Abstract
Background/Aim: Viral infection often exacerbates proteinuria, which has been suggested to be due to antiviral responses of podocytes. We examined the effect of polyinosinic-polycytidylic acid (polyIC) on the expression of retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) in differentiated human podocytes in culture. Materials and Methods: The podocytes were treated with 2 ng/ml to 500 μg/ml of polyIC for 3 to 36 h, and also transfected with siRNA against RIG-I and MDA5. F-actin staining was performed to assess actin reorganization. Results: PolyIC induced the expression of RIG-I and MDA5 in dose- and time-dependent manner, accompanied with interferon-β (IFN-β) and interleukin-6 (IL-6) up-regulation and actin reorganization. Temporal knockdown of RIG-I by siRNA decreased IFN-β expression, while MDA5 siRNA inhibited IFN-β and IL-6 expression. Actin reorganization was attenuated by RIG-I and MDA5 knockdown. Conclusion: RIG-I and MDA5 may play a role in the antiviral responses of podocytes
Keywords: Podocyte, polyIC, RIG-I, MDA5, innate immunity
Viral infection often causes or exacerbates glomerular diseases, which may be due to immune responses of the intrinsic kidney cells (1). The innate immune system serves as a first-line host defense (2). The pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns expressed by pathogenic microbes. The PRR family comprises four different classes: Toll-like receptors (TLRs) and C-type lectin receptors, which are transmembrane proteins, and retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) and NOD-like receptors, which are cytoplasmic proteins (3). When PRRs recognize microbial components, type I IFN production is induced and nuclear factor-kappa B (NF-ĸB) is activated (2,4), leading to inflammatory responses.
Among the PRRs, the expression of TLRs and their role in various kidney diseases have been relatively well explored, for example, TLR7 and TLR9 are involved in lupus nephritis (5), TLR3 and TLR4 in minimal change disease (6), and TLR3 in hepatitis C-associated glomerulonephritis (7). However, there is limited information on the expression of RLRs in the kidney.
The RLR family commonly contains the DExD/H motif, which encodes an RNA helicase, and a C-terminus domain, which recognizes RNA (8). RIG-I preferentially recognizes negative-stranded RNA, including that of influenza viruses and rhabdoviruses, whereas MDA5 recognizes positive-stranded RNA, including that of picornaviruses, and relatively long RNA (8).
PolyIC is a synthetic double-stranded RNA (dsRNA), which has been widely used to mimic viral infection. It is recognized by several TLRs as well as RLRs (9). It has been shown to increase RIG-I expression and to up-regulate cytokine and chemokine expression in mesangial cells (10,11). In glomerular endothelial cells (GEnC), polyIC induces RIG-I expression, along with inflammatory cytokines, chemokines, and type I IFN, and increases GEnC permeability (12). In the present study, we hypothesized that RIG-I and MDA5 expression is induced by polyIC in podocytes, which subsequently leads to podocyte damage.
Materials and Methods
Cell culture. Immortalized human podocytes were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS), 1% insulin-transferrin-selenium-A supplement (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were grown at 33˚C in 95% air and 5% CO2, and then were differentiated by incubating at 37˚C for 10 days. They were kept in 1% FBS for 24 h, treated with 2 ng/ml to 500 μg/ml of polyIC for 3 to 36 h, and used between passages 15 and 18.
Small interfering RNA. Cultured podocytes were transfected with small interfering RNAs (siRNAs) that target RIG-I and MDA5. A siRNA targeted to an irrelevant mRNA served as the nonspecific control. Cells were transfected with 50 nM siRNA in a medium without antibiotics for 24 h using the Dharmafect transfection reagent (GE Healthcare, Little Chalfont, UK), in accordance with the manufacturer’s protocol. Then, the cells were further incubated for 24 h in the presence or absence of polyIC. Target gene depletion was determined using quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Analysis of mRNA expression by qRT-PCR. The QIA shredder and RNeasy Protect Mini Kit (QIAGEN, Valencia, CA, USA) were utilized for total RNA extraction from the cells. RNA was transcribed into the first-strand cDNA with the Omniscript RT kit (QIAGEN) in accordance with the manufacturer’s protocol. qRT-PCR was performed using an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA) with TaqMan Universal PCR Master Mix (Applied Biosystems). Specific primers and probes (Applied Biosystems) were acquired for human glyceraldehyde-3-phosphatasedehydrogenase (GAPDH) (Assay ID:Hs99999905_m1), RIG-I (Hs00204833_m1), MDA5 (Hs0107
0332_m1), IL-6 (Hs00985638_g1), and IFN-β(Hs0107958_s1) detection. Gene expression results were normalized by GAPDH expression levels.
Western blot analysis. Western blot analysis was performed as described in previous reports (13). Briefly, the cells were lysed using Laemmli reducing sample buffer, and then the lysates were subjected to polyacrylamide gel electrophoresis. The proteins were blotted onto polyvinylidene fluoride membranes, which were then blocked and probed with an anti-RIG-I (1:10,000), anti-MDA5 (1:2,000), or anti-actin (1:3,000) antibody. After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody. A chemiluminescent substrate was used for detection.
Immunofluorescence study. Glass-bottom culture dishes (MatTec, Ashland, MA, USA) were utilized for podocyte growth and differentiation. Cells were pretreated with 1% reduced serum for 24 h and incubated in the presence of polyIC for another 24 h. Then, they were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 10 min. For F-actin staining, cells were subjected to fixation and incubated with an Alexa Fluor 488-conjugated phalloidin (1:500) (Molecular Probes, Carlsbad, CA, USA) for 1 h and imaged using a fluorescence microscope (model BZ-X700; Keyence).
Statistical analysis. All data were expressed as mean±standard deviation, which were then compared using Student’s independent sample t-test or one-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference test and two-way repeated measures ANOVA. p<0.05 was considered statistically significant.
Results
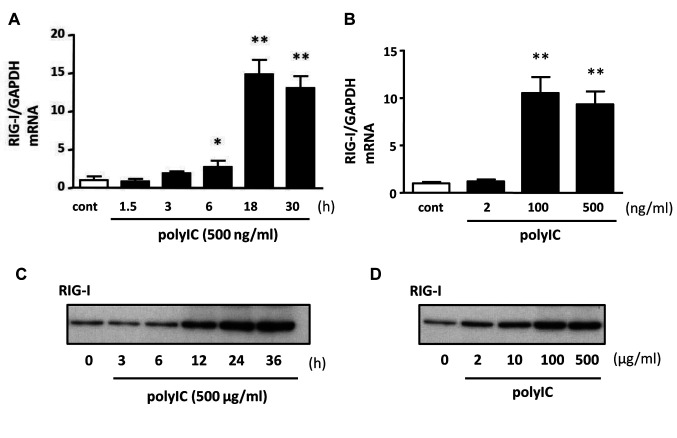
RIG-I expression after treatment with polyIC. To examine the effect of polyIC on human podocytes, we performed qRT-PCR (Figure 1A and B) and western blot (Figure 1C and D) analyses. The RIG-I mRNA expression levels started increasing at 6 h and were significantly elevated at 18 h (Figure 1A) of treatment with polyIC (500 ng/ml). In the dose-response experiment, RIG-I mRNA expression levels were increased following treatment with 100 ng/ml polyIC and remained high after 500 ng/ml polyIC treatment (Figure 1B). RIG-I protein expression levels increased after polyIC treatment in a time- (500 μg/ml, Figure 1C) and dose (Figure 1D)-dependent manner.
Figure 1. Polyinosinic-polycytidylic acid (PolyIC) induced retinoic acid-inducible gene-I (RIG-I) expression on cultured human podocytes. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) (A, B) and western blot (C, D) analysis showed an increased expression of RIG-I after polyIC treatment. 500 ng/ml polyIC up-regulated mRNA of RIG-I (A) and 500 μg/ml PolyIC induced protein of RIG-I (C) in time dependent manner. Similarly, polyIC induced mRNA (B) and protein (D) of RIG-I in dose dependent manner. GAPDH was used as an internal control. The results represent the means±SEM (n=3 wells). *p<0.05; **p<0.001 versus control.
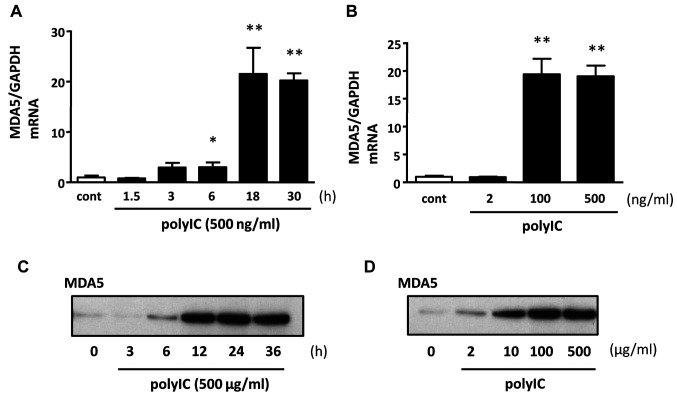
The expression of MDA5 after treatment with PolyIC. Next, we analyzed the expression of MDA5 in podocytes treated with polyIC (Figure 2). Gene expression levels increased following treatment with 500 ng/ml polyIC treatment for 18 h (Figure 2A), and by 100 ng/ml polyIC (Figure 2B). PolyIC up-regulated the protein levels of MDA-5 in a time (500 μg/ml, Figure 2C) and dose (Figure 2D)-dependent manner.
Figure 2. PolyIC induced melanoma differentiation-associated gene 5 (MDA5) expression on cultured human podocytes. qRT-PCR (A, B) and western blot (C, D) analysis showed an increased expression of MDA5 after polyIC treatment. Five hundred ng/ml polyIC up-regulated mRNA of MDA5 (A) and 500 μg/ml PolyIC induced protein of MDA5 (C) in a time-dependent manner. Similarly, polyIC induced mRNA (B) and protein (D) of MDA5 in dose dependent manner. GAPDH was used as an internal control. The results represent the means±SEM (n=3 wells). *p<0.05; **p<0.001 versus control.
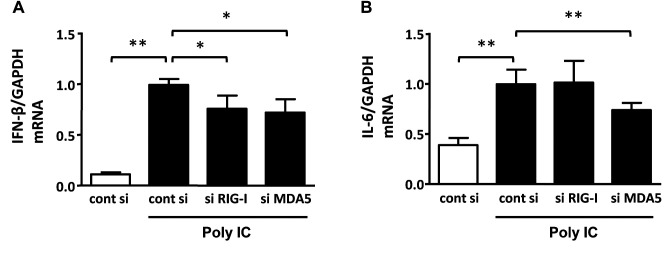
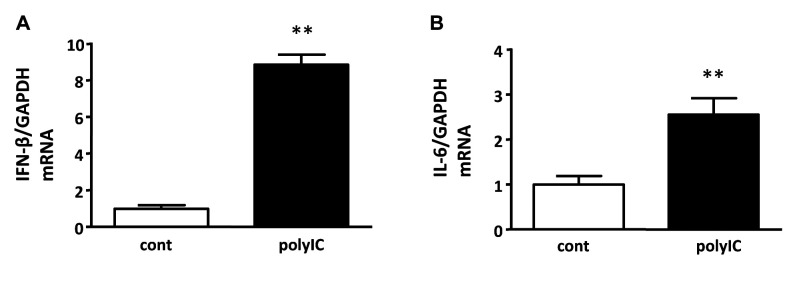
The expression of inflammatory cytokines after treatment with PolyIC. Increased expression of RIG-I and MDA5 leads to up-regulation of transcription factors, including NF-ĸB, which promotes the production of inflammatory cytokines. Therefore, we examined whether polyIC induces the gene expression of inflammatory cytokines such as IFN-β and IL-6. Treatment of differentiated podocytes with 500 ng/ml polyIC for 6 h increased the mRNA expression of IFN-β and IL-6 (Figures 3A and B).
Effect of small interfering RNA of RIG-I and MDA5. To assess the effect of RLR signaling pathway, we used siRNA to silence RIG-I and MDA5. The gene expression of IFN-β was partially but significantly decreased by knockdown of RIG-I and MDA5 (Figure 4A). However, IL-6 expression was partially suppressed by the knockdown of MDA5 but not of RIG-I (Figure 4B).
Figure 4. Knockdown of RIG-I and MDA5 with siRNA inhibited the expression of inflammatory cytokines by polyIC. Cultured human podocytes were transfected with non-targeting (control) siRNA, RIG-I, and MDA5 siRNA using Dharmafect Reagent®. PolyIC (500 ng/ml) was added and incubated for 6 h. qRT-PCR analysis showed the gene expression levels of IFN-β (A) and IL-6 (B). GAPDH was used as an internal control. The results represent the means±SEM (n=3 wells). *p<0.05; **p<0.001.
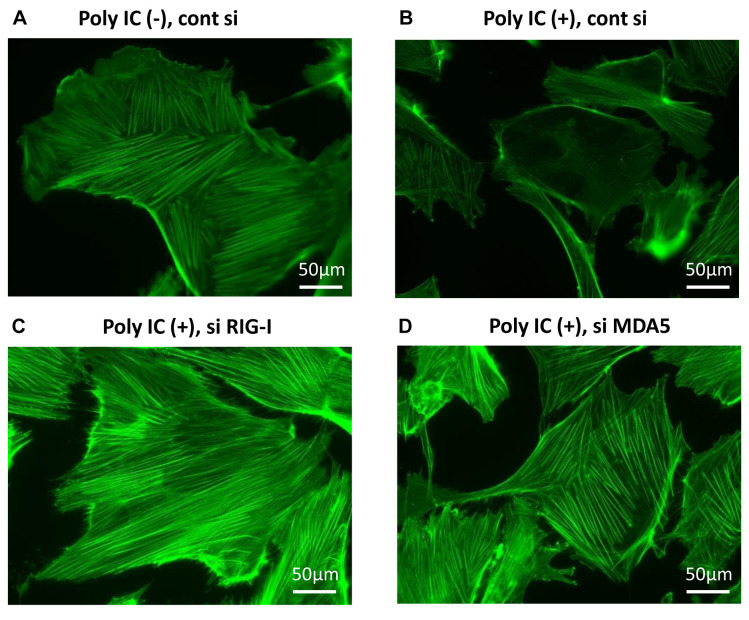
PolyIC-induced actin reorganization was partially attenuated by RIG-I and MDA5 knockdown. An intact actin cytoskeleton is essential for maintaining the normal shape and function of podocytes. Actin reorganization, histologically observed as foot process effacement, is associated with proteinuria. Differentiated human podocytes have characteristic central stress fibers composed of actin filaments (Figure 5A). PolyIC treatment induced the disruption of actin cytoskeleton (Figure 5B), which was partly attenuated by the temporal knockdown of RIG-I and MDA5 by siRNA (Figures 5C and D).
Figure 5. PolyIC-induced actin reorganization was partially attenuated by RIG-I and MDA5 knockdown. F-actin representative microscopic images in human podocytes. Podocytes were transfected with non-targeting siRNA (cont si), RIG-I siRNA, or MDA5 siRNA, followed by treatment with 500 ng/ml poly IC for 24 h. At 72 h post-transfection, podocytes were fixed and stained with phalloidin to detect F-actin. Magnification 400×. Bar represents 50 μm.
Discussion
Viral infection often causes or exacerbates glomerular diseases. In some cases, host-derived antiviral antibodies have been suggested as the mechanism of the pathogenesis; however, a direct cytopathogenic effect on glomerular cells has been suggested in HIV-associated focal segmental glomerulosclerosis (1). Besides, in steroid-responsive nephrotic syndrome, at least 50% of relapses are triggered by viral upper respiratory tract infections (1), suggesting a direct effect of viral infection on podocyte damage.
The role of innate immunity in podocyte damage has been previously investigated (4,14). Lipopolysaccharide (LPS), a TLR4 ligand, has been shown to induce proteinuria and podocyte damage (14). Furthermore, LPS and polyIC, both of which are also TLR3 ligands, induce CD80 expression in podocytes (4,15). It has been reported that urinary CD80 was increased in minimal change disease (16). However, urinary CD80 is not available for the clinical diagnosis of minimal change disease.
Previous reports have shown that polyIC induces the up-regulation of inflammatory cytokines and podocyte actin reorganization, which is dependent on CD80 (4). Furthermore, polyIC has been shown to induce transient proteinuria and glomerular CD80 production in mice (15), indicating that innate immunity activation via PRRs is closely related to proteinuria. In the present study, we showed that polyIC induces RIG-I and MDA5 expression. Moreover, the polyIC-induced increase in IL-6 was RIG-I-dependent, and the increase in IFN-β levels and actin reorganization were both RIG-I- and MDA5-dependent. Consistently, Flür et al. have reported that in mesangial cells, polyIC-induced IL-6 expression was inhibited by knockdown of MDA5, but not by knockdown of RIG-I (11).
The actin cytoskeleton is essential for the normal function of podocytes. The reorganization of actin cytoskeleton in podocytes is generally accepted as podocyte damage, leading to foot process effacement, which is commonly observed in many proteinuric conditions (17). In macrophages, a relationship between RIG-I and actin cytoskeleton has been suggested. RIG-I accumulated in membrane ruffles, clearly colocalizing with F-actin (18). In our study, polyIC-induced actin reorganization was reduced by the knockdown of both RIG-I and MDA5. RIG-I may have a direct and significant role in the pathogenesis of actin reorganization; presumably, multiple mechanisms exist in the pathogenesis of actin reorganization, and RIG-I and MDA5 play a significant role at least in part.
There are some limitations in our study. First, this is an in vitro study. During a viral infection, many systemic reactions and direct cytopathogenic actions occur in the kidney. Our study only focused on podocytes. In addition, phalloidin staining is a generally accepted method for podocyte injury; however, it lacks objectivity and quantitativity. Secondly, we tested only some of the numerous changes occurring upon stimulation with polyIC. Besides, we did not investigate the interplay between TLRs and RLRs nor the interplay between RIG-I and MDA5 in this study. Our results warrant the importance of further studies on the role of RIG-I and MDA5 in podocytes.
In conclusion, our study showed that polyIC remarkably increased the expression of RIG-I and MDA5 in differentiated human podocytes in culture. PolyIC induced the up-regulation of inflammatory cytokines and podocyte actin reorganization, which were partly attenuated by the temporal knockdown of RIG-I and MDA5, suggesting a possible role of RIG-I and MDA5 in the antiviral responses of podocytes.
Conflicts of Interest
The Authors have no conflicts of interest directly relevant to the content of this article.
Authors’ Contributions
M.S. and T.I conceived of the presented idea. M.N., I.N., M.S., T.I., D.N., K.K., M.H., N.M., M.N., Y.K., T.F. and R.M. carried out the experiments and performed the analytic calculations. N.N. and H.T. helped supervise the project. M.N., I.N. and M.S. wrote the manuscript. All Authors approved the final manuscript.
Acknowledgements
The Authors would like to thank Prof. Moin A Saleem, University of Bristol, UK for kindly providing human podocytes. The Authors also thank Yoshiko Shutto-Uchita for her excellent technical support
Figure 3. polyIC increased the gene expression of inflammatory cytokines. Cultured human podocytes were treated with 500 ng/ml polyIC for 6 h. qRT-PCR analysis showed that the gene expression levels of interferon -β (IFN-β) (A) and interleukin-6 (IL-6) (B) were increased by polyIC treatment. GAPDH was used as an internal control. The results represent the means±SEM (n=3 wells). *p<0.05; **p<0.001 versus control.
References
- 1.Lai AS, Lai KN. Viral nephropathy. Nat Clin Pract Nephrol. 2006;2(5):254–262. doi: 10.1038/ncpneph0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Wymer DT, Yamabe H, Mathieson PW, Saleem MA, Garin EH, Johnson RJ. Toll-like receptor 3 ligands induce cd80 expression in human podocytes via an nf-kappab-dependent pathway. Nephrol Dial Transplant. 2012;27(1):81–89. doi: 10.1093/ndt/gfr271. [DOI] [PubMed] [Google Scholar]
- 5.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of tlr7 and tlr9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37(12):3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 6.Mishra OP, Kumar R, Narayan G, Srivastava P, Abhinay A, Prasad R, Singh A, Batra VV. Toll-like receptor 3 (tlr-3), tlr-4 and cd80 expression in peripheral blood mononuclear cells and urinary cd80 levels in children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2017;32(8):1355–1361. doi: 10.1007/s00467-017-3613-8. [DOI] [PubMed] [Google Scholar]
- 7.Wornle M, Schmid H, Banas B, Merkle M, Henger A, Roeder M, Blattner S, Bock E, Kretzler M, Grone HJ, Schlondorff D. Novel role of toll-like receptor 3 in hepatitis c-associated glomerulonephritis. Am J Pathol. 2006;168(2):370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisse M, Ly H. Comparative structure and function analysis of the rig-i-like receptors: Rig-i and mda5. Front Immunol. 2019;10:1586. doi: 10.3389/fimmu.2019.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Imaizumi T, Tanaka H, Matsumiya T, Yoshida H, Tanji K, Tsuruga K, Oki E, Aizawa-Yashiro T, Ito E, Satoh K. Retinoic acid-inducible gene-i is induced by double-stranded rna and regulates the expression of cc chemokine ligand (ccl) 5 in human mesangial cells. Nephrol Dial Transplant. 2010;25(11):3534–3539. doi: 10.1093/ndt/gfq270. [DOI] [PubMed] [Google Scholar]
- 11.Flur K, Allam R, Zecher D, Kulkarni OP, Lichtnekert J, Schwarz M, Beutler B, Vielhauer V, Anders HJ. Viral rna induces type i interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: Implications for viral infection-associated glomerulonephritis. Am J Pathol. 2009;175(5):2014–2022. doi: 10.2353/ajpath.2009.080585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagele H, Allam R, Pawar RD, Anders HJ. Double-stranded rna activates type i interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (rig)-1. Nephrol Dial Transplant. 2009;24(11):3312–3318. doi: 10.1093/ndt/gfp339. [DOI] [PubMed] [Google Scholar]
- 13.Imaizumi T, Aizawa-Yashiro T, Watanabe S, Matsumiya T, Yoshida H, Tatsuta T, Xing F, Meng P, Hayakari R, Tsuruga K, Tanaka H. Tlr4 signaling induces retinoic acid-inducible gene-i and melanoma differentiation-associated gene 5 in mesangial cells. J Nephrol. 2013;26(5):886–893. doi: 10.5301/jn.5000254. [DOI] [PubMed] [Google Scholar]
- 14.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of b7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, Lanaspa MA, Rivard C, Maruyama S, Garin EH, Johnson RJ. Toll-like receptor 3 ligand, polyic, induces proteinuria and glomerular cd80, and increases urinary cd80 in mice. Nephrol Dial Transplant. 2013;28(6):1439–1446. doi: 10.1093/ndt/gfs543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ. Urinary cd80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20(2):260–266. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundel P, Reiser J. Proteinuria: An enzymatic disease of the podocyte. Kidney Int. 2010;77(7):571–580. doi: 10.1038/ki.2009.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong L, Sun L, Zhang H, Liu Q, Liu Y, Qin L, Shi G, Hu JH, Xu A, Sun YP, Li D, Shi YF, Zang JW, Zhu J, Chen Z, Wang ZG, Ge BX. An essential role for rig-i in toll-like receptor-stimulated phagocytosis. Cell Host Microbe. 2009;6(2):150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]