Abstract
The pine wilt disease (PWD), for which no effective treatment is available at the moment, is a constant threat to Pinus spp. plantations worldwide, being responsible for significant economic and environmental losses every year. It has been demonstrated that elicitation with chitosan increases plant tolerance to the pinewood nematode (PWN) Bursaphelenchus xylophilus, the causal agent of the PWD, but the biochemical and genetic aspects underlying this response have not been explored. To understand the influence of chitosan in Pinus pinaster tolerance against PWN, a low-molecular-weight (327 kDa) chitosan was applied to mock- and PWN-inoculated plants. Nematode population, malondialdehyde (MDA), catalase, carotenoids, anthocyanins, phenolic compounds, lignin and gene expression related to oxidative stress (thioredoxin 1, TRX) and plant defence (defensin, DEF, and a-farnesene synthase, AFS), were analysed at 1, 7, 14, 21 and 28 days post-inoculation (dpi). At 28 dpi, PWN-infected plants elicited with chitosan showed a sixfold lower nematode population when compared to non-elicited plants. Higher levels of MDA, catalase, carotenoids, anthocyanins, phenolic compounds, and lignin were detected in chitosan-elicited plants following infection. The expression levels of DEF gene were higher in elicited plants, while TRX and AFS expression was lower, possibly due to the disease containment-effect of chitosan. Combined, we conclude that chitosan induces pine defences against PWD via modulation of metabolic and transcriptomic mechanisms related with plant antioxidant system.
Subject terms: Biotechnology, Microbiology, Plant sciences, Environmental sciences
Introduction
Bursaphelenchus xylophilus, commonly known as the pinewood nematode (PWN), is the etiological agent of the pine wilt disease (PWD), which affects several Pinus spp., particularly the maritime pine P. pinaster1. The PWD leads to important economic losses in the timber and wood industries, and increases the costs of disease management procedures and control, along with their inherent environmental impacts2–4. Currently, large areas of forests damaged by PWD are located in Japan, South Korea, China, and southern Europe, and these are estimated to increase by 50% in the next 50 years5.
Following infection, nematodes move and feed on plant resin canals leading to loss of water conductivity in stems and, subsequently, decreased transpiration and photosynthesis in leaves, resulting in generalized oxidative damage6,7. The production of hydrogen peroxide (H2O2) and superoxide ion radical (O2·−), for example, has been associated with increased B. xylophilus pathogenicity8. To counteract the harmful effects of these molecules, plants activate several antioxidant enzymes, such as superoxide dismutase, peroxidases and catalase. However, if excessive oxidative stress occurs, plant cells may accumulate malondialdehyde (MDA), a secondary product of cell wall lipid peroxidation9, whose content was already demonstrated to increase in plants inoculated with PWN1. In addition to the production of these metabolites, soluble phenolic compounds and lignin synthesis have also been implicated in plant defence against pathogens10,11. Phenolic compounds production and accumulation are associated with the browning of the leaf tissues injured by the PWN12–14, but the role of these secondary metabolites is still not fully understood. In addition, lignin biosynthesis was found to occur in stem tissues during the advanced stages of the PWD, and constitutive lignin was suggested to be a mechanical barrier against nematode invasion, conferring higher plant tolerance to the pathogens15,16. These enzymatic and other biochemical responses occur as soon as a few hours after infection, and are activated when a group of genes related to plant resistance recognizes pathogen effectors, initiating a resistance response17. In fact, genes related to secondary metabolite biosynthesis (α-farnesene synthase, AFS), defence against pathogens (defensins, DEF) and oxidative stress (thioredoxin, TRX) were found to be highly expressed in pine trees within a few hours after PWN infection18, especially when infected with virulent strains of B. xylophilus16.
Prevention is undoubtedly the best approach to reduce PWD incidence and different strategies have been suggested to avoid or treat the disease by: targeting the nematode itself, the vector, the host, or a combination of all three. Several synthetic compounds have already been developed to control the PWD, but many of them are toxic to the environment, labour intensive to apply, and expensive19. Previous studies suggested that chitosan can be used to enhance plant defence against bacteria20,21, fungi22 and nematodes23,24. Chitosan acts as a plant growth promoter, stimulating responses associated with both primary and secondary metabolism, including: carbon and nitrogen metabolism, primary photochemistry and photosynthesis, the tricarboxylic acid cycle, and terpenoid and phenolic compounds biosynthesis25. For example, carotenoids, one of the most important plant terpenoids, were found to be increased in basil and strawberry tissues following chitosan-elicitation26,27. Phenolic compounds, which play very important functions during pathogen infection (by e.g., providing mechanical strength through cell wall lignification processes) and in preventing oxidative stress, were also demonstrated to increase following elicitation with chitosan28. In fact, total phenolic content, and anthocyanins in particular, have been found to accumulate with chitosan treatment in leaf tissues of lemon balm and basil, and in cell cultures of grape vines29–31. Chitosan application also enhances the accumulation of several enzymatic antioxidants, including catalase, decreasing malondialdehyde (MDA) levels in leaf tissues thus improving plant antioxidant status30,32.
The use of chitosan in plant protection against pests and pathogens presents various advantages compared with the currently employed control compounds, as it is physically and biologically functional, biodegradable and biocompatible with tissues and cells33,34. Khalil et al.23 described the nematicidal activity of chitosan with different molecular weights (MW) against Meloidogyne incognita in tomato seedlings, reporting that low MW had the highest efficacy in controlling pathogen progression. Previous studies on the effect of chitosan as a control agent against the PWN demonstrated that low MW components had the highest nematicidal effect, reducing nematode density inside P. pinaster tissues up to 24 days post-inoculation (dpi)24. More recently, it has been demonstrated that P. pinaster and P. pinea supplemented with diazotrophic bacteria and a chitosan-producing fungus, Cunninghamella elegans, showed a 36-fold reduction of nematode colonization when compared to non-supplemented plants, as well as improved photosynthetic pigments, water content and phenolic compounds biosynthesis35. Despite the promising pieces of evidence that chitosan may be a useful tool in the control of the PWD, the physiological, metabolic and genetic mechanisms induced by chitosan application to PWN-infected Pinus spp. have not been addressed yet. The lack of knowledge regarding the regulatory mechanisms involved in the crosstalk between Pinus spp. plants and PWN after plant elicitation with chitosan greatly hinders the efforts to develop evidence-based, sustainable and affordable control methods against the PWD.
As such, in the present work, we hypothesized that the protective action of chitosan in PWN-infected plants might be due to systemic acquired resistance-related responses associated with a readjustment of the plant’s oxidative status. To test this hypothesis, the potential of low MW chitosan against the PWD in one-year-old P. pinaster plants was analysed, through the evaluation of the effect of chitosan on (1) nematode population dynamics, (2) plant antioxidative system and secondary metabolites accumulation, and (3) gene expression related to oxidative stress and defence responses.
Results
Nematode population in infected plants
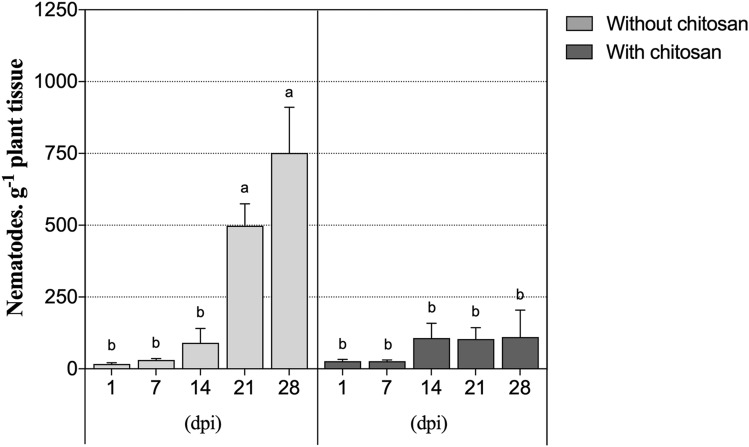
Total nematode count inside stem tissues of inoculated plants without chitosan elicitation significantly increased by 44-fold from 1 to 28 dpi, indicating a successful infection and multiplication of PWN inside plant tissues over time (Fig. 1). In contrast, with chitosan elicitation the number of nematodes was low and statistically identical over the entire experimental period. In fact, only about 0.14-fold of the number of nematodes found in non-elicited plants were recovered from plants inoculated in the presence of chitosan, being the effect of the chitosan treatment significant (P < 0.0001) (Table 1).
Figure 1.
Nematode population in plant tissues. Nematode density in P. pinaster stem tissues 1, 7, 14, 21 and 28 days post-inoculation (dpi). Plants were inoculated with the pinewood nematode (PWN) B. xylophilus 14 days following the application of 0.5% acetic acid (without chitosan, non-elicited controls) or a chitosan solution to plants’ substrate. Data represents the difference between the averages of PWN-inoculated and water-inoculated plants ± SE, and different letters indicate statistically different means at P < 0.05.
Table 1.
Effect of chitosan treatment, time-points of analysis (days post-inoculation, DPI) and their interaction in the physiological, biochemical and gene expression analyses of control and inoculated P. pinaster plants, following elicitation with chitosan.
| Chitosan treatment | DPI | Interaction | ||||
|---|---|---|---|---|---|---|
| Nematode population | F(1,32) | P value | F(4,32) | P value | F(4,32) | P value |
| 32.60 | < 0.0001 | 19.75 | < 0.0001 | 13.42 | < 0.0001 | |
| MDA | F(1, 26) | P value | F(4, 26) | P value | F(4, 26) | P value |
| 13.94 | 0.0009 | 14.89 | < 0.0001 | 0.7780 | 0.5496 | |
| Catalase activity | F(1, 40) | P value | F(4, 40) | P value | F(4, 40) | P value |
| 37.18 | < 0.0001 | 34.58 | < 0.0001 | 12.16 | < 0.0001 | |
| Carotenoids | F(1, 35) | P value | F(4, 35) | P value | F(4, 35) | P value |
| 0.013 | 0.9116 | 13.02 | < 0.0001 | 2.906 | 0.0355 | |
| Anthocyanins | F(1, 30) | P value | F(4, 30) | P value | F(4, 30) | P value |
| 0.324 | 0.5737 | 25.21 | < 0.0001 | 4.488 | 0.0058 | |
| Total Polyphenols | F(1, 33) | P value | F(4, 33) | P value | F(4, 33) | P value |
| 19.24 | 0.0001 | 14.25 | < 0.0001 | 1.219 | 0.3215 | |
| Lignin | F(1,31) | P value | F(4, 31) | P value | F(4, 31) | P value |
| 7.172 | 0.012 | 11.45 | < 0.0001 | 2.415 | 0.0699 | |
| Gene expression | F(1,20) | P value | F(4, 20) | P value | F(4, 20) | P value |
| TRX1 | 34.37 | < 0.0001 | 72.75 | < 0.0001 | 42.92 | < 0.0001 |
| DEF | 7.522 | 0.0125 | 10.83 | < 0.0001 | 15.53 | < 0.0001 |
| AFS | 10.10 | 0.0047 | 29.26 | < 0.0001 | 20.77 | < 0.0001 |
Oxidative stress-related mechanisms
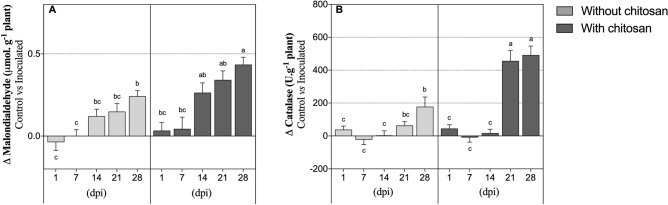
Plants inoculated with PWN displayed a progressive and significant increase in MDA concentration throughout the experimental period, regardless of chitosan elicitation (Fig. 2A), with a significant effect of the chitosan treatment and the DPI (Table 1). In fact, at the end of the experimental period, PWN-inoculated plants elicited with chitosan had significantly higher MDA concentration than non-elicited plants (by 1.8-fold). Similarly to MDA, during the first 14 days of infection, catalase activity was not significantly affected in PWN-infected plants, regardless of chitosan elicitation, which remained similar to catalase levels in water-inoculated plants (Fig. 2B). Non-elicited inoculated plants presented a progressive increase in catalase levels and, at 28 dpi, these were 4.5-fold significantly higher than at 1 dpi. In chitosan-elicited plants, a more drastic and significant increase was registered after 14 dpi, with catalase activity increasing by 25-fold at 21 dpi, resulting in a significant interaction between chitosan treatment and DPI (Table 1).
Figure 2.
Lipid peroxidation and antioxidant activity. Malondialdehyde concentration and catalase activity in P. pinaster leaf tissues 1, 7, 14, 21 and 28 days post-inoculation (dpi) with the pinewood nematode (PWN) B. xylophilus. Plants were inoculated 14 days following the application of 0.5% acetic acid (without chitosan, non-elicited controls) or a chitosan solution to plants’ substrate. Data represents the difference between the averages of PWN-inoculated and water-inoculated plants ± SE, and different letters indicate statistically different means at P < 0.05.
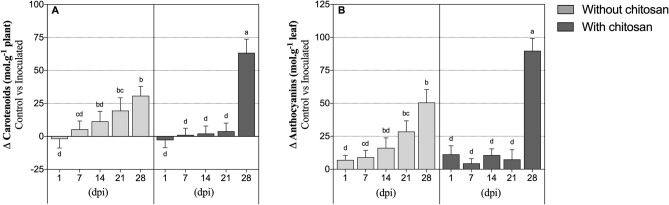
Plants behaved similarly regarding the accumulation of carotenoids (Fig. 3A) and anthocyanins (Fig. 3B). From 1 to 28 dpi PWN-infected plants without chitosan elicitation showed a progressive and significant increase of 15- and sevenfold in carotenoid and anthocyanin concentrations, respectively. In chitosan-elicited plants, carotenoid and anthocyanin concentrations did not vary significantly between control and PWN-infected plants. Hence, no significant effect was found for the chitosan treatment for both analytes (Table 1). However, their concentration abruptly increased at 28 dpi by 21-fold in carotenoids concentration and 13-fold in anthocyanins concentration (when compared to the 21 dpi time-point), with a significant effect of the DPI and its interaction with the chitosan treatment (Table 1).
Figure 3.
Carotenoids and anthocyanins. Carotenoids and anthocyanins concentrations in P. pinaster leaf tissues 1, 7, 14, 21 and 28 days post-inoculation (dpi) with the pinewood nematode (PWN) B. xylophilus. Plants were inoculated 14 days following the application of 0.5% acetic acid (without chitosan, non-elicited controls) or a chitosan solution to plants’ substrate. Data represents the difference between the averages of PWN-inoculated and water-inoculated plants ± SE, and different letters indicate statistically different means at P < 0.05.
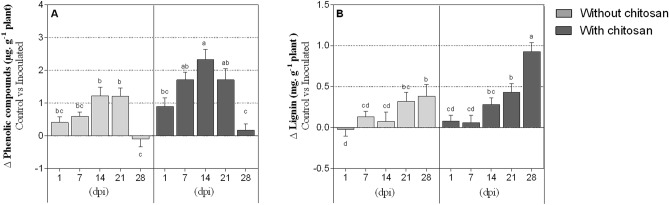
For all time-points, total phenolic concentration was about two times higher in PWN-infected plants with chitosan elicitation (P < 0.0001), when compared to non-elicited plants (Fig. 4A, Table 1). At 14 dpi, plants treated with chitosan presented the highest levels of total phenolic compounds. Moreover, in both elicited and non-elicited plants, polyphenols concentration was drastically reduced in infected plants at the end of the assay (28 dpi), returning to concentration levels similar to non-infected plants. In general, lignin concentration was similar between PWN-infected plants regardless of chitosan elicitation (Fig. 4B). However, at 28 dpi, inoculated plants showed increased lignin concentration (as compared with water-inoculated control plants), particularly with chitosan elicitation (by ca. twofold). No significant interaction between chitosan treatment and DPI was found for both total phenolic compounds and lignin accumulation (Table 1).
Figure 4.
Total phenolic compounds and lignin. Total phenolic compounds and lignin concentrations in P. pinaster stem tissues 1, 7, 14, 21 and 28 days post-inoculation (dpi) with the pinewood nematode (PWN) B. xylophilus. Plants were inoculated 14 days following the application of 0.5% acetic acid (without chitosan, non-elicited controls) or a chitosan solution to plants’ substrate. Data represents the difference between the averages of PWN-inoculated and water-inoculated plants ± SE, and different letters indicate statistically different means at P < 0.05.
Relative expression of stress-related genes
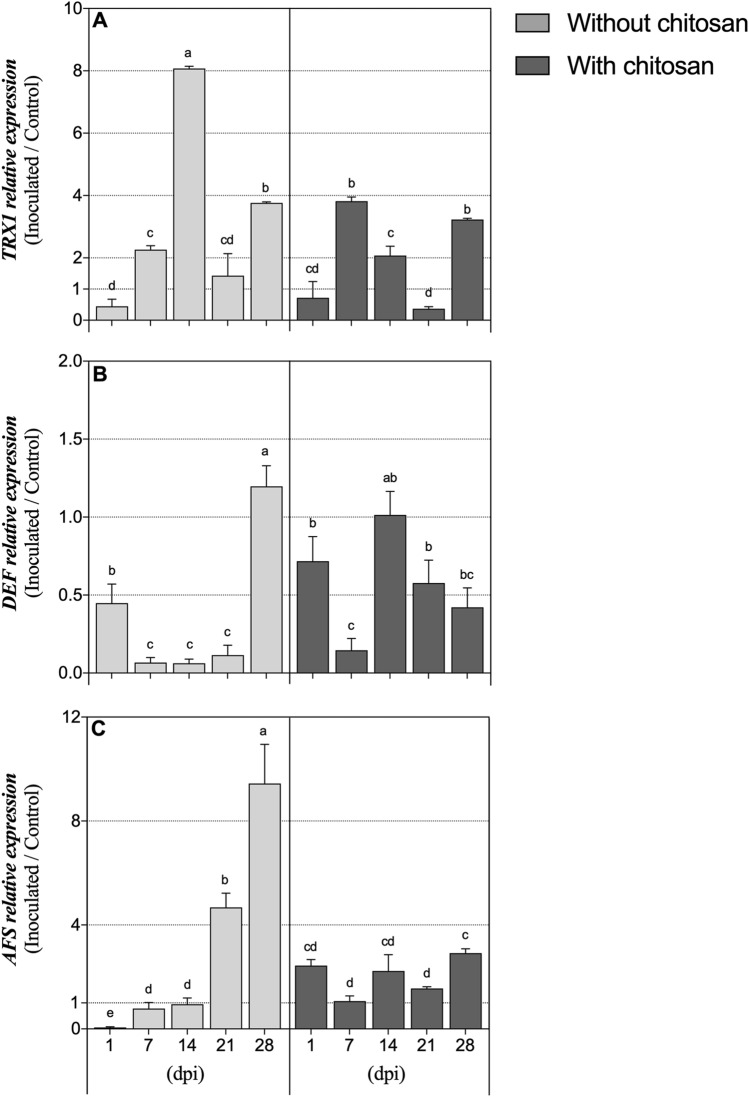
Regarding the expression of TRX1 gene (Fig. 5A), non-elicited PWN-infected plants showed an increase by more than twofold at 7 dpi, which was followed by an eightfold increase at 14 dpi, when compared to 1 dpi. The expression decreased to less than half of the values seen at 14 dpi, and remained at this level until the end of the experiment. For elicited PWN-infected plants, TRX1 relative expression was also significantly increased at 7 dpi, however, it immediately decreased in the following time-points and was generally lower than the expression values in non-elicited plants. Following PWN infection, DEF expression (Fig. 5B) was differently modulated throughout the experiment depending on the chitosan treatment (P < 0.05). In non-elicited plants, from 1 to 7 dpi it was significantly reduced to almost null values and maintained at this level until 21 dpi, until its expression increased again, by about twofold, at 28 dpi. In elicited plants, there was a significant decrease in DEF expression at 7 dpi, but at 14 dpi its expression increased again, remaining unaltered until the end of the experimental period. Chitosan elicitation significantly impacted the expression pattern of AFS (Fig. 5C), with non-elicited PWN-infected plants having a progressive increase in its expression over time, reaching the highest value at 28 dpi (ninefold higher than at 1 dpi). Elicited plants showed constant and relatively low AFS expression throughout the experiment, which was twofold higher in PWN-infected plants than in controls. Chitosan treatment and DPI had a significant effect in the expression of all genes, also having a significant interaction among them (Table 1).
Figure 5.
Stress-related gene expression. Fold expression of genes thioredoxin H1(TRX1) (A), defensin (DEF) (B) and α-farnesene synthase (AFS) (C) in plants inoculated with B. xylophilus relative to their expression in water-inoculated plants, 1, 7, 14, 21 and 28 days post-inoculation (dpi). Plants were inoculated 14 days following the application of 0.5% acetic acid (without chitosan, non-elicited controls) or a chitosan solution to plants’ substrate. Data represents the difference between the averages of PWN-inoculated and water-inoculated plants ± SE, and different letters indicate statistically different means at P < 0.05.
Discussion
When the PWN infects susceptible plant species, there is a primary phase of the disease (early phase), where the nematode population remains low, without producing any visual symptoms. When the second phase develops (advanced stage), PWN population increases exponentially, beginning a more destructive phase for the plant due to nematode feeding and reproduction on the epithelial cells6,7,36. Previous histological and biochemical works on the PWN population dynamics demonstrated that the PWD progresses strongly between 7 and 14 days after infection37,38, which is in accordance to what was observed in the present work in the absence of chitosan elicitation. It has been previously shown that chitosan application prevents nematode multiplication in plant tissues over time24, and reduces the number of juveniles in soil39. The present work seems to attest for the promising role of chitosan elicitation of P. pinaster plants against the PWD, as the nematode population remained at low levels throughout the entire experimental period, indicating that this compound prevented, or, at least, slowed disease progression.
In susceptible Pinus spp. host plants, PWN damages xylem and phloem parenchyma and cortex cells, which can compromise water transport, lead to the peroxidation of the unsaturated lipids of cellular membranes and to ROS formation, culminating in leaf necrosis and often in plant death7,40. MDA, a secondary compound of lipid peroxidation reactions, is frequently used as an indicator of cell damage induced by free radicals41. Interestingly, despite preventing nematode reproduction in plants tissues, chitosan did not prevent cellular damage, leading to increased MDA accumulation. This could be a result of the abrupt induction of plant defence mechanisms upon PWN infection, with consequent formation of ROS42. To counteract the negative effects of intracellular ROS levels, increased or de novo synthesis of several antioxidant enzymes occur. One of those enzymes, catalase, can capture H2O2 and convert it in water and oxygen molecules, which do not have a damaging impact to plant cells43,44. Here, following PWN inoculation, catalase activity was significantly induced throughout the progression of the disease, although more drastically in chitosan-elicited plants, putatively representing the activation of plants’ antioxidant defences. Hydrogen peroxide-mediated oxidative burst has been observed in several plant species supplemented with chitosan, and it is generally acknowledged that it is required for chitosan-induced defence responses25,45. Increased H2O2 content in chitosan-elicited plants in generally accompanied by the accumulation of several antioxidant enzymes, among which catalase and peroxidase seem to be the most important46–48. The application of chitosan may, therefore, lead to a stronger, and possibly more efficient, antioxidant response against PWN infection, which may contribute to an increased tolerance against the pathogen.
Carotenoids and anthocyanins are metabolites involved in plant protection by preventing photosystem damage, participating in signalling, ROS scavenging and inhibiting the growth of noxious microorganisms49–51. It appears that their concentration increased in a similar manner to the PWN population, MDA concentration and catalase activity, as they gradually increased in inoculated plants (as compared to the controls), in both elicited and non-elicited plants. Previous studies have reported that chitosan-elicitation promotes plant defences through increased activity of several defence-related enzymes, e.g. phenylalanine ammonia-lyase, involved in the biosynthesis of phenolic compounds and terpenoids (which include, among several others, carotenoids and anthocyanins)52,53.We hypothesise that increased concentrations of carotenoids and anthocyanins in PWN-infected tissues, especially by the end of the experimental period, may be a coping mechanism to prevent further cellular damage caused by the pathogen54. As observed for catalase, chitosan elicitation seems to promote the accumulation of these metabolites, thus corroborating their potential in improving plants’ antioxidant defences. Along with carotenoids and anthocyanins biosynthesis, phenolics and lignin are also involved in plant resistance to biotic stress15,55,56. In general, total phenolic compounds concentration increased at 14 dpi (with statistical significance in PWN-infected elicited plants), which seems to correspond to the beginning of the advanced stage of the PWD. This is concordant with a previously reported positive relationship found between nematode migration and phenolics concentration57. In the case of lignin, chitosan-elicited plants had higher accumulation than non-elicited ones, which may indicate an intensified tissue lignification after pathogen infection, possibly increasing plant tolerance to the pathogen58. Chitosan also induces a generalized H2O2-mediated hypersensitive response, involved in cell wall lignification processes that confer protection against pathogen-induced mechanical damage30,59. The higher accumulation of carotenoids, anthocyanins, phenolics and lignin observed in the present study, particularly during the later stages of PWN infection and with chitosan elicitation, are in agreement in these previous works, and attest the potential of chitosan elicitation in promoting Pinus spp. defences against the pathogen.
In plants, thioredoxins act as signalling molecules through their reduction-regulatory properties in response to pathogen attacks60,61. In the present work, significant overexpression of TRX1 was observed in non-elicited plants at 14 dpi, corroborating the hypothesis that the advanced stage of the PWD was triggered around this time-point. In PWN-infected elicited plants, TRX1 expression levels were not induced at this time-point, which is coherent with the lower nematode population and increased antioxidative activity previously discussed. Plant defensins, on the other hand, are usually associated with antimicrobial and antifungal activity, constituting the first defensive line against pathogens62,63. It is interesting to note that, here, DEF expression pattern was very distinct between chitosan-elicited and non-elicited plants following PWN infection. It seems that, without chitosan elicitation, plants were not able to activate the expression of this gene as the PWN population progressed, whereas in elicited plants DEF expression was significantly increased along time, possibly contributing to the lower extent of disease progression observed. Also involved in plant defence is α-farnesene (AFS), an herbivore-induced plant volatile, usually increased in plants suffering pathogen attacks, being key in disease resistance18,64. Here, non-elicited plants which showed increased nematode population over time, also displayed higher levels of AFS expression, particularly at 28 dpi, when the highest number of nematodes were registered. Contrastingly, chitosan-elicited plants had lower levels of AFS expression, possibly due to the low nematode progression and multiplication within their tissues.
In general, chitosan increased P. pinaster tolerance to the PWN, resulting in decreased nematode density, increased accumulation of metabolites involved in antioxidant activity, and differential expression of stress-related gene expression. This further supports the potential of chitosan in the prevention and/or treatment of the PWD in Pinus spp., which should be further explored and considered for the development of sustainable disease control strategies.
Methods
Plant maintenance and chitosan elicitation
One-year-old (40–50 cm height) P. pinaster plants were maintained in a growth chamber (Fitoclima 10 000 EHF; Aralab, Rio de Mouro, Portugal) under a 16 h light/8 h darkness photoperiod at 25/18 °C, respectively, and 80% relative humidity. Photon flux density during the day was 380 µmol m−2 s−1. Plants were kept in a commercial substrate (COMPO SANA universal substrate; Compo GmbH, Münster, Germany) composed of (mg L-1): 200–450 N; 200–500 P2O5; 300–550 K2O, pH 5.0–6.5. Plants were watered weekly until field capacity.
An acidic solution (0.5% of acetic acid, pH 6.0) of 4.4% chitosan (molecular weight 327 kDa, deacetylation degree ≥ 75%, Ref.: 448869, Sigma-Aldrich, Missouri, USA) was prepared and left shaking for 24 h before use. A single chitosan treatment was applied to a group of 75 plants by adding 80 mL the chitosan solution to the substrate of each plant. An additional group of 75 plants served as non-elicited control, where a 0.5% solution of acetic acid (no chitosan) was added to the soil.
Nematode culture and plant inoculation
Fourteen days following plant elicitation, a virulent strain of PWN (65 GO, isolated from Góis, Portugal) was inoculated into plant stems. Nematodes were maintained in agar plates with Botrytis cinerea mycelia for 14 days, after which they were extracted from the growing media using the Baermann funnel technic65. Plant inoculation was performed with 2 000 nematodes at ca. 3 cm from the top of each plant as described by Nunes da Silva et al.24.
Experimental design and plant sampling
A total of 150 P. pinaster plants were used in this experiment. Chitosan was applied to a group of 75 plants, in which 25 were mock-inoculated with water (non-inoculated controls) and 50 were inoculated with PWN. An additional group of 75 plants served as non-elicited control, where a 0.5% solution of acetic acid (without chitosan) was added to the substrate: 25 plants were mock-inoculated with water (non-inoculated controls) and 50 were inoculated with PWN. At 1, 7, 14, 21 and 28 days post-inoculation (dpi) five biological replicates of each treatment were sampled. At each time-point, leaves were recovered for the quantification of lipid peroxidation (MDA), catalase, carotenoids and anthocyanins, and stems were used for total soluble phenolics and lignin quantification and gene expression analysis. Five additional PWN-inoculated plants were sampled at the same time-points for whole-stem PWN quantification.
Nematode quantification in plant tissues
In each time-point, five inoculated plants from each treatment were randomly selected for PWN quantification. Leaves were removed, and the entire stem was cut into small portions (ca. 0.5 cm) and used for nematode quantification using the Baermann funnel technique for 24 h at 25 °C. Nematode density in stem tissues was estimated taking into consideration whole stem fresh weight.
Lipid peroxidation and catalase
Quantification of MDA, a sub-product of lipid peroxidation, was performed by mixing vigorously 100 mg of leaf tissue previously homogenized in liquid nitrogen with 10 mL of 0.5% thiobarbituric acid in 20% of trichloroacetic acid and incubated at 100 °C for 30 min. After the incubation period, the reaction was terminated by transferring the tubes into ice. Samples were centrifuged during 10 min at 5 000 g and the supernatant was filtered, after which absorbances were measured at 450, 532 and 600 nm and MDA estimated as described by Yang et al.66.
Quantification of catalase activity was performed following the protocol by Ruley et al.67, adding 1.5 mL of phosphate buffer 1 M to 100 mg of leaf tissue previously homogenized with liquid nitrogen. Samples were vigorously mixed for 2 min, centrifuged at 5 000 g for 10 min at 4 °C and the supernatant was recovered and diluted 3 times with phosphate buffer. Three hundred and forty-four microlitres of a 73 mM H2O2 solution (in 0.5 M Tris/HCl pH 7.0) were then added to 666 μL of sample extract. Enzyme activity was measured for 3 min at 240 nm. One unit of enzyme corresponded to a decrease of 0.001 in absorbance.
Plant secondary metabolites
For carotenoids and anthocyanins quantification, leaves sampled as previously described (100 mg) were homogenized with liquid nitrogen and extracted with 10 mL of cold acetone/Tris buffer solution at 1 M (80:20 v/v, pH 7.8). Samples were incubated at 4 °C for 72 h and absorbances were recorded at 470, 537, 647 and 663 nm. Metabolite concentration was calculated as described by Sims and Gamon68.
Total soluble phenolic compounds were quantified as in Azevedo et al.69. Lyophilized leaf tissues previously homogenized with liquid nitrogen (100 mg) were extracted with 5 mL of methanol for 24 h in the dark at 4 °C. One hundred μL of sample extract were mixed with 5 mL of ultrapure water and 0.5 mL of Folin-Denis reagent, stirred vigorously and incubated at room temperature for 5 min. After incubation, 1.5 mL of sodium carbonate at 35% (w/v) was mixed with each sample and let to react in the dark for 2 h, after which 2.9 mL of ultrapure water were added and absorbances were determined at 760 nm. Soluble phenolic compounds were determined using a quercetin calibration curve.
Lignin was determined by the acetyl bromide method70 using the leaf biomass used for phenolics extraction, which was subjected to sequential 24 h extractions with water, acetone and hexane. Samples were dried at 60 °C for 48 h, and 10 mg of biomass were mixed with 500 µL of glacial acetic acid and 500 µL of acetyl bromide 25%. The mixture was digested at 50 °C for 2 h with vigorous stirring, after which samples were centrifuged for 10 min at 15 000 g. One hundred microlitre of sample were recovered to a new microcentrifuge tube and homogenized with 200 µL of glacial acetic acid, 150 µL of NaOH 0.3 M, 50 µL of hydroxylamine hydrochloride 0.5 M and 500 µL of glacial acetic. Absorbances were recorded at 280 nm and lignin concentration in each sample was determined through a standard calibration curve.
Gene expression analysis
From the five biological replicates from each treatment, three were randomly selected for gene expression analysis. After stem tissue homogenization with liquid nitrogen, RNA extraction was performed using the RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany) following the manufacturer’s protocol. RNA quality and quantity were determined spectrophotometrically and single-strand cDNA was synthesised using iScrip cDNA Synthesis Kit (Bio-Rad Laboratories, Inc, California, USA) following the manufacturer’s instructions. The transcript levels of genes encoding proteins related to oxidative stress (thioredoxin 1, TRX) and plant defence (defensin, DEF; α-farnesene synthase, AFS) were analysed. Primer sequences were retrieved from available published literature18. Reactions of RT-qPCR were carried out in a thermal cycler CFX96 Touch Deep Well Real-Time PCR Detection System using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc, California, USA) and data visualized with the software Bio-Rad CFX Manager 3.1. The amplification protocol cycle was 95 °C for 3 min and 40 cycles at 95 °C for 15 s, 30 s at 54 °C and 71 °C for 30 s. The comparative CT method (ΔΔCT) was used for the relative quantification of gene expression values using the geometric mean of the expression of the control transcripts ubiquitin and 18S ribosomal RNA genes, and the plants inoculated with water (non-inoculated controls) as reference sample71. For each sample and target gene, two technical replicates were analysed.
Statistical analysis
Data were analysed using GraphPad Prism v6.0 software (GraphPad Software, California, USA) and significant differences between chitosan treatments were determined using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test with P = 0.05. Due to the large number of datasets, and to facilitate result viewing and discussion, results concerning MDA, catalase, carotenoids, anthocyanins, phenolic compounds and lignin are presented as the difference between the averages of PWN- and water-inoculated plants. Bars showing positive values represent an increase in PWN-inoculated plants relatively to water-inoculated plants, whereas negative results represent a decrease. Propagation of uncertainty was used to calculate the standard error of each value.
Acknowledgements
This article was supported by the project “POINTERS - Interactions between nematodes and host pine trees: the discovery of sustainable alternatives for the management of the pine wilt disease”, funded by the Competitiveness and Internationalization Operational Program (POCI-01-0145- FEDER-031999) and by Fundação para a Ciência e a Tecnologia under its OE component (PTDC/ASP-SIL/31999/2017). The authors would like to thank Dr. Manuel Mota for providing the nematode strain HF. This work was also supported by National Funds from Fundação para a Ciência e a Tecnologia through the scientific collaboration under the Project UID/Multi/50016/2019, and through A.L.V.’s scholarship funded by the Galician government (Ref.: 530 IN606B).
Author contributions
M.N.S. and C.S.S. designed the experimental setup and M.W.V. coordinated the funding project of this study. M.N.S., C.S.S. and A.C. maintained and inoculated the plants and conducted the analytical procedures. All authors participated in result analysis, critical discussion and writing, and approved the submitted version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Marta Nunes da Silva and Carla S. Santos.
References
- 1.Nunes da Silva M, Solla A, Sampedro L, Zas R, Vasconcelos M. Susceptibility to the pinewood nematode (PWN) of four pine species involved in potential range expansion across Europe. Tree Physiol. 2015;35(9):987–999. doi: 10.1093/treephys/tpv046. [DOI] [PubMed] [Google Scholar]
- 2.Mamiya Y. Pine wilt disease. In: Mota M, Vieira P, editors. Pine Wilt Disease in Japan. New York: Springer; 2004. pp. 9–20. [Google Scholar]
- 3.Yang L, Wang Z, Hou Y, Han R, Sun Y. Effects of Cu2+ on wheat seedlings exposed to enhanced ultraviolet-b radiation. Am. J. Plant Sci. 2014;5:3060–3065. doi: 10.4236/ajps.2014.520322. [DOI] [Google Scholar]
- 4.Shimazu, M. Current status on research and management of pine wilt disease in Japan. Current status on research and management of pine wilt disease, International Symposium, October 20. Korea Forest Research Institute, Seoul, Korea: 1–18 (2006).
- 5.Hirata A, Nakamura K, Nakao K, Kominami Y, Tanaka N, Ohashi H, Takano K, Takeuchi W, Matsui T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE. 2017;12:e0182837. doi: 10.1371/journal.pone.0182837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroda K. Pine wilt disease. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Physiological Incidences Related to Symptom Development and Wilting Mechanism. New York: Springer; 2008. pp. 204–222. [Google Scholar]
- 7.Yamada T. Pine wilt disease. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Biochemical Responses in Pine Trees Affected by Pine Wilt Disease. New York: Springer; 2008. pp. 223–234. [Google Scholar]
- 8.Zhang W, Zhao L, Zhou J, Yu H, Zhang C, Lv Y, Lin Z, Hu S, Zou Z, Sun J. Enhancement of oxidative stress contributes to increased pathogenicity of the invasive pine wood nematode. Philos. Trans. R. Soc. Lond. B. 2019;374:20180323. doi: 10.1098/rstb.2018.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and toichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 10.Treutter D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006;4:147–157. doi: 10.1007/s10311-006-0068-8. [DOI] [Google Scholar]
- 11.Liu Q, Luo L, Zheng L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018;19(2):335. doi: 10.3390/ijms19020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamiya Y. Inoculation of the first year pine (Pinus densiflora) seedlings with Bursaphelenchus lignicolus and the histopathology of diseased seedlings. J. Jap. For. Soc. 1980;62:176–183. [Google Scholar]
- 13.Myers RF. Cambium destruction in conifers caused by pinewood nematodes. J. Nematol. 1986;18:398–402. [PMC free article] [PubMed] [Google Scholar]
- 14.Futai K. Abnormal metabolites in pine wood nematode-inoculated Japanese black pine. Jap. J. Nematol. 2003;33:45–56. doi: 10.3725/jjn1993.33.2_45. [DOI] [Google Scholar]
- 15.Kawaguchi E. Relationship between the anatomical characteristics of cortical resin canals and migration of Bursaphelenchus xylophilus in stem cuttings of Pinus thunbergii seedlings. J. Jap. Soc. Hort. Sci. 2006;88:240–244. doi: 10.4005/jjfs.88.240. [DOI] [Google Scholar]
- 16.Lee IH, Han H, Koh YH, Kim IS, Lee SW, Shim D. Comparative transcriptome analysis of Pinus densiflora following inoculation with pathogenic (Bursaphelenchus xylophilus) or non-pathogenic nematodes (B. thailandae) Sci. Rep. 2019;9:12180. doi: 10.1038/s41598-019-48660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffer R, Gorg R, Jarosch B, Beckhove U, Bahrenberg G, Kogel KH, Schulze-Lefert P. Tissue dependence and differential cordycepin sensitivity of race-specific resistance responses in the barley-powdery mildew interaction. Mol. Plant-Microbe Interact. 1997;10:830–839. doi: 10.1094/MPMI.1997.10.7.830. [DOI] [Google Scholar]
- 18.Santos CS, Pinheiro M, Silva A, Egas C, Vasconcelos MW. Searching for resistance genes to Bursaphelenchus xylophilus using high throughput screening. BMC Genom. 2012;13:599. doi: 10.1186/1471-2164-13-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamata N. Pine wilt disease. In: Zhao BG, Futai K, Sutherland JR, Takeuchi Y, editors. Integrated Pest Management of Pine Wilt Disease in Japan: Tactics and Strategies. New York: Springer; 2008. pp. 304–322. [Google Scholar]
- 20.Tikhonov VE, Stepnova EA, Babak VG, Yamskov IA, Palma-Guerrero J, Jansson HB. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/- derivatives. Carbohydr. Polym. 2006;64:66–72. doi: 10.1016/j.carbpol.2005.10.021. [DOI] [Google Scholar]
- 21.Rabea EI, Steurbaut W. Chemically modified chitosans as antimicrobial agents against some plant pathogenic bacteria and fungi. Plant Protect. Sci. 2010;4:149–158. doi: 10.17221/9/2009-PPS. [DOI] [Google Scholar]
- 22.Trotel-Aziz P, Couderchet M, Vernet G, Aziz A. Chitosan stimulates defense reactions in grape vine leaves and inhibits development of Botrytis cinerea. Eur. J. Plant Pathol. 2006;114:405–413. doi: 10.1007/s10658-006-0005-5. [DOI] [Google Scholar]
- 23.Khalil MS, Badawy MEI. Nematicidal activity of a biopolymer chitosan at different molecular weights against root-knot nematode Meloidogyne incognita. Plant Protect. Sci. 2012;48(4):170–178. doi: 10.17221/46/2011-PPS. [DOI] [Google Scholar]
- 24.Nunes da Silva M, Cardoso AR, Ferreira D, Brito M, Pintado ME, Vasconcelos MW. Chitosan as a biocontrol agent against the pinewood nematode (Bursaphelenchus xylophilus) For. Pathol. 2014;44(5):420–423. doi: 10.1111/efp.12136. [DOI] [Google Scholar]
- 25.Mukhtar Ahmed KB, Khan MM, Siddiqui H, Jahan A. Chitosan and its oligosaccharides, a promising option for sustainable crop production: A review. Carbohydr. Polym. 2020;227:115331. doi: 10.1016/j.carbpol.2019.115331. [DOI] [PubMed] [Google Scholar]
- 26.Pirbalouti AG, Malekpoor F, Salimi A, Golparvar A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017;217:114–122. doi: 10.1016/j.scienta.2017.01.031. [DOI] [Google Scholar]
- 27.Rahman M, Mukta JA, Sabir AA, Gupta DR, Mohi-Ud-Din M, Hasanuzzaman M, Islam MT. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE. 2018;13(9):e0203769. doi: 10.1371/journal.pone.0203769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehregan M, Mehrafarin A, Labbafi MR, Naghdi Badi H. Effect of different concentrations of chitosan biostimulant on biochemical and morphophysiological traits of stevia plant (Stevia rebaudiana Bertoni) J. Med. Plants Res. 2017;2(62):169–181. [Google Scholar]
- 29.Cai Z, Kastell A, Mewis I, Knorr D, Smetanska I. Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. 2011;108:401–409. doi: 10.1007/s11240-011-0051-3. [DOI] [Google Scholar]
- 30.Hawrylak-Nowak B, Dresler S, Rubinowska K, Matraszek-Gawron R. Eliciting effect of foliar application of chitosan lactate on the phytochemical properties of Ocimum basilicum. L and Melissa officinalis L. Food Chem. 2020;342:128358. doi: 10.1016/j.foodchem.2020.128358. [DOI] [PubMed] [Google Scholar]
- 31.Singh RK, Martins V, Soares B, Castro I, Falco V. Chitosan application in vineyards (Vitis vinifera L. cv. Tinto Cão) induces accumulation of anthocyanins and other phenolics in berries, mediated by modifications in the transcription of secondary metabolism genes. Int. J. Mol. Sci. 2020;21(1):306. doi: 10.3390/ijms21010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zong H, Liu S, Xing R, Chen X, Li P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017;138:271–278. doi: 10.1016/j.ecoenv.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Badawy MEI, Rabea EI. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011;2011:460381. doi: 10.1155/2011/460381. [DOI] [Google Scholar]
- 34.Rendina N, Nuzzaci M, Scopa A, Cuypers A, Sofo A. Chitosan-elicited defense responses in Cucumber mosaic virus (CMV)-infected tomato plants. J. Plant Pysiol. 2019;234–235:9–17. doi: 10.1016/j.jplph.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Nunes da Silva M, Pintado M, Sarmento B, Stamford NP, Vasconcelos MW. A biofertilizer with diazotrophic bacteria and a filamentous fungus increases Pinus pinaster tolerance to the pinewood nematode (Bursaphelenchus xylophilus) Biol. Control. 2019;132:72–80. doi: 10.1016/j.biocontrol.2019.01.013. [DOI] [Google Scholar]
- 36.Myers RF. Pathogenesis in pine wilt caused by pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 1988;20:236–244. [PMC free article] [PubMed] [Google Scholar]
- 37.Son J, Komatsu M, Matsushita N, Hogetsu T. Migration of pine wood nematodes in the tissues of Pinus thunbergii. J. For. Res. 2009;15(3):186–193. doi: 10.1007/s10310-009-0171-3. [DOI] [Google Scholar]
- 38.Trindade, C.M.P.P. Avaliação da expressão de genes relacionados com a susceptibilidade a Bursaphelenchus xylophilus, agente causal da doença da murchidão dos pinheiros (pine wilt disease) em Pinus pinaster Ait e Pinus yunannensis Franch. Master’s thesis in Celular and Molecular Biotechnology, University of Lisbon, Faculty of Sciences (Lisbon, Portugal, 2012).
- 39.El-Sayed SM, Mahdy ME. Effect of chitosan on root-knot nematode, Meloidogyne javanica on tomato plants. Int. J. Chem. Tech. Res. 2015;7(4):1985–1992. [Google Scholar]
- 40.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 41.Santos CS, Ozgur R, Uzilday B, Turkan I, Roriz M, Rangel AO, Carvalho SM, Vasconcelos M. Understanding the role of the antioxidant system and the tetrapyrrole cycle in iron deficiency chlorosis. Plants. 2019;8(9):348. doi: 10.3390/plants8090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal GK, Rakwal R, Tamogami S, Yonekurad M, Kubo A, Saji H. Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol. Biochem. 2002;40:1061–1069. doi: 10.1016/S0981-9428(02)01471-7. [DOI] [Google Scholar]
- 43.Tavares JTQ, Silva CL, Carvalho LA, Silva MA, Santos CMG. Estabilidade do ácido ascórbico em suco de laranja submetido a diferentes tratamentos. Magistra. 2000;12:1–2. [Google Scholar]
- 44.Barreiros LBS, David JM, David JP. Estresse oxidativo: relação entre geração de espécies reativas e defesas do organismo. Quím. Nova. 2006;29:113–123. doi: 10.1590/S0100-40422006000100021. [DOI] [Google Scholar]
- 45.Zhao T, Wang J, Wang Y, Sun JW, Cao Y. Effects of reactive oxygen species metabolic system on soybean (Glycine max) under exogenous chitosan to ozone stress. Bull. Environ. Contam. Toxicol. 2010;85:59–63. doi: 10.1007/s00128-010-0039-4. [DOI] [PubMed] [Google Scholar]
- 46.Ferri M, Tassoni A, Franceschetti M, Righetti L, Naldrett MJ, Bagni N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics. 2009;9:610–624. doi: 10.1002/pmic.200800386. [DOI] [PubMed] [Google Scholar]
- 47.Povero G, Loreti E, Pucciariello C, Santaniello A, Tommaso DD, Tommaso GD, Kapetis D, Zolezzi F, Piaggesi A, Perata P. Transcript profiling of chitosan-treated Arabidopsis seedlings. J. Plant Res. 2010;124:619–629. doi: 10.1007/s10265-010-0399-1. [DOI] [PubMed] [Google Scholar]
- 48.Pongprayoon W, Roytrakul S, Pichayangkura R, Chadchawan S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L) Plant Growth Regul. 2013;70:159–173. doi: 10.1007/s10725-013-9789-4. [DOI] [Google Scholar]
- 49.Schaefer HM, Rentzsch M, Breuer M. Anthocyanins reduce fungal growth in fruits. Nat. Prod. Commun. 2008;3(8):1267–1272. [Google Scholar]
- 50.Nishino A, Yasui H, Maoka T. Reaction of paprika carotenoids, capsanthin and capsrubin, with reactive oxygen species. J. Agric. Food Chem. 2016;64:4786–4792. doi: 10.1021/acs.jafc.6b01706. [DOI] [PubMed] [Google Scholar]
- 51.Elkhouni A, Rabhi M, Ivanov AG, Krol M, Zorrig W, Smaoui A, Abdelly C, Huner NPA. Structural and functional integrity of Sulla carnosa photosynthetic apparatus under iron deficiency conditions. Plant Biol. 2018;20:415–425. doi: 10.1111/plb.12684. [DOI] [PubMed] [Google Scholar]
- 52.Kim HJ, Chen F, Wang X, Rajapakse NC. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.) J. Agric. Food Chem. 2005;53(9):3696–3701. doi: 10.1021/jf0480804. [DOI] [PubMed] [Google Scholar]
- 53.Pichyangkura R, Chadchawan S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015;196:49–65. doi: 10.1016/j.scienta.2015.09.031. [DOI] [Google Scholar]
- 54.Nakajima J, Tanaka I, Seo S, Yamazaki M, Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J. Biomed. Biotechnol. 2004;2004(5):241–247. doi: 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuroda H, Goto S, Kazumi E, Kuroda K. The expressed genes of Japanese red pine (Pinus densiflora) involved in the pine wilt disease severity. BMC Proc. 2011;5(7):92. doi: 10.1186/1753-6561-5-S7-P92. [DOI] [Google Scholar]
- 56.Gaspar D, Trindade C, Usié A, Meireles B, Barbosa P, Fortes AM, Pesquita C, Costa RL. Expression profiling in Pinus pinaster in response to infection with the pine wood nematode Bursaphelenchus xylophilus. Forest. 2017;8(8):279. doi: 10.3390/f8080279. [DOI] [Google Scholar]
- 57.Zas R, Moreira X, Ramos M, Lima M, Silva MN, Solla A, Vasconcelos M, Sampedro L. Intraspecific variation of anatomical and chemical defensive traits in Maritime pine (Pinus pinaster) as factors in susceptibility to the pinewood nematode (Bursaphelenchus xylophilus) Trees. 2014;29:663–673. doi: 10.1007/s00468-014-1143-6. [DOI] [Google Scholar]
- 58.Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W. Genome-wide characterization of the lignification toolbox in arabidopsis. Plant Physiol. 2003;133(3):1051–1071. doi: 10.1104/pp.103.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucini L, Baccolo G, Rouphael Y, Colla G, Bavaresco L, Trevisan M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochem. 2018;156:1–8. doi: 10.1016/j.phytochem.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Jacquot JP, Lancelin JM, Meyer Y. Thioredoxins: Structure and function in plant cells. New Phytol. 1997;136:543–570. doi: 10.1046/j.1469-8137.1997.00784.x. [DOI] [PubMed] [Google Scholar]
- 61.Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld J. The arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 2004;134:1006–1016. doi: 10.1104/pp.103.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kovaleva V, Krynytskyy H, Gout I, Gout R. Recombinant expression, affinity purification and functional characterization of Scots pine defensin 1. Appl. Microbiol. Biotechnol. 2011;89:1093–1101. doi: 10.1007/s00253-010-2935-2. [DOI] [PubMed] [Google Scholar]
- 63.Zamany A, Liu JJ, Ekramoddoullah A, Sniezko R. Antifungal activity of a Pinus monticola antimicrobial peptide 1 (Pm-AMP1) and its accumulation in western white pine infected with Cronartium ribicola. Can. J. Microbiol. 2011;57:667–679. doi: 10.1139/w11-046. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Zeng L, Liao Y, Li J, Tang J, Yang Z. Formation of α-farnesene in tea (Camellia sinensis) leaves induced by herbivore-derived wounding and its effect on neighbouring tea plants. Int. J. Mol. Sci. 2019;20:4151. doi: 10.3390/ijms20174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baermann G. Eine einfache methode zur auffindung von Anchylostomum-(Nematoden)-larven in erdproben. Geneeskundig Tijdschrift Nederlands Indië. 1917;57:131–133. [Google Scholar]
- 66.Yang B. Pine wilt disease. In: Mota M, Vieira P, editors. The history, dispersal and potential threat of pine wood nematode in China. New York: Springer; 2004. pp. 21–24. [Google Scholar]
- 67.Ruley AT, Sharma NC, Sahi SV. Antioxidant defence in a lead accumulating plant, Sesbania drummondii. Plant Physiol. 2004;42:899–906. doi: 10.1016/j.plaphy.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Sims DA, Gamon JA. Relationships between leaf pigment content and sprectral reflectance across a wide range of species, lead structures and development stages. Remote Sens. Environ. 2002;81:337–354. doi: 10.1016/S0034-4257(02)00010-X. [DOI] [Google Scholar]
- 69.Azevedo, H.A.Q.P. Contributions to the study of the Pinus pinaster-Botrytis cinerea interaction. Ph.D. dissertation in Biology, Minho University (Braga, Portugal, 2005).
- 70.Fukushima RS, Hatfield RD. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agric. Food Chem. 2001;49(7):3133–3139. doi: 10.1021/jf010449r. [DOI] [PubMed] [Google Scholar]
- 71.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]