Abstract
Purpose
The goal of our retrospective single tertiary academic medical center investigation was to examine the added diagnostic value and clinical impact of 68Ga-DOTATATE PET/CT in the therapeutic management of patients with neuroendocrine tumors (NETs).
Methods
Imaging database was queried for all “PET-DOTATATE” examinations performed at our tertiary care academic institution using MONTAGE™. The patient’s clinical history and recent prior imaging were reviewed. The additional diagnostic value and clinical management impact of 68Ga-DOTATATE were assessed through retrospective chart review.
Results
A total of 81 68Ga-DOTATATE PET/CT scans in 74 patients were found, and 11 patients were excluded from analysis as they had no prior imaging available for comparison, with resultant analysis cohort of 63 patients. Six patients had 2 or more 68Ga-DOTATATE PET/CT examinations. The most common primary diagnosis was undifferentiated NET (63.5%), followed by carcinoid (27.0%), paraganglioma (4.8%), insulinoma (3.2%), and pheochromocytoma (1.6%). The primary sites of disease from the most to the least common were the pancreas (36.5%), small bowel (22.2%), unknown primary (15.9%), lung (6.3%), large bowel (6.3%), and mesentery (4.8%), and other locations accounted for 7.9%. In patients who had prior imaging available for comparison, there were new lesions identified on 68Ga-DOTATATE PET/CT in 21 patients (33.3%) that were not identified on other prior imaging modalities. Of these patients, 5 underwent subsequent MRI and 1 had a repeat 68Ga-DOTATATE PET/CT to further characterize new lesions seen. Moreover, 15 patients (23.8%) had a change in treatment plan, including altering medical therapy in 9 patients, change in planned extent of surgical management in 5 patients, and cancelation of a planned primary tumor resection in 1 patient with metastatic disease.
Conclusion
Our retrospective cohort demonstrated that 68Ga-DOTATATE PET/CT improves lesion detection over conventional imaging in 33.3% and impacts the therapeutic management in 23.8% of patients with NET.
Keywords: Neuroendocrine, Tumor, 68Ga-DOTATATE, PET, Somatostatin
Introduction
Neuroendocrine tumors (NETs) are malignancies arising from neural crest cells, which can occur nearly anywhere in the body, primarily the gastrointestinal, pancreas, and pulmonary systems, and present with a wide variety of symptoms depending on their biochemical function. NETs are often heterogeneous and can harbor various tumor grades, which impact management, response to treatment, and outcome [1, 2]. NETs generally overexpress somatostatin receptors (SSTR) on their cell surfaces, which allows for targeted molecular imaging and therapy [3, 4]. Conventional nuclear medicine method to image NETs has relied on single-photon scintigraphy with radiolabeled somatostatin analogs (111In-pentetreotide, octreoscan) but this technique is limited by relatively low spatial resolution, low sensitivity, and high background activity [5]. 68Ga-1,4,7,10-tetrazyzcyclododecane-1,4,7,10-tetraacetic acid (DOTA)-octreotate (68Ga-DOTATATE or NetSpot™) with the high affinity for somatostatin receptor 2 (SSTR 2) was the first approved positron emission tomography (PET) imaging agent for NETs by the US Food and Drug Administration (FDA) in 2016 [6, 7]. More recently, 68Ga-DOTATOC and 64Cu-DOTATATE (Detectnet™) were approved by the FDA in 2019 and 2020, respectively.
A review by Mojtahedi et al. described advantages of 68Ga-DOTATATE PET when compared to other traditional imaging studies [8]. 68Ga-DOTATATE has higher sensitivity (97%, 95% confidence interval [CI]: 82–100%) compared to 111In-pentetreotide imaging (65%, 95%CI: 64–94%) [9, 10]. A systematic review and meta-analysis of 68Ga-DOTATATE in pulmonary and gastroenteropancreatic NETs showed both high sensitivity and specificity, with an estimated sensitivity of 90.9% (95% CI: 81.4–96.4%) and specificity 90.6% (95% CI: 77.8–96.1%) [11]. Janssen et al. evaluated the use of 68Ga-DOTATATE specifically in head and neck paragangliomas and found that significantly more lesions were detected compared to other imaging agents. 68Ga-DOTATATE detected 37 or 38 known lesions in 20 different patients, compared to only 23 lesions by CT and MRI and 24 by 18F-fluorodeoxyglucose (FDG) PET/CT (p < 0.01); 68Ga-DOTATATE was also able to detect 7 new lesions which were not previously known [12]. 68Ga-DOTATATE has an added benefit of prognostic utility in NETs. Tirosh et al. showed that higher tumor volumes as depicted on 68Ga-DOTATATE PET/CT correlated independently with progression-free survival and disease-related mortality [13].
In view of the above supportive results, the goal of our retrospective single tertiary academic medical center investigation was to examine the added diagnostic value and clinical impact of 68Ga-DOTATATE PET/CT in the therapeutic management of patients with NETs.
Methods and Materials
Patient Selection
Institutional Review Board approval was obtained for this retrospective cohort study which adhered to regulations of the Health Insurance Portability and Accountability Act. Imaging database was queried for all “PET-DOTATATE” examinations performed at our institution from July 2018 to January 2020 using MONTAGE™ (Montage Healthcare Solutions, Inc., Philadelphia, PA). Studies were excluded if the indications were not clearly for neuroendocrine tumor evaluation.
Image Acquisition and Analysis
Patients were scanned supine on a PET/CT scanner (Biograph Duo LSO; Siemens) approximately 60 min following the administration of 185 MBq (5 mCi) of 68Ga-DOTATATE through a peripheral vein. Low-dose CT (pitch, 1.0; 90–130 mAs; 130 kVp) with only oral contrast was performed for attenuation correction of PET images and lesion localization. PET was performed from skull base to mid thighs for 4 min per bed position. Attenuation-corrected PET images were viewed on a color high-resolution monitor with MIM™ (MIM Software, Inc., Cleveland, OH). Images were interpreted by a board-certified nuclear medicine physician with extensive experience in interpreting 68Ga-DOTATATE PET/CT examinations and unblinded to other relevant correlative imaging and clinical information. All other imaging reports signed by subspecialty board-certified interpreters were retrieved electronically and documented.
Impact on Patient Management and Data Analysis
Retrospective analysis of clinical data from patients with 68Ga-DOTATATE PET/CT examinations was performed. Patients without prior imaging were censored from data analysis as it was deemed impossible to determine an added benefit of 68Ga-DOTATATE PET/CT without available previous imaging studies. The patient’s clinical history and all prior imaging were reviewed. The additional diagnostic value and management impact of 68Ga-DOTATATE PET/CT were assessed by electronic chart review. Changes in management were defined as (1) change in therapy including change or initiation of chemotherapy and/or somatostatin receptor-targeted treatment including 177Lu-DOTATATE peptide-receptor radionuclide therapy (PRRT), or (2) change in surgical resection either in extent or cancelation of planned surgery prior to 68Ga-DOTATATE PET/CT.
Results
Patient Selection
A total of 81 examinations in 74 patients (44.4% female). Of 74 patients, 11 had no prior imaging for comparison purposes and were excluded from further analysis. The average age was 60.3 years with interquartile range of 12.5 years. The most common primary tumor types included undifferentiated NET (63.5%), carcinoid (27.0%), paraganglioma (4.8%), insulinoma (3.2%), and pheochromocytoma (1.6%) (Table 1). The most common organs or anatomic areas of tumor involvement included the pancreas (36.5%), small bowel (22.2%), lung (6.3%), large bowel (4.8%), mesentery (6.3%), and other sites including the stomach and adrenal glands accounting for 7.9%. The primary tumor site was unknown in 10 patients (15.9%). In 2 of the 10 patients with tumor of unknown primary origin, 68Ga-DOTATATE PET/CT was able to identify the likely primary tumor.
Table 1.
Patient demographics including primary disease site and tumor type
| Demographic | # Patients, (%) |
|---|---|
| Total patients | 74 |
| Excluded patients | 11 |
| Patients included in analysis | 63 |
| Male | 35 (55.6) |
| Female | 28 (44.4) |
| Site of primary | |
| Pancreas | 23 (36.5) |
| Small bowel | 14 (22.2) |
| Unknown | 10 (15.9) |
| Lung | 4 (6.3) |
| Mesentery | 4 (6.3) |
| Large bowel/appendix | 3 (4.8) |
| Other (gastric, adrenal, etc.) | 5 (7.9) |
| Type of primary | |
| Undifferentiated NET | 40 (63.5) |
| Carcinoid | 17 (27.0) |
| Paraganglioma | 3 (4.8) |
| Insulinoma | 2 (3.2) |
| Pheochromocytoma | 1 (1.6) |
Imaging Findings and Impact on Patient Management
Sixty-three patients had at least 1 prior imaging study (CT, MRI, FDG PET/CT, or Octreoscan). Forty-eight (76.2%) of these patients had a prior CT examination, 16 (25.8%) had prior FDG PET/CT, 16 (25.8%) had a prior MRI, 14 (22.2%) had a prior Octreoscan, and 1 (1.6%) had a prior 131I-meta-iodobenzylguanadine (131I-MIBG) scan. Thirty-five patients (55.6%) had any combination of 2 or more of the above imaging studies prior to the 68Ga-DOTATATE PET/CT.
Imaging findings were concordant in 42 patients (66.7%) and discordant (new lesions identified) in 21 patients (33.3%). Of these 21 patients, 6 went on to have advanced imaging studies to further evaluate suspected lesions; these included MRI in 5 patients (1 for evaluation of a brain lesion, 1 for a cervical spine lesion, 1 for a thoracic spine lesion, 1 for a lumbar spine lesion and 1 for a lesion in the pancreatic region since the location was not typical for physiologic uncinate process uptake), and 1 patient was scheduled for a repeat 68Ga-DOTATATE PET/CT to evaluate disease stability before consideration for change in treatment plan. A total of 15 patients (23.8%) had a change in therapeutic management; 9 patients had a change in medical therapy alone, including 7 for referral and/or initiation of PRRT (3 patients had not been on any therapy, 3 were previously treated with sandostatin alone and 1 had been treated with an sandostatin and everolimus). Of the other 2 patients with change in medical management, 1 had a change from no prior therapy to octreotide analog (sandostatin) alone while the other had a change from no prior therapy to chemotherapy (everolimus) alone. Six patients had a change in planned extent of surgical management (including 1 of 2 patients with unknown primary tumor). Of these patients, 1 was scheduled for thyroidectomy for an incidentally detected thyroid lesion, 3 were scheduled for partial or total pancreatectomy, and 1 was scheduled for a partial small bowel and mesenteric mass resection, which was considered to be the primary malignancy. Cancelation of a scheduled surgery occurred in 1 patient who was found to have metastatic disease not seen on prior imaging studies (Table 2).
Table 2.
Imaging findings on 68Ga-DOTATATE PET/CT compared to prior imaging studies and type and number of confirmed or tentative changes to clinical management
| Imaging findings | # Patients, % |
|---|---|
| Patients with prior imaging | 63 |
| Concordant | 42 (66.7) |
| Discordant & new | 21 (33.3) |
| Confirmed or tentative changes in clinical management | |
| Follow-up imaging | 6 (9.5) |
| Treatment change | 15 (23.8) |
| Medical | 9 (14.3) |
| PRRT | − 7 (11.1) |
| Chemotherapy (everolimus) | − 1 (1.6) |
| Octreotide agonist (sandostatin) | − 1 (1.6) |
| Surgical | 6 (9.5) |
| Thyroidectomy | − 1 (1.6) |
| Partial/total pancreatectomy | − 3 (4.8) |
| Small bowel/ mesentery resection | − 1 (1.6) |
| Surgery cancelation | − 1 (1.6) |
Figures 1, 2, and 3 show representative examples of prior imaging studies and follow-up 68Ga-DOTATAT PET/CT revealing new lesions. Subsequent advanced imaging studies are also shown confirming the new lesions. Six patients had both FDG PET/CT and 68Ga-DOTATATE PET/CT studies within a 12-month period. In 1 patient with history of prior lung carcinoid, a prior FDG PET/CT showed a hypermetabolic mass in the medial left lung, corresponding to known primary tumor, without evidence of metastatic disease. A 68Ga-DOTATATE examination 2 months later showed multiple hepatic lesions which were suspicious for metastatic disease. The patient went on to have an abdominal MRI which confirmed the metastatic liver lesions (Fig. 4).
Fig. 1.
55-year-old male with a history of pancreatic NET with prior pancreatectomy who had planned surgical resection of a solitary peripancreatic lesion seen on surveillance CT. Representative pre-operative CT imaging showing only a 2.2 cm peripancreatic lesion but otherwise no additional sites of disease (a). Follow-up 68Ga-DOTATATE PET/CT (b and c) examination revealed multiple osseous, and liver and nodal metastases which were also confirmed on an Octreoscan (d). The planned surgery was canceled, and the patients were treated medically
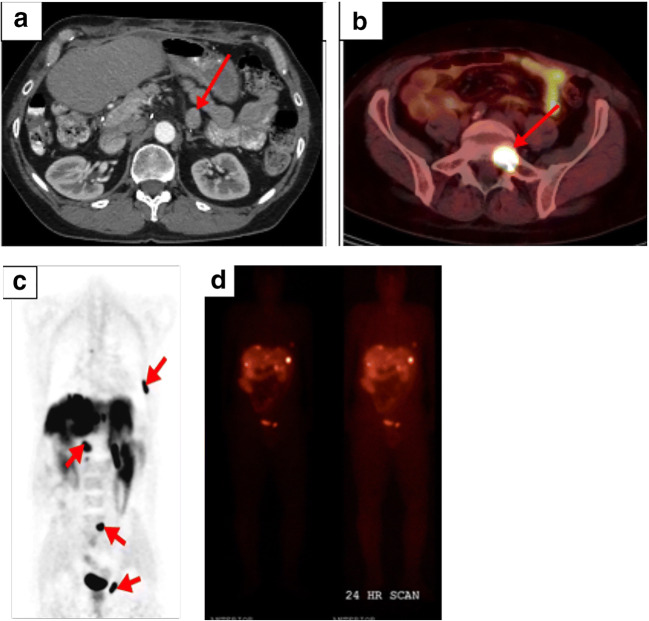
Fig. 2.
59-year-old male with known pancreatic NET status post-distal pancreatectomy found to have liver metastases on surveillance CT but no suspicious pancreatic lesion. Subsequent 68Ga-DOTATATE PET/CT revealed increased uptake in the mid pancreatic body (a). Follow-up T2 HASTE abdominal MRI showed a corresponding partly cystic mass in the mid pancreas (b)
Fig. 3.
79-year-old female with a history of multiple liver masses suspicious for metastatic disease. Initial CT showed multiple liver lesions without a definite primary lesion (a). Follow-up 68Ga-DOTATATE PET /CT revealed increased uptake in the distal pancreas compatible with primary NET and confirmed high radiotracer avidity in the multiple hepatic metastases (b). This patient was referred for PRRT with 177Lu-DOTATATE
Fig. 4.
59-year-old female with history of lung carcinoid undergoing routine surveillance imaging. Initial FDG PET/CT did not show evidence for metastatic disease (a). Follow-up 68Ga-DOTATATE PET/CT revealed multiple hepatic lesions, suspicious for metastatic disease (b). Contrast-enhanced fat-saturated T1-weighted MRI of the abdomen confirmed enhancing hepatic lesions (c). Note that the smaller lesions seen on the 68Ga-DOTATATE PET/CT are at ill-defined on the MRI
Discussion
Our aim in this retrospective investigation was to examine our own heterogeneous clinical data at a single tertiary academic medical center with regard to impact on management decisions, which provided valuable independent information to our patients, clinicians, and hospital administration. In case of detection of occult primary tumor, our localization rate of 20% (2 of 10 patients) was similar to those reported in previous studies (10–22%) [14, 15]. However, 68Ga-DOTATATE PET/CT provides additional benefit in the imaging evaluation and management of NETs compared to conventional imaging methods [16–19]. 68Ga-DOTATATE PET/CT has been previously shown to improve assessment for disease extent compared to conventional imaging (100% versus 68–83%, respectively) [20]. Additionally, Hofman et al. showed high impact (defined as inter-modality treatment change) in management in 47% of patients. Similarly, Srirajaskanthan et al. showed a change in patient management in 70.6% of patients who had additional lesions seen on 68Ga-DOTATATE PET/CT [21]. In the review by Mojtahedi et al., clinical management was changed in 70.6–81% of patients when compared to Octreoscan alone [8]. Interestingly, in our study, management change occurred in 23.8% of patients, which is similar to previously reported data by Deppen et al., who reported a change in management in 37% of their patients [9]. Reasons for the lower percentage of change in clinical management in our study compared to those reported previously may include (1) our comparison of 68Ga-DOTATATE PET/CT to multiple prior imaging studies including CT and MRI versus 111In-octreotide alone and (2) a more strict definition of change in clinical management to include only confirmed or tentative changes to medical and/or surgical therapy.
Recently, particular attention has been placed on the utility of dual tracer PET/CT evaluation of NETs with both FDG and 68Ga-DOTATATE [22, 23]. It has been shown that high-grade NETs tend to have low or absent SSTR expression and higher glucose metabolism with upregulation of glucose transporters [24]. In our cohort of 6 patients who underwent both FDG PET/CT and 68Ga-DOTATATE PET/CT, the combined imaging information was consequential only in 1 patient who had a FDG-avid poorly differentiated lung neuroendocrine tumor but also had co-existent well-differentiated metastatic hepatic lesions on 68Ga-DOTATATE PET/CT. 68Ga-DOTATATE PET/CT has additional utility in identifying disease in patient with suspected NETs with prior negative imaging. Shell et al. showed that 68Ga-DOTATATE PET/CT was able to detect disease in 18% of patients with biochemical evidence of disease and 64% of biochemically negative patients which lead to a change in clinical management in 50% and 71% of patients, respectively [25].
Another advantage of 68Ga-DOTATATE PET/CT over other NET imaging modalities (including functional and receptor-based imaging studies) is the ability to help identify or select patients who may be candidates for PRRT with 177Lu-DOTATATE (Lutathera™) [26, 27]. Since 68Ga-DOTATATE and 177Lu-DOTATATE share a common receptor target, a pre-therapy 68Ga-DOTATATE PET/CT can identify disease targets for PRRT with 177Lu-DOTATATE [28]. Additionally, follow-up studies with 68Ga-DOTATATE PET/CT may also be useful for treatment response evaluation [29].
We acknowledge the limitations of our investigation in view of its retrospective single-center design. There were multiple interpreters for various imaging studies and imaging findings were not all biopsied for verification due to practical and ethical constraints. Nevertheless, our study reflects the typical tertiary academic center clinical workflow. Additionally, management changes may not necessarily lead to improved outcomes cost effectively. Larger prospective clinical investigations with well-defined outcome measures and cost-benefit analyses will be needed.
Conclusion
68Ga-DOTATATE PET/CT provides competitive advantage over other conventional imaging methods in the evaluation of NETs by detecting new lesions in 33.3% of patients. By improving disease detection including identification of unknown primaries or metastases, use of 68Ga-DOTATATE PET/CT led to changes in therapeutic management of 23.8% of patients. An additional added benefit of the 68Ga-DOTATATE PET/CT is identification of patients who may benefit from PRRT with the theranostic pair 177Lu-DOTATATE. Future studies will establish whether changes in clinical management translate into cost-effective improved patient outcome.
Funding
The study was supported in part by a grant from the US National Cancer Institute, National Institutes of Health, P30-CA014089 (USC Norris Comprehensive Cancer Center).
Compliance with Ethical Standards
Conflict of Interest
Redmond-Craig Anderson, Erik M. Velez, Bhushan Desai, and Hossein Jadvar declare no conflict of interest.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Redmond-Craig Anderson, Email: redmond.anderson@med.usc.edu.
Erik M. Velez, Email: erik.velez@med.usc.edu
Bhushan Desai, Email: bhushan.desai@med.usc.edu.
Hossein Jadvar, Email: jadvar@med.usc.edu.
References
- 1.Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19:991–1002. doi: 10.1016/j.neo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunz PL. Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol. 2015;33:1855–1863. doi: 10.1200/JCO.2014.60.2532. [DOI] [PubMed] [Google Scholar]
- 3.Bodei L, Ambrosini V, Herrmann K, Modlin I. Current concepts in 68Ga-DOTATATE imaging of neuroendocrine neoplasms: interpretation, biodistribution, dosimetry, and molecular strategies. J Nucl Med. 2017;58:1718–1726. doi: 10.2967/jnumed.116.186361. [DOI] [PubMed] [Google Scholar]
- 4.Hope TA, Bergland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59:66–74. doi: 10.2967/jnumed.117.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell JE, Sherman SK, Menda Y, Wang D, O'Dorisio TM, Howe JR. Limitations of somatostatin scintigraphy in primary small bowel neuroendocrine tumors. J Surg Res. 2015;190:548–553. doi: 10.1016/j.jss.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2014;55:389–398. doi: 10.1177/0284185113496679. [DOI] [PubMed] [Google Scholar]
- 7.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–993. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 8.Mojtahedi A, Thamake S, Tworowska I, Ranganthan D, Delpasand ES. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging. 2014;4:426–434. [PMC free article] [PubMed] [Google Scholar]
- 9.Walker R, Deppen S, Smith G, Shi C, Leman J, Clanton J, et al. 68Ga-DOTATATE PET/CT imaging of indeterminate pulmonary nodules and lung cancer. PLoS One. 2017;12:9–11. doi: 10.1371/journal.pone.0171301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Łapińska G, Bryszewska M, Fijołek-Warszewska A, Gudzirska IK, Ochman P, Sackiewicz-Slaby A. The diagnostic role of 68Ga-DOTATATE PET/CT in the detection of neuroendocrine tumours. Nucl Med Rev Cent Eat Eur. 2011;14:16–20. doi: 10.5603/NMR.2011.0004. [DOI] [PubMed] [Google Scholar]
- 11.Deppen SA, Liu E, Blume JD, Calnton J, Shi C, Jones-Jackson LB, et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016;57:708–714. doi: 10.2967/jnumed.115.163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen I, Chen CC, Taieb D, Patronas NJ, Millo CM, Adams KT, et al. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared with other functional imaging modalities and CT/MRI. J Nucl Med. 2016;57:186–191. doi: 10.2967/jnumed.115.161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirosh A, Papadakis GZ, Millo C, Hammoud D, Sadowski SM, Herscovitch P, et al. Prognostic utility of total 68Ga-DOTATATE-avid tumor volume in patients with neuroendocrine tumors. Gastroenterology. 2018;154:998–1008. doi: 10.1053/j.gastro.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polish A, Vergo MT, Agulnik M. Management of neuroendocrine tumors of unknown origin. J Natl Compr Cancer Netw. 2011;9:1397–1402. doi: 10.6004/jnccn.2011.0118. [DOI] [PubMed] [Google Scholar]
- 15.Alexandraki K, Angelousi A, Boutzios G, Kyriakopoulos G, Rontogianni D, Kaltsas G. Management of neuroendocrine tumors of unknown primary. Rev Endocr Metab Disord. 2017;18:423–431. doi: 10.1007/s11154-017-9437-9. [DOI] [PubMed] [Google Scholar]
- 16.Sanli Y, Garg I, Kandathil A, Kendi T, Zanetti TK, Kuyumcu S, et al. Neuroendocrine tumor diagnosis and management: 68Ga-DOTATATE PET/CT. AJR Am J Roentgenol. 2018;211:267–277. doi: 10.2214/AJR.18.19881. [DOI] [PubMed] [Google Scholar]
- 17.Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–516. doi: 10.1148/rg.352140164. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann K, Czernin J, Wolin EM, Gupta P, Barrio M, Guiterrez A, et al. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: the referring physician’s perspective. J Nucl Med. 2015;56:70–75. doi: 10.2967/jnumed.114.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calais J, Czernin J, Eiber M, Fendler WP, Gartmann J, Heaney AP, et al. Most of the intended management changes after 68Ga-DOTATATE PET/CT are implemented. J Nucl Med. 2017;58:1793–1796. doi: 10.2967/jnumed.117.192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ, et al. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 21.Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med. 2010;51:875–882. doi: 10.2967/jnumed.109.066134. [DOI] [PubMed] [Google Scholar]
- 22.Jha A, Ling A, Millo C, Gupta G, Viana B, Lin FI, et al. Superiority of 68Ga-DOTATATE over 18F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx)-related pheochromocytoma and paraganglioma in the pediatric population. Eur J Nucl Med Mol Imaging. 2018;45:787–797. doi: 10.1007/s00259-017-3896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatkar D, Utpat K, Basu S, Joshi JM. Dual tracer pet imaging (68Ga-dotatate and 18F-FDG) features in pulmonary carcinoid: correlation with tumor proliferation index. Indian J Nucl Med. 2017;32:39–41. doi: 10.4103/0972-3919.198476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (Dota-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 25.Shell J, Keutgen XM, Millo C, Nilobul N, Patel D, Sadowski S, et al. 68Ga DOTATATE scanning in symptomatic patients with negative anatomic imaging but suspected neuroendocrine tumor. Int J Endocr Oncol. 2018;5:IJE04. doi: 10.2217/ije-2017-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soydal Ç, Peker A, Özkan E, Kucuk ON, Kir MK. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with Lu-177 DOTATATE. Turkish J Med Sci. 2016;46:409–413. doi: 10.3906/sag-1412-11. [DOI] [PubMed] [Google Scholar]
- 27.Hennrich U, Kopka K. Lutathera®: The first FDA-and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals (Basel) 2019;12:114. doi: 10.3390/ph12030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baum RP, Kulkarni HR. Theranostics: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy - the Bad Berka experience. Theranostics. 2012;2:437–447. doi: 10.7150/thno.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirosh A, Kebebew E. The utility of 68Ga-DOTATATE positron-emission tomography/computed tomography in the diagnosis, management, follow-up and prognosis of neuroendocrine tumors. Future Oncol. 2018;14:111–122. doi: 10.2217/fon-2017-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]