New strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with several dominant mutations in the spike protein have been identified recently, and crucial issues associated with the possible reinfection of recovered patients and the efficiencies of vaccines designed based on epidemic strains in early 2020. Here, we provide evidence that the sera collected from convalescent COVID-19 patients in early 2020 and rhesus macaques vaccinated with receptor-binding domain (RBD)-based nanoparticles efficiently neutralize viral variants of D614G and B.1.1.7 but weakly neutralize those of 501Y.V2, displaying a warning to recovered patients and developed vaccines.
During the last 2 months, two new epidemic SARS-CoV-2 variants named 501Y.V2 and B.1.1.7 have rapidly spread across South Africa and the United Kingdom, respectively.1,2 In the 501Y.V2 variant, the spike protein harbors three pivotal mutations (K417N, E484K and N501Y) within the RBD; this variant emerged in early August and became the dominant lineage in early November in South Africa.1 The spike protein of the B.1.1.7 lineage harbors 8 mutations: one mutation (N501Y) within the RBD, three mutations (ΔH69/V70, ΔY144 and A570D) within S1, and four mutations (P681H, T716I, S982A, and D1118H) within S2.2 The B.1.1.7 variant was isolated in late September and accounted for over 60% of cases in the United Kingdom in early December.3
Both 501Y.V2 and B.1.1.7 variants harbor the early epidemic D614G mutation within the spike protein.4 Several reports demonstrate that the D614G mutation enhances SARS-CoV-2 infectivity and transmission by increasing the functional spike density on the virion.5–8 However, the D614G mutation does not alter the binding affinity to hACE2 or the susceptibility to neutralizing antibodies.5,7,9 The mutant virus with glycine at residue 614 (Spike-G614 virus) also becomes more sensitive to sera from Spike-D614-vaccinated mice, nonhuman primates (NHPs) and humans, and convalescent sera as well as from RBD-specific monoclonal antibodies.6 Another shared mutation of 501Y.V2 and B.1.1.7 is N501Y within the RBD. Mutational scanning of the RBD and correlated experiments in mice indicate that N501F and N501Y enhance ACE2 binding affinity and increase virulence, respectively.10,11
Although both 501Y.V2 and B.1.1.7 exhibit high transmissibility, there are no reports that indicate whether mutations within both lineages enhance infectivity and impair antibody neutralization. Here, we performed neutralizing assays with convalescent sera from COVID-19-infected patients collected before April 15, 2020 and sera from RBD nanoparticle vaccine-immunized rhesus macaques against various mutated spike-packaged pseudotyped viruses. Our results demonstrated that most of the current vaccines based on the original D614 spike sequence should have potent protection against the new round of pandemic-mutated SARS-CoV-2.
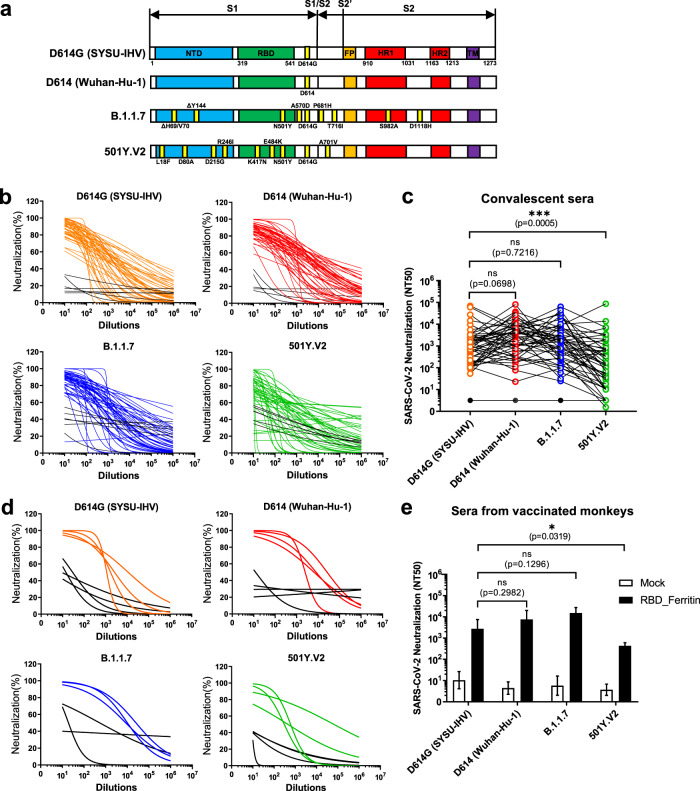
The evolution of the 501Y.V2 and B.1.1.7 lineages have been speculated to be driven by convalescent plasma therapy for chronically infected individuals.12 Immunodeficient or immunosuppressed patients who are chronically infected with SARS-CoV-2 are treated with convalescent plasma. The deficiency of natural immune responses in these patients and the treatment of high antibody concentrations in convalescent plasma give high selective pressures on the intrapatient virus population, which could drive the genetic divergences of 501Y.V2 and B.1.1.7. In addition, many mutations within the spike protein of 501Y.V2 and B.1.1.7 are located in the RBD region, which harbors many pivotal epitopes that elicit neutralizing antibodies.13 To evaluate whether the current dominant SARS-CoV-2 variants could escape neutralization from wild-type strain-derived antibodies, we first constructed various spike protein-expressing plasmids derived from G614 SARS-CoV-2 (SYSU-IHV), D614 virus (Wuhan-Hu-1), B.1.1.7, and 501Y.V2 to package the pseudotyped SARS-CoV-2 S/HIV-1 viruses (Fig. 1a). The luciferase gene is incorporated into the HIV-1 vector and can be expressed after pseudotyped virus infection.14 We incubated various pseudotyped SARS-CoV-2 S/HIV-1 viruses with convalescent sera from 49 COVID-19 patients,15 followed by infection and luciferase reporter assays (Table S1). All 49 sera were collected in the cities Guangzhou and Zhuhai in South China before April 15, 2020, which could exclude the possible epidemic of mutated SARS-CoV-2 strains. The antibodies in convalescent sera efficiently neutralized pseudotyped SARS-CoV-2 D614G and B.1.1.7. Although these sera were also capable of neutralizing 501Y.V2, the neutralizing titers were significantly low against this strain (Fig. 1b and c).
Fig. 1.
Neutralizing potential of sera from convalescent patients and RBD nanoparticle-vaccinated rhesus macaques against SARS-CoV-2 variants. a Schematic of the spike protein of SARS-CoV-2 variants, including G614, D614, B.1.1.7, and 501Y.V2. Mutation types and positions are indicated next to each backbone. b Different pseudotyped viruses were incubated with serially diluted sera from convalescent COVID-19 patients and healthy controls to detect neutralizing antibodies. Neutralization potency was calculated by measuring the luciferase activity of infected hACE2-HeLa cells and plotted as a neutralization curve. Black lines represent the sera from healthy donors. c NT50 of each convalescent serum against each pseudotyped virus. d Different pseudotyped viruses were incubated with serially diluted sera from nanoparticle-vaccinated rhesus macaques to detect neutralizing antibodies. Neutralization potency was plotted as a neutralization curve. e NT50 of each serum from nanoparticle-vaccinated rhesus macaques against each pseudotyped virus. The NT50 titer in (c) and (e) was defined as the reciprocal of serum dilution at which nAbs caused 50% inhibition of infection. Data in (c) were analyzed by the Friedman test with Dunn’s multiple comparisons test (n = 49). ***p < 0.001. Data in (e) represented as the mean ± SEM (n = 4). Adjusted p values were calculated by two-way ANOVA with Tukey’s multiple comparisons test *p < 0.05
Another major concern is whether these variants would turn to resistance to the protection of existing vaccines, as most of the current vaccines were based on the spike protein of viruses that were prevalent earlier in the epidemic.16 Recently, we reported an efficient ferritin-based nanoparticle vaccine that simultaneously presented 24 copies of the RBD and elicited robust protective immune responses against the wild-type SARS-CoV-2 strain.17 Various pseudotyped viruses were incubated with sera from rhesus macaques vaccinated with RBD nanoparticles to evaluate their neutralizing potential against the new variants. Consistent with the sera from convalescent COVID-19 patients, the sera from rhesus macaques efficiently neutralized variants D614G and B.1.1.7; however, their neutralization efficiency against 501Y.V2 was significantly low (Fig. 1d and e).
Based on the above results, we believe that the risk of recovered individuals being infected once again by the mutants D614G and B.1.1.7 is much lower than that by the mutant 501Y.V2. Although the spike protein of both B.1.1.7 and 501Y.V2 acquired multiple mutations, part of which were even within the RBD, some mutations impaired the binding of neutralizing antibodies in the sera from early convalescent individuals, while some mutations did not. With the decrease in neutralizing antibodies in the individuals who recovered from COVID-19 early in 2020, they might become especially susceptible to infection by the 501Y.V2 stain.
Supportively, we found that the sera from RBD nanoparticle-vaccinated rhesus macaques efficiently neutralized the B.1.1.7 pseudotyped virus but weakly neutralized the 501Y.V2 pseudotyped virus. The RBD nanoparticle vaccine we tested was established based upon the RBD of a strain that was prevalent early in the epidemic (Wuhan-Hu-1). While the single mutation N501Y in the RBD region of B.1.1.7 does not affect the efficiency of neutralizing antibodies, the combination of the 3 mutations (K417N, E484K, and N501Y) in the RBD region of 501Y.V2 might result in the decreased interaction between the neutralizing antibodies and spike protein. Taken together, these results show that as mutations accumulate in the RBD, spike proteins may acquire an antigenic shift that enable SARS-CoV-2 variants to eventually resist the current vaccines. Therefore, intensive monitoring of virus mutations and timely adjustment of the designed vaccines are required to control the viral pandemic.
Supplementary information
Acknowledgements
This work was supported by the National Special Research Program of China for Important Infectious Diseases (2018ZX10302103 and 2017ZX10202102), the Special 2019-nCoV Program of Natural Science Foundation of China (NSFC) (82041002), the Special 2019-nCoV Project of National Key Research and Development Program of China (2020YFC0841400), the Special 2019-nCoV Project of Research and Development Program of Guangdong (2020B111123001), the Important Key Program of NSFC (81730060), and the Joint-innovation Program in Healthcare for Special Scientific Research Projects of Guangzhou (201803040002) to H.Z. This work was also supported by the National Postdoctoral Program for Innovative Talents and the General Program of China Postdoctoral Science Foundation (BX20190398 and 2019M663215) to X.M.
Author contributions
Conceptualization, R.L., X.M., X.H., and H.Z.; methodology, R.L., X.M., and H.Z.; validation, X.H. and H.Z.; formal analysis, R.L., X.M., X.H., and H.Z.; investigation, R.L., X.M., J.D., Q.C., W.L., Z.P., Y.Q., Y.L., X.H., and H.Z.; resources, R.L., X.M., and H.Z.; data curation, X.M., X.H., and H.Z.; writing–original draft, X.M. and H.Z.; writing–reviewing and editing, X.M., X.H., and H.Z.; supervision, X.H. and H.Z.; project administration, X.M., X.H., and H.Z.; and funding acquisition, X.M. and H.Z.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Rong Li, Xiancai Ma
Contributor Information
Xin He, Email: hexin59@mail.sysu.edu.cn.
Hui Zhang, Email: zhangh92@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00641-8.
References
- 1.Tegally, H. et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv, 2020.2012.2021.20248640, 10.1101/2020.12.21.20248640 (2020).
- 2.Kemp, S. et al. Recurrent emergence and transmission of a SARS-CoV-2 Spike deletion ΔH69/ΔV70. bioRxiv, 2020.2012.2014.422555, 10.1101/2020.12.14.422555 (2020).
- 3.Page, A. J. et al. Large scale sequencing of SARS-CoV-2 genomes from one region allows detailed epidemiology and enables local outbreak management. medRxiv, 2020.2009.2028.20201475, 10.1101/2020.09.28.20201475 (2020). [DOI] [PMC free article] [PubMed]
- 4.Volz, E. et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell, 10.1016/j.cell.2020.11.020 (2020). [DOI] [PMC free article] [PubMed]
- 5.Zhang L, et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020;11:6013. doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissman, D. et al. D614G Spike Mutation Increases SARS CoV-2 Susceptibility to Neutralization. Cell Host & Microbe, 10.1016/j.chom.2020.11.012 (2020). [DOI] [PMC free article] [PubMed]
- 7.Hou YJ, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plante, J. A. et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature, 10.1038/s41586-020-2895-3 (2020). [DOI] [PMC free article] [PubMed]
- 9.Garcia-Beltran, W. F. et al. COVID-19 neutralizing antibodies predict disease severity and survival. Cell, 10.1016/j.cell.2020.12.015 (2020). [DOI] [PMC free article] [PubMed]
- 10.Gu H, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starr TN, et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295–1310.e1220. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemp, S. et al. Neutralising antibodies drive Spike mediated SARS-CoV-2 evasion. medRxiv, 2020.2012.2005.20241927, 10.1101/2020.12.05.20241927 (2020).
- 13.Yuan M, et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, et al. Recovered COVID-19 patients with recurrent viral RNA exhibit lower levels of anti-RBD antibodies. Cell. Mol. Immunol. 2020;17:1098–1100. doi: 10.1038/s41423-020-00528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, et al. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity. 2020;53:1315–1330.e1319. doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.