Abstract
Contemporary theory that emphasizes the roles of oxytocin and vasopressin in mammalian sociality has been shaped by seminal vole research that revealed interspecific variation in neuroendocrine circuitry by mating system. However, substantial challenges exist in interpreting and translating these rodent findings to other mammalian groups, including humans, making research on nonhuman primates crucial. Both monogamous and non-monogamous species exist within Eulemur, a genus of strepsirrhine primate, offering a rare opportunity to broaden a comparative perspective on oxytocin and vasopressin neurocircuitry with increased evolutionary relevance to humans. We performed oxytocin and arginine vasopressin 1a receptor autoradiography on 12 Eulemur brains from seven closely related species to (1) characterize receptor distributions across the genus, and (2) examine differences between monogamous and non-monogamous species in regions part of putative “pair-bonding circuits”. We find some binding patterns across Eulemur reminiscent of olfactory-guided rodents, but others congruent with more visually oriented anthropoids, consistent with lemurs occupying an ‘intermediary’ evolutionary niche between haplorhine primates and other mammalian groups. We find little evidence of a “pair-bonding circuit” in Eulemur akin to those proposed in previous rodent or primate research. Mapping neuropeptide receptors in these nontraditional species questions existing assumptions and informs proposed evolutionary explanations about the biological bases of monogamy.
Subject terms: Anthropology, Social evolution, Social behaviour, Social neuroscience, Brain, Psychology
Introduction
“Undoubtedly there are numerous molecular and neurobiological pathways that could evolve to support pair-bond formation between mates, and different species may have achieved similar behaviors through a process of convergent evolution involving different circuits… Nevertheless, it is intriguing to consider the possibility that similar mechanisms may underlie the formation of pair bonds in both humans and rodents.” –Young and Wang (2004, p. 1052).
Behavioral biologists are keenly interested in the evolved mechanisms that underlie diversity in social systems. Oxytocin and vasopressin, two closely related neuropeptides, have been promising hormonal candidates in the study of social systems due to their socioregulatory functions spanning a range of behavior in a wide variety of taxa1. Beyond their conserved physiological roles, key behavioral and cognitive processes modulated by these nonapeptides include pair bonding and mating2–5, parental care6,7, stress coping8,9, aggression10,11, social reward12, and social recognition13,14. Many hypotheses regarding the mechanisms by which oxytocin and vasopressin modulate attachment behavior, and even mating systems, stem from comparative rodent work examining underlying neural circuits. Hypotheses arising from this work posed the exciting possibility that mechanisms of neuropeptide function might generalize to humans; nevertheless, primate models that would provide important, evolutionarily relevant tests of these mechanisms have been underutilized15,16. Here, we examine neuroanatomical distributions of oxytocin and vasopressin receptors for the first time in strepsirrhine primates, taking advantage of the distinct interspecific variation in social system within a single genus to test the influential hypothesis that differences in receptor distributions reflect differences in mating system.
Only one type of oxytocin receptor, the G protein-coupled receptor OXTR, has been characterized thus far, but vasopressin has three known receptors (AVPR1a, AVPR1b and AVPR2;17). Of these receptors, AVPR1a is present centrally and is thought to mediate the majority of vasopressin’s social effects17. In foundational neuroanatomical studies on the Microtus genus of voles, researchers used in vitro receptor autoradiography to examine both OXTR and AVPR1a. Voles emerged as exceptional comparative models because both monogamous and non-monogamous species exist within the same genus, allowing researchers to examine neurobiological differences between phylogenetically proximate, yet socially divergent species. While the neural distributions of vasopressin and oxytocin immunoreactive fibers are relatively conserved across vertebrates1, receptor distributions can vary substantially between even closely related species. Indeed, researchers found striking species-level differences in the distribution of OXTR and AVPR1a between monogamous prairie voles (Microtus ochrogaster) and promiscuous montane and meadow voles (Microtus montanus and Microtus pennsylvanicus, respectively)18–21, linking these differences in neuropeptide receptor distributions to interspecific differences in the ability to form pair bonds4,18. This approach has since been broadly applied to examine the evolved functions of oxytocin and vasopressin in rodent social behavior (e.g.22,23) and has established rodent models as central to understanding oxytocin’s purportedly conserved social functions across mammals, including humans4,24,25.
Developments within the field of oxytocin and vasopressin research have also revealed substantial challenges to the interpretation and translation of findings from rodent models to other mammalian groups. Prairie voles exhibit substantial diversity in their mating tactics26 and their central distribution patterns of nonapeptide receptors27–29. Reducing any species’ socioecology and neurobiology to sets of strictly canalized components might oversimplify the underlying mechanisms and limit insights. More generally, behavioral endocrinologists and neuroscientists have long raised concerns about the field’s reliance on a small set of model organisms30–32. Additionally, research identifying unique aspects of human neurobiology33,34 challenges the potential translatability of rodent models. Nonhuman primates might thus serve as valuable bridges from rodent to human biology and sociality15,16.
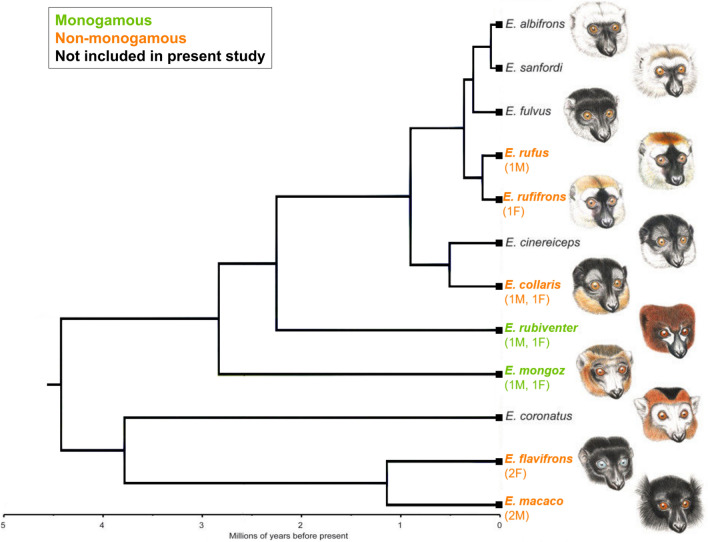
The Eulemur genus of strepsirrhine primates represents a unique and powerful test system for this research area for two reasons. First, with regard to their morphology and sensory adaptations, lemurs are seen as occupying a ‘transitional’ evolutionary niche between rodents and the more-often studied anthropoid primates35,36. Features shared with rodents include enhanced olfactory reliance, acuity, and chemical communication, including a functional vomeronasal organ; features shared with haplorhine primates include forward-facing eyes and visual elaboration35. Despite being our most distant primate relatives, lemurs are approximately only half the genetic distance away from humans as are rodents37. Second, and crucially, Eulemur is the sole primate analogue to Microtus in terms of containing both monogamous and non-monogamous species in a single genus. The 12 extant species of Eulemur are all cathemeral, arboreal, and seasonal breeders; they are generally frugivorous and sympatric; and they are collectively found in almost all forest habitats across Madagascar38. Phylogenetic reconstructions have revealed group-living (with male dispersal and female philopatry) as the ancestral form of social organization in this clade39). Social monogamy and pair-bonding (which need not entail genetic monogamy)40, a year-round arrangement whereby a male–female pair lives in a small family group, defends a shared territory via mutual scent-marking, and jointly cares for young across several seasons, has evolved either once or twice, giving rise to E. rubriventer and E. mongoz as the two monogamous species in this genus39,41–43. All other species are non-monogamous, live in larger social groups, and exhibit varying degrees of promiscuous mating; they also lack the behavioral signatures of social pair-bonds seen in monogamous species39,42. Along with evidence of behavioral bifurcation, phylogenetic evidence of recent species divergence in Eulemur39 supports a consideration of two distinct categories of social systems across the genus. Thus, Eulemur presents us with the opportunity to examine how a conspicuous split in mating systems between closely related primate species is predicted by neuropeptide circuitry.
Because the neurobiology of the oxytocin and vasopressin systems has yet to be characterized in any strepsirrhine primate, we begin with a broad ‘discovery’ aim (1). Across species, we expect to find conserved binding to both neuropeptide receptors in certain specific regions. Parallel to the findings of OXTR/AVPR1a expression along regions of the olfactory pathway in rodents19,20,44, OXTRs in regions of visual processing and attention have consistently been found in anthropoid primates45. In strepsirrhines, we expect to find binding in nuclei involved in both perceptual modalities. Additionally, in anthropoid primates, AVPR1a generally has been found to be more widely distributed than OXTRs46–48—we predict a similar pattern in strepsirrhines.
With foundational neuroanatomical information in hand, we address our more targeted aim (2) to test variation in these receptors as a function of mating system. We investigate whether binding patterns in the brains of monogamous versus non-monogamous Eulemur differ in several regions of hypothesized “pair-bonding circuits”. Based on correlational and experimental evidence in voles4,18,19 linking neuropeptide receptor expression in specific nuclei to pair-bond formation, key OXTR regions include the medial amygdala, nucleus accumbens, and prefrontal cortex, and key AVPR1a regions include the ventral pallidum, lateral septum, and bed nucleus of the stria terminalis. Based on reported neuropeptide receptor distributions and patterns of central glucose uptake upon partner separation in monogamous coppery titi monkeys (Plecturocebus cupreus)49, additional key regions for either OXTR or AVPR1a include the hippocampus, lateral septum, and central amygdala. Examining differences in these predicted regions as a function of mating system, as well as any additional differences specific to Eulemur, will provide a powerful test of the role of neuropeptide receptor organization in predicting social diversity.
Methods
Specimens
Frozen, unfixed brain specimens derived from 12 individual Eulemur subjects (6 M, 6 F), representing two distinct mating systems among seven closely related species (see Fig. 1 for numbers of specimens per sex and species). Members of Eulemur range from vulnerable to critically endangered50. The specimens were thus obtained from the tissue bank of the Duke Lemur Center (DLC) in Durham, NC, which is the only facility outside of Madagascar to house and/or breed several of these species. All individuals whose brains were obtained had been housed socially, primarily in adult male–female pairs, until their death from natural causes or veterinary euthanization (all for non-neurological reasons; age of death ranged from 16.6 to 34.0 years of age).
Figure 1.
The mating system classification of seven Eulemur species at the Duke Lemur Center and their phylogenetic relationships, adapted from39,51. The number and sex of specimens from each focal species is denoted in parentheses. The source figure51 is published under a creative commons attribution license.
Of the 12 specimens, 11 were hemispheres, and one was a whole brain. Owing to variability in location of the midline bisections, and tissue integrity around the edges, some brain regions (such as the midline thalamic nuclei and reticulotegmental nucleus; see below) were either absent or non-quantifiable in some specimens. Additionally, the olfactory bulbs and brainstem nuclei (such as the inferior olive and spinal trigeminal nucleus) were only quantifiable in a subset of subjects (see below).
Tissue preparation
We blocked brain specimens coronally on dry ice, before wrapping them tightly in aluminum foil and storing them at − 80 °C until sectioning. We brought hemisphere blocks up to − 20 °C for sectioning at 20 µm on one of two cryostats. We mounted tissue sections on SuperFrost Plus slides (Brain Research Labs, Newton, MA) and stored them in a sealed slide box with a desiccant packet at − 80 °C until their use in receptor autoradiography.
Receptor autoradiography
For receptor autoradiography, we used a competitive oxytocin and vasopressin receptor binding protocol developed and optimized for primate tissue by Freeman et al.46 in rhesus macaques (Macaca mulatta) and validated to selectively reveal OXTR or AVPR1a binding sites in postmortem brain tissue from common marmosets (Callithrix jacchus;45), coppery titi monkeys [47], and humans48,52. After lightly fixing the tissue sections in 0.1% paraformaldehyde and rinsing with Tris buffer, we incubated them with either the OXTR radioligand 125I-ornithine vasotocin analogue (125I-OVTA; PerkinElmer, Waltham, MA) or the AVPR1a radioligand 125I-linear vasopressin antagonist (125I-LVA; PerkinElmer, Waltham, MA). We co-incubated sets of three adjacent sections in three different conditions: (i) 50 pM radioligand alone, (ii) 50 pM radioligand plus 1 nM SR49059 (Tocris, Minneapolis, MN), an AVPR1a antagonist, and (iii) 50 pM radioligand plus 100 nM ALS-II-69 (donated by ALS; see53), an OXTR antagonist. Accordingly, set (i) could be compared to sets (ii) and (iii) to show regions of selective binding. After incubation, we washed the slides with Tris buffer, dipped them in ddH2O, air dried them, and exposed them to BioMax MR film (Kodak, Rochester, NY) for four days with a set of ten125I autoradiographic standards (American Radiolabeled Chemicals, St. Louis, MO). After film development, we quantified receptor density directly from films without image enhancement.
Because no labelled brain atlas exists for any Eulemur species (or any member of the Lemuridae family), we delineated brain regions for image analysis by counterstaining slides for acetylcholinesterase (AChE) following a modified protocol from Lim et al.20 that has been shown to amplify signal in tissue previously used for receptor autoradiography.
We quantified the optical binding density (OBD) of the autoradiogram images on a light box with MCID Core Digital Densitometry software (Cambridge, UK). First, we determined a flat field correction for luminosity. Then, we loaded the optical binding values from the set of125I autoradiographic standards into the software and used them to generate a standard curve from which OBD values of brain regions of interest could be interpolated. To determine neuroanatomical landmarks and identify regions, we compared images to the sets of AChE counterstained slides, as well as to two atlases of rhesus macaque brains (54,55; www.brainmuseum.org) and an atlas of the adult human brain56. We made three separate measurements per brain region with identifiable OXTR/AVPR1a binding.
Analyses
Our statistical analyses proceeded in three stages. First, we validated the competitive binding protocol using paired t-tests to compare results from each of the ‘competitor binding’ conditions to the ‘radioligand alone’ condition, in four representative regions as well as across all measured regions. In these analyses, we considered measurements from monogamous and non-monogamous animals together. We next performed Welch's t-tests to identify regions with appreciable selective binding of OXTR (125I-OVTA + SR49059) or AVPR1a (125I-LVA + ALS-II-69). Results in this section are presented as mean ± SEM estimated disintegrations per minute per milligram (dpm/mg). Lastly, to examine differences as a function of mating system, we used linear mixed models that contained replicate OBD measurements nested within individual animals as a random effect. These analyses included sex as a factor in the mixed model; however, the exclusion of sex had no substantive effect on any results presented below, indicating a lack of significant differences in binding profiles between the sexes. We performed separate models for individual regions that had either (a) been previously implicated as key areas for rodent and/or primate pair bonding, or (b) showed dense neuropeptide binding in our exploratory analyses. We report results for mating system differences as effect sizes in Cohen’s d, with positive values of d representing greater binding in specimens from monogamous lemurs. The data and corresponding R code needed to reproduce our results are publicly available at https://osf.io/rymz5/.
Results
Selectivity of radioligands
The radioligands125I-OVTA and125I-LVA produced distinct patterns of binding in Eulemur brains (Fig. S1). As in anthropoids, strepsirrhine brains required competitive binding with the AVPR1a antagonist to allow accurately identifying regions of OXTR binding. At the concentration used in our assay,125I-OVTA labelled both OXTR and AVPR1a. Both the AVPR1a antagonist, SR49059, and the OXTR antagonist, ALS-II-69, significantly reduced125I-OVTA binding in the central amygdala (CeA), nucleus accumbens (NAcc), and spinal trigeminal nucleus (Sp5) (Table S1). In contrast, the AVPR1a antagonist significantly reduced125I-LVA binding in the CeA, Sp5, and primary visual cortex (V1), whereas the OXTR antagonist did not reduce125I-LVA binding in these regions (Table S2). This selective reduction in125I-LVA binding by the AVPR1a antagonist showed that125I-LVA binds selectively to AVPR1a and not to OXTR in Eulemur species, while125I-OVTA appears to be able to bind to both receptor subtypes in some regions. Alternatively, SR49059 and ALS-II-69 may have different affinities for Eulemur OXTR and AVPR1a at the concentrations used in this study. Fig. S1 shows the overall efficacy of the antagonists for displacing radioligand binding. Based on these results, below we present values for sections incubated with both the radioligand and the opposing receptor antagonist:125I-OVTA + SR49059, and125I-LVA + ALS-II-69.
OXTR distribution Across Eulemur brains
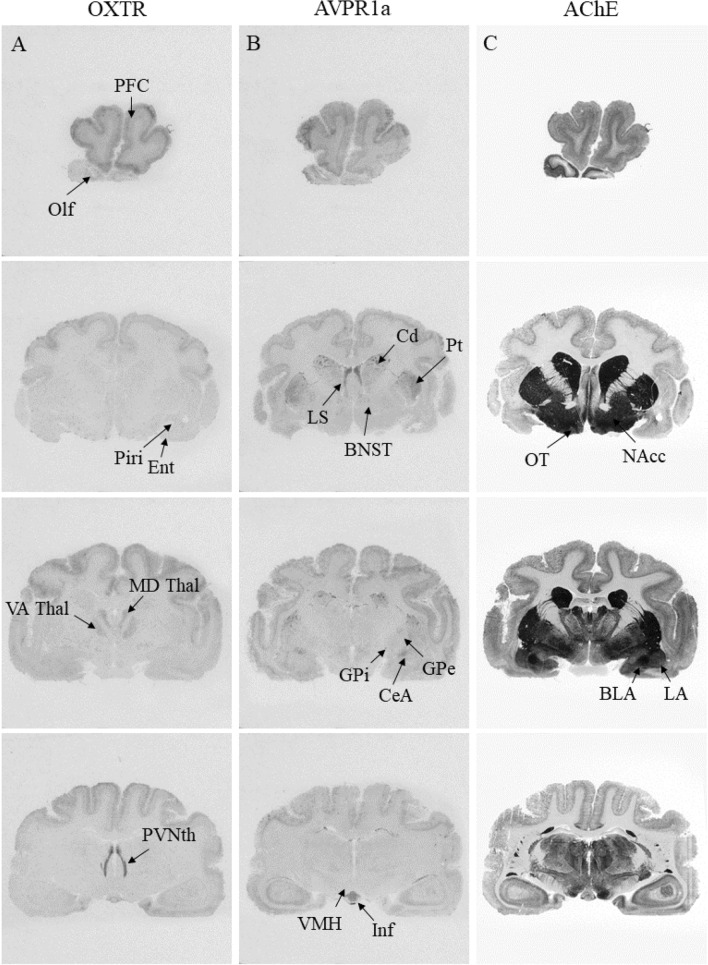
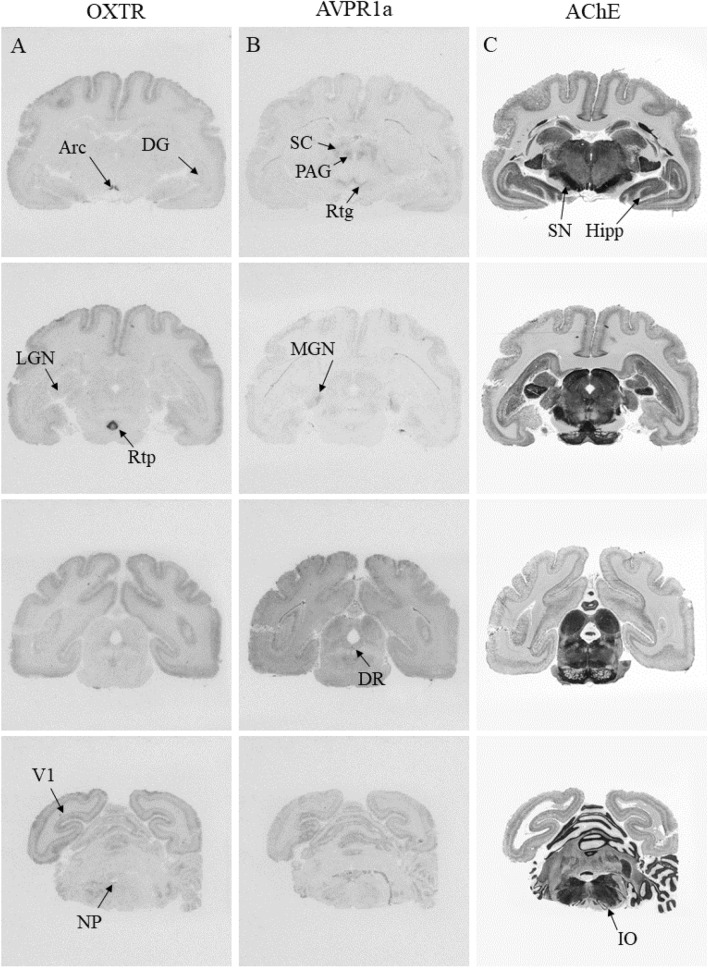
Strong125I-OVTA binding in the presence of the AVPR1a antagonist was restricted to few areas (Figs. 2A, 3A,D), including the paraventricular nucleus of the thalamus (PVNth; 343.48 ± 39.17), V1 (86.66 ± 17.33), prefrontal cortex (PFC; 76.82 ± 12.50), mediodorsal thalamus (MD Thal; 72.66 ± 32.97), and olfactory bulb (Olf; 61.99 ± 26.88; this region was only present in specimens from non-monogamous species; Figs. 2, 4). We observed modest OXTR binding in the hypothalamus (arcuate nucleus [Arc]: 55.00 ± 19.40; ventromedial hypothalamus [VMH]: 34.36 ± 12.53), striatum (caudate [Cd]: 37.58 ± 7.56; putamen [Pt]: 33.43 ± 6.26; NAcc: 19.20 ± 6.31), and assorted brainstem nuclei (nucleus prepositus [NP]: 52.07 ± 8.37; Sp5: 43.86 ± 9.51). We found low levels of binding in the olfactory tubercle (OT), piriform cortex (Pir), entorhinal cortex (EC), globus pallidus external and internal segments (GPe / GPi), various amygdalar nuclei (CeA; LA; BLA), hippocampal formation (Hipp), lateral geniculate nucleus (LGN), and dorsal raphé nucleus (DR). Lastly, there were also notable null results: Unlike binding in vole species18, we observed no OXTR radioligand binding in the lateral septum (LS) or bed nucleus of the stria terminalis (BNST) of any Eulemur specimen (Figs. 2A, 3A). Unlike previous findings in multiple non-human primate species46,47, we did not detect OXTR radioligand binding in the nucleus basalis of Meynert.
Figure 2.
Distribution of OXTR (A) and AVPR1a (B) in sequential coronal sections from the brain of one representative non-monogamous Eulemur individual (E. macaco), aligned with acetylcholinesterase (AChE) counterstain (C). Panels 1–2.
Figure 3.
Distribution of OXTR (A, D) and AVPR1a (B, E) in sequential coronal sections from the brain of one representative monogamous Eulemur individual (E. rubriventer), aligned with acetylcholinesterase (AChE) counterstain (C, F).
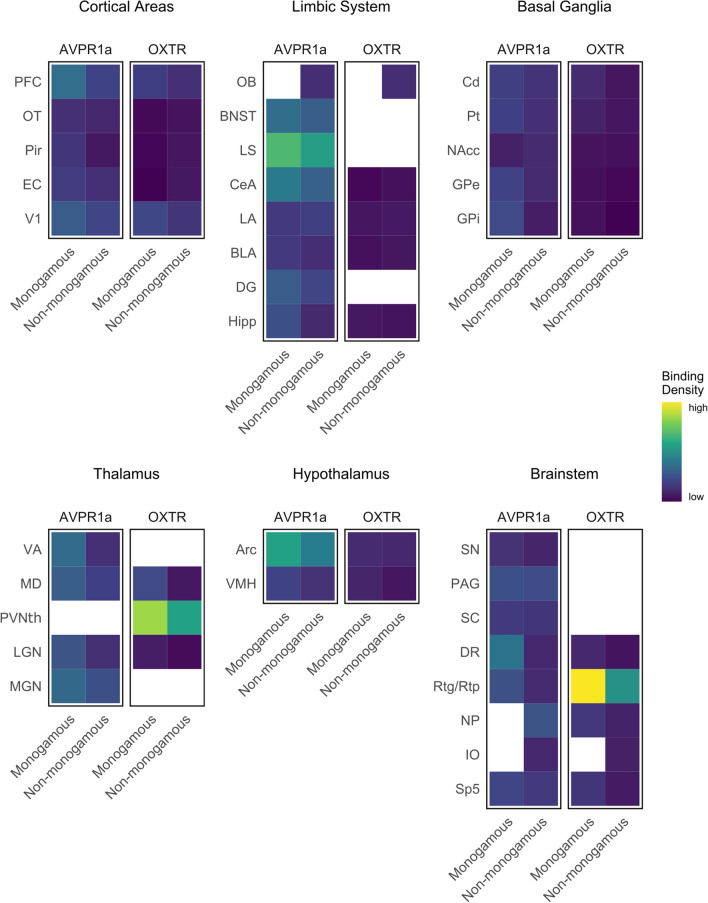
Figure 4.
Summary of relative binding densities by brain region in Eulemur species. Columns depict average binding density across brain specimens from monogamous and non-monogamous species, respectively. Blank cells indicate no measurement available for radioligand binding in that region.
AVPR1a distribution across Eulemur brains
Relative to OXTR binding patterns in Eulemur brains, AVPR1a binding was much more widespread and showed greater average binding across regions (Figs. 2B, 3B,E). In the presence of the OXTR antagonist, we found dense125I-LVA binding in specimens across mating systems in the PFC (126.33 ± 17.43) and V1 (116.80 ± 7.92), the Arc (231.13 ± 43.40), along with several areas of the limbic system (LS: 299.54 ± 45.03; BNST: 162.10 ± 13.73; CeA: 170.28 ± 13.38), thalamus (MD 111.70 ± 20.97; medial geniculate [MGN]: 137.80 ± 18.57), and brainstem (periaqueductal gray [PAG]: 119.40 ± 15.77; Sp5: 88.38 ± 18.56). We found moderate binding in the basal ganglia (Cd: 78.20 ± 13.37; Pt: 77.75 ± 11.57; NAcc: 53.76 ± 12.44; GPe: 72.39 ± 16.95; GPi: 64.18 ± 19.73), LGN (88.26 ± 23.38), olfactory cortex (OT: 54.43 ± 12.44; EC: 73.83 ± 17.20; Pir: 39.41 ± 10.24), VMH (79.18 ± 13.56), and other areas of the limbic system (LA: 90.97 ± 7.94; BLA: 67.99 ± 14.23; Hipp: 72.46.31 ± 17.36) and brainstem (SC; 78.80 ± 10.38; SN; 53.23 ± 15.32) (Figs. 2 and 3).
Binding patterns as a function of mating system
We targeted candidate regions of hypothesized ‘pair-bonding circuits’ in rodents (MeA, NAcc, PFC, LS and BNST;4) and titi monkeys (LS, CeA, and Hipp;49) in our comparisons of mating system-related differences in Eulemur OXTR/AVPR1a binding. Contra Insel and Shapiro’s18 consistent findings of greater OXTR binding in monogamous specimens, we found no evidence that OXTR binding patterns in the Eulemur amygdala differed significantly between specimens from monogamous vs. non-monogamous species (Figs. 2, 3, 4). We did not observe significant binding in the medial amygdala of any specimens, and in other amygdalar nuclei where OXTR was present, differences between mating systems were non-significant and inconsistent in direction (d ranging from − 0.25 to 0.33). Similarly, and somewhat surprisingly, we observed no significant differences in OXTR binding patterns in the NAcc (d = 0.13), Hipp (d = 0.43), or PFC (d = 0.68).
We also observed no statistically significant differences in AVPR1a binding in the regions of interest that were targeted in this analysis for their hypothesized roles in rodent or titi monkey pair-bonding4,49. Also divergent from findings in rodents, but consistent with findings in haplorhine primates (e.g.,47), we observed no binding in the ventral pallidum of any Eulemur specimen. Furthermore, in regions where we observed binding, including the LS, BNST and Hipp, there were no significant differences as a function of mating system. Differences were small to medium for the LS (d = 0.41) and BNST (d = 0.59), and larger for the Hipp (d = 1.03), but all p > 0.05 (Figs. 2, 3, 4).
We next examined if there were differences by mating system in any of the regions in which we observed OXTR and/or AVPR1a binding. The only region where we observed significant OXTR differences by mating system was the reticulotegmental nucleus, which showed stronger binding in specimens from monogamous than non-monogamous species (d = 3.71, p = 0.021; Fig. 4). For AVPR1a, we observed a significant difference in the ventral anterior thalamus (VA; d = 1.28, p = 0.025), dorsal raphé nucleus (DR; d = 1.49, p = 0.010), and PFC (d = 1.45, p = 0.028), with specimens from monogamous species again showing stronger binding in all three regions compared to their counterparts from non-monogamous species (Fig. 4).
Discussion
As the first study to investigate neuropeptide receptor distribution in strepsirrhine primates, we document binding patterns of both oxytocin and vasopressin in members of the Eulemur clade that fall between those of classic rodent models (e.g.18,19) and those of more recently characterized haplorhine primates46,47,57. This intermediacy may have functional implications for lemurs’ evolutionary specializations, potentially reflecting the comparatively variable role of these neuropeptides in sensory ecology (e.g.13,58). As the first primate study to directly compare neuropeptide receptor binding between brain specimens from monogamous and non-monogamous species of the same genus, our findings also fill a critical gap in knowledge of how variation in neuroanatomy reflects variation in primate mating systems or sociality. Beyond simply representing another data point in the domain of comparative neurology, findings from our study of Eulemur question the universality of classic vole models and suggest a revisitation of their implications for humans.
Like rodents, lemurs show olfactory specialization59, which is prominently displayed in their use of scent to convey a wide array of reproductive and social information60,61. Some degree of similarity in the involvement of OXTRs in processing chemically encoded socio-reproductive information in these taxa is suggested by the diffuse binding of both OXTR and AVPR1a in the olfactory bulbs and olfactory tubercle, and by dense binding of AVPR1a in the CeA and BNST (across mating systems). Likewise, AVPR1a binding has been found in the olfactory bulb of platyrrhine primates (e.g. common marmosets;57) that also rely extensively on olfactory communication62; similar binding has not been reported in less olfactory-oriented catarrhine primates.
Relative to other placental mammals, vision is exceptionally well-developed in primates, but less so in strepsirrhines than in haplorhines. In catarrhines, for example, trichromacy63,64 and visual gaze are particularly important in reproductive and social communication65,66. Consistent with previous work in haplorhine primates, we found OXTR expression in V1 and the LGN across species, and AVPR1a expression in these and additional areas related to visual attention (i.e., Rtg, SC, and amygdalar nuclei); nevertheless, binding in Eulemur was less widespread than that observed in haplorhine primates (e.g.46,47). With regard to sensory pathways, therefore, our results are consistent with lemur neuroanatomy representing a bridge between odor-reliant rodents and vision-reliant haplorhines.
Intermediary patterns were also evident in other pathways. For instance, consistent with findings in some rodent species (singing mice:23; prairie and montane voles:19), but unlike findings in haplorhine primates, we observed dense AVPR1a expression in the MGN of lemurs. Because the MGN is an essential auditory relay nucleus—receiving input from the inferior colliculus and projecting to the auditory cortices—our findings potentially implicate vasopressin in another sensory modality in Eulemur; it is possible that vasopressin plays a modulatory role in the processing of vocal communication or emotionally valent sounds.
Relative to haplorhines, additional patterns of receptor binding in Eulemur show both similarities and striking reversals. In Eulemur, we observed strong AVPR1a binding, but diffuse or modest OXTR binding, in both the striatum and hippocampal regions. This striatal pattern is comparable to that seen in coppery titi monkeys47, but it contrasts with the dense OXTR expression found in both rodents67 and marmosets57. Hippocampal patterns in Eulemur are reversed from that observed in titi monkeys47. Oxytocin acting on OXTRs in the NAcc is necessary for pair-bond formation in voles4. Precise functions of oxytocin or vasopressin within the hippocampal formation remain to be identified68, but there is some evidence that they modulate the encoding and consolidation of socially relevant memories69,70. In any event, our divergent results in these regions suggest that neural mechanisms of pair bonding in lemurs may differ substantially from other mammalian groups studied thus far.
Regarding the influential hypothesis that interspecific variation in specific populations of receptors reflects variation in social organization or mating system, our results did not reveal comparable differences to the striking findings previously reported for monogamous and non-monogamous vole species. For instance, in Insel and Shapiro18, the effect size for a mating system difference in OXTR was d = 2.23 in the NAcc and d = 2.06 for the LA; in Insel et al.19, the mating system difference in AVPR1a was d = 3.66 for the LS and d = 2.81 for the BNST. In Eulemur, despite a sample size that matched these classic vole studies, differences between mating systems, for either neuropeptide, were almost uniformly non-significant (with much smaller effect sizes; all d < 0.8) in all regions of a hypothesized rodent ‘pair-bonding circuit’. Our results do not support the suggestion that OXTR/AVPR1a differences in key dopaminergic areas separate monogamous from non-monogamous species4. When expanding our comparisons across the entire brain, however, we observed some significant differences between mating systems, including in the Rtp for OXTR and the VA Thal, DR, and PFC for AVPR1a.
How should one interpret these mixed results? Regarding null findings, we note that exhausting the available bank of Eulemur brain tissue at the Duke Lemur Center nevertheless left us with limited statistical power to detect differences in individual regions as a function of mating system. While large differences comparable in magnitude to those reported in Insel and Shapiro18 and Insel et al.19 would be detectable with our sample—indeed, we matched the sample size from these classic studies—more modest differences may have been missed. Regarding exploratory positive findings, we first caution that examining numerous regions increases the potential for false-positives, and that there is a lack of information about the functional significance for many of these differences. For instance, whereas the presence of OXTR in the pontine reticular areas of Eulemur and rhesus macaques46 suggests a possible conserved function of oxytocin in this region, it is unclear how differences in this region that controls horizontal gaze and saccadic eye movement would be involved in differences in social bonding behavior. Although the ventral anterior thalamus has important functions in spatial memory and learning71, it has not been specifically implicated in pair-bonding processes. That said, other findings more readily yield potential interpretations. First, the AVPR1a difference we observed in the DR, a source of serotonin and a region involved in reward-seeking and reward-tracking behavior72, suggests that some of the effects of vasopressin on social behavior may owe to activation of the DR serotonin system73. If so, monogamous Eulemur may have developed denser populations of AVPR1a to bolster serotonergic functions of social reward behavior that foster the creation of pair bonds. Second, rather than observing OXTR binding differences in the PFC—a key area generating the reinforcing, hedonic properties of pair-bonding behavior and mating in rodents4—we instead found a difference in AVPR1a binding in this region. Perhaps some of the mechanisms mediated by oxytocin in rodents are carried out by the structurally similar vasopressin in primates—a suggestion that has been hypothesized and substantiated in several previous studies45.
Collectively, mixed findings for mating system differences, like the aforementioned binding patterns found across lemur species, are consistent with the existence of distinctive mechanisms for the formation of monogamous mating systems in Eulemur. In questioning the universality of these mechanisms across mammalian groups, our findings in this domain can also be considered within the broader context of psychological oxytocin research, which is similarly marked by interpretive challenges and heterogeneous findings (e.g.74). We suggest that expanding the toolkits available to researchers, including broadening the animal models studied, will likely continue to reveal unexpected findings that require modification to existing theory (a point echoed by behavioral ecologists; e.g.75).
Providing context to our results is the fact that numerous factors other than species identity influence an individual’s oxytocin and vasopressin neurocircuitry. Neurobiology is not static throughout the lifespan, but rather may vary seasonally, with social circumstance, and with age or life-history stage (e.g.52). Thus, while receptor distributions can differ widely between species and social systems18,19,22, they might also differ substantially within individuals of the same species or mating system. Indeed, Phelps and Young27 report intraspecific variation in AVPR1a binding among prairie voles often comparable to or greater than interspecific variation (for a recent example of experience-dependent, intraspecific OXTR patterns in a primate model, see68). Nevertheless, these same authors also report less variation in regions regulating social bonding, relative to those unrelated to social bonding—a pattern consistent with natural selection winnowing neuropeptide expression in these former regions. We also observed substantial intraspecific and within-mating system variation in Eulemur (see individual-level estimates of receptor profiles in Table S3)—given our limited sample size per species, it is unclear to what extent this might be explained by season-level, individual-level, and/or species-level differences. In Eulemur, some areas previously identified as key to social bonding—such as nuclei of the amygdala and the BNST—showed relatively small coefficients of variation within mating systems, consistent with27, even though they did not differ significantly between mating systems. Other regions that showed relatively little variation within Eulemur mating systems, such as the primary visual cortex and SC, were not the same ones identified as part of a pair-bonding circuit in rodent studies, but they are consistently identified as sites of OXTR and AVPR1a in nonhuman primate studies46,47. Perhaps neuropeptide binding in regions responsible for processing visual information are important targets of stabilizing selection in primates, regardless of the underlying mating system.
As in the classic vole studies18,19, we categorized our Eulemur species as belonging to one of two broad mating systems, based on extant information about their wild counterparts39. On the one hand, we cannot rule out the possibility that group size reductions, selective reproduction, or long-term pair housing in captivity may have contributed to ‘monogamous-like’ receptor binding profiles across species in our sample, potentially minimizing differences by mating-system category. On the other hand, one might expect such a ‘flattening’ influence to lead to similar receptor profiles across individuals and species, but this does not reflect our results, which are more accurately characterized by a large degree of within-mating system variation. More generally, we believe our results complement the recognition of substantial, natural heterogeneity in social behavior, within or between species, under the general umbrella of ‘monogamous’ or ‘non-monogamous’. Pair-living, pair-bonding, and genetic monogamy are overlapping, yet constitute distinct components of a monogamous mating system that are often conflated40,76. Different configurations of these components across ‘monogamous’ species could conceivably create different neuropeptide receptor distributions. Importantly, we note that flexibility in putative mating systems is likely the norm, rather than the exception in animal models. Even the seemingly well-characterized mating system of prairie voles contains surprises revealed only upon extensive observation in naturalistic settings26. In some cases, differences in neuropeptide receptor distributions may be detectable in spite of intraspecific (or within-mating system) social variation, but this may less common than previously assumed.
Conclusion
Our analyses of the oxytocin and vasopressin receptor distributions throughout the Eulemur genus break ground into a previously unstudied neurobiological system and question a popular and foundational neurobiological explanation for the differences between monogamous and non-monogamous species. We find in lemurs some elements of neuropeptide expression seen in rodents (e.g. binding in olfactory regions) and other elements more commonly found in haplorhine primates (e.g. binding along visual pathways), consistent with other lines of evidence suggesting the intermediary evolutionary niche occupied by lemurs between other mammalian groups and haplorhine primates. While previous researchers often note the possibility that different mammalian lineages have developed mating systems via distinct neurobiological mechanisms (e.g. 4), much of the impact and appeal of rodent studies has come from the enticing possibility that conserved mechanisms related to oxytocin and vasopressin may help explain how human pair bonds are formed. We show that circuits identified as key to pair bonding in rodents cannot simply be invoked to explain primate pair bonding. Our research on the lemur oxytocin system, as part of burgeoning body of work across a range of nonhuman primates, also has important implications for translational research, as it provides a glimpse into the diversity by which these neuropeptides may have their manifold effects on social behavior.
Supplementary Information
Acknowledgements
We are indebted to Brian Horman, Genna St. Armour, Jordan Walker, and Tyler Beauchamp for their assistance with tissue sectioning and quantification, Leonard White and Jenna McHenry for their comments on a previous version of this manuscript, and staff at the Duke Lemur Center for assistance with acquiring brain specimens. This research was supported by: the National Science Foundation (SBE-1808803 to NMG and CMD); the National Institute of Mental Health (NIMH R21MH115680 to SMF and KLB); Duke University Research Support (to CMD); the Josiah Charles Trent Memorial Foundation Endowment Fund, Duke Institute for Brain Sciences, and the Duke Lemur Center Director’s Fund (to NMG); and the Duke Office of Undergraduate Research Support and Charles Lafitte Foundation for Research (to AS). This is DLC publication #1471.
Abbreviations
- Arc
Arcuate nucleus
- BLA
Basolateral amygdala
- BNST
Bed nucleus of the stria terminalis
- CeA
Central amygdala
- Cd
Caudate
- DG
Dentate gyrus
- DR
Dorsal raphe nucleus
- EC
Entorhinal cortex
- GPe
Globus pallidus external segment
- GPi
Globus pallidus internal segment
- Hipp
Hippocampal formation
- IO
Inferior olive
- LA
Lateral amygdala
- LGN
Lateral geniculate nucleus
- LS
Lateral septum
- MD
Medial dorsal thalamus
- MGN
Medial geniculate nucleus
- NAcc
Nucleus accumbens
- NP
Nucleus prepositus
- OB
Olfactory bulb
- OT
Olfactory tubercle
- PAG
Periaqueductal gray
- PFC
Prefrontal cortex
- Pir
Piriform cortex
- Pt
Putamen
- PVNth
Paraventricular nucleus of the thalamus
- Rtg/Rtp
Reticulotegmental/reticulopontine nucleus
- SC
Superior colliculus
- SN
Substantia nigra
- V1
Primary visual cortex
- VA
Ventral anterior thalamus
- VMH
Ventromedial hypothalamus
Author contributions
N.M.G., S.M.F., and C.M.D. designed research; N.M.G. and A.S. prepared specimens; N.M.G., A.S., S.M.F., and M.C.P. conducted experiments; H.B.P. and K.L.B. provided equipment and analytic tools; N.M.G. and A.S. analyzed data; all authors contributed to the writing of the manuscript.
Data availability
The data and corresponding R code needed to reproduce our results are publicly available at https://osf.io/rymz5/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83342-6.
References
- 1.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter CS. Oxytocin and sexual behavior. Neurosci. Biobehav. Rev. 1992;16(2):131–144. doi: 10.1016/S0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 3.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113(5):1071–1079. doi: 10.1037/0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 4.Young LJ, Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 5.Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm. Behav. 2012;61(3):266–276. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Young LJ, De Vries GJ, Insel TR. Voles and vasopressin: a review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog. Brain Res. 1998;119:483–499. doi: 10.1016/S0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- 7.Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Cavanaugh J, Carp SB, Rock CM, French JA. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology. 2016;66:22–30. doi: 10.1016/j.psyneuen.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potegal M, Ferris CF. Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin-receptor antagonist. Aggress. Behav. 1989;15(4):311–320. doi: 10.1002/ab.2480150406. [DOI] [Google Scholar]
- 11.De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 12.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopatina OL, Komleva YK, Gorina YV, Higashida H, Salmina AB. Neurobiological aspects of face recognition: the role of oxytocin. Front. Behav. Neurosci. 2018;12:195. doi: 10.3389/fnbeh.2018.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman SM, Bales KL. Oxytocin, vasopressin, and primate behavior: diversity and insight. Am. J. Primatol. 2018;80(10):e22919. doi: 10.1002/ajp.22919. [DOI] [PubMed] [Google Scholar]
- 16.Putnam PT, Young LJ, Gothard KM. Bridging the gap between rodents and humans: the role of non-human primates in oxytocin research. Am. J. Primatol. 2018;80(10):e22756. doi: 10.1002/ajp.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell, H. K., & Young, W. S. Oxytocin and vasopressin: genetics and behavioral implications. In Handbook of Neurochemistry and Molecular Neurobiology 573–607 (Boston, MA, Springer US, 2006).
- 18.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. USA. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci. 1994;14(9):5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J. Comp. Neurol. 2004;468(4):555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- 21.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci. Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J. Comp. Neurol. 2008;507(6):1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- 23.Campbell P, Ophir AG, Phelps SM. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J. Comp. Neurol. 2009;516(4):321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- 24.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-H. [DOI] [PubMed] [Google Scholar]
- 25.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madrid, J. E., Parker, K. J., & Ophir, A. G. Variation, plasticity, and alternative mating tactics: Revisiting what we know about the socially monogamous prairie vole. In Advances in the Study of Behavior 203–42 (Elsevier, 2020).
- 27.Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J. Comp. Neurol. 2003;466(4):564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- 28.King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the oxytocin receptor gene predicts brain region–specific expression and social attachment. Biol. Psychiatry. 2016;80(2):160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018;19(11):643–654. doi: 10.1038/s41583-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beach FA. The Snark was a Boojum. Am. Psychol. 1950;5(4):115–124. doi: 10.1037/h0056510. [DOI] [Google Scholar]
- 31.Preuss TM. Taking the measure of diversity: comparative alternatives to the model-animal paradigm in cortical neuroscience. Brain Behav. Evol. 2000;55(6):287–299. doi: 10.1159/000006664. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RR. An updated field guide for snark hunting: Comparative contributions to behavioral neuroendocrinology in the era of model organisms. Horm. Behav. 2020;122(104742):104742. doi: 10.1016/j.yhbeh.2020.104742. [DOI] [PubMed] [Google Scholar]
- 33.Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, et al. Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat. Neurosci. 2018;21(9):1185–1195. doi: 10.1038/s41593-018-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573(7772):61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleagle JG. Primate Adaptation and Evolution. 3. San Diego: CA, Academic Press; 2013. [Google Scholar]
- 36.Hozer C, Pifferi F, Aujard F, Perret M. The biological clock in gray mouse lemur: Adaptive, evolutionary and aging considerations in an emerging non-human primate model. Front Physiol. 2019;10:1033. doi: 10.3389/fphys.2019.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezran C, Karanewsky CJ, Pendleton JL, Sholtz A, Krasnow MR, Willick J, et al. The mouse lemur, a genetic model organism for primate biology, behavior, and health. Genetics. 2017;206(2):651–664. doi: 10.1534/genetics.116.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ossi K, Kamilar JM. Environmental and phylogenetic correlates of Eulemur behavior and ecology (Primates: Lemuridae) Behav. Ecol. Sociobiol. 2006;61(1):53–64. doi: 10.1007/s00265-006-0236-7. [DOI] [Google Scholar]
- 39.Kappeler PM, Fichtel C. The evolution of Eulemur social organization. Int. J. Primatol. 2016;37(1):10–28. doi: 10.1007/s10764-015-9873-x. [DOI] [Google Scholar]
- 40.Tecot SR, Singletary B, Eadie E. Why, “monogamy” isn’t good enough: pair-living, pair-bonding, and monogamy. Am. J. Primatol. 2016;78(3):340–354. doi: 10.1002/ajp.22412. [DOI] [PubMed] [Google Scholar]
- 41.Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- 42.Singletary B, Tecot S. Signaling across the senses: a captive case study in pair-bonded red-bellied lemurs (Eulemur rubriventer) at the Duke Lemur Center, NC. USA. Primates. 2019;60(6):499–505. doi: 10.1007/s10329-019-00770-9. [DOI] [PubMed] [Google Scholar]
- 43.Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479(7372):219–222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- 44.Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464(7287):413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol. 2016;28(4). [DOI] [PMC free article] [PubMed]
- 46.Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, et al. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman SM, Smith AL, Goodman MM, Bales KL. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Soc. Neurosci. 2017;12(2):113–123. doi: 10.1080/17470919.2016.1156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bales KL, Arias Del Razo R, Conklin QA, Hartman S, Mayer HS, Rogers FD, et al. Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. Yale J. Biol. Med. 2017;90(3):373–387. [PMC free article] [PubMed] [Google Scholar]
- 50.The IUCN Red List of Threatened Species. 2020–2 [cited 2020 Jul 9]. Available from: https://www.iucnredlist.org
- 51.Markolf M, Kappeler PM. Phylogeographic analysis of the true lemurs (genus Eulemur) underlines the role of river catchments for the evolution of micro-endemism in Madagascar. Front Zool. 2013;10(1):70. doi: 10.1186/1742-9994-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman SM, Palumbo MC, Lawrence RH, Smith AL, Goodman MM, Bales KL. Effect of age and autism spectrum disorder on oxytocin receptor density in the human basal forebrain and midbrain. Transl. Psychiatry. 2018;8(1):257. doi: 10.1038/s41398-018-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith AL, Freeman SM, Barnhart TE, Abbott DH, Ahlers EO, Kukis DL, et al. Initial investigation of three selective and potent small molecule oxytocin receptor PET ligands in New World monkeys. Bioorg. Med. Chem. Lett. 2016;26(14):3370–3375. doi: 10.1016/j.bmcl.2016.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakker R, Tiesinga P, Kötter R. The Scalable Brain Atlas: Instant web-based access to public brain atlases and related content. Neuroinformatics. 2015;13(3):353–366. doi: 10.1007/s12021-014-9258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, et al. The INIA19 template and NeuroMaps atlas for primate brain image parcellation and spatial normalization. Front. Neuroinform. 2012;6:27. doi: 10.3389/fninf.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding, S.-L., Royall, J. J., Sunkin, S. M., Ng, L., Facer, B. A. C., Lesnar, P. et al. Comprehensive cellular-resolution atlas of the adult human brain: adult human brain atlas. J. Comp. Neurol.524(16), Spc1–Spc1 (2016). [DOI] [PMC free article] [PubMed]
- 57.Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci. Lett. 2009;461(3):217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Wacker D, Ludwig M. The role of vasopressin in olfactory and visual processing. Cell Tissue Res. 2019;375(1):201–215. doi: 10.1007/s00441-018-2867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heritage S. Modeling olfactory bulb evolution through primate phylogeny. PLoS ONE. 2014;9(11):e113904. doi: 10.1371/journal.pone.0113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drea CM. D’scent of man: a comparative survey of primate chemosignaling in relation to sex. Horm. Behav. 2015;68:117–133. doi: 10.1016/j.yhbeh.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Drea CM. Design, delivery and perception of condition-dependent chemical signals in strepsirrhine primates: implications for human olfactory communication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1800;2020(375):20190264. doi: 10.1098/rstb.2019.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazaro-Perea C, Snowdon CT, de Fátima AM. Scent-marking behavior in wild groups of common marmosets (Callithrix jacchus) Behav. Ecol. Sociobiol. 1999;46(5):313–324. doi: 10.1007/s002650050625. [DOI] [Google Scholar]
- 63.Changizi MA, Zhang Q, Shimojo S. Bare skin, blood and the evolution of primate colour vision. Biol. Lett. 2006;2(2):217–221. doi: 10.1098/rsbl.2006.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez AA, Morris MR. Sexual selection and trichromatic color vision in primates: statistical support for the preexisting-bias hypothesis. Am. Nat. 2007;170(1):10–20. doi: 10.1086/518566. [DOI] [PubMed] [Google Scholar]
- 65.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 2000;24(6):581–604. doi: 10.1016/S0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 66.Shepherd SV, Platt ML. Spontaneous social orienting and gaze following in ringtailed lemurs (Lemur catta) Anim. Cogn. 2008;11(1):13–20. doi: 10.1007/s10071-007-0083-6. [DOI] [PubMed] [Google Scholar]
- 67.Freeman AR, Aulino EA, Caldwell HK, Ophir AG. Comparison of the distribution of oxytocin and vasopressin 1a receptors in rodents reveals conserved and derived patterns of nonapeptide evolution. J. Neuroendocrinol. 2020;32(4):e12828. doi: 10.1111/jne.12828. [DOI] [PubMed] [Google Scholar]
- 68.Baxter A, Anderson M, Seelke AM, Kinnally EL, Freeman SM, Bales KL. Oxytocin receptor binding in the titi monkey hippocampal formation is associated with parental status and partner affiliation. Sci. Rep. 2020;10(1):1–14. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cilz NI, Cymerblit-Sabba A, Young WS. Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav. 2019;18(1):e12535. doi: 10.1111/gbb.12535. [DOI] [PubMed] [Google Scholar]
- 70.Pagani JH, Zhao M, Cui Z, Avram SKW, Caruana DA, Dudek SM, et al. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry. 2015;20(4):490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front. Syst. Neurosci. 2013;7:45. doi: 10.3389/fnsys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura K. The role of the dorsal raphé nucleus in reward-seeking behavior. Front. Integr. Neurosci. 2013;7:60. doi: 10.3389/fnint.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rood BD, Beck SG. Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience. 2014;260:205–216. doi: 10.1016/j.neuroscience.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mierop A, Mikolajczak M, Stahl C, Béna J, Luminet O, Lane A, et al. How can intranasal oxytocin research be trusted? A systematic review of the interactive effects of intranasal oxytocin on psychosocial outcomes. Perspect. Psychol. Sci. 2020;15(5):1228–1242. doi: 10.1177/1745691620921525. [DOI] [PubMed] [Google Scholar]
- 75.Rosenthal MF, Gertler M, Hamilton AD, Prasad S, Andrade MCB. Taxonomic bias in animal behavior publications. Anim. Behav. 2017;127:83–89. doi: 10.1016/j.anbehav.2017.02.017. [DOI] [Google Scholar]
- 76.Huck M, Di Fiore A, Fernandez-Duque E. Of apples and oranges? The evolution of “monogamy” in non-human primates. Front. Ecol. Evol. 2020;7:472. doi: 10.3389/fevo.2019.00472. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and corresponding R code needed to reproduce our results are publicly available at https://osf.io/rymz5/.