Graphical Abstract
Letter to the Editor
Since the development of single cell RNA-sequencing (scRNA-seq) technology, characterization of the heterogeneous cardiac cellulome has been a primary goal. Major avenues of research have focused on development of the embryonic heart and the impact of injury on the transcriptome of adult cardiac cells. In this regard, a comprehensive overview of scRNA-seq procedures and application in cardiac cells was recently published in the Journal of Molecular and Cellular Cardiology1. Compared to cardiomyocytes, cardiac fibroblasts are more easily investigated by scRNA-seq due to their size and viability post-digestion. ScRNA-seq and subsequent differential gene expression has identified distinct cardiac fibroblast subpopulations highlighting their heterogeneity within the myocardium2,3. Cardiac fibroblasts arbitrate extracellular matrix (ECM) deposition in the remodeling myocardium in pathological disease states such as hypertension and myocardial infarction (MI). Thus, the identity and function of cardiac fibroblast subpopulations is important for elucidation of novel therapeutic targets. Cardiac fibroblasts are much more dynamic than previously thought and targeting specific subpopulations is a viable approach for limiting the extent of cardiac fibrosis with both acute and chronic injury.
Independent studies of MI in the normotensive heart have elucidated novel markers for activated fibroblasts including scx, thbs44, and ckap42 that are upregulated in fibrogenic cardiac fibroblast subpopulations. The post-MI time course has been utilized to characterize general shifts in fibroblast phenotypes using bulk RNA-seq identifying pro-inflammatory, proliferative, and neo-homeostatic transitions5. In a similar time course approach, scRNA-seq revealed that an early shift to proliferation, reduced fibrinolysis, and increased angiotensin II (Ang II) responsiveness leads to a higher rate of cardiac rupture in hypertension-prone mice three days post-MI6. Just as important as the identification of candidate markers and targets is the time frame for efficacious drug delivery. The genetic makeup and relative proportion of cardiac fibroblast subpopulations holds valuable information for limiting cardiac fibrosis in both normotensive and hypertensive cardiac injury.
Hypertension is a major risk factor for cardiovascular disease-related mortality given that 20% of people with heart failure7, ~35% with ST-elevation MI (STEMI), and ~75% with Non-ST-elevation MI (NSTEMI)8 had antecedent high blood pressure. Nearly 50% of Americans are diagnosed with high blood pressure7, thus highlighting the critical need to determine the impact of hypertension on cardiac fibroblast subpopulations. Resident cardiac fibroblasts expand and activate in the hypertensive and pressure overloaded heart, likely producing altered fibroblast subpopulations that mediate the resultant cardiac remodeling (Figure 1). Our laboratory has shown that transiently inhibiting the renin angiotensin system (RAS) produces a suppressed fibrogenic cardiac fibroblast phenotype that persists even after cessation of treatment in spontaneously hypertensive rats9. Based on prior work from our laboratory and others, we have proposed that associated with hypertension, fibroblast hyperplasia leads to an expansion of a pathological subset of fibroblasts that is also susceptible to RAS inhibitor-induced apoptosis10. Thus, it may be that the cardioprotection conferred by RAS inhibition is due, in part, to apoptosis of a susceptible fibroblast subpopulation that is particularly fibrogenic10. This hypothesis that hypertension produces pathological subsets of resident cardiac fibroblasts that predispose the heart to fibrotic remodeling and adverse events was supported by recent work from McLellan et al3 who evaluated the impact of Ang II infusion on cardiac cell populations. ScRNA-seq analysis identified nine subpopulations of cardiac fibroblasts that included two fibrogenic pools (Cilp+ and Thbs4+) that were expanded following Ang II infusion3. These subpopulations of fibroblasts were associated with ECM and wound healing, thus highlighting them as potential targets for intervention.
Figure 1.
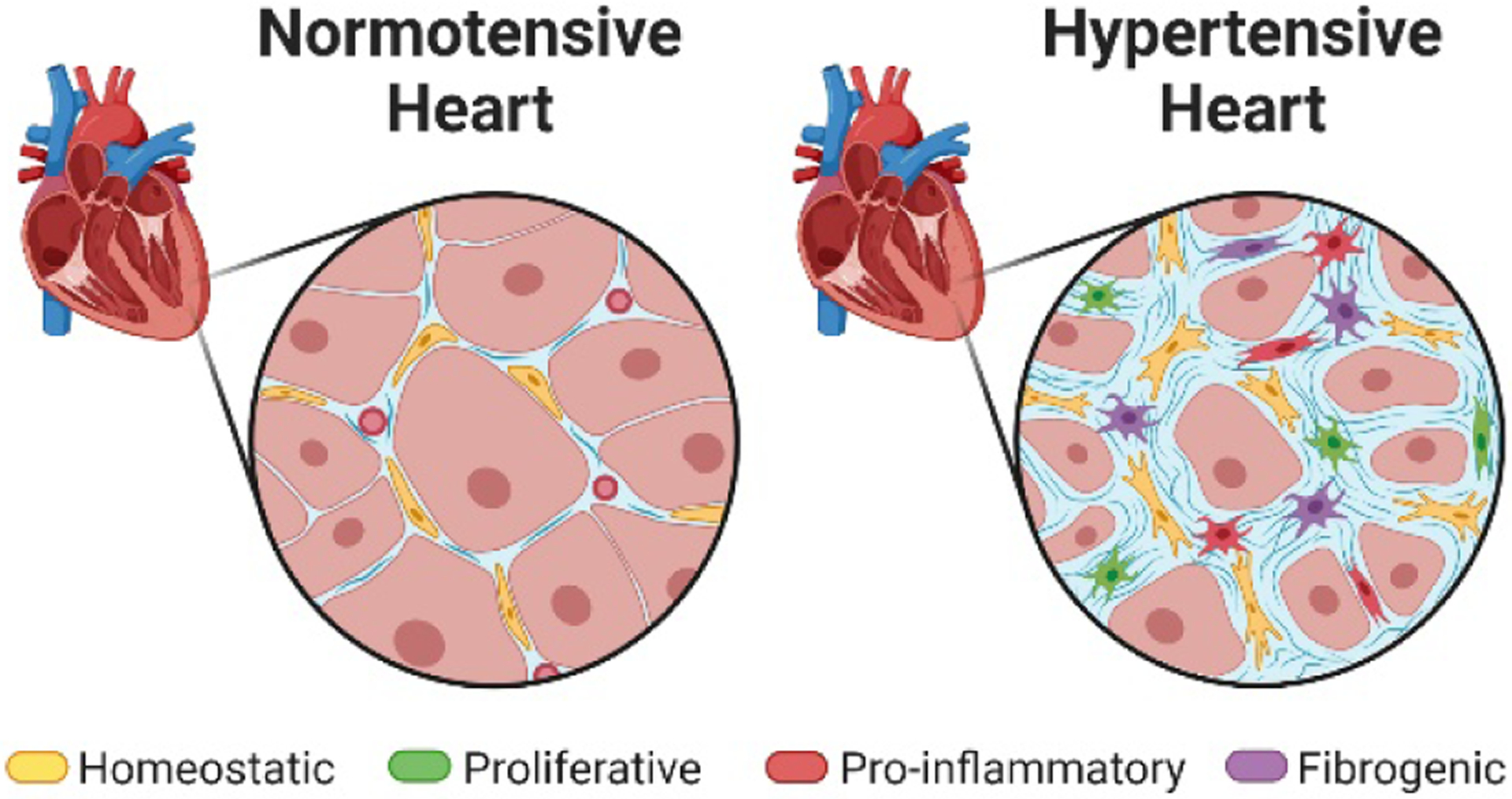
In the hypertensive and hypertrophied heart, resident cardiac fibroblasts undergo hyperplasia and activation resulting in a more fibrogenic phenotype that promotes fibrotic remodeling. Created with BioRender.com.
In conclusion, identification, localization, and evaluation of relative abundance of various subpopulations of fibroblasts in the hypertensive heart is important for developing targeted therapies that remove or inhibit particularly fibrogenic fibroblasts, while still allowing for maintenance of the ECM and appropriate wound healing response after injury. The key to treating or preventing heart failure is not stopping but limiting the extent and duration of cardiac fibroblast activation in the post-MI and pressure overloaded heart. Given the risk that hypertension presents for both increased prevalence of and worsened outcomes following cardiac injury, cardiac fibroblasts in the hypertensive heart are understudied. Using scRNA-seq as a tool for profiling subpopulations of cardiac fibroblasts and understanding the ways in which they are impacted by hypertension would fill important knowledge gaps that will be extremely valuable in the development of novel anti-fibrotic therapies.
Acknowledgements
The authors thank the National Institutes of Health NHLBI HL141165 (TMH); and the American Heart Association 19AIREA34460000 (TMH) and 19POST34410055 (AMG) for research funding.
Footnotes
Declarations of interest: none
Disclosures
None
References
- 1.Yifan C, Fan Y, Jun P. Visualization of cardiovascular development, physiology and disease at the single-cell level: Opportunities and future challenges. Journal of molecular and cellular cardiology. 2020;142:80–92. [DOI] [PubMed] [Google Scholar]
- 2.Gladka MM, Molenaar B, De Ruiter H, Van Der Elst S, Tsui H, Versteeg D, Lacraz GP, Huibers MM, Van Oudenaarden A, Van Rooij E. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation. 2018;138(2):166–180. [DOI] [PubMed] [Google Scholar]
- 3.McLellan MA, Skelly DA, Dona MSI, Squiers GT, Farrugia GE, Gaynor TL, Cohen CD, Pandey R, Diep H, Vinh A, Rosenthal NA, Pinto AR. High-Resolution Transcriptomic Profiling of the Heart During Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and Hypertrophy. Circulation.0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub-Lis K, Ho JW, Nordon RE, Harvey RP. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouton AJ, Ma Y, Rivera Gonzalez OJ, Daseke MJ, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY, Lindsey ML. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic research in cardiology. 2019;114(2):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forte E, Skelly DA, Chen M, Daigle S, Morelli KA, Hon O, Philip VM, Costa MW, Rosenthal NA, Furtado MB. Dynamic Interstitial Cell Response during Myocardial Infarction Predicts Resilience to Rupture in Genetically Diverse Mice. Cell Rep. 2020;30(9):3149–3163.e3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 8.Picariello C, Lazzeri C, Attanà P, Chiostri M, Gensini GF, Valente S. The impact of hypertension on patients with acute coronary syndromes. Int J Hypertens. 2011;2011:563657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza KM, Biwer LA, Madhavpeddi L, Ramaiah P, Shahid W, Hale TM. Persistent change in cardiac fibroblast physiology after transient ACE inhibition. American journal of physiology Heart and circulatory physiology. 2015;309(8):H1346–1353. [DOI] [PubMed] [Google Scholar]
- 10.Hale TM. Persistent phenotypic shift in cardiac fibroblasts: impact of transient renin angiotensin system inhibition. Journal of molecular and cellular cardiology. 2016;93:125–132. [DOI] [PubMed] [Google Scholar]


