Abstract
Background/Aim: Arsenic trioxide (As2O3) is an environmental pollutant. However, the detailed mechanisms about As2O3-induced loss of endothelial integrity are unknown. This study aimed at investigating how As2O3 causes endothelial dysfunction and whether baicalin can reverse such dysfunction. Materials and Methods: Human umbilical vein endothelial cells (HUVECs) were used to examine As2O3-induced oxidative stress, and apoptosis. The influence of baicalin on As2O3-induced endothelial dysfunction were investigated. Results: The viability of HUVECs was inhibited by As2O3 and cells underwent apoptosis. As2O3 treatment increased NADPH oxidase activity, and elevated the level of reactive oxygen species (ROS). Formamidopyrimidine DNA-glycosylase- and endonuclease III-digestible adducts were accumulated. Baicalin reversed As2O3-induced apoptosis and As2O3-suppressed cell viability. Baicalin caused a decrease in NADPH oxidase activity, and re-balanced the ROS level. As2O3-induced formamidopyrimidine DNA-glycosylase- and endonuclease III-digestible adducts were down-regulated. Conclusion: Baicalin was found to have the potential capacity to protect endothelial cells from As2O3-induced cytotoxicity.
Keywords: Apoptosis, arsenic trioxide, baicalin, human umbilical vein endothelial cells, oxidative damage
Arsenic has been recognized as a major inorganic pollutant, and drinking contaminated groundwater is a major source of arsenic toxicity for humans. The level of inorganic arsenic in contaminated groundwater has been found to be as high as 5300 μg/l in Latin America and Asian countries (1-5). From the viewpoint of epidemiology, mounting evidence has shown that chronic exposure to inorganic arsenic from drinking water is closely related to many human disorders, including atherosclerosis (6), diabetes mellitus (7), hypertension (8), ischemic heart disease (9,10) peripheral vascular disease (11), and cancer (12,13). However, extremely little information is available in the literature on the effects of inorganic arsenic exposure on the vascular system. Translational scientists are also interested in potential anti-toxic traditional Chinese medicine for protection against these disorders caused by chronic exposure to arsenic. Endothelial cells in blood vessels are the key targets of vasculopathy caused by inorganic arsenic exposure. Arsenic has been reported to increase vascular oxidative stress, which has been reported to promote cell apoptosis and dysfunction of endothelial cells (14). Oxidative stress is thought to be a major part of the intracellular mechanism of arsenic toxicity (15,16), while its effects on DNA has, as far as we are aware, never been studied.
Scutellaria baicalensis Georgi has been used as traditional Chinese medicine for more than 2,000 years, and has been reported to have antioxidant, anti-inflammatory, antibacterial, and immune-enhancing properties (17-21). Baicalin is a flavonoid representing the major bioactive component from the roots of Scutellaria baicalensis Georgi and is reported to have a series of pharmacological activities, including antioxidant, anti-inflammatory, antiviral, anti-human immunodeficiency virus and antitumor properties (17,22-27). However, to our knowledge, it has never been studied whether it may protect from arsenic toxicity. The aim of the present study was to evaluate the effects of As2O3 exposure on endothelial apoptosis and oxidative stress, and to investigate whether baicalin can effectively prevent such oxidative endothelial damage.
Materials and Methods
Cell culture and chemicals. Human umbilical vein endothelial cells (HUVECs) (CRL-1730; American Type Culture Collection, Manassas, VA, USA) were maintained in RPMI-1640 Medium (Hyclone, South Logan, UT, USA), supplemented with 10% fetal calf serum. Baicalin, As2O3, dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and propidium iodide (PI) were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and Aldrich Chemical Co. (Milwaukee, WI, USA). Baicalin was freshly dissolved in DMSO and the final concentration of DMSO in any experimental treatment was less than 0.05%.
Cell viability assay. Cell viability were performed by tetrazolium 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay as previously published (28,29). Briefly, HUVECs were cultured on 96-well plates at a density of 3×104 cells/well, and then exposed to 0-20 μM As2O3, with or without pre-treatment of 0-200 μg/ml baicalin for 1 h prior to As2O3 exposure. After As2O3 exposure, the cells were re-incubated with 0.5 mg/ml MTT. The plates were further incubated in the dark for 4 h at 37˚C. The color intensity was then measured at 570 nm using a Multiskan MS ELISA reader (Labsystems, Helsinki, Finland). Each experiment was repeated at least in triplicate.
Cell apoptosis measurement. HUVECs were seeded in 10-cm dishes at 2×106/ml and treated with 0-20 μM As2O3, with or without 0-200 μg/ml baicalin pre-treatment, and cells were incubated for 24 h. Then the cells were fixed with 70% ethanol and incubated with PI buffer [4 μg/ml PI, 0.5 μg/ml RNase, and 1% Triton X-100 in phosphate-buffered saline (PBS)] for 30 min in the dark. After the fixing and staining processes, the HUVECs were filtered through a 40-μm nylon filter and 10,000 HUVECs were analyzed for appearance of sub-G1 phase cells using a FACS Calibur instrument (BD Biosciences, San Jose, CA, USA) equipped with Cell Quest software as described elsewhere (30,31). The sub-G1 population was deemed to be representative of the percentage of cells in apoptosis. Each experiment was repeated at least in triplicate.
Measurement of ROS production. Cells were plated at a density of 2×105 cells/well into 12-well plates and incubated with 0-20 μM As2O3 alone, 0-200 μg/ml baicalin alone, or pre-treatment 0-200 μg/ml of baicalin followed by co-treatment for 12 h. The cells were harvested, re-suspended in 10 μM 2',7'-dichlorodihydrofluorescein diacetate, incubated at 37˚C for 30 min and then analyzed by flow cytometry (FACS Calibur; BD Bioscience). Results are expressed as a fold of the value of the control and each experiment was repeated at least in triplicate.
Detection of NADPH oxidase activity. HUVECs were plated at a density of 2×105 cells/well into 12-well plates and incubated with 0-20 μM As2O3 alone, 0-200 μg/ml baicalin alone, or pre-treatment 0-200 μg/ml of baicalin followed by co-treatment for 24 h, and were washed with PBS. Then cells were scraped from the plate in 1 ml of PBS and centrifuged for 10 min at 4˚C at 9,100 × g. The supernatant was discarded and the pellet was resuspended to measure the activity of NADPH oxidase. The reaction buffer contained 100 mM of Tris-HCl, 1 mM of EDTA and 0.2 mM of NADPH. NADPH oxidase activity was calculated by monitoring the alteration in absorbance at 340 nm. Each experiment was repeated at least in triplicate.
Formamidopyrimidine DNA-glycosylase (FPG) and endonuclease III-incorporated comet assay. A glass microscope slide was placed on a hot plate at 60˚C, and 100 μl of 1% normal-melting point agarose at 65˚C was firstly applied. A coverslip was applied immediately and the slide was put on ice. Then the coverslips were removed. A second layer of agarose containing HUVECs in a volume of 80 μl was applied. This second layer was prepared by mixing 400 μl of 1.5% low-melting point agarose with 200 μl of PBS containing HUVECs. A coverslip was applied to each slide with HUVECs. After the gel had solidified, the coverslips were removed. In a similar manner as the first layer, 100 μl of 1.5% low-melting-point agarose was then applied as a third layer.
For enzyme digestion, as performed previously (32,33), slides were washed after lysis treatment with distilled water then incubated at 37˚C for 30 min in enzyme reaction buffer. Then one unit of FPG or one unit of endonuclease III in 10 μl of enzyme buffers supplied by Trevigen (Gaithersburg, MD, USA) [FDG: 10 mM HEPES-KOH (pH 7.4), 100 mM KCl; endonuclease III: 10 mM EDTA, 0.1 mg/ml bovine serum albumin; and 10 mM HEPES-KOH (pH 7.4), 100 mM KCl, and 10 mM EDTA] was added. A coverslip was applied and the slides were further incubated at 37˚C for 2 h.
After removing coverslips, slides were immersed in cell lysis solution (2.5 M NaCl, 100 mM EDTA and 10 mM Tris, pH adjusted to 10.0 with NaOH, and 1% N-lauroylsarcosine, 1% Triton X-100, and 10% DMSO added immediately before use) at 4˚C for 18 h. DNA was totally denatured by incubating in 0.3 M NaOH, 1 mM EDTA pH 13.4 for 20 min. Electrophoresis was performed at 25 V, 300 mA for 25 min using a tank. The slides were stained by adding 12.5 μl of 200x SYBR Green I. A coverslip was applied and the comets were observed under a fluorescence microscope (wavelength 450-490 nm, farb teiler 510 nm, long-pass filter 520 nm). The comet moment was calculated using the formula ∑0→n [(amount of DNA at distance X)×(distance X)]/total DNA.
Statistical analysis. The results were expressed as the mean±standard error of the mean of at least three independent experiments in which triplicate samples were performed. Statistical significance was assessed using Student’s t-test and one-way ANOVA followed by post hoc test using SPSS (version 15.0) software (SPSS Inc., Chicago, IL, USA). A value of p<0.05 was considered statistically significant.
Results
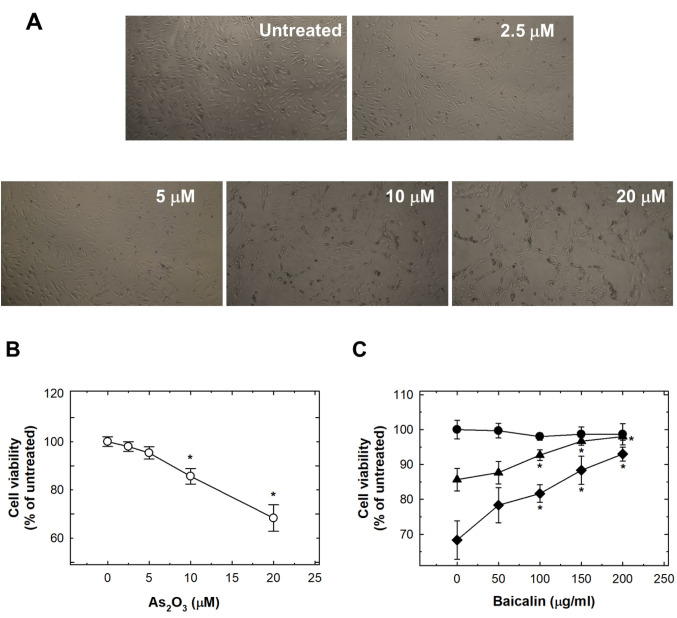
As2O3-induced cytotoxicity and apoptosis were suppressed by baicalin in HUVECs. Originally, we found that As2O3 induced cell cytotoxicity, with dose-dependent reduction of cell viability (Figure 1A). Treatment of HUVECs with 10 μM and 20 μM reduced viability by 14.3% and 31.7%, respectively (Figure 1B). Pre-treatment with baicalin restored cell viability to almost normal levels (Figure 1C).
Figure 1. The effects of As2O3 and baicalin on viability of human umbilical vein endothelial cells (HUVECs). A: The effects of 24-h treatment of HUVECs with 0-20 μM As2O3. HUVECs were photographed under a microscope at ×40, representative images are shown. B: The quantification of the effect of 0-20 μM As2O3 treatment for 24 h in HUVECs. C: The effect on HUVEC viability after pretreatment with baicalin for 1 h, and cotreatment of baicalin with 20 μM (◆), 10 μM (▲) or 0 μM (●) As2O3 for 24 h. Data are the mean±SD for three independent experiments. *Significantly different at p<0.05 compared with untreated control cells.
Treatment with As2O3 at 5 μM or more for 24 h was capable of inducing a significant sub G1 population of cells, i.e. significant apoptosis (Figure 2A). Pre-treatment with baicalin abrogated the activation of apoptosis dose-dependently (Figure 2B).
Figure 2. The effects of As2O3 and baicalin on apoptosis of human umbilical vein endothelial cells (HUVECs). A: The effects of 24-h treatment with 0-20 μM As2O3 on HUVECs. Apoptotic cells were detected by flow cytometry by the appearance of a sub-G1 cell population. B: The effect on apoptosis of HUVECs after pretreatment with baicalin for 1 h, and co-treatment of baicalin with 20 μM (◆), 10 μM (▲) or 0 μM (●) As2O3 for 24 h. Data are the mean±SD for three independent experiments. *Significantly different at p<0.05 compared with untreated cells.
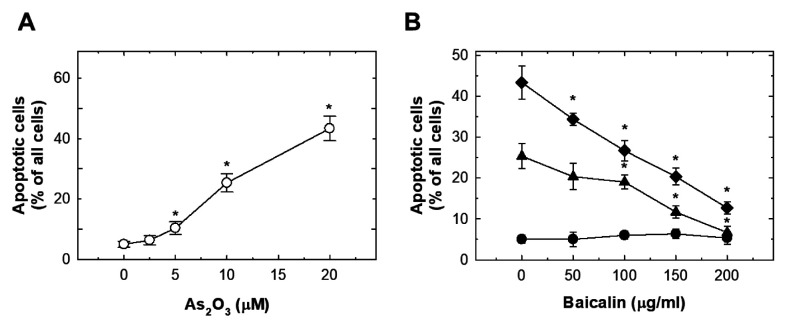
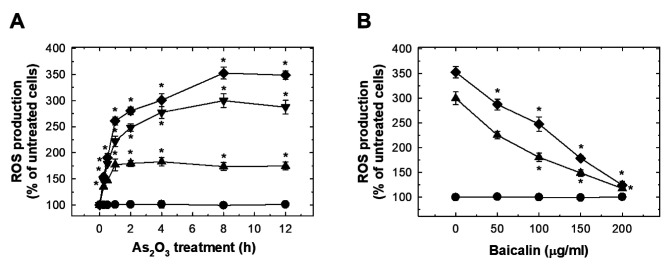
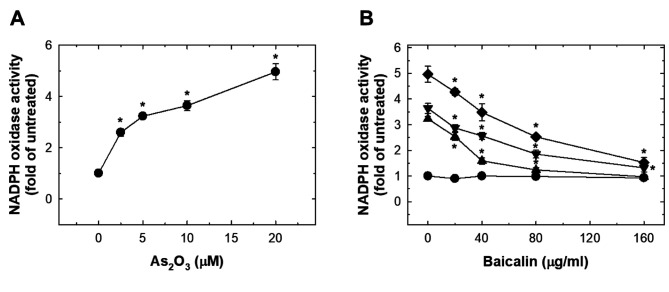
As2O3 induced oxidative stress in HUVECs which was suppressed by baicalin. As2O3 treatment dose-dependently induced significant ROS production at 15 min (Figure 3A). The peak ROS level was reached at 8 h, and plateau at 12 h (Figure 3A). The effect of As2O3 treatment at 10 and 20 μM on ROS was suppressed by pre-treatment with baicalin (Figure 3B). Exposure to As2O3 increased NADPH oxidase activity dose-dependently (Figure 4A). Pre-treatment with baicalin abrogated As2O3-induced activation of NADPH oxidase (Figure 4B).
Figure 3. The effects of As2O3 and baicalin on reactive oxygen species (ROS) production in human umbilical vein endothelial cells (HUVECs). A: HUVECs were treated with 0, 5, 10, and 20 μM As2O3 for the indicated time, and ROS were detected with 2',7'-dichlorodihydrofluorescein diacetate. B: The effect on ROS production in HUVECs after pretreatment with baicalin for 1 h, and co-treatment of baicalin with 20 μM (◆), 10 μM (▼), 5 μM (▲) or 0 μM (●) As2O3 for 12 h. Data are the mean±SD for three independent experiments. *Significantly different at p<0.05 compared with untreated cells.
Figure 4. The effects of As2O3 and baicalin on NADPH oxidase activity in HUVECs. A: HUVECs were treated with 0-20 μM As2O3 for 24 h and NADPH oxidase activity was evaluated as fold that of untreated cells. B: The effect on NADPH oxidase activity in HUVECs after pretreatment of baicalin for 1 h, and co-treatment of baicalin with 20 μM (◆), 10 μM (▼), 5 μM (▲) or 0 μM (●) As2O3 for 24 h. Data are the mean±SD for three independent experiments. *Significantly different at p<0.05 compared with untreated cells.
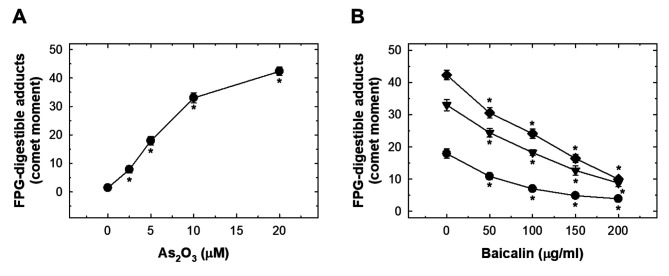
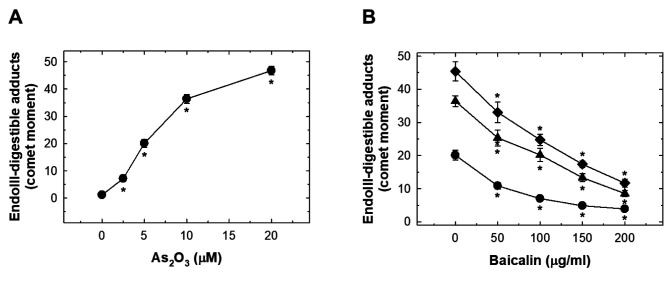
As2O3 induced oxidative FPG- and endonuclease III-digestible DNA adducts in HUVECs which was suppressed by baicalin. As2O3 treatment induced a significant level of FPG- and endonuclease III-digestible adducts (Figures Figure 5A and Figure 6A). The levels of these oxidative adducts were effectively reduced by pre-treatment with baicalin but did not reach basal levels (Figures Figure 5B and Figure 6B).
Figure 5. The effects of As2O3 and baicalin on formamidopyrimidine-DNA glycosylase (FPG)-digestible adducts in human umbilical vein endothelial cells (HUVECs). A: HUVECs were treated with 0-20 μM As2O3 for 24 h and FPG-digestible adducts produced were detected with comet assay. The data are presented as comet moments. B: The effect on FPG-digestible adducts in HUVECs after pretreatment with baicalin for 1 h, and cotreatment of baicalin with 20 μM (◆), 10 μM (▼) or 0 μM (●) As2O3 for 24 h. Data are the mean±SD for three independent experiments. *Significantly different at p<0.05 compared with untreated cells.
Figure 6. The effects of As2O3 and baicalin on endonuclease III-digestible adducts in human umbilical vein endothelial cells (HUVECs). A: HUVECs were treated with 0-20 μM As2O3 for 24 h and FPG-digestible adducts produced were detected with comet assay. The data are presented as comet moments. B: The effect on endonuclease III-digestible adducts in HUVECs after pretreatment with baicalin for 1 h, and co-treatment of baicalin with 20 μM (◆), 10 μM (▼) or 0 μM (●) As2O3 for 24 h. Data are the mean±SD for three independent experiments. *Significantly different at p<0.05 compared with untreated cells.
Discussion
Vascular endothelium not only serves as a physical monolayer between blood and tissue but plays an important role in detection and reaction to blood-borne stresses. Impaired endothelial function maybe an irreversible process in the initiation event of various cardiovascular diseases and it is worth determining the practical cardio-protective effects of Chinese traditional medicine, such as baicalin. Arsenic exposure induced HUVEC dysfunction through pro-apoptotic and oxidative stress, which might be responsible for its adverse effects on blood vessels. The protective role of Chinese traditional medicine may be of great help in prevention of cardiovascular disorders. In this study, the effects of baicalin on As2O3-induced endothelial cell apoptosis, oxidative stress and oxidative DNA adduct production were revealed for the first time.
Increased endothelial cell apoptosis may contribute to the loss of endothelial integrity and lead to vascular disorders (34,35). Our study confirmed a significant increase in HUVEC apoptosis when cells were treated with As2O3, while pre-treatment with baicalin reduced As2O3-induced HUVEC apoptosis (Figure 2B). The degree of As2O3-induced apoptosis was not as strong as in Yu et al.’s finding, reporting that 5 μM As2O3 was capable suppressed cell viability by about 40% of (36). Our results are consistent with Ma et al.’s finding that 5 μM As2O3 induced apoptosis in less than 20% of HUVECs (37). The As2O3 induced generation of ROS was rapidly detected, and sustained for as long as 12 h (Figure 3A). Pre-treatment with baicalin did not alter the level of ROS itself but did suppress ROS production induced by As2O3. A number of risk factors for cardiovascular disorders were reported to be associated with increased vascular ROS (38,39). Our studies also showed that As2O3 activated NADPH oxidase, which was reversed by pre-treatment with baicalin. Activation of NADPH oxidase may be the major mechanism related to As2O3-mediated intracellular generation of ROS.
One of the highlights of this was the detection of FPG-digestible adducts and endonuclease III-digestible adducts. FPG specifically cleaves oxidized purines, including 8-oxoguanine, 5-hydroxycytosine, 5-hydroxyuracil, 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine, and 4,6-diamino-5-formamidopyrimidine (40), while endonuclease III specifically cleaves oxidized pyrimidines, including thymine glycol, 5,6-dihydrothymine, 5-hydroxydihydrothymine, 5-hydroxycytosine, 5-hydroxyuracil, and uracil glycol (41). We also demonstrated that baicalin pre-treatment reduced the formation of As2O3-induced oxidative adducts, although the detailed mechanisms of this need further investigation.
In conclusion, at a non-toxic dose, baicalin is of reducing the As2O3-induced apoptosis, oxidative stress and DNA adducts. The underlying mechanisms may provide novel insights into potential clinical cardioprotective effects.
Conflicts of Interest
The Authors declare no conflicts of interest in regard to this study.
Authors’ Contributions
C.L.T. and C.W.T. conceived and designed the experiments. W.S.C. and C.W.T. performed the experiments. J.C.L. and T.C.H. analyzed the data. C.L.T. and J.C.L. contributed reagents/materials/analysis tools. C.W.T. and D.T.B. wrote the article.
Acknowledgements
Yun-Chi Wang and Yu-Chen Hsiau helped in the experiments and analysis. This study was supported mainly by grants from Taichung Veterans General Hospital (TCVGH-VHCY1098604).
References
- 1.Jadhav SV, Bringas E, Yadav GD, Rathod VK, Ortiz I, Marathe KV. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J Environ Manage. 2015;162:306–325. doi: 10.1016/j.jenvman.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Guzman A, Nava JL, Coreno O, Rodriguez I, Gutierrez S. Arsenic and fluoride removal from groundwater by electro-coagulation using a continuous filter-press reactor. Chemosphere. 2016;144:2113–2120. doi: 10.1016/j.chemosphere.2015.10.108. [DOI] [PubMed] [Google Scholar]
- 3.Pi K, Wang Y, Xie X, Su C, Ma T, Li J, Liu Y. Hydrogeochemistry of co-occurring geogenic arsenic, fluoride and iodine in groundwater at Datong Basin, northern China. J Hazard Mater. 2015;300:652–661. doi: 10.1016/j.jhazmat.2015.07.080. [DOI] [PubMed] [Google Scholar]
- 4.Rasool A, Xiao T, Baig ZT, Masood S, Mostofa KM, Iqbal M. Co-occurrence of arsenic and fluoride in the groundwater of Punjab, Pakistan: Source discrimination and health risk assessment. Environ Sci Pollut Res Int. 2015;22(24):19729–19746. doi: 10.1007/s11356-015-5159-2. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Horta C, Ballinas-Casarrubias L, Sanchez-Ramirez B, Ishida MC, Barrera-Hernandez A, Gutierrez-Torres D, Zacarias OL, Saunders RJ, Drobna Z, Mendez MA, Garcia-Vargas G, Loomis D, Styblo M, Del Razo LM. A concurrent exposure to arsenic and fluoride from drinking water in Chihuahua, Mexico. Int J Environ Res Public Health. 2015;12(5):4587–4601. doi: 10.3390/ijerph120504587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh YC, Lien LM, Chung WT, Hsieh FI, Hsieh PF, Wu MM, Tseng HP, Chiou HY, Chen CJ. Significantly increased risk of carotid atherosclerosis with arsenic exposure and polymorphisms in arsenic metabolism genes. Environ Res. 2011;111(6):804–810. doi: 10.1016/j.envres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Brauner EV, Nordsborg RB, Andersen ZJ, Tjonneland A, Loft S, Raaschou-Nielsen O. Long-term exposure to low-level arsenic in drinking water and diabetes incidence: A prospective study of the diet, cancer and health cohort. Environ Health Perspect. 2014;122(10):1059–1065. doi: 10.1289/ehp.1408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: A systematic review. Environ Health Perspect. 2012;120(4):494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, Shaheen I, Sarwar G, Ahmed A, Islam T, Slavkovich V, Rundek T, Demmer RT, Desvarieux M, Ahsan H. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am J Epidemiol. 2013;178(3):372–381. doi: 10.1093/aje/kwt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CH. Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis. 2008;199(1):12–18. doi: 10.1016/j.atherosclerosis.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Zimta AA, Schitcu V, Gurzau E, Stavaru C, Manda G, Szedlacsek S, Berindan-Neagoe I. Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ Res. 2019;178:108700. doi: 10.1016/j.envres.2019.108700. [DOI] [PubMed] [Google Scholar]
- 13.Wei S, Zhang H, Tao S. A review of arsenic exposure and lung cancer. Toxicol Res. 2019;8(3):319–327. doi: 10.1039/c8tx00298c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi N, Flora G, Kushwaha P, Flora SJ. Alpha-lipoic acid protects oxidative stress, changes in cholinergic system and tissue histopathology during co-exposure to arsenic-dichlorvos in rats. Environ Toxicol Pharmacol. 2014;37(1):7–23. doi: 10.1016/j.etap.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Kesavan M, Sarath TS, Kannan K, Suresh S, Gupta P, Vijayakaran K, Sankar P, Kurade NP, Mishra SK, Sarkar SN. Atorvastatin restores arsenic-induced vascular dysfunction in rats: Modulation of nitric oxide signaling and inflammatory mediators. Toxicol Appl Pharmacol. 2014;280(1):107–116. doi: 10.1016/j.taap.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci Biotechnol Biochem. 2006;70(10):2371–2380. doi: 10.1271/bbb.50698. [DOI] [PubMed] [Google Scholar]
- 18.Jeong K, Shin YC, Park S, Park JS, Kim N, Um JY, Go H, Sun S, Lee S, Park W, Choi Y, Song Y, Kim G, Jeon C, Park J, Lee K, Bang O, Ko SG. Ethanol extract of Scutellaria baicalensis Georgi prevents oxidative damage and neuroinflammation and memorial impairments in artificial senescence mice. J Biomed Sci. 2011;18(1):14. doi: 10.1186/1423-0127-18-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C, Ji GE. Bioconversion of flavones during fermentation in milk containing Scutellaria baicalensis extract by Lactobacillus brevis. J Microbiol Biotechnol. 2013;23(10):1422–1427. doi: 10.4014/jmb.1305.05001. [DOI] [PubMed] [Google Scholar]
- 20.Yoon JJ, Jeong JW, Choi EO, Kim MJ, Hwang-Bo H, Kim HJ, Hong SH, Park C, Lee DH, Choi YH. Protective effects of Scutellaria baicalensis Georgi against hydrogen peroxide-induced DNA damage and apoptosis in HaCaT human skin keratinocytes. EXCLI J. 2017;16:426–438. doi: 10.17179/excli2016-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Yang ZY, Tang RC. Bioactive and UV protective silk materials containing baicalin – The multifunctional plant extract from Scutellaria baicalensis Georgi. Mater Sci Eng C Mater Biol Appl. 2016;67:336–344. doi: 10.1016/j.msec.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Chuah AM, Yamaguchi T, Takamura H, Matoba T. Antioxidant activity of traditional Chinese medicinal herbal materials. Food Sci Technol Res. 2008;14(2):205–210. doi: 10.3136/fstr.14.205. [DOI] [Google Scholar]
- 23.Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, Kang J, Wang JM. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000;49(3):295–306. doi: 10.1016/s0162-3109(00)00244-7. [DOI] [PubMed] [Google Scholar]
- 24.Li BQ, Fu T, Yan YD, Baylor NW, Ruscetti FW, Kung HF. Inhibition of HIV infection by baicalin–a flavonoid compound purified from Chinese herbal medicine. Cell Mol Biol Res. 1993;39(2):119–124. [PubMed] [Google Scholar]
- 25.Pan TL, Wang PW, Leu YL, Wu TH, Wu TS. Inhibitory effects of Scutellaria baicalensis extract on hepatic stellate cells through inducing G2/M cell-cycle arrest and activating ERK-dependent apoptosis via BAX and caspase pathway. J Ethnopharmacol. 2012;139(3):829–837. doi: 10.1016/j.jep.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Park JR, Lee MC, Moon SC, Kim J, Ha KT, Park EJ, Hong C, Seo BD, Kim BJ. Scutellaria baicalensis Georgi induces caspase-dependent apoptosis via mitogen-activated protein kinase activation and the generation of reactive oxygen species signaling pathways in MCF-7 breast cancer cells. Mol Med Rep. 2017;16(2):2302–2308. doi: 10.3892/mmr.2017.6798. [DOI] [PubMed] [Google Scholar]
- 27.Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z, Peng J. Scutellaria barbata D. Don inhibits tumor angiogenesis via suppression of Hedgehog pathway in a mouse model of colorectal cancer. Int J Mol Sci. 2012;13(8):9419–9430. doi: 10.3390/ijms13089419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu SW, Hsu PC, Chang WS, Yu CC, Wang YC, Yang JS, Tsai FJ, Chen KY, Tsai CW, Bau DT. Protective effects of valproic acid on 6-hydroxydopamine-induced neuroinjury. Environ Toxicol. 2020;35(8):840–848. doi: 10.1002/tox.22920. [DOI] [PubMed] [Google Scholar]
- 29.Chang WS, Lin EY, Hsu SW, Hu PS, Chuang CL, Liao CH, Fu CK, Su CH, Gong CL, Hsiao CL, Bau DT, Tsai CW. Baicalin scavenged reactive oxygen species and protected human keratinocytes against UVB-induced cytotoxicity. In Vivo. 2016;30(5):605–610. [PubMed] [Google Scholar]
- 30.Lin CC, Chen KB, Tsai CH, Tsai FJ, Huang CY, Tang CH, Yang JS, Hsu YM, Peng SF, Chung JG. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via RAS/AKT/ NF-kappaB signaling pathways. J Food Biochem. 2019;43(7):e12902. doi: 10.1111/jfbc.12902. [DOI] [PubMed] [Google Scholar]
- 31.Wu KM, Hsu YM, Ying MC, Tsai FJ, Tsai CH, Chung JG, Yang JS, Tang CH, Cheng LY, Su PH, Viswanadha VP, Kuo WW, Huang CY. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr Metab. 2019;16:36. doi: 10.1186/s12986-019-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SC, Chen SF, Lee YM, Chuang CL, Bau DT, Lin SS. Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In Vivo. 2013;27(6):707–714. [PubMed] [Google Scholar]
- 33.Tsai CW, Yang MD, Hsia TC, Chang WS, Hsu CM, Hsieh YH, Chung JG, Bau DT. Dithiothreitol enhanced arsenic-trioxide-induced cell apoptosis in cultured oral cancer cells via mitochondrial dysfunction and endoplasmic reticulum stress. Environ Toxicol. 2017;32(1):17–27. doi: 10.1002/tox.22208. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Shen H, Xu M, Liu O, Zhao L, Liu S, Guo Z, Du J. FRP inhibits ox-LDL-induced endothelial cell apoptosis through an AKT-NF-{kappa}B-BCL-2 pathway and inhibits endothelial cell apoptosis in an apoE-knockout mouse model. Am J Physiol Endocrinol Metab. 2010;299(3):E351–E363. doi: 10.1152/ajpendo.00005.2010. [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Xiang G, Lu J, Xiang L, Dong J, Mei W. TRAIL protects against endothelium injury in diabetes via AKT-eNOS signaling. Atherosclerosis. 2014;237(2):718–724. doi: 10.1016/j.atherosclerosis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Yu CX, Zhang YY, Wu XY, Tang HX, Liang XQ, Xue ZM, Xue YD, Li J, Zhu H, Huo R, Ban T. Transient receptor potential melastatin 4 contributes to early-stage endothelial injury induced by arsenic trioxide. Toxicol Lett. 2019;312:98–108. doi: 10.1016/j.toxlet.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y, Ma Z, Yin S, Yan X, Wang J. Arsenic and fluoride induce apoptosis, inflammation and oxidative stress in cultured human umbilical vein endothelial cells. Chemosphere. 2017;167:454–461. doi: 10.1016/j.chemosphere.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 38.States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tousoulis D, Briasoulis A, Papageorgiou N, Tsioufis C, Tsiamis E, Toutouzas K, Stefanadis C. Oxidative stress and endothelial function: therapeutic interventions. Recent Pat Cardiovasc Drug Discov. 2011;6(2):103–114. doi: 10.2174/157489011795933819. [DOI] [PubMed] [Google Scholar]
- 40.Nickoloff JA, Spirio LN, Reynolds RJ. A comparison of calcium phosphate coprecipitation and electroporation. Implications for studies on the genetic effects of DNA damage. Mol Biotechnol. 1998;10(2):93–101. doi: 10.1007/BF02760857. [DOI] [PubMed] [Google Scholar]
- 41.Sarker AH, Ikeda S, Nakano H, Terato H, Ide H, Imai K, Akiyama K, Tsutsui K, Bo Z, Kubo K, Yamamoto K, Yasui A, Yoshida MC, Seki S. Cloning and characterization of a mouse homologue (mNthl1) of Escherichia coli endonuclease III. J Mol Biol. 1998;282(4):761–774. doi: 10.1006/jmbi.1998.2042. [DOI] [PubMed] [Google Scholar]