Abstract
Understanding how injury to the central nervous system induces de novo neurogenesis in animals would help promote regeneration in humans. Regenerative neurogenesis could originate from glia and glial neuron-glia antigen-2 (NG2) may sense injury-induced neuronal signals, but these are unknown. Here, we used Drosophila to search for genes functionally related to the NG2 homologue kon-tiki (kon), and identified Islet Antigen-2 (Ia-2), required in neurons for insulin secretion. Both loss and over-expression of ia-2 induced neural stem cell gene expression, injury increased ia-2 expression and induced ectopic neural stem cells. Using genetic analysis and lineage tracing, we demonstrate that Ia-2 and Kon regulate Drosophila insulin-like peptide 6 (Dilp-6) to induce glial proliferation and neural stem cells from glia. Ectopic neural stem cells can divide, and limited de novo neurogenesis could be traced back to glial cells. Altogether, Ia-2 and Dilp-6 drive a neuron-glia relay that restores glia and reprogrammes glia into neural stem cells for regeneration.
Research organism: D. melanogaster
Introduction
The central nervous system (CNS) can regenerate after injury in some animals, and this involves de novo neurogenesis (Tanaka and Ferretti, 2009). Newly formed neurons integrate into functional neural circuits, enabling the recovery of function and behaviour, which is how CNS regeneration is measured (Tanaka and Ferretti, 2009). The human CNS does not regenerate after injury. However, in principle it could, as we continue to produce new neurons throughout life that integrate into functional circuits (Tanaka and Ferretti, 2009; Gage, 2019). Through understanding the molecular mechanisms underlying natural regenerative neurogenesis in animals, we might be able to provoke de novo neurogenesis in the human CNS to promote regeneration after damage or neurodegenerative diseases. Regenerative neurogenesis across animals may reflect an ancestral, evolutionarily conserved genetic mechanism, which manifests itself to various degrees in regenerating and non-regenerating animals (Tanaka and Ferretti, 2009). Accordingly, it may be possible to discover molecular mechanisms of injury-induced neurogenesis in the fruit-fly Drosophila, which is a powerful genetic model organism.
Regenerative neurogenesis could occur through activation of quiescent neural stem cells, de-differentiation of neurons or glia, or direct conversion of glia to neurons (Tanaka and Ferretti, 2009; Falk and Götz, 2017). Across many regenerating animals, new neurons originate mostly from glial cells (Tanaka and Ferretti, 2009; Falk and Götz, 2017). In the mammalian CNS, radial glial cells behave like neural stem cells to produce neurons during development. Remarkably, whereas NG2-glia (also known as oligodendrocyte progenitor cells, OPCs) produce only glia (oligodendrocytes and astrocytes) in development, they can also produce neurons in the adult and upon injury (Dimou and Götz, 2014; Falk and Götz, 2017; Valny et al., 2017; Du et al., 2021) – although this remains controversial. Discovering the molecular mechanisms of a neurogenic response of glia is of paramount urgency.
NG2-glia are progenitor cells in the adult human brain, constituting 5–10% of total CNS cells, and remain proliferative throughout life (Dimou and Götz, 2014). In development, NG2-glia are progenitors of astrocytes, OPCs, and oligodendrocytes, but postnatally and upon injury they can also produce neurons (Dimou and Götz, 2014; Torper et al., 2015; Falk and Götz, 2017; Valny et al., 2017; Du et al., 2021). They can also be directly reprogrammed into neurons that integrate into functional circuits (Torper et al., 2015; Pereira et al., 2017). The diversity and functions of NG2-glia are not yet fully understood, but they are particularly close to neurons. They receive and respond to action potentials generating calcium signals, they monitor and modulate the state of neural circuits by regulating channels and secreting chondroitin sulphate proteoglycan perineural nets, and they also induce their own proliferation to generate more NG2-glia, astrocytes that sustain neuronal physiology, and oligodendrocytes that enwrap axons (Dimou and Götz, 2014; Sakry and Trotter, 2016; Sun et al., 2016; Du et al., 2021). NG2-glia have key roles in brain plasticity, homeostasis, and repair in close interaction with neurons (Dimou and Götz, 2014; Sakry and Trotter, 2016; Du et al., 2021), but to what extent this depends on the NG2 gene and protein, is not known.
NG2 (also known as chondroitin sulphate proteoglycan 4, CSPG4) is expressed by NG2-glia and pericytes, but not by oligodendrocytes, neurons, or astrocytes (Cahoy et al., 2008). NG2 is a transmembrane protein that can be cleaved upon neuronal stimulation to release a large secreted extracellular domain and an intracellular domain (Sakry et al., 2014; Sakry and Trotter, 2016). The intracellular domain (ICD, NG2ICD) is mostly cytoplasmic, and it induces protein translation and cell cycle progression (Nayak et al., 2018). NG2ICD lacks a DNA binding domain and therefore does not function as a transcription factor, but it has a nuclear WW4 domain and nuclear localisation signals and can regulate gene expression (Sakry et al., 2015; Sakry and Trotter, 2016; Nayak et al., 2018). It is thought that NG2 functions as a receptor, triggering nuclear signalling in response to ligands or partners (Sakry et al., 2014; Sakry and Trotter, 2016). NG2 protein is abundant in proliferating NG2-glia and glioma (Sakry et al., 2015; Sakry and Trotter, 2016; Nayak et al., 2018). It is also required for OPC proliferation and migration in development and in response to injury (Kucharova and Stallcup, 2010; Kucharova et al., 2011; Binamé et al., 2013). Given the close relationship of NG2-glia with neurons, it is anticipated that key partners of NG2 are produced from neurons, but these remain largely unknown.
The fruit-fly Drosophila is particularly powerful for discovering novel molecular mechanisms. The Drosophila NG2 homologue is called kon-tiki (kon) or perdido (Estrada et al., 2007; Schnorrer et al., 2007; Pérez-Moreno et al., 2017). Kon functions in glia, promotes glial proliferation and glial cell fate determination in development and upon injury, and promotes glial regeneration and CNS injury repair (Losada-Perez et al., 2016). Kon works in concert with the receptor Notch and the transcription factor Prospero (Pros) to drive the glial regenerative response to CNS injury (Kato et al., 2011; Losada-Perez et al., 2016). It is normally found in low levels in the larval CNS, but injury induces a Notch-dependent increase in kon expression in glia (Losada-Perez et al., 2016). Together, Notch signalling and Kon induce glial proliferation. Kon also initiates neuropile glial differentiation and pros expression, and Pros maintains glial cell differentiation (Griffiths and Hidalgo, 2004; Kato et al., 2011; Losada-Perez et al., 2016). This glial regenerative response to injury is homeostatic and time-limited, as two negative feedback loops halt it: Kon represses Notch, and Pros represses kon expression, preventing further cell division (Kato et al., 2011; Losada-Perez et al., 2016). The relationship between these genes is also conserved in the mouse, where the homologue of pros, Prox1, is expressed together with Notch1 in NG2-glia (Kato et al., 2015). Following cell division, Prox1 represses NG2-glia proliferation and promotes oligodendrocyte differentiation (Kato et al., 2015). Together, Notch, Kon, and Pros form a homeostatic gene network that sustains neuropile glial integrity throughout life and drives glial regeneration upon injury (Hidalgo and Logan, 2017; Kato et al., 2018). As Kon is upregulated upon injury and provokes glial proliferation and differentiation, it is the key driver of the glial regenerative response to CNS injury.
A critical missing link to understand CNS regeneration was the identification of neuronal partners of glial NG2/Kon that could induce regenerative neurogenesis. We had observed that injury to the Drosophila larval CNS also resulted in spontaneous, yet incomplete, repair of the axonal neuropile (Kato et al., 2011). This strongly suggested that injury might also induce neuronal events, such as axonal regrowth or generation of new neurons. Thus, we asked whether Kon may interact with neuronal factors that could contribute to regenerative neurogenesis after injury. Here, we report that relay of insulin signalling involving neuronal Ia-2 and glial Kon drives in vivo reprogramming of neuropile glia into neural stem cells.
Results
Genetic analysis reveals Ia-2 is a key neuronal factor interacting with Kon
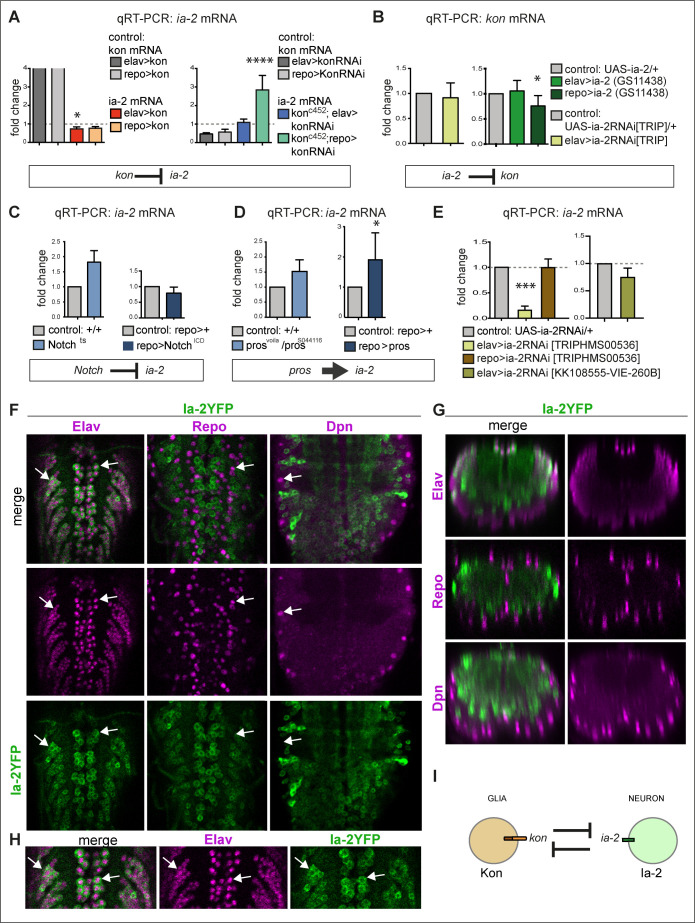
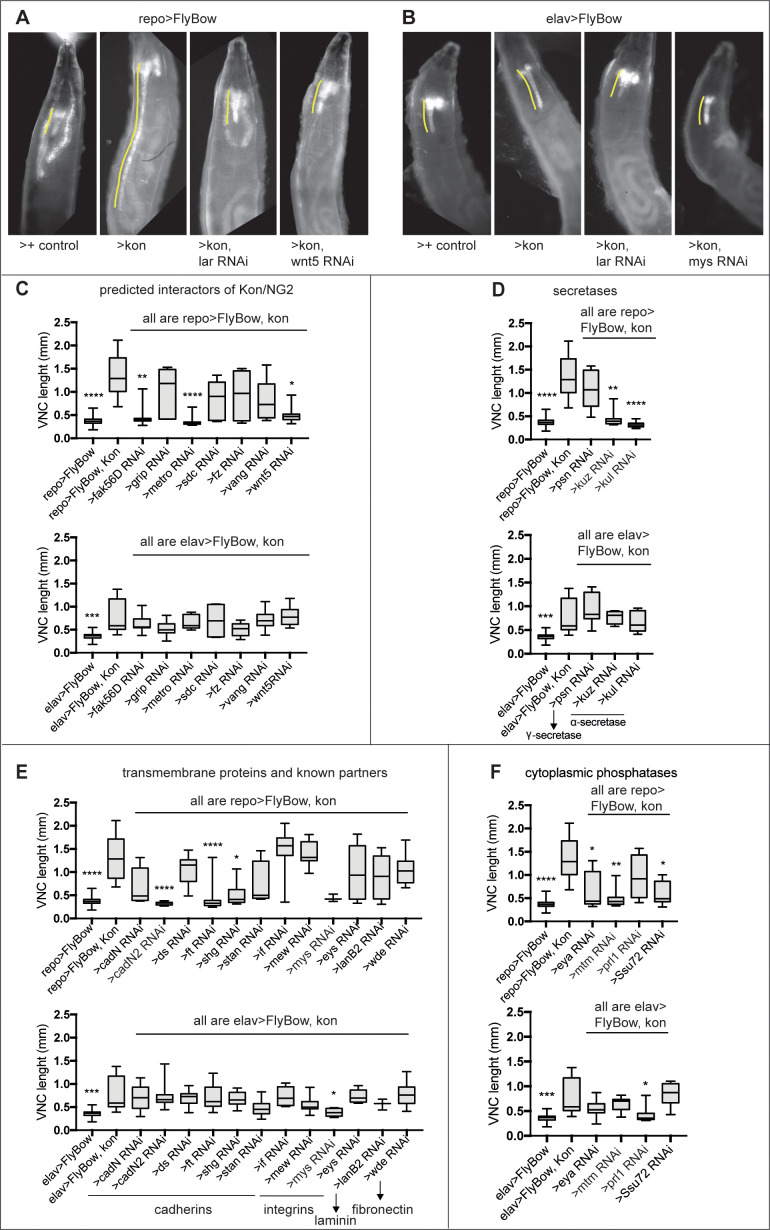
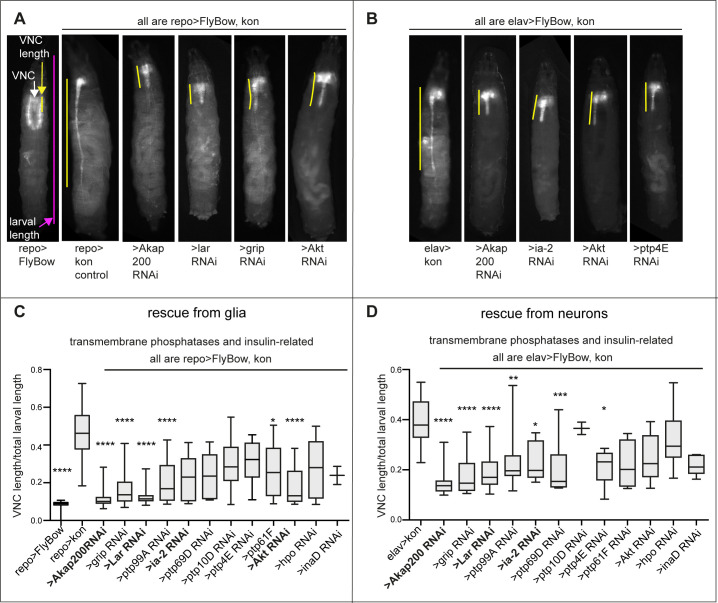
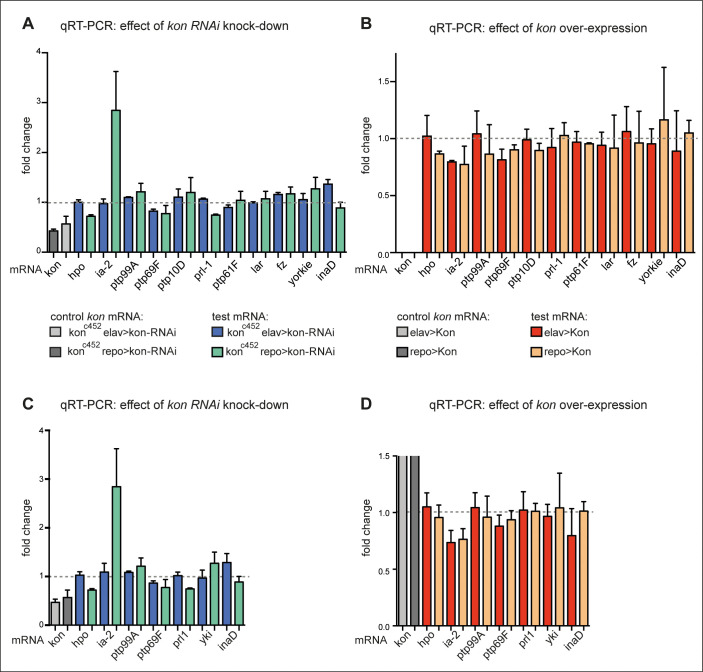
To search for neuronal factors that might interact with glial kon, we carried out genetic screens aimed at identifying genes expressed in neurons that had non-autonomous effects on glia. We exploited the fact that overexpression of kon elongates the larval ventral nerve cord (VNC) (Losada-Perez et al., 2016), and tested whether RNAi knock-down of candidate genes in neurons or glia could rescue this phenotype (Figure 1—figure supplements 1 and 2). To validate the approach, we first tested genes predicted or known to interact with kon and/or NG2 (Schnorrer et al., 2007; Pérez-Moreno et al., 2017). Indeed, knock-down of known interactors, such as integrins (Pérez-Moreno et al., 2017), factors involved in Notch signalling (e.g. Mtm, Akap200), secretases (i.e. kuz, kul) that cleave both Notch and NG2/Kon (Sakry and Trotter, 2016), and phosphatases Prl1 and Ptp99A (Song et al., 2012), all rescued the phenotype, validating the approach (Figure 1—figure supplements 1 and 2). We tested knock-down of other genes encoding phosphatases and transmembrane proteins expressed in neurons. Knocking-down phosphatases ptp99A, ptp69D, and ptp4E from neurons rescued the phenotype, but most prominent was knock-down of phosphatase lar, a negative regulator of insulin signalling (Figure 1—figure supplement 2A–D). Notably, knock-down of other insulin related factors including Akt and ia-2 also caused some rescue (Figure 1—figure supplement 2A–D). However, multiple genes can affect VNC length, and these rescue phenotypes may not necessarily reflect specific gene interactions. Thus, we next asked whether altering kon function affected the expression of a group of genes selected from the above screens. Kon can influence gene expression, as kon mutations cause loss of glial gene expression (Losada-Perez et al., 2016). Using quantitative real-time reverse transcription PCR (qRT-PCR) on dissected larval CNSs, we found that kon knock-down in neurons (with konc452, elavGAL4>UAS-konRNAi) or glia (with konc452, repoGAL4>UAS-konRNAi) had no effect on the expression of most phosphatases, including lar, or other tested genes. By contrast, it resulted in an approximately three fold increase in ia-2 mRNA levels (Figure 1—figure supplement 3A). Conversely, overexpression of full-length kon in either neurons or glia downregulated ia-2 mRNA levels by 25% (Figure 1—figure supplement 3B). We validated these results by increasing the repeats of the most promising subset of genes (Figure 1—figure supplement 3C,D), and this confirmed the strongest effect of kon loss of function (LOF) and gain of function (GOF) on ia-2 (Figure 1A). Accordingly, Kon function in glia prevents ia-2 expression. Next, we asked whether knock-down or overexpression of ia-2 in neurons (with elavGAL4) had any effect on kon mRNA levels, but none did (Figure 1B). However, overexpression of ia-2 in glia (with repoGAL4>ia-2[GS11438]) decreased kon mRNA levels (Figure 1B). As Kon functions in glia (Losada-Perez et al., 2016), these data indicated that kon and ia-2 restrict each other’s expression to glia or neurons, respectively, and/or that Ia-2 is restricted to neurons. Either way, these data showed that ia-2 and kon interact genetically.
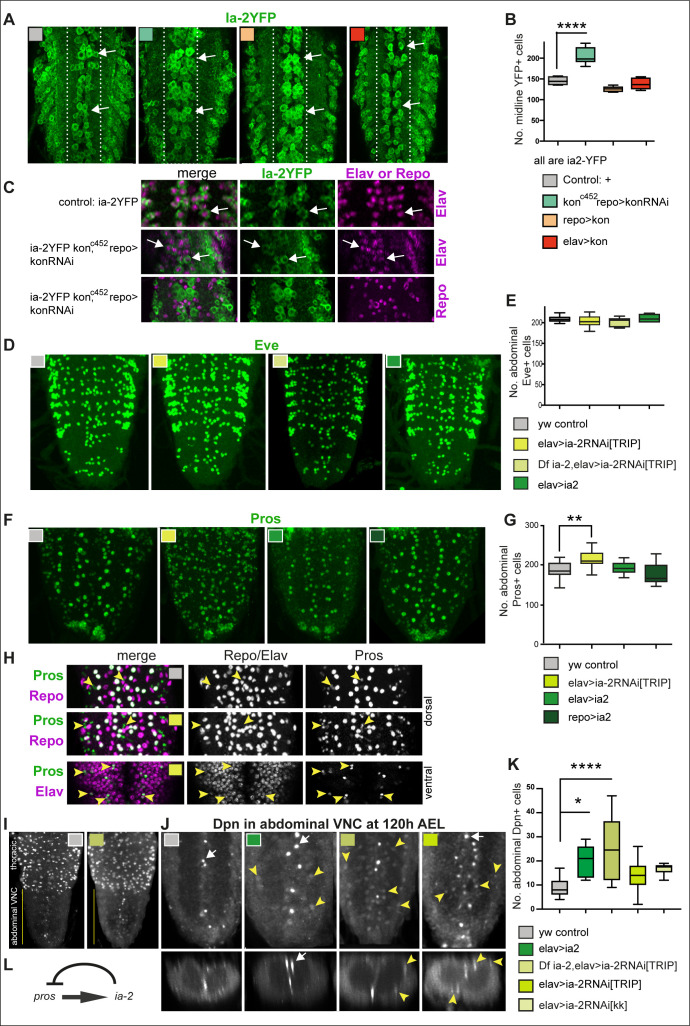
Figure 1. ia-2 interacts genetically with kon, Notch, and pros.
(A) Quantitative real-time PCR (qRT-PCR) showing that gain of kon function reduced ia-2 mRNA levels by 25% (one-way ANOVA p=0.045), whereas loss of kon function in glia caused practically a threefold increase in ia-2 mRNA levels (genotype: konc452/UASkonRNAi; repoGAL4/+; one-way ANOVA p<0.0001). Post hoc Dunnett’s test multiple comparisons to control. N = 4 replicates. (B) qRT-PCR showing that overexpression of ia-2 in glia downregulated kon mRNA levels. Left: Unpaired Student's t-test with Welch correction p=0.457. Right: one-way ANOVA p<0.045, post hoc Dunnett’s test multiple comparisons to control. N = 4–6 replicates. (C) Ia-2 is functionally related to Notch: qRT-PCR showing that ia-2 mRNA levels increased in Nts mutant larvae at the restrictive temperature of 25°C. Unpaired Student's t-test with Welch correction. Left: p=0.4123; Right: p=0.2182. N = 3 replicates. (D) ia-2 is functionally related to pros: qRT-PCR showing that overexpression of pros in glia increased ia-2 mRNA levels by twofold. Unpaired Student's t-test with Welch correction. Left: p=0.1368; Right: p=0.0428. N = 3 replicates. (E) qRT-PCR showing that UAS-ia-2 RNAi[TRIPHMS00536] knock-down in neurons (with elavGAL4) lowered ia-2 mRNA levels to 20%, whereas in glia it has no effect, meaning that ia-2 is expressed in neurons. A second UAS-ia-2RNAi[KK108555-VIE-260B] line lowered mRNA levels by 25%. One-way ANOVA p=0.0004, post hoc multiple comparisons to control Dunnett’s test. N = 3 replicates. (F–H) Fusion protein Ia-2YFP revealed expression exclusively in neurons, as all Ia-2YFP+ cells were also Elav+, but Repo− and Dpn−. Genotype: ia-2[CPTI100013]. N = 4–16 larval ventral nerve cords (VNCs). (I) Illustration showing that kon and ia-2 functions are restricted to glia and neurons, respectively, and they mutually exclude each other. (G) Transverse views; (F and H) horizontal views; (H) higher magnification views. With more than two sample types, asterisks indicate multiple comparison post hoc tests to controls: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. For full genotypes and further statistical analysis details, see Supplementary file 1.
Figure 1—figure supplement 1. Modifier genetic screens identify genes interacting with kon.
Figure 1—figure supplement 2. Modifier candidate genetic screens identify genes encoding transmembrane phosphatases and insulin signalling factors as interacting with kon.
Figure 1—figure supplement 3. Loss and gain of kon function prominently affected ia-2 expression.
Our genetic and qRT-PCR based screens had identified genetic interactions between kon and lar, and Akt and ia-2. LAR is involved in neuronal axon guidance and is responsible for de-phosphorylating, and thus inactivating insulin receptor signalling (Mooney et al., 1997; Wills et al., 1999). Akt is a key effector of insulin receptor signalling downstream (van der Heide et al., 2006). Ia-2 is a highly evolutionarily conserved phosphatase-dead transmembrane protein phosphatase required in dense core vesicles for the secretion of insulin, insulin-related factor-1 (IGF-1) and neurotransmitters; it also has synaptic functions and influences behaviour and learning (Cai et al., 2001; Harashima et al., 2005; Hu et al., 2005; Henquin et al., 2008; Cai et al., 2009; Nishimura et al., 2010; Cai et al., 2011; Carmona et al., 2014). Rather unexpectedly, our findings suggested that Kon is involved in insulin signalling.
To ask whether and how Ia-2 might relate to the Kon-Notch-Pros glial regenerative gene network, we tested whether LOF or GOF for Notch or pros might affect ia-2 expression. With qRT-PCR on dissected larval CNSs, we found that Notchts mutants had an almost twofold increase in ia-2 expression, whereas NotchICD overexpression in glia (repoGAL4>NotchICD) caused no significant effect (Figure 1C). Like Kon, Notch also functions in glia (Griffiths and Hidalgo, 2004; Kato et al., 2011; Losada-Perez et al., 2016), thus the genetic inference is that ia-2 expression in glia is prevented by Notch. Ia-2 mRNA levels increased slightly (albeit not significantly) in pros mutant larvae, and most prominently, when pros was overexpressed in glia (Figure 1D). The loss of function phenotype could be indirect: in glial cells Pros and Notch depend on each other (Griffiths and Hidalgo, 2004; Kato et al., 2011), thus loss of pros causes the downregulation of Notch, which would increase ia-2 expression. Instead, the stronger effect of pros GOF on ia-2 indicated that Pros could directly regulate ia-2 expression. Importantly, Pros is a transcription factor found in glia, type I and II neuroblasts, ganglion mother cells (GMCs), and some neurons (Bayraktar et al., 2010). Thus, Pros could regulate ia-2 expression in any of these cell types. Most importantly, these data meant that ia-2 participates in the kon, Notch, pros gene network that drives the regenerative response to CNS injury.
The above data suggested that ia-2 expression is normally excluded from glia. To test what cells express ia-2, we knocked-down ia-2 with RNAi in either neurons or glia and measured ia-2 mRNA levels with qRT-PCR in dissected larval CNSs. ia-2-RNAi knock-down in glia (repoGAL4>UASia-2RNAiTRIPHMS00536) had no effect, however knock-down in neurons (elavGAL4>UASia-2RNAiTRIPHMS00536) downregulated ia-2 transcripts to about 20% of wild-type levels (Figure 1E). A second UAS-ia-2 RNAi line (line UAS-ia-2RNAiKK108555-VIE-260B) had a milder effect, but still reduced ia-2 expression by 25% (Figure 1E). These data meant that ia-2 is expressed in neurons. To visualise ia-2 expression in vivo, we used a transgenic protein fusion of Ia-2 to yellow fluorescent protein (YFP), Ia-2YFPCPTI100013 (Lowe et al., 2014; Lye et al., 2014), from now on called Ia-2YFP. Ia-2YFP+ cells did not have the glial marker Repo (Figure 1F,G). They did not have Deadpan (Dpn) either (Figure 1F,G), which is the general marker for neuroblasts as well as intermediate neural progenitors of type II neuroblast lineages (Boone and Doe, 2008). All Ia-2YFP+ cells had the pan-neuronal marker Elav (Figure 1F–H). This demonstrated that ia-2 is expressed exclusively in neurons.
Altogether, these data showed that Ia-2 and Kon function within the regenerative gene network and are restricted to neurons and glia, respectively (Figure 1I).
Alterations in Ia-2 levels induced ectopic cells expressing the neural stem cell marker Dpn
Next, we carried out a functional analysis of ia-2 in the CNS. As kon knock-down increased ia-2 mRNA levels, we sought to verify this using Ia-2YFP. Ia-2YFP+ appeared undistinguishable from wild type when kon was knocked-down in glia (konc452/ia-2YFP; repoGAL4>kon-RNAi) (Figure 2A). However, as Ia-2YFP is normally in all neurons, a potential effect could have been missed. Thus, we focused on the midline, where a limited number of dorsal Ia-2YFP+ neurons can be counted. Indeed, kon loss of function in glia increased the number of Ia-2YFP+ cells along the midline (Figure 2A,B). The ectopic ia-2YFP cells had the pan-neuronal marker Elav and did not have the glial marker Repo (Figure 2C), meaning they were neurons. Ia-2YFP+ midline cells were unaffected by kon overexpression in either neurons or glia (Figure 2A,B, elavGAL4>kon and repoGAL4>kon). Thus, in the absence of kon, ectopic Ia-2YFP+ neurons were found at the midline. Loss of kon function prevents glial differentiation (Losada-Perez et al., 2016) and could result in more Ia-2YFP+ neurons also in other locations. The increase in neurons could explain why ia-2 mRNA levels increased with kon loss of function (see Figure 1A). However, the mRNA levels for ptp99A, −69F, and 10D (Figure 1—figure supplement 3), also known to function in neurons, were not increased. Either way, these data confirmed that Kon and Ia-2 are mutually exclusive in glia and neurons, respectively.
Figure 2. ia-2 influences neural cell fate non-autonomously.
(A and B) Loss of kon function in glia (konc452/UASkonRNAi; repoGAL4/+) increased the number of Ia-2YFP+ cells along the midline. One-way ANOVA p<0.0001, post hoc Tukey’s test. N = 5–8 ventral nerve cords (VNCs). (C) The ectopic Ia-2YFP+ cells in kon loss of function were Elav+ and not Repo+. N = 5–7 VNCs. (D and E) Neither loss nor gain of ia-2 function affected the number of Eve+ neurons. One-way ANOVA p=0. 2374. N = 7–12 VNCs. (F and G) Loss of ia-2 function (elavGAL4>UASia-2RNAi[TRIPHSM00536]) increased Pros+ cell number, and supernumerary cells were small. Kruskal–Wallis ANOVA p=0.0003, post hoc Dunnett’s test. N = 8–26 VNCs. (H) Small Pros+ cells in ia-2 knock-down (genotype as in F and G) did not have the glial marker Repo, but could have the neuronal marker Elav. (I–K) Dpn+ cells visualised at 120 hr AEL, after developmental neuroblasts have disappeared. (I) Dpn signal in thorax was strong as normal, and in abdomen lower Dpn was found ectopically in ia-2 loss or gain of function. (J and K) Both loss and gain of ia-2 function increased the number of abdominal Dpn+ cells, which also were in ectopic locations. (L) Genetic inference: ia-2 negatively regulates pros, most likely non-autonomously. All images are horizontal views, except for I (bottom row) which are transverse views. One-way ANOVA p=0.0002, post hoc Dunnett. N = 7–15. Data shown in box-plot s: box represents 50% values around with median, and whiskers 25% top and bottom values. Asterisks indicate multiple comparison post hoc tests to a fixed control: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. For further statistical analysis details, see Supplementary file 1.
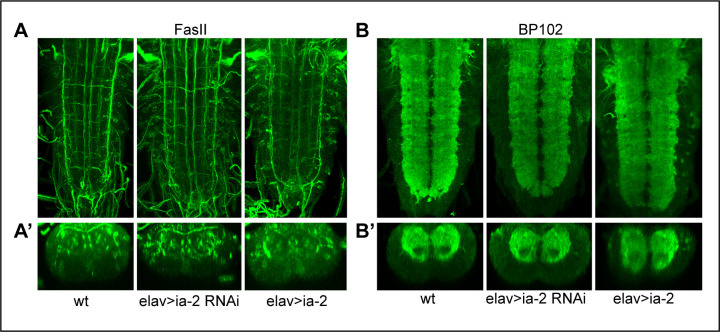
Figure 2—figure supplement 1. Alterations in ia-2 levels cause no obvious neuronal phenotypes.
To ask what function Ia-2 might have in neurons, we altered ia-2 expression and visualised the effect using standard neuronal markers. ia-2 knock-down in neurons (elavGAL4>ia-2RNAiTRIPHMS00536) had no detectable effects on FasII or BP102 (Figure 2—figure supplement 1A,B). It did not change Eve+ neuron number either (Figure 2D,E). As Pros activates ia-2 expression (Figure 1D), we asked whether ia-2 might affect Pros. Overexpression of ia-2 in either neurons or glia had no effect on Pros+ cells (Figure 2F,G). By contrast, ia-2 knock-down in neurons (elavGAL4>ia-2RNAi TRIPHMS00536) increased the number of small Pros+ cells (Figure 2F,G). GMCs and neurons are generally smaller than glia. In fact, these small Pros+ cells lacked the glial marker Repo, but had the neuronal marker Elav (Figure 2H). Altogether, data showed that reduced ia-2 function caused ectopic Pros+ neurons, and potentially also GMCs. Genetic inference is that ia-2 represses pros (Figure 2L).
Pros is normally found in the nuclei of some neurons, some glia (astrocytes and midline), and all GMCs. In neuroblasts, Pros is cytoplasmic, and nuclear translocation of Pros drives the switch from neural stem cell (neuroblast) to progenitor (GMC) cell fate (Choksi et al., 2006). One of the target genes repressed by Pros is the marker common to all Drosophila neural stem cells, Dpn (Choksi et al., 2006). Thus, we asked whether altering ia-2 function might affect Dpn. Dpn was visualised in normally high levels, in thoracic neuroblasts of third instar larvae (Figure 2I). Both ia-2 GOF (elav>ia-2) and LOF (Df(2L)ED7733/+; elav>ia-2RNAi TRIPHMS00536), and elavGAL4 knock-down using two different RNAi lines, UAS-ia-2RNAi TRIPHMS00536 and UAS-ia-2RNA KK108555-VIE-260B, in neurons increased the total number of abdominal VNC Dpn+ cells (Figure 2I–K). Supernumerary abdominal Dpn+ cells were located at ectopic positions, along the midline and around the neuropile, positions normally occupied by glial cells (Figure 2J). The ectopic Dpn+ cells were distinct from normal abdominal larval neural stem cells, which are ventro-lateral and further away from the neuropile. Furthermore, they were visualised 120 hr after egg laying (AEL), after the disappearance of developmental abdominal neural stem cells. Dpn levels in these ectopic abdominal cells were lower than in thoracic Dpn+ neuroblasts (compare with Figure 2I). Altogether, alterations in the levels of neuronal Ia-2 induced neural stem cell marker expression ectopically.
These data showed that interference with normal neuronal Ia-2 levels upregulated Pros (a marker for progenitor cells, neurons, and glia) and Dpn (the general neural stem cell marker). This effect was non-autonomous, as neurons themselves seemed unaffected. As Ia-2 and Kon are functionally related but confined to either neurons or glia, respectively, this suggested that communication between neurons and glia was involved in inducing an ectopic neural stem cell state.
Injury induces ia-2 expression and a regenerative neurogenic response
Above data had shown that altering ia-2 levels upregulated the neural stem cell marker Dpn (Figure 2I–K). Since CNS injury induced the upregulation of kon expression (Losada-Perez et al., 2016), we asked whether injury might affect ia-2 expression and, consequently, induce a neurogenic response. To this end, crush injury was carried out at 74–76 hr AEL in early third-instar larval VNCs labelled with the endoplasmic reticulum GFP marker G9 (Figure 3A–C), using a previously established protocol (Losada-Perez et al., 2016). qRT-PCR in injured VNCs revealed approximately a twofold increase in ia-2 mRNA levels at 5–7 hr post-injury, which recovered homeostatically by 24 hr post-injury (Figure 3B). This paralleled the effect of injury on kon expression (Losada-Perez et al., 2016). Thus, CNS injury caused an increase in ia-2 expression.
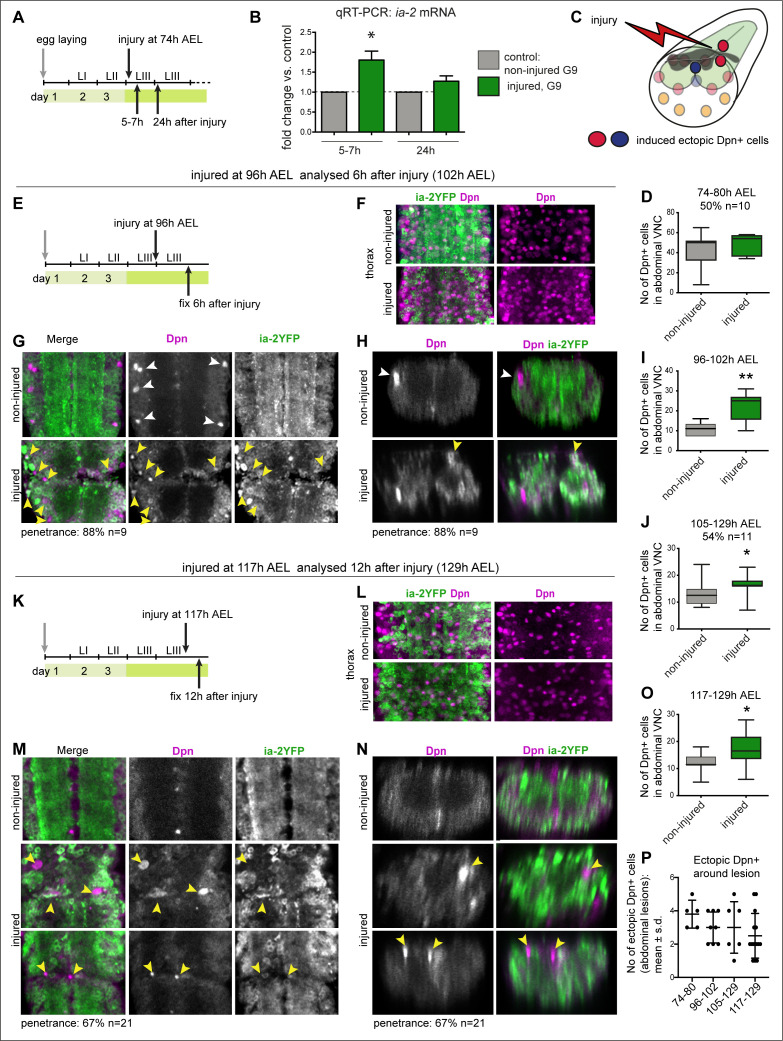
Figure 3. Injury induced ia-2 expression and ectopic Dpn+ cells.
(A, E, and K) Time course of crush-injury experiments in the larval abdominal ventral nerve cord (VNC), indicating the age of the larvae (after egg laying, AEL) when crush was applied (top arrows), followed by various recovery periods, and when they were dissected and fixed (bottom arrows). (C) Diagram showing that crush injury induced ectopic Dpn+ cells. (A, B, and D) Crush injury in the larval abdominal VNC at 74 hr AEL: (B) increased the levels of ia-2 mRNA at 5–7 hr post-injury, which recovered homeostatically by 24 hr, detected by qRT-PCR. N = 3 biological replicates. (D) Induced ectopic abdominal Dpn+ cells by 5–7 hr post-injury (74–80 hr AEL) in half of injured VNCs (penetrance 50%, N = 10), and increased (albeit not significantly) the total number of abdominal Dpn+ cells inthose samples. Mann–Whitney U-test, p=0.24. (F and L) Thoracic Ia-2YFP and Dpn signal were normal in thoracic neuroblasts, in samples shown in (G and H) and (M and N), respectively. (E–I) Injury at 96 hr AEL caused ectopic abdominal Dpn+ cells by 6 hr post-injury (arrowheads). Most Dpn+ cells were Ia-2YFP−. At this stage, some developmental neuroblasts could still remain (white arrowheads), but ectopic abdominal Dpn+ cells were dorsal (yellow arrowheads, H). Most injured VNCs had ectopic abdominal Dpn+ cells (88% penetrance N=9). (I) Unpaired Student's t-test p=0.0063. (J) Injury at 105 hr AEL and fixation at 129 hr AEL, when no developmental neuroblasts remain, induced a significant increase in abdominal Dpn+ cells (54.5% penetrance N = 11). Mann–Whitney U-test p=0.0375. (K–O) Injury at 117 hr AEL caused ectopic abdominal Dpn+ cells by 12 hr post-injury (129 hr AEL). . Ectopic abdominal Dpn+ cells were found in ectopic dorsal positions (yellow arrowheads, N). This stage is devoid of developmental neural stem cells. Over two thirds of injured VNCs had ectopic Dpn+ cells (67% penetrance N=21). (O) Student's t-test p=0.0302. (I,J,O) All abdominal Dpn+ cells were counted in all injured samples. (P) Temporal profile of number of ectopic Dpn+ cells surrounding the lesions, number in X-axis indicate time points of injury and fixation. (F, G, L, and M) Horizontal views, (H and N) transverse views. (D, I, J, O ) Graphs show box-plots . (P) Shows dot plots, with mean and error bars (±s.d.) indicated. *p<0.05, **p<0.01. For full genotypes and further statistical analysis details, see Supplementary file 1.
Since increased ia-2 levels induced ectopic Dpn+ cells (Figure 2I–K), and ia-2 was upregulated in injury, we asked whether injury induced neural stem cells. We focused on the abdominal VNC only, which has three neuroblasts per hemi-segment in ventro-lateral positions, in early third instar larvae. Crush injury in the abdominal VNC at 74–76 hr AEL resulted in ectopic abdominal Dpn+ cells by 5–7 hr later (Figure 3A,D). These were more numerous than the normal developmental abdominal larval neuroblasts, and included cells located in dorsal positions, which are not normally occupied by them (see Sousa-Nunes et al., 2011; Froldi et al., 2015). The numerous Dpn+ cells could correspond to injury-induced divisions of neuroblasts normally found during larval development. To test whether injury might induce ectopic neural stem cells distinct from developmental neuroblasts, we next carried out crush injury at three later time points: (1) at 96 hr AEL and analysed the VNCs 6 hr post-injury (PI, 102 hr AEL), when in control VNCs, abdominal hemi-segments have 0 or 1 Dpn+ cells remaining (Figure 3E–I); (2) at 105 hr and analysed 24 hr PI (129 hr AEL), when in controls there are no ventro-lateral neuroblasts, only Dpn+ cells along the midline (Figure 3J); and (3) at 117 hr AEL and analysed the VNCs 12 hr PI (129 hr AEL), taking advantage of the delayed pupariation of injured larvae (Figure 3K–O). At 129 hr AEL there were no remaining abdominal ventro-lateral neural stem cells in intact controls, only some Dpn+ cells along the midline (Figure 3M,N). Normal thoracic Dpn+ neuroblasts were present in all samples (Figure 3F,L). Injury induced at the three later time points caused ectopic abdominal Dpn+ cells at the lesion site (Figure 3G,H,M,N,P). Most, ectopic Dpn+ cells lacked Ia-2YFP (Figure 3G,H,M,N). Importantly, most ectopic abdominal Dpn+ cells surrounded the neuropile, and some were dorsal, in positions never occupied by developmental neural stem cells (Figure 3H,N). Ectopic abdominal Dpn+ cells were located surrounding the lesions (Figure 3G,M,P). To take into account that Dpn might also be induced in cells at a distance from the lesion site, we also counted the total number of abdominal Dpn+ cells in all injured samples (Figure 3G–O). The number of abdominal Dpn+ cells increased significantly in injured samples compared to controls (Figure 3I,J,O). The incidence of ectopic Dpn+ cells at the injury site decreased as larval age at the time of injury increased (Figure 3P). This most likely means that in older injured larvae there was not enough time between injury and pupariation for cells to divide further. Altogether, these data showed that injury induced ectopic neural stem cells that were distinct from developmental neuroblasts. Since ia-2 levels increased upon injury, and ia-2 GOF induced neural stem cells, this suggested that ia-2 was responsible for the increase in Dpn+ cells caused by injury.
Neuronal Ia-2 and glial Kon regulate Dilp-6
The above data raised the question of how Ia-2 might induce ectopic neural stem cells. Ia-2 is highly evolutionarily conserved and it functions in dense core vesicles to release insulin and neurotransmitters (Harashima et al., 2005; Kim et al., 2008; Nishimura et al., 2010; Cai et al., 2011). There are eight Drosophila insulin-like-peptides (Dilps) and Ia-2 affects only Dilp-6 (Kim et al., 2008). dilp-6 is expressed in cortex and blood–brain barrier CNS glia, and activates neural stem cell proliferation following a period of quiescence in normal larval development (Chell and Brand, 2010; Sousa-Nunes et al., 2011). Thus, we asked whether the increase in Dpn+ cells in ia-2 LOF and GOF observed above involved dilp-6.
We first visualised dilp-6 expressing cells in wandering larvae using dilp6-GAL4 (Chell and Brand, 2010; Sousa-Nunes et al., 2011) to drive expression of the nuclear reporter Histone-YFP (His-YFP). Most dilp-6>his-YFP+ cells were also Repo+, but they did not surround the neuropile and lacked the neuropile glial marker Pros (Figure 4A,B). Therefore, most dilp-6 expressing cells in the abdominal larval VNC were cortex and surface glia, as previously reported (Chell and Brand, 2010; Sousa-Nunes et al., 2011). Some dilp6>his-YFP+ cells were Repo− Elav+ and thus were neurons (Figure 4A,B). Therefore, dilp-6 is expressed in some neurons per VNC segment and mostly in non-neuropile glia.
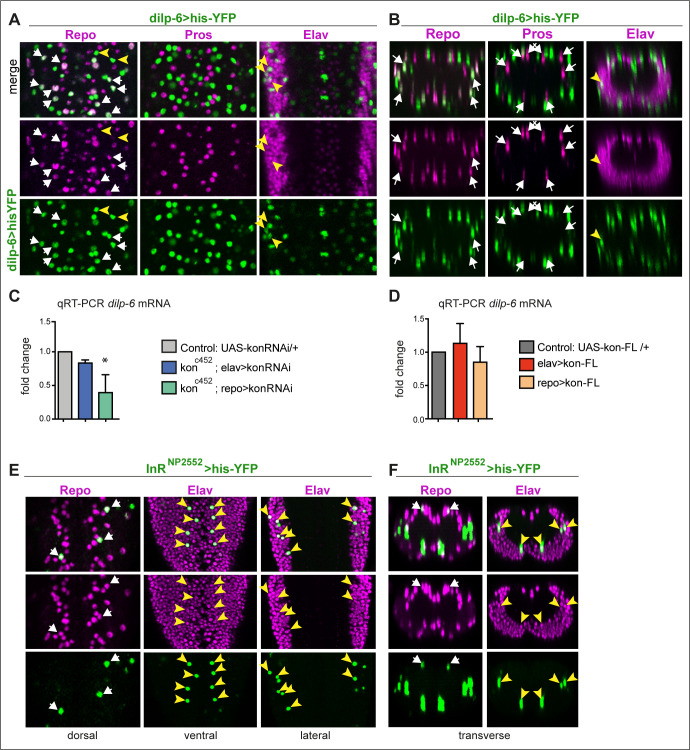
Figure 4. dilp-6 is expressed in neurons and cortex glia and received by neuropile glia.
(A and B) Dilp-6GAL4>UAShisYFP cells are mostly Repo+ Pros− glia that do not surround the neuropile (white arrows), and from position appear to be cortex and surface glia. No YFP+ cells have Pros. Some cells are Repo− Pros− Elav+ (yellow arrowheads) meaning they are neurons. (C and D) qRT-PCRs showing that: (C) kon knock-down in glia (konc452/UASkonRNAi; repoGAL4/+) downregulates dilp-6 mRNA levels; (D) overexpression on kon does not cause a significant effect. N = 3 replicates for both. (C and D) One-way ANOVA, only differences in (C) for dilp-6 mRNA significant p=0.0362, *p<0.05. (E and F) inR expression visualised with reporter InRNP2552GAL4> UAShistoneYFP is expressed stochastically in some dorsal Repo+ neurople glia (white arrows), and other glia, and in some Elav+ neurons (yellow arrowheads). (A and E) Horizontal views of the abdominal ventral nerve cord (VNC); (B and F) transverse views. For full genotypes and further statistical analysis details, see Supplementary file 1.
Kon function is required for glial cell fate (Losada-Perez et al., 2016), so we used qRT-PCR to ask whether altering Kon levels might affect dilp-6 expression. Overexpression of full-length kon mildly increased dilp-6 mRNA levels (albeit not significantly), but kon knock-down in glia significantly reduced dilp-6 mRNA levels (Figure 4C,D). This effect could be indirect, as glial proliferation and differentiation are impaired with kon loss of function (Losada-Perez et al., 2016), or perhaps Kon regulates dilp-6 expression. Either way, dilp-6 expression depends on kon in glia.
Dilp-6 is a ligand for the insulin receptor (InR), which functions at least in CNS neurons and neuroblasts (Fernandez et al., 1995; Song et al., 2003; Sousa-Nunes et al., 2011; Fernandes et al., 2017). To revisit what cells might receive Dilp-6, we visualised InR expression, using multiple available GAL4 lines to drive his-YFP, and tested co-localisation with glial and neuronal markers. At 72 hr AEL, InRNP2552>his-YFP+ cells comprised some Elav+ neurons and some Repo+ glia, including dorsal neuropile glia and surface glia (Figure 4E,F). The distribution was stochastic, most likely due to the insertion of GAL4 into an intron. To verify whether InR is expressed in glia, we searched published single cell RNAseq data from the larval CNS (Brunet Avalos et al., 2019). Indeed, of 152 Repo+ glial cells, 97 were also InR+, that is, 63% of glial cells express InR. Thus, InR is expressed in both neurons and glia.
Altogether, these data indicated that in the third instar larva Dilp-6 is produced and secreted by Ia-2 from some neurons, it is mostly produced in non-neuropile glia, and it is received by InR in neurons and glia, including neuropile glia.
A positive neuron-glia communication loop boosts Dilp-6 production from glia
The above data strongly suggested that a neuron-glia communication loop might serve to amplify Dilp-6. A limiting step could be Kon, as glial dilp-6 expression depends on kon. Kon is required for glial gene expression (Losada-Perez et al., 2016), but whether this depends on the nuclear translocation of its intracellular domain, KonICD, is unknown. In Drosophila, Kon had been reported to lack a nuclear localisation signal (Schnorrer et al., 2007). In mammals, NG2ICD positively regulates the expression of multiple genes, including downstream targets of mTOR (Sakry et al., 2015; Nayak et al., 2018), but whether this requires nuclear NG2ICD is also unknown. Altogether, whether NG2 or Kon regulates glial gene expression through nuclear events remained unsolved. Thus, to ask whether KonICD might function in the nucleus, we generated a HA-tagged form of KonICD (KonICD-HA). Glial overexpression of konICD-HA (repoGAL4>UAS-KonICD-HA) revealed distribution of anti-HA in glial cytoplasms and in nuclei, co-localising with the glial nuclear transcription factor Repo, in both embryos and larvae (Figure 5A and Figure 5—figure supplement 1). Thus, KonICD is distributed in the cytoplasm and nucleus, from where it could regulate gene expression.
Figure 5. Ia-2, Kon, and Dilp-6 are linked though a neuron-glia communication loop.
(A) Overexpressed HA-tagged KonICD in glia (repoGAL4>UASkonICD::HA) visualised with anti-HA antibodies in third instar wandering larvae, localises to both glial cytoplasms and nuclei (arrows). (B and C) Overexpression of the intracellular domain of kon (konICD) or dilp-6 increased glial cell number, visualised with repoGAL4>UAShistone-YFP, and quantified automatically with DeadEasy in (C). Overexpression of a dominant negative form of the insulin receptor rescues the increase in cell number caused by Dilp-6 (repo>hisYFP, dilp-6, InRDN), meaning that autocrine InR signalling regulates glial proliferation. Box-plots. One-way ANOVA p<0.0001, post hoc Tukey’s test multiple comparisons between all samples. N = 15–28 ventral nerve cords (VNCs). (D and E) Third star larvae at 72 hr AEL to visualise abdominal developmental neuroblasts: kon-RNAi knock-down in neural stem cells with insGAL4 does not affect Dpn+ cell number. Box-plots. Unpaired Student's t-test, p=0.3111. N = 10 VNCs. (F) Illustration summarising that a positive feedback autocrine loop involving Dilp-6, InR, and Kon promotes both glial proliferation and Dilp-6 production. All images are horizontal views. Asterisks refer to multiple comparison post hoc tests, all samples vs. all: **p<0.01, ****p<0.0001. All graphs show box-plots. For full genotypes and further statistical analysis details, see Supplementary file 1.
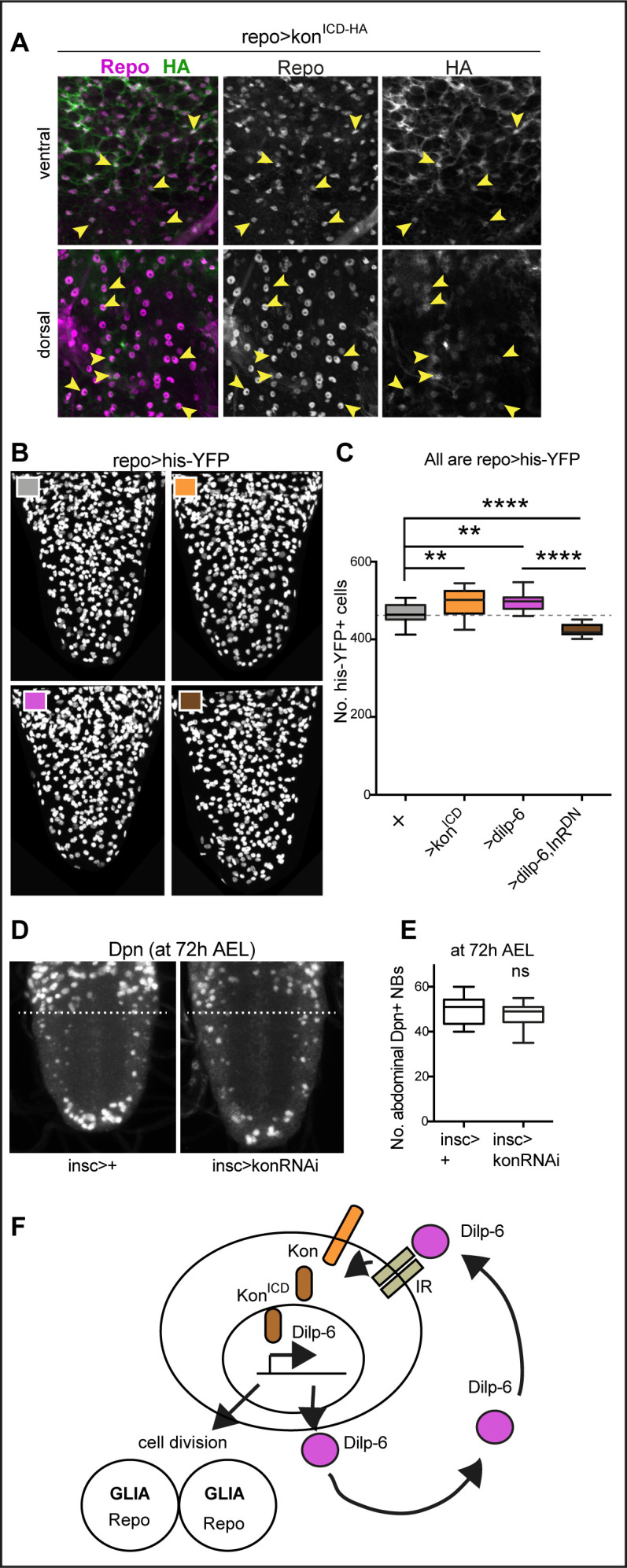
Figure 5—figure supplement 1. Over-expressed HA-tagged konICD localised to nuclei in embryos.
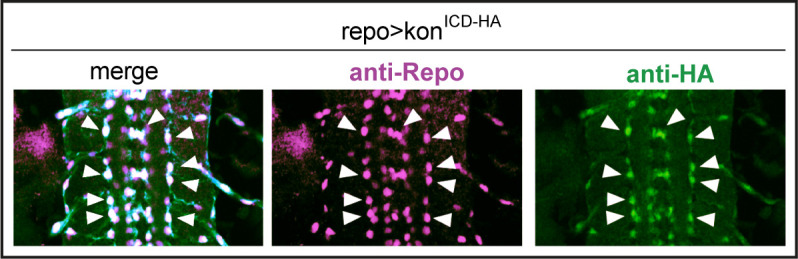
Next, we asked whether KonICD is functional. Since NG2 and Kon are responsible for glial proliferation both in mammals and Drosophila (Kucharova and Stallcup, 2010; Losada-Perez et al., 2016), we used glial cell number as a read-out of KonICD function. First, we tested whether cleaved KonICD could induce glial proliferation, like full-length Kon does (Losada-Perez et al., 2016). We overexpressed konICD in glia and automatically counted glial cells labelled with the nuclear marker his-YFP, using DeadEasy software (Forero et al., 2012). Overexpression of konICD in glia increased glial cell number (UAShisYFP; repoGAL4>UASkonICD, Figure 5B,C), meaning that KonICD can induce glial proliferation. As full-length Kon also promotes glial proliferation (Losada-Perez et al., 2016), these data meant that full-length Kon is normally cleaved, releasing KonICD to promote glial proliferation.
In principle, Dilp-6 amplification could occur if it was first secreted from neurons by Ia-2 to activate InR in glia, and InR signalling in turn drove the Kon-dependent upregulation of dilp-6 expression in glia (Figure 5F). To test whether Dilp-6 activates InR in glia, which activates Kon, we asked: (1) whether overexpression of dilp-6 could mimic the increase in glial cell number caused by KonICD, and (2) whether this could be rescued by overexpression of a dominant negative form of the insulin receptor (InRDN) in glia. We found that overexpression of dilp-6 in glial cells increased glial cell number comparably to KonICD (Figure 5B,C). Furthermore, this was rescued with concomitant overexpression of InRDN in glia (Figure 5B,C). These data meant that Dilp-6 activates InR signalling in glia and induces glial proliferation.
Dilp-6 and InR signalling reactivate quiescent developmental neural stem cells (Chell and Brand, 2010; Sousa-Nunes et al., 2011), but Kon functions in glia (Losada-Perez et al., 2016). To further verify whether Kon function is restricted to glia, we asked whether Kon might also be required in neural stem cells during development at 72 hr AEL, when normally there are neural stem cells in both thorax and abdomen of larvae (Figure 5D). RNAi kon knock-down in neural stem cells with inscutable-GAL4 (ins-GAL4>UAS-konRNAi) did not affect the number or distribution of abdominal developmental Dpn+ cells at 72 hr AEL (Figure 5D,E), meaning that Kon is not required for neural stem cell development. Since glial proliferation depends on Kon (Losada-Perez et al., 2016), the fact that dilp-6 alone could reproduce the increase in cell number caused by konICD, and this depended on InR in glia, strongly suggested that InR signalling can activate Kon cleavage downstream in glia.
To conclude, altogether these data suggested that Ia-2 triggers the release of Dilp-6 from neurons, which then is received by glial cells, where InR signalling activates Kon, which in turn induces glial proliferation enabling further production of Dilp-6. Thus, a non-autonomous relay from neuronal Ia-2 to glial Kon promotes glial proliferation and induces a positive feedback loop that amplifies Dilp-6 production from glia (Figure 5F).
Ia-2 and Dilp-6 can induce neural stem cells from glia
So far, our data had shown that: alterations in Ia-2 levels caused either by genetic manipulation or injury induced ectopic neural stem cells; Ia-2 is required for the neuronal secretion of Dilp-6, which is received and amplified in cortex glia under the control of Kon; and secreted Dilp-6 is received by InR also in neuropile glia. As Dilp-6 activates quiescent developmental neural stem cells (Chell and Brand, 2010; Sousa-Nunes et al., 2011), this raised the question of whether the Ia-2-Kon-Dilp-6 loop not only produced more glia but could also induce a neurogenic response from glia.
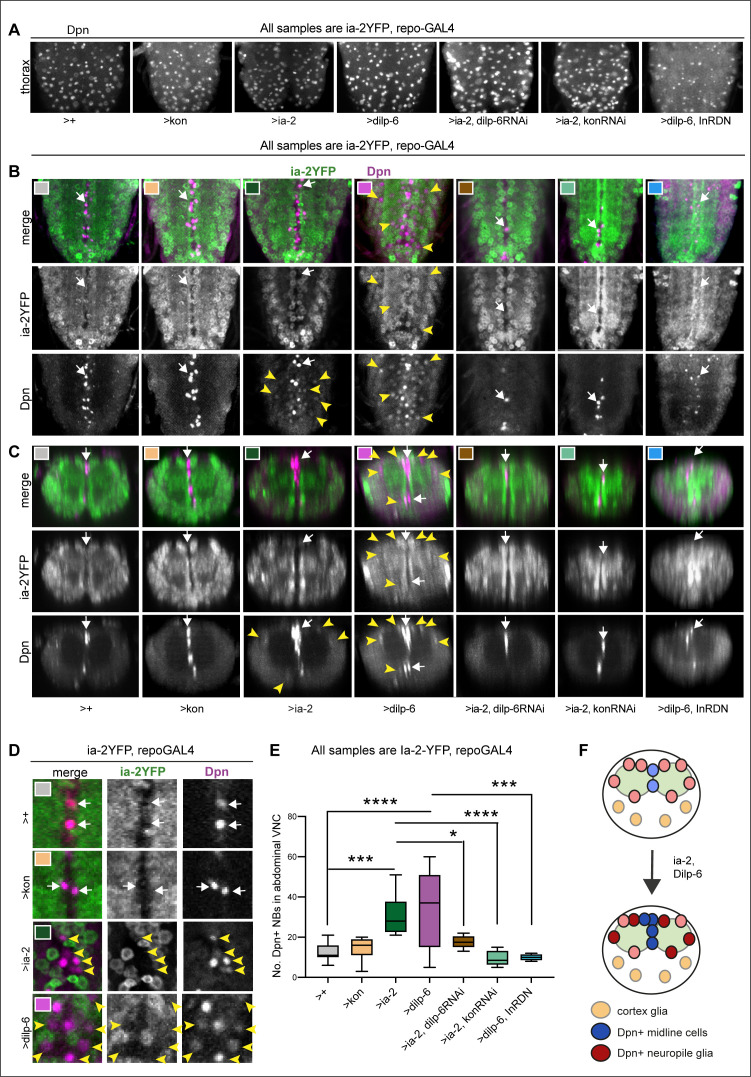
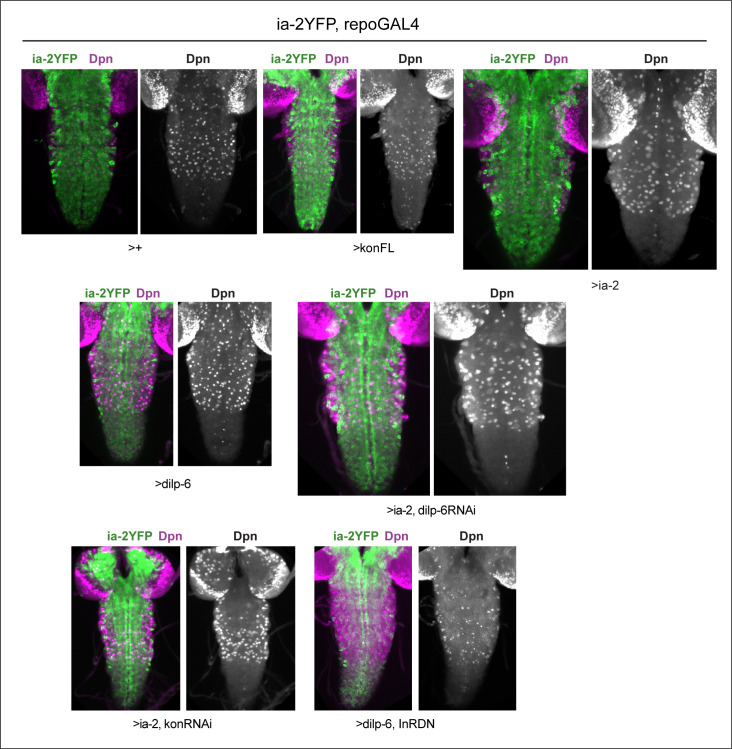
To ask whether Kon, Ia-2, or Dilp-6 could be responsible for inducing ectopic neural stem cells from glia, we overexpressed them in glia (with repoGAL4), and analysed Dpn at 120 hr AEL, after the disappearance of developmental abdominal neural stem cells. Dpn was detected normally in thoracic neuroblasts in all samples (Figure 6A and Figure 6—figure supplement 1). Interestingly, overexpression of a dominant negative form of the InR (InRDN) in glia together with dilp-6 reduced the levels of Dpn in thoracic neuroblasts (Figure 6A).
Figure 6. Ia-2 and Dilp-6 induce ectopic neural stem cells from InR signalling in glia.
All samples were analysed at 120 hr AEL, after disappearance of abdominal developmental neuroblasts. (A) Dpn signal in thorax was normally strong and clear, except with the overexpression of both dilp-6 and InRDN in glia, which reduced Dpn levels and NB size. (B and C) Overexpression of ia-2 and dilp-6, but not kon-full-length, induced Dpn+ cells in the abdominal ventral nerve cord (VNC), at the midline and in lateral positions. Ectopic abdominal Dpn was at lower levels than normal thoracic signal in NBs. (D) Ectopic Dpn+ cells did not express Ia-2YFP (arrowheads). (E) Overexpression of ia-2 or dilp-6 increased abdominal Dpn+ cell number. Quantification of all abdominal VNC Dpn+ cells, and genetic epistasis analysis showing that: the increase in Dpn+ cell number caused by ia-2 overexpression was rescued by dilp-6 RNAi and kon-RNAi knock-down in glia, meaning that ia-2 requires Dilp-6 and glial Kon to induce Dpn; and preventing insulin signalling with InRDN in glia rescued the increase in Dpn+ cell number caused by dilp-6 overexpression, meaning that Dilp-6 induced Dpn via InR signalling in glia. One-way ANOVA p<0.0001, post hoc Tukey’s test multiple comparisons all samples vs. all. N = 5–13 VNCs. (F) Illustration showing that Ia-2 and Dilp-6 can induce Dpn via InR signalling in glial cells. (A and B) Horizontal views; (C) transverse views; (D) higher magnification. Graphs show quantifications in box-plots. Asterisks refer to multiple comparison post hoc tests: *p<0.05, ***p<0.0001, ****p<0.0001. For full genotypes and further statistical analysis details, see Supplementary file 1.
Figure 6—figure supplement 1. Dpn in thoracic neuroblasts of specimens shown in Figure 6.
Figure 6—figure supplement 2. KonICD does not induce dilp-6 nor dpn expression.
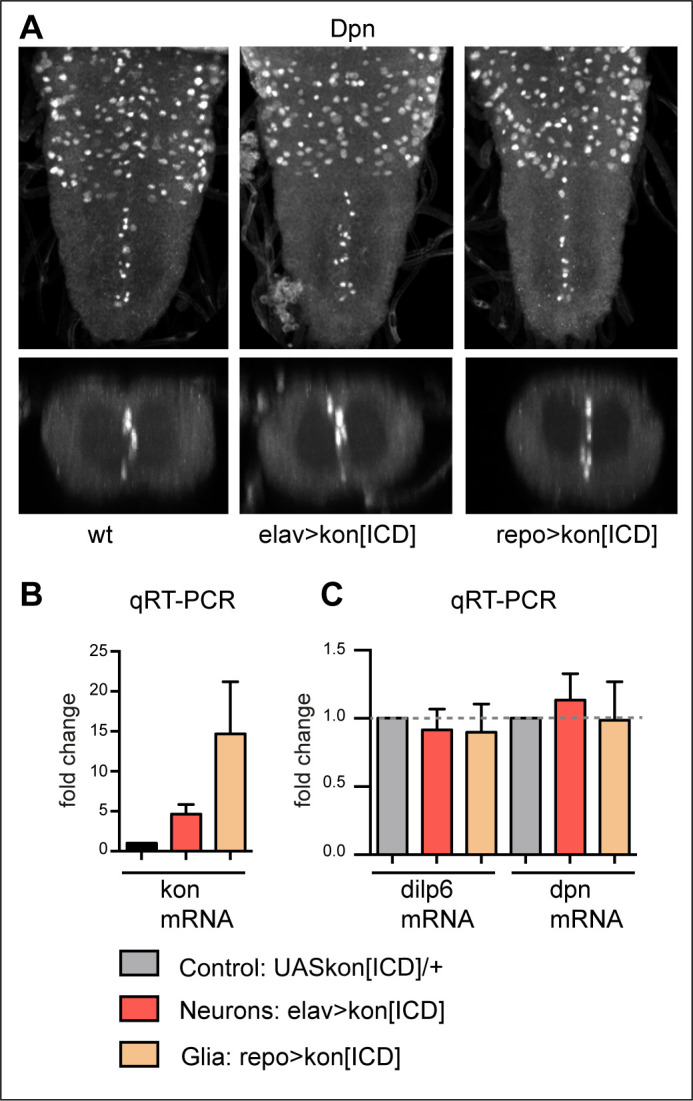
Overexpression of kon-FL did not induce ectopic abdominal Dpn+ cells (Figure 6B–E and Figure 6—figure supplement 1). To test whether KonICD might be required instead, we overexpressed konICD in glia, but it did not induce ectopic Dpn+ cells either (Figure 6—figure supplement 2A). We verified whether repo>KonICD resulted in increased konICD expression, and it did, between 15- and 20-fold (Figure 6—figure supplement 2B). However, konICD overexpression in glia (repo>konICD) did not significantly alter mRNA levels for dilp-6 nor dpn either (Figure 6—figure supplement 2C). These data meant that KonICD does not directly function as a transcription factor. Instead, it may function as a co-factor of an unknown transcription factor. KonICD could also participate in the nuclear import–export shuttle, as loss of kon function prevented nuclear translocation of Repo (Losada-Perez et al., 2016). Thus, most likely Kon regulates dilp-6 indirectly by influencing glial cell fate and it does not induce ectopic Dpn+ cells.
By contrast, overexpression of ia-2 induced ectopic abdominal Dpn+ cells prominently along the midline but also in lateral locations surrounding the neuropile, ordinarily occupied by glia (Figure 6B–E and Figure 6—figure supplement 1). Overexpression of dilp-6 had a stronger effect, and there were many ectopic Dpn+ cells surrounding the neuropile (Figure 6B–E and Figure 6—figure supplement 1). Dpn levels in ectopic cells were generally lower than in normal thoracic neural stem cells. These data showed that both Ia-2 and Dilp-6 can induce dpn expression, potentially in glia. However, Kon alone cannot, meaning that insulin signalling is required to induce neural stem cells. Since Ia-2 drives Dilp-6 production and secretion, this suggested that ultimately Dilp-6 induced ectopic neural stem cells.
To further test whether Ia-2 upregulated dpn ectopically via Dilp-6, we carried out epistasis analysis. Overexpression of ia-2 together with dilp-6 knock-down in glia (ia-2YFP, repoGAL4>UAS-ia-2, UAS-dilp-6RNAi), rescued the number of abdominal Dpn+ cells (Figure 6A–E and Figure 6—figure supplement 1), demonstrating that Ia-2 induces ectopic neural stem cells via Dilp-6. Furthermore, overexpression of ia-2 together with kon RNAi in glia (ia2-YFP, repoGAL4>UAS-ia-2, UAS-konRNAi) also rescued the Dpn+ phenotype (Figure 6B–E and Figure 6—figure supplement 1), confirming that dilp-6 expression depends on Kon in glia (see Figure 4C) and that Kon and Dilp-6 engage in a positive feedback loop (see Figure 5). Finally, the ectopic Dpn+ phenotype was also rescued by overexpression of dilp-6 together with InRDN in glia (Figure 6B–E and Figure 6—figure supplement 1, ia-2YFP repoGAL4>UAS-dilp6, UAS-InRDN), meaning that ectopic neural stem cells depend on InR signalling in glia. Together, these data showed that Ia-2 induces ectopic abdominal neural stem cells via Dilp-6 and InR signalling in glia, and that ectopic Dpn cells originated from glia (Figure 6F).
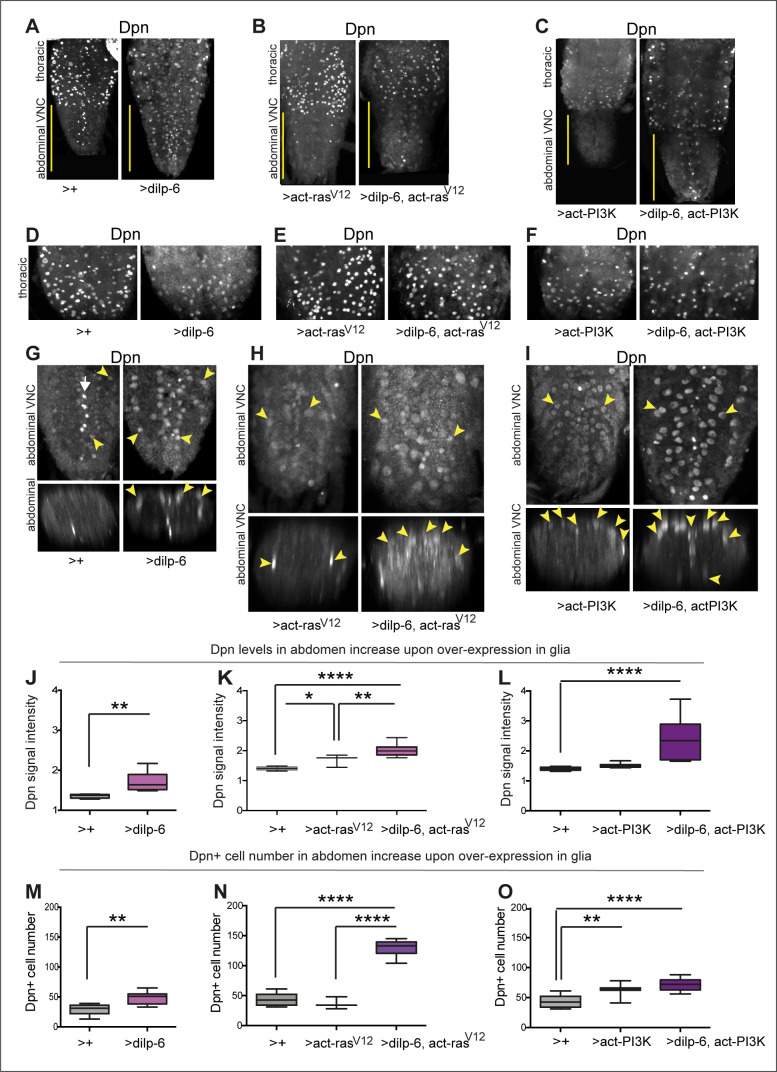
The observation that Ia-2 and Dilp-6 could induce neural stem cell marker expression from glia was important. Thus, we sought to further verify it in two ways. Firstly, we used a second anti-Dpn antibody aliquot, from the Wang Lab (Huang and Wang, 2018; Zhang et al., 2019), as well as their protocol. This revealed thoracic Dpn signal in normal neuroblasts, in late third instar larvae (Figure 7A–F). In the abdomen, Dpn could be detected in some cells at low levels in ectopic positions corresponding to neuropile glia (Figure 7A,G). Ectopic abdominal Dpn levels increased significantly when dilp-6 was overexpressed in glia (with repoGAL4, Figure 7A,G,J). dilp-6 overexpression also increased abdominal Dpn+ cell number (Figure 7G,M), reflecting either higher levels and/or that Dpn+ cell proliferated. Secondly, we asked whether directly manipulating downstream effectors of insulin signalling – Ras and PI3Kinase – in glia might also influence dpn expression. Since InR signalling can trigger multiple signalling pathways downstream, we overexpressed dilp-6 together with the activated forms of Ras (RasV12) and PI3Kinase (Dp110CAAX, hereby called activated PI3K). In order to prevent potential embryonic lethality, to separate Dilp-6 function from its developmental function activating quiescent neuroblasts, and to separate further developmental neuroblasts from ectopic Dpn in glia, we used tubGAL80ts to conditionally overexpress these factors in glia after larval L1 hatching. Samples were analysed in late third instar larvae, just before pupariation, when no normal neuroblasts remain in the abdomen. Dpn was normal in thoracic neuroblasts, with genotype-specific modulation (Figure 7A–F). In the abdominal VNC, overexpression of activated rasV12 in glia (tubGAL80ts, repo>act-rasV12) increased the levels of ectopic Dpn (Figure 7B,H,K), and joint overexpression of dilp-6 and activated-rasV12 (tubGAL80ts, repo>dilp6, act-rasV12) increased Dpn levels further (over twofold, Figure 7B,H,K). Remarkably, whereas activated RasV12 did not alter abdominal Dpn+ cell number, together Dilp-6 RasV12 resulted in a dramatic threefold increase (Figure 7B,H,N). It appeared that more than all glial cells now expressed dpn. These ectopic Dpn+ cells were located throughout the VNC, also invading and disrupting the neuropile (Figure 7H). Thus, activated Ras with Dilp-6 induced Dpn+ cell proliferation. Overexpression of activated PI3K in glia (tubGAL80ts, repo>act-PI3K) had no effect on abdominal Dpn+ levels (Figure 7I,L), but levels increased nearly fourfold when dilp-6 was also overexpressed (tubGAL80ts, repo>dilp-6, act-PI3K) (Figure 7I,L). Interestingly, and contrary to RasV12, activated PI3K induced Dpn only surrounding the neuropile, in positions normally occupied by neuropile glia (astrocytes, ensheathing glia, and midline glia). Co-overexpression of dilp-6 act-PI3K increased the number of Dpn+ cells (Figure 7I,O), but not as dramatically as Dilp-6 RasV12. These data demonstrated that insulin signalling induces dpn expression ectopically in late larval glial cells and promotes their proliferation.
Figure 7. Activation of Ras or PI3K downstream of insulin signalling in glia induces ectopic Dpn+ cells.
(A–C) Full ventral nerve cord (VNC) projections of VNCs showing normal thoracic levels of Dpn, and genotype-specific modulations, using anti-Dpn from Wang Lab. (D–F) Projections of thoracic sections, showing Dpn signal in normal NBs. (G, J, and M) Overexpression of dilp-6 with repoGAL4 induced ectopic abdominal Dpn (G). Some ectopic Dpn+ cells were also observed at low levels in control samples. (J) Dilp-6 increased ectopic abdominal Dpn levels, unpaired Student's t-test; and (M) abdominal Dpn+ cell number, Mann–Whitney U-test. (H, I, K, L, N, and O) tubGAL80ts repoGAL4 was used to overexpress dilp-6 together with activated rasV12 or PI3K in glia to prevent embryonic lethality (48 hr 18°C, followed by 30°C until dissection ). (H, K, and N) Overexpression of dilp-6 with activated rasV12 induced ectopic Dpn in possibly all glia (H). Ectopic Dpn+ cells were large and neuropile integrity was disrupted. (K) Ectopic abdominal Dpn levels increased. One-way ANOVA, p<0.0001, Tukey’s multiple comparison test. (N) Abdominal Dpn+ cell number increased threefold, indicating widespread Dpn+ cell proliferation. One-way ANOVA, p<0.0001, Tukey’s multiple comparison test. (I, L, and O) Overexpression of dilp-6 with activated PI3K induced ectopic abdominal Dpn in neuropile glia only (astrocytes and midline glia). Cells and VNC were large. (L) Ectopic abdominal Dpn levels increased. One-way ANOVA, p<0.0001, Tukey’s multiple comparison test. (O) Abdominal Dpn+ cell number increased, One-Way ANOVA p<0.0001, Tukey’s multiple comparison test. Graphs show quantifications in box-plots. Asterisks refer to multiple comparison tests: *p<0.05, **p<0.01, ****p<0.0001. N=3-10 VNCs. For full genotypes, sample sizes and further statistical analysis details, see Supplementary file 1.
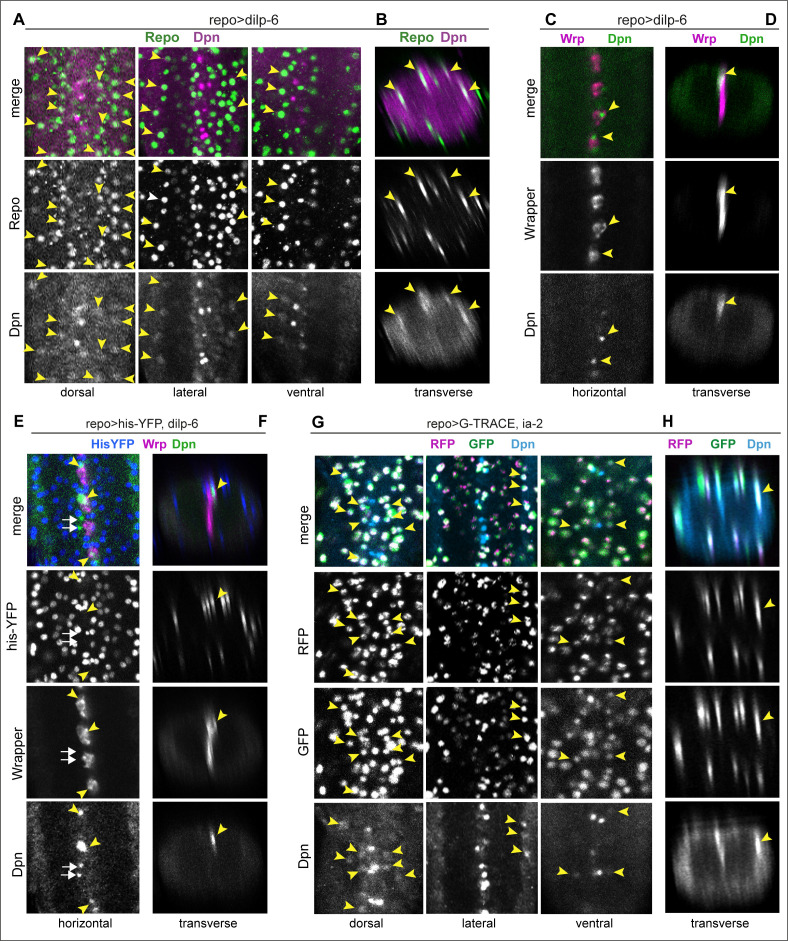
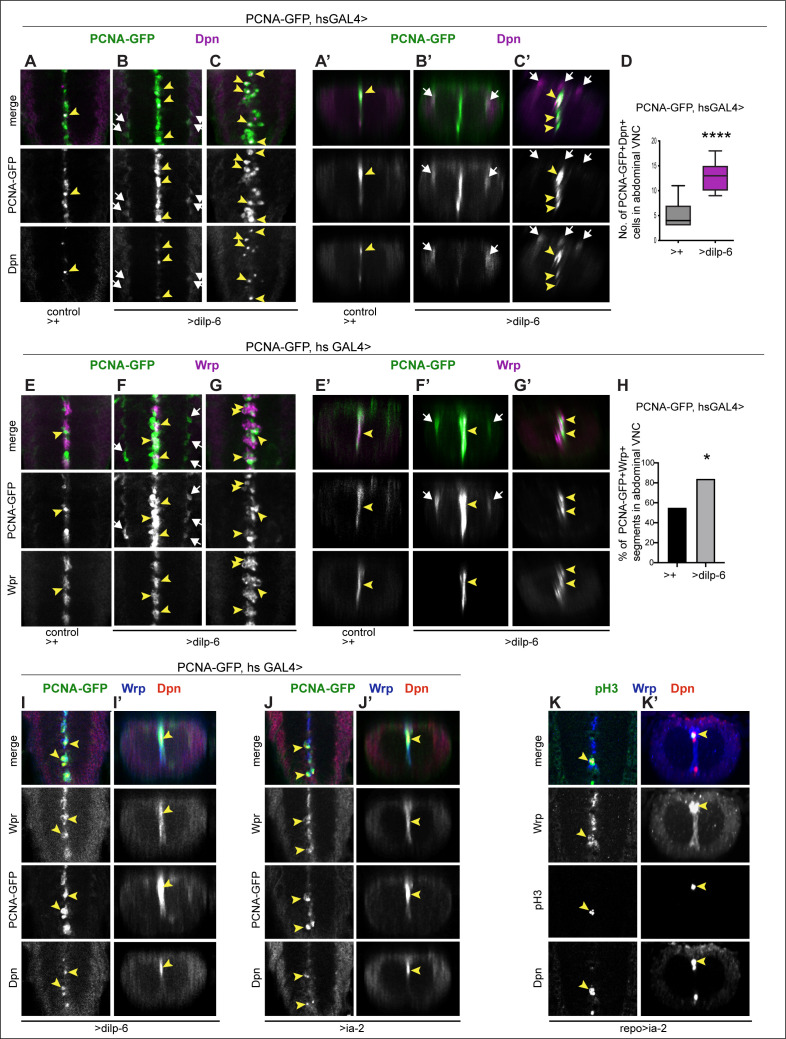
To further test whether the ectopic Dpn+ cells originated from glia, we first asked whether ectopic abdominal Dpn colocalised with the glial marker Repo, in larvae at 120 hr AEL, after the disappearance of developmental abdominal neural stem cells. Lateral ectopic Dpn+ cells observed with dilp-6 overexpression were Repo+ (Figure 8A,B), consistent with originating from glial cells. Dpn levels were lower than in normal neural stem cells. By contrast, ectopic midline Dpn+ cells were not Repo+. Midline glia do not normally express repo, but express wrapper (wrp). Overexpression of dilp-6 resulted in Dpn+ cells along the midline that also had Wrp (Figure 8C,D), showing that ectopic midline Dpn+ cells were midline glia. Thus, there are two distinct populations of ectopic Dpn+ cells: latero/dorsal Repo+ around the neuropile and midline Wrp+ cells, meaning that Dpn was induced in neuropile glia (class known as ‘astrocytes’) and midline glia.
Figure 8. Ia-2 and Dilp-6 induced ectopic neural stem cells originate from glia.
All samples were analysed at 120 hr AEL, after disappearance of abdominal developmental neuroblasts. (A and B) Overexpression of dilp-6 from glia (repoGAL4>UAS-dilp-6) induced Dpn expression in Repo+ neuropile glial cells (arrowheads). N = 10 ventral nerve cords (VNCs). (C and D) Overexpressed dilp-6 also induced Dpn in Wrp+ midline glia (arrowheads). N = 6 VNCs. (E and F) When dilp-6 was overexpressed, and all glia except midline glia were visualised with nuclear repoGAL4>Histone-YFP and midline glia with anti-Wrp, Dpn+ YFP−Wrp− cells were found, which therefore were not glia (white arrows; yellow arrowheads point to Dpn+Wrp+ cells). N = 6 VNCs. (G and H) G-TRACE expression in glia with repoGAL4 revealed with GFP cells that were originally glia or originated from a glial cell lineage, even if they switched off the glial repo promoter, and with RFP newly generated glial cells. Dpn colocalised in neuropile glia with both GFP and RFP, meaning that Dpn+ cells originated from glia, and at that point in time these cells still retained active the glial repo promoter. N = 8 VNCs. (A, C, E, and G) Horizontal and (B, D, F, and H) transverse views. For full genotypes and sample sizes, see Supplementary file 1.
However, not all Dpn+ cells were Repo+ or Wrp+, as some did not express either of these markers (Figure 8E,F, white arrows; genotype: repoGAL4>his-YFP, dilp-6). This could mean that either some ectopic Dpn+ did not originate from glia, or that as glial cells reprogrammed into neural stem cells, they switched off glial gene expression. To test whether ectopic neural stem cells originated from glia, we used the cell-lineage marker G-TRACE. This GAL4-dependent tool results in the permanent labelling of GAL4/UAS-expressing cells and their lineage. Thus, as glial cells become neural stem cells, the glial repo promoter would be switched off, but G-TRACE would enable their visualisation as well as that of all their progeny cells. Cells that were originally glia but may no longer be so would be labelled in green (GFP+), and recently specified glial cells would be labelled in red (RFP+). G-TRACE expression in glia with repoGAL4 together with dilp-6 caused larval lethality and thus could not be analysed. By contrast, overexpression of both G-TRACE and ia-2 in glia (repoGAL4>G-TRACE, ia-2) revealed G-TRACE+ Dpn+ cells around the neuropile, at 120 hr AEL (Figure 8G,H). Most, if not all, of these cells had GFP, but also RFP (Figure 8G,H). These data demonstrate that ectopic Dpn+ originates from glial cells. Since RFP was also present, glial cell fate had not been suppressed, and instead glial cells may have been in the process of reprogramming.
Altogether, these data showed that Ia-2 and Dilp-6 can induce de novo formation of neural stem cells from neuropile and midline glial cells.
In vivo reprogrammed glial cells can divide and generate neurons
To ask whether ectopic neural stem cells can divide to generate neurons, we used the S-phase marker PCNA-GFP, and overexpressed dilp-6 specifically at the third instar larva using GAL4 under the control of a heat-shock promoter. We heat-shocked larvae at 110.5 hr AEL at 37°C for 30 min, and then kept them at 25°C for 9 hr, when they were dissected and fixed, to visualise Dpn+ and PCNA-GFP at 120 hr AEL. In control wandering third instar larvae, a few PCNA-GFP+ cells could be observed along the midline, but not in lateral positions (Figure 9A,A’). Overexpressed dilp-6 resulted in ectopic Dpn+ PCNA-GFP+ cells in lateral positions around the neuropile (Figure 9B,B’,D), as well as along the midline (Figure 9C,C’,D). Some of the dividing midline cells were glia, as Dilp-6 overexpression resulted in PCNA-GFP+Wrp+ cells along the midline (Figure 9E–H), and in an increase in Wrp+ cells (Figure 9G). Thus, Dilp-6 overexpression induced proliferation and increased the number of both Repo+ glia (see Figure 5) and Wrp+ midline glia (Figure 9E–H). Upon overexpression of either dilp-6 or ia-2, some of the dividing PCNAGFP+ Wrp+ cells also had Dpn (PCNA-GFP, hsGAL4>ia-2, or dilp-6, Figure 9I–J’), showing that Dpn+ cells of glial origin can divide. To further verify whether Wrp+ Dpn+ cells could divide, we used the mitotic marker anti-phospho-Histone-H3. Overexpression of ia-2 in glia (repo>Ia-2) induced proliferation of Wrp+ Dpn+ cells, as labelled with pH3 (Figure 9K,K’). These data demonstrate that Ia-2 and Dilp-6 glial-reprogrammed neural stem cells can divide.
Figure 9. Ia-2 and Dilp-6 induced ectopic neural stem cells can divide.
All samples were analysed at 120 hr AEL, after disappearance of abdominal developmental neuroblasts. (A–C’ and E–G’) Cell proliferation was visualised with the S-phase marker PCNA-GFP, quantification in (D and H). dilp-6 expression was induced in all cells with heat-shock-GAL4, raising the temperature to 37°C for 30 min at the end of the third instar larval stage at 110.5 hr AEL, and then larvae were kept at 25°C for 9 hr, visualising Dpn+ and PCNA-GFP at 120 hr AEL. (A–C’) Overexpression of dilp-6 resulted in Dpn+ PCNA-GFP+ cells laterally around the neuropile (B and B’ white arrows) and along the midline (C and C’ yellow arrowheads), showing that these ectopic Dpn+ cells were in S-phase. Quantification box-plots in (D), Student's t-test. There were also some Dpn+ cells that were not dividing (white arrows in C’). (E–G’) Overexpression of dilp-6 resulted in PCNA-GFP+ Wrp+ midline glia (yellow arrowheads) that therefore were dividing. In (G) there is a notable increase in the number of Wrp+ cells. In (E and E’) lateral PCNA-GFP+Wrp−Dpn+ cells around the neuropile (white arrows) most likely correspond to neuropile glia. (H) Quantification showing phenotypic penetrance: percentage of segmentally repeated Wrp+ cell clusters that contain PCNAGFP+ cells. Fisher’s exact test p=0.0276. (I–J’) Overexpression of either dilp-6 or ia-2 with hsGAL4 upregulated the S-phase marker PCNA-GFP in Wrp+ Dpn+ midline cells, meaning these ectopic Dpn+ cells were dividing. Penetrance: >dilp-6 25% N = 4; >ia-2: 18% N = 11 ventral nerve cords (VNCs). (K and K’) Overexpression of ia-2 in glia with repoGAL4 induced non-autonomously proliferation of ectopic Wrp+ Dpn+ cells, visualised with the mitotic marker pH3. Penetrance: 60% N = 10 VNCs.
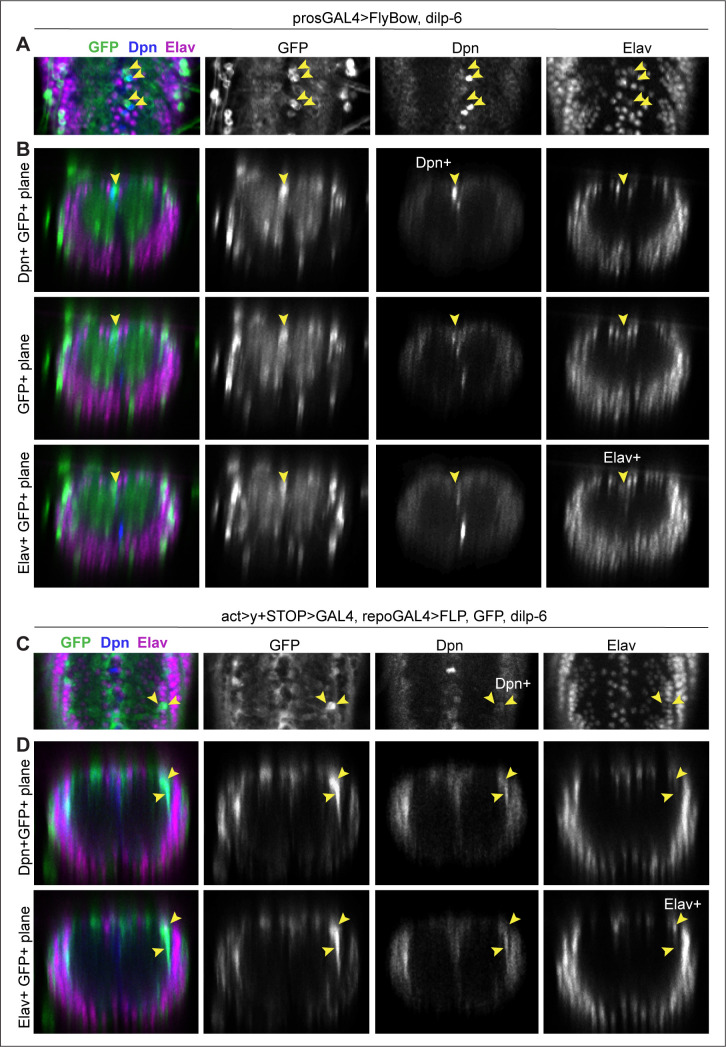
To ask whether the reprogrammed, proliferating Dpn+ cells might result in de novo neurogenesis, we first visualised cells using the pros-promoter, which drives expression in neural stem cells, GMCs, neurons, and glia. We reasoned that this promoter would be less likely to be silenced through a cell-state transition. FlyBow was used as a reporter to visualise pros expressing cells. Interestingly, this also revealed that the small Pros+ cells are generally neurons (Figure 10A). Overexpression of dilp-6 with pros-GAL4 (prosvoilaGAL4>UAS-FlyBow, UASdilp-6) resulted in groups of GFP+ cells (at 120 hr AEL) that comprised one GFP+ cell, one GFP+Dpn+ Elav− cell, and one GFP+Dpn− Elav+ cell (Figure 10A,B). These data were consistent with Dilp-6 reprogrammed glia becoming neurogenic.
Figure 10. Neurons were detected from glial-derived neural stem cells.
All samples were analysed at 120 hr AEL, after disappearance of abdominal developmental neuroblasts. (A and B) prosGAL4>FlyBow can reveal expression of neural stem cells, ganglion mother cells, neurons, and glia. Overexpression of dilp-6 with prosGAL4 resulted in clusters of 3 GFP+ cells along the midline that comprised one GFP+Dpn+ neural stem cell (top row in B), a GF+Dpn−Elav− progeny cell (middle row, B) and one GFP+Elav+ progeny neuron (bottom row, B). (C and D) Progeny cells of a glial cell-lineage were visualised with GFP, expressed originally under the control of the glial repo promoter, then switched using Flipase, to the permanent actin promoter activated only in glial cells (act>y+STOP>UAS-GFP/UAS-FLP; repoGAL4/UAS-Dilp-6). Overexpression of dilp-6 resulted in clusters of two to three GFP+ cells that comprised a GFP+Dpn+ neural stem cell and two progeny GFP+Elav+ neuronal progeny cells. (A and C) Horizontal and (B and D) transverse views. For full genotypes, sample sizes, and statistical details, see Supplementary file 1.
To further verify that neurons could be generated by Dilp-6 from glia, we used a lineage-tracing method. We overexpressed dilp-6 and flippase (FLP) in glia, to flip-out a stop codon placed between the actin promoter and GAL4, to swap the expression of the reporter GFP from being controlled by the glial repo promoter, to the constant actin promoter (actin>y+STOP>GAL4 UAS-GFP/UAS-FLP; repoGAL4/dilp-6). Thus, as reprogrammed glial cells switched off the glial repo promoter and switched on neural stem cell gene expression, they and their progeny cells would still be visible with GFP. Larvae were analysed at 120 hr AEL. In this genetic background, overexpression of dilp-6 resulted in lateral ectopic Dpn+ cells that were also GFP+ (Figure 10C,D, at 120 hr AEL). This showed that, like with Ia-2 and G-TRACE (Figure 8G,H), ectopic Dpn+ cells induced by Dilp-6 originated from glia. Furthermore, there were groups of two to three GFP+ cells, some of which were Elav+, meaning they were neurons (Figure 10C,D). Importantly, GFP+ Elav+ cells were found near ectopic Dpn+ cells (Figure 10C,D). These data meant that glial-derived Dpn+ cells could produce neurons. We did not find any larger clusters, suggesting that neurogenesis was limited. Pupariation occurs soon after 120 hr AEL, potentially limiting and altering further cellular events.
Discussion
A critical missing link to understand how to induce CNS regeneration in non-regenerating animals such as humans had been to identify factors that interact with NG2 to induce regenerative neurogenesis. NG2-glia are abundant progenitor cells present throughout life in the adult human brain and can respond to injury (Dimou and Götz, 2014; Torper et al., 2015; Valny et al., 2017). Thus, they are the ideal cell type to manipulate to promote regeneration. However, whether NG2-glia can give rise to neurons is highly debated, and potential mechanisms remained unknown (Dimou and Götz, 2014; Viganò and Dimou, 2016; Falk and Götz, 2017; Valny et al., 2017; Du et al., 2021). Here, using Drosophila in vivo functional genetic analysis we have identified neuronal Ia-2 as a genetic interactor of the NG2 homologue Kon, and show that it can induce a neurogenic response from glial cells via insulin signalling.
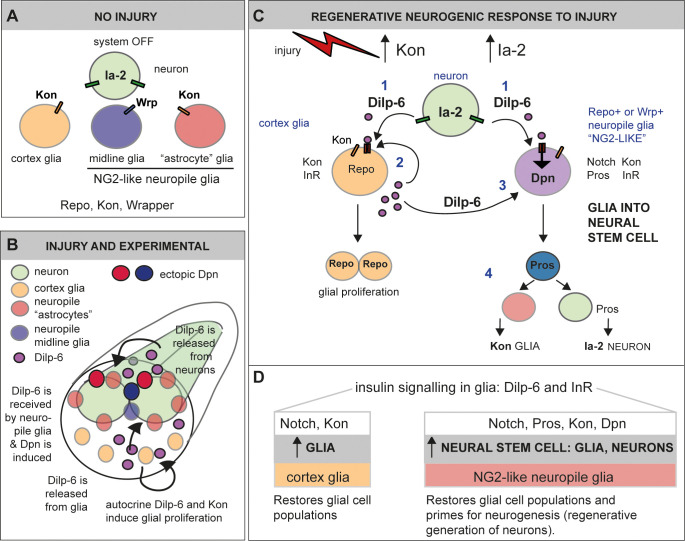
We provide evidence that Ia-2, Kon, and Dilp-6 induce a regenerative neurogenic response from glia (Figure 11). In the un-injured CNS, Kon and Ia-2 are restricted to glia and neurons, respectively (Figure 11A). Ia-2 is required for neuronal Dilp-6 secretion (Cai et al., 2001; Kim et al., 2008), Dilp-6 is produced by some neurons and mostly glia, and its production depends mostly on Kon regulated glia. Alterations in Ia-2 levels, increased Dilp-6, and concerted activation of Ras or PI3Kinase downstream of insulin signalling induced ectopic neural stem cells from glia. Both loss and gain of ia-2 function induced ectopic Dpn cells. Ia-2 depends on Pros and in turn negatively regulates Pros. Pros controls the switch from neural stem cell to progenitor state (Choksi et al., 2006). In this way, cell–cell interactions involving Ia-2 can influence neural progenitor cell fate. ia-2 loss of function would also cause a decrease in Dilp-6 secretion from neurons, but not from glia, as kon mRNA levels were unaffected, and dilp-6 expression depends mostly on glial kon. As neuronal Ia-2 and glial Kon mutually exclude each other, perhaps loss of ia-2 function might increase kon-dependent Dilp-6 production. As Ia-2 is required for Dilp-6 secretion (Cai et al., 2001; Harashima et al., 2005; Kim et al., 2008), ia-2 GOF would increase Dilp-6 release triggering the Dilp-6 amplification loop. Conceivably, either way Dilp-6 increased and this induced Dpn. Upon injury, levels of kon (Losada-Perez et al., 2016) and ia-2 expression increased (Figure 11C). Ia-2 drives secretion of Dilp-6 from neurons, Dilp-6 is received by glia, and a positive feedback amplification loop drives the further Kon and InR dependent production of Dilp-6 from cortex glia (Figure 11B, C). Dilp-6 can then both promote glial proliferation to generate more glia and induce the neural stem cell marker Dpn in neuropile glia – the subset known as ‘Drosophila astrocytes’ and midline glia (Figure 11B,C). Ectopic Dpn+ cells were induced from glia both upon injury and genetic manipulation of Ia-2, Dilp-6, Ras, and PI3Kinase. Importantly, these glial-derived neural stem cells could divide, as revealed by the S-phase marker PCNA-GFP and the mitotic marker pH3, and could generate neurons, albeit to a rather limited extent. Altogether, Dilp-6 is relayed from neurons to cortex and then to neuropile glia. This neuron-glia communication relay could enable concerted glio- and neuro-genesis, matching interacting cell populations for regeneration (Figure 11C,D). Interestingly, Dilp-6 is also involved in non-autonomous relays between distinct CNS cell populations to activate neural stem cells and induce neuronal differentiation in development (Sousa-Nunes et al., 2011; Fernandes et al., 2017).
Figure 11. Ia-2 and Dilp-6 drive a regenerative neurogenic response to central nervous system (CNS) injury.
(A) In the abdominal larval ventral nerve cord (VNC), neurons have Ia-2, glia have Kon, and Ia-2 and Kon are mutually exclusive; non-midline glia have the transcription factor Repo and midline glia the membrane protein Wrapper. In the normal, uninjured abdominal VNC, InR is in glial cells and some neurons; Ia-2 expression is constantly present in neurons; kon is switched off, and there are no neural stem cells (neuroblasts). (B) Diagram showing that Dilp-6 can be secreted from neurons, amplified and secreted by cortex glia, and received by all glial types. Dilp-6 production and secretion depend on Kon and Ia-2, which increase in injury. (C) Injury to the abdominal VNC provokes a dramatic surge in Ia-2 and Kon. This drives the initial secretion of Dilp-6 from neurons (1). Secreted Dilp-6 binds InR in glia, and InR signalling may facilitate cleavage and activation of Kon. KonICD activates glial proliferation (2). In an autocrine Kon and InR dependent manner, Dilp-6 sets off a positive feedback loop that amplifies Dilp-6 production from cortex glia (2). Once secreted, Dilp-6 and InR signalling cause the upregulation of Dpn+ in neuropile glia – including Notch+ Pros+ lateral (astrocytes) and Wrp+ midline glia (3). Neuropile glia can stochastically switch on Dpn. Glial-derived Dpn+ neural stem cells can divide and generate new neurons – although to a rather limited extent (4). After cell division, Kon may determine whether daughter cells become glia, to the exclusion of Ia-2. (D) Insulin signalling involving Ia-2, Dilp-6, and InR can increase cell number of various glial cell types – including cortex glia, neuropile astrocytes and midline glia – induce neural stem cells, and potentially generate new neurons. The neurogenic potential of glia may depend on the availability of Notch and Pros and the downregulation of Kon. Together, these genes can potentially induce neurogenesis and gliogenesis, matching cell populations for regeneration.
We have demonstrated that ectopic neural stem cells originate from glia. Regenerative neurogenesis could occur via direct conversion of glia into neurons, glial de-differentiation, or neuronal de-differentiation. Neuronal de-differentiation occurs both in mammals and in Drosophila (Froldi et al., 2015). However, in most animals, neural stem cells in the adult CNS and upon injury are generally distinct from developmental ones, and can originate from hemocytes, but most often, glial cells (Tanaka and Ferretti, 2009; Dimou and Götz, 2014; Falk and Götz, 2017; Simões and Rhiner, 2017; Du et al., 2021). In the mammalian brain, radial glia in the hippocampus respond to environmental challenge by dividing asymmetrically to produce neural progenitors that produce neurons (Shtaya et al., 2018); and astrocytes and NG2-glia can generate neurons, particularly in response to stroke, excitoxic injury, and genetic manipulations (Heinrich et al., 2014; Dimou and Gallo, 2015; Péron and Berninger, 2015; Du et al., 2021). Furthermore, genetic manipulation can lead to the direct conversion of NG2-glia into neurons (Torper et al., 2015; Pereira et al., 2017). Our findings that Dilp-6 and InR signalling can induce dpn expression are reminiscent of their functions in the induction of neural stem cells from quiescent progenitors in development (Chell and Brand, 2010; Sousa-Nunes et al., 2011; Gil-Ranedo et al., 2019). However, the Dpn+ cells induced upon injury and after development are distinct from the developmental neural stem cells normally induced by Dilp-6 in multiple ways. Firstly, in injuries carried out in third instar larvae, the induced neural stem cells were more numerous than normal neural stem cells. Secondly, in injuries carried out late in wandering larvae, Dpn+ cells were found after normal developmental neural stem cells have been eliminated through apoptosis (Bello et al., 2003). Thirdly, Dpn+ cells were found in dorsal ectopic locations not normally occupied by developmental neural stem cells. In all injury and genetic manipulation experiments involving overexpression of either ia-2, dilp-6, or PI3K, ectopic Dpn+ cells were located along the midline and surrounding the neuropile, in positions normally occupied by glia. Remarkably, concerted overexpression of ras and dilp-6 induced Dpn in potentially all glial cells and more, consistently with further Dpn+ cell proliferation. Consistent with our findings, ectopic neuroblasts were also observed upon co-expression of activated rasV12 and knock-down of PTEN in glia, within glioma models in Drosophila (Gangwani et al., 2020). We demonstrated that ectopic Dpn+ originated from glia, most particularly neuropile glia (midline glia and ‘Drosophila astrocytes’). Firstly, ectopic Dpn+ cells did not have Ia-2YFP, which is expressed in all neurons. Secondly, overexpression of ia-2 or dilp-6, alone or in combination with ras and PI3K, in glia dramatically increased Dpn levels, meaning that insulin signalling induces dpn expression in glia. Thirdly, ectopic Dpn+ cells surrounding the neuropile occupied positions of astrocytes and had the pan-glial marker Repo, and Repo− Dpn+ along the midline had the midline glia marker Wrp. Fourthly, the glial origin of the ectopic Dpn+ cells was demonstrated using two cell-lineage tracing methods (G-TRACE and glial activation of the actin promoter) whereby the expression initiated from the glia repo promoter was turned permanent despite cell state transitions. Consistently with our findings, TRAP-RNA analysis of the normal third instar larva revealed expression of dpn and multiple genes involved in neuroblast polarity, asymmetric cell division, neuroblast proliferation, and neurogenesis in glia (Huang et al., 2015). And single cell RNAseq analysis of the larval CNS revealed that in normal larvae some Repo+ glial cells can express dpn, or other neuroblast markers like wor and ase (Brunet Avalos et al., 2019). Our findings show that basal or potential expression of neuroblast genes in glia is switched on and amplified by insulin signalling. We conclude that Ia-2 and Dilp-6 could reprogramme glial cells in vivo into neural stem cells.
Our data showed that the ectopic ia-2 and dilp-6 induced neural stem cells could divide and generate neurons. In fact, concomitant overexpression of dilp-6 and PI3K, and most prominently dilp-6 and ras, dramatically increased Dpn+ cell number. Dilp-6 induced glial-derived Dpn+ cells could express the S-phase marker PCNA-GFP, and Ia-2 induced Wrp+ Dpn+ cells that were pH3+ in mitosis. We could not detect mitotic cells surrounding the neuropile, but mitosis is brief, and could have easily been missed. The Dilp-6 induced ectopic Dpn+ cells could generate neurons that could be traced with GFP expression from their glial origin. Thus, ectopic neural stem cells induced by Dilp-6 can divide and produce neuronal progeny cells. However, the clusters of GFP+ cells originating from the in vivo reprogrammed glial cells were rather small, indicating that although neurogenesis was possible in late larvae, it was extremely constrained. This could be due to the fact that in the third instar larva, time is rather limited by pupariation. Injury and genetic manipulation in late larvae may not allow sufficient time for cell lineages to progress, before pupariation starts. Pupariation and metamorphosis bring in a different cellular context, which could interfere with regenerative neuronal differentiation. Alternatively, Ia-2 and Dpn may not be sufficient to carry neurogenesis through either. For instance, gain of ia-2 function resulted only in Dpn+ but not Pros+ or Eve+ cells, suggesting that Ia-2 and Dpn are not sufficient for neuroblasts to progress to GMCs and neurons. In fact, ectopic Dpn+ cells still had Repo. Furthermore, we did not detect other ectopic neuroblast markers, such as Wor or Ase in glia. Nevertheless, RNA seq data revealed expression of neuroblast markers, including dpn, wor, and ase in some glia in normal larval CNS, meaning they could potentially be further regulated (Brunet Avalos et al., 2019; Huang et al., 2015). Still, to generate neurons, glia may not only require the expression of neural stem cell markers like dpn, but also perhaps receive other yet unknown signals (Figure 11B). In mammals, injury creates a distinct cellular environment that prompts glial cells to generate different cell types than in the un-injured CNS. For instance, elevated Sox-2 is sufficient to directly reprogramme NG2-glia into neurons, but only upon injury (Heinrich et al., 2014). Whereas during normal development NG2-glial cells may only produce oligodendrocyte lineage cells, upon injury they can also produce astrocytes and neurons (Dimou and Gallo, 2015; Huang et al., 2018). This suggests that there are injury-induced cues for neuronal differentiation. In the future, it will be compelling to find out what signals could enhance neurogenesis from glial cells reprogrammed in vivo by insulin signalling.
Our work has revealed a novel molecular mechanism driving a regenerative neurogenic response from glia, involving Kon/NG2 and insulin signalling. Ia-2 induces an initial secretion of Dilp-6 from neurons, Dilp-6 is received by glia, and a positive feedback loop amplifies the Kon-dependent production of Dilp-6 by cortex glia, Dilp-6 is then relayed to neuropile glia, resulting in the in vivo reprogramming of glial cells into neural stem cells (Figure 11C). This mechanism can induce both glial regeneration and neural stem cells from glia, potentially also neurons, matching interacting neuronal and glial cell populations. The incidence of neuropile glia conversion to Dpn+ cells was variable, meaning the process is stochastic. However, all glia converted when activated Ras or PI3K were combined with Dilp-6, meaning levels of insulin signalling matter. Such a mechanism may also operate in mammals. In fact, Ia-2 has universal functions in dense core vesicles to release insulin (Cai et al., 2001; Harashima et al., 2005; Kim et al., 2008; Nishimura et al., 2010; Cai et al., 2011). Insulin-like growth factor 1 (IGF-1) induces the production of astrocytes, oligodendrocytes, and neurons from progenitor cells in the adult brain, in response to exercise (Nieto-Estévez et al., 2016; Mir et al., 2017). The transcription factor Sox-2 that can switch astrocytes to neural stem cells and produce neurons is a downstream effector of InR/AKT signalling (Mir et al., 2017). NG2 also interacts with downstream components of the InR signalling pathway (e.g. PI3K-Akt-mTOR) to promote cell cycle progression and regulate the expression of its downstream effectors in a positive feedback loop (Sakry et al., 2015; Nayak et al., 2018). Together, all of these findings indicate that Ia-2, NG2/Kon, and insulin signalling have a common function across animals in reprogramming glial cells into becoming neural stem cells.
Intriguingly, dpn was mostly induced in neuropile associated glial cells and was only induced in other glial types with overexpression of active RasV12 together with Dilp-6. Thus, perhaps prominently neuropile glia have neurogenic potential. Of the neuropile glia, Drosophila ‘astrocytes’ and midline glia express Notch, pros, and kon, as well as InR. The cells frequently called ‘astrocytes’ share features with mammalian NG2-glia (Losada-Perez et al., 2016; Hidalgo and Logan, 2017; Kato et al., 2018). In mammals, the combination of Notch1, Prox1, and NG2 is unique to NG2-glia and is absent from astrocytes (Cahoy et al., 2008). Perhaps Ia-2 and Dilp-6 can only induce neural stem cells from NG2-like glia bearing this combination of factors. Notch activates glial proliferation and kon expression in Drosophila (Losada-Perez et al., 2016), and in the mammalian CNS, Notch promotes NG2-glia proliferation and maintains the progenitor state (Yamamoto et al., 2001; Ables et al., 2010; Piccin et al., 2013; Falk and Götz, 2017). In Drosophila, Notch and Pros also regulate dpn expression: Notch activates dpn expression promoting stemness, and Pros inhibits it, promoting transition to GMC and neuron (Vaessin et al., 1991; San-Juán and Baonza, 2011; Babaoğlan et al., 2013; Bi and Kuang, 2015). Thus, only glial cells with Notch and Pros may be poised to modulate stemness and neuronal differentiation. We showed that InR is expressed in neuropile glia, which was confirmed by publically available single cell RNAseq data (Brunet Avalos et al., 2019). Insulin signalling represses FoxO, which represses dpn, and thus ultimately activates dpn expression (Siegrist et al., 2010). As Notch and insulin signalling positively regulate dpn expression (Vaessin et al., 1991; Siegrist et al., 2010; San-Juán and Baonza, 2011; Babaoğlan et al., 2013; Bi and Kuang, 2015), and injury induces a Notch-dependent upregulation of Kon (Losada-Perez et al., 2016), which enables dilp-6 expression, and of Ia-2, which secretes Dilp-6, our data indicate that Notch-Kon/NG2-insulin synergy triggers the activation of dpn expression. Importantly, we found no evidence that Kon functions in neural stem cells. Thus, perhaps induced neural stem cells can generate only glia from daughter cells that inherit Kon, on which Repo and glial cell fate depend, or generate neurons, from daughter cells that lack Kon, but have Pros, on which Ia-2 depends (Figure 11C). Thus, upon injury, Notch, Pros, Kon/NG2, Ia-2, and insulin signalling function together to enable the regenerative production of both glial cells and neural stem cells from glia (Figure 11C,D). Intriguingly, developmental neural stem cells are thought to be eliminated through upregulation of Pros, induction of cell cycle exit, and terminal differentiation into glia (Maurange et al., 2008). Our findings imply that such termination may not be final.
To conclude, a neuron-glia communication relay involving Ia-2, Dilp-6, Kon, and InR is responsible for the induction of neural stem cells from glia, their proliferation, and limited neurogenesis. Neuronal Ia-2 and Dilp-6 trigger two distinct responses in glia: (1) in cortex glial cells, insulin signalling boosts Kon-dependent amplification of Dilp-6, glial proliferation, and glial regeneration. (2) In neuropile-associated NG2-like glial cells, insulin signalling unlocks a neurogenic response, inducing neural stem cell fate. As a result, these genes can drive the production of both glial cells and neurons after injury, enabling the matching of interacting cell populations, which is essential for regeneration.
Materials and methods
Fly stocks and genetics
Fly stocks used are listed in Key Resources Table. Stocks carrying combinations of overexpression and RNAi, or RNAi and mutants, etc., were generated by conventional genetics. Nts mutants were raised at 18°C to enable normal embryogenesis and switched to 25°C from larval hatching to the third instar larval wandering stage to cause N loss of function. For experiments in Figure 7B–D,F,G,I,J,L,M, eggs from crosses to tubGAL80ts repoGAL4 were kept at 18°C for 48 hr and moved to 30°C after L1 larval hatching. For all experiments, larvae bearing balancer chromosomes were identified by either using the fluorescent balancers CyO Dfd-YFP and TM6B Dfd-YFP or using the fused balancer SM6a- TM6B Tb−, which balances both the second and third chromosomes, and discarded. For the genetic screens, larvae with fluorescent VNCs (i.e. repoGAL4>UAS-FlyBow or elavGAL4>UAS-FlyBow) were selected.
Crush injury in the larval VNC
Crush injury in the larval CNS was carried out as previously reported (Losada-Perez et al., 2016), and only lesions in the abdominal VNC were analysed. Larval collections were staged by putting the G0 flies in an egg laying chamber for 2 hr, then collecting the F1 larve some time later, as indicated next. Larvae were placed on a chilled petri-dish with agar over ice. Crush injury was carried out by pinching with fine forceps the GFP-bearing VNCs under UV light using a fluorescence dissecting microscope: (1) at 74–76 hr AEL; VNCs were then left to carry on developing at 25°C and were dissected either 5–7 hr or 24 hr post-injury (PI); (2) at 96 hr, kept at 25°C and dissected and fixed 6 hr PI; (3) at 105 hr AEL, kept at 25°C and dissected 24 hr PI; (4) at 117 hr AEL, kept at 25°C, and dissected 12 hr PI. Dissected and fixed VNC were then processed for antibody stainings following standard procedures.
Molecular cloning
The UAS-konICD-HA construct was generated from EST LD31354 via PCR amplification with Kappa HiFi PCR kit (Peqlab) and subsequent cloning using the Gateway cloning system (Invitrogen) according to manufacturer's instructions. Primers used were konICD fwd comprising the CACC-sequence at the 5’-end (CCACAGGAAACTGAGAAAGCACAAGGC) for direct cloning of the PCR product (482 bp) into the entry vector pENTR/D-Topo, and konICD rev (AAACCTTACACCCAATACTGATTCC) including the endogenous stop-codon, underlined. Destination vector was pTHW for tagging the ICD on the N-terminus with HA, including a 5xUAS cassette and P-element ends for transformation. These destination vectors were developed by the Murphy-Lab at Carnegie Institution of Science, Baltimore, MD, USA, and can be obtained from the Drosophila Genomics Resource Center at Indiana University, USA. Transformant fly strains were generated by BestGene Inc, Chino Hills, CA, USA following a standard tranposase-mediated germline transformation protocol.
A UAS-ia-2 construct was generated using Gateway cloning (Invitrogen, as above). Ia-2 cDNA was generated by reverse-transcription PCR of purified mRNA from Oregon R flies and cloned into pDONR. Subsequently, a standard PCR amplification was performed using Phusion High-Fidelity (Fisher Scientific), primers Ia-2F (5’-ATGGCACGCAATGTACAACAACGGC) and ia-2-stopR (5’-CTTCTTCGCCTGCTTCGCCGATTTG), and the resulting PCR product (3918 bp) was cloned into pGEM-T Easy Vector (Promega). Subsequently, a Phusion High Fidelity PCR amplification was carried out using Gateway primers Ia-2attB F1 (5’-ggggacaagtttgtacaaaaaagcaggcttcATGGCACGCAATGTACAACAACGGC) and Ia-2attB R1 (5’-ggggaccactttgtacaagaaagctgggtcCTTCTTCGCCTGCTTCGCCGATTTG), and plasmid pGEM-ia-2 as template. Using Gateway cloning, the PCR product (3979 bp) was cloned first into pDONR221 and subsequently into the pUAS-gw-attB destination vector, for ϕC31 transgenesis. The construct was injected by BestGene Inc to generate transgenic flies bearing UAS-ia-2 at the attP2 landing site.
Quantitative real time reverse transcription PCR (qRT-PCR)
qRT-PCR was preformed according to standard methods and as previously described (Losada-Perez et al., 2016), with the following alteration. For each sample, 10 third instar larvae were used per genotype per replicate. At least three independent biological replicates were performed for all experiments other than in Figure 1—figure supplement 3A and B where two replicates were carried out on all candidates and those of interest where taken forward to carry out two further replicates. For a list of the primers used in this study please see Key Resources Table below.
Immunostainings
Immunostainings were carried out following standard procedures, with the following modifications. For anti-Dpn, we used: (1) 20 min dissection in PBS into 4% formaldehyde on ice followed by 45 min fixation at room temperature (anti-Dpn from J. Skeath); (2) 5 min dissection in PBS into 4% formaldehyde on ice, followed by 10 min fixation at room temperature (anti-Dpn from H. Wang) (Huang and Wang, 2018; Zhang et al., 2019). The following primary antibodies were used: mouse anti-Repo (1:100, DSHB); guinea pig anti-Repo (1:1000, Ben Altenhein); rat anti-Elav (1:250, DSHB); mouse anti-FasII ID4 (1:500, DSHB); mouse anti-Prospero (1:250, DSHB); guinea pig anti-Dpn (1:1000, gift of J. Skeath); guinead pig anti-Dpn (1: 1000 gift of H. Wang); mouse anti-Eve 3C10 (1:20, DSHB); rabbit anti-phospho-histone-H3 (1:250); rabbit anti-HA (1:1600, Cell Signalling Technology); rabbit anti-GFP at 1:250 (Molecular Probes); mouse anti-Wrapper (1:5, gift of G. Tear). Secondary antibodies were Alexa conjugated: Donkey anti-rabbit 488 (1:250, Molecular Probes), goat anti-rabbit 488 (1:250, Molecular Probes), goat anti-rabbit 647 (1:250, Molecular Probes), goat anti-mouse 488 (1:250, Molecular Probes), goat anti-mouse 546 (1:250, Molecular Probes), goat anti-mouse 647 (1:250, Molecular Probes), goat anti-rat 546 (1:250, Molecular Probes), goat anti-guinea pig 488 (1:250, Molecular Probes), goat anti-guinea pig 633 (1:250, Molecular Probes), and goat anti-rat 647 and 660 (1:250, Molecular Probes).
Microscopy and imaging
Image data were acquired using a Zeiss LSM710 laser scanning confocal microscope, with 25× lens and 1.00 zoom, an Olympus Fluoview FV1000, 20× lens, and a Leica SP8 laser scanning confocal microscope, with a 20× lens, 1.25 zoom. All images were taken with resolution 512 × 512 or 1024 × 1024, step 0.96 μm and 1–3× averaging for all samples except for cell counting with DeadEasy that have no averaging.
Images were analysed using ImageJ. Images of horizontal sections are projections from the stacks of confocal images that span the thickness of the entire VNC, using ImageJ. Transverse views were generated using the Reslice option. Images were processed using Adobe Creative Suite 6 Photoshop and compiled with Adobe Illustrator.
Automatic cell counting
Glial cells labelled either with anti-Repo or with repoGAL4>UAShistone-YFP were counted automatically in 3D across the thickness of the VNC using DeadEasy Larval Glia software, as previously described. Prospero+ and Dpn+ cells were counted manually in 3D (i.e. not in projections), as the signal was noisy for DeadEasy.
Statistical analysis
Statistical analysis was carried out using Graphpad Prism. All data in this work are continuous, except for the PCNAGFP data in Figure 9H which are categorical. The latter were analysed with a non-parametric Fisher’s exact test. For all other data, tests to determine whether data were distributed normally and variances were equal were initially carried out, and thereafter if so, parametric one-way ANOVA tests were carried out when comparing more than two sample types group. Multiple comparison corrections were carried out with post hoc Dunnett tests comparisons to set controls, or Bonferroni comparisons of all samples against all. Box plots were used to represent the distribution of continuous data, where the line within the box represents the median of the data distribution, the box comprises the 25 percentiles above and below the median, and the whiskers the lowest and highest 25 percentiles.
Acknowledgements
We thank our labs and C Rezaval for discussions and comments on the manuscript; S Corneliussen, T Schunke, and S Dietz for technical help; Y Fan, A Gould, Y Jan, J Skeath, F Schnorrer, and H Wang for reagents; A Di Maio and Birmingham Advanced Light Microscopy for assistance; Bloomington Drosophila Stock Centre for fruit-flies and Developmental Studies Hybridoma Bank, Iowa for antibodies.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Alicia Hidalgo, Email: a.hidalgo@bham.ac.uk.
Hugo J Bellen, Baylor College of Medicine, United States.
Utpal Banerjee, University of California, Los Angeles, United States.
Funding Information
This paper was supported by the following grants:
Biotechnology and Biological Sciences Research Council BB/L008343/1 to Neale J Harrison, Marta Moreira, Alicia Hidalgo.
Biotechnology and Biological Sciences Research Council BB/R00871X/1 to Marta Moreira, Alicia Hidalgo.
Biotechnology and Biological Sciences Research Council MIBTP Studentship to Elizabeth Connolly.
MSCA TOLKEDA to Jun Sun, Alicia Hidalgo.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Supervision, Validation, Investigation, Visualization, Methodology, Writing - review and editing.
Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - review and editing.
Data curation, Formal analysis, Validation, Investigation, Visualization, Writing - review and editing.
Formal analysis, Validation, Investigation, Visualization, Writing - review and editing.
Conceptualization, Data curation, Formal analysis, Supervision, Validation, Investigation, Visualization, Writing - review and editing.
Resources, Methodology, Writing - review and editing.
Resources, Data curation, Methodology, Writing - review and editing.
Formal analysis.
Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Additional files
This table contains full genotypes for all experiments, sample sizes used, and statistical analysis details including normality tests, tests applied, and multiple comparison correction tests.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
The following previously published datasets were used:
Avalos CB, Maier GL, Bruggmann R, Sprecher SG. 2019. Single cell transcriptome atlas of the Drosophila larval brain. NCBI Gene Expression Omnibus. GSE134722
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. Journal of Neuroscience. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaoğlan AB, Housden BE, Furriols M, Bray SJ. Deadpan contributes to the robustness of the notch response. PLOS ONE. 2013;8:e75632. doi: 10.1371/journal.pone.0075632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Boone JQ, Drummond ML, Doe CQ. Drosophila type II neuroblast lineages keep Prospero levels low to generate large clones that contribute to the adult brain central complex. Neural Development. 2010;5:26. doi: 10.1186/1749-8104-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello BC, Hirth F, Gould AP. A pulse of the Drosophila hox protein Abdominal-A schedules the end of neural proliferation via neuroblast apoptosis. Neuron. 2003;37:209–219. doi: 10.1016/S0896-6273(02)01181-9. [DOI] [PubMed] [Google Scholar]
- Bi P, Kuang S. Notch signaling as a novel regulator of metabolism. Trends in Endocrinology & Metabolism. 2015;26:248–255. doi: 10.1016/j.tem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binamé F, Sakry D, Dimou L, Jolivel V, Trotter J. NG2 regulates directional migration of oligodendrocyte precursor cells via rho GTPases and polarity complex proteins. Journal of Neuroscience. 2013;33:10858–10874. doi: 10.1523/JNEUROSCI.5010-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Developmental Neurobiology. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet Avalos C, Maier GL, Bruggmann R, Sprecher SG. Single cell transcriptome atlas of the Drosophila larval brain. eLife. 2019;8:e50354. doi: 10.7554/eLife.50354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. Journal of Neuroscience. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Krause MW, Odenwald WF, Toyama R, Notkins AL. The IA-2 gene family: homologs in Caenorhabditis elegans, Drosophila and zebrafish. Diabetologia. 2001;44:81–88. doi: 10.1007/s001250051583. [DOI] [PubMed] [Google Scholar]
- Cai T, Hirai H, Fukushige T, Yu P, Zhang G, Notkins AL, Krause M. Loss of the transcriptional repressor PAG-3/Gfi-1 results in enhanced neurosecretion that is dependent on the dense-core vesicle membrane protein IDA-1/IA-2. PLOS Genet. 2009;5:e1000447. doi: 10.1371/journal.pgen.1000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Hirai H, Zhang G, Zhang M, Takahashi N, Kasai H, Satin LS, Leapman RD, Notkins AL. Deletion of Ia-2 and/or Ia-2β in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia. 2011;54:2347–2357. doi: 10.1007/s00125-011-2221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona GN, Nishimura T, Schindler CW, Panlilio LV, Notkins AL. The dense core vesicle protein IA-2, but not IA-2β, is required for active avoidance learning. Neuroscience. 2014;269:35–42. doi: 10.1016/j.neuroscience.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell JM, Brand AH. Nutrition-responsive Glia control exit of neural stem cells from quiescence. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Developmental Cell. 2006;11:775–789. doi: 10.1016/j.devcel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Götz M. Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiological Reviews. 2014;94:709–737. doi: 10.1152/physrev.00036.2013. [DOI] [PubMed] [Google Scholar]
- Du X, Zhang Z, Zhou H, Zhou J. Differential modulators of NG2-Glia differentiation into neurons and Glia and their crosstalk. Cellular and Molecular Neurobiology. 2021;41:1–15. doi: 10.1007/s10571-020-00843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B, Gisselbrecht SS, Michelson AM. The transmembrane protein perdido interacts with grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development. 2007;134:4469–4478. doi: 10.1242/dev.014027. [DOI] [PubMed] [Google Scholar]
- Falk S, Götz M. Glial control of neurogenesis. Current Opinion in Neurobiology. 2017;47:188–195. doi: 10.1016/j.conb.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Fernandes VM, Chen Z, Rossi AM, Zipfel J, Desplan C. Glia relay differentiation cues to coordinate neuronal development in Drosophila. Science. 2017;357:886–891. doi: 10.1126/science.aan3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. The EMBO Journal. 1995;14:3373–3384. doi: 10.1002/j.1460-2075.1995.tb07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero MG, Kato K, Hidalgo A. Automatic cell counting in vivo in the larval nervous system of Drosophila. Journal of Microscopy. 2012;246:202–212. doi: 10.1111/j.1365-2818.2012.03608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froldi F, Szuperak M, Weng CF, Shi W, Papenfuss AT, Cheng LY. The transcription factor Nerfin-1 prevents reversion of neurons into neural stem cells. Genes & Development. 2015;29:129–143. doi: 10.1101/gad.250282.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Adult neurogenesis in mammals. Science. 2019;364:827–828. doi: 10.1126/science.aav6885. [DOI] [PubMed] [Google Scholar]
- Gangwani K, Snigdha K, Kango-Singh M. Tep1 regulates yki activity in neural stem cells in Drosophila glioma model. Frontiers in Cell and Developmental Biology. 2020;8:306. doi: 10.3389/fcell.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Ranedo J, Gonzaga E, Jaworek KJ, Berger C, Bossing T, Barros CS. STRIPAK members orchestrate hippo and insulin receptor signaling to promote neural stem cell reactivation. Cell Reports. 2019;27:2921–2933. doi: 10.1016/j.celrep.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RL, Hidalgo A. Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. The EMBO Journal. 2004;23:2440–2450. doi: 10.1038/sj.emboj.7600258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S, Clark A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. PNAS. 2005;102:8704–8709. doi: 10.1073/pnas.0408887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C, Bergami M, Gascón S, Lepier A, Viganò F, Dimou L, Sutor B, Berninger B, Götz M. Sox2-Mediated conversion of NG2 Glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J-C, Nenquin M, Szollosi A, Kubosaki A, Louis Notkins A. Insulin secretion in islets from mice with a double knockout for the dense core vesicle proteins islet antigen-2 (IA-2) and IA-2β. Journal of Endocrinology. 2008;196:573–581. doi: 10.1677/JOE-07-0496. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Logan A. Go and stop signals for glial regeneration. Current Opinion in Neurobiology. 2017;47:182–187. doi: 10.1016/j.conb.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YF, Zhang HL, Cai T, Harashima S, Notkins AL. "The IA-2 interactome.". Diabetologia. 2005;48:2576–2581. doi: 10.1007/s00125-005-0037-y. [DOI] [PubMed] [Google Scholar]
- Huang Y, Ng FS, Jackson FR. Comparison of Larval and Adult Drosophila Astrocytes Reveals Stage-Specific Gene Expression Profiles. G3: Genes, Genomes, Genetics. 2015;5:551–558. doi: 10.1534/g3.114.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Bai X, Stopper L, Catalin B, Cartarozzi LP, Scheller A, Kirchhoff F. During development NG2 glial cells of the spinal cord are restricted to the oligodendrocyte lineage, but generate astrocytes upon acute injury. Neuroscience. 2018;385:154–165. doi: 10.1016/j.neuroscience.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Huang J, Wang H. Hsp83/Hsp90 physically associates with insulin receptor to promote neural stem cell reactivation. Stem Cell Reports. 2018;11:883–896. doi: 10.1016/j.stemcr.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Forero MG, Fenton JC, Hidalgo A. The glial regenerative response to central nervous system injury is enabled by pros-notch and pros-NFκB feedback. PLOS Biology. 2011;9:e1001133. doi: 10.1371/journal.pbio.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Konno D, Berry M, Matsuzaki F, Logan A, Hidalgo A. Prox1 inhibits proliferation and is required for differentiation of the oligodendrocyte cell lineage in the mouse. PLOS ONE. 2015;10:e0145334. doi: 10.1371/journal.pone.0145334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Losada-Perez M, Hidalgo A. Gene network underlying the glial regenerative response to central nervous system injury. Developmental Dynamics. 2018;247:85–93. doi: 10.1002/dvdy.24565. [DOI] [PubMed] [Google Scholar]
- Kim J, Bang H, Ko S, Jung I, Hong H, Kim-Ha J. Drosophila ia2 modulates secretion of insulin-like peptide. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2008;151:180–184. doi: 10.1016/j.cbpa.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB. Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. Journal of Neuroinflammation. 2011;8:158. doi: 10.1186/1742-2094-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharova K, Stallcup WB. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience. 2010;166:185–194. doi: 10.1016/j.neuroscience.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada-Perez M, Harrison N, Hidalgo A. Molecular mechanism of central nervous system repair by the Drosophila NG2 homologue kon-tiki. Journal of Cell Biology. 2016;214:587–601. doi: 10.1083/jcb.201603054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe N, Rees JS, Roote J, Ryder E, Armean IM, Johnson G, Drummond E, Spriggs H, Drummond J, Magbanua JP, Naylor H, Sanson B, Bastock R, Huelsmann S, Trovisco V, Landgraf M, Knowles-Barley S, Armstrong JD, White-Cooper H, Hansen C, Phillips RG, Lilley KS, Russell S, St Johnston D, UK Drosophila Protein Trap Screening Consortium Analysis of the expression patterns, subcellular localisations and interaction partners of Drosophila proteins using a pigP protein trap library. Development. 2014;141:3994–4005. doi: 10.1242/dev.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye CM, Naylor HW, Sanson B. Subcellular localisations of the CPTI collection of YFP-tagged proteins in Drosophila embryos. Development. 2014;141:4006–4017. doi: 10.1242/dev.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Mir S, Cai W, Carlson SW, Saatman KE, Andres DA. IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Scientific Reports. 2017;7:3283. doi: 10.1038/s41598-017-03641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Kulas DT, Bleyle LA, Novak JS. The protein tyrosine phosphatase LAR has a major impact on insulin receptor dephosphorylation. Biochemical and Biophysical Research Communications. 1997;235:709–712. doi: 10.1006/bbrc.1997.6889. [DOI] [PubMed] [Google Scholar]
- Nayak T, Trotter J, Sakry D. The intracellular cleavage product of the NG2 proteoglycan modulates translation and Cell-Cycle kinetics via effects on mTORC1/FMRP signaling. Frontiers in Cellular Neuroscience. 2018;12:231. doi: 10.3389/fncel.2018.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Estévez V, Defterali Ç, Vicario-Abejón C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Frontiers in Neuroscience. 2016;10:52. doi: 10.3389/fnins.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Harashima S, Yafang H, Notkins AL. IA-2 modulates dopamine secretion in PC12 cells. Molecular and Cellular Endocrinology. 2010;315:81–86. doi: 10.1016/j.mce.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Birtele M, Shrigley S, Benitez JA, Hedlund E, Parmar M, Ottosson DR. Direct reprogramming of resident NG2 Glia into neurons with properties of Fast-Spiking Parvalbumin-Containing interneurons. Stem Cell Reports. 2017;9:742–751. doi: 10.1016/j.stemcr.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Moreno JJ, Espina-Zambrano AG, García-Calderón CB, Estrada B. Kon-tiki enhances PS2 integrin adhesion and localizes its ligand, thrombospondin, in the myotendinous junction. Journal of Cell Science. 2017;130:950–962. doi: 10.1242/jcs.197459. [DOI] [PubMed] [Google Scholar]
- Péron S, Berninger B. Reawakening the sleeping beauty in the adult brain: neurogenesis from parenchymal Glia. Current Opinion in Genetics & Development. 2015;34:46–53. doi: 10.1016/j.gde.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Piccin D, Yu F, Morshead CM. Notch signaling imparts and preserves neural stem characteristics in the adult brain. Stem Cells and Development. 2013;22:1541–1550. doi: 10.1089/scd.2012.0390. [DOI] [PubMed] [Google Scholar]
- Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Binamé F, Perera SS, Endres K, Lutz B, Radyushkin K, Trotter J, Mittmann T. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLOS Biology. 2014;12:e1001993. doi: 10.1371/journal.pbio.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Yigit H, Dimou L, Trotter J. Oligodendrocyte precursor cells synthesize neuromodulatory factors. PLOS ONE. 2015;10:e0127222. doi: 10.1371/journal.pone.0127222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Trotter J. The role of the NG2 proteoglycan in OPC and CNS network function. Brain Research. 2016;1638:161–166. doi: 10.1016/j.brainres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- San-Juán BP, Baonza A. The bHLH factor deadpan is a direct target of notch signaling and regulates neuroblast self-renewal in Drosophila. Developmental Biology. 2011;352:70–82. doi: 10.1016/j.ydbio.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Kalchhauser I, Dickson BJ. The transmembrane protein Kon-tiki couples to dgrip to mediate myotube targeting in Drosophila. Developmental Cell. 2007;12:751–766. doi: 10.1016/j.devcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Shtaya A, Sadek AR, Zaben M, Seifert G, Pringle A, Steinhäuser C, Gray WP. AMPA receptors and seizures mediate hippocampal radial glia-like stem cell proliferation. Glia. 2018;66:2397–2413. doi: 10.1002/glia.23479. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Haque NS, Chen CH, Hay BA, Hariharan IK. Inactivation of both foxo and reaper promotes long-term adult neurogenesis in Drosophila. Current Biology. 2010;20:643–648. doi: 10.1016/j.cub.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões AR, Rhiner C. A Cold-Blooded view on adult neurogenesis. Frontiers in Neuroscience. 2017;11:327. doi: 10.3389/fnins.2017.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Wu L, Chen Z, Kohanski RA, Pick L. Axons guided by insulin receptor in Drosophila visual system. Science. 2003;300:502–505. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the akt pathway involving pten and microRNA bantam. Genes & Development. 2012;26:1612–1625. doi: 10.1101/gad.193243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471:508–512. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Matthews EA, Nicolas V, Schoch S, Dietrich D. NG2 glial cells integrate synaptic input in global and dendritic calcium signals. eLife. 2016;5:e16262. doi: 10.7554/eLife.16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nature Reviews Neuroscience. 2009;10:713–723. doi: 10.1038/nrn2707. [DOI] [PubMed] [Google Scholar]
- Torper O, Ottosson DR, Pereira M, Lau S, Cardoso T, Grealish S, Parmar M. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell Reports. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessin H, Grell E, Wolff E, Bier E, Jan LY, Jan YN. Prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell. 1991;67:941–953. doi: 10.1016/0092-8674(91)90367-8. [DOI] [PubMed] [Google Scholar]
- Valny M, Honsa P, Kriska J, Anderova M. Multipotency and therapeutic potential of NG2 cells. Biochemical Pharmacology. 2017;141:42–55. doi: 10.1016/j.bcp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Progress in Neurobiology. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Viganò F, Dimou L. The heterogeneous nature of NG2-glia. Brain Research. 2016;1638:129–137. doi: 10.1016/j.brainres.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase abl and its substrate enabled collaborate with the receptor phosphatase dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/S0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Nagao M, Sugimori M, Kosako H, Nakatomi H, Yamamoto N, Takebayashi H, Nabeshima Y, Kitamura T, Weinmaster G, Nakamura K, Nakafuku M. Transcription factor expression and Notch-dependent regulation of neural progenitors in the adult rat spinal cord. The Journal of Neuroscience. 2001;21:9814–9823. doi: 10.1523/JNEUROSCI.21-24-09814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Koe CT, Tan YS, Ho J, Tan P, Yu F, Sung WK, Wang H. The integrator complex prevents dedifferentiation of intermediate neural progenitors back into neural stem cells. Cell Reports. 2019;27:987–996. doi: 10.1016/j.celrep.2019.03.089. [DOI] [PubMed] [Google Scholar]