Abstract
The incidence of neurosyphilis has declined since effective penicillin therapy against Treponema pallidum was introduced. However, the diagnosis of neurosyphilis early in the disease course is very important in order to select appropriate antibiotic therapy. We report brain MRI, SPECT with Tc-99m ECD, and PET with F-18 FDG findings before antibiotic therapy in a neurosyphilis patient with neurological symptoms. The cerebral cortices showed hypoperfusion with a patchy distribution on SPECT and foci with high signal intensity on MRI, suggesting ischemia. Brain PET showed areas with hypometabolism in the temporoparietal lobes bilaterally.
Keywords: Neurosyphilis; Magnetic resonance imaging; Tomography, emission-computed, single-photon; Positron-emission tomography
Introduction
The incidence of neurosyphilis has declined since the introduction of penicillin therapy against Treponema pallidum. Neurological symptoms improve markedly after starting an appropriate treatment in early neurosyphilis [1]. Therefore, an early diagnosis and timely antibiotic treatment are important. Previous brain MRI findings in neurosyphilis showed white matter abnormalities in cerebral parenchyma and atrophy in various degrees [2, 3]. However, only a few case reports exist describing imaging findings of brain SPECT which were performed before antibiotic therapy in neurosyphilitic patients [4, 5]. In addition, there are fewer reports that describe similar patterns reported by brain MRI, SPECT, and PET imaging findings in the previous cases.
Herein, we report the imaging characteristics of brain MRI, SPECT, and PET in a neurosyphilitic patient before undergoing antibiotic therapy.
Case Report
A 39-year-old man was admitted to our neurology department for disorientation, progressive memory disturbance, impaired concentration, altered personality, and hand tremor. The patient had coitus with many sexual partners for years. He denied having medical history for sexually transmitted disease including syphilis or human immunodeficiency virus (HIV) infections and was unware of any cutaneous symptoms. Serological testing revealed a rapid plasma reagin (RPR) with a titer of 1:374 and reactive Treponema pallidum hemagglutination (TPHA). TPHA and fluorescent treponemal antibody absorption (FTA-ABS) were positive in cerebrospinal fluid (CSF). The serologic tests for the main sexually transmitted infections (HIV, hepatitis B or C viruses) were negative.
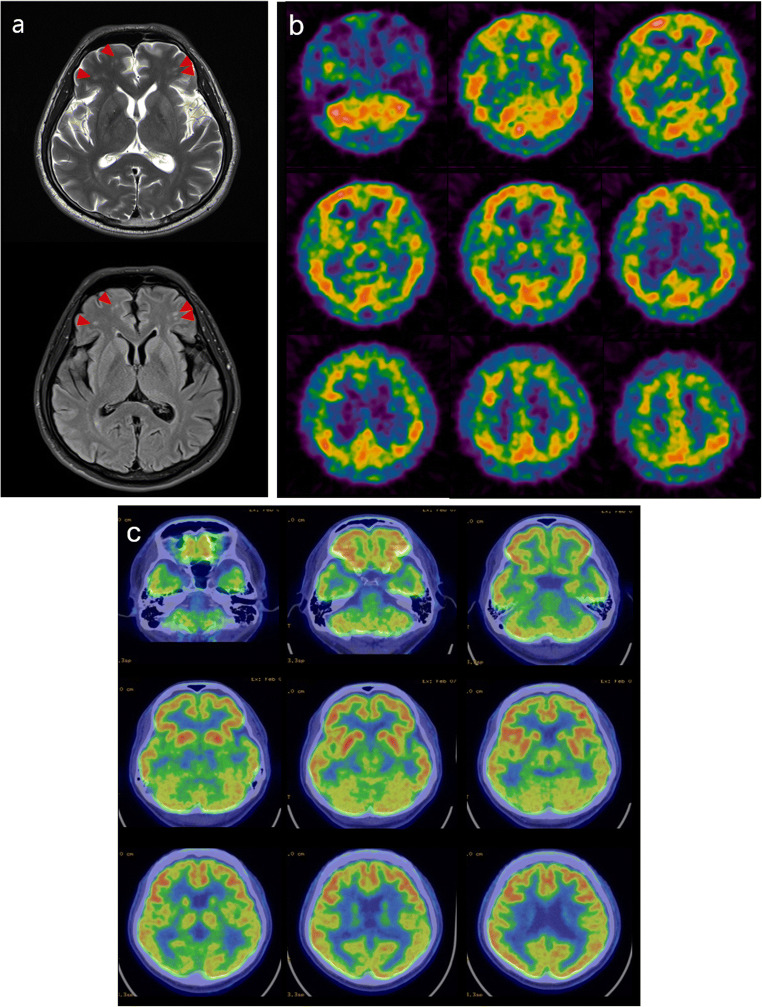
At the time of admission, a brain MRI was performed. T2-weighted MRI and fluid attenuated inversion recovery (FLAIR) images revealed multiple high signal intensity foci in the cerebral white matter, suggesting cerebral small-vessel disease (Fig. 1a). Although the diagnosis of neurosyphilis should have been suspected, SPECT with Tc-99m ethyl cysteinate dimer (ECD) and PET with F-18 fluorodeoxyglucose (FDG) were performed before treatment to rule out other infectious and non-infectious inflammatory disease, dementia, toxic and metabolic disease, and primary psychiatric disease. SPECT images were obtained 15 min after intravenous injection of 555 MBq of Tc-99m ECD using a dual-head gamma camera (NM630, GE Healthcare, Milwaukee, WI) equipped with a low-energy, high-resolution, parallel-hole collimator. Brain SPECT showed multifocal hypoperfusion with a patchy distribution throughout the cerebral cortices (Fig. 1b). The next day, brain PET images were acquired 40 min after intravenous administration of 185 MBq of F-18 FDG using a PET/CT scanner (Discovery STE, GE Healthcare, Milwaukee, WI). Brain PET showed decreased F-18 FDG uptake in the temporoparietal cortex and thalamus bilaterally (Fig. 1c). The final diagnosis of neurosyphilis was made, and the patient received intravenous penicillin G treatment. The patient’s neurologic symptoms gradually improved and RPR titer declined following treatment with penicillin. One year after the start of high-dose penicillin treatment, the patient was examined again. The RPR titer declined, and neurologic symptoms were markedly improved, but impaired concentration still remained. The follow-up SPECT revealed improvement of cerebral perfusion (Fig. 2).
Fig. 1.
a T2-weighted MRI and fluid attenuated inversion recovery (FLAIR) images revealed multiple high signal intensity foci (arrowheads) in the cerebral white matter. b Brain SPECT showed multifocal hypoperfusion with a patchy distribution throughout the cerebral cortices. c Brain PET showed hypometabolism in temporoparietal cortex and thalamus bilaterally
Fig. 2.
Follow-up SPECT showed improvement of cerebral perfusion
Discussion
We have described the brain MRI, SPECT, and PET imaging findings of a neurosyphilis patient prior to the antibiotic treatment. Our case highlights interesting issues about differential diagnosis of neurosyphilis. Our patient’s symptoms shared some of the common early manifestations of Alzheimer’s disease and primary psychiatric disease, which could lead to misdiagnosis. In addition, when taking his medical history, he denied having syphilitic infection, and it was difficult to assess the typical clinical manifestations such as genital chancre and cutaneous symptoms because of his impaired cognitive function. Therefore, in young patients with neurologic symptoms such as personal changes, mood disorders, delusion, or aggression, brain imaging may be useful as they can be differentiated from other neurologic and psychiatric diseases.
It is not clear whether the cerebral perfusion is increased or decreased in neurosyphilis patients, although most reports indicate hypoperfusion in the cerebrum on brain SPECT [5–7]. We observed multifocal hypoperfusion in the cerebral cortices, and brain MRI revealed high signal intensity on T2-weighted and FLAIR images. It has been postulated that the neurological symptoms in neurosyphilis are closely related to the cerebral vessels and blood flow [4, 6, 8]. Brain PET performed before treatment showed decreased glucose metabolism in bilateral temporal lobes. Previous FDG PET studies in neurosyphilis showed different metabolic patterns from early Alzheimer’s disease, such as increased or decreased metabolism in the temporal or frontal lobes [9–11]. A mismatch of cerebral perfusion and glucose metabolism is likely that deterioration of glucose metabolism occurs after impairment of cerebral blood flow. Another speculation is that the inflammatory process reduces cerebral perfusion and the involvement of the temporal lobe in the default mode network results in temporal hypometabolism [11]. Our finding on the thalamic hypometabolism is in line with that of a previous case, which studied PET and MRI findings in a patient with neurosyphilis [12]. Our findings could be helpful in the differentiation of neurosyphilis from Alzheimer’s disease. The disturbance of thalamic-cortical circuitry may be associated with cerebral hypoperfusion. It is known that the thalamus is involved in the regulation of cortical excitability [13–15]. Thus, the disturbance of thalamic-cortical circuitry may be associated with hypometabolism of cerebral cortex and thalamus and cause psychiatric and emotional disorders in the patients with neurosyphilis.
Conclusion
This case demonstrates that nuclear medicine imaging modalities could aid in the differential diagnosis of neurosyphilis when neurological symptoms are observed in young patients.
Funding
This research was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (2020R1C1C1007254).
Compliance with Ethical Standards
Conflict of Interest
Eun Kyoung Choi, Young Do Kim, Hyeonseok Jeong, Yong-An Chung, Jin Kyoung Oh, and In-Uk Song declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration as revised in 2013 and its later amendments or comparable ethical standards.
Informed Consent
The requirement to obtain informed consent was waived.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eun Kyoung Choi, Email: eet0224@gmail.com.
Young Do Kim, Email: limbic@catholic.ac.kr.
Hyeonseok Jeong, Email: hsjeong@catholic.ac.kr.
Yong-An Chung, Email: yongan@catholic.ac.kr.
Jin Kyoung Oh, Email: mirriam@catholic.ac.kr.
In-Uk Song, Email: siuy@catholic.ac.kr.
References
- 1.Hahn RD, Webster B, Weickhardt G, Thomas E, Timberlake W, Solomon H, et al. Penicillin treatment of general paresis (dementia paralytica) AMA Arch Neurol Psychiatry. 1959;81:557–590. doi: 10.1001/archneurpsyc.1959.02340170023003. [DOI] [PubMed] [Google Scholar]
- 2.Peng F, Hu X, Zhong X, Wei Q, Jiang Y, Bao J, et al. CT and MR findings in HIV-negative neurosyphilis. Eur J Radiol. 2008;66:1–6. doi: 10.1016/j.ejrad.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y, Wei M, Huang Y, Jiang W, Liu X, Xia F, et al. Clinical presentation and imaging of general paresis due to neurosyphilis in patients negative for human immunodeficiency virus. J Clin Neurosci. 2010;17:308–310. doi: 10.1016/j.jocn.2009.07.092. [DOI] [PubMed] [Google Scholar]
- 4.Kitabayashi Y, Ueda H, Narumoto J, Nakamura K, Kita H, Tsuchida H, et al. Cerebral blood flow changes in general paresis following penicillin treatment: a longitudinal single photon emission computed tomography study. Psychiatry Clin Neurosci. 2002;56:65–70. doi: 10.1046/j.1440-1819.2002.00930.x. [DOI] [PubMed] [Google Scholar]
- 5.Morikawa M, Kosaka J, Imai T, Ohsawa H, Iida J, Kishimoto T. A case of general paresis showing marked treatment-associated improvement of cerebellar blood flow by quantitative imaging analysis. Ann Nucl Med. 2002;16:71–74. doi: 10.1007/BF02995296. [DOI] [PubMed] [Google Scholar]
- 6.Kawai N, Baba A, Mizukami K, Sakai T, Shiraishi H, Koizumi J. CT, MR, and SPECT findings in a general paresis. Comput Med Imaging Graph. 1994;18:461–465. doi: 10.1016/0895-6111(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 7.Denays R, Collier A, Rubinstein M, Atsama P. A 51-year-old woman with disorientation and amnesia. Lancet. 1999;354:1786. doi: 10.1016/S0140-6736(99)09151-5. [DOI] [PubMed] [Google Scholar]
- 8.Tien RD, Gean-Marton AD, Mark AS. Neurosyphilis in HIV carriers: MR findings in six patients. AJR Am J Roentgenol. 1992;158:1325–1328. doi: 10.2214/ajr.158.6.1590135. [DOI] [PubMed] [Google Scholar]
- 9.Scheid R, Voltz R, Vetter T, Sabri O, von Cramon DY. Neurosyphilis and paraneoplastic limbic encephalitis: important differential diagnoses. J Neurol. 2005;252:1129–1132. doi: 10.1007/s00415-005-0812-1. [DOI] [PubMed] [Google Scholar]
- 10.Pichler R, Doppler S, Szalay E, Hertl C, Knell U, Winkler J. SPECT and FDG-PET in diagnostics of neurolues. Wien Klin Wochenschr. 2008;120:20–23. doi: 10.1007/s00508-008-1036-z. [DOI] [PubMed] [Google Scholar]
- 11.Verjans S, Van Laere K, Vandenberghe R. Neurosyphilis mimicking young-onset Alzheimer's disease: a case report explaining the pitfalls of FDG-PET. Acta Neurol Belg. 2016;116:207–210. doi: 10.1007/s13760-015-0508-y. [DOI] [PubMed] [Google Scholar]
- 12.Park YA, Ann JW, Jeon SH, Chung EJ, Kim EG, Park JH, et al. Radiographic features in a patient diagnosed with neurosyphilis. J Neurosonol Neuroimaging. 2019;11:173–177. doi: 10.31728/jnn.2019.00069. [DOI] [Google Scholar]
- 13.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172(4):687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Leenders KL, Hajek M, Maguire P, Missimer J, Wieser HG. Thalamic glucose metabolism in temporal lobe epilepsy measured with 18F-FDG positron emission tomography (PET) Epilepsy Res. 1997;28(3):233–243. doi: 10.1016/S0920-1211(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 15.Rose JE, Woolsey CN. Organization of the mammalian thalamus and its relationships to the cerebral cortex. Electroencephalogr Clin Neurophysiol. 1949;1(4):391–403. doi: 10.1016/0013-4694(49)90212-6. [DOI] [PubMed] [Google Scholar]