Summary
Extant land plants consist of two deeply divergent groups, tracheophytes and bryophytes, which shared a common ancestor some 500 million years ago. While information about vascular plants and the two of the three lineages of bryophytes, the mosses and liverworts, is steadily accumulating, the biology of hornworts is poorly explored. Yet, as the sister group to liverworts and mosses, hornworts are critical in understanding the evolution of key land plant traits. Until recently, there was no hornwort model species amenable to systematic experimental investigation, which hampered detailed insight into the molecular biology and genetics of this unique group of land plants. The emerging hornwort model species, Anthoceros agrestis is instrumental in our efforts to better understand not only hornwort biology but also fundamental questions of land plant evolution. To this end, here we provide an overview of hornwort biology and current research on the model plant A. agrestis to highlight its potential in answering key questions of land plant biology and evolution.
Keywords: bryophytes, hornworts, Anthoceros, evolution, development, chloroplast, symbiosis
I. Introduction
Land plants (embryophytes) evolved from streptophyte algae (charophytes) some 500 million years ago (Delwiche & Cooper, 2015; Harholt et al., 2016). The colonization of land by plants led to their diversification into the monophyletic group of bryophytes and the tracheophytes (vascular plants) (Renzaglia et al. 2000; Nishiyama et al., 2004; Leebens-Mack et al. 2019). Bryophytes include liverworts, mosses, and hornworts, while tracheophytes consist of lycophytes, ferns, gymnosperms and angiosperms. The hornworts are the smallest and least diverse clade within bryophytes, consisting of approximately 220 species (Söderström et al., 2016) that are geographically widespread primarily in the tropical areas (Villarreal et al. 2014). Hornworts comprise the division Anthocerotophyta, the name of which is derived from three Greek words: άνθος / anthos (meaning flower), κέρας / ceras (meaning horn) and φυτό / phyto (meaning plant) and refers to the horn-like shape of the hornwort sporophyte (the multicellular diploid phase of the life cycle).
Inference about the common ancestor of land plants is ambiguous. This is, in part, due to the deep divergence of the three groups of bryophytes, as well as bryophytes and tracheophytes, that provided ample time for independent gains/losses of genes to occur. Comparison of developmental, physiological and molecular features of hornworts with those of mosses and liverworts will provide a more accurate picture of the nature of the common ancestor of bryophytes and that of all land plants. It will also help to understand the diversity and molecular basis of evolution and development across bryophytes and tracheophytes.
Hornworts also possess a large number of unique traits that are not found in any other land plants. These include: (i) Zygote and sporophyte development: The orientation of the first zygote division is longitudinal unlike other land plants except only leptosporangiate ferns (Johnson and Renzaglia 2009). The sporophyte differs from mosses and liverworts in that it lacks a seta and continuously and progressively produces spores upwardly from a basal meristem, being essentially a growing sporangium. Sporophytes bear stomata, which may be homologous to those of tracheophytes (Renzaglia et al. 2017; Harris et al. 2020). (ii) Chloroplast: It is the only extant land plant lineage, together with some lycophytes (Liu et al., 2020), that has a single (or just a few) per cell. The chloroplast of several hornwort species may contain a pyrenoid, a structure also found in many streptophyte algae and other algal lineages but not in other land plants (Li et al., 2017; Meyer et al., 2017). Hornwort plastid genomes have also one of the highest RNA editing rates amongst land plants (Small et al., 2019) (iii) Symbiosis: Hornworts establish symbiotic relationships with endophytic cyanobacteria (Renzaglia et al., 2009) and various fungal partners (mycorrhiza) (Desirò et al., 2013). A small number of studies provide insight into hornwort morphology and growth (Proskauer, 1948a,b; Renzaglia 1978), however, detailed molecular and genetic studies examining hornwort development are lacking.
Here, we review our current understanding of hornwort biology and development, highlighting the role that Anthoceros agrestis could play in shedding light on key innovations during land plant evolution.
II. The hornwort model species Anthoceros agrestis
First used by Micheli (1729) but properly designated by Linnaeus in 1753, Anthoceros L. was the first described and with 60 clearly-delineated species (Söderström et al., 2016), it is the most species rich hornwort genus. Anthoceros agrestis Paton is emerging as the model system for the study of hornwort biology (Szövényi et al., 2015). It is an annual species with a wide northern temperate distribution and has key features that make it amenable to laboratory study. This plant is small, easy to propagate, and has a small genome size and sexual reproduction is facilitated in the laboratory because the plants are monoicous (male and female reproductive organs are produced on the same individual) (Proskauer, 1948a,b; Szövényi et al., 2015). A. agrestis has been adopted as a plant model by research groups around the world to study biological processes such as the evolution of circadian clocks (Linde et al., 2017), microbial type terpene synthase biochemistry (Xiong et al., 2018), and RNA editing (Gerke et al., 2019).
1. A. agrestis morphology and life cycle
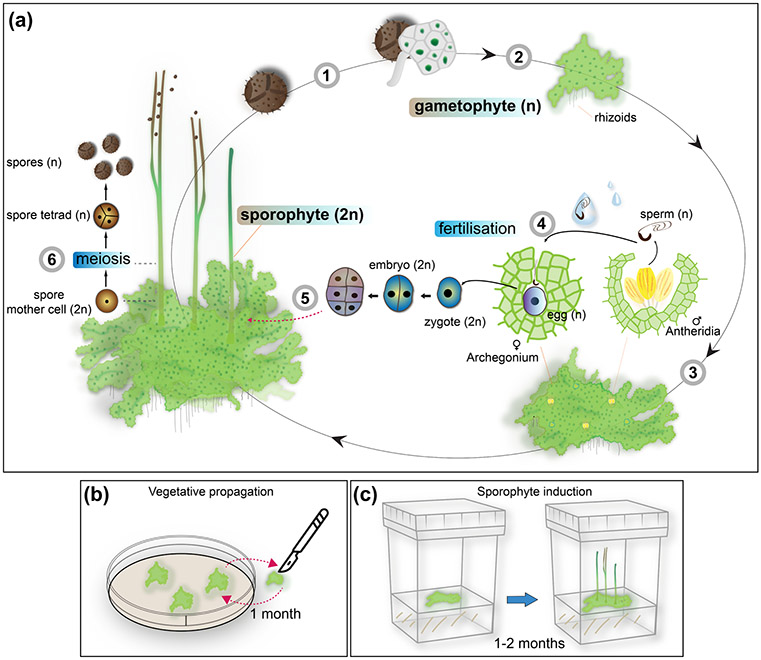
Similar to all bryophytes, the life cycle of A. agrestis is dominated by the haploid gametophyte phase (Fig. 1). The gametophyte of A. agrestis is thalloid, often a rosette, with irregularly dissected margins and dorsal lamellae (Fig. 2a, b & d). The thallus lacks organised external appendages and specialised internal tissue differentiation except for mucilage canals (Fig. 2e) that form by separation between cells and Nostoc cavities that are colonised by cyanobacteria (Renzaglia et al., 2009). Rhizoids develop on the ventral midline of the thallus (Fig. 2b & c). A. agrestis is monoecious, with male (antheridia) (Fig. 2h, f & g) and female (archegonia) (Fig. 2j & m) reproductive organs embedded in the thallus, differing from those in liverworts and mosses that are superficial. Antheridia are sunken in groups of four to 16 in chambers along the dorsal midline of the thallus. Each cluster of antheridia develops from a subepidermal cell in the apical notch (invaginated thallus margins where stem cells reside) (Campbell, 1918; Renzaglia et al., 2009). The overlying epidermal cell forms the roof of the antheridial chamber that is two cells thick. Archegonia are also embedded in the thallus and they develop usually behind the growing point of the thallus. An archegonium is composed of neck canal cells and a ventral canal cell with an egg surrounded by thallus cells (Fig. 2j). When ready for fertilization, the neck canal cells and ventral canal cells disintegrate and the cover cells dissociate, leaving a canal for sperm cells to swim down to the egg (Fig. 2m). Antheridia typically develop and mature before archegonia. Similar to mosses, liverworts and lycophytes fertilization takes place via biflagellate motile sperms (Fig. 2i) that swim to the egg via water and fuse, forming the diploid zygote. The embryo develops within the gametophyte and gives rise to the sporophyte (Fig. 2d & k). Like other bryophytes, the hornwort sporophyte is matrotrophic, meaning it develops on and is nourished by the gametophyte. Unlike other plants, the sporophyte grows from a basal meristem that is established in the early stages of its development. The hornwort sporophyte is an elongated cylinder with no branching and, similar to most mosses, it possesses stomata (Fig. 2k & n). Sporogenous tissue is continually produced, meiosis is always occurring in a progressive and spatial, but not temporal, fashion, and all stages of spore differentiation are visible along the length of the sporophyte. There are no parallels to this development in any extant plant group nor in the fossil record (Renzaglia et al. 2000; Villarreal and Renzaglia 2015). At maturity the sporophyte splits below the apex and releases the spores (Fig. 2o). Spores germinate (Fig. 2a), typically resulting in a globose sporeling that forms an apical cell and develops into the thalloid gametophyte. Under low light conditions, the sporeling of A. agrestis forms a germ tube and has a short protonemal stage that is a single cell and produces the sporeling at its tip (Fig. 2a bottom) (Wada et al. 1984).
Fig. 1: Life and laboratory cycle of the hornwort A. agrestis.
(a) A. agrestis has two life cycle phases. A dominant haploid phase called gametophyte and a diploid phase called sporophyte. The life cycle of A. agrestis starts with the germination of the haploid spores (1) which develop into an irregularly shaped thallus (2). A. agrestis is monoecious, with both male and female reproductive organs present on the same individual. Male (antheridia) and female (archegonia) reproductive organs are embedded in the thallus and mitotically produce sperm and egg, respectively (3). Biflagellated motile sperm cells swim in water to the archegonium where the egg is fertilised (4). The resulting diploid zygote divides first by a longitudinal division and subsequent divisions to form the embryo, which is initially composed of three tiers. The bottom tier produces the foot. The middle tier gives rise to the basal meristem and the top tier forms the tip of the sporophyte (5). The sporophyte develops within the gametophyte and is nourished through the placenta, the junction between foot and gametophyte cells. Meiosis and sporogenesis occur progressively from the base of the sporophyte upwardly, leading to spore formation: sporogenous tissue at the base of the sporophyte produces spore mother cells that, via meiosis, produce spore tetrads and spores that are released at the tip where the sporophyte separates into two valves. (6). n: haploid, 2n: diploid.
(b - c) Laboratory cycle of A. agrestis: (b) Plants can be easily propagated in axenic culture by transferring small thallus fragments (typically 1 mm x 1 mm) onto plates with fresh growth media using sterile scalpels. (c) In laboratory conditions A. agrestis sporophyte induction can be achieved in 1-2 months under axenic conditions using a small thallus fragment as starting material.
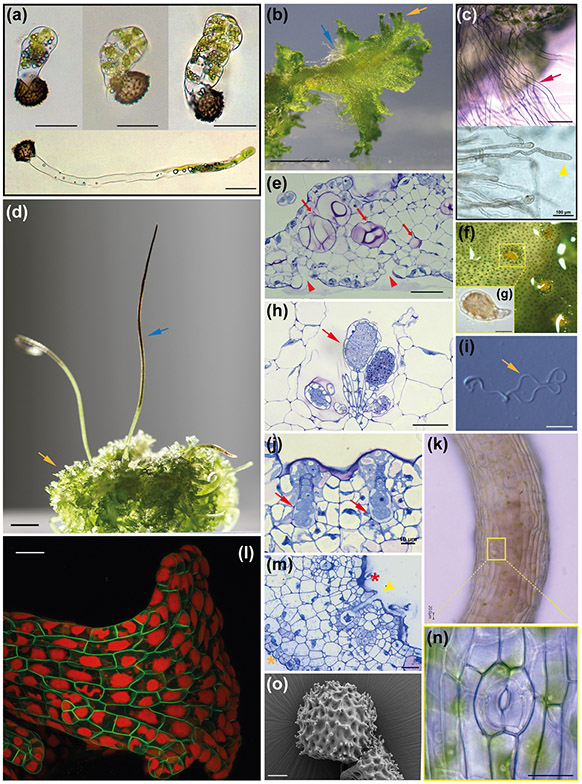
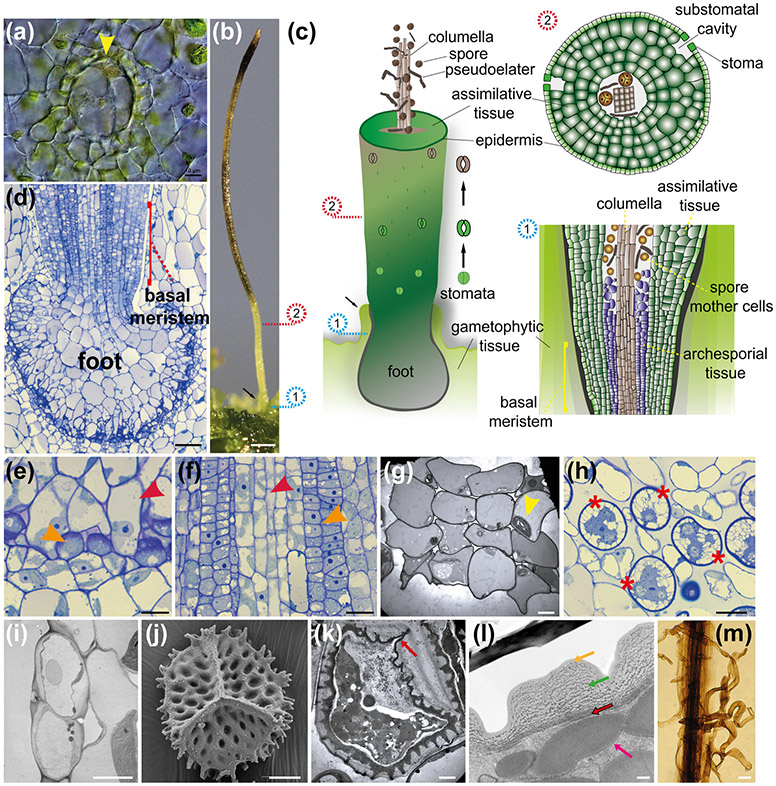
Fig. 2: Key morphological features of A. agrestis.
(a) Light micrograph (LM) of germinating spores. Upper three images, successive stages in globose sporeling production. Lowermost: under low light conditions spore germination involves a germ tube, a long single-celled filament that develops a terminate globose sporeling. Scale bars: 50 μm. (b) Surface view of the irregularly shaped thallus. Blue arrow: rhizoids. Orange arrow: wavy thallus edge. Scale bar: 2.0 mm. (c) Top: LM of single-celled rhizoids on the ventral thallus (red arrow). Scale bar: 150 μm. Bottom: LM of single-celled rhizoid tips (yellow arrowhead) Scale bar: 100 μm (d) Sporophytes (blue arrow) growing on the gametophyte (yellow arrow). Scale bar: 3.0 mm. (e) LM of longitudinal section of thallus with mucilage canals (red arrows). Mucilage clefts on the ventral side indicated with red arrowheads. Scale bar: 50 μm. (h) LM of antheridia (red arrow) in an antheridial chamber in longitudinal section. Scale bar: 50 μm. (f) Surface view of antheridial chamber with yellow antheridia embedded in the dorsal thallus. Scale bar: 10 μm (h). (g) Antheridium removed from chamber in (f) showing antheridial body with sperm cells inside and stalk to the lower right. LM of two archegonia embedded in the dorsal thallus in longitudinal section showing from the base up: egg cell, ventral canal cell, neck cells and cover cells. Scale bar: 10 μm. (i) LM of biflagellate sperm. Coiled cell body is on the left and the flagella are on the right (yellow arrow). Scale bar: 5.0 μm. (m) LM longitudinal section of thallus with an open archegonium containing only the egg cell near the apical notch. Dorsal side (red asterisk) and ventral side (orange asterisk). Scale bar: 25 μm. (k) Sporophyte with stomata. Scale bar: 20 μm. (l) Confocal fluorescence microscopy image of transgenic gametophyte showing single plastids in each cell. Green: green fluorescent protein localised in the plasma membrane expressed under the CaMV 35S promoter. Red: chlorophyll autofluorescence. Scale bar 50 μm. (m) (n) Higher magnification LM of a stoma with two guard cells surrounding a pore. Scale bar: 10 μm. (o) Scanning electron micrograph (SEM) of distal side of a spinose spore. Scale bar: 10 μm.
2. A. agrestis laboratory cycle
A. agrestis plants have a size of approximately 0.5 - 1 cm in laboratory conditions, and can be maintained by vegetative (clonal) propagation under axenic conditions (Fig. 1b). There is no requirement for elaborate growth media or specialised growth facilities. Plants can routinely be propagated by transferring, on a monthly basis, small thallus fragments (usually 1 mm x 1 mm) on petri dishes containing media with a source of nitrogen, potassium, calcium, magnesium and ferrous (such as BCD or 1/10 KNOP media (Szövényi et al., 2015)). A carbon source supplementation is not necessary, however, addition of 2% (w/v) of sucrose can enhance growth. Plants do not tolerate high light intensity (growth is optimal when light intensity is below 1500 lux), however, photoperiod is not crucial with an 8 hours light : 16 hours dark regime being preferable. Two different isolates are currently available, the Oxford (originally from Scotland with cultures established at Oxford University) and the Bonn strain (originally from Germany). The conditions for successful induction of gametangia and subsequent sporophyte production have been determined (Szövényi et al., 2015). The entire life cycle of A. agrestis, from spore to spore, can be completed within 3 months under laboratory conditions, which is similar to other established bryophyte model species such as the liverwort Marchantia polymorpha. Sporophyte induction is more efficient in the Bonn than the Oxford isolate. It can be achieved by transferring plants (thallus) grown for 1 month at 22 °C to 16 °C under an 8 hours light: 16 hours dark photoperiod regime. Approximately a month later antheridia produced in the thallus are visible as yellow-orange spots. For fertilisation, the addition of water on the thallus is necessary. Sporophytes develop after an additional month (Fig. 1c). Protocols for DNA and RNA extraction are also available despite hornworts appearing to have high polysaccharide and polyphenol content, which usually make nucleic acid extraction challenging. Finally, a simple Agrobacterium mediated transformation technique is currently under optimisation, with fluorescent proteins such as the green fluorescent protein (GFP) being successfully expressed in A. agrestis plants (Fig. 2l). The tissue used for transformation is the haploid thallus, thus there is no need for crosses to establish inbred lines like in many tracheophyte plant model systems.
3. A. agrestis genome
The genomes of the two A. agrestis isolates have been sequenced (Li et al., 2020) and a high quality genome assembly has been generated with an estimated genome size of 126.1 Mb (Oxford isolate) and 124.5 Mb (Bonn isolate), amongst the smallest in land plants (Bowman et al., 2017; Pellicer et al., 2018). The number of predicted protein-coding genes varies between 24,700-25,800 with an estimated chromosome number of six (Li et al., 2020). There is no evidence of whole-genome duplication but 36-38% of the genome is estimated to be composed of repetitive and transposable elements, with the most abundant being Long Terminal Repeats (LTR) retrotransposons. A whole genome assembly is also available for Anthoceros punctatus (Li et al., 2020) and Anthoceros angustatus (Zhang et al., 2020). The A. punctatus genome is about 10-20 Mb larger than the A. agrestis genomes mainly due to repeat expansions. Nevertheless, the A. agrestis and A. punctatus genomes are largely collinear with a very similar gene complement while fewer genes were predicted for A. angustatus. Hornworts also have the smallest set of transcription associated proteins (TAP) (Wilhelmsson et al., 2017) among all land plant groups sequenced to date. Therefore, the A. agrestis genome represents an appropriate model system for genetic studies owing to its small and paralog poor genome.
III. Phylogeny of land plants and hornworts
The monophyly of tracheophytes (lycophytes, ferns, gymnosperms and angiosperms) is well-supported, while the interrelationships among bryophyte lineages and tracheophytes has been the subject of a long-standing debate (Nishiyama et al., 2004; Qiu et al. 2006; Wickett et al., 2014; Morris et al., 2018).
Until recently the widely accepted hypothesis was that bryophytes are paraphyletic, with liverworts, mosses, and hornworts successive sister lineages to tracheophytes (Qiu et al. 2006) (Fig. 3a). However, several recent phylogenomic studies challenged this view. In particular, the monophyly of liverworts and mosses (Setaphyta) is well-supported, with hornworts either sister to Setaphyta (i.e. bryophyte monophyly) or to all land plants (Morris et al., 2018; Renzaglia et al. 2000, 2018) (Fig. 3b & c). Monophyly of bryophytes was further supported by the analysis of over 1000 plant transcriptomes (Leebens-Mack et al., 2019) and by two more studies using whole genome information (Harris et al., 2020; Li et al., 2020; Zhang et al., 2020). Therefore, there is mounting evidence that extant bryophytes are monophyletic with hornworts sister to a moss and liverwort clade (Fig. 3c). The above-mentioned analyses rejected the hypothesis that liverworts are sister to all other extant land plant lineages as proposed by Qiu et al. 2006.
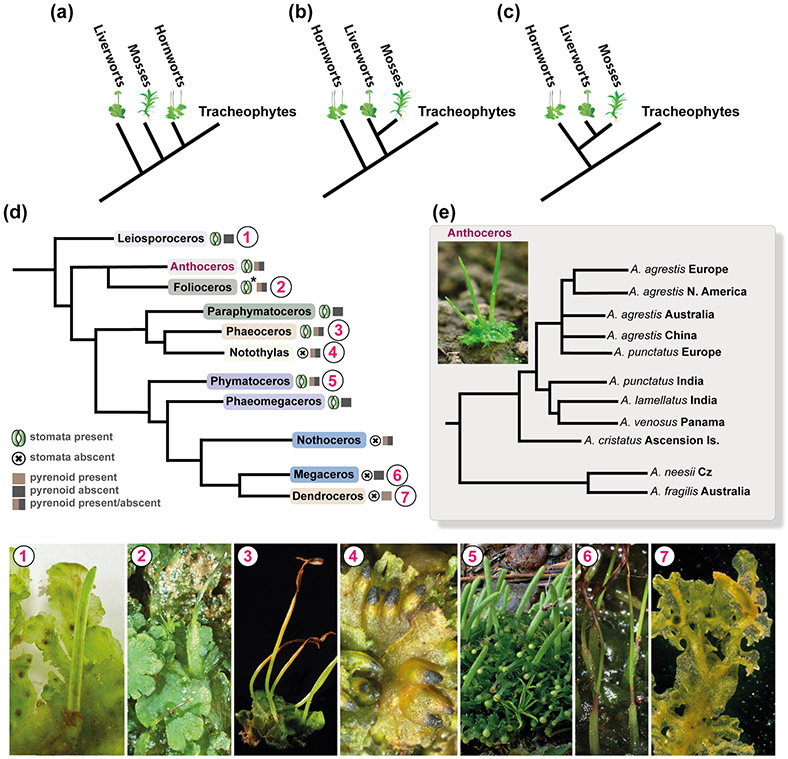
Fig. 3: Phylogeny of land plants and hornworts.
(a-c) Competing hypotheses about the phylogenetic position of hornworts among land plants. (a) Liverworts, mosses and hornworts are successive sister lineages to tracheophytes (Qiu et al. 2006). (b) Hornworts sister to all other land plants with liverworts and mosses monophyletic (Wickett et al., 2014). (c) Monophyletic bryophytes with hornworts sister to Setaphyta that include mosses and liverworts (Li et al., 2020; Renzanglia et al., 2018). (d) Phylogeny of hornworts based on Villarreal & Renner (2012), numbered circles next to the names in the phylogenetic tree correspond to species example images below. Phaeoceros photo credit: John Baker, University of Oxford. Presence and/or absence of stomata and pyrenoid is indicated next to each genera name. * exception: stomata absent in Folioceros incurvus (Renzaglia et al., 2009; Villarreal & Renner, 2012). (e) Phylogeny of the Anthoceros agrestis/Anthoceros punctatus group based on Dawes et al., 2020.
On the other hand, hornwort phylogeny is less controversial. Traditionally, a series of morphological characters were used for resolving hornwort phylogenies: thallus shape, chloroplast number per cell, presence and morphology of pyrenoid, stomata and colour of spore wall (Cargill et al., 2005). Several recent molecular analyses provided more robust insight into hornwort phylogenetic relationships and pointed to the limitations of morphological characters in clarifying relationships (Duff et al., 2007; Villarreal & Renner, 2012, 2013). Hornworts comprise 11 genera: Leiosporoceros, Anthoceros, Folioceros, Paraphymatoceros, Phaeoceros, Notothylas, Phymatoceros, Phaeomegaceros, Nothoceros, Megaceros and Dendroceros, that are placed into five orders: Leiosporocerotales, Anthocerotales, Notothyladales, Phymatocerotales, Dendrocerotales (Fig. 3d) (Villarreal & Renner, 2012). Leiosporoceros is sister to all other hornworts and the Anthocerotaceae, which includes Anthoceros and Folioceros, is sister to the remaining taxa (Söderström et al., 2016). A single and recent (less than 1 Ma) origin of the European A. agrestis has been suggested (Dawes et al., 2020) (Fig. 3e).
IV. Hornwort development
All extant representatives of streptophyte algae, the sister group of land plants, possess a haplontic life cycle in which mitotic divisions are restricted to the haploid phase that produces gametes and the diploid phase is represented by a single cell (zygote) undergoing meiosis. In contrast, mitotic divisions occur in both haploid and diploid phases of all land plants leading to the alternation of multicellular haploid and multicellular diploid phases, referred to as a haplodiplontic life cycle. In contrast to the dominant gametophyte and dependent sporophyte in bryophytes, the free-living sporophyte in tracheophytes has progressively increased in complexity, with the gametophyte reduced to just a few cells in seed plants. In flowering plants, the male and female gametophytes are represented by the pollen and the embryo sac, respectively (Niklas & Kutschera, 2009).
Because all extant taxa of streptophyte algae are haplontic, it is assumed that the origin of the haplodiplontic life cycle and that of the multicellular sporophyte phase are tightly linked to the evolution of land plants (Langdale, 2008; Bowman et al., 2016; Kenrick, 2018). The land plant sporophyte has undergone major morphological and physiological changes during evolution (Harrison, 2017; Szövényi et al., 2019). In the bryophyte lineage this generation is developmentally simple and unbranched while in the tracheophyte lineage it is a highly variable and often elaborate plant body with a wide array of organs and tissue systems. Because hornworts are sister to Setaphyta and sporophytic features of the three bryophyte clades are highly divergent, hornworts are key to reveal shared sporophytic characters with Setaphyta, and thereby provide information on the sporophytic complexity of the common ancestor of bryophytes. Moreover, hornworts are critical to better understand the evolutionary mechanisms leading to the increased complexity and dominance of the sporophytic phase in tracheophytes (Villarreal & Renzaglia, 2015). To facilitate future evolutionary studies focused on early land plants, we describe the anatomy and development of the hornwort gametophyte and sporophyte phases in the sections that follow. Comparisons are made with the model liverwort M. polymorpha and the model moss Physcomitrium patens (formerly Physcomitrella patens), and our current understanding of the genetic control of development in these plants is discussed.
1. Gametophyte
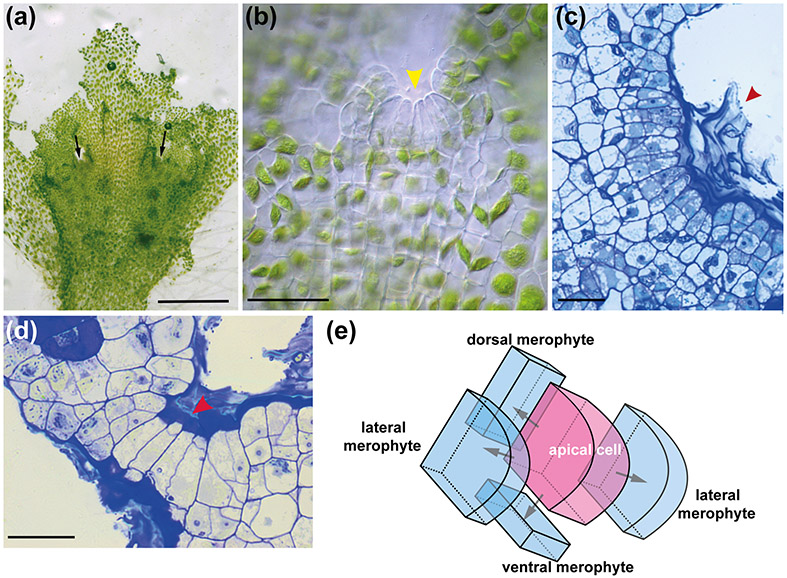
In the A. agrestis gametophyte there are multiple wedge-shaped apical cells (stem cells) with four cutting faces in notches around the thallus (Fig. 4 a-e). Derivatives from the apical cells divide and form a flattened orbicular gametophyte with clearly defined dorsal and ventral sides (Renzaglia 1978) and wavy margins. Dorsal derivatives give rise to the gametangia (Fig. 2h & m) and upper thallus while ventral derivatives form the rhizoids, Nostoc cavities and lower thallus region (Fig. 2c & e). Lateral derivatives produce tissue that “fuses” adjacent growing notches forming the rosette. In the model liverwort M. polymorpha the thallus has a single apical notch (or two if branching) and basal (ventral and dorsal) derivatives divide more than the two lateral derivatives, making the thallus elongate to form a dichotomously-branched strap-shaped gametophyte (Shimamura 2016).
Fig. 4 : A. agrestis gametophyte apical growth.
(a) Surface view of A. agrestis gametophyte. Arrows indicate apical notches. Scale bar: 1.0 mm (b) LM surface view of apical notch (yellow arrowhead) showing row of apical cell and immediate derivatives and single chloroplasts in older cells. Scale bar: 50 μm (c) LM surface section of apical notch covered by mucilage (arrowhead). Scale bar: 50 μm. (d) LM transverse section of thallus showing four rectangular cells that include the apical cell (red arrowhead) and three immediate derivatives in a growing notch covered by mucilage. The more abundant cells on either side are from divisions in the lateral derivatives. Scale bar: 50 μm. (e) Schematic representation of gametophyte apical cell (pink) with four cutting faces and four derivatives (blue).
In the model moss P. patens, gametophyte development involves the production of filamentous protonemata directly from germinating spores. Protonemata grow from their tips and side-branch initial cells differentiate into buds that develop single tetrahedral apical cells that divide to produce leafy shoots called gametophores (Kofuji & Hasebe, 2014). Each derivative from the three cutting faces of the apical cell produces a leaflet giving rise to the spiralled arrangement of leaflets on the gametophore (Renzaglia et al., 2018).
Several studies are attempting to shed light on gametophytic growth of bryophytes by focusing on the role of CLAVATA3/EMBRYO SURROUNDING REGION-related (CLE) and CLAVATA1 (CLV1) genes. CLE genes are found in all land plants studied (but not in charophytes) (Fig. 5) and can be categorised into two major subclasses: TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR-like (TDIF-like) and CLAVATA3-like (CLV3-like) (Hirakawa & Bowman, 2015; Hirakawa & Sawa, 2019). TDIF-like peptides regulate stem cell maintenance in the vasculature (Hirakawa et al., 2008) while CLV3-like peptides control stem cell maintenance in the shoot apical meristem (SAM) and partly in the root meristem (Barton, 2010; Kim et al., 2017). TDIF and CLV3 act via receptors: TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR RECEPTOR (TDR) for TDIF and CLAVATA1 for CLV3 (Hirakawa & Sawa, 2019).
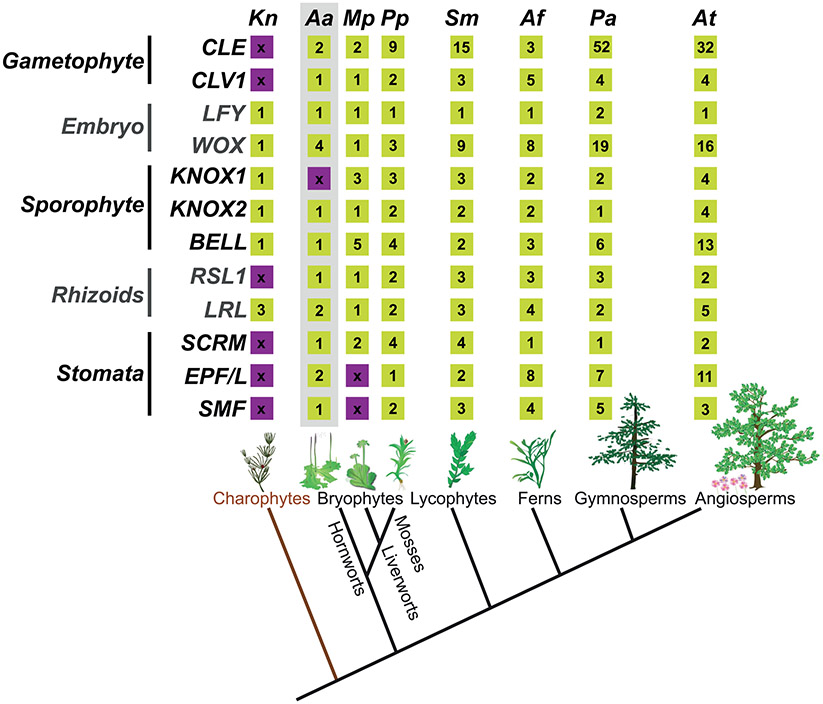
Fig. 5: Key developmental genes of land plants.
Key genes controlling, gametophyte, embryo, sporophyte, rhizoid and stomata development in bryophytes. Bottom: Phylogenetic relationships of the major lineages of land plants illustrating the monophyly of bryophytes, lycophytes, ferns, gymnosperms and angiosperms (tracheophytes) (Li et al., 2020). CLE, refers to the number of CLE signalling peptide encoding genes. Kn: Klebsormidium nitens, Aa: Anthoceros agrestis, Mp: Marchantia polymorpha, Pp: Physcomitrella patens, Sm: Selaginella moellendorffii, Af: Azolla filiculoides, Pa: Picea abies and At: Arabidopsis thaliana. Numbers in yellow boxes indicate the number of homologs in the corresponding species genome. Purple boxes with “x” indicate the absence of the homolog (Miwa et al., 2009; Bowman et al., 2017; Li et al., 2018; Whitewoods et al., 2018; Zhang et al., 2020; Li et al., 2020; Nystedt et al., 2013) .
The genome of M. polymorpha has two CLE homologs, MpCLE1/TDIF and MpCLE2/CLV3, as well as homologs of their respective receptors, MpTDR and MpCLV1. MpCLE1 and its receptor gene MpTDR are expressed in distinct patterns across the gametophyte apical notch and act as a negative regulator of gametophytic apical cell meristematic activity (Hirakawa et al., 2019). In the moss P. patens, there are nine (Goad et al. 2017) or seven (Whitewoods et al., 2018; Whitewoods, 2020) potential CLE genes (Fig. 5) and two CLV1 genes. All CLE genes belong to the CLV3-like subclass while genes of the TDIF-like subclass are absent. Three CLE genes and their receptors PpCLV1a, PpCLV1b and PpRPK2 are co-expressed in the gametophyte shoot regulating orientation of stem cell division planes during the transition from 2D to 3D growth in the gametophyte (Whitewoods et al., 2018). The role of CLV1 in regulating cell division plane orientation in P. patens has been shown to be shared with Arabidopsis thaliana but not with M. polymorpha. A. agrestis has one TDIF-like and one CLE encoding gene and a single CLV1 (Fig. 5) and it can be speculated that they may play a role in gametophyte growth regulation similar to other bryophytes.
Additional genes seem to be part of the network regulating apical cell development in P. patens, such as the DEFECTIVE KERNEL 1 (DEK1) (Perroud et al., 2020) and the NO GAMETOPHORES 1 (NOG1) (Moody et al., 2018) and genes encoding polycomb group (PcG) and PIN proteins (Bennett et al., 2014). For example, the P. patens Fertilization Independent Endosperm (PpFIE) gene which encodes a PcG protein, is expressed only in the gametophyte apical cells and in cells that undergo fate transition. In the absence of PpFIE, gametophore meristems overproliferate but also fail to further develop and reach the reproductive phase. This illustrates the key role of PpFIE in the regulation of differentiation and proliferation of gametophytic stem cells in P. patens (Mosquna et al., 2009). Similar observations were made on knock outs involving the P. patens CURLY LEAF (CLF), another PcG protein (Okano et al., 2009). A. agrestis has homologs of NOG1, DEK1, PpCLF and PpFIE (Li et al., 2020). To what extent these genes are also involved in the regulation of gametophyte apical growth in A. agrestis needs to be confirmed by functional studies.
2. Rhizoids
Tracheophytes have specialised, complex multicellular structures on the sporophyte called roots that are involved in water and nutrient uptake, anchoring the plant to a substrate and mediating symbiosis (Jones & Dolan, 2012). Roots have outgrowths of epidermal cells called root hairs that effectively increase the total surface area for water absorption. Bryophytes on the other hand lack roots and have instead simple tip-growing filamentous cells, called rhizoids (Fig. 2b & c) which are unicellular in hornworts and liverworts but multicellular in mosses. Rhizoids and root hairs serve a similar function allowing nutrient absorption from the soil and anchorage (Jones & Dolan, 2012). In all bryophytes including hornworts rhizoid development is restricted to the gametophyte. All hornworts have unbranched rhizoids with the exception of Megaceros and Nothoceros in which rhizoids branch at the tip (Renzaglia, 1978).
Class 1 ROOT HAIR DEFECTIVE SIX-LIKE (RSL) genes regulate root hair development in the sporophyte of A. thaliana and rhizoid development in the gametophyte of bryophytes M. polymorpha and P. patens (Catarino et al., 2016; Menand et al., 2007; Proust et al., 2016). Similarly, LOTUS JAPONICUS ROOTHAIRLESS1-LIKE (LRL) group XI bHLH transcription factors also play a key role in the regulation of root hair and rhizoid development in M. polymorpha, P. patens and A. thaliana (Tam et al., 2015).
RSL-LRL genetic networks have been repeatedly deployed to control rhizoid or root hair development in different land plant groups during evolution. A. agrestis has a single class 1 RSL homolog and two RLR homologs (Fig. 5). It is very likely that the same set of genes regulate rhizoid development in A. agrestis. It must be noted though that not all elements of the genetic network that controls rhizoid development are likely to be conserved. FEW RHIZOIDS1 (MpFRH1), a microRNA that acts as a negative regulator of the class 1 RSL gene in M. polymorpha, has recently been identified (Honkanen et al., 2018; Thamm et al., 2020). This finding demonstrated that the negative regulators of class 1 RSL genes, at least in liverworts, are different from those in A. thaliana where class 1 RSL genes are negatively regulated by a Class IV homeodomain-leucine-zipper protein AtGL2 (Di Cristina et al., 1996; Bernhardt et al., 2003; Lin et al., 2015).
3. Embryo
In hornworts, after fertilisation the first division of the zygote is parallel to the longitudinal axis of the archegonium (Fig. 6a) (Renzaglia 1978). This is unlike other bryophytes in which the initial division is transversal. Leptosporangiate ferns also have longitudinal first divisions in the zygote and this may be related to hornworts and ferns having zygotes and embryos in an embedded archegonium (Shaw & Renzaglia, 2004; Johnson & Renzaglia 2009). Subsequent divisions give rise to the embryo which is composed of three tiers (Fig. 2a) (Ligrone et al., 2012). The first (lowest) tier produces the foot early in development of the sporophyte. The second (middle) tier gives rise to the meristematic area of the sporophyte, referred to as the basal meristem. Finally, the third (top) tier gives rise to the tip of the sporophyte capsule. The first and the third-tier cells stop dividing early in sporophyte development.
Fig. 6: A. agrestis embryo and sporophyte.
(a) Differential interference contrast image of embryo with first longitudinal division (yellow arrowhead). Scale bar 10 μm. (b) Sporophyte. Spores mature progressively from the bottom to the top of the sporophyte. Involucre indicated with black arrow. Scale bar: 0.5 mm. (c) Schematic representation of sporophyte. The sporophyte has a foot, a basal meristem, the columella, a spore layer with pseudoelaters (for spore dispersal), a multicellular assimilative layer, and stomata. Stomata successive developmental stages are shown next to the sporophyte. Opening of the pore occurs near the base of the sporophyte then guard cells wall thickens, guard cells collapse toward the upper part of the sporophyte, allowing dehydration and dehiscence into two valves. Involucre indicated with black arrow. Numbered circles indicate the relative position on the sporophyte that corresponds to the cartoons on the right of panel: 1. Schematic representation of longitudinal section directly above the foot showing the basal meristem and differentiating sporogenous tissue. 2. Schematic representation of transverse section showing from centre to outside, columella, spores, pseudoelaters, assimilative tissue, epidermis and stomata with substomatal cavities. (d) LM longitudinal section of bulbous foot and basal meristem surrounded by the involucre. The placenta consists of elongated haustorial foot cells adjacent to small gametophyte cells. Scale bar: 50 μm. (e) Enlargement of placental cells in (d) showing smooth walled haustorial cells (red arrowhead) intermixed with gametophyte cells (orange arrowhead) with conspicuous cell wall ingrowths (orange arrowhead) Scale bar: 10 μm (f) LM longitudinal section of archesporial tissue (orange arrowhead) surrounding columella (red arrowhead) directly above the basal meristem. Scale bar: 20 μm (g) Transmission electron microscopy (TEM) image of columella in cross section showing 4 by 4 arrangement of the 16 living cells that contain dense cytosol and chloroplast (yellow arrowhead). Scale bar: 4.0 μm (h) LM section showing spore mother cells (red asterisks) with three of four large starch-filled plastids in four poles and central nucleus preparing for meiosis. Scale bar: 20 μm (i) TEM of dying and collapsing stoma (brown in (c)) showing thickened walls, inner and outer ledges of guard cells, and substomatal cavity. This section is on the polar end of the guard cells, away from the pore. Scale bar: 5 μm (j) SEM of a proximal surface of spore with a defined trilete mark. Scale bar: 10 μm. (k) TEM of spore in a tetrad still surrounded by the spore mother cell wall showing three-layered wall with ornamentation. The aperture on the proximal wall where spores in a tetrad meet each other has a thick intine and includes the trilete mark (red arrow). Scale bar 4.0 μm. (l) TEM of mature spore wall composed of outer exine (orange arrow), thick inner exine with compressed globular sporopollenin (green arrow) and thin intine that is much like a primary cell wall (red arrow). Protein bodies fill the spore (pink arrow). Scale bar 0.5 μm. (m) LM of dissected columella with elongated multicellular pseudoelaters attached. Scale bars: 25 μm.
In P. patens FLORICAULA/LEAFY (FLO/LFY) is required for the first cell division in the zygote (Maizel et al., 2005; Tanahashi et al., 2005). P. patens has two LFY genes, PpLFY1 and PpLFY2, (Tanahashi et al., 2005) and in loss-of-function mutants the zygote nucleus does not divide after fertilization. Notably, P. patens LFY does not complement LFY mutants in A. thaliana indicating that LFY function in angiosperms has diverged from that in bryophytes (Tanahashi et al., 2005). It has been demonstrated that these differences in LFY function can be attributed to specific amino acid substitutions in the DNA binding domain of the LFY protein (Maizel et al., 2005). A. agrestis has a single LFY homolog (Fig. 5) and its expression seems to be more pronounced in the gametophyte stages (Li et al., 2020) unlike the predominantly sporophytic expression in P. patens. Future functional analysis will elucidate its potential role in embryo development in hornworts.
WUS-related homeobox (WOX) genes (Haecker et al., 2004) regulate key aspects of plant development, such as stem cell maintenance (Laux et al., 1996; Kamiya et al., 2003; Hirakawa et al., 2010 ), and zygote and embryo development (Breuninger et al., 2008; Ueda et al., 2011). WOX genes can be grouped into three subclasses (Zhang et al., 2017; Wu et al., 2019): the WUSCHEL (WUS) clade, WOX9 clade and WOX13 clade. All WOX genes in the P. patens genome belong to the WOX13 clade (van der Graaff et al., 2009). P. patens zygotes lacking activity of the two WOX13-like genes are unable to elongate and initiate the apical cell of the embryo (Sakakibara et al., 2014). This function is different from the A. thaliana WOX13 gene which promotes replum formation in the fruit (Romera-Branchat et al., 2013) and WOX14 which promotes vascular cell differentiation (Denis et al., 2017). The A. agrestis genome has four WOX13 clade members (Fig. 5). WOX13-like 1 is specifically expressed in sporophytes while WOX13-like 2, 3 and 4 are expressed in both the gametophyte and sporophyte (Li et al., 2020) and may have diverse roles in stem cell maintenance and embryo development.
4. Sporophyte
A single gametophyte thallus can produce multiple sporophytes (Fig. 1d). Similar to other bryophyte groups the hornwort sporophyte is largely dependent on the gametophyte (Renzaglia et al., 2000). The A. agrestis sporophyte grows from a basal meristem (Fig. 6b-d) that continuously produces new sporophytic tissue upwardly and eventually gives rise to spores and pseudoelaters. Spores mature progressively from the bottom to the top of the sporophyte. The basal meristem remains active throughout the entire life of the sporophyte (Renzaglia et al., 2009). At maturity, the sporophyte is composed of (from the centre to the outer layer) (Fig. 6c): the columella, sporogenous tissue, assimilative (photosynthetic) tissue and epidermis. The columella is important in spore dispersal and is composed of 16 cells that are living until sporophyte dehiscence (Fig. 6c, f & g). From the basal meristem (Fig. 6d), two layers of cells begin to differentiate between the columella and assimilative tissue, forming the archesporium that gives rise to the sporogenous tissue, including spore mother cells and sterile pseudoelaters (Fig. 6c, f & h). Sporogenesis begins with the differentiation and enlargement of rounded spore mother cells (Fig. 6c) that undergo meiosis and develop into the spores (Fig. 6j & k). Pseudoelaters are interspersed among sporogenous cells, they are multicellular and do not undergo meiosis. Spore maturation involves the development of a three-layered spore wall (Fig. 6l) that consists of a thin outer layer followed by a thick inner layer with globular sporopollenin (called outer and inner exine respectively), and an innermost layer (called intine) similar to the primary cell wall in composition except that it contains callose (Renzaglia al. 2020). The proximal spore surface (where spores of the tetrad meet) has an aperture with thickened intine, which is the site of germination (Fig. 6j & k). Spore tetrads are surrounded by the spore mother cell wall until late in development when all wall layers are compacted (Fig. 6l). Storage material in the spores consists of protein and oils (Fig. 6l).
The basis of the sporophyte is surrounded by gametophytic tissue called involucre (Fig. 6b & c) and is anchored in the gametophyte by the bulbous foot that contains peripheral cells (called haustorial) that elongate into gametophytic cells forming the placenta (Fig. 6d & e). Unlike the vast majority of land plants, cell wall ingrowths typical of transfer cells in hornworts are restricted to gametophyte cells of the placenta (Fig. 6d & e). Sporophyte haustorial cells have thin smooth walls and penetrate the surrounding gametophytic tissue allowing efficient transfer of nutrients from the gametophyte to the sporophyte (Fig. 6d & e) (Ligrone & Renzaglia, 1990).
In P. patens the sporophyte initially grows from an apical cell that forms in the first few cell divisions of the embryo, but apical cell activity ceases after approximately 12 cell divisions (Sakakibara et al., 2008). A new multicellular meristem, called the intercalary or seta meristem, is then formed in the middle of the sporophyte (Sakakibara et al., 2008). Meristematic activity of the seta meristem terminates and is followed by expansion of the sporangium or capsule. The sporophyte of the liverwort M. polymorpha does not possess a well-defined meristematic region, and cell divisions occur throughout the developing tissue (Shimamura, 2016). In tracheophytes, the sporophyte grows from the shoot apical meristem (SAM) and root apical meristem, that are composed of one, two or numerous stem cells (Harrison, 2017). The mechanisms by which apical stem cell activity evolved in tracheophyte sporophytes remain elusive. It has been hypothesised that the tracheophyte SAM evolved from an embryonic apical meristem, similar to the one present in extant mosses (Albert, 1999). Transcriptomic data suggest that tracheophyte meristems may have evolved independently in lycophytes, ferns and seed plants (Frank et al., 2015). It has also been hypothesised that the hornwort basal meristem evolved into the tracheophyte SAM by displacement to the shoot apex (Ligrone et al., 2012).
A series of studies indicates that a small family of transcription factors called KNOTTED1-LIKE HOMEOBOX (KNOX) genes played a key role in the evolution of the land plant sporophyte (Hay & Tsiantis, 2010). KNOX genes are found in all green plant lineages, from chlorophytes to angiosperms. Insight into the role of KNOX genes in lineages that diverged before plants colonized the land was provided by a study in the chlorophyte alga Chlamydomonas reinhardtii (Lee et al., 2008). C. reinhardtii has two types of gametes, plus and minus, that express the KNOX protein GAMETE SPECIFIC MINUS 1 (GSM1) and the BELL protein GAMETE SPECIFIC PLUS 1 (GSP1), respectively. GSM1 and GSP1 proteins accumulate in the gametes, physically interact with each other upon gametic fusion and translocate from the cytosol to the nucleus to initiate zygote development.
KNOX genes have diversified into two subfamilies, class 1 and class 2 (Fig. 5). The duplication leading to class 1 and class 2 KNOX genes occurred within the ancestor of land plants and charophytes since homologs for both classes have been identified in the charophytes Spirogyra pratensis (Frangedakis et al., 2017) and Klebsormidium nitens (Hori et al., 2014).
In P. patens class 1 KNOX genes acquired functions to control meristematic activity in the sporophyte (Sakakibara et al., 2008; Coudert et al., 2019) and class 2 KNOX genes evolved to maintain the diploid state through suppression of the gametophytic development program (Sakakibara et al., 2013). Similar to chlorophytes, land plant KNOX proteins function via the formation of heterodimers with BELL proteins. One of the four BELL genes in P. patens, PpBELL1, is necessary and sufficient for sporophyte development (Horst et al., 2016). In M. polymorpha a class 1 KNOX protein is expressed in the egg and is necessary for the formation of the zygote via interaction with two paternally inherited BELL proteins (MpBELL3 and/or MpBELL4) (Dierschke et al., 2020), a function similar to the KNOX function in C. reinhardtii. The class 1 KNOX, another MpBELL1 and class 2 KNOX genes, are also upregulated in the developing sporophytes of M. polymorpha suggesting a role in sporophyte development (Frank & Scanlon, 2015; Flores-Sandoval et al., 2018; Hisanaga et al., 2020). Collectively, these observations indicate that the ancestral mechanism to initiate zygote development in C. reinhardtii was retained in M. polymorpha but also diversified to control sporophyte development in both M. polymorpha similar to P. patens. The function of KNOX genes further diversified during the evolution of tracheophytes to control shoot meristem establishment and maintenance as indicated by studies in the lycophyte Selaginella kraussiana and A. thaliana (Harrison et al., 2005). Hornworts have only a single KNOX gene that belongs to the class 2 subfamily and a single BELL gene (Fig. 5). The presence of a single KNOX gene in the hornwort A. agrestis is an intriguing finding given that other bryophytes and charophytes carry both class 1 and 2 KNOX genes. A possible scenario is that the absence of class 1 KNOX genes in A. agrestis represents a secondary loss within hornworts, or at least within the genus Anthoceros. The function of class 2 KNOX genes in A. agrestis is unknown and may be key to understanding how the function of class 1 and 2 genes has diversified during land plant evolution.
5. Stomata development
Most hornworts have stomata on their sporophytes (Fig. 2k & n and Fig. 6c). Recent studies suggest that stomata in hornworts are involved in sporophyte dehydration and spore dispersal (Renzaglia et al., 2017; Pressel et al., 2018). Stomata develop at the base of the sporophyte and initially consist of two guard cells covering a liquid filled intercellular space (ICS). Opening of the pore occurs near the base of the sporophyte before guard cells wall thickens, which allows the liquid inside the ICS to evaporate, favouring the dehydration and dehiscence of the sporophyte (Fig. 6c & i). There is still an open debate on the different functions of stomata between tracheophytes and bryophytes. Tracheophytes have gas filled ICSs, surrounded by guard cells which open and close in response to environmental cues, preventing water loss and optimising CO2 assimilation. In contrast, stomata of hornworts remain open after the pore is formed and they die and collapse prior to spore maturation (Merced & Renzaglia 2017; Renzaglia et al. 2017). It is hypothesized that this allows for the evaporation of the liquid inside the intercellular spaces and to promote spore maturation and dispersal. Hornwort stomata lack physiological response to exogenous signals such as abscisic acid, water availability and CO2 (Pressel et al., 2018). The small changes in the size of the aperture are probably the result of desiccation and not active closing of the pore. Different composition of guard cell walls in hornworts compared with A. thaliana supports the inability of hornwort stomata to regulate the opening and closing of the pore (Merced & Renzaglia 2019).
Stomatal development is regulated by a set of genes in A. thaliana (Chater et al., 2016, 2017), including the basic helix-loop-helix (bHLH) transcription factors SPCH, MUTE and FAMA (SMF), ICE/SCREAM (SCRM), the signal peptide EPIDERMAL PATTERNING FACTOR (EPF) and its receptors ERECTA and TOO MANY MOUTHS (TMM) (Chater et al., 2016; Lee & Bergmann, 2019). The origin of stomata (single or multiple) and the evolution of the developmental mechanism/functionality of stomata across land plants are in debate (Chater et al., 2017; Renzaglia et al. 2020). Recently, it was hypothesised that the common ancestor of embryophytes possessed the core genetic toolkit for stomata development, which were then lost or reduced during the evolution of bryophytes (Harris et al., 2020). A gene encoding a protein similar to SPCH/MUTE/FAMA/SMF was present in the common ancestor of embryophytes. A duplication event led to its divergence into a SPCH/MUTE and a FAMA/SMF clade. The FAMA/SMF clade further diversified into a FAMA and a SMF clade before the divergence of tracheophytes and bryophytes. SMF was lost in most tracheophytes whereas FAMA and SPCH/MUTE were lost in all bryophytes (Harris et al., 2020). ERECTA, SCRM, EPF and TMM homologs were also present in the common ancestor of embryophytes Harris et al., 2020). In line with this hypothesis, orthologs of SMF, SCRM, ERECTA, EPF, and TMM are present in A. agrestis (Fig. 5). SMF, SCRM, TMM and EPF are expressed in the sporophyte, suggesting that they may have similar roles in stomata development (Li et al., 2020). Genetic studies in A. agrestis will help to elucidate the evolution of stomata in hornworts and across land plants.
V. The hornwort chloroplast
1. Monoplastidy
Chloroplasts evolved from an endosymbiotic event between a eukaryotic host and a photosynthetic prokaryote, more than a billion years ago (Jensen & Leister, 2014). Unique amongst land plants, all hornworts have one (or just a few) chloroplast(s) per cell (Fig. 7) with the exception of a small number of lycophyte species (Vaughn et al. 1992; de Vries & Gould, 2018; Liu et al., 2020). All other land plants have several chloroplasts per vegetative cell. The increase in number of chloroplasts per cell and the subsequent reduction in their size increases the surface area to volume ratio of the chloroplast which leads to an enhanced photosynthetic efficiency (Xiong et al., 2017). Another hypothesis states that many small chloroplasts allows movement and rearrangement within the cell for more effective light acclimation (Trojan & Gabrys, 1996; Königer et al., 2008), and reduces the chance of damage to the photosystem II complex under high-light conditions (Park et al., 1996). If one organelle is damaged, a cell has many more chloroplasts as back-up. The evolution of multiple chloroplasts per cell (polyplastidity) is poorly understood. The complete loss of the peptidoglycan (PG) layer of the chloroplast might be linked with the switch from mono- to polyplastidy in land plants, but it cannot be the only driving factor because the PG layer is absent in rhodophytes (Grosche & Rensing, 2017), which are predominantly monoplastidic. Hornworts are therefore a key group in understanding the evolution of polyplastidy in land plants.
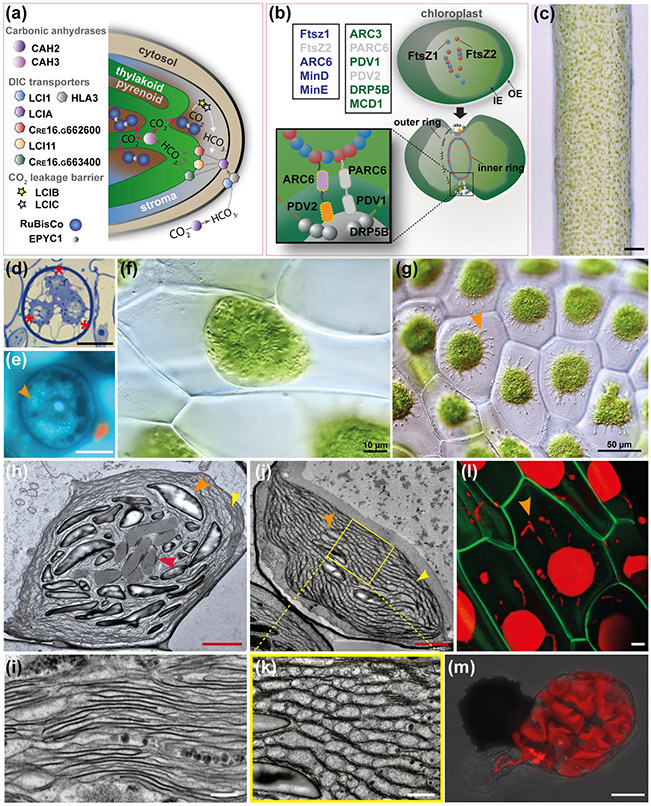
Fig. 7: A. agrestis chloroplast.
(a) Schematic representation of C. reinhardtii CCM model. The carbonic anhydrase (CAH2) converts CO2 into HCO3− (dicarboxylate (DIC)) in the periplasmic space. DIC is pumped across membranes via DIC transporters localised in the plasma membrane (low-CO2 inducible 1 (LCI1) and high light activated 3 (HLA3)), the chloroplast envelope (low-CO2 inducible A (LCIA)) and the thylakoid membrane (low-CO2 inducible 11 (LCI11), Cre16.g662600 and Cre16.g663400). The carbonic anhydrase 3 (CAH3) in the thylakoid lumen converts HCO3− into CO2. supplied to RuBiSCo. EPYC1 is acting as glue between RuBiSCo units in the pyrenoid. The low-CO2 inducible B and C (LCIB/C) proteins are thought to form a molecular “ring” around the pyrenoid that acts as a barrier to CO2 leakage transferring CO2 back to the thylakoid via the DIC pumps. (b) Top left boxes: Genes of prokaryotic origin (in blue) and genes of land plant origin (in green) involved in chloroplast division in A. thaliana. Genes absent in A. agrestis genome are in grey. Top right and bottom: Schematic representation of the key elements of chloroplast division machinery in A. thaliana (Chen et al., 2018). FtsZ1 and FtsZ2 self-assemble and then recruit ARC6, PARC6, PDV1, PDV2 and DRP5B forming a ring around the chloroplast that mediates its division. IE: inner envelope, OE: outer envelope. (c) Surface view of sporophyte showing chloroplasts. Scale bar: 100 μm. (d) LM section of spore mother cell with plastids (three out of four visible) at poles indicated with asterisks. Scale bar: 15 μm (e) LM of spore mother cell stained with DAPI showing DNA in central nucleus and plastids (orange arrowhead). Scale bar: 15 μm (f) Cells of young gametophyte tissue with a single chloroplast and central pyrenoid surrounded by starch grains. Scale bar: 10 μm (g) Cells of mature gametophyte tissue with single chloroplasts that have several protrusions that are likely stromules (orange arrowhead). Scale bar: 50 μm. (h-k) TEMs of chloroplasts of A. agrestis. (h) Chloroplast in an assimilative cell of sporophyte near intercellular space, with pyrenoid (red arrowhead) traversed by thylakoids and small grana (orange arrowhead). Starch granules indicated by yellow arrowhead and thylakoids enlarged in (i) at yellow arrowhead. Scale bar: 2.0 μm. (i) Details of thylakoids and grana showing absence of end membranes. Scale bar: 0.5 μm. (j&k) Sporophyte chloroplast in an assimilative cell near intercellular space traversed by channel thylakoids and grana stacks (yellow arrowhead). Starch grains indicated by orange arrowhead. Pyrenoid is not in this non-median section. Scale bar: 500 nm. (k) Higher magnification (scale bar: 0.5 μm) of region indicated in (j) showing channel thylakoids (red arrowhead). Scale bar: 2.0 μm. (l) Confocal microscopy image of A. agrestis transgenic gametophyte. Green: green fluorescent protein localised in the plasma membrane expressed under the CaMV 35S promoter. Red: chlorophyll autofluorescence. Orange arrowhead indicates chloroplast protrusions likely to be stromules. Scale bar: 10 μm (m) Confocal microscopy image of a germinating spore highlighting the wavy 3D structure of the chloroplast. Red: chlorophyll autofluorescence. Scale bar: 20 μm.
Unlike the evolution of the mechanism that led to polyplastidy, the molecular mechanisms of plastid division in land plants is well understood (Fig. 7b) (Chen et al., 2018; Okazaki et al., 2010). Cell division, both mitosis and meiosis, in hornworts involves plastid divisions that are closely linked with the nuclear division (Figs. 7d & e) (Brown & Lemmon 1990, 1993). In meiosis, the large starch-filled plastids migrate to the four poles and form the focal points for the quadripolar spindle (Fig. 7e). Throughout the process, plastid DNA is visible and abundant (Fig. 7d). A series of genes are known to be involved in plastid division machinery in A. thaliana including, the Filamentous Temperature Sensitive Z 1 (FtsZ1), FtsZ2, Accumulation and Replication of Chloroplast 6 (ARC6), Dynamin-related protein 5B (DRP5B), Septum site-determining protein MinC, E and D, Plastid Division protein 2 (PDV2), Plastid Division protein 1 (PDV1), Multiple Chloroplast Division site 1, Paralogue of ARC6 (PARC6), Accumulation and Replication of Chloroplast 3 (ARC3) (Fig. 6e). The A. agrestis genome lacks homologs of PARC6 and PDV1 consistent with the current understanding that they diversified after tracheophytes diverged, however A. agrestis also lacks an FtsZ2 homologue (Fig. 7b) (Li et al., 2020). FtsZ2 knock-out mutants in P. patens and A. thaliana (Martin et al., 2009; Schmitz et al., 2009) result in cells that contain a single chloroplast. This raises the question of whether the lack of FtsZ2 in A. agrestis is related to its monoplastidy. FtsZ2 is also absent in the streptophyte algae Mesostigma viride, Chlorokybus atmophyticus and C. braunii but not in K. nitens and Zygnematophyceae (Hori et al., 2014; Nishiyama et al., 2018; Cheng et al., 2019; Wang et al., 2020). It is possible that FtsZ2 was present in the last common ancestor of land plants and streptophyte algae and then was lost several times or FtsZ2 had independent origins.
Cells of mature gametophyte tissue have tubular protrusions on the chloroplast, potentially stromules (Fig. 7g & l). Stromules have been found to be involved in various functions such as protein trafficking and effector-triggered immunity (Caplan et al., 2015; Hanson & Hines, 2018).
2. The pyrenoid
Hornworts are the only land plant group that has pyrenoids (Fig. 3d and Fig. 7i & j) (Villarreal & Renner, 2012), which are otherwise common in most unicellular algae (Meyer et al., 2017). The pyrenoid is an unbound proteinaceous specialised compartment within the chloroplast that is mainly composed of the enzyme RuBisCO (Meyer et al., 2017).
Most of our current understanding about pyrenoids is based on studies in the alga C. reinhardtii. In aquatic environments a carbon concentrating mechanism (CCM) is necessary for RuBisCO to function efficiently since CO2 diffuses 10,000 times slower in water than in air (Machingura & Moroney, 2018). The CCM mechanism in C. reinhardtii (Fig. 7a) involves a carbonic anhydrase (CAH2) in the periplasmic space that converts CO2 into bicarbonate (HCO3−). Then transport proteins, both on the cell (low-CO2 inducible 1 (LCI1) and high light activated 3 (HLA3)), chloroplast (low-CO2 inducible A (LCIA), Cre16.g662600 and Cre16.g663400) and thylakoid (low-CO2 inducible 1 (LCI11)) membranes, pump bicarbonate (HCO3−) from the environment, into the cell and into the chloroplast thylakoids (Wang et al., 2015). In the chloroplast thylakoids, CAH3 converts HCO3− into CO2 (Aspatwar et al., 2018) resulting in an up to a 50-fold increase of CO2 concentration in the pyrenoids and improving the photosynthetic efficiency of RuBisCO. In the pyrenoid of C. reinhardtii, RuBisCO is scaffolded by the Essential Pyrenoid Component 1 (EPYC1) protein (Mackinder et al., 2016) which functions as a “glue”. The remaining proteins that compose the algal pyrenoid include RuBisCO-interacting proteins, photosystem I (PSI) assembly factor candidates, and inorganic carbon flux components (Mackinder et al., 2017).
One main difference between hornworts and algal chloroplast is the presence of grana (Fig. 7h-k), stacked thylakoids, that compartmentalize chloroplast space, are enriched in photosystem II (PSII) and allow more efficient light capturing (Wilsenach, 1963; Burr, 1970; Renzaglia et al., 2009). Grana in hornworts lack the highly-curved end membranes that are common in the chloroplast of other land plants, suggesting biochemical differences (Fig. 7i) (Vaughn et al. 1992). Another unique feature of the hornwort chloroplast is the presence of thylakoids that connect adjacent grana, at a right angle to the long axis of the granum, called channel thylakoids. Channel thylakoids function in separating space within the chloroplast stroma (Fig. 7k) and are enriched in PSI. In the majority of hornworts, pyrenoids have multiple subunits traversed by thylakoids and may or may not be encircled by an outer starch sheath (Fig. 7h). In A. agrestis, the pyrenoid in vegetative cells consists of lens-shaped electron dense units delineated by thylakoids and small grana (Fig. 7h). On an anatomical level there is considerable variability in chloroplasts across hornworts. A notable example is the epiphytic Dendroceros that possesses star-shaped plastids with a large central pyrenoid containing globular units. Pyrenoidless species include Paraphymatoceros, Megaceros and some Nothoceros (up to 14 plastids per cell). In Leiosporoceros, the sister taxon to the remaining hornworts, the pyrenoidless chloroplasts have a central aggregation of massive grana, which may represent the ancestral condition in the clade.
Hornwort pyrenoids evolved independently from streptophyte algae pyrenoids (Villarreal & Renner, 2012). Genomic and transcriptomic data show that some C. reinhardtii CCM components, such as the CAH3, the LCI11 and the low-CO2 inducible B (LCIB) genes (Fig. 7a), have putative homologs in hornworts (Li et al., 2020). In C. reinhardtii, LCIB proteins localize around pyrenoids and are hypothesized to prevent CO2 leakage from the pyrenoids (Jin et al., 2016; Yamano et al., 2010) (Fig. 7a). However, several other C. reinhardtii pyrenoid related genes, such as the EPYC1, are absent in the genome of A. agrestis. To what extent hornwort pyrenoids share more common traits with streptophyte algae pyrenoids remains to be explored.
3. RNA editing
Hornworts have one of the highest levels of chloroplast RNA editing amongst all land plants studied to date (Kugita et al., 2003). RNA editing is a form of nucleotide sequence alteration that occurs at the transcriptional level in the chloroplast (Small et al., 2019). RNA editing converts cytidines (C) to uridines (U) (C-to-U, called canonical RNA editing) or uridines to cytidines (U-to-C, called reverse editing) in mRNAs before their translation. As a consequence, a sense codon can be converted into a more evolutionary conserved one or a start/stop codon to a sense codon (Tillich et al., 2006). Organelles in A. agrestis feature high amounts of RNA editing with altogether more than 1,100 sites of C-to-U and 1,300 sites of U-to-C editing (Gerke et al., 2019).
The nuclear genome also reveals over 1,400 genes for PPR proteins (Li et al., 2020). PPR proteins are a group of RNA-binding proteins which play critical roles in post-transcriptional gene regulation in plant chloroplasts (Barkan & Small, 2014). Preliminary data suggest that the few land plant lineages capable of reverse editing have evolved special types of PPR proteins providing strong candidates for future studies (Gerke et al., 2019; Gutmann et al., 2020). A. agrestis provides an exceptional system to study the poorly known mechanisms and the evolution of reverse editing in land plants.
VI. Symbiosis
Hornworts establish two types of symbiotic relationships: with cyanobacteria and with arbuscular mycorrhizal fungi (AMF) (Fig. 8).
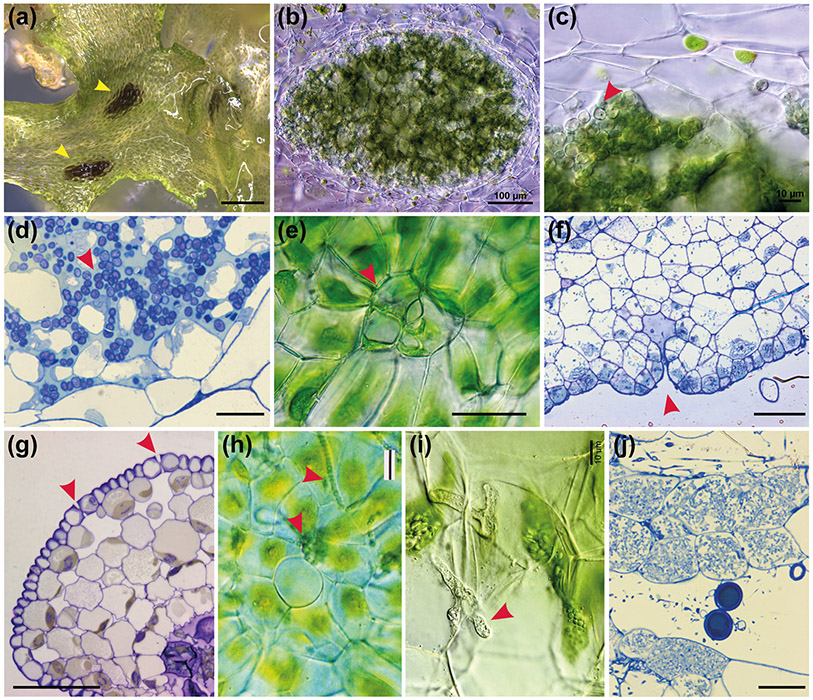
Fig. 8: Hornwort symbiotic relationships.
(a) Surface view of A. puncatus thallus colonised by cyanobacteria (yellow arrowheads). Scale bar: 450 μm. (b-c) Hand sections of A. punctatus thallus showing ellipsoidal cavities colonised by cyanobacteria. In (c) cyanobacteria indicated with red arrowhead Scale bars: 100 μm (b) and 10 μm (c). (d) LM section of Nostoc colony showing algal cells (red arrowhead) with intermingling gametophyte cells in A. agrestis. Scale bar: 10 μm (e) LM surface view of ventral mucilage cleft. Scale bar: 15 μm. (f) Longitudinal section of a mucilage cleft (red arrowhead) leading to small intercellular space near apical notch of A. agrestis. Scale bar: 50 μm (g) LM transverse section of sporophyte showing guard cell in epidermis that lead to substomatal cavities. Guard cells are larger than epidermal cells and have differentially thickened cell walls with inner and outer ledges and are different from mucilage cleft cells in (f) that have evenly thickened walls. Scale bar: 50 μm. (h) LM showing surface view of a mucilage cleft and attracted cyanobacteria just entered the cleft in Phaeoceros carolinianus. Scale bar:20 μm (i, j) Hornwort symbiotic relationship with arbuscular mycorrhizal fungi. Hand section LM of P. carolinianus thallus cells with fungal hyphae (red arrowhead). Scale bar: 10 μm. (j) LM section of gametophyte cells containing vesicles (circles) and arbuscules (masses of hyphae in cells). Scale bar: 20 μm
1. Cyanobacteria symbiosis
Cyanobacteria are prokaryotes possessing the ability to perform photosynthesis and to fix atmospheric nitrogen thus providing the host with usable nitrogen (Adams & Duggan, 2008). The earliest evidence of cyanobacteria growing within the tissue of a land plant comes from Aglaophyton major fossils (Krings et al., 2009). This finding suggests that land plant - cyanobacteria associations are probably at least 400 Ma old. Symbiosis of land plants with nitrogen-fixing cyanobacteria is uncommon, however, in hornwort endophytic associations are ubiquitous (Fig. 8a, b, c, d & h) (Renzaglia et al., 2009). The other bryophyte group that has an endophytic symbiotic relationship with cyanobacteria are liverworts (Adams, 2002), but this is rare as it occurs in only two closely related genera: Blasia and Cavicularia (Meeks, 1998). Cyanobacterial endosymbiosis is also found in the water fern Azolla (Whitton, 1993), cycads (Lindblad & Bergman, 2018), and Gunnera (an angiosperm) (Bergman et al., 1992).
Cyanobacteria that establish symbiotic relationships with plants are primarily members of the polyphyletic genus Nostoc (Dodds et al., 1995; Rai et al., 2002). Amongst all the land plant associations with cyanobacteria most of the research has been done on hornworts, using A. punctatus (Fig. 8a, b & c) and the cyanobacterium Nostoc punctiforme as the study system (Meeks, 2003). Recent transcriptomic data from both A. punctatus and A. agrestis growing with or without N. punctiforme, identified 40 candidate genes that may play a role in the symbiotic relationship (Li et al., 2020). Those genes include receptor kinases, transcription factors, and transporters.
2. Mucilage cleft
An innovation of the hornwort gametophyte was mucilage clefts (Fig. 2e and Fig. 8e, f & h). Mucilage clefts are usually two-celled epidermal structures that provide a pore for Nostoc cyanobacteria symbionts to enter and lead to a small mucilage-filled cavity (Renzaglia et al., 2009) (Fig. 8f). Superficially they bear a resemblance to sporophyte stomata in that there are typically two cells surrounding a pore (compare Fig. 2n and Fig. 8e). However, unlike guard cells, the cells surrounding the opening in mucilage clefts are not specialized and lack cell wall ledges and differential thickenings (Fig. 8f & g). Mucilage clefts are ephemeral and once Nostoc cyanobacteria enter the cleft, the epidermal cells increase in size and number and close the opening to the outside (Renzaglia 1978). The Nostoc and gametophyte cells proliferate in synchrony, producing an ellipsoidal colony with intermingling algal and hornwort cells (Fig. 8a, b, c & d). The presence of pores in both generations of A. agrestis resembles observations made on the fossil plant A. major (Krings et al., 2009).
An EPF-like gene belonging to the EPFL4-6 clade was found in A. agrestis genome, which is specifically expressed in gametophytes (Li et al., 2020). This raises the possibility that the A. agrestis EPF-like gene is involved in mucilage cleft formation, possibly controlling separation between cells to make a pore and perhaps the production of a small intercellular space comparable to the substomatal cavity.
3. Arbuscular Mycorrhizal Fungi
The symbiotic relationship with arbuscular mycorrhizal fungi (AMF) (Fig. 8i & j) is a key innovation underlying plant colonisation of terrestrial environments. The funarrgal endophytes of A. agrestis include Mucoromycotina and/or Glomeromycota (Desirò et al., 2013; Villarreal et al., 2017). AMF are present in the majority of extant land plants. Recent studies have identified a series of genes in the angiosperms that regulate the establishment and maintenance of AMF symbiosis. All the key angiosperm AMF symbiosis genes have orthologs in A. agrestis genome (Li et al., 2020).
VII. Photoreceptors
Hornworts harbor a unique chimeric photoreceptor called neochrome, that is composed of a red/far-red-sensing module from phytochrome and a blue-sensing phototropin (Li et al., 2014, 2015a,b). Neochrome was initially discovered in ferns (Nozue et al., 1998) and was considered a key innovation enabling ferns to diversify in the low-light angiosperm canopies (Kawai et al., 2003; Schneider et al., 2004). Through transcriptome- and genome-mining, Li et al (2014) found that among land plants, neochrome is restricted to ferns and hornworts, and they demonstrated that fern neochrome sequences were phylogenetically nested within those of hornworts. Such nested relationship suggests that fern neochrome was horizontally acquired from hornworts. Interestingly, hornwort phototropin lacks introns, similar to neochrome but unlike all other phototropin genes that typically have >20 introns (Li et al., 2014, 2015b). This implies that neochrome likely originated in hornworts, possibly through a retrotransposition event.
The function of neochrome in hornworts is unknown. In ferns, neochrome integrates red/far-red and blue light to orchestrate phototropism and chloroplast relocation. However, no phototropic response has been recorded in hornworts, and since most cells contain only a single chloroplast that occupy a large portion of the cellular space, it is unclear how directional chloroplast movement is possible or even necessary. On the other hand, hornwort chloroplasts can contract and expand in response to light intensity (Burr, 1969; Li et al., 2014); whether this is mediated by neochrome awaits future studies.
VIII. Conclusions
The development of A. agrestis as a hornwort experimental system, the sequencing of its genome and the availability of genetic manipulation methods, will greatly facilitate efforts towards a more comprehensive study of the mechanisms underpinning land plant evolution. It will also provide detailed understanding of hornwort biology.
Synthetic biology approaches (Liu & Neal Stewart, 2015; Benning & Sweetlove, 2016) can also be employed to engineer hornwort traits in crops. Engineering pyrenoids for example into plants with agronomic value, has the potential to increase carbon fixation and therefore increase crop yield (Li et al., 2017). Similarly, the hornwort-cyanobacteria symbiosis may hold the key to engineer crops with enhanced yield without increasing the amount of synthetic fertilizer. It is envisioned that detailed insights into the biology of hornworts have great potential to contribute to various fields of synthetic biology.
Acknowledgements
National Science Foundation (NSF) grants DEB1831428 and IOS1923011 to F.-W.L. Swiss National Science Foundation grants 160004, 131726 and 184826 to P.S.; NSF grant DUE-1758497 and NIH 5R25GM107760-07 to KSR; funding from the Georges and Antoine Claraz Foundation to P.S. and M.W.; funding from The Forschungskredit and the University Research Priority Program ‘Evolution in Action’ of the University of Zurich to M.W. and P.S.; Japan Society for the Promotion of Science (JSPS) grant nos KAKENHI 26650143 and 18K06367 to K.S.
REFERENCES
- Adams DG. 2002. Cyanobacteria in symbiosis with hornworts and liverworts In: Rai AN, Bergman B, Rasmussen U, eds. Cyanobacteria in Symbiosis. Springer, Dordrecht, 117–135. [Google Scholar]
- Adams DG, Duggan PS. 2008. Cyanobacteria-bryophyte symbioses. Journal of experimental botany 59: 1047–1058. [DOI] [PubMed] [Google Scholar]
- Albert VA. 1999. Shoot apical meristems and floral patterning: an evolutionary perspective. Trends in Plant Science 4: 84–86. [Google Scholar]
- Aspatwar A, Susanna H, Seppo P. 2018. An update on the metabolic roles of carbonic anhydrases in the model alga Chlamydomonas reinhardtii. Metabolites 8: 22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I. 2014. Pentatricopeptide repeat proteins in plants. Annual Review of Plant Biology 65: 415–442. [DOI] [PubMed] [Google Scholar]
- Barton MK. 2010. Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Developmental biology 341: 95–113. [DOI] [PubMed] [Google Scholar]
- Bennett TA, Liu MM, Aoyama T, Bierfreund NM, Braun M, Coudert Y, Dennis RJ, O’Connor D, Wang XY, White CD, et al. 2014. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Current biology 24: 2776–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C, Sweetlove L. 2016. Synthetic biology for basic and applied plant research. The Plant Journal 87: 3–4. [DOI] [PubMed] [Google Scholar]
- Bergman B, Johansson C, Soderback E. 1992. The Nostoc-Gunnera symbiosis. New Phytologist 122: 379–400. [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J. 2003. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–299. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Sakakibara K, Furumizu C, Dierschke T. 2016. Evolution in the cycles of life. Annual Review of Genetics 50: 133–154. [DOI] [PubMed] [Google Scholar]
- Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. 2008. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Developmental cell 14: 867–876. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. 1990. Monoplastidic cell division in lower land plants. American Journal of Botany 77: 559–571. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. 1993. Diversity of cell division in simple land plants holds clues to evolution of the mitotic and cytokinetic apparatus in higher plants. Mem. Torrey Bot. Club 25: 45–62. [Google Scholar]
- Burr FA. 1969. Chloroplast structure and division in Megaceros species. The Bryologist. 72: 200–209. [Google Scholar]
- Burr FA. 1970. Phylogenetic Transitions in the chloroplasts of the Anthocerotales. I. The number and ultrastructure of the mature plastids. American Journal of Botany 57: 97–110. [Google Scholar]
- Campbell DH. 1918. The structure and development of mosses and ferns (archegoniate). Third edition. New York, USA: Macmillan. [Google Scholar]
- Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP. 2015. Chloroplast stromules function during innate immunity. Developmental Cell 34: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill DC, Christine Cargill D, Renzaglia KS, Villarreal JC, Joel Duff R. 2005. Generic concepts within hornworts: historical review, contemporary insights and future directions. Australian Systematic Botany 18: 7. [Google Scholar]
- Catarino B, Hetherington AJ, Emms DM, Kelly S, Dolan L. 2016. The stepwise increase in the number of transcription factor families in the precambrian predated the diversification of plants on land. Molecular Biology and Evolution 33: 2815–2819. [DOI] [PubMed] [Google Scholar]
- Chater CCC, Caine RS, Fleming AJ, Gray JE. 2017. Origins and evolution of stomatal development. Plant physiology 174: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater CC, Caine RS, Tomek M, Wallace S, Kamisugi Y, Cuming AC, Lang D, MacAlister CA, Casson S, Bergmann DC, et al. 2016. Origin and function of stomata in the moss Physcomitrella patens. Nature plants 2: 16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, MacCready JS, Ducat DC, Osteryoung KW. 2018. The molecular machinery of chloroplast division. Plant physiology 176: 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Xian W, Fu Y, Marin B, Keller J, Wu T, Sun W, Li X, Xu Y, Zhang Y, et al. 2019. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 179: 1057–1067.e14. [DOI] [PubMed] [Google Scholar]
- Coudert Y, Novák O, Harrison CJ. 2019. A KNOX-Cytokinin regulatory module predates the origin of indeterminate vascular plants. Current biology 29: 2743–2750.e5. [DOI] [PubMed] [Google Scholar]
- Dawes TN, Villarreal JCA, Szövényi P, Bisang I, Li FW, Hauser DA, Quandt D, Cargill DC, Forrest LL. 2020. Molecular data shows a recent European origin of the model bryophyte Anthoceros agrestis. Plant Systematics and Evolution, 306: 49 10.1007/s00606-020-01676-6. [DOI] [Google Scholar]
- Delwiche CF, Cooper ED. 2015. The evolutionary origin of a terrestrial flora. Current Biology: 25: R899–910. [DOI] [PubMed] [Google Scholar]
- Denis E, Kbiri N, Mary V, Claisse G, Conde E Silva N, Kreis M, Deveaux Y. 2017. WOX14 promotes bioactive gibberellin synthesis and vascular cell differentiation in Arabidopsis. The Plant journal: for cell and molecular biology 90: 560–572. [DOI] [PubMed] [Google Scholar]
- Dierschke T, Flores-Sandoval E, Rast-Somssich MI, Althoff F, Zachgo S, Bowman JL. 2020. Gamete-specific expression of TALE class HD genes activates the diploid sporophyte program in Marchantia polymorpha. bioRxiv. doi: 10.1101/2020.04.06.027821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desirò A, Duckett JG, Pressel S, Villarreal JC, Bidartondo MI. 2013. Fungal symbioses in hornworts: a chequered history. Proceedings. Biological sciences / The Royal Society 280: 20130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, Ruberti I, Morelli G. 1996. The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. The Plant journal: for cell and molecular biology 10: 393–402. [DOI] [PubMed] [Google Scholar]
- Dodds WK, Gudder DA, Mollenhauer D. 1995. The ecology of Nostoc. Journal of Phycology 31: 2–18. [Google Scholar]
- Duckett JG, Pressel S. 2018. The evolution of the stomatal apparatus: intercellular spaces and sporophyte water relations in bryophytes-two ignored dimensions. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 373: 20160498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff RJ, Villarreal JC, Cargill CD, Renzaglia KS. 2007. Progress and challenges toward developing a phylogeny and classification of the hornworts. The Bryologist 110: 214–243. [Google Scholar]
- Flores-Sandoval E, Romani F, Bowman JL. 2018. Co-expression and transcriptome analysis of Marchantia polymorpha transcription factors supports class C ARFs as independent actors of an ancient auxin regulatory module. Frontiers in Plant Science 9: 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangedakis E, Saint-Marcoux D, Moody LA, Rabbinowitsch E, Langdale JA. 2017. Nonreciprocal complementation of KNOX gene function in land plants. The New Phytologist 216: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MH, Edwards MB, Schultz ER, McKain MR, Fei Z, Sørensen I, Rose JKC, Scanlon MJ. 2015. Dissecting the molecular signatures of apical cell-type shoot meristems from two ancient land plant lineages. New Phytologist 207: 893–904. [DOI] [PubMed] [Google Scholar]
- Frank MH, Scanlon MJ. 2015. Transcriptomic evidence for the evolution of shoot meristem function in sporophyte-dominant land plants through concerted selection of ancestral gametophytic and sporophytic genetic programs. Molecular biology and evolution 32: 3033. [DOI] [PubMed] [Google Scholar]
- Gerke P, Szövényi P, Neubauer A, Lenz H, Gutmann B, McDowell R, Small I, Schallenberg-Rüdinger M, Knoop V. 2019. Towards a plant model for enigmatic U-to-C RNA editing: the organelle genomes, transcriptomes, editomes and candidate RNA editing factors in the hornwort Anthoceros agrestis. New Phytologist 225:1974–1992. [DOI] [PubMed] [Google Scholar]
- Goad DM, Zhu C, Kellogg EA. 2017. Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. The New phytologist 216: 605–616. [DOI] [PubMed] [Google Scholar]
- Grosche C, Rensing SA. 2017. Three rings for the evolution of plastid shape: a tale of land plant FtsZ. Protoplasma 254: 1879–1885. [DOI] [PubMed] [Google Scholar]
- van der Graaff E, Laux T, Rensing SA. 2009. The WUS homeobox-containing (WOX) protein family. Genome biology 10: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann B, Royan S, Schallenberg-Rüdinger M, Lenz H, Castleden IR, McDowell R, Vacher MA, Tonti-Filippini J, Bond CS, Knoop V, et al. 2020. The expansion and diversification of pentatricopeptide repeat RNA-editing factors in plants. Molecular plant 13: 215–230. [DOI] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. 2004. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668. [DOI] [PubMed] [Google Scholar]
- Hanson MR, Hines KM. 2018. Stromules: probing formation and function. Plant physiology 176: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J, Moestrup Ø, Ulvskov P. 2016. Why plants were terrestrial from the beginning. Trends in plant science 21: 96–101. [DOI] [PubMed] [Google Scholar]
- Harris BJ, Harrison CJ, Hetherington AM, Williams TA. 2020. Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Current biology 30: 2001–2012.e2. [DOI] [PubMed] [Google Scholar]
- Harrison JC. 2017. Development and genetics in the evolution of land plant body plans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 372: 20150490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Corley SB, Moylan EC, Alexander DL, Scotland RW, Langdale JA. 2005. Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature 434: 509–514. [DOI] [PubMed] [Google Scholar]
- Hay A, Tsiantis M. 2010. KNOX genes: versatile regulators of plant development and diversity. Development 137: 3153–3165. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Bowman JL. 2015. A Role of TDIF peptide signaling in vascular cell differentiation is conserved among euphyllophytes. Frontiers in plant science 6: 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H. 2010. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. The Plant cell 22: 2618–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Sawa S. 2019. Diverse function of plant peptide hormones in local signalling and development. Current opinion in plant biology 51: 81–87. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H. 2008. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proceedings of the National Academy of Sciences of the United States of America 105: 15208–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga T, Fujimoto S, Cui Y, Sato K, Sano R, Yamaoka S, Kohchi T, Berger F, Nakajima K. 2020. Deep evolutionary origin of gamete-directed zygote activation by KNOX/BELL transcription factors in green plants. bioRxiv. doi: 10.1101/2020.04.08.031930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Uchida N, Yamaguchi YL, Tabata R, Ishida S, Ishizaki K, Nishihama R, Kohchi T, Sawa S, Bowman JL. 2019. Control of proliferation in the haploid meristem by CLE peptide signaling in Marchantia polymorpha. PLoS genetics 15: e1007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen S, Thamm A, Arteaga-Vazquez MA, Dolan L. 2018. Negative regulation of conserved RSL class I bHLH transcription factors evolved independently among land plants. eLife 7: e38529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Maruyama F, Fujisawa T, Togashi T, Yamamoto N, Seo M, Sato S, Yamada T, Mori H, Tajima N, et al. 2014. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nature communications 5: 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NA, Katz A, Pereman I, Decker EL, Ohad N, Reski R. 2016. A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nature Plants 2: 15209. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Leister D. 2014. Chloroplast evolution, structure and functions. F1000prime reports 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Sun J, Wunder T, Tang D, Cousins AB, Sze SK, Mueller-Cajar O, Gao Y-G. 2016. Structural insights into the LCIB protein family reveals a new group of β-carbonic anhydrases. Proceedings of the National Academy of Sciences of the United States of America 113: 14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GP, Renzaglia KS. 2009. Evaluating the diversity of pteridophyte embryology in the light of recent phylogenetic analyses leads to new inferences on character evolution. Plant Systematics and Evolution 283: 149–164. [Google Scholar]
- Jones VAS, Dolan L. 2012. The evolution of root hairs and rhizoids. Annals of botany 110: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Nagasaki H, Morikami A, Sato Y, Matsuoka M. 2003. Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. The Plant journal: for cell and molecular biology 35: 429–441. [DOI] [PubMed] [Google Scholar]
- Kawai H, Kanegae TT, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada MM. 2003. Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421: 287–290. [DOI] [PubMed] [Google Scholar]
- Kenrick P 2018. Changing expressions: a hypothesis for the origin of the vascular plant life cycle. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 373: 20170149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-J, Wu C-Y, Yu H-M, Sheen J, Lee H. 2017. Dual CLAVATA3 peptides in Arabidopsis shoot stem cell signaling. Journal of plant biology 60: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji R, Hasebe M. 2014. Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Current opinion in plant biology 17: 13–21. [DOI] [PubMed] [Google Scholar]
- Königer M, Delamaide JA, Marlow ED, Harris GC. 2008. Arabidopsis thaliana leaves with altered chloroplast numbers and chloroplast movement exhibit impaired adjustments to both low and high light. Journal of experimental botany 59: 2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings M, Hass H, Kerp H, Taylor TN, Agerer R, Dotzler N. 2009. Endophytic cyanobacteria in a 400-million-yr-old land plant: A scenario for the origin of a symbiosis? Review of Palaeobotany and Palynology 153: 62–69. [Google Scholar]
- Kugita M, Yamamoto Y, Fujikawa T, Matsumoto T, Yoshinaga K. 2003. RNA editing in hornwort chloroplasts makes more than half the genes functional. Nucleic acids research 31: 2417–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA. 2008. Evolution of developmental mechanisms in plants. Current opinion in genetics & development 18: 368–373. [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jürgens G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Leebens-Mack JH, Barker MS, Carpenter EJ, Deyholos MK, Gitzendanner MA, Others 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LR, Bergmann DC. 2019. The plant stomatal lineage at a glance. Journal of cell science 132: jcs228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lin H, Joo S, Goodenough U. 2008. Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell 133: 829–840. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. 2012. Major transitions in the evolution of early land plants: a bryological perspective. Annals of Botany 109: 851–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, Renzaglia KS. 1990. The sporophyte-gametophyte junction in the hornwort, Dendroceros tubercularis Hatt (Anthocerotophyta). New Phytologist 114: 497–505. [DOI] [PubMed] [Google Scholar]
- Li F-W, Brouwer P, Carretero-Paulet L, Cheng S, de Vries J, Delaux PM, Eily A, Koppers N, Kuo LY, Li Z, et al. 2018. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nature Plants 4: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-W, Melkonian M, Rothfels CJ, Villarreal JC, Stevenson DW, Graham SW, Wong GK-S, Pryer KM, Mathews S. 2015a. Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nature communications 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-W, Nishiyama T, Waller M, Frangedakis E, Keller J, Li Z, Fernandez-Pozo N, Barker MS, Bennett T, Blázquez MA, et al. 2020. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nature plants 6: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-W, Rothfels CJ, Melkonian M, Villarreal JC, Stevenson DW, Graham SW, Wong GK-S, Mathews S, Pryer KM. 2015b. The origin and evolution of phototropins. Frontiers in plant science 6: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-W, Villarreal Aguilar JC, Kelly S, Rothfels CJ, Melkonian M, Frangedakis E, Ruhsam M, Sigel EM, Der JP, Pittermann J, et al. 2014. Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proceedings of the National Academy of Sciences of the United States of America 111: 6672–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-W, Villarreal Aguilar JC, Szövényi P. 2017. Hornworts: An overlooked window into carbon-concentrating mechanisms. Trends in plant science 22: 275–277. [DOI] [PubMed] [Google Scholar]
- Lindblad P, Bergman B. 2018. The Cycad-Cyanobacterial Symbiosis In: Rai AN, ed. CRC Handbook of Symbiotic Cyanobacteria. Boca Raton: CRC Press, 137–159. [Google Scholar]
- Linde A-M, Eklund DM, Kubota A, Pederson ERA, Holm K, Gyllenstrand N, Nishihama R, Cronberg N, Muranaka T, Oyama T, et al. 2017. Early evolution of the land plant circadian clock. New phytologist 216: 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Ohashi Y, Kato M, Tsuge T, Gu H, Qu L-J, Aoyama T. 2015. GLABRA2 directly suppresses basic helix-loop-helix transcription factor genes with diverse functions in root hair development. The Plant cell 27: 2894–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Neal Stewart C. 2015. Plant synthetic biology. Trends in Plant Science 20: 309–317. [DOI] [PubMed] [Google Scholar]
- Liu J-W, Li S-F, Wu C-T, Valdespino IA, Ho J-F, Wu Y-H, Chang H-M, Guu T-Y, Kao M-F, Chesson C, et al. 2020. Gigantic chloroplasts, including bizonoplasts, are common in shade-adapted species of the ancient vascular plant family Selaginellaceae. American journal of botany 107: 562–576. [DOI] [PubMed] [Google Scholar]
- Machingura MC, Moroney JV. 2018. Closing the circle. eLife 7: e42507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinder LCM, Chen C, Leib RD, Patena W, Blum SR, Rodman M, Ramundo S, Adams CM, Jonikas MC. 2017. A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 171: 133–147.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinder LCM, Meyer MT, Mettler-Altmann T, Chen VK, Mitchell MC, Caspari O, Freeman Rosenzweig ES, Pallesen L, Reeves G, Itakura A, et al. 2016. A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proceedings of the National Academy of Sciences of the United States of America 113: 5958–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A, Busch MA, Tanahashi T, Perkovic J, Kato M, Hasebe M, Weigel D. 2005. The floral regulator LEAFY evolves by substitutions in the DNA binding domain. Science 308: 260–263. [DOI] [PubMed] [Google Scholar]
- Martin A, Lang D, Hanke ST, Mueller SJX, Sarnighausen E, Vervliet-Scheebaum M, Reski R. 2009. Targeted gene knockouts reveal overlapping functions of the five Physcomitrella patens FtsZ isoforms in chloroplast division, chloroplast shaping, cell patterning, plant development, and gravity sensing. Molecular Plant 2: 1359–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J 1998. Symbiosis between Nitrogen-Fixing Cyanobacteria and Plants. BioScience 48: 266–276. [Google Scholar]
- Meeks JC. 2003. Symbiotic interactions between Nostoc punctiforme, a multicellular cyanobacterium, and the hornwort Anthoceros punctatus. Symbiosis 35:55–71 [Google Scholar]
- Menand B, Yi K, Jouannic S, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L. 2007. An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316: 1477–1480. [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2017. Structure, function and evolution of stomata from a bryological perspective. Bryophyte Diversity and Evolution 39: 7. [Google Scholar]
- Merced A, Renzaglia KS. 2019. Contrasting pectin polymers in guard cell walls of Arabidopsis and the hornwort Phaeoceros reflect physiological differences. Annals of botany 123: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MT, Whittaker C, Griffiths H. 2017. The algal pyrenoid: key unanswered questions. Journal of experimental botany 68: 3739–3749. [DOI] [PubMed] [Google Scholar]
- Micheli PA 1927. Nova plantarum genera. Florentiae: Typis Bernardi Paperinii. [Google Scholar]
- Miwa H, Tamaki T, Fukuda H, Sawa S. 2009. Evolution of CLE signaling: origins of the CLV1 and SOL2/CRN receptor diversity. Plant signaling & behavior 4: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody LA, Kelly S, Rabbinowitsch E, Langdale JA. 2018. Genetic regulation of the 2D to 3D Growth transition in the moss Physcomitrella patens. Current biology 28: 473–478.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ. 2018. The timescale of early land plant evolution. Proceedings of the National Academy of Sciences of the United States of America 115: E2274–E2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquna A, Katz A, Decker EL, Rensing SA, Reski R, Ohad N. 2009. Regulation of stem cell maintenance by the Polycomb protein FIE has been conserved during land plant evolution. Development 136: 2433–2444. [DOI] [PubMed] [Google Scholar]
- Niklas KJ, Kutschera U. 2009. The evolutionary development of plant body plans. Functional Plant Biology 36: 682. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Wolf PG, Kugita M, Sinclair RB, Sugita M, Sugiura C, Wakasugi T, Yamada K, Yoshinaga K, Yamaguchi K, et al. 2004. Chloroplast phylogeny indicates that bryophytes are monophyletic. Molecular biology and evolution 21: 1813–1819. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sakayama H, de Vries J, Buschmann H, Saint-Marcoux D, Ullrich KK, Haas FB, Vanderstraeten L, Becker D, Lang D, et al. 2018. The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 174: 448–464.e24. [DOI] [PubMed] [Google Scholar]
- Nozue K, Kanegae T, Imaizumi T, Fukuda S, Okamoto H, Yeh KC, Lagarias JC, Wada M. 1998. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proceedings of the National Academy of Sciences of the United States of America 95: 15826–15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt B, Street NR, Wetterbom A, Zuccolo A, Lin Y-C, Scofield DG, Vezzi F, Delhomme N, Giacomello S, Alexeyenko A, et al. 2013. The Norway spruce genome sequence and conifer genome evolution. Nature 497: 579–584. [DOI] [PubMed] [Google Scholar]
- Okano Y, Aono N, Hiwatashi Y, Murata T, Nishiyama T, Ishikawa T, Kubo M, Hasebe M. 2009. A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution. Proceedings of the National Academy of Sciences of the United States of America 106: 16321–16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Kabeya Y, Miyagishima S-Y. 2010. The evolution of the regulatory mechanism of chloroplast division. Plant Signaling & Behavior 5: 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YI, Chow WS, Anderson JM. 1996. Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant physiology 111: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer J, Hidalgo O, Dodsworth S, Leitch IJ. 2018. Genome size diversity and its impact on the evolution of land plants. Genes 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud P, Meyberg R, Demko V, Quatrano RS, Olsen O, Rensing SA. 2020. DEK1 displays a strong subcellular polarity during Physcomitrella patens 3D growth. New Phytologist 226: 1029–1041. [DOI] [PubMed] [Google Scholar]
- Pressel S, Renzaglia KS, Dicky Clymo RS, Duckett JG. 2018. Hornwort stomata do not respond actively to exogenous and environmental cues. Annals of botany 122: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskauer J 1948a. Studies on the morphology of Anthoceros. II1. Annals of Botany 12: 427–439. [Google Scholar]
- Proskauer J 1948b. Studies on the morphology of Anthoceros. I1. Annals of Botany 12: 237–265. [Google Scholar]
- Proust H, Honkanen S, Jones VAS, Morieri G, Prescott H, Kelly S, Ishizaki K, Kohchi T, Dolan L. 2016. RSL Class I genes controlled the development of epidermal structures in the common ancestor of land plants. Current biology 26: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y-L, Li L, Wang B, Chen Z, Knoop V, Groth-Malonek M, Dombrovska O, Lee J, Kent L, Rest J, et al. 2006. The deepest divergences in land plants inferred from phylogenomic evidence. Proceedings of the National Academy of Sciences of the United States of America 103: 15511–15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai AN, Bergman B, Rasmussen U. 2002. Cyanobacteria in symbiosis. Netherlands: Kluwer, Dordrecht. [Google Scholar]
- Renzaglia KS. 1978. A comparative morphology and developmental anatomy of the Anthocerotophyta. Journal of the Hattori Botanical Laboratory 44: 31–90. [Google Scholar]
- Renzaglia KS, Browning WB, Merced A. 2020. With over 60 independent losses, stomata are expendable in mosses. Frontiers in Plant Science 11: 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Duff RJT, Nickrent DL, Garbary DJ. 2000. Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 355: 769–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Villarreal JC, Duff RJ. 2009. New insights into morphology, anatomy, and systematics of hornworts In: Goffinet B, Shaw J, eds. Bryophyte Biology. Cambridge: Cambridge University Press, 139–171. [Google Scholar]
- Renzaglia KS, Villarreal JC, Garbary DJ. 2018. Morphology supports -the setaphyte hypothesis: mosses plus liverworts form a natural group. Bryophyte Diversity and Evolution 40: 11. [Google Scholar]
- Renzaglia KS, Villarreal JC, Piatkowski BT, Lucas JR, Merced A. 2017. Hornwort stomata: architecture and fate shared with 400-Million-year-old fossil plants without leaves. Plant physiology 174: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera-Branchat M, Ripoll JJ, Yanofsky MF, Pelaz S. 2013. The WOX 13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. The Plant journal: for cell and molecular biology 73: 37–49. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Ando S, Yip HK, Tamada Y, Hiwatashi Y, Murata T, Deguchi H, Hasebe M, Bowman JL. 2013. KNOX2 genes regulate the haploid-to-diploid morphological transition in land plants. Science 339: 1067–1070. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Nishiyama T, Deguchi H, Hasebe M. 2008. Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella patens but do function in sporophyte development. Evolution & Development 10: 555–566. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Reisewitz P, Aoyama T, Friedrich T, Ando S, Sato Y, Tamada Y, Nishiyama T, Hiwatashi Y, Kurata T, et al. 2014. WOX13-like genes are required for reprogramming of leaf and protoplast cells into stem cells in the moss Physcomitrella patens. Development 141: 1660–1670. [DOI] [PubMed] [Google Scholar]
- Schmitz AJ, Glynn JM, Bradley JS, Stokes KD, Osteryoung KW. 2009. Arabidopsis FtsZ2-1 and FtsZ2-2 are functionally redundant, but FtsZbased plastid division is not essential for chloroplast partitioning or plant growth and development. Molecular Plant 2: 1211–1222. [DOI] [PubMed] [Google Scholar]
- Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallón S, Lupia R. 2004. Ferns diversified in the shadow of angiosperms. Nature 428: 553–557. [DOI] [PubMed] [Google Scholar]
- Shaw J, Renzaglia K. 2004. Phylogeny and diversification of bryophytes. American journal of botany 91: 1557–1581. [DOI] [PubMed] [Google Scholar]
- Shimamura M 2016. Marchantia polymorpha: Taxonomy, phylogeny andmMorphology of a model system. Plant & cell physiology 57: 230–256. [DOI] [PubMed] [Google Scholar]
- Small ID, Schallenberg-Rüdinger M, Takenaka M, Mireau H, Ostersetzer-Biran O. 2019. Plant organellar RNA editing: what 30 years of research has revealed. The Plant journal: for cell and molecular biology 101: 1040–1056. [DOI] [PubMed] [Google Scholar]
- Söderström L, Hagborg A, von Konrat M, Bartholomew-Began S, Bell D, Briscoe L, Brown E, Cargill DC, Costa DP, Crandall-Stotler BJ, et al. 2016. World checklist of hornworts and liverworts. PhytoKeys: 1–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szövényi P, Frangedakis E, Ricca M, Quandt D, Wicke S, Langdale JA. 2015. Establishment of Anthoceros agrestis as a model species for studying the biology of hornworts. BMC plant biology 15: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szövényi P, Waller M, Kirbis A. 2019. Evolution of the plant body plan. Current topics in developmental biology 131: 1–34. [DOI] [PubMed] [Google Scholar]
- Tam THY, Catarino B, Dolan L. 2015. Conserved regulatory mechanism controls the development of cells with rooting functions in land plants. Proceedings of the National Academy of Sciences of the United States of America 112: E3959–E3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi T, Sumikawa N, Kato M, Hasebe M. 2005. Diversification of gene function: homologs of the floral regulator FLO/LFY control the first zygotic cell division in the moss Physcomitrella patens. Development 132: 1727–1736. [DOI] [PubMed] [Google Scholar]
- Thamm A, Saunders TE, Dolan L. 2020. MpFEW RHIZOIDS1 miRNA-mediated lateral inhibition controlsrhizoid cell patterning in Marchantia polymorpha. Current biology: CB 30: 1905–1915.e4. [DOI] [PubMed] [Google Scholar]
- Tillich M, Lehwark P, Morton BR, Maier UG. 2006. The evolution of chloroplast RNA editing. Molecular Biology and Evolution 23: 1912–1921. [DOI] [PubMed] [Google Scholar]
- Trojan A, Gabrys H. 1996. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiology 111: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Zhang Z, Laux T. 2011. Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development. Developmental cell 20: 264–270. [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Ligrone R, Owen HA, Hasegawa J, Campbell EO, Renzaglia KS, Monge-Najera J. 1992. The anthocerote chloroplast: a review. New phytologist 120: 169–190. [Google Scholar]
- Villarreal JC, Cargill DC, Hagborg A, Soderstrom L, Renzaglia KS. 2014. A synthesis of hornwort diversity: Patterns, causes and future work. Phytotaxa 9: 150–166. [Google Scholar]
- Villarreal JC, Duckett JG, Pressel S. 2017. Morphology, ultrastructure and phylogenetic affinities of the single-island endemic Anthoceros cristatus Steph. (Ascension Island). Journal of Bryology 39: 226–234. [Google Scholar]
- Villarreal JC, Renner SS. 2012. Hornwort pyrenoids, carbon-concentrating structures, evolved and were lost at least five times during the last 100 million years. Proceedings of the National Academy of Sciences of the United States of America 109: 18873–18878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal JC, Renner SS. 2013. Correlates of monoicy and dioicy in hornworts, the apparent sister group to vascular plants. BMC evolutionary biology 13: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal JC, Renzaglia KS. 2015. The hornworts: important advancements in early land plant evolution. Journal of Bryology 37: 157–170. [Google Scholar]
- de Vries J, Gould SB. 2018. The monoplastidic bottleneck in algae and plant evolution. Journal of cell science 131: jcs203414. [DOI] [PubMed] [Google Scholar]
- Wada K, Hirabayashi Y, Saito W. 1984. Light germination of Anthoceros miyabeanus spores. The Botanical Magazine Tokyo 97: 369–379. [Google Scholar]
- Wang Y, Stessman DJ, Spalding MH. 2015. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: how Chlamydomonas works against the gradient. Plant Journal 82: 429–448. [DOI] [PubMed] [Google Scholar]
- Wang S, Li L, Li H, Sahu SK, Wang H, Xu Y, Xian W, Song B, Liang H, Cheng S, et al. 2020. Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nature Plants 6: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitewoods CD, Cammarata J, Venza ZN, Sang S, Crook AD, Aoyama T, Wang XY, Waller M, Kamisugi Y, Cuming AC, et al. 2018. CLAVATA was a genetic novelty for the morphological innovation of 3D growth in land plants. Current Biology 28: 2365–2376.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitewoods CD. 2020. Evolution of CLE peptide signalling. Seminars in cell & developmental biology. 10.1016/j.semcdb.2020.04.022 [DOI] [PubMed] [Google Scholar]
- Whitton BA. 1993. Cyanobacteria and Azolla Jones DG, ed. Exploitation of Microorganisms. Springer, Dordrecht: 137–167. [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter E, Matasci N, Ayyampalayam S, Barker MS, Burleigh JG, Gitzendanner MA, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences of the United States of America 111: E4859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson PKI, Mühlich C, Ullrich KK, Rensing SA. 2017. Comprehensive genome-wide classification reveals that many plant-specific transcription factors evolved in streptophyte algae. Genome biology and evolution 9: 3384–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsenach R 1963. Differentiation of the chloroplast of Anthoceros. The Journal of cell biology 18: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-C, Li F-W, Kramer EM. 2019. Large-scale phylogenomic analysis suggests three ancient superclades of the WUSCHEL-RELATED HOMEOBOX transcription factor family in plants. PloS one 14: e0223521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Fu J, Köllner TG, Chen X, Jia Q, Guo H, Qian P, Guo H, Wu G, Chen F. 2018. Biochemical characterization of microbial type terpene synthases in two closely related species of hornworts, Anthoceros punctatus and Anthoceros agrestis. Phytochemistry 149: 116–122. [DOI] [PubMed] [Google Scholar]
- Xiong D, Huang J, Peng S, Li Y. 2017. A few enlarged chloroplasts are less efficient in photosynthesis than a large population of small chloroplasts in Arabidopsis thaliana. Scientific reports 7: 5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Tsujikawa T, Hatano K, Ozawa S-I, Takahashi Y, Fukuzawa H. 2010. Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant & cell physiology 51: 1453–1468. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fu X-X, Li R-Q, Zhao X, Liu Y, Li M-H, Zwaenepoel A, Ma H, Goffinet B, Guan Y-L, et al. 2020. The hornwort genome and early land plant evolution. Nature plants 6: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiao Y, Jiao H, Zhao H, Zhu Y-X. 2017. Two-step functional innovation of the stem-cell factors WUS/WOX5 during plant evolution. Molecular biology and evolution 34: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]