Abstract
Objectives
To assess the combined role of tumor vascularity, estimated from perfusion MRI, and MGMT methylation status on overall survival (OS) in patients with glioblastoma.
Methods
A multicentric international dataset including 96 patients from NCT03439332 clinical study were used to study the prognostic relationships between MGMT and perfusion markers. Relative cerebral blood volume (rCBV) in the most vascularized tumor regions was automatically obtained from preoperative MRIs using ONCOhabitats online analysis service. Cox survival regression models and stratification strategies were conducted to define a subpopulation that is particularly favored by MGMT methylation in terms of OS.
Results
rCBV distributions did not differ significantly (p > 0.05) in the methylated and the non-methylated subpopulations. In patients with moderately vascularized tumors (rCBV < 10.73), MGMT methylation was a positive predictive factor for OS (HR = 2.73, p = 0.003, AUC = 0.70). In patients with highly vascularized tumors (rCBV > 10.73), however, there was no significant effect of MGMT methylation (HR = 1.72, p = 0.10, AUC = 0.56).
Conclusions
Our results indicate the existence of complementary prognostic information provided by MGMT methylation and rCBV. Perfusion markers could identify a subpopulation of patients who will benefit the most from MGMT methylation. Not considering this information may lead to bias in the interpretation of clinical studies.
Key Points
• MRI perfusion provides complementary prognostic information to MGMT methylation.
• MGMT methylation improves prognosis in glioblastoma patients with moderate vascular profile.
• Failure to consider these relations may lead to bias in the interpretation of clinical studies.
Electronic supplementary material
The online version of this article (10.1007/s00330-020-07297-4) contains supplementary material, which is available to authorized users.
Keywords: Perfusion imaging, Glioblastoma, O(6)-Methylguanine-DNA methyltransferase, Prognostic factors, Temozolomide
Introduction
Gliomas are the most common malignant primary central nervous system tumors, with an estimated annual incidence of 3.21 per 100,000 individuals in the USA [1]. About half of all newly diagnosed gliomas are classified as glioblastoma, which is the most malignant type of brain cancer. Glioblastoma pathology is characterized by angiogenesis, highly infiltrative growth, and cellular heterogeneity [2]. Despite an aggressive therapeutic approach combining maximum safe resection with radiotherapy (RT) plus concomitant and adjuvant temozolomide (TMZ), prognosis is poor, with median overall survival (OS) duration of approximately 14 months [3]. In 2016, the World Health Organization introduced molecular parameters along with histology to describe the interpatient glioblastoma heterogeneity associated with differential prognosis and responses to therapy [4].
Adequate clinical and molecular biomarkers are needed for accurate estimations of prognosis and optimal treatment selections. Inactivation through promoter methylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene, which impairs the ability to repair DNA damaged induced by alkylating agents such as TMZ [5], has been described as a relevant biomarker for clinical decision-making in glioblastoma treatment. In a post hoc analysis of a phase III trial, MGMT promoter methylation was associated with a 2-year survival increase in TMZ-treated glioblastoma patients from 14 to 46% [6]. Additional studies have also described not only a predictive, but also a prognostic role of MGMT methylation for glioblastoma patients [7, 8]. Current guidelines support the use of MGMT methylation as a predictive biomarker in patients older than 70 years with isocitrate dehydrogenase (IDH) wild-type grade IV gliomas [8].
Despite the well-documented impact of MGMT methylation on the prognosis of glioblastoma patients treated with TMZ, the survival of these patients is not explained by this factor alone. Tumor vascularity, for instance, is strongly associated with glioma transformation (i.e. grade progression), poorer survival [9], sensitivity to RT, and effectiveness of bloodborne delivery of nutrients and chemotherapy [10].
MRI perfusion–based parameters correlate with tumor vascularity and properties of vessels [11, 12]. Numerous studies show that the vascularity as defined by MRI perfusion is a prognostic factor for glioblastoma even prior to initial surgery [13–15]. Perfusion parameters, such as relative cerebral blood volume (rCBV) in the enhancing tumor areas, are among the most consistently recognized independent predictors of survival [9]. Novel approaches based on artificial intelligence [15, 16] have been proposed to calculate perfusion-based biomarkers, based not on the entire enhancing lesion but on more homogeneous regions (habitats), thereby improving not only the prognostic capacity but also the reproducibility of the results [13, 17, 18].
In this study, we aimed at evaluating whether the rCBV is a modulating factor of the prognostic effect of MGMT methylation status in patients with glioblastoma treated with TMZ.
Materials and methods
Patient cohort
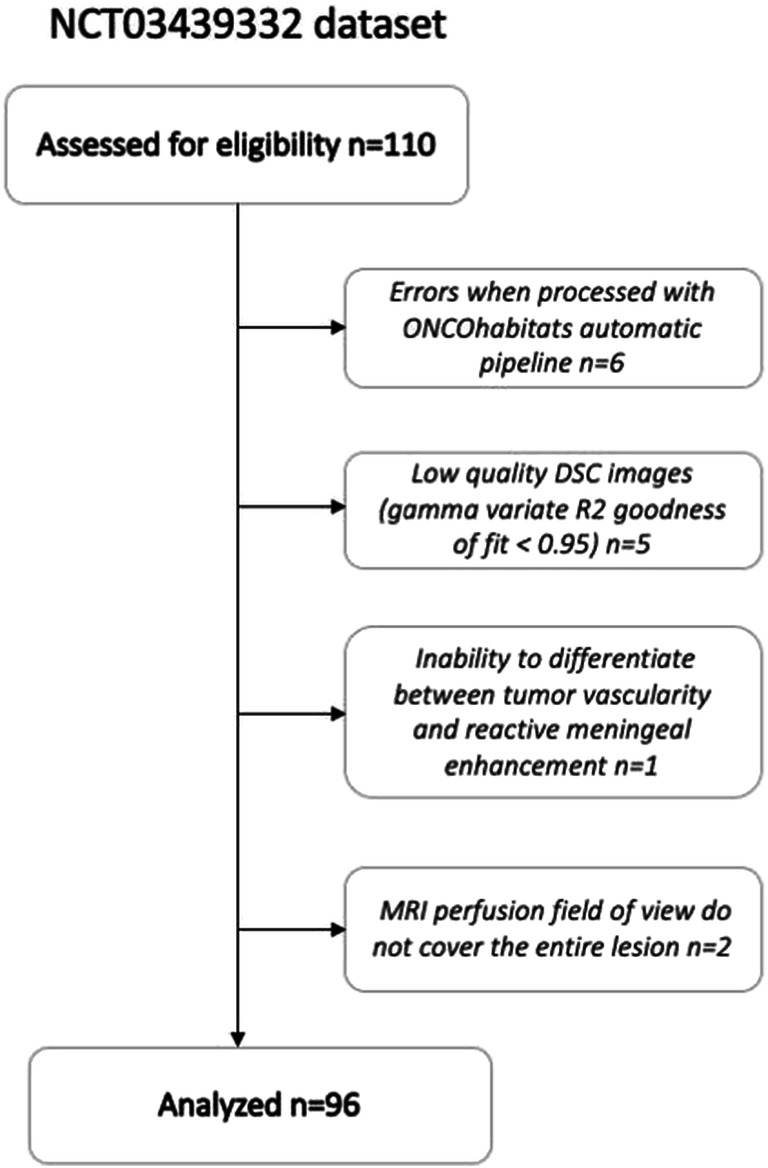
The patient cohort has compiled by the multicenter and international retrospective clinical study NCT03439332 [19]. A total of 110 cases from this dataset with MRI perfusion studies were included in the current study (Fig. 1). The NCT03439332 multicenter international dataset included patients treated at seven European hospitals from four countries: Hospital Clinic, Barcelona, Spain; Hospital Universitario Vall d’Hebron, Barcelona, Spain; Hospital Universitario de La Ribera, Alzira, Spain; Hospital de Manises, Manises, Spain; Azienda Ospedaliero-Universitaria di Parma, Parma, Italy; Oslo University Hospital, Oslo, Norway; and Centre Hospitalier Universitaire de Liège, Liège, Belgium. Patients were diagnosed with glioblastoma grade IV WHO with histopathological confirmation and followed Stupp standard treatment. The extent of resection was assessed in each center by expert neurosurgeons and radiologists based on the postsurgical MRI study findings. A material transfer agreement was approved by all the participating centers and an acceptance report was issued by the Ethical Committee of each center. The Universitat Politècnica de València institutional ethical board also approved this retrospective study. All methods were performed in accordance with the relevant guidelines and regulations or Declaration of Helsinki.
Fig. 1.
CONSORT diagrams for the NTC3439332 dataset
IDH1 mutation and MGMT methylation status assessment
MGMT methylation status was assessed in each center by pyrosequencing using a cut-off value between 9 and 10%, except for the cases from the Hospital Clinic in Barcelona where MGMT methylation status was assessed by methylation-specific polymerase chain reaction. The IDH1 mutation status was assessed in each center by immunostains using the IDH1 R132H antibody.
MRI data acquisition protocol
Pre-surgical standard-of-care MRI examinations including pre- and post-gadolinium T1-weighted MRI as well as T2-weighted, T2-fluid-attenuated inversion recovery (FLAIR), and dynamic susceptibility contrast (DSC) T2* perfusion-weighted sequences were collected from each participating center. All the MRIs were obtained by 1.5-T or 3-T scanners, using different MRI acquisition protocols in each participating center. A summary of the MRI acquisition protocol used by each center is presented in Supplementary Materials Table S-I.
MRI data preprocessing
Anatomical MRI acquisitions were preprocessed using the following pipeline: (1) voxel isotropic resampling, (2) denoising, (3) rigid intra-patient registration, (4) affine registration to MNI space [20], (5) brain extraction, and (6) magnetic field inhomogeneity correction. First, image resampling at 1 mm3 was performed through linear interpolation. Next, denoising was carried out using the adaptive non-local means filter [21] with a search window of 7 × 7 × 7 voxels and a patch window of 3 × 3 × 3 voxels. Registration was performed with ANTs software [22] and Mutual Information, using the T1c MRI as reference. Brain extraction was performed through a convolutional neural network with a U-Net architecture trained on a dataset of 160 T1c MRIs with glioblastomas manually annotated and validated by several independent neuroimaging researchers with more than 5 years of experience. Finally, magnetic field inhomogeneities were corrected with the N4 software using the previously computed intra-cranial mask [23].
Lesion segmentation
Glioblastoma tissue segmentation was performed by means of 3D patch-based convolutional neural networks [24]. A U-Net Res-Net architecture of 5 levels with long-term skip connections with 16, 32, 64, 128, and 256 filters at each level, respectively, was used to perform the segmentation. At each level, a simple block and a residual block were chained together. A simple block consists of a convolution + batch normalization + ReLu activation function. A residual block consists of a convolution + batch normalization + ReLu + convolution + batch normalization + residual connection summation + ReLu activation function. Convolutions were performed with isotropic kernels of size 3 × 3 × 3, while batch normalization momentum was fixed to 0.9. Max-pooling layers with pooling size and stride 2 × 2 × 2 were employed in the contracting path to sequentially condensate the relevant features of the input patches, while transpose convolutions were used in the expanding path to rearrange and project the latent features to the original size. The network works with patches of 32 × 32 × 32 with 3 channels corresponding to the T1c, T2, and FLAIR sequences. Adam optimizer with cross-entropy loss-function was used to train the network. The lesion segmentation network was trained on BraTS dataset [25–27] including 260 MRI studies segmented manually, by one to four raters, following the same annotation protocol, and their annotations were approved by experienced neuroradiologists. Based on a blind independent validation dataset provided by BraTS international challenge, the results of this segmentation method obtain a median DICE of 0.85 on delineating the enhancing tumor region and a DICE of 0.92 in delineating the whole tumor region [28].
DSC perfusion quantification
Quantification of rCBV and relative cerebral blood flow (rCBF) hemodynamic indices was performed employing standard techniques proposed in the literature [29]. rCBV was calculated by numerical integration of the area under the curve of the T2* concentration-time signal, while rCBF was obtained by means of the block-circulant singular value decomposition (SVD) devolution technique [29]. Gamma-variate curve-fitting and Boxerman technique [30] were used to correct for T2 and T1 leakage effects. The mean transit time was computed from the central limit theorem by dividing rCBV/rCBF. The arterial input function (AIF) was automatically detected by means of an iterative divide-and-conquer approach, using the peak height, the time-to-peak, and the full-width at half maximum as features to detect arterial shape-like signals [24].
Definition of the vascular marker
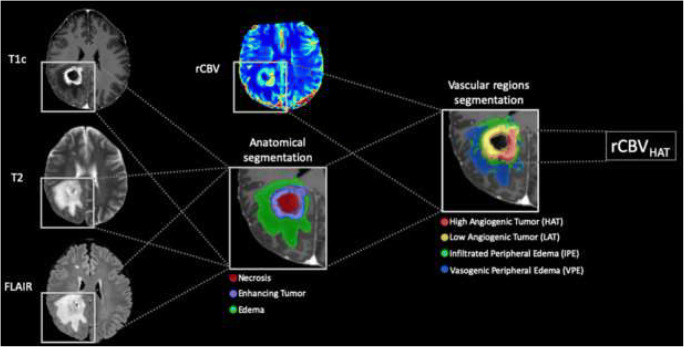
The vascular marker used was based on the rCBV map obtained from the DSC MRI sequence. Specifically, we used the 90th percentile of rCBV values at the highly angiogenic tumor (HAT) region of the tumor (rCBVHAT) as a robust indicator of the maximum perfusion value of the region. The HAT region was defined based on rCBV and rCBF maps following the methodology proposed by Juan-Albarracín et al [15] and implemented as an open service in [24]. A schema of the methodology used to compute the rCBVHAT is presented in Fig. 2. This methodology has been shown to obtain comparable rCBV values between centers using different clinical protocols, imaging protocols, or scanner models as reported in [17].
Fig. 2.
Schema of the methodology used to compute the relative cerebral blood volume at the high angiogenic tumor (rCBVHAT) region
Stratification of responsive patients
The stratification of responsive patients was made based on the MGMT methylation and tumor vascularity (i.e., rCBVHAT) markers. We generated two subpopulations, namely (1) the MGMT methylated, moderately vascularized tumors and (2) the MGMT unmethylated, highly vascularized tumors. Highly and moderately vascular glioblastomas were determined by a rCBVHAT threshold (rCBVth) value. The rCBVth value was defined as the median rCBVHAT of the study cohort.
Statistical analyses
To analyze whether MGMT methylation was associated with differences in rCBVHAT values, we compared the rCBVHAT values for methylated and unmethylated MGMT populations using a non-parametric Mann-Whitney U test. In addition, we assessed the complementary prognostic information provided by MGMT methylation and rCBVHAT, by computing three different Cox proportional hazards regressions: (1) The first regression took into account only MGMT methylation status plus a set of relevant clinical variables (i.e., age at diagnostic, gender, and the extent of resection); (2) The second regression took into account only the rCBVHAT value in the HAT habitat, plus the clinical variables; (3) The last regression was performed using both the MGMT methylation status and the rCBVHAT value, plus the clinical variables. In order to take advantage of as many cases as possible for the Cox analysis, we used a mean imputation strategy [31] in cases that did not have information on the extent of resection. The goodness of fit of each of survival models was evaluated through the concordance statistic as defined by Harrell et al [32].
As suggested in the literature [6], we performed a Kaplan-Meier survival analysis to assess differences in OS between patient subpopulations. The log-rank test was performed to test for significant differences in OS. For censored cases, we set the date of censorship to the last date of contact with the patient or, in cases where this information was not available, the date of the last MRI exam.
Finally, to reduce biases in survival analyses, we analyzed whether the IDH1 mutation was related to MGMT methylation by performing a Fisher test, and whether IDH1-mutated tumors have significantly different rCBVHAT values performing a Mann-Whitney U test, using those cases where IDH1 information was available.
Software
The MRI data analysis was performed using ONCOhabitats [24], a freely available online service (www.onchabitats.upv.es). It provides a fully automated pipeline for the analysis of glioblastoma MRI studies including pre-processing, lesion segmentation, DSC perfusion quantification, and high angiogenic tumor delineation services, among others. Statistical analysis was performed with MATLAB (R2019a) and R (v3.6.0) [33].
Results
Characterization of patient cohorts
Of the 110 patients included from the NCT03439332 dataset, six exams (patients) were excluded because of processing errors with the automatic processing pipeline, five exams were excluded due to noise or MR artifacts that precluded DSC quantification (gamma-variate R2 goodness of fit < 0.95), one exam was excluded due to inability to differentiate between tumor vascularity and reactive meningeal enhancement, and two cases were excluded because MRI perfusion field of view did not cover the entire lesion (see Fig. 1). Of the remaining 96 patients, 43 (44.8%) presented the MGMT methylated. With a median follow-up of 391 days, median OS was 402 days; 15 patients were alive at the last data cut-off date and were censored in the survival analyses. Table 1 includes the most relevant demographic, molecular, and clinical features of the study cohort and summarizes the patients recruited. No significant relationships have been found between IDH1 mutation and MGMT methylation status (p = 0.39), nor between IDH1 mutation and rCBVHAT values (p = 0.15).
Table 1.
Summary of NCT03439332 study cohort
| Initial no. of patients | 110 |
| Excluded no. of patients | 14 (13%) |
| Included no. of patients | 96 (87%) |
| Gender (f/m) | 30/66 |
| Age at diagnosis (years) | 59.1 [32, 81] |
| Median survival (days) | 402 [43, 1229] |
| IDH1 | |
| -Mutated | 5 |
| -Wild type | 58 |
| -Unknown | 33 |
| MGMT | |
| -Methylated | 43 |
| -Unmethylated | 53 |
| Resection | |
| -Gross total | 45 |
| -Subtotal | 41 |
| -Biopsy | 8 |
| -Unknown | 2 |
| Location | |
| -Frontal | 36 |
| -Parietal | 16 |
| -Temporal | 31 |
| -Occipital | 2 |
| -Unknown | 11 |
Non-significant association between MGMT and rCBVHAT
Median rCBVHAT in the whole population was 10.73. Median rCBVHAT values in methylated and non-methylated MGMT subpopulations were 11.06 and 10.35, respectively. rCBVHAT distributions did not differ significantly (Mann-Whitney U of 1062; n = 43 and n = 53, respectively, p > 0.05 two-tailed) in the methylated and the non-methylated subpopulations.
MGMT methylation status and rCBVHAT provide complementary prognostic information
In order to evaluate the association between rCBVHAT (as a continuous variable) and MGMT methylation status with OS, we initially constructed three Cox proportional hazards (Cox-PH) regression models. The results of the three Cox-PH regression models obtained from NCT03439332 dataset are shown in Table 2. Results of the two first models including rCBVHAT or MGMT status independently showed a significant association of MGMT status (HR: 2.01; 95% CI: 1.21–3.34; p = 0.007) with OS, but a non-significant association of rCBVHAT (HR: 1.05; 95% CI: 0.99–1.11; p = 0.102) with OS. When evaluating both parameters in the multivariable Cox-PH model (Table 2), both were independently associated with OS: MGMT status (HR: 2.12; 95% CI: 1.27–3.52; p = 0.004) and rCBVHAT (HR: 1.06; 95% CI: 1.00–1.12; p = 0.049). The performance of the model improved by increasing the concordance, decreasing the p values of each variable, and increasing the hazard ratios (HR), suggesting that MGMT methylation and rCBVHAT provide complementary prognostic information.
Table 2.
Results of the three Cox proportional hazards regression models obtained from NCT03439332 dataset
| Clinical variable | Excluding rCBVHAT | Excluding MGMT meth. | All clinical variables | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at diagnostic | 1.00 [0.97, 1.02] | 0.948 | 0.99 [0.97, 1.02] | 0.629 | 0.97 [0.99, 1.02] | 0.695 |
| Gender | 0.88 [0.51, 1.53] | 0.653 | 1.03 [0.61, 1.75] | 0.910 | 0.83 [0.48, 1.44] | 0.512 |
| Resection | 1.79 [0.86, 3.73] | 0.119 | 2.16 [1.02, 4.54] | 0.043* | 1.84 [0.87, 3.86] | 0.109 |
| MGMT meth. | 2.01 [1.21, 3.34] | 0.007* | - | - | 2.12 [0.27, 3.52] | 0.004* |
| rCBVHAT | - | - | 1.05 [0.99, 1.11] | 0.102 | 1.06 [1.00, 1.12] | 0.049* |
| Concordance | 0.59 | 0.59 | 0.61 | |||
* Indicates significant difference (p < 0.05)
rCBVHAT improves the capability of MGMT to stratify responsive patients
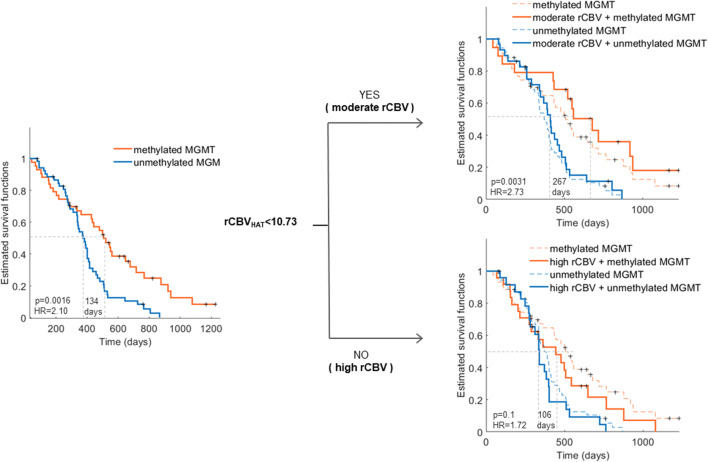
We then evaluated the impact of MGMT methylation status in patients with highly and moderately vascularized tumors, as defined by the rCBVHAT values. rCBVth, defined as the median rCBVHAT of the study cohort, was established at rCBVth = 10.73.
We then analyzed differences in OS between patients with methylated and unmethylated MGMT promoters for the entire cohort, patients with highly vascularized tumors (rCBVHAT ≥ 10.73), and patients with moderately vascularized tumors (rCBVHAT < 10.73). In the cohort, 48 (50%) and 48 (50%) patients presented with highly and moderately vascularized tumors, respectively.
We observed a significant association of MGMT methylation and OS (HR: 2.1 [95% CI: 1.33–3.33]; p = 0.002) (Table 3). In patients with moderately vascularized tumors (rCBVHAT < 10.73), the association between MGMT methylation and OS was higher (HR: 2.73 [95% CI: 1.40–5.32]; p = 0.003) than in the general population. Finally, there was no association between MGMT methylation and OS in patients with high vascularity (rCBVHAT ≥ 10.73) (HR: 1.72 [95% CI: 0.90–3.27]; p = 0.10). Kaplan-Meier curves for the four subpopulations are presented in Fig. 3.
Table 3.
Results of the log-rank test of the Kaplan-Meier analysis for NCT03439332 cohort: (1) all, (2) moderate vascular (rCBVHAT < rCBVth), and (3) high vascular (rCBVHAT > rCBVth). For each subpopulation, the mean OS and number of patients with methylated MGMT and unmethylated MGMT are presented. Additionally, differences between OS (days), hazard ratios, area under the curve (AUC), and log-rank test resulting p value are presented
| No. of patients | Median OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Meth. MGMT | Unmeth. MGMT | Meth. MGMT | Unmeth. MGMT | ΔOS | HR [95% CI] | p | AUC | |
| All | 43 | 53 | 507 | 373 | 134 | 2.10 [1.33–3.33] | 0.0016 | 0.61 |
| Moderate rCBV | 19 | 29 | 678 | 411 | 267 | 2.73 [1.40–5.32] | 0.0031 | 0.70 |
| High rCBV | 24 | 24 | 444 | 338 | 106 | 1.72 [0.90–3.27] | 0.10 | 0.56 |
Fig. 3.
Kaplan-Meier curves showing the results of the stratification between moderate (rCBVHAT < 10.73) and high (rCBVHAT > 10.73) vascular subpopulations
Discussion
In this study, we present a potential role of tumor vascularity in the prognostic impact of MGMT methylation status in glioblastoma patients. As was expected from the literature, we observe a significant prognostic impact of MGMT methylation in glioblastoma patients treated with TMZ included in the NCT03439332 multicenter international dataset. Moreover, our results show that tumor vascularity may identify a subgroup of patients that exhibit a greater benefit from MGMT methylation. Once glioblastoma patients are dichotomized into highly and moderately vascularized tumor subgroups using rCBV, we observe a highly significant impact of MGMT status in patients with moderately vascularized tumors and only a non-significant trend in patients with highly vascularized tumors. From the comparison of the three multiparametric Cox survival analyses based on MGMT and rCBVHAT, we conclude that the information provided by both variables are relevant and complementary in predicting the prognosis of glioblastoma patients treated with TMZ. This implies that MGMT methylation assessment may provide incomplete information regarding prognosis, and that adding the characterization of tumor vascularization may improve estimation of responsiveness to TMZ. We propose a new classification of patients based on tumor vascularity, derived from the NCT03439332 multicenter international dataset. Our study proves the feasibility of using perfusion MRIs to identify subpopulations of glioblastoma patients with a higher likelihood of a beneficial effect of standard Stupp treatment (i.e., including TMZ), patients with methylated MGMT, and moderate vascularization in the contrast-enhancing tumor areas. On the contrary, patients with highly vascularized tumors will probably benefit less from TMZ, independently of the MGMT methylation status. We hypothesize that tumors with a lower vascularization may be potentially less aggressive, with a lower prevalence of molecular aberrations that may confer resistance to alkylating agents in the presence of a methylated MGMT.
Different studies have evaluated possible associations between MGMT methylation and tumor vascularity. A study by Hempel et al [34] (n = 100 including 24 glioblastomas) found significantly higher rCBV values in IDH wild-type glioblastomas with a methylated MGMT than in those with an unmethylated MGMT. Chahal et al [35] found higher levels of vascular endothelial growth factor receptor 1 (VEGFR-1) in unmethylated compared with methylated MGMT cells, hypothesizing that this should lead to an increased vascularization of the tumor. However, another small sample study (n = 24) by Moon et al [36] concluded that rCBV did not differ significantly between methylated and unmethylated MGMT tumors. In our study, based on a larger cohort, no significant relationship was observed between MGMT methylation and rCBVHAT. Due to the influence of MGMT on the effectiveness of therapies with antineoplastic drugs of alkylating agent class (i.e., TMZ), several studies suggest avoiding these therapies in those patients with non-methylated MGMT [6, 37]. The conclusions of this study further suggest that to obtain the most significant benefit, we should select among patients with methylated MGMT those with moderate vascularity.
The main limitation of the study is the lack of a complete molecular profile for all cases. Several studies already showed the importance of combining MGMT methylation status with other features when determining the patients who will benefit the most from it. Having methylated MGMT is an advantage especially when TERT expression is high [38] and progression-free survival is higher when both MGMT and mutant p53 expression are low [39]. Also, MGMT methylation displays better survival prediction performance when combined with IDH1 mutations [40]. In this study, we have chosen to use CBV as the most relevant perfusion parameter for the analysis of the prognostic value of vascularity. However, in future work, it would be interesting to extend the study to include complementary markers derived from morphological [41], molecular [42], dynamic contrast-enhanced, and vessel architecture [43] imaging sequences that could help us to understand the complex vascular phenomena behind the results of this study.
This study demonstrates that combining MGMT methylation status together with the rCBVHAT value obtained completely automatically from standard-of-care MRI studies through an online and open image analysis service (https://www.oncohabitats.upv.es) can provide a more reliable prognostic indicator for designing patient stratification strategies. This ensures the reproducibility of the obtained results and the immediate applicability of the proposed conclusions in the clinical trial setting. In conclusion, we consider that information of rCBVHAT and MGMT methylation status should be included in randomization/stratification strategies of new clinical trials. Due to the joint implications of these two factors on patient survival, failure to consider these factors may lead to significant bias in the interpretation of the results from such studies.
Electronic supplementary material
(DOCX 23 kb)
Acknowledgments
We sincerely thank the collaboration of the different hospitals that have participated in the NCT03439332 multicenter international study: Hospital Clinic, Barcelona, Spain; Hospital Universitario Vall d’Hebron, Barcelona, Spain; Hospital Universitario de La Ribera, Alzira, Spain; Hospital de Manises, Manises, Spain; Azienda Ospedaliero-Universitaria di Parma, Parma, Italy; Oslo University Hospital, Oslo, Norway; and Centre Hospitalier Universitaire de Liège, Liège, Belgium.
Abbreviations
- AUC
Area under the curve
- DSC
Dynamic susceptibility contrast
- FLAIR
Fluid-attenuated inversion recovery
- HAT
Highly angiogenic tumor
- HR
Hazard ratio
- IDH
Isocitrate dehydrogenase
- KPS
Karnofsky Performance Status
- MGMT
O(6)-Methylguanine-DNA methyltransferase
- MS-MLPA
Methylation-specific multiplex ligation-dependent probe amplification
- MSP
Methylation-specific polymerase chain reaction
- OS
Overall survival
- rCBF
Relative cerebral blood flow
- rCBV
Relative cerebral blood volume
- RT
Radiotherapy
- TMZ
Temozolomide
Funding
Open Access funding provided by University of Oslo (incl Oslo University Hospital). This study has received funding from MTS4up project (National Plan for Scientific and Technical Research and Innovation 2013-2016, No. DPI2016-80054-R) (JMGG); H2020-SC1-2016-CNECT Project (No. 727560) (JMGG), H2020-SC1-BHC-2018-2020 (No. 825750) (JMGG), the European Research Council (ERC) under the European Union’s Horizon 2020 (Grant Agreement No. 758657), the South-Eastern Norway Regional Health Authority Grants 2017073 and 2013069, the Research Council of Norway Grants 261984 (KEE). M.A.T was supported by Programa Estatal de Promoción del Talento y su Empleabilidad en I+D+i (DPI2016-80054-R). E.F.G was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (No. 844646).
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Juan Miguel García Gómez, full professor at Universitat Politècnica de València (juanmig@upv.es).
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained. A material transfer agreement was approved by all the participating centers and an acceptance report was issued by the Ethical Committee of each center. The Universitat Politècnica de València institutional ethical board also approved this retrospective study.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in [16].
In the present study, we used a subset of the NCT03439332 multicenter study cohort. The NCT03439332 cohort was used in [16] to validate the primary endpoint of the NCT03439332 study, which is to validate the robust association between vascular habitats and patient prognosis in glioblastoma. In the present study, however, we use a subset of these data to study the joint influence of vascularity, estimated by perfusion MRI, and MGMT methylation on the survival of patients with GBM. MGMT methylation was not considered in the study of Álvarez-Torres et al [16]; moreover, the objective, results, and conclusions do not overlap between the current and previous studies.
[16] Álvarez-Torres MDM, Juan-Albarracín J, Fuster-Garcia E, et al (2019) Robust association between vascular habitats and patient prognosis in glioblastoma: an international multicenter study. J Magn Reson Imaging. 10.1002/jmri.26958
Methodology
•retrospective
•observational
•multicenter study
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60:166–193. 10.3322/caac.20069 [DOI] [PMC free article] [PubMed]
- 4.Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed]
- 5.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 8.Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116–121. doi: 10.1093/neuonc/nop020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell SM, Elliott R, Forshaw D, Golfinos JG, Nelson PK, Kelly PJ (2009) Glioma vascularity correlates with reduced patient survival and increased malignancy. Surg Neurol 72:242–246; discussion 246-247. 10.1016/j.surneu.2008.11.012 [DOI] [PubMed]
- 10.Batchelor TT, Gerstner ER, Emblem KE et al (2013) Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A 110:19059–19064. 10.1073/pnas.1318022110 [DOI] [PMC free article] [PubMed]
- 11.Ulyte A, Katsaros VK, Liouta E, et al. Prognostic value of preoperative dynamic contrast-enhanced MRI perfusion parameters for high-grade glioma patients. Neuroradiology. 2016;58:1197–1208. doi: 10.1007/s00234-016-1741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo R-E, Yun TJ, Hwang I, et al. Arterial spin labeling perfusion-weighted imaging aids in prediction of molecular biomarkers and survival in glioblastomas. Eur Radiol. 2020;30:1202–1211. doi: 10.1007/s00330-019-06379-2. [DOI] [PubMed] [Google Scholar]
- 13.Fuster-Garcia E, Juan-Albarracín J, García-Ferrando GA, et al. Improving the estimation of prognosis for glioblastoma patients by MR based hemodynamic tissue signatures. NMR Biomed. 2018;31:e4006. doi: 10.1002/nbm.4006. [DOI] [PubMed] [Google Scholar]
- 14.Hou BL, Wen S, Katsevman GA et al (2018) Magnetic resonance imaging parameters and their impact on survival of patients with glioblastoma: tumor perfusion predicts survival. World Neurosurg. 10.1016/j.wneu.2018.12.085 [DOI] [PMC free article] [PubMed]
- 15.Juan-Albarracín J, Fuster-Garcia E, Pérez-Girbés A, et al. Glioblastoma: vascular habitats detected at preoperative dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging predict survival. Radiology. 2018;287:944–954. doi: 10.1148/radiol.2017170845. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y-CC, Ackerstaff E, Tschudi Y, et al. Delineation of tumor habitats based on dynamic contrast enhanced MRI. Sci Rep. 2017;7:9746. doi: 10.1038/s41598-017-09932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Álvarez-Torres MDM, Juan-Albarracín J, Fuster-Garcia E et al (2019) Robust association between vascular habitats and patient prognosis in glioblastoma: an international multicenter study. J Magn Reson Imaging. 10.1002/jmri.26958 [DOI] [PubMed]
- 18.Wu H, Tong H, Du X et al (2020) Vascular habitat analysis based on dynamic susceptibility contrast perfusion MRI predicts IDH mutation status and prognosis in high-grade gliomas. Eur Radiol. 10.1007/s00330-020-06702-2 [DOI] [PubMed]
- 19.Multicentre validation of how vascular biomarkers from tumor can predict the survival of the patient with glioblastoma - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03439332. Accessed 24 Apr 2019
- 20.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 21.Manjón JV, Coupé P, Martí-Bonmatí L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- 22.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed]
- 23.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan-Albarracín J, Fuster-Garcia E, García-Ferrando GA, García-Gómez JM. ONCOhabitats: a system for glioblastoma heterogeneity assessment through MRI. Int J Med Inform. 2019;128:53–61. doi: 10.1016/j.ijmedinf.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Menze BH, Jakab A, Bauer S, et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS) IEEE Trans Med Imaging. 2015;34:1993–2024. doi: 10.1109/TMI.2014.2377694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakas S, Akbari H, Sotiras A, et al. Advancing the cancer genome atlas glioma MRI collections with expert segmentation labels and radiomic features. Sci Data. 2017;4:170117. doi: 10.1038/sdata.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakas S, Reyes M, Jakab A, et al (2018) Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. ArXiv181102629
- 28.Juan-Albarracín J, Fuster-Garcia E, del Mar Álvarez-Torres M, et al. ONCOhabitats glioma segmentation model. In: Crimi A, Bakas S, et al., editors. Brain lesion: glioma, multiple sclerosis, stroke and traumatic brain injuries. Cham: Springer International Publishing; 2020. pp. 295–303. [Google Scholar]
- 29.Knutsson L, Ståhlberg F, Wirestam R. Absolute quantification of perfusion using dynamic susceptibility contrast MRI: pitfalls and possibilities. MAGMA. 2010;23:1–21. doi: 10.1007/s10334-009-0190-2. [DOI] [PubMed] [Google Scholar]
- 30.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 31.Little RJA, Rubin DB (2002) Statistical analysis with missing data. John Wiley & Sons, New York [etc.]
- 32.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team (v3.6.0) R: a language and environment for statistical computing
- 34.Hempel J-M, Schittenhelm J, Klose U et al (2018) In vivo molecular profiling of human glioma. Clin Neuroradiol. 10.1007/s00062-018-0676-2 [DOI] [PubMed]
- 35.Chahal M, Xu Y, Lesniak D, et al. MGMT modulates glioblastoma angiogenesis and response to the tyrosine kinase inhibitor sunitinib. Neuro Oncol. 2010;12:822–833. doi: 10.1093/neuonc/noq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon W-J, Choi JW, Roh HG, Lim SD, Koh YC (2012) Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology 54:555–563. 10.1007/s00234-011-0947-y [DOI] [PubMed]
- 37.Li Q, Guo J, Wang W, Wang D. Relationship between MGMT gene expression and treatment effectiveness and prognosis in glioma. Oncol Lett. 2017;14:229–233. doi: 10.3892/ol.2017.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen HN, Lie A, Li T, et al. Human TERT promoter mutation enables survival advantage from MGMT promoter methylation in IDH1 wild-type primary glioblastoma treated by standard chemoradiotherapy. Neuro Oncol. 2017;19:394–404. doi: 10.1093/neuonc/now189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Jiang T, Li G, Wang Z. Impact of p53 status to response of temozolomide in low MGMT expression glioblastomas: preliminary results. Neurol Res. 2008;30:567–570. doi: 10.1179/174313208X297913. [DOI] [PubMed] [Google Scholar]
- 40.Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014;16:1263–1273. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z-C, Bai H, Sun Q, et al. Multiregional radiomics features from multiparametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: a multicentre study. Eur Radiol. 2018;28:3640–3650. doi: 10.1007/s00330-017-5302-1. [DOI] [PubMed] [Google Scholar]
- 42.Jiang S, Rui Q, Wang Y, et al. Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics. Eur Radiol. 2018;28:2115–2123. doi: 10.1007/s00330-017-5182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19:1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)