Abstract
Background/Aim: We hypothesised that the prognostic nutrition index (PNI) is useful for evaluating host immunity and response to immune checkpoint inhibitors. We investigated the effect of PNI on nivolumab monotherapy efficacy in advanced or recurrent gastric cancer (GC) or gastro-oesophageal junction cancer (GOC) patients. Patients and Methods: We retrospectively examined 110 patients, divided them into a high-PNI group and a low-PNI group, and compared treatment efficacy, adverse events (AEs), and survival between the groups. Results: Median overall survival (OS) was significantly longer in the high-PNI group than in the low-PNI group (205 vs. 109 days; p < 0.001). Multivariate analysis revealed that low PNI was an independent risk factor for OS (hazard ratio=2.398; 95% confidence interval=1.384-4.154; p=0.002). The overall response rate and frequency of AEs were not significantly different between the groups. Conclusion: PNI could be a useful prognostic factor in GC or GOC patients undergoing nivolumab monotherapy.
Keywords: Prognostic nutrition index, nivolumab, gastric cancer, gastro-oesophageal junction cancer
Gastric cancer (GC) and gastroesophageal junction cancer (GOC) are the world’s fifth most frequently diagnosed cancer and the third leading cause of cancer-related deaths annually, respectively (1). For advanced or recurrent GC and GOC patients, systemic chemotherapy is crucial to achieve palliation of symptoms and improve survival outcomes. The first-line standard chemotherapy for unresectable advanced or recurrent GC patients includes fluoropyrimidine plus a platinum agent (2-4). For patients who are refractory or intolerant to these first-line therapies, paclitaxel plus ramucirumab is recommended as the second-line standard chemotherapy (5,6).
Nivolumab is an immune checkpoint inhibitor (ICI) and anticancer agent that increases the lymphocyte activity of cancer cells by inhibiting programmed death-1 (7). In 2017, the results of the ATTRACTION-2 study demonstrated the efficacy of nivolumab monotherapy in advanced or recurrent GC or GOC patients after second-line chemotherapy (8), and nivolumab monotherapy became one of the third-line standard chemotherapeutic drugs (2).
There was variation in the response to nivolumab in advanced or recurrent GC and GOC patients in the ATTRACTION-2 study. Nivolumab monotherapy is extremely effective in some patients, wherein the overall response rate and disease control rate were 11.2% and 40.3%, respectively. However, some patients show poor response to nivolumab monotherapy (8,9), which warrants the importance of identifying predictors of response to nivolumab monotherapy. Thus far, programmed death ligand-1 expression and cluster of differentiation 8+ T-cell infiltration (10,11), tumour mutational burden (12), high microsatellite instability frequency (13,14), and Epstein-Bar virus infection (15,16) have been reported as useful biomarkers that predict the effects of ICIs, including nivolumab. However, the evaluation of host immunity may be useful for predicting the response to nivolumab in advanced or recurrent GC and GOC patients.
Prognostic nutrition index (PNI), determined based on serum albumin levels and peripheral blood lymphocyte counts, was developed for predicting the risk of postoperative complications mainly in surgical patients by assessing the preoperative nutritional status (17). PNI reflected the nutritional and immunological status of cancer patients and was useful as a predictor of prognosis in patients with various cancers (18-21). Cancer progression deteriorates the nutritional status, affecting serum albumin levels and leading to a compromised host immune status (22). Furthermore, lymphocytes, which are ICI targets, suppress tumours and play a role in tumour immunity. Hence, lymphocyte counts are widely used as an index of immunocompetence (23,24). Therefore, we hypothesised that PNI, determined based on serum albumin levels and peripheral blood lymphocyte counts, might be useful as an index for evaluating host immunity and as a predictor of response to ICIs. This study aimed to investigate the effect of pre-treatment PNI on the efficacy of nivolumab monotherapy in advanced or recurrent GC or GOC patients.
Patients and Methods
Ethical approval. This study was approved by the Institutional Review Board of Kanagawa Cancer Center before the study was initiated (approval number: epidemiological study-69).
Patients. We retrospectively examined consecutive advanced or recurrent GC or GOC patients who underwent nivolumab monotherapy at Kanagawa Cancer Center. Patients were selected from our institutional database between October 2015 and December 2019. Inclusion criteria were defined as patients with 1) histologically proven GC or GOC (adenocarcinoma), 2) advanced or recurrent cancer, and 3) history of nivolumab monotherapy. The exclusion criterion was a history of any other cancers.
Assessment of response and adverse events after nivolumab monotherapy. GC or GOC patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 0/1 received a standard nivolumab dose (3 mg/kg or 240 mg) intravenously every 2 weeks in one cycle until disease progression, including clinical deterioration, unacceptable toxicity, or patient’s refusal. The response was assessed using the Response Evaluation Criteria in Solid Tumours (RECIST) ver. 1.1 (25). Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events ver. 5.0.
Definition of PNI. PNI was calculated as 10 × serum albumin level (g/dl) + 0.005× total lymphocyte count (per mm3) using data collected from blood investigations performed before initiating the first nivolumab cycle (17). In line with previous studies, patients with a PNI of <40 and ≥40 were classified into the low-PNI and high-PNI groups, respectively (18-22).
Statistical analyses. We compared patients’ characteristics using Fisher’s exact test and chi-square test. We defined overall survival (OS) as the time from initiating nivolumab therapy to death from any cause, and progression-free survival (PFS) as the time from initiating nivolumab therapy to disease progression. The survival rate was analysed using Kaplan–Meier curves, and differences in survival rates were assessed using the log-rank test. A Cox proportional-hazards regression model was used for univariate and multivariate analyses. All statistical analyses were performed using EZR ver. 1.37 (Jichi University, Tochigi, Japan). Two-sided p-values were calculated, and a p-value <0.05 was considered statistically significant.
Results
Patients. This study included 110 patients. Sixty-five and 45 patients were classified into the high-PNI and low-PNI groups, respectively.
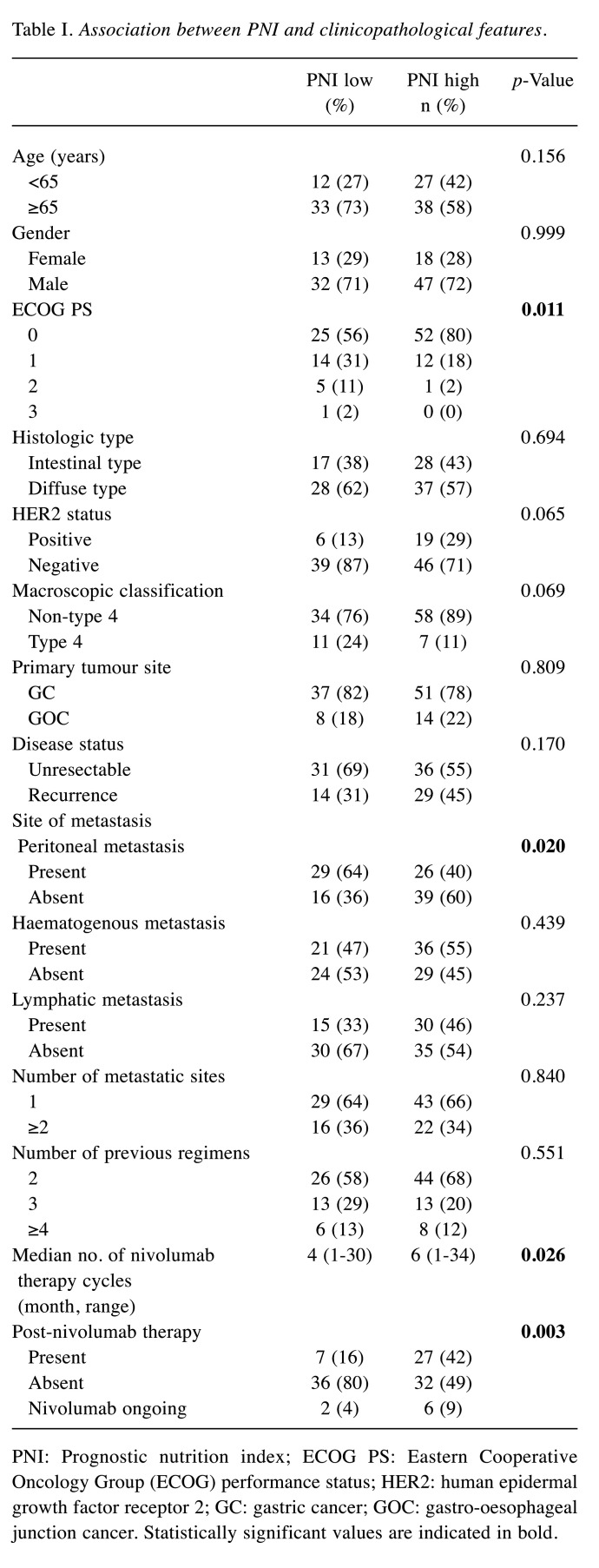
Association between PNI and clinicopathological features. There was a significant association between PNI and ECOG PS, rate of peritoneal metastasis, number of courses of nivolumab monotherapy, and number of patients undergoing post-nivolumab chemotherapy. No significant differences were observed in terms of age, sex, histologic type, human epidermal growth factor receptor 2 status, primary tumour site, disease status, rate of haematogenous metastasis and lymphatic metastasis, and number of metastatic sites (Table I).
Table I. Association between PNI and clinicopathological features.
PNI: Prognostic nutrition index; ECOG PS: Eastern Cooperative Oncology Group (ECOG) performance status; HER2: human epidermal growth factor receptor 2; GC: gastric cancer; GOC: gastro-oesophageal junction cancer. Statistically significant values are indicated in bold
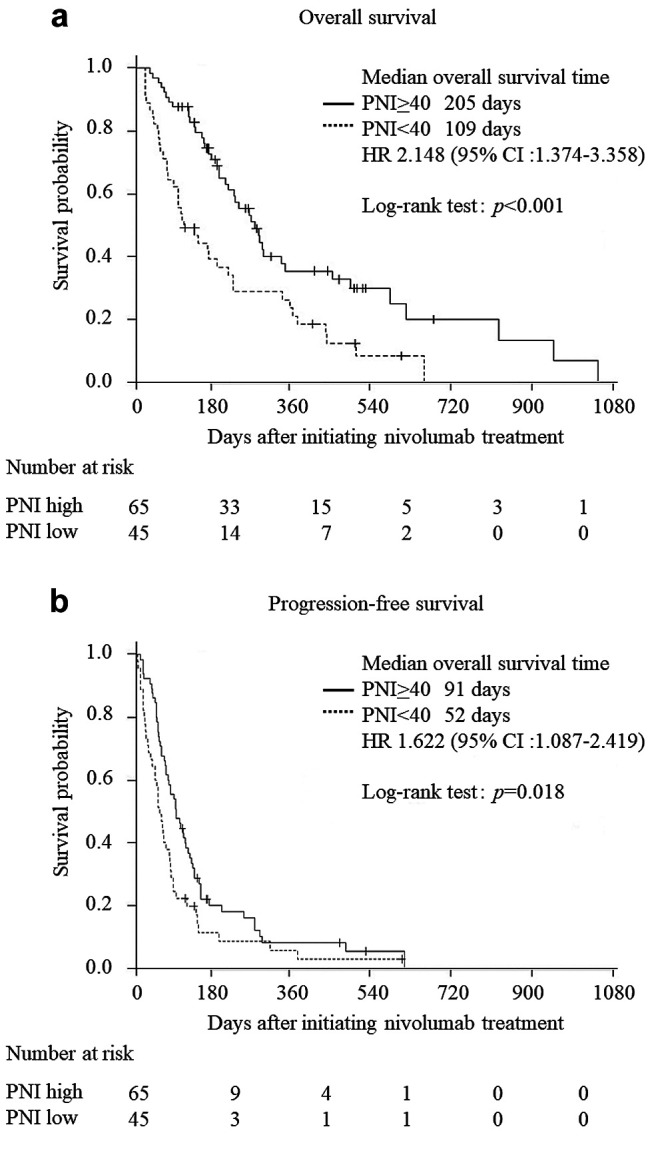
OS and PFS in the high-PNI and low-PNI groups. The median OS rates in the high-PNI and low-PNI groups were 205 and 109 days, respectively. OS was significantly better in the high-PNI group than in the low-PNI group (p<0.001 using the log-rank test, Figure 1A). The median PFS rates in the PNI and low-PNI groups were 91 and 52 days, respectively. PFS was significantly better in the high-PNI group than in the low-PNI group (p=0.018 using the log-rank test, Figure 1B).
Figure 1. Kaplan–Meier curves for overall survival (OS) and progressionfree survival (PFS). A) Kaplan–Meier curves for OS. The median OS of patients in the PNI≥40 group was 205 days, and that of patients in the PNI<40 group was 109 days. OS was significantly better in the PNI≥40 group than in the PNI<40 group (p<0.001). B) Kaplan–Meier curves for PFS. The median PFS of patients in the PNI≥40 group was 91 days, and that of patients in the PNI<40 group was 52 days. PFS was significantly better in the PNI≥40 group than in the PNI<40 group (p=0.018).
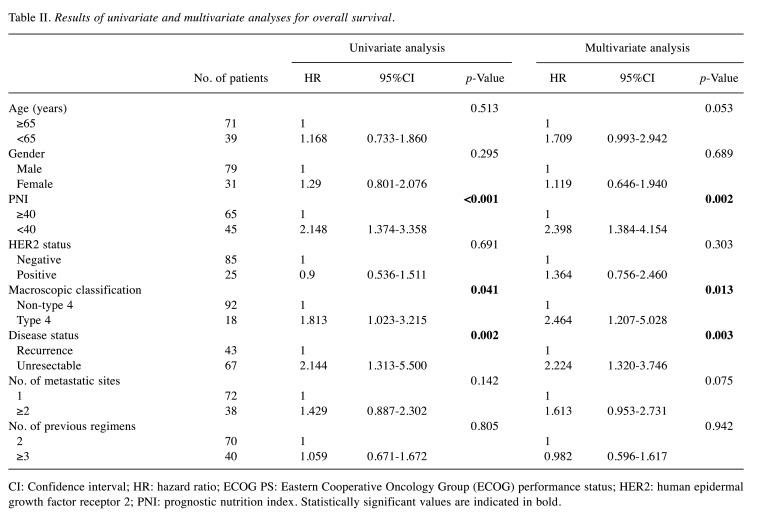
Univariate and multivariate analyses for OS in GC or GOC patients who received nivolumab monotherapy. Univariate analyses revealed OS, ECOG PS, PNI, macroscopic classification, and presence of metastatic lesions as significant factors for OS. Multivariate analysis revealed ECOG PS, PNI, macroscopic classification, and the presence of metastatic lesions as independent predictive factors for OS (Table II).
Table II. Results of univariate and multivariate analyses for overall survival.
CI: Confidence interval; HR: hazard ratio; ECOG PS: Eastern Cooperative Oncology Group (ECOG) performance status; HER2: human epidermal growth factor receptor 2; PNI: prognostic nutrition index. Statistically significant values are indicated in bold
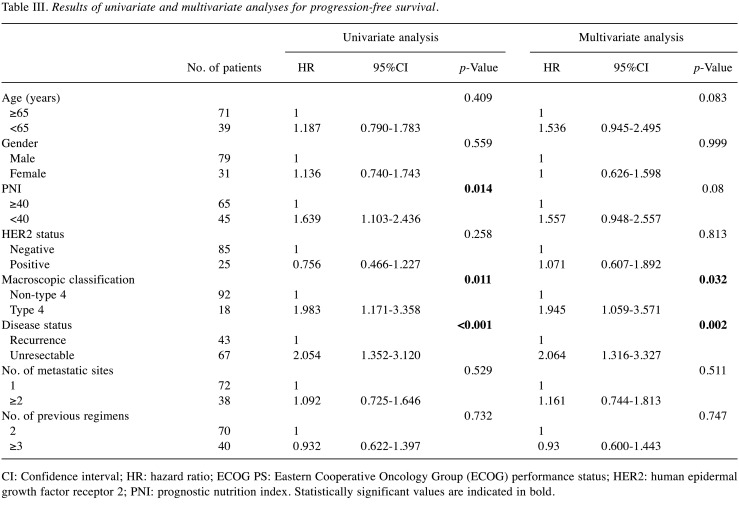
Univariate and multivariate analyses for PFS in GC or GOC patients who received nivolumab monotherapy. Univariate analyses revealed PFS, PNI, macroscopic classification, and the presence of metastatic lesions as significant factors for PFS. Multivariate analysis revealed ECOG PS, macroscopic classification, and the presence of metastatic lesions as independent risk factors for PFS. However, PNI was not a significant factor for PFS (p=0.080, Table III).
Table III. Results of univariate and multivariate analyses for progression-free survival.
CI: Confidence interval; HR: hazard ratio; ECOG PS: Eastern Cooperative Oncology Group (ECOG) performance status; HER2: human epidermal growth factor receptor 2; PNI: prognostic nutrition index. Statistically significant values are indicated in bold
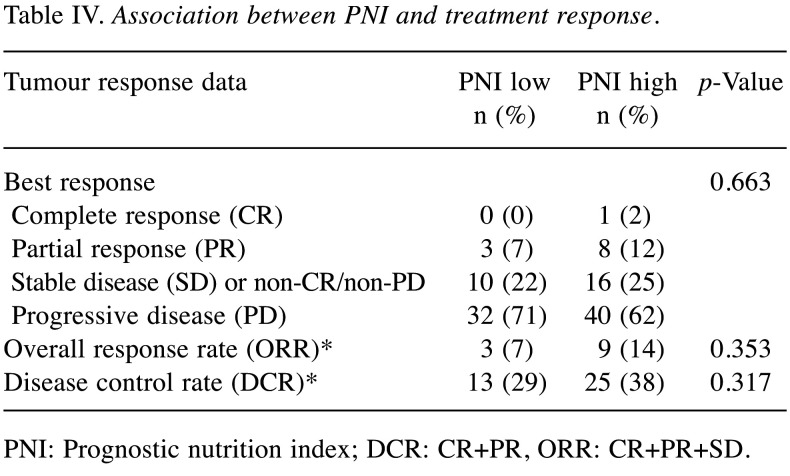
Association between PNI and response to nivolumab monotherapy. The overall response rates (ORRs) were 7% (3/45) and 14% (9/65) in the high-PNI and low-PNI groups, respectively, showing an insignificant difference (p=0.353, Table II). The disease control rates (DCRs) were 29% (13/45) and 38% (25/65) in the low-PNI and high-PNI groups, respectively, showing an insignificant difference (p=0.317, Table IV).
Table IV. Association between PNI and treatment response.
PNI: Prognostic nutrition index; DCR: CR+PR, ORR: CR+PR+SD
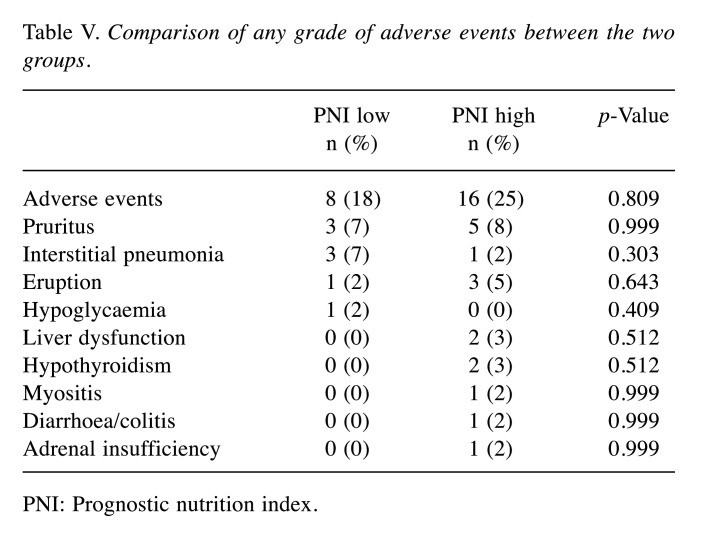
AEs by nivolumab monotherapy in the high-PNI and low-PNI groups. No difference was observed in all immune-related AEs between the two groups (Table V). There were no treatment-related deaths during the study.
Table V. Comparison of any grade of adverse events between the two groups.
PNI: Prognostic nutrition index
Discussion
We investigated the effects of PNI on survival outcomes in advanced or recurrent GC or GOC patients who underwent nivolumab monotherapy. The ORR, DCR, and rate of AEs were not significantly different between the high-PNI and low-PNI groups. PNI was an independent predictive factor of OS in these patients.
To understand the reason why PNI was an important factor for OS in advanced or recurrent GC or GOC patients who underwent nivolumab monotherapy, several factors were considered. First, the nutritional status is reflected by PNI. It includes the serum albumin level, which is a well-known indicator of the nutritional status (26). Metabolic abnormalities caused by malnutrition reduce the therapeutic effect of ICIs (27-32). Second, lymphocyte function is also an important factor. The peripheral blood lymphocyte count, which plays an important role in tumour immune response, reflects the immunological status of hosts (33,34).
Only a single study besides the present one examined the association between PNI and survival in GC patients undergoing nivolumab monotherapy. PNI after administering nivolumab, and not PNI before nivolumab therapy, was a risk factor for prognosis (35). In this study, we investigated whether PNI administered before nivolumab therapy could be a biomarker for treatment response and demonstrated that the high-PNI group had worse outcomes than the low-PNI group.
Regarding PFS, Kaplan–Meier curves were clearly separated, and PFS was significantly better in the high-PNI group compared to the low-PNI group. However, PNI was not an independent predictive factor for PFS. Regarding the reasons, first, the number of AEs may have been inadequate to detect a significant difference in PFS. Second, the accurate determination of progression and pseudo-progression of the disease in patients treated with nivolumab could be difficult, and there could be some bias in clinician’s decisions regarding disease progression and post-nivolumab treatment indication (36).
This study had some limitations. First, this was a retrospective study performed in a single institution. To generalise this result, a multicentre study is necessary. Second, the number of patients and number of AEs were small. Thus, we could not include some possible factors, including postoperative therapy and peritoneal metastasis, in the multivariate analysis. Third, optimal cut-off values for PNI in the truest sense remain unknown. In this study, we defined PNI for clinically significant malnutrition at below 40 based on an original investigation and previous reports (17-21).
In conclusion, PNI might be a useful independent prognostic factor in advanced or recurrent GC or GOC patients undergoing nivolumab monotherapy.
Conflicts of Interest
All Authors have no conflicts of interest or financial ties to disclose in relation to this study.
Authors’ Contributions
Concept and study design were contributed by H. Watanabe and T. Yamada. Data collection and literature search were performed by H. Watanabe, K. Komori, K. Hara, and T. Oshima. Data analysis was performed by H. Watanabe, T. Yamada, and T. Oshima. Interpretation of data was performed by all 20 investigators. Drafting the article and preparation of figures were carried out by H. Watanabe, T. Yamada, and T. Oshima. Finally, this article was revised and approved by all 20 investigators. Thus, all Authors actively participated in this study.
Acknowledgements
The Authors extend their sincere thanks to the patients, their families, and the site staff.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma. Tokyo: Kanehara. 2017;the 15th edition (in Japanese) doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–1312. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- 4.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:38–49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 6.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATT RAC TION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tam JG, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Kang YK, Boku N. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update date. Gastric Cancer. 2020;23:510–519. doi: 10.1007/s10120-019-01034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh J, Ock CY, Kim JW, Nam SK, Kwak Y, Yun S, Ahn SH, Park DJ, Kim HH, Kim WH, Lee HS. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer. Oncotarget. 2017;18:26356–26367. doi: 10.18632/oncotarget.15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspsy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;27:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;21:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA.Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YY, Noh SH, Cheong JH. Molecular dimensions of gastric cancer: Translational and clinical perspectives. J Pathol Trans Med. 2016;50:1–9. doi: 10.4132/jptm.2015.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SY, Park C, Kim HJ, Park J, Hwang J, Kim JI, Choi MG, Kim S, Kim KM, Kang MS. Deregulation of immune response genes in patients with Epstein-Barr virus-associated gastric cancer and outcomes. Gastroenterology. 2015;148:137–147. doi: 10.1053/j.gastro.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 18.Mohri T, Mohri Y, Shigemori T, Takeuchi K, Itoh Y, Kato T. Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J Surg Oncol. 2016;14:170. doi: 10.1186/s12957-016-0920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Wang Y. The prognostic nutritional index is prognostic factor of gynecological cancer: A systematic review and meta-analysis. Int J Surg. 2019;67:79–86. doi: 10.1016/j.ijsu.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Shen J, Liu R, Feng Z, Zhang C, Ling L, Chen L. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: A systematic review and meta-analysis. Int J Biol Markers. 2018;33:372–378. doi: 10.1177/1724600818799876. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, Ma B, Wang Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. 2016;42:1176–1182. doi: 10.1016/j.ejso.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda A, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. British J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 23.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber C, Bobek N, Kuball J, Thaler S, Hoffarth S, Huber C, Theobald M, Schuler M. Inhibitors of apoptosis confer resistance to tumour suppression by adoptively transplanted cytotoxic T-lymphocytes in vitro and in vivo. Cell Death Differ. 2005;12:317–325. doi: 10.1038/sj.cdd.4401563. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41:64–69. doi: 10.1080/01635581.2001.9680613. [DOI] [PubMed] [Google Scholar]
- 27.Brocco D, Di Marino P, Graandonia A. From cachexia to obesity: the role of host metabolism in cancer immunotherapy. Curr Opin Support Palliat Care. 2019;13:305–310. doi: 10.1097/SPC.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 28.Ohba T, Takamori S, Toyozawa R, Nosaki K, Umeyama Y, Haratake N, Miura N, Yamaguchi M, Taguchi K, Seto T, Shimokawa M, Takenoyama M. Prognostic impact of the controlling nutritional status score in patients with non-small cell lung cancer treated with pembrolizumab. J Thorac Dis. 2019;11:3757–3768. doi: 10.21037/jtd.2019.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CS, Devoe CE, Zhu X, Fishbein JS, Seetharamu N. Pretreatment nutritional status and response to checkpoint inhibitors in lung cancer. Lung Cancer. 2020;9:LMT31. doi: 10.2217/lmt-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishioka N, Uchino J, Hirai S, Kitayama Y, Yoshikawa A, Okura N, Tanimura K, Harita S, Imabayashi T, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K. Association of sarcopenia with and efficacy of Anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. J Clin Med. 2019;8:450. doi: 10.3390/jcm8040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, Jain L, Mayawala K, Kang J, Ebbinghaus SW, Sinha V, de Alwis DP, Stone JA. Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res. 2018;24:5841–5849. doi: 10.1158/1078-0432.CCR-18-0415. [DOI] [PubMed] [Google Scholar]
- 32.Flint TR, Fearon DT, Janowitz T. Connecting the metabolic and immune responses to cancer. Trends Mol Med. 2017;23:451–464. doi: 10.1016/j.molmed.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 35.Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today. 2020 doi: 10.1007/s00595-020-02048-w. [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]