Abstract
Thiol-disulphide homeostasis (TDH) is a new parameter indicating oxidative stress that plays a role in the pathogenesis of various clinical disorders. Our study planned to investigate TDH in COVID-19 patients.
Age and gender-matched healthy subjects (n = 70) and COVID-19 patients (n = 144) were included in the study. In addition to the routine laboratory parameters of the groups, their native thiol (NT), total thiol (TT) and disulphide levels were measured. Primarily, we compared COVID-19 patients to the healthy control group for inflammatory parameters, NT, TT and disulphide levels. Then, COVID-19 patients were divided into two groups according to the severity of the disease as mild to moderate and severe COVID-19, and the three groups were compared with each other. Predictive value of thiol parameters in the diagnosis of COVID-19 and in the determining its severity, and its correlation with presence and duration of symptoms were investigated.
Severe COVID-19 patients had lower NT and TT levels compared with healthy controls and mild to moderate patients (P < 0.001 for both). The results of ROC analysis show that the greatest AUC was IL-6 and NT (AUC = 0.97, AUC = 0.96, respectively) between control and COVID-19 patients, while it was CRP and NT (AUC = 0.85, AUC = 0.83) between mild to moderate and severe patients. A negative correlation was found between duration of symptoms of dyspnoea, cough, fever, and sore throat and NT (r = −0.45, P = 0.017, r = −0.418, P < 0.001, r = −0.131, P = 0.084, r = −0.452, P = 0.040, respectively).
NT and TT levels have a strong predictive value in the diagnosis of COVID-19 and in determining disease severity. Our results support that changing TDH parameters appears to have an important role in disease pathogenesis and it can be used in clinical management of patients.
Keywords: COVID-19, Oxidative stress, Thiol-disulphide homeostasis, Native thiol, Clinical severity
Graphical abstract
1. Introduction
The new coronavirus disease (COVID-19), resulting in a wide range of effect from mild disease to severe infection resulting in mortality, continues to threaten public health since the last days of 2019. While many patients are in mild form, severe illness develops in 14% of cases, and critical illness in 5%. Many works have been published demonstrating the correlation between the rise of cytokine and the development of severe disease during various changes in laboratory parameters and the course of the disease [[1], [2], [3]].
While the COVID-19 pathogenesis has not yet been fully illuminated, there is evidence in the literature pointing to possible mechanisms such as the down-regulation of the angiotensin conversion enzyme 2 (ACE2) receptor, systematic inflammatory response, T-cell overactivity, hypoxia, antibody-dependent enhancement (ADE), and cross-reactivity to antibodies developed against pneumocytes [4,5]. Especially in cases of severe disease, “cytokine storms” which develop as a result of excessive cytokine due to damaged tissue are held responsible [3,6]. In addition to the overproduction of cytokine, it is known that oxidative stress (OS), which develops as a result of reactive oxygen species (ROS) and the depletion of antioxidant mechanisms, plays an important role in viral replication and the pathogenesis of subsequent virus-associated disease [[7], [8], [9]]. In animal experiments, determination of the rise in ROS levels and decrease in antioxidant defense during severe acute respiratory syndrome coronavirus (SARS-CoV) infection supports the conjecture that OS can play a role in pathogenesis in SARS-CoV-2 infection [10]. OS plays a key role in the development of lung fibrosis and cardiovascular complications, the need for mechanical ventilation, and rise in mortality among COVID-19 patients [11,12]. For this reason, “oxidative storm” should be researched in the pathogenesis of the disease.
OS that develops as a result of ROS overproduction causes oxidative damage in cell structures such as lipids, membranes, proteins, and nucleic acids. ROS affects the sulfhydryl groups (-SH) of these cell structures. The negative effects ROS on tissues are the neutralization of organic sulfur derivatives through antioxidants such as thiols. Thiols have a private role for antioxidant defense in the human body [13]. ROSs attach to thiols and thiols become disulphides. Disulphides can be regressed back to thiols, whereby thiol-disulphide homeostasis (TDH) can be maintained [14,15]. The deterioration of TDH plays a role in many diseases such as diabetes mellitus, hypertension, cardiovascular diseases, hyperemesis gravidarum, brucellosis, Crimean-Congo Hemorrhagic fever, and tonsillopharyngitis [16]. The deterioration of TDH can be shown through changes in the levels of oxidant or antioxidant molecules. While there are a limited number of studies examining the effect of ROS production on the severe clinical course of COVID-19 patients, there have been no studies done to examine the clinical effect of changes in antioxidant molecule levels such as thiol. Considering the available data, we predict that thiol levels – as antioxidant molecules – are lower in COVID-19 patients compared to healthy people, and that depending on the severity of the disease, thiol levels will decrease as disease severity increases. In this study, it was planned to compare primarily COVID-19 patients with healthy controls, as well as patients with mild to moderate COVID-19 and patients with severe COVID-19 in terms of thiol level within themselves. It was planned to compare NT levels with other inflammatory markers in predicting the disease and clinical severity of the disease.
2. Materials and methods
2.1. Study design
This prospective cohort study was conducted on adult patients (>18 years of age) hospitalized with the diagnosis of COVID-19 at the Ankara City Hospital Infectious Diseases and Clinical Microbiology clinic between 31 March 2020 and 30 May 2020 and in age-matched healthy control group. The study was approved by the Turkish Ministry of Health and Ankara City Hospital Ethical Committee. Demographic characteristics such as age, gender, comorbidities, symptoms and onset times, vital signs, laboratory tests obtained upon hospital admission of patients and the need for intensive care unit (ICU) follow-up were recorded. We classified the patients as mild illness, pneumonia, severe pneumonia, acute respiratory distress syndrome (ARDS), and sepsis/septic shock according to the clinical severity of the disease based on WHO guidelines [17]. Patients were divided into two groups as mild to moderate patients (mild illness/pneumonia) and severe patients (severe pneumonia, ARDS, sepsis and septic shock). Control group were selected among age-matched healthy people who have no infectious symptoms and a history of cardiovascular disease, cancer, kidney disease, liver disorder, diabetes, or rheumatologic disorder. Among both control and COVID-19 patients, those who use antioxidants, fish-oil, supplemental vitamins or iron supplements were excluded from the study.
2.2. Sample collection and processing
Thiol levels were studied from serum samples taken simultaneously with routine laboratory parameters in the COVID-19 patient group and control group. Blood samples were collected from patient groups within the first 24 hours of admission. Thiol levels were studied from venous blood samples collected in plain tubes. The serum was separated after centrifugation at 1500 × g for 10 minutes and stored at −80°C refrigerator until analysis. All samples were performed at the same loop. The native thiol (NT), disulphide and total thiol (TT) levels were measured by an automated spectrophotometric method developed by Erel and Neselioğlu [18]. While TT includes both reduced and oxidized thiols, native thiol reflects only reduced thiols. Half of the difference of TT and NT was accepted as disulphide levels.
Laboratory parameters (white blood cell count, lymphocyte count, neutrophil lymphocyte ratio (NLR), C-reactive protein (CRP), ferritin, interleukin-6 (IL-6)) and thiol-disulphide homeostasis parameters (NT, TT, and disulphide levels) were first compared between COVID-19 patients group and healthy control group. And also these parameters were compared between three groups, mild to moderate COVID-19 patients, severe COVID-19 patients and healthy control group. The AUC for each of these parameters was calculated for its diagnostic value in COVID-19 infection and its role in determining disease severity.
2.3. Statistical analyses
Statistical analysis was performed using IBM SPSS V.20 software version. Descriptive statistics were presented as frequency and percentages for categorical variables and as mean ± standard deviation (SD) or median (minimum-maximum values) for continuous variables. Student's t-test was used for normally distributed data and the Mann Whitney U test for non-normal distribution in comparison of continuous variables. Kruskal-Wallis and ANOVA were conducted to compare the parameters among groups. Chi-square test was used for comparing categorical variables if parametric conditions were met. A p-value of less than 0.05 was considered statistically significant. AUCs were calculated with ROC Curve analysis in order to evaluate cut-off points for continuous variables. Correlation analyses for symptom recovery time were done by using Spearman's correlation test.
3. Results
One hundred forty-four COVID-19 patients and 70 control patients were included in the study. The average age of COVID-19 patients was 49.6, 65.2% of them were male; 41% had at least one comorbidity. Hypertension (17.3%), diabetes mellitus (13.1%) and coronary artery disease (5.5%) were the comorbidities encountered most often. 93.8% of patients were symptomatic, with cough (66.7%), fever (52%), dyspnea (29%) and fatigue (25.7%) determined as the most frequent symptoms. At hospital admission, 81.3% (117) of patients were mild to moderate patients while 18.7% (27) were severe patients. While 20.8% (30) of patients were taken to the intensive care, 6.2% (9) of patients were exitus (Table 1 ).
Table 1.
Baseline demographic and clinical characteristics of COVID-19 patients.
| Characteristics | COVID-19 patients (n = 144) |
|---|---|
| Age, years (mean ± SD) | 49.6 ± 18.3 |
| Sex, male | 94 (65.2%) |
| Any comorbidity | 59 (41.0%) |
| Hypertension | 25 (17.3%) |
| Diabetes | 19 (13.1%) |
| Coronary artery disease | 8 (5.5%) |
| Other | 19 (13.1%) |
| Symptoms | |
| Any Symptoms | 135 (93.8%) |
| Cough | 96 (66.7%) |
| Fever | 75 (52.0%) |
| Dyspnea | 43 (29.0%) |
| Fatigue | 37 (25.7%) |
| Sore throat | 19 (13.2%) |
| Time between symptom onset and hospitalization, day (median, min-max) | 3 (1–21) |
| Diseases severity | |
| Mild to moderate patients | 117 (81.3%) |
| Severe patients | 27 (18.7%) |
| Hospitalization in intensive care unit | 30 (20.8%) |
| Outcome, death | 9 (6.2%) |
ST: Standard deviation.
In comparison of COVID-19 patients and control group, there was no significant difference in terms of age and gender (p < 0.05). NLR, CRP, ferritin, and IL-6 levels were higher among COVID-19 patients than in control patients, while lymphocyte counts were lower (p < 0.001 for all). COVID-19 patients had lower NT and TT levels compared with the control groups, while disulphide levels were higher (P < 0.001, P < 0.001, and P = 0.029, respectively) (Table 2 ).
Table 2.
Comparison of inflammatory markers, native thiol, total thiol and disulphide levels between COVID-19 patients and control group.
| Control group |
COVID-19 patients |
P value | |
|---|---|---|---|
| (n = 70) | (n = 144) | ||
| Inflammatory marker levels, median (IQR) | |||
| White blood cell count (/mm³) | 6270 (3510) | 6300 (4065) | 0.892 |
| Lymphocyte count (/mm³) | 1880 (837) | 1090 (760) | <0.001 |
| NLR | 1.83 (1.07) | 3.08 (3.67) | <0.001 |
| CRP (g/L) | 0.002 (0.006) | 0.017 (0.092) | <0.001 |
| Ferritin (μg/L) | 35 (66.5) | 226 (303.5) | <0.001 |
| IL-6 (pg/ml) | 3.65 (2.4) | 15 (23.0) | <0.001 |
| Thiol-disulphide homeostasis parameters, mean ± SD | |||
| NT level (μmol/L) | 419.8 ± 55.9 | 242.1 ± 90.0 | <0.001 |
| TT level (μmol/L) | 459.1 ± 60.1 | 285.3 ± 95.0 | <0.001 |
| Disulphide level (μmol/L) | 19.69 ± 4.9 | 21.61 ± 6.4 | 0.029 |
IQR: interquartile range, NLR: Neutrophil to lymphocyte ratio, CRP: C-reactive protein, IL-6: Interleukin 6, NT: native thiol, TT: Total thiol, SD: Standard deviation.
While lenfosit count, NT, TT, disulphide levels were low, NLR, CRP, ferritin, IL-6 levels were high in severe COVID-19 patients compared to mild to moderate COVID-19 patients (Table 3 ). NT and TT levels were significantly low in severe COVID-19 patients compared to control group and mild to moderate COVID-19 patients (P < 0.001 for all) (Table 3, Fig. 1 ). Disulphide levels were significantly low in severe COVID-19 patients compared to mild to moderate COVID-19 patients (P < 0.001).
Table 3.
Comparison of inflammatory markers, native thiol, total thiol and disulphide levels between the control group, mild to moderate COVID-19 patients and severe COVID-19 patients.
| Group 1 Control | Group 2 Mild to moderate COVID-19 patients | Group 3 Severe COVID-19 patients | *p-value | Comparison of groups | Post hoc p-value | |
|---|---|---|---|---|---|---|
| Inflammatory marker levels, median (IQR) | ||||||
| White blood cell count (/mm³) | 6270 (3510) | 6330 (3680) | 5750 (5769) | 0.985 | 1 vs 2 | NS |
| 1 vs 3 | NS | |||||
| 2 vs 3 | NS | |||||
| Lymphocyte count (/mm³) | 1880 (837) | 1140 (860) | 910 (485) | <0.001 | 1 vs 2 | <0.001 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | 0.033 | |||||
| NLR | 1.83 (1.04) | 2.97 (3.04) | 4.97 (6.83) | <0.001 | 1 vs 2 | <0.001 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | 0.062 | |||||
| CRP (g/L) | 0.002 (0.0063) | 0.01 (0.0258) | 0.139 (0.1675) | <0.001 | 1 vs 2 | <0.001 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | <0.001 | |||||
| Ferritin (μg/L) | 35 (66.50) | 170 (263.75) | 395.6 (340.40) | <0.001 | 1 vs 2 | <0.001 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | 0.014 | |||||
| IL-6 (pg/ml) | 3.6 (2.4) | 10.6 (17.9) | 34.5 (31.7) | <0.001 | 1 vs 2 | 0.024 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | 0.006 | |||||
| Thiol-disulphide homeostasis parameters, mean ± SD | ||||||
| NT level (μmol/L) | 419.75 ± 55.88 | 260.71 ± 83.08 | 156.62 ± 69.75 | <0.001 | 1 vs 2 | <0.001 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | <0.001 | |||||
| TT level (μmol/L) | 459.12 ± 60.05 | 305.73 ± 86.40 | 192.00 ± 75.20 | <0.001 | 1 vs 2 | <0.001 |
| 1 vs 3 | <0.001 | |||||
| 2 vs 3 | <0.001 | |||||
| Disulphide level (μmol/L) | 19.69 ± 4.91 | 22.50 ± 6.19 | 17.54 ± 5.83 | <0.001 | 1 vs 2 | 0.004 |
| 1 vs 3 | 0.329 | |||||
| 2 vs 3 | <0.001 | |||||
IQR: interquartile range, NLR: Neutrophil to lymphocyte ratio, CRP: C-reactive protein, IL-6: Interleukin 6, NT: native thiol, TT: Total thiol, SD: Standard deviation, NS: Not significant. *Indicates a significant statistical difference with p < 0.05 (Kruskal-Wallis or ANOVA).
Fig. 1.
Serum native thiol levels of control groups, mild to moderate and severe COVID-19 patients.
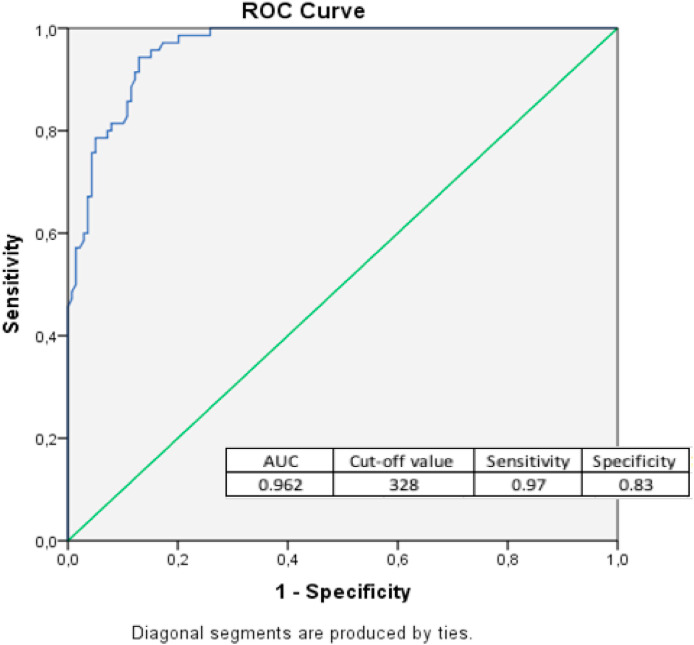
ROC analysis showed the largest AUC for IL-6, NT and TT (AUC = 0.972, AUC = 0.962, AUC = 0.950, respectively) to distinguish COVID-19 patients from control groups (Table 4 ). A cut-off value of 328 μmol/L for NT has a 97% sensitivity and 83% specificity (Fig. 2 ).
Table 4.
Evaluation of diagnostic predictive value of inflammatory parameters and native thiol, total thiol, disulphide in COVID-19 patients compared to control group.
| Cut-off value | AUC (95%CI) | Sensitivity | Specificity | P | |
|---|---|---|---|---|---|
| Inflammatory markers | |||||
| Lymphocyte count | 1390 | 0.817 | 0.71 | 0.79 | <0.001 |
| NLR | 2.71 | 0.762 | 0.60 | 0.86 | <0.001 |
| CRP | 0.02 | 0.781 | 0.48 | 0.10 | <0.001 |
| Ferritin | 191 | 0.842 | 0.53 | 0.10 | <0.001 |
| IL-6 | 6.40 | 0.972 | 0.98 | 0.96 | 0.002 |
| Thiol-disulphide homeostasis parameters | |||||
| NT | 328 | 0.962 | 0.97 | 0.83 | <0.001 |
| TT | 376 | 0.950 | 0.83 | 0.96 | <0.001 |
| Disulphide | 25.25 | 0.412 | 0.27 | 0.87 | 0.038 |
AUC: Area under curve, NLR: Neutrophil to lymphocyte ratio, CRP: C-reactive protein, IL-6: Interleukin 6, NT: Native thiol, TT: Total thiol.
Fig. 2.
AUC of native thiol in the distinction between COVID-19 patients and control group (Using receiver operating characteristic (ROC) curves).
NT and TT levels were found to be significantly lower in patients with fever (P = 0.025 and 0.021, respectively) and dyspnea symptoms (P = 0.003 and 0.002, respectively) (Table 5 ). Duration of symptoms for dyspnea, cough, fever, and sore throat were 6, 5.1, 3.6, and 3 days on average. There was a negative correlation between the duration of dyspnea, cough, fever, sore throat and NT levels (r = − 0.45, P = 0.017, r = −0.42, P < 0.001, r = −0.13, P = 0.084, r = −0.45, P = 0.040, respectively) (Table 6 ).
Table 5.
Relation between symptoms of COVID-19 patients and native thiol, total thiol and disulphide levels.
| Native Thiol | Total Thiol | Disulphide | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptoms (n) | Yes | No | P | Yes | No | P | Yes | No | P |
| Fever (75) | 225.3 ± 81.94 | 259.5 ± 95.18 | 0.025 | 267.1 ± 87.66 | 304.2 ± 99.17 | 0.021 | 11.2 ± 9.20 | 10 ± 5.63 | 0.38 |
| Dyspnea (43) | 207.07 ± 91.43 | 256.68 ± 85.67 | 0.003 | 246.48 ± 97.88 | 301.51 ± 89.31 | 0.002 | 12.25 ± 11.78 | 9.91 ± 4.96 | 0.22 |
| Cough (96) | 239.55 ± 90.54 | 246.93 ± 89.67 | 0.64 | 282.56 ± 95.17 | 290.59 ± 95.35 | 0.63 | 10.98 ± 8.89 | 9.86 ± 4.34 | 0.41 |
| Sore throat (19) | 268.26 ± 102.03 | 237.74 ± 87.63 | 0.15 | 320.00 ± 104.98 | 279.78 ± 92.56 | 0.086 | 11.57 ± 7.54 | 10.45 ± 7.70 | 0.55 |
Table 6.
Correlation analysis of native thiol, total thiol and disulfide values with duration of symptoms.
| Symptoms (n) | Duration of symptom, days (min-max) | Native thiol |
Total thiol |
Disulphide |
|||
|---|---|---|---|---|---|---|---|
| r value | P value | r value | P value | r value | P value | ||
| Dyspnea (n = 43) | 6 (1–24) | −0.45 | 0.017 | −0.47 | 0.019 | - 0.39 | 0.035 |
| Cough (n = 96) | 5.1 (1–22) | −0.42 | <0.001 | −0.41 | <0.001 | −0.09 | 0.446 |
| Fever (n = 75) | 3.6 (1–16) | −0.13 | 0.084 | −0.20 | 0.081 | −0.10 | 0.445 |
| Sore throat (n = 19) | 3 (1–6) | −0.45 | 0.040 | −0.47 | 0,031 | −0.22 | 0.388 |
The AUCs for white blood cell, lymphocyte count, NLR, CRP, ferritin, IL-6, NT, TT and disulphide are given in Table 7, Table 8 . According to the results, IL-6 and NT are the best parameters to distinguish control and mild to moderate patients. CRP and NT are the best parameters to distinguish between mild to moderate and severe patients.
Table 7.
Predictive performance for inflammatory markers, native thiol, total thiol and disulphide to distinguish control group from mild to moderate patients.
| Control group and mild to moderate patients | ||
|---|---|---|
| AUC | P | |
| Inflammatory markers | ||
| White blood cell | 0.53 | 0.430 |
| Lymphocyte count (/mm³) | 0.79 | <0.001 |
| NLR | 0.72 | 0.210 |
| CRP (g/L) | 0.82 | 0.069 |
| Ferritin (μg/L) | 0.94 | 0.012 |
| IL-6 (pg/ml) | 0.97 | 0.007 |
| Thiol-disulphide homeostasis parameters | ||
| NT | 0.95 | <0.001 |
| TT | 0.94 | <0.001 |
| Disulphide | 0.64 | 0.002 |
AUC: Area under curve, NLR: Neutrophil to lymphocyte ratio, CRP: C-reactive protein, IL-6: Interleukin 6, NT: Native thiol, TT: Total thiol.
Table 8.
Predictive performance for inflammatory markers, native thiol, total thiol and disulphide to distinguish mild to moderate COVID-19 patients from severe COVID-19 patients.
| Mild to moderate and severe patients | ||
|---|---|---|
| AUC | P | |
| Inflammatory markers | ||
| White blood cell | 0.51 | 0.892 |
| Lymphocyte count (/mm³) | 0.68 | 0.006 |
| NLR | 0.74 | 0.023 |
| CRP (g/L) | 0.85 | 0.001 |
| Ferritin (μg/L) | 0.65 | 0.138 |
| IL-6 (pg/ml) | 0.81 | 0.001 |
| Thiol-disulphide homeostasis parameters | ||
| NT | 0.83 | <0.001 |
| TT | 0.82 | <0.001 |
| Disulphide | 0.73 | <0.001 |
AUC: Area under curve, NLR: Neutrophil to lymphocyte ratio, CRP: C-reactive protein, IL-6: Interleukin 6, NT: Native thiol, TT: Total thiol.
The cut-off values of NT levels to distinguish patients from the control group and to predict the severity of COVID-19 patients are shown in Table 9 . In the distinction of the healthy and the patients using NT testing, the AUC was found to be 0.962 (95% CI: 0.940–0.983) (Fig. 2), while AUC in evaluating disease severity was found to be 0.83 (95% CI: 0.75–0.92).
Table 9.
Cut-off value, sensitivity, and specificity of native thiol levels to predict severity of COVID-19 patients.
| Between control group and mild to moderate patients | Between mild to moderate and severe patients | |
|---|---|---|
| Cut-off value of NT levels (μmol/L) | 349.5 | 189.0 |
| AUC (95% CI) | 0.95 (0.93–0.99) | 0.83 (0.75–0.92) |
| Sensitivity | 0.90 | 0.79 |
| Specificity | 0.86 | 0.72 |
| P value | <0.001 | <0.001 |
NT: Native thiol. AUC: Area under the curve. CI: Confidence interval.
4. Discussion
There are few studies investigating the role of oxidative stress in the pathogenesis of COVID-19, despite it has been shown to play a role in the pathogenesis of many infections [[19], [20], [21]]. In lung autopsies, neutrophile infiltrations in pulmonary capillaries, neutrophile extravasation in air cells and neutrophilic mucositis were determined [22]. This supports the expected symptom finding of increased ROS production as a result of neutrophile infiltration in COVID-19 patients just as in other viral infections [19]. NT and TT are antioxidant molecules found in the body that neutralize the effects of ROS. The produced ROS bonds with the sulfhydryl groups (-SS) of natural antioxidants found in the body and reduces them to disulphides. In COVID-19 infection, NT and TT levels are expected to decrease and disulphide levels to increase. In this study, the NT and TT levels of COVID-19 patients were lower compared to the control group while their disulphide levels were higher; these findings indicate that increased oxidative stress and deteriorated TDH will be effective in the pathogenesis of COVID-19. Additionally, the negative correlation between symptom duration and NT levels supports the consumption of antioxidant molecules while fighting the infection. It has been shown that NT and disulphide levels in severe patients were lower compared to the mild to moderate patient group. It has been postulated that the determination of a decrease instead of the expected rise in disulphide levels is related to the elimination of thiol which can be turned into disulphides due to increased production of ROS. However, more research is necessary on this topic.
The decrease in NT levels in this study is indicative of not just the presence of the disease but is also predictive of disease severity. Predicting cases that may show a severe course on admission to the hospital will provide early intervention advantage in the management of patients. Previous studies have shown the relation between D-dimer, ferritin, and IL-6 with the severity of the disease [23,24]. In our study, it was determined that a single-time measurement of thiol levels is highly indicative of predicting disease severity, like IL-6. Thiol measurement using the automated spectrophotometric method developed by Erel and Neşelioğlu yields results in a cheaper and must faster (<10 minutes) manner compared to the IL-6 test. In a period where patient numbers are on the rise, it is a fast and cost-effective test in the diagnosis of COVID-19 and in determining clinical severity. While the IL-6 cost per test is 0.972 EUR, the cost of NT per test is 0.043 EUR at our hospital (market rates from 22/10/2020 have been used; 1 TRY = 0.108 EUR).
In severe patients with negative SARS-CoV-2 PCR testing, diagnostic tests like thorax computed tomography and others support the diagnosis. However, it is not always easy to distinguish mild to moderate COVID-19 patients from those who are not ill. For this reason, there is a need for additional parameters that can be used to both begin treatments early and to separate mild to moderate COVID-19 patients from those who are not COVID-19 patients. In our study, it was observed that NT levels were highly conducive to separating the mild to moderate patient group from the control group.
There are studies on the use of antioxidant molecules in the treatment of COVID-19 patients and their clinical benefit [[25], [26], [27], [28]]. Glutathione, some trace elements like zinc and selenium, vitamin E, C, D, melatonin, carotenoids and polyphenols are among the molecules used as antioxidants. Glutathione, which includes the cysteine amino acid, has been shown to be effective in the viral infections such as influenza, herpes simplex virus [29]. The low levels of NT and TT we observed in our study also explains the benefit of antioxidants in treatments. Additionally, the negative correlation between symptom duration and NT levels is supportive of the use of thiol derivatives in both the treatment of the disease as well as symptomatic treatments.
The role of Nox2, an important ROS, in COVID-19 was investigated in a recently published article. Nox2 activity was found to be higher in COVID-19 patients compared to the control group and patients with ICU need compared to the patients without ICU need [30]. These findings demonstrate that oxidative stress can be effective in the pathogenesis of COVID-19 and support our study showing a decrease in antioxidant molecules that neutralize oxidative molecules.
Our study has some limitations; the number of patients is relatively low and the relation between comorbidities and thiols has not been studied. Additionally, other oxidative stress markers such as total peroxide, malondialdehyde, serum paraoxonase has not been compared with NT, TT and disulphide levels.
5. Conclusions
In conclusion, increasing evidence such as rising ROS production, decreased antioxidant molecules and demonstration that antioxidant treatments have clinical benefits in COVID-19 treatment supports the role of oxidative stress in the pathogenesis of COVID-19. Our study has shown that NT levels, which play an antioxidant role by neutralizing the increase in ROS, decrease in COVID-19 infection and have a negative correlation with symptom duration, and the level of NT on admission is a highly sensitive and cost-effective marker in COVID-19. As the first publication examining the relation between NT, TT, disulphide levels in COVID-19 patients, we hope to contribute to the literature by helping to illuminate the pathogenesis and to develop new treatment methods.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Zheng M., Gao Y., Wang G., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. Published 2020 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497e506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4) doi: 10.3390/v12040372. Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y.S., Lin C.F., Fang Y.T., Kuo Y.M., Liao P.C., Yeh T.M., et al. Antibody to severe acute respiratory syndrome (SARS)-associated coronavirus spike protein domain 2 cross-reacts with lung epithelial cells and causes cytotoxicity. Clin. Exp. Immunol. 2005;141(3):500–508. doi: 10.1111/j.1365-2249.2005.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls, Treasure Island (FL): StatPearls Publishing; 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 7.Esen R., Aslan M., Kucukoglu M.E., Cıkman A., Yakan U., Sunnetcioglu M., et al. Serum paraoxonase activity, total thiols levels, and oxidative status in patients with acute brucellosis. Wien Klin. Wochenschr. 2015;127(11–12):427–433. doi: 10.1007/s00508-015-0720-z. [DOI] [PubMed] [Google Scholar]
- 8.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180e183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khomich O.A., Kochetkov S.N., Bartosch B., et al. Redox biology of respiratory viral infections. Viruses. 2018;10:E392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Brand J.M.A., Haagmans B.L., van Riel D., et al. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. J. Comp. Pathol. 2014;151:83e112. doi: 10.1016/j.jcpa.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loffredo L., Violi F. COVID-19 and cardiovascular injury: a role for oxidative stress and antioxidant treatment? Int. J. Cardiol. 2020;312:136. doi: 10.1016/j.ijcard.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Can melatonin reduce the severity of COVID-19 pandemic? [published online ahead of print, 2020 Apr 29] Int. Rev. Immunol. 2020:1–10. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 13.Sen C.K., Packer L. Thiol homeostasis and supplements in phys ical exercise. Am. J. Clin. Nutr. 2000;72(2 Suppl) doi: 10.1016/j.ijcard.2020.04.066. 653–69. Loffredo L, Violi F. COVID-19 and cardiovascular injury: A role for oxidative stress and antioxidant treatment?. Int J Cardiol. 2020;312:136. [DOI] [PubMed] [Google Scholar]
- 14.Cremers C.M., Jakob U. Oxidant sensing by reversible disulfide bond formation. J. Biol. Chem. 2013;288(37):26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones D.P., Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic. Biol. Med. 2009;47(10):1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erel Ö., Erdoğan S. Thiol disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk. J. Med. Sci. 2020 doi: 10.3906/sag-1912-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance V 1.2. 13. 2020. https://apps.who.int/iris/rest/bitstreams/1272156 March, available at:
- 18.Erel O., Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014;47(18):326–332. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Laforge M., Elbim C., Frère C., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso A.A., Del Prete A., Lazzarino A.I. Hydrogen peroxide and viralinfections: a literature review with research hypothesis definition in rela-tion to the current COVID-19 pandemic. Med. Hypotheses. 2020;144:109910. doi: 10.1016/j.mehy.2020.109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascarella G., Strumia A., Piliego C., et al. COVID-19 diagnosis and management: a comprehensive review. J. Intern. Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y.R., Cao Q.D., Hong Z.S., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. Published 2020 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slominski R.M., Stefan J., Athar M., et al. COVID-19 and Vitamin D: a lesson from the skin [published online ahead of print, 2020 Aug 11] Exp. Dermatol. 2020 doi: 10.1111/exd.14170. 10.1111/exd.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J.Z., Zhang R.Y., Bai J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int. J. Cardiol. 2020;312:137–138. doi: 10.1016/j.ijcard.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shneider A., Kudriavtsev A., Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020;39(4):153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 28.Ntyonga-Pono M.P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35(Suppl 2):12. doi: 10.11604/pamj.2020.35.2.22877. Published 2020 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraternale A., Paoletti M.F., Casabianca A., Nencioni L., Garaci E., Palamara A.T., et al. GSH and analogs in antiviral therapy. Mol. Aspect. Med. 2009;30(1–2):99–110. doi: 10.1016/j.mam.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Violi F., Oliva A., Cangemi R., et al. Nox2 activation in covid-19. Redox Biol. 2020;36:101655. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]