Abstract
Atrial fibrillation (AF) occurrence and maintenance is associated with progressive remodeling of electrophysiological (repolarization and conduction) and 3D structural (fibrosis, fiber orientations, and wall thickness) features of the human atria. Significant diversity in AF etiology leads to heterogeneous arrhythmogenic electrophysiological and structural substrates within the 3D structure of the human atria. Since current clinical methods have yet to fully resolve the patient-specific arrhythmogenic substrates, mechanism-based AF treatments remain underdeveloped. Here, we review current knowledge from in-vivo, ex-vivo, and in-vitro human heart studies, and discuss how these studies may provide new insights on the synergy of atrial electrophysiological and 3D structural features in AF maintenance. In-vitro studies on surgically acquired human atrial samples provide a great opportunity to study a wide spectrum of AF pathology, including functional changes in single-cell action potentials, ion channels, and gene/protein expression. However, limited size of the samples prevents evaluation of heterogeneous AF substrates and reentrant mechanisms. In contrast, coronary-perfused ex-vivo human hearts can be studied with state-of-the-art functional and structural technologies, such as high-resolution near-infrared optical mapping and contrast-enhanced MRI. These imaging modalities can resolve atrial arrhythmogenic substrates and their role in reentrant mechanisms maintaining AF and validate clinical approaches. Nonetheless, longitudinal studies are not feasible in explanted human hearts. As no approach is perfect, we suggest that combining the strengths of direct human atrial studies with the high fidelity approaches available in the laboratory and in realistic patient-specific computer models would elucidate deeper knowledge of AF mechanisms. We propose that a comprehensive translational pipeline from ex-vivo human heart studies to longitudinal clinically relevant AF animal studies and finally to clinical trials is necessary to identify patient-specific arrhythmogenic substrates and develop novel AF treatments.
Keywords: atrial fibrillation, electrophysiology, fibrosis, near-infrared optical mapping, ex-vivo human heart
Graphical Abstract
Introduction
Atrial fibrillation (AF) is a significant cause of age-related morbidity and mortality in the United States [1, 2]. Concurrence of AF with structural heart diseases, such as heart failure (HF), myocardial infarction (MI), and hypertension (HTN) is common; these interactions are particularly concerning due to increased deleterious effects, rising prevalence, and resistance to available treatments [3, 4]. Moreover, comorbidities can directly contribute to the remodeling of atrial myocardium and development of arrhythmogenic electrophysiological (EP) and structural substrates for AF occurrence and maintenance. Despite moderate success of interventional and pharmacological therapies, efficacy of conventional AF treatments is limited along with several side effects [5]. While pharmacological and mechanism-based ablation treatments [6, 7] have been developed to treat AF, the road to complete freedom from AF is still hampered with several limitations. The major limitations of AF treatments may arise from a limited understanding of human AF mechanisms in part due to technically insufficient mapping approaches to identify AF sources and/or patient-specific AF substrates. In addition, these deficits make it difficult to know whether efforts to maintain sinus rhythm (SR) are an exercise in futility.
A general understanding of AF mechanisms is that AF initiation requires triggers such as spontaneous extra beats that may lead to the formation of self-sustained reentry in the presence of bi-atrial arrhythmogenic EP (i.e. heterogeneously shortened refractoriness and slow conduction) and/or structural (i.e. enlarged atria, varied wall thickness, transmural myofiber twists, and fibrosis) substrates [8, 9]. Extra-pulmonary veins (PV) arrhythmogenic substrates can vary in a patient-specific manner depending on the nature and severity of the disease as well as underlying comorbidities [10–12]. Most AF ablation treatment strategies have focused on the elimination of AF triggers, which primarily originate from the PVs, and they do not require extensive bi-atrial mapping procedures [13]. Conventional anatomical based PV isolation (PVI) has shown to be more effective in treatment for paroxysmal AF (pAF), which lasts from several minutes to days, but PVI demonstrates lower efficacy in persistent AF (perAF) and is almost ineffective in long-standing perAF, which lasts for more than one year [14–16]. The low success of PVI may stem from progressive bi-atrial remodeling underlying AF maintenance. Indeed, during the last decade, different clinical mapping approaches have shown that the limited number of localized substrate-associated reentrant sources may maintain AF. The number of drivers may also progressively increase from ~1–3 in pAF, ~2–4 in perAF and ~3–6 or more drivers in long-standing perAF patients [6, 17, 18]. These studies also showed that the targeted ablation of the localized substrate-associated drivers in addition to PVI may increase success and suggested the crucial importance of localized drivers in maintaining all types of AF [19]. This review primarily addresses the mechanisms underlying AF maintenance and will not discuss pathophysiology of AF triggers, which has been reviewed previously in detail by several groups [20–22].
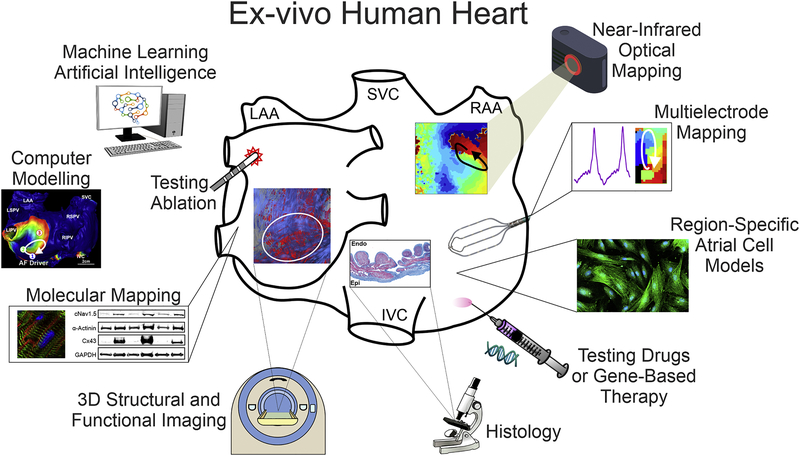
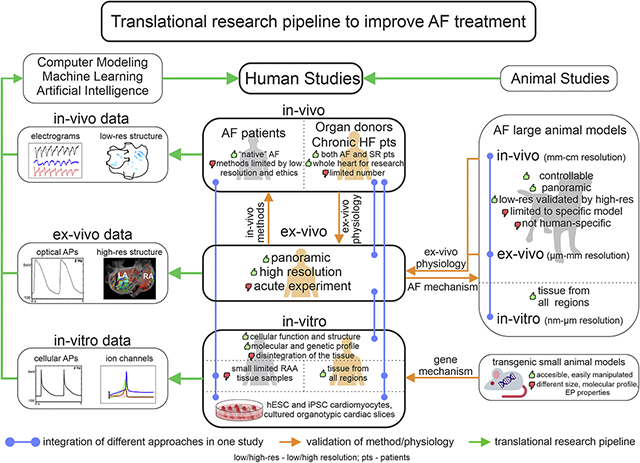
There is an urgent need for better identification and treatment approaches of extra-PV, patient-specific EP-structural AF substrates. Animal models have been widely used to reveal AF mechanisms at all three levels (in-vitro, ex-vivo, and in-vivo); however, animal AF substrates are not always similar to those found in humans due to significant EP and structural differences, which limit the applicability of findings from experimental animal models to humans [23, 24]. The complex and heterogeneous makeup of patient-specific 3D arrhythmogenic substrate and limitations of clinical methods for their identification may explain the frequent contradictions in clinical research on AF mechanisms. However, many of these limitations can be resolved by the ex-vivo coronary-perfused human heart approach, which allows the integration of state-of-the-art high-resolution functional and 3D structural imaging technologies with the submillimeter resolution required to resolve heart-specific intramural EP-structural AF mechanisms (Figure 1). We suggest that determination of patient-specific AF mechanisms requires a comprehensive multi-scale understanding of both human heart electrophysiology and structure, from in-vivo and ex-vivo to in-vitro and in-silico studies (Graphical Abstract).
Figure 1. Advantages of Integrated Explanted Human Heart Approach.
Ex-vivo human heart provides multiple advantages over in-vitro single cell and in-vivo studies of the human heart and allows for integration of molecular mapping, histology validated high-resolution 3D structural imaging, standard clinical electrode mapping, and near-infrared optical mapping with subsequent utilization of machine learning and computer modeling. In addition, cellular studies, drug testing, and gene-based therapy, as well as clinical ablation techniques can be utilized to define AF mechanisms and develop patient-specific therapy.
Abbreviations are as follows: LAA/RAA – left and right atrial appendages; IVC/SVC – inferior and superior vena cava; Endo/Epi – endocardial, epicardial.
Part 1: Approaches to study human atrial electrophysiology: Overview
Several methods have been employed in-vivo, in-vitro, and ex-vivo to study EP features (activation, conduction, and repolarization) and their contribution to AF pathophysiology. Activation and repolarization can be recorded in-vitro and ex-vivo by intracellular microelectrodes, which represent a gold standard in the field, and in-vivo by monophasic action potentials (MAPs). In addition, single-cell studies utilize patch-clamp techniques for studying action potentials and ion currents. Furthermore, electrical activation and conduction can be evaluated by contact and non-contact cardiac electrograms (EGMs). Along with EGMs, ex-vivo studies can also implement high-resolution optical mapping to panoramically record activation, repolarization, and conduction [25, 26]. Below we will discuss the applicability of these methods to different types of studies as well as their pros and cons.
a. In-vivo clinical studies
In 1988, Boineau et al. [27] were among the first to perform in-vivo epicardial multi-electrode mapping and demonstrate different patterns of impulse propagation during SR and left atrial (LA) escape rhythm. In-vivo EGMs record local activation times from multiple locations, which can be used to create activation maps. Activation maps help visualize propagation patterns and the origin of normal or pathological activity as well as allow the measurement of conduction velocity (CV). In addition, electrodes can be used for different pacing maneuvers to evaluate rate-dependent changes in MAPs and CV, to measure effective refractory periods (ERP), which correlate with action potential duration (APD) [28, 29], and to induce or entrain atrial arrhythmias, including AF. EGM amplitude can also identify low-voltage zones that may represent atrial fibrotic scars [30], whereas EGM multicomponent fractionations may represent local conduction blocks, epi-endocardial dyssynchrony, or reentrant activity [31–33].
In-vivo MAP recordings can be performed with a specific suction [34] or contact [35] electrode. This type of signal has a similar shape and duration as transmembrane action potentials (APs) recorded with intracellular electrodes [34–36] (Figure 2A). Although MAP differs from AP and involves injury potential, this type of signal closely replicates transmembrane AP and allows evaluating local repolarization and its changes during pacing and pharmacological tests. Furthermore, MAP recordings more accurate represent AF rate and local activation time compared to bipolar EGMs, which often overestimate fractionation and dominant frequency due to noise and artifacts [33, 37].
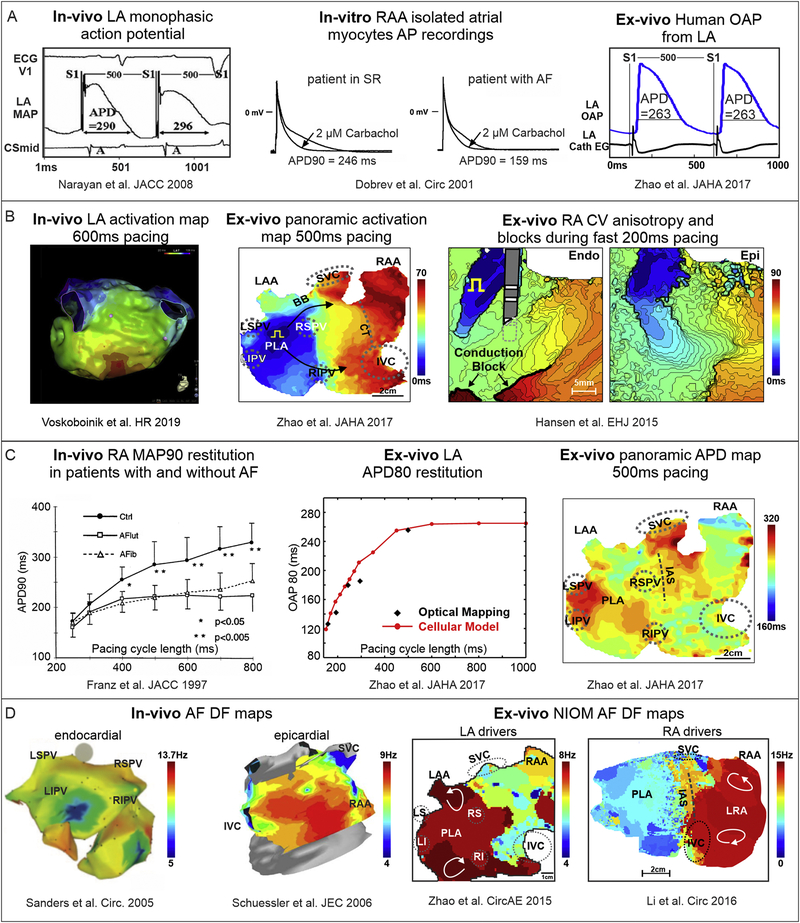
Figure 2. Human atrial electrophysiological features evaluated in-vivo, in-vitro and ex-vivo by different methods.
A. Action potential recordings using in-vivo, in-vitro single cell, and ex-vivo human heart methods. Adapted from Narayan et al. 2008 [119]; Dobrev et al. 2001 [120]; Zhao et al 2017 [113]. B. In-vivo and ex-vivo CV measurements during pacing. Right: Ex-vivo optical mapping revealed endocardial (Endo) vs. epicardial (Epi) conduction blocks during fast atrial pacing. Adapted from Voskoboinik et al. 2019 [49]; Zhao et al 2017 [113]; Hansen et al. 2018 [77]. C. Left: In-vivo and ex-vivo APD restitution curves. Right: Panoramic ex-vivo APD map of the intact atria. Adapted from Franz et al. 1997 [117]; Zhao et al 2017 [113].
D. In-vivo and ex-vivo dominant frequency (DF) mapping during AF. Left: In-vivo endocardial and epicardial DF maps from electrode recordings in persistent AF patients. Right: Ex-vivo DF map during sustained AF showing stable regions of reentry in areas with high DF in human RA and LA. Adapted from Sanders et al. 2005 [129]; Schuessler et al. 2006 [128]; Zhao et al. 2015 [75]; Li et al., 2016 [57]. Abbreviations are as follows: AF – atrial fibrillation; APD – action potential duration; CV – conduction velocity; DF – dominant frequency; EG - electrogram; ECG – electrocardiogram; IAS – interatrial septum; IVC/SVC – inferior and superior vena cava; LA/RA – left and right atria; LIPV/LSPV/RIPV/RSPV – left, right, superior, inferior pulmonary veins; LRA – left right atria; MAP – monophasic action potential; NIOM – near-infrared optical imaging; OAP – optical action potential; PLA – posterior left atrium; SR – sinus rhythm.
Pros:
Clinical studies have revealed AF-related EP changes, such as heterogeneous ERP, APD shortening [38–40], and a rate-dependent decrease in APD and CV [41, 42]. Panoramic activation and voltage mapping can also visualize focal and reentrant sources of AF [18] and fibrotic scars, resulting in improvement of AF treatment [43]. In addition, various mapping techniques can be combined with structural imaging methods such as contrast-enhanced magnetic resonance imaging (CE-MRI) and computed tomography (CT). Unlike animal studies, clinical research focuses on human AF substrates that evolve over many years and are affected by multiple comorbidities.
Cons:
At the same time, diversity in AF patients’ clinical histories may lead to a variety of patient-specific substrates and AF mechanisms causing discrepancies in results and debates in the field. Another limitation is low resolution of clinical visualization and mapping methods compared to experimental in-vivo and ex-vivo technologies. As EGM recordings primarily represent surface-only activation, intramural conduction can be missed and misinterpreted even during SR [8, 44]. EGM morphology and amplitude are influenced by the direction of activation, catheter angle, wall thickness, and tissue contact [45, 46], which may lead to improper evaluation of activation pattern during AF. Moreover, discrepancies exist in clinical CV measurements. For example, mean CV across the LA can vary from 40 to 220 cm/s in different clinical studies [47–49]. MAP is a single-electrode technique, and its signal quality is dependent on catheter angle and requires secure contact with tissue.
b. In-vitro tissue and cellular studies
Trautwein et al. [50] were the first to record atrial cellular AP parameters, including excitability, resting membrane potential, and repolarization in superfused myocardial fibers from human atrial tissue biopsies. Enzymatic cell isolation and single-cell ion current recording (patch-clamp) techniques [51, 52] opened up new methods to study cell-specific EP characteristics that are altered in AF, including key ion channel currents, ion channel expression, and direct AP morphology, including AP upstroke and APD.
Pros:
In atrial cells isolated from AF patients, major ion channels conducting sodium, calcium, and potassium currents can be studied free from autonomic nervous system inputs [53]. Atrial tissues also provide molecular profiles including RNA, microRNA, and protein levels that can identify alterations in AF [53, 54]. Studies of isolated atrial myocytes allow evaluation of alterations in intracellular calcium fluxes that can lead to ectopic sources/triggers of AF including early afterdepolarizations and delayed afterdepolarizations [21]. They can also provide mechanistic insights on the heterogeneous shortening of atrial APD and ERP seen in pAF and perAF patients and chamber-specific differences in sensitivities to autonomic mediators and agonists (e.g. epinephrine, isoproterenol, and acetylcholine) and endogenic metabolites (e.g. adenosine) [55–57]. In addition to ion-channel remodeling, intracellular structural remodeling, including loss of T-tubules and cardiomyocyte hypertrophy, is also associated with AF [58]. Furthermore, studies of single atrial myofibrils reveal translational and post-translational changes of multiple myofilament proteins, which contribute not only to AF perpetuation, but also to atrial dilatation and contractile dysfunction [59]. Isolated cardiomyocytes offer in-vitro systems to address cell-specific alterations. In addition to atrial cardiomyocytes, isolated and cultured atrial fibroblasts are utilized to study cellular sources of atrial fibrosis [60]. Along with fibroblasts from AF animal models, non-transformed human atrial fibroblast cell lines generated with genetic transfer of proliferation genes are also available, which provide a cellular system with high proliferative and migratory rates to study mechanisms of atrial arrhythmogenic fibrosis [61]. In addition to fibrosis mechanisms, isolated human atrial fibroblast models can be used to test anti-fibrotic interventions as well as pharmacological screening for patient-specific drug selection.
Cons:
Inter-cellular mechanisms that mediate formation and propagation of ectopic activation and reentry across the 3D myocardium, including myocardial fibrosis and myofiber orientation, cannot be studied in single cells. A majority of the atrial samples are from right atrial appendage (RAA) tissue, which is not a common location of AF triggers or drivers, and may not represent the heterogeneous remodeling across the whole atria. As with clinical studies, diversity between study populations may cause discrepancies between EP findings [62]. Moreover, the in-vitro approach lacks systemic neurohormonal interferences. Some limitations of human single cell studies can be overcome by micro-engineered cellular and tissue systems [63, 64] and ultrathin (100–400μm) human myocardial slices, which offer living multicellular systems of intermediate complexity to study CV, arrhythmogenicity, contractility, and to perform molecular-biochemical analyses and drug testing [65–67], thereby filling the gap between in-vivo and isolated cell studies. Human induced pluripotent stem cells (hiPSCs) [68–70] have also been used as AF multicellular platforms since they can be differentiated from individual AF patients [71]. Recent advances have developed novel approaches to selectively stimulate the hiPSC differentiation towards an atrial lineage [72]. Although these multicellular iPSC systems can be cultured and studied for longer periods, accurate reproduction of the EP parameters of adult human atrial myocytes is still an ongoing avenue of research (e.g. slower dV/dtmax of upstroke, slower CV (5 vs 60–100 cm/sec), depolarized resting potentials) [71].
c. Ex-vivo human heart studies
The isolated ex-vivo human heart approach represents a powerful platform, where multiple clinical and experimental methods can be integrated to study human-specific mechanisms of automaticity, conduction, and repolarization, and link them with the underlying structure and a molecular profile to evaluate arrhythmia mechanisms (Figure 1). In fact, in 1970, before detailed atrial activation patterns were mapped in patients with multielectrode mapping (MEM) [27], Durrer et al. successfully recovered physiological function of several explanted human hearts and provided the first EGM measurements of conduction patterns across both atria during SR and pacing, which revealed preferential conduction across Bachmann’s bundle [73]. With the development of explanted human heart programs at Washington University St. Louis [74], and later at The Ohio State University [75], ex-vivo human hearts were rigorously studied with high-resolution optical mapping methods with voltage-sensitive dyes. These methods can provide detailed information on ex-vivo cardiac conduction and repolarization at the tissue level with unprecedented submillimeter resolution [25]. For instance, newly developed near-infrared optical mapping (NIOM) with non-toxic voltage-sensitive dyes (e.g di-4-ANDBQBS) [76, 77], compared to conventional green-red dyes (e.g. di-4-ANNEPSs or RH237), surpasses surface-only mapping as it can collect signals from intramural layers of the human atrial tissue (~4mm). Explanted human hearts can be acquired alive and cardioplegically arrested from cardiac transplantation patients with advanced HF and from organ donor organizations. Ex-vivo human heart NIOM studies can successfully replicate in-vivo-like atrial EP patterns [27], and rigorously validate several clinically recorded parameters including heart rate during intrinsic SR (ranging from 56 to 116 beats per minute [74, 78–82]), sinoatrial conduction times (range 58 to 82ms), right atrial conduction velocities (70 to 160 cm/s), and repolarization (200–300 ms).
Pros:
Panoramic and transmural NIOM approaches can collect signals from subsurface intramural layers, including the SAN [82] and atrioventricular node [76], and were able to observe intramural reentry during AF [83]. Ex-vivo human hearts permit the combination of clinical mapping techniques with gold standard ex-vivo optical mapping, 3D imaging including CE-MRI and micro-CT, and detailed histological and molecular mapping (Figure 1). Thus, integrated ex-vivo studies could provide the missing link that validates the accuracy of current in-vivo techniques and clarifies the false positive and false negative artifacts of limited electrode resolution and data processing [26]. Importantly, in the ex-vivo human heart experiments, it is feasible to modulate the APD and conduction of the human atria with clinically related pharmacological tools (e.g. adenosine, isoproterenol, etc.) in order to reveal the mechanism of arrhythmias, such as AF [57]. Ex-vivo human hearts are either from heart transplant patients with varying heart failure etiologies, or from organ donors with and without cardiac comorbidities (e.g. MI, obstructive sleep apnea (OSA) and HTN) and risk factors (e.g. alcohol and drug abuse). Out of 197 hearts (19 – 74 y. o., 52±12.9 y.o., 39% female, 95 donors and 102 HF transplant hearts) acquired and studied by our group in the last seven years. Importantly, at least 30% of the hearts had documented AF history (55.7 ±11.9 y.o, 24% female). Thus, the ex-vivo approach provides a unique opportunity for testing the efficacy and safety of antiarrhythmic therapies, including targeted ablation and novel antiarrhythmic drugs, directly on human hearts with AF history without risk to patients [83].
Cons:
Ex-vivo human hearts are limited in number. In addition, the ex-vivo mapping procedure is extremely challenging, as it requires 24/7 coordination between surgical and research teams to obtain cardioplegically arrested hearts from hospital surgery rooms. Explanted hearts must then be studied at any time of the day or night. Furthermore, researchers must follow very rigorous experimental protocols to maintain proper physiological function of ex-vivo coronary-perfused human atria. Ex-vivo hearts do not experience systemic neurohormonal interferences and are mechanically unloaded. The duration of ex-vivo experiments is usually limited to a few hours (up to 12 hours) in order to keep heart property integrity, which does not allow chronic experiments.
Part 2: Electrophysiological features of the atrial myocardium
Evaluation of atrial arrhythmogenic substrates requires an introduction to the concept of wavelength for reentry [84]: the general criteria for estimating favorable conditions for sustained reentry in the myocardium [85]. The wavelength of reentry provides an estimate of the shortest possible length of the reentry path and is directly proportional to both ERP and CV. Slowing of the CV and/or shortening of the ERP would reduce the wavelength of reentry and increase the likelihood of self-sustaining reentry. Here we will review how changes in CV and ERP contribute to human AF.
a. Conduction
Cardiac CV is dictated by myofiber orientation, wall thickness, and fibrotic tissue architecture. Pioneering studies by Spach et al. [86, 87] of human atrial tissue revealed direction-dependent anisotropy of CV: fast propagation of conduction along the long axis of human atrial myocytes and slow conduction propagation across myobundles. At the cellular level, atrial CV depends on the density of fast sodium channels and the electrical coupling between atrial myocytes via the high conductance gap junction consisting of connexin proteins (Cx43 and Cx40). Gap junctions in the atrial myocardium are located predominantly at the end of cardiomyocytes, which explains fast conduction along the long axis of the cells [88].
As atrial conduction relies on inter-cellular electrical connectivity to ensure a rapid and homogeneous spread of electrical activation across the atrial myocardium, any disturbances in these components by structural or molecular remodeling can result in CV slowing, which leads to a higher predisposition to AF. Indeed, although the absolute numbers from clinical studies may vary from 40 to 220 cm/s [42, 48, 89, 90], there is a common trend of slower CV in diseased atria of AF patients [42, 91], especially in those with risk factors, such as aging [47], obesity [92], and alcohol consumption [49] (Figure 2B). Our recent ex-vivo NIOM study [93] has shown that the averaged atrial CV in human hearts with history of AF is slower (67±11 cm/s) than without AF (100±14 cm/s), which is comparable with clinical data.
Inherited mutations and disease-induced molecular remodeling of sodium channels and connexins are the main reasons for conduction impairments in AF. The SCN5A gene encodes Nav1.5, which is the most abundant sodium channel type in the atrial myocardium. SCN5A mutations are associated with familial lone AF [94]. Some examples of SCN5A mutations include Brugada [95] and long QT [96, 97] syndromes, which have a higher prevalence of AF among young adults than in the general population. Moreover, acquired cardiac sodium channel abnormalities have also been observed: AF patients show a reduced peak sodium current (INa) and lower expression of Nav1.5 compared to patients in SR [98]. Human data on AF-related connexin expression changes are controversial. While some studies report that AF is associated with Cx40 upregulation [99–101], others show the reverse trend. For example, atrial tissues from pAF patients showed reduced Cx40 expression and increased heterogeneity of Cx40 distribution, chronic AF (cAF) patients also showed reduction of Cx40 expression [102]. In another study of cAF patients, AF complexity calculated using the amount of separately propagating waves was found to be inversely correlated with atrial Cx40 expression [103]. Lastly, patients with AF and mitral valve disease showed downregulation and abnormal phosphorylation of Cx40 compared to healthy controls [104].
Spatial heterogeneity and anisotropy of CV are present even in the healthy myocardium and are both important for the effective synchronized contraction of heart chambers [105, 106]. However, pathological structural and molecular remodeling can significantly enhance this heterogeneity and lead to arrhythmogenic conduction blocks, which act as an AF substrate in the presence of triggers such as extra-systolic beats [107, 108]. Slow CV and anisotropic conduction blocks in diseased human atria can be detected in ex-vivo human NIOM studies, which can provide both high resolution and panoramic activation patterns exemplifying the local CV heterogeneity seen clinically (Figure 2B) [57, 77]. Integration of NIOM with CE-MRI shows that architectural discontinuities between small intramural myobundles underlie enhanced CV anisotropy and conduction blocks. In isolated RA from 63 y.o. patient with atrial septal defect, Spach et al. revealed CV anisotropy in fibrotically insulated myobundles up to 10:1, which led to microreentry [107]. These findings are in agreement with clinical studies, which show that fibrotic scars revealed by Late Gadolinium Enhanced (LGE)-MRI [109] and voltage mapping [90, 110] underlie CV heterogeneity and AF propensity.
Another important feature of CV is the rate-dependent slowing of CV (or CV restitution) and pathologic adaption of CV during the fast rhythm of AF, which can further promote AF (“AF begets AF”) [111]. In their study, Weber et al. [112] described that AF patients have a pronounced rate-dependent decrease of mean LA CV. Another study [90] showed that rate-dependent slowing of CV is more prominent in areas of low voltage signal; moreover, 72% of confirmed AF drivers were located in the regions of steepest CV restitution. Narayan and colleagues [41] demonstrated that during restitution (before AF onset) CV slows down in the regions where reentrant AF drivers are identified and a flattened CV restitution curve correlates with the inability to induce AF. Our ex-vivo NIOM studies observed a similar trend in CV restitution curves in diseased human hearts [113] (Figure 2B).
Conduction dissociation between the epicardial and endocardial layers is another arrhythmogenic substrate. As conduction propagates across the complex 3D atrial wall, it leads to local epi-endocardial activation dyssynchrony. This can be observed clinically with simultaneous epi-endocardial studies of the RA [32, 114, 115], even during SR, and is more prevalent at faster activation rates during pacing or AF (Figure 2B). Of note, the distance traveled by excitation waves measured on the atrial surface by clinical electrodes may not accurately account for intramural conduction within the 3D atrial wall, and thus, CV can be underestimated. One solution to this obstacle is simultaneous epi-endocardial optical mapping. This intramural mapping technique reveals that epi-endocardial activation dyssynchrony seen with electrodes, during pacing and AF, may not always represent discontinuity but can be related with slower transmural conduction within the human atrial wall [77]. NIOM also visualizes that local transmural conditions blocks seen during CV restitution are one of the arrhythmogenic features with the propensity to develop reentrant drivers [77].
b. Effective refractory period and repolarization
The other half of the equation for wavelength of reentry, local APD/ERP, has been demonstrated to be an important determinant of perAF initiation and maintenance. In general, clinical MAP90 (duration of MAP at 90% of repolarization) during 500–600ms pacing is reported to be in the range of 215–315ms and 250–325ms for RA and LA, respectively, in patients with and without AF history [39, 41, 116–119]. Ex-vivo human atrial optical mapping [57] shows similar APD80 in RA (290±45ms) and LA (307±24ms) (Figure 2A). Patients with atrial arrhythmias (chronic atrial flutter and AF) have shorter APDs than control patients (245±39 versus 325±41ms), as demonstrated by Franz et al. [117] (Figure 2C). ERP values correlate with APD and have been shown to be shorter in perAF than in pAF patients (204±28 versus 242±34ms) [119].
The APD shortening in AF has been directly documented in isolated human atrial myocytes. Dobrev et al. showed that APD90 in SR patients is 202±18ms vs 149±17ms in patients with cAF [120] (Figure 2A). Van Wagoner et al. [121] showed that the delayed rectifier potassium current density and the expression of Kv1.5 are decreased in cells isolated from the atrial appendages of AF patients. Workman et al. [122] reported for the first time that ERP in RAA isolated myocytes from cAF patients are shorter versus patients in SR (104±15 versus 203±16ms). The APD/ERP-shortening in cells from cAF patients was associated with significantly reduced density of L-type Ca2+ and transient outward K+ (Ito) currents and increased inward rectifier K+ current (IK1). The ultra-rapid delayed rectifier potassium current (IKur) was identified in human cardiomyocytes isolated from RAA, which is absent in the ventricle [123]. IKur was shown to play a role in the repolarization phase of human atrial AP and has been suggested as a potential antiarrhythmic target to prolong APD in cAF [124, 125]; however, the efficacy might depend on the extent of ion-channel remodeling in AF hearts.
In addition to changes in CV, spatial heterogeneity in repolarization and refractoriness within the atrial chambers are present in human hearts with and without AF [48]. Choi et al. [126] reported that MAP90 values in the septum (246±21ms) are significantly higher than in the RA lateral wall (224±33ms) and the RAA (223±24ms at 600ms pacing). The minimal MAP90 or ERP was shorter and the dispersion of MAPD90 was higher in patients with paroxysmal AF [39] and perAF versus non AF patients [38].
Our ex-vivo human heart study also revealed that AF induction by rapid atrial pacing requires short APD values (206±44ms versus 245±34ms in non-inducible hearts) and high APD dispersion in the RA [57]. During fast arrhythmias, such as AF, the AF cycle length (AFCL) or dominant frequency (DF) can be used to approximate the local ERP of atrial tissue [127]. Remarkably, the averaged DF (~7Hz) seen in ex-vivo human heart experiments reproduces the averaged DF (6–8Hz) reported in many clinical studies [93, 128, 129] (Figure 2D). While AFCL is variable between the right and left human atria [130, 131], the regions with shortest AFCL may correlate with the AF drivers’ location. Indeed, ex-vivo NIOM revealed the presence of reentrant drivers in regions with the shortest local APDs and with the fastest DF (~7.6–13.8Hz) [75, 77, 93]. This finding is also supported by detailed clinical catheter mapping studies showing the discrete RA locations with the fastest DF (13.7Hz and 13.25Hz) during permanent AF [129] (Figure 2).
Similar to CV, altered rate-dependent changes of APD/ERP have been observed in both pAF and perAF patients. Franz et al. [132] first reported that APD varies with the preceding stimulus interval in the human heart, which is reduced in premature beats and increases as the stimulus interval is lengthened. Furthermore, Workman et al. [122] report that ERP and repolarization rate-dependent shortening in isolated cardiomyocytes from patients with AF is attenuated in comparison to patients in SR. Narayan et al. [119] demonstrate that the steep slope (>1) of APD restitution contributes to the initiation of paroxysmal AF. Subsequent studies by this group suggested that atrial APD alternans at both slower and faster rates may represent a substrate in perAF and usually precede AF induction [118]. Kim et al. [40] found that the spatial dispersion of APD restitution in patients with cAF was greater than in paroxysmal AF and control subjects, indicating that the heterogeneity of APD restitution in the atrium plays an important role in the persistence of AF. These data suggest that inhomogeneous shortening of atrial refractoriness is one of the arrhythmogenic features that contribute to AF development.
Both vagal stimulation and endogenous adenosine promote reentrant AF due to shortening ERP/APD by activating an inward-rectifier K+-current (IK,ACh/Ado) via muscarinic (M2R) and adenosine A1 receptors (A1R) [120]. In addition to M2R, Heijman et al. recently showed that IK,ACh may also be regulated by the activation of M1Rs and upregulated in cAF patients [133]. Importantly, IK,ACh in cAF patients was found to be constitutively active even without presence of acetylcholine, which could lead to arrhythmogenic APD shortening [120]. In ex-vivo human hearts, activation of IK,Ado by adenosine perfusion induced heterogeneous APD shortening with significantly greater effects in the RA than LA (17.4±11.7% versus 6.3±5.5% at 10 μmol/L) [57]. Tebbenjohanns et al. [134] previously reported that intravenous bolus of 6 mg and 12 mg of adenosine induced 19% and 27% APD shortening respectively at 500 ms pacing in RA, which is similar to ex-vivo findings. A recent clinical mapping study showed that adenosine bolus also shortens AFCL in perAF patients up to 38% in RA and 10±11% in LA, which may assist in mapping AF drivers [93]. In the ex-vivo hearts with more pronounced and heterogeneous APD shortening induced by adenosine perfusion, restitution pacing resulted in sustained AF maintained by localized reentrant AF drivers [57]. Using isolated cardiomyocytes from human atrial appendages, Voigt et al. showed that GIRK1 and GIRK4 proteins (which form IK, ACh/Ado channels) expression is higher in RA versus LA, and IK,ACh is 70% larger in RA versus LA in SR patients [135]. Ex-vivo, the heterogeneous distribution of A1R across the entire atria [57] could also explain the predominant shortening of APD caused by adenosine in RA versus LA, which is seen in AF patients [134].
Part 3. Structural substrates as fingerprints of AF drivers
In addition to EP and molecular features, human atrial 3D myofiber anatomy governs the conduction patterns during both sinus rhythm and AF [75], but its role in AF is often underestimated [87, 128, 136, 137]. Structural features of the human atria, such as chamber size, wall thickness gradient, atrial fibrosis, epi-endocardial transmural twists in myofiber orientations, and the 3D architecture itself, represent structural substrates that contribute AF (Figure 3B).
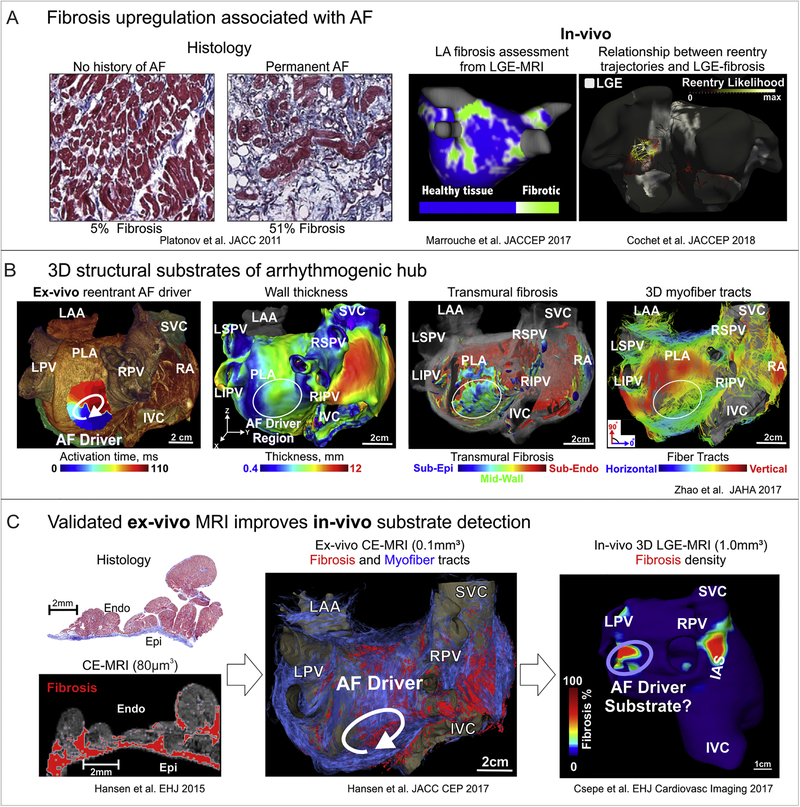
Figure 3. Structural substrates of human AF.
A. Evaluation of atrial fibrosis by histology and LGE-MRI. Left to right: Fibrosis is upregulated in association with AF in comparison to patients with no history of AF by histology. In-vivo studies showed that the extent of atrial fibrosis was a significant predictor of ablation failure, and likelihood of AF reentrant mechanism increases in areas of high enhancement. Adapted from Platonov et al.2011 [152]; Marrouche et al. 2017 [10, 172]; Cochet et al. 2018 [12]. B. Arrhythmogenic 3D structural substrates in AF driver maintenance. From left to right: Reentrant AF driver identified optically overlapped with 3D CE-MRI anatomy. Bi-atrial wall thickness variations, transmural 3D fibrosis distribution (sub-epi (blue), mid-wall (green), and sub-endo (red)) as well as 3D myofiber tracts created arrhythmogenic hubs for reentrant AF drivers. Adapted from Zhao et al 2017 [113]. C. Histological validation improves ex-vivo and in-vivo fibrosis MRI analysis. Ex-vivo 3D CE-MRI reconstruction shows fibrosis in red and myofibers in blue. In-vivo 3D CE-MRI shows fibrosis islands in possible driver regions. Adapted from Hansen et al. 2015, 2017 [8, 77]; Csepe et al. 2017 [180]. Abbreviations AF – atrial fibrillation; LGE-MRI – late gadolinium enhanced magnetic resonance imaging; IVC/SVC –inferior and superior vena cava; LA/RA – left and right atria; LAA –left atrial appendage; LIPV/LSPV/RIPV/RSPV – left, right, superior, inferior pulmonary veins; CE-MRI – contrast enhanced magnetic resonance imaging; PLA –posterior left atrium
Atrial chambers dilation is one of the risk factors of AF incidence and reoccurrence after cardiac ablation [138–140]. This occurs because large atria may support maintenance of reentry and several localized reentries without pronounced wavelength shortening necessary in smaller atria. Larger atria may also allow multiple reentrant wavelets, which supports the critical mass theory of AF [141]. Further, clinical studies suggest that the majority of AF patients [2] have subclinical or diagnosed cardiac diseases (e.g. HTN, MI, HF) with structural remodeling, including increased fibrosis, atrial dilation, and heterogeneous hypertrophy [142–146]. Structural cardiac diseases, such as HTN [147] and HF [148] have been associated with an increased occurrence of AF compared to healthier hearts.
The natural 3D complexity of the diseased human atrial wall, consisting of a web of fibrotically insulated myobundles [75, 77, 113, 142–144, 149], is predisposed to complex intramural conduction, resulting in dissociation between epi- versus endocardial surface activation. Histological cadaver studies [150], high-resolution (~100μm) ex-vivo 3D CE-MRI [77, 149, 151], and micro-CT [75] of human atria show significant variations in atrial wall thickness, ranging from about 1 to 10mm.
In addition to wall thickness, heterogeneous transmural fibrotic remodeling, which occurs in a patient-specific manner due to aging and cardiac diseases, significantly increases the natural complexity of the human atrial wall. Spach et al. [87] found that fibrotic strands create a substrate for reentry within very small areas due to highly anisotropic conduction. Studies from Kottkamp and colleagues [142] suggest that the phenomena of fibrotic atrial cardiomyopathy may accompany or even precede AF, independent of other co-morbidities. Platonov et al. [152], using histological analysis of autopsy specimens, showed that patients with paroxysmal or permanent AF have 2–3 times (up to 50%) greater fibrosis than patients without AF history (5–10%) (Figure 3A). Furthermore, LGE-MRI can be used as a non-invasive, clinical quantitative technique to estimate the extent of atrial fibrotic tissue. The DECAAF Trial [10], a multi-center prospective trial that employed LGE-MRI, shows that the extent of atrial fibrosis is a significant predictor of ablation failure, suggesting there may be a population with extensive fibrotic remodeling whose mechanism of AF maintenance is resistant to current anatomy-based ablation strategies targeting known sites for triggers. Recent studies also demonstrate that in addition to fibrosis, epicardial adipose tissue plays a role in AF progression as it may lead to heterogeneous conduction slowing [153].
Another important component of AF structural substrate is 3D myofiber orientation [75, 77, 113, 154]. The almost perpendicular myofiber orientation of the sub-epicardium and sub-endocardium [77, 150], in combination with their separation by intramural fibrosis, leads to nearly two separate atrial layers that are connected by intramural bundles. The uncoupling of atrial layers is exacerbated by AF progression, and cardiac comorbidities are observed to cause significant differences between local epicardial versus endocardial electrode recordings, especially during fast AF rhythm [114, 155, 156].
Thus, the complex 3D human atrial anatomy in diseased hearts has region-specific transmural myofiber divergence [75, 77, 113, 157], wall thickness variation, and interstitial 3D fibrotic insulation that create a complicated web of fine muscular ridges. This complex human atrial architecture has a significant effect on the local conduction direction and speed, which provides a substrate of arrhythmogenic conduction blocks for AF maintenance.
Part 4. Features of AF pathophysiology and maintenance mechanisms
These unique EP as well as complex anatomical attributes of the human heart have greatly impeded the identification and visualization of AF mechanisms. Several studies investigating human AF mechanisms have reported drastic variations in EP and structural features across patient cohorts, which could be another reason why after a century of research, the mechanisms maintaining human AF are still highly debated [1, 52, 158]. Currently, the most evidence-based theories for the mechanism of AF maintenance are localized AF drivers [77, 93] and self-replicating multi-wavelets [159]. However, the specific mechanism of AF drivers/sources discussed in the current literature [6, 17, 18, 57, 75, 77, 136, 160–163] remains ambiguous in humans [26].
The theory of perAF maintained by a limited number of stable reentrant drivers has recently gained a lot of attention [9], although Thomas Lewis [164] proposed this AF mechanism nearly 100 years ago. In 1991, Cox et al.[165] multi-electrode mapping study demonstrated for the first time that a single reentrant circuit in the RA can maintain pacing-induced AF in patients undergoing open chest procedures (Figure 4A). In the past decade, several clinical groups [17, 18, 166] identified localized drivers in paroxysmal, persistent, and long-standing perAF patients using a diverse set of clinical mapping tools [6, 18, 167, 168]. These studies also demonstrated an improvement in AF treatment by successful ablation of AF driver regions. However, neither epicardial nor endocardial surface-only electrode mapping [115, 169] has the ability to visualize AF mechanisms below the surface of the highly complex 3D atrial wall [26]. Recent ex-vivo human atria transmural optical mapping studies [57, 77] support clinical findings by directly demonstrating that human AF may be driven by microanatomic reentrant AF drivers within the atrial wall. Moreover, the integration of NIOM and contact MEM show that intramural conduction may cause microanatomic reentrant AF drivers to be visualized by MEM as complete or partial reentry and spatially stable or unstable breakthroughs (Figure 4B). Thus, these findings reconcile with several clinical electrode mapping studies [115, 155, 160, 170] that show stable or unstable breakthroughs instead of reentrant AF drivers.
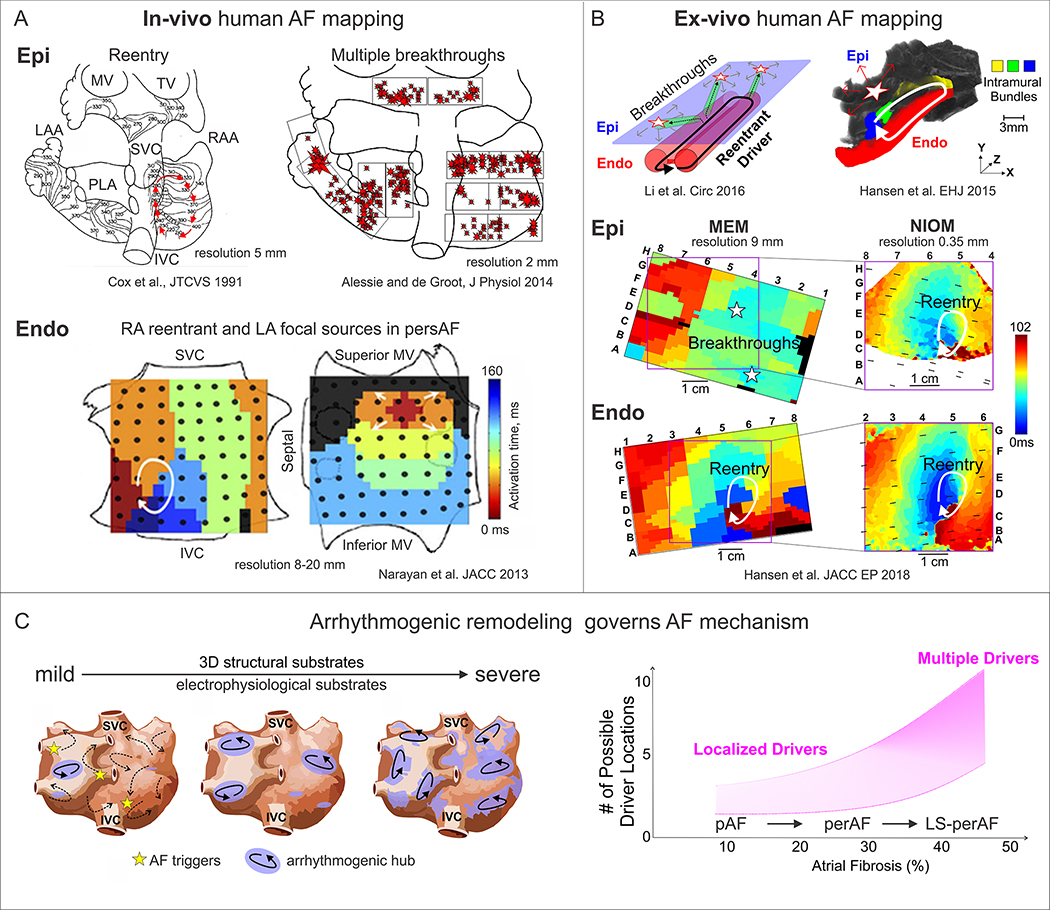
Figure 4. Human AF mapping and evolution of AF mechanisms.
A. In-vivo epicardial and endocardial mapping visualized different mechanisms of AF maintenance. Adapted from Cox et al.,1991 [165]; Alessie et al., 2014 [170]; Narayan et al., 2013 [169]. B. Dual sided ex-vivo NIOM integrated with clinical multielectrode mapping (MEM) to validate clinical mapping methods and reveal AF driver mechanism. NIOM resolves intramural reentry, which is seen as multiple breakthroughs by clinical epicardial MEM. AF driver denoted by white arrow on Endo view, while star denotes breakthrough visualization on Epi view. Adapted from Hansen et al., 2015, 2018 [77, 83]; Li et al. 2016 [57]. C. Left, progressive electrophysiological and structural remodeling governs AF mechanism. Right, graph showing that the number of AF drivers may depend on the severity of fibrotic remodeling. Abbreviations: Epi – epicardial, Endo – endocardial; AF –atrial fibrillation; FIRM – focal impulse and rotor mapping; MEM – multielectrode mapping; MV – mitral valve; TV – tricuspid valve; LAA/RAA – left and right atrial appendages; NIOM – near-infrared optical mapping; pAF/perAF/LS-perAF – paroxysmal/ persistent AF/long-standing persistent AF; IVC/SVC – inferior and superior vena cava.
As stated before, fibrosis is one of the main components of AF structural substrates. Although the pattern, content, and specific location of atrial fibrosis leading to stabilization of reentrant AF drivers is still unclear [77, 142, 171, 172], most clinical studies have shown that reentrant AF drivers tend to be co-localized within regions of increased fibrotic tissue content [12, 77, 83] (Figure 3A). For example, a recent clinical study using body surface electrocardiographic imaging (ECGI) [12] found that the extent of LGE-MRI atrial fibrosis correlates with the number of reentrant regions (Figure 3A). Similarly, some in-vivo studies have attempted to correlate arrhythmogenic substrate areas observed by LGE-MRI to the functional mapping of AF drivers, but the results have been controversial [12, 171]. However, percent of regional fibrosis may not be sufficient for identifying fibrotic substrates in the perAF remodeled atria to guide targeted ablation. Recent experimental [17, 173] and computational studies [174, 175] suggest that fibrotic architecture in the border zones of dense fibrosis may be more arrhythmogenic than the dense patch itself. However, the low resolution of clinical LGE-MRI is currently unable to resolve 3D fibrosis architecture, myofiber orientation, and wall thickness gradient. Thus, the specific role of these structural features in AF maintenance [2, 142–144, 176–178] remains clinically unexplored. Furthermore, regional atrial interstitial fibrosis detection by LGE-MRI requires patient-specific histological validation [143, 179], which is limited to ex-vivo human studies [77, 180] (Figure 3C).
At the same time, ex-vivo transmural mapping integrated with high-resolution CE-MRI (80μm3) allows for the direct study of region-specific structural arrhythmogenic substrates of reentrant AF drivers [77]. Specifically, reentrant AF drivers in our recent study were anchored to 3D fibrotically-insulated microanatomic tracks (~15×6mm surface projection and ~3mm depth), which represent heart-specific fibrotic arrhythmogenic hubs. Histologically-validated 3D CE-MRI of optically-mapped AF driver regions suggests that the combination of intramural fibrotic strands, misaligned epi-endocardial myofibers (myofiber discontinuity), and varying atrial wall thickness create patient-specific anisotropic pathways. These pathways create microanatomic tracks of localized reentrant AF drivers of varying number, size, and distribution in diseased human hearts (Figure 3B). Moreover, ex-vivo human atria studies [150, 173] suggest that intramural fibrosis affecting the path of conduction could be more arrhythmogenic in contrast to fibrosis that naturally covers the epicardial or endocardial surfaces.
Heterogeneous bi-atrial structural and EP remodeling together with patient-specific 3D atrial architecture may provide region-specific substrates for spatially stable AF drivers [8, 113]. The number and temporal stability of these reentrant AF drivers may depend on the severity of heterogeneous fibrotic and EP remodeling as well as temporal variations of EP properties due to autonomic tone. A recent translational ex-vivo to in-vivo study by Hansen et al. [93] suggested that persistent AF can be maintained by reentrant drivers within a fibrotic arrhythmogenic hub composed of multiple reentry pathways, allowing beat-to-beat variability to obscure activation mapping. The tracks may include a primary common path for preferential conduction in addition to several possible return paths to complete the reentry. The study also shows that these common paths, which may be hidden from surface only MEM, can be revealed with pharmacological challenges, such as adenosine. Notably, ablating these common reentrant paths terminated persistent AF, indicating that modulating driver physiology is a feasible tool to help guiding catheter ablation.
Multiple epicardial and endocardial clinical MEM studies have also shown that patients may have both LA drivers and RA drivers in the majority of perAF and long-standing perAF cases [6, 7, 11, 160]. Miller et al. reported that 85% of patients with perAF have AF drivers in the RA [7]. Importantly, clinical MRI [12, 181, 182] and postmortem histological [152] studies indicate that fibrosis is upregulated in both LA and RA in AF patients. However, it is currently unknown if RA drivers have similar or different structural substrates or “fingerprints” as LA drivers [11]. Our ex-vivo studies suggest that RA drivers are mainly located near connections between the crista terminalis and fibrotic insulated pectinate bundles, where abrupt changes in wall thickness are observed [83, 93]. However, rigorous studies directly comparing structural and EP substrates (ERP, CV) of LA versus RA drivers are required to elucidate their differences and clinical relevance.
Furthermore, studies using 64-electode clinical FIRM (focal impulse and rotor mapping) [17, 18] and body-surface ECVUE phase mapping [6] found the number of drivers was higher in patients with perAF and long-standing perAF than pAF and that more advanced forms of AF had greater numbers of RA drivers than less perAF (Figure 4C). In general, both approaches revealed a limited number of spatially distinct, primarily reentrant, drivers that become more prevalent with progression/duration of AF, thereby suggesting an important role of patient-specific structural and EP remodeling in the development and localization of AF drivers. These clinical findings are similar to those of recent high-resolution panoramic optical mapping studies of intact explanted human atria, which revealed two or more simultaneous, competing, localized reentrant drivers during sustained AF [75, 93]. These studies also suggest that driver stability is mainly dictated by the functional (ERP and CV) and structural composition of each driver’s arrhythmogenic hub.
The variety of human AF mechanisms suggests that treatment approaches also must be different. Therefore, in order to successfully target these variations in patients with multiple unstable drivers, approaches more extensive than targeted ablation may be required, including the surgical bi-atrial Maze procedure [183]. For example, several studies [184, 185] showed relatively poor outcomes of driver ablation, which can be explained not only by insufficient mapping techniques, but also, by the fact that an evolved substrate is already capable of maintaining multiple drivers. Furthermore, procedures targeting both atrial chambers (e.g. gold-standard Cox-Maze IV procedure) are more effective than those which target the LA only [186].
Part 5. Integration and cross-validation of mapping modalities to fill the gaps in human AF mechanistic understanding and patient treatment
a. Ex-vivo human heart models
The data discussed thus far directly support the critical need to resolve the discrepancies regarding human AF mechanisms. The limitations of AF mapping in the clinical setting provide at best a highly interpolated view of surface-only activation that is prone to multiple interpretations [6, 18, 115, 160]. These varying interpretations of AF mechanisms stem from a lack of commonly accepted standards in the AF driver definition, clinical mapping techniques and signal processing methods. Similarly, atrial fibrosis detection by clinical LGE-MRI has not been directly validated by histology [179]. Moreover, the low resolution of clinical LGE-MRI cannot differentiate between various types of fibrosis (diffuse fibrosis, interstitial fibrosis, patchy fibrosis). This can explain the conflicting results that have been reported from clinical studies trying to correlate MRI-detected fibrosis with AF driver locations. These limitations emphasize a critical need to develop approaches to cross-validate findings between clinical and experimental methods in order to arrive at a consensus regarding human AF mechanisms.
Our lab has recently utilized ex-vivo NIOM of intact atria to validate clinical MEM approaches, including FIRMap 64-electode basket and modified basket catheters [83]. In this combined mapping approach, the ability of FIRM to identify AF driver location and activation pattern was validated by NIOM AF driver identification in the same heart simultaneously. The study revealed that both reentrant and breakthrough/focal AF driver patterns visualized by surface-only clinical MEM can represent projections of 3D intramural microanatomic reentry paths. Importantly, integration of NIOM and MEM in the ex-vivo setting can validate and help optimize novel catheter designs before they are used in patients as we recently showed in our studies [83, 187, 188].
Another significant limitation of clinical MEM is the signal processing of phase-based mapping, which often falsely identifies drivers at locations of passive rotation and wave break [189]. This technical limitation could be overcome by integrating MEM with 3D fibrotic structural data (e.g. intramural fibrosis), which may further enhance accuracy of true AF driver detection by eliminating false positive drivers and improve the efficacy of driver targeted ablation. However, as we already mentioned, assessing the 3D architecture of intramural fibrosis in human atria may prove difficult for LGE-MRI [10] with a clinical resolution of 1.25mm3. In contrast, a considerably higher resolution of ~0.1mm3 can be achieved in the ex-vivo human atria.
In summary, high-resolution ex-vivo human heart studies [83] have shown that reentrant AF drivers have unique, identifiable structural substrates and activation patterns. While clinical evidence suggests that this phenomenon may perpetuate AF in patients similarly to ex-vivo human hearts, direct translation of ex-vivo findings is difficult unless the same heart can be studied both ex-vivo and in-vivo. Since the logistics make in-vivo to ex-vivo study of the human heart impossible, large animal models of perAF present an opportunity for direct translation and validation of mapping techniques and testing novel treatment approaches.
b. Animal models: advantages, limitations, and comparison with human data
Large animal models of AF can be used to study the 3D structure and function of the same heart in-vivo and ex-vivo. These models can provide an excellent platform for studying AF mechanisms, validating clinical AF driver mapping, and testing novel drug treatments. However, before interpreting data from large animal models, the limitations must be understood. A recent review by Hucker and colleagues [24] provides a detailed overview of the pros and cons of small and large animal models of AF. Multiple animal models [21, 52, 190–192] have been developed to unmask structural substrates of AF [142], including an increase in fibrotic tissue content [2, 21, 193]. However, the extent of fibrotic remodeling observed in animal models, even with AF sustained for several months is generally less (5–10%) than that reported in perAF and long-standing perAF patients (up to 30–50%) (Figure 3A) [152]. Due to this observed difference, perAF animal models mainly rely on EP remodeling (e.g. refractoriness shortening) associated with tachypacing and AF [116, 194–207] rather than structural remodeling, which may not be a realistic representation of complex human AF mechanisms. For example, atrial APD/ERP values are usually shorter in large animal models (70–150ms) [24] compared to perAF patients (200–230ms) regardless of their comparable atria size (except in the equine AF model: ERP ≈ 225–275ms, but the heart size is five times larger [208]). In short-term tachypaced animal models, a shorter ERP, a smaller wavelength, and a lesser fibrotic tissue content may predispose to a type of AF, which results from multiple unstable reentries/wavelets rather than the spatially stable localized drivers seen in perAF patients.
Therefore, when choosing the optimal animal model of AF for clinical EP mapping and LGE-MRI validation, it is important to consider the interspecies variations in atrial anatomy and substrates. For example, the proportions of human atrial anatomical regions, such as a smaller atrial appendage to free wall ratio in both atria, differ from that of commonly used sheep models [75, 150, 154]. In contrast to sheep and pig atria, canine atria are more comparable to that of a human [209] due to more similar overall shape, structure proportions, and EP properties [24, 210, 211]. As such, a chronic canine AF model may be more suitable for translational in-vivo MRI studies than other large animal models [212]. Recently, our group used a canine model to successfully conduct in-vivo high-resolution NIOM, which was integrated with novel high-density MEM to visualize atrial conduction during SR, atrial pacing, and during AF [187]. Importantly, subsequent ex-vivo mapping of the same canine heart showed very similar activation, repolarization, and conduction patterns as seen in-vivo. In this study, in-vivo to ex-vivo mapping of the same heart bridged the theoretical divide that questioned the reproducibility of in-vivo phenomena in ex-vivo settings. Furthermore, the study also showed that high-resolution ex-vivo CE-MRI, correlated with histological analysis, can validate fibrotic AF substrates on in-vivo LGE-MRI of the same heart. Together, these findings suggest that if an AF mechanism is visualized similarly both in-vivo and ex-vivo conditions in the same canine heart, it is likely that the AF mechanisms seen in ex-vivo human hearts may faithfully reproduce AF mechanisms in patients. This study also opens the door to the promising potential application of in-vivo high-resolution NIOM to overcome the limitations of clinical surface MEM and validate the current MEM and LGE-MRI methods to resolve patient-specific AF mechanisms.
c. Patient-specific computer models
In addition to animal models, the unique abilities of computational simulations can be leveraged in the study of human AF mechanisms and treatment [8]. Computational simulations based on in-vivo or ex-vivo AF mapping of human hearts are able to reproduce AF mechanisms [113, 213]. Importantly, critical aspects of AF mechanisms, including conduction/repolarization, ion channels, calcium handling proteins/receptors, and structural features [154, 214], can be altered in a systematic and controlled fashion that allows to differentiate the relative contribution of each variable, which is not possible to do clinically or experimentally. Additionally, 3D computer models can also allow for the development of personalized treatments, such as targeted ablation planning or antiarrhythmic drug selection. Various human atrial computer models have been developed from detailed single-cell modeling [215] to patient-specific 3D models based on clinical imaging data [216]. However, computer models derived from low-resolution clinical LGE-MRI scans and generic human or animal EP properties may not precisely reproduce human AF mechanisms [217, 218]. Recently, to help overcome these limitations, models integrating structural and EP data derived from the combination of submillimeter high-resolution ex-vivo imaging of human atria [113] and in-vitro cellular/molecular data have been developed [219]. Such models can improve identification of the most critical arrhythmogenic EP-structural substrates for AF drivers, which can eventually guide targeted ablation.
d. Artificial intelligence on the hunt for AF drivers
One potential solution to improve AF treatment is related to the widespread adoption of machine learning (ML) techniques. Multiple studies [220–222] have utilized ML and deep learning approaches to identify atrial rhythm on electrocardiograms. Several groups have utilized ML for identifying the location of simulated AF drivers [223], but the feasibility of these algorithms remains limited due to simplicity and inaccuracy of cellular models. The Narayan group has recently utilized an ML approach to classify intracardiac electrical patterns during AF based on FIRM basket catheter phase maps [225]. The main limitation is the imprecision of AF driver visualization by clinical methods such as MEM or ECGI, which may impair the quality of ML training sets by both false positives and false negatives [83, 224]. In contrast, the ex-vivo human atrial approach can provide precise data on driver locations correctly identified by NIOM. In our recent study [188], we demonstrated that pre-trained ML algorithms allow efficient classification of EGM recordings based on their Fourier spectrum features into drivers versus non-driver and correctly identified 86% of AF driver locations with clinical MEM. We propose that ML and deep learning approaches, validated by ex-vivo human heart data, can improve the detection accuracy of AF arrhythmogenic substrates and reentrant drivers in clinical settings.
Part 6. Future Directions
Despite significant improvement in understanding AF pathophysiology and novel AF treatment development, there remain many unresolved questions and gaps in knowledge concerning these topics. We believe that new methods and approaches will be able to answer some of these questions in the near future.
New integrated in-vivo to ex-vivo studies should also define molecular profiles of AF driver substrates in different regions of LA and RA to gain a more complete understanding of chamber-specific arrhythmogenic substrates and develop substrate-specific drug and gene therapies for treatment of AF.
Moreover, deeper understanding of the interplay between AF and high-risk factors of AF (such as HTN, OSA, coronary artery disease, etc.), as well as gender and race, may allow for the classification of AF “phenotypes” and mechanisms. That can result in development of more patient-specific treatment approaches and prevention of further disease progression.
A promising direction towards to patient-specific treatment is identifying reliable biomarkers of AF occurrence, progression and post-ablation reoccurrence to facilitate risk assessment, personalized treatment and longitudinal surveillance [226–228].
Similarly, genetic studies like genome-wide association studies (GWAS) can define groups of patients in whom genetic predisposition prevails over other factors (such as comorbidities or risk factors). In addition, the results of GWAS provide not only new genetic markers and insights into biological pathways underlying AF, but also identify new targets for personalized AF prevention and treatment [229].
As discussed previously, although spatially stable reentrant drivers were directly shown to maintain AF in ex-vivo human hearts and by clinical MEM studies [93], the success rates of extra-PV driver ablation AF treatment vary across different centers [19]. With the contradicting results of extensive extra-PV ablation studies, some attribute the lack of success as evidence against the presence of spatially stable AF drivers. However, it may be explained by the fact that clinical MEM did not identify all AF drivers and/or due to an inadequate ablation of the driver’s substrate. In order to improve long-term AF driver ablation outcomes there is an urgent need not only for novel clinical mapping tools but also for driver-specific ablation approaches. Ideally, these approaches should be able to resolve subsurface intramural atrial activation and to accurately define AF drivers’ arrhythmogenic hubs and then create efficient and safe transmural lesions. Irreversible electroporation ablation approach may hold great promise in this regard [230].
Conclusion
An integrated mapping approach, which incorporates in-vivo, ex-vivo, and in-vitro human heart studies together with in-silico and large animal models, allows mutual validation of the electrostructural features of the human atria to identify all possible AF mechanisms. Ex-vivo human atrial integrated approach, where optical and clinical mapping techniques combined with new artificial intelligence-based approaches can define EP and structural features of AF drivers, may represent a robust and efficient translational platform to improve AF treatment. This may lead to more accurate clinical identification of AF driver location, and thus, provide more efficient targeted ablation. New ablation techniques, pharmacological interventions, and gene therapy strategies may be tested in ex-vivo human heart experiments and, subsequently, optimized through heart-specific computer simulations of AF without any risks to patients.
Atrial fibrillation (AF) occurrence and maintenance is associated with progressive remodeling of electrophysiological (repolarization and conduction) and 53D structural (fibrosis, fiber orientations, and wall thickness) features of the human atria. Significant diversity in AF etiology leads to heterogeneous arrhythmogenic electrophysiological and structural substrates within the 3D structure of the human atria. Since current clinical methods have yet to fully resolve the patient-specific arrhythmogenic substrates, mechanism-based AF treatments remain underdeveloped. Here, we review current knowledge from in-vivo, ex-vivo, and in-vitro human heart studies, and discuss how these studies may provide new insights on the synergy of atrial electrophysiological and 3D structural features in AF maintenance. In-vitro studies on surgically acquired human atrial samples provide a great opportunity to study a wide spectrum of AF pathology, including functional changes in single-cell action potentials, ion channels, and gene/protein expression. However, limited size of the samples prevents evaluation of heterogeneous AF substrates and reentrant mechanisms. In contrast, coronary-perfused ex-vivo human hearts can be studied with state-of-the-art functional and structural technologies, such as high-resolution near-infrared optical mapping and contrast-enhanced MRI. These imaging modalities can resolve atrial arrhythmogenic substrates and their role in reentrant mechanisms maintaining AF and validate clinical approaches. Nonetheless, longitudinal studies are not feasible in explanted human hearts. As no approach is perfect, we suggest that combining the strengths of direct human atrial studies with the high fidelity approaches available in the laboratory and in realistic patient-specific computer models would elucidate deeper knowledge of AF mechanisms. We propose that a comprehensive translational pipeline from ex-vivo human heart studies to longitudinal clinically relevant AF animal studies and finally to clinical trials is necessary to identify patient-specific arrhythmogenic substrates and develop novel AF treatments.
Highlights:
Electrical and structural bi-atrial remodeling form patient-specific AF substrates.
AF can be maintained by reentrant drivers within a fibrotic arrhythmogenic hub.
Number of AF drivers increases with the progression of remodeling.
AF treatment may depend on the AF progression and patient’s comorbidities.
Ex-vivo human heart is an efficient platform to reveal human AF mechanisms.
Acknowledgments:
We thank the Lifeline of Ohio Organ Procurement Organization and the Division of Cardiac Surgery at The OSU Wexner Medical Center for providing the explanted human hearts.
Sources of Funding: National Institute of Health [grant numbers HL115580 and HL135109] and the Bob and Corrine Frick Center for Heart Failure and Arrhythmia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nishida K, Nattel S, Atrial fibrillation compendium: historical context and detailed translational perspective on an important clinical problem, Circ. Res 114(9) (2014) 1447–1452. [DOI] [PubMed] [Google Scholar]
- [2].Wyse DG, Van Gelder IC, Ellinor PT, Go AS, Kalman JM, Narayan SM, et al. , Lone Atrial Fibrillation: Does it Exist?, J. Am. Coll. Cardiol 63(17) (2014) 1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pedersen OD, Abildstrom SZ, Ottesen MM, Rask-Madsen C, Bagger H, Kober L, et al. , Increased risk of sudden and non-sudden cardiovascular death in patients with atrial fibrillation/flutter following acute myocardial infarction, Eur Heart J 27(3) (2006) 290–5. [DOI] [PubMed] [Google Scholar]
- [4].Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ, Comorbidity of atrial fibrillation and heart failure, Nat Rev Cardiol 13(3) (2016) 131–47. [DOI] [PubMed] [Google Scholar]
- [5].Woods CE, Olgin J, Atrial fibrillation therapy now and in the future: drugs, biologicals, and ablation, Circ. Res 114(9) (2014) 1532–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, et al. , Driver domains in persistent atrial fibrillation, Circulation 130(7) (2014) 530–538. [DOI] [PubMed] [Google Scholar]
- [7].Miller JM, Kalra V, Das MK, Jain R, Garlie JB, Brewster JA, et al. , Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation: The Indiana University FIRM Registry, J. Am. Coll. Cardiol 69(10) (2017) 1247–1256. [DOI] [PubMed] [Google Scholar]
- [8].Hansen BJ, Zhao J, Fedorov VV, Fibrosis and Atrial Fibrillation: Computerized and Optical Mapping; A View into the Human Atria at Submillimeter Resolution, JACC Clin Electrophysiol 3(6) (2017) 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nattel S, Dobrev D, Controversies About Atrial Fibrillation Mechanisms: Aiming for Order in Chaos and Whether it Matters, Circ. Res 120(9) (2017) 1396–1398. [DOI] [PubMed] [Google Scholar]
- [10].Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. , Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study, JAMA 311(5) (2014) 498–506. [DOI] [PubMed] [Google Scholar]
- [11].Friedman DJ, Liu P, Barnett AS, Campbell KB, Jackson KP, Bahnson TD, et al. , Obstructive sleep apnea is associated with increased rotor burden in patients undergoing focal impulse and rotor modification guided atrial fibrillation ablation, Europace (2017) doi: 10.1093/europace/eux248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cochet H, Dubois R, Yamashita S, Al JN, Berte B, Sellal JM, et al. , Relationship Between Fibrosis Detected on Late Gadolinium-Enhanced Cardiac Magnetic Resonance and Re-Entrant Activity Assessed With Electrocardiographic Imaging in Human Persistent Atrial Fibrillation, JACC. Clin. Electrophysiol 4(1) (2018) 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. , Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins, N. Engl. J Med 339(10) (1998) 659–666. [DOI] [PubMed] [Google Scholar]
- [14].Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM, Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials, Circ Arrhythm Electrophysiol 2(6) (2009) 626–33. [DOI] [PubMed] [Google Scholar]
- [15].Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. , Approaches to catheter ablation for persistent atrial fibrillation, N. Engl. J. Med 372(19) (2015) 1812–1822. [DOI] [PubMed] [Google Scholar]
- [16].Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, et al. , Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review, Heart Rhythm 7(6) (2010) 835–846. [DOI] [PubMed] [Google Scholar]
- [17].Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, et al. , Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry, J. Cardiovasc. Electrophysiol 25(9) (2014) 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM, Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial, J. Am. Coll. Cardiol 60(7) (2012) 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baykaner T, Rogers AJ, Meckler GL, Zaman J, Navara R, Rodrigo M, et al. , Clinical Implications of Ablation of Drivers for Atrial Fibrillation: A Systematic Review and Meta-Analysis, Circ. Arrhythm. Electrophysiol 11(5) (2018) e006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sanchez-Quintana D, Lopez-Minguez JR, Pizarro G, Murillo M, Cabrera JA, Triggers and anatomical substrates in the genesis and perpetuation of atrial fibrillation, Curr Cardiol Rev 8(4) (2012) 310–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, et al. , The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis, Cardiovasc. Res 109(4) (2016) 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nattel S, Dobrev D, Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation, Nat Rev Cardiol 13(10) (2016) 575–90. [DOI] [PubMed] [Google Scholar]
- [23].Clauss S, Bleyer C, Schuttler D, Tomsits P, Renner S, Klymiuk N, et al. , Animal models of arrhythmia: classic electrophysiology to genetically modified large animals, Nat Rev Cardiol 16(8) (2019) 457–475. [DOI] [PubMed] [Google Scholar]
- [24].Schüttler D, Bapat A, Kääb S, Lee K, Tomsits P, Clauss S, et al. , Animal Models of Atrial Fibrillation, Circulation Research 127(1) (2020) 91–110. [DOI] [PubMed] [Google Scholar]
- [25].Herron TJ, Lee P, Jalife J, Optical imaging of voltage and calcium in cardiac cells & tissues, Circ Res 110(4) (2012) 609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hansen BJ, Csepe TA, Zhao J, Ignozzi AJ, Hummel JD, Fedorov VV, Maintenance of Atrial Fibrillation: Are Reentrant Drivers With Spatial Stability the Key?, Circ. Arrhythm. Electrophysiol 9(10) (2016) e004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boineau JP, Canavan TE, Schuessler RB, Cain ME, Corr PB, Cox JL, Demonstration of a widely distributed atrial pacemaker complex in the human heart, Circulation 77(6) (1988) 1221–1237. [DOI] [PubMed] [Google Scholar]
- [28].Bode F, Kilborn M, Karasik P, Franz MR, The repolarization-excitability relationship in the human right atrium is unaffected by cycle length, recording site and prior arrhythmias, J Am Coll Cardiol 37(3) (2001) 920–5. [DOI] [PubMed] [Google Scholar]
- [29].Morgan JM, Cunningham AD, Rowland E, Relationship of the effective refractory period and monophasic action potential duration after a step increase in pacing frequency, Pacing Clin Electrophysiol 13(8) (1990) 1002–8. [DOI] [PubMed] [Google Scholar]
- [30].Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, et al. , Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure, J Am Coll Cardiol 45(2) (2005) 285–92. [DOI] [PubMed] [Google Scholar]
- [31].Konings KT, Smeets JL, Penn OC, Wellens HJ, Allessie MA, Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans, Circulation 95(5) (1997) 1231–41:. [DOI] [PubMed] [Google Scholar]
- [32].van der Does L, Knops P, Teuwen CP, Serban C, Starreveld R, Lanters EAH, et al. , Unipolar atrial electrogram morphology from an epicardial and endocardial perspective, Heart Rhythm 15(6) (2018) 879–887. [DOI] [PubMed] [Google Scholar]
- [33].Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, et al. , Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies, Heart Rhythm 8(2) (2011) 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hoffman BF, Cranefield PF, Lepeschkin E, Surawicz B, Herrlich HC, Comparison of cardiac monophasic action potentials recorded by intracellular and suction electrodes, Am J Physiol 196(6) (1959) 1297–301. [DOI] [PubMed] [Google Scholar]
- [35].Franz MR, Burkhoff D, Spurgeon H, Weisfeldt ML, Lakatta EG, In vitro validation of a new cardiac catheter technique for recording monophasic action potentials, Eur Heart J 7(1) (1986) 34–41. [DOI] [PubMed] [Google Scholar]
- [36].Ino T, Karagueuzian HS, Hong K, Meesmann M, Mandel WJ, Peter T, Relation of monophasic action potential recorded with contact electrode to underlying transmembrane action potential properties in isolated cardiac tissues: a systematic microelectrode validation study, Cardiovasc Res 22(4) (1988) 255–64. [DOI] [PubMed] [Google Scholar]
- [37].Narayan SM, Franz MR, Quantifying fractionation and rate in human atrial fibrillation using monophasic action potentials: implications for substrate mapping, Europace 9 Suppl 6 (2007) vi89–95. [DOI] [PubMed] [Google Scholar]
- [38].Pandozi C, Bianconi L, Villani M, Gentilucci G, Castro A, Altamura G, et al. , Electrophysiological characteristics of the human atria after cardioversion of persistent atrial fibrillation, Circulation 98(25) (1998) 2860–2865. [DOI] [PubMed] [Google Scholar]
- [39].Sekiya J, Ohnishi Y, Inoue T, Yokoyama M, Monophasic action potentials of the right atrium in patients with paroxysmal atrial fibrillation, Jpn. Circ. J 65(10) (2001) 893–896. [DOI] [PubMed] [Google Scholar]
- [40].Kim BS, Kim YH, Hwang GS, Pak HN, Lee SC, Shim WJ, et al. , Action potential duration restitution kinetics in human atrial fibrillation, J Am Coll Cardiol 39(8) (2002) 1329–36. [DOI] [PubMed] [Google Scholar]
- [41].Lalani GG, Schricker A, Gibson M, Rostamian A, Krummen DE, Narayan SM, Atrial conduction slows immediately before the onset of human atrial fibrillation: a bi-atrial contact mapping study of transitions to atrial fibrillation, J Am Coll Cardiol 59(6) (2012) 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zheng Y, Xia Y, Carlson J, Kongstad O, Yuan S, Atrial average conduction velocity in patients with and without paroxysmal atrial fibrillation, Clin Physiol Funct Imaging 37(6) (2017) 596–601. [DOI] [PubMed] [Google Scholar]
- [43].Blandino A, Bianchi F, Grossi S, Biondi-Zoccai G, Conte MR, Gaido L, et al. , Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis, Pacing Clin Electrophysiol 40(2) (2017) 199–212. [DOI] [PubMed] [Google Scholar]
- [44].Bromberg BI, Hand DE, Schuessler RB, Boineau JP, Primary negativity does not predict dominant pacemaker location: implications for sinoatrial conduction, Am J Physiol 269(3 Pt 2) (1995) H877–87. [DOI] [PubMed] [Google Scholar]
- [45].Anter E, Josephson ME, Bipolar voltage amplitude: What does it really mean?, Heart Rhythm 13(1) (2016) 326–7. [DOI] [PubMed] [Google Scholar]
- [46].de Bakker JM, Electrogram recording and analyzing techniques to optimize selection of target sites for ablation of cardiac arrhythmias, Pacing Clin Electrophysiol 42(12) (2019) 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kojodjojo P, Kanagaratnam P, Segal OR, Hussain W, Peters NS, The effects of carbenoxolone on human myocardial conduction: a tool to investigate the role of gap junctional uncoupling in human arrhythmogenesis, J. Am. Coll. Cardiol 48(6) (2006) 1242–1249. [DOI] [PubMed] [Google Scholar]
- [48].Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, et al. , Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”, J Am Coll Cardiol 53(14) (2009) 1182–91. [DOI] [PubMed] [Google Scholar]
- [49].Voskoboinik A, Wong G, Lee G, Nalliah C, Hawson J, Prabhu S, et al. , Moderate alcohol consumption is associated with atrial electrical and structural changes: Insights from high-density left atrial electroanatomic mapping, Heart Rhythm 16(2) (2019) 251–259. [DOI] [PubMed] [Google Scholar]
- [50].Trautwein W, Kassebaum DG, Nelson RM, Hechthh, Electrophysiological study of human heart muscle, Circ Res 10 (1962) 306–12. [DOI] [PubMed] [Google Scholar]
- [51].Rosen MR, Wit AL, Hoffman BF, Electrophysiology and pharmacology of cardiac arrhythmias. I. Cellular electrophysiology of the mammalian heart, Am Heart J 88(3) (1974) 380–5. [DOI] [PubMed] [Google Scholar]
- [52].Heijman J, Voigt N, Nattel S, Dobrev D, Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression, Circ. Res 114(9) (2014) 1483–1499. [DOI] [PubMed] [Google Scholar]
- [53].Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, et al. , Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation, Circulation 103(5) (2001) 684–90. [DOI] [PubMed] [Google Scholar]
- [54].Chiang DY, Zhang M, Voigt N, Alsina KM, Jakob H, Martin JF, et al. , Identification of microRNA-mRNA dysregulations in paroxysmal atrial fibrillation, Int J Cardiol 184 (2015) 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nakai T, Watanabe I, Kunimoto S, Kojima T, Kondo K, Saito S, et al. , Electrophysiological effect of adenosine triphosphate and adenosine on atrial and ventricular action potential duration in humans, Jpn. Circ. J 64(6) (2000) 430–435. [DOI] [PubMed] [Google Scholar]
- [56].Botteron GW, Smith JM, Spatial and temporal inhomogeneity of adenosine’s effect on atrial refractoriness in humans: using atrial fibrillation to probe atrial refractoriness, J. Cardiovasc. Electrophysiol 5(6) (1994) 477–484. [DOI] [PubMed] [Google Scholar]
- [57].Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, et al. , Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart, Circulation 134(6) (2016) 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D’Hooge J, et al. , Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation, Circ Res 105(9) (2009) 876–85. [DOI] [PubMed] [Google Scholar]
- [59].Belus A, Piroddi N, Ferrantini C, Tesi C, Cazorla O, Toniolo L, et al. , Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils, Circ Res 107(1) (2010) 144–52. [DOI] [PubMed] [Google Scholar]
- [60].Nattel S, Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation, JACC Clin Electrophysiol 3(5) (2017) 425–435. [DOI] [PubMed] [Google Scholar]
- [61].Kunzel SR, Rausch JSE, Schaffer C, Hoffmann M, Kunzel K, Klapproth E, et al. , Modeling atrial fibrosis in vitro-Generation and characterization of a novel human atrial fibroblast cell line, FEBS Open Bio 10(7) (2020) 1210–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Krueger MW, Dorn A, Keller DU, Holmqvist F, Carlson J, Platonov PG, et al. , In-silico modeling of atrial repolarization in normal and atrial fibrillation remodeled state, Med Biol Eng Comput 51(10) (2013) 1105–19. [DOI] [PubMed] [Google Scholar]
- [63].Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, et al. , Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes, Nat Commun 11(1) (2020) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ribeiro AJS, Guth BD, Engwall M, Eldridge S, Foley CM, Guo L, et al. , Considerations for an In Vitro, Cell-Based Testing Platform for Detection of Drug-Induced Inotropic Effects in Early Drug Development. Part 2: Designing and Fabricating Microsystems for Assaying Cardiac Contractility With Physiological Relevance Using Human iPSC-Cardiomyocytes, Front Pharmacol 10 (2019) 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Perbellini F, Thum T, Living myocardial slices: a novel multicellular model for cardiac translational research, Eur Heart J 41(25) (2020) 2405–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, et al. , Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials, Sci Rep 6 (2016) 28798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].George SA, Brennan JA, Efimov IR, Preclinical Cardiac Electrophysiology Assessment by Dual Voltage and Calcium Optical Mapping of Human Organotypic Cardiac Slices, J Vis Exp (160) (2020). [DOI] [PubMed] [Google Scholar]
- [68].Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. , Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature 556(7700) (2018) 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ronaldson-Bouchard K, Yeager K, Teles D, Chen T, Ma S, Song L, et al. , Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype, Nat Protoc 14(10) (2019) 2781–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, et al. , Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes, Stem Cell Reports 10(3) (2018) 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].van Gorp PRR, Trines SA, Pijnappels DA, de Vries AAF, Multicellular In vitro Models of Cardiac Arrhythmias: Focus on Atrial Fibrillation, Front Cardiovasc Med 7 (2020) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, et al. , Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals, Cell Res 21(4) (2011) 579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Durrer D, Dam RT, van, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC, Total excitation of the isolated human heart, Circulation 41(6) (1970) 899–912. [DOI] [PubMed] [Google Scholar]
- [74].Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, et al. , Optical mapping of the isolated coronary-perfused human sinus node, J Am. Coll. Cardiol 56(17) (2010) 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhao J, Hansen BJ, Csepe TA, Lim P, Wang Y, Williams M, et al. , Integration of High-Resolution Optical Mapping and 3-Dimensional Micro-Computed Tomographic Imaging to Resolve the Structural Basis of Atrial Conduction in the Human Heart, Circ Arrhythm Electrophysiol 8(6) (2015) 1514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fedorov VV, Ambrosi CM, Kostecki G, Hucker WJ, Glukhov AV, Wuskell JP, et al. , Anatomic Localization and Autonomic Modulation of AV Junctional Rhythm in Failing Human Hearts, Circ. Arrhythm. Electrophysiol 4(4) (2011) 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, et al. , Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts, Eur. Heart J 36(35) (2015) 2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jose AD, Collison D, The normal range and determinants of the intrinsic heart rate in man, Cardiovasc. Res 4(2) (1970) 160–167. [DOI] [PubMed] [Google Scholar]
- [79].Alboni P, Malcarne C, Pedroni P, Masoni A, Narula OS, Electrophysiology of normal sinus node with and without autonomic blockade, Circulation 65(6) (1982) 1236–1242. [DOI] [PubMed] [Google Scholar]
- [80].Christou DD, Seals DR, Decreased maximal heart rate with aging is related to reduced {beta}-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate, J Appl Physiol (1985) 105(1) (2008) 24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nwazue VC, Paranjape SY, Black BK, Biaggioni I, Diedrich A, Dupont WD, et al. , Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity, J Am Heart Assoc 3(2) (2014) e000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li N, Kalyanasundaram A, Hansen BJ, Artiga EJ, Sharma R, Abudulwahed SH, et al. , Impaired neuronal sodium channels cause intranodal conduction failure and reentrant arrhythmias in human sinoatrial node, Nat. Commun 11(1) (2020) 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin S, Wang Y, et al. , Human Atrial Fibrillation Drivers Resolved With Integrated Functional and Structural Imaging to Benefit Clinical Mapping, JACC Clin Electrophysiol 4(12) (2018) 1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wiener N, Rosenblueth A, The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle, Arch. Inst. Cardiologia de Mexico 16(3–4) (1946) 205–265. [PubMed] [Google Scholar]
- [85].Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ, Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs, Circ Res 62(2) (1988) 395–410:. [DOI] [PubMed] [Google Scholar]
- [86].Spach MS, Dolber PC, Heidlage JF, Kootsey JM, Johnson EA, Propagating depolarization in anisotropic human and canine cardiac muscle: apparent directional differences in membrane capacitance. A simplified model for selective directional effects of modifying the sodium conductance on Vmax, tau foot, and the propagation safety factor, Circ. Res 60(2) (1987) 206–219. [DOI] [PubMed] [Google Scholar]
- [87].Spach MS, Dolber PC, Heidlage JF, Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation, Circ. Res 62(4) (1988) 811–832. [DOI] [PubMed] [Google Scholar]
- [88].van der Velden HM, Jongsma HJ, Cardiac gap junctions and connexins: their role in atrial fibrillation and potential as therapeutic targets, Cardiovasc Res 54(2) (2002) 270–9. [DOI] [PubMed] [Google Scholar]
- [89].Hansson A, Holm M, Blomstrom P, Johansson R, Luhrs C, Brandt J, et al. , Right atrial free wall conduction velocity and degree of anisotropy in patients with stable sinus rhythm studied during open heart surgery, Eur. Heart J 19(2) (1998) 293–300. [DOI] [PubMed] [Google Scholar]
- [90].Honarbakhsh S, Schilling RJ, Finlay M, Keating E, Ullah W, Hunter RJ, STAR mapping method to identify driving sites in persistent atrial fibrillation: Application through sequential mapping, J. Cardiovasc. Electrophysiol 30(12) (2019) 2694–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wong CX, Stiles MK, John B, Brooks AG, Lau DH, Dimitri H, et al. , Direction-dependent conduction in lone atrial fibrillation, Heart Rhythm 7(9) (2010) 1192–9. [DOI] [PubMed] [Google Scholar]
- [92].Schram-Serban C, Heida A, Roos-Serote MC, Knops P, Kik C, Brundel B, et al. , Heterogeneity in Conduction Underlies Obesity-Related Atrial Fibrillation Vulnerability, Circ Arrhythm Electrophysiol 13(5) (2020) e008161. [DOI] [PubMed] [Google Scholar]
- [93].Hansen BJ, Zhao J, Helfrich KM, Li N, Iancau A, Zolotarev A, et al. , Unmasking arrhythmogenic hubs of reentry driving persistent atrial fibrillation for patient-specific treatment, J Am Heart Assoc (2020), In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, Oka Y, et al. , A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation, J Am Coll Cardiol 52(16) (2008) 1326–34. [DOI] [PubMed] [Google Scholar]
- [95].Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. , Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association, Circulation 111(5) (2005) 659–70. [DOI] [PubMed] [Google Scholar]
- [96].Johnson JN, Tester DJ, Perry J, Salisbury BA, Reed CR, Ackerman MJ, Prevalence of early-onset atrial fibrillation in congenital long QT syndrome, Heart Rhythm 5(5) (2008) 704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Platonov PG, McNitt S, Polonsky B, Rosero SZ, Zareba W, Atrial Fibrillation in Long QT Syndrome by Genotype, Circ Arrhythm Electrophysiol 12(10) (2019) e007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, et al. , Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium, J Am Coll Cardiol 55(21) (2010) 2330–42. [DOI] [PubMed] [Google Scholar]
- [99].Dupont E, Ko Y, Rothery S, Coppen SR, Baghai M, Haw M, et al. , The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation, Circulation 103(6) (2001) 842–9. [DOI] [PubMed] [Google Scholar]
- [100].Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, et al. , Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria, J Am Coll Cardiol 38(3) (2001) 883–91. [DOI] [PubMed] [Google Scholar]
- [101].Wetzel U, Boldt A, Lauschke J, Weigl J, Schirdewahn P, Dorszewski A, et al. , Expression of connexins 40 and 43 in human left atrium in atrial fibrillation of different aetiologies, Heart 91(2) (2005) 166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gemel J, Levy AE, Simon AR, Bennett KB, Ai X, Akhter S, et al. , Connexin40 abnormalities and atrial fibrillation in the human heart, J Mol Cell Cardiol 76 (2014) 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kanagaratnam P, Cherian A, Stanbridge RD, Glenville B, Severs NJ, Peters NS, Relationship between connexins and atrial activation during human atrial fibrillation, J Cardiovasc Electrophysiol 15(2) (2004) 206–16. [DOI] [PubMed] [Google Scholar]
- [104].Nao T, Ohkusa T, Hisamatsu Y, Inoue N, Matsumoto T, Yamada J, et al. , Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm, Am J Cardiol 91(6) (2003) 678–83. [DOI] [PubMed] [Google Scholar]
- [105].Ho SY, Anderson RH, Sanchez-Quintana D, Atrial structure and fibres: morphologic bases of atrial conduction, Cardiovasc Res 54(2) (2002) 325–36. [DOI] [PubMed] [Google Scholar]
- [106].Markides V, Schilling RJ, Ho SY, Chow AW, Davies DW, Peters NS, Characterization of left atrial activation in the intact human heart, Circulation 107(5) (2003) 733–9. [DOI] [PubMed] [Google Scholar]
- [107].Spach MS, Josephson ME, Initiating reentry: the role of nonuniform anisotropy in small circuits, J Cardiovasc Electrophysiol 5(2) (1994) 182–209. [DOI] [PubMed] [Google Scholar]
- [108].Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, et al. , Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis, Circulation 104(25) (2001) 3069–75. [DOI] [PubMed] [Google Scholar]
- [109].Fukumoto K, Habibi M, Ipek EG, Zahid S, Khurram IM, Zimmerman SL, et al. , Association of Left Atrial Local Conduction Velocity With Late Gadolinium Enhancement on Cardiac Magnetic Resonance in Patients With Atrial Fibrillation, Circ Arrhythm Electrophysiol 9(3) (2016) e002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Miyamoto K, Tsuchiya T, Narita S, Yamaguchi T, Nagamoto Y, Ando S, et al. , Bipolar electrogram amplitudes in the left atrium are related to local conduction velocity in patients with atrial fibrillation, Europace 11(12) (2009) 1597–605. [DOI] [PubMed] [Google Scholar]
- [111].Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA, Atrial fibrillation begets atrial fibrillation. a study in awake chronically instrumented goats, Circulation 92(7) (1995) 1954–68:. [DOI] [PubMed] [Google Scholar]
- [112].Weber FM, Luik A, Schilling C, Seemann G, Krueger MW, Lorenz C, et al. , Conduction velocity restitution of the human atrium--an efficient measurement protocol for clinical electrophysiological studies, IEEE Trans. Biomed. Eng 58(9) (2011) 2648–2655. [DOI] [PubMed] [Google Scholar]
- [113].Zhao J, Hansen BJ, Wang Y, Csepe TA, Sul LV, Tang A, et al. , Three-dimensional Integrated Functional, Structural, and Computational Mapping to Define the Structural “Fingerprints” of Heart-Specific Atrial Fibrillation Drivers in Human Heart Ex Vivo, J. Am. Heart Assoc 6(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Parameswaran R, Teuwen CP, Watts T, Nalliah CJ, Royse A, Goldblatt J, et al. , Functional Atrial Endocardial-Epicardial Dissociation in Patients With Structural Heart Disease Undergoing Cardiac Surgery, JACC Clin Electrophysiol 6(1) (2020) 34–44. [DOI] [PubMed] [Google Scholar]
- [115].de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops P, et al. , Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans, Circ Arrhythm Electrophysiol 9(5) (2016) e003648. [DOI] [PubMed] [Google Scholar]
- [116].Kofune T, Watanabe I, Okubo K, Okumura Y, Masaki R, Shindo A, et al. , Effect of IKr blocker nifekalant on atrial action potential duration after successful internal cardioversion of chronic atrial fibrillation, Pacing Clin. Electrophysiol 28(5) (2005) 391–396. [DOI] [PubMed] [Google Scholar]
- [117].Franz MR, Karasik PL, Li C, Moubarak J, Chavez M, Electrical remodeling of the human atrium: similar effects in patients with chronic atrial fibrillation and atrial flutter, J. Am. Coll. Cardiol 30(7) (1997) 1785–1792. [DOI] [PubMed] [Google Scholar]
- [118].Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE, Repolarization alternans reveals vulnerability to human atrial fibrillation, Circulation 123(25) (2011) 2922–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Narayan SM, Kazi D, Krummen DE, Rappel WJ, Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats, J. Am. Coll. Cardiol 52(15) (2008) 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, et al. , Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials, Circulation 104(21) (2001) 2551–7. [DOI] [PubMed] [Google Scholar]
- [121].Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM, Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation, Circ Res 80(6) (1997) 772–81. [DOI] [PubMed] [Google Scholar]
- [122].Workman AJ, Kane KA, Rankin AC, The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation, Cardiovasc. Res 52(2) (2001) 226–235. [DOI] [PubMed] [Google Scholar]
- [123].Wang Z, Fermini B, Nattel S, Delayed rectifier outward current and repolarization in human atrial myocytes, Circ. Res 73(2) (1993) 276–285. [DOI] [PubMed] [Google Scholar]
- [124].Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, et al. , Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation, Circulation 110(16) (2004) 2299–2306. [DOI] [PubMed] [Google Scholar]
- [125].Courtemanche M, Ramirez RJ, Nattel S, Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model, Am. J. Physiol 275(1 Pt 2) (1998) H301–H321. [DOI] [PubMed] [Google Scholar]
- [126].Choi KJ, Kim J, Kim SH, Nam GB, Kim YH, Increased dispersion of atrial repolarization in Brugada syndrome, Europace 13(11) (2011) 1619–24. [DOI] [PubMed] [Google Scholar]
- [127].Kim KB, Rodefeld MD, Schuessler RB, Cox JL, Boineau JP, Relationship between local atrial fibrillation interval and refractory period in the isolated canine atrium, Circulation 94(11) (1996) 2961–2967. [DOI] [PubMed] [Google Scholar]
- [128].Schuessler RB, Kay MW, Melby SJ, Branham BH, Boineau JP, Damiano RJ Jr., Spatial and temporal stability of the dominant frequency of activation in human atrial fibrillation, J Electrocardiol 39(4 Suppl) (2006) S7–12. [DOI] [PubMed] [Google Scholar]
- [129].Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, et al. , Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans, Circulation 112(6) (2005) 789–797. [DOI] [PubMed] [Google Scholar]
- [130].Hasebe H, Yoshida K, Iida M, Hatano N, Muramatsu T, Aonuma K, Right-to-left frequency gradient during atrial fibrillation initiated by right atrial ectopies and its augmentation by adenosine triphosphate: Implications of right atrial fibrillation, Heart Rhythm 13(2) (2016) 354–63. [DOI] [PubMed] [Google Scholar]
- [131].Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, et al. , Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism, Circulation 114(23) (2006) 2434–2442. [DOI] [PubMed] [Google Scholar]
- [132].Franz MR, Schaefer J, Schottler M, Seed WA, Noble MI, Electrical and mechanical restitution of the human heart at different rates of stimulation, Circ. Res 53(6) (1983) 815–822. [DOI] [PubMed] [Google Scholar]
- [133].Heijman J, Kirchner D, Kunze F, Chretien EM, Michel-Reher MB, Voigt N, et al. , Muscarinic type-1 receptors contribute to IK,ACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation, Int J Cardiol 255 (2018) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tebbenjohanns J, Schumacher B, Pfeiffer D, Jung W, Luderitz B, Dose and rate-dependent effects of adenosine on atrial action potential duration in humans, J. Interv. Card Electrophysiol 1(1) (1997) 33–37. [DOI] [PubMed] [Google Scholar]
- [135].Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, et al. , Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation, Circ. Arrhythm. Electrophysiol 3(5) (2010) 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Quintanilla JG, Perez-Villacastin J, Perez-Castellano N, Pandit SV, Berenfeld O, Jalife J, et al. , Mechanistic Approaches to Detect, Target, and Ablate the Drivers of Atrial Fibrillation, Circ. Arrhythm. Electrophysiol 9(1) (2016) e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A, et al. , Role of endoepicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation, Prog Biophys Mol Biol 115(2–3) (2014) 173–185. [DOI] [PubMed] [Google Scholar]
- [138].Bajraktari G, Bytyci I, Henein MY, Left atrial structure and function predictors of recurrent fibrillation after catheter ablation: a systematic review and meta-analysis, Clin Physiol Funct Imaging 40(1) (2020) 1–13. [DOI] [PubMed] [Google Scholar]
- [139].Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, et al. , Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women, Mayo Clin Proc 76(5) (2001) 467–75. [DOI] [PubMed] [Google Scholar]
- [140].Seko Y, Kato T, Haruna T, Izumi T, Miyamoto S, Nakane E, et al. , Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling, Sci Rep 8(1) (2018) 6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Byrd GD, Prasad SM, Ripplinger CM, Cassilly TR, Schuessler RB, Boineau JP, et al. , Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis, Circulation 112(9 Suppl) (2005) I7–13. [DOI] [PubMed] [Google Scholar]
- [142].Kottkamp H, Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications, J. Cardiovasc. Electrophysiol 23(7) (2012) 797–799. [DOI] [PubMed] [Google Scholar]
- [143].Kottkamp H, Bender R, Berg J, Catheter ablation of atrial fibrillation: how to modify the substrate?, J. Am. Coll. Cardiol 65(2) (2015) 196–206. [DOI] [PubMed] [Google Scholar]
- [144].Krul SP, Berger WR, Smit NW, van Amersfoorth SC, Driessen AH, van Boven WJ, et al. , Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation, Circ. Arrhythm. Electrophysiol 8(2) (2015) 288–295. [DOI] [PubMed] [Google Scholar]
- [145].de JS, van Veen TA, van Rijen HV, de Bakker JM, Fibrosis and cardiac arrhythmias, J. Cardiovasc. Pharmacol 57(6) (2011) 630–638. [DOI] [PubMed] [Google Scholar]
- [146].Nguyen TP, Qu Z, Weiss JN, Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils, J. Mol. Cell Cardiol 70 (2014) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Medi C, Kalman JM, Spence SJ, Teh AW, Lee G, Bader I, et al. , Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation, J. Cardiovasc. Electrophysiol 22(12) (2011) 1317–1324. [DOI] [PubMed] [Google Scholar]
- [148].Aldhoon B, Kucera T, Smorodinova N, Martinek J, Melenovsky V, Kautzner J, Associations between cardiac fibrosis and permanent atrial fibrillation in advanced heart failure, Physiol Res 62(3) (2013) 247–255. [DOI] [PubMed] [Google Scholar]
- [149].Wang Y, Xiong Z, Nalar A, Hansen BJ, Kharche S, Seemann G, et al. , A robust computational framework for estimating 3D Bi-Atrial chamber wall thickness, Comput Biol Med 114 (2019) 103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Sanchez-Quintana D, Pizarro G, Lopez-Minguez JR, Ho SY, Cabrera JA, Standardized review of atrial anatomy for cardiac electrophysiologists, J. Cardiovasc. Transl. Res 6(2) (2013) 124–144. [DOI] [PubMed] [Google Scholar]
- [151].Roy A, Varela M, Aslanidi O, Image-Based Computational Evaluation of the Effects of Atrial Wall Thickness and Fibrosis on Re-entrant Drivers for Atrial Fibrillation, Front Physiol 9 (2018) 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY, Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age, J. Am. Coll. Cardiol 58(21) (2011) 2225–2232. [DOI] [PubMed] [Google Scholar]
- [153].Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. , Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality, J Am Coll Cardiol 76(10) (2020) 1197–1211. [DOI] [PubMed] [Google Scholar]
- [154].Zhao J, Butters TD, Zhang H, Pullan AJ, LeGrice IJ, Sands GB, et al. , An image-based model of atrial muscular architecture: effects of structural anisotropy on electrical activation, Circ. Arrhythm. Electrophysiol 5(2) (2012) 361–370. [DOI] [PubMed] [Google Scholar]
- [155].de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, et al. , Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough, Circulation 122(17) (2010) 1674–1682. [DOI] [PubMed] [Google Scholar]
- [156].Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL, et al. , Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium, Circulation 88(1) (1993) 250–263. [DOI] [PubMed] [Google Scholar]
- [157].Pashakhanloo F, Herzka DA, Ashikaga H, Mori S, Gai N, Bluemke DA, et al. , Myofiber Architecture of the Human Atria as Revealed by Submillimeter Diffusion Tensor Imaging, Circ Arrhythm Electrophysiol 9(4) (2016) e004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Efimov IR, Fedorov VV, Chessboard of atrial fibrillation: reentry or focus? Single or multiple source(s)? Neurogenic or myogenic?, Am J Physiol Heart Circ. Physiol 289(3) (2005) H977–H979. [DOI] [PubMed] [Google Scholar]
- [159].Moe GK, Abildskov JA, Atrial fibrillation as a self-sustained arrhythmia independent of focal discharge, Am Heart J 58 (1959) 59–70. [DOI] [PubMed] [Google Scholar]
- [160].Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL, Simultaneous Biatrial High-Density (510–512 Electrodes) Epicardial Mapping of Persistent and Long-Standing Persistent Atrial Fibrillation in Patients: New Insights Into the Mechanism of Its Maintenance, Circulation 132(22) (2015) 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Haissaguerre M, Hocini M, Sanders P, Takahashi Y, Rotter M, Sacher F, et al. , Localized sources maintaining atrial fibrillation organized by prior ablation, Circulation 113(5) (2006) 616–625. [DOI] [PubMed] [Google Scholar]
- [162].Atienza F, Almendral J, Ormaetxe JM, Moya A, Martinez-Alday JD, Hernandez-Madrid A, et al. , Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial, J. Am. Coll. Cardiol 64(23) (2014) 2455–2467. [DOI] [PubMed] [Google Scholar]
- [163].Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, et al. , Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations, Circulation 110(21) (2004) 3293–3299. [DOI] [PubMed] [Google Scholar]
- [164].Lewis T, Feil HS, Stroud WD, Observations upon flutter and fibrillation. Part II, The nature of auricular flutter., Heart VII(4) (1920) 191–345. [Google Scholar]
- [165].Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, et al. , The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation, J Thorac Cardiovasc Surg 101(3) (1991) 406–26. [PubMed] [Google Scholar]
- [166].Hummel J, Michaud G, Hoyt R, DeLurgio D, Rasekh A, Kusumoto F, et al. , Phased RF ablation in persistent atrial fibrillation, Heart Rhythm 11(2) (2014) 202–209. [DOI] [PubMed] [Google Scholar]
- [167].Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, et al. , Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias, J. Cardiovasc. Electrophysiol 16(11) (2005) 1138–1147. [DOI] [PubMed] [Google Scholar]
- [168].Dubois R, Shah AJ, Hocini M, Denis A, Derval N, Cochet H, et al. , Non-invasive cardiac mapping in clinical practice: Application to the ablation of cardiac arrhythmias, J. Electrocardiol 48(6) (2015) 966–974. [DOI] [PubMed] [Google Scholar]
- [169].Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM, Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation), J. Am. Coll. Cardiol 62(2) (2013) 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Allessie M, de Groot N, CrossTalk opposing view: Rotors have not been demonstrated to be the drivers of atrial fibrillation, J Physiol 592(15) (2014) 3167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Chrispin J, Gucuk IE, Zahid S, Prakosa A, Habibi M, Spragg D, et al. , Lack of regional association between atrial late gadolinium enhancement on cardiac magnetic resonance and atrial fibrillation rotors, Heart Rhythm 13(3) (2016) 654–660. [DOI] [PubMed] [Google Scholar]
- [172].Higuchi K, Cates J, Gardner G, Morris A, Burgon NS, Akoum N, et al. , The Spatial Distribution of Late Gadolinium Enhancement of Left Atrial Magnetic Resonance Imaging in Patients With Atrial Fibrillation, JACC Clin Electrophysiol 4(1) (2018) 49–58. [DOI] [PubMed] [Google Scholar]
- [173].Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, et al. , Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity, Eur. Heart J 35(2) (2014) 86–97. [DOI] [PubMed] [Google Scholar]
- [174].Morgan R, Colman MA, Chubb H, Seemann G, Aslanidi OV, Slow Conduction in the Border Zones of Patchy Fibrosis Stabilizes the Drivers for Atrial Fibrillation: Insights from Multi-Scale Human Atrial Modeling, Front Physiol 7 (2016) 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Saha M, Roney CH, Bayer JD, Meo M, Cochet H, Dubois R, et al. , Wavelength and Fibrosis Affect Phase Singularity Locations During Atrial Fibrillation, Front Physiol 9 (2018) 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Burstein B, Nattel S, Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation, J. Am. Coll. Cardiol 51(8) (2008) 802–809. [DOI] [PubMed] [Google Scholar]
- [177].Mahnkopf C, Badger TJ, Burgon NS, Daccarett M, Haslam TS, Badger CT, et al. , Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation, Heart Rhythm 7(10) (2010) 1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Cochet H, Scherr D, Zellerhoff S, Sacher F, Derval N, Denis A, et al. , Atrial Structure and Function 5 Years After Successful Ablation for Persistent Atrial Fibrillation: An MRI Study, J. Cardiovasc. Electrophysiol (2014) 671–679. [DOI] [PubMed] [Google Scholar]
- [179].Sramko M, Kautzner J, Atrial Fibrosis: An Illusion or a True Key to Successful Ablation of Atrial Fibrillation?, J. Am. Coll. Cardiol 65(22) (2015) 2465. [DOI] [PubMed] [Google Scholar]
- [180].Csepe TA, Zhao J, Sul LV, Wang Y, Hansen BJ, Li N, et al. , Novel application of 3D contrast-enhanced CMR to define fibrotic structure of the human sinoatrial node in vivo, Eur Heart J Cardiovasc Imaging 18(8) (2017) 862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Akoum N, McGann C, Vergara G, Badger T, Ranjan R, Mahnkopf C, et al. , Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant, J. Cardiovasc. Electrophysiol 23(1) (2012) 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Sohns C, Lemes C, Metzner A, Fink T, Chmelevsky M, Maurer T, et al. , First-in-Man Analysis of the Relationship Between Electrical Rotors From Noninvasive Panoramic Mapping and Atrial Fibrosis From Magnetic Resonance Imaging in Patients With Persistent Atrial Fibrillation, Circ. Arrhythm. Electrophysiol 10(8) (2017). [DOI] [PubMed] [Google Scholar]
- [183].Cox JL, Churyla A, Malaisrie SC, Pham DT, Kruse J, Kislitsina ON, et al. , A Hybrid Maze Procedure for Long-Standing Persistent Atrial Fibrillation, Ann Thorac Surg 107(2) (2019) 610–618. [DOI] [PubMed] [Google Scholar]
- [184].Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, et al. , Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience, Heart Rhythm 13(3) (2016) 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Berntsen RF, Haland TF, Skardal R, Holm T, Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: A within-patient controlled study with implanted cardiac monitoring, Heart Rhythm 13(9) (2016) 1768–1774. [DOI] [PubMed] [Google Scholar]
- [186].Cox JL, Malaisrie SC, Kislitsina ON, McCarthy PM, The electrophysiologic basis for lesions of the contemporary Maze operation, J Thorac Cardiovasc Surg 157(2) (2019) 584–590. [DOI] [PubMed] [Google Scholar]
- [187].Hansen BJ, Li N, Helfrich KM, Abudulwahed SH, Artiga EJ, Joseph ME, et al. , First In Vivo Use of High-Resolution Near-Infrared Optical Mapping to Assess Atrial Activation During Sinus Rhythm and Atrial Fibrillation in a Large Animal Model, Circ-Arrhythmia Elec 11(12) (2018) e006870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zolotarev AM, Hansen BJ, Ivanova EA, Helfrich KM, Li N, Janssen PML, et al. , Optical Mapping-Validated Machine Learning Improves Atrial Fibrillation Driver Detection by Multi-Electrode Mapping, Circ Arrhythm Electrophysiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Podziemski P, Zeemering S, Kuklik P, van HA, Maesen B, Maessen J, et al. , Rotors Detected by Phase Analysis of Filtered, Epicardial Atrial Fibrillation Electrograms Colocalize With Regions of Conduction Block, Circ. Arrhythm. Electrophysiol 11(10) (2018) e005858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Nishida K, Michael G, Dobrev D, Nattel S, Animal models for atrial fibrillation: clinical insights and scientific opportunities, Europace 12(2) (2010) 160–172. [DOI] [PubMed] [Google Scholar]
- [191].Chorro FJ, Such-Belenguer L, Lopez-Merino V, [Animal models of cardiovascular disease], Rev. Esp. Cardiol 62(1) (2009) 69–84. [PubMed] [Google Scholar]
- [192].Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, et al. , Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation, Circulation 129(14) (2014) 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Schotten U, Verheule S, Kirchhof P, Goette A, Pathophysiological mechanisms of atrial fibrillation: a translational appraisal, Physiol Rev 91(1) (2011) 265–325. [DOI] [PubMed] [Google Scholar]
- [194].Li D, Fareh S, Leung TK, Nattel S, Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort, Circulation 100(1) (1999) 87–95. [DOI] [PubMed] [Google Scholar]
- [195].Liang Z, Liu LF, Chen XP, Shi XM, Guo HY, Lin K, et al. , Establishment of a model of renal impairment with mild renal insufficiency associated with atrial fibrillation in canines, PLoS. One 9(8) (2014) e105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Morillo CA, Klein GJ, Jones DL, Guiraudon CM, Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation, Circulation 91(5) (1995) 1588–1595. [DOI] [PubMed] [Google Scholar]
- [197].Everett TH, Wilson EE, Verheule S, Guerra JM, Foreman S, Olgin JE, Structural atrial remodeling alters the substrate and spatiotemporal organization of atrial fibrillation: a comparison in canine models of structural and electrical atrial remodeling, Am. J. Physiol Heart Circ. Physiol 291(6) (2006) H2911–H2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Zhao J, Xu W, Yun F, Zhao H, Li W, Gong Y, et al. , Chronic obstructive sleep apnea causes atrial remodeling in canines: mechanisms and implications, Basic Res. Cardiol 109(5) (2014) 427. [DOI] [PubMed] [Google Scholar]
- [199].Ausma J, Van Der Velden HM, Lenders MH, Van Ankeren EP, Jongsma HJ, Ramaekers FC, et al. , Reverse Structural and Gap-Junctional Remodeling After Prolonged Atrial Fibrillation in the Goat, Circulation 107(15) (2003) 2051–2058. [DOI] [PubMed] [Google Scholar]
- [200].Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, et al. , Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat, J Mol. Cell Cardiol 33(12) (2001) 2083–2094. [DOI] [PubMed] [Google Scholar]
- [201].Schoonderwoerd BA, Van Gelder IC, van Veldhuisen DJ, Tieleman RG, Grandjean JG, Bel KJ, et al. , Electrical remodeling and atrial dilation during atrial tachycardia are influenced by ventricular rate: role of developing tachycardiomyopathy, J. Cardiovasc. Electrophysiol 12(12) (2001) 1404–1410. [DOI] [PubMed] [Google Scholar]
- [202].Remes J, van Brakel TJ, Bolotin G, Garber C, de Jong MM, van der Veen FH, et al. , Persistent atrial fibrillation in a goat model of chronic left atrial overload, J. Thorac. Cardiovasc. Surg 136(4) (2008) 1005–1011. [DOI] [PubMed] [Google Scholar]
- [203].Angel N, Li LI, MacLeod RS, Marrouche N, Ranjan R, Dosdall DJ, Diverse Fibrosis Architecture and Premature Stimulation Facilitate Initiation of Reentrant Activity Following Chronic Atrial Fibrillation, J. Cardiovasc. Electrophysiol 26(12) (2015) 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, et al. , Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity, J. Am. Coll. Cardiol 66(1) (2015) 1–11. [DOI] [PubMed] [Google Scholar]
- [205].Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Brooks AG, Worthington M, et al. , Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model, Heart Rhythm 7(9) (2010) 1282–1290. [DOI] [PubMed] [Google Scholar]
- [206].Anne W, Willems R, Holemans P, Beckers F, Roskams T, Lenaerts I, et al. , Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model, J. Mol. Cell Cardiol 43(2) (2007) 148–158. [DOI] [PubMed] [Google Scholar]
- [207].Osaka T, Yamazaki M, Yokoyama E, Ito A, Kodama I, Sotalol reverses remodeled action potential in patients with chronic atrial fibrillation but does not prevent arrhythmia recurrence, J. Cardiovasc. Electrophysiol 15(8) (2004) 877–884. [DOI] [PubMed] [Google Scholar]
- [208].Fenner MF, Carstensen H, Dalgas Nissen S, Melis Hesselkilde E, Scott Lunddahl C, Adler Hess Jensen M, et al. , Effect of selective IK,ACh inhibition by XAF-1407 in an equine model of tachypacing-induced persistent atrial fibrillation, Br J Pharmacol 177(16) (2020) 3778–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Csepe TA, Kalyanasundaram A, Hansen BJ, Zhao J, Fedorov VV, Fibrosis: a structural modulator of sinoatrial node physiology and dysfunction, Front Physiol 6 (2015) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH, Anatomy of the pig heart: comparisons with normal human cardiac structure, J Anat 193 ( Pt 1) (1998) 105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].DiVincenti L Jr., Westcott R, Lee C, Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass, J Am Assoc Lab Anim Sci 53(5) (2014) 439–48. [PMC free article] [PubMed] [Google Scholar]
- [212].Dosdall DJ, Ranjan R, Higuchi K, Kholmovski E, Angel N, Li L, et al. , Chronic atrial fibrillation causes left ventricular dysfunction in dogs but not goats: experience with dogs, goats, and pigs, Am. J. Physiol Heart Circ. Physiol 305(5) (2013) H725–H731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Boyle PM, Zahid S, Trayanova NA, Towards personalized computational modelling of the fibrotic substrate for atrial arrhythmia, Europace 18(suppl 4) (2016) iv136–iv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Smaill BH, Zhao J, Trew ML, Three-dimensional impulse propagation in myocardium: arrhythmogenic mechanisms at the tissue level, Circ. Res 112(5) (2013) 834–848. [DOI] [PubMed] [Google Scholar]
- [215].Bai J, Lo A, Gladding PA, Stiles MK, Fedorov VV, Zhao J, In silico investigation of the mechanisms underlying atrial fibrillation due to impaired Pitx2, PLoS Comput Biol 16(2) (2020) e1007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Ali RL, Hakim JB, Boyle PM, Zahid S, Sivasambu B, Marine JE, et al. , Arrhythmogenic propensity of the fibrotic substrate after atrial fibrillation ablation: a longitudinal study using magnetic resonance imaging-based atrial models, Cardiovasc Res 115(12) (2019) 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zhao J, Butters TD, Zhang H, LeGrice IJ, Sands GB, Smaill BH, Image-based model of atrial anatomy and electrical activation: a computational platform for investigating atrial arrhythmia, IEEE Trans. Med. Imaging 32(1) (2013) 18–27. [DOI] [PubMed] [Google Scholar]
- [218].Trayanova NA, Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management, Circ. Res 114(9) (2014) 1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Lo ACY, Bai J, Gladding PA, Fedorov VV, Zhao J, Afterdepolarizations and abnormal calcium handling in atrial myocytes with modulated SERCA uptake: a sensitivity analysis of calcium handling channels, Philos Trans A Math Phys Eng Sci 378(2173) (2020) 20190557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, et al. , Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network, Nat. Med 25(1) (2019) 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Xiong Z, Nash MP, Cheng E, Fedorov VV, Stiles MK, Zhao J, ECG signal classification for the detection of cardiac arrhythmias using a convolutional recurrent neural network, Physiol Meas 39(9) (2018) 094006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. , An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction, Lancet 394(10201) (2019) 861–867. [DOI] [PubMed] [Google Scholar]
- [223].McGillivray MF, Cheng W, Peters NS, Christensen K, Machine learning methods for locating re-entrant drivers from electrograms in a model of atrial fibrillation, R. Soc. Open. Sci 5(4) (2018) 172434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Vijayakumar R, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y, Methodology Considerations in Phase Mapping of Human Cardiac Arrhythmias, Circ. Arrhythm. Electrophysiol 9(11) (2016) e004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Alhusseini MI, Abuzaid F, Rogers AJ, Zaman JAB, Baykaner T, Clopton P, et al. , Machine Learning to Classify Intracardiac Electrical Patterns During Atrial Fibrillation: Machine Learning of Atrial Fibrillation, Circ Arrhythm Electrophysiol 13(8) (2020) e008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Li N, Brundel B, Inflammasomes and Proteostasis Novel Molecular Mechanisms Associated With Atrial Fibrillation, Circ Res 127(1) (2020) 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Begg GA, Holden AV, Lip GY, Plein S, Tayebjee MH, Assessment of atrial fibrosis for the rhythm control of atrial fibrillation, Int J Cardiol 220 (2016) 155–61. [DOI] [PubMed] [Google Scholar]
- [228].Komal S, Yin JJ, Wang SH, Huang CZ, Tao HL, Dong JZ, et al. , MicroRNAs: Emerging biomarkers for atrial fibrillation, J Cardiol 74(6) (2019) 475–482. [DOI] [PubMed] [Google Scholar]
- [229].Roselli C, Rienstra M, Ellinor PT, Genetics of Atrial Fibrillation in 2020: GWAS, Genome Sequencing, Polygenic Risk, and Beyond, Circ Res 127(1) (2020) 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Loh P, van Es R, Groen MHA, Neven K, Kassenberg W, Wittkampf FHM, et al. , Pulmonary Vein Isolation with Single Pulse Irreversible Electroporation: A First in Human Study in 10 Patients with Atrial Fibrillation, Circ Arrhythm Electrophysiol (2020). [DOI] [PubMed] [Google Scholar]