Abstract
Reactive oxygen species (ROS)-dependent production of ROS underlies sustained oxidative stress, which has been implicated in the pathogenesis of cardiovascular diseases such as hypertension, aortic aneurysm, hypercholesterolaemia, atherosclerosis, diabetic vascular complications, cardiac ischaemia–reperfusion injury, myocardial infarction, heart failure and cardiac arrhythmias. Interactions between different oxidases or oxidase systems have been intensively investigated for their roles in inducing sustained oxidative stress. In this Review, we discuss the latest data on the pathobiology of each oxidase component, the complex crosstalk between different oxidase components and the consequences of this crosstalk in mediating cardiovascular disease processes, focusing on the central role of particular NADPH oxidase (NOX) isoforms that are activated in specific cardiovascular diseases. An improved understanding of these mechanisms might facilitate the development of novel therapeutic agents targeting these oxidase systems and their interactions, which could be effective in the prevention and treatment of cardiovascular disorders.
Accumulating evidence indicates that the major enzymatic sources of reactive oxygen species (ROS) in the cardiovascular system are NADPH oxidase (NOX), uncoupled endothelial nitric oxide synthase (eNOS; also known as NOS3), mitochondria and xanthine oxidase (XO)1. NOX is distinct from other enzymatic sources because its primary function is to produce ROS. Low levels of ROS produced by certain NOX isoforms (such as NOX2) have been implicated in physiological processes, including cell proliferation, migration, differentiation and cytoskeletal organization2. However, excessive production of ROS from activated NOXs contributes to cardiovascular pathogenesis. Of note, NOX-derived ROS, such as superoxide and hydrogen peroxide (H2O2), can trigger ROS production through the activation of other enzymatic systems3–8. For example, ROS produced from NOX can induce oxidative inactivation of tetrahydrobiopterin (H4B), an essential cofactor for eNOS, resulting in eNOS uncoupling and the production of superoxide rather than nitric oxide (NO)9–37. In addition, ROS can stimulate the conversion of xanthine dehydrogenase (XDH) to XO by oxidation of the sulfhydryl residue. ROS produced by NOX can also cause mitochondrial DNA damage, oxidation of components of the membrane permeability transition pore and opening of the redox-sensitive mitochondrial ATP-sensitive K+ channel (mitoKATP), all of which contribute to mitochondrial uncoupling and ROS production1–7,38–42. Important mechanistic pathways of ROS amplification or propagation to mediate cardiovascular pathogenesis, particularly those centred on NOX-dependent uncoupling of eNOS and consequent mitochondrial dysfunction, are shown in FIG. 1. Indeed, NOX has emerged as the primary oxidase system underlying oxidative stress in vascular diseases, such as hypertension43, aortic aneurysms34,44, hypercholesterolaemia45, atherosclerosis46,47 and diabetic vascular complications46,47, as well as in cardiac diseases, including ischaemia–reperfusion (IR) injury48, myocardial infarction (MI)49,50, heart failure51,52 and cardiac arrhythmias53. In this Review, we discuss the crosstalk between NOXs and the other ROS-generating systems in the pathogenesis of cardiovascular diseases (CVDs), the targeting of which could reveal novel therapeutic strategies for the treatment and prevention of CVDs.
Fig. 1 |. NADPH oxidase-dependent oxidase crosstalk in the pathogenesis of cardiovascular diseases.
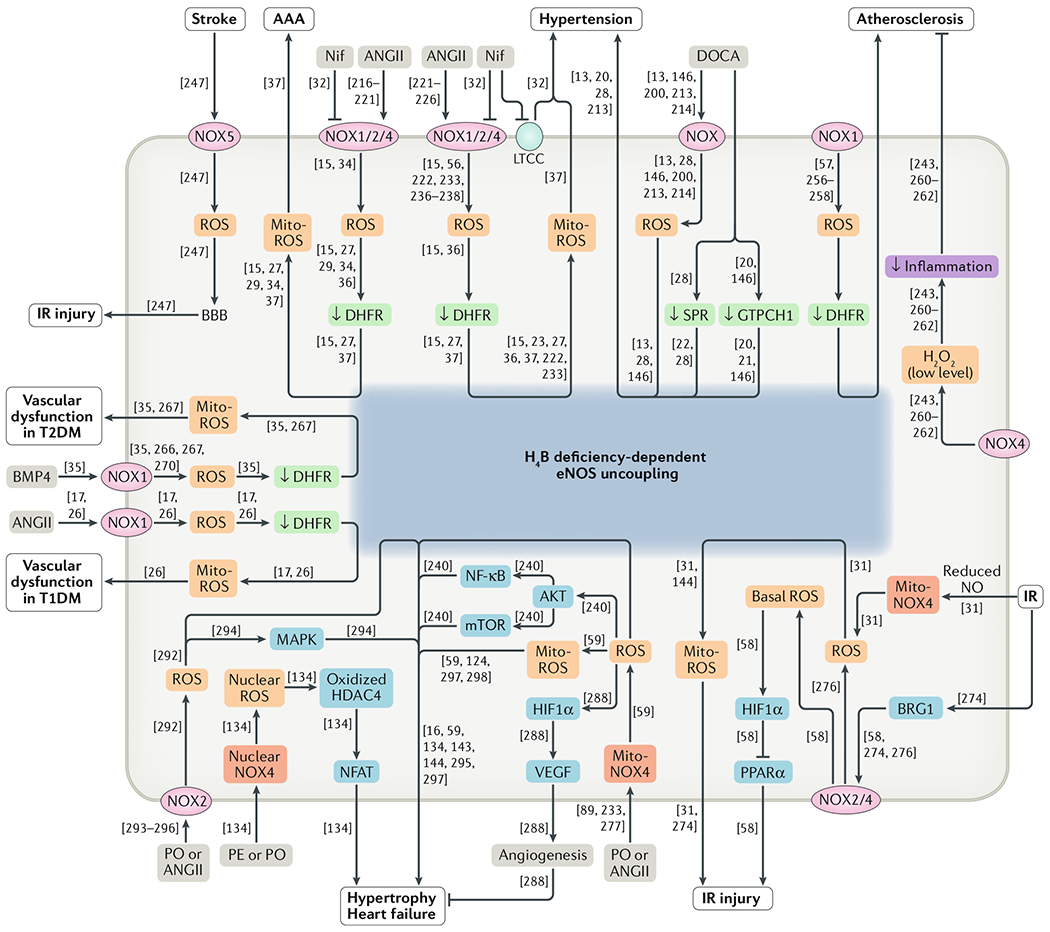
NADPH oxidase (NOX)-derived reactive oxygen species (ROS) production induces endothelial nitric oxide synthase (eNOS) uncoupling and mitochondrial dysfunction, resulting in sustained oxidative stress and the development of cardiovascular diseases. Reference numbers are given in square brackets. AAA, abdominal aortic aneurysm; AKT, RACα serine/threonine-protein kinase; ANGII, angiotensin II; BBB, blood–brain barrier; BMP4, bone morphogenetic protein 4; BRG1, transcription activator BRG1; DHFR, dihydrofolate reductase; DOCA, deoxycorticosterone acetate; GTPCH1, GTP cyclohydrolase 1; H2O2, hydrogen peroxide; H4B, tetrahydrobiopterin; HDAC4, histone deacetylase 4; HIF1α, hypoxia-inducible factor 1α; IR, ischaemia–reperfusion; LTCC, L-type calcium channel; MAPK, mitogen-activated protein kinase; Mito, mitochondrial; Mito-ROS, mitochondria-derived reactive oxygen species; mTOR, mechanistic target of rapamycin; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; N if, nifedipine; NO, nitric oxide; PE, phenylephrine; PO, pressure overload; PPARα, peroxisome proliferator-activated receptor-α; SPR, sepiapterin reductase; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; VEGF, vascular endothelial growth factor.
Oxidases in CVD pathogenesis
NOX family of enzymes
Accumulating evidence indicates that NOXs are the predominant sources of ROS in CVDs1,5–8,34,43–55. Genetic modifications of NOX isoforms have specific effects on cardiovascular phenotypes in animal models26,56–60, indicating a central role of NOXs in the development of CVDs.
Discovery.
The first member of the NOX family of enzymes to be discovered was NOX2 (also known as gp91phox or cytochrome b-245 heavy chain); NOX2 was discovered in phagocytes as the enzyme complex underlying the oxidative burst in response to the invasion of microorganisms61,62. In 1978, the protein responsible for ROS production in phagocytes was found to be cytochrome b558 (composed of NOX2 and p22phox (also known as cytochrome b-245 light chain))63,64. After the successful cloning of NOX2 in 1986, other subunits and isoforms of NOXs were identified and cloned between 1986 and 2006 (REFS65–84). So far, seven isoforms of NOXs (NOX1–NOX5, dual oxidase 1 (DUOX1) and DUOX2) have been identified. The historical discovery and characterization of the NOX family oxidases have been thoroughly reviewed previously85 and are summarized in BOX 1. The development of pharmaceutical inhibitors of the NOXs is summarized in BOX 2, and the latest agents are discussed below. The genetic modification of NOXs in animal models of CVDs is summarized in BOX 3.
Box 1 |. Identification of NOXs and subunits.
1960s–1970s
1980s
1990s
2000s
DUOX, dual oxidase; DUOXA, dual oxidase maturation factor; EC, endothelial cell; NOX, NADPH oxidase; NOXA1, NADPH oxidase activator 1; NOXO1, NADPH oxidase organizer 1; VSMC, vascular smooth muscle cell.
Box 2 |. Development of NOX inhibitors.
1980s
1990s
2000s
2010–2012
2013–2015
2016–2019
AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride; apocynin, 4′-hydroxy-3′-methoxyacetophenone; DPI, diphenyleneiodonium; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; NOX, NADPH oxidase; NOXA1, NADPH oxidase activator 1.
Box 3 |. Transgenic NOX animal models of CVDs.
2000s
2010–2012
Nox2−/y (atherosclerosis) (2010)258
Nox4−/− (heart failure) (2010)288
Cardiac-specific Nox4−/− (heart failure) (2010)59
Cardiac-specific Nox4-Tg, cardiac-specific Nox4-DN-Tg (heart failure) (2010)89
EC-specific Nox4-Tg (hypertension) (2011)241
Nox1−/y (diabetic vascular function) (2012)26
EC-specific Nox2-Tg (atherosclerosis) (2012)259
Ncf1−/− (diabetes) (2012)26
2013–2015
Nox1−/y (atherosclerosis) (2013)257
Nox2−/y, Nox4−/−, cardiac-specific Nox4−/−, Nox2−/y plus cardiac-specific Nox4−/− (ischaemia–reperfusion injury) (2013)58
Cardiac-specific Nox4-Tg, cardiac-specific Nox4-DN-Tg (ischaemia–reperfusion injury) (2013–2014)58,276
Nox4 or Nox4-DN transient overexpression (arrhythmia) (2014)60
VSMC-specific Cyba-Tg, VSMC-specific Cyba−/− (obesity and diabetes) (2014)267
Nox4−/− (atherosclerosis) (2015)261
EC-specific Nox4-Tg (atherosclerosis) (2015)262
Podocyte-specific human NOX5-Tg (hypertension, diabetic nephropathy) (2014)248
2016–2019
Nox4−/− (hypertension) (2016)249
Ncf1−/−, Nox1−/y, Nox2−/y, Nox4−/− (abdominal aortic aneurysm) (2017)34
VSMC-specific human NOX5-Tg (diabetic nephropathy) (2017)246
Nox4−/− (hypertension) (2018)238
VSMC-specific human NOX5-Tg (vasorelaxation) (2018)245
EC-specific human NOX5-knock-in (stroke) (2019)247
CVD, cardiovascular disease; DN, dominant negative; EC, endothelial cell; NOX, NADPH oxidase; Tg, transgenic; VSMC, vascular smooth muscle cell.
Structure.
NOXs are multi-transmembrane proteins (NOX1–NOX5 are six-transmembrane proteins, whereas DUOX1 and DUOX2 are seven-transmembrane proteins), with the C-terminus exposed to the cytosol. NOXs share common structural domains, including six conserved transmembrane domains, four conserved haem-binding histidines, the FAD-binding domain and the NADPH-binding domain80. NOXs sequentially transfer electrons from NADPH to FAD, haem groups and then to molecular oxygen, leading to superoxide production86. Mutation of one proline residue in the NADPH-binding domain inactivates NOX2 (Pro415 in human NOX2)87, NOX3 (Pro413 in human NOX3)88 and NOX4 (Pro437 in human NOX4)60,89, indicating an important role of the NADPH-binding domain in the activation of NOXs. Of note, both NOX1 and NOX2 (also known as CYBB) are located on chromosome X, whereas other NOX genes are located on autosomes.
The crystal structures of the NOXs have been reported. In 2009, the crystal structure of the N-terminal regulatory domain of a plant NOX in rice (a homologue of mammalian NOX2) was published90. Plant NOX proteins have a cytosolic N-terminal region with two EF hands that bind to Ca2+ (REF.90). These motifs are absent from the mammalian NOX2, but are present in NOX5, DUOX1 and DUOX2 (REF.90). In 2017, the crystal structures of the FAD-binding and NADPH-binding domains (known as the C-terminal cytosolic dehydrogenase (DH) domain when combined) of NOX5 from Cylindrospermum stagnale were reported91. Of note, this DH domain is common to all seven members of the NOX family91. The C-terminus was shown to function as a toggle switch and to regulate access ofthe NADPH to NOX91. The structure of the NADPH-binding domain reported in this NOX5 DH domain is very similar to that of the NADPH-binding domain of human NOX2 (REF.91) previously deposited in the RCSB Protein Data Bank (ID: 3A1F).
Activation.
Each NOX isoform contains one catalytic subunit and other subunits, except for NOX5, which consists of one catalytic subunit alone. As the only membrane-bound subunit, p22phox is required for the stability and activation of NOX1–NOX4 (REF.92). Given that NOX2 was the first NOX isoform to be discovered and has been the subject of more mechanistic studies of activation, we first discuss the activation of this isoform. Under resting conditions, NOX2 and p22phox locate at the membrane as an inactive complex, whereas the p40phox (also known as neutrophil cytosol factor 4), p67phox (also known as neutrophil cytosol factor 2) and p47phox (also known as neutrophil cytosol factor 1) subunits are in the cytosol56,93. Activation of NOX2 also requires the small GTPase p21-RAC1 (also known as Ras-related C3 botulinum toxin substrate 1) to assemble with NOX2 on the membrane for full activity. Whereas RAC1 is ubiquitously distributed, RAC2 is reportedly required for the activation of NOX2 in differentiated granulocytes derived from the HL60 cell line and in neutrophils94–96. Upon NOX2 activation, RAC1 or RAC2 is recruited to the membrane, followed by recruitment of other cytosolic components. p47phox is then phosphorylated by protein kinase C (PKC)97–99 and translocated to the membrane, together with p67phox and p40phox. Next, phosphorylation of p47phox leads to a conformational change in its structure and subsequent interaction with p22phox, when the tandem SRC homology 3 (SH3) domain in p47phox can bind to the proline-rich region in the cytosolic C-terminus of p22phox (REF.100). These assembly processes result in the activation of NOX2. The initial ROS production (especially of H2O2) activates proto-oncogene tyrosine-protein kinase Src, leading to epidermal growth factor receptor (EGFR) transactivation and PI3K-dependent activation of RAC1, which further amplifies NOX2 activation39,101.
NOX1 activation also requires the assembly of multiple subunits85. In the process of NOX1 activation, either NADPH oxidase organizer 1 (NOXO1) or p47phox can be phosphorylated by PKC and translocated to the membrane to bind to p22phox (REFS26,92). Another difference in NOX1 activation compared with that of NOX2 is the replacement of p67phox with an alternative subunit, NADPH oxidase activator 1 (NOXA1)85,92.
Owing to the limited expression of NOX3 (only in fetal tissue and the inner ear), the mechanism of NOX3 activation has been studied only in overexpression systems43,80,92. Activation of NOX3 reportedly requires p22phox (REFS88,92). In the presence of p22phox, NOX3 is active without cytosolic subunits88,92. Interestingly, the activity of NOX3 can be increased by RAC1 and the subunits NOXO1–p47phox and NOXA1–p67phox (REFS88,92).
The activation of NOX4 does not require cytosolic regulatory subunits other than the membrane partner p22phox. NOX4 is mainly regulated at the expression level92,102,103. Polymerase δ-interacting protein 2 (POLDIP2) has been shown to associate with p22phox and to regulate NOX4 activity in vascular smooth muscle cells104. POLDIP2 increases NOX4 enzymatic activity and ROS production, leading to increased focal adhesion turnover and vascular smooth muscle cell migration104.
NOX5 is unique among NOX isoforms in that it contains an N-terminal calmodulin-like domain with four binding sites for Ca2+ (EF hands)105–107. The activation of NOX5 is Ca2+-dependent and does not require interaction with known subunits106,108. In response to an increase in Ca2+ concentration, the N-terminus of NOX5 undergoes conformational changes and exposes its hydrophobic patch105. This patch provides an interface for intramolecular interaction between the N-terminus and the C-terminus, resulting in the activation of NOX5 (REF.105). In the C-terminus, NOX5 has a binding site for the Ca2+-modulated and Ca2+-binding protein calmodulin109. Calmodulin is reported to bind to NOX5 in a Ca2+-dependent fashion, resulting in increased Ca2+ sensitivity of NOX5 (REFS109,110). The activity of NOX5 can also be positively regulated through phosphorylation by PKC (at Ser490, Ser494 and Thr498), Ca2+/calmodulin-dependent protein kinase II (CaMKII; at Ser475, Thr494, Ser498, Ser502 and Ser659) and mitogen-activated protein kinases (MAPKs; at Ser498)111–114.
DUOX1 and DUOX2 are composed of the basic NOX5-like structure, but fused with an additional transmembrane domain and an extracellular N-terminus102,115. The association of DUOX1 with dual oxidase maturation factor 1 (DUOXA1) and of DUOX2 with DUOXA2 enables the translocation of DUOX1 and DUOX2 from the endoplasmic reticulum to the plasma membrane102,115. DUOX1 and DUOX2 are activated by the binding of Ca2+ to their intracellular domain102,115.
The composition of all the NOX isoforms is summarized in FIG. 2. In summary, the activation of NOX1 requires p22phox, RAC1, p47phox and/or NOXO1, and NOXA1. The activation of NOX2 requires p22phox, RAC1 or RAC2, p47phox, p67phox and p40phox. The activation of NOX4 requires p22phox, and the activity of NOX4 can also be regulated by POLDIP2. The activation of NOX5 is primarily dependent on Ca2+. DUOX1 and DUOX2 require DUOXA1 and DUOXA2, respectively, and Ca2+ for their activation, and are not expressed in the cardiovascular system.
Fig. 2 |. Composition and cell-specific expression and activity of NOX isoforms in the cardiovascular system.
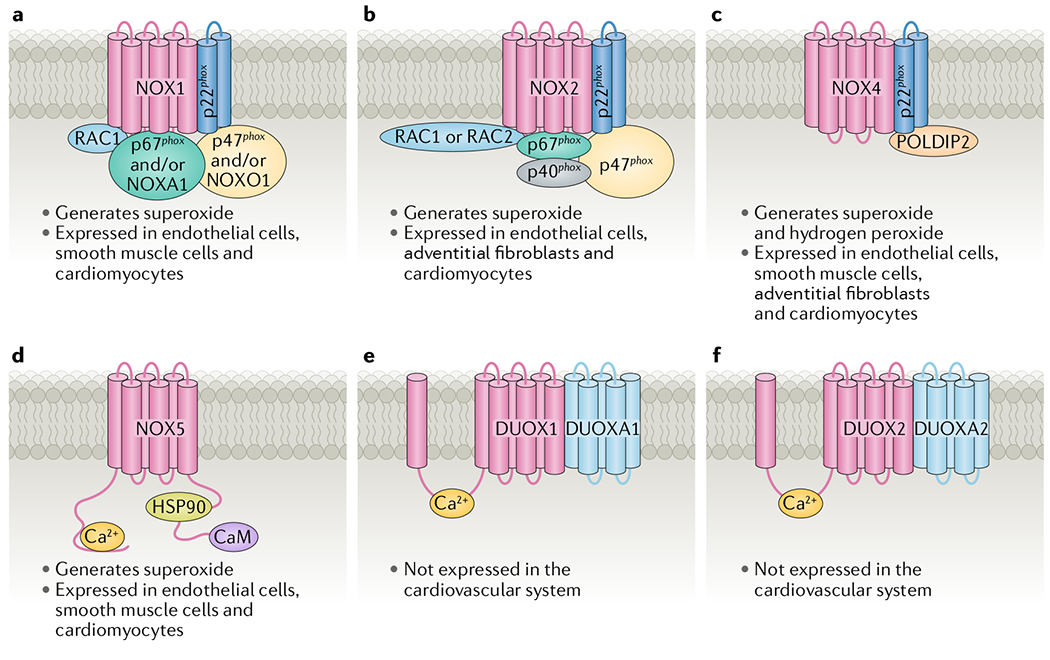
a | NADPH oxidase 1 (NOX1). b | NOX2. c | NOX4. d | NOX5. e | Dual oxidase 1 (DUOX1). f | DUOX2. CaM, calmodulin; DUOXA, dual oxidase maturation factor; HSP90, heat shock protein 90; NOXA1, NADPH oxidase activator 1; NOXO1, NADPH oxidase organizer 1; POLDIP2, polymerase δ-interacting protein 2.
Subcellular localization.
The NOX isoforms each have a specific cellular expression pattern and subcellular localization that determines the types of ROS from each isoform detectable by currently available techniques116. NOX1, NOX2, NOX4 and NOX5 are expressed in cardiovascular cells51,103,117. Endothelial cells contain NOX1 (REF.118), NOX2 (REF.119), NOX4 (REF.118) and NOX5 (REFS108,120). Vascular smooth muscle cells express NOX1 (REF.73), NOX4 (REF.121) and NOX5 (REFS79,120). Cardiomyocytes express NOX1 (REFS122,123), NOX2 (REFS124,125), NOX4 (REF.89) and NOX5 (REF.126). The cell-specific expression of NOX isoforms in the cardiovascular system is summarized in FIG. 2.
The subcellular localization of NOX isoforms varies between cell types. NOX2 is localized at the perinuclear cytoskeleton63 and endoplasmic reticulum93, whereas NOX4 (REFS93,127,128) and NOX5 (REF.108) are localized at the endoplasmic reticulum in endothelial cells. NOX1 is localized in the caveolae of vascular smooth muscle cells129. Interestingly, NOX4 has been reported to localize in the nucleus, focal adhesions and stress fibres in vascular smooth muscle cells under normal conditions129–131 and might translocate to the endoplasmic reticulum in hypertension132. NOX5 was found in the plasma membrane in vascular smooth muscle cells47. In cardiomyocytes, NOX2 is localized in the plasma membrane and the cytosol125, whereas NOX4 is localized in the mitochondria133 and nuclei134.
NOX1, NOX2 and NOX5 produce superoxide directly. Distinct from other NOX isoforms, NOX4 has been shown to produce H2O2 through the rapid dis-mutation of superoxide into H2O2 because of a highly conserved histidine residue in NOX4 (REFS135–137). Conversely, NOX4 production of H2O2 is thought to be the consequence of the localization of NOX4 at the mitochondria in cardiomyocytes and at the endoplasmic reticulum in endothelial cells; superoxide cannot cross the membranes of these subcellular organelles, so only the superoxide dismutated product, H2O2, is releasable to the cytoplasm and detectable by currently available methods138.
Importantly, one of the major consequences of NOX activation is the activation of other oxidase systems to sustain oxidative stress in a process known as ROS-dependent ROS production. These secondary oxidase systems include, but are not limited to, uncoupled eNOS, dysfunctional mitochondria, XO and the endoplasmic reticulum.
Uncoupled eNOS
There are three isoforms of nitric oxide synthase (NOS): eNOS, neuronal NOS (nNOS; also known as NOS1) and inducible NOS (iNOS; also known as NOS2). For the synthesis of NO, l-arginine is required as the substrate, whereas molecular oxygen and reduced NADPH (harbouring one extra electron) are required as co-substrates. H4B is an essential cofactor for the synthesis of NO because its presence stabilizes the dimeric state of eNOS. l-arginine, H4B, haem and molecular oxygen bind to the N-terminal oxygenase domain of eNOS, whereas NADPH binds to its C-terminal reductase domain. Under physiological conditions, eNOS catalyses electron transfer from reduced NADPH of one monomer to the haem-containing oxygenase domain of the other monomer. At this site, oxygen is reduced by the electrons and incorporated into the terminal guanidine group of l-arginine to generate NO and l-citrulline. eNOS exists as a dimer under normal conditions; however, when H4B is deficient because of oxidative inactivation, the dimer breaks down, resulting in electron transfer to the molecular oxygen to generate superoxide instead of NO9–11,15,17,22,23,26–29,31,34,35,37,139,140. This state is referred tO as eNOS uncoupling.
H4B can be generated through two enzymatic path-ways: the de novo synthetic pathway and the salvage pathway, which regenerates H4B from its oxidized form, dihydrobiopterin (H2B). In the de novo synthesis pathway, H4B is generated from GTP sequentially by the enzymes GTP cyclohydrolase 1 (GTPCH1; the rate-limiting synthetic enzyme), 6-pyruvoyl tetrahydrobiopterin synthase and sepiapterin reductase (SPR)24,141. H4B can also be regenerated from its oxidized form H2B in a process catalysed by the rate-limiting, salvage enzyme dihydrofolate reductase (DHFR); H2B can be converted from the exogenous precursor sepiapterin by SPR.
eNOS uncoupling can occur downstream of NOX activation. Activated NOXs produce ROS, which leads to H4B deficiency and eNOS uncoupling15. The crosstalk and interaction between NOXs and eNOS uncoupling in CVDs is discussed below.
H4B deficiency-induced eNOS uncoupling has been implicated in various CVDs, including hypertension and aortic aneurysms13,21,27,29,37, atherosclerosis18, diabetes mellitus17,26,142,143, cardiac IR injury144 and heart failure16,145. Specifically, H4B deficiency and eNOS uncoupling can be induced through DHFR depletion. Knockdown of Dhfr leads to eNOS uncoupling15. Dhfr+/− mice (the homozygous knockout is embryonically lethal) have reduced H4B levels in the aorta at baseline and a low-level eNOS uncoupling that is well compensated for37, similar to what is observed in Apoe−/− mice12,29 and hph-1 mice (a model of GTPCH1 deficiency)27,32. However, angiotensin II infusion into Dhfr+/− mice resulted in marked hypertension and development of abdominal aortic aneurysm (AAA)37. Conversely, upregulation of DHFR recoupled eNOS in animals with hypertension and aortic aneurysms23,27,36 or diabetes26, details of which are discussed in the following section.
Additionally, H4B deficiency and eNOS uncoupling can be caused by deficiency of SPR or GTPCH1, as shown in deoxycorticosterone acetate (DOCA)–salt hypertensive mice20,21,28,146. Overexpression of GTPCH1 restored the H4B level and recoupled eNOS in DOCA–salt hypertensive mice20,146. These data indicate that modulation of H4B metabolic enzymes might be a robust strategy to recouple eNOS as a therapeutic strategy in CVDs.
In addition to H4B deficiency, other mechanisms have been implicated in inducing eNOS uncoupling99. All three isoforms of NOS have a zinc tetrathiolate (ZnS4) cluster at the dimer interface147–149. Oxidants (such as peroxynitrite and hypochlorous acid) disrupt the ZnS4 cluster of eNOS and result in eNOS uncoupling150,151. In addition, S-glutathionylation of cysteine residues of eNOS has been shown to induce eNOS uncoupling152. In particular, S-glutathionylation of aortic eNOS was increased in animal models of hypertension152, nitrate tolerance153,154 and streptozotocin (STZ)-induced diabetes155. Normalization of S-glutathionylation of eNOS in these models reduced eNOS uncoupling and improved vasorelaxation152–155.
In addition to eNOS uncoupling, uncoupling of nNOS and iNOS has been reported. The first report suggesting that NOS might produce ROS was in the early 1990s, when purified nNOS produced superoxide (then converted to H2O2 by superoxide dismutase (SOD)) owing to H4B or l-arginine deficiency156,157. Later, iNOS was shown to catalyse superoxide production under l-arginine-depleted conditions or H4B deficiency158,159. Of note, deficiency of l-arginine is rare under physiological conditions.
Dysfunctional mitochondria
Mitochondria are the cellular energy factory where ATP is synthesized by oxidative phosphorylation43. This process relies on a proton gradient generated by the mitochondrial electron transport chain. The electron transport chain comprises a series of complexes that pump protons across the mitochondrial inner membrane to generate a proton gradient, whilst transferring electrons from electron donors (NADH or succinate from the citric acid cycle) to oxygen to generate water. Under normal conditions, electron transportation is efficient and the electron leak is maintained at low, physiological levels160.
Under conditions of oxidative stress, mitoKATP is activated by redox-sensitive PKC to transduce redox signals from the cytosol to the mitochondria39,161,162. Opening of the mitoKATP increases the K+ influx into the mitochondrial matrix, leading to mitochondrial ROS production from the electron transport chain163–165. Incubation with 5-hydroxydecanoate, a specific inhibitor of mitoKATP, prevented mitochondrial ROS production, suggesting that K+ influx has an important role in regulating mitochondrial ROS production41,166. At the same time, more electron donors were generated from the citric acid cycle and were pushed into the electron transport chain167. Under these conditions, the mitochondrial electron transport chain generates superoxide through electron leakage, when electrons react with oxygen to form superoxide167,168. In addition, mitochondrial DNA is damaged by oxidative stress169, which causes ROS production and apoptosis. The generated superoxide is then rapidly dismutated into H2O2 by the mitochondrial isoform of SOD (SOD2), followed by diffusion out of the mitochondria167,170–172. Complex I and complex III are reported to be the major sites at which superoxide is generated164,167,169.
Dysfunctional mitochondria are considered the intracellular source of ROS in various CVDs. Mitochondrial dysfunction has been reported in hypertension173, atherosclerosis174, diabetes175,176, heart failure177–179 and AAA37. The mitochondria-targeted antioxidant MitoQ attenuated cardiac hypertrophy in stroke-prone spontaneously hypertensive rats173. MitoQ also reduced ROS production and leukocyte–endothelial cell interactions in leukocytes isolated from patients with diabetes180. Sod2+/− mice with apolipoprotein E deficiency had greater impairment of vessel relaxation and increased formation of atherosclerotic lesions compared with Apoe−/− mice174,181, implying a critical role of mitochondrial ROS in the development of vascular dysfunction. Overexpression of mitochondrial brown fat uncoupling protein 1 (UCP1) disrupted the mitochondrial electron transport chain and completely inhibited hyperglycaemia-induced mitochondrial superoxide production in mice182. Attenuation of mitochondrial ROS by the mitochondria-targeted peptide antioxidant SS-31 preserved insulin sensitivity in rats fed a high-fat diet176. In mice, inhibition of mitochondrial ROS production by SS-31 or genetic transfer of catalase targeted to the mitochondria prevented angiotensin II-induced cardiac hypertrophy and diastolic dysfunction178,183. Of note, NOX-derived ROS have been shown to enter the mitochondria and promote electron leak and mitochondrial ROS production4,41, suggesting that dysfunctional mitochondria lie downstream of NOXs.
Xanthine oxidase
Xanthine oxidoreductase is an enzyme initially synthesized in the dehydrogenase form (XDH), which can be rapidly converted into the oxidase form (XO) by oxidation. XDH and XO are interconvertible. Xanthine oxidoreductase is involved in the last two reactions of the purine degradation pathway, converting hypoxan-thine to xanthine and then touric acid184–186. In these reactions, XDH favours NAD+ as the electron acceptor and generates NADH, whereas XO uses oxygen as an electron acceptor and generates superoxide.
Studies suggest that XO is involved in the progression of various CVDs. The administration of an XO inhibitor was beneficial in animal models of hypertension187–189, myocardial IR injury190,191 and chronic heart failure192,193. However, use of an XO inhibitor (300–600 mg per day) did not show benefits in patients with hypertension or chronic heart failure194–196. A retrospective analysis in patients with hyperuricaemia and acute MI suggested that the combination of an XO inhibitor and an angiotensin-converting enzyme (ACE) inhibitor protected against major cardiovascular events (death or hospitalization for cardiovascular causes) after acute MI compared with treatment with an ACE inhibitor alone197. These data suggest that XO inhibition might have limited beneficial effects only in patients with hyperuricaemia and CVDs. Given that inhibition of NOX activity suppresses XO activation and superoxide production198, the roles of NOX–XO crosstalk in the pathogenesis of CVDs are discussed below.
Cardiovascular oxidase crosstalk
NOXs have been shown to be the primary oxidases activated in the cardiovascular system, but accumulating data indicate that complex crosstalk exists between NOXs and other ROS-generating enzymes or enzymatic systems, including uncoupled eNOS, dysfunctional mitochondria and XO. These secondary oxidase systems can also activate NOXs and/or each other. The interactions between these oxidases in the cardiovascular system are introduced in this section; the contributions of oxidase crosstalk to particular CVDs are then discussed in detail in the next section.
NOXs and uncoupled eNOS
Transient exposure (30 min) of bovine endothelial cells to angiotensin II in vitro increased the production of superoxide, which was attenuated by the RAC1 inhibitor NSC23766, indicating NOX-derived ROS production15. However, after 24 h of angiotensin II treatment, superoxide production was completely blocked by administration of l-NAME (a NOS inhibitor), whereas NSC23766 did not significantly reduce superoxide production15. These data suggest that uncoupled eNOS is predominantly responsible for ROS production after prolonged exposure of endothelial cells to angiotensin II, and that eNOS uncoupling occurs as a consequence of angiotensin II-induced activation of NOX15.
NOX activation induces uncoupling of eNOS through E2F1-dependent, E2F2-dependent or E2F3a-dependent downregulation of Dhfr expression15,36. In bovine endothelial cells, angiotensin II-induced NOX activation leads to H2O2 production15. In turn, H2O2 downregulated the expression of E2F1, E2F2 and E2F3a, the main transcription factors required to activate Dhfr transcription in endothelial cells36. As a result, the expression and activity of DHFR were attenuated, leading to persistent H4B deficiency and eNOS uncoupling15,36. In mouse models, angiotensin II infusion induces endothelial DHFR deficiency and eNOS uncoupling27,29,32. Restoration of endothelial DHFR expression and activity with oral folic acid administration or in vivo transfection of Dhfr recoupled eNOS and improved NO bioavailability in angiotensin II-infused animals, resulting in lowered blood pressure15,27,29. Similarly, adenovirus-delivered E2F1 overexpression in mice significantly increased DHFR protein abundance and H4B bioavailability and recoupled eNOS36. NO bioavailability was also restored, resulting in reduced blood pressure36. These data reveal a novel pathway of NOX–H2O2–E2F–DHFR-dependent regulation of eNOS uncoupling and its role in elevating blood pressure.
In addition, eNOS uncoupling develops in DOCA–salt hypertensive mice and rats and is associated with H4B deficiency13,20,28,199. This deficiency has been shown to result from decreased SPR expression and GTPCH1 activity, both of which lead to impaired H4B bioavailability86,88. NOX activity was also reported to be upregulated in DOCA–salt hypertensive mice200. Application of the NOX inhibitor 4’-hydroxy-3’-methoxyacetophenone (apocynin; later shown to be a nonspecific inhibitor of all flavin-containing enzymes, including NOXs) or deletion of p47phox restored H4B bioavailability and eNOS coupling in DOCA–salt hypertensive mice13,28. These data demonstrate an upstream role of NOXs in eNOS uncoupling in a salt-sensitive model of hypertension. Of note, different H4B metabolic enzymes are involved in different types of hypertension, with DHFR deficiency underlying eNOS uncoupling and hypertension in angiotensin II-infused mice, and SPR and GTPCH1 deficiency accounting for hypertension in DOCA–salt hypertensive animals.
The interaction between NOXs and uncoupled eNOS has also been studied in STZ-injected animals. STZ injection in mice downregulated aortic DHFR expression and H4B bioavailability resulting in eNOS uncoupling17,26. Attenuation of angiotensin II signalling in STZ-injected mice by oral administration of the angiotensin II receptor type 1 (AT1) blocker candesartan or the ACE inhibitor captopril recoupled eNOS through inhibition of NOX activity and restoration of DHFR protein expression17. Further investigation demonstrated that knockout of either Nox1 or Ncf1 (encoding p47phox), or in vivo knockdown of Noxo1 by RNA interference, improved endothelium-dependent vasodilatation in STZ-induced diabetic mice26. This improvement was attributed to recoupling of eNOS as a result of the restoration of DHFR function and H4B bioavailability26. These data strongly implicate a selective role of NOX1 in activating eNOS uncoupling via angiotensin II signalling in STZ-injected type 1 diabetic mice. By contrast, in db/db type 2 diabetic mice, infusion of the bone morphogenetic protein 4 (BMP4) antagonist noggin attenuated eNOS uncoupling through inhibition of NOX1 (REF.35). Together, these results strongly indicate NOX-dependent uncoupling of eNOS through NOX-derived ROS production and oxidation of H4B.
NOXs and mitochondria
Angiotensin II-induced NOX activation has been reported to induce mitochondrial ROS production and mitochondrial dysfunction in endothelial cells41,201,202. Inhibition of NOX activity with apocynin or with small interfering RNA (siRNA) targeted to Cyba (encoding p22phox) in bovine endothelial cells in vitro reduced angiotensin II-provoked mitochondrial ROS production, indicating NOX-dependent modulation of mitochondrial dysfunction41,202. This modulation seems to be mediated by uncoupling of eNOS. Treatment with the NOS inhibitor l-NAME prevented angiotensin II-induced mitochondrial dysfunction41. Angiotensin II-stimulated mitochondrial ROS production is also reported to involve the opening of mitoKATP in both endothelial cells and vascular smooth muscle cells41,166.
Conversely, feedback regulation of mitochondria on NOXs has also been reported. Opening of mitoKATP by treatment with diazoxide results in NOX activation166. Moreover, treatment with the mitoKATP-specific inhibitor 5-hydroxydecanoate reduced superoxide production (generated by NOXs and uncoupled eNOS) in angiotensin II-treated endothelial cells in vitro, suggesting feedback regulation of NOX and eNOS activity by mitoKATP (REFS38,39,41). As discussed above, PKC and Src induce NOX activation through p47phox phosphorylation and the EGFR–PI3K–RAC1 axis, respectively39,97–99,101. In mice, direct clearance of mitochondrial superoxide by either overexpression of SOD2 or the administration of the mitochondria-targeted antioxidant MitoTEMPO inhibited NOX activity in endothelial cells203. Of note,SOD2 or MitoTEMPO had no effects on basal NOX activity and inhibited NOX activation only in angiotensin II-stimulated cells39,203.
Uncoupled eNOS and mitochondria
An interaction between uncoupled eNOS and mitochondria has been reported in endothelial cells41. Superoxide reacts with NO to form peroxynitrite, which can damage mitochondria through oxidation of membrane lipids and electron transport chain complexes204,205. Administration of uric acid, a scavenger of peroxynitrite, or l-NAME protected against angiotensin II-induced mitochondrial dysfunction in cultured endothelial cells, indicating eNOS-dependent mitochondrial dysfunction41. Angiotensin II-infused Dhfr+/− mice have dramatically increased mitochondrial superoxide production in the aorta, suggesting that DHFR deficiency-dependent eNOS uncoupling induces mitochondrial dysfunction37. An upstream role of uncoupled eNOS in mediating mitochondrial dysfunction has also been reported in the heart206. Uncoupling of eNOS induced by treatment of mice with 2,4-diamino-6-hydroxypyrimidine (DAHP; an inhibitor of GTPCH1) resulted in H4B depletion, impaired mitochondrial function in the heart, and cardiac contractile dysfunction206. In mice with cardiac IR injury, treatment with sepiapterin (a precursor of H4B) recoupled eNOS to reduce mitochondrial superoxide production, resulting in preserved cardiac mitochondrial function and cardiac function31.
Conversely, mitochondrial ROS production might also regulate eNOS coupling–uncoupling activity. In humans, restoration of mitochondrial electron transport by supplementation with antioxidant coenzyme Q10 recoupled eNOS and resulted in improved endothelial function in diabetes and atherosclerosis207,208. Additionally, inhibition of mitoKATP by 5-hydroxydecanoate completely restored NO production in angiotensin II-treated endothelial cells41. Although eNOS uncoupling was not directly measured in this study, restored NO production indicated improved eNOS function and a reduced uncoupling status41.
NOXs, XO and mitochondria
Apocynin treatment reportedly prevented XO activation and superoxide production in IR-injured guinea pig hearts198. However, inhibition of XO by allopurinol or tungsten did not modulate NOX activity198, suggesting that XO acts downstream of NOX in IR injury.
An interaction between XO and mitochondria has been reported in vivo. In a rat model of cocaine-induced cardiac dysfunction, treatment with allopurinol significantly reduced mitochondrial ROS production and improved cardiac function209. In left ventricular cardiomyocytes isolated from adult rats, application of the mitochondrial inhibitor MitoQ prevented stretch-induced XO activation, indicating a self-perpetuating cycle between XO and mitochondria210. The mechanisms might involve hypoxanthine, a metabolic product of ADP and AMP, both of which are produced by the breakdown of ATP from mitochondria210. Hypoxanthine reacts with XO to produce superoxide, which in turn causes damage to mitochondria210.
Oxidase crosstalk in CVDs
Emerging evidence indicates that oxidase crosstalk is a major mechanism underlying sustained oxidative stress during cardiovascular pathogenesis. The primary oxidase system that is first activated seems to be NOXs, which can activate downstream oxidases or oxidase systems, such as uncoupled eNOS, dysfunctional mitochondria or XO, resulting in secondary production of ROS. The detailed molecular pathways and pathophysiological relevance of the oxidase crosstalk in CVDs, including hypertension, AAA, hypercholesterolaemia, atherosclerosis, diabetic vascular dysfunction, cardiac IR injury, heart failure and cardiac arrhythmias, are discussed below. The investigations of animal models with genetic modifications of various NOX isoforms and subunits discussed in this section are summarized in TABLE 1.
Table 1.
NADPH oxidase genetically modified animal models
| Gene | Type of modification | Genetic modification | Disease model | Experimental resultsa | Refs |
|---|---|---|---|---|---|
| Nox1 | Global knockout | Nox1−/y | ANGII infusion | Attenuated ANGII-induced hypertension | 222,233 |
| Nox1−/y on hph-1 background233 | ANGII infusion | Prevented eNOS uncoupling in the aorta and reduced incidence of AAA | 34 | ||
| Nox1−/y on Apoe−/− background233 | High-fat diet or STZ injection | Reduced superoxide production in the aorta and reduced aortic lesion formation | 256,257 | ||
| Nox1−/y (REF.233! | STZ injection | Inhibited aortic eNOS uncoupling and improved endothelium-dependent vasorelaxation | 26 | ||
| Vascular smooth muscle transgene | Overexpression of human NOX1 in vascular smooth muscle cells | ANGII infusion | Increased aortic superoxide production and exaggerated increase in BP in ANGII-treated animals | 235 | |
| Nox2 (Cybb) | Global knockout | Nox2−/y | ANGII infusion | Inhibited ANGII-induced superoxide production in the aorta and reduction or no change in BP at baseline or in response to ANGII infusion | 56,372 |
| Nox2−/y on hph-1 background | ANGII infusion | Prevented eNOS uncoupling in the aorta and reduced incidence of AAA | 34 | ||
| Nox2−/y on Apoe−/− background373 | High-fat diet | Reduced aortic superoxide production and reduced aortic lesion formation | 258 | ||
| Nox2−/y | Cardiac IR injury | Reduced cardiac superoxide production, attenuated cardiomyocyte apoptosis and reduced IR injury in the heart | 58 | ||
| Nox2−/y plus cardiac-specific Nox4−/− | Cardiac IR injury | Reduced superoxide production, increased cardiomyocyte apoptosis and increased IR injury in the heart | 58 | ||
| Nox2−/y | ANGII infusion or TAC | Inhibited ANGII-induced myocardial NOX activity or superoxide production, reduced cardiac fibrosis in ANGII-infused animals, and no change or attenuated cardiac hypertrophy | 292,294,372 | ||
| Endothelial transgene | Overexpression of human NOX2 in endothelial cells | ANGII infusion | Increased NOX activity or superoxide production, increased BP in ANGII-infused animals(0.3–0.4 mg/kg per day) and no change in ANGII-induced hypertension (1.1 mg/kg per day) | 236,237,372 | |
| Overexpression of human NOX2 in endothelial cells on Apoe−/− background236 | ANGII infusion | Increased endothelial superoxide production at baseline and no change in atherosclerotic lesion formation | 259 | ||
| Overexpression of human NOX2 in endothelial cells237 | ANGII infusion or left coronary artery ligation | No change in hypertrophy or infarct area | 275,372 | ||
| Cardiac transgene | Overexpression of human NOX2 in cardiomyocytes | Left coronary artery ligation | No change in infarct area and same mortality | 275 | |
| Nox4 | Global knockout | Nox4−/− | ANGII infusion | No change or decreased BP in response to ANGII infusion | 238,239 |
| Nox4−/− in Dahl salt-sensitive rats | 4.0% NaCldiet | Reduced kidney oxidative stress and attenuated BP in salt diet-treated animals | 249 | ||
| Nox4−/− on hph-1 background | ANGII infusion | Prevented eNOS uncoupling in the aorta and reduced incidence of AAA | 34 | ||
| Nox4−/− on Apoe−/− or LDlr−/− background | High-fat diet with orwithout partial carotid artery ligation, or STZ injection | Increased aortic superoxide production and increased atherosclerotic lesion formation | 243,260,261 | ||
| Nox4−/− | Cardiac IR injury | Reduced cardiac superoxide production, inhibited cardiomyocyte apoptosis and protected from IR injury in the heart | 58 | ||
| Nox4−/− | Suprarenal aortic constriction or TAC | Exaggerated cardiac fibrosis and increased cardiac hypertrophy | 134,288 | ||
| Knockdown of Nox4 by in vivo siRNA injection | Cardiac IR injury (Langendorff) | Recoupled eNOS in IR-injured heart, attenuated mitochondrialsuperoxide production and reduced cardiac IR injury | 31 | ||
| Cardiac knockout | Cardiac-specific Nox4−/− | Cardiac IR injury | Reduced cardiac superoxide production, inhibited cardiomyocyte apoptosis and protected from IR injury in the heart | 58 | |
| Cardiac-specific Nox4−/− | Phenylephrine infusion or TAC | Reduced cardiac hypertrophy | 59,134 | ||
| Global transient overexpression | Overexpression of human NOX4 | NA | Induced arrhythmic phenotype in zebrafish embryos | 60 | |
| Overexpression of dominant-negative form of human NOX4 (P437H) | NA | Abrogated arrhythmic phenotype in zebrafish embryos | 60 | ||
| Endothelial transgene | Overexpression of Nox4 in endothelial cells | ANGII infusion | Elevated H2O2 production in endothelial cells, improved endothelial-dependent vasodilatation and reduced BP in ANGII-infused animals | 241 | |
| Overexpression of Nox4 in endothelial cells on Apoe−/− background | High-fat and high-cholesterol diet | Attenuated atherosclerosis | 262 | ||
| Cardiac transgene | Inducible overexpression of Nox4 in cardiomyocytes | ANGII infusion | No change in mean BP in response to ANGII infusion | 240 | |
| Overexpression of Nox4 in cardiomyocytes89 | Cardiac IR injury (in vivo or Langendorff) | Increased ROS production and no alteration or exacerbation in cardiac IR injury | 58,276 | ||
| Overexpression of dominant-negative form of Nox4 (P437H) in cardiomyocytes89 | Cardiac IR injury (in vivo or Langendorff) | Increased superoxide production and increased IR injury in the heart | 58,276 | ||
| Overexpression of Nox4 in cardiomyocytes | Ageing, TAC or phenylephrine or ANGII infusion | Increased ROS production and apoptosis, diminished left ventricle function and increased hypertrophy (at cellular level of cardiomyocytes and at the organ level) | 59,89,134,240 | ||
| Overexpression of Nox4 in cardiomyocytes | Suprarenal aortic constriction | Increased H2O2 production in the heart at baseline, increased myocardial angiogenesis, protected from cardiac dysfunction and fibrosis, and reduced cardiac hypertrophy | 288 | ||
| Overexpression of dominant-negative form of Nox4 (P437H) in cardiomyocytes89 | Ageing | Decreased superoxide production in left ventricle and no change in cardiac fibrosis or apoptosis | 89 | ||
| Nox5 | Endothelial knock-in | Knock-in of human NOX5 in endothelial cells | Cardiac IR injury, MI orbrain IR injury (stroke–reperfusion) | No change in BP at baseline, no change in cardiac infarctsize or cardiac function aftercardiac IR injury or MI, increased ROS production after stroke, increased blood–brain barrier leakage and increased infarctsize after brain IR injury | 247 |
| Vascular smooth muscle overexpression | Overexpression of human NOX5 in vascular smooth muscle cells | STZ injection | Increased ROS production and increased diabetic nephropathy | 246 | |
| Vascular smooth muscle overexpression | Overexpression of human NOX5 in vascular smooth muscle cells | ANGII infusion | Increased ROS in the vessels and the heart, impaired endothelium-dependent vasorelaxation and no change in BP at baseline or in response to ANGII infusion | 245 | |
| Podocyte overexpression | Overexpression of human NOX5 in podocytes | STZ injection | Increased ROS production, increased BP at baseline plus further increase in response to STZ and increased renal damage in response to STZ | 248 | |
| Cyba (encoding p22phox) | Vascular smooth muscle knockout | Smooth muscle-specific Cyba−/− | High-fat diet | Reduced perivascular inflammation | 267 |
| Vascular smooth muscle overexpression | Overexpression of Cyba in vascular smooth muscle cells | ANGII infusion | Increased aortic H2O2 production and exaggerated BP increase in ANGII-treated animals | 234 | |
| Overexpression of Cyba invascular smooth muscle cells | High-fat diet | Increased skeletal mitochondrialsuperoxide production and impaired skeletal mitochondrial function | 267 | ||
| Ncf1 (encoding p47phox) | Global knockout | Ncf1−/− (REF.374) | DOCA–salt, 1% saline, left kidney removal | Increased aortic H4B bioavailability, reduced aortic superoxide and H2O2 production, and reduced BP | 13 |
| Ncf−/− on hph-1 background | ANGII infusion | Prevented eNOS uncoupling in the aorta and reduced incidence of AAA | 34 | ||
| Ncf1−/− on Apoe−/− background | High-fat, atherogenic diet | Reduced aortic ROS production and decreased lesion formation | 57 | ||
| Ncf1−/− | STZ injection | Recoupling of eNOS in STZ-injected animals | 26 | ||
| Noxo1 | Global knockdown | Knockdown of Noxo1 by in vivo siRNA injection | STZ injection | Recoupling of eNOS in STZ-injected animals | 26 |
AAA, abdominal aortic aneurysm; ANGII, angiotensin II; BP, blood pressure; DOCA, deoxycorticosterone acetate; eNOS, endothelial nitric oxide synthase; H2O2, hydrogen peroxide; IR, ischaemia–reperfusion; MI, myocardial infarction; NA, not applicable; NOX, NADPH oxidase; ROS, reactive oxygen species; siRNA, small interfering RNA; STZ, streptozotocin; TAC, transverse aortic constriction.
Compared with nongenetically modified animals on the same genetic background and with the same treatment.
Hypertension
Increased vascular ROS production in hypertension has long been reported in animal models treated with angiotensin II211,212, DOCA–salt28,213,214 or l-NAME215. The association between elevated vascular ROS production and hypertension has also been reported in animals with genetic modifications or in inbred strains, including Dahl salt-sensitive rats216 and spontaneously hypertensive rats217. NOX and uncoupled eNOS have important roles in the elevation of blood pressure23,27,36,218–220. Angiotensin II is a potent vasoconstrictive peptide that induces hypertension through the activation of vascular NOX and NOX-derived ROS15,23,27,32,37,211. Specifically, increased levels of Nox1, Nox2 and Nox4 mRNA were reported in aortas from angiotensin II-infused animals221,222. In vitro, angiotensin II has been shown to upregulate Nox1 mRNA and NOX1 protein levels as well as Nox4 mRNA levels in vascular smooth muscle cells223,224 and to upregulate both NOX2 and NOX4 protein levels and activity in endothelial cells225,226. Expression of NOX5 was also found to be upregulated by angiotensin II in human cultured endothelial cells227. Previous studies have demonstrated that angiotensin II activates NOX via AT1-dependent phosphorylation of p47phox through a signalling pathway involving Src, PKC and phospholipase D101,228,229. Conversely, Src-induced EGFR transactivation and PI3K activation lead to the activation of RAC1, an essential event in the activation of NOX101. The detailed mechanisms of activation of NOX by angiotensin II have been previously reviewed5,230–232.
Infusion of angiotensin II increases aortic superoxide production and blood pressure in wild-type mice212,222,233. Overexpression of p22phox in vascular smooth muscle cells exacerbated angiotensin II-induced hypertension in mice234. Overexpression of NOX1 in vascular smooth muscle further increased angiotensin II-induced aortic superoxide production and hypertension in mice, both of which were corrected by administration of the antioxidant Tempol235. Deletion of Nox1 attenuated oxidative stress and hypertension in angiotensin II-infused mice222,233. Given that NOX1 was shown to be upregulated by angiotensin II only in vascular smooth muscle cells, and not in endothelial cells223,224, vascular smooth muscle NOX1 might have a more important role than endothelial NOX1 in the development of hypertension. Moreover, knockout of Nox2 in mice attenuated aortic superoxide production and blood-pressure elevation in response to angiotensin II56. Endothelium-specific overexpression of NOX2 in mice exacerbated the angiotensin II-induced increase in blood pressure236,237. Although NOX2 was not reported to be regulated by angiotensin II in vascular smooth muscle cells, endothelial NOX2 might, however, be involved in the regulation of angiotensin II-induced hypertension.
The role of NOX4 in hypertension has been studied, with inconsistent results. Nox4−/− mice had a lower blood pressure increase in response to angiotensin II infusion than wild-type mice238. However, another study showed that inducible deletion of Nox4 had no effect on basal blood pressure or angiotensin II-induced hypertension (conditional Nox4 deletion after initiation of angiotensin II infusion)239. These results suggest that NOX4 might be involved in the initiation rather than the maintenance of angiotensin II-induced hypertension in mice. Interestingly, cardiac-specific overexpression of NOX4 did not modulate blood pressure in angiotensin II-infused mice240, suggesting that vascular rather than cardiac NOX4 has an important role in the regulation of blood pressure. Contrary to the results in Nox4−/− mice, endothelium-specific overexpression of NOX4 in mice decreased the angiotensin II-induced rise in blood pressure owing to increased H2O2 production and endothelium-dependent vasorelaxation241. Perhaps these results can be interpreted as a compensatory response of H2O2-dependent vasodilatation on blood pressure242–244.
Rodents do not have NOX5. In transgenic mice with vascular smooth muscle-specific expression of human NOX5, baseline blood pressure levels and the angiotensin II-induced elevation in blood pressure were not different from levels in wild-type mice245,246. Given that angiotensin II induces the upregulation of NOX5 expression and activity in human cultured endothelial cells, investigation of endothelial NOX5 in angiotensin II-dependent hypertension in patients is important227. Basal blood pressure levels in mice with endothelial knock-in of human NOX5 with the use of a Tie2 promoter was not different from that in wild-type animals247. Interestingly, transgenic mice expressing human NOX5 specifically in podocytes had elevated blood pressure, which was further exacerbated by STZ-induced diabetes owing to severe renal damage248. Taken together, these data suggest that activation of NOXs has an important role in the development of angiotensin II-induced hypertension in animal models. Global knockout of either Nox1, Nox2 or Nox4 protected against angiotensin II-induced hypertension56,222,233,238, implicating an upstream role of NOX1, NOX2 and/or NOX4 in the development of this type of hypertension.
In addition to angiotensin II-dependent hypertension, NOXs have been shown to be involved in the regulation of blood pressure in other models of hypertension. In mice and rats with DOCA–salt hypertension, aortic expression of p22phox and superoxide production were both increased28,213. Treatment with apocynin significantly reduced superoxide production and decreased blood pressure in these animals28,213. Global deletion of Ncf1 abrogated aortic superoxide production and hypertension in DOCA–salt mice13. In addition, deletion of Nox4 has been shown to attenuate renal oxidative stress and hypertension in Dahl salt-sensitive rats249.
As discussed above, NOX-dependent ROS production leads to eNOS uncoupling. In vivo evidence also supports the upstream role of NOX in inducing eNOS uncoupling in the development of hypertension222,233. Deletion of Nox1 protected against vascular dysfunction and hypertension in response to angiotensin II infusion in mice34,37,222,233. Interestingly, administration of l-NAME diminished the protective effects of Nox1 deletion222, strongly indicating an intermediate role of eNOS uncoupling in the NOX1-triggered development of angiotensin II-induced hypertension. Moreover, recoupling of eNOS has been reported to attenuate hypertension in angiotensin II-infused or DOCA–salt-treated mice13,20,27,33,36. Adenovirus-mediated overexpression of E2F1 led to eNOS recoupling and normalized blood pressure in angiotensin II-infused mice36. Endothelial overexpression of GTPCH1 improved H4B bioavailability, recoupled eNOS and reduced blood pressure in DOCA–salt-treated mice20. Direct supplementation of H4B recoupled eNOS and reduced blood pressure in DOCA–salt mice13, hph-1 mice have a mutation in Gch1 (encoding GTPCH1) and have a phenotype of modest eNOS uncoupling that is well compensated for by H2O2-dependent vasodilatation27. Blood pressure in these mice is elevated by only 10 mmHg at baseline compared with wild-type mice27. With angiotensin II infusion, eNOS uncoupling was tripled in hph-1 mice, which resulted in severe vascular remodelling and the formation of AAA27. Oral administration of folic acid to restore endothelial DHFR function and recouple eNOS normalized blood pressure in angiotensin II-infused wild-type mice and prevented aneurysm-related blood pressure decline in hph-1 mice27. These findings suggest that NOX-dependent eNOS uncoupling has an important role in the development of hypertension and AAA (discussed further below).
Dhfr+/− mice have an exaggerated elevation in blood pressure in response to angiotensin II infusion owing to exacerbated eNOS uncoupling activity and mitochondrial dysfunction37. Administration of MitoTEMPO in these mice significantly reduced angiotensin II-induced high blood pressure and AAA formation37, implicating eNOS uncoupling-dependent mitochondrial dysfunction in the development of hypertension and AAA.
In SOD2-deficient mice, angiotensin II-induced NOX activation, eNOS uncoupling and high blood pressure were further elevated compared with wild-type animals receiving the same treatment, suggesting crosstalk between mitochondrial ROS, NOX activation and eNOS uncoupling250. Importantly, angiotensin II-induced NOX activation, eNOS uncoupling and hypertension can be blocked by inhibition of the mitochondrial membrane permeability transition pore (by cyclophilin D deficiency or sanglifehrin A treatment), implying an essential role of mitochondrial ROS in the modulation of NOX activity and eNOS uncoupling in angiotensin II-induced hypertension250.
Aortic aneurysms
Whereas a certain degree of eNOS uncoupling mediates hypertension, more extensive eNOS uncoupling induces the formation of AAA. As mentioned above, angiotensin II-infused hph-1 mice had a threefold increase in eNOS uncoupling, accompanied by a 79% incidence of AAA and a 14% rate of AAA rupture within 2 weeks27. This mouse is a novel and robust model of AAA. The traditional models of AAA, such as angiotensin II-infused Apoe−/− mice, usually take 4 weeks to develop AAA, and the aneurysms rarely rupture. Administration of folic acid, which restores endothelial DHFR expression and activity to recouple eNOS, completely prevented the development of AAA in angiotensin II-infused hph-1 mice27, indicating a causal role of uncoupled eNOS in the formation of AAA. In addition, eNOS uncoupling mediates AAA formation in the angiotensin II-infused Apoe−/− mouse model of AAA29. Similar to hph-1 mice, Apoe−/− mice have minimal eNOS uncoupling activity at baseline and a compensated phenotype of normal vasodilatation27,29. With angiotensin II infusion, aortic eNOS uncoupling was markedly increased in these mice, accompanied by severe vascular remodelling and the development of AAA (92% incidence)29. Recoupling of eNOS by restoration of endothelial DHFR function through folic acid supplementation substantially reduced angiotensin II-induced AAA formation to 22%29. In Dhfr+/− mice, angiotensin II infusion also resulted in a significantly higher incidence of AAA compared with wild-type littermates with the same genetic background37. These findings strongly indicate a central causal role of eNOS uncoupling in the development of AAA. Furthermore, we have established in mice that circulating H4B levels can be used as a novel and powerful biomarker for AAA development and response to treatment30. Circulating levels of H4B are accurately and linearly correlated with aortic H4B levels in angiotensin II-treated hph-1 mice and Apoe−/− mice30. Reduced circulating H4B levels are associated with an increased incidence of AAA, whereas prevention of AAA with folic acid dietary supplementation is associated with fully restored circulating H4B levels30.
As an upstream activator of uncoupled eNOS, the NOX family has been studied for their roles in the formation of AAA. NOX activity was upregulated in aortic tissues from AAA in patients251. NOX inhibitors (diphenyliodonium and apocynin) potently reduced superoxide production in patients with AAA, indicating an important role of NOXs in AAA formation252,253. Expression of p22phox, p47phox, NOX2 and NOX5 was found to be upregulated in AAA in patients251,252. Two novel NOX4 mutations were identified in patients with AAA34. These mutations are associated with a markedly increased H2O2 production34. In hph-1 mice, deletion of Nox1, Nox2, Nox4 or Ncf1 was sufficient to prevent AAA formation with angiotensin II infusion34. Consistent with our previous findings of a critical role of eNOS uncoupling in AAA formation in angiotensin II-infused hph-1 mice27, deletion of Nox1, Nox2, Nox4 or Ncf1 on the hph-1 background restored endothelial DHFR function and recoupled eNOS to attenuate AAA formation34. These data establish an essential role of NOX1-dependent, NOX2-dependent and/or NOX4-dependent eNOS uncoupling in the development of AAA. Recoupling of eNOS by targeting DHFR deficiency or NOX1, NOX2, or NOX4 might be a novel therapeutic approach for the prevention and treatment of AAA.
Our findings in Dhfr+/− mice have shown that mitochondria act downstream of eNOS uncoupling in modulating the development of AAA37. Application of MitoTEMPO completely blocked the development of AAA in angiotensin II-infused Dhfr+/− mice37. These data indicate that eNOS uncoupling induces AAA formation through mitochondrial dysfunction, targeting of which might be a novel and effective therapeutic strategy for AAA. Consistent with the notion that eNOS uncoupling has a causal role in AAA formation, we have shown that two doses of nifedipine (an L-type Ca2+-channel blocker) treatment prevented AAA formation in angiotensin II-infused hph-1 mice via inhibition of NOX activity and eNOS uncoupling32. Whereas a low dose of nifedipine is ineffective in reducing blood pressure, a high dose of nifedipine is effective in reducing both blood pressure and the formation of AAA in mice. These data indicate that nifedipine might be a particularly useful treatment for patients with coexisting hypertension and AAA.
Hypercholesterolaemia and atherosclerosis
NOX-derived oxidative stress has been shown to be a major mediator of atherosclerosis254. LDL oxidation, a major event during early atherogenesis, can be induced by NOX-derived ROS255. Roles of NOXs in atherosclerosis have been investigated in genetically modified animal models on the background of Apoe−/−, a widely used model of atherogenesis. In Apoe−/− mice, knockout of Ncf1 protected against lesion formation, which suggests that either NOX1 or NOX2 or both are required for the development of atherosclerosis57. Specifically, deletion of Nox1 in Apoe−/− mice reduced aortic superoxide production, macrophage infiltration and lesion formation256,257. Administration of GKT137831, an inhibitor of NOX1 and NOX4, had similar effects to those of Nox1 deletion in Apoe−/− mice257. Likewise, deletion of Nox2 in Apoe−/− mice resulted in decreased aortic superoxide production, reduced lesion formation and increased NO bioavailability258. Of note, endothelium-specific overexpression of NOX2 did not further accelerate the progression of atherosclerosis in Apoe−/− mice, although superoxide production and macrophage recruitment were increased259. These results indicate that NOX1 and NOX2 have critical roles in atherogenesis, and cell type-specific contributions of NOXs and NOX-derived ROS warrant further investigation.
Several studies have produced evidence to support a protective role of NOX4 in atherosclerosis243,260–262. Global Nox4 knockout or induced deletion of Nox4 increased atherosclerosis in Apoe−/− mice260,261. Although H2O2 production was reduced, increased inflammation, macrophage accumulation and fibrosis were noted in the aortas of Nox4−/−Apoe−/− mice260,261. The researchers concluded that NOX4-produced H2O2 has anti-atherosclerotic functions260,261. In accordance with these results, endothelial overexpression of NOX4 protected Apoe−/− mice from the formation of atherosclerotic lesions, primarily through attenuated inflammatory responses262. In Ldlr−/− mice, deletion of Nox4 resulted in increased atherosclerotic lesion formation mediated by H2O2 deficiency and endothelial dysfunction243. Together, these results suggest that NOX4-derived H2O2 might mediate beneficial effects in atherosclerosis via inhibition of inflammation, which is contrary to the deleterious effects of ROS produced by NOX1 and NOX2. These findings in mouse models are in agreement with the findings that NOX4 mRNA levels were decreased whereas NOX1 mRNA levels were increased in endarterectomy specimens from patients with CVDs (compared with individuals without CVDs) or diabetes (compared with individuals without diabetes)260.
Finally, NOX5 is an important source of ROS in atherosclerosis. NOX5 is localized in the lesion area (in both endothelial and vascular smooth muscle cells) in the coronary arteries from patients with coronary artery disease undergoing cardiac transplantation120. Expression of NOX5 is very low in coronary arteries from patients undergoing cardiac transplantation who do not have coronary artery disease; however, NOX5 expression (both mRNA levels and protein levels) is significantly upregulated in patients with coronary artery disease undergoing cardiac transplantation120. Moreover, NOX5 has been shown to increase the proliferation of vascular smooth muscle cells, further suggesting a role of NOX5 in atherosclerosis263. So far, no direct evidence on the role of NOX5 in atherogenesis is available from animal models because rodents do not have NOX5.
Of note, eNOS uncoupling occurs in Apoe−/− mice12,14,29. Endothelial transgenesis of eNOS was reported to increase the formation of atherosclerotic lesions in Apoer−/− mice owing to increased eNOS uncoupling18,264. Strategies to recouple eNOS by supplementation with H4B or endothelial-specific overexpression of GTPCH1 significantly reduced lesion formation in Apoe−/− mice, accompanied by decreased superoxide production, improved vasorelaxation and NO bioavailability, and reduced inflammation14,18,19,25,264. Angiotensin II induces atherogenesis in Apoe−/− mice, which was attenuated by the upregulation of eNOS phosphorylation and NO production when animals were fed with mitochondria-targeted aesculetin (6,7-dihydroxycoumarin)265, implying a beneficial effect of eNOS recoupling on the prevention of atherogenesis in Apoer−/− mice. Angiotensin II infusion causes eNOS uncoupling in Apoe−/− mice, resulting in AAA formation29. In addition, NOX activation and NOX-derived ROS mediate angiotensin II-induced eNOS uncoupling in hph-1 mice, leading to hypertension and AAA27,32,34. However, direct evidence as to whether eNOS uncoupling in atherosclerosis lies downstream of NOX activation requires further investigation.
Diabetic vascular complications
NOX activation has been implicated in endothelial dysfunction in diabetes. Expression of NOX1 was induced by high glucose levels in human aortic endothelial cells in vitro in accordance with upregulation of superoxide production257. The superoxide production induced by high glucose levels was attenuated by NOX1 siRNA or GKT137831 (REF.257). NOX activation has also been reported in animal models of both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). In particular, we have shown that NOX1 protein levels were upregulated threefold in T1DM26. Knockdown of Nox1 or Noxo1 or deletion of Ncf1 reduced eNOS uncoupling in STZ-induced diabetic animals26. However, knockout of Nox2 or knockdown of Nox4 did not alter eNOS uncoupling26. Taken together, these data indicate a NOX1-specific induction of eNOS uncoupling in T1DM. Nox1 knockout also reversed the impaired endothelium-dependent vasodilatation in T1DM26. Blocking angiotensin II signalling in vivo with an AT1 receptor antagonist or an ACE inhibitor attenuated NOX activity and eNOS uncoupling in STZ-treated T1DM mice17. In addition, Nox1 deletion resulted in eNOS recoupling through preservation of DHFR function and restoration of H4B bioavailability26. These findings indicate a pathological role of the angiotensin II–NOX1–eNOS uncoupling axis in the induction of vascular dysfunction in T1DM.
The roles of NOX activation and eNOS uncoupling in vascular dysfunction and inflammation have also been studied in db/db mice, a model of T2DM. These mice have impaired vascular relaxation compared with wild-type littermates, which is restored by treatment with apocynin266. Aortic mRNA and protein levels of p22phox and NOX1, but not NOX2, were elevated in db/db mice compared with wild-type mice, accompanied by increased superoxide production and impaired vasorelaxation35,266, suggesting a role of NOX1 activation in inducing vascular dysfunction in T2DM. Additionally, eNOS uncoupling has been reported in db/db mice35, indicating that NOX1-dependent eNOS uncoupling might be the cause of endothelial dysfunction in T2DM. However, the upstream mechanism of NOX1 activation in T2DM is different from that in T1DM. Circulating BMP4 levels were robustly elevated in both db/db mice and wild-type mice fed a high-fat diet (in contrast to the elevated angiotensin II levels in plasma observed in T1DM mice)35. Interestingly, noggin, a BMP4 antagonist, attenuates eNOS uncoupling and endothelial dysfunction in db/db mice35, indicating BMP4-dependent eNOS uncoupling. siRNA targeting Nox1 blocked BMP4-induced eNOS uncoupling35. These findings suggest that BMP4–NOX1 mediates eNOS uncoupling in T2DM. Furthermore, upregulation of the levels of the inflammatory regulators prostaglandin G/H synthase 2 (also known as COX2) and vascular cell adhesion protein 1 (VCAM1) in db/db mice was significantly blocked by noggin infusion35. Of note, BMP4-dependent COX2 upregulation was normalized by administration of sepiapterin, an eNOS-recoupling agent, indicating that COX2 lies downstream of BMP4-induced uncoupling of eNOS35. Taken together, these findings demonstrate that BMP4–NOX1-dependent eNOS uncoupling and subsequent COX2–VCAM1 activation mediate vascular dysfunction and inflammation in T2DM.
To examine whether vascular NOX-derived oxidative stress has a role in the development of obesity and metabolic syndrome, our group used transgenic mice with vascular smooth muscle-specific expression of Cyba that were fed a high-fat diet242,267. Of note, the Cyba transgene increased vascular superoxide and H2O2 production at baseline, which has a compensatory vasodilatory effect, such that the animals had no obvious pathological phenotype but a minimally increased blood pressure at baseline242. When fed a high-fat diet, these transgenic mice developed marked obesity, insulin resistance, leptin resistance and glucose intolerance compared with wild-type mice267. The underlying mechanisms involve mitochondrial dysfunction and elevated mitochondrial ROS production in skeletal muscle, impaired spontaneous activity, as well as increased adipogenesis and perivascular inflammation267. Targeted deletion of Cyba in vascular smooth muscle prevented obesity and leptin resistance induced by a high-fat diet via restoration of skeletal muscle mitochondrial function and attenuation of adipogenesis and perivascular inflammation267. These findings are paradigm-shifting in establishing a novel concept that vascular-driven oxidative stress is a cause of obesity and metabolic syndrome rather than a consequence.
Diabetic vascular complications have been examined in both large vessels (such as the aorta) and small vessels (such as skeletal muscle arterioles and adipose microvessels). In human primary isolated skeletal muscle arterioles and human adipose microvascular endothelial cells, treatment with insulin induced vascular dysfunction via VAS2870-inhibitable superoxide production and eNOS uncoupling268. In this study, VAS2870 was used as a NOX2 inhibitor268. Although VAS2870 was later shown to be an inhibitor of all NOXs269, these data suggest that insulin induces vascular dysfunction via NOX activation and superoxide production in small vessels. A thorough examination of three types of microvessels in db/db mice demonstrated that NOX activity (measured by lucigenin chemiluminescence in the presence of NADPH) was increased in diabetes in coronary arteries, mesenteric resistance arteries and femoral arteries270. This increased NOX activity was blocked by in vivo Cyba-targeted siRNA or SOD treatment270. In vivo knockdown of Cyba with siRNA also improved vascular relaxation in the three types of microvessels270, again implying an intermediate role of NOX-dependent ROS production in mediating vascular dysfunction in diabetes.
Cardiac IR injury and MI
Cardiac IR induces ROS production when oxygen supply is restored after an ischaemic event271–273. Data have shown that NOX isoforms have important roles in IR injury. NOX2 protein levels were found to be elevated in cardiomyocytes from individuals who had died from acute MI125. In animal models, increased protein expression of NOX2 and NOX4 has been reported during IR (30 min of ischaemia followed by 24 h of reperfusion for in vivo experiments; 20–25 min of ischaemia followed by 1 h of reperfusion for ex vivo experiments)31,58. Reduced infarct size after IR was reported in mice with global knockout of Nox2 or Nox4 via inhibition of superoxide production58. Endothelial-specific deletion of Smarca4, which encodes the transcription factor BRG1 that regulates Nox2 and Nox4 transcription, attenuated IR-induced Nox2 and Nox4 expression, reduced superoxide production and decreased infarct size274, indicating that endothelial NOX2 and NOX4 might have an important role in cardioprotection against IR injury. However, overexpression of NOX2 in cardiomyocytes or endothelial cells had no effect on infarct size in a mouse model of MI at 4 weeks275. Conversely, cardiac transgenesis of Nox4 in mice increased oxidative stress and infarct size in response to IR using an ex vivo Langendorff system276. Consistently, protection from IR injury in mice with cardiac-specific Nox4 knockout has been reported58. In accordance with these findings, we have shown that inhibition of NOX4 in vivo with the use of siRNA attenuated IR-induced infarct size in mice31. IR-induced upregulation of NOX4 levels increased cardiac ROS production, eNOS uncoupling and mitochondrial dysfunction, resulting in cardiac injury31. However, we did not observe cardioprotection in Nox1−/− or Nox2−/− mice subjected to IR31, indicating a NOX4-specific role in IR injury. Our data established a critical role of the NOX4–uncoupled eNOS–mitochondrial dysfunction axis in mediating IR-induced cardiac injury31. Infusion of netrin-1 into Langendorff-perfused mouse hearts stimulates NO production from coupled eNOS, resulting in NO-dependent inhibition of NOX4, reduced oxidative stress, preserved mitochondrial function and markedly reduced infarct size31.
Intriguingly, global Nox2 knockout combined with cardiac-specific Nox4 knockout resulted in increased cardiac injury in response to IR, despite reduced superoxide production58. A similar phenotype was observed in transgenic mice with cardiac-specific expression of a dominant-negative form of NOX4, which has been shown to suppress both NOX4 and NOX2 in cardiomyocytes58,276. The researchers suggested that mild downregulation of oxidative stress (by Nox2 or Nox4 deletion) is protective, whereas marked downregulation of oxidative stress (by combined Nox2 and Nox4 knockout or overexpression of a dominant-negative form of NOX4) increases cardiomyocyte death58. Markedly reducing the levels of oxidative stress is thought to lead to reduced levels of hypoxia-inducible factor 1α (HIF1α) and increased levels of peroxisome proliferator-activated receptor-α (PPARα) after IR58,277. Elevated levels of PPARα then stimulate free fatty acid metabolism, which in turn induces triglyceride accumulation in the heart and lipotoxicity58. Taken together, these studies suggest that a basal level of ROS can be cardioprotective and is required to maintain cardiac homeostasis, although high levels of ROS are deleterious and can result in MI58,276,278.
Treatment with folic acid is reported to ameliorate ROS production and attenuate IR injury in rats144, suggesting an important role of eNOS recoupling in cardiac protection against IR. Our studies have shown that NOX4, but not NOX1 or NOX2, was significantly upregulated and activated by IR, resulting in eNOS uncoupling, mitochondrial dysfunction and cardiac injury31. Given that NO effectively downregulates NOX4 expression, loss of NO as a result of initial ROS production during IR leads to persistent NOX4 expression and activity31. We have previously reported that netrin-1 prevents IR-induced cardiac injury through elevated NO production ex vivo and in vivo279–281. This effect is via netrin receptor DCC-dependent activation of ERK1 (also known as MAPK3), ERK2 (also known as MAPK1) and eNOS279,282. Netrin-1–NO signalling also robustly inhibits the E3 ubiquitin-protein ligases SIAH1 and SIAH2 (which mediate the proteasome-dependent degradation of the netrin receptor DCC) thereby potentiating netrin-1-induced cardioprotection283. In addition, netrin-1 inhibits post-MI autophagy to limit cardiac remodelling31. Therefore, netrin-1 abrogates IR-induced cardiac mitochondrial dysfunction and infarction through NO-dependent downregulation of Nox4 expression and recoupling of eNOS31. These data indicate important crosstalk between NOX4, uncoupled eNOS and mitochondrial dysfunction in mediating cardiac IR injury, the interruption of which by netrin-1 is cardioprotective. Moreover, small netrin-1-derived peptides (9–11 amino acids) are highly effective in protecting against IR injury via production of NO, making these peptides a pharmacologically novel approach for the treatment of acute MI284.
NOX5 is absent in rodents, but transgenic animals with the human isoform have been created. In a humanized mouse model with endothelial-specific (Tie2 promoter-driven) knock-in of human NOX5, the IR-induced infarct size in the heart was not significantly different from that in wild-type controls, but the brain infarct size after stroke was increased through ROS-dependent blood–brain barrier leakage247.
Heartfailure
Heart failure is a chronic, progressive condition that often occurs as a result of maladaptive changes to compensate for cardiac hypertrophy285. The role of different NOX isoforms in the development of cardiac hypertrophy has been assessed in various genetically modified animal models, mostly focusing on NOX2 and NOX4 (REFS286,287). Multiple research groups have reported that NOX4 expression in the heart is upregulated in response to 2–4 weeks of transverse aortic constriction (TAC) to induce pressure overload, or after phenylephrine or angiotensin II infusion89,134,240,288. Indeed, cardiac-specific knockout of Nox4 was found to be effective in attenuating TAC-induced or phenylephrine-induced cardiac hypertrophy59,134, and cardiac-specific overexpression of Nox4 potentiated the hypertrophic phenotype59,134. In animals with TAC-induced cardiac hypertrophy, NOX4 activation leads to mitochondrial superoxide production, resulting in apoptosis and cardiac dysfunction59. TAC-dependent and phenylephrine-dependent upregulation of NOX4 leads to superoxide accumulation in the nuclei and oxidation of histone deacetylase 4 (HDAC4)134. The oxidation of cysteine residues in HDAC4 induces cardiac hypertrophy through activation of nuclear factor of activated T cells (NFAT)134,289. Cardiac-specific overexpression of Nox4 in mice potentiated angiotensin II-induced cardiac hypertrophy, which was significantly inhibited by GKT137831 administration240. The mechanisms of angiotensin II-induced hypertrophy involve upregulation of NOX4 levels, NOX4-dependent ROS production and subsequent increased phosphorylation of RACα serine/threonine-protein kinase (AKT)240. Phosphorylation of the two downstream effectors of AKT, mechanistic target of rapamycin (mTOR) and nuclear factor-κB (NF-κB; specifically, the p65 subunit), was upregulated in the hearts of angiotensin II-infused mice240.
Angiotensin II has also been shown to promote H2O2 production in isolated cardiomyocytes290. In vivo generation of H2O2 in the heart induced heart failure in rats291. In this study, cardiac H2O2 was produced from the conversion of orally administered d-alanine to pyruvate, catalysed by a virally delivered d-amino acid oxidase (driven by the Tnnt2 promoter)291. Interestingly, studies have shown that ageing or TAC impaired cardiac function in mice with cardiac-specific overexpression of Nox4, without significant changes in cardiac hypertrophy at the organ level48,78. However, left ventricular cardiomyocyte cross-sectional size was increased in Nox4-transgenic mice59,89, suggesting a compensatory response of the heart against cardiac dysfunction. The inconsistent phenotypes of Nox4 transgenesis in cardiac hypertrophy models might be caused by different time points of Nox4 expression induction in these animal models59,89,240. In the inducible transgenic model (in cardiomyocytes), Nox4 expression was induced 7 days before angiotensin II infusion240. In this model of transient overexpression of Nox4 in the heart, NOX4 exacerbated angiotensin II-induced hypertrophy via increased ROS production240. By contrast, in animals with embryonic cardiac overexpression of Nox4, no further exaggeration in hypertrophy was developed after TAC, suggesting that adaptation to Nox4 overexpression had been established in these animals59,89,134.
Of note, another research group observed that global knockout of Nox4 exaggerated hypertrophy during exposure to chronic pressure overload (6 weeks of suprarenal aortic constriction)288. Deletion of Nox4 inhibited expression of Hif1a and Vegf, which blocked angiogenesis of myocardial capillaries and contributed to hypertrophy288. In addition to the different strategies applied to induce hypertrophy, the discrepancy between this global knockout model288 and the previously discussed cardiac-specific knockout model59,134 suggests that NOX4 in different cell types might have opposing roles in the development of hypertrophy, which warrant further investigation.
The role of NOX2 in the development of cardiac hypertrophy has also been studied. At a lower dose of angiotensin II infusion (0.3 mg/kg per day), which did not alter blood pressure, Nox2 deletion protected against angiotensin II-induced cardiac hypertrophy through reduced ROS production292. The protein level of NOX2 was reportedly not modulated by angiotensin II in the mouse heart240. These data suggest that angiotensin II induces hypertrophy through activation of NOX2 rather than upregulation of its expression level. Moreover, cardiac NOX2 protein levels were reported to be upregulated after aortic banding293,294 or MI295,296. Of note, NOX2 protein levels were elevated in left ventricular myocardial tissue from patients with end-stage heart failure and dilated cardiomyopathy compared with that of individuals without heart failure294. The same research group has reported that deletion of Nox2 in mice prevented TAC-induced oxidative stress and development of hypertrophy through inhibition of MAPK signalling294, suggesting a pathogenic role of NOX2 in heart failure. Similarly, a potential role of NOX2 in MI-induced hypertrophy was also studied in genetically modified Nox2 mice. Global knockout of Nox2 protected against post-MI cardiac hypertrophy and restored cardiac function295. As expected, cardiac-specific overexpression of Nox2 increased chronic MI (left coronary artery ligation)-induced cardiomyocyte hypertrophy compared with wild-type littermates, in accordance with elevated superoxide production, whereas endothelial-targeted overexpression of Nox2 did not alter cardiac remodelling after MI275, indicating that cardiac but not endothelial NOX2 is involved in cardiac remodelling after MI.
eNOS uncoupling has been reported to occur in mouse hearts in response to TAC16,297. Supplementation with H4B recoupled eNOS in wild-type mice after TAC, whereas knockout of Nos3 (encoding eNOS) reduced TAC-induced hypertrophy and cardiac remodelling16,145. These results are consistent with the observation that NOX2-dependent and NOX4-dependent ROS production has an important role in the development of cardiac hypertrophy and that NOX-derived ROS lead to eNOS uncoupling. Another downstream effector of NOX-derived ROS is mitochondria. Excessive ROS production derived from mitochondria has been shown to contribute to heart failure124,179,297–299. Treatment of a healthy myocardium sample from dogs with antimycin A (a mitochondrial complex III inhibitor) reproduced the increase in superoxide production seen in the failing heart298. In angiotensin II-infused animals, ROS scavenging with N-acetylcysteine was less effective than mitochondria-targeted scavenging with peptide SS-31 in the prevention of cardiac hypertrophy, suggesting that mitochondrial ROS have an important role in modulating cardiac remodelling in angiotensin II-infused animals183,300. A positive correlation has been reported between myocardial ROS and left ventricular contractile dysfunction298. In a guinea pig model, heart failure was induced by ascending aorta constriction and daily infusion of the β-adrenergic agonist isoprenaline299,301. With an adenovirus-delivered H2O2 sensor (roGFP-ORP1) targeting the cytoplasm and mitochondria, the researchers showed that levels of both cytoplasmic and mitochondrial ROS were significantly increased in freshly isolated cardiomyocytes from failing hearts compared with cardiomyocytes from sham control hearts299,302. Administration of MitoTEMPO to clear mitochondrial ROS effectively normalized both cytoplasmic and mitochondrial ROS in cardiomyocytes from failing hearts299, indicating crosstalk between mitochondria and oxidases in the cytoplasm. Administration of MitoTEMPO from the day of ascending aorta constriction or after heart failure is already present (3 weeks after the surgery) prevented or reversed heart failure299, suggesting an important role of mitochondrial ROS in the development of heart failure. Taken together, these results indicate that eNOS uncoupling and mitochondrial ROS production contribute to NOX activation-induced pathogenesis of heart failure.
Cardiac arrhythmias
Cardiac arrhythmia is associated with elevated production of ROS. A growing number of studies have shown that NOXs are emerging sources of excessive production of ROS in the pathogenesis of arrhythmia53,103,303,304. A study in pigs showed increased NOX2 expression after MI in line with the development of arrhythmia, whereas reducing NOX2 protein levels by acute unloading of the left ventricle decreased the incidence of arrhythmia296. Atrial fibrillation (AF) is the most common cardiac arrhythmia. Expression of NOX2 and NOX4 and production of ROS were found to be upregulated in atrial tissue from patients with AF compared with that from individuals in sinus rhythm303,304. We have previously reported upregulated NOX4 expression and H2O2 levels (the detectable product of NOX4) in the cardiac tissues of patients with AF, whereas NOX2 expression was not significantly changed304. However, a potential causal role of NOX4 activation in the development of AF remained uncertain. To address this question, we examined arrhythmogenesis in zebrafish embryos with acute induction of nox4 (REF.60). Overexpression of nox4, instead of nox2, induced ROS production and cardiac arrhythmia (in the form of irregular heartbeats) in zebrafish embryos, both of which were blocked by nox4 antisense morpholino oligonucleotide co-injection, treatment with poly(ethylene glycol)-SOD or NOX4 inhibitors 6-(dimethylamino)fulvene, fulvene-5 or proton sponge blue60. Overexpression of Nox4-P437H protein, a dominant negative form of Nox4, did not induce ROS production or an arrhythmic phenotype60. Nox4 overexpression induced arrhythmia through ROS-dependent activation of CaMKII60. These data demonstrate a causal role of Nox4-derived ROS in arrhythmogenesis in zebrafish60. The emerging roles of NOXs as a source of ROS production in inducing the development of AF has been previously reviewed53.
Moreover, uncoupled eNOS and mitochondrial dysfunction have been reported to be involved in cardiac arrhythmia. Pretreatment with a NOS inhibitor significantly (although to a lower extent than a NOX inhibitor) reduced superoxide production in atrial homogenates from patients with AF, implicating uncoupling of eNOS303. Additionally, a mitochondrial complex I inhibitor lowered the basal levels of superoxide production in atrial homogenates from patients with AF, suggesting that mitochondrial ROS might contribute to the development of AF303. These data suggest that uncoupled eNOS and mitochondrial dysfunction are likely to underlie the development of AF downstream of NOX activation.
NOX inhibitors
Given the critical roles of NOXs in the pathogenesis of CVDs, they have been considered as prominent targets for the development of novel therapeutic agents305. The long quest to develop specific NOX inhibitors is illustrated in BOX 2.
Small-molecule inhibitors
Initially, several nonspecific small molecules were used as NOX inhibitors306; diphenyleneiodonium (DPI) and apocynin are the most widely studied28,213,307–310. DPI is a general, nonreversible inhibitor of all NOX isoforms with an inhibitory constant (Ki) of 10–70 nmol/l (REFS311–313). However, off-target effects have been reported with DPI, which also inhibits other ROS-generating enzymes and systems, including XO, NOS and the mitochondrial electron transport chain314–318. Apocynin inhibits the membrane translocation of p47phox and p67phox (REFS102,319–321) in leukocytes and was initially considered to be a specific inhibitor of NOXs (but was later found to inhibit all flavin-containing enzymes). Apocynin is activated after the formation of apocynin dimers in leukocytes321,322; however, in endothelial and smooth muscle cells, these dimers do not form and, therefore, apocynin is not activated in these cell types322. Nevertheless, apocynin has intrinsic antioxidant activity as a ROS scavenger, which explains the inhibitory effects of apocynin treatment on ROS levels in endothelial cells and smooth muscle cells102,318,322,323.
Other small-molecule NOX inhibitors have also been investigated and used. 4-(2-Aminoethyl)-benzenesulfonyl fluoride, an inhibitor of serine proteases, has been used as a NOX inhibitor324,325. As a nonspecific inhibitor, 4-(2-aminoethyl)-benzenesulfonyl fluoride has low inhibitory potency for NOX (100 μmol/l) compared with DPI (10 μmol/l) and is, therefore, not frequently used102,326. A synthetic polyphenol, S17834, reduced tumour necrosis factor-induced NOX activity in endothelial cells and inhibited atherosclerotic lesion formation in the aortas of Apoe−/− and Ldlr−/− mice327,328. S17834, which targets 5’ AMP-activated protein kinase102, did not change superoxide production by XO and is not a scavenger of superoxide. Statins — inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase that are used for the lowering of blood lipid levels — inhibit NOX activity by targeting RAC1 (REFS329–332). However, statins also upregulate eNOS and NO production333. NSC23766, a RAC1 inhibitor, has been used as a NOX inhibitor15,334,335. VAS2870 and VAS3947 have also been used as NOX inhibitors336–338. Whereas VAS2870 inhibits NOX2, NOX4 and NOX5, VAS3947 targets NOX1, NOX2 and NOX4 (REF.102). The lack of specificity of these two inhibitors has limited their use for isoform-specific evaluation of NOXs in disease development. An inhibitor thought to be selective for NOX1, 2-acetylphenothiazine (known as ML171), was identified by high-throughput screening316,323; however, this inhibitor was soon demonstrated to inactivate all NOX isoforms, including NOX1, and interferes with the Amplex Red Assay for H2O2 detection339. Owing to their chemical similarity to DPI, triphenylmethane dyes have been tested for their NOX inhibitory capacity340. Brilliant green and gentian violet have displayed effective inhibition of NOX2 and NOX4, and imipramine blue inhibited NOX4-derived ROS in vitro341. In addition, fulvene derivatives have been synthesized and been shown to inhibit NOX2 and NOX4 activity in vivo342. In particular, 6-(dimethylamino) fulvene, fulvene-5 and proton sponge blue blocked NOX4-induced ROS production in vivo60.
Peptide-based inhibitors
Peptide-based inhibitors have been designed to target NOXs. NOX2 docking sequence (ds)-tat (originally known as gp91ds-tat) is a chimeric peptide designed to interfere with the interaction between NOX2 and p47phox (REF.343). NOX2ds-tat contains nine amino acids (NOX2ds) corresponding to the human and mouse NOX2 sequence but with one substituted amino acid (isoleucine for valine at position 89)343. To ensure the in vivo delivery of this peptide, a specific nine-amino acid peptide of the human immunodeficiency virus coat (HIV-tat) was linked343. NOX2ds-tat completely inhibited angiotensin II-induced aortic superoxide production and blood pressure increase, suggesting effective attenuation of angiotensin II-induced NOX activation343. The specificity of peptide NOX2ds has been examined by the same researchers, who showed that NOX2ds did not change the activity of NOX1, NOX4 or XO; NOX2ds is, therefore, considered to be a specific inhibitor of NOX2 (REF.344). Similarly, an 11-amino acid peptide, NOXA1ds, which mimics the activation domain of NOXA1, was designed to block NOX1 activity (without fusion to another peptide to facilitate cellular delivery)345. In this peptide, the phenylalanine at position 199 was changed to alanine345. NOXA1ds disrupted the association between NOX1 and NOXA1 and specifically inhibited NOX1-dependent superoxide production, without changing ROS production by NOX2, NOX4, NOX5 or XO345. NOXA1ds significantly inhibited hypoxia-induced or angiotensin II-induced ROS production in endothelial cells and vascular smooth muscle cells, respectively132,345. NOXA1ds also blocked vascular endothelial growth factor (VEGF)-induced wound healing in cultured endothelial cells345. Given that both NOX2ds-tat and NOXA1ds are peptide-based inhibitors that are subject to degradation in the gut, further modifications might be needed to improve their oral bioavailability and efficacy345,346. Given the limitations of the existing agents, the development of new classes of NOX inhibitor is urgently needed. Ideally, an agent should inhibit a specific NOX isoform, because different NOX isoforms are selectively involved in the pathogenesis of different CVDs.
Small-molecule inhibitors of GKT family
Several novel small molecules have been identified and characterized as NOX inhibitors316,347–350. These inhibitors seem to target NOX isoforms selectively. During a high-throughput screening campaign, several potent pyrazolopyridine dione derivatives were identified as NOX4 inhibitors347. Following investigation of the structure–activity relationship around the pyrazolopyridine dione core, two NOX inhibitors (GKT136901 and GKT137831) were discovered347. These inhibitors preferentially target NOX1, NOX4 and NOX5 (Ki = 10–100 nmol/l) over other NOX isoforms (Ki > 1 μmol/l for NOX2 and Ki > 100 μmol/l for XO)311–313,351,352. In one report, vascular smooth muscle cell-specific overexpression of human NOX5 increased vascular contractile function, which was blocked by administration of N-acetylcysteine, a ROS scavenger, but not by GKT137831 (10 μmol/l)245. These data suggest that GKT137831 preferentially targets NOX1 and NOX4 over NOX5 (REF.245). GKT136901 and GKT137831 did not show off-target effects when tested on G protein-coupled receptors, kinases, ion channels and other ROS-producing and redox-sensitive enzymes312,313, making them very specific inhibitors of NOX1 and NOX4. Further studies have shown that GKT136901 did not interact with NO, superoxide or hydroxyl radicals353, but scavenged peroxynitrite through direct interaction353 and decreased the Amplex Red fluorescent signal102, weakening its potential as a selective and direct NOX inhibitor, although the clearance of free radicals might be beneficial. GKT136901 was also reported to inhibit DUOX-dependent ROS production at micromolar concentrations in cells354. The capacity of GKT137831 to clear free radicals directly has not been reported.
Both GKT136901 and GKT137831 showed high plasma concentrations in vivo347,355. The half-life of GKT137831 is longer than that of GKT136901 in both rodents and humans355,356. Oral administration of GKT136901 (10 mg/kg of body mass) in Apoer−/− mice fed a high-fat diet reduced aortic lesion formation to a similar level to that previously reported in Apoe−/−Nox1−/− double knockout animals256,357. This decrease was accompanied by reduced aortic superoxide production and systematic oxidative stress in GKT136901-treated Apoe−/− mice357. Likewise, administration of GKT137831 (60 mg/kg of body mass) in STZ-induced diabetic Apoe−/− mice completely reduced aortic lesion size to basal levels257,358. Similarly, treatment with GKT137831 mimicked the anti-atherosclerotic effect of Nox1 deletion in reducing aortic superoxide production, aortic inflammation and fibrosis257,358. In the same study, Nox4 deletion was also examined but had no effect on atherosclerosis257, indicating the potency of GKT137831 for NOX1 inhibition and a selective role of NOX1 in atherogenesis in this model. This observation is consistent with our findings that NOX1 has an essential role in inducing vascular dysfunction in mice with either T1DM or T2DM26,35.
Moreover, the effect of GKT137831 has been compared with that of Nox4 deletion on cardiac remodelling in vivo. Angiotensin II is known to induce cardiac remodelling in wild-type mice51,52,240. In mice with cardiac-specific overexpression of Nox4, infusion of angiotensin II induced severe cardiac remodelling and fibrosis through ROS production and the AKT–mTOR and NF-κB signalling pathways240. Administration of GKT137831 (40 mg/kg per day) abolished the increase in oxidative stress, suppressed AKT–mTOR and NF-κB signalling, and attenuated cardiac remodelling and fibrosis240. These data suggest that GKT137831 is a potent inhibitor of NOX4 in vivo.
The effects of GKT compounds have also been examined in angiogenesis. In mouse cultured primary lung endothelial cells, either administration of GKT136901 or Nox1 deletion inhibited VEGF-induced ROS production311. In vivo data in mice showed that GKT136901 administration (40 mg/kg per day) was more potent than Nox1 deletion in inhibiting angiogenesis in implanted Lewis lung carcinoma 1 tumours311. In addition, GKT137831 is currently under phase II clinical testing in patients with T2DM and albuminuria (NCT02010242) and patients with primary biliary cholangitis receiving ursodeoxycholic acid (NCT03226067). Therefore, GKT137831 is one of the most promising NOX inhibitors to date.
Newly reported inhibitors
GLX compounds were identified as NOX inhibitors by high-throughput screening348,349. GLX351322 was reported to inhibit NOX1, NOX2, NOX4 and NOX5 (REFS348,349). However, its lack of specificity greatly reduced its potential application. Two novel GLX compounds (GLX481372 and GLX7013114) have been identified as NOX4 inhibitors and tested in vitro349. These GLX compounds were also identified by high-throughput screening and were developed in a structure–activity relationship campaign349. Whereas GLX481372 selectively targets NOX4 and NOX5, GLX7013114 is, so far, the first-reported highly selective inhibitor of NOX4 (REF.349); GLX7013114 at concentrations <1 μmol/l reportedly inhibits only NOX4 (REF.349). GLX7013114 protects islet cells against high glucose+palmitate and cytokine-induced cell death349. The researchers believe that GLX7013114 targets a unique domain of NOX4 that makes it selective in inhibiting NOX4, whereas previous NOX inhibitors target a common site shared by more than one NOX isoform349. GLX7013114 did not show activity as a direct ROS scavenger or as an inhibitor of XO or glucose oxidase349.
GSK2795039 is the first small molecule identified that selectively inhibits NOX2 over other NOX isoforms350. GSK2795039 does not show inhibition of PKC, XO or eNOS at concentrations that are efficacious for NOX2 inhibition350. Systemic administration of GSK2795039 (intraperitoneal injection of 100 mg/kg) mimics the effect of Nox2 deletion in the attenuation of ROS production in mice in vivo350. Further investigation is needed to characterize GSK2795039 as a selective inhibitor of NOX2. A nonspecific NOX inhibitor, APX-115, has been shown to inhibit NOX1, NOX2 and NOX4 in animal models in vivo and to protect against kidney injury in animal models of T1DM and T2DM359–361.
Epigenetic modulation of NOXs
Methylation-dependent and acetylation-dependent regulation of NOX gene expression has been studied. Methylation-mediated downregulation of DUOX1, DUOX2 and NOX5 expression has been reported in cancer cells362–364. Incubation with a methyltransferase inhibitor (5′-aza-2′-deoxycytidine) increased DUOX1, DUOX2 and NOX5 expression in cancer cells362,363.
Conversely, deficiency of the histone acetyltransferase KAT2A dramatically downregulated NOX2 transcription and superoxide production365. In addition, inhibition of HDACs with pharmacological inhibitors (scriptaid, suberoylanilide hydroxamic acid, trichostatin A and valproic acid) reduced NOX1, NOX2, NOX4 and NOX5 mRNA levels and ROS production366–369. The underlying mechanism involves HDAC inhibitors decreasing binding of the histone acetyltransferase p300 to the NOX promoter regions, reducing accessibility of RNA polymerase II and attenuating transcription efficiency368. HDAC inhibitors also increased expression of SOD3 through acetylation and methylation of histones in its promoter region367. Therefore, epigenetic modulation regulates oxidative stress via both NOXs and SOD.
Surprisingly, application of suberoylanilide hydroxamic acid (an HDAC inhibitor) inhibited STZ-induced upregulation of Nox1, Nox2 and Nox4 expression as well as ROS production in mouse aorta369. This change is caused by inhibition of glucose-stimulated interaction ofHDAC1, HDAC2 and p300 with the promoter regions of NOX1, NOX4 and NOX5, and of glucose-stimulated acetylation and NOX1, NOX4 and NOX5 transcription in human vascular smooth muscle cells369. In endothelial cells, high glucose levels activate hydroxymethylating enzymes, leading to increased levels of 5-hydroxymethylcytosine (generated from 5-methylcytosine) and its binding to the RAC1 promoter region as well as activation of RAC1 transcription in diabetes370. Moreover, in an animal model of cardiac IR injury, macrophage expression of myocardin-related transcription factor A recruited the histone acetyltransferase KAT8 to the promoters of Nox1, Nox2 and Nox4 to activate transcription371. Inhibition of KAT8 with MG149 significantly downregulated Nox1 and Nox4 expression and ROS production and restored myocardial function in mice exposed to IR injury371. These studies have revealed an emerging mechanism of epigenetic regulation of NOX gene transcription and NOX-derived ROS production in CVDs369–371, the targeting of which might provide alternative therapeutic strategies to NOX inhibition.
In addition to the challenge of developing isoform-specific inhibitors of NOXs, it is important to note that basal ROS production is required for many physiological processes, as discussed above2,58. Removing basal levels of ROS might compromise these physiological functions. Furthermore, tissue-specific or cell-specific roles of NOX isoforms need to be targeted in different cardiovascular conditions. Therefore, details of NOX inhibitor application (that is, timing, dosing and cell-specific or tissue-specific targeting) are of great importance in the prevention and treatmentof CVDs.
Conclusions
During the pathogenesis of CVDs, ROS are generated by various sources, particularly NOXs. The interactions between different ROS-producing systems demonstrate a critical role of NOXs and NOX-dependent activation of secondary oxidase systems in sustaining oxidative stress, leading to the development of CVDs. Upon activation, NOX-derived ROS induce eNOS uncoupling, mitochondrial dysfunction and, to a lesser extent, XO activation, resulting in further release of ROS and tissue injury. Systematic evaluations of interactions between ROS-producing systems have provided new insights into the mechanistic details of CVDs, especially isoform-specific activation of NOXs under different disease conditions. Targeting specific NOX isoforms selectively to correct eNOS uncoupling and mitochondrial dysfunction might prove to be highly beneficial as a novel therapeutic strategy for the treatment of various CVDs, including hypertension, aortic aneurysms, diabetic vascular dysfunction, atherosclerosis, cardiac IR injury, heart failure and cardiac arrhythmias. Therefore, the development of novel, effective and isoform-specific NOX inhibitors, as well as the development of novel strategies targeting uncoupled eNOS and mitochondrial dysfunction, are essential in realizing the therapeutic value of targeting NOX isoforms and downstream oxidase systems for the prevention and treatment of CVDs.
Key points.
Activation of NADPH oxidase (NOX) has a critical role in the pathogenesis of cardiovascular diseases.
Activation of NOX induces activation of downstream secondary oxidase systems, including uncoupled endothelial nitric oxide synthase, dysfunctional mitochondria and xanthine oxidase.
Crosstalk between oxidases or oxidase systems sustains oxidative stress to mediate the development of cardiovascular diseases.
Targeting NOXs as well as interactions between NOXs and secondary oxidase systems might be a novel therapeutic strategy for the prevention and treatment of cardiovascular diseases.
Acknowledgements
The authors are supported by an AHA Postdoctoral Fellowship Award #14POST20380996 (Y.Z.), NIH National Heart, Lung, and Blood Institute (NHLBI) grants HL077440, HL088975 and HL119968, and an AHA Established Investigator Award 12EIA8990025 (H.C.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Cai H & Harrison DG Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res 87, 840–844 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Brown DI & Griendling KK Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res 116, 531–549 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorov DB, Filburn CR, Klotz LO, Zweier JL & Sollott SJ Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med 192, 1001–1014 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinkevich NS & Gutterman DD ROS-induced ROS release in vascular biology: redox-redox signaling. Am. J. Physiol. Heart Circ. Physiol 301, H647–H653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai H, Griendling KK & Harrison DG The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci 24, 471–478 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Cai H Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res 68, 26–36 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Cai H NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ. Res 96, 818–822 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Youn JY, Siu KL, Li Q, Harrison DG & Cai H in Systems biology of free radicals and antioxidants (ed. Laher I) 849–876 (Springer, Berlin, Heidelberg, 2014). [Google Scholar]
- 9.Wever RM, van Dam T, van Rijn HJ, de Groot F & Rabelink TJ Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun 237, 340–344 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Vasquez-Vivar J et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl Acad. Sci. USA 95, 9220–9225 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Y, Tsai AL, Berka V & Zweier JL Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J. Biol. Chem 273, 25804–25808 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Laursen JB et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103, 1282–1288 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Landmesser U et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest 111, 1201–1209 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alp NJ, McAteer MA, Khoo J, Choudhury RP & Channon KM Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol 24, 445–450 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Chalupsky K & Cai H Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 102, 9056–9061 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takimoto E et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Invest 115, 1221–1231 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oak JH & Cai H Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes 56, 118–126 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Takaya T et al. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol 27, 1632–1637 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Hattori Y et al. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler. Thromb. Vasc. Biol 27, 865–870 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Du YH, Guan YY, Alp NJ, Channon KM & Chen AF Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117, 1045–1054 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Wang S et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension 52, 484–490 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L et al. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol 297, H331–H339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Chalupsky K, Stefani E & Cai H Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J. Mol. Cell. Cardiol 47, 752–760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabtree MJ & Channon KM Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25, 81–88 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Chen W, Rezvan A, Jo H & Harrison DG Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler. Thromb. Vasc. Biol 31, 1547–1554 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn JY, Gao L & Cai H The p47phox− and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 55, 2069–2079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L et al. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid. Hypertension 59, 158–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn JY et al. Endothelium-specific sepiapterin reductase deficiency in DOCA–salt hypertension. Am. J. Physiol. Heart Circ. Physiol 302, H2243–H2249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siu KL, Miao XN & Cai H Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice. PLOS ONE 9, e88899 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siu KL & Cai H Circulating tetrahydrobiopterin as a novel biomarker for abdominal aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol 307, H1559–H1564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siu KL, Lotz C, Ping P & Cai H Netrin-1 abrogates ischemia/reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-dependent attenuation of NOX4 activation and recoupling of NOS. J. Mol. Cell. Cardiol 78, 174–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao XN, Siu KL & Cai H Nifedipine attenuation of abdominal aortic aneurysm in hypertensive and non-hypertensive mice: mechanisms and implications. J. Mol. Cell. Cardiol 87, 152–159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Youn JY & Cai H Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens 33, 1128–1136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siu KL et al. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol 11, 118–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youn JY, Zhou J & Cai H Bone morphogenic protein 4 mediates NOX1-dependent eNOS uncoupling, endothelial dysfunction, and COX2 induction in type 2 diabetes mellitus. Mol. Endocrinol 29, 1123–1133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H et al. Novel treatment of hypertension by specifically targeting E2F for restoration of endothelial dihydrofolate reductase and eNOS function under oxidative stress. Hypertension 73, 179–189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q et al. Knockout of dihydrofolate reductase in mice induces hypertension and abdominal aortic aneurysm via mitochondrial dysfunction. Redox Biol 24, 101185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daiber A Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 1797, 897–906 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Dikalov S Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med 51, 1289–1301 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daiber A et al. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol 174, 1670–1689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doughan AK, Harrison DG & Dikalov SI Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res 102, 488–496 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Zhang DX et al. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ. Res 89, 1177–1183 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Loperena R & Harrison DG Oxidative stress and hypertensive diseases. Med. Clin. North Am 101, 169–193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kigawa Y et al. NADPH oxidase deficiency exacerbates angiotensin II-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol 34, 2413–2420 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Forstermann U, Xia N & Li H Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res 120, 713–735 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Amanso AM & Griendling KK Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front. Biosci 4, 1044–1064 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konior A, Schramm A, Czesnikiewicz-Guzik M & Guzik TJ NADPH oxidases in vascular pathology. Antioxid. Redox Signal 20, 2794–2814 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushima S, Tsutsui H & Sadoshima J Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med 24, 202–205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahles T & Brandes RP NADPH oxidases as therapeutic targets in ischemic stroke. Cell. Mol. Life Sci 69, 2345–2363 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carbone F et al. Pathophysiology and treatments of oxidative injury in ischemic stroke: focus on the phagocytic nadph oxidase 2. Antioxid. Redox Signal 23, 460–489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Perino A, Ghigo A, Hirsch E & Shah AM NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid. Redox Signal 18, 1024–1041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sag CM, Santos CX & Shah AM Redox regulation of cardiac hypertrophy. J. Mol. Cell. Cardiol 73, 103–111 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Youn JY et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J. Mol. Cell. Cardiol 62, 72–79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guzik TJ & Harrison DG Vascular NADPH oxidases as drug targets for novel antioxidant strategies. DrugDiscov. Today 11,524–533 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Briones AM & Touyz RM Oxidative stress and hypertension: current concepts. Curr. Hypertens. Rep 12, 135–142 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Wang HD et al. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res 88, 947–953 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Barry-Lane PA et al. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J. Clin. Invest 108, 1513–1522 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsushima S et al. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor 1α and upregulation of peroxisome proliferator-activated receptor-α. Circ. Res 112, 1135–1149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuroda J et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl Acad. Sci. USA 107, 15565–15570 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y et al. NADPH oxidase 4 induces cardiac arrhythmic phenotype in zebrafish. J. Biol. Chem 289, 23200–23208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyer GY, Islam MF & Quastel JH Biochemical aspects of phagocytosis. Nature 192, 535–541 (1961). [Google Scholar]
- 62.Rossi F & Zatti M Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia 20, 21–23 (1964). [DOI] [PubMed] [Google Scholar]
- 63.Segal AW & Jones OT Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276, 515–517 (1978). [DOI] [PubMed] [Google Scholar]
- 64.Segal AW, Jones OT, Webster D & Allison AC Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet 2, 446–449 (1978). [DOI] [PubMed] [Google Scholar]
- 65.Royer-Pokora B et al. Cloning the gene for an inherited human disorder–chronic granulomatous disease–on the basis of its chromosomal location. Nature 322, 32–38 (1986). [DOI] [PubMed] [Google Scholar]
- 66.Dinauer MC, Orkin SH, Brown R, Jesaitis AJ & Parkos CA The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327, 717–720 (1987). [DOI] [PubMed] [Google Scholar]
- 67.Nunoi H, Rotrosen D, Gallin JI & Malech HL Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science 242, 1298–1301 (1988). [DOI] [PubMed] [Google Scholar]
- 68.Volpp BD, Nauseef WM & Clark RA Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 242, 1295–1297 (1988). [DOI] [PubMed] [Google Scholar]
- 69.Abo A et al. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353, 668–670 (1991). [DOI] [PubMed] [Google Scholar]
- 70.Knaus UG, Heyworth PG, Evans T, Curnutte JT & Bokoch GM Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 254, 1512–1515 (1991). [DOI] [PubMed] [Google Scholar]
- 71.Wientjes FB, Hsuan JJ, Totty NF & Segal AW p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem. J 296, 557–561 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Griendling KK, Minieri CA, Ollerenshaw JD & Alexander RW Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res 74, 1141–1148 (1994). [DOI] [PubMed] [Google Scholar]
- 73.Suh YA et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401,79–82 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Banfi B et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287, 138–142 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Kikuchi H, Hikage M, Miyashita H & Fukumoto M NADPH oxidase subunit, gp91phox homologue, preferentially expressed in human colon epithelial cells. Gene 254, 237–243 (2000). [DOI] [PubMed] [Google Scholar]
- 76.Geiszt M, Kopp JB, Varnai P & Leto TL Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl Acad. Sci. USA 97, 8010–8014 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Deken X et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem 275, 23227–23233 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Edens WA et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol 154, 879–891 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banfi B et al. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem 276, 37594–37601 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Cheng G, Cao Z, Xu X, van Meir EG & Lambeth JD Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269, 131–140 (2001). [DOI] [PubMed] [Google Scholar]
- 81.Banfi B, Clark RA, Steger K & Krause KH Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem 278, 3510–3513 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Geiszt M, Lekstrom K, Witta J & Leto TL Proteins homologous to p47phox and p67phox support superoxide-production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem 278, 20006–20012 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Takeya R et al. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem 278, 25234–25246 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Grasberger H & Refetoff S Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem 281, 18269–18272 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Bedard K & Krause KH The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev 87, 245–313 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Gimenez M, Schickling BM, Lopes LR & Miller FJ Jr. Nox1 in cardiovascular diseases: regulation and pathophysiology. Clin. Sci 130, 151–165 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Dinauer MC, Curnutte JT, Rosen H & Orkin SH A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J. Clin. Invest 84, 2012–2016 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ueno N, Takeya R, Miyano K, Kikuchi H & Sumimoto H The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem 280, 23328–23339 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Ago T et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res 106, 1253–1264 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oda T et al. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J. Biol. Chem 285, 1435–1445 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Magnani F et al. Crystal structures and atomic model of NADPH oxidase. Proc. Natl Acad. Sci. USA 114, 6764–6769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brandes RP, Weissmann N & Schroder K Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med 76, 208–226 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Van Buul JD, Fernandez-Borja M, Anthony EC & Hordijk PL Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal 7, 308–317 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Mizuno T et al. Regulation of the superoxide-generating NADPH oxidase by a small GTP-binding protein and its stimulatory and inhibitory GDP/GTP exchange proteins. J. Biol. Chem 267, 10215–10218 (1992). [PubMed] [Google Scholar]
- 95.Kwong CH, Malech HL, Rotrosen D & Leto TL Regulation of the human neutrophil NADPH oxidase by rho-related G-proteins. Biochemistry 32, 5711–5717 (1993). [DOI] [PubMed] [Google Scholar]
- 96.Kim C & Dinauer MC Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J. Immunol 166, 1223–1232 (2001). [DOI] [PubMed] [Google Scholar]
- 97.Fontayne A, Dang PM, Gougerot-Pocidalo MA & El-Benna J Phosphorylation of p47phox sites by PKC α, βII, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41,7743–7750 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Kitada M et al. Translocation of glomerular p47phox and p67phox by protein kinase C-β activation is required for oxidative stress in diabetic nephropathy. Diabetes 52, 2603–2614 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Schulz E, Wenzel P, Munzel T & Daiber A Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal 20, 308–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Groemping Y, Lapouge K, Smerdon SJ & Rittinger K Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113, 343–355 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Seshiah PN et al. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res 91,406–413 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Altenhofer S, Radermacher KA, Kleikers PW, Wingler K & Schmidt HH Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal 23, 406–427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sahoo S, Meijles DN & Pagano PJ NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin. Sci 130, 317–335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lyle AN et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res 105, 249–259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banfi B et al. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem 279, 18583–18591 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Jha JC, Watson AMD, Mathew G, de Vos LC & Jandeleit-Dahm K The emerging role of NADPH oxidase NOX5 in vascular disease. Clin. Sci 1 31, 981–990 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Chen F, Yin C, Dimitropoulou C & Fulton DJ Cloning, characteristics, and functional analysis of rabbit NADPH oxidase 5. Front. Physiol 7, 284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.BelAiba RS et al. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med 42, 446–459 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Chen F, Wang Y, Barman S & Fulton DJ Enzymatic regulation and functional relevance of NOX5. Curr. Pharm. Des 21, 5999–6008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tirone F & Cox JA NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett 581, 1202–1208 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Chen F et al. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLOS ONE 9, e88405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandey D, Gratton JP, Rafikov R, Black SM & Fulton DJ Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharmacol 80, 407–415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pandey D & Fulton DJ Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am. J. Physiol. Heart Circ. Physiol 300, H1336–H1344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montezano AC et al. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr. Opin. Nephrol. Hypertens 24, 425–433 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambeth JD, Kawahara T & Diebold B Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med 43, 319–331 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lassegue B, San Martin A & Griendling KK Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res 110, 1364–1390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brandes RP & Schroder K Differential vascular functions of Nox family NADPH oxidases. Curr. Opin. Lipidol 19, 513–518 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Ago T et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke 36, 1040–1046 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Gorlach A et al. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res 87, 26–32 (2000). [DOI] [PubMed] [Google Scholar]
- 120.Guzik TJ et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol 52, 1803–1809 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N & Drummond GR The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res 65, 495–504 (2005). [DOI] [PubMed] [Google Scholar]
- 122.Matsuno K et al. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radic. Biol. Med 53, 1718–1728 (2012). [DOI] [PubMed] [Google Scholar]
- 123.Morawietz H & Bornstein SR Leptin, endothelin, NADPH oxidase, and heart failure. Hypertension 47, e20 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Heymes C et al. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol 41, 2164–2171 (2003). [DOI] [PubMed] [Google Scholar]
- 125.Krijnen PA et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J. Clin. Pathol 56, 194–199 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hahn NE et al. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am. J. Pathol 180, 2222–2229 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Chen K, Kirber MT, Xiao H, Yang Y & Keaney JF Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol 181, 1129–1139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu RF, Ma Z, Liu Z & Terada LS Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol 30, 3553–3568 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD & Griendling KK Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol 24, 677–683 (2004). [DOI] [PubMed] [Google Scholar]
- 130.Clempus RE et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol 27, 42–48 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perrotta I, Sciangula A, Perrotta E, Donato G & Cassese M Ultrastructural analysis and electron microscopic localization of Nox4 in healthy and atherosclerotic human aorta. Ultrastruct. Pathol 35, 1–6 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Camargo LL et al. Vascular NOX (NADPH oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension 72, 235–246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ago T et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109, 227–233 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Matsushima S et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res 112, 651–663 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dikalov SI et al. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med 45, 1340–1351 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Helmcke I, Heumuller S, Tikkanen R, Schroder K & Brandes RP Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox. Signal 11, 1279–1287 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Takac I et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem 286, 13304–13313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai H, Dikalov S, Griendling KK & Harrison DG Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods Mol. Med 139, 293–311 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Schulz E, Jansen T, Wenzel P, Daiber A & Munzel T Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal 10, 1115–1126 (2008). [DOI] [PubMed] [Google Scholar]
- 140.Forstermann U & Li H Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol 164, 213–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thony B, Auerbach G & Blau N Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J 347, 1–16 (2000). [PMC free article] [PubMed] [Google Scholar]
- 142.Hink U et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res 88, E14–E22 (2001). [DOI] [PubMed] [Google Scholar]
- 143.Faria AM, Papadimitriou A, Silva KC, Lopes de Faria JM & Lopes de Faria JB Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 61, 1838–1847 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moens AL et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation 117, 1810–1819 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moens AL et al. Bi-modal dose-dependent cardiac response to tetrahydrobiopterin in pressure-overload induced hypertrophy and heart failure. J. Mol. Cell. Cardiol 51,564–569 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng JS et al. Gene transfer of human guanosine 5’-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation 108, 1238–1245 (2003). [DOI] [PubMed] [Google Scholar]
- 147.Raman CS et al. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell 95, 939–950 (1998). [DOI] [PubMed] [Google Scholar]
- 148.Li H et al. Crystal structures of zinc-free and -bound heme domain of human inducible nitric-oxide synthase. Implications for dimer stability and comparison with endothelial nitric-oxide synthase. J. Biol. Chem 274, 21276–21284 (1999). [DOI] [PubMed] [Google Scholar]
- 149.Hemmens B, Goessler W, Schmidt K & Mayer B Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric-oxide synthase. J. Biol. Chem 275, 35786–35791 (2000). [DOI] [PubMed] [Google Scholar]
- 150.Zou MH, Shi C & Cohen RA Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest 109, 817–826 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu J, Xie Z, Reece R, Pimental D & Zou MH Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol 26, 2688–2695 (2006). [DOI] [PubMed] [Google Scholar]
- 152.Chen CA et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468, 1115–1118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Knorr M et al. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol 31,2223–2231 (2011). [DOI] [PubMed] [Google Scholar]
- 154.Oelze M et al. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur. Heart J 34, 3206–3216 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Schuhmacher S et al. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes 60, 2608–2616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heinzel B, John M, Klatt P, Bohme E & Mayer B Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem. J 281,627–630 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pou S, Pou WS, Bredt DS, Snyder SH & Rosen GM Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem 267, 24173–24176 (1992). [PubMed] [Google Scholar]
- 158.Xia Y, Dawson VL, Dawson TM, Snyder SH & Zweier JL Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc. Natl Acad. Sci. USA 93, 6770–6774 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loughran PA et al. Monomeric inducible nitric oxide synthase localizes to peroxisomes in hepatocytes. Proc. Natl Acad. Sci. USA 102, 13837–13842 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chance B, Sies H & Boveris A Hydroperoxide metabolism in mammalian organs. Physiol. Rev 59, 527–605 (1979). [DOI] [PubMed] [Google Scholar]
- 161.Trono D, Laus MN, Soccio M, Alfarano M & Pastore D Modulation of potassium channel activity in the balance of ROS and ATP production by durum wheat mitochondria — an amazing defense tool against hyperosmotic stress. Front. Plant Sci 6, 1072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Queliconi BB, Wojtovich AP, Nadtochiy SM, Kowaltowski AJ & Brookes PS Redox regulation of the mitochondrial KATP channel in cardioprotection. Biochim. Biophys. Acta 1813, 1309–1315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Oldenburg O, Cohen MV, Yellon DM & Downey JM Mitochondrial KATP channels: role in cardioprotection. Cardiovasc. Res 55, 429–437 (2002). [DOI] [PubMed] [Google Scholar]
- 164.Malinska D, Mirandola SR & Kunz WS Mitochondrial potassium channels and reactive oxygen species. FEBS Lett 584, 2043–2048 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Brandes R P Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45, 847–848 (2005). [DOI] [PubMed] [Google Scholar]
- 166.Kimura S et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 45, 438–444 (2005). [DOI] [PubMed] [Google Scholar]
- 167.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR & Brand MD Mitochondrial proton and electron leaks. Essays Biochem 47, 53–67 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Madamanchi NR & Runge MS Mitochondrial dysfunction in atherosclerosis. Circ. Res 100, 460–473 (2007). [DOI] [PubMed] [Google Scholar]
- 170.Cadenas E, Boveris A, Ragan CI & Stoppani AO Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome C reductase from beef-heart mitochondria. Arch. Biochem. Biophys 180, 248–257 (1977). [DOI] [PubMed] [Google Scholar]
- 171.Han D, Williams E & Cadenas E Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J 353, 411–416 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ago T, Kuroda J, Kamouchi M, Sadoshima J & Kitazono T Pathophysiological roles of NADPH oxidase/NOX family proteins in the vascular system. Review and perspective. Circ. J 75, 1791–1800 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Graham D et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54, 322–328 (2009). [DOI] [PubMed] [Google Scholar]
- 174.Ballinger SW et al. Mitochondrial integrity and function in atherogenesis. Circulation 106, 544–549 (2002). [DOI] [PubMed] [Google Scholar]
- 175.Chen J, Stimpson SE, Fernandez-Bueno GA & Mathews CE Mitochondrial reactive oxygen species and type 1 diabetes. Antioxid. Redox Signal 29, 1361–1372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anderson EJ et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest 119, 573–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ide T et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res 85, 357–363 (1999). [DOI] [PubMed] [Google Scholar]
- 178.Dai DF et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gaq overexpression-induced heart failure. Circ. Res 108, 837–846 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Munzel T, Gori T, Keaney JF Jr, Maack C & Daiber A Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J 36, 2555–2564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Escribano-Lopez I et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol 10, 200–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ohashi M, Runge MS, Faraci FM & Heistad DD MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol 26, 2331–2336 (2006). [DOI] [PubMed] [Google Scholar]
- 182.Nishikawa T et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 (2000). [DOI] [PubMed] [Google Scholar]
- 183.Dai DF et al. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol 58, 73–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hille R & Nishino T Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J 9, 995–1003 (1995). [PubMed] [Google Scholar]
- 185.Christen S, Bifrare YD, Siegenthaler C, Leib SL & Tauber MG Marked elevation in cortical urate and xanthine oxidoreductase activity in experimental bacterial meningitis. Brain Res 900, 244–251 (2001). [DOI] [PubMed] [Google Scholar]
- 186.Nagler RM, Klein I, Zarzhevsky N, Drigues N & Reznick AZ Characterization of the differentiated antioxidant profile of human saliva. Free Radic. Biol. Med 32, 268–277 (2002). [DOI] [PubMed] [Google Scholar]
- 187.Nakazono K et al. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl Acad. Sci. USA 88, 10045–10048 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suzuki H et al. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl Acad. Sci. USA 95, 4754–4759 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Swei A, Lacy F, Delano FA, Parks DA & Schmid-Schonbein GW A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation 6, 179–187 (1999). [PubMed] [Google Scholar]
- 190.Montor SG, Thoolen MJ, Mackin WM & Timmermans PB Effect of azapropazone and allopurinol on myocardial infarct size in rats. Eur. J. Pharmacol 140, 203–207 (1987). [DOI] [PubMed] [Google Scholar]
- 191.Li GR & Ferrier GR Effects of allopurinol on reperfusion arrhythmias in isolated ventricles. Am. J. Physiol 263, H341–H348 (1992). [DOI] [PubMed] [Google Scholar]
- 192.Stull LB, Leppo MK, Szweda L, Gao WD & Marban E Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ. Res 95, 1005–1011 (2004). [DOI] [PubMed] [Google Scholar]
- 193.Engberding N et al. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation 110, 2175–2179 (2004). [DOI] [PubMed] [Google Scholar]
- 194.Segal MS et al. The effect of the addition of allopurinol on blood pressure control in African Americans treated with a thiazide-like diuretic. J. Am. Soc. Hypertens 9, 610–619.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hare JM et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol 51, 2301–2309 (2008). [DOI] [PubMed] [Google Scholar]
- 196.Alem MM, Alshehri AM, Cahusac PM & Walters MR Effect of xanthine oxidase inhibition on arterial stiffness in patients with chronic heart failure. Clin. Med. Insights Cardiol 12, 1179546818779584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Borghi C et al. Effects of the concomitant administration of xanthine oxidase inhibitors with zofenopril or other ACE-inhibitors in post-myocardial infarction patients: a meta-analysis of individual data of four randomized, double-blind, prospective studies. BMC Cardiovasc. Disord 18, 112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Duda M, Konior A, Klemenska E & Beresewicz A Preconditioning protects endothelium by preventing ET-1-induced activation of NADPH oxidase and xanthine oxidase in postischemic heart. J. Mol. Cell. Cardiol 42, 400–410 (2007). [DOI] [PubMed] [Google Scholar]
- 199.Zhao Q, Zhang J & Wang H PGC-αa overexpression suppresses blood pressure elevation in DOCA–salt hypertensive mice. Biosci. Rep 35, e00217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Callera GE, Tostes RC, Yogi A, Montezano AC & Touyz RM Endothelin-1-induced oxidative stress in DOCA–salt hypertension involves NADPH-oxidase-independent mechanisms. Clin. Sci 110, 243–253 (2006). [DOI] [PubMed] [Google Scholar]
- 201.Pain T et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ. Res 87, 460–466 (2000). [DOI] [PubMed] [Google Scholar]
- 202.Lassegue B & Griendling KK NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol 30, 653–661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dikalova AE et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res 107, 106–116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rubbo H et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem 269, 26066–26075 (1994). [PubMed] [Google Scholar]
- 205.Ebadi M & Sharma SK Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid. Redox Signal 5, 319–335 (2003). [DOI] [PubMed] [Google Scholar]
- 206.Ceylan-Isik AF et al. Metallothionein abrog ates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension 53, 1023–1031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Watts GF et al. Coenzyme Q10 improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia 45, 420–426 (2002). [DOI] [PubMed] [Google Scholar]
- 208.Chew GT & Watts GF Coenzyme Q10 and diabetic endotheliopathy: oxidative stress and the ‘recoupling hypothesis’. QJM 97, 537–548 (2004). [DOI] [PubMed] [Google Scholar]
- 209.Vergeade A et al. Xanthine oxidase contributes to mitochondrial ROS generation in an experimental model of cocaine-induced diastolic dysfunction. J. Cardiovasc. Pharmacol 60, 538–543 (2012). [DOI] [PubMed] [Google Scholar]
- 210.Gladden JD et al. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radic. Biol. Med 51, 1975–1984 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rajagopalan S et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest 97, 1916–1923 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fukui T et al. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res 80, 45–51 (1997). [DOI] [PubMed] [Google Scholar]
- 213.Beswick RA, Dorrance AM, Leite R & Webb RC NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38, 1107–1111 (2001). [DOI] [PubMed] [Google Scholar]
- 214.Wu R, Millette E, Wu L & de Champlain J Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J. Hypertens 19, 741–748 (2001). [DOI] [PubMed] [Google Scholar]
- 215.Bauersachs J et al. Hydralazine prevents endothelial dysfunction, but not the increase in superoxide production in nitric oxide-deficient hypertension. Eur. J. Pharmacol 362, 77–81 (1998). [DOI] [PubMed] [Google Scholar]
- 216.Kobori H & Nishiyama A Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem. Biophys. Res. Commun 315, 746–750 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zalba G et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 35, 1055–1061 (2000). [DOI] [PubMed] [Google Scholar]
- 218.Delles C, Miller WH & Dominiczak AF Targeting reactive oxygen species in hypertension. Antioxid. Redox Signal 10, 1061–1077 (2008). [DOI] [PubMed] [Google Scholar]
- 219.Sedeek M, Hebert RL, Kennedy CR, Burns KD & Touyz RM Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr. Opin. Nephrol. Hypertens 18, 122–127 (2009). [DOI] [PubMed] [Google Scholar]
- 220.Takac I, Schroder K & Brandes RP The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr. Hypertens. Rep 14, 70–78 (2012). [DOI] [PubMed] [Google Scholar]
- 221.Higashi M et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P) H oxidase system. Circ. Res 93, 767–775 (2003). [DOI] [PubMed] [Google Scholar]
- 222.Matsuno K et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112, 2677–2685 (2005). [DOI] [PubMed] [Google Scholar]
- 223.Wingler K et al. Upregulation of the vascular NAD(P) H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic. Biol. Med 31, 1456–1464 (2001). [DOI] [PubMed] [Google Scholar]
- 224.Zhao Q, Zhang J & Wang H PGC-1α limits angiotensin II-induced rat vascular smooth muscle cells proliferation via attenuating NOX1-mediated generation of reactive oxygen species. Biosci. Rep 35, e00252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liang GZ et al. ClC-3 promotes angiotensin II-induced reactive oxygen species production in endothelial cells by facilitating Nox2 NADPH oxidase complex formation. Acta Pharmacol. Sin 39, 1725–1734 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yamagishi S, Nakamura K, Ueda S, Kato S & Imaizumi T Pigment epithelium-derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti-oxidative mechanism of PEDF. Cell Tissue Res 320, 437–445 (2005). [DOI] [PubMed] [Google Scholar]
- 227.Montezano AC et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ. Res 106, 1363–1373 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Touyz RM, Yao G & Schiffrin EL c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol 23, 981–987 (2003). [DOI] [PubMed] [Google Scholar]
- 229.Touyz RM & Schiffrin EL Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34, 976–982 (1999). [DOI] [PubMed] [Google Scholar]
- 230.Touyz RM & Schiffrin EL Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev 52, 639–672 (2000). [PubMed] [Google Scholar]
- 231.Garrido AM & Griendling KK NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol 302, 148–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nguyen Dinh Cat A, Montezano AC, Burger D & Touyz RM Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal 19, 1110–1120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gavazzi G et al. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580, 497–504 (2006). [DOI] [PubMed] [Google Scholar]
- 234.Weber DS et al. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol 288, H37–H42 (2005). [DOI] [PubMed] [Google Scholar]
- 235.Dikalova A et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112, 2668–2676 (2005). [DOI] [PubMed] [Google Scholar]
- 236.Bendall JK et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ. Res 100, 1016–1025 (2007). [DOI] [PubMed] [Google Scholar]
- 237.Murdoch CE et al. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol 106, 527–538 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bouabout G et al. Nox4 genetic inhibition in experimental hypertension and metabolic syndrome. Arch. Cardiovasc. Dis 111, 41–52 (2018). [DOI] [PubMed] [Google Scholar]
- 239.Schroder K et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res 110, 1217–1225 (2012). [DOI] [PubMed] [Google Scholar]
- 240.Zhao QD et al. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation 131, 643–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ray R et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol 31, 1368–1376 (2011). [DOI] [PubMed] [Google Scholar]
- 242.Laude K et al. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am. J. Physiol. Heart Circ. Physiol 288, H7–H12 (2005). [DOI] [PubMed] [Google Scholar]
- 243.Langbein H et al. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J 37, 1753–1761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Drummond GR & Sobey CG Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol. Metab 25, 452–463 (2014). [DOI] [PubMed] [Google Scholar]
- 245.Montezano AC et al. NADPH oxidase 5 is a pro-contractile nox isoform and a point of cross-talk for calcium and redox signaling-implications in vascular function. J. Am. Heart Assoc 7, e009388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jha JC et al. NADPH oxidase NOX5 accelerates renal injury in diabetic nephropathy. Diabetes 66, 2691–2703 (2017). [DOI] [PubMed] [Google Scholar]
- 247.Casas AI et al. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J. Clin. Invest 130, 1772–1778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Holterman CE et al. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J. Am. Soc. Nephrol 25, 784–797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cowley AW Jr. et al. Evidence of the importance of NOX4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67, 440–450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kroller-Schon S et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species — studies in white blood cells and in animal models. Antioxid. Redox Signal 20, 247–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Miller FJ Jr. et al. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol 22, 560–565 (2002). [DOI] [PubMed] [Google Scholar]
- 252.Guzik B et al. Mechanisms of oxidative stress in human aortic aneurysms — association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol 168, 2389–2396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McCormick ML, Gavrila D & Weintraub NL Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol 27, 461–469 (2007). [DOI] [PubMed] [Google Scholar]
- 254.Streeter J, Thiel W, Brieger K & Miller FJ Opportunity NOX: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc. Ther 31, 125–137 (2013). [DOI] [PubMed] [Google Scholar]
- 255.Aviram M, Rosenblat M, Etzioni A & Levy R Activation of NADPH oxidase required for macrophage-mediated oxidation of low-density lipoprotein. Metabolism 45, 1069–1079 (1996). [DOI] [PubMed] [Google Scholar]
- 256.Sheehan AL et al. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216, 321–326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gray SP et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127, 1888–1902 (2013). [DOI] [PubMed] [Google Scholar]
- 258.Judkins CP et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol. Heart Circ. Physiol 298, H24–H32 (2010). [DOI] [PubMed] [Google Scholar]
- 259.Douglas G et al. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE−/− mice. Cardiovasc. Res 94, 20–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gray SP et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol 36, 295–307 (2016). [DOI] [PubMed] [Google Scholar]
- 261.Schurmann C et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J 36, 3447–3456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Craige SM et al. Endothelial NADPH oxidase 4 protects ApoE−/− mice from atherosclerotic lesions. Free Radic. Biol. Med 89, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jay DB et al. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med 45, 329–335 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ozaki M et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J. Clin. Invest 110, 331–340 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Karnewar S et al. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci. Rep 6, 24108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.San Martin A et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol 292, H2073–H2082 (2007). [DOI] [PubMed] [Google Scholar]
- 267.Youn JY et al. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 63, 2344–2355 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mahmoud AM et al. Nox2 contributes to hyperinsulinemia-induced redox imbalance and impaired vascular function. Redox Biol 13, 288–300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wingler K et al. VAS2870 is a pan-NADPH oxidase inhibitor. Cell. Mol. Life Sci 69, 3159–3160 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kassan M et al. Enhanced p22phox expression impairs vascular function through p38 and ERK1/2 MAP kinase-dependent mechanisms in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol 306, H972–H980 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Maxwell SR & Lip GY Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int. J. Cardiol 58, 95–117 (1997). [DOI] [PubMed] [Google Scholar]
- 272.Eltzschig HK & Collard CD Vascular ischaemia and reperfusion injury. Br. Med. Bull 70, 71–86 (2004). [DOI] [PubMed] [Google Scholar]
- 273.Brandes RP, Weissmann N & Schroder K NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med 49, 687–706 (2010). [DOI] [PubMed] [Google Scholar]
- 274.Li Z et al. BRG1 regulates NOX gene transcription in endothelial cells and contributes to cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis 1864, 3477–3486 (2018). [DOI] [PubMed] [Google Scholar]
- 275.Sirker A et al. Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. J. Mol. Cell. Cardiol 98, 11–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yu Q et al. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J. Am. Heart Assoc 3, e000555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Narravula S & Colgan SP Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor a expression during hypoxia. J. Immunol 166, 7543–7548 (2001). [DOI] [PubMed] [Google Scholar]
- 278.Braunersreuther V & Jaquet V Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr. Pharm. Biotechnol 13, 97–114 (2012). [DOI] [PubMed] [Google Scholar]
- 279.Zhang J & Cai H Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J. Mol. Cell. Cardiol 48, 1060–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bouhidel JO et al. Netrin-1 improves post-injury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial integrity, while attenuating autophagy. Biochim. Biophys. Acta 1852, 277–289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bouhidel JO, Wang P, Li Q & Cai H Pharmacological postconditioning treatment of myocardial infarction with netrin-1. Front. Biosci 19, 566–570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nguyen A & Cai H Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc. Natl Acad. Sci. USA 103, 6530–6535 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li Q, Wang P, Ye K & Cai H Central role of SIAH inhibition in DCC-dependent cardioprotection provoked by netrin-1/NO. Proc. Natl Acad. Sci. USA 112, 899–904 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li Q & Cai H Induction of cardioprotection by small netrin-1-derived peptides. Am. J. Physiol. Cell Physiol 309, C100–C106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Octavia Y, Brunner-La Rocca HP & Moens AL NADPH oxidase-dependent oxidative stress in the failing heart: from pathogenic roles to therapeutic approach. Free Radic. Biol. Med 52, 291–297 (2012). [DOI] [PubMed] [Google Scholar]
- 286.Sirker A, Zhang M & Shah AM NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res. Cardiol 106, 735–747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Maejima Y, Kuroda J, Matsushima S, Ago T & Sadoshima J Regulation of myocardial growth and death by NADPH oxidase. J. Mol. Cell. Cardiol 50, 408–416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang M et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl Acad. Sci. USA 107, 18121–18126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Burgoyne JR, Mongue-Din H, Eaton P & Shah AM Redox signaling in cardiac physiology and pathology. Circ. Res 111, 1091–1106 (2012). [DOI] [PubMed] [Google Scholar]
- 290.Sartoretto JL, Kalwa H, Pluth MD, Lippard SJ & Michel T Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc. Natl Acad. Sci. USA 108, 15792–15797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Steinhorn B et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun 9, 4044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bendall JK, Cave AC, Heymes C, Gall N & Shah AM Pivotal role of a gp91 phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105, 293–296 (2002). [DOI] [PubMed] [Google Scholar]
- 293.Li JM, Gall NP, Grieve DJ, Chen M & Shah AM Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 40, 477–484 (2002). [DOI] [PubMed] [Google Scholar]
- 294.Parajuli N, Patel VB, Wang W, Basu R & Oudit GY Loss of NOX2 (gp91 phox) prevents oxidative stress and progression to advanced heart failure. Clin. Sci 127, 331–340 (2014). [DOI] [PubMed] [Google Scholar]
- 295.Looi YH et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51, 319–325 (2008). [DOI] [PubMed] [Google Scholar]
- 296.Ishikawa K et al. Acute left ventricular unloading reduces atrial stretch and inhibits atrial arrhythmias. J. Am. Coll. Cardiol 72, 738–750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Takimoto E & Kass DA Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248 (2007). [DOI] [PubMed] [Google Scholar]
- 298.Ide T et al. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ. Res 86, 152–157 (2000). [DOI] [PubMed] [Google Scholar]
- 299.Dey S, DeMazumder D, Sidor A, Foster DB & O’Rourke B Mitochondrial ROS drive sudden cardiac death and chronic proteome remodeling in heart failure. Circ. Res 123, 356–371 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Maack C & Bohm M Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J. Am. Coll. Cardiol 58, 83–86 (2011). [DOI] [PubMed] [Google Scholar]
- 301.Liu T et al. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ. Res 115, 44–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Meyer AJ & Dick TP Fluorescent protein-based redox probes. Antioxid. Redox Signal 13, 621–650 (2010). [DOI] [PubMed] [Google Scholar]
- 303.Kim YM et al. A myocardial Nox2 containing NAD(P) H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res 97, 629–636 (2005). [DOI] [PubMed] [Google Scholar]
- 304.Zhang J et al. NOX4-dependent hydrogen peroxide overproduction in human atrial fibrillation and HL-1 atrial cells: relationship to hypertension. Front. Physiol 3, 140 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Schramm A, Matusik P, Osmenda G & Guzik TJ Targeting NADPH oxidases in vascular pharmacology. Vasc. Pharmacol 56, 216–231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wingler K et al. NOX1, 2, 4, 5: counting out oxidative stress. Br. J. Pharmacol 164, 866–883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cross AR & Jones OT The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J 237, 111–116 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ellis JA, Mayer SJ & Jones OT The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem. J 251, 887–891 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H & Labadie RP Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic. Biol. Med 8, 251–258 (1990). [DOI] [PubMed] [Google Scholar]
- 310.Suzuki Y, Wang W, Vu TH & Raffin TA Effect of NADPH oxidase inhibition on endothelial cell ELAM-1 mRNA expression. Biochem. Biophys. Res. Commun 184, 1339–1343 (1992). [DOI] [PubMed] [Google Scholar]
- 311.Garrido-Urbani S et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARa mediated mechanism. PLOS ONE 6, e14665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sedeek M et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol 299, F1348–F1358 (2010). [DOI] [PubMed] [Google Scholar]
- 313.Aoyama T et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56, 2316–2327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.O’Donnell VB, Smith GC & Jones OT Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol. Pharmacol 46, 778–785 (1994). [PubMed] [Google Scholar]
- 315.O’Donnell BV, Tew DG, Jones OT & England PJ Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J 290, 41–49 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gianni D et al. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol 5, 981–993 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Altenhofer S et al. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci 69, 2327–2343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Maraldi T Natural compounds as modulators of NADPH oxidases. Oxid. Med. Cell. Longev 2013, 271602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Barbieri SS et al. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic. Biol. Med 37, 156–165 (2004). [DOI] [PubMed] [Google Scholar]
- 320.Stolk J, Hiltermann TJ, Dijkman JH & Verhoeven AJ Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol 11, 95–102 (1994). [DOI] [PubMed] [Google Scholar]
- 321.Williams HC & Griendling KK NADPH oxidase inhibitors: new antihypertensive agents? J. Cardiovasc. Pharmacol 50, 9–16 (2007). [DOI] [PubMed] [Google Scholar]
- 322.Tanriverdi LH et al. Inhibition of NADPH oxidase by apocynin promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress in rats. Free. Radic. Res 51, 772–786 (2017). [DOI] [PubMed] [Google Scholar]
- 323.Drummond GR, Selemidis S, Griendling KK & Sobey CG Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. DrugDiscov 10, 453–471 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Remold-O’Donnell H & Parent D Downregulation of neutrophil CD43 by opsonized zymosan. Blood 85, 337–342 (1995). [PubMed] [Google Scholar]
- 325.Diatchuk V, Lotan O, Koshkin V, Wikstroem P & Pick E Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J. Biol. Chem 272, 13292–13301 (1997). [DOI] [PubMed] [Google Scholar]
- 326.Wartenberg M et al. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBSLett 579, 4541–4549 (2005). [DOI] [PubMed] [Google Scholar]
- 327.Cayatte AJ et al. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arterioscler. Thromb. Vase. Biol 21, 1577–1584 (2001). [DOI] [PubMed] [Google Scholar]
- 328.Zang M et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55, 2180–2191 (2006). [DOI] [PubMed] [Google Scholar]
- 329.Delbosc S et al. Statins, 3-hydiOxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J. Cardiovasc. Pharmacol 40, 611–617 (2002). [DOI] [PubMed] [Google Scholar]
- 330.Wassmann S et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vase. Biol 22, 300–305 (2002). [DOI] [PubMed] [Google Scholar]
- 331.Wei YM et al. Attenuation by statins of membrane raft-redox signaling in coronary arterial endothelium. J. Pharmacol. Exp. Then 345, 170–179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kwok JMF, Ma CCH & Ma S Recent development in the effects of statins on cardiovascular disease through Rac1 and NADPH oxidase. Vase. Pharmacol 58, 21–30 (2013). [DOI] [PubMed] [Google Scholar]
- 333.Shiga N et al. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ. Res 96, 1014–1021 (2005). [DOI] [PubMed] [Google Scholar]
- 334.Gao Y, Dickerson JB, Guo R, Zheng J & Zheng Y Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl Acad. Sci. USA 101, 7618–7623 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Youn JY, Nguyen A & Cai H Inhibition of XO or NOX attenuates diethylstilbestrol-induced endothelial nitric oxide deficiency without affecting its effects on LNCaP cell invasion and apoptosis. Clin. Sci 123, 509–518(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.ten Freyhaus H et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res 71, 331–341 (2006). [DOI] [PubMed] [Google Scholar]
- 337.Stielow C et al. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem. Biophys. Res. Commun 344, 200–205 (2006). [DOI] [PubMed] [Google Scholar]
- 338.Wind S et al. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br. J. Pharmacol 161, 885–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Seredenina T et al. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radio. Biol. Med 86, 239–249 (2015). [DOI] [PubMed] [Google Scholar]
- 340.Perry BN et al. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J. Invest. Dermatol 126, 2316–2322 (2006). [DOI] [PubMed] [Google Scholar]
- 341.Munson JM et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl Med 4, 127ra36 (2012). [DOI] [PubMed] [Google Scholar]
- 342.Bhandarkar SS et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J. Clin. Invest 119, 2359–2365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rey FE, Cifuentes ME, Kiarash A, Quinn MT & Pagano PJ Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ. Res 89, 408–414 (2001). [DOI] [PubMed] [Google Scholar]
- 344.Csanyi G et al. Nox2 B-looppeptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radio. Biol. Med 51, 1116–1125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ranayhossaini DJ et al. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J. Biol. Chem 288, 36437–36450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Cifuentes-Pagano E, Csanyi G & Pagano PJ NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HIS. Cell. Mol. Life Sci 69, 2315–2325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Laleu B et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J. Med. Chem 53, 7715–7730 (2010). [DOI] [PubMed] [Google Scholar]
- 348.Anvari E, Wikstrom P, Walum E & Welsh N The novel NADPH oxidase 4 inhibitor GLX351322 counteracts glucose intolerance in high-fat diet-treated C57BL76 mice. Free Radio. Res 49, 1308–1318 (2015). [DOI] [PubMed] [Google Scholar]
- 349.Wang X et al. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLOS ONE 13, e0204271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hirano K et al. Discovery of gsk2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid. Redox Signal 23, 358–374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Musset B et al. NOX5 in human spermatozoa: expression, function, and regulation. J. Biol. Chem 287, 9376–9388 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Jiang JX et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic. Biol. Med 53, 289–296 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Schildknecht S et al. The NOX 1/4 inhibitor GKT 136901 as selective and direct scavenger of peroxynitrite. Curr. Med. Chem 21, 365–376 (2014). [DOI] [PubMed] [Google Scholar]
- 354.Strengert M et al. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza A virus infection. Antioxid. Redox Signal 20, 2695–2709 (2014). [DOI] [PubMed] [Google Scholar]
- 355.Gorin Y et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol 308, F1276–F1287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Teixeira G et al. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br. J. Pharmacol 174, 1647–1669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Vendrov AE et al. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J. Biol. Chem 285, 26545–26557 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Di Marco E et al. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe−/− mice. Diabetologia 57, 633–642 (2014). [DOI] [PubMed] [Google Scholar]
- 359.Joo JH et al. A novel pyrazole derivative protects from ovariectomy-induced osteoporosis through the inhibition of NADPH oxidase. Sci. Rep 6, 22389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Cha JJ et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Invest 97, 419–431 (2017). [DOI] [PubMed] [Google Scholar]
- 361.Dorotea D et al. A pan-NADPH oxidase inhibitor ameliorates kidney injury in type 1 diabetic rats. Pharmacology 102, 180–189 (2018). [DOI] [PubMed] [Google Scholar]
- 362.Luxen S, Belinsky SA & Knaus UG Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68, 1037–1045 (2008). [DOI] [PubMed] [Google Scholar]
- 363.Shames DS et al. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLOS MED 3, e486 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hayes P & Knaus UG Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxid. Redox Signal 18, 1937–1945 (2013). [DOI] [PubMed] [Google Scholar]
- 365.Kikuchi H, Kuribayashi F, Kiwaki N, Takami Y & Nakayama T GCN5 regulates the superoxide-generating system in leukocytes via controlling gp91 - phox gene expression. J. Immunol 186, 3015–3022 (2011). [DOI] [PubMed] [Google Scholar]
- 366.Siuda D et al. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res. Cardiol 107, 283 (2012). [DOI] [PubMed] [Google Scholar]
- 367.Zelko IN & Folz RJ Regulation of oxidative stress in pulmonary artery endothelium: modulation of extracellular superoxide dismutase and NOX4 expression using histone deacetylase class I inhibitors. Am. J. Respir. Cell Mol. Biol 53, 513–524 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Chen F et al. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic. Biol. Med 99, 167–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Manea SA et al. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol 16, 332–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Duraisamy AJ, Mishra M, Kowluru A & Kowluru RA Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci 59, 4831–4840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Yu L et al. Megakaryocytic leukemia 1 bridges epigenetic activation of NADPH oxidase in macrophages to cardiac ischemia-re perfusion injury. Circulation 138, 2820–2836(2018). [DOI] [PubMed] [Google Scholar]
- 372.Murdoch CE et al. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through piOinflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol 63, 2734–2741 (2014). [DOI] [PubMed] [Google Scholar]
- 373.Pollock JD et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet 9, 202–209 (1995). [DOI] [PubMed] [Google Scholar]
- 374.Jackson SH, Gallin JI & Holland SM The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med 182, 751–758 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]


