Abstract
Background/Aim: Eosinophils are blood cells responsible for response against parasites and allergens. Εοsinophil to lymphocyte ratio (ELR) is a biomarker for inflammatory conditions. Our aim was to evaluate the role of eosinophils and ELR in COVID-19 patients. Patients and Methods: The study included 96 patients hospitalized with COVID-19. They were classified into moderate to severe cases and critical cases. Eosinophils and ELR were determined in both groups, in patients that died or survived and were correlated to duration of hospitalization. Results: There was a statistically significant decrease in eosinophils and ELR between patients that died and patients that survived (p<0.05), and in mean values of the two biomarkers (p<0.05 for eosinophils and p<0.05 for ELR) between patients hospitalized for more or less than 15 days among those with moderate to severe disease. Conclusion: Lower eosinophil counts and ERL could probably predict worse outcome in COVID-19 patients.
Keywords: Eosinophils, lymphocyte, ratio, biomarker, prediction, COVID-19
Eosinophils are a type of white blood cells and an immune system component against multicellular parasites. Along with mast cells and basophils, they control mechanisms related to allergy and asthma. These granulocytes develop during hematopoiesis in the bone marrow, migrate into blood where they differentiated and do not multiply (1).
The role of eosinophils against respiratory viruses has been investigated in numerous studies (2-4). Eosinophil-activated cytokines, such as pre-formed interleukin-2 (IL-2), interleukin-12 (IL-12), and interferon-c (IFN-c) which are typical T-helper 1 (Th1) cytokines involved in antiviral responses, produce molecules with antiviral activity, including RNases and reactive nitrogen species and they contain receptors allowing the recognition of viruses (2-4). In addition, eosinophils can migrate to lymph nodes, where they can present antigens to T cells (2). Eosinophil antiviral immune response has been described against some respiratory viruses, including respiratory syncytial virus (3) and influenza (4). Eosinophils express numerous toll-like receptors (TLRs) including those that participate in viral recognition such as TLR-3, TLR-7, and TLR-9 (5). They also produce nitric oxide (NO) by inducible NO synthase, that inhibits viral replication through various mechanisms (6).
Coronavirus belongs to a large virus family, which members cause common cold and serious illnesses, such as Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). The 2019 coronavirus disease (COVID-19) was found to be responsible for unexplained viral pneumonia in Wuhan, China, in December 2019 and on March 11, 2020, the World Health Organization (WHO) characterized the outbreak as a pandemic (7). The estimated case-fatality rate for COVID-19 is about 3.5%, similar to Spanish influenza and higher than that of seasonal influenza. Over 80% of COVID-19 cases have mild symptoms and 10-20% of COVID-19 cases proceed to a severe illness. In cases of critical illness, patients progress rapidly to acute respiratory failure, acute respiratory distress syndrome, metabolic acidosis, bleeding disorder and sepsis (8).
The role of eosinophils in COVID-19 has been reported. Peripheral blood eosinophil counts have been described as an effective and efficient indicator for diagnosis, evaluation and prognosis of COVID-19 patients (9). Persistent eosinopenia after admission has been associated with high disease severity and low rates of recovery (10). It has been also reported that dynamic changes in routine blood parameters, including eosinophils, might be prognostic markers for COVID-19 patients and the evaluation of treatment efficacy (11).
The eοsinophil to lymphocyte ratio (ELR) is a novel marker, evaluated in several inflammatory conditions and malignancies. A high ELR is associated with cigarette smoking and may be useful indicator of systemic inflammation activity, even in healthy smokers (12). The ELR has been reported to be helpful in differentiating patients with bronchial asthma with and without nonsteroidal anti-inflammatory drug (NSAID) hypersensitivity (13). Moreover, increased ELR portend worse overall survival in endometrial cancer, especially in patients characterized as a high-risk group (14). Additionally, ELR has been associated with prolonged overall survival in patients with locally advanced pancreatic cancer undergoing chemoradiotherapy (15).
Our aim was to evaluate the role of peripheral eosinophils and ELR in COVID-19 patients.
Patients and Methods
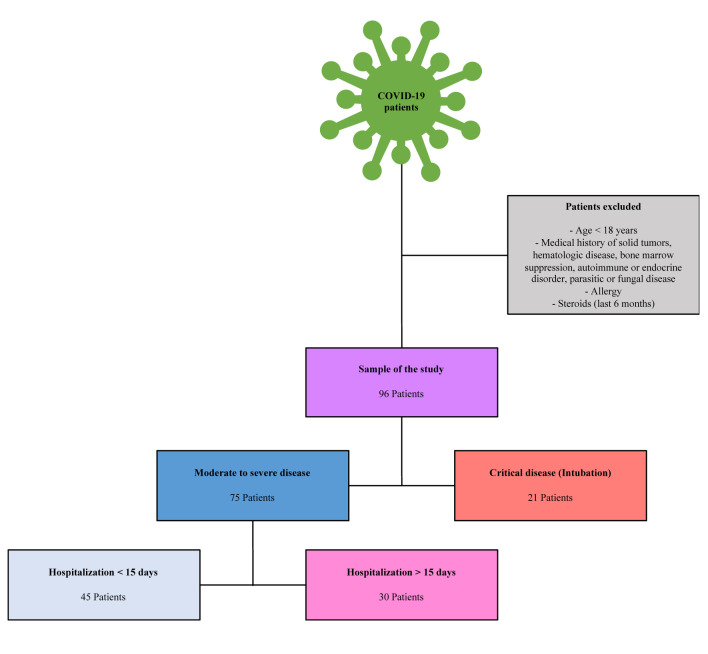
Study design. The study involved patients hospitalized with clinical presentation of lower respiratory infection that were tested positive for COVID-19 by real-time reverse transcription polymerase chain reaction assay (RT-PCR test), which is the gold standard for the diagnosis of the disease. The exclusion criteria were: patients <18 years old, patients with a history of solid tumors or hematological malignancy, patients suffering from disease or receiving therapy that suppresses the bone marrow activity, patients with autoimmune and endocrine disorders, patients with parasitic and fungal diseases, patients with allergic disorders and patients received steroids within the previous six months. All these clinical conditions can affect peripheral eosinophil counts. Whole blood samples were collected from the patients on admission. Complete blood counts, which included total white blood cells, neutrophils, lymphocytes and eosinophils were obtained using the automated ΧΕ 2100 hematology analyzer (Sysmex Corporation, Kobe, Japan). ELR was calculated as the ratio of the eosinophils to lymphocytes. The patients were classified in two groups (16). The first group included moderate to severe cases and the second group included critical cases that underwent intubation. The first group was furthermore divided in two subgroups: The first included 45 patients with duration of hospitalization less than 15 days and the second included 30 patients with duration of hospitalization more than 15 days. This classification was based on WHO guidance (16). Peripheral eosinophil counts and ELR were determined in both groups, compared between patients who died and patients who survived and correlated to duration of hospitalization.
Figure 1 summarizes the patients selection criteria and flow chart of our study.
Figure 1. Patients selection criteria and flow chart of the current study.
Statistical analysis. The data was analyzed with the use of SPSS software (version 13.0: SPSS Inc, Chicago, IL, USA). Differences between the independent groups were assessed by Student’s t-test and one-way analysis of variance. Data are presented as mean±SD. Statistical significance was set at p<0.05 level (two-tailed).
Results
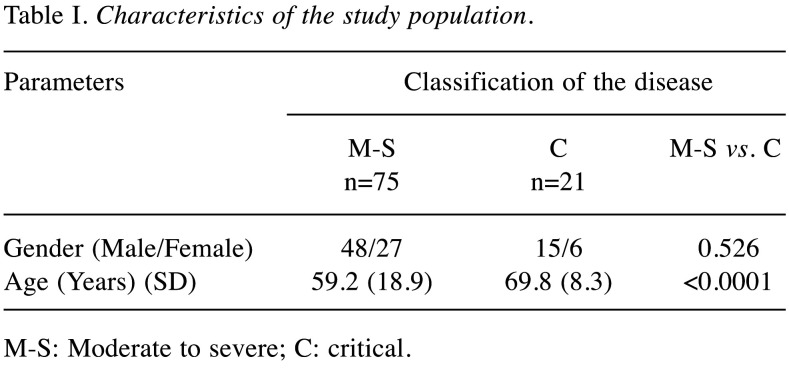
A total of 96 patients, 63 males and 33 females, with a mean age of 59.64±19.08 years with lower respiratory infection and positive RT-PCR test for COVID-19, hospitalized from March 2020 to May 2020, were included. Seventy-five patients (48 males and 27 females) had moderate to severe disease and 21 patients (15 males and 6 females) had critical disease and were intubated. Table I summarizes the characteristics of the study population.
Table I. Characteristics of the study population.
M-S: Moderate to severe; C: critical
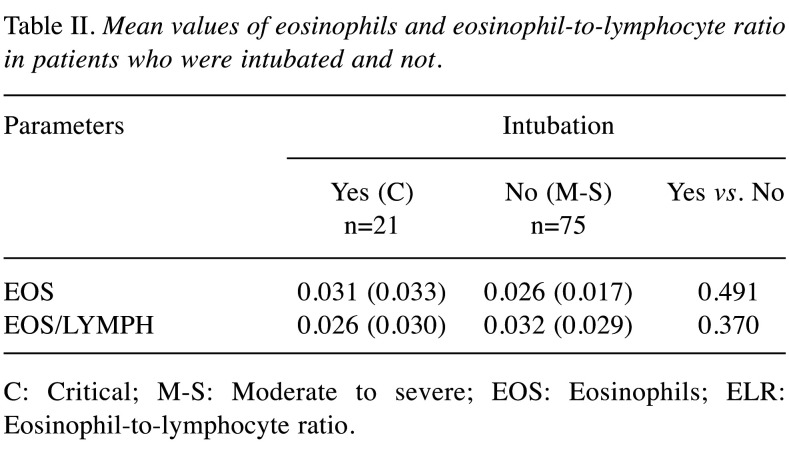
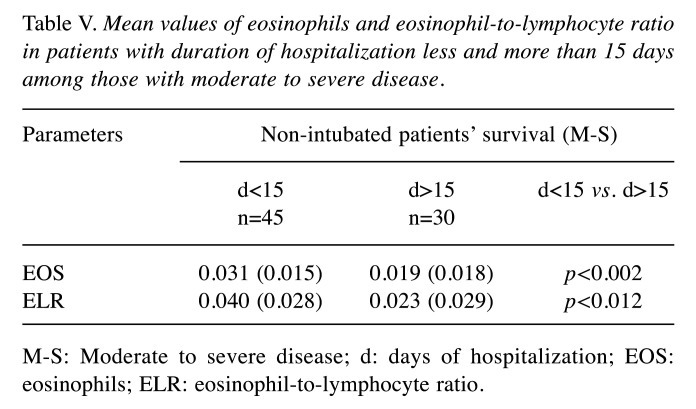
The mean value of absolute eosinophil number was 0.026±0.017 K/μl in patients with moderate to severe disease and 0.031±0.033 K/μl in patients that were intubated (p=0.491) (Figure 2). The mean value of ELR was 0.026±0.030 in patients with moderate to severe disease and 0.032±0.029 in patients that were intubated (p=0.370) (Table II, Figure 3).
Figure 2. Mean value of eosinophil count in patients with moderate to severe disease and in patients who were intubated or not. EOS: Eosinophils.
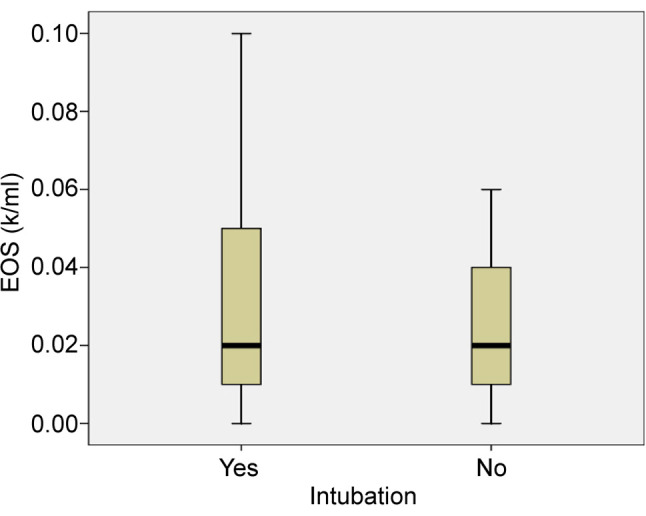
Table II. Mean values of eosinophils and eosinophil-to-lymphocyte ratio in patients who were intubated and not.
C: Critical; M-S: Moderate to severe; EOS: Eosinophils; ELR: Eosinophil-to-lymphocyte ratio
Figure 3. Mean value of eosinophil to lymphocyte ratio (ELR) in patients with moderate to severe disease and in patients who were intubated or not. 28, 83: Outliers. ELR: Eosinophil to lymphocyte ratio.

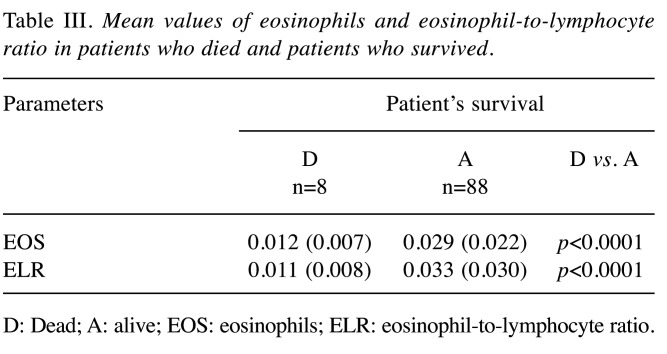
From the 96 patients, 8 patients died. All patients that died had critical illness and were intubated. The mean value of absolute eosinophil number was 0.012±0.007 K/μl in patients who died and 0.029±0.022 K/μl in patients that survived (p<0.0001) (Figure 4). The mean value of ELR was 0.011±0.008 in patients who died and 0.033±0.030 in patients who survived (p<0.0001) (Table III, Figure 5).
Figure 4. Mean value of eosinophil count in patients who died and in patients who survived. 7: Outlier. EOS: Eosinophils.
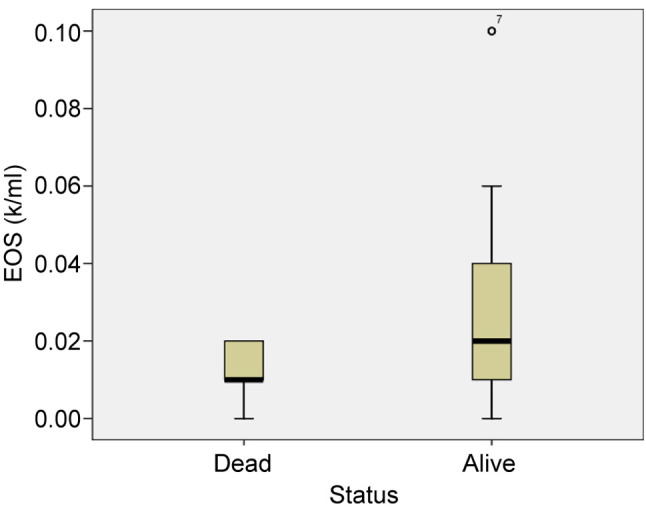
Table III. Mean values of eosinophils and eosinophil-to-lymphocyte ratio in patients who died and patients who survived.
D: Dead; A: alive; EOS: eosinophils; ELR: eosinophil-to-lymphocyte ratio
Figure 5. The mean value of eosinophil to lymphocyte ratio (ELR) in patients who died and in patients who survived. 28, 83: Outliers. ELR: Eosinophil to lymphocyte ratio.
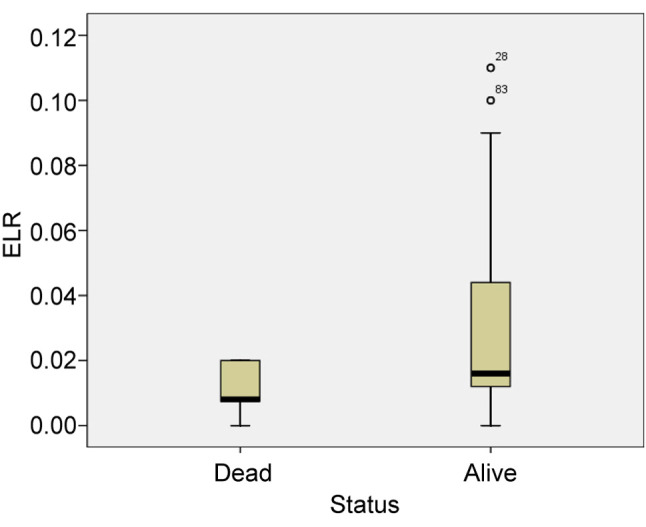
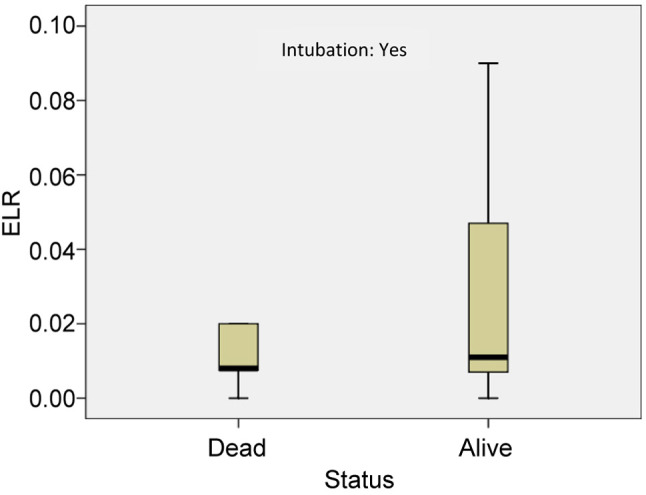
Furthermore, the mean value of absolute eosinophil number was 0.012±0.007 K/μl in patients who were intubated and died and 0.043±0.037 K/μl in patients who survived (p<0.05) (Figure 6). The mean value of ELR was 0.011±0.008 in patients who died and 0.035±0.035 in patients who survived (p<0.005) (Table IV, Figure 7).
Figure 6. Mean value of eosinophil count in patients who were intubated and died and in patients who were intubated and survived. EOS: Eosinophils.
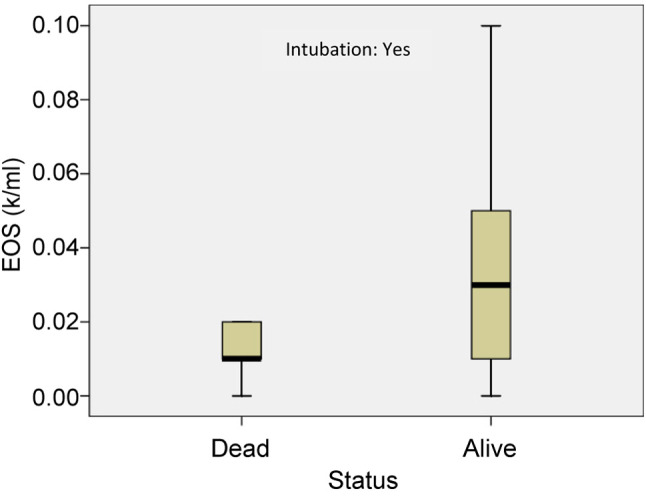
Table IV. Mean values of eosinophils and eosinophil-to-lymphocyte ratio in patients who died and patients who survived among intubated.
C: Critical; D: dead; A: alive; EOS: eosinophils; ELR: eosinophil-tolymphocyte ratio
Figure 7. The mean value of eosinophil to lymphocyte ratio (ELR) in patients who were intubated and died and patients who were intubated and survived. ELR: Eosinophil to lymphocyte ratio.
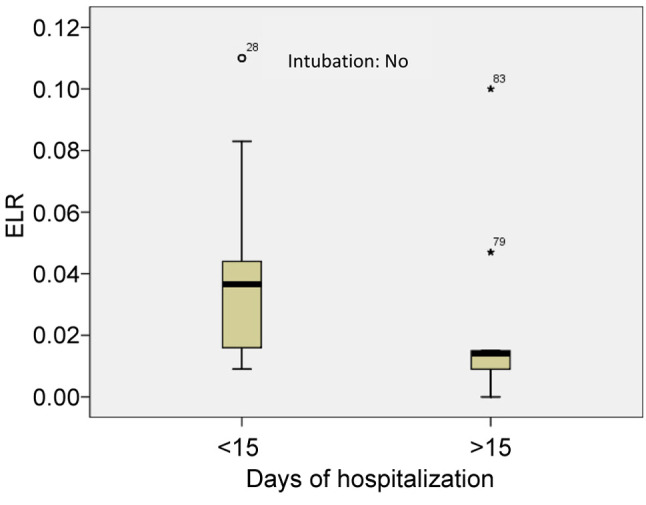
As mentioned above, the patients with moderate to severe disease were divided in two groups. The first group included 45 patients with a duration of hospitalization of less than 15 days and the second group included 30 patients with duration of hospitalization of more than 15 days. The mean value of absolute eosinophil number was 0.012±0.007 K/μl in the first group and 0.019±0.018 K/μl in the second group (p<0.005) (Figure 8). The mean value of ELR was 0.040±0.028 in the first group and 0.023±0.029 in the second group (p<0.005) (Table V, Figure 9).
Figure 8. Mean value of eosinophil count in patients with a duration of hospitalization of less than 15 days and in patients with hospitalization of more than 15 days (patients with moderate to severe disease). 79: Extreme value, 83: Outlier. EOS: Eosinophils.
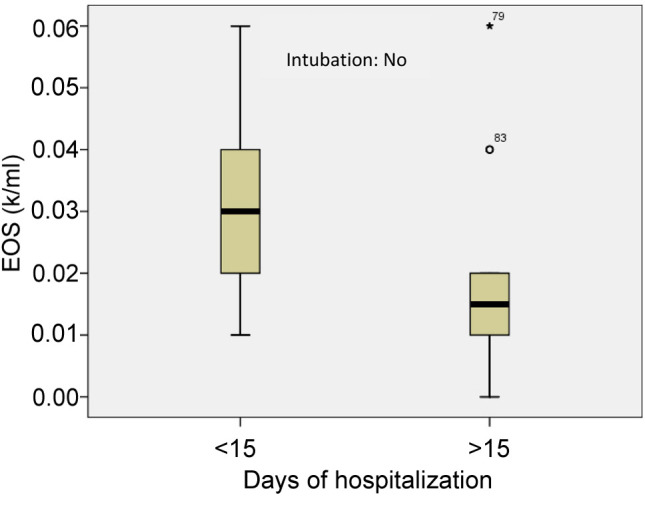
Table V. Mean values of eosinophils and eosinophil-to-lymphocyte ratio in patients with duration of hospitalization less and more than 15 days among those with moderate to severe disease.
M-S: Moderate to severe disease; d: days of hospitalization; EOS: eosinophils; ELR: eosinophil-to-lymphocyte ratio
Figure 9. Mean value of eosinophil to lymphocyte ratio (ELR) in patients with a duration of hospitalization of less than 15 days and in patients with hospitalization of more than 15 days (patients with moderate to severe disease). 79, 83: Extreme values, 28: Outlier. ELR: Eosinophil to lymphocyte ratio.
A total of 96 patients, 63 males and 33 females, with a mean age of 59.64±19.08 years with lower respiratory infection and positive RT-PCR test for COVID-19, hospitalized from March 2020 to May 2020, were included. Seventy-five patients (48 males and 27 females) had moderate to severe disease and 21 patients (15 males and 6 females) had critical disease and were intubated. Table I summarizes the characteristics of the study population.
The mean value of absolute eosinophil number was 0.026±0.017 K/μl in patients with moderate to severe disease and 0.031±0.033 K/μl in patients that were intubated (p=0.491) (Figure 2). The mean value of ELR was 0.026±0.030 in patients with moderate to severe disease and 0.032±0.029 in patients that were intubated (p=0.370) (Table II, Figure 3).
From the 96 patients, 8 patients died. All patients that died had critical illness and were intubated. The mean value of absolute eosinophil number was 0.012±0.007 K/μl in patients who died and 0.029±0.022 K/μl in patients that survived (p<0.0001) (Figure 4). The mean value of ELR was 0.011±0.008 in patients who died and 0.033±0.030 in patients who survived (p<0.0001) (Table III, Figure 5).
Furthermore, the mean value of absolute eosinophil number was 0.012±0.007 K/μl in patients who were intubated and died and 0.043±0.037 K/μl in patients who survived (p<0.05) (Figure 6). The mean value of ELR was 0.011±0.008 in patients who died and 0.035±0.035 in patients who survived (p<0.005) (Table IV, Figure 7).
As mentioned above, the patients with moderate to severe disease were divided in two groups. The first group included 45 patients with a duration of hospitalization of less than 15 days and the second group included 30 patients with duration of hospitalization of more than 15 days. The mean value of absolute eosinophil number was 0.012±0.007 K/μl in the first group and 0.019±0.018 K/μl in the second group (p<0.005) (Figure 8). The mean value of ELR was 0.040±0.028 in the first group and 0.023±0.029 in the second group (p<0.005) (Table V, Figure 9).
Discussion
According to our results, we can conclude that lower values of peripheral eosinophil counts and lower ELR are associated with worse outcome and longer duration of hospitalization of patients with COVID-19. To our knowledge, the current study is the first to evaluate ELR in the clinical course of COVID-19. However, several studies have evaluated the role of eosinophils in patients with COVID-19. Santotoribio et al., have reported that blood counts of eosinophils can be used to differentiate patients with and without COVID-19 and as a biomarker for the diagnosis of suspected COVID-19 in adult patients (17). Zhao et al., have found significant association between eosinopenia and COVID-19 severity (p=0.006) (18). Du et al., have examined the clinical features of 85 fatal cases of COVID-19 in two hospitals in Wuhan and concluded that eosinopenia may indicate a poor prognosis (19).
Sun et al., have found that dynamic surveillance of peripheral blood cell counts especially eosinophils helps in the prediction of severe COVID-19 cases (20). Lu et al., in their case report of a severe COVID-19 patient, have noticed that eosinophils were at extremely low levels within the first 10 days after admission and their number recovered at 12 days after admission, earlier than other biomarkers, which might be of great importance for disease progression (11). Wang et al., in a study of 45 moderate and severe COVID-19 cases at Jingzhou Central Hospital, found that the absolute number of peripheral eosinophils in severe cases was significantly lower than that in moderate cases (p<0.05) (21). Finally, Chen et al., in their retrospective study including 548 patients with COVID-19 with clarified outcome (discharged or deceased) from a national cohort in China, have reported that on admission, the counts of eosinophils was markedly decreased, especially in severe/critical and fatal patients (22).
The pathophysiology for eosinopenia in COVID-19 remains unclear but is thought to be multifactorial, including suppression of the bone marrow eosinophil production, inhibition of eosinophilopoiesis, reduced expression of chemokine receptors, and eosinophil apoptosis driven by type 1 interferons (IFNs) released during the infection (23). In addition, no eosinophil recruitment into the lung tissue has been reported in studies with samples from alive patients with COVID-19 (24) or patients who have deceased from this entity (25). Moreover, postmortem analysis of pulmonary tissue from patients who died from COVID-19 demonstrated signs of acute respiratory distress syndrome that was mostly dominated by lymphocytes (26). Besides, it is thought that eosinophil exhaustion is associated with neutralization of virus by eosinophil-derived enzymes and that the intereleukin-33 (IL-33) pathway is affected by the virus. IL-33 is responsible for eosinophil activation in the airways and bone marrow and ciliated epithelial cells, as the first target of coronavirus, are IL-33 positive epithelial cells (18).
Our study has some potential limitations. It consists a single-center retrospective study, with relatively small sample size of patients that does not permit to generalize the results.
In Greece, the number of the patients affected from the novel coronavirus was low at the first wave of the infection (27). A further well-controlled, multicenter prospective study is needed to clarify the possible role of eosinophils and ELR as biomarkers in the clinical course of patients with COVID-19.
Conclusion
Lower values of peripheral eosinophil count and ERL are associated with worse outcome in patients with COVID-19 and could probably predict longer duration of hospitalization in patients with moderate to severe disease. More studies are needed to determine the role of these biomarkers in the clinical course of patients with COVID-19.
Conflicts of Interest
All the Authors declare that there are no conflicts of interest regarding this study.
Authors’ Contributions
Nikolaos Garmpis, Christos Damaskos and Anna Garmpi designed the study. Nikolaos Gravvanis and Stamatis Velonias collected the clinical data of the patients from their institution. Evangelos Diamantis, Paraskevi Farmaki, Chrysovalantis V. Papageorgiou, Sotiria Makrodimitri, Zoi Damaskou, Georgia-Eleni Korrou, Lourdes-Victoria Quiles-Sanchez, Alexandros Patsouras, Dimitrios Lamprinos and Athanasia Stelianidi performed the literature search and collected the data. Nikolaos Trakas performed the statistical analysis. Vasiliki E. Georgakopoulou wrote the article. Serena Valsami, Dimitrios Dimitroulis, Pagona Sklapani, Efstathios A. Antoniou, Konstantinos Kontzoglou and Lampros Nikolidakis offered scientific advice. Nikolaos Garmpis, Christos Damaskos, Athanasios Syllaios, Georgios Marinos, Georgia Vogiatzi, Georgios Kyriakos and Spyridon Savvanis revised the article. Anna Garmpi critically revised the article and was the supervisor.
References
- 1.Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2012;4(2):68–79. doi: 10.4168/aair.2012.4.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores-Torres AS, Salinas-Carmona MC, Salinas E, Rosas-Taraco AG. Eosinophils and respiratory viruses. Viral Immunol. 2019;32(5):198–207. doi: 10.1089/vim.2018.0150. [DOI] [PubMed] [Google Scholar]
- 3.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110(5):1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 4.Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, Surman SL, Lee JJ, Hurwitz JL, Thomas PG, McCullers JA. Eosinophils promote antiviral immunity in mice infected with influenza A virus. J Immunol. 2017;198(8):3214–3226. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37(1):85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Cader MS, Amarasinghe A, Abdul-Careem MF. Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses. Arch Virol. 2016;161(8):2075–2086. doi: 10.1007/s00705-016-2904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z, Wang X. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang MC, Park YK, Kim BO, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20(1):445. doi: 10.1186/s12879-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie G, Ding F, Han L, Yin D, Lu H, Zhang M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2020 doi: 10.1111/all.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanni F, Akker E, Zaman MM, Figueroa N, Tharian B, Hupart KH. Eosinopenia and COVID-19. J Am Osteopath Assoc. 2020 doi: 10.7556/jaoa.2020.091. [DOI] [PubMed] [Google Scholar]
- 11.Lu G, Wang J. Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin Chim Acta. 2020;508:98–102. doi: 10.1016/j.cca.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Çekici Y, Yılmaz M, Seçen Ö. New inflammatory indicators: Association of high eosinophil-to-lymphocyte ratio and low lymphocyte-to-monocyte ratio with smoking. J Int Med Res. 2019;47(9):4292–4303. doi: 10.1177/0300060519862077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branicka O, Rogala B, Glück J. Eosinophil/neutrophil/platelet-to-lymphocyte ratios in various types of immediate hypersensitivity to NSAIDs: A preliminary study. Int Arch Allergy Immunol. 2020;181(10):774–782. doi: 10.1159/000509116. [DOI] [PubMed] [Google Scholar]
- 14.Holub K, Biete A. New pre-treatment eosinophil-related ratios as prognostic biomarkers for survival outcomes in endometrial cancer. BMC Cancer. 2018;18(1):1280. doi: 10.1186/s12885-018-5131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holub K, Conill C. Τhe impact of inflammatory biomarkers on overall survival of patients with pancreatic cancer treated with chemoradiation. Ann Oncol. 2018;29(Suppl 5):P-135. doi: 10.1093/annonc/mdy151.134. [DOI] [Google Scholar]
- 16.WHO global: Clinical management of COVID-19: Interim guidance. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19 [Last accessed on November 20, 2020]
- 17.Santotoribio JD, Nuñez-Jurado D, Lepe-Balsalobre E. Evaluation of routine blood tests for diagnosis of suspected coronavirus disease 2019. Clin Lab. 2020;66(9) doi: 10.7754/Clin.Lab.2020.200522. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Zhang YP, Yang X, Liu X. Eosinopenia is associated with greater severity in patients with coronavirus disease 2019. Allergy. 2020 doi: 10.1111/all.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Cai X, Wang H, He G, Lin Y, Lu B, Chen C, Pan Y, Hu X. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Deng R, Gou L, Fu Z, Zhang X, Shao F, Wang G, Fu W, Xiao J, Ding X, Li T, Xiao X, Li C. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. 2020;8(9):593. doi: 10.21037/atm-20-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, Xie J, Guan W, Liang W, Ni Z, Hu Y, Liu L, Shan H, Lei C, Peng Y, Wei L, Liu Y, Hu Y, Peng P, Wang J, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Zheng J, Zhang N, Li Y, He J, Li J, Li S, Zhong N, Medical Treatment Expert Group for COVID-19 Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146(1):89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butterfield JH. Treatment of hypereosinophilic syndromes with prednisone, hydroxyurea, and interferon. Immunol Allergy Clin North Am. 2007;27(3):493–518. doi: 10.1016/j.iac.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damaskos C, Garmpi A, Georgakopoulou VE, Farmaki P, Diamantis E, Dimitroulis D, Valsami S, Kontzoglou K, Antoniou EA, Damaskou Z, Nikolidakis L, Syllaios A, Marinos G, Trakas N, Garmpis N. COVID-19: Do it like Greece. Why Greece is coping with COVID-19 better than other countries. Pan Afr Med J. 2020;35(Suppl 2):109. doi: 10.11604/pamj.supp.2020.35.109.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]