Abstract
Background:
Prenatal depression has lasting effects on offspring development, including later mental illness risk. Maternal responses to depression include inflammation and HPA-axis stimulation. Effects on development of cerebral inhibitory neurocircuits may differ for female and male fetuses.
Methods:
Mothers (N = 181) were assessed periodically, beginning at 16 weeks gestation, on the Center for Epidemiological Studies of Depression Scale (CESD). Maternal prenatal C-reactive protein (CRP) and hair cortisol and cortisone levels were determined. Cortisone was determined in neonatal hair. Development of cerebral inhibitory neurocircuits was assessed in 162 1-month-old newborns by inhibition of P50 electrophysiological responses to repeated sounds.
Results:
Maternal depression was associated with decreased newborn P50 inhibition in both sexes. Maternal CRP levels were significantly associated with depression only in male-fetus pregnancies and with decreased newborn P50 inhibition only in males. Maternal cortisol levels were significantly associated with depression only in female-fetus pregnancies and with decreased newborn P50 inhibition only in females. In male-fetus pregnancies compared to female-fetus pregnancies, cortisol was more robustly metabolized to cortisone, which does not activate cortisol receptors.
Conclusion:
The study finds sex-specific associations of CRP and cortisol levels with prenatal depression in women and with decreased development of newborn P50 inhibition. Sex-based differences in maternal response to depression with inflammation or cortisol and their developmental effects may reflect evolutionary influences to promote survival in adversity. Decreased newborn P50 inhibition is associated with later childhood behavioral problems, and decreased P50 inhibition is a pathophysiological feature of several mental illnesses.
Keywords: Depression, Pregnancy, Fetus, Hydroxycortisone, C-reactive protein, Inflammation
Introduction
Prenatal maternal depression’s effects are implicated in the child’s future risk for schizophrenia, attention deficit disorder, autism spectrum disorder, and depression, adding to familial genetic risk (1-4). Cognitive and behavioral effects, as well as brain structural effects, have been characterized in the years after birth (5-11). This study investigated the association of prenatal depression, together with its associated maternal inflammation and cortisol secretion, on the development of a specific inhibitory brain function that is assessed in newborns.
Animal models of prenatal inflammation and cortisol secretion find effects on the development of the hippocampus, neocortex, amygdala, and particularly inhibitory interneurons (12-16). Many of these interneurons develop in human fetuses between 12-20 weeks in gestation (17-18). Optogenetic inactivation of limbic neocortical interneurons decreases the animal’s social interactions and its attention (19). The hippocampus is a source for the P50 wave of the human cerebral auditory-evoked response (20). Its analogue in rodent models is generated by hippocampal pyramidal neurons, and its inhibition in response to repeated sounds involves activation of hippocampal inhibitory interneurons (21). Inhibition of the P50 response can be recorded in 1 month-old-newborns and adds to other phenotypes that are assessed before or soon after birth, to gauge prenatal effects on central nervous system development (5-6, 22-29).
The present cohort was recruited from prenatal clinic admissions in a metropolitan community hospital that serves disadvantaged women for a study designed to examine a wide range of prenatal influences on fetal brain development of cerebral inhibition and early childhood behavior. The study included infection, substance use, adversity, depression, and stress as factors to be assessed (Table 1). Depression is associated with both inflammation and increased cortisol (30). CRP is a marker of the mother’s hepatic response to inflammation from a range of causes including depression (31). Cortisol levels have been measured in saliva, plasma, and maternal hair during pregnancy (23,32-33). We reported in another cohort that maternal hair cortisol levels mediate the relationship between prenatal stress and premature delivery (33). In the placenta, 11-β-hydroxysteroid-dehydrogenase-2 (11BHSD2) irreversibly converts cortisol to cortisone, which does not activate cortisol receptors (34). Male placentas have higher 11BHSD2 (35-36). We measured cortisone levels in neonatal hair, to assess cortisol conversion to cortisone during gestation (37).
Table 1.
Differences between mothers and offspring by maternal depression rating.
| Center for Epidemiological Studies of Depression (CESD) 16 weeks gestation |
CESD≥16 N = 61 |
CESD<16 N =101 |
Significance | ||
|---|---|---|---|---|---|
| N | % | N | % | Fishers P | |
| Infection in first trimester, viral or bacterial brought to medical attention or endorsed by mother as moderate to severe | 31 | 51 | 35 | 35 | 0.049 |
| Obesity pre-pregnancy BMI≥30 | 19 | 31 | 28 | 28 | 0.7 |
| Alcohol use at 16 wks. gestation | 16 | 25 | 0 | 0 | <0.001 |
| Marijuana use at 16 wks. gestation | 14 | 23 | 11 | 11 | 0.046 |
| Tobacco use at 16 wks. Gestation | 7 | 11 | 5 | 5 | 0.13 |
| Biological father present | 39 | 64 | 80 | 80 | 0.04 |
| European-American | 42 | 33 | 84 | 67 | |
| African-American | 12 | 55 | 10 | 45 | 0.091 |
| Native-American | 8 | 57 | 6 | 43 | 0.091 |
| Hispanic | 34 | 56 | 48 | 48 | 0.3 |
| Antidepressant use | 11 | 18 | 11 | 11 | 0.24 |
| Fetal Sex (male) | 34 | 56 | 47 | 47 | 0.3 |
| Mean | SD | Mean | SD | t test P | |
| Maternal age yr | 29.0 | 6.4 | 30.3 | 5.6 | 0.2 |
| Adverse Childhood Experiences (ACE) | 3.42 | 2.70 | 2.07 | 2.05 | 0.003 |
| Perceived Stress Scale at | 29.4 | 5.9 | 20.2 | 7.1 | <0.001 |
| 16 wks gestation | |||||
| State Trait-Anxiety Inventory-State at 16 wks gestation | 43.0 | 10.9 | 31.3 | 8.0 | <0.001 |
| Male fetus | N = 34 | N = 47 | |||
| Gestational age at birth d | 273.8 | 13.5 | 273.8 | 17.5 | 0.9 |
| Birth weight g | 3260 | 585 | 3272 | 591 | 0.9 |
| Breast feeding N (%) | 29 | 88% | 44 | 94% | 0.7 |
| Maternal C-reactive protein (CRP) 16 weeks gestation | 10.1 | 8.0 | 6.2 | 5.8 | 0.016 |
| Maternal Cortisol log 2nd Trimester log pg/mg hair | 0.86 | 0.39 | 0.88 | 0.35 | 0.7 |
| Maternal Cortisone 2nd Trimester pg/mg hair | 14.7 | 6.0 | 28.4 | 39.5 | 0.2 |
| Neonatal Cortisol log pg/mg hair at birth | 2.04 | 0.38 | 2.15 | 0.49 | 0.3 |
| Neonatal Cortisone pg/mg hair at birth | 73.5 | 49.2 | 133.0 | 85.8 | 0.045 |
| P50S2μV adjusted for P50S1μV 1 month of age | 1.10 | 0.08 | 0.70 | 0.07 | <0.001 |
| Female fetus | N = 27 | N = 54 | |||
| Gestational age at birth d | 270.5 | 12.1 | 271.6 | 21.3 | 0.8 |
| Birth weight g | 3004 | 483 | 3083 | 675 | 0.6 |
| Breast feeding N (%) | 25 | 93% | 48 | 89% | 0.7 |
| Maternal C-reactive protein (CRP) 16 weeks gestation | 9.3 | 7.2 | 10.1 | 8.4 | 0.7 |
| Maternal Cortisol | 1.06 | 0.58 | 0.81 | 0.47 | 0.045 |
| 2nd Trimester log pg/mg hair | |||||
| Maternal Cortisone 2nd Trimester pg/mg hair | 18.5 | 9.8 | 10.4 | 5.2 | 0.007 |
| Neonatal Cortisol log pg/mg hair at birth | 2.11 | 0.28 | 2.15 | 0.34 | 0.6 |
| Neonatal Cortisone pg/mg hair at birth | 65.3 | 17.9 | 95.3 | 48.9 | 0.08 |
| P50S2μV adjusted for P50S1μV 1 month of age | 0.93 | 0.09 | 0.71 | 0.06 | 0.04 |
African-Americans and Native-Americans are compared with European Americans. Ethnicity and race are self reported.
We have previously shown that both infection and cannabis use decrease development of P50 inhibition in this cohort and that increased maternal CRP from infection in gestation is associated with decreased newborn P50 inhibition in male, but not female newborns (38-40). This is the first report from this cohort to characterize the interaction of maternal depression with fetal sex on both CRP and cortisol levels and the first to characterize the interaction of depression and cortisol levels with fetal sex on newborn P50 inhibition.
The interaction of prenatal depression with fetal sex on maternal CRP and cortisol levels has not been previously reported. A large survey of pregnant women reported that cortisol levels were higher in pregnancies with female fetuses and that CESD rating had no effect, but the interaction between depression and fetal sex was not assessed (41). Another report also found higher basal cortisol response and arousal in women carrying female fetuses, but that study did not address depression (42). Other studies of prenatal depression and CRP levels did not examine the effect of fetal sex and found inconsistent effects (43-44). Asthma and its corticosteroid treatment is a potential model for the interaction of fetal sex, cortisol, and inflammation. Placentas of female fetuses, but not male fetuses, of women who use corticosteroids for asthma during pregnancy had cytokine levels inversely correlated with cord blood cortisol levels (45). A study in depressed women sampling plasma cortisol and cytokines between 16 and 26 weeks gestation reported a similar inverse relationship, but did not assess effects of fetal sex (32). We hypothesized that maternal depression and fetal sex would interact, with higher cortisol levels in female-fetus pregnancies and lower CRP levels reflecting decreased inflammation. The opposite, lower cortisol levels and higher CRP levels were hypothesized in male-fetus pregnancies.
We hypothesized that decreased newborn P50 inhibition would be associated with maternal depression, based on P50 inhibition association with other maternal insults (38-40). We hypothesized 16 weeks gestation to be the critical time period, based on the embryology of inhibitory interneurons responsible for P50 inhibition in this gestational window (17-18). Animal model studies report that hippocampal neurons are more responsive to gestational inflammation in males and to glucocorticoids in females (13-14). Human studies have found greater effects of prenatal inflammation in male offspring and greater effects of maternal cortisol in female offspring (23,26,29,46-48), but other studies have not reported sex effects (49-50). Based on these studies, we hypothesized that male newborn P50 inhibition would be more sensitive to maternal CRP elevation, and that female newborn P50 inhibition would be more sensitive to maternal cortisol elevation.
Methods and Materials
1. Maternal assessment and recruitment
Women (N = 181) were enrolled from a public safety-net prenatal clinic at 14-16 weeks gestation from July 2013 until July 2016. Gestational age at enrollment was assessed by first ultrasound (51). Exclusions were fetal anomaly, major maternal medical morbidity, and steroid therapy. The Colorado Multiple Institution Review Board approved the study; all mothers, and fathers if available, gave informed consent. Participants were asked to participate in a study of stress on their child’s development.
Self-ratings on Adverse Childhood Experiences (ACE), Center for Epidemiological Studies of Depression (CESD), State-Trait Anxiety Inventory-State Version (STAI-S), and the Perceived Stress Scale (PSS) were acquired in structured interviews by trained staff. The CESD is particularly sensitive across multiple socio-economic, ethnic and racial groups, compared to other depression scales (52-54). The 181 women at 16 weeks (range 15-17 weeks) had mean CESD ratings 13.9 (SD 9.5); 19 dropped out within several weeks of enrollment. Mothers who brought their newborns for newborn P50 recording, N = 162, had mean CESD 13.9 (SD 9.3). The most common reason for dropout was the mother’s move to another city (Figure S1). Further subject assessments are in the supplement.
2. Maternal and newborn hair cortisol and cortisone
Hair (2.5 cm) was cut from the posterior vertex region as close to the scalp as possible at 28 weeks, which integrates levels from 15-28 weeks of gestation, encompassing the 16-week CESD rating (55). Excessively dyed or shampooed hair was not used (56). The correlation between maternal hair cortisol levels and the Area under the Curve for salivary cortisol, sampled 30 min before waking, before lunch, and 10 hours after waking for 3 days in the 2nd trimester is r2 = 0.34, P = 0.04 (57). Hair samples were obtained from 141 neonates. Neonatal hair at birth is formed during the last trimester and measures fetal production of cortisone (37). Cortisone levels were available from only 75 infants for technical reasons. The mass spectrometry-based assay is described in the supplement.
3. Maternal CRP
Plasma CRP at 16 weeks gestation (range 15-17 weeks) was obtained mid-morning and assayed by Beckman-Coulter high sensitivity immunoassays. The lower level of detection was 0.1 mg/L with CV<5%.
4. Neonatal physiological recording of cerebral inhibition
Newborns were studied one month after birth adjusted for gestational age. The mean age was 28.0 days (SD 9.7), range 14-59. Vertex electroencephalogram, electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants napped (22). Recording of the cerebral auditory evoked potential P50, a positive EEG wave 50ms post-stimulus, occurred in the second active sleep episode, the precursor of REM sleep, identified by low voltage desynchronized vertex activity with the absence of K-complexes, change in respiration, and large eye movements with submental atonia (58). The second active sleep episode was reached approximately 45 minutes after sleep onset. In adults, P50 inhibition in REM and waking are equivalent (59).
Two identical binaural auditory stimuli SPL 85dB were delivered 500 ms apart at 10s intervals to elicit P50S1 and P50S2. Responses to 96 stimulus pairs were averaged, using a Gaussian-based algorithm that accounts for variance or jitter in response latency (60). P50 inhibition is often assessed as amplitude ratios P50S2/P50S1 or (P50S1-P50S2)/P50S1 (61). However, the skew inherent in ratios limits power for correlation with risk factors. P50S2 amplitude, covaried for P50S1, which is normally distributed, was therefore used as the parameter to assess cerebral inhibition, as validated in a recent meta-analysis (62). Detailed methods have been previously reported (63-64).
5. Statistical analyses
Neonatal P50S2 amplitude covaried for P50S1μV was the principal outcome. Shapiro-Wilks tests did not find a significant deviation from normal distribution. Hair cortisol levels were skewed and therefore were log10-transformed. General Linear Models and multiple regressions analyzed child sex as a fixed effect and CESD, cortisol, cortisone, and CRP levels as continuous covariates (Table S1). Maternal age was used as a covariate for CESD ratings, CRP, and cortisol levels, to detect confounding maternal socioeconomic influences, including education and occupation, which are correlated with maternal age (38). Gestational age at birth and at newborn P50 recording were covariates to control for maturation differences affecting P50 amplitudes. Previously reported CRP associations were re-analyzed to use the same covariates as other analyses in this study. Spearman’s ρ was used for non-parametric correlations. α = 0.05 two-tailed was the measure of significance.
Results
CESD 16-week depression ratings were positively correlated with lifetime history of DSM-5 Major Depressive Disorder (ρ = 0.289, P < 0.001) and Bipolar Disorder (ρ = 0.192, P = 0.014). CESD ratings ≥ 16 are consistent with DSM-5 Major Depressive Disorder and were recorded for 38% of the women. In comparison, 36% of child-bearing age women in a survey of Los Angeles County had CESD ratings ≥ 16 (65-66). Women with CESD ratings ≥16 differed from less depressed women in their rates of infection, substance use, single parenthood, Adverse Childhood Experiences, stress, and anxiety (Table 1). There was no difference in CESD ratings between pregnancies with female and male fetuses (Table S1).
Child sex, maternal CESD depression ratings, and newborn P50S2μV
Higher maternal 16-week CESD ratings were positively associated with higher newborn P50S2μV (Fdf1,111 = 6.636, P = .011; β = 0.237; Table S2). Maternal PSS stress and STAI-S anxiety ratings were not significant in this analysis. Only the 16-week CESD rating was significant when considered with CESD ratings later in gestation, including CESD rating at 46 weeks gestation (postpartum) (Table 2). No differences noted in Table 1 between women based on their CESD rating were significant when considered with CESD ratings, including gestational infection, use of alcohol, cannabis, or nicotine, obesity (pre-pregnancy BMI ≥ 30), absence of father, number of Adverse Childhood Experiences, or African American and Native American ethnicity (Table S3). There was no interaction between child sex and the association of CESD rating on newborn P50S2μV (Fig. 1; Table S4).
Table 2.
Correlations between CESD Depression ratings, newborn P50 parameters, and maternal CRP and cortisol
| CESD Depression Scale 16 wks |
Newborn P50S2μ adjusted for P50S1μ |
Newborn P50S2μ adjusted for P50S1μ Correlation controlled for CESD 16 wks |
Maternal C- reactive Protein, male fetus, mg/L 16 wks |
Maternal hair cortisol log pg/mg, female fetus 2nd trimester |
|
|---|---|---|---|---|---|
| Prenatal | |||||
| CESD 16 wks | -- | 0.301** | -- | 0.363** | 0.293** |
| STAI-S 16 wks | 0.214** | 0.161* | −0.022 | −0.014 | 0.264** |
| PSS 16 wks | 0.212** | 0.192* | −0.020 | 0.230* | 0.348** |
| CESD 22 wks | 0.695** | 0.224** | −0.060 | 0.294* | 0.061 |
| CESD 28 wks | 0.660** | 0.172* | −0.042 | 0.074 | 0.189 |
| CESD 34 wks | 0.648** | 0.175* | −0.055 | 0.288* | 0.189 |
| CESD 40 wks | 0.585** | 0.192* | −0.025 | 0.111 | 0.049 |
| Postnatal | |||||
| CESD 46 wks | 0.524** | 0.157* | −0.033 | 0.012 | 0.143 |
Pearson’s r
P<0.05
P<0.01
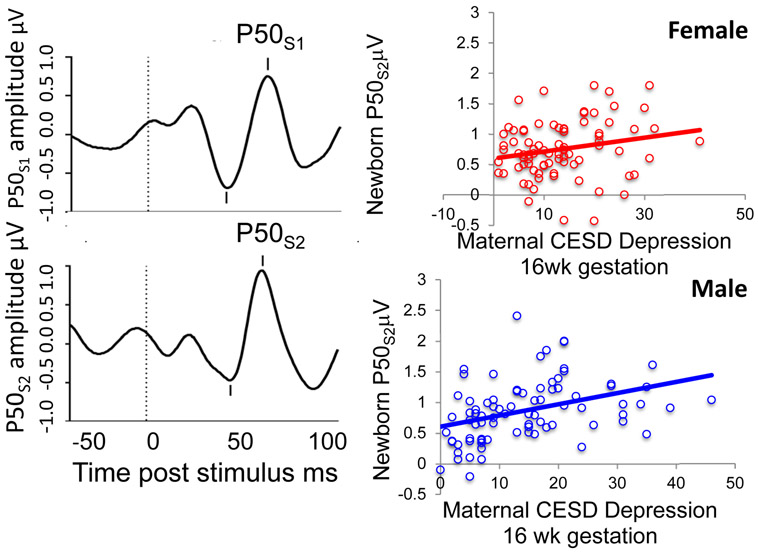
Figure 1.
Left: P50 evoked potentials of an individual 1-month-old female recorded from the scalp vertex in response to paired sounds, 500 ms apart. This child was born full term to a 23-year-old woman with CESD = 18. Maternal 2nd trimester hair cortisol was 34.2pg/mg, the upper 12th%ile for women with female fetuses. Plasma C-Reactive Protein was 12.2mg/L. P50 amplitude (μV) is measured from the most positive peak (mark above wave) to the preceding negativity (mark below wave). The waves are the averaged responses to 96 pairs of stimuli delivered during the newborn’s active sleep. This newborn had little suppression of P50S2μV, compared to P50S1μV. Her P50S2μV adjusted for P50S1μV is 1.35μV, the upper 14th%tile of female newborn amplitudes. Right plots: Significant positive associations for all male and female newborns of maternal depression CESD rating with newborn P50S2μV adjusted for P50S1μV, female β = 0.211, P = 0.021, male β = 0.316, P < 0.001.
Child sex, maternal C-reactive Protein (CRP), and newborn P50S2μV
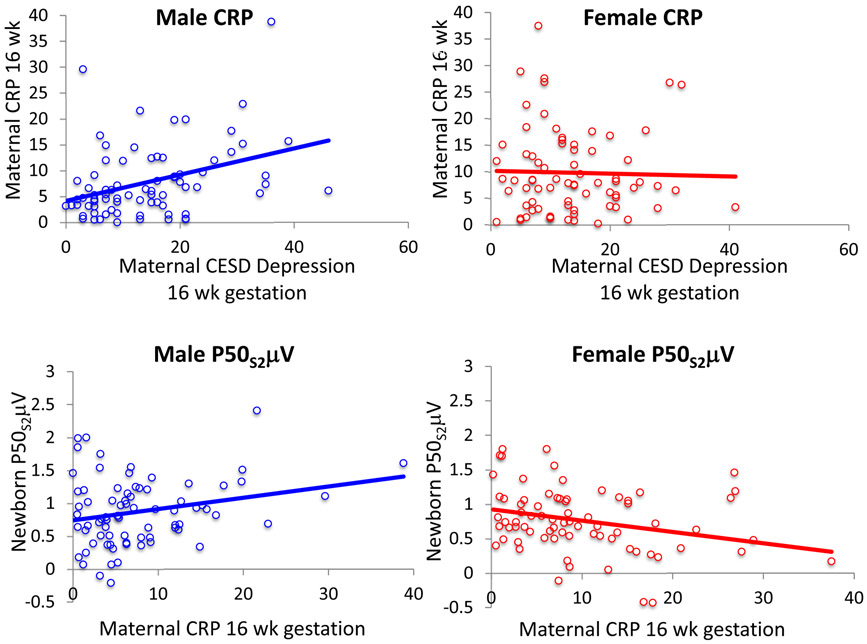
For CRP level at 16 weeks gestation, there was a significant interaction between maternal CESD rating at 16 weeks gestation and the sex of the fetus, Fdf1,142 = 4.64, P = 0.03 (Table S5). For women with male fetuses, CESD rating was positively related to 16-week CRP levels, β = 0.330, P = 0.006, in a multivariate analysis that considered maternal age, obesity (pre-pregnancy BMI ≥ 30), and infection (Fig. 2). For women with CESD rating ≥16 with male fetuses, 16-week gestation CRP levels, 10.1 mg/L (SD 8.0), were higher compared to women with CESD rating <16, 6.2 mg/L (SD 5.8), P = 0.016 (Table 1). For newborn P50S2μV, there was a significant interaction between maternal CRP levels and the sex of the child, Fdf1,142 = 8.47, P = 0.004 (Table S6). Higher CRP levels were positively associated with greater male newborn P50S2μV (β = 0.190, P = 0.046), as previously reported (40).
Figure 2.
Top plots. Significant positive association of maternal CESD depression rating and maternal C-Reactive Protein (CRP) at 16 weeks gestation for pregnancies with male fetuses β = 0.330, P = 0.006, but not with female fetuses. Bottom plots: The association of maternal CRP levels with newborn P50S2μV adjusted for P50S1μV was positive for male newborns β = 0.190, P = 0.046, and negative for female newborns β = −0.206, P = 0.020. The effects of CRP on P50S2μV were published previously (40).
For women with female fetuses, CESD ratings were not related to 16-week CRP levels in the multivariate analysis, β = −0.013, P = 0.9. Infection reported at 16 weeks gestation was a positive factor, β = 0.273, P = 0.024. For female newborns, higher CRP levels were inversely associated with lower P50S2μV, β = −0.206, P = 0.020, as previously reported (40).
Child sex, maternal depression and cortisol
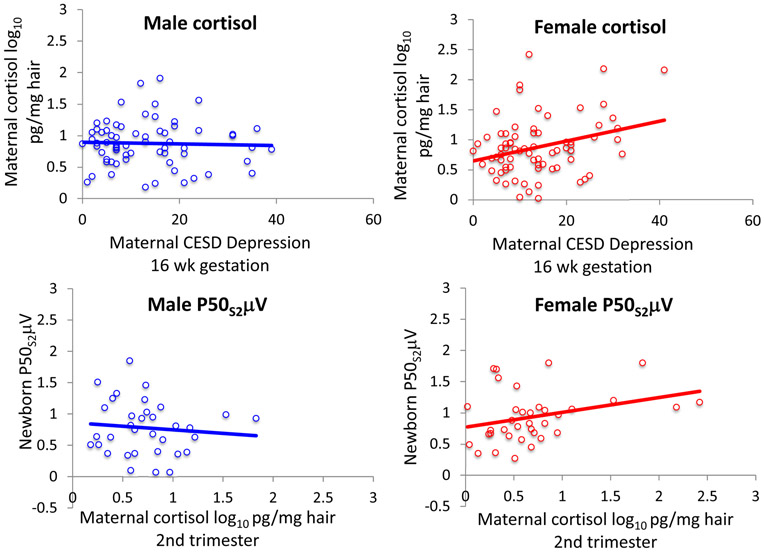
For maternal 2nd trimester cortisol levels, there was a significant interaction between CESD rating at 16 weeks gestation and fetal sex, Fdf 1,131 = 5.49, P = 0.021 (Table S7). For women with male fetuses, cortisol levels were not related to CESD depression ratings, β = β0.052, P = 0.7. For women with female fetuses, CESD depression ratings were positively related to their 2nd trimester cortisol levels (β = 0.320, P = 0.010; Fig. 3). Maternal age, obesity (pre-pregnancy BMI≥30), and infection were not significant factors. For women with CESD rating≥16 with female fetuses, 2nd trimester hair cortisol levels were significantly higher 1.06 log pg/mg (0.58) compared to women with CESD rating <16, 0.81 log pg/mg (SD 0.47), P = 0.045 (Table 1).
Figure 3.
Top plots. The association of maternal CESD depression rating and 2nd trimester maternal hair cortisol for pregnancies with males fetuses was not significant, but it was positive for pregnancies with female fetuses β = 0.320, P = 0.010. Bottom plots: The association of maternal 2nd trimester hair cortisol with newborn P50S2μV adjusted for P50S1μV for male newborns was not significant, but it was positive for female newborns β = 0.312, P = 0.020.
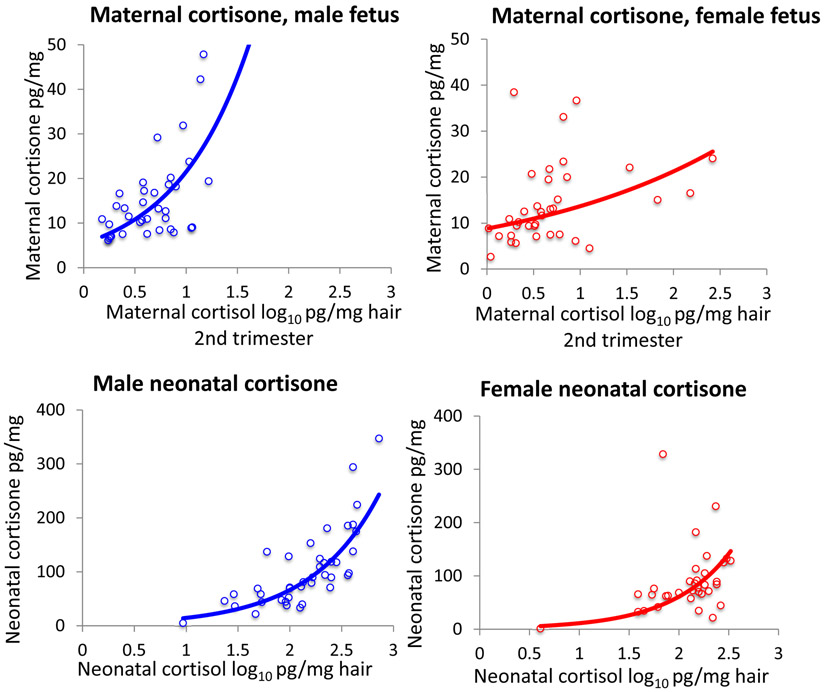
Fetal sex, maternal and neonatal hair cortisol and cortisone
Cortisone levels in 2nd trimester maternal hair relative to cortisol levels were higher in women with male fetuses, exponential association r2 = 0.569, P < 0.001, than in women with female fetuses, r2 = 0.157, P = 0.017; child sex * 2nd trimester hair cortisol Fdf1,65 = 43.4, P < 0.001. Neonatal hair cortisone relative to neonatal hair cortisol was higher in males, exponential association r2 = 0.660, P < 0.001 than in females, exponential association r2 = 0.477, P < 0.001; child sex * neonatal hair cortisol Fdf1,70 = 4.85, P = 0.031 (Figure 4).
Figure 4.
Relationships of cortisol to cortisone in mothers and neonates. Top: Relationship of maternal 2nd trimester cortisone hair levels to maternal cortisol in women with male fetuses, exponential association, r2 = 0.569, P < 0.001) and female fetuses, exponential association r2 = 0.157, P = 0.017. Bottom: Relationship of neonatal hair cortisone to neonatal hair cortisol in males, exponential association r2 = 0.660, P < 0.001, and in females, exponential association r2 = 0.477, P < 0.001.
Child sex, maternal cortisol, neonatal cortisone and newborn P50S2μV
For newborn P50S2μV, there was a significant interaction between child sex, maternal 2nd trimester hair cortisol, and neonatal hair cortisone levels, Fdf1,58 = 3.36, P = 0.042 (Table S8). For males the association of 2nd trimester maternal cortisol with newborn P50S2μV was not significant, β = −0.037, P = 0.8. Higher male neonatal hair cortisone levels were inversely correlated with lower P50S2μV, β = −0.499, P = 0.002. Maternal cortisol was positively associated with greater female newborn P50S2μV, β = 0.312, P = 0.020 (Fig. 3). For females, the association of neonatal hair cortisone with P50S2μV was non-significant, neonatal hair cortisone, β = −0.126, P = 0.4. The association of maternal CESD depression ratings with newborn female P50S2μV became non-significant when co-varied for maternal 2nd trimester cortisol (β = 0.100, P = 0.5); cortisol remained significant in this analysis (β = 0.290, P = 0.048).
Effects of antidepressants during gestation
Antidepressants were used by 22 women during gestation (14%). Use was significantly positively associated with higher CESD rating at 16 weeks gestation, 18.7 (SD 12.4), compared to no antidepressant use, 13.2 (SD 8.7), P = 0.012. As gestation progressed, the association with CESD ratings became non-significant. There were no significant effects of antidepressant use, controlled for CESD rating at 16 weeks gestation, on gestational CRP, maternal hair cortisol, or newborn P50 amplitude (Table 3).
Table 3.
Effects of antidepressants on maternal and child parameters.
| Measure mean (SD) |
Antidepressant N = 22 |
None N = 139 |
Significance P = |
|---|---|---|---|
| CESD Depression scale 16 wks gestation | 18.7 (12.4) | 13.2 (8.7) | 0.012 |
| CESD 22 wks | 17.0 (11.8) | 12.6 (9.1) | 0.045 |
| CESD 28 wks | 15.4 (9.0) | 13.7 (9.6) | 0.4 |
| CESD 34 wks | 16.9 (11.8) | 13.5 (9.8) | 0.1 |
| CESD 40 wks | 14.0 (7.7) | 11.5 (8.8) | 0.2 |
| CESD 46 wks (postpartum) | 14.4 (8.7) | 10.7 (9.2) | 0.1 |
| Analyses of antidepressants controlled for CESD 16 weeks gestation, mean (SE) | |||
| P50S2μV adjusted for P50S1μV | 0.84 (1.02) | 0.82 (0.40) | 0.9 |
| Male fetus C-reactive Protein 16 weeks gestation mg/L | 9.24 (1.0) | 7.49 (0.93) | 0.4 |
| Female fetus cortisol 2nd trimester log pg/mg hair | 1.14 (0.16) | 0.86 (0.06) | 0.1 |
Discussion
Maternal depression at 16 weeks gestation was positively associated with higher amplitudes of the newborn P50 response to a repeated sound stimulus, which indicates poorer development of cerebral inhibitory neurocircuit function. Ratings of maternal stress, anxiety, and adverse childhood experience, as well as depression later in gestation, were not significantly associated when considered with 16-week CESD ratings. The association of maternal depression with either CRP or cortisol levels depended upon the sex of the fetus. Depression in mothers with male fetuses was positively associated with increased CRP levels, and in mothers with female fetuses, depression was positively associated with increased cortisol. These findings are summarized in Table S9. The timing of negative effects of prenatal maternal CRP on male newborn P50 inhibition and cortisol on female newborn P50 inhibition is consonant with other evidence that this period is when both inflammation and cortisol appear to be most pathogenic, when interneuron differentiation is occurring (17-18,49,67).
Evidence for mediation by cortisol of the effects of maternal prenatal depression on female newborn P50 inhibition was observed with Baron and Kenny’s criteria (68). A multivariate analysis showed a positive cortisol association with greater P50S2μV and loss of significance of the maternal CESD association. The multivariate association was predicated on positive univariate associations of (i) maternal CESD with newborn P50S2μV, (ii) maternal CESD depression with cortisol levels, and (iii) maternal cortisol levels with newborn P50S2μV (68). The corresponding analysis with depression, CRP levels, and P50S2μV in male newborns showed similar positive univariate associations between CESD, CRP levels, and P50S2μV, but the multivariate analysis of effects of depression and CRP levels continued to show significant positive association of depression with P50S2μV. Unlike cortisol, which is pathogenic itself, CRP is only an indicator of inflammatory response and not a pathogenic agent per se, which may account for the failure to find mediation.
The fetal-sex specific association of the mother’s depression with either CRP or cortisol, and the sex-specific association of CRP and cortisol with development of cerebral inhibition may be related. Male placentas in early gestation have higher levels of 11-β-hydroxysteroid-dehydrogenase-2 (11BHSD2), which irreversibly converts cortisol to cortisone (34-36). Cortisone does not activate the cortisol receptor and thus effects of maternal cortisol are diminished (34). Higher male neonatal cortisone hair levels, presumably reflecting this irreversible fetal 11BHSD2 activity, were associated with lower P50S2μV. However, in the mother, cortisone is converted back to cortisol in the hypothalamus by 11-β-hydroxysteroid-dehydrogenase-1 (11BHSD1), which is a reversible enzyme (34). Increased hypothalamic cortisol in mothers with male fetuses may then suppress the association of cortisol with her depression (34). Depression and stress are associated with increased methylation of 11BHSD2 (25,69). Methylation is associated with decreased placenta 11BHSD2 protein expression in pregnancies with female, but not male fetuses (70), which leaves the female fetus more exposed to maternal cortisol.
CRP levels and depression were positively associated only in mothers with male fetuses. However, there are positive associations of CRP with prenatal infection in mothers with either sex fetus, because viral RNA or bacterial lipopolysaccharide activates a vigorous maternal inflammatory response regardless of fetal sex (38). Inflammation in depression, in the absence of an external antigen, may occur predominantly with male fetuses because of the sex-discordance with the mother, analogous to increased inflammation in female hosts from grafts of male tissue (71). Female placentas are also protected from inflammation by several mechanisms, including higher expression of human chorionic gonadotropin genes, increased expression of X-chromosome genes, differences in serotonin signaling, and differences in torr-receptors (6,72-74).
Possible advantages of the differences between male and female pregnancies in fetal and placental development have been considered from an evolutionary perspective (75-78). Male fetuses are larger, which prepares them to be stronger and support more aggressive behavior. Their development is resistant to the effects of the mother’s HPA-axis activation during stress, because of the effects of 11BHSD2 in the placenta. However, their placentas are smaller and their gestation is more vulnerable to other gestational insults that invoke inflammation, which can result in decreased male births in adverse conditions like epidemics (6, 75-78). Female placentas are larger and presumably better able to support the smaller female fetus, perhaps insuring that more females survive adversity in utero to bear their own progeny (79). One mechanism by which the mother limits female fetal growth is to increase cortisol during stressful circumstances (78). The smaller fetus survives but may be more vulnerable to postnatal problems in HPA axis regulation, including those associated with depression (77).
Antidepressant treatment had no direct positive or negative association with newborn P50 inhibition. Any association depended upon the current CESD depression rating (Table 3). The finding corroborates our report in another cohort that newborn P50 inhibition is increased by antidepressant treatment of maternal depression and provides further evidence that this aspect of development is not impeded by antidepressant treatment (80).
Limitations of correlational observations include the possibility of reverse causality and undetected mediating variables. The increased female birth rate in stressed women could explain their higher cortisol response (6). However, there were no differences in CESD ratings in women with female or male fetuses in this cohort. Depression co-existed with many other factors in each woman’s pregnancy. These factors, including prenatal infection, substance use, adverse childhood experiences, and postnatal depression have significant impact on fetal development. They were included as variables in the present analyses of the association with maternal depression, which remained significant. The mothers rated their own depression. However, patient self-ratings of depression are found to be more reliable than clinician diagnoses (81). This study also has limitations in its assessment of inflammatory and corticosteroid effects on the fetus. CRP is only an indirect marker of inflammatory response. We cannot be certain how much cortisol actually reaches the fetus during the 2nd trimester from the mother’s level; it is estimated from radiotracers as 25% (82). Neonatal hair cortisone was used to indicate the placenta’s 11BHSD2 activity, but this hair is formed in the 3rd trimester when 11BHSD2 levels change in anticipation of parturition (37).
The P50 response is generated in hippocampus, where inhibitory interneurons are responsible for the decrement in its amplitude in response to repeated sounds, which we assessed in this study as P50S2μV co-varied for P50S1μV (62). In the offspring of mothers with increased prenatal anxiety, the hippocampus was not smaller at birth, but growth decreased in the first 6 months of life related to both pre and postnatal maternal anxiety (27). Another study imaged the fetal hippocampus at 24 and 40 weeks gestation and found decreased volume in fetuses of mothers with anxiety and decreased creatine and membrane choline levels in fetuses of mothers with depression, indicative of decreased cellular development (28). Newborn fractional anisotropy (FA) in the uncinate fasiculus near the hippocampus, associated with axonal development, was decreased in relation to increased prenatal maternal Interleukin-6, but increased more rapidly thereafter (29). Another study had similar findings in mothers related to their prenatal anxiety (26). Sex-specific effects were observed in offspring of mothers with higher prenatal depression and anxiety, with lower FA females and higher FA in males in white matter adjacent to the hippocampus. There are no specific studies of interneuron development with maternal gestation depression. Pathological developmental effects on interneurons in high-risk prenatal conditions, which may or may not include maternal depression, can be observed in post mortem brain tissue from individuals with schizophrenia. Some interneurons fail to migrate from the subplate into the temporal lobe, and they do not fully develop the mature chloride transporter necessary for their inhibitory function (83-84).
Newborn P50 inhibition is a putative translational bridge between pathophysiological associations of maternal depression with fetal development of inhibitory neurons of the hippocampus and later behavioral problems including increased risk for later mental illness. P50 inhibition is a consistent trait in individuals from newborn assessment to 4 years of age (64). The continuity of newborn with adult P50 inhibition is unknown, but decreased P50 inhibition is common in both adult children and siblings of probands with schizophrenia and infants of a parent with schizophrenia, as we and another group have shown (60, 85-86). In this cohort, we previously reported that higher newborn P50S2μV, indicative of poor P50 inhibition, was positively associated with behavioral differences at 3 months of age, as assessed by the Infant Behavior Questionnaire-R/SF (40). Female infants with higher P50S2μV were less active and quieter. Male infants with higher P50S2μV were fussier, harder to engage, and less able to seek comfort from caregivers. In two other cohorts, we assessed children at 3.5 years of age using the Child Behavior Checklist for age 1½-5 years (87-88). Problems with attention and social withdrawal were positively associated with poorer newborn P50 inhibition. Parents whose children developed schizophrenia as young adults report similar levels of attention and social withdrawal problems on the Child Behavior Checklist when their children were as young as 2 years of age (89). Poorer P50 inhibition is positively associated with problems in attention in adults with schizophrenia as well as in adults without mental illness (90-91). Problems in the self-regulation of temperament and behavior in infancy can thus have enduring consequences as these individuals develop into adulthood (92).
Supplementary Material
Acknowledgments.
Supported by grants from the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development K12HD001271-11 [to M.C.H.] and National Center for Advancing Translational Sciences UL1TR001082 [to all investigators], and by the Institute for Children’s Mental Disorders and The Anschutz Foundation.
The late Randal G. Ross, M.D., initiated this study. C. Neill Epperson commented on a draft of the manuscript.
Footnotes
Disclosure. Dr. Freedman is a consultant to Minerva Pharmaceuticals. The other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mäki P, Riekki T, Miettunen J, Isohanni M, Jones PB, Graham K Murray GK, et al. (2010): Schizophrenia in the offspring of antenatally depressed mothers in the northern Finland 1966 birth cohort: relationship to family history of psychosis. Am J Psychiatry 167: 70–79. [DOI] [PubMed] [Google Scholar]
- 2.Weissman MM, Prusoff BA, Gammon GE, Merikangas KR, Leckman JF, Kidd KK (1984): Psychopathology in the children (ages 6–18) of depressed and normal parents. J Am Acad Child Adolesc Psychiatry 23: 78–84. [DOI] [PubMed] [Google Scholar]
- 3.Sagiv SK, Epstein JN, Bellinger DC, Korrick SA (2013): Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. J Attent Disord 17: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberlander TF, Zwaigenbaum L (2017): Disentangling maternal depression and antidepressant use during pregnancy as risks for autism in children. JAMA 317: 1533–1534. [DOI] [PubMed] [Google Scholar]
- 5.Dean DC, Planalp EM, Wooten W, Kecskemeti SR, Adluru N, Cory K, Schmidt CK et al. (2018): Association of prenatal maternal depression and anxiety symptoms with infant white matter microstructure. JAMA Pediatr 172: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh K, McCormack CA, Webster R, Pinto A, Lee S, Feng T et al. (2019): Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc Natl Acad Sci 116: 23996–24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker ED, Jaffee SR, Uher R, Maughan B (2011): The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety 28:696–702. [DOI] [PubMed] [Google Scholar]
- 8.Evans J, Melotti R, Heron J, Ramchandani P, Wiles N, Murray L, et al. (2012): The timing of maternal depressive symptoms and child cognitive development: a longitudinal study. J Child Psychol Psychiatry 53:632–640. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen M, Luoma I, Salmelin R, Tamminen T (2012): A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. J Affect Disord 136:680–692. [DOI] [PubMed] [Google Scholar]
- 10.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. (2011): Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci 108:14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandman CA, Buss C, Head K, Davis EP (2015): Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry 77: 234–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M (2012): Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun 26: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuloaga DG, Carbone DL, Quihuis A, Hiroi R, Chong DL, Handa RJ (2012): Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. J Neurosci Res 90: 1403–1412. [DOI] [PubMed] [Google Scholar]
- 14.Owen D, Setiawan E, Li A, McCabe L, Matthews SG (2004): Regulation of N-methyl-D-aspartate receptor subunit expression in the fetal guinea pig brain. Biol Reprod 71: 676–683. [DOI] [PubMed] [Google Scholar]
- 15.Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL et al. (2016): Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry 21: 956–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasistha NA, Pardo-Navarro M, Gasthaus J, Weijers D, Müller MK, García-González D, et al. (2019): Maternal inflammation has a profound effect on cortical interneuron development in a stage and subtype-specific manner. Mol Psychiatry doi: 10.1038/s41380-019-0539-5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayatti N, Moss JA, Sun L. (2008): A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cerebral Cortex 18:1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zecevic N, Hu F, Jakovcevski I (2011): Cortical interneurons in the developing human neocortex. Dev Neurobiol 71: 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, et al. (2011): Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goff WR, Williamson PD, Vangilder JC, Allison T, Fisher TC (1990): Neural origins of long latency evoked potentials recorded from the depth and from the cortical surface of the brain in man. Prog Clin Neurophysiol 7:126–145. [Google Scholar]
- 21.Miller CL, Freedman R (1995): The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neurosci 69: 371–381. [DOI] [PubMed] [Google Scholar]
- 22.Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R (2003): Early postnatal development of sensory gating. Neuroreport 14: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham AM, Rasmussen JM, Entringer S, Ward EB, Rudolph MD, Gilmore JH et al. (2019): Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry 85: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham AM, Rasmussen JM, Rudolph MD, Heim CM , Gilmore JH, Styner M et al. (2018): Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry 83: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B (2016): Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am J Psychiatry 173: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rifkin-Graboi A, Meaney MJ, Chen H, Bai J, Hameed WB, Tint MT et al. (2015): Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J Am Acad Child Adolesc Psychiatry 54: 313–321. [DOI] [PubMed] [Google Scholar]
- 27.Qiu A, Rifkin-Graboi A, Chen H, et al. (2013): Maternal anxiety and infants' hippocampal development: timing matters. Transl Psychiatry 3:e306. doi: 10.1038/tp.2013.79, 10.1038/tp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Lu YC, Jacobs M, et al. (2020): Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA netw open 2020;3(1):e1919940. doi: 10.1001/jamanetworkopen.2019.19940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen JM, Graham AM, Entringer S, et al. (2019): Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage 185:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan V, Nestler EJ (2010): Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 167:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howren MB, Lamkin DM, Suls J (2009): Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. [DOI] [PubMed] [Google Scholar]
- 32.Shelton MM, Schminkey DL, Groer MW (2015): Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biol Res Nurs 17:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, Ross RG (2016): Measures of maternal stress and mood in relation to preterm birth. Obstet Gynecol 127:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman K, Holmes M, Seckl J (2013). 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93: 1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun K, Yang K, Challis JR (1997): Differential expression of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. J Clin Endocrin Metab 82:300–305. [DOI] [PubMed] [Google Scholar]
- 36.Mericq V, Medina P, Kakarieka E, et al. (2009): Differences in expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 and 2 in human placentas of term pregnancies according to birth weight and gender. Eur J Endocrin 161:419–425. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman MC, D'Anna-Hernandez K, Benitez P, Ross RG, Laudenslager ML (2017): Cortisol during human fetal life: Characterization of a method for processing small quantities of newborn hair from 26 to 42 weeks gestation. Develop Psychobiol 59:123–127. [DOI] [PubMed] [Google Scholar]
- 38.Freedman R, Hunter SK, Law AJ, Wagner BD, D’Alessandro A, Christians U et al. (2019): Higher gestational choline levels in maternal infection are protective for infant brain development. J Pediatr 208:198–206.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman MC, Hunter SK, D'Alessandro A, Noonan K, Wyrwa A, Freedman R (2019): Interaction of maternal choline levels and prenatal Marijuana's effects on the offspring. Psychol Med doi: 10.1017/S003329171900179X [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter SK, Hoffman MC, D’Alessandro A, Noonan K, Wyrwa A, Freedman R, et al. (2019): Male fetus susceptibility to maternal inflammation: C-reactive protein and brain development. Psychol Med 10.1017/S0033291719003313 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bleker LS, Roseboom TJ, Vrijkotte TG, Reynolds RM, de Rooij SR (2017): Determinants of cortisol during pregnancy - The ABCD cohort. Psychoneuroendocrinol 83:172–181. [DOI] [PubMed] [Google Scholar]
- 42.Giesbrecht GF, Campbell T, Letourneau N (2015): APrON Study Team. Sexually dimorphic adaptations in basal maternal stress physiology during pregnancy and implications for fetal development. Psychoneuroendocrinol 56:168–178. [DOI] [PubMed] [Google Scholar]
- 43.Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN (2012): Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol 94:202–209. [DOI] [PubMed] [Google Scholar]
- 44.Azar R, Mercer D (2013): Mild depressive symptoms are associated with elevated C-reactive protein and proinflammatory cytokine levels during early to midgestation: a prospective pilot study. J Womens Health 22:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott NM, Hodyl NA, Murphy VE, et al. (2009): Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol 182:1411–1420. [DOI] [PubMed] [Google Scholar]
- 46.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA (2012): Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Nat Acad Sci 109:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli J-AL, Remington AG, Tsuang MT, et al. (2014): Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol Med 44: 3249–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braithwaite EC, Murphy SE, Ramchandani PG, Hill J (2017): Associations between biological markers of prenatal stress and infant negative emotionality are specific to sex. Psychoneuroendocrinol 86:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomaki S, Leiviska J, Kellendonk C, et al. (2014): Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry 171: 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allswede DM, Yolken RH, Buka DL, Cannon TD (2020): Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study. Lancet Psychiatry 7: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ACOG (American College of Obstetricians and Gynecologists), Society for Maternal-Fetal Medicine. Guidance 700: Methods for estimating the due data. 2017; https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/methods-for-estimating-the-due-date
- 52.Radloff LS (1977): The CES-D scale: A self-report depression scale for research in the general population. Applied Psychol Measurement 1: 385–401. [Google Scholar]
- 53.Naughton MJ, Wiklund I (1993): A critical review of dimension-specific measures of health-related quality of life in cross-cultural research. Qual Life Res 2: 397–432. [DOI] [PubMed] [Google Scholar]
- 54.Eaton WW, Kessler LG (1981): Rates of symptoms of depression in a national sample. Am J Epidemiol 114: 528–538. [DOI] [PubMed] [Google Scholar]
- 55.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L (2009): Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrin 34: 32–37. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman MC, Karban LV, Benitez P, Goodteacher A, Laudenslager ML (2014): Chemical processing and shampooing impact cortisol measured in human hair. Clin Invest Med– Med Clin Exper 37: E252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML (2011): Hair cortisol levels as a retrospective marker of hypothalamic- pituitary axis activity throughout pregnancy: comparison to salivary cortisol Physiol Behav 104: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anders T, Emde R, Parmelee A; A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. Los Angeles: UCLA Brain Information Service, NINDS Neurological Information Network, 1971 [Google Scholar]
- 59.Griffith JM, Freedman R (1995). Normalization of the auditory P50 gating deficit of schizophrenic patients after non-REM but not REM sleep. Psychiatry Res 56: 271–278. [DOI] [PubMed] [Google Scholar]
- 60.Freedman R, Adler LE, Waldo M, Myles-Worsley M, Nagamoto HT, Miller C, et al. (1996): Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects: Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry 53: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 61.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A et al. (1997): Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Nat Acad Sci 94: 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freedman R, Olsen-Dufour AM, Olincy A, Consortium on the Genetics of Schizophrenia (2020): P50 inhibitory sensory gating in schizophrenia: analysis of recent studies. Schizophr Res 218:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunter SK, Corral N, Ponicsan H, Ross RG (2008): Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport 19:79–82. [DOI] [PubMed] [Google Scholar]
- 64.Hunter SK, Gillow SJ, Ross RG (2015): Stability of P50 auditory sensory gating during sleep from infancy to 4 years of age. Brain Cogn 94: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frerichs RR, Aneschensel CS, Clark VA (1981). Prevalence of depression in Los Angeles County. Am J Epdemiol 113: 691–699. [DOI] [PubMed] [Google Scholar]
- 66.Aneshensel CS, Frerichs RR, Clark VA(1981). Family roles and sex differences in depression. J Health Social Behav 22: 379–393 [PubMed] [Google Scholar]
- 67.Davis EP, Sandman CA (2010): The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev 81: 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baron RM, Kenny DA (1986): The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Personal Soc Psychol 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 69.Stroud LR, Papandonatos GD, Parade SH, Salisbury AL, Phipps MG, Lester BM, et al. (2016). Prenatal major depressive disorder, placenta glucocorticoid and serotonergic signaling, and infant cortisol response. Psychosom Med 78: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green BB, Armstrong DA, Lesseur C, Paquette AG, Guerin DJ, Kwan LE, et al. (2015): The role of placental 11-beta hydroxysteroid dehydrogenase type 1 and type 2 methylation on gene expression and infant birth weight. Biol Reprod 92:149. doi: 10.1095/biolreprod.115.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makinson R, Lloyd K, Rayasam A, McKee S, Brown A, Barila G, et al. (2017): Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain Behav Immun 66: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT (2014): Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal–maternal interface. Mol Hum Reprod 20: 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nugent BM, O’Donnell CM, Epperson CN, Bale T (2018): Placental HEK27me3 establishes female resilience to prenatal insults. Nat Commun 9:2555. doi: 10.1038/s41467-018-04992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Missig G, Robbins JO, Mokler EL, McCullough KM, Bilbo SD, McDougle CJ, et al. (2019): Sex-dependent neurobiological features of prenatal immune activation via TLR7. Mol Psychiatry doi: 10.1038/s41380-018-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haig D (1993). Genetic conflicts in human pregnancy. Quarterly Rev Biol 68:495–532. [DOI] [PubMed] [Google Scholar]
- 76.Wells JC (2000): Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol 202: 65–76. [DOI] [PubMed] [Google Scholar]
- 77.Sandman CA, Glynn LM, Davis EP (2013): Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res 75:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clifton V (2010). Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31(Suppl): S33–S39. [DOI] [PubMed] [Google Scholar]
- 79.Murphy VE, Zakar T, Smith R, Giles WB, Gibson PG, Clifton VL (2002) Reduced 11beta-hydroxysteroid dehydrogenase type 2 activity is associated with decreased birth weight centile in pregnancies complicated by asthma. J Clin Endocrinol Metab 87:1660–1668. [DOI] [PubMed] [Google Scholar]
- 80.Hunter SK, Mendoza JH, D'Anna K, Zerbe GO, McCarthy L, Hoffman C, et al. (2012): Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am J Psychiatry 169: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narrow WE, Clarke DE, Kuramato J, Kraemer HC, Kupfer DJ, Greiner L, et al. (2013): DSM-5 field trials in the United States and Canada, part III: development and reliability testing of a cross-cutting symptom assessment for DSM-5. Am J Psychiatry 170: 71–82. [DOI] [PubMed] [Google Scholar]
- 82.Beitins IZ, Bayard F, Ances IG, Kowarski A, Megeon CJ (1973): The metabolic clearance tate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediat Res 7:509–519. [DOI] [PubMed] [Google Scholar]
- 83.Akbarian S, Vinuela A, Kim JJ, Potkin SG, Bunney WE Jr, Jones EG (1993): Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry 50:178–187. [DOI] [PubMed] [Google Scholar]
- 84.Hyde TM, Lipska BK, Ali T, et al. (2011): Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 31:11088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG (2011): Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: Parental psychosis, maternal depression, and nicotine use. Schizophr Bull 37: 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith E, Crawford Y, Thomas M, Reid V (2018): Schizotypy and sensory gating: A 6-month-old EEG study. Schizophr Bull 44: S301–S302. [Google Scholar]
- 87.Ross RG, Hunter SK, Hoffman MC, McCarthy L, Chambers BM, Law AJ et al. (2016): Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatry 173: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutchison AK, Hunter SK, Wagner BD, Calvin EA, Zerbe GO, Ross RG (2007): Diminished infant P50 sensory gating predicts increased 40-month-old attention, anxiety/depression, and externalizing symptoms. J Atten Disord 21:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossi A, Pollice R, Daneluzzo E, Marinangeli MG, Stratta P (2000): Behavioral neurodevelopment abnormalities and schizophrenic disorder: a retrospective evaluation with the Childhood Behavior Checklist (CBCL). Schizophr Res 44: 121–128. [DOI] [PubMed] [Google Scholar]
- 90.Hamilton HK, William TJ, Ventura J, et al. (2018): Clinical and cognitive significance of auditory sensory processing deficits in schizophrenia. Am J Psychiatry 175:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wan L, Friedman BH, Boutros NN, Crawford HJ (2008): P50 sensory gating and attentional performance. Int J Psychophysiol 67:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang A, Crawford H, Morales S, Degnan KA, Pine DS, Fox NA (2020): Infant behavioral inhibition predicts personality and social outcomes three decades later. Proc Nat Acad Sci 10.1073/pnas.1917376117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.