Abstract
Background
Currently, diabetic peripheral neuropathy (DPN) is one of the most severe complications of diabetes mellitus (DM). Despite the seriousness of this problem, limited evidence is available on the prevalence of diabetic peripheral neuropathy among patients with diabetes mellitus in Ethiopia. In Ethiopia, there were no updated studies that estimate the national prevalence of DPN. Hence, this systematic review and meta-analysis provided a national prevalence of diabetic peripheral neuropathy among patients with diabetes mellitus in Ethiopia.
Methods
This study was submitted for registration with the International Prospective Register of Systematic Reviews (PROSPERO) in March 2020 and accepted with the registration number CRD42020173831. Different database searching engines were searched online to retrieve related articles, including PubMed, Scopus, Google Scholar, African Journals Online, World Health Organization (WHO) Afro Library, and Cochrane Review. The reviewers used the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline in the reviewing process. In this systematic review and meta-analysis, all published and unpublished articles were analyzed. The reviewers used the random effects model to estimate the pooled prevalence of diabetic peripheral neuropathy among diabetes mellitus patients. The reviewers conducted the statistical analysis using the R version 3.5.3 and RStudio version 1.2.5033 software for Windows. The reviewers evaluated the heterogeneity across the included studies by the inconsistency index (I2). The reviewers examined the publication bias by the funnel plot.
Results
The search of the databases produced 245 papers. After checking the inclusion and exclusion criteria, 38 articles with 14029 total patients with diabetes mellitus were found suitable for the review. Except for three (retrospective cohort study), all studies were cross-sectional. The overall pooled prevalence of diabetic peripheral neuropathy was 22% (95% CI 18% to 26%). The subgroup analysis of diabetic peripheral neuropathy among patients with diabetes in the different regions was 23% (95% CI 17% to 29%) in Addis Ababa, 27% (95% CI 16% to 38%) in Oromia, 16% (95% CI 14% to 18%) in South nation and nationalities, and 15% (95% CI 6% to 24%) in Amhara.
Conclusions
More than one-fifth of patients with diabetes have diabetic peripheral neuropathy. According to this study, the prevalence of diabetic peripheral neuropathy in Ethiopia is considerably high. This evidence suggests that attention should be given to patients with diabetes in monitoring patients' blood glucose.
1. Introduction
Currently, half a billion people are living with diabetes worldwide and will increase by 578 million (25%) in 2030 and 700 million (51%) in 2045 [1].
Diabetes mellitus (DM) is a metabolic disorder that can predispose to diseases of the heart, blood vessels, lungs, kidneys, and nerves. Such damage may result in a decreased blood flow to the feet, which is coupled with neuropathy to the nerves—raising the risk of foot ulcers, inflammation, and subsequent limb amputation [2].
One of the most severe complications of DM is diabetic polyneuropathy (DPN). The lifetime prevalence of DPN was 50% [3–5]. DPN is associated with a significant reduction in quality of life and poses treatment challenges to the practicing physician [4]. It is also the cause of impairment due to foot ulceration and amputation, disruption of the gait, and damage involved with dropping. About 20 to 30 percent of DPN patients suffer from neuropathic pain [4–6].
The average estimated expense for diabetes medication, health insurance of DPN, and debilitating DPN is $6632, $12492, and $30755, respectively [7].
In Ethiopia, the prevalence of DPN is estimated to be in the range of 1.9–53.6% that shows a difference across different geographical settings and time [8, 9]. Many factors contribute to this huge difference, including study quality, study design, ethnic differences, and different diagnostic methods. Reliable estimates of DPN prevalence are required to enhance awareness of the prevention and management of DPN in patients with diabetes. Therefore, the primary purpose of this systematic review and meta-analysis is to determine the prevalence of DPN among patients with diabetes mellitus in Ethiopia.
2. Materials and Methods
2.1. Setting
Ethiopia is an East African country containing ten regions, namely, Tigray; Afar; Amhara; Oromia; Somali; Benishangul-Gumuz; Southern Nations, Nationalities, and People's Region (SNNPR), Gambella; Sidama Harari; and two urban administrative states (Addis Ababa City administration and Dire Dawa City administration).
2.2. Search Strategy and Information Sources
A search strategy was implemented using electronic databases (PubMed/MEDLINE, Embase, Google Scholar, Web of Science, Cochran Library, Africa-Wide Information, World Health Organization (WHO) Afro Library, and African Index Medicus) from inception to October 2020.
The reviewers have checked the presence of a precursor systematic review and protocol on the topic of interest via searching for different databases. The included databases were the Cochrane Database of Systematic Reviews, Joanna Briggs Institute Database of Systematic Review and Implementation Reports (JBI-DSRIR), the national health center review and dissemination database, health technology assessment (HTA), the Campbell Collaboration Library, and Evidence for Policy and Practice Information (EPPI-Centre).
The reviewers have developed a literature search technique using the headings of the medical subject headings (Met) and Boolean (AND/OR) operator. The reviewers have used the combination of key terms “DPN”, “Diabetic peripheral neuropathy”, “Diabetes mellitus”, “Diabetic complication”, “Macro and micro-vascular diabetic complication”, “diabetic polyneuropathy”, “Ethiopia”, “systematic review”, and protocols. The search from the above databases confirmed that there was no systematic review and/or protocol on the topic of interest.
2.3. Data Extraction, Selection, and Process
Based on the inclusion and exclusion criteria, the reviewers have developed a tool to guide the screening and selection process. This tool was piloted and revised before data extraction begins. The search results were first uploaded to the EndNote software to remove duplicates.
Two blind and independent reviewers extracted the data using a preconceived and standardized data collection format. The two reviewers (DBT and GGG) screened the titles, abstracts, and full-text search results to identify potentially eligible studies. Where necessary, the authors were contacted for additional information to confirm the eligibility of the studies. Disagreements between the reviewers were resolved by discussion with the help of a third independent reviewer. Where there is missing information, the reviewers contacted the corresponding author of the study to request the missing data. A maximum of three emails was sent to the corresponding author of the retrieved studies to request additional information before excluding the article. For studies appearing in more than one publication, we considered the most recent and comprehensive studies and those with the largest sample size. For surveys appearing in one article with multiple surveys conducted at different time points, we treated each as a separate study.
The components of the data extraction format included the information on the year of publication, region, authors, and country; setting, objective, and study design; diagnostic criteria of DPN; prevalence; or incidence of DPN.
2.4. Criteria for Considering Studies for the Review
2.4.1. Inclusion Criteria
The inclusion criteria are as follows:
Design: all observational studies.
Population: study participants of at least 18 years of age with type 1 or 2 DM.
Publication status: published and unpublished studies.
Settings: all health institution-based studies.
Language: articles in the English language.
Publication or report year: studies conducted from 1976 to 2020.
Method of diagnosis: all studies, regardless of the method used for diagnosing DPN.
Intervention(s)/exposure(s): patients taking any form of antidiabetic medication.
Outcome: the primary outcome is the prevalence of DPN among diabetes mellitus patients in Ethiopia. It is defined by international consensus guidelines as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after exclusion of other causes” [10, 11].
2.4.2. Exclusion Criteria
The exclusion criteria are as follows: case report and case series studies, studies that lack relevant data needed to compute the prevalence of DPN, studies on pregnancy-related diabetes mellitus, and studies on children and adolescents < 18 years.
2.5. Risk of Bias and Quality Assessment of the Included Studies
Methodological quality and risk of bias assessments were performed by two reviewers (DBT and GGG), blindly and independently. The reviewers maintained the blinding reviewing method using the Covidence software that allows/obligates each reviewer to work without knowing the other reviewer's choice. This helps to diminish errors and the risk of bias in the selection of the studies. Disagreements between the reviewers were resolved by discussion and where necessary involving a third author.
For each included study, we estimated the precision (C) or margin of error, considering the sample size (SS) and the observed prevalence (p) of DPN using the formula:
| (1) |
where z was the z value fixed at 1.96 across studies (corresponding to 95% confidence interval). The desirable margin of error is 5% (0.05) or lower.
The methodological quality was evaluated using the Newcastle-Ottawa Scale. The scale is primarily formulated by a star allocation system, assigning a maximum of 10 stars for the risk of bias in three areas: a selection of study groups (4 or 5 stars), comparability of groups (2 stars), and ascertainment of the outcome of interest or the exposure (3 stars). No validation study provides a cutoff score for rating low-quality studies. We arbitrarily established 0–3, 4–6, and 7–10 stars considered at high, moderate, and low risk of bias, respectively [12].
2.6. Data Analysis and Presentation of Results
The reviewers have registered this review in PROSPERO with the registration number (CRD42020173831), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines have been used to report the results [13]. The data were analyzed using the R version 3.5.3 and RStudio version 1.2.5003 software. The pooled prevalence of diabetic peripheral neuropathy was estimated using forest plots and the extent of statistical heterogeneity between studies. Statistical heterogeneity was assessed using the standard chi-squared test (Cochran Q test) and quantified by calculating the I2 statistics (with values of 25%, 50%, and 75% assumed to be representative of low, medium, and high heterogeneity, respectively) [14]. There was clinical heterogeneity between the included studies. Consequently, we used a random effects meta-analysis to estimate the overall pooled prevalence of DPN in Ethiopia. The reviewers used a funnel plot to evaluate publication bias and the 95% confidence interval (CI) to describe the results.
3. Results
3.1. Screening Flow
Figure 1 is a flow diagram outlining the process of identification and selection of the included studies. The included databases and number of included studies thereof were PubMed (63), Scopus (46), Google Scholar (107), and the World Health Organization (WHO) Afro Library (29). Of these studies, 101 are duplicates and removed. Subsequently, we screened 144 titles and abstracts and excluded 66 irrelevant papers. Then, based on the predefined criteria and quality assessment, 38 full-text articles with 14029 total patients with diabetes were included in this systematic review and meta-analysis. The detailed steps of the screening process are shown in a PRISMA flow chart (Figure 1).
Figure 1.
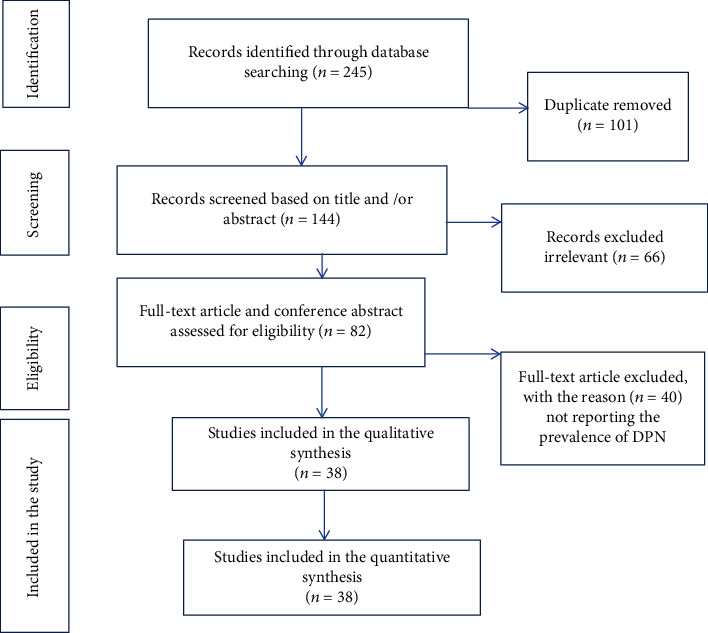
Flow chart diagram showing the selection of articles for systemic review and meta-analysis of DPN among patients with diabetes in Ethiopia, 2020.
3.2. Study Characteristics
More than one-third (16, 42.1%) of the studies were from studies conducted in the capital city of Ethiopia (Addis Ababa), 9 (28.9%) in Oromia, 7 (18.2%) in Amhara, 2 (5.26%) in South Nation and Nationalities, and 1 (2.6%) in Tigray. Except for three studies (cohort), all the rest were cross-sectional. Of these studies, six studies were among patients with type 2 diabetes mellitus, and the rest were conducted on type 1 and type 2 diabetes mellitus. The quality of each primary study assessed using the Newcastle-Ottawa Scale shows no considerable risk. Therefore, all the included studies considered in this systematic review and meta-analysis were with a low risk of bias (Table 1).
Table 1.
Characteristics of studies included in the systematic review and meta-analysis of the prevalence of DPN among adult patients with diabetes in Ethiopia, 2020.
| Author | Publication year | Data collection year | Study region | Types of hospital | Types of DM | SD | Sample size | Number of patients with DPN by type of DM | Prevalence (%) | Methods for detecting DPN | Quality assessment (based on NOS) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Total | Type I | Type II | Total | ||||||||||
| Worku et al. [15] | 2010 | October 2008 | Oromia | Referral | Types I and II | CS | 116 | 189 | 305 | 19 | 71 | 90 | 29.5 | MNSI assessment | 8 |
| Gudina et al. [16] | 2011 | From August to November 2009 | Oromia | Referral | Types I and II | CS | 117 | 212 | 329 | NM | NM | 27 | 8.2 | DNS instrument | 7 |
| Ejigu [17] | 2000 | September 1996 to July 1997 | AA | Referral | Types I and II | RDS | 112 | 171 | 283 | 25 | 75 | 100 | 35.3 | DNE instrument | 9 |
| Tsehay et al. [18] | 2014 | From May to June 2014 | AA | Referral | Type II | CS | 321 | 73 | 73 | 22.7 | DNE instrument | 7 | |||
| Abejew et al. [19] | 2015 | From April to May 2013 | Amhara | Referral | Types I and II | CS | NM | NM | 216 | NM | NM | 13 | 6 | DNS instrument | 6 |
| Gizaw et al. [20] | 2015 | Between January 2010 and December 2013 | AA | Referral | Types I and II | RDS | 122 | 401 | 523 | 20 | 89 | 109 | 20.8 | DNE instrument | 8 |
| Feleke and Enquselassie [21] | 2005 | From February 2002 to July 2003 | AA | Mixed | Types I and II | CS | 50 | 179 | 229 | NM | NM | 20 | 10 | DNE instrument | 7 |
| Wild et al. [22] | 2000 | 1993 | Different regions | Mixed | Type II | CS | — | 1386 | 1386 | — | 146 | 146 | 10.5 | MNSI assessment | 8 |
| Gebre and Assefa [23] | 2019 | From April 1 to April 2019 | SNNPR | General | Types I and II | CS | 160 | 178 | 338 | NM | NM | 50 | 14.8 | NSS, NDS | 7 |
| Korsa et al. [24] | 2019 | Between March and April 2016 | Oromia | Mixed | Types I and II | CS | 148 | 109 | 257 | NM | NM | 8 | 3.11 | NSS, NDS | 6 |
| Lebeta et al. [25] | 2017 | From April to May 2015 | Amara | Referral | Type II | CS | — | 344 | 344 | — | 344 | 27 | 7.8 | NSS, NDS | 9 |
| Ahmed et al. [26] | 2018 | From July to August 2015 | Oromia | Referral | Types I and II | CS | 70 | 90 | 160 | NM | NM | 10 | 6.3 | DNS instrument | 7 |
| Fiseha and Belete[27] | 2018 | From January 2008 to August 2015 | SNNPR | Referral | Types I and II | CO | 187 | 495 | 682 | NM | NM | 116 | 17 | DNS instrument | 8 |
| Jember et al. [9] | 2017 | From February 2016 to June 30 2016 | Amara | Referral | Types I and II | CS | 164 | 204 | 368 | 84 | 108 | 192 | 52.2 | DNS instrument | 8 |
| Tilahun et al. [28] | 2017 | October to December of 2015 | Oromia | Referral | Types I and II | CS | 98 | 138 | 236 | NM | NM | 60 | 25.4 | NSS, NDS | 7 |
| Tesfaye [29] | 2014 | From October to November 2013 | AA | Referral | Types I and II | CS | 56 | 191 | 247 | NM | NM | 25 | 10.1 | Clinical examination | 6 |
| Jarso et al. [30] | 2011 | December 2009 to February 2010 | AA | Referral | Types I and II | CS | 104 | 280 | 384 | 86 | 99 | 185 | 48.2 | DNS instrument | 9 |
| Lester [31] | 1984 | 1982 | AA | Referral | Types I and II | CS | NM | NM | 849 | NM | NM | 235 | 27.8 | DNS instrument | 8 |
| Fiseha and Belete [27] | 2011 | 2010 | AA | Referral | Types I and II | CS | NM | NM | 724 | NM | NM | 90 | 12.4 | Clinical examination | 9 |
| Gill et al. [32] | 2008 | 2007 | AA | Referral | Types I and II | CS | NM | NM | 108 | NM | NM | 43 | 41 | Clinical examination | 7 |
| Fasil et al. [33] | 2019 | February–March 2017 | Amara | Referral | Types I and II | CS | NM | NM | 367 | NM | NM | 29 | 7.9 | DNS assessment | 8 |
| Lester [34] | 1983 | 1983 | AA | Referral | Types I and II | CS | NM | NM | 809 | NM | NM | 47 | 5.8 | DNE instrument | 7 |
| Lester et al. [35] | 1976 | 1976 | AA | Referral | Types I and II | CS | NM | NM | 404 | NM | NM | 38 | 9.4 | DNS assessment | 6 |
| Teshager [36] | 2016 | March–April 2016 | Amhara | Referral | Types I and II | CS | 178 | 238 | 416 | NM | NM | 14 | 3.4 | DNS assessment | 9 |
| Kefeany [37] | 2017 | March–May 2017 | AA | Referral | Type II | CS | — | 121 | 121 | — | 49 | 49 | 40.5 | NSS, NDS | 8 |
| Kassahun et al. [38] | 2016 | February–April 2014 | Oromia | Referral | Type II | CS | — | 309 | 309 | — | 146 | 146 | 47.2 | DNE instrument | 8 |
| Eticha et al. [39] | 2016 | February 2015 | Tigray | Referral | Type II | CS | — | 384 | 384 | — | 125 | 125 | 32.6 | NSS, NDS | 7 |
| Cheneke et al. [40] | 2016 | From May to July 2012 | Oromia | Referral | Types I and II | CS | NM | NM | 148 | NM | NM | 18 | 12.2 | Clinical examination | 6 |
| Shiels et al. [8] | 2018 | From October 2016 to January 2017 | AA | Referral (police) | Type II | CS | — | 414 | 414 | — | 8 | 8 | 1.9 | NSS, NDS | 8 |
| Mariam et al. [41] | 2017 | From March to April 2016 | Amhara | Referral | Types I and II | CS | 110 | 169 | 279 | NM | NM | 28 | 10 | NSS, NDS | 7 |
| Demoz et al. [42] | 2019 | From August 2017 to July 2018 | AA | Referral | Type II | CS | — | 357 | 357 | — | 167 | 167 | 46.8 | Clinical examination | 7 |
| Yigazu and Deese [43] | 2017 | From February to March 2016 | Oromia | General | Type II | CS | — | 174 | 174 | — | 31 | 31 | 17.8 | Clinical examination | 8 |
| Woldu et al. [44] | 2018 | March 2017 | Amhara | Referral | Type II | CS | — | 341 | 341 | — | 64 | 64 | 18.8 | DNE instrument | 9 |
| Abdissa et al. [45] | 2019 | June to August 2019 | Oromia | Referral | Types I and II | CS | 48 | 229 | 277 | NM | NM | 129 | 46.6 | NSS, NDS | 9 |
| Tamura et al. [46] | 2020 | March 1 to April 28, 2019 | AA | Referral | Type II | CS | — | 346 | 346 | — | 53 | 53 | 15.3 | MNSI assessment | 7 |
| Abdissa et al. [47] | 2020 | September to November 2019 | Oromia | Referral | Type II | CS | — | 336 | 336 | — | 196 | 196 | 53.6 | DNS instrument | 8 |
| Woldemariam et al. [48] | 2020 | March to May 2019 | AA | Referral | Types I and II | CS | NM | NM | 161 | NM | NM | 44 | 27.3 | DNE instrument | 9 |
| YimamAhmed et al. [49] | 2020 | February to March 2019 | Oromia | Referral | Types I and II | CS | 24 | 76 | 100 | NM | NM | 45 | 45 | DNE instrument | 9 |
AA: Addis Ababa; SNNPR: South Nation Nationalities and People Representative; NM: not mentioned; CS: cross-sectional; SD: study design; RDS: retrospective descriptive study; CO: cohort; NOS: Newcastle-Ottawa Scale; NSS: neuropathy symptom. The score: DNS: diabetic neuropathy symptom; DNE: diabetic neuropathy examination; MNSI: Michigan Neuropathy Screening Instrument.
3.3. The Pooled Prevalence of DPN
The pooled prevalence of DPN among patients with diabetes in Ethiopia is 22% (95% CI 18-26%). The heterogeneity test shown in I2 with 98% indicates high heterogeneity. The prevalence of diabetic peripheral neuropathy is varying widely in the studies. This heterogeneity might be due to differences in the diagnostic criteria employed and the different defining criteria of DPN used (Figure 2).
Figure 2.

Forest plot for the pooled prevalence of DPN from 38 observational studies.
A funnel test was used to test for publication bias. And it showed the presence of publication bias. Therefore, there are unpublished data that can modify the prevalence of DPN (Figure 3).
Figure 3.

Funnel plot showing evidence of publication bias across studies.
3.4. Subgroup Analysis of DPN by Region of the Country
Based on the subgroup analysis of DPN among patients with diabetes attending hospitals in Addis Ababa, the pooled point estimate was 23% (with 95% CI; 17 to 29) (Figure 4).
Figure 4.

Forest plot of DPN in Addis Ababa.
Based on a subgroup analysis of DPN among patients with diabetes attending Oromia's hospitals, the pooled point estimate was 27% (with 95% CI; 16 to 38) (Figure 5).
Figure 5.
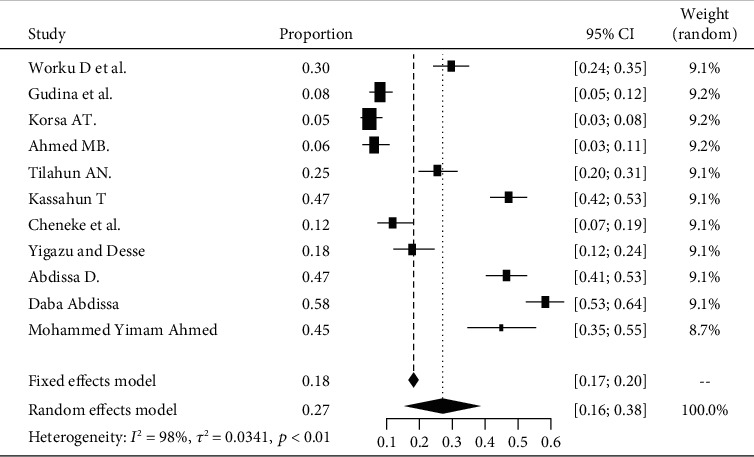
Forest plot of DPN in the Oromia region.
Based on the subgroup analysis of DPN among patients with diabetes attending South Nation and Nationalities' hospitals, the pooled point estimate was 16% (with 95% CI; 14 to 18) (Figure 6).
Figure 6.

Forest plot of DPN in the south region.
Based on the subgroup analysis of DPN among patients with diabetes attending Amhara's hospitals, the pooled point estimate was 15% (with 95% CI; 6 to 24) (Figure 7).
Figure 7.

Forest plot of DPN in the Amhara region.
4. Discussion
The reviewers have performed the present systematic review and meta-analysis to produce pooled estimates of nationwide results of peripheral neuropathy in Ethiopia among patients with diabetes. This review emphasized the burden of peripheral neuropathy among patients with diabetes in Ethiopia for a better understanding of the medical condition that can help in mitigating the problem of peripheral neuropathy in the country.
This meta-analysis showed the pooled estimate of diabetic peripheral neuropathy in Ethiopia to be standing at 22% (with 95% CI: 18-26). Individually, there was a variety in the prevalence of peripheral neuropathy in the studies included in our work which ranged from 1.9% as reported by Jember et al. [9] to 53.6% by Abdissa [47]. The reason for these variations could be due to the types of diabetes and assessment methods. The estimates of DPN are more common in patients with type 2 diabetes compared to those with type 1 diabetes (53% in type 2 diabetes) in the meta-analysis reported from Iran [50] versus type 1 (28.2% in type 1) [51]. As we estimated both in one, this truth is supported by one systematic review, reported in its subgroup analysis; the higher prevalence of peripheral neuropathy was lesser in patients with type 2 diabetes (38.8%) than in patients with type 1 diabetes (17.5%) [52]. The overall pooled prevalence of peripheral neuropathy in this study (22%) was consistent with a study reported from Arab countries that had a pooled prevalence of diabetic peripheral neuropathy of 18% (with a 95% CI: 0.09-0.34) [52]. Similarly, our finding was also comparable with a systematic review and meta-analysis reported from Oceania (23.2%, 95% CI: 2.68-76) [53]. This similarity might be due to the type of diabetes in our work conducted on both type 1 and type 2 diabetes, and the meta-analysis reported from Iran [52] focused on studies which included only patients with type 1 diabetes.
On the other hand, the result found in this study is lower as compared to other previous systematic review and meta-analysis studies that estimated the prevalence of peripheral neuropathy in patients with diabetes: 46% from the African region [54], 53% from Iran [50], and 31.6%, 32.2%, and 48.1% from America, Asia, and Europe, respectively, with an overall prevalence of 35.7% [53]. Similarly, the result was smaller than the results found in other meta-analyses conducted among patients with type 1 DM which showed a prevalence of 28.2% in Iran [51] and 30% in studies conducted in multination [55]. Our findings were higher compared with a worldwide estimate of DPN prevalence among patients with diabetes (8.1%–12.2%) [56].
To look at the heterogeneity of the studies included in this review, we conducted subgroup analysis by regions, and we found that a study done in Addis Ababa had the highest pooled point estimate of peripheral neuropathy in patients with diabetes, accounting for 23% (95% CI: 16, 30), and the lowest prevalence was reported from the Amhara region (15%, 95% CI: 6, 24). This discrepancy could be due to the difference in health care settings such as tertiary and primary health care that may cause a difference in screening and assessment practice and quality of service for diabetes patients with peripheral neuropathy. Some health care facilities may be enriched with the specialty that works more on better assessment or good practice on prevention strategies or well found with diagnostic instruments and severity of diabetes. Moreover, it may also be because there are no uniform assessment criteria or diagnostic method in clinical practice in all health care settings or due to the use by different clinicians of their unique clinical expertise in examining and individualizing their patients.
Our findings from the meta-analysis have implications in clinical practice as it can contribute to giving attention to the prevention and care of patients with diabetes. This pooled point of estimates for peripheral neuropathy in patients with diabetes provides updated evidence to advance prevention strategy, serves as key indicators of patient safety, and reflects the quality of the health care service and appropriate treatment strategy for peripheral neuropathy in patients with diabetes. The finding of the prevalence of diabetic peripheral neuropathy improves with good glucose control; use of adherence interventions, such as patient education and counseling on how to self-monitor blood glucose; and lifestyle modification interventions, such as exercise, weight reduction, and healthy diet. Therefore, the glycemic control strategy should be kept in mind that in addition to prescribing appropriate antidiabetic medicines and diabetes care practice, they need to include resources that help patients overcome individual challenges to reduce the development of such chronic diabetic complications using self-care practice. The implication of this study, particularly the pronounced variation between studies (1.9% to 53.6%), reflects patients with diabetes which require developing standards for management and implementing endorsed guidelines for clinical practice.
5. Limitations of This Study
We conducted this review with rigor and standards of the art. So far, this is the first systematic review and meta-analysis that draws a clear picture of the pooled point of prevalence for peripheral neuropathy in patients with diabetes in Ethiopia. However, our work has certain limitations. Among these limitations are studies that did not describe the type of diabetes, and this could induce errors by finding untrue substantial associations when combining such studies affected by confounding. It was challenging to do a subgroup analysis for studies conducted among patients with type 1 and type 2 diabetes and those subjected to a high degree of heterogeneity among studies as we could not report by type of diabetes. But the random effects model was used to obtain the pooled results that can minimize such heterogeneity among studies. Additionally, the defining criteria for DPN differed from one to another study. This could also affect the results of the meta-analysis with far-reaching clinical heterogeneity across studies. The presence of publication bias was an additional limitation.
6. Conclusion
Our work systematically summarises the prevalence of peripheral neuropathy among patients with diabetes in Ethiopia that revealed that more than one-fifth of the patients with diabetes had developed peripheral neuropathy. Thus, our finding suggests that the prevalence of peripheral neuropathy is high at the national level but still lower than other previous studies reported from different countries. The Ministry of Health, health policymakers, clinicians, and other health care providers should strengthen the quality of health care service for patients with diabetes to reduce the development of peripheral neuropathy. The Ministry of Health should develop a context-based intervention and preventive strategies to reduce the burden of diabetes-related complications, particularly DPN.
Abbreviations
- DM:
Diabetes mellitus
- DPN:
Diabetic peripheral neuropathy
- PRISMA-P:
Preferred Reporting Items for Systematic reviews and Meta-Analysis protocol.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
DBT, GTG, AH, WA, TGH, and GTD developed the protocol and are involved in the design, selection of study, data extraction, statistical analysis, and development of the initial drafts of the manuscript. GG, GM, and KZ are involved in data extraction, quality assessment, statistical analysis, and revision. TM, DBT, GTD, and GGG prepared the final draft of the manuscript. All authors read and approved the final manuscript.
Supplementary Materials
The supplementary material includes the appendix about the search strategy and information sources.
References
- 1.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice. 2019;157, article 107843 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Roglic G. WHO global report on diabetes: a summary. International Journal of Noncommunicable Diseases. 2016;1(1):p. 3. doi: 10.4103/2468-8827.184853. [DOI] [Google Scholar]
- 3.Singh R., Kishore L., Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacological Research. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Tesfaye S., Vileikyte L., Rayman G., et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes/Metabolism Research and Reviews. 2011;27(7):629–638. doi: 10.1002/dmrr.1225. [DOI] [PubMed] [Google Scholar]
- 5.Tesfaye S., Boulton A. J., Dyck P. J., et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan B. C., Cheng H. T., Stables C. L., Smith A. L., Feldman E. L. Diabetic neuropathy: clinical manifestations and current treatments. The Lancet Neurology. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadosky A., Mardekian J., Parsons B., Hopps M., Bienen E. J., Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. Journal of Diabetes and its Complications. 2015;29(2):212–217. doi: 10.1016/j.jdiacomp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Shiels T., Abebaw M., Bilal A. I., Tesfaye T. Treatment pattern and factors associated with blood pressure and fasting plasma glucose control among patients with type 2 diabetes mellitus in Police Referral Hospital in Ethiopia. Ethiopian Journal of Health Sciences. 2018;28(4):461–472. doi: 10.4314/ejhs.v28i4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jember G., Melsew Y. A., Fisseha B., Sany K., Gelaw A. Y., Janakiraman B. Peripheral sensory neuropathy and associated factors among adult diabetes mellitus patients in Bahr Dar, Ethiopia. Journal of Diabetes & Metabolic Disorders. 2017;16(1) doi: 10.1186/s40200-017-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton A. J. Guidelines for diagnosis and outpatient management of diabetic peripheral neuropathy. European Association for the Study of Diabetes, Neurodiab. Diabetes & Metabolism. 1998;24 [PubMed] [Google Scholar]
- 11.Boulton A. J., Malik R. A., Arezzo J. C., Sosenko J. M. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 12.Shea B. J., Reeves B. C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358, article j4008 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W-65–W-94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worku D., Hamza L., Woldemichael K. Patterns of diabetic complications at Jimma University Specialized Hospital, southwest Ethiopia. Ethiopian Journal of Health Sciences. 2011;20(1):33–39. doi: 10.4314/ejhs.v20i1.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudina E. K., Amade S. T., Tesfamichael F. A., Ram R. Assessment of quality of care given to diabetic patients at Jimma University Specialized Hospital diabetes follow-up clinic, Jimma, Ethiopia. BMC Endocrine disorders. 2011;11(1) doi: 10.1186/1472-6823-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ejigu A. Brief communication: patterns of chronic complications of diabetic patients in Menelik II hospital, Ethiopia. Ethiopian Journal of Health Development. 2000;14(1):113–116. doi: 10.4314/ejhd.v14i1.9938. [DOI] [Google Scholar]
- 18.Tsehay T., Engidawork E., Ahmed A. Assessment of antidiabetic medication adherence and its determinants among ambulatory patients with type 2 diabetes at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethopia. The Journal of Pharmacy and Alternative Medicine. 2016;11 [Google Scholar]
- 19.Abejew A. A., Belay A. Z., Kerie M. W. Diabetic complications among adult diabetic patients of a tertiary hospital in northeast Ethiopia. Advances in Public Health. 2015;2015:7. doi: 10.1155/2015/290920. [DOI] [Google Scholar]
- 20.Gizaw M., Harries A. D., Ade S., et al. Diabetes mellitus in Addis Ababa, Ethiopia: admissions, complications, and outcomes in a large referral hospital. Public Health Action. 2015;5(1):74–78. doi: 10.5588/pha.14.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feleke Y., Enquselassie F. An assessment of the health care system for diabetes in Addis Ababa, Ethiopia. Ethiopian Journal of Health Development. 2005;19(3):203–210. doi: 10.4314/ejhd.v19i3.9999. [DOI] [Google Scholar]
- 22.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 23.Gebre B. B., Assefa Z. M. magnitude and associated factors of diabetic complication among diabetic patients attending Gurage zone hospitals, South West Ethiopia. BMC Research Notes. 2019;12(1):p. 780. doi: 10.1186/s13104-019-4808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korsa A. T., Genemo E. S., Bayisa H. G., Dedefo M. G. Diabetes mellitus complications and associated factors among adult diabetic patients in selected hospitals of West Ethiopia. The Open Cardiovascular Medicine Journal. 2019;13(1):41–48. doi: 10.2174/1874192401913010041. [DOI] [Google Scholar]
- 25.Lebeta R., Argaw Z., Walle B. Prevalence of diabetic complications and its associated factors among diabetes mellitus patients attending diabetes mellitus clinics; institution based cross sectional study. American Journal of Health Research. 2017;5(2):p. 38. doi: 10.11648/j.ajhr.20170502.13. [DOI] [Google Scholar]
- 26.MB A., E Y., G T. Diabetic complications among follow-up patients: a cross-sectional study at Jimma University Specialized Hospital diabetic clinic. Journal of Clinical and Molecular Endocrinology. 2018;3(1):p. 45. doi: 10.21767/2572-5432.100045. [DOI] [Google Scholar]
- 27.Fiseha T., Belete A. G. Diabetes mellitus and its associated factors among human immunodeficiency virus-infected patients on antiretroviral therapy in Northeast Ethiopia. BMC Research Notes. 2019;12(1, article 372) doi: 10.1186/s13104-019-4402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AN T., C W., GM T., et al. Major micro vascular complications and associated risk factors among diabetic outpatients in Southwest Ethiopia. Endocrinology & Metabolic Syndrome. 2017;6(4):p. 272. doi: 10.4172/2161-1017.1000272. [DOI] [Google Scholar]
- 29.Tesfaye D. J. Coexistence of chronic complications among diabetic patients at Nigist Eleni Mohammed Memorial Hospital, Hosanna, south Ethiopia. Open Access Library Journal. 2015;2(1):p. 1. [Google Scholar]
- 30.Jarso G., Ahmed A., Feleke Y. The prevalence, clinical features and management of periphral neuropathy among diabetic patients in Tikur Anbessa and St. Paul's Specialized University Hospitals, Addis Ababa, Ethiopia. Ethiopian medical journal. 2011;49(4):299–311. [PubMed] [Google Scholar]
- 31.Lester F. T. The clinical pattern of diabetes mellitus in Ethiopians. Diabetes Care. 1984;7(1):6–11. doi: 10.2337/diacare.7.1.6. [DOI] [PubMed] [Google Scholar]
- 32.Gill G., Gebrekidan A., English P., Wile D., Tesfaye S. Diabetic complications, and glycaemic control in remote North Africa. QJM. 2008;101(10):793–798. doi: 10.1093/qjmed/hcn096. [DOI] [PubMed] [Google Scholar]
- 33.Fasil A., Biadgo B., Abebe M. Glycemic control and diabetes complications among diabetes mellitus patients attending at University of Gondar Hospital, Northwest Ethiopia. Diabetes, Metabolic Syndrome, and Obesity: Targets and Therapy. 2019;12:75–83. doi: 10.2147/dmso.s185614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lester F. T. Long-standing diabetes mellitus in Ethiopia: a survey of 105 patients. Diabetologia. 1983;25(3):222–225. doi: 10.1007/BF00279932. [DOI] [PubMed] [Google Scholar]
- 35.Lester F. T., Abdulkadir J., Larson D., Quana'a P. Diabetes mellitus: clinical features in 404 Ethiopians. Ethiopian Medical Journal. 1976;14(4):185–198. [PubMed] [Google Scholar]
- 36.Teshager W. Prevalence of depression, and associated factors among adult diabetic patients attending outpatient department, at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia, 2016. Addis Ababa University; 2016. [Google Scholar]
- 37.Kefeany G. Status of vitamin B12 level among type 2 diabetes mellitus patients on metformin treatment attending diabetic clinic of the Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Addis Ababa University; 2017. [Google Scholar]
- 38.Kassahun T., Eshetie T., Gesesew H. Factors associated with glycemic control among adult patients with type 2 diabetes mellitus: a cross-sectional survey in Ethiopia. BMC Research Notes. 2016;9(1, article 78) doi: 10.1186/s13104-016-1896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eticha T., Mulu A., Gebretsadik H., Kahsay G., Ali D. Y. Factors associated with poor glycemic control in type 2 diabetic patients investigated at Ayder Referral Hospital, Mekelle, Ethiopia. International Journal of Pharmacy and Pharmaceutical Research (IJPPR) 2016;6(3):160–171. [Google Scholar]
- 40.Cheneke W., Suleman S., Yemane T., Abebe G. Assessment of glycemic control using glycated hemoglobin among diabetic patients in Jimma University Specialized Hospital, Ethiopia. BMC Research Notes. 2016;9(1):p. 96. doi: 10.1186/s13104-016-1921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariam T. G., Alemayehu A., Tesfaye E., et al. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the University of Gondar Referral Hospital, North West Ethiopia, 2016: institutional-based cross-sectional study. Journal of diabetes research. 2017;2017:1–8. doi: 10.1155/2017/2879249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demoz G. T., Gebremariam A., Yifter H., et al. Predictors of poor glycemic control among patients with type 2 diabetes on follow-up care at a tertiary healthcare setting in Ethiopia. BMC Research Notes. 2019;12(1):p. 207. doi: 10.1186/s13104-019-4248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yigazu D. M., Desse T. A. Glycemic control and associated factors among type 2 diabetic patients at Shanan Gibe Hospital, Southwest Ethiopia. BMC Research Notes. 2017;10(1):p. 597. doi: 10.1186/s13104-017-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alebachew Woldu M., Diriba Wami C. Factors associated with poor glycemic control among patients with type 2 diabetes mellitus in Ambo Hospital, Ambo; Ethiopia. Endocrinology & Metabolic Syndrome. 2014;3(4) doi: 10.4172/2161-1017.1000143. [DOI] [Google Scholar]
- 45.Abdissa D., Adugna T., Gerema U., Dereje D. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients on follow-up clinic at Jimma Medical Center, Southwest Ethiopia, 2019: an institutional-based cross-sectional study. Journal of Diabetes Research. 2020;2020:6. doi: 10.1155/2020/4106383.4106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamru K., Aga F., Berhanie E., Aynalem Y. A., Shiferaw W. S. Incidence of diabetic nephropathy in patients with type 2 diabetes mellitus at a tertiary healthcare setting in Ethiopia. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(5):1077–1083. doi: 10.1016/j.dsx.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Abdissa D., Hamba N., Kene K., et al. Prevalence and determinants of peripheral neuropathy among type 2 adult diabetes patients attending Jimma University Medical Center, Southwest Ethiopia, 2019, an Institutional-Based Cross-Sectional Study. Journal of Diabetes Research. 2020;2020:8. doi: 10.1155/2020/9562920.9562920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woldemariam G. T., Atnafu N. T., Radie Y. T., et al. Determinants of diabetic foot ulcer among adult patients with diabetes attending the diabetic clinic in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia: unmatched case-control study. Diabetes, Metabolic Syndrome, and Obesity: Targets and Therapy. 2020;13:3739–3747. doi: 10.2147/DMSO.S265988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.YimamAhmed M., Ejigu S. H., Zeleke A. Z., Hassen M. Y. Glycemic control, diabetes complications, and their determinants among ambulatory diabetes mellitus patients in Southwest Ethiopia: a prospective cross-sectional study. Diabetes, Metabolic Syndrome, and Obesity: Targets and Therapy. 2020;13:1089–1095. doi: 10.2147/DMSO.S227664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobhani S., Asayesh H., Sharifi F., et al. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. Journal of Diabetes & Metabolic Disorders. 2014;13(1):p. 97. doi: 10.1186/s40200-014-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasigh A., Abdi A., Borji M., Tarjoman A. The prevalence of neuropathy among type 1 diabetic adolescents in Iran: a systematic review and meta-analysis. International Journal of Adolescent Medicine and Health. 2019 doi: 10.1515/ijamh-2018-0223. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Motal U. M., Abdelalim E. M., Abou-Saleh H., Zayed H. Neuropathy of type 1 diabetes in the Arab world: a systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2017;127:172–180. doi: 10.1016/j.diabres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 53.de Souza R., Debiasi D., Ceretta L. B., Simões P. W., Tuon L. Meta-analysis and meta-regression of the prevalence of diabetic peripheral neuropathy among patients with type 2 diabetes mellitus. International Archives of Medicine. 2016;9 doi: 10.3823/1936. [DOI] [Google Scholar]
- 54.Shiferaw W. S., Akalu T. Y., Work Y., Aynalem Y. A. Prevalence of diabetic peripheral neuropathy in Africa: a systematic review and meta-analysis. BMC Endocrine Disorders. 2020;20(1):p. 49. doi: 10.1186/s12902-020-0534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun J., Wang Y., Zhang X., Zhu S., He H. Prevalence of peripheral neuropathy in patients with diabetes: a systematic review and meta-analysis. Primary Care Diabetes. 2020;14(5):435–444. doi: 10.1016/j.pcd.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Said G. Diabetic neuropathy--a review. Nature Clinical Practice Neurology. 2007;3(6):331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material includes the appendix about the search strategy and information sources.


