Abstract
Endogenous self-reactive autoantibodies (AAs) recognize a range of G-protein coupled receptors (GPCRs). They are frequently associated with cardiovascular, neurologic, and autoimmune disorders, and in some cases directly impact disease progression. Many GPCR-AAs modulate receptor signaling, but molecular details of their modulatory activity are not well understood. Technological advances have provided great insight into GPCR biology, which now facilitates deeper understanding of GPCR-AA function at the molecular level. Most GPCR-AAs are allosteric modulators and exhibit a broad range of pharmacological properties, altering both receptor signaling and trafficking. Understanding GPCR-AAs is not only important for defining how these unusual GPCR modulators function in disease, but also provides insight into the potential use and limitations of employing therapeutic antibodies to modulate GPCR signaling.
Keywords: G protein-coupled receptor, autoantibody, autoimmunity, allosteric modulation
Antibodies as GPCR therapeutics
Over one hundred G-protein coupled receptors (GPCRs) are targeted by approved pharmaceuticals, making the family one of the most successful classes of drug targets [1]. Although biopharmaceuticals have gained popularity for therapeutic targeting of many other cell-surface molecules [2], GPCRs are almost exclusively targeted by small molecules. The unique pharmacokinetic and pharmacodynamic profiles and exceptional target specificity of biotherapeutics make antibodies compelling alternatives to small molecule drugs [3]. For example, antibodies do not readily enter the central nervous system, permitting selective targeting of peripheral receptors, such as the adenosine A2a receptor, which plays distinct roles in neuronal and cardiac processes and immunity [4]. Additionally, antibodies can trigger targeted cytotoxic immune responses through their Fc regions, which may be desirable in certain indications. Current drug discovery efforts have produced therapeutic antibodies targeting GPCRs with large substrate binding extracellular domains, which can serve as the antibody binding site, and chemokine receptors [5]. However, identifying functional antibodies remains highly challenging for the majority of GPCRs that lack large ectodomains and instead recognize their ligands directly within their transmembrane domains and extracellular loops. With few examples available, the capabilities and limitations of using antibodies to modulate GPCR signaling are largely unclear.
Endogenous self-antigen reacting antibodies, known as autoantibodies (AAs, see Glossary), have been detected for a wide range of GPCRs, including members of the adrenergic, muscarinic, angiotensin, and metabotropic glutamate families (Figure 1, Supplementary Table 1). The majority of AAs reported to date independently act as agonists and activate GPCRs, but they can both increase and decrease the efficacy of orthosteric agonists. In some cases, GPCR-AAs induce non-canonical receptor-mediated activities. With the ability to alter a receptor’s endogenous biology, GPCR-AAs are often associated with disease and can be pathogenic. GPCR-AAs could serve as scaffolds for future therapeutic antibody discovery efforts and may also provide valuable insight into how antibodies can be used to effectively modulate GPCR function.
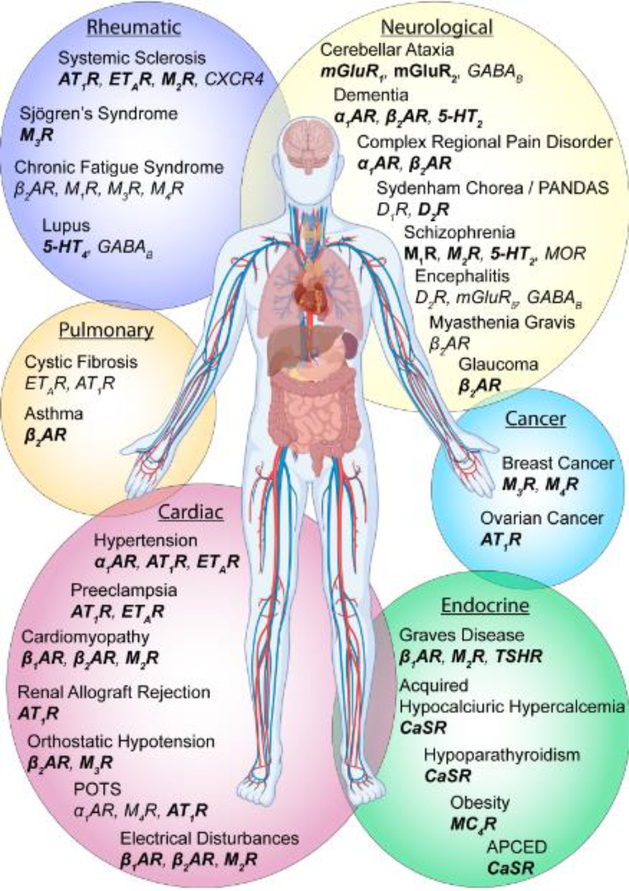
Figure 1. GPCR-AAs are detected in a variety of conditions.
GPCR-AAs are reported for 26 different GPCRs including the 5-HT2 and 5-HT4 serotonin receptors, α1, β1, and β2 adrenergic receptors (β1AR, β2AR, α1AR), angiotensin II type I receptor (AT1R), calcium sensing receptor (CaSR), endothelin type A receptor (ETAR), GABAB receptor, M1, M2, M3, and M4 muscarinic acetylcholine receptors (M1R, M2R, M3R, M4R), melanocortin-4 receptor (MC4R), metabotropic glutamate receptors 1, 2, and 5 (mGluR1, mGluR2, mGluR5), thyroid stimulating hormone receptor (TSHR), and μ-opioid receptor (MOR). GPCR-AAs are typically detected with ELISA, cell-staining, radioligand binding, or functional bioassays. GPCR-AAs that influence receptor-mediated signaling events are bolded. See Supplementary Table 1 for additional information on AA epitopes and functional effects of AAs.
Generation of autoantibodies
Antibody-mediated immune response
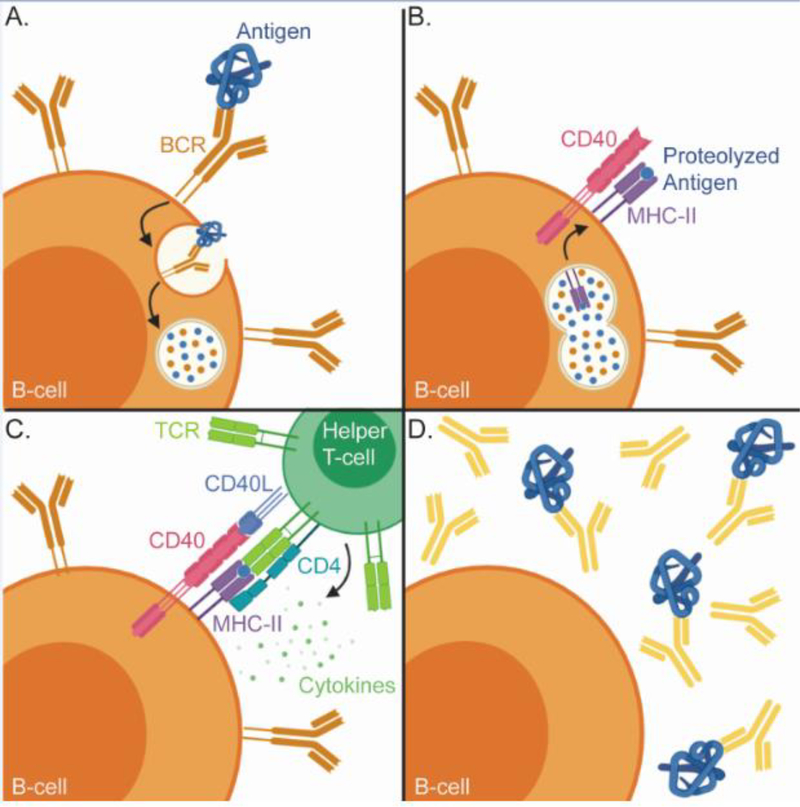
B-cells display a massive repertoire of antibody clones in the form of B cell receptors (BCRs), which bind to specific antigens through their complementary determining regions (CDRs). Recognition of a protein antigen by the BCR induces internalization of the antibody-antigen complex and proteolysis of the antigen (Figure 2). Antigen peptides are displayed on the surface of B-cells by the Class II major histocompatibility complex (MHC-II). Recognition of the MHC-II linked peptide by a peptide specific T-cell receptor induces a signaling cascade, which stimulates B-cell proliferation, antibody affinity maturation, antibody class switching, and the secretion of antibodies into circulation [6].
Figure 2. Antibody production requires input from B-cells and T-cells.
A) Antigens are recognized by the B-cell receptor (BCR), a membrane tethered antibody. The bound antigen is internalized and proteolyzed. B) Proteolyzed antigen is loaded in to the class II MHC and trafficked to the B-cell surface. C) The MHC-II bound peptide is recognized by a peptide specific T-cell receptor on the surface of a CD4+ helper T-cell. A series of co-activating interactions between the T-cell and B-cell, such as the engagement of CD40 with CD40L, trigger the release of cytokines, which initiates B-cell activation and antibody maturation. D) Activated B-cells differentiate into antibody secreting plasma cells to immediately respond to the antigen and memory B-cells, which preserve the immune response.
In order to suppress inappropriate immune responses to endogenous proteins, developing B-cells and T-cells undergo clonal selection to remove self-reactive B-cell and T-cell receptors. The autoimmune regulator (AIRE) transcription factor promotes low level expression of many proteins not typically resident in the thymus allowing T-cell receptors to broadly sample endogenous peptides. Self-reactive T-cells are eliminated [7]. Similarly, contact with a self-antigen in early B-cell development triggers genetic recombination of the antibody to reduce self-reactivity. If self-reactivity persists, apoptosis occurs [8].
GPCR-AAs in healthy individuals
Clonal selection of the immune repertoire requires a fine balance; self-reactivity must be avoided without relinquishing the ability to effectively respond to a diverse range of antigens. Despite clonal selection, some self-reactive T-cells and B-cells leave the thymus and bone marrow and many are maintained in an anergic state through peripheral tolerance mechanisms [9]. Still, low levels of “natural” AAs are present in healthy individuals, including antibodies recognizing GPCRs [11]. Some GPCR AAs detected in healthy individuals are apparently non-functional [12–15]. However, AAs recognizing the μ-opioid (MOR) and δ-opioid (DOR) receptors, isolated from therapeutic intravenous immunoglobulin (IVIG) pooled from thousands of apparently healthy donors or single healthy donors, activate G-protein signaling [16–18]. AAs targeting the endothelin type A receptor (ETAR) in healthy individuals promote neutrophil migration through a receptor dependent mechanism[11]. Furthermore, “natural” AAs targeting the CCR5 chemokine receptor, a coreceptor for HIV-1, are found in a subset of healthy individuals and some individuals who are infected with HIV and do not receive anti-retroviral therapy, but do not progress to AIDS [19, 20]. CCR5-AAs inhibit binding of the endogenous ligand Mip1β (CCL4) to the receptor [20] and block HIV viral entry [19, 21, 22] neutralizing viral infection [20, 23, 24]. Thus, not all GPCR-AAs are pathogenic and, in some cases, can be beneficial.
Loss of self-tolerance and production of GPCR-AAs
The majority of disease-associated GPCR-AAs arise through B-cell activation and the subsequent antibody maturation process. The production of high-affinity AAs can result from cross-reactivity between foreign and self-antigens (molecular mimicry), alterations to the self-antigen through post-translational modification, exposure of “immunologically privileged” antigens through tissue damage, increased inflammatory signals, or deficiencies in self-tolerance mechanisms [7, 8, 10]. Several of these mechanisms are linked to the production of GPCR-AAs. Placental damage and exposure to inflammatory cytokines stimulates the production of AAs targeting the angiotensin II type I receptor (AT1R) in a rat model of preeclampsia [25], a pregnancy-related hypetensive disease and a leading cause of maternal and fetal morbidity [26]. Molecular mimicry between ribosomal proteins from the parasite Trypanosoma cruzi (T. cruzi), the causative agent of Chagas’ disease, and the β1 adrenergic receptor (β1AR) produce antibodies cross reacting with T. cruzi proteins and the β1AR [27–29]. Similarly, exposure to Streptococcus pyogenes produces antibodies recognizing both bacterial antigens and the D2 dopamine receptor (D2R) [30]. Mutations in the transcription factor AIRE, which regulates T-cell receptor self-tolerance, results in autoimmune polyendocrine syndrome type 1 (APS1, also known as APCED). Individuals with this syndrome are prone to developing AAs for the calcium-sensing receptor (CaSR), which alter calcium homeostasis and cause hypoparathyroidism [31].
The events triggering the production of many GPCR-AAs are unidentified. For example, AAs targeting the metabotropic glutamate receptor 5 (mGluR5) are linked to neurological symptoms such as memory loss in individuals with Hodgkin’s lymphoma [32, 33]. These cognitive symptoms, which are likely caused by mGluR5-AAs, decline with successful cancer treatment [32]. Therefore, mGluR5-AA production may be paraneoplastic and occur as a response to aberrant receptor expression on the cancer cells [32, 33], but the exact antibody generating stimulus remains unknown. In other cases, the ongoing immune response to a self-antigen can trigger the production of AAs for additional self-antigens through “epitope spreading” [34], which may account for correlating levels of GPCR-AAs targeting multiple receptors in some diseases [11].
Pathological role of GPCR targeting autoantibodies
As GPCRs regulate many aspects of biology (Box 1), alterations to canonical GPCR signaling mechanisms by AAs often have pathological consequences. In order to classify an AA as a causative agent of disease, an AA must recapitulate features of the disease in an animal model as defined by Witebsky’s postulates for autoimmune diseases [35]. Titers of GPCR-AAs are often low and the sequence of most GPCR-AAs are unknown making it difficult to perform such experiments with endogenous AAs. Often evidence for the pathological role of AAs is gathered by replicating the immune response through exposure of an animal to antibody-accessible extracellular regions of the receptor to produce AAs that share many of the activities of the human AA (AA-mimics) (Table 1). AA-mimics produced via immunization of animals with peptides derived from the extracellular loops of β1AR induce many of the pathologies found in individuals with β1AR-AA associated cardiomyopathy, confirming β1AR-AAs as cardiotoxic [36, 37]. More complete evidence for a causative role of β1AR-AAs in cardiomyopathy comes from passive transfer experiments, where exposure of healthy mice to β1AR-AAs induces cardiac damage [37]. Similar experiments implicate AT1R-AAs in the development of preeclampsia [38], as transfer of AT1R-AAs from a preeclamptic human patient to healthy pregnant mice induced many of the symptoms of preeclampsia. Blockade of the AA interaction with AT1R prevents the development of the disease [39]. Similarly, mice treated with AAs targeting the metabotropic glutamate receptor 1 (mGluR1) from individuals with coordination deficiencies caused by paraneoplastic cerebellar ataxia develop analagous neurological sympotoms [40].
Box 1. Canonical GPCR Signaling.
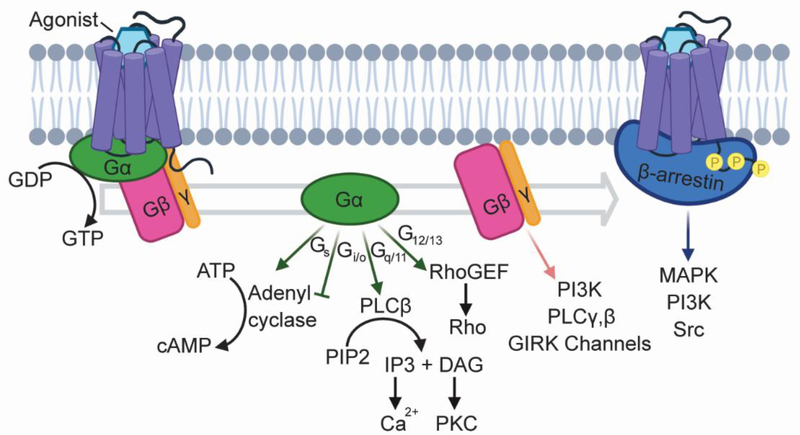
GPCRs are dynamic proteins that fluctuate between a range of functional states with varying affinities both orthosteric ligands and intracellular signal transducers. In canonical signaling pathways, the binding of an endogenous or synthetic agonist to the receptor triggers a conformational change within the receptor’s seven transmembrane domain, which recruits heterotrimeric G-proteins containing Gα, Gβ, and Gγ subunits and promotes exchange of GDP to GTP in the Gα subunit. The activated G-proteins dissociate from the receptor and induce a series downstream signaling events to elicit a biological response (Box 1, Figure I). G-protein signaling is attenuated through receptor phosphorylation by GPCR kinases (GRKs) and the recruitment of β-arrestins, which sterically hinder interactions between GPCRs and G-proteins and promote receptor internalization. In addition to their role in desensitization, β-arrestins initiate G-protein independent signaling cascades (Box 1, Figure I). Agonists that preferentially activate G-protein dependent or independent signaling pathways and are known as “biased” agonists.
Box 1, Figure I. GPCRs induce a variety of downstream signaling cascades.
GPCRs are stimulated by a wide range of agonists, including small molecules, peptides, and proteins such as antibodies, and they regulate many biological processes. Downstream signaling is mediated by the Gα, Gβ, and Gγ subunits of heterotrimeric G-proteins. Sixteen Gα subunits are encoded by four families (Gs, Gi/o, Gq/11, G12/13), each of which engages a distinct signaling cascade [75]. Additionally, four different Gβ subunits and twelve Gγ subunits stimulate additional signaling pathways [136]. Recruitment of β-arrestins suppress G-protein signaling, and initiates G-protein independent signaling cascades, primarily through MAP kinase (MAPK) pathways [137].
Table 1.
GPCR-AAs identified as pathogenic
| Receptor | Syndrome | Method | Pathology | Reference |
|---|---|---|---|---|
| β1AR | Cardiomyopathy | ECL2 peptide immunization | Increased heart weight Enlarged left and right ventricles Decreased right and left ventricle wall thickness Cardiac degeneration and inflammation |
[36] |
| ECL2 peptide immunization | Left ventricle hypertrophy | [116] | ||
| ECL2 peptide immunization | Left ventricle dilation and dysfunction Increased heart mass |
[37] | ||
| Passive transfer | Left ventricle dilation and dysfunction Increased heart mass |
[37] | ||
| ECL2 peptide immunization | Left ventricle dilation Decrease in fractional shortening | [117] | ||
| ECL2 peptide immunization | Cardiac electrical remodeling | [118] | ||
| M2R | Atrial fibrillation | ECL2 peptide immunization | Altered cardiac electrophysiology Atrial fibrosis | [119] |
| Cardiomyopathy | ECL2 peptide immunization | Enlarged right ventricle Decreased right ventricle wall thickness Cardiac degeneration and inflammation |
[36] | |
| ECL2 peptide immunization | Decreased myocardial contractility Decreased diastolic function Ventricle dilation and wall thinning |
[120] | ||
| ECL2 plasmid immunization | Reduced left ventricular wall thickness Reduced ejection fraction |
[121] | ||
| Passive transfer | Reduced left ventricular wall thickness Reduced ejection fraction |
[121] | ||
| M3R | Sjögren’s Syndrome | Passive transfer | Overactive bladder | [122] |
| Passive transfer | Decreased saliva volumes | [123] | ||
| AT1R | Cardiovascular Disease | ECL2 peptide immunization | Cardiac hypertrophy Increased blood pressure Increased heart rate |
[124] |
| Preeclampsia | Passive transfer | Hypertension Proteinuria Glomular endotheliosis Placental abnormalities Small fetus size Increased levels of soluble fms-related tyrosine kinase-1 (sFlt1) |
[39] | |
| Passive transfer | Increased blood pressure Production of endothelin-1 | [125] | ||
| Passive transfer | Increased sensitivity to AngII | [126] | ||
| Passive transfer | Increased blood pressure Increased sFlt1 Increased sEndoglin |
[127] | ||
| Passive transfer | Increased blood pressure Increased oxidative stress |
[128] | ||
| Passive transfer | Increased blood pressure Increased sensitivity to AngII |
[129] | ||
| ETAR | Pulmonary arterial hypertension | Passive transfer | Diminished peripheral vasculature Dilated pulmonary arteries Right ventricular hypertrophy Vascular remodeling |
[130] |
| MC4R | Obesity | N-terminal peptide immunization | Increased body weight Increased food intake Increased plasma triglyceride levels |
[131] |
| Passive transfer | Increased food intake | [131] | ||
| Passive transfer | Increased food intake | [132] | ||
| TSHR | Grave’s Disease | Passive transfer | Thyroid hormone secretion | [133] |
| Passive transfer | Thyroid hormone secretion Inhibition of thyroid hormone secretion | [134] | ||
| mGluR1 | Cerebellar ataxia | Passive transfer | Ataxia | [40] |
Effects of AAs at the molcular level
Identification of extracellular epitopes
Binding and neutralization experiments have defined epitopes on the extracelullar surface of GPCRs involved in AA binding. AAs interacting with class C or glycohormone GPCRs can activate or inhibit receptor function through interactions with the receptor’s large N-terminal ectodomains. Interaction of an AA with the thyroid-stimulating hormone receptor (TSHR) ectodomain activates the receptor and causes hyperthyroidism associated with Graves’ disease. A second TSHR-AA binds a distinct, yet overlapping, region of the ectodomain and suppresses basal signaling, which results in hypothyroidism (Figure 3a) [41, 42]. A third type of TSHR-AA interacts with a region of the receptor that undergoes intramolecular cleavage, interrupting processing of the receptor and consequently function [43]. Similarly, AAs binding the extracellular venus flytrap domain of the class C CaSR can positively or negatively regulate receptor function resulting in either hypocalcemia or hypercalcemia [44, 45].
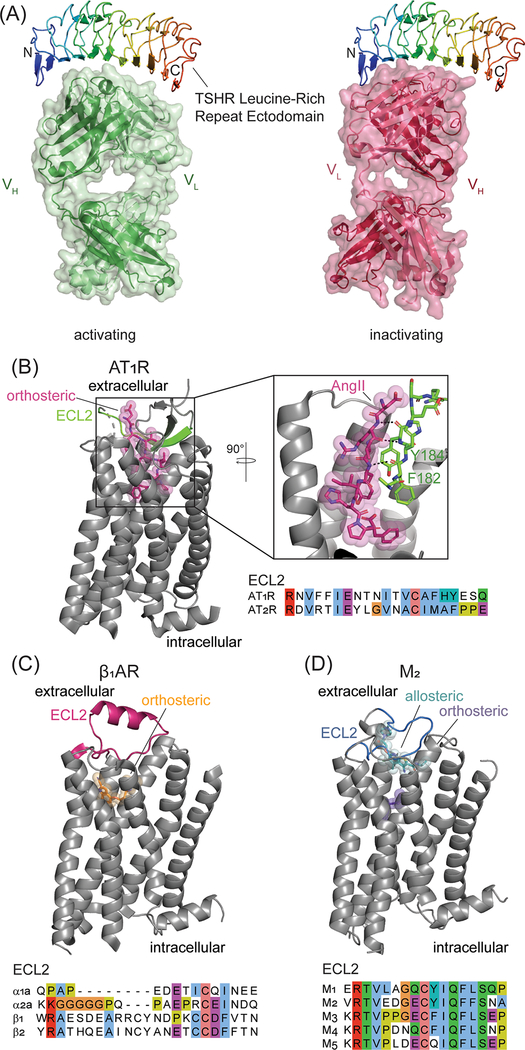
Figure 3. Antigen recognition by GPCR-AAs.
A) The TSHR contains an N-terminal leucine rich repeat (LRR) ectodomain (colored as a rainbow, from blue (N-terminus) to red (C-terminus)) that binds the receptor’s endogenous agonist thyroid stimulating hormone (TSH). TSHR-AAs interfere with TSH binding by interacting with the LRR. Despite recognizing adjacent regions, TSHR-AAs both activate (PDB: 3G04 [41]) and suppress (PDB: 2XWT [42]) receptor signaling. B-D) AA epitopes and ECL2 sequence alignments. B) Phe182 and Tyr184 in the ECL2 epitope (green) recognized by AT1R-AAs interacts with the receptor’s endogenous peptide agonist angiotensin II (pink) through backbone interactions (PDB: 6OS0 [56]). C) ECL2 (pink, PDB: 2VT4 [135]), a common epitope for adrenergic receptor AAs is highly divergent among receptor subtypes. D) The ECL2 binding site for M2-AAs (blue, PDB: 4MQT [58]) overlaps with a known allosteric regulatory site (teal) for muscarinic receptors
Class A GPCRs, which mostly lack structured ectodomains, have an N-terminus of variable length and three extracellular loops (ECLs) accessible to antibodies. ECL1 is short and portions of the loop are buried, including a conseved tryptophan that packs against a conserved disufide bond between ECL2 and transmembrane helix three. ECL2 is the largest of all three ECLs and one of the the most distinct features of Class A GPCRs both in sequence and structure (Figure 3b–d). Largely exposed, ECL2 lines the entrance to the orthosteric ligand binding site and can rearrange upon agonist binding. In some cases, such as in many peptide receptors, ECL2 directly interacts with agonists. ECL3 is variable and exposed, but typically short, sometimes unstructured, and not associated with activation-related conformational changes.
All three ECLs have been implicated in AA binding to class A GPCRs. The most predominant epitope for functional AAs is ECL2 (Figure 3b–d, Supplementary Table 1) [46–53], but interactions with ECL1 and 3 are crucial for the function of a few AAs [18, 46, 53–55]. As ECL2 is variable in sequence even among subtypes of receptors, AAs may readily achieve subtype selectivity. AT1R-AAs from women with preeclampsia interact with the C-terminal portion of ECL2 (Figure 3b, amino acids 181–187) [51] that directly interacts with the endogenous peptide ligand angiotensin II (AngII) (Figure 3b) [56]. While this suggests that AT1R-AAs in individuals with preeclampsia may directly interfere with the binding of orthosteric ligands, ECL2 binding AT1R-AAs from hypertensive patients are not reported to effect AngII binding [57]. β1AR-AAs studied in individuals with Chagas’ cardiomyopathy and dilated cardiomyopathy bind to adjacent regions of ECL2 (amino acids 201–205 and 206–218, Figure 3c) [27, 46], and confer similar physiological effects (Figure 3c). Binding studies with AAs for the M2 muscarinic receptor (M2R) demonstrate that M2R-AAs are competitive with the allosteric modulator gallamine, which occupies a well-characterized allosteric site for muscarinic receptors [58–61], and partially reverse gallamine’s ability to attenuate orthosteric ligand dissociation [62]. Allosteric modulators of muscarinic receptors mediate their effects by binding to the extracellular vestibule of the orthosteric ligand binding site, which includes ECL2 (Figure 3d). The ECL2 binding M2R-AAs may share some of the functionalities of muscarinic allosteric modulators and alter access to the orthosteric site, but as M2R-AAs do not slow orthosteric ligand dissociation to the same extent as gallamine the AAs may not fully occupy the allosteric site [62].
GPCR-AAs as allosteric modulators
Allosteric ligands influence coupling of GPCRs to G-proteins and β-arrestins by altering the ability of agonists or antagonists to bind to the orthosteric site or directly influencing the receptor’s global conformation (Box 2) [63], conferring distinct pharmacological properties to allosteric modulators. While extensive pharmacological characterization is needed to fully define how AAs influence GPCR function, several observations support an allosteric mechanism as a general feature of most GPCR-targeting AAs. Generally, activating AAs for adrenergic and muscarinic receptors displace radiolabeled antagonists in a non-competitive manner, typically decreasing the number of available antagonist binding sites with little to no effect on antagonist affinity [64–71]. This means that these AAs do not directly compete for the orthosteric site of the receptor, but likely induce the receptor to more frequently sample active or active-like receptor states that are not compatible with antagonist binding and favor transducer binding. At equilibrium, this manifests as an overall reduction in antagonist-accessible binding sites. Further support for the possibility of AA’s directly influencing receptor conformation is seen in agonist competition binding experiments performed in the presence of G-proteins and AAs. G-proteins typically produce an additional high-affinity agonist binding state in addition to that of the lower-affinity uncoupled receptor [72]. Similar to exposure to an orthosteric agonist or nucleotide addition, preincubation of the M2R with M2R-AAs fully leads to release of the G-protein and diminishes the high affinity G-protein-coupled component of agonist binding [67]. In addition, BRET studies demonstrate AA-induced conformational change in the β1AR [13].
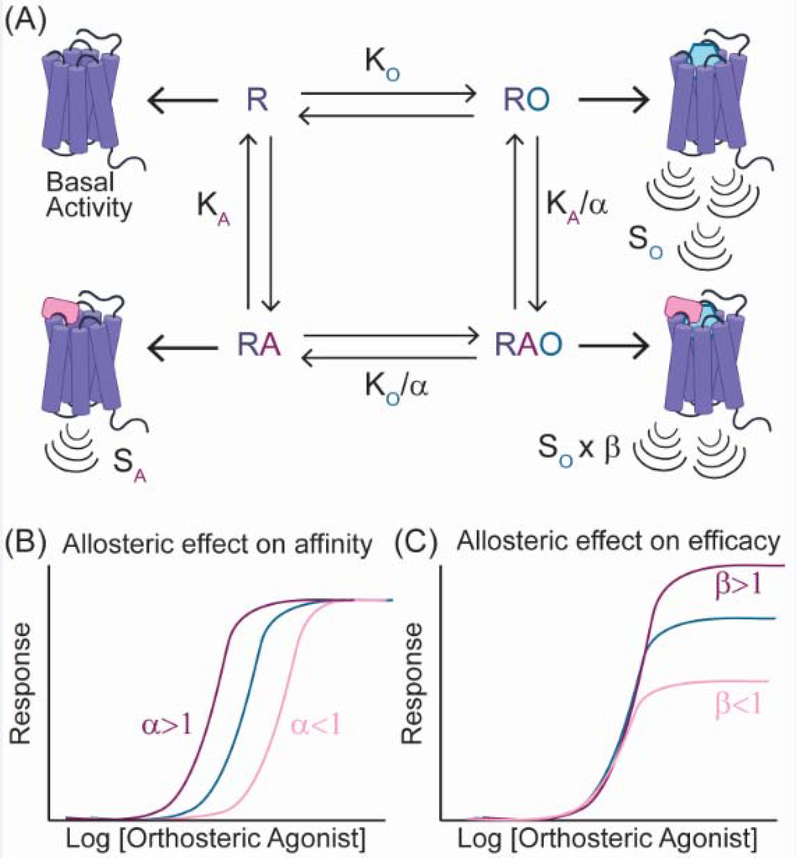
Box 2. Allosteric modulation of GPCR signaling.
By binding outside the orthosteric binding site, allosteric modulators can both positively and negatively influence the affinity of orthosteric ligands and modulate a receptor’s ability to couple transducers. The combination of these independent effects yields varied outcomes on the intrinsic efficacy (signaling strength) of an orthosteric ligand. In the operational model describing orthosteric ligand-receptor interactions, the effects of allosteric modulators on ligand affinity and the transducer coupling are described by the cooperativity factors α and β (Box 2, Figure I) [63]. Individually, coupling of the allosteric modulator or orthosteric ligand elicit distinct signaling response. Combined, the allosteric and orthosteric ligand can influence one another to elicit a new signaling response, that differs from the allosteric or orthosteric ligand alone. The cooperativity factors for allosteric modulators are dependent on the identity of the orthosteric ligand, which can result in varied effects on the efficacy of different orthosteric agonists or in the absence of ligand.
Box 2, Figure I. Influence of allosteric molecules on the operational model of ligand-receptor interactions.
A) The cooperativity factors α and β define the effects of the allosteric modulator (A) on the orthosteric ligand (O). α is a measure of the effect of the orthosteric and allosteric ligands on their respective equilibrium constants (KO, KA). β describes the effect of the allosteric modulator on the signaling response (S). B) When α>1 (purple) the allosteric ligand potentiates signaling, whereas α<1 (pink) suppresses signaling in response to the orthosteric ligand. When α=1 the allosteric modulator has no effect on the activity of the orthosteric ligand (blue). C) β describes the effect of the allosteric modulator on the efficacy or intensity of the signaling response (blue). When β>1 the level of signaling is increased (purple), but when β<1 a decreased response occurs (pink). The values of α and β are independent of one another; an allosteric ligand with α>1 can have a β<1.
Like other allosteric modulators, the effects of AAs in the presence of an orthosteric agonist are distinct from those on the unliganded receptor (Supplementary Table 1). Activating GPCR-AAs can both positively and negatively modulate the activity of orthosteric agonists (Box 2). Nearly all functional β1AR-AAs recognize an ECL2 epitope and result in activation of adenylyl cyclase. Despite these shared features, the effects of β1AR-AAs on ligand efficacy diverge, with most suppressing [65, 68, 73, 74], but some enhancing [68, 69] agonist-induced cAMP production.
Unique pharmacology of GPCR-AAs
AAs influence G-protein selectivity
Many GPCRs display a preference for one of four classes of Gα transducer subunits (Gs, Gi/o, Gq/11, G12/13), but can exhibit varying degrees of promiscuity [75, 76]. Like orthosteric ligands, AAs can influence G-protein selectivity. The β2AR primarily activates Gs-mediated signaling to stimulate cAMP production, but it can also couple to Gi [77]. While the majority of β2AR-AAs stimulate cAMP [47, 78], β2AR-AAs derived from a subset of individuals with heart failure decreased cAMP production [79]. As the addition of pertussis toxin (PTX), a Gi inhibitor, suppressed AA activity, these β2AR-AAs are not simply acting as inverse agonists, but rather activate Gi [79]. An analogous negative inotropic effect induced by β2AR-AAs on myocardial contractility was reversed by β2AR antagonists [80]. In this example, Gi-mediated activity is suspected, but not confirmed. Protein kinase A (PKA)-mediated phosphorylation of β2AR has been shown to promote coupling to Gi [77], suggesting that the AA may stabilize a receptor state that promotes PKA phosphorylation. β2AR-AAs that activate either Gs or Gi bind to ECL2 [47, 78, 79]. This suggests that antibodies binding to similar extracellular regions of a GPCR can exhibit divergent signaling biology. AAs recognizing the 5-hydroxytryptamine 2A receptor (5-HT2A) also alter G-protein selectivity. Some 5-HT2A-AAs activate Gq signaling pathways to cause neurotoxic effects [81, 82]. However, AAs from a subset of individuals with schizophrenia elicit 5-HT2A-mediated neuroprotection, which is sensitive to PTX, indicative of Gi coupling [81]. Finally, an AA from an individual with acquired hypocalciuric hypercalcemia targeting the CaSR, which activates both Gq and Gi, potentiates the Gq-mediated generation of inositol phosphate, but suppresses Gi-mediated ERK 1/2 phosphorylation [83]. Effects of the “Gq-biased” CaSR-AAs, which bind to the receptor’s extracellular venus flytrap domain, are reversed by binding of the CaSR positive allosteric modulator cincalet to the receptor’s 7-TM domain [84], further linking the action of ectodomain binding AAs to transducer coupling.
Activation of non-canonical signaling pathways by AAs
In addition to altering canonical G-protein-mediated pathways, AAs can support signaling through mechanisms that differ from orthosteric agonists. For example, a β1AR-AA delays activation of downstream MAP kinase signaling. Unlike orthosteric agonists, β1AR-AA-induced ERK 1/2 phosphorylation is sensitive to a Src kinase inhibitor [85], suggestive of Gicoupled Gβγ-dependent MAPK activation [86]. However, PTX is not inhibitory, indicating that the AA is invoking an alternative MAPK activation network. Although MAPK activity is not dependent on receptor endocytosis [85], this β1AR-AA may support β-arrestin based MAPK activity, which also stimulates ERK activity through Src [87]. While the mechanism of β1AR-AA induced ERK activation is not fully understood, it is clear that it differs from those of orthosteric agonists.
GPCR-AAs prolong signaling
Activated GPCRs normally undergo both rapid desensitization that suppresses G-protein signaling cascades and long-term desensitization that provides temporal control to signaling responses and results in tachyphylaxis in response to clinically used agonists [88, 89]. Rapid desensitization is largely mediated by the recruitment of arrestin and subsequent receptor internalization, whereas long-term desensitization is controlled by receptor expression and degradation. For typical agonists, rapid desensitization occurs within minutes of receptor activation. AAs targeting the β1AR, α1AR, M2R, and ETAR sustain GPCR signaling for hours and do not undergo rapid desensitization [49, 74, 90]. Prolonged signaling by AAs is not indefinite, as long-term desensitization mechanisms may still function [91]. Deficiencies in short-term desensitization could arise from reduced engagement of β-arrestins, which is characteristic of G-protein-biased signaling. Overall, the effects of AAs on β-arrestin recruitment are not well established and appear to be quite variable. Murine β1AR-AA mimics and M2R-AAs from individuals with Chagas’ disease are incapable of recruiting β-arrestins [92, 93], but an M3R-AA from an individual with postural hypotension promotes β-arrestin recruitment [78]. Similarly, AT1R-AAs from preeclamptic women do not support β-arrestin recruitment [94], but AT1R-AAs from individuals with postural orthostatic tachycardia syndrome (POTS) stimulate recruitment of β-arrestin [95]. The lack of β-arrestin recruitment to the AT1R is surprising as it strongly couples to β-arrestins in response to agonist-induced signaling [96] and G-protein biased orthosteric ligands for the AT1R retain the ability to recruit arrestin [97]. This discrepancy in β-arrestin recruitment by AAs could be explained by different compositions of polyclonal AAs in the two patient groups or perhaps reduced or delayed β-arrestin recruitment mechanism, which may not be observed in all assay setups. Reports of the effect of AAs on receptor internalization are also varied with AAs for the same receptor both causing and suppressing internalization [13, 49, 67, 98].
Effect of GPCR-AAs on receptor trafficking
Once internalized, interactions between a GPCR and β-arrestins influence canonical receptor trafficking, with weak interactions between a receptor and β-arrestins promoting rapid recycling back to the membrane and strong interactions that prolonging endosomal residence times prior to recycling or degradation [96]. AAs, which permit receptor internalization, also influence receptor trafficking. However, the role of β-arrestins in AA-mediated receptor internalization is often undefined. Compared to endogenous agonists that stimulate rapid CCR5 internalization and recycling [99], CCR5-AAs induce slow clathrin-mediated internalization, depleting CCR5 from the cell surface over 48 hours [24]. Protein synthesis is required for the repopulation of CCR5 at the cell surface, suggesting that internalized AA-CCR5 complexes are not recycled [100]. Similarly, AA-mediated internalization of β1AR results in a loss of receptor recycling and presumably receptor degradation [98]. A loss of receptor recycling pathways may account for the downregulation of mGluR5 expression after exposure to AAs [101].
Receptor dimerization in AA-induced signaling
While the role of oligomerization in endogenous GPCR signaling is debated [102], dimerization is required for the agonistic activity of several AAs. Conversion of a dimeric β1AR-AA to a monovalent Fab fragment results in a loss of agonistic activity [103]. Similarly, M2R-AA Fab fragments do not reduce the efficacy of orthosteric agonists like full-length AAs [93]. An analogous phenomenon is observed with murine monoclonal AA-mimics for β1AR [92], β2AR [104], and M2R [105], where Fab fragments do not possess the agonistic effects of the full-length antibodies. In some cases, the AA Fab fragment acts as a receptor antagonist [104]. Crosslinking the AA-Fabs with an anti-mouse IgG restored the AA’s agonistic effects [104, 105], confirming the oligomeric requirement for AA-induced signaling. However, the specific interaction between the AA and receptor is crucial for activity, as the addition of a receptor-dimerizing anti-FLAG antibody is not sufficient for activation of FLAG-tagged β1AR [92]. The functional requirement for receptor dimerization in AA-mediated receptor activation is surprising, since monomeric β2AR is fully capable of activating G-proteins [106]. While additional biochemical and structural analysis is needed to understand the role dimerization plays in AA-mediated signaling, receptor activation by AAs could be similar to activation of dimeric class C GPCRs, where interactions between the ectodomain and ECL2, and between transmembrane helix 6 of each receptor monomer stabilize the active state [107–109]. Still, receptor dimerization is not necessary for all AA-mediated signaling, as a murine monoclonal antibody that cross-reacts with ECL2 of both β1AR and M2R acts as an agonist as a full-length antibody and a monomeric Fab fragment [110].
Concluding Remarks and Future Perspectives
Decades of research have identified and provided initial characterization of functionally modulatory GPCR-AAs. Still, there are countless unanswered questions regarding the mechanistic basis of GPCR-AA action and pathological role of GPCR-AAs (see Outstanding Questions). Conflicting reports on the prevalence of GPCR-AAs complicates correlating the presence of AAs with disease. Some discrepancies are a result of experimental differences [111]. Much work has relied on peptide-based ELISA assays, which are subject to false positives due to polyreactivity [69], will not detect antibodies recognizing three-dimensional conformational epitopes, and are not reflective GPCR-AA bioactivity [112]. Thus, the true frequency of functional GPCR-AAs is unknown. The majority of GPCR-AAs described in the literature are antibody agonists. It is possible that GPCR-AA antagonists exist but have largely gone undetected however, since they may not show any clear effect in a signaling assay in the absence of agonist. The increased use of ELISAs with membrane extracts containing over-expressed receptors [14] and development of standardized GPCR-AA ELISA assays (Cell-Trend) is a step towards consistency and may also allow identification of antagonistic or functionally neutral AAs. However, functional assays are critical for determining the potential pathogenicity of AAs. As many AAs are not abundant, increased application of highly sensitive secondary messenger assays [113] or assays relying on amplified signals, such as transcriptional readouts or bioassays are ideal for identifying functional AAs in patient samples. Defining the functionality of GPCR-AAs is especially essential for understanding the significance of “natural” GPCR-AAs detected in healthy individuals.
Outstanding Questions.
What is the true prevalence of functional AAs in both healthy individuals and disease states?
How are interactions with AAs and the extracellular loops transduced through the receptor core to activate G-proteins?
Do AAs stabilize receptor conformations that differ from orthosteric agonist bound states?
Why is dimerization often required for AA-mediated receptor activation?
Do AAs frequently induce biased signaling?
Are there distinct trafficking pathways for internalized AA-GPCR complexes?
Like other allosteric modulators, GPCR-AAs have distinct and complex pharmacological properties. The development of new technologies for studying both antibodies and GPCRs will certainly facilitate in depth mechanistic studies of GPCR-AAs to fully understand their biology. The majority of work characterizing GPCR-AAs has been performed with polyclonal patient samples or animal AA mimics produced through immunization. Limited access to patient-derived samples puts limitations on both the quantity and types of experiments used to study GPCR-AAs. Single-cell techniques commonly used to identify and clone rare virus-neutralizing antibodies [114] in combination with advances in preparing GPCR antigens for antibody discovery efforts [5] could rapidly identify patient-derived monoclonal GPCR-AA. Unlimited access to patient-derived monoclonal GPCR-AAs willenable detailed structure-function studies using lipidic cubic phase crystallography and serial crystallography techniques, as well as electron cryo-microscopy (Cryo-EM) [115], to determine the mechanism by which antibodies activate GPCRs. Additionally, access to monoclonal samples will facilitate full pharmacological profiling of GPCR-AAs
Overall, GPCR-AAs are an untapped resource for studying how allosteric antibodies can influence GPCR biology. While prior research has provided many intriguing hints regarding their function, additional insight into the action of GPCR-AAs could provide essential information for the development of antibody therapeutics, including non-canonical effects on receptor signaling and trafficking that could be advantageous or detrimental to drug development.
Supplementary Material
Highlights.
Self-reactive antibodies (autoantibodies or AAs) are produced when there is a breakdown in the immune system’s self-tolerance mechanisms and have been detected for twenty-six GPCRs. Despite considerable interest in therapeutic modulation of GPCRs with antibodies, little is known about the molecular function of AAs.
The vast majority of AAs described to date are functional and activate GPCR signaling, uncoupling receptors from endogenous signaling networks. Such modulation of signaling is often deleterious and many GPCR-AAs are either known to be pathogenic or associated with disease.
AAs act allosterically and possess unique pharmacological properties, which often diverge from orthosteric agonists.
A dimeric antibody is often required for AA-induced receptor activation, suggesting that AAs may invoke an activation mechanism distinct from orthosteric agonists.
Acknowledgments
We would like to thank Morgan Gilman and Niranjan Varma for critical reading of the manuscript. Figures were prepared using BioRender. This work was supported by National Institutes of Health (NIH) grant R21HD101596 (A.C.K.) and a Merck Postdoctoral Fellowship from the Helen Hay Whitney Foundation (M.A.S).
GLOSSARY
- Affinity maturation
activated B-cells undergo hypermutation producing antibodies with increased affinity for the targeted antigen
- Agonist
a molecule that activates a receptor, stimulating a cellular response
- Allosteric
a binding site on a receptor that differs from where the endogenous ligand binds
- Autoantibody
an endogenous antibody that recognizes a “self” protein, most frequently associated with autoimmune diseases
- Biased signaling
a phenomenon where an agonist preferentially activates one signaling outcome over another
- Chagas’ disease
a parasitic infection characterized by acute flu like symptoms, which can cause chronic cardiovascular damage
- Class A GPCR
the largest family of GPCRs, which share sequence homology to rhodopsin. Members include aminergic, peptide, chemokine, and olfactory receptors
- Class C GPCR
A small class of GPCRs containing the metabotropic glutamate receptors (mGluRs) and GABAB receptors, which contain large venus fly-trap ectodomains and are obligate dimers
- Class switching
activated B-cells can alter the isotype of the non-variable region of an antibody through recombination, altering the antibodies function in the immune response
- Efficacy
the maximum strength of a signaling response
- Enzyme-linked immunosorbent assay (ELISA)
A method to detect the presence of an antibody for a specific antigen
- Fab
a monomeric fragment encoding the variable regions of an antibody required for antigen interactions
- Natural autoantibodies
self-reactive antibodies present in healthy individuals
- Paraneoplastic
arising from cancer
Footnotes
Disclaimer Statement
A.C.K. is a cofounder and consultant for Tectonic Therapeutic Inc. and for the Institute for Protein Innovation, a non-profit research institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hauser AS et al. (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16 (12), 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodgers KR and Chou RC (2016) Therapeutic monoclonal antibodies and derivatives: Historical perspectives and future directions. Biotechnol Adv 34 (6), 1149–1158. [DOI] [PubMed] [Google Scholar]
- 3.Hutchings CJ et al. (2017) Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat Rev Drug Discov 16 (9), 661. [DOI] [PubMed] [Google Scholar]
- 4.Fredholm BB et al. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev 63 (1), 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchings CJ (2020) A review of antibody-based therapeutics targeting G protein-coupled receptors: an update. Expert Opin Biol Ther, 1–11. [DOI] [PubMed] [Google Scholar]
- 6.Parkin J and Cohen B (2001) An overview of the immune system. Lancet 357 (9270), 1777–89. [DOI] [PubMed] [Google Scholar]
- 7.Theofilopoulos AN et al. (2017) The multiple pathways to autoimmunity. Nat Immunol 18 (7), 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemazee D (2017) Mechanisms of central tolerance for B cells. Nat Rev Immunol 17 (5), 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards DM et al. (2016) Re-examining the nature and function of self-reactive T cells. Trends Immunol 37 (2), 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkon K and Casali P (2008) Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 4 (9), 491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabral-Marques O et al. (2018) GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun 9 (1), 5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiale PA et al. (1995) High prevalence of antibodies against β1- and β2-adrenoceptors in patients with primary electrical cardiac abnormalities. J Am Coll Cardiol 26 (4), 864–9. [DOI] [PubMed] [Google Scholar]
- 13.Bornholz B et al. (2013) Impact of human autoantibodies on β1-adrenergic receptor conformation, activity, and internalization. Cardiovasc Res 97 (3), 472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riemekasten G et al. (2011) Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis 70 (3), 530–6. [DOI] [PubMed] [Google Scholar]
- 15.Fu LX et al. (1993) Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest 91 (5), 1964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganathan P et al. (2009) Autoantibodies to the Δ-opioid receptor function as opioid agonists and display immunomodulatory activity. J Neuroimmunol 217 (1–2), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mace G et al. (1999) Isolation and characterization of natural human IgG with a morphine-like activity. Eur J Immunol 29 (3), 997–1003. [DOI] [PubMed] [Google Scholar]
- 18.Mace G et al. (1999) Morphine-like activity of natural human IgG autoantibodies is because of binding to the first and third extracellular loops of the μ-opioid receptor. J Biol Chem 274 (29), 20079–82. [DOI] [PubMed] [Google Scholar]
- 19.Bouhlal H et al. (2001) Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J Immunol 166 (12), 7606–11. [DOI] [PubMed] [Google Scholar]
- 20.Lopalco L et al. (2000) CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 In vitro. J Immunol 164 (6), 3426–33. [DOI] [PubMed] [Google Scholar]
- 21.Bouhlal H et al. (2005) Natural antibodies to CCR5 from breast milk block infection of macrophages and dendritic cells with primary R5-tropic HIV-1. J Immunol 174 (11), 7202–9. [DOI] [PubMed] [Google Scholar]
- 22.Bomsel M et al. (2007) Natural mucosal antibodies reactive with first extracellular loop of CCR5 inhibit HIV-1 transport across human epithelial cells. AIDS 21 (1), 13–22. [DOI] [PubMed] [Google Scholar]
- 23.Barassi C et al. (2004) CCR5-specific mucosal IgA in saliva and genital fluids of HIV-exposed seronegative subjects. Blood 104 (7), 2205–6. [DOI] [PubMed] [Google Scholar]
- 24.Pastori C et al. (2006) Long-lasting CCR5 internalization by antibodies in a subset of long-term nonprogressors: a possible protective effect against disease progression. Blood 107 (12), 4825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaMarca B et al. (2008) Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52 (6), 1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mol BWJ et al. (2016) Pre-eclampsia. Lancet 387 (10022), 999–1011. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari I et al. (1995) Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human β1-adrenergic receptor. J Exp Med 182 (1), 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smulski C et al. (2006) Structural basis of the cross-reaction between an antibody to the Trypanosoma cruzi ribosomal P2beta protein and the human beta1 adrenergic receptor. FASEB J 20 (9), 1396–406. [DOI] [PubMed] [Google Scholar]
- 29.Mahler E et al. (2001) A monoclonal antibody against the immunodominant epitope of the ribosomal P2β protein of Trypanosoma cruzi interacts with the human β1-adrenergic receptor. Eur J Immunol 31 (7), 2210–6. [DOI] [PubMed] [Google Scholar]
- 30.Cox CJ et al. (2013) Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol 191 (11), 5524–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavalas NG et al. (2007) The calcium-sensing receptor is a target of autoantibodies in patients with autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab 92 (6), 2107–14. [DOI] [PubMed] [Google Scholar]
- 32.Lancaster E et al. (2011) Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 77 (18), 1698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mat A et al. (2013) Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology 80 (14), 1349–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanderlugt CL and Miller SD (2002) Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol 2 (2), 85–95. [DOI] [PubMed] [Google Scholar]
- 35.Witebsky E et al. (1957) Chronic thyroiditis and autoimmunization. J Am Med Assoc 164 (13), 1439–47. [DOI] [PubMed] [Google Scholar]
- 36.Matsui S et al. (1997) Peptides derived from cardiovascular G-protein-coupled receptors induce morphological cardiomyopathic changes in immunized rabbits. J Mol Cell Cardiol 29 (2), 641–55. [DOI] [PubMed] [Google Scholar]
- 37.Jahns R et al. (2004) Direct evidence for a β1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest 113 (10), 1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqui AH et al. (2010) Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 55 (2), 386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou CC et al. (2008) Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 14 (8), 855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sillevis Smitt P et al. (2000) Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 342 (1), 21–7. [DOI] [PubMed] [Google Scholar]
- 41.Sanders J et al. (2007) Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17 (5), 395–410. [DOI] [PubMed] [Google Scholar]
- 42.Sanders P et al. (2011) Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol 46 (2), 81–99. [DOI] [PubMed] [Google Scholar]
- 43.Morshed SA et al. (2010) Neutral antibodies to the TSH receptor are present in Graves’ disease and regulate selective signaling cascades. Endocrinology 151 (11), 5537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kifor O et al. (2004) Activating antibodies to the calcium-sensing receptor in two patients with autoimmune hypoparathyroidism. J Clin Endocrinol Metab 89 (2), 548–56. [DOI] [PubMed] [Google Scholar]
- 45.Kifor O et al. (2003) A syndrome of hypocalciuric hypercalcemia caused by autoantibodies directed at the calcium-sensing receptor. J Clin Endocrinol Metab 88 (1), 60–72. [DOI] [PubMed] [Google Scholar]
- 46.Wallukat G et al. (1995) Anti-β1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol 27 (1), 397–406. [DOI] [PubMed] [Google Scholar]
- 47.Li H et al. (2013) Implications of a vasodilatory human monoclonal autoantibody in postural hypotension. J Biol Chem 288 (42), 30734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu ML et al. (1994) Functional autoimmune epitope on α1-adrenergic receptors in patients with malignant hypertension. Lancet 344 (8938), 1660–3. [DOI] [PubMed] [Google Scholar]
- 49.Wallukat G et al. (1999) Autoantibodies against M2 muscarinic receptors in patients with cardiomyopathy display non-desensitized agonist-like effects. Life Sci 64 (6–7), 465–9. [DOI] [PubMed] [Google Scholar]
- 50.Eftekhari P et al. (2000) Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol 30 (10), 2782–90. [DOI] [PubMed] [Google Scholar]
- 51.Wallukat G et al. (1999) Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103 (7), 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velloso EP et al. (2016) Identification of a novel agonist-like autoantibody in preeclamptic patients. Am J Hypertens 29 (3), 405–12. [DOI] [PubMed] [Google Scholar]
- 53.Tsuboi H et al. (2010) New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren’s syndrome. Clin Exp Immunol 162 (1), 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karczewski P et al. (2012) Agonistic autoantibodies to the α1 -adrenergic receptor and the β2 -adrenergic receptor in Alzheimer’s and vascular dementia. Scand J Immunol 75 (5), 524–30. [DOI] [PubMed] [Google Scholar]
- 55.Koo NY et al. (2008) Functional epitope of muscarinic type 3 receptor which interacts with autoantibodies from Sjögren’s syndrome patients. Rheumatology (Oxford) 47 (6), 828–33. [DOI] [PubMed] [Google Scholar]
- 56.Wingler LM et al. (2020) Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science 367 (6480), 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu ML et al. (2000) Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens 18 (7), 945–53. [DOI] [PubMed] [Google Scholar]
- 58.Kruse AC et al. (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504 (7478), 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voigtlander U et al. (2003) Allosteric site on muscarinic acetylcholine receptors: identification of two amino acids in the muscarinic M2 receptor that account entirely for the M2/M5 subtype selectivities of some structurally diverse allosteric ligands in N-methylscopolamine-occupied receptors. Mol Pharmacol 64 (1), 21–31. [DOI] [PubMed] [Google Scholar]
- 60.May LT et al. (2007) Structure-function studies of allosteric agonism at M2 muscarinic acetylcholine receptors. Mol Pharmacol 72 (2), 463–76. [DOI] [PubMed] [Google Scholar]
- 61.Leppik RA et al. (1994) Role of acidic amino acids in the allosteric modulation by gallamine of antagonist binding at the M2 muscarinic acetylcholine receptor. Mol Pharmacol 45 (5), 983–90. [PubMed] [Google Scholar]
- 62.Hernandez CC et al. (2008) Autoantibodies enhance agonist action and binding to cardiac muscarinic receptors in chronic Chagas’ disease. J Recept Signal Transduct Res 28 (4), 375–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christopoulos A and Kenakin T (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54 (2), 323–74. [DOI] [PubMed] [Google Scholar]
- 64.Sterin-Borda L et al. (1986) Chagasic IgG binds and interacts with cardiac β adrenoceptor-coupled adenylate cyclase system. Int J Immunopharmacol 8 (6), 581–8. [DOI] [PubMed] [Google Scholar]
- 65.Limas CJ et al. (1989) Autoantibodies against β-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res 64 (1), 97–103. [DOI] [PubMed] [Google Scholar]
- 66.Magnusson Y et al. (1990) Mapping of a functional autoimmune epitope on the β1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest 86 (5), 1658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leiros CP et al. (1997) Desensitization and sequestration of human M2 muscarinic acetylcholine receptors by autoantibodies from patients with Chagas’ disease. J Biol Chem 272 (20), 12989–93. [DOI] [PubMed] [Google Scholar]
- 68.Jahns R et al. (2000) Modulation of β1-adrenoceptor activity by domain-specific antibodies and heart failure-associated autoantibodies. J Am Coll Cardiol 36 (4), 1280–7. [DOI] [PubMed] [Google Scholar]
- 69.Jahns R et al. (1999) Autoantibodies activating human β1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation 99 (5), 649–54. [DOI] [PubMed] [Google Scholar]
- 70.Sterin-Borda L et al. (1990) Human chagasic IgG interacting with lymphocyte neurotransmitter receptors triggers intracellular signal transduction. FASEB J 4 (6), 1661–7. [DOI] [PubMed] [Google Scholar]
- 71.Bacman S et al. (1996) Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjögren’s syndrome. Clin Exp Immunol 104 (3), 454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Lean A et al. (1980) A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J Biol Chem 255 (15), 7108–17. [PubMed] [Google Scholar]
- 73.Limas CJ et al. (1990) Influence of anti-β-receptor antibodies on cardiac adenylate cyclase in patients with idiopathic dilated cardiomyopathy. Am Heart J 119 (6), 1322–8. [DOI] [PubMed] [Google Scholar]
- 74.Magnusson Y et al. (1994) Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the β1-adrenoceptor with positive chronotropic effect. Circulation 89 (6), 2760–7. [DOI] [PubMed] [Google Scholar]
- 75.Inoue A et al. (2019) Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell 177 (7), 1933–1947 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olsen RHJ et al. (2020) TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat Chem Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daaka Y et al. (1997) Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390 (6655), 88–91. [DOI] [PubMed] [Google Scholar]
- 78.Li H et al. (2012) Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension 59 (2), 402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao N et al. (2018) β2-adrenergic receptor autoantibodies alleviated myocardial damage induced by β1-adrenergic receptor autoantibodies in heart failure. Cardiovasc Res 114 (11), 1487–1498. [DOI] [PubMed] [Google Scholar]
- 80.Stavrakis S et al. (2011) Opposing cardiac effects of autoantibody activation of β-adrenergic and M2 muscarinic receptors in cardiac-related diseases. Int J Cardiol 148 (3), 331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimering MB and Nadkarni SG (2019) Schizophrenia plasma autoantibodies promote ‘biased agonism’ at the 5-Hydroxytryptamine 2A receptor: neurotoxicity is positively modulated by metabotropic glutamate 2/3 receptor agonism. Endocrinol Diabetes Metab J 3 (4). [PMC free article] [PubMed] [Google Scholar]
- 82.Zimering MB (2017) Diabetes autoantibodies mediate neural- and endothelial cell-inhibitory effects fia 5-Hydroxytryptamine- 2 receptor coupled to phospholipase C/inositol triphosphate/Ca2+ pathway. J Endocrinol Diabetes 4 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makita N et al. (2007) An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations. Proc Natl Acad Sci U S A 104 (13), 5443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makita N et al. (2019) Cinacalcet corrects biased allosteric modulation of CaSR by AHH autoantibody. JCI Insight 4 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tutor AS et al. (2007) Anti- β1-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc Res 76 (1), 51–60. [DOI] [PubMed] [Google Scholar]
- 86.Luttrell LM et al. (1996) Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem 271 (32), 19443–50. [DOI] [PubMed] [Google Scholar]
- 87.Luttrell LM et al. (1999) β-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283 (5402), 655–61. [DOI] [PubMed] [Google Scholar]
- 88.Rajagopal S and Shenoy SK (2018) GPCR desensitization: Acute and prolonged phases. Cell Signal 41, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams JT et al. (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65 (1), 223–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wallukat G et al. (2007) Agonistic autoantibodies against the endothelin 1 ETA - and α1-adrenergic- receptor in the sera of patients with idiopathic pulmonary arterial hypertension. (Abstract). Circulation 116 (suppl_16). [Google Scholar]
- 91.Podlowski S et al. (1998) Agonistic anti-β1-adrenergic receptor autoantibodies from cardiomyopathy patients reduce the beta1-adrenergic receptor expression in neonatal rat cardiomyocytes. Circulation 98 (22), 2470–6. [DOI] [PubMed] [Google Scholar]
- 92.Hutchings CJ et al. (2014) Monoclonal anti-β1-adrenergic receptor antibodies activate G protein signaling in the absence of beta-arrestin recruitment. MAbs 6 (1), 246–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beltrame SP et al. (2020) Impairment of agonist-induced M2 muscarinic receptor activation by autoantibodies from chagasic patients with cardiovascular dysautonomia. Clin Immunol 212, 108346. [DOI] [PubMed] [Google Scholar]
- 94.Bian J et al. (2019) Limited AT1 receptor internalization is a novel mechanism underlying sustained vasoconstriction induced by AT1 receptor autoantibody from preeclampsia. J Am Heart Assoc 8 (6), e011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu X et al. (2018) Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J Am Heart Assoc 7 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oakley RH et al. (2000) Differential affinities of visual arrestin, β–arrestin1, and β–arrestin2 for G-protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275 (22), 17201–10. [DOI] [PubMed] [Google Scholar]
- 97.Strachan RT et al. (2014) Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). J Biol Chem 289 (20), 14211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Limas CJ et al. (1991) Effect of antireceptor antibodies in dilated cardiomyopathy on the cycling of cardiac β receptors. Am Heart J 122 (1 Pt 1), 108–14. [DOI] [PubMed] [Google Scholar]
- 99.Mueller A et al. (2002) Pathways for internalization and recycling of the chemokine receptor CCR5. Blood 99 (3), 785–91. [DOI] [PubMed] [Google Scholar]
- 100.Venuti A et al. (2015) ERK1-Based Pathway as a New Selective Mechanism To Modulate CCR5 with Natural Antibodies. J Immunol 195 (7), 3045–57. [DOI] [PubMed] [Google Scholar]
- 101.Spatola M et al. (2018) Encephalitis with mGluR5 antibodies: Symptoms and antibody effects. Neurology 90 (22), e1964–e1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milligan G (2009) G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol 158 (1), 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Christ T et al. (2001) Autoantibodies against the β1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol 33 (8), 1515–25. [DOI] [PubMed] [Google Scholar]
- 104.Mijares A et al. (2000) From agonist to antagonist: Fab fragments of an agonist-like monoclonal anti-β2-adrenoceptor antibody behave as antagonists. Mol Pharmacol 58 (2), 373–9. [DOI] [PubMed] [Google Scholar]
- 105.Elies R et al. (1998) Immunochemical and functional characterization of an agonist-like monoclonal antibody against the M2 acetylcholine receptor. Eur J Biochem 251 (3), 659–66. [DOI] [PubMed] [Google Scholar]
- 106.Whorton MR et al. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A 104 (18), 7682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koehl A et al. (2019) Structural insights into the activation of metabotropic glutamate receptors. Nature 566 (7742), 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaye H et al. (2020) Structural basis of the activation of a metabotropic GABA receptor. Nature 584 (7820), 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mao C et al. (2020) Cryo-EM structures of inactive and active GABAB receptor. Cell Res 30 (7), 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cremaschi G et al. (2004) Modulatory effects on myocardial physiology induced by an anti-Trypanosoma cruzi monoclonal antibody involve recognition of major antigenic epitopes from β1-adrenergic and M2-muscarinic cholinergic receptors without requiring receptor cross-linking. J Neuroimmunol 153 (1–2), 99–107. [DOI] [PubMed] [Google Scholar]
- 111.Kamel R et al. (2005) Autoantibodies against the serotoninergic 5-HT4 receptor and congenital heart block: a reassessment. J Autoimmun 25 (1), 72–6. [DOI] [PubMed] [Google Scholar]
- 112.Limas CJ et al. (1992) Assessment of immune modulation of β-adrenergic pathways in human dilated cardiomyopathy: influence of methodologic factors. Am Heart J 123 (4 Pt 1), 967–70. [DOI] [PubMed] [Google Scholar]
- 113.Nikolaev VO et al. (2007) A novel fluorescence method for the rapid detection of functional β1-adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol 50 (5), 423–31. [DOI] [PubMed] [Google Scholar]
- 114.Tiller T et al. (2008) Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods 329 (1–2), 112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishchenko A et al. (2018) Structural biology of G protein-coupled receptors: new opportunities from XFELs and cryoEM. Curr Opin Struct Biol 51, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iwata M et al. (2001) Autoimmunity against the second extracellular loop of β1-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res 88 (6), 578–86. [DOI] [PubMed] [Google Scholar]
- 117.Buvall L et al. (2006) Phenotype of early cardiomyopathic changes induced by active immunization of rats with a synthetic peptide corresponding to the second extracellular loop of the human β-adrenergic receptor. Clin Exp Immunol 143 (2), 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukuda Y et al. (2004) Autoimmunity against the second extracellular loop of β1-adrenergic receptors induces early afterdepolarization and decreases in K-channel density in rabbits. J Am Coll Cardiol 43 (6), 1090–100. [DOI] [PubMed] [Google Scholar]
- 119.Hong CM et al. (2009) Effects of autoantibodies against M2 muscarinic acetylcholine receptors on rabbit atria in vivo. Cardiology 112 (3), 180–7. [DOI] [PubMed] [Google Scholar]
- 120.Zhang S et al. (2015) Mitochondrial Ultrastructural Alterations and Declined M2 Receptor Density Were Involved in Cardiac Dysfunction in Rats after Long Term Treatment with Autoantibodies against M2 Muscarinic Receptor. PLoS One 10 (6), e0129563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribeiro KC et al. (2018) Immunization with plasmids encoding M2 acetylcholine muscarinic receptor epitopes impairs cardiac function in mice and induces autophagy in the myocardium. Autoimmunity 51 (5), 245–257. [DOI] [PubMed] [Google Scholar]
- 122.Wang F et al. (2004) Passive transfer of Sjogren’s syndrome IgG produces the pathophysiology of overactive bladder. Arthritis Rheum 50 (11), 3637–45. [DOI] [PubMed] [Google Scholar]
- 123.Robinson CP et al. (1998) Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren’s syndrome. Proc Natl Acad Sci U S A 95 (13), 7538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jin Z et al. (2011) Changes in cardiac structure and function in rats immunized by angiotensin type 1 receptor peptides. Acta Biochim Biophys Sin (Shanghai) 43 (12), 970–6. [DOI] [PubMed] [Google Scholar]
- 125.LaMarca B et al. (2009) Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54 (4), 905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wenzel K et al. (2011) Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58 (1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parrish MR et al. (2010) The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23 (8), 911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parrish MR et al. (2011) Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens 24 (7), 835–40. [DOI] [PubMed] [Google Scholar]
- 129.Brewer J et al. (2013) Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 62 (5), 886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo L et al. (2015) Anti-endothelin receptor type A autoantibodies in systemic lupus erythematosus-associated pulmonary arterial hypertension. Arthritis Rheumatol 67 (9), 2394–402. [DOI] [PubMed] [Google Scholar]
- 131.Peter JC et al. (2007) Antibodies against the melanocortin-4 receptor act as inverse agonists in vitro and in vivo. Am J Physiol Regul Integr Comp Physiol 292 (6), R2151–8. [DOI] [PubMed] [Google Scholar]
- 132.Peter JC et al. (2009) Anti-melanocortin-4 receptor autoantibodies in obesity. J Clin Endocrinol Metab 94 (3), 793–800. [DOI] [PubMed] [Google Scholar]
- 133.McKenzie JM (1962) Fractionation of plasma containing the long acting thyroid stimulator. J Biol Chem 237, 3571–2. [PubMed] [Google Scholar]
- 134.Valente WA et al. (1982) Monoclonal antibodies to the thyrotropin receptor: stimulating and blocking antibodies derived from the lymphocytes of patients with Graves disease. Proc Natl Acad Sci U S A 79 (21), 6680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Warne T et al. (2008) Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454 (7203), 486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smrcka AV and Fisher I (2019) G-protein βγ subunits as multi-functional scaffolds and transducers in G-protein-coupled receptor signaling. Cell Mol Life Sci 76 (22), 4447–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeWire SM et al. (2007) β-arrestins and cell signaling. Annu Rev Physiol 69, 483–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.