Abstract
Background/Aim: Curcumin is a polyphenol that exerts a variety of pharmacological activities and plays an anti-cancer role in many cancer cells. It was recently reported that gasdermin E (GSDME) is involved in the progression of pyroptosis. Materials and Methods: HepG2 cells were treated with various concentrations of curcumin and cell viability was examined using MTT assay, apoptosis was analysed using flow cytometry, reactive oxygen species (ROS) levels using dihydroethidium, LDH release using an LDH cytotoxicity assay, and protein expression using western blot. Results: Curcumin increased the expression of the GSDME N-terminus and proteins involved in pyrolysis, promoted HspG2 cell pyrolysis and increased intracellular ROS levels. Moreover, inhibition of the production of intracellular ROS with n-acetylcysteine (NAC) improved the degree of apoptosis and pyrolysis induced by curcumin. Conclusion: Curcumin induces HspG2 cell death by increasing apoptosis and pyroptosis, and ROS play a key role in this process. This study improves our understanding of the potential anti-cancer properties of curcumin in liver cancer.
Keywords: Curcumin, GSDME, liver cancer, proptosis, ROS
Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione (diferuloy l methane)] is a type of polyphenol extracted from the rhizomes of ginger plants, such as turmeric and zedoary (1), which has been shown to display various pharmacological activities, including anti-inflammatory (2), antioxidant (3), hypolipidemic (4), anti-tumor (5), and immune regulatory (6). Previous studies have also demonstrated that curcumin exerts anti-cancer effects in many types of cancer cells, including stomach (7), colon (8), lung (9), liver (10) and breast (11). In addition, it has been reported that curcumin can affect the cellular redox balance, resulting in increased cellular reactive oxygen species (ROS) production (12). Excessive ROS can directly or indirectly affect the regulation of anti- or pro-apoptotic proteins by Bcl-2 family members, leading to cellular apoptosis (13). Moreover, ROS accumulation has been shown to induce cellular apoptosis, necroptosis, endoplasmic reticulum stress, and autophagy in various cancer cells (14). Therefore, curcumin may also affect cell death in liver cancer cells.
Pyroptosis is a recently discovered type of programmed cell death that depends on the activation of the inflammation-related caspases 1, 4, 5, and 11, as well as apoptosis-related caspase-3 (15). The morphology of cells undergoing pyroptosis differs from that in apoptosis or necrosis, and is characterized by cell swelling, membrane breakage, pore formation, and osmotic lysis (16). It was recently reported that gasdermin E (GSDME) is involved in the progression of pyroptosis (17). In particular, activated caspase-3 was found to cleave GSDME between the N-terminus (GSMDE-N) and C-terminus under certain apoptotic stimuli, thus relieving the self-inhibition of the C-terminus and releasing the active GSDME-N-terminus as well as the pore-forming domain (PFD). Consequently, non-selective pores are formed in the cell membrane, leading to cell swelling, membrane rupture, and cell pyrolysis (18). However, the effect of curcumin on GSDME during pyroptotic cell death in liver cancer cells remains unclear.
In this study, the effect of curcumin on cell death was evaluated by examining the viability, apoptosis, pyroptosis, and expression of related proteins in HepG2 liver cancer cells treated with curcumin. Together, the findings of this study provide new insights into the potential anti-cancer properties of curcumin in liver cancer.
Materials and Methods
Chemicals and materials. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and Annexin V apoptosis detection kit FITC were purchased from Invitrogen (Carlsbad, CA, USA). Penicillin-streptomycin liquid (P/S), 1 × tris-buffered saline and Tween 20 (TSBT) buffer solution, n-acetylcysteine (NAC), and Hoechst 32258 were obtained from Solarbio Life Sciences (Beijing, China). Propidium iodide (PI) staining kit, horseradish peroxidase (HP)-conjugated goat anti-rabbit IgG and anti-mouse IgG were purchased from Sangon Biotech (Shanghai, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and curcumin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Six- and ninety-six-well plates were purchased from NEST Biotechnology (Wuxi, Jiangsu, PR China). Dihydroethidium (DHE) and LDH cytotoxicity assay kits were obtained from Beyotime Biotechnology (Shanghai, PR China). Anti-Bax (ab32503), anti-Bcl-2 (ab32124), anti-caspase-3 (ab32351), anti-DFN45/GSDME (ab215191), and anti-α tubulin (ab7291) antibodies were purchased from Abcam (Cambridge, MA, USA). The chemiluminescence detection system was obtained from GE Healthcare Life Sciences (Chalfont, UK). The UV MAX kinetic microplate reader was purchased from Molecular Devices (LLC, Sunnyvale, CA, USA). Fluorescence microscopy was performed using an EVOS®xl core cell culture microscope from the Advanced Microscopy Group (Paisley, Scotland). Flow cytometry was performed using a FACSCalibur instrument from BD Biosciences (Franklin Lakes, NJ, USA). Nitrocellulose membranes were obtained from Millipore (Bedford, MA, USA).
Cell culture. HepG2 liver cancer cells were cultured in DMEM supplemented with 10% FBS and 1% P/S, and maintained in a humidified incubator at 37˚C with 5% CO2.
Cell viability assay. Cell viability was analyzed using an MTT assay. Briefly, HepG2 cells were seeded in 96-well plates (1×104 cells/well) and treated with curcumin for 12 h. Next, 10 μl (0.5 mg/ml) of MTT was added to each well and incubated for 2 h at 37˚C in 5% CO2. After the supernatant had been removed, 100 μl of dimethyl sulfoxide was added and absorbance was measured at 490 nm using a UV MAX kinetic microplate reader.
Apoptosis detection. HepG2 cells were seeded in 6-well plates (2 × 105 cells/well) and treated with curcumin. To detect apoptosis, cells were prepared using an Annexin V apoptosis detection kit FITC according to the manufacturer’s protocol and analyzed using fluorescence microscopy and flow cytometry.
ROS detection. HepG2 cells were seeded in 6-well plates (2×105 cells/well) and treated with curcumin. Changes in cellular ROS levels were determined using DHE (1 μM/ml) and nuclei were visualized qualitatively under a microscope after 20 min of incubation with Hoechst 32258 dye (2 μg/ml).
Detection of LDH release. HepG2 cells were seeded in 96-well plates (1×105 cells/well) and treated with curcumin. Changes in LDH release were determined using an LDH cytotoxicity assay. Absorbance was measured at 490 nm using a UV MAX kinetic microplate reader, according to the manufacturer's instructions.
Lytic cell death assay. HepG2 cells were seeded in 6-well plates (2×105 cells/well) and treated with curcumin. Lytic cell death was measured using PI incorporation, as described previously. Nuclei were visualized using a fluorescence microscope after incubation with Hoechst 33342 dye (2 μg/ml) and PI for 20 min at room temperature (19).
Western blot analysis. Cell lysate proteins were separated using 12 % sodium dodecyl sulfate-polyacrylamide gels and transferred onto nitrocellulose membranes, which were blotted with primary antibodies against Bax, Bcl-2, caspase-3, GSDME, and α-tubulin at 4˚C for 12 h. The membranes were then washed five times with TBST and incubated with HP-conjugated goat anti-rabbit or anti-mouse IgG for 2 h at room temperature. After excess antibodies were removed by washing with TBST, specific binding was detected using a chemiluminescence detection system according to the manufacturer's protocol.
Statistical analysis. Data from at least three independent experiments are presented as the mean±standard error of the mean (SEM). Two-way analysis of variance (ANOVA) was used to analyze changes over time and differences between groups in each experiment. For most experiments, Tukey's post hoc test (α=0.05) was used to determine statistical significance among two groups. All statistical analyses were conducted using SPSS software (version 25; IBM Corp). Differences were considered statistically significant if the p-value was less than 0.05 (*p<0.05, **p<0.01, ***p<0.001).
Results
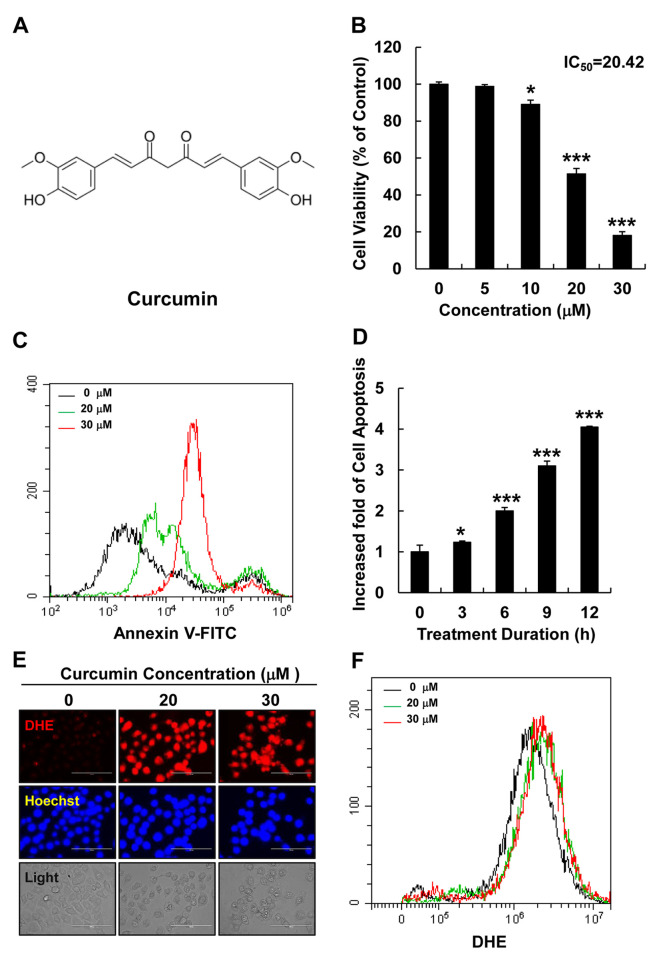
Curcumin increased cellular ROS levels and induced apoptosis in HepG2 cells. The chemical structure of curcumin is shown in Figure 1A. To determine its effect on HepG2 cell viability, cells were treated with various concentrations of curcumin (0, 5, 10, 20, or 30 μM) for 12 h and cell viability was examined using an MTT assay. Curcumin significantly reduced the viability of HepG2 cells in a dose-dependent manner (Figure 1B). Furthermore, the effect of curcumin on apoptosis induction was investigated by treating HepG2 cells with different curcumin concentrations (0, 20, or 30 μM) for 12 h and with 20 μM of curcumin for different durations (0, 3, 6, 9, 12, or 24 h). Cellular apoptosis was evaluated using an Annexin-V FITC detection kit and flow cytometry, and revealed that curcumin significantly increased apoptosis in HepG2 cells in a dose- and time-dependent manner (Figure 1C and D). The effect of curcumin on the cellular ROS levels was also examined using fluorescence microscopy and flow cytometry by staining with DHE (a superoxide detection marker) after HepG2 cells had been treated with different curcumin concentrations (0, 20, or 30 μM). Curcumin significantly increased intracellular ROS levels in HepG2 cells (Figure 1E and F).
Figure 1. Curcumin increased cellular ROS levels and induced apoptosis in HepG2 liver cancer cells. (A) The chemical structural of curcumin. (B) Cell viability was analyzed using MTT assay, in HepG2 cells treated with curcumin (0, 5, 10, 20, or 30 μM) for 12 h. (C) Cellular apoptosis was measured using flow cytometry in HepG2 cells treated with curcumin (0, 20, or 30 μM) for 12 h. Black line: without curcumin; green line: 20 μM curcumin; red line: 30 μM curcumin. (D) Cellular apoptosis was measured by flow cytometry in HepG2 cells treated with curcumin at different times (0, 3, 6, or 12 h). The fold increase in apoptotic cells is presented as the mean±SEM (*p<0.05, **p<0.01). (E) Cellular ROS levels were detected by fluorescence microscopy in DHE (red)- and Hoechst (blue)-stained HepG2 cells treated with curcumin (0, 20, or 30 μM) for 12 h (scale bar=100 μm). (F) Cellular ROS levels were detected by fluorescence microscopy in DHE-stained HepG2 cells treated with curcumin (0, 20, or 30 μM) for 12 h.
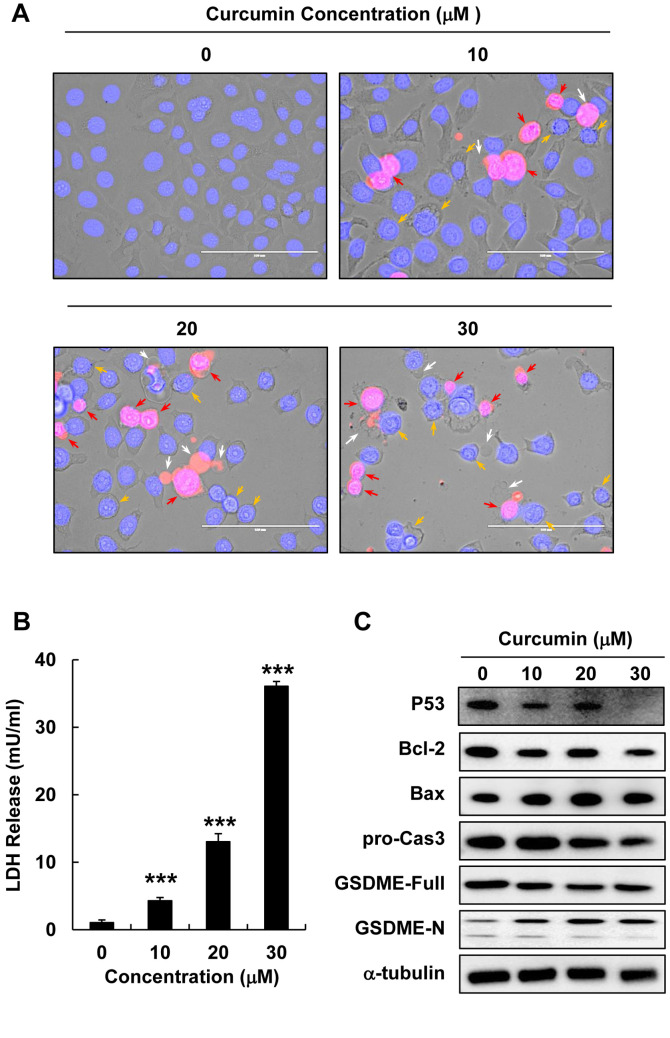
Curcumin induced lytic cell death and pyroptosis in HepG2 cells. To elucidate the features of cell death induced by curcumin, we performed PI and Hoechst staining in HepG2 cells treated with different curcumin concentrations (0, 10, 20, or 30 μM) for 12 h. The observation of morphological changes using a fluorescent microscope revealed swelling, membranolysis, and the formation of numerous pyroptotic bodies in curcumin-treated HepG2 cells (Figure 2A). LDH release is a marker of cell death that is closely related to cell membrane destruction; therefore, we examined LDH release in HepG2 cells treated with various curcumin concentrations (0, 10, 20, or 30 μM) for 12 h. Curcumin significantly increased LDH release in HepG2 cells in a dose-dependent manner (Figure 2B), indicating that curcumin treatment increases cell death. In addition, western blotting analysis revealed that curcumin treatment significantly down-regulated pro-caspase 3 and Bcl-2 (anti-apoptotic) protein expression but up-regulated Bax (pro-apoptotic) protein expression in HepG2 cells (Figure 2C and D). Next, we measured the expression of the GSDME protein, which is a known marker of pyroptosis, after curcumin treatment, and found that increasing curcumin concentrations down-regulated full length GSDME and up-regulated GSDME-N (Figure 2C and D). Thus, curcumin treatment appears to increase pyroptosis in HepG2 cells.
Figure 2. Curcumin induced lytic cell death and pyroptosis in HepG2 cells. HepG2 cells were treated with curcumin (0, 10, 20, or 30 μM) for 12 h. (A) Morphological changes and pyroptosis were observed using fluorescence microscopy in PI (red)- and Hoechst (blue)-stained HepG2 cells treated with curcumin (0, 10, 20, or 30 μM) (scale bar=100 μm). (B) LDH release in HepG2 cells treated with curcumin (0, 10, 20, or 30 μM). (C) Bcl-2, Bax, pro-caspase3, GSDME-Full, and GSDME-N protein expression detected using western blot analysis in HepG2 cells treated with curcumin (0, 10, 20, or 30 μM). (D) Quantitative analysis of Bcl-2, pro-caspase3, and GSDME-N protein expression presented as the mean±SEM of three different samples (*p<0.05, **p<0.01, ***p<0.001).
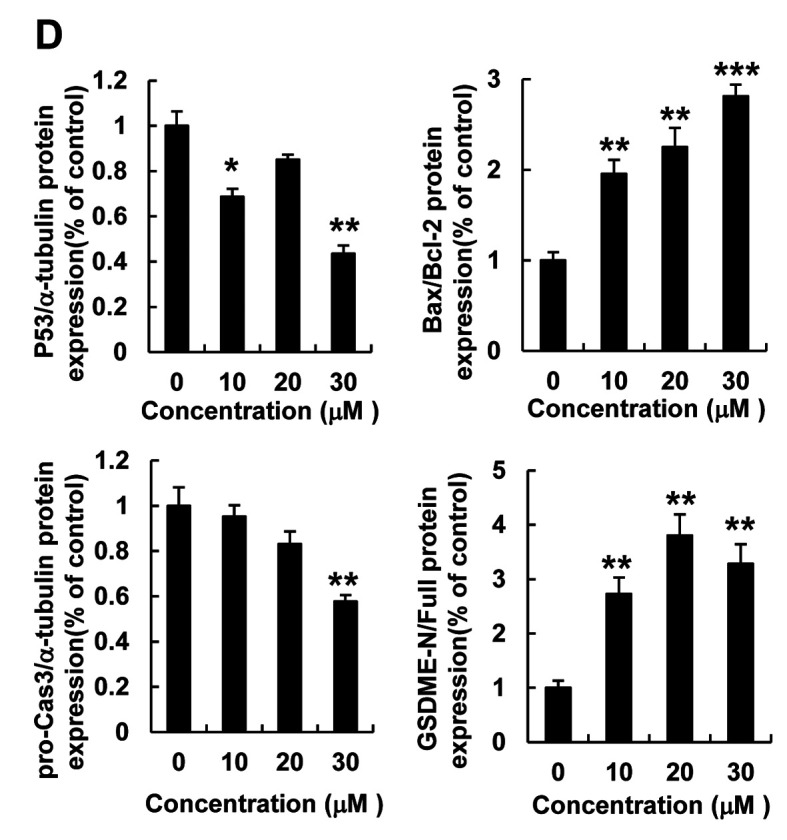
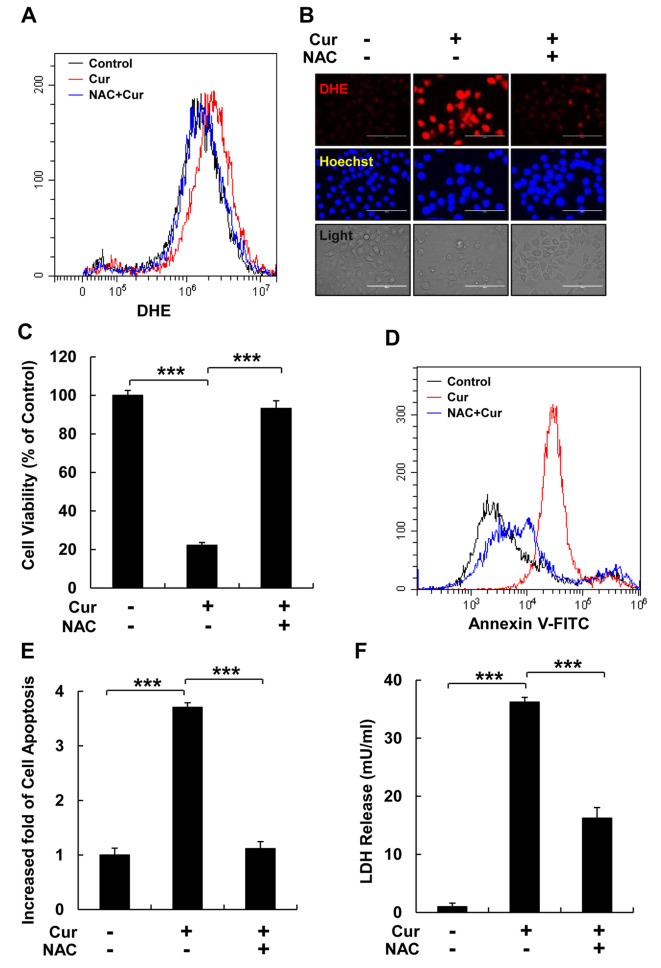
ROS inhibition reduced cellular apoptosis and LDH release. To verify the regulatory effect of curcumin-induced ROS levels on cell death, HspG2 cells were pre-treated with NAC (a ROS scavenger) for 30 min and then, treated with curcumin for 12 h. Flow cytometry and fluorescence microscopy analyses revealed that NAC treatment significantly reduced curcumin-stimulated cellular ROS levels, as detected using DHE staining (Figure 3A and B). In addition, NAC treatment reversed the reduction in cell viability induced by curcumin in HepG2 cells (Figure 3C); therefore, we examined the effect of NAC on curcumin-induced cellular apoptosis and pyroptosis in HepG2 cells. NAC treatment significantly reduced curcumin-induced cell apoptosis and LDH release in HepG2 cells (Figure 3D-F).
Figure 3. ROS inhibition reduces cellular apoptosis and LDH release. HepG2 cells were pre-treated with NAC, a ROS scavenger, for 30 min. (A) Cellular ROS levels were detected using flow cytometry with DHE staining. Black: no treatment; red: curcumin treatment; blue: NAC pretreatment and curcumin treatment. (B) Cellular ROS were detected using a fluorescence micrograph with DHE (red) and Hoechst (blue) staining (scale bar=100 μm). (C) Cell viability was analyzed using an MTT assay. (D) Cellular apoptosis was detected using flow cytometry and Annexin V - FITC staining of HepG2 cells. Black: normal cells; red: curcumin treatment; blue: NAC pretreatment and curcumin treatment. (E) Quantitative analysis of cellular apoptosis shown in (D). (F) LDH production in HepG2 cells following three different treatments, as indicated. Data represent the mean±SEM of three different samples (***p<0.001).
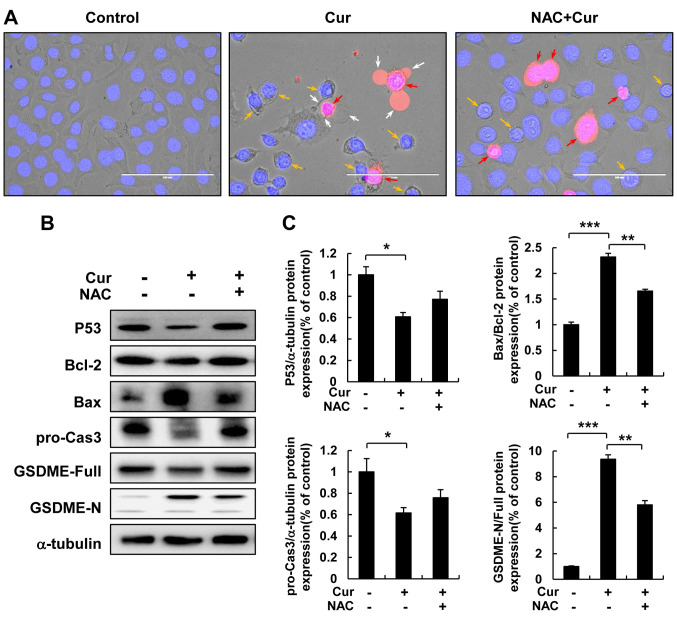
ROS play a key role in curcumin-induced HepG2 cell pyroptosis. To verify the regulatory role of ROS in curcumin-induced pyroptosis, HepG2 cells were pre-treated with NAC for 30 min, treated with curcumin for 12 h, and morphological changes were observed using fluorescence microscopy after staining with PI and Hoechst (Figure 4A). NAC treatment reversed curcumin-induced cell swelling, membranolysis, and pyroptotic body formation in HepG2 cells; therefore, we examined protein expression in HepG2 cells following curcumin and NAC treatment to identify the molecular mechanism of ROS in curcumin-induced pyroptosis. Interestingly, NAC treatment reversed the down-regulation of pro-caspase 3 and Bcl-2 and up-regulation of Bax protein induced by curcumin in HepG2 cells, as well as the down-regulation of full length GSDME and up-regulation of GSDME-N (Figure 4B and C).
Figure 4. ROS inhibition reduces lytic cell death and pyroptosis. HepG2 cells were pretreated with NAC for 30 min and then, treated with curcumin for 12 h. (A) Morphological changes and pyroptosis were observed using fluorescence microscopy of PI (red)- and Hoechst (blue)-stained HepG2 cells treated with curcumin (30 μM) (scale bar=100 μm). (B) Bcl-2, Bax, pro-caspase3, GSDME-Full, and GSDME-N protein expression was detected using western blot analysis in HepG2 cells treated with curcumin. (C) Quantitative analysis of Bcl-2, pro-caspase3, and GSDME-N protein expression presented as the mean±SEM of three different samples (*p<0.05, **p <0.01, ***p<0.001).
Discussion
Liver cancer is one of the most common tumors worldwide, with around one million individuals being diagnosed each year (20). Current treatments for liver cancer mainly involve surgical resection and chemotherapy (21); however, both of these methods have limitations. For instance, surgical resection can only be conducted in a small proportion of patients, and chemotherapy can also kill normal cells in addition to cancer cells, causing adverse side effects. Therefore, it is necessary to find novel, safe and effective treatment strategies for liver cancer.
The use of herbal medicines has been recorded in pharmacological papers dating back to ancient Korea and China (22); however, traditional Chinese herbal medicine has attracted interest from scientists due to their high quantity and variety, good safety, and low price (23). In-depth research has revealed that Korean and Chinese herbal medicines and their extracts can exert good inhibitory effects against cancer at all stages of development (24). For instance, quercetin is a natural flavonoid that can inhibit proliferation and metastasis of a variety of tumor cells (25), while salidroside has been shown to inhibit the proliferation of A549 lung cancer cells (26). Similarly, Salvia miltiorrhiza can inhibit the over-expression of the proto-oncogene c-myc (27), and curcumin has been found to inhibit cancer cell proliferation by inhibiting DNA synthesis (28). Thus, Chinese herbal medicine extracts can exert a variety of anti-cancer effects.
Curcumin is a type of polyphenol extracted from the rhizome of ginger plants that has been shown to exert anti-cancer effects, such as inhibition of cell proliferation and induction of apoptosis via ROS (29). Consistent with previous research, our study demonstrated that curcumin can effectively induce apoptosis in HepG2 liver cancer cells in a time- and concentration-dependent manner. Moreover, morphological observation revealed that curcumin treatment causes cells to swell, form pores, and release LDH, suggesting that curcumin induces pyrolysis in HepG2 cells.
Pyroptosis is a recently discovered form of programmed cell death characterized by swelling that results in cell membrane rupture, the release of cell contents, and the activation of a strong inflammatory response. Previous studies have indicated that small-molecule drugs can induce pyrolysis by mediating various cell signaling pathways involving proteins of the GSDM family (30). In this study, we found that curcumin can increase GSDME-N expression as well as the expression of proteins involved in pyrolysis, thereby promoting pyrolysis. In addition, we revealed that curcumin can increase intracellular ROS levels, suggesting that inhibition f intracellular ROS production can promote the degree of apoptosis and pyrolysis induced by curcumin. Taken together, our results demonstrate that curcumin can promote apoptosis and pyroptosis in liver cancer cells by regulating ROS production, thus exerting anticancer effects.
In conclusion, our results suggest that curcumin can induce liver cancer cell death by increasing apoptosis and pyroptosis, and that ROS play a key role in this process. In addition, curcumin activates the GSDME-related scorch death and mitochondria-dependent apoptosis signaling pathways. Thus, curcumin may be a potential new strategy for treating liver cancer.
Conflicts of Interest
The Authors declare that there are no conflicts of interest regarding this study.
Authors’ Contributions
Conceptualization: WFL, YXG, TK, HNS. Methodology: TK, HNS Software: TK, HNS. Validation: TK, HNS. Formal Analysis: HFL, FLS, WLL, DQC, DPX, CXR. Investigation: WFL, YXG, TK, HNS. Resources: TK, HNS. Data Curation: TK, HNS. Writing – Original Draft: WFL, YXG, TK, HNS. Writing – Review & Editing: TK, HNS. Visualization: TK, HNS. Supervision: TK, HNS. Project Administration: TK, HNS. Funding Acquisition: TK. All Authors read and approved the final article.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A2052417) and by grants from the Korean Research Institute of Bioscience and Biotechnology Research Initiative Program (KRIBB) (RBM0112011), Republic of Korea.
References
- 1.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 3.Abrahams S, Haylett WL, Johnson G, Carr JA, Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013;39(1):101–121. doi: 10.1002/biof.1072. [DOI] [PubMed] [Google Scholar]
- 5.Unlu A, Nayir E, Dogukan Kalenderoglu M, Kirca O, Ozdogan M. Curcumin (turmeric) and cancer. J buon. 2016;21(5):1050–1060. [PubMed] [Google Scholar]
- 6.Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit Rev Food Sci Nutr. 2019;59(1):89–101. doi: 10.1080/10408398.2017.1358139. [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Zhang S, Liu C, Liu X. Curcumin promoted mir-34a expression and suppressed proliferation of gastric cancer cells. Cancer Biother Radiopharm. 2019;34(10):634–641. doi: 10.1089/cbr.2019.2874. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Zhao B, Xiong P, Wang C, Zhang J, Tian X, Huang Y. Curcumin induces autophagy via inhibition of yes-associated protein (yap) in human colon cancer cells. Med Sci Monit. 2018;24:7035–7042. doi: 10.12659/MSM.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Mohd Tajuddin WNB, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic understanding of curcumin’s therapeutic effects in lung cancer. Nutrients. 2019;11(12):2989. doi: 10.3390/nu11122989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren B, Luo S, Tian X, Jiang Z, Zou G, Xu F, Yin T, Huang Y, Liu J. Curcumin inhibits liver cancer by inhibiting damp molecule hsp70 and tlr4 signaling. Oncol Rep. 2018;40(2):895–901. doi: 10.3892/or.2018.6485. [DOI] [PubMed] [Google Scholar]
- 11.Calaf GM, Ponce-Cusi R, Carrión F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol Rep. 2018;40(4):2381–2388. doi: 10.3892/or.2018.6603. [DOI] [PubMed] [Google Scholar]
- 12.Larasati YA, Yoneda-Kato N, Nakamae I, Yokoyama T, Meiyanto E, Kato JY. Curcumin targets multiple enzymes involved in the ros metabolic pathway to suppress tumor cell growth. Sci Rep. 2018;8(1):2039. doi: 10.1038/s41598-018-20179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong SJ, Low IC, Pervaiz S. Mitochondrial ros and involvement of bcl-2 as a mitochondrial ros regulator. Mitochondrion. 2014;19(Pt A):39–48. doi: 10.1016/j.mito.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9(1):49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 15.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Frank D, Vince JE. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs SB, Miao EA. Gasdermins: Effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ, Zeng CY, Mai FY, Hu B, Ouyang DY. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in a549 lung cancer cells via caspase-3/gsdme activation. Apoptosis. 2019;24(3-4):312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 20.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects onpatient prognosis. Gastroenterology. 2017;152(4):745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 21.Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5(9):a021535. doi: 10.1101/cshperspect.a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng P, Li J, Chen Y, Zhang L. The structures and biological functions of polysaccharides from traditional chinese herbs. Prog Mol Biol Transl Sci. 2019;163:423–444. doi: 10.1016/bs.pmbts.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh A, Zhao K. Herb-drug interactions of commonly used chinese medicinal herbs. Int Rev Neurobiol. 2017;135:197–232. doi: 10.1016/bs.irn.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Tay KC, Tan LT, Chan CK, Hong SL, Chan KG, Yap WH, Pusparajah P, Lee LH, Goh BH. Formononetin: A review of its anticancer potentials and mechanisms. Front Pharmacol. 2019;10:820. doi: 10.3389/fphar.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, Mubarak MS. Anticancer potential of quercetin: A comprehensive review. Phytother Res. 2018;32(11):2109–2130. doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 26.Ren M, Xu W, Xu T. Salidroside represses proliferation, migration and invasion of human lung cancer cells through akt and mek/erk signal pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):1014–1021. doi: 10.1080/21691401.2019.1584566. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Zhu R, Zheng J, Chen C, Huang C, Ma J, Xu C, Zhai W, Zheng J. Cryptotanshinone inhibits proliferation yet induces apoptosis by suppressing stat3 signals in renal cell carcinoma. Oncotarget. 2017;8(30):50023–50033. doi: 10.18632/oncotarget.18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting CY, Wang HE, Yu CC, Liu HC, Liu YC, Chiang IT. Curcumin triggers DNA damage and inhibits expression of DNA repair proteins in human lung cancer cells. Anticancer Res. 2015;35(7):3867–3873. [PubMed] [Google Scholar]
- 29.Yu T, Dohl J, Elenberg F, Chen Y, Deuster P. Curcumin induces concentration-dependent alterations in mitochondrial function through ros in c2c12 mouse myoblasts. J Cell Physiol. 2019;234(5):6371–6381. doi: 10.1002/jcp.27370. [DOI] [PubMed] [Google Scholar]
- 30.Xia S. Biological mechanisms and therapeutic relevance of the gasdermin family. Mol Aspects Med. 2020 doi: 10.1016/j.mam.2020.100890. [DOI] [PMC free article] [PubMed] [Google Scholar]