Abstract
Purpose:
Few studies have examined the geometry of endovascular mechanical thrombectomy pathways. Here we examine the tortuosity and angulations of catheter pathways from the aortic arch to the termination of the internal carotid artery (ICA) and its association with thrombectomy performance.
Methods:
We included 100 consecutive anterior circulation large vessel occlusion thrombectomy patients over 12 months. Computed Tomography angiograms (CTA) were used for 3D segmentation of catheter pathway from the aortic arch to ICA termination. Tortuosity index (TI) and angulations of the catheter pathway were measured in a semiautomated fashion. TI and angulation degree were compared between sides and correlated with age and procedural measures.
Results:
We analyzed 188 catheter pathways in 100 patients. Severe angulation (≤30°) was present in 5.8% and 39.4% of common carotid artery (CCA) and extracranial ICA segments, respectively. Five pathways (2.6%) had 360° loop. CCA and extracranial ICA tortuosity had a weak but significant correlation with age (r=0.17, 0.21, p-value= 0.05, 0.02 respectively), time from groin puncture to the site of occlusion (r=0.29, 0.25, p-values= 0.008, 0.026 respectively) and fluoroscopy time (r= 0.022, 0.31, p-values= 0.016, 0.001 respectively). There was a significant difference in the pattern of angulation (p-value=0.04) and tortuosity between right and left side in CCA segment (TI= 0.20 ± 0.086 vs. 0.15 ± 0.82, p-value<0.001).
Conclusions:
There was a significant difference in CCA angulation between right and left sides. TI of extracranial CCA and ICA correlated with age and influenced time from groin puncture to the occlusion site and total fluoroscopy time.
Keywords: Tortuosity index, endovascular mechanical thrombectomy, Stroke, Large vessel occlusion, angulation, arterial loop
INTRODUCTION
The HERMES collaboration analysis of the recent randomized trials of endovascular mechanical thrombectomy (EVT) in patients with acute ischemic stroke (AIS) from emergent large vessel occlusion (ELVO) confirmed a strong association between earlier recanalization and improved clinical outcomes.1 Limited data is available on the prevalence of cervical and intracranial anatomical variants in patients with AIS from ELVO, and on how these influence the effectiveness of EVT. Prior work has focused mainly on surgical landmarks to assist preoperative planning, providing inadequate information on the distribution of neuroendovascular-relevant features such as vessel tortuosity and angulation.2, 3 For certain neuroendovascular procedures, such as carotid artery stenting, unfavorable arch anatomy affects the performance of the procedure.4, 5 Emerging data indicates that the effectiveness of thrombectomy may be influenced by the degree of intracranial tortuosity.6, 7
Earlier studies have attempted to quantify the extracranial carotid artery tortuosity using ultrasound, or two-dimensional digital subtraction angiography (DSA) or computed tomography angiographic (CTA) images of patients undergoing endovascular interventions.8, 9 However, the accuracy of these studies is limited due to the lack of 3-dimensional (3D) analysis. The knowledge of population-specific anatomical variants of large vessel tortuosity in patients with AIS treated with EVT may help in improving catheter systems to successfully navigate through the tortuous vessels, design of 3D models for training and treatment planning, and tailoring design of clinical trials towards a specific population of stroke patients with certain anatomical variants.
To our knowledge, no previous study has quantified the tortuosity of catheter pathway from aortic arch till common sites of occlusion in the middle cerebral artery (MCA). In this study, we sought to describe the geometry of the catheter pathway from the aortic arch to MCA in consecutive patients undergoing endovascular mechanical thrombectomy using a semi-automated method of measuring the curves and tortuosity. We then aimed to determine the association between tortuosity and time required to achieve access to the site of occlusion.
METHODS
Study Population and Data Acquisition
This retrospective study was approved by the University at Buffalo Institutional Review Board (UBIRB). Consecutive patients with AIS from anterior circulation ELVO treated with EVT over 12 months (December, 2016 to November, 2017) at a comprehensive stroke center were included in the study. Patients who had transfemoral mechanical thrombectomy for ELVO were included in the study. We excluded patients who did not have a baseline (pre-procedure) head/neck CTA or if accurate segmentation was not possible due to poor contrast opacification. The following data were collected: age, gender, cerebrovascular risk factors, time of symptom onset (or time patient last known at baseline if the exact time of onset was unknown), presence and location of ELVO on CTA, immediate angiographic findings. All EVT angiographic studies and operative reports were reviewed to collect the time of groin puncture, time of establishing guide catheter access, and fluoroscopy times. Angiographic recanalization was defined using a modified Thrombolysis in Cerebral Infarction (TICI) score.10 TICI 2b/3 was defined as successful recanalization. First pass effect was defined as a single pass/use of the device, TICI 2c/3 recanalization, and no use of rescue therapy.11 Aortic arch type was classified according to previously published classification as: bovine or types one two and three.12
CT Acquisition:
CTA data was obtained using 2, 2012 Aquilion ONE CT units (Canon Medical Systems Corporation, Otawara, Japan) Neuro ONE protocol. Nineteen scan volumes, were obtained between 1.5 and 3.2s apart over a period of 49.3s. Each volume consisted of 320 slices of 0.5mm resolution. Contrast agent, Omnipaque (350 i.e., 50 mL) was injected at a rate of 5mL/s. Tube voltage of 80 kVp, CT dose indices were 15.3–44.4 mGy, dose length products were 244.5–709.8 mGy×cm.
Image Processing and Calculation of Tortuosity
Step 1 -. Segmentation of Vessel Path from CTA.
The CTA Digital Imaging and Communications in Medicine (DICOM) files were de-identified and imported into segmentation software (Materialise Mimics Medical Version 21.0.0.406) which is a 510(k)- cleared software package (clearance number K073468). The arterial vasculature in the region of interest was segmented using a combination of thresholding, region growing, and use of the split mask function for optimal visualization (Figure 1A). The focus of segmentation included the aortic arch, brachiocephalic artery, bilateral common carotid arteries, bilateral ICA including its extracranial and intracranial segments up to the ICA terminus. The segmented vasculature was then converted to a StereoLithography (STL) file format using the Mimics identified ―optimal resolution‖ to calculate part from the segmented mask.
Figure 1.
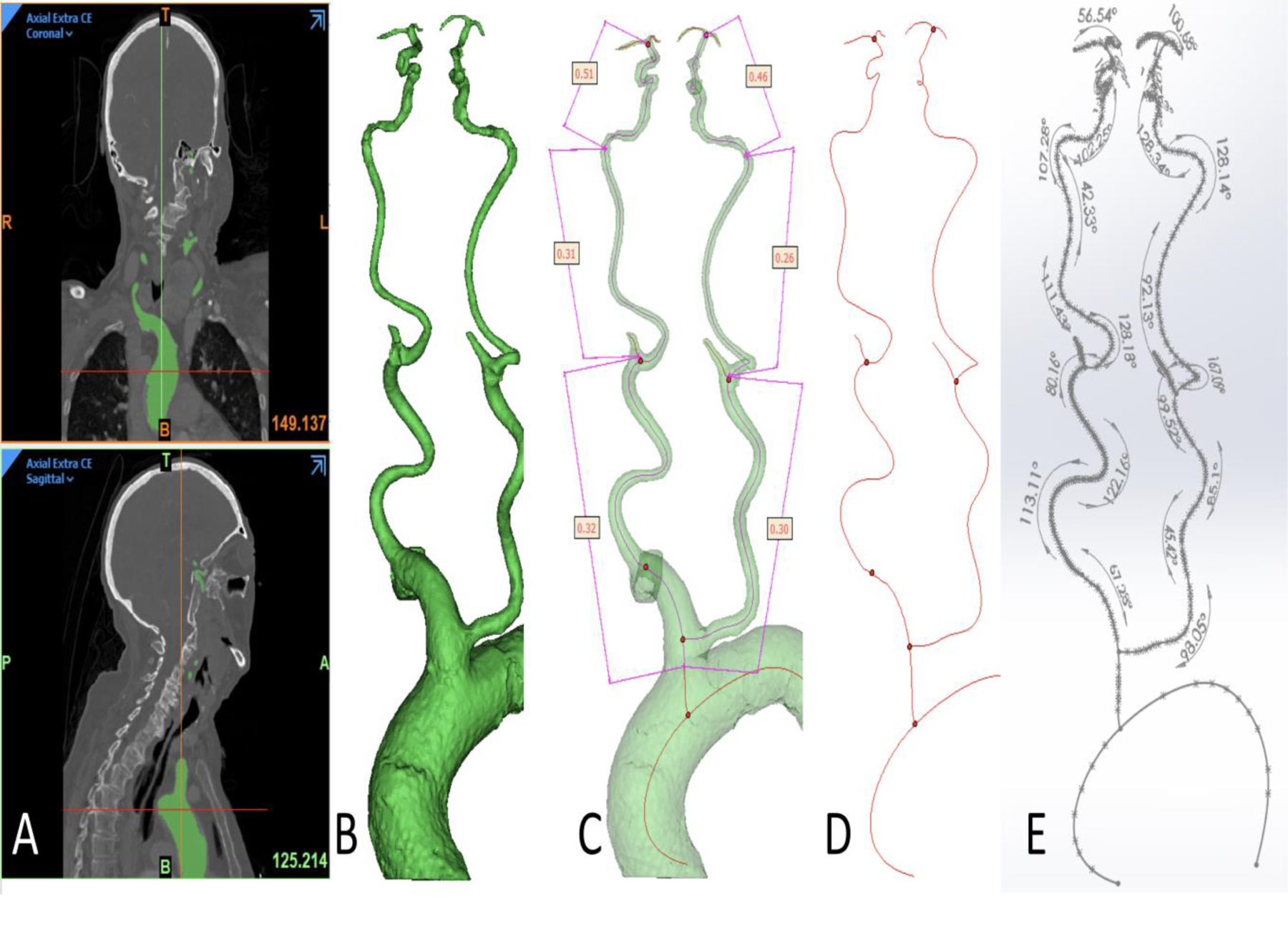
Image processing and measurement of tortuosity.
(A) Segmentation was performed using cranio-cervical CT angiogram.
(B) A 3D reconstruction was obtained including aortic arch, bilateral common and internal carotid arteries (ICA) till ICA bifurcation with length of different segments.
(C) Tortuosity index being taken bilaterally for the following 3 segments: common carotid artery, cervical ICA and intracranial ICA.
(D) A center line was obtained and exported to SolidWorks software(Dassault Systemes, Vélizy-Villacoublay, France)..
(E) Angle of each curve was calculated on SolidWorks (Dassault Systemes, Vélizy-Villacoublay, France).
Step 2 -. Vessel Path Centerline Computation & Measurement Preparation.
Centerlines were fit to the arterial pathway STL. A wrap function set at the highest resolution (the pixel size of the imaging) was used on the STL to ensure a smooth surface file for centerline computation. The centerline properties were exported as .txt files for further analysis (Figure 1D)
Step 3 –. Measurements.
The measurements were performed using 3D Sketch tools in Solidworks (Dassault Systemes, Vélizy-Villacoublay, France). For each measurement location, the minimum curvatures were determined by visually identifying the sharpest curve from the centerline in 3-dimensional space using SolidWorks (Dassault Systemes, Vélizy-Villacoublay, France) curvature combs as a guide (Figure 1E). The pathway was divided into three main segments i.e., aortic arch takeoff (brachiocephalic artery for right side pathway, common carotid artery [CCA] for left side) CCA till its bifurcation, extracranial ICA (from CCA bifurcation to ICA entry into the skull) and intracranial ICA to the bifurcation into MCA and anterior cerebral artery. Measurement transcription was independently verified. Tortuosity index (TI) was calculated for each segment of each carotid pathway using the formula: Tortuosity Index = 1 - (straight line distance/distance along the centerline). The same definition has been used in previous studies.13, 14 Since the tortuosity index measurements were automated an inter-rater variability was not assessed. The quality of 3 segmentation was evaluated and approved by the attending interventional neuroradiologist.
Data Analysis
Categorical data were presented as percentage and proportions. Shapiro-Wilk test was used to assess the distribution of continuous data. We report the median and interquartile range for data with skewed distribution and means and standard deviation to report data with normal distribution. Mean degrees of angle at each curve was reported with standard deviation for right and left side, and for each segment of the EVT pathway. We used Spearman’s correlation to estimate the association between tortuosity index with age, fluoroscopy time and time required to access the site of occlusion. A p-value of less than 0.05 was considered significant. Angulations were categorized into mild (>60°), moderate (30–60°) or severe (<30°) based on the previously described classification systems.15, 16 The analysis needs 15–20 minutes.
RESULTS
During the study period 156 patients underwent mechanical thrombectomy for ELVO. One hundred consecutive patients were included in the study. The mean age of the population was 72.5 ± 16.2 years, and 52% were female. The mean arrival NIHSS score was 13.7±6.3. The rate of successful revascularization was 86% with EVT. The mean time from groin puncture to establishing access to the occlusion target side was 23.5±12.4 minutes. Baseline clinical characteristics of the study cohort are presented in Table 1.
Table 1.
Clinical and procedure related details of study population
| Variables | N (%) or Mean ± SD |
|---|---|
| Age | 72.5±15.2 |
| Female | 52(52) |
| NIHSS at presentation | 13.7±6.3 |
| Successful revascularization (TICI 2b or more) | 86 (86) |
| Bovine Arch | 25 (25) |
| Type 3 arch | 15 (15) |
| Type 2 arch | 41(41) |
| Failure to Access ICA | 1 (1) |
| Failure to Access MCA | 1 (1) |
| Number of Passes | 1.64 ± 1.05 |
| First Pass Effect | 54 (54) |
| Groin puncture to target time, min | 23.5±12.4 |
| Fluoroscopy Time, min | 37.7±24.4 |
Abbreviations: CCA, common carotid artery; ICA, internal carotids artery; NIHSS, national institute of health stroke scale; SD, standard deviation; TICI, thrombolysis in cerebral infarction.
The overall distribution of the degree angulation of the CCA and extracranial ICA segments is summarized in Table 2. 77.6% of patients had mild angulation (>60°) angulation in the CCA segment. Moderate and severe angulation was present in 16.5% and 5.8% of cases, respectively. In the extracranial ICA segment mild, moderate and severe extracranial ICA angulation were observed in 18.1%, 44.1% and 39.4% of cases respectively. Five carotid pathways (2.6%) had arterial 360 degree loop. There was significant difference in the degree of angulation between the right and left side for CCA and but no difference was seen for extracranial ICA segments (P=0.04 and 0.67, respectively, Table 2). Supplementary table 1 provides a more detailed description of the number and degree of curves, angulation for the right and left side separately.
Table 2.
Angulation of the CCA and extracranial ICA segments
| Overall, % | Right side, % | Left side, % | P value | |
|---|---|---|---|---|
| CCA segment* | ||||
| Mild angulation (>60°) | 77.6 | 85 | 70.2 | 0.04 |
| Moderate angulation (31–60°) | 16.5 | 11.7 | 21.3 | |
| Severe angulation (≤30°) | 5.8 | 3.2 | 8.5 | |
| Extracranial ICA segment | ||||
| Mild angulation (>60°) | 18.1 | 15.9 | 20.2 | 0.67 |
| Moderate angulation (31–60°) | 44.14 | 46.8 | 41.5 | |
| Severe angulation (≤30°) | 39.4 | 37.2 | 38.3 | |
Abbreviations: CCA, common carotid artery; ICA, internal carotids artery.
Brachiocephalic segment is included into right CCA pathway measurement
The tortuosity index of the CCA segment was 0.17± 0.008 with significant difference between the right and left sides (0.20±0.086 vs. 0.15± 0.082, respectively, P-value <0.001). The cervical and intracranial ICA segments tortuosity were similar between the two sides (P=0.4 and 0.2, respectively). Tortuosity index values for the CCA, extracranial and intracranial ICA segments are listed in Table 3 and presented in figure 2. The distribution of tortuosity index values and diagrams illustrating segment anatomy are depicted in figure 3.
Table 3.
Tortuosity of Different segments of thrombectomy access pathway
| Segment | Tortuosity index, Right side | Tortuosity index, Left side | Overall Tortuosity index | P-value |
|---|---|---|---|---|
| CCA segment* | 0.20 ± 0.086 | 0.15 ± 0.82 | 0.17 ± 0.088 | <0.001 |
| Cervical ICA | 0.17 ± 0.87 | 0.18 ± 0.079 | 0.18 ±0.83 | 0.4 |
| Intracranial ICA | 0.45 ± 0.086 | 0.47 ± 0.70 | 0.46 ± 0.79 | 0.2 |
Abbreviations: CCA, common carotid artery; ICA, internal carotids artery.
Brachiocephalic segment is included into right CCA pathway measurement
Figure 2.
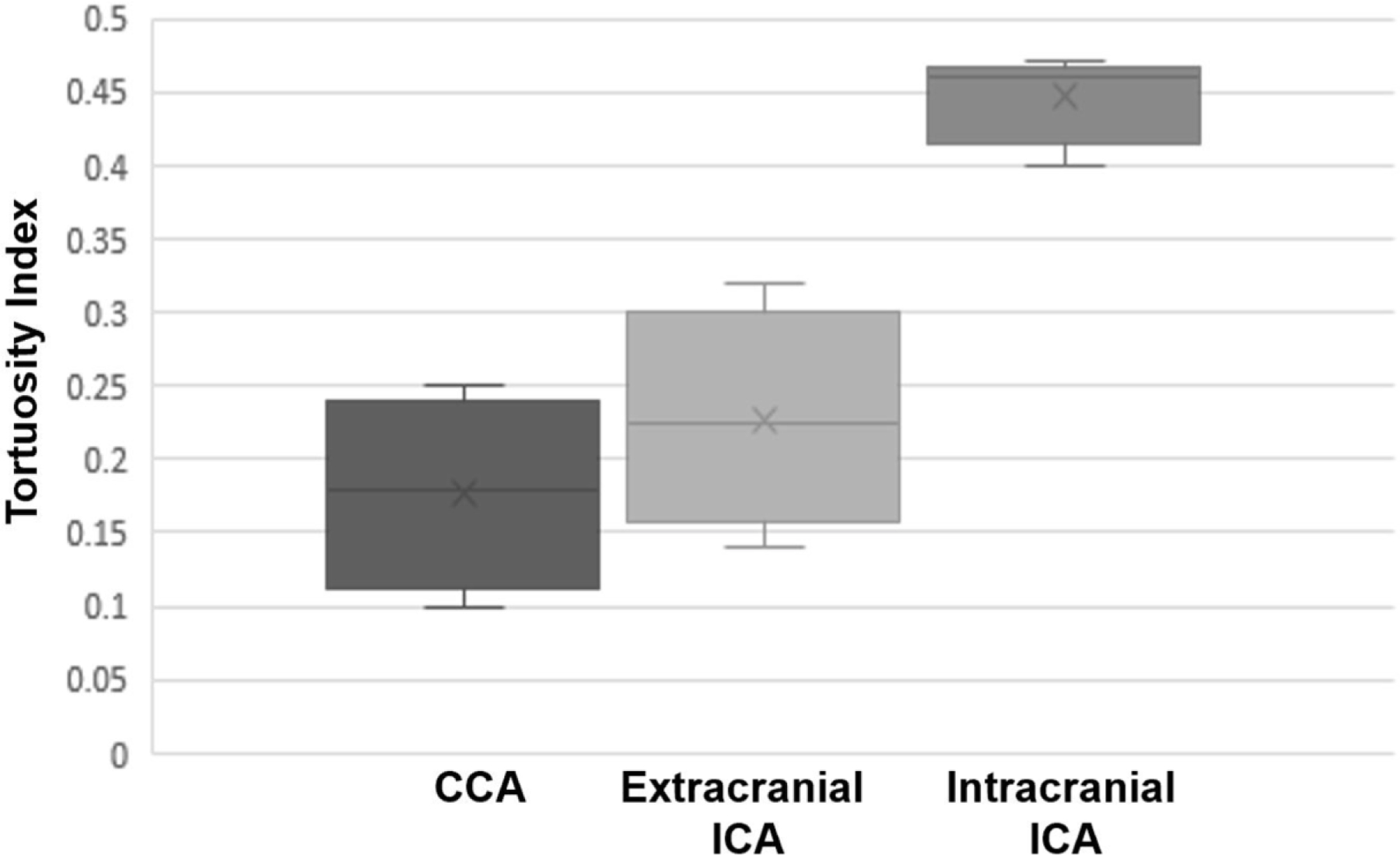
Box and Whisker plot for tortuosity of access pathway from aortic arch to common carotid bifurcation, cervical internal carotid artery (ICA) and intracranial ICA. Cross represents median value while upper and lower limits of the box are representative of interquartile range.
Figure 3.
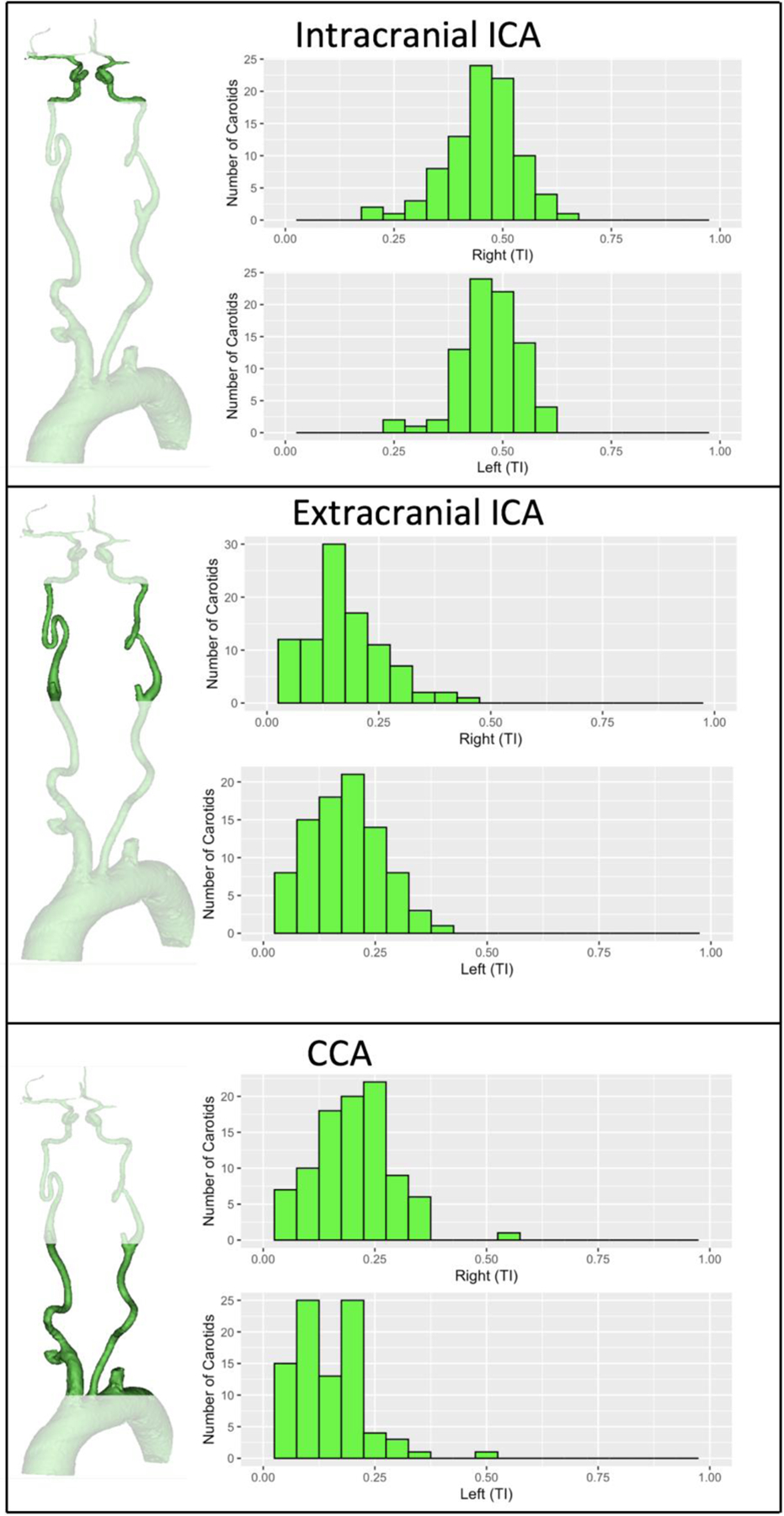
Population distribution of carotid tortuosity.
Illustrations and histograms illustrating the 3 anatomical segments analyzed and spread of their tortuosity index values. CCA segment on the right side included Brachiocephalic artery.
There was a weak but statistically significant correlation of age with CCA and extracranial ICA tortuosity index (r= 0.17, P=0.05 and r=0.21, P value= 0.02 respectively; Table 4). We also observed significant though weak correlation between tortuosity of CCA bifurcation and extracranial ICA tortuosity with time from groin puncture to establishing access to the target site of occlusion (r= 0.295, P=0.008 and r=0.251, P=0.026, respectively; Table 4). There was no difference between intracranial tortuosity with respect to the number of thrombectomy device passes (P=0.6). Of these aspirations first approach was used in 24 (24%) cases while stent retriever was used as primary approach in 76 (76%) cases. Neuron Max (Penumbra, California) was the most frequently used catheter (n=50) followed by Benchmark (Penumbra, California) (n=36) and Fubuki (Asahi, Tokyo) (n=14). Solitaire (Medtronic, Minnesota) was used in 51 cases; Trevo (Stryker, Michigan) in 18 cases and Embotrap (Cerenovus, Tokyo) was used in a total of 7 cases. ACE 068 (Penumbra, California) was used in 20 cases, while Sofia plus (MicroVention Inc., California) was used in 4 cases.
Table 4.
Correlation of tortuosity indices with age, fluoroscopy time and time from groin puncture to access of occlusion site
| Age | Groin Puncture to Site of Occlusion | Fluoroscopy Time | ||||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| CCA tortuosity | 0.17 | 0.05 | 0.29 | 0.008 | 0.224 | 0.016 |
| Extracranial ICA tortuosity | 0.21 | 0.02 | 0.25 | 0.026 | 0.31 | 0.001 |
| Intracranial ICA tortuosity | 0.04 | 0.35 | 0.02 | 0.85 | 0.03 | 0.37 |
Abbreviations: CCA, common carotid artery; ICA, internal carotid artery; r = correlation co-efficient
There was a significant correlation of CCA and extracranial ICA tortuosity indexes with total fluoroscopy time (r= 0.22, P=0.016 and r=0.31, P = 0.001, respectively; Table 4). Tortuosity index of CCA was significantly higher in patients who required more than 30 minutes to access (30.4% cases) the target lesion (0.21 ± 0.1 vs 0.15 ± 0.1, P =0.002). Proportions of type 2 (52% vs. 35%) and type 3 aortic arch (17% vs. 15%), bovine arch (26.1% vs. 23.3%) and mean sternal notch to vertex height (30 vs. 29.3 cm) were not significantly different between delayed and rapid access groups (p-values, 0.26, 0.79 and 0.22 respectively). In 2 cases access to the occlusion site failed. Both these cases had high tortuosity index in common carotid and extracranial ICA segments. i.e. case 1, left M1 occlusion; 0.37 and 0.24 while case 2, right M1 occlusion (0.4 and 0.30, for CCA and extracranial ICA respectively.
DISCUSSION
The anatomic variants knowledge of the carotid artery segments in patients with ELVO stroke and the impact of vessel tortuosity on the efficacy of mechanical thrombectomy is limited. Failure or delay in establishing arterial access for effective EVT negatively affects clinical outcome. In the prospective Bernese Stroke Registry of 592 AIS patients (93% with anterior circulation) with an intention to perform stent-retriever thrombectomy, one-third of reperfusion failures were due to the inability to reach the target occlusion.17 Such failures were found to occur mainly due to cervical vessel tortuosity or failed catheterization because of difficult aortic arch anatomy.17 However, the overall rate of failure to achieve target occlusion is relatively low. In the Bernese registry, cervical artery access catheterization failure occurred in 20 out of 529 patients (3%).17
Delay rather than a failure to reach target occlusion is more common in daily clinical practice. In our series of 100 cases, failure to establish access to the target lesion occurred in only 2% of cases, whereas delay of 30 min or longer to reach target lesion was encountered in 30.4% of cases. Both these cases had high tortuosity index in common carotid and extracranial ICA segments. One of the cases with failed access had 360-degree loop had failure. Multiple studies have shown that more prolonged thrombectomy procedures lead to lower rates of functional independence and higher rates of intracranial hemorrhage.18–20 One of the key challenges in studying how carotid tortuosity affects thrombectomy procedure is the difficulty to determine and classify the degree of angulation given the wide variety of anatomical variants of the carotid artery. Previously studies have relied predominantly on a qualitative 2-dimensional analysis in measuring angles, which is subject to significant error resulting in either under- or overcalling the degree of angulation or vessel kinking. Kaymaz et al. presented their results of consecutive 76 patients with stroke treated with thrombectomy by measuring tortuosity from the centerline of blood flow in inclined sagittal and coronal planes of CTA source images.21 Similarly, measurement of intracranial tortuosity to evaluate its effect on the performance of thrombectomy devices utilized 2-dimensional views of catheter angiography or CTA rather than 3-dimensional analysis.6, 7
In this study, we demonstrate that TI of CCA-Brachiocephalic segment was significantly associated with prolonged access time. We also compared arch type, bovine variant, and sternal notch to vertex height between delayed and rapid access groups. Proportion of type 2 (52% vs. 35%) and type 3 aortic arch (17% vs. 15%), bovine arch (26.1% vs. 23.3%) and mean sternal notch to vertex height (30 vs. 29.3 cm) were not significantly associated with delayed access times (p-values, 0.26, 0.79 and 0.22 respectively).
To our knowledge, we were able for the first time to accurately classify anatomical variants of carotid tortuosity in patients treated with thrombectomy. These data can have several important clinical implications. 3d-printed phantoms are increasingly being used for the development and testing of new thrombectomy devices and approaches. Yet, these phantoms may not accurately represent the typical clinical variants of patients treated with thrombectomy. The knowledge of most common anatomical variants of the CCA, extracranial and intracranial ICA can help create phantoms that accurately represent population-specific rather anatomical variants. Another potential application of the approach and knowledge gained in this study is its incorporation in refining robotic-assisted neurovascular interventions, including stroke thrombectomy procedures. While minimally invasive endovascular robot surgery for neurointerventions is currently in its infancy, early experience in patients treated with carotid stenting and aneurysm embolization indicates its feasibility as a tool to treat acute stroke from LVO.22 3D segmentation coupled with machine learning tools could be applied to calculate optimal pathways for devices such as catheters and guidewires.
The retrospective nature of our study may have limited the accuracy of data collection. Although the technical data were collected after reviewing the fluoroscopy records of the entire thrombectomy procedure for each case, prospectively collected records would be more accurate in ensuring that critical procedural details are captured. Second, the data on the segmentation of the MCA were not collected. MCA tortuosity will be a focus of a separate project given the significant variability of the MCA patterns and the complexity of analysis needed to study how MCA tortuosity affects stent retriever and aspiration thrombectomy, considering the variety of techniques and devices used nowadays. However, the findings of this study are not applicable radial approach since radial cases were not included in the study. Additionally, since the technique is time consuming, which limits its application in acute setting.
CONCLUSION
Utilizing CTA data and 3D segmentation, we were able to describe tortuosity and angulations of catheter pathway from aortic arch to ICA termination. TI of extracranial CCA and ICA correlated with age and influenced time from groin puncture to the site of occlusion and total fluoroscopy time. There was significant difference in the pattern of CCA angulation between right and left sides. Severe angulation was present in 5.8% and 39.4% of CCA and extracranial ICA segments, respectively. Mean time to clot was 23 min, however in 30% cases it was over 30 min which correlated with tortuosity index. To summarize, tortuosity significantly prolongs time to revascularization in some cases. These findings me be applied to the design of new devices and experimental models of endovascular stroke thrombectomy.
Supplementary Material
Funding:
This work was supported in part by the NIH grant 1R21NS109575
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest:
Levy- Shareholder/Ownership interests: NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care (formerly the Stroke Project), Rebound Therapeutics, StimMed, Three Rivers Medical; National Principal Investigator/Steering Committees: Medtronic (merged with Covidien Neurovascular) SWIFT Prime and SWIFT Direct Trials; Honoraria: Medtronic (training and lectures); Consultant: Claret Medical, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, Rebound, StimMed; Advisory Board: Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical, Endostream Medical; Site Principal Investigator: CONFIDENCE study (MicroVention), STRATIS Study—Sub I (Medtronic).
Siddiqui- Research grant: NIH/NINDS 1R01NS091075 as a co-investigator for “Virtual Intervention of Intracranial Aneurysms;” financial interest/investor/stock options/ownership: Amnis Therapeutics, Apama Medical, Blink TBI Inc., Buffalo Technology Partners Inc., Cardinal Consultants, Cerebrotech Medical Systems, Inc. Cognition Medical, Endostream Medical Ltd., Imperative Care, International Medical Distribution Partners, Neurovascular Diagnostics Inc., Q’Apel Medical Inc, Rebound Therapeutics Corp., Rist Neurovascular Inc., Serenity Medical Inc., Silk Road Medical, StimMed, Synchron, Three Rivers Medical Inc., Viseon Spine Inc; Consultant/advisory board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA Inc., Cerebrotech Medical Systems Inc., Cerenovus, Corindus Inc., Endostream Medical Ltd., Guidepoint Global Consulting, Imperative Care, Integra LifeSciences Corp., Medtronic, MicroVention, Northwest University–DSMB Chair for HEAT Trial, Penumbra, Q’Apel Medical Inc., Rapid Medical, Rebound Therapeutics Corp., Serenity Medical Inc., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, W.L. Gore & Associates; Principal investigator/steering comment of the following trials: Cerenovus NAPA and ARISE II; Medtronic SWIFT PRIME and SWIFT DIRECT; MicroVention FRED & CONFIDENCE; MUSC POSITIVE; and Penumbra 3D Separator, COMPASS, and INVEST.
Borlongan - Funded and received royalties and stock options from Astellas, Asterias, Sanbio, Athersys, KMPHC, and International Stem Cell Corporation; and also received consultant compensation for Chiesi Farmaceutici.
Mokin- Consultant: Canon, Cerebrotech, Imperative care. Investor: Serenity Medical.
None for others
Ethical approval:
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent:
Informed consent was obtained from all individual participants included in the study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016;316:1279–1288 [DOI] [PubMed] [Google Scholar]
- 2.Shen XH, Xue HD, Chen Y, Wang M, Mirjalili SA, Zhang ZH, et al. A reassessment of cervical surface anatomy via ct scan in an adult population. Clin Anat 2017;30:330–335 [DOI] [PubMed] [Google Scholar]
- 3.Zenteno M, Leeb A, Moscote-Salazar LR. Anatomic variations of the internal carotid artery: Implications for the neurologic endovascular therapist. Bol Asoc Med P R 2013;105:70–75 [PubMed] [Google Scholar]
- 4.Dumont TM, Mokin M, Wach MM, Drummond PS, Siddiqui AH, Levy EI, et al. Understanding risk factors for perioperative ischemic events with carotid stenting: Is patient age over 80 years or is unfavorable arch anatomy to blame? J Neurointerv Surg 2014;6:219–224 [DOI] [PubMed] [Google Scholar]
- 5.Werner M, Bausback Y, Braunlich S, Ulrich M, Piorkowski M, Friedenberger J, et al. Anatomic variables contributing to a higher periprocedural incidence of stroke and tia in carotid artery stenting: Single center experience of 833 consecutive cases. Catheter Cardiovasc Interv 2012;80:321–328 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Yamagami H, Todo K, Kuramoto Y, Ishikawa T, Imamura H, et al. Correlation of middle cerebral artery tortuosity with successful recanalization using the merci retrieval system with or without adjunctive treatments. Neurol Med Chir (Tokyo) 2014;54:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwaiger BJ, Gersing AS, Zimmer C, Prothmann S. The curved mca: Influence of vessel anatomy on recanalization results of mechanical thrombectomy after acute ischemic stroke. AJNR Am J Neuroradiol 2015;36:971–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrino L, Prencipe G, Vairo F. [dolicho-arteriopathies (kinking, coiling, tortuoosity) of the carotid arteries: Study by color doppler ultrasonography]. Minerva Cardioangiol 1998;46:69–76 [PubMed] [Google Scholar]
- 9.Kim ST, Brinjikji W, Lehman VT, Carr CM, Luetmer PH, Rydberg CH. Association between carotid artery tortuosity and carotid dissection: A case-control study. J Neurosurg Sci 2018;62:413–417 [DOI] [PubMed] [Google Scholar]
- 10.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013;44:2650–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al. First pass effect: A new measure for stroke thrombectomy devices. Stroke 2018;49:660–666 [DOI] [PubMed] [Google Scholar]
- 12.Madhwal S, Rajagopal V, Bhatt DL, Bajzer CT, Whitlow P, Kapadia SR. Predictors of difficult carotid stenting as determined by aortic arch angiography. J Invasive Cardiol 2008;20:200–204 [PubMed] [Google Scholar]
- 13.Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation 2011;124:388–396 [DOI] [PubMed] [Google Scholar]
- 14.Dougherty G, Varro J. A quantitative index for the measurement of the tortuosity of blood vessels. Med Eng Phys 2000;22:567–574 [DOI] [PubMed] [Google Scholar]
- 15.Togay-Isikay C, Kim J, Betterman K, Andrews C, Meads D, Tesh P, et al. Carotid artery tortuosity, kinking, coiling: Stroke risk factor, marker, or curiosity? Acta Neurol Belg 2005;105:68–72 [PubMed] [Google Scholar]
- 16.Weibel J, Fields WS. Tortuosity, coiling, and kinking of the internal carotid artery. I. Etiology and radiographic anatomy. Neurology 1965;15:7–18 [DOI] [PubMed] [Google Scholar]
- 17.Kaesmacher J, Gralla J, Mosimann PJ, Zibold F, Heldner MR, Piechowiak E, et al. Reasons for reperfusion failures in stent-retriever-based thrombectomy: Registry analysis and proposal of a classification system. AJNR Am J Neuroradiol 2018;39:1848–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alawieh A, Vargas J, Fargen KM, Langley EF, Starke RM, De Leacy R, et al. Impact of procedure time on outcomes of thrombectomy for stroke. J Am Coll Cardiol 2019;73:879–890 [DOI] [PubMed] [Google Scholar]
- 19.Alawieh A, Pierce AK, Vargas J, Turk AS, Turner RD, Chaudry MI, et al. The golden 35 min of stroke intervention with adapt: Effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointerv Surg 2018;10:213–220 [DOI] [PubMed] [Google Scholar]
- 20.Spiotta AM, Vargas J, Turner R, Chaudry MI, Battenhouse H, Turk AS. The golden hour of stroke intervention: Effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointerv Surg 2014;6:511–516 [DOI] [PubMed] [Google Scholar]
- 21.Kaymaz ZO, Nikoubashman O, Brockmann MA, Wiesmann M, Brockmann C. Influence of carotid tortuosity on internal carotid artery access time in the treatment of acute ischemic stroke. Interv Neuroradiol 2017;23:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albuquerque FC, Hirsch JA, Chen M, Fiorella D. Robotics in neurointervention: The promise and the reality. J Neurointerv Surg 2020;12:333–334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


