Abstract
Objectives:
Despite the proven efficacy of endovascular thrombectomy (EVT) for large vessel occlusion stroke, over half treated remain functionally disabled or die. Infarct topography may have implications for prognostication, patient selection, and the development of tissue-specific neuroprotective agents. We sought to quantify white matter injury in anterior circulation acute infarcts post-EVT to understand its significance and identify its determinants.
Materials/Methods:
Demographics, history, presentation, and outcomes for consecutive patients treated with EVT were recorded in a prospectively maintained database at a single center. Acute infarct masks were coregistered to standard space. Standard atlases of white matter, cortex, and basal ganglia were used to determine region-specific infarct volumes.
Results:
167 individuals were identified with median age 69 years and 53% women. 85% achieved adequate reperfusion (TICI 2b-3) after EVT; 43% achieved 90-day functional independence (mRS 0-2). Median infarct volumes were 45cc (IQR 18-122) for total, 17cc (6-49) for white matter, 21cc (4-53) for cortex, and 5cc (1-8) for basal ganglia. The odds of 90-day mRS 0-2 were reduced in patients with larger white matter infarct volume (cc, OR=0.89, 95%CI=0.81-0.96), independent of cortex infarct volume, basal ganglia infarct volume, age, NIHSS, and TICI 2b-3 reperfusion. Reperfusion-to-MRI time was associated with white matter infarct volume (hr, β=0.119, p=0.017), but not cortical or basal ganglia infarct volume.
Conclusions:
These data quantitatively describe region-specific infarct volumes after EVT and suggest the clinical relevance of white matter infarct volume as a predictor of long-term outcomes. Further study is warranted to examine delayed white matter infarction and the significance of specific white matter tracts.
Keywords: Ischemic stroke, Large vessel occlusion, Endovascular thrombectomy, Infarct topography, White matter
Introduction:
Endovascular thrombectomy (EVT) has been shown to be a powerful intervention that has revolutionized the care of large vessel occlusion (LVO) acute ischemic stroke, recognized as the most devastating subtype.[1-4] However, even among those treated, 54% remain functionally disabled or are dead at 90 days.[1] It is important to understand the mechanisms of EVT at the level of the brain parenchyma, beyond revascularization, to understand complex tissue-level pathophysiology and variability in outcomes. Acute infarct topography, or a description of brain infarct patterns with regions involved, may have implications for EVT patient selection, outcome prediction, and the development of novel tissue-specific neuroprotective/neuroreparative agents.[6-8] In the pre-EVT era, several small studies have demonstrated that both infarct size and location are related to long-term outcomes.[9-12] However, routine clinical measures of infarct topography in the EVT era, such as Alberta Stroke Program Early Computed Tomography Score (ASPECTS), are only crude estimations.[13,14]
White matter may play a key role in outcomes after acute ischemic stroke, both by its direct acute infarction and by its extent of baseline injury.[15-18] The pathophysiology of ischemia, infarction, and repair of white matter is becoming increasingly understood.[19-21] Compared to gray matter, white matter can survive longer at a given degree of hypoperfusion [22] and undergoes different chemical cascades during ischemia.[25,26]A recent study showed that qualitative sparing of white matter in post-EVT infarcts, half identified by less sensitive CT imaging, was associated with improved outcomes at 90 days.[27] To better understand outcomes after EVT, we sought to quantitatively assess the degree of white matter injury involved in post-EVT acute infarcts by magnetic resonance imaging (MRI), understand its significance for outcomes, and identify its determinants.
Methods:
This study was approved by the Massachusetts General Hospital institutional review board. Informed consent was waived based on minimal patient risk and practical inability to perform the study without the waiver. The data that support the findings of this study will be made available from the corresponding author upon reasonable request and pending approval of local institutional review board.
We retrospectively identified acute anterior circulation LVO stroke patients who underwent EVT from a prospectively maintained database at a single referral center from January 2011 to September 2019.[28] This database includes demographic information, past medical history, clinical presentation, treatments, and outcomes for consecutive patients treated with EVT. Presenting NIH Stroke Scale (NIHSS) score was determined as described,[29] with higher numbers reflecting increased clinical stroke severity. Alteplase treatment decisions were guideline based at the discretion of a vascular neurologist. EVT treatment decisions were at the discretion of a vascular neurologist and neurointerventionalist. Cervical internal carotid artery (ICA) disease was defined as severe stenosis (>70%) or occlusion related to atherosclerosis or dissection.[30,31] Thrombolysis in Cerebral Infarction (TICI) scores were determined by a neurointerventionalist using the modified scale: 2a partial filling <50%, 2b partial filling ≥50%, 3 complete perfusion.[32] Adequate reperfusion was considered TICI 2b-3. Intracerebral hemorrhage (ICH) was defined as any symptomatic or asymptomatic PH1 or PH2 by ECASS criteria during the hospitalization.[33] 90-day modified Rankin Scale (mRS) score was obtained by telephone call and available for 87% of patients.[34,35] Functional independence was defined as mRS 0-2.
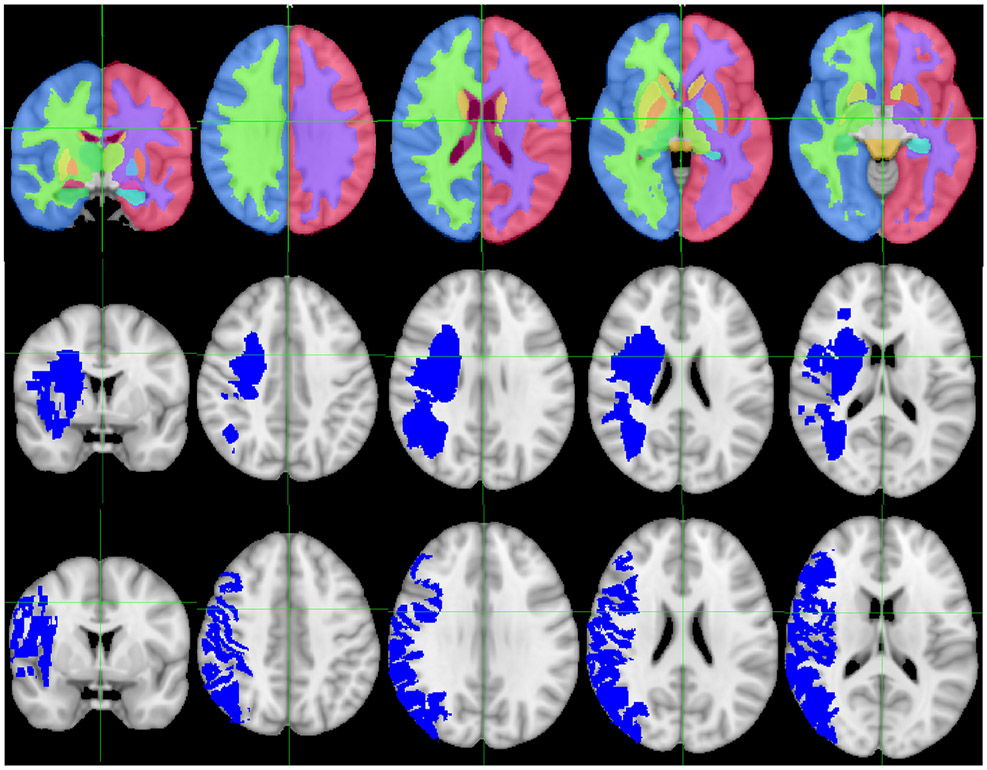
Patients with available post-EVT MRIs were identified retrospectively. Clinical MRIs were downloaded with OsiriX (Pixmeo). Diffusion-weighted imaging (DWI) sequences were obtained on a Siemens 3T MRI or a GE 1.5T MRI with echo time 60-120 ms, repetition time 5300-5600 ms, and slice thickness 5 mm with a 1 mm gap. Infarct lesion masks were manually traced using Slicer 4.8.1 (Brigham and Women’s Hospital)[36] by a vascular neurologist blinded to clinical data. RegLSM (University of Calgary) was used to register the DWI sequences to MNI-152 space.[37] The registration procedure consisted of linear registration followed by nonlinear registration using the Elastix toolbox. Registered infarct masks were overlaid with the Harvard-Oxford subcortical structural atlas to determine quantitative structure-specific infarct volumes using FSL (FMRIB Analysis Group) (Fig. 1).[38] All individual DWI sequences and DWI infarct registrations were visually inspected for appropriate registration blinded to clinical data. Those with significant artifacts and/or poor registration were excluded (n=23).
Figure 1.
Region-specific infarct volumes. Top row: Sections through the Harvard-Oxford subcortical atlas, demonstrating white matter, cortex, and basal ganglia regions, overlaid on the MNI 152 standard brain. Middle row: Sections through an example of an infarct affecting primarily white matter overlaid on the MNI 152 standard brain. Bottom row: Sections through an example of an infarct affecting primarily cortex overlaid on the MNI 152 standard brain.
Median values with interquartile range (IQR) were reported for continuous variables. Percent and count were reported for categorical variables. Differences were assessed using nonparametric Wilcoxon rank-sum for continuous variables and Fisher’s Exact tests for categorical variables. Logistic regression analyses were performed to assess associations with 90-day functional independence, and linear regression analyses were performed to assess for associations with natural logarithm-transformed infarct volumes. While we aimed to limit the transformation of data when not required for statistical tests, infarct volumes were natural log-transformed for use in linear regressions given they were not normally distributed. Variables with pre-specified significance of p<0.10 in univariable analysis were subsequently included in multivariable models. Two-tailed P values <0.05 were interpreted as statistically significant. Analyses were performed with SPSS version 23.0 (IBM Corp).
Results:
381 consecutive patients underwent EVT for anterior LVO during the study period. Post-EVT MRI was available for 190 and obtained at a median of 24 h (IQR 18-46) after the procedure. Of these, 167 (87%) had available DWI imaging that was traceable and registered adequately. Among these 167 patients, the median age was 69 years (IQR 59-79), 53% were women, and 59% had left-sided infarcts. Risk factors included hypertension (66%), diabetes (17%), atrial fibrillation (30%), and prior stroke/transient ischemic attack (16%). 55% were treated with intravenous alteplase; 85% achieved TICI 2b-3 reperfusion after EVT. At 90 days, 43% achieved functional independence (Table 1).
Table 1.
Demographics, medical history, clinical presentations, outcomes, and infarct topography for large vessel occlusion stroke patients treated with endovascular thrombectomy (EVT). Infarct topography quantified on MRI describes brain regions involved in acute infarcts after EVT; percentages are represented as both of total infarct and of brain structure. Abbreviations: TIA transient ischemic attack, NIHSS NIH Stroke Scale, ICA internal carotid artery, M1 first segment of middle cerebral artery, M2 second segment, LKW last known well, TICI Thrombolysis in Cerebral Infarction, MRI magnetic resonance imaging, ICH intracerebral hemorrhage, mRS modified Rankin Scale, cc cubic centimeter, IQR interquartile range.
| Median/Count | IQR/Percent | |
|---|---|---|
| Age, Years | 69 | 59-79 |
| Female | 89 | 53% |
| Hypertension | 110 | 66% |
| Diabetes | 29 | 17% |
| Atrial Fibrillation | 50 | 30% |
| Stroke/TIA History | 27 | 16% |
| Coronary Artery Disease | 31 | 19% |
| Smoking | 31 | 19% |
| Presenting NIHSS | 16 | 13-20 |
| Cervical ICA Disease | 29 | 17% |
| Intracranial Occlusion | ||
| ICA Terminus | 32 | 19% |
| M1 | 107 | 64% |
| M2 | 27 | 16% |
| Alteplase | 92 | 55% |
| LKW-Alteplase Time, Min | 116 | 80-169 |
| LKW-Groin Time, Min | 255 | 169-376 |
| LKW-Recanalization Time, Min | 289 | 208-410 |
| Groin-Recanalization Time, Min | 38 | 22-64 |
| TICI 2b-3 Recanalization | 142 | 85% |
| Groin-MRI Time, Hr | 24 | 18-46 |
| Recanalization-MRI Time, Hr | 23 | 16-39 |
| Left Side Infarct | 99 | 59% |
| Bilateral Infarct | 6 | 4% |
| ICH | 5 | 3% |
| 90d mRS 0-2 | 62 | 43% |
| Total Infarct Volume, cc | 45 | 18-122 |
| White Matter | ||
| Infarct Volume, cc | 17 | 6-49 |
| % of Total Infarct | 39% | 33%-47% |
| % of Ipsilateral White Matter | 7% | 3%-20% |
| Cortex | ||
| Infarct Volume, cc | 21 | 4-53 |
| % of Total Infarct | 42% | 23%-52% |
| % of Ipsilateral Cortex | 4% | 1%-10% |
| Basal Ganglia | ||
| Infarct Volume, cc | 5 | 1-8 |
| % of Total Infarct | 7% | 3%-21% |
| % of Ipsilateral Basal Ganglia | 39 | 11%-61% |
The median post-EVT total infarct volume was 45 cc (IQR 18-122). Anterior LVO infarct sub-region volumes were notable for median white matter infarct volume 17 cc (IQR 6-49), cortex infarct volume 21 cc (IQR 4-53), and basal ganglia infarct volume 5cc (IQR 1-8). The median percentage of total infarct that was white matter was 39% (IQR 33-47%), total infarct that was cortex was 42% (IQR 23-52%), and total infarct that was basal ganglia was 7% (IQR 3-21%). The median percentage of ipsilateral white matter that was infarcted was 7% (IQR 3-20%), ipsilateral cortex that was infarcted was 4% (IQR 1-10%), and ipsilateral basal ganglia that was infarcted was 39% (11-61%) (Table 1).
Greater post-EVT anterior circulation sub-region infarct volumes (white matter, cortex, basal ganglia) decreased the odds of 90-day functional independence, as did older age, more coronary artery disease, higher presenting NIHSS, and lower rates of TICI 2b-3 reperfusion in univariable analyses (Table 2). In a multivariable model including infarct volumes from each anterior circulation region and other variables with associations in univariable analyses, only greater post-EVT white matter infarct volume (cc, OR=0.89, 95%CI=0.81,0.96) and older age (years, OR=0.92, 95%CI=0.89,0.96) independently decreased the odds of functional independence at 90-days (Table 2). When analyzing infarct volume sub-regions, we included five sensitivity analyses confirming similar results when white matter and cortical infarct volumes were included separately in different multivariable models given the concern for collinearity and when white matter infarct was included as percentage of total infarct instead of absolute volume (Supplemental Table 1). When comparing quartiles of white matter infarct volume, there were significant differences in the proportion who reached 90-day functional independence (p<0.0001): 68% for the lowest volume first quartile (<6.0cc), 66% for the second quartile (6.0-16.8cc), 32% for the third quartile (16.8-49.4cc), 8% for the fourth quartile (>49.4cc). Furthermore, we included an additional sensitivity analysis using the outcome 90- day mRS 0-3 rather than 0-2; this confirmed similar results supporting these findings are robust (Supplemental Table 2).
Table 2.
Greater post-EVT white matter infarct volume independently decreased the odds of 90-day functional independence. Anterior circulation sub-region infarct volumes (white matter, cortex, basal ganglia), older age, more coronary artery disease, higher presenting NIHSS, and lower rates of TICI 2b-3 reperfusion decreased the odds of 90-day functional independence in univariable analyses. In a multivariable model including infarct volumes from each region and these other identified candidate variables, only greater post-EVT white matter infarct volume and older age independently decreased the odds of functional independence at 90-days. Abbreviations: TIA transient ischemic attack, NIHSS NIH Stroke Scale, ICA internal carotid artery, M1 first segment of middle cerebral artery, LKW last known well, TICI Thrombolysis in Cerebral Infarction, OR odds ratio, CI confidence interval.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age, Years | 0.956 (0.932,0.980) | <0.0001 | 0.920 (0.885,0.958) | <0.0001 |
| Female | 0.792 (0.409,1.530) | 0.487 | ||
| Hypertension | 0.567 (0.282,1.141) | 0.112 | ||
| Diabetes | 0.620 (0.247,1.559) | 0.310 | ||
| Atrial Fibrillation | 0.703 (0.343,1.442) | 0.337 | ||
| Stroke/TIA History | 0.711 (0.291,1.736) | 0.454 | ||
| Coronary Disease | 0.332 (0.132,0.835) | 0.019 | 0.456 (0.141,1.472) | 0.189 |
| Smoking | 1.429 (0.610,3.346) | 0.411 | ||
| Presenting NIHSS | 0.927 (0.862,0.996) | 0.039 | 0.941 (0.848,1.045) | 0.255 |
| Cervical ICA Disease | 0.872 (0.362,2.097) | 0.759 | ||
| ICA Terminus Occlusion | 1.183 (0.504,2.775) | 0.699 | ||
| M1 Occlusion | 0.926 (0.464,1.848) | 0.827 | ||
| Alteplase | 1.377 (0.708,2.678) | 0.346 | ||
| LKW-Alteplase Time, Min | 0.998 (0.991,1.006) | 0.663 | ||
| LKW-Groin Time, Min | 1.000 (0.998,1001) | 0.447 | ||
| Groin-Recanalization Time, Min | 0.993 (0.984,1.002) | 0.147 | ||
| TICI 2b-3 | 6.243 (1.763,22.11) | 0.005 | 3.723 (0.549,25.25) | 0.178 |
| Left Side Infarct | 1.649 (0.835,3.256) | 0.150 | ||
| Bilateral Infarct | 2.059 (0.334,12.72) | 0.437 | ||
| White Matter Infarct Volume, cc | 0.953 (0.933,0.972) | <0.0001 | 0.886 (0.814,0.964) | 0.005 |
| Cortical Infarct Volume, cc | 0.970 (0.957,0.983) | <0.0001 | 1.041 (0.988,1.098) | 0.130 |
| Basal Ganglia Infarct Volume, cc | 0.916 (0.838,1.001) | 0.053 | 1.033 (0.866,1.233) | 0.717 |
We subsequently explored potential determinants of post-EVT white matter infarct volume with univariable analyses (Table 3). While there were several associated variables, when including each in a multivariable model controlling for total infarct volume (to identify unique determinants of white matter infarct volume), only total infarct volume (cc, β=0.755, p<0.0001) and recanalization-to-MRI time (hr, β=0.119, p=0.017) were independently associated with white matter infarct volume. When total infarct volume was controlled for, no significant association was found with TICI 2b-3, last known well-to-groin time, groin-to-recanalization time, age, sex, atrial fibrillation, or cervical ICA disease (Table 3). Similarly, in a multivariable model for possible determinants of post-EVT cortical infarct volume, only total infarct volume was associated (cc, β=0.697, p<0.0001) (Supplemental Table 3). In a multivariable model for possible determinants of post-EVT basal ganglia infarct volume, total infarct volume (cc, β=0.270, p<0.0001), NIHSS (β=0.227, p=0.002) and first segment middle cerebral artery (M1) occlusion location (β =0.228, p=0.001) were positively independently associated, while age was negatively independently associated (years, β =−0.204, p=0.004) (Supplemental Table 4).
Table 3.
Determinants of white matter infarct volume after endovascular thrombectomy among large vessel occlusion stroke patients. Several variables were associated with greater natural log-transformed white matter infarct volumes in univariable analyses. In a multivariable model including these variables and controlling for total infarct volume (to identify unique determinants of white matter infarct volume), only greater total infarct volume and recanalization-to-MRI time were independently associated with greater white matter infarct volume. Abbreviations: TIA transient ischemic attack, NIHSS NIH Stroke Scale, ICA internal carotid artery, M1 first segment of middle cerebral artery, LKW last known well, TICI Thrombolysis in Cerebral Infarction, MRI magnetic resonance imaging, β beta parameter estimate.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| β | P | β | P | |
| Age, Years | −0.147 | 0.059 | −0.024 | 0.648 |
| Female | −0.131 | 0.091 | −0.016 | 0.737 |
| Hypertension | −0.029 | 0.711 | ||
| Diabetes | 0.100 | 0.199 | ||
| Atrial Fibrillation | −0.176 | 0.023 | −0.046 | 0.377 |
| Stroke/TIA History | −0.060 | 0.443 | ||
| Coronary Artery Disease | −0.070 | 0.366 | ||
| Smoking | 0.020 | 0.802 | ||
| Presenting NIHSS | 0.037 | 0.635 | ||
| Cervical ICA Disease | 0.148 | 0.057 | 0.086 | 0.085 |
| ICA Terminus Occlusion | −0.014 | 0.855 | ||
| M1 Occlusion | −0.016 | 0.839 | ||
| Alteplase | −0.033 | 0.674 | ||
| LKW-Alteplase Time, Min | 0.158 | 0.134 | ||
| LKW-Groin Time, Min | 0.156 | 0.044 | 0.071 | 0.137 |
| Groin-Recanalization Time, Min | 0.306 | <0.0001 | 0.033 | 0.530 |
| TICI 2b-3 | −0.371 | <0.0001 | −0.026 | 0.624 |
| Recanalization-MRI Time, Hr | 0.243 | 0.003 | 0.119 | 0.017 |
| Total Infarct Volume, cc | 0.826 | <0.0001 | 0.755 | <0.0001 |
Discussion:
In this analysis of anterior circulation LVO stroke patients, greater post-EVT white matter infarct volume was associated with decreased odds of functional independence at 90 days when included in a model controlling for volumes of infarct in other anterior circulation brain regions, age, stroke severity, and adequate reperfusion status. Furthermore, we quantified, for the first time, regional infarct volumes on MRI after EVT, showing infarcted white matter was median 17cc (39% total infarct).
The present study represents the first quantitative description of region-specific infarct volumes after EVT. The median total infarct volume was 45 cc (IQR 18-122). This is similar to that observed in a randomized trial, which showed a median volume of 38 cc (IQR 17-128) for those undergoing general anesthesia and 22 cc (8-65) for those undergoing conscious sedation.[39] However, there were differences as the trial measured FLAIR sequences at 48-72 hours and the present study quantified DWI sequences registered in standard space. In the present study, white matter comprised a median of 39% (IQR 33-47%) of the total post-EVT infarct volume. White matter survives longer at a given degree of hypoperfusion[22] and has a 40% lower perfusion level that results in irreversible infarction compared to gray matter.[23,24] One study showed that 36% of post-EVT infarcts qualitatively spared white matter, defined as ≤5 lesions of infarcted white matter ≤3 mm in diameter.[27] However, for half of the patients CT was utilized for infarct assessment, which is less sensitive than MRI.
Post-EVT white matter infarct volume may be among the strongest determinants of 90-day disability. In the present study, it was independently associated with poor outcome in a multivariable model controlling for infarct volumes in other anterior regions, TICI score, age, and NIHSS. A prior study showed that qualitative sparing of white matter in post-EVT infarcts after EVT was associated with 90-day functional independence.[27] Similar to the present study, their multivariable model for 90-day functional independence showed that when white matter infarction was included, the only other variable that remained significantly associated was age (NIHSS, alteplase, TICI 2b-3, total infarct volume were not significant). However, their assessment was not quantitative, and half of the patients were assessed by CT. In the pre-EVT era, a study using voxel-based lesion symptom mapping showed areas with high influence on mRS included the corona radiata and internal capsule.[10] Together these findings underscore the importance of acute white matter infarction for long-term outcomes after anterior LVO stroke.
In addition to outcome prediction, these data may have implications for patient selection for EVT. White matter infarct volume may be a consideration among other factors, although this requires further study. For example, patients with minimal white matter infarction, despite borderline total infarct volume, may stand to benefit from EVT. Furthermore, these data may inform preclinical studies of neuroprotective/neuroreparative agents that are desperately needed when reperfusion is delayed, incomplete, or not possible.[6,7] An underestimation of white matter injury in human stroke has been proposed, among other explanations, to underlie the translation failure of neuroprotective agents developed in rodent models.[40-42] While the human brain is composed nearly of 50% white matter, most rodent brains are composed of only 15% suggesting white matter injury is not adequately modeled with conventional rodent territorial infarcts.[43] The present study illustrates the importance of infarcted white matter in humans and provides compelling evidence to utilize preclinical models that study white matter infarction specifically in the development of novel agents.
Understanding the mechanisms involved and the factors that predispose to white matter infarction is key to prevent its occurrence in the setting of LVO and subsequently augment its repair. Our analyses suggest several variables may be associated with white matter infarct volume, including adequate reperfusion (TICI 2b-3), last known well-to-groin time, groin-recanalization time, age, sex, atrial fibrillation, and cervical ICA disease. However, when including these variables in a multivariable model controlling for total infarct volume, none were significant. Another study showed that potential determinants of white matter sparing included time to recanalization and adequate reperfusion, but the authors did not assess white matter infarct quantitatively nor control for total infarct volume in their model.[27] While vessel occlusion location was not associated with white matter infarct volume, the M1 location was associated with basal ganglia infarct volume likely related to occlusion of M1 perforators. Unexpectedly, reperfusion-to-MRI time was significantly associated with white matter infarct volume, even when controlling for total infarct volume. This time epoch was not associated with cortical infarct volume or basal ganglia infarct volume, suggesting there may be a time-dependent slow infarction specific to white matter even after EVT. White matter survives longer than gray matter at any given degree of hypoperfusion,[22] likely related to different metabolic demands and different chemical cascades triggered by ischemia.[25,26] PET[44] and MRI[45,46] studies have confirmed this in human patients. However, this has not been described after EVT. If confirmed in futures studies, this may represent a novel therapeutic target.
This study has several limitations, largely due to its retrospective design. Treatment and imaging decisions were at the discretion of the treating clinicians. However, baseline patient demographics, medical history, clinical presentation, and outcomes, including adequate reperfusion and 90-day disability, were similar to randomized EVT trials[1-3] underscoring the generalizability of this study. Secondly, while attempts were made to control for multiple variables using statistical models, these exploratory results require confirmation in independent studies. There is also inherent error in the registration process of clinical scans to standard MNI-152 space. However, our successful registration rate was high (87%) despite our stringent inspection and exclusion of poor registrations. Further, registration error is unlikely to affect interpretation because of the relatively large infarct volumes in this study. While clinical scans are usually of lower quality than those obtained exclusively for research, they increase the translatability of the results. Another potential limitation is the inclusion of patients over a time frame that spans several landmark trials that changed the standard of care for LVO treatment. Despite this, there is unlikely to be an effect on the study’s interpretation since the variable of interest is post-EVT infarct volume. We also include TICI score as a covariate to control for improving techniques over time. While all patients included in the study underwent post-EVT MRI, the median recanalization-to-MRI time was 23 hours so the chronic final lesion size may differ slightly. Repeat imaging after the acute hospital admission was not available as it is not often clinically indicated. Finally, the association of recanalization-to-MRI time and white matter infarct volume was unexpected and may suggest the white matter infarct volumes reported are an under-estimation. Future studies using larger cohorts are needed to explore this relationship in detail.
Conclusion:
These data support that white matter infarct volume may be among the most important determinants of 90-day functional independence and quantitatively describe region-specific infarct volumes on MRI after EVT for the first time. These results underscore the potential significance of white matter acute infarction for neuroprognostication and have implications for treatment selection and the development of novel neuroprotective/neuroreparative agents. Further study is warranted to examine delayed white matter infarction after EVT and the significance of specific white matter tracts for outcomes.
Supplementary Material
Acknowledgments
Joyce A McIntyre maintained the prospective Massachusetts General Hospital stroke database. The National Institutes of Health, National Institute of Neurological Disorders and Stroke supported this work (RWR by R25 NS065743; NSR by R01 NS082285, R01 NS086905, U19 NS115388). There are no relevant competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2017;378:11–21. doi: 10.1056/NEJMoa1706442 [doi] [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018;378:708–18. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regenhardt RW, Biseko MR, Shayo AF, et al. Opportunities for intervention: Stroke treatments, disability and mortality in urban Tanzania. Int J Qual Heal Care 2019;31:385–92. doi: 10.1093/intqhc/mzy188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinman JD, Rost NS, Leung TW, et al. Principles of precision medicine in stroke. J Neurol Neurosurg Psychiatry 2017;88:54–61. doi: 10.1136/jnnp-2016-314587 [DOI] [PubMed] [Google Scholar]
- 6.Regenhardt RW, Takase H, Lo EH, et al. Translating concepts of neural repair after stroke: Structural and functional targets for recovery. Restor Neurol Neurosci 2020;38:67–92. doi: 10.3233/rnn-190978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regenhardt RW, Das AS, Stapleton CJ, et al. Blood pressure and penumbral sustenance in stroke from large vessel occlusion. Front. Neurol 2017;8. doi: 10.3389/fneur.2017.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etherton MR, Rost NS, Wu O. Infarct topography and functional outcomes. J Cereb Blood Flow Metab 2018;38:1517–32. doi: 10.1177/0271678X17700666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan TG, Demchuk A, Srikanth V, et al. Proof of Concept Study: Relating Infarct Location to Stroke Disability in the NINDS rt-PA Trial. Cerebrovasc Dis 2013;35:560–5. doi: 10.1159/000351147 [DOI] [PubMed] [Google Scholar]
- 10.Cheng B, Forkert ND, Zavaglia M, et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke 2014;45:1695–702. doi: 10.1161/STROKEAHA.114.005152 [DOI] [PubMed] [Google Scholar]
- 11.Menezes NM, Ay H, Wang Zhu M, et al. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke 2007;38:194–7. doi: 10.1161/01.STR.0000251792.76080.45 [DOI] [PubMed] [Google Scholar]
- 12.Beloosesky Y, Streifler JY, Burstin A, et al. The importance of brain infarct size and location in predicting outcome after stroke. Age Ageing 1995;24:515–8. doi: 10.1093/ageing/24.6.515 [DOI] [PubMed] [Google Scholar]
- 13.Sheth SA, Malhotra K, Liebeskind DS, et al. Regional Contributions to Poststroke Disability in Endovascular Therapy. Interv Neurol 2018;7:533–43. doi: 10.1159/000492400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–4. doi:S0140673600022376 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Rost NS, Cougo P, Lorenzano S, et al. Diffuse microvascular dysfunction and loss of white matter integrity predict poor outcomes in patients with acute ischemic stroke. J Cereb Blood Flow Metab 2018;38:75–86. doi: 10.1177/0271678X17706449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etherton MR, Wu O, Cougo P, et al. Integrity of normal-appearing white matter and functional outcomes after acute ischemic stroke. Neurology 2017;88:1701–8. doi: 10.1212/WNL.0000000000003890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atchaneeyasakul K, Leslie-Mazwi T, Donahue K, et al. White Matter Hyperintensity Volume and Outcome of Mechanical Thrombectomy With Stentriever in Acute Ischemic Stroke. Stroke 2017;48:2892–4. doi: 10.1161/STROKEAHA.117.018653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulouis G, Bricout N, Benhassen W, et al. White matter hyperintensity burden in patients with ischemic stroke treated with thrombectomy. Neurology 2019;93:e1498–506. doi: 10.1212/WNL.0000000000008317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regenhardt RW, Das AS, Lo EH, et al. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol. 2018;75:1273–81. doi: 10.1001/jamaneurol.2018.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regenhardt RW, Das AS, Ohtomo R, et al. Pathophysiology of Lacunar Stroke: History’s Mysteries and Modern Interpretations. J. Stroke Cerebrovasc. Dis 2019;28:2079–97. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takase H, Regenhardt RW. Motor tract reorganization after acute central nervous system injury: a translational perspective. Neural Regen Res. 2021. June;16(6):1144–1149. doi: 10.4103/1673-5374.300330. PMID: 33269763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcoux FW, Morawetz RB, Crowell RM, et al. Differential regional vulnerability in transient focal cerebral ischemia. Stroke 1982;13:339–46. doi: 10.1161/01.STR.13.3.339 [DOI] [PubMed] [Google Scholar]
- 23.Arakawa S, Wright PM, Koga M, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke 2006;37:1211–6. doi: 10.1161/01.STR.0000217258.63925.6b [DOI] [PubMed] [Google Scholar]
- 24.Murphy BD, Fox AJ, Lee DH, et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology 2008;247:818–25. doi: 10.1148/radiol.2473070551 [DOI] [PubMed] [Google Scholar]
- 25.Omata N, Murata T, Maruoka N, et al. Different mechanisms of hypoxic injury on white matter and gray matter as revealed by dynamic changes in glucose metabolism in rats. Neurosci Lett 2003;353:148–52. [DOI] [PubMed] [Google Scholar]
- 26.Nishizaki T, Yamauchi R, Tanimoto M, et al. Effects of temperature on the oxygen consumption in thin slices from different brain regions. Neurosci Lett 1988;86:301–5. [DOI] [PubMed] [Google Scholar]
- 27.Kleine JF, Kaesmacher M, Wiestler B, et al. Tissue-Selective Salvage of the White Matter by Successful Endovascular Stroke Therapy. Stroke 2017;48:2776–83. doi: 10.1161/STROKEAHA.117.017903 [DOI] [PubMed] [Google Scholar]
- 28.Regenhardt RW, Mecca AP, Flavin SA, et al. Delays in the Air or Ground Transfer of Patients for Endovascular Thrombectomy. Stroke 2018;49:1419–25. doi: 10.1161/STROKEAHA.118.020618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Group NI of ND and S rt-PSS. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–7. doi: 10.1056/NEJM199512143332401 [doi] [DOI] [PubMed] [Google Scholar]
- 30.Regenhardt RW, Etherton MR, Das AS, et al. Infarct Growth despite Endovascular Thrombectomy Recanalization in Large Vessel Occlusive Stroke. J Neuroimaging Published Online First: 2020. doi: 10.1111/jon.12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolan NM, Regenhardt RW, Koch MJ, Raymond SB, Stapleton CJ, Rabinov JD, Silverman SB, Leslie-Mazwi TM, Patel AB. Treatment Approaches and Outcomes for Acute Anterior Circulation Stroke Patients with Tandem Lesions. J Stroke Cerebrovasc Dis. 2021. February;30(2):105478. doi: 10.1016/j.jstrokecerebrovasdis.2020.105478. Epub 2020 Nov 26. PMID: 33248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on Angiographic Revascularization Grading Standards for Acute Ischemic Stroke. Stroke 2013;44:2650–63. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–25. doi: 10.1001/jama.1995.03530130023023 [DOI] [PubMed] [Google Scholar]
- 34.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. doi: 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 35.Regenhardt RW, Young MJ, Etherton MR, et al. Toward a more inclusive paradigm: thrombectomy for stroke patients with pre-existing disabilities. J Neurointerv Surg 2020;:neurintsurg-2020-016783. doi: 10.1136/neurintsurg-2020-016783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slicer, https://www.slicer.org/ (accessed 5 May 2020).
- 37.Aben HP, Biessels GJ, Weaver NA, et al. Extent to Which Network Hubs Are Affected by Ischemic Stroke Predicts Cognitive Recovery. Stroke 2019;50:2768–74. doi: 10.1161/STROKEAHA.119.025637 [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuroimage 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 39.Simonsen CZ, Yoo AJ, Sørensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke a randomized clinical trial. JAMA Neurol 2018;75:470–7. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx 2004;1:36–45. doi: 10.1602/neurorx.1.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeyaseelan K, Lim KY, Armugam A. Neuroprotectants in stroke therapy. Expert Opin Pharmacother 2008;9:887–900. doi: 10.1517/14656566.9.6.887 [DOI] [PubMed] [Google Scholar]
- 42.Lo EH, Ning M. Mechanisms and challenges in translational stroke research. J Investig Med 2016;64:827–9. doi: 10.1136/jim-2016-000104 [DOI] [PubMed] [Google Scholar]
- 43.Goldberg MP, Ransom BR. New light on white matter. Stroke 2003;34:330–2. doi: 10.1161/01.STR.0000054048.22626.B9 [DOI] [PubMed] [Google Scholar]
- 44.Falcao ALE, Reutens DC, Markus R, et al. The resistance to ischemia of white and gray matter after stroke. Ann Neurol 2004;56:695–701. doi: 10.1002/ana.20265 [DOI] [PubMed] [Google Scholar]
- 45.Koga M, Reutens DC, Wright P, et al. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke 2005;36:2132–7. doi: 10.1161/01.STR.0000181066.23213.8f [DOI] [PubMed] [Google Scholar]
- 46.Bristow MS, Simon JE, Brown RA, et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab 2005;25:1280–7. doi: 10.1038/sj.jcbfm.9600135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.