Fig. 1. Molecular contributors to prostate cancer aggressiveness.
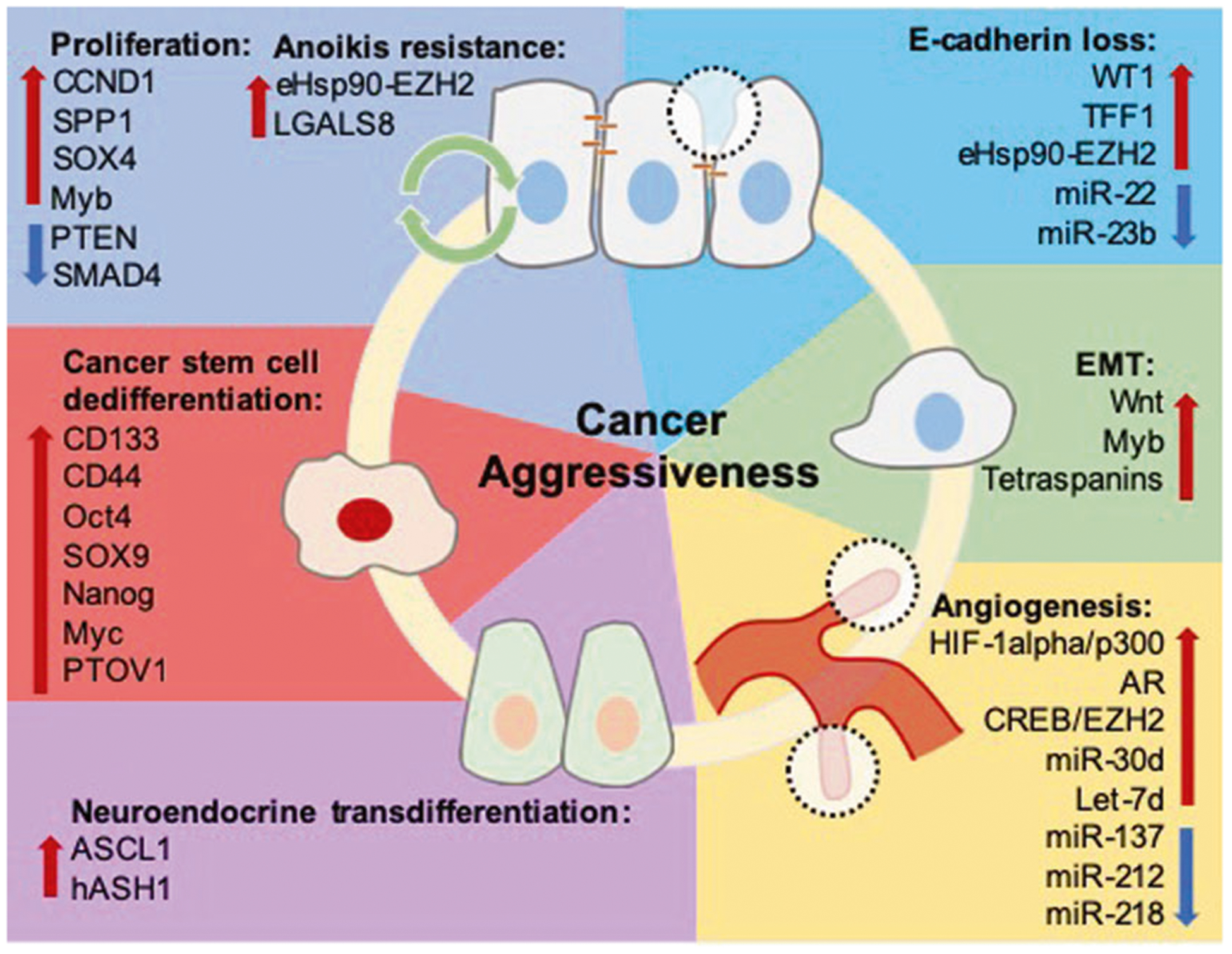
Tumor proliferation, apoptosis/anoikis evasion, and EMT are functional contributing process to tumor recurrence, invasion, and metastasis. Genes including CCND1, SPP1, SOX4, and Myb promote tumor proliferation and survival, with Myb also demonstrating a role in enhancing survival in the context of ADT-resistance. SMAD4 and PTEN are known tumor suppressors, and decreased expression of these genes is known to increase prostate cancer growth and invasion. Resistance to anoikis-related cell death is crucial for tumor survival in the circulation during metastasis. Overactivation of the eHsp90-EZH2 pathway and overexpression of LGALS8 have been shown to promote tumor cell survival and invasion. WT1 and TFF1 promote EMT by decreasing E-cadherin expression via direct transcriptional modulation, and eHsp90 upregulates expression of EZH2, which decreases E-cadherin expression by direct interaction with the E-cadherin promoter to enhance prostate cancer invasion. miR-22 and miR-23b have tumor suppressor roles and their loss is associated with decreased E-cadherin expression. Consequential to E-cadherin loss, β-catenin is released from the plasma membrane and canonical Wnt signaling is activated, leading to acquisition of invasive properties. Myb is associated with promotion of filopodia formation, and tetraspanins such as TSPAN1 and CD151 are also associated with increased expression of classical EMT machinery, promoting cellular motility and dissolution of cell polarity. VEGFA is a direct target of HIF-1α and is transcriptionally regulated by AR, and the HIF-1α/p300 pathway and AR-signaling promotes VEGFA production. In the context of ADT-resistance, the CREB/EZH2 axis promotes vascularization by allowing tumor cells to bypass androgen dependent angiogenesis. miR-30d and let-7d are pro-angiogenic and associated with increased tumor vascularity, while miR-137, miR-212, and miR-218 have inhibitory roles in angiogenesis and are frequently downregulated in prostate cancer. Finally, transdifferentiation and acquisition of a NE phenotype is a critical means of achieving ADT-resistance. ASCL1 and hASH1 are cell fate regulators that respond to decreased androgen signaling by downstream formation of NE characteristics. CSCs exhibit resistance to chemotherapy, and CD133+ cells demonstrating DTX-resistance often have increased CD44, Oct4, SOX9, and Nanog expression. Expression of renewal and stemness genes such as Nanog and Myc are also induced by PTOV1 during CSC formation.
