Abstract
Diagnosis and management of pediatric retinal conditions such as retinopathy of prematurity (ROP) have been evolving significantly with the availability of new technology and treatments. New imaging systems, telemedicine, tele-education, and anti‒vascular endothelial growth factor (VEGF) intravitreal pharmacotherapy are all changing the way we diagnose and deliver care to children with pediatric retinal disease. Fluorescein angiography and optical coherence tomography have the potential to improve our diagnosis and management of disease, and with improvements in retinal imaging, telemedicine is becoming more feasible. Telemedicine, tele-education, and computer-based image analysis may overcome many of the challenges we face in providing adequate care and access for children with pediatric retinal disease. Treatment options have also expanded with the use of anti-VEGF therapy. Although the use of intravitreal anti-VEGF for ROP has been documented in the literature for more than a decade, many questions still remain about its safety in the pediatric patient population. Several ongoing prospective studies are exploring the utility of anti-VEGF agents for ROP, with attention to the optimal dose of drug, systemic safety, and our understanding of recurrence of disease. This review aims to provide an update on current diagnostic and therapeutic modalities, focusing predominantly on the role of anti-VEGF therapy, for the management of ROP and other pediatric retinal vascular diseases.
Keywords: anti-VEGF therapy, retinopathy of prematurity, pediatric retina
Management of pediatric retinal conditions has traditionally been dependent on examination findings from ophthalmoscopy, in an era when access to pediatric retinal imaging was more limited than today. Currently, however, management paradigms are shifting as novel techniques, technologies, and clinical evidence emerge.
Intravitreal anti‒vascular endothelial growth factor (anti-VEGF) agents such as bevacizumab, ranibizumab, aflibercept, and conbercept have been shown to be effective in retinal vascular disorders. In adults, anti-VEGF therapy has revolutionized the management of conditions such as exudative age-related macular degeneration and diabetic retinopathy. The use of anti-VEGF in retinopathy of prematurity (ROP) or other pediatric retinal conditions, however, is less well defined.
Retinopathy of prematurity is a leading cause of childhood blindness worldwide that provides a relevant example of a pediatric retinal condition whose management has historically and continues to change. Clinical evidence and experience with anti-VEGF in pediatric retinal diseases is most established with ROP. Other pediatric retinal diseases with reported clinical use of anti-VEGF include Norrie disease,1 familial exudative vitreoretinopathy (FEVR),2,3 Coats disease,4,5 and incontinentia pigmenti.6
In this review, we discuss the potential impact of recent and future advances in diagnostic and therapeutic modalities, in particular the role of anti-VEGF therapy, on the management of ROP and other pediatric retinal conditions.
ROP—CLASSIFICATION AND SCREENING
Initially reported as “retrolental fibroplasia” in 1944,7 ROP clinically presents with peripheral retinal avascularity and can subsequently develop neovascular proliferation with possible progression to retinal detachment.8–11 Risk factors for ROP include low birth weight of 1500 g or less, gestational age (GA) of 32 weeks or less, and exposure to unmonitored supplemental oxygen.12,13 Extended high levels of oxygen lead to an initial hyperoxic vaso-obliterative phase followed by a hypoxic vasoproliferative phase in the immature retina.14 Although prolonged oxygen exposure is considered to be a major risk factor in the development of ROP, intraventricular hemorrhage, sepsis, hyperglycemia, blood transfusions, and respiratory distress syndrome have been reported as other associated risk factors.15,16
Globally, ROP has presented in 3 distinct epidemics since Terry’s original description.12 The first epidemic in the 1940s and 1950s was driven by the use of unmonitored supplemental oxygen in premature babies in highly developed countries. Increased access and quality of intensive neonatal care resulted in improved survival outcomes of increasingly premature and extremely low birth weight infants, leading to the second epidemic in the 1970s. The third epidemic that began approximately in the late 1990s involves mostly middle income countries due to a combination of both increasing access to neonatal intensive care and risk factors that lead to smaller premature babies being born.12,17
Retinopathy of prematurity can be classified based on location of the disease in the retina, extent of vasculature involved, and staging as indicated by the International Classification of ROP (ICROP) (Table 1).10 Plus disease is a term used when retinal vascular dilation and arteriolar tortuosity exceed that of a standard fundus photograph published by ICROP, which has important implications for clinical management.9 Cases where posterior pole vascular abnormalities are present but are not deemed to qualify as plus disease are termed pre-plus disease. A rapidly progressing form of ROP, aggressive posterior ROP (APROP), is defined as ROP typically in zone I or posterior zone II, plus disease, and rapid vascular changes that can result in stage 5 ROP without typical progression through stages 1‒3.
TABLE 1.
International Classification of Retinopathy of Prematurity Disease Classification
| Location |
| Three concentric zones each centered on the optic disc |
| Zone I: circle with a radius of twice the distance from the optic disc to the fovea |
| Zone II: from edge of zone I out to encompassing the nasal ora |
| Zone III: residual crescent of retina anterior to zone II |
| Severity |
| Stage 1: demarcation line that separates avascular and vascular retina |
| Stage 2: elevated ridge that has grown in height, width, volume, and extends out of the retinal plane that may or may not have fibrovascular proliferation |
| Stage 3: extraretinal fibrovascular proliferation |
| Stage 4: partial retinal detachment |
| Stage 4A: extrafoveal partial retinal detachment |
| Stage 4B: partial retinal detachment including the fovea |
| Stage 5: total retinal detachment |
| Plus disease: presence of arterial tortuosity and venous dilation in the posterior pole greater than or equal to that of a standard photograph published by the ICROP |
| Pre-plus disease: presence of arterial tortuosity and venous dilation insufficient to be defined as plus disease |
Current guidelines in the United States recommend ROP screening for infants with a birth weight of 1500 g or less, GA of 30 weeks or less, or infants more than 1500 g or more than 30 weeks of GA that are high risk for ROP. High-risk characteristics may include necrotizing enterocolitis, intraventricular hemorrhage, sepsis, and bronchopulmonary dysplasia. Retinal screening examinations are performed using binocular indirect ophthalmoscopy by a qualified ophthalmologist.10,18 Time of initiation of first ROP screening examination is based on postmenstrual age (PMA), which has been shown to correlate better with the onset of ROP as compared with postnatal age alone.19 Recommended duration for follow-up examinations to continue varies according to ROP classification and severity (Table 2).19,20
TABLE 2.
Timing of Initial Eye Examinations
| Gestational Age at Birth (weeks) | Postmenstrual Gestational Age at Initial Exam (weeks) | Chronologic Age at Initial Exam (weeks) |
|---|---|---|
| 22 | 31 | 9 |
| 23 | 31 | 8 |
| 24 | 31 | 7 |
| 25 | 31 | 6 |
| 26 | 31 | 5 |
| 27 | 31 | 4 |
| 28 | 32 | 4 |
| 29 | 33 | 4 |
| 30 | 34 | 4 |
| 31 | 35 | 4 |
| 32 | 36 | 4 |
HISTORICAL AND CURRENT TREATMENT—CRYOTHERAPY AND LASER PHOTOCOAGULATION
Landmark studies such as Cryotherapy for ROP (CRYO-ROP)11 in 1988 and Early Treatment for ROP (ETROP)21 in 2004 have largely guided the management of ROP thus far. The CRYO-ROP study demonstrated utility of treatment with cryoablation of peripheral immature avascular retina in patients with morphologic changes that corresponded to a more than 50% risk of unfavorable outcome, termed threshold ROP (at least 5 contiguous or 8 cumulative clock hours of stage 3 ROP in zones I or II in the presence of plus disease).11,22 In the 15-year follow-up study, there remained a decrease of more than 40% in unfavorable structural outcomes and a decrease of 30% in unfavorable visual acuity outcomes such as retinal detachments, retinal degenerative conditions, pigment abnormalities, cataracts, glaucoma, band keratopathy, and microphthalmos.23 Diode laser photocoagulation therapy, shown to be as effective as cryoablation, subsequently was adopted as the preferred method of ablation, with lower rates of lid edema, conjunctival hyperemia, and chemosis.24,25 Indications for treatment of ROP were redefined by ETROP as type 1 ROP (zone I ROP at any stage with plus disease, zone I ROP stage 3 without plus disease, and zone II stage 2 or 3 with plus disease).21 Type 2 ROP, as defined by ETROP, was determined to require close follow-up. Treatment of type 1 ROP led to a reduction of unfavorable visual acuity outcomes from 19.8% to 14.3% and structural findings from 15.6% to 9.0%. In comparison with eyes treated with cryotherapy, 5-year follow-up of eyes treated with laser had better structural and functional outcome, better visual acuity, and less myopia compared with eyes treated with cryotherapy.26,27 Odds that eyes treated with laser would have a best corrected visual acuity of 20/50 or better were almost 7 times those of eyes treated with cryotherapy with less macular dragging and less long-term morbidity.24
EVOLVING MANAGEMENT—TREATMENT OF ROP OUTSIDE CURRENT GUIDELINES
Current treatment guidelines are largely based on findings from ETROP (Table 3). Gupta et al28 have recently demonstrated that experts often perform treatment for infants with less than type 1 ROP because of other examination findings or logistical concerns. In this multicenter retrospective review of eyes that were treated for ROP in the Imaging and Informatics for ROP (i-ROP) study, 9.5% of these eyes had a clinical diagnosis milder than type 1 ROP. Indications that prompted experts to recommend and administer treatment in cases milder than type 1 ROP included 1) eyes with active ROP with the contralateral eye being treated for type 1 ROP, 2) vitreous hemorrhage, 3) persistent ROP at an advanced PMA, and 4) concerning structural changes at risk for future complications. Concerning structural changes included tangential traction with temporal vessel straightening concerning for macular dragging and thick stage 3 membranes with anterior-posterior traction concerning for progression to stage 4 ROP. Results from this study illustrate that clinical judgment is particularly important in situations outside of evidence-based treatment guidelines and reflect recognition of the need for continuing evolution of the management paradigm.
TABLE 3.
International Classification of Retinopathy of Prematurity Treatment and Follow-Up Guidelines
| Treatment recommended |
| 1. Zone I ROP at any stage with plus disease |
| 2. Zone I ROP stage 3 without plus disease |
| 3. Zone II stage 2 or 3 with plus disease |
| Follow-up examination interval |
| 1 week or less |
| Zone I: immature retina, stage 1 or 2 ROP |
| Zone II: stage 3 ROP |
| Presence or suspected presence of APROP |
| 1–2 weeks |
| Zone I: unequivocally regressing ROP |
| Posterior zone II: immature retina |
| Zone II: stage 2 ROP |
| 2 weeks |
| Zone II: immature retina, stage 1 ROP, unequivocally regressing ROP |
| 2–3 weeks |
| Zone II: stage 1 ROP |
| Zone III: stage 1 or 2 ROP, regressing ROP |
| Screening is discontinued if the following are present |
| 1. Zone III vascularization without previous zone I or II disease |
| 2. 360 degrees of full retinal vascularization |
| 3. Postmenstrual age of 50 weeks without prethreshold disease |
| 4. Regression of ROP in zone III without abnormal vascular tissue that can reactivate in zones II or III |
ROLE OF IMAGING IN THE EVOLUTION OF PEDIATRIC RETINAL SCREENING, DIAGNOSIS, AND MANAGEMENT
The management of ROP continues to change as we gain further understanding of the disease with the advent of new diagnostic and therapeutic modalities. Imaging systems such as RetCam (Natus Medical, Pleasanton, CA), Panocam (Visunex Medical Systems, Fremont, CA), 3nethra neo (Forus Health, Bengaluru, India), Optomap imaging systems (Optos, Marlborough, MA), and ICON (Phoenix Clinical, Pleasanton, CA) have allowed for photographic documentation of pathology and the development of telemedicine systems in ROP (Table 4). Fluorescein angiography (FA) and optical coherence tomography (OCT) have been shown to aid in the diagnostic evaluation of the ROP patient. Image montaging has also been reported to increase accuracy and intergrader agreement in ROP diagnosis,29 and automated computer-based image analysis has already been shown to be comparable to human expert image interpretation in the detection of plus disease.30–33
TABLE 4.
Retinal Fundus Imaging Systems
| Imaging System | Manufacturer | Primary Uses | Mydriasis | Field of View |
|---|---|---|---|---|
| RetCam | Natus Medical, Pleasanton, CA | Contact fundus imaging | Mydriatic | 130 degrees |
| Panocam | Visunex Medical Systems, Fremont, CA | Contact fundus imaging | Mydriatic | 130 degrees |
| 3nethra neo | Forus Health, Bengaluru, India | Contact fundus imaging | Mydriatic | 120 degrees |
| ICON | Phoenix Clinical, Pleasanton, CA | Contact fundus imaging | Mydriatic | 100 degrees |
| Optomap | Optos, Marlborough, MA | Noncontact fundus imaging | Nonmydriatic | 200 degrees |
All of these advances in retinal imaging have led to further advances of pediatric retinal screening, evaluation, diagnosis, documentation, communication of findings, and clinical management. Fundus imaging systems enable the acquisition of high-resolution fundus images during examinations under anesthesia.34 Fluorescein angiography is a relatively safe procedure in the pediatric population, and the combination of fundus imaging with FA has been shown to enable the detection of vascular changes that were not detected by indirect ophthalmoscopy in ROP.35 In the latter study by Patel et al,35 FA significantly improved sensitivity of diagnosis of stage 2 or worse, stage 3 or worse, pre-plus or worse, and type 2 ROP or worse disease. The presence of retinal neovascularization in stage 3 disease is often clinically significant, and FA may be particularly sensitive in the diagnosis of stage 3 ROP, increasing sensitivity from 39.8% to 74.1%. Additionally, FA has been shown to be particularly adept at detecting changes such as arteriovenous shunts, capillary loss, arteriolar leakage, and retinal ischemia.36,37 Presence of these findings on FA can precede diagnosis of prethreshold disease and may predict progression of aggressive ROP at an earlier stage.38 Furthermore, FA has been shown to be important in the evaluation and management of Coats disease,39 FEVR, sickle cell retinopathy,40 and ocular tumors.35,41
Ultra-widefield imaging systems such as the 200-degree field of view noncontact, nonmydriatic Optomap imaging systems (Optos, Marlborough, MA) are often successful in children older than 3 years old and have also been shown to be successful in infants.42 When combined with FA, ultra-widefield imaging has been shown to aid in the evaluation, documentation, and management of peripheral retinal pathology in various pediatric retinal conditions.42
Computer-generated mosaic photographs collate multiple fundus photographs into 1 single photograph that matches or exceeds the field of view of individual widefield images. Compared with the interpretation of multiple individual photographs, inclusion of the collated fundus areas within a single mosaic photograph has been shown to increase diagnostic accuracy and intergrader agreement of certain categories of ROP.29
TELEMEDICINE AND COMPUTER-BASED IMAGE ANALYSIS
Multiple surveys, including a 2006 survey from the American Academy of Ophthalmology, indicate that fewer ophthalmologists are willing to manage ROP in the future.13 Therefore, given the potential shortage of skilled examiners for ROP, it will be important to find ways to improve access to care for children at risk of ROP. Telemedicine has been promoted as a platform to reduce the burden of bedside ROP screening examinations for physicians. Ancillary healthcare staff can effectively obtain high-quality fundus imaging on patients, and subsequent remote interpretation in a centralized reading center may create a more efficient system of ROP care with larger geographical reach and increasing cost effectiveness.43–45
Telemedicine for ROP has been shown to be efficient and have good sensitivity and specificity.46,47 In 1 study, diagnosis of ROP by physicians using telemedicine images took less time (1‒1.75 minutes) in comparison with ophthalmoscopy (4.17‒6.63 minutes).48 Intergrader agreement in telemedicine imaging studies has been consistently high.49,50 Nevertheless, there is variability, even among experts, in plus disease diagnosis and in defining cut off points for vascular abnormality, making defining plus disease and pre-plus disease difficult. The ETROP trial determined that plus disease was the most significant ICROP parameter in the determination of treatment-requiring ROP. A continuous severity score may help by providing a range rather than specific classifications.18,51 Computer-based image analysis programs such as ROPtool and programs from the i-ROP consortium have the potential for diagnosing plus disease along with expert image graders. These automated programs identify retinal vessels in fundus photographs, generate vascular tortuosity and dilation values, and may have an important role to play in the determination of plus disease in image analysis, especially if human intergrader agreement remains low. Deep learning algorithms are also being applied to further automate and improve computer image analysis, which may significantly alter the manner in which ROP and pediatric retinal diseases are managed.
Additionally, tele-education systems may have a role to play in the education of trainees and healthcare staff in the management of ROP and pediatric retinal disease. The Global Education Network for ROP has used web-based systems to improve ROP education in the United States and internationally.43,52–53 Providing remote access to training and assessing an individual’s competency in image-based diagnosis may allow for the establishment and certification of a standardized level of care before participation in a telemedicine screening program.
Telemedicine for ROP holds the potential to overcome many challenges associated with ROP screening, diagnosis, and management. However, wider implementation of telemedicine requires the establishment of standardized protocols for image capture and training for both photographers and image graders to ensure consistent delivery of high-quality and reliable programs.
INTRAVITREAL ANTI-VEGF PHARMACOTHERAPY FOR ROP
Reported advantages of anti-VEGF pharmacotherapy over laser photocoagulation include less time for treatment administration, less stress on the infant, faster improvement in plus disease and regression of ROP, less treatment-related destruction of the peripheral retina, lower risk of myopia, and monotherapy treatment for zone I ROP or APROP.54–58 However, widespread acceptance of anti-VEGF therapy has been limited due to the potential risk of higher rates of late recurrence (Fig. 1), the need for an extended follow-up period, persistent peripheral avascular retina after treatment, membrane contraction with retinal detachment, and delayed onset retinal detachment.59–64 Bevacizumab has also been shown to be detectable in the serum for 8‒12 weeks after intravitreal injection, raising concern for possible systemic adverse events.65
FIGURE 1.
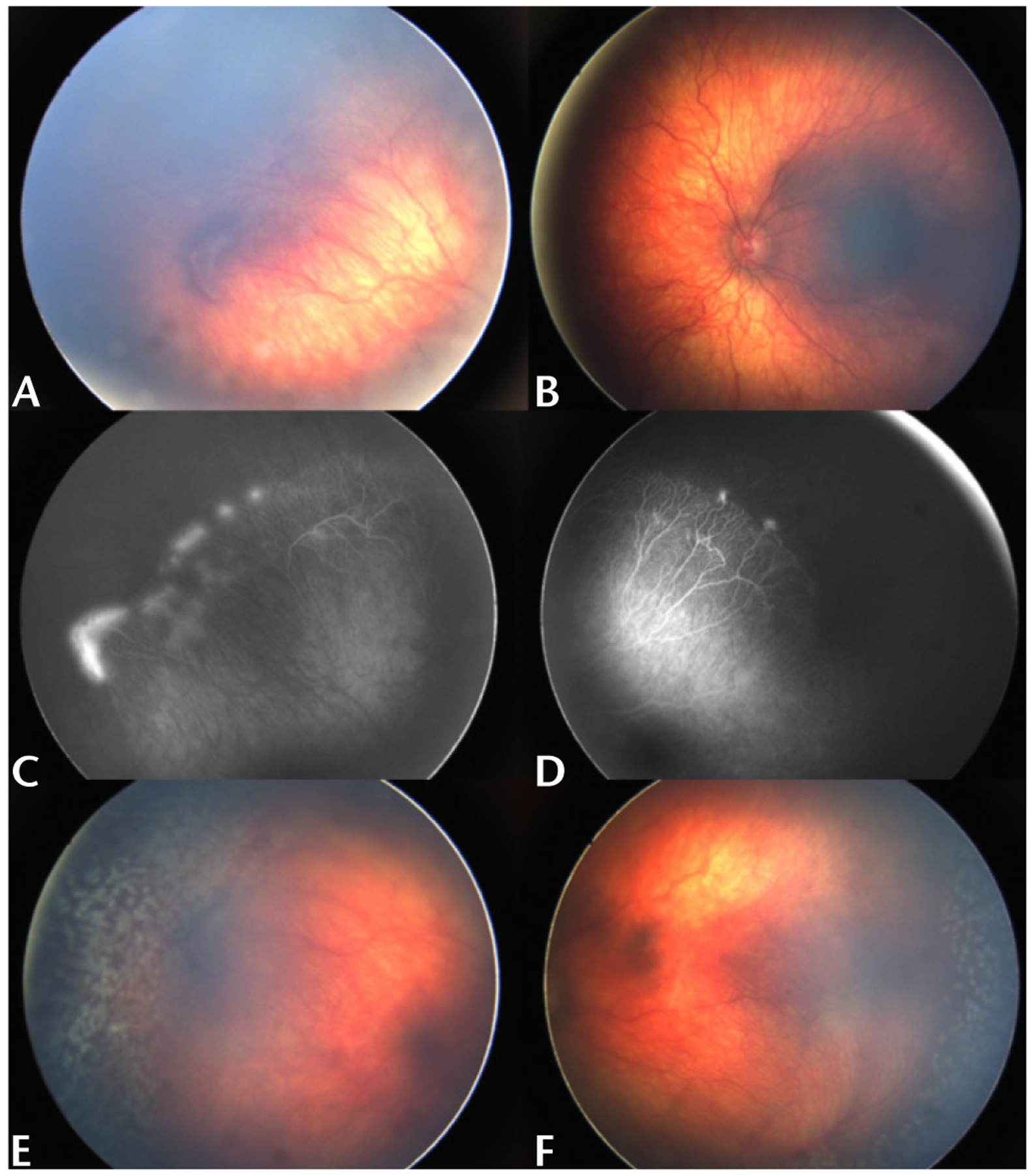
Late recurrence of active ROP after anti-VEGF injections. A 15-month-old male (GA 24 2/7 weeks) received a total of 3 intravitreal bevacizumab injections each in both eyes at an outside institution. Most recent injections were at 12 months chronological age. Examination was notable for zone II stage 3 ROP without plus disease in the right (A, C) and left (B, D) eyes. Given the patient’s age and history of multiple injections with late recurrence, laser photocoagulation was administered to both eyes (E, F).
A 2017 report by the American Academy of Ophthalmology on the use of anti-VEGFs for primary treatment of ROP noted no level I evidence on the subject,54–58 which by predefined standard criteria required masking when outcomes are subjective. Six prospective randomized controlled trials and 7 retrospective studies were considered to provide level II and III evidence, respectively.
Response to Treatment, Retreatment Rate, Outcomes, and Follow-Up Examinations
Level II Evidence—Prospective Randomized Trials
The Bevacizumab Eliminates the Angiogenic Threat of Retinopathy (BEAT-ROP) study in 2011 compared 0.625 mg of intravitreal bevacizumab monotherapy with laser photocoagulation in 150 infants with bilateral stage 3 with plus ROP in zone I or posterior zone II.54 The primary outcome measure was treatment-requiring recurrence by 54 weeks’ PMA. The authors report a 4% retreatment rate in the bevacizumab group, compared with 22% in the laser group. This statistically significant difference was noted to be true for zone I ROP, and not zone II ROP, on stratified result analysis. However, limitations of the study included a lack of standardized laser protocol and a higher level of treatment failure in eyes treated with laser photocoagulation compared with other study populations.
In 2012, Autrata et al55 in the Czech Republic compared 0.3 mg intravitreal pegaptinib with laser photocoagulation against laser photocoagulation only in 76 infants with stage 3 with plus ROP in zone I or posterior zone II. Study outcomes were anatomic status and rate of recurrence requiring treatment by 55 weeks’ PMA. An unfavorable anatomic outcome was defined as stage 4A or worse ROP. When combination therapy was used, the authors reported lower recurrence of stage 3 ROP (14.6% versus 50%), higher favorable anatomic outcomes (89.7% versus 60.7%), faster resolution of plus disease (mean, 1.3 weeks versus 3.6 weeks), shorter time to growth of retinal vessels into the peripheral retina (mean, 2.2 weeks versus 3.6 weeks), and longer time to recurrence (mean ± standard deviation, 15.1 ± 4.1 weeks versus 5.9 ± 4.8 weeks).
In 2014, Moran et al56 compared 1.25 mg bevacizumab against laser photocoagulation in the contralateral eye in 14 patients with bilateral stage 3 with plus ROP in zone I or posterior zone II. The authors report later retreatment in eyes treated with bevacizumab (3 eyes; 50, 51, and 52 weeks) compared with laser (1 eye, 37 weeks). Pediatric examination with assessment of developmental status and magnetic resonance imaging at 1 and 2 years of age revealed no systemic adverse events associated with bevacizumab therapy. No ocular adverse events were reported to be associated with bevacizumab therapy.
Lepore et al66 in 2014 compared 0.5 mg bevacizumab against laser therapy in the fellow eye of 13 infants with stage 3 ROP in zone I. Six eyes of 3 infants had plus disease at the time of treatment. All eyes treated with bevacizumab had favorable anatomic outcomes at 9 months, whereas 2 eyes treated with laser progressed to retinal detachment. Color fundus and FA images at 9 months PMA revealed abnormalities such as retinal vascular branching, peripheral retinal shunt vessels, persistent avascular retina, absence of foveal avascular zone, posterior hyperfluorescent lesions, or linear choroidal filling patterns in all eyes treated with bevacizumab but not in the majority of eyes treated with laser.
In 2016, Zhang et al67 compared 0.3 mg intravitreal ranibizumab against laser photocoagulation therapy in 50 infants with stage 2 or 3 ROP with plus disease in zone II. Initial regression of ROP occurred in all eyes treated with ranibizumab, whereas both eyes of 1 patient treated with laser did not exhibit initial response to treatment. However, 52% of eyes treated with ranibizumab subsequently developed recurrence and underwent laser photocoagulation retreatment.
Level III Evidence—Retrospective Comparative Case Series
In 2010, Lee et al68 compared the combination of 0.5 mg bevacizumab and laser photocoagulation versus laser photocoagulation alone in 8 patients with bilateral moderate to severe ROP. Eyes diagnosed with more severe vascular activity received combination treatment. The authors report faster resolution of plus disease, regression of fibrovascular tissue, and earlier vascularization into the peripheral retina with combination treatment.
In 2015, Hwang et al69 compared 0.625 mg bevacizumab and laser photocoagulation in 54 eyes of 28 patients with type 1 ROP. Recurrence of ROP occurred in 3 of 22 eyes (14%) treated with bevacizumab and 1 of 32 eyes (3%) treated with laser. All recurrences in both groups occurred in eyes with zone I ROP. No progression to retinal detachment or macular ectopia occurred in eyes treated with bevacizumab. In eyes treated with laser, 1 eye progressed to stage 5 ROP, and 5 eyes developed macular ectopia.
In 2015, Isaac et al70 compared 0.625 mg bevacizumab and laser therapy in 45 eyes of 25 patients with type 1 ROP. All eyes had favorable anatomic outcomes.
In 2016, Mueller et al71 compared 0.625 mg bevacizumab (37 patients) with laser photocoagulation (17 patients with type 1 ROP). Time to complete regression between treatment groups was significantly lower for eyes with posterior ROP treated with bevacizumab (median, 9 days versus 57 days) but not significantly different for peripheral zone II ROP. Recurrence of ROP was noted in 12% of eyes treated with bevacizumab, whereas none were seen in eyes treated with laser.
Gunay et al72 in 2016 compared 0.625 mg bevacizumab versus 0.25 mg ranibizumab versus laser photocoagulation. Recurrence of ROP was highest in infants treated with ranibizumab (50%), followed by bevacizumab (5.5%) and laser (1.8%). Mean time to recurrence was longer for bevacizumab (14 weeks) compared with ranibizumab (9 weeks). This study additionally reported good therapeutic response with no complications or recurrences in eyes treated with bevacizumab or laser, when bevacizumab was preferentially chosen for eyes with zone I or posterior zone II ROP and laser for eyes with anterior zone II ROP.73
Additionally, Isaac et al70 reported that there was a significant increase in the number of follow-up examinations in eyes treated with bevacizumab (16 ± 6 visits versus 6 ± 3 visits), even when both groups had high rates of treatment success.
Short-term efficacy and ocular safety of anti-VEGF treatment for ROP has been shown to be good and comparable with laser therapy, although systemic and long-term risks are less established for anti-VEGF therapy. Anti-VEGF treatment may be particularly useful in certain cases where quicker regression may be clinically beneficial, such as zone I disease and APROP. Studies have reported variable rates of recurrence and most studies have shown that use of anti-VEGF therapy requires longer and more diligent follow-up examination schedules. However, an exact definition for recurrence in each paper was not specifically stated and there is currently no consensus agreement by ROP experts on the definition of recurrence of ROP after intravitreal anti-VEGF therapy. Therefore, it must be noted that recurrence, in currently published literature, may be defined in a number of ways, including recurrence of disease, disease that requires retreatment, or even disease that does not resolve with treatment.
Refractive Outcomes
A 2014 follow-up study on refractive outcomes of BEAT-ROP patients by Geloneck et al57 reported lower spherical equivalent refractive errors in the bevacizumab group for patients treated for zone I [−1.51 ± 3.42 diopters (D) versus −8.44 ± 7.57 D] and zone II (−0.58 ± 2.53 D versus −5.83 ± 5.87 D) disease. Very high myopia of at least 8 D was less frequent in eyes treated with bevacizumab (3.8% zone I, 1.7% zone II versus 51.4% zone I, 36.4% zone II).
In 2013, Harder et al74 compared bevacizumab (0.375 mg or 0.625 mg based on practice preference) with laser photocoagulation in 25 patients with type 1 ROP. Less myopia was seen in eyes treated with bevacizumab (−1.04 ± 4.24 D) compared with laser (−4.41 ± 5.50 D). In 2014 Geloneck et al57 compared bevacizumab with laser therapy in 131 infants originally enrolled in the BEAT-ROP study. More very high myopia (at least −8.00 D) was reported in zone I of laser treated eyes (51.4%) compared with eyes treated with bevacizumab (3.8%). The 2015 Hwang et al69 study reported less myopia (mean spherical equivalent, −2.4 D at 22.4 months versus −5.3 D at 37.1 months) in eyes treated with bevacizumab, but this was only significant for eyes with zone II ROP and not zone I ROP. The 2015 Isaac et al70 study reported a higher but nonsignificant prevalence of myopia in eyes treated with laser. Gunay et al72 reported that the prevalence of emmetropia was highest in eyes treated with anti-VEGF (50.9% bevacizumab, 45.5% ranibizumab) compared with laser (16.3%). Zone I ROP was associated with development of myopia and high myopia regardless of treatment with anti-VEGF or laser.
Overall, there is consistent evidence reporting lower rates of myopia, high myopia, and astigmatism with anti-VEGF as compared with laser.
Lowest Effective Dose
A variety of doses of bevacizumab has been used in the treatment of ROP, with little consensus as to the lowest effective dose required to promote regression of disease. In a phase 1 dosing study, Wallace et al75 reported success in 11 of 11 eyes at 0.25 mg, 14 of 14 eyes at 0.125 mg, 21 of 24 eyes at 0.063 mg, and 9 of 9 eyes at 0.031 mg. The authors conclude that demonstration of effectiveness of treatment at doses as low as 0.031 mg should be further investigated to theoretically reduce local and systemic adverse effects associated with higher medication doses.
Clinical trials that have investigated alternative ranibizumab doses include the phase 2 Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in ROP (CARE-ROP, 0.12 mg versus 0.20 mg) study in Germany and the international multicenter phase 3 Ranibizumab Compared with Laser Therapy for the Treatment of Infants Born Prematurely with ROP (RAINBOW, 0.1 mg versus 0.2 mg versus laser) study.
Systemic Adverse Effects
Neurodevelopmental outcomes have been studied by a number of groups. Araz-Ersan et al76 found no difference in mean cognitive, language, or motor scores when comparing bevacizumab-treated infants to laser therapy‒treated infants on the Bayley Scales of Infant Development test. Furthermore, Lien et al77 reported no difference in mental or psychomotor impairment when comparing bevacizumab monotherapy with laser monotherapy, but patients with combination therapy of laser and bevacizumab were noted to have higher incidence of mental or psychomotor impairment. Morin et al78 reported lower median motor composite scores and 3.1 times higher odds of having neurodevelopmental disability in bevacizumab-treated infants. However, these studies were not randomized, and further studies should be performed to better elucidate the difference between prematurity itself and anti-VEGF treatment as the causative factor for neurodevelopmental outcomes.
INTRAVITREAL ANTI-VEGF THERAPY FOR NON-ROP PEDIATRIC RETINAL CONDITIONS
Bevacizumab has been reported to be a useful adjunct to laser photocoagulation in the successful treatment of Coats disease.5 However, reports have also cautioned against the use of bevacizumab in Coats disease due to a possible association with the development of vitreoretinal fibrosis and potentially tractional retinal detachment, when used in combination with laser or cryotherapy.
Development of vitreoretinal traction has also been associated with the use of anti-VEGF therapy (pegaptanib sodium) in FEVR.2 However, the authors report that visual acuity can improve after surgical release of traction and that anti-VEGF is a potential treatment when refractory to standard therapy. A separate case report describes rapid resolution of FEVR with bevacizumab.3
Intravitreal bevacizumab treatment was reported in an infant with Norrie disease born at 34 weeks via planned preterm delivery and treated on day 2 of life with laser photocoagulation of avascular retina, sparing foveal and macular regions, along with 0.75 mg intravitreal bevacizumab.1 Repeat injections of intravitreal bevacizumab were administered at 16 (right eye) and 20 (left eye) weeks of age, when preretinal hemorrhages were observed. The patient demonstrated Teller visual acuities of 20/350 bilaterally at 9 months. Retinal detachment was not present at 10 months of age, although the fovea remained avascular bilaterally.
Intravitreal bevacizumab was reported to lead to complete resolution of neovascular tissue in 2 incontinential pigmenti patients, although 1 required laser treatment for recurrence 7 months after injection.6
ADVANCES IN SURGICAL TECHNIQUES
Surgical advances in the management of ROP and pediatric retinal conditions include advances in areas such as pharmacologic vitreolysis and microincision vitrectomy surgery. A posterior vitreous detachment is more difficult to induce intraoperatively in pediatric patients due to a formed and strongly adherent vitreous. Pharmacologic vitreolysis with intravitreal ocriplasmin before surgical vitrectomy has been investigated as a possible method to facilitate this but was not reported to exhibit clear efficacy.79
Microincision vitrectomy surgery has been advancing with increasingly smaller instrumentation. Smaller gauge surgery may allow for more predictable sutureless vitrectomy and may decrease postoperative morbidity, whereas larger instruments may allow for better efficiency and performance. Three-dimensional displays and endoscopic visualization are newer technologies that may be applied to microincision vitrectomy surgery, and intraoperative OCT allows for intraoperative assessment and confirmation of retinal microarchitecture.
Immediate sequential bilateral vitreoretinal surgery (ISBVS) was reported to be feasible and safe and may be considered in pediatric patients requiring bilateral vitreoretinal surgery.80 Indications for ISBVS as found in the study were rapidly progressive disease, systemic morbidity placing the child at high anesthesia risk, and residence remote from surgery location.
CONCLUSIONS
The diagnosis and management of pediatric retinal conditions has improved with the availability of new technology and treatments. We are seeing a new era in pediatric retina with the development of new retinal imaging technologies, telemedicine programs, computer-based image analysis, adoption of deep learning algorithms, and microsurgical advances. We are now able to visualize pathology in ways we were unable to before. Pharmacotherapy with anti-VEGF is also playing a more important role in the management of pediatric retinal disease.
As we may begin to see a new epidemic of ROP in preterm infants,81 we are reminded of the persistent clinical challenges that remain with the management of pediatric retinal conditions. Increasing rates of premature births, lack of qualified examiners, and suboptimal access to highly specialized care for pediatric retina occur on a global scale. Future international collaborative efforts will aid with comprehending and adopting developing technologies and guide new management standards for the future.
Acknowledgments
Support provided by a unrestricted departmental grant from Research to Prevent Blindness (R.V.P.C., R.C., D.D., S.N.P., K.E.J., S.O., M.F.C.); National Institutes of Health R01 EY019474 (R.V.P.C., M.F.C., J.P.C., S.O., K.E.J.); National Science Foundation SCH-1622679 (R.V.P.C., M.F.C., J.P.C., S.O.); National Institutes of Health P30 EY001792 Core Grant for Vision Research (R.V.P.C., R.C., K.E.J., D.D.).
Footnotes
The authors have no other funding or conflicts of interest to declare.
REFERENCES
- 1.Sisk RA, Hufnagel RB, Bandi S, et al. Planned preterm delivery and treatment of retinal neovascularization in Norrie disease. Ophthalmology. 2014;121:1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quiram PA, Drenser KA, Lai MM, et al. Treatment of vascularly active familiar exudative vitreoretinopathy with pegaptanib sodium (Macugen). Retina. 2008;28:S8–S12. [DOI] [PubMed] [Google Scholar]
- 3.Tagami M, Kusuhara S, Honda S, et al. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246:1787–1789. [DOI] [PubMed] [Google Scholar]
- 4.Ramasubramanian A, Shields CL. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2011;96:356–359. [DOI] [PubMed] [Google Scholar]
- 5.Stergiou PK, Symeonidis C, Dimitrakos SA. Coats’ disease: treatment with intravitreal bevacizumab and laser photocoagulation. Acta Ophthalmol. 2009;87:687–688. [DOI] [PubMed] [Google Scholar]
- 6.Shah PK, Bachu S, Narendran V, et al. Intravitreal bevacizumab for incontinentia pigmenti. J Pediatr Ophthalmol Strabismus. 2013;50:e52–e54. [DOI] [PubMed] [Google Scholar]
- 7.Terry TL. Retrolental fibroplasia in the premature infant: V. Further studies on fibroplastic overgrowth of the persistent tunica vasculosa lentis. Trans Am Ophthalmol Soc. 1944;42:383–396. [PMC free article] [PubMed] [Google Scholar]
- 8.The Committee for the Classification of Retinopathy of Prematurity. An International Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102:1130–1134. [DOI] [PubMed] [Google Scholar]
- 9.Patz A. An International Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1987;105:905. [DOI] [PubMed] [Google Scholar]
- 10.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999. [DOI] [PubMed] [Google Scholar]
- 11.Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1988;106:471–479. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. [DOI] [PubMed] [Google Scholar]
- 13.Kemper AR, Freedman SF, Wallace DK. Retinopathy of prematurity care: patterns of care and workforce analysis. J AAPOS. 2008;12:344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ML, Guo L, Smith LEH, et al. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics. 2010;125: e1483–e1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles JB, Ganthier R, Appiah AP. Incidence and characteristics of retinopathy of prematurity in a low-income inner-city population. Ophthalmology. 1991;98:14–17. [DOI] [PubMed] [Google Scholar]
- 16.Suelves AM, Shulman JP. Current screening and treatments in retinopathy of prematurity in the US. Eye Brain. 2016;8:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave HB, Gordillo L, Yang Z, et al. The societal burden of blindness secondary to retinopathy of prematurity in Lima, Peru. Am J Ophthalmol. 2012;154:750–755. [DOI] [PubMed] [Google Scholar]
- 18.Chiang MF. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125:875. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–576. [DOI] [PubMed] [Google Scholar]
- 20.Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131: 189–195. [DOI] [PubMed] [Google Scholar]
- 21.Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–248; discussion 248–250. [PMC free article] [PubMed] [Google Scholar]
- 22.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: natural history ROP: ocular outcome at 5 1/2 years in premature infants with birth weights less than 1251 g. Arch Ophthalmol. 2002;120:595–599. [DOI] [PubMed] [Google Scholar]
- 23.Palmer EA, Hardy RJ, Dobson V, et al. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123: 311–318. [DOI] [PubMed] [Google Scholar]
- 24.McNamara JA, Tasman W, Vander JF, et al. Diode laser photocoagulation for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1992; 110:1714–1716. [DOI] [PubMed] [Google Scholar]
- 25.Connolly BP, McNamara JA, Sharma S, et al. A comparison of laser photocoagulation with trans-scleral cryotherapy in the treatment of threshold retinopathy of prematurity. Ophthalmology. 1998;105:1628–1631. [DOI] [PubMed] [Google Scholar]
- 26.Connolly BP, Ng EYJ, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 2. Refractive outcome. Ophthalmology. 2002;109:936–941. [DOI] [PubMed] [Google Scholar]
- 27.Shalev B, Farr AK, Repka MX. Randomized comparison of diode laser photocoagulation versus cryotherapy for threshold retinopathy of prematurity: seven-year outcome. Am J Ophthalmol. 2001;132:76–80. [DOI] [PubMed] [Google Scholar]
- 28.Gupta MP, Chan RVP, Anzures R, et al. Practice patterns in retinopathy of prematurity treatment for disease milder than recommended by guidelines. Am J Ophthalmol. 2016;163:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SN, Klufas MA, Douglas CE, et al. Influence of computer-generated mosaic photographs on retinopathy of prematurity diagnosis and management. JAMA Ophthalmol. 2016;134:1283–1289. [DOI] [PubMed] [Google Scholar]
- 30.Campbell JP, Ataer-Cansizoglu E, Bolon-Canedo V, et al. Expert diagnosis of plus disease in retinopathy of prematurity from computer-based image analysis. JAMA Ophthalmol. 2016;134:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittenberg LA, Jonsson NJ, Chan RVP, et al. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012;49:11–19; quiz 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbey AM, Besirli CG, Musch DC, et al. Evaluation of screening for retinopathy of prematurity by ROPtool or a lay reader. Ophthalmology. 2016;123:385–390. [DOI] [PubMed] [Google Scholar]
- 33.Chiang MF, Gelman R, Martinez-Perez ME, et al. Image analysis for retinopathy of prematurity diagnosis. J AAPOS. 2009;13:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chee RI, Patel SN, Jonas KE, et al. Current trends in telemedicine for retinopathy of prematurity. Vision Pan-America, The Pan-American Journal of Ophthalmology. 2017;16:7–11. [Google Scholar]
- 35.Klufas MA, Patel SN, Ryan MC, et al. Influence of fluorescein angiography on the diagnosis and management of retinopathy of prematurity. Ophthalmology. 2015;122:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng EYJ, Lanigan B, O’Keefe M. Fundus fluorescein angiography in the screening for and management of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2006;43:85–90. [DOI] [PubMed] [Google Scholar]
- 37.Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography. Ophthalmology. 2009;116:1377–1382. [DOI] [PubMed] [Google Scholar]
- 38.Zepeda-Romero LC, Oregon-Miranda AA, Lizarraga-Barrón DS, et al. Early retinopathy of prematurity findings identified with fluorescein angiography. Graefes Arch Clin Exp Ophthalmol. 2013;251:2093–2097. [DOI] [PubMed] [Google Scholar]
- 39.Koozekanani DD, Connor TB Jr, Wirostko WJ. RetCam II fluorescein angiography to guide treatment and diagnosis of Coats disease. Ophthalmic Surg Lasers Imaging. March 9, 2010. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Hero M, Harding SP, Riva CE, et al. Photographic and angiographic characterization of the retina of Kenyan children with severe malaria. Arch Ophthalmol. 1997;115:997–1003. [DOI] [PubMed] [Google Scholar]
- 41.Shields JA, Reichstein D, Mashayekhi A, et al. Retinal vasoproliferative tumors in ocular conditions of childhood. J AAPOS. 2012;16:6–9. [DOI] [PubMed] [Google Scholar]
- 42.Fung THM, Muqit MMK, Mordant DJ, et al. Noncontact high-resolution ultra-wide-field oral fluorescein angiography in premature infants with retinopathy of prematurity. JAMA Ophthalmol. 2014;132:108–110. [DOI] [PubMed] [Google Scholar]
- 43.Campbell JP, Swan R, Jonas K, et al. Implementation and evaluation of a tele-education system for the diagnosis of ophthalmic disease by international trainees. AMIA Annu Symp Proc. 2015;2015:366–375. [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson KM, Scott KE, Graff Zivin J, et al. Cost-utility analysis of telemedicine and ophthalmoscopy for retinopathy of prematurity management. Arch Ophthalmol. 2008;126:493–499. [DOI] [PubMed] [Google Scholar]
- 45.Vinekar A, Gilbert C, Dogra M, et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014;62:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdul Aziz AA, Isaac M, Tehrani NN. Using telemedicine to screen for retinopathy of prematurity. CMAJ. 2014;186:1012–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skalet AH, Quinn GE, Ying GS, et al. Telemedicine screening for retinopathy of prematurity in developing countries using digital retinal images: a feasibility project. J AAPOS. 2008;12:252–258. [DOI] [PubMed] [Google Scholar]
- 48.Richter GM, Sun G, Lee TC, et al. Speed of telemedicine vs ophthalmoscopy for retinopathy of prematurity diagnosis. Am J Ophthalmol. 2009;148: 136–142.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott KE, Kim DY, Wang L, et al. Telemedical diagnosis of retinopathy of prematurity intraphysician agreement between ophthalmoscopic examination and image-based interpretation. Ophthalmology. 2008;115: 1222–1228.e3. [DOI] [PubMed] [Google Scholar]
- 50.Chiang MF, Wang L, Busuioc M, et al. Telemedical retinopathy of prematurity diagnosis: accuracy, reliability, and image quality. Arch Ophthalmol. 2007;125:1531–1538. [DOI] [PubMed] [Google Scholar]
- 51.Campbell JP, Kalpathy-Cramer J, Erdogmus D, et al. Plus disease in retinopathy of prematurity: a continuous spectrum of vascular abnormality as a basis of diagnostic variability. Ophthalmology. 2016;123:2338–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan RV, Patel SN, Ryan MC, et al. The Global Education Network for Retinopathy of Prematurity (Gen-Rop): development, implementation, and evaluation of a novel tele-education system. Trans Am Ophthalmol Soc. 2015;113:T2. [PMC free article] [PubMed] [Google Scholar]
- 53.Patel SN, Martinez-Castellanos MA, Berrones-Medina D, et al. ; GEN-ROP, i-ROP Research Consortium. Assessment of a tele-education system to enhance retinopathy of prematurity training by international ophthalmologists-in-training in Mexico. Ophthalmology. 2017;124:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Autrata R, Krejcírová I, Senková K, et al. Intravitreal pegaptanib combined with diode laser therapy for stage 3+ retinopathy of prematurity in zone I and posterior zone II. Eur J Ophthalmol. 2012;22:687–694. [DOI] [PubMed] [Google Scholar]
- 56.Moran S, O’Keefe M, Hartnett C, et al. Bevacizumab versus diode laser in stage 3 posterior retinopathy of prematurity. Acta Ophthalmol. 2014;92: e496–e497. [DOI] [PubMed] [Google Scholar]
- 57.Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1327–1333. [DOI] [PubMed] [Google Scholar]
- 58.VanderVeen DK, Melia M, Yang MB, et al. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:619–633. [DOI] [PubMed] [Google Scholar]
- 59.Yonekawa Y, Wu WC, Nitulescu CE, et al. Progressive retinal detachment in infants with retinopathy of prematurity treated with intravitreal bevacizumab or ranibizumab. Retina. May 3, 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 60.Hu J, Blair MP, Shapiro MJ, et al. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–1006. [DOI] [PubMed] [Google Scholar]
- 61.Hu Q, Bai Y, Chen X, et al. Recurrence of retinopathy of prematurity in zone II stage 3+ after ranibizumab treatment: a retrospective study. J Ophthalmol. 2017;2017:5078565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, et al. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J AAPOS. 2014;18:120–123. [DOI] [PubMed] [Google Scholar]
- 63.Mintz-Hittner HA, Geloneck MM. Review of effects of anti-VEGF treatment on refractive error. Eye Brain. 2016;8:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tahija SG, Hersetyati R, Lam GC, et al. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol. 2014;98:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–333.e1. [DOI] [PubMed] [Google Scholar]
- 66.Lepore D, Quinn GE, Molle F, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity. Ophthalmology. 2014;121: 2212–2219. [DOI] [PubMed] [Google Scholar]
- 67.Zhang G, Yang M, Zeng J, et al. Comparison of intravitreal injection of ranibizumab versus laser therapy for zone II treatment-requiring retinopathy of prematurity. Retina. 2017;37:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JY, Chae JB, Yang SJ, et al. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010;248:1257–1262. [DOI] [PubMed] [Google Scholar]
- 69.Hwang CK, Hubbard GB, Hutchinson AK, et al. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015;122:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Isaac M, Mireskandari K, Tehrani N. Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. J AAPOS. 2015;19:140–144. [DOI] [PubMed] [Google Scholar]
- 71.Mueller B, Salchow DJ, Waffenschmidt E, et al. Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. Br J Ophthalmol. June 14, 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 72.Gunay M, Sukgen EA, Celik G, et al. Comparison of bevacizumab, ranibizumab, and laser photocoagulation in the treatment of retinopathy of prematurity in Turkey. Curr Eye Res. 2017;42:462–469. [DOI] [PubMed] [Google Scholar]
- 73.Gunay M, Celik G, Tuten A, et al. Characteristics of severe retinopathy of prematurity in infants with birth weight above 1500 grams at a referral center in Turkey. PLoS One. 2016;11:e0161692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harder BC, Schlichtenbrede FC, von Baltz S, et al. Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol. 2013;155:1119–1124.e1. [DOI] [PubMed] [Google Scholar]
- 75.Wallace DK, Kraker RT, Freedman SF, et al. Assessment of lower doses of intravitreous bevacizumab for retinopathy of prematurity: a phase 1 dosing study. JAMA Ophthalmol. 2017;135:654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araz-Ersan B, Kir N, Tuncer S, et al. Preliminary anatomical and neurodevelopmental outcomes of intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Curr Eye Res. 2015;40:585–591. [DOI] [PubMed] [Google Scholar]
- 77.Lien R, Yu MH, Hsu KH, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS One. 2016;11:e0148019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137:e20153218. [DOI] [PubMed] [Google Scholar]
- 79.Drenser K, Girach A, Capone A Jr. A randomized, placebo-controlled study of intravitreal ocriplasmin in pediatric patients scheduled for vitrectomy. Retina. 2016;36:565–575. [DOI] [PubMed] [Google Scholar]
- 80.Yonekawa Y, Wu WC, Kusaka S, et al. Immediate sequential bilateral pediatric vitreoretinal surgery: an international multicenter study. Ophthalmology. 2016;123:1802–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cayabyab R, Ramanathan R. Retinopathy of prematurity: therapeutic strategies based on pathophysiology. Neonatology. 2016;109:369–376. [DOI] [PubMed] [Google Scholar]


