can-17-3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeiser HH, Stiborovà M, Arlt VM (2009) Chemical and molecular basis of the carcinogenicity of Aristolochia plants. Curr Opin Drug Discov Devel 12(1):141–148 [PubMed] [Google Scholar]
- Schmidt R, Baumann F, Knüpfer H, et al. (2004) CYP3A4, CYP2C9 and CYP2B6 expression and ifosfamide turnover in breast cancer tissue microsomes. Br J Cancer 90(4):911–916 doi: 10.1038/sj.bjc.6601492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Cascorbi I, Schunck WH, Roots I (2001) Differential metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1 variants. Carcinogenesis 22(3):453–459 doi: 10.1093/carcin/22.3.453 [DOI] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Ericksen SS, et al. (2004) Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol 67(8):1445–1457 doi: 10.1016/j.bcp.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Roots I (2003) St. John’s wort extracts and some of their constituents potently inhibit ultimate carcinogen formation from benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1. Cancer Res 63(22):8062–8068 [PubMed] [Google Scholar]
- Schwarz D, Roots I (2003) In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem Biophys Res Commun 303(3):902–907 doi: 10.1016/s0006-291x(03)00435-2 [DOI] [PubMed] [Google Scholar]
- Seaton MJ, Follansbee MH, Bond JA (1995) Oxidation of 1,2-epoxy-3-butene to 1,2:3,4-diepoxybutane by cDNA-expressed human cytochromes P450 2E1 and 3A4 and human, mouse and rat liver microsomes. Carcinogenesis 16(10):2287–2293 doi: 10.1093/carcin/16.10.2287 [DOI] [PubMed] [Google Scholar]
- Seidel A, Soballa VJ, Raab G, et al. (1998) Regio- and stereoselectivity in the metabolism of benzo[c]phenanthrene mediated by genetically engineered V79 Chinese hamster cells expressing rat and human cytochromes P450. Environ Toxicol Pharmacol 5(3):179–196 doi: 10.1016/s1382-6689(97)10073-4 [DOI] [PubMed] [Google Scholar]
- Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF (2003) The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine Tob Res 5(6):891–899 doi: 10.1080/14622200310001615231 [DOI] [PubMed] [Google Scholar]
- Shebley M, Jushchyshyn MI, Hollenberg PF (2006) Selective pathways for the metabolism of phencyclidine by cytochrome P450 2B enzymes: identification of electrophilic metabolites, glutathione, and N-acetylcysteine adducts. Drug Metab Dispos 34(3):375–383 doi: 10.1124/dmd.105.007047 [DOI] [PubMed] [Google Scholar]
- Shen C, Meng Q, Zhang G, Hu W (2008) Rifampicin exacerbates isoniazid-induced toxicity in human but not in rat hepatocytes in tissue-like cultures. Br J Pharmacol 153(4):784–791 doi: 10.1038/sj.bjp.0707611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Marchick MR, Davis MR, Doss GA, Pohl LR (1999) Metabolic activation of diclofenac by human cytochrome P450 3A4: role of 5-hydroxydiclofenac. Chem Res Toxicol 12(2):214–222 doi: 10.1021/tx9802365 [DOI] [PubMed] [Google Scholar]
- Shet MS, McPhaul M, Fisher CW, Stallings NR, Estabrook RW (1997) Metabolism of the antiandrogenic drug (flutamide) by human CYP1A2. Drug Metab Dispos 25(11):1298–1303 [PubMed] [Google Scholar]
- Shibutani S, Bonala RR, Rosenquist T, et al. (2010) Detoxification of aristolochic acid I by O-demethylation: less nephrotoxicity and genotoxicity of aristolochic acid Ia in rodents. Int J Cancer 127(5):1021–1027 doi: 10.1002/ijc.25141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield KD, Ferlay J, Jemal A, et al. (2017) The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin 67(1):51–64 doi: 10.3322/caac.21384 [DOI] [PubMed] [Google Scholar]
- Shimada T (2006) Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet 21(4):257–276 doi: 10.2133/dmpk.21.257 [DOI] [PubMed] [Google Scholar]
- Shimada T, El-Bayoumy K, Upadhyaya P, Sutter TR, Guengerich FP, Yamazaki H (1997a) Inhibition of human cytochrome P450-catalyzed oxidations of xenobiotics and procarcinogens by synthetic organoselenium compounds. Cancer Res 57(21):4757–4764 [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y (2004) Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci 95(1):1–6 doi: 10.1111/j.1349-7006.2004.tb03162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Gillam EMJ, Sandhu P, Guo Z, Tukey RH, Guengerich FP (1994) Activation of procarcinogens by human cytochrome P450 enzymes expressed in Escherichia coli. Simplified bacterial systems for genotoxicity assays. Carcinogenesis 15(11):2523–2529 doi: 10.1093/carcin/15.11.2523 [DOI] [PubMed] [Google Scholar]
- Shimada T, Gillam EM, Sutter TR, Strickland PT, Guengerich FP, Yamazaki H (1997b) Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metab Dispos 25(5):617–622 [PubMed] [Google Scholar]
- Shimada T, Guengerich FP (1989) Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A 86(2):462–465 doi: 10.1073/pnas.86.2.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Guengerich FP (1991) Activation of amino-a-carboline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and a copper phthalocyanine cellulose extract of cigarette smoke condensate by cytochrome P-450 enzymes in rat and human liver microsomes. Cancer Res 51(19):5284–5291 [PubMed] [Google Scholar]
- Shimada T, Guengerich FP (2006) Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol 19(2):288–294 doi: 10.1021/tx050291v [DOI] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, et al. (1996) Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res 56(13):2979–2984 [PubMed] [Google Scholar]
- Shimada T, Murayama N, Yamazaki H, et al. (2013) Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem Res Toxicol 26(4):529–537 doi: 10.1021/tx3004906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Oda Y, Gillam EMJ, Guengerich FP, Inoue K (2001a) Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos 29(9):1176–1182 [PubMed] [Google Scholar]
- Shimada T, Watanabe J, Inoue K, Guengerich FP, Gillam EMJ (2001b) Specificity of 17b-oestradiol and benzo[a]pyrene oxidation by polymorphic human cytochrome P4501B1 variants substituted at residues 48, 119 and 432. Xenobiotica 31(3):163–176 doi: 10.1080/00498250110043490 [DOI] [PubMed] [Google Scholar]
- Shimada T, Watanabe J, Kawajiri K, et al. (1999) Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis 20(8):1607–1613 doi: 10.1093/carcin/20.8.1607 [DOI] [PubMed] [Google Scholar]
- Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP, Gillam EMJ (1998) Recombinant human cytochrome P450 1B1 expression in Escherichia coli. Arch Biochem Biophys 357(1):111–120 doi: 10.1006/abbi.1998.0808 [DOI] [PubMed] [Google Scholar]
- Shin JG, Kane K, Flockhart DA (2001) Potent inhibition of CYP2D6 by haloperidol metabolites: stereoselective inhibition by reduced haloperidol. Br J Clin Pharmacol 51(1):45–52 doi: 10.1046/j.1365-2125.2001.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou M, Gonzalez FJ, Gelboin HV (1996a) Stereoselective epoxidation and hydration at the K-region of polycyclic aromatic hydrocarbons by cDNA-expressed cytochromes P450 1A1, 1A2, and epoxide hydrolase. Biochemistry 35(49):15807–15813 doi: 10.1021/bi962042z [DOI] [PubMed] [Google Scholar]
- Shou M, Korzekwa KR, Brooks EN, Krausz KW, Gonzalez FJ, Gelboin HV (1997) Role of human hepatic cytochrome P450 1A2 and 3A4 in the metabolic activation of estrone. Carcinogenesis 18(1):207–214 doi: 10.1093/carcin/18.1.207 [DOI] [PubMed] [Google Scholar]
- Shou M, Korzekwa KR, Crespi CL, Gonzalez FJ, Gelboin HV (1994) The role of 12 cDNA-expressed human, rodent, and rabbit cytochromes P450 in the metabolism of benzo[a]pyrene and benzo[a]pyrene trans-7,8-dihydrodiol. Mol Carcinog 10(3):159–168 doi: 10.1002/mc.2940100307 [DOI] [PubMed] [Google Scholar]
- Shou M, Korzekwa KR, Krausz KW, et al. (1996b) Specificity of cDNA-expressed human and rodent cytochrome P450s in the oxidative metabolism of the potent carcinogen 7,12-dimethylbenz[a]anthracene. Mol Carcinog 17(4):241–249 doi: [DOI] [PubMed] [Google Scholar]
- Shou M, Krausz KW, Gonzalez FJ, Gelboin HV (1996c) Metabolic activation of the potent carcinogen dibenzo[a,l]pyrene by human recombinant cytochromes P450, lung and liver microsomes. Carcinogenesis 17(11):2429–2433 doi: 10.1093/carcin/17.11.2429 [DOI] [PubMed] [Google Scholar]
- Simarro Doorten Y, Nijmeijer S, de Nijs-Tjon L, Fink-Gremmels J (2006) Metabolism-mediated ochratoxin A genotoxicity in the single-cell gel electrophoresis (Comet) assay. Food Chem Toxicol 44(2):261–270 doi: 10.1016/j.fct.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Sinclair J, Jeffery E, Wrighton S, et al. (1998) Alcohol-mediated increases in acetaminophen hepatotoxicity: role of CYP2E and CYP3A. Biochem Pharmacol 55(10):1557–1565 doi: 10.1016/s0006-2952(97)00656-4 [DOI] [PubMed] [Google Scholar]
- Smith GB, Bend JR, Bedard LL, Reid KR, Petsikas D, Massey TE (2003a) Biotransformation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in peripheral human lung microsomes. Drug Metab Dispos 31(9):1134–1141 doi: 10.1124/dmd.31.9.1134 [DOI] [PubMed] [Google Scholar]
- Smith KS, Smith PL, Heady TN, Trugman JM, Harman WD, Macdonald TL (2003b) In vitro metabolism of tolcapone to reactive intermediates: relevance to tolcapone liver toxicity. Chem Res Toxicol 16(2):123–128 doi: 10.1021/tx025569n [DOI] [PubMed] [Google Scholar]
- Smith TJ, Guo Z, Gonzalez FJ, Guengerich FP, Stoner GD, Yang CS (1992) Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human lung and liver microsomes and cytochromes P-450 expressed in hepatoma cells. Cancer Res 52(7):1757–1763 [PubMed] [Google Scholar]
- Smith TJ, Guo Z, Guengerich FP, Yang CS (1996) Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by human cytochrome P450 1A2 and its inhibition by phenethyl isothiocyanate. Carcinogenesis 17(4):809–813 doi: 10.1093/carcin/17.4.809 [DOI] [PubMed] [Google Scholar]
- Smith TJ, Stoner GD, Yang CS (1995) Activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human lung microsomes by cytochromes P450, lipoxygenase, and hydroperoxides. Cancer Res 55(23):5566–5573 [PubMed] [Google Scholar]
- Spink BC, Pang S, Pentecost BT, Spink DC (2002a) Induction of cytochrome P450 1B1 in MDA-MB-231 human breast cancer cells by non-ortho-substituted polychlorinated biphenyls. Toxicol In Vitro 16(6):695–704 doi: 10.1016/s0887-2333(02)00091-7 [DOI] [PubMed] [Google Scholar]
- Spink DC, Katz BH, Hussain MM, et al. (2002b) Induction of CYP1A1 and CYP1B1 in T-47D human breast cancer cells by benzo[a]pyrene is diminished by arsenite. Drug Metab Dispos 30(3):262–269 doi: 10.1124/dmd.30.3.262 [DOI] [PubMed] [Google Scholar]
- Spink DC, Spink BC, Cao JQ, et al. (1998) Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 19(2):291–298 doi: 10.1093/carcin/19.2.291 [DOI] [PubMed] [Google Scholar]
- Spink DC, Spink BC, Cao JQ, et al. (1997) Induction of cytochrome P450 1B1 and catechol estrogen metabolism in ACHN human renal adenocarcinoma cells. J Steroid Biochem Mol Biol 62(2–3):223–232 doi: 10.1016/s0960-0760(97)00024-1 [DOI] [PubMed] [Google Scholar]
- Spracklin DK, Hankins DC, Fisher JM, Thummel KE, Kharasch ED (1997) Cytochrome P450 2E1 is the principal catalyst of human oxidative halothane metabolism in vitro. J Pharmacol Exp Ther 281(1):400–411 [PubMed] [Google Scholar]
- Spracklin DK, Kharasch ED (1998) Human halothane reduction in vitro by cytochrome P450 2A6 and 3A4: identification of low and high KM isoforms. Drug Metab Dispos 26(6):605–607 [PubMed] [Google Scholar]
- Spracklin DK, Thummel KE, Kharasch ED (1996) Human reductive halothane metabolism in vitro is catalyzed by cytochrome P450 2A6 and 3A4. Drug Metab Dispos 24(9):976–983 [PubMed] [Google Scholar]
- Staretz ME, Murphy SE, Patten CJ, et al. (1997) Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N´- nitrosonornicotine in human hepatic microsomes. Drug Metab Dispos 25(2):154–162 [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, et al. (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95(23):1758–1764 doi: 10.1093/jnci/djg108 [DOI] [PubMed] [Google Scholar]
- Stiborová M, Bárta F, Levová K, et al. (2015) A mechanism of O-demethylation of aristolochic acid I by cytochromes P450 and their contributions to this reaction in human and rat livers: experimental and theoretical approaches. Int J Mol Sci 16(11):27561–27575 doi: 10.3390/ijms161126047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiborová M, Bieler CA, Wiessler M, Frei E (2001a) The anticancer agent ellipticine on activation by cytochrome P450 forms covalent DNA adducts. Biochem Pharmacol 62(12):1675–1684 doi: 10.1016/s0006-2952(01)00806-1 [DOI] [PubMed] [Google Scholar]
- Stiborová M, Borek-Dohalská L, Hodek P, Mráz J, Frei E (2002) New selective inhibitors of cytochromes P450 2B and their application to antimutagenesis of tamoxifen. Arch Biochem Biophys 403(1):41–49 doi: 10.1016/s0003-9861(02)00259-x [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Arlt VM, Schmeiser HH (2014) Knockout and humanized mice as suitable tools to identify enzymes metabolizing the human carcinogen aristolochic acid. Xenobiotica 44(2):135–145 doi: 10.3109/00498254.2013.848310 [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Hodek P, Wiessler M, Schmeiser HH (2005) Human hepatic and renal microsomes, cytochromes P450 1A1/2, NADPH:cytochrome P450 reductase and prostaglandin H synthase mediate the formation of aristolochic acid-DNA adducts found in patients with urothelial cancer. Int J Cancer 113(2):189–197 doi: 10.1002/ijc.20564 [DOI] [PubMed] [Google Scholar]
- Stiborová M, Frei E, Wiessler M, Schmeiser HH (2001b) Human enzymes involved in the metabolic activation of carcinogenic aristolochic acids: evidence for reductive activation by cytochromes P450 1A1 and 1A2. Chem Res Toxicol 14(8):1128–1137 doi: 10.1021/tx010059z [DOI] [PubMed] [Google Scholar]
- Stiborová M, Levová K, Bárta F, et al. (2012a) Bioactivation versus detoxication of the urothelial carcinogen aristolochic acid I by human cytochrome P450 1A1 and 1A2. Toxicol Sci 125(2):345–358 doi: 10.1093/toxsci/kfr306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiborová M, Martínek V, Frei E, Arlt VM, Schmeiser HH (2013) Enzymes metabolizing aristolochic acid and their contribution to the development of aristolochic acid nephropathy and urothelial cancer. Curr Drug Metab 14(6):695–705 doi: 10.2174/1389200211314060006 [DOI] [PubMed] [Google Scholar]
- Stiborová M, Poljaková J, Martínková E, et al. (2012b) Ellipticine oxidation and DNA adduct formation in human hepatocytes is catalyzed by human cytochromes P450 and enhanced by cytochrome b5. Toxicology 302(2–3):233–241 doi: 10.1016/j.tox.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Stiborová M, Sejbal J, Borek-Dohalská L, et al. (2004) The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res 64(22):8374–8380 doi: 10.1158/0008-5472.Can-04-2202 [DOI] [PubMed] [Google Scholar]
- Styles JA, Davies A, Lim CK, et al. (1994) Genotoxicity of tamoxifen, tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis 15(1):5–9 doi: 10.1093/carcin/15.1.5 [DOI] [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X (2000) Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 60(18):5074–5079 [PubMed] [Google Scholar]
- Takada K, Arefayene M, Desta Z, et al. (2004) Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum 50(7):2202–2210 doi: 10.1002/art.20338 [DOI] [PubMed] [Google Scholar]
- Takagi T, Ishii H, Takahashi H, et al. (1983) Potentiation of halothane hepatotoxicity by chronic ethanol administration in rat: an animal model of halothane hepatitis. Pharmacol Biochem Behav 18 Suppl 1:461–465 doi: 10.1016/0091-3057(83)90218-6 [DOI] [PubMed] [Google Scholar]
- Takemoto K, Yamazaki H, Nakajima M, Yokoi T (2002) Genotoxic activation of benzophenone and its two metabolites by human cytochrome P450s in SOS/umu assay. Mutat Res 519(1–2):199–204 doi: 10.1016/s1383-5718(02)00141-9 [DOI] [PubMed] [Google Scholar]
- Tan SC, New LS, Chan EC (2008) Prevention of acetaminophen (APAP)-induced hepatotoxicity by leflunomide via inhibition of APAP biotransformation to N-acetyl-p-benzoquinone imine. Toxicol Lett 180(3):174–181 doi: 10.1016/j.toxlet.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Tang J, Cao Y, Rose RL, et al. (2001) Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab Dispos 29(9):1201–1204 [PubMed] [Google Scholar]
- Tang W (2003) The metabolism of diclofenac--enzymology and toxicology perspectives. Curr Drug Metab 4(4):319–329 doi: 10.2174/1389200033489398 [DOI] [PubMed] [Google Scholar]
- Tang W, Stearns RA, Wang RW, Chiu SH, Baillie TA (1999) Roles of human hepatic cytochrome P450s 2C9 and 3A4 in the metabolic activation of diclofenac. Chem Res Toxicol 12(2):192–199 doi: 10.1021/tx9802217 [DOI] [PubMed] [Google Scholar]
- Teiber JF, Hollenberg PF (2000) Identification of the human liver microsomal cytochrome P450s involved in the metabolism of N-nitrosodi-n-propylamine. Carcinogenesis 21(8):1559–1566 [PubMed] [Google Scholar]
- Teitelbaum AM, McDonald MG, Kowalski JP, et al. (2019) Influence of stereochemistry on the bioactivation and glucuronidation of 4-ipomeanol. J Pharmacol Exp Ther 368(2):308–316 doi: 10.1124/jpet.118.249771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettey JN, Maggs JL, Rapeport WG, Pirmohamed M, Park BK (2001) Enzyme-induction dependent bioactivation of troglitazone and troglitazone quinone in vivo. Chem Res Toxicol 14(8):965–974 doi: 10.1021/tx0001981 [DOI] [PubMed] [Google Scholar]
- Thornton-Manning J, Appleton ML, Gonzalez FJ, Yost GS (1996) Metabolism of 3-methylindole by vaccinia-expressed P450 enzymes: correlation of 3-methyleneindolenine formation and protein-binding. J Pharmacol Exp Ther 276(1):21–29 [PubMed] [Google Scholar]
- Thummel KE, Slattery JT, Ro H, et al. (2000) Ethanol and production of the hepatotoxic metabolite of acetaminophen in healthy adults. Clin Pharmacol Ther 67(6):591–599 doi: 10.1067/mcp.2000.106574 [DOI] [PubMed] [Google Scholar]
- Travica S, Pors K, Loadman PM, et al. (2013) Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clin Cancer Res 19(11):2952–2961 doi: 10.1158/1078-0432.Ccr-13-0238 [DOI] [PubMed] [Google Scholar]
- Tsou HH, Ko HT, Chen CT, et al. (2019) Betel quid containing safrole enhances metabolic activation of tobacco specific 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Environ Pollut 251:13–21 doi: 10.1016/j.envpol.2019.04.080 [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Yokoi T (2005) Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227(2):115–124 doi: 10.1016/j.canlet.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Tu M, Li L, Lei H, et al. (2014) Involvement of organic cation transporter 1 and CYP3A4 in retrorsine-induced toxicity. Toxicology 322:34–42 doi: 10.1016/j.tox.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Constable A, Fay LB, Guengerich FP (1999) Interspecies differences in metabolism of heterocyclic aromatic amines by rat and human P450 1A2. Cancer Lett 143(2):109–112 doi: 10.1016/s0304-3835(99)00137-8 [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Constable A, Richoz J, et al. (1998) Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem Res Toxicol 11(8):925–936 doi: 10.1021/tx980022n [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Guengerich FP, Guillouzo A, Langouët S (2002) Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P450 1A2. Mutat Res 506–507:187–195 doi: 10.1016/s0027-5107(02)00165-3 [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Parisod V, Huynh-Ba T, Langouët S, Guengerich FP (2001) Regioselective differences in C(8)- and N-oxidation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline by human and rat liver microsomes and cytochromes P450 1A2. Chem Res Toxicol 14(7):901–911 doi: 10.1021/tx010035s [DOI] [PubMed] [Google Scholar]
- Ueng Y-F, Hsieh CH, Don MJ, Chi CW, Ho LK (2004) Identification of the main human cytochrome P450 enzymes involved in safrole 1’-hydroxylation. Chem Res Toxicol 17(8):1151–1156 doi: 10.1021/tx030055p [DOI] [PubMed] [Google Scholar]
- Ueng Y-F, Kuwabara T, Chun Y-J, Guengerich FP (1997) Cooperativity in oxidations catalyzed by cytochrome P450 3A4. Biochemistry 36(2):370–381 doi: 10.1021/bi962359z [DOI] [PubMed] [Google Scholar]
- Ueng Y-F, Shimada T, Yamazaki H, Guengerich FP (1995) Oxidation of aflatoxin B1 by bacterial recombinant human cytochrome P450 enzymes. Chem Res Toxicol 8(2):218–225 doi: 10.1021/tx00044a006 [DOI] [PubMed] [Google Scholar]
- Uno T, Obe Y, Ogura C, et al. (2013) Metabolism of 7-ethoxycoumarin, safrole, flavanone and hydroxyflavanone by cytochrome P450 2A6 variants. Biopharm Drug Dispos 34(2):87–97 doi: 10.1002/bdd.1825 [DOI] [PubMed] [Google Scholar]
- Usuki E, Van der Schyf CJ, Castagnoli N Jr. (1998) Metabolism of haloperidol and its tetrahydropyridine dehydration product HPTP. Drug Metab Rev 30(4):809–826 doi: 10.3109/03602539808996331 [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, van der Bruggen M, van der Linden J, van den Berg M (2003) Effects of several dioxin-like compounds on estrogen metabolism in the malignant MCF-7 and nontumorigenic MCF-10A human mammary epithelial cell lines. Toxicol Appl Pharmacol 190(3):241–250 doi: 10.1016/s0041-008x(03)00166-2 [DOI] [PubMed] [Google Scholar]
- Van Vleet TR, Klein PJ, Coulombe RA Jr. (2001) Metabolism of aflatoxin B1 by normal human bronchial epithelial cells. J Toxicol Environ Health A 63(7):525–540 doi: 10.1080/15287390152410156 [DOI] [PubMed] [Google Scholar]
- Van Vleet TR, Klein PJ, Coulombe RA Jr. (2002a) Metabolism and cytotoxicity of aflatoxin B1 in cytochrome P-450-expressing human lung cells. J Toxicol Environ Health A 65(12):853–867 doi: 10.1080/00984100290071216 [DOI] [PubMed] [Google Scholar]
- Van Vleet TR, Macé K, Coulombe RA Jr. (2002b) Comparative aflatoxin B1 activation and cytotoxicity in human bronchial cells expressing cytochromes P450 1A2 and 3A4. Cancer Res 62(1):105–112 [PubMed] [Google Scholar]
- von Weymarn LB, Chun JA, Hollenberg PF (2006) Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: potential for chemoprevention in smokers. Carcinogenesis 27(4):782–790 doi: 10.1093/carcin/bgi301 [DOI] [PubMed] [Google Scholar]
- Walker D, Flinois JP, Monkman SC, et al. (1994) Identification of the major human hepatic cytochrome P450 involved in activation and N-dechloroethylation of ifosfamide. Biochem Pharmacol 47(7):1157–1163 doi: 10.1016/0006-2952(94)90387-5 [DOI] [PubMed] [Google Scholar]
- Walsky RL, Obach RS (2007) A comparison of 2-phenyl-2-(1-piperidinyl)propane (PPP), 1,1´,1´´-phosphinothioylidynetrisaziridine (thioTEPA), clopidogrel, and ticlopidine as selective inactivators of human cytochrome P450 2B6. Drug Metab Dispos 35(11):2053–2059 doi: 10.1124/dmd.107.015883 [DOI] [PubMed] [Google Scholar]
- Wang H, Dick R, Yin H, et al. (1998) Structure-function relationships of human liver cytochromes P450 3A: aflatoxin B1 metabolism as a probe. Biochemistry 37(36):12536–12545 doi: 10.1021/bi980895g [DOI] [PubMed] [Google Scholar]
- Wang YP, Yan J, Fu PP, Chou MW (2005) Human liver microsomal reduction of pyrrolizidine alkaloid N-oxides to form the corresponding carcinogenic parent alkaloid. Toxicol Lett 155(3):411–420 doi: 10.1016/j.toxlet.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Ward S, Back DJ (1993) Metabolism of gestodene in human liver cytosol and microsomes in vitro. J Steroid Biochem Mol Biol 46(2):235–243 doi: 10.1016/0960-0760(93)90299-c [DOI] [PubMed] [Google Scholar]
- Watanabe J, Shimada T, Gillam EMJ, et al. (2000) Association of CYP1B1 genetic polymorphism with incidence to breast and lung cancer. Pharmacogenetics 10(1):25–33 doi: 10.1097/00008571-200002000-00004 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Watanabe N, Maruyama S, Kawashiro T (2015) Comparative metabolic study between two selective estrogen receptor modulators, toremifene and tamoxifen, in human liver microsomes. Drug Metab Pharmacokinet 30(5):325–333 doi: 10.1016/j.dmpk.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Weems JM, Lamb JG, D’Agostino J, Ding X, Yost GS (2010) Potent mutagenicity of 3-methylindole requires pulmonary cytochrome P450-mediated bioactivation: a comparison to the prototype cigarette smoke mutagens B(a)P and NNK. Chem Res Toxicol 23(11):1682–1690 doi: 10.1021/tx100147z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems JM, Yost GS (2010) 3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem Res Toxicol 23(3):696–704 doi: 10.1021/tx9004506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Ren Z, Shu XO, et al. (2007) Expression of cytochrome P450 1B1 and catechol-O-methyltransferase in breast tissue and their associations with breast cancer risk. Cancer Epidemiol Biomarkers Prev 16(5):917–920 doi: 10.1158/1055-9965.Epi-06-1032 [DOI] [PubMed] [Google Scholar]
- White IN, De Matteis F (2001) The role of CYP forms in the metabolism and metabolic activation of HCFCs and other halocarbons. Toxicol Lett 124(1–3):121–128 doi: 10.1016/s0378-4274(00)00288-5 [DOI] [PubMed] [Google Scholar]
- White IN, De Matteis F, Gibbs AH, et al. (1995) Species differences in the covalent binding of [14C]tamoxifen to liver microsomes and the forms of cytochrome P450 involved. Biochem Pharmacol 49(8):1035–1042 doi: 10.1016/0006-2952(95)98498-x [DOI] [PubMed] [Google Scholar]
- Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH (2000) Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis 21(9):1683–1689 doi: 10.1093/carcin/21.9.1683 [DOI] [PubMed] [Google Scholar]
- Williams JA, Ring BJ, Cantrell VE, et al. (2002) Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos 30(8):883–891 doi: 10.1124/dmd.30.8.883 [DOI] [PubMed] [Google Scholar]
- Williams JA, Stone EM, Millar BC, Gusterson BA, Grover PL, Phillips DH (1998) Determination of the enzymes responsible for activation of the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline in the human breast. Pharmacogenetics 8(6):519–528 doi: 10.1097/00008571-199812000-00009 [DOI] [PubMed] [Google Scholar]
- Williams JM, Murphy S, Burke M, Roman RJ (2010) 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56(4):336–344 doi: 10.1097/FJC.0b013e3181f04b1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter HR, Wang Y, Unadkat JD (2000) CYP2C8/9 mediate dapsone N-hydroxylation at clinical concentrations of dapsone. Drug Metab Dispos 28(8):865–868 [PubMed] [Google Scholar]
- Wolkenstein P, Tan C, Lecoeur S, et al. (1998) Covalent binding of carbamazepine reactive metabolites to P450 isoforms present in the skin. Chem-Biol Interact 113(1):39–50 doi: 10.1016/s0009-2797(98)00021-0 [DOI] [PubMed] [Google Scholar]
- Wong HL, Murphy SE, Hecht SS (2005a) Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N´-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem Res Toxicol 18(1):61–69 doi: 10.1021/tx0497696 [DOI] [PubMed] [Google Scholar]
- Wong HL, Zhang X, Zhang QY, et al. (2005b) Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem Res Toxicol 18(6):913–918 doi: 10.1021/tx0500777 [DOI] [PubMed] [Google Scholar]
- Wu J, Dong H, Cai Z, Yu Y (1997) Stable expression of human cytochrome CYP2B6 and CYP1A1 in Chinese hamster CHL cells: their use in micronucleus assays. Chin Med Sci J 12(3):148–155 [PubMed] [Google Scholar]
- Wu Z-L, Sohl CD, Shimada T, Guengerich FP (2006) Recombinant enzymes overexpressed in bacteria show broad catalytic specificity of human cytochrome P450 2W1 and limited activity of human cytochrome P450 2S1. Mol Pharmacol 69(6):2007–2014 doi: 10.1124/mol.106.023648 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Shinkyo R, Guengerich FP (2011) Cytochrome P450 2S1 is reduced by NADPH-cytochrome P450 reductase. Drug Metab Dispos 39(6):944–946 doi: 10.1124/dmd.111.039321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Wang HF, Wang Q, et al. (2001) Influence of P450 3A4 SRS-2 residues on cooperativity and/or regioselectivity of aflatoxin B1 oxidation. Chem Res Toxicol 14(5):483–491 doi: 10.1021/tx000218z [DOI] [PubMed] [Google Scholar]
- Yager JD (2000) Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr(27):67–73 doi: 10.1093/oxfordjournals.jncimonographs.a024245 [DOI] [PubMed] [Google Scholar]
- Yakkundi A, McErlane V, Murray M, et al. (2006) Tumor-selective drug activation: a GDEPT approach utilizing cytochrome P450 1A1 and AQ4N. Cancer Gene Ther 13(6):598–605 doi: 10.1038/sj.cgt.7700933 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Inoue K, Chiba K, et al. (1998a) Comparative studies on the catalytic roles of cytochrome P450 2C9 and its Cys- and Leu-variants in the oxidation of warfarin, flurbiprofen, and diclofenac by human liver microsomes. Biochem Pharmacol 56(2):243–251 doi: 10.1016/s0006-2952(98)00133-6 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Inui Y, Wrighton SA, Guengerich FP, Shimada T (1995) Procarcinogen activation by cytochrome P450 3A4 and 3A5 expressed in Escherichia coli and by human liver microsomes. Carcinogenesis 16(9):2167–2170 doi: 10.1093/carcin/16.9.2167 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Inui Y, Yun C-H, Guengerich FP, Shimada T (1992) Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis 13(10):1789–1794 doi: 10.1093/carcin/13.10.1789 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Mimura M, Oda Y, et al. (1993) Roles of different forms of cytochrome P450 in the activation of the promutagen 6-aminochrysene to genotoxic metabolites in human liver microsomes. Carcinogenesis 14(7):1271–1278 doi: 10.1093/carcin/14.7.1271 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shaw PM, Guengerich FP, Shimada T (1998b) Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol 11(6):659–665 doi: 10.1021/tx970217f [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shibata A, Suzuki M, et al. (1999) Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-450 2C8 and P-450 3A4 in human liver microsomes. Drug Metab Dispos 27(11):1260–1266 [PubMed] [Google Scholar]
- Yamazaki Y, Fujita K, Nakayama K, et al. (2004) Establishment of ten strains of genetically engineered Salmonella typhimurium TA1538 each co-expressing a form of human cytochrome P450 with NADPH-cytochrome P450 reductase sensitive to various promutagens. Mutat Res 562(1–2):151–162 doi: 10.1016/j.mrgentox.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhong HM, Maher N, et al. (2005) Bioactivation of 4-methylphenol (p-cresol) via cytochrome P450-mediated aromatic oxidation in human liver microsomes. Drug Metab Dispos 33(12):1867–1876 doi: 10.1124/dmd.105.006387 [DOI] [PubMed] [Google Scholar]
- Yang AH, Zhang L, Zhi DX, Liu WL, Gao X, He X (2018) Identification and analysis of the reactive metabolites related to the hepatotoxicity of safrole. Xenobiotica 48(11):1164–1172 doi: 10.1080/00498254.2017.1399227 [DOI] [PubMed] [Google Scholar]
- Yang CS, Chhabra SK, Hong JY, Smith TJ (2001) Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr 131(3s):1041s–1045s doi: 10.1093/jn/131.3.1041S [DOI] [PubMed] [Google Scholar]
- Yang G, Nowsheen S, Aziz K, Georgakilas AG (2013) Toxicity and adverse effects of tamoxifen and other anti-estrogen drugs. Pharmacol Ther 139(3):392–404 doi: 10.1016/j.pharmthera.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Yang MX, Cederbaum AI (1997) Characterization of cytochrome P450 2E1 turnover in transfected HepG2 cells expressing human CYP2E1. Arch Biochem Biophys 341(1):25–33 doi: 10.1006/abbi.1997.9907 [DOI] [PubMed] [Google Scholar]
- Yang XJ, Lu HY, Li ZY, et al. (2012) Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 300(3):138–148 doi: 10.1016/j.tox.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N (2016) Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol 4(2):131–142 doi: 10.14218/jcth.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Nguyen N, Famourzadeh M, et al. (2001) The contribution of UDP-glucuronosyltransferase 1A9 on CYP1A2-mediated genotoxicity by aromatic and heterocyclic amines. Carcinogenesis 22(6):943–950 doi: 10.1093/carcin/22.6.943 [DOI] [PubMed] [Google Scholar]
- Yukinaga H, Takami T, Shioyama SH, et al. (2007) Identification of cytochrome P450 3A4 modification site with reactive metabolite using linear ion trap-Fourier transform mass spectrometry. Chem Res Toxicol 20(10):1373–1378 doi: 10.1021/tx700165q [DOI] [PubMed] [Google Scholar]
- Yun C-H, Shimada T, Guengerich FP (1991) Purification and characterization of human liver microsomal cytochrome P-450 2A6. Mol Pharmacol 40(5):679–685 [PubMed] [Google Scholar]
- Yun C-H, Shimada T, Guengerich FP (1992) Roles of human liver cytochrome P450 2C and 3A enzymes in the 3-hydroxylation of benzo(a)pyrene. Cancer Res 52(7):1868–1874 [PubMed] [Google Scholar]
- Zaher H, Buters JT, Ward JM, et al. (1998) Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol 152(1):193–199 doi: 10.1006/taap.1998.8501 [DOI] [PubMed] [Google Scholar]
- Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E (2006) The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem Res Toxicol 19(1):164–172 doi: 10.1021/tx050229y [DOI] [PubMed] [Google Scholar]
- Zahno A, Bouitbir J, Maseneni S, Lindinger PW, Brecht K, Krähenbühl S (2013) Hepatocellular toxicity of clopidogrel: mechanisms and risk factors. Free Radic Biol Med 65:208–216 doi: 10.1016/j.freeradbiomed.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Zand R, Nelson SD, Slattery JT, et al. (1993) Inhibition and induction of cytochrome P450 2E1-catalyzed oxidation by isoniazid in humans. Clin Pharmacol Ther 54(2):142–149 doi: 10.1038/clpt.1993.125 [DOI] [PubMed] [Google Scholar]
- Zeldin DC, DuBois RN, Falck JR, Capdevila JH (1995) Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys 322(1):76–86 doi: 10.1006/abbi.1995.1438 [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Moomaw CR, Jesse N, et al. (1996) Biochemical characterization of the human liver cytochrome P450 arachidonic acid epoxygenase pathway. Arch Biochem Biophys 330(1):87–96 doi: 10.1006/abbi.1996.0229 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Wang L, Yang F, et al. (2016) The mechanism and risk factors of clopidogrel-induced liver injury. Drug Chem Toxicol 39(4):367–374 doi: 10.3109/01480545.2015.1122606 [DOI] [PubMed] [Google Scholar]
- Zhang C, Booz GW, Yu Q, He X, Wang S, Fan F (2018) Conflicting roles of 20-HETE in hypertension and renal end organ damage. Eur J Pharmacol 833:190–200 doi: 10.1016/j.ejphar.2018.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, D’Agostino J, Wu H, et al. (2007) CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Pharmacol Exp Ther 323(2):570–578 doi: 10.1124/jpet.107.127068 [DOI] [PubMed] [Google Scholar]
- Zhang X, Su T, Zhang QY, et al. (2002) Genetic polymorphisms of the human CYP2A13 gene: identification of single-nucleotide polymorphisms and functional characterization of an Arg257Cys variant. J Pharmacol Exp Ther 302(2):416–423 doi: 10.1124/jpet.302.2.416 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang X, Wang Y, et al. (2013) Cytochrome P450 2A13 is an efficient enzyme in metabolic activation of aflatoxin G1 in human bronchial epithelial cells. Arch Toxicol 87(9):1697–1707 doi: 10.1007/s00204-013-1108-3 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wan D, Yang J, Hammock BD, Ortiz de Montellano PR (2016) Catalytic activities of tumor-specific human cytochrome P450 CYP2W1 toward endogenous substrates. Drug Metab Dispos 44(5):771–780 doi: 10.1124/dmd.116.069633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Josephy PD, Kim D, Guengerich FP (2004) Functional characterization of four allelic variants of human cytochrome P450 1A2. Arch Biochem Biophys 422(1):23–30 doi: 10.1016/j.abb.2003.11.019 [DOI] [PubMed] [Google Scholar]
- Zhou L, Erickson RR, Hardwick JP, Park SS, Wrighton SA, Holtzman JL (1997) Catalysis of the cysteine conjugation and protein binding of acetaminophen by microsomes from a human lymphoblast line transfected with the cDNAs of various forms of human cytochrome P450. J Pharmacol Exp Ther 281(2):785–790 [PubMed] [Google Scholar]
- Zhou D, Lu Y, Steiner MS, Dalton JT (2000) Cytochrome P-450 2C9 sensitizes human prostate tumor cells to cyclophosphamide via a bystander effect. Antimicrob Agents Chemother 44(10):2659–2663 doi: 10.1128/aac.44.10.2659-2663.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BT, Lee AJ (2005) NADPH-dependent metabolism of 17b-estradiol and estrone to polar and nonpolar metabolites by human tissues and cytochrome P450 isoforms. Steroids 70(4):225–244 doi: 10.1016/j.steroids.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Zhu BT, Roy D, Liehr JG (1993) The carcinogenic activity of ethinyl estrogens is determined by both their hormonal characteristics and their conversion to catechol metabolites. Endocrinology 132(2):577–583 doi: 10.1210/endo.132.2.8381068 [DOI] [PubMed] [Google Scholar]


