Abstract
Fungal diseases contribute significantly to morbidity and mortality in humans. Although recent research has improved our understanding of the complex and dynamic interplay that occurs between pathogenic fungi and the human host, much remains to be elucidated concerning the molecular mechanisms that drive fungal pathogenicity and host responses to fungal infections. In recent times, there has been a significant increase in studies investigating the immunological functions of microbial-induced host cell death. In addition, pathogens use many strategies to manipulate host cell death pathways to facilitate their survival and dissemination. This review will focus on the mechanisms of host programmed cell death that occur during opportunistic fungal infections and explore how cell death pathways may affect immunity towards pathogenic fungi.
Keywords: Programmed cell death, fungal infection, apoptosis, necroptosis, pyroptosis, ETosis
Host cell death and opportunistic fungal diseases
Host cell death is a fundamental defence mechanism that occurs in response to microbial infection. Indeed, biological processes including apoptosis and regulated necrosis are important processes that can influence the outcome of a microbial insult [1]. Pathogens can also benefit from host cell death, as destruction of the cellular niche can facilitate infection of neighbouring cells, evasion of immune cells and acquisition of nutrients. Thus, to maintain their replicative niche within the host, pathogenic microbes (see Glossary) have developed multiple mechanisms to manipulate host cell death and survival pathways [1, 2].
Although investigation of programmed cell death has been undertaken predominantly in the context of viral and bacterial infections, there is evidence suggesting that apoptosis and regulated necrosis (e.g., necroptosis, pyroptosis and Extracellular Trap cell death (ETosis)) play a key role in the interplay between pathogenic fungi and host cells. Fungi are responsible for a wide variety of diseases, ranging from superficial mucosal infection and allergies to life-threatening systemic mycoses. The high incidence of fungal infections is largely attributable to an increase in the number of immunocompromised individuals, including HIV/AIDS patients, cancer patients, and organ transplant recipients [3, 4]. Candida, Cryptococcus, Aspergillus and Pneumocystis species are the most common opportunistic fungal pathogens of humans (Table 1) and cause high rates of morbidity and mortality. Furthermore, treatment options in the clinical setting are limited and the incidence of drug-resistant infections is rising [5]. Of note, during the past decades, significant progress has been made in our understanding of the host immune responses to human pathogenic fungi (Box 1). Thus, further investigation of the pathogenic mechanisms and the complex interplay between pathogenic fungi and host cells may eventually lead to the development of new efficient antifungal treatments. This review will describe the different forms of regulated cell death pathways employed by the host or induced by opportunistic fungi and explore the role of programmed cell death in host responses to the three key human fungal pathogens (Candida, Cryptococcus and Aspergillus).
Table 1.
Common opportunistic human fungal pathogens.
| Fungus | Source | Main virulence traits | Spectrum of diseases |
|---|---|---|---|
| Candida albicans |
Endogenous: part of the normal commensal microbiota of the skin, oral cavity, vagina and GI tract. Exogenous: nosocomial (hands of health care workers, contaminated medical devices). Invasion occurs following iatrogenic breaks of the mucosal barriers. |
- Dimorphism. - Adhesins and invasins. - Candidalysin. - Secreted hydrolases. - Biofilm formation. - Metabolic adaptation. - Thermotolerance. |
- Mucocutaneous infection (oropharyngeal and skin infections, vaginitis). - Hematogenously disseminated infection. |
| Cryptococcus neoformans | Inhalation of spores or dry yeast from environmental sources (soil, pigeon droppings, decaying wood). | - Polysaccharide capsule. - Extracellular vesicles. - Proteases and phospholipases. - Mannitol and melanin production. - Thermotolerance. |
- Pneumonia. - Meningitis. - Hematogenously disseminated infection. |
| Aspergillus fumigatus | Inhalation of ubiquitous spores. | - Melanin, gliotoxin and other secondary metabolites. - Thermotolerance. - Nutrient uptake. - Secreted proteases. |
- Lung diseases. - Hematogenously disseminated infection. |
| Pneumocystis jirovecii | No known environmental reservoir. Propagation within its host, which may serve as the natural reservoir. |
- Specific virulence factors are poorly defined. | - Pneumonia. |
Box 1 -. Protective antifungal immune responses.
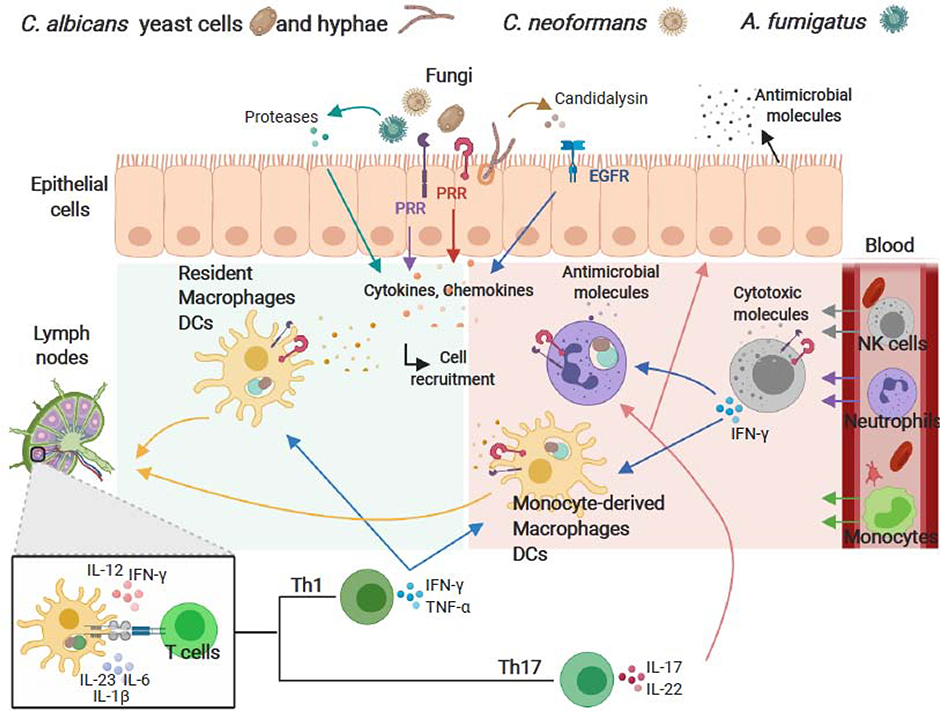
The coordinated action of both innate and adaptive immunity is required for an optimal antifungal host response (Figure I). Impairment of immune responses, breaches in the skin or mucosal barrier, and a disturbance of the host by external or internal factors can promote fungal overgrowth and disseminated infection.
Skin and mucosal surfaces are recognized as the first line of defence against fungi. Epithelial cells play a pivotal role in sensing the presence of fungi and providing signals that activate host defence mechanisms. The interaction between fungi and epithelial cells occurs initially through the recognition of fungal cell wall components and secreted proteases by a variety of pattern recognition receptors (PRRs) (for instance, Toll-like (TLR), NOD-like (NLR) and C-type lectin-like (CLR) receptors). Furthermore, epithelial cells express non-classical receptors (for instance, epidermal growth factor receptor (EGFR)) that can be activated in response to C. albicans hyphal formation and candidalysin secreted from C. albicans hyphae. Following activation, epithelial cells secrete antimicrobial molecules and orchestrate an inflammatory response to activate and recruit myeloid cells. Similarly, following ligand binding, PRRs initiate complex signalling cascades in innate myeloid cells (monocytes, macrophages, neutrophils, dendritic cells (DCs) and natural killer (NK) cells) that culminate in phagocytosis, production of reactive oxygen species (ROS), release of cytotoxic and antimicrobial molecules, cytokines, chemokines, and recruitment of circulating leukocytes. Thus, the interplay between epithelial cells and resident and infiltrating immune cells, acting in concert with effector molecules, provide protection through phagocytosis, growth inhibition and direct fungal clearance. Furthermore, cytokine responses, maturation of antigen presenting cells following fungal uptake and the transport of fungal antigens by DCs to the draining lymph nodes are important in directing adaptive CD4+ T helper (Th) cell responses to fungal pathogens. Th1 responses, characterized by the production of tumour necrosis factor (TNF)-α and interferon (IFN)-γ which promote the activation and the fungicidal activities of phagocytes, are essential for host resistance against the majority of fungal pathogens. Th17-based responses are also important effectors in antifungal immunity, particularly at mucosal surfaces. Th17 cells release interleukin (IL)-17 and IL-22 that promote neutrophil recruitment, prompt epithelial cells to release antimicrobial peptides, and induce barrier repair.
Apoptosis during fungal infections
Apoptosis is a highly complex form of programmed cell death, involving an energy-dependent cascade of molecular and cellular events (Box 2). It represents a vital part of the immune response to pathogens, which leads to the destruction of the intracellular niche of microbial replication. Furthermore, elimination of pathogen-containing apoptotic bodies by secondary phagocytes and presentation of antigens derived from apoptotic material by dendritic cells (DCs) represent important antimicrobial effector mechanisms [6]. Pathogenic fungi have therefore evolved multiple distinct mechanisms for modulating host cell apoptosis. Notably, the demise of key immune effector cells by apoptosis represents a central mechanism to evade host defences and ensure pathogen survival (Figure 1).
Box 2 – Molecular and cellular events of apoptotic cell death.
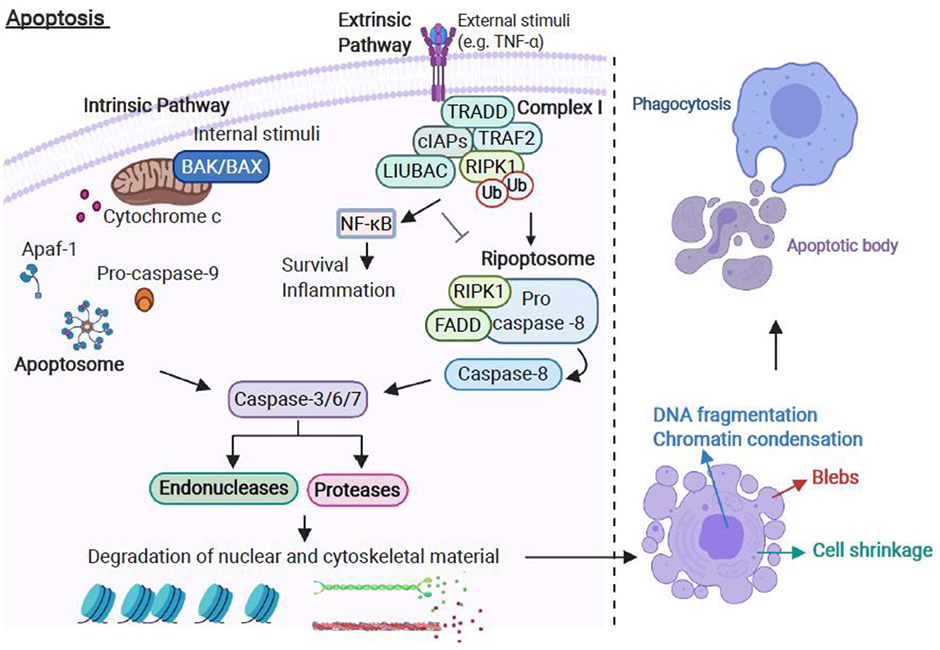
Apoptosis can be initiated through extrinsic and intrinsic pathways (Figure II). The extrinsic pathway is triggered when death ligands bind to death receptors [84]. The best characterised death receptor and its cognate ligand are tumour necrosis factor receptor 1 (TNFR1) and TNFα, respectively [85]. Binding of TNFα to TNFR1 results in recruitment of the adaptor TNFR1-associated death domain protein (TRADD), receptor interacting protein kinase 1 (RIPK1), TNF-receptor-associated factor 2 (TRAF2), cellular inhibitors of apoptosis (cIAPs) and the linear ubiquitin chain assembly complex (LIUBAC), forming Complex I [85]. cIAPs mediate ubiquitination of RIPK1, which stimulates activation of nuclear factor (NF)-κB signalling, thus Complex I favours pro-inflammatory signalling via NF-κB activation [85]. In the absence of cIAPs, Complex I dissociates and a ripoptosome complex composed of RIPK1, Fas-associated protein with death domain (FADD) and pro-caspase-8 forms in the cytosol [86]. The binding between FADD and pro-caspase-8 promotes the autocatalytic activation of pro-caspase-8, potentiating extrinsic apoptosis [86].
Intrinsic apoptosis is induced by numerous triggers (e.g., radiation, hypoxia, free radicals and toxins) that generate intracellular signals through the host mitochondria. All intrinsic apoptotic inducers trigger mitochondrial outer membrane permeabilization (MOMP) and release of pro-apoptotic proteins from the mitochondrial intermembrane space into the cytosol of the cell [87]. Cytochrome c is a major pro-apoptotic protein that binds Apaf-1 and pro-caspase-9 to form the apoptosome complex. Once formed, the apoptosome activates pro-caspase-9 [87]. The intrinsic apoptotic pathway is regulated by members of the B cell lymphoma-2 (Bcl-2) family [88]. The Bcl-2 family of proteins contain pro- and anti-apoptotic factors that control MOMP and hence release of cytochrome c. The ratio of pro- and anti-apoptotic Bcl proteins expressed within a cell determines whether the pro-apoptotic proteins Bcl-2-associated X protein (BAX) and Bcl-2-antagonist/killer (BAK) are activated [88]. Once activated, BAX and BAK oligomerize and form pores to cause MOMP, cytochrome c release and apoptosome formation, leading to caspase-9 activation [88].
Both extrinsic and intrinsic apoptotic pathways converge and activate the execution phase of cell death. The execution phase initiates with the activation of effector caspases (including caspase-3, −6 and −7) that, in turn, activate endonucleases and proteases leading to DNA fragmentation and chromatin condensation, nuclear fragmentation, cell shrinkage, formation of plasma membrane blebs and apoptotic bodies, and the display of phosphatidylserine (PS) on the outer leaflet of the plasma membrane [89]. Externalisation of PS facilitates phagocyte uptake and allows the clearance of apoptotic cells without eliciting an inflammatory response [89].
Figure 1. Induction and manipulation of apoptosis in the host-pathogenic fungi interaction.
(A) Binding of C. albicans secreted aspartyl proteinases (Saps) to host integrin leads to fungal endocytosis and lysosomal permeabilization in epithelial cells. In macrophages, C. albicans phospholipomannan (PLM) induces Bad dephosphorylation, which recruits Bcl-2 and leads to mitochondrial dysfunction and caspase activation. Epithelial damage and manipulation of macrophage apoptosis favour fungal colonization and infection. C. albicans also counteracts host apoptotic cell death by activating the PI3K/Akt survival pathways to facilitate intracellular replication and dissemination. The host-induced anti-apoptotic response likely contributes to host cell/tissue integrity. (B) C. neoformans promotes apoptosis of macrophages and T cells by inducing the expression of FAS/FASL and DR4/TRAIL, thereby evading host defences. (C) A. fumigatus counteracts host apoptosis by activating PI3K/Akt signalling to escape from phagocyte killing and facilitate intracellular replication/dissemination. A. fumigatus gliotoxin induces epithelial apoptosis via JNK signalling, which activates Bim-Bak-dependent mitochondrial apoptotic machinery. Apoptosis-mediated barrier disruption promotes fungal invasion, facilitating A. fumigatus infection. Gliotoxin also induces apoptosis of dendritic cells.
Abbreviations: Bcl-2 associated agonist of cell death (Bad); B cell lymphoma-2 (Bcl-2); cytochrome C (cytC); phosphatidylinositol-3-kinase (PI3K)/kinase B (Akt); Fas-associated protein with death domain (FADD); death receptor 4 (DR4); Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL); c-Jun N-terminal kinase (JNK); Bcl-2 interacting mediator of cell death (Bim); Bcl-2-antagonist/killer (BAK).
Apoptosis and Candida
The induction or manipulation of apoptosis by Candida albicans has been reported in both immune and non-immune cells (Figure 1). For instance, endocytosis of C. albicans (and not of the non-pathogenic yeast Saccharomyces cerevisiae) and phospholipomannan, a sphingolipid that is present on the cell-wall surface of C. albicans yeast cells and shed upon contact with host cells, were observed to induce apoptosis of murine macrophages [7]. Although macrophages phagocytose and rapidly kill S. cerevisiae [8], treatment of macrophages with C. albicans phospholipomannan together with S. cerevisiae promoted yeast survival [7]. This suggests that phospholipomannan-induced apoptosis following C. albicans endocytosis may favour fungal escape from macrophage killing, although further experiments (for instance, the inhibition of the apoptotic pathway prior to Candida infection) are needed to specifically address this issue. Similarly, mucosal lesions that result from the apoptosis of epithelial cells may destroy epithelial immunity and promote fungal pathogenesis. Consistently, the glycan moieties and secreted aspartyl proteases (Saps) of C. albicans trigger apoptosis in oral and lung epithelial cells [9, 10].
Transcriptomic and proteomic data also reveal the up-regulation of anti-apoptotic genes and the activation of phosphatidylinositol-3-kinase (PI3K)/kinase B (Akt) survival pathway in both epithelial cells and macrophages in response to C. albicans [11, 12]. However, whether anti-apoptotic mechanisms are induced by the fungus or the host is unclear. Indeed, while the maintenance of epithelial barrier integrity plays an important role in defence against invading pathogens, fungi may inhibit host apoptotic signalling to promote their replication and dissemination.
More recently, IL-23 has been shown to play a critical role in the protection against systemic candidiasis by preventing myeloid cell death via apoptosis, independently of the IL-23/IL-17 axis or lymphoid cells [13]. Indeed, a higher fungal load in the kidneys and the rapid loss by apoptosis of the myeloid inflammatory infiltrates, which critically contribute to fungal control, were observed in IL-23−/− compared to wild type mice following C. albicans infection. However, similar findings were not observed when mice were infected with a yeast-locked mutant of C. albicans. Similarly, no difference in the viability of neutrophils isolated from IL-23 pathway-sufficient and -deficient mice was observed after in vitro challenge with viable C. albicans hyphae [13]. Together, these results suggest that a strong inflammatory environment during the course of systemic infection with virulent C. albicans is required for the IL-23-dependent protection from myeloid cell apoptosis. However, whether IL-23 induces a pro-survival signal or negatively regulates factors that promote apoptosis of myeloid cells remains to be determined.
Apoptosis and Cryptococcus
Cryptococcus neoformans can destroy host immune cells by extrinsic and intrinsic apoptotic pathways as an immune evasion strategy (Figure 1). The capsule of C. neoformans mediates the inhibition of T cell responses by inducing apoptosis both in vitro and in vivo. Glucuronoxylomannan (GXM), the principal constituent of the C. neoformans capsule, suppresses T cell-mediated immune responses through the induction of FAS ligand (FASL) on macrophages, which in turn triggers FAS-mediated apoptosis of T cells through the cooperation of both caspase-8 and −9 pathways [14]. Unlike GXM, Galactoxylomannan (GalXM) can mediate direct cytotoxicity to T lymphocytes through the up-regulation of FAS/FASL and caspase-8 activation, culminating in apoptotic cell death [15]. Moreover, GalXM induces immunological paralysis in mice by apoptotic ablation of B lymphocytes [16].
Interaction of C. neoformans with macrophages is also crucial in the pathogenesis of cryptococcosis, and regulation of apoptosis in innate immune cells by infecting fungus has been demonstrated to occur via different mechanisms. GXM and GalXM induce apoptosis through a FAS/FASL interaction in murine macrophages [17]. More recently, an NF-κB-dependent induction of extrinsic and intrinsic apoptosis in macrophages was demonstrated in mice infected with C. neoformans [18]. Together, these reports suggest that apoptosis of innate and adaptive immune cells by capsular polysaccharides represents a pivotal mechanism used by C. neoformans to inhibit the host immune response.
Apoptosis and Aspergillus
Manipulation of host cell apoptosis has also been reported during infections with Aspergillus fumigatus (Figure 1). Alveolar phagocytes and airway epithelial cells constitute the first lines of defence against inhaled A. fumigatus conidia and apoptosis of infected cells is crucial to prevent the spread of the infection. Notably, inhibition of apoptosis and activation of the PI3K/Akt survival pathway in response to the conidial component dihydroxynaphthalene (DHN)-melanin have been demonstrated in bothhuman lung epithelial cells and macrophages [19, 20]. Furthermore, DHN-melanin enhances the survival of conidia against phagocytes [19, 20], thus providing evidence of a potential correlation between the inhibition of apoptosis and the pathogenesis of A. fumigatus.
A number of studies have highlighted the role of gliotoxin, a secondary metabolite produced during hyphal growth, in the induction of apoptosis during aspergillosis [21–23]. Gliotoxin concentrations below those typically found in patients with invasive aspergillosis induced caspase-3 activation and apoptosis of human dendritic cells [22]. Apoptosis of antigen presenting cells may thus contribute to the impairment of antifungal defence during Aspergillus infections. Subsequent work elucidated the biochemical pathway involved in gliotoxin-induced apoptosis in mice and humans. JNK-dependent phosphorylation of Bid at three different sites drives Bak activation, which in turn triggers apoptosis in fibroblasts and lung epithelial cells upon gliotoxin stimulation [21, 23]. Importantly, Bak knockout mice are less susceptible to A. fumigatus infection, providing compelling evidence that gliotoxin-induced apoptosis plays an important role in the pathogenesis of invasive aspergillosis [23].
Significance of apoptosis during opportunistic fungal infections
To date, data support an important role for apoptosis against microbial infections [24]. Candida, Cryptococcus and Aspergillus seem to employ common strategies to manipulate this important pathway to benefit their own survival. The three opportunistic pathogens hijack the host apoptotic machinery to induce the death of immune effector cells and evade host defence. Toxin (e.g., gliotoxin in Aspergillus infection), secreted proteases (e.g., Saps in Candida infection) or other virulence factors (e.g., capsular constituents in Cryptococcus infection) appear capable of directly activating the host apoptotic machinery. However, C. albicans and A. fumigatus can also activate a PI3K/Akt survival pathway and promote host cell survival. This observation is reminiscent of pro-survival and anti-apoptotic mechanisms that have been described in the context of viral [25] and bacterial infections [26, 27], which appear central to pathogenesis. Therefore, not only viruses and obligate intracellular bacteria, which are highly dependent on host cell survival, but many other human pathogens activate cell survival pathways in order to maintain their replicative compartment, further suggesting that this may represent a more widespread strategy. C. albicans and A. fumigatus may activate PI3K/Akt and inhibit apoptosis in order to provide a protective niche for yeast cells/conidial survival and dissemination within the host. Furthermore, both fungi possess the ability to form filamentous hyphae, which represents the main virulence factor associated with the pathogenesis of Candida and Aspergillus diseases. Since yeast cells and conidia may be more susceptible to host defence mechanisms than hyphae, it might also be speculated that activation of survival pathways and inhibition of host apoptosis may provide temporary protection against phagocyte killing thus allowing the fungus to filament and escape from immune surveillance.
Necroptosis during fungal infections
Necroptosis is a pro-inflammatory necrosis-like programmed cell death pathway (Box 3) that is critical for immune activation following injury. Necroptosis can be triggered by ligation of specific cell surface receptors, toxins, excessive ROS and numerous cellular stresses [28]. However, whether necroptosis favours anti-microbial immunity or is detrimental to the host has yet to be clarified and is consequently under intense investigation [28]. Recent studies have addressed the role of necroptosis in Candida and Aspergillus infection (Figure 2). In contrast, evidence for the induction of necroptosis and its role during Cryptococcus infection is still lacking. Internalization of C. neoformans by brain endothelial cells was observed to lead to cell stress, loss of plasma membrane integrity and inflammation, which may be indicative of necroptotic cell death [29]. However, further analysis with the use of biochemical biomarkers for necroptosis or specific inhibitors is required to confirm whether Cryptococcus may use necroptosis to induce endothelial cell injury to favour its migration across the blood-brain barrier. More recently, RIPK3 was reported to play an important role in the protection of mice against cryptococcal infection [30]. RIPK3 but not MLKL-deficient mice exhibited higher mortality than wild type controls following pulmonary infection with C. neoformans due to an excessive accumulation of neutrophils, an over exuberant inflammatory response, and lung injury. Moreover, no differences in the level of cellular damage were observed between macrophages isolated from Ripk3−/− and wild type mice, following in vitro cryptococcal challenge [30]. These results confirm that RIPK3 play an important role in pulmonary immune responses to cryptococcal infection, although the mechanism of action appears to be independent of necroptosis.
Box 3 – Programmed necrosis pathways: necroptosis, pyroptosis and ETosis.
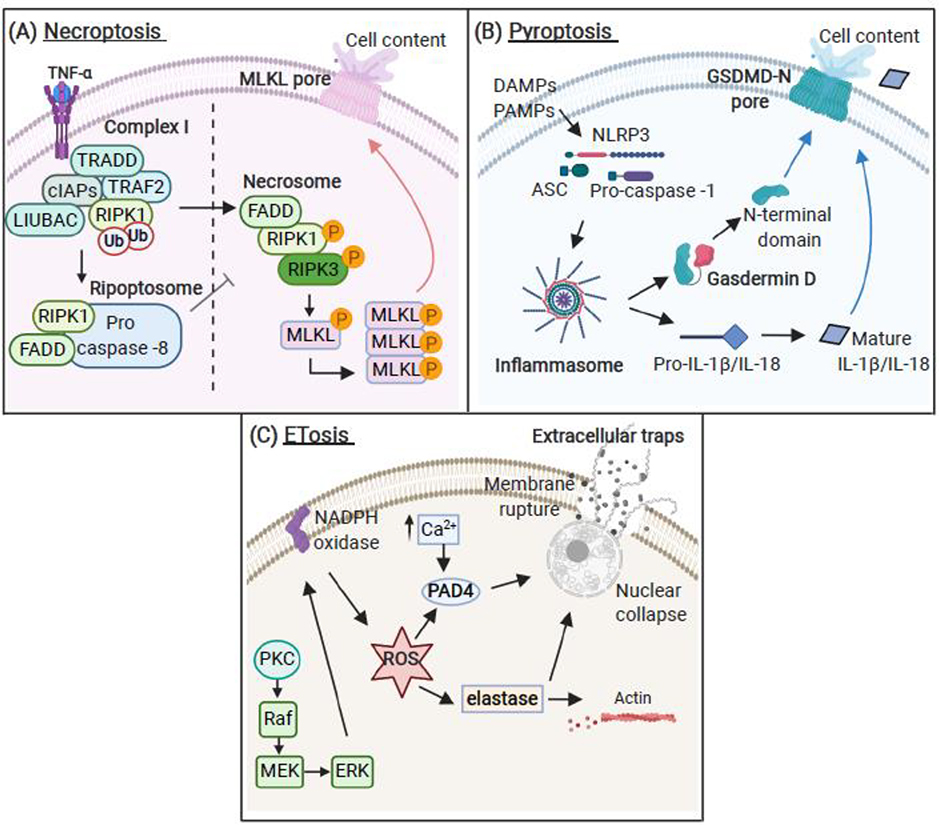
The majority of necroptosis research has focussed on TNF-α signalling (Figure IIIA). Necroptosis initially proceeds identically to extrinsic apoptosis [85]. However, in the absence of active caspase-8, RIPK1 dissociates from the ripoptosome to form the necrosome complex with receptor interacting protein kinase 3 (RIPK3) [28]. Caspase-8 cleaves and inactivates RIPK1 [90] and RIPK3 [91], thus acting as a negative regulator of necrosome assembly. RIPK1 and RIPK3 interact through RIP homotypic interaction motifs [92], which trigger auto- and trans-phosphorylations events between RIPK1 and RIPK3, resulting in necrosome activation [93]. RIPK3 phosphorylation is important for recruitment and phosphorylation of the mixed lineage kinase domain-like protein (MLKL). MLKL phosphorylation stimulates its oligomerisation and insertion into the plasma membrane causing permeabilization and cell death [94].
Pyroptosis is activated through inflammasomes, which serve as intracellular signalling platforms [39] (Figure IIIB). Assembly of the NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome, in response to pathogen- or damage-associated molecular patterns (PAMPs/DAMPs), involves recruitment of the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase‐1, resulting in caspase-1 activation [95]. Caspase-1 cleaves the immature pro-inflammatory cytokines pro-IL-1β and pro-IL-18, which are secreted in their active form [95]. Another recently discovered inflammasome target is the pore-forming protein gasdermin D (GSDMD), which is required for the execution of pyroptotic cell death [96]. Caspase-1 cleaves GSDMD, releasing the GSDMD N-terminus, which translocates to the inner leaflet of the plasma membrane and forms membrane pores, thereby dissipating cellular ionic gradients and resulting in lytic cell death [97].
The mechanisms regulating ET release predominantly derives from neutrophil studies (Figure IIIC). Suicidal NETosis can be initiated through the activation of the protein kinase C (PKC)/rapidly accelerated fibrosarcoma (Raf)/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway, which mediates ROS production through the activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [98]. ROS induce the release of neutrophil elastase (NE) and other proteases from azurophilic granules in a myeloperoxidase (MPO)-dependent manner [99]. NE induces actin degradation and then translocates to the nucleus, where it promotes proteolysis of nuclear histones and chromatin decondensation [99]. Studies also suggest that peptidylarginine deiminase 4 (PAD4)-mediated posttranslational citrullination of histones is required for chromatin decondensation, nuclear collapse and ET release [100]. Finally, disintegration of the nuclear envelope and a mixing of granular proteins into the decondensed chromatin network precede the rupture of the plasma membrane and the release of ETs into the extracellular milieu [75].
Figure 2. Induction and manipulation of programmed necrosis by human fungal pathogens.
Human pathogenic fungi induce regulated necrosis through necroptosis, pyroptosis and ETosis. (A) C. albicans triggers necroptotic RIPK1/RIPK3/MLKL signalling in macrophages via dectin-1 and CARD9. A. fumigatus induces a calcineurin-dependent cell death, which exhibits hallmarks of necroptosis and favours the lateral transfer of germinating conidia to bystander macrophages (metaforosis). (B) Phagocytosis-induced C. albicans cell wall remodelling in macrophages drives NLRP3/ASC/caspase-1 inflammasome activation and pyroptotic cell death. While the fungus evades macrophage-mediated immune responses, pyroptotic inflammation drives immune cell recruitment (e.g., neutrophils). Internalization of opsonized C. neoformans by dendritic cells induces pyroptosis via canonical NLRP3/ASC/caspase-1 or non-canonical NLRP3/ASC/caspase-8 inflammasome activation.
Caspase-1 negatively regulates non-canonical inflammasome activation, which is activated in the absence of active caspase-1. (C) NETosis: C. albicans cell wall components and secreted molecules activate neutrophil receptors (CD11b, CD11a, CR3, Dectin-1/2, CD14 and TLRs) to induce NET release. A. fumigatus-induced NETosis is predominantly mediated by the CR3 receptor. NETosis occurs via NADPH oxidase- and/or PAD4-dependent (full arrows) or independent (dashed arrows) mechanisms. NETs mediate potent antifungal activity against C. albicans and A. fumigatus. C. neoformans cell wall polysaccharides inhibit NETosis, which prevents fungal killing. MoET/METosis: C. albicans activates ETosis in monocytes and macrophages (MoETs/METs). MoET release occurs via NADPH and PAD4-dependent mechanisms, while MET release appears to occur independently of NADPH oxidase activity. EETosis: A. fumigatus activation of eosinophil CD11b triggers EET release via Syk- and PAD4-dependent but NADPH oxidase-independent mechanisms.
Abbreviations: caspase recruitment domain-containing protein 9 (CARD9); receptor interacting protein kinase 1 (RIPK1); receptor interacting protein kinase 3 (RIPK3); mixed lineage kinase domain-like protein (MLKL); nod-like receptor pyrin domain containing 3 (NLRP3); adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC); complement receptor 3 (CR3); cluster of differentiation 11b (CD11b); toll-like receptors (TLRs); neutrophil extracellular traps (NETs); nicotinamide adenine dinucleotide phosphate (NADPH); peptidylarginine deiminase 4 (PAD4); monocytes extracellular traps (MoETs); macrophage extracellular traps (METs); spleen tyrosine kinase (Syk); eosinophils extracellular traps (EETs).
Necroptosis and Candida
The β-glucan receptor dectin-1 was recently established in the induction of necroptosis in response to C. albicans [31]. Live C. albicans triggered the RIPK1/RIPK3/MLKL necroptotic cell death pathway in human and murine macrophages in the absence of caspase-8 activity. In addition, necroptotic cell death occurred independently of autocrine TNF-α, but instead was activated by the adaptor molecule caspase recruitment domain-containing protein-9 (CARD-9) upon binding of C. albicans to dectin-1. Importantly, mice lacking RIPK1, RIPK3 or MLKL showed increased susceptibility to C. albicans infection (decreased survival and increased fungal load in kidneys, livers and spleens) compared with wild-type controls [31], suggesting that necroptosis contributes to host defence against Candida.
Necroptosis and Aspergillus
A recent study elegantly demonstrated the lateral transfer of germinating A. fumigatus between macrophages in a process termed “metaforosis” [32]. Metaforosis of A. fumigatus occurred in macrophages that exhibited hallmarks of necroptosis [33]. Pre-incubation of macrophages with a pan-caspase inhibitor (an inhibitor of caspase-dependent apoptosis that increases necroptosis) caused an increase in the amount of A. fumigatus metaforosis. In contrast, pre-incubating macrophages with necrostatin-1 (specific inhibitor of necroptosis) was observed to inhibit both cell death and metaforosis of conidia [33]. Importantly, necroptotic-like macrophages that transferred conidia to neighbouring cells had a greater capacity to control the germination of conidia compared to dying macrophages where conidia were not transferred. Metaforosis was observed to occur in monocyte-derived macrophages and human alveolar macrophages isolated from bronchoalveolar lavage (BAL) fluid and was calcineurin-dependent [33]. Therefore, necroptotic-like programmed cell death may represent a mechanism to control fungal growth and dissemination, which may be impaired in transplant patients treated with calcineurin inhibitors.
Sensitization to A. fumigatus increases the risk for allergic airway diseases, including severe asthma [34], which are associated with the activity of the pro-inflammatory alarmin IL-33 [35]. Recently, the release of a biologically active IL-33 precursor was observed in vitro following stimulation of human keratinocyte and murine fibrosarcoma cell lines with TNF-α, a second mitochondrial-derived activator of caspases (SMAC) mimetic and a pan-caspase inhibitor, which are known to trigger necroptosis. Accordingly, necroptosis was shown to exacerbate airway inflammation in a mouse model of Aspergillus extract-induced asthma [36]. Indeed, when pre-treated with the necroptosis inhibitor GW80, Aspergillus sensitized asthma mice exhibited reduced levels of IL-33 in BAL fluid and a decrease in the recruitment of eosinophils and CD4+ T cells to the bronchoalveolar space [36]. However, whether Aspergillus infection may induce necroptosis in epithelial cells or fibroblasts has yet to be demonstrated definitively.
Significance of necroptosis during opportunistic fungal infections
While recent evidence highlights necroptosis as an important defence mechanism against viral infections, it does not support a prominent role for necroptosis against bacterial infections [1]. Similarly, to date, only C. albicans and A. fumigatus have been demonstrated to trigger necroptosis in macrophages, which appear to exert antifungal activity. However, while an in vivo study has confirmed a protective role of necroptosis against Candida infection, further studies using knockout mice are required to determine its role in Aspergillus infection. Furthermore, it is important to note the pro-inflammatory functions of necroptosis and the contribution of different cell types in the in vivo setting. For instance, while necroptotic macrophages may be important in controlling Aspergillus growth and dissemination, the release of pro-inflammatory mediators from immune or non-immune cells following necroptosis may drive pathological inflammation and exacerbate Aspergillus-associated respiratory disorders. In contrast, current in vivo data does not support a role for necroptosis during Cryptococcus infection. RIPK3, but not the executioner of necroptosis MLKL, provides protection against Cryptococcus, and this may be due to the existence of necroptosis independent-inflammatory and immune functions of RIPK3 [37, 38]. This also highlights the importance of inactivating multiple necroptosis components before attributing a role to necroptosis in disease.
Pyroptosis during fungal infections
Similar to necroptosis, pyroptosis is an inflammatory form of regulated lytic cell death [39]. However, unlike necroptosis, pyroptosis is induced by inflammasome activation (Box 3). Recently, increasing evidence has suggested a critical role for pyroptosis in mediating immunity to infections and clearance of pathogens, particularly in the context of bacterial diseases [1, 39]. Of note, fungal pathogens can stimulate inflammasome assembly [40] and inflammasome activation leads to pyroptosis in the context of Candida and Cryptococcus infection (Figure 2).
Pyroptosis and Candida
Our current understanding of fungal-induced activation and modulation of pyroptosis is based largely on studies using human cell lines and mice in response to Candida infection (Figure 2). Murine macrophages undergo pyroptosis in response to Candida species capable of altering their morphology from yeast to filamentous cells [41]. Candida-induced pyroptosis was shown to be dependent on NLRP3/ASC/Caspase-1 by the use of pharmacological inhibitors and macrophages isolated from caspase-1 and ASC deficient mice [41]. Similarly, the use of time-lapse microscopy also demonstrated that pyroptosis occurs at early time points following phagocytosis of C. albicans by macrophages, and accounts for around 20–30% of macrophage cell death in vitro [42]. Notably, both studies showed that mutant C. albicans strains lacking functional genes involved in the regulation of ergosterol biosynthesis (upc2Δ/Δ) or in the control of morphogenesis and cell wall integrity (srb9Δ/Δ), but still able to form filaments, induced less pyroptotic cell death than the wild-type strain following macrophage challenge [41, 42]. These observations suggest that fungal filamentation per se is not the sole driver of pyroptosis and some additional biophysical/biochemical features of hyphae are required to fully activate pyroptotic cell death. More recently, the importance of hyphae as a key virulence determinant in the induction of pyroptosis has been shown following infection of macrophages with C. albicans isolates originating from different body sites in patients and healthy individuals [43]. In this study, C. albicans-induced NLRP3-dependent pyroptosis correlated with the ability of the clinical isolates to form hyphae [43]. In addition, C. albicans ahr1Δ/Δ and stp2Δ/Δ mutants that are incapable of neutralizing macrophage lysosomal acidification and switching from yeast to hyphae fail to trigger pyroptosis [44]. Notably, the transcription factor Ahr1p controls the expression of ECE1 [45] from which the hypha-associated peptide toxin candidalysin is produced [46], and the transcription factor Stp2p controls the uptake and utilization of amino acids [47]. However, whether macrophage pyroptotic cell death occurs in response to lysosomal dysfunction or because of hypha-associated factors remains to be determined. The importance of hyphal morphogenesis in the induction of pyroptosis has recently been placed under scrutiny [48, 49]. Indeed, analysis of a C. albicans mutant library identified strains that are defective in hyphal development but still capable of inducing pyroptosis [48, 49]. Cell wall remodeling and exposure of glycosylated proteins rather than filamentation, were thought to be essential for fungal-induced pyroptosis upon internalization by host macrophages [48, 49]. Furthermore, fungal activation of TLRs and CLRs was necessary for the induction of IL-1β and NLRP3 transcripts (priming step) but not sufficient to trigger inflammasome activation (activation step) and pyroptosis. In contrast, Candida cell wall remodeling, in the absence of phagolysosomal rupture, was dispensable for the priming step but crucial for inflammasome activation and pyroptosis, thus suggesting that priming and activation can be decoupled in response to C. albicans [49]. However, how the signal moves from the phagolysosome to cytosolic NLRP3 remains unknown.
Pyroptosis and Cryptococcus
The mechanism of pyroptotic cell death in response to C. neoformans infection has recently been elucidated in DCs (Figure 2) [50]. Phagocytosis of opsonized or acapsular C. neoformans can trigger either the canonical NLRP3/ASC/caspase-1 or non-canonical NLRP3/ASC/caspase-8 inflammasome in DCs. More specifically, activation of the non-canonical inflammasome accounts for IL-1β release by DCs in the absence of caspase-1. Similarly, C. neoformans was observed to induce pyroptosis preferentially through a caspase-1-dependent mechanism in wild-type cells. However, the fungus also possesses the ability to trigger pyroptosis through the non-canonical/caspase-8 inflammasome in DCs lacking caspase-1 [50]. Furthermore, as observed for C. albicans, remodeling of the acapsular C. neoformans cell surface following phagocytosis is a key driver of pyroptotic cell death in macrophages [48]. However, the precise role of pyroptosis during Cryptococcus infection is still unknown.
Significance of pyroptosis during opportunistic fungal infections
Although growing evidence demonstrates that C. albicans and C. neoformans trigger pyroptosis, there is no direct proof ascribing precise functions for pyroptosis during fungal infection. However, macrophages derived from casp-1−/− and casp-11−/− mice were more resistant to killing by C. albicans following early infection, suggesting that pyroptotic cell death may provide an escape route for the pathogen [42]. In vitro infection of macrophages with C. albicans in the presence of potassium, which inhibited ASC speck formation (used as a readout of inflammasome activation and pyroptosis), also correlated with decreased fungal CFU levels [49]. Furthermore, reduced neutrophil recruitment was observed in the kidneys of mice infected with C. albicans strains that did not induce ASC speck formation and pyroptosis [49]. However, whether C. albicans activates pyroptosis to escape from macrophage-mediated immune surveillance and/or whether pyroptosis represents an important host defense mechanism that stimulates the recruitment of immune effector cells, remains unknown and largely speculative. Similarly to other microbial infections [1], an accurate analysis of the role of pyroptosis during fungal infections may be limited by the presence of confounding factors when using in vivo knockout models (for example inflammasome, caspase-1 or IL-1β/IL-18-deficient mice). Indeed, inflammasome activation and IL-1β/IL-18 secretion greatly contribute in a variety of protective host responses against pathogenic fungi [40]. Likewise, inactivation of GSDMD inhibits pyroptosis but also limits the release of IL-1β/IL-18. Thus, we still have a limited understanding of the role of pyroptosis during infections, although emerging findings, mainly derived from engineered bacterial models [1], seem to support a direct anti-microbial role for pyroptosis during bacterial infections.
ETosis during fungal infections
ETosis is a recently identified programmed cell death pathway characterised by the active release of extracellular traps (ETs), comprising a network of chromatins (DNA) attached to antimicrobial peptides and enzymes [51]. The release of ETs was first observed with neutrophils (NETs) [52] but more recently, ETs have also been reported in mast cells (MCETs) [53], eosinophils (EETs) [54], basophils (BETs) [55] and monocytes/macrophages (MoETs/METs) [56, 57]. Diverse pathogens and chemicals trigger the release of ETs and multiple pathways are emerging as regulators of ETosis (Box 3). Although a primary role for ETosis appears to be the entrapment and destruction of pathogens [58], the release of ETs is linked to the pathogenesis of several human diseases [58], and its precise function is still a matter of debate. Irrespective, human fungal pathogens induce the release of ETs from neutrophils, macrophages, monocytes and eosinophils (Figure 2).
ETosis and Candida
While the killing of C. albicans yeast cells by phagocytes occurs predominantly via phagocytosis and oxidative burst mechanisms, large hyphae can be more efficiently trapped and killed by NETosis [59]. Phagocytosis negatively regulates NETosis, thus avoiding unnecessary NET release when the pathogen is still small enough to be engulfed [59]. However, whether NETosis is induced exclusively by hyphae, or by hyphae and yeast cells remains controversial [59–63]. Similarly, ROS-dependent [62, 64] and -independent [60, 64, 65] mechanisms of NETosis have also been reported. Several C. albicans cell wall components such as β-glucan, mannans, cell wall-tethered Sap9p/10p and secreted Sap4p/6p serve as potent inducers of NETosis [64, 65]. Most importantly, in vivo studies with mice lacking key regulatory molecules of NETosis clearly show the importance of NET release in protection against C. albicans infection [59, 60, 66]. For instance, unlike wild-type mice, MPO deficient mice (incapable of NET formation) succumbed to C. albicans infection [59]. Furthermore, injection of the PAD4 inhibitor GSK484 in a C. albicans peritonitis model was observed to favour the spread of C. albicans from the peritoneal cavity to the kidneys [60]. Urban et al., demonstrated that calprotectin (a cytoplasmic dimer of calcium-binding proteins S100A8 and S100A9) is released from neutrophils and tightly binds to NETs upon C. albicans infection, both in vitro and in vivo [66]. The potent antifungal property of NET-bound calprotectin was highlighted during pulmonary and subcutaneous C. albicans infection. While wild-type mice survived C. albicans infections, S100A9 knockout mice succumbed to pulmonary candidiasis with a concomitant spread of infection from subcutaneous to apical skin layers, thus confirming the pivotal antifungal activity of NET-associated calprotectin during C. albicans infections [66].
It was recently demonstrated that ETs released by eosinophils following stimulation with the common NET inducer phorbol 12-myristate 13-acetate (PMA) captured C. albicans in vitro [67]. C. albicans also induced the release of ETs containing DNA, histones, lysozyme and MPO from murine J774A.1 and peritoneal macrophages in a NADPH-independent fashion. These METs efficiently captured the fungus but did not exhibit effective microbicidal activity [68]. More recently, however, a higher percentage of MET formation was observed in murine macrophages following challenge with both heat-killed and live C. albicans when the multiplicity of infection was increased [57]. Interestingly, degradation of MET-associated DNA using DNase significantly reduced fungal killing, indicating that METs contribute to fungal eradication and the secretion of DNase by C. albicans may represent an important immune escape mechanism [57]. Furthermore, the antifungal activity of ETosis was confirmed in human monocytes [56]. MoETs, primarily composed of MPO, elastase, citrullinated histone h3 and lactoferrin, were extruded onto the surface of C. albicans to immobilize the fungus and reduce fungal growth [56].
ETosis and Cryptococcus
A recent study has shown that only an acapsular strain of C. neoformans lacking the polysaccharide GXM induces NET release with fungicidal activity through a ROS- and PAD4-dependent mechanism [69]. In contrast, wild-type yeast and GXM, which fail to trigger NET formation, also inhibit NETosis upon stimulation of neutrophils with PMA [69]. Inhibition of NETosis may, therefore, represent an important mechanism of Cryptococcus escape from immune surveillance (Figure 2).
ETosis and Aspergillus
While NETosis represents an important mechanism in C. albicans hyphal killing, several independent observations suggest that NET release by human and murine neutrophils in response to A. fumigatus does not contribute to fungal killing. Rather, NET release represents a defence mechanism used by the host to inhibit conidial germination, trap the fungus, reduce fungal growth and, ultimately, confine the infection [70–72] (Figure 2). NETosis of human neutrophils occurs similarly in response to A. fumigatus and the less pathogenic species A. nidulans, although the latter show more susceptibility to NET-mediated fungal killing [73]. A. fumigatus produces higher amounts of cell wall-associated galactosaminogalactan (GAG), a virulence factor composed of varying amounts of galactose and N-acetyl-galactosamine (GalNAc) [73]. Of note, A. nidulans overexpressing GalNAc shows enhanced resistance to NETs and virulence comparable to that of A. fumigatus, suggesting that cell wall-bound GAG enhances the resistance of A. fumigatus to NETosis-induced fungal damage [73]. Similar to C. albicans [64, 65], complement receptor 3 (CR3: CD11b/CD18) plays an important role in A. fumigatus recognition and NET release [71, 74]. In contrast, although ROS-dependent and -independent mechanisms have been reported for C. albicans-induced NETosis, both in vivo and in vitro studies support the role of NADPH activity for NET induction during Aspergillus infection [71, 74]. This finding is corroborated by studies with neutrophils from Chronic Granulomatous Disease (CGD) patients with impaired NADPH oxidase function, which do not form NETs following challenge with Aspergillus [73, 75]. Importantly, complementation of NADPH function by gene therapy in CGD patients restores NET formation and leads to the control of fungal growth [76, 77].
More recently, EETs were identified in bronchial secretions obtained from patients with allergic bronchopulmonary aspergillosis (ABPA) [78]. Notably, A. fumigatus was observed to induce the release of EETs from human eosinophils in vitro through the activation of CD11b and Syk in a NAPDH-independent fashion. However, EETs did not contribute to A. fumigatus killing [78]. Whether A. fumigatus-induced EETs are linked with immune protection or contribute to the pathogenesis of ABPA is currently unknown.
Significance of ETosis during opportunistic fungal infections
ETosis appears to be an effective defence mechanism employed by immune cells to protect the host against opportunistic fungal pathogens. Candida, acapsular Cryptococcus and Aspergillus induce ET release with fungicidal activity by a number of immune cells. Furthermore, capsular polysaccharides (Cryptococcus infection), or secreted DNAses and cell wall components (Candida and Aspergillus infection) likely target ET formation and activity, respectively, to minimize the antifungal ET effects and evade host immune defences. Therefore, the exploration and identification of other virulence factors targeting ETosis would be helpful to further understand the molecular basis of fungal pathogenesis. However, although ETs may be critical against fungal infections, it is less clear how ETs contribute to antibacterial and antiviral immunity [1, 58]. Furthermore, ET release is associated with an increasing number of pathological conditions and there are examples, mainly in the context of viral infections, where ET release results in immunopathology [1, 58]. Although ET structures have been identified in patients with ABPA, whether fungal-induced ETosis contributes to inflammatory diseases is still largely unknown and is thus an important direction for future research.
Concluding remarks
Pathogen-induced cell death may occur by a variety of complex mechanisms including apoptosis, necrosis, necroptosis, pyroptosis and ETosis. Host–pathogen interactions and their role in cell death are highly complex, involving a fine balance between pro- and anti-death strategies for both host and pathogen. The study of fungal-induced programmed host cell death has gained much attention with the recognition that this phenomenon may not be an incidental occurrence during infection, but rather, a controlled process with significant implications for disease pathogenesis and host responses. The outcome of host cell death in shaping immune responses in the context of fungal infection appears to depend greatly on several variables such as host cell type, fungal species and strains, specific fungal moieties and secreted molecules. Future detailed investigations will better elucidate the specific host cell death protective mechanisms as well as the spectrum of strategies used by pathogenic fungi to manipulate host cell death and survival (see Outstanding Questions). Large-scale screenings of mutants will help to identify specific virulence determinants required to activate/manipulate host cell death mechanisms and establish infection. Inactivation of these virulence factors will also improve our understanding of the precise function of regulated cell death during fungal infection. Although much effort has been focused on studying programmed cell death in mononuclear phagocytes, opportunistic fungi can disseminate into many tissues and interact with various cell types, which can differently react to the pathogens. Moreover, lytic forms of programmed cell death, if not tightly regulated, can lead to chronic inflammation and disease. Understanding whether and how fungal-induced regulated necrosis drives inflammatory conditions is key to developing effective interventions to preserve health and combat diseases. Towards this goal, the identification of fungal strains that fail to manipulate regulated necrosis pathways, the use of many different types of animal models of fungal infection, and pharmacological inhibitor/genetic deletion of key host cell death regulators will provide important features to uncover associations between opportunistic fungi and inflammatory diseases. To date, the scarce knowledge in mechanistic and precise host cell death molecular pathways that are manipulated by fungal pathogens certainly represents an important limitation. In this regard, integrated approach that combine transcriptomics, phosphoproteomics and genetic or chemical inactivation may aid the identification of host molecular targets which can be treated with existing or novel drugs. Intensive investigation into the molecular mechanisms of apoptosis in cancer cells has led to the identification of compounds that show efficacy in patients with cancer [79]. A similar strategy aimed at targeting anti-apoptotic pathways, which are employed by Legionella to replicate within host cells, efficiently abrogated bacterial replication and prevented lethal lung infections in mice [80]. Targeting cellular inhibitor of apoptosis also promoted the killing of HBV-infected hepatocytes in preclinical infection models [81]. On the other hand, inhibiting the demise of immune cells by apoptosis during severe infections improved the survival in sepsis [82]. Although many proof-of-concept studies exist, whether therapeutic approaches aiming at targeting cell death pathways will be beneficial during infections is only just being investigated. Nonetheless, it is reasonable to envisage that a comprehensive understanding of the role and mechanisms of host cell death in different host–fungi interactions may provide a rational basis for the design of future therapeutic interventions to improve outcome in patients who are at risk from these life-threatening infections. Indeed, invasive fungal diseases cause unacceptably high rates of morbidity and mortality, and resistance to antifungals therapy is an escalating global issue [5]. Efficacious therapy is compounded in the host since fungal pathogens are eukaryotes. Accordingly, the majority of molecules that are toxic to fungi are also toxic to humans. Hence, complementary therapies that target immune pathways to manipulate and enhance host defence mechanisms against fungal pathogens (i.e. cytokine therapy, adoptive T cell therapy, and potential vaccine) [83] are becoming more appealing and attractive strategies for the treatment of fungal diseases. Finally, perhaps the greatest future challenge will be the exploration of host-targeted therapy of fungal infections in well-designed clinical trials.
Outstanding Questions.
What are the key fungal virulence factors leading to the activation, inhibition or modulation of programmed cell death mechanisms? What are the host receptors and the precise molecular events involved in the activation/manipulation of programmed host cell death by human fungal pathogens?
What is the role of Cryptococcus-induced pyroptosis in vivo? Does pyroptosis play a role in host defense against Cryptococcus infection and immunopathology?
Does fungal-induced programmed necrosis correlate with inflammatory-driven disease?
What is the role of eosinophil extracellular traps (EETs) in Aspergillus infection? Does the release of EETs contribute to allergic bronchopulmonary aspergillosis?
Does the C. albicans toxin candidalysin induce cytolysis by a programmed cell death mechanism? Do other fungal pathogens also secrete toxins to induce programmed cell death?
Why is the ROS- and PAD4-dependency of ETosis highly variable during fungal infection?
What are the specific signalling pathways leading to ET release in fungal infection?
Can programmed cell death pathways be manipulated therapeutically to treat life-threatening fungal infections?
Figure I.
Protective antifungal immune responses.
Figure II.
Molecular and cellular events of apoptotic cell death.
Figure III.
Programmed necrosis pathways: necroptosis, pyroptosis and ETosis
Highlights.
Programmed cell death pathways play a key role in infection and immunity and influence host disease outcome.
The host triggers lytic or non-lytic programmed cell death pathways in response to opportunistic fungal pathogens to fight infection.
Human pathogenic fungi target specific host cell death mechanisms to perturb innate and adaptive immune cell function. Indeed, fungal pathogens can induce or inhibit cell death pathways to promote their survival and dissemination.
Deciphering the complex interplay between pathogenic fungi, host cell death mechanisms and immune responses will provide insight into new therapeutic approaches to control life-threatening fungal diseases.
Acknowledgements
We apologize to our colleagues whose work we could not cite owing to space limitations.
This work was supported by grants from the Wellcome Trust (214229_Z_18_Z), Biotechnology & Biological Sciences Research Council (BB/N014677/1), National Institutes of Health (R37-DE022550) and the NIH Research at Guys and St. Thomas’s NHS Foundation Trust and the King’s College London Biomedical Research Centre (IS-BRC-1215-20006).
Figures were created with BioRender.
Glossary
- Candidalysin
the first cytolytic peptide toxin identified in any human fungal pathogen. Candidalysin is secreted by the pathogenic hyphal form of C. albicans and facilitates pathogenicity by acting as a classical virulence factor, but also contributes to antifungal immune responses. Candidalysin is generated from its parent protein (Ece1p) via sequential enzymatic processing by fungal kexins and is secreted from hyphae.
- CFU
colony-forming units. Viable fungi (often obtained from infected experimental models) that are cultured and counted as discrete colonies on nutrient agar plates. CFU are often used to quantify fungal burdens in infected tissue.
- Gliotoxin
sulfur-containing fungal secondary metabolite, belonging to the epipolythiodioxopiperazine class of toxins, produced by various filamentous fungi. Important virulence factor of the human pathogenic fungus A. fumigatus. Circulating gliotoxin (>100 ng/mL) is often detectable in the serum of individuals with documented invasive aspergillosis.
- Glycan moieties
polysaccharide or carbohydrate chains covalently linked to lipid or protein molecules (glycoconjugates). Polysaccharides such as glucans and chitin are major components of the fungal cell wall. Glycans contribute to fungal growth, morphology, cell wall integrity and participate in the host-pathogen interaction.
- Hyphae
long, tubular branching structures with parallel cell walls that are produced by many fungi. The C. albicans yeast-to-hypha morphological switch is triggered by numerous host stimuli and is typically associated with mucosal pathogenicity.
- Metaforosis
a form of macrophage-programmed necrosis during which phagocytosed A. fumigatus germinating conidia are transferred to live bystander macrophages. Metaforosis is dependent on calcineurin activation and facilitates the successful control of fungal germination in recipient macrophages.
- Pathogenic fungi
eukaryotic microbes that cause disease in humans or other organisms. Human pathogenic fungi include commensal microorganisms, which are normal constituents of the human microflora, or environmental microbes that reside in specific environmental niches. Pathogenic fungi possess determinants of virulence that can contribute to both damage and activation of host immune defences. Few pathogenic fungi can cause disease in healthy individuals while most fungal infections occur in immunocompromised patients.
- Pathogenic microbes
microorganisms that have the ability to cause disease (infection). Pathogenic microbes are very diverse and consist of viruses and both prokaryotic (bacteria) and eukaryotic (fungi, protozoa) organisms. The abilities to adhere, invade, and cause damage to host cells and tissues are common strategies employed by pathogenic microbes to establish infections.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jorgensen I et al. (2017) Programmed cell death as a defence against infection. Nat Rev Immunol 17 (3), 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashida H et al. (2011) Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol 195 (6), 931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bongomin F et al. (2017) Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel) 3 (4), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(2017) Stop neglecting fungi. Nat Microbiol 2, 17120. [DOI] [PubMed] [Google Scholar]
- 5.Berman J and Krysan DJ (2020) Drug resistance and tolerance in fungi. Nat Rev Microbiol 18 (6), 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doran AC et al. (2020) Efferocytosis in health and disease. Nat Rev Immunol 20 (4), 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibata-Ombetta S et al. (2003) Candida albicans phospholipomannan promotes survival of phagocytosed yeasts through modulation of bad phosphorylation and macrophage apoptosis. J Biol Chem 278 (15), 13086–93. [DOI] [PubMed] [Google Scholar]
- 8.Ibata-Ombetta S et al. (2001) Role of extracellular signal-regulated protein kinase cascade in macrophage killing of Candida albicans. J Leukoc Biol 70 (1), 149–54. [PubMed] [Google Scholar]
- 9.Wu H et al. (2013) Candida albicans secreted aspartic proteases 4–6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism. FASEB J 27 (6), 2132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagener J et al. (2012) Glycosylation of Candida albicans cell wall proteins is critical for induction of innate immune responses and apoptosis of epithelial cells. PLoS One 7 (11), e50518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reales-Calderon JA et al. (2013) Candida albicans induces pro-inflammatory and anti-apoptotic signals in macrophages as revealed by quantitative proteomics and phosphoproteomics. J Proteomics 91, 106–35. [DOI] [PubMed] [Google Scholar]
- 12.Moyes DL et al. (2014) Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J Infect Dis 209 (11), 1816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nur S et al. (2019) IL-23 supports host defense against systemic Candida albicans infection by ensuring myeloid cell survival. PLoS Pathog 15 (12), e1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monari C et al. (2008) Capsular polysaccharide induction of apoptosis by intrinsic and extrinsic mechanisms. Cell Microbiol 10 (10), 2129–37. [DOI] [PubMed] [Google Scholar]
- 15.Pericolini E et al. (2006) Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol 8 (2), 267–75. [DOI] [PubMed] [Google Scholar]
- 16.De Jesus M et al. (2009) Galactoxylomannan-mediated immunological paralysis results from specific B cell depletion in the context of widespread immune system damage. J Immunol 183 (6), 3885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villena SN et al. (2008) Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol 10 (6), 1274–85. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Abdallah M et al. (2012) Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-kappaB. PLoS Pathog 8 (3), e1002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin S et al. (2014) Melanin dependent survival of Aspergillus fumigatus conidia in lung epithelial cells. Int J Med Microbiol 304 (5–6), 626–36. [DOI] [PubMed] [Google Scholar]
- 20.Volling K et al. (2011) Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling. Cell Microbiol 13 (8), 1130–48. [DOI] [PubMed] [Google Scholar]
- 21.Geissler A et al. (2013) Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ 20 (10), 1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanzani M et al. (2005) Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 105 (6), 2258–65. [DOI] [PubMed] [Google Scholar]
- 23.Pardo J et al. (2006) The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J Cell Biol 174 (4), 509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M and Dixit VM (2010) Manipulation of host cell death pathways during microbial infections. Cell Host Microbe 8 (1), 44–54. [DOI] [PubMed] [Google Scholar]
- 25.Diehl N and Schaal H (2013) Make yourself at home: viral hijacking of the PI3K/Akt signaling pathway. Viruses 5 (12), 3192–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behar SM and Briken V (2019) Apoptosis inhibition by intracellular bacteria and its consequence on host immunity. Curr Opin Immunol 60, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson KS and Aw R (2016) The Commonalities in Bacterial Effector Inhibition of Apoptosis. Trends Microbiol 24 (8), 665–680. [DOI] [PubMed] [Google Scholar]
- 28.Weinlich R et al. (2017) Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18 (2), 127–136. [DOI] [PubMed] [Google Scholar]
- 29.Vu K et al. (2013) Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect Immun 81 (9), 3139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fa Z et al. (2017) RIPK3/Fas-Associated Death Domain Axis Regulates Pulmonary Immunopathology to Cryptococcal Infection Independent of Necroptosis. Front Immunol 8, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao M et al. (2019) Dectin-1-induced RIPK1 and RIPK3 activation protects host against Candida albicans infection. Cell Death Differ 26 (12), 2622–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong-James D et al. (2018) From phagocytosis to metaforosis: Calcineurin’s deadly role in innate processing of fungi. PLoS Pathog 14 (1), e1006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah A et al. (2016) Calcineurin Orchestrates Lateral Transfer of Aspergillus fumigatus during Macrophage Cell Death. Am J Respir Crit Care Med 194 (9), 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denning DW et al. (2006) The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 27 (3), 615–26. [DOI] [PubMed] [Google Scholar]
- 35.Chan BCL et al. (2019) IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front Immunol 10, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlomovitz I et al. (2019) Necroptosis directly induces the release of full-length biologically active IL-33 in vitro and in an inflammatory disease model. FEBS J 286 (3), 507–522. [DOI] [PubMed] [Google Scholar]
- 37.Moriwaki K et al. (2014) The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 41 (4), 567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton K et al. (2016) RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23 (9), 1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Man SM et al. (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277 (1), 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavares AH et al. (2015) Turning Up the Heat: Inflammasome Activation by Fungal Pathogens. PLoS Pathog 11 (7), e1004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellington M et al. (2014) Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13 (2), 329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uwamahoro N et al. (2014) The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio 5 (2), e00003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucey TM et al. (2020) Metabolic competition between host and pathogen dictates inflammasome responses to fungal infection. PLoS Pathog 16 (8), e1008695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vylkova S and Lorenz MC (2017) Phagosomal Neutralization by the Fungal Pathogen Candida albicans Induces Macrophage Pyroptosis. Infect Immun 85 (2), e00832–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruben S et al. (2020) Ahr1 and Tup1 Contribute to the Transcriptional Control of Virulence-Associated Genes in Candida albicans. mBio 11 (2), e00206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moyes DL et al. (2016) Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532 (7597), 64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez P and Ljungdahl PO (2005) Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol Cell Biol 25 (21), 9435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Meara TR et al. (2015) Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun 6, 6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Meara TR et al. (2018) High-Throughput Screening Identifies Genes Required for Candida albicans Induction of Macrophage Pyroptosis. mBio 9 (4), e01581–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen M et al. (2015) Internalized Cryptococcus neoformans Activates the Canonical Caspase-1 and the Noncanonical Caspase-8 Inflammasomes. J Immunol 195 (10), 4962–72. [DOI] [PubMed] [Google Scholar]
- 51.Wartha F and Henriques-Normark B (2008) ETosis: a novel cell death pathway. Sci Signal 1 (21), p e25. [DOI] [PubMed] [Google Scholar]
- 52.Brinkmann V et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303 (5663), 1532–5. [DOI] [PubMed] [Google Scholar]
- 53.von Kockritz-Blickwede M et al. (2008) Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111 (6), 3070–80. [DOI] [PubMed] [Google Scholar]
- 54.Ueki S et al. (2013) Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 121 (11), 2074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morshed M et al. (2014) NADPH oxidase-independent formation of extracellular DNA traps by basophils. J Immunol 192 (11), 5314–23. [DOI] [PubMed] [Google Scholar]
- 56.Halder LD et al. (2016) Factor H Binds to Extracellular DNA Traps Released from Human Blood Monocytes in Response to Candida albicans. Front Immunol 7, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loureiro A et al. (2019) Relevance of Macrophage Extracellular Traps in C. albicans Killing. Front Immunol 10, 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papayannopoulos V (2018) Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18 (2), 134–147. [DOI] [PubMed] [Google Scholar]
- 59.Branzk N et al. (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15 (11), 1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu SY et al. (2019) Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog 15 (11), e1008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guiducci E et al. (2018) Candida albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4. Front Immunol 9, 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kenno S et al. (2016) Autophagy and Reactive Oxygen Species Are Involved in Neutrophil Extracellular Traps Release Induced by C. albicans Morphotypes. Front Microbiol 7, 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urban CF et al. (2006) Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8 (4), 668–76. [DOI] [PubMed] [Google Scholar]
- 64.Zawrotniak M et al. (2017) Aspartic Proteases and Major Cell Wall Components in Candida albicans Trigger the Release of Neutrophil Extracellular Traps. Front Cell Infect Microbiol 7, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrd AS et al. (2013) An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol 190 (8), 4136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urban CF et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5 (10), e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueki S et al. (2016) Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol 137 (1), 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu P et al. (2014) Escherichia coli and Candida albicans induced macrophage extracellular trap-like structures with limited microbicidal activity. PLoS One 9 (2), e90042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocha JD et al. (2015) Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep 5, 8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gazendam RP et al. (2016) Human Neutrophils Use Different Mechanisms To Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects. J Immunol 196 (3), 1272–83. [DOI] [PubMed] [Google Scholar]
- 71.Silva JC et al. (2020) Mac-1 triggers neutrophil DNA extracellular trap formation to Aspergillus fumigatus independently of PAD4 histone citrullination. J Leukoc Biol 107 (1), 69–83. [DOI] [PubMed] [Google Scholar]
- 72.Bruns S et al. (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6 (4), e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee MJ et al. (2015) The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog 11 (10), e1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark HL et al. (2018) Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and β-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent Only on CR3. Front Immunol 9, 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs TA et al. (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176 (2), 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bianchi M et al. (2011) Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol 127 (5), 1243–52 e7. [DOI] [PubMed] [Google Scholar]
- 77.Bianchi M et al. (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114 (13), 2619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muniz VS et al. (2018) Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol 141 (2), 571–585 e7. [DOI] [PubMed] [Google Scholar]
- 79.Ashkenazi A et al. (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16 (4), 273–284. [DOI] [PubMed] [Google Scholar]
- 80.Speir M et al. (2016) Eliminating Legionella by inhibiting BCL-XL to induce macrophage apoptosis. Nat Microbiol 1, 15034. [DOI] [PubMed] [Google Scholar]
- 81.Ebert G et al. (2015) Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proc Natl Acad Sci U S A 112 (18), 5803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao C et al. (2019) Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis 10 (10), 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Armstrong-James D et al. (2017) Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect Dis 17 (12), e393–e402. [DOI] [PubMed] [Google Scholar]
- 84.Seyrek K et al. (2020) Controlling Cell Death through Post-translational Modifications of DED Proteins. Trends Cell Biol 30 (5), 354–369. [DOI] [PubMed] [Google Scholar]
- 85.Kalliolias GD and Ivashkiv LB (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12 (1), 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tenev T et al. (2011) The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 43 (3), 432–48. [DOI] [PubMed] [Google Scholar]
- 87.Bock FJ and Tait SWG (2020) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21 (2), 85–100. [DOI] [PubMed] [Google Scholar]
- 88.Kale J et al. (2018) BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ 25 (1), 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor RC et al. (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9 (3), 231–41. [DOI] [PubMed] [Google Scholar]
- 90.Lin Y et al. (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13 (19), 2514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng S et al. (2007) Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19 (10), 2056–67. [DOI] [PubMed] [Google Scholar]
- 92.Li J et al. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150 (2), 339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho YS et al. (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137 (6), 1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai Z et al. (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16 (1), 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo H et al. (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21 (7), 677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi J et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526 (7575), 660–5. [DOI] [PubMed] [Google Scholar]
- 97.Liu X et al. (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535 (7610), 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hakkim A et al. (2011) Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 7 (2), 75–7. [DOI] [PubMed] [Google Scholar]
- 99.Metzler KD et al. (2014) A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 8 (3), 883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y et al. (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184 (2), 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]