Abstract
Industrial wastewater discharges pose an environmental risk. Here, the effectiveness of an up-flow vertical hybrid system, operating with synthetic and industrial wastewater was investigated, as a new approach to perform nitrification/denitrification and desulfurization within a single reactor. The hybrid reactor is divided in two reaction zones, the oxic and anoxic. The removal of chemical oxygen demand (COD), ammonium, and sulfide was investigated, highlighting changes in microbial diversity. The reactor was evaluated at hydraulic residence time (HRT) of 1.6 days, and its performance throughout 180 days is presented in four stages. In stages I–II, high COD and ammonium removal was obtained with synthetic wastewater. In stage-III, sulfide-rich synthetic wastewater did not alter the system, attaining COD, ammonium, and sulfide removal efficiencies of 81, 99.5, and 99.7%, respectively. In the last stage, a mixture of effluents was fed into the reactor at loading rates of 277 mg COD/L-d, 46.5 mg NH4+-N /L-d, and 15 mg HS−-S /L-d. Sulfide and ammonium removals were 100% and 99.9%, respectively. However, low COD removal was observed, being of 51%, and the system removed 97% in terms of BOD5. The structure and microbial diversity also changed. Sulfide feeding, induced the proliferation of sulfur oxidizers like Thiomiscropira and Thiobacillus. Industrial wastewater enhanced the abundance of Pseudomonas (15.53%) and favored the proliferation of new bacteria of the genus Truepera (2.98%) and Alicyclipilus (7.56%). This is the first study reporting simultaneous nitrification/denitrification and desulfurization to remove ammonium, COD and sulfide from complex industrial wastewater using an up-flow vertical hybrid reactor.
Keywords: Oxic-anoxic, Wastewater, Hybrid, Desulfurization, Community
Introduction
Industrial wastewater discharges polluted with sulfide, chemical oxygen demand (COD) and ammonium, present an environmental risk. Sulfide is a toxic pollutant that in high concentrations can block respiration in humans or even alter microbial activity (Hou et al. 2018). Additionally, sulfide and organic matter in receiving water bodies decrease the concentration of dissolved oxygen affecting fish and other organisms, whereas ammonium promotes eutrophication (Veeresh et al. 2005). There are several methods to treat industrial wastewater that vary in their efficiency. For example, physicochemical and adsorption treatments although efficient, only transfer pollutants from one side to another, implying no real solution to water treatment. On the other hand, advanced treatments (e.g., electro-oxidation, Fenton, UV-Fenton) have been useful to completely remove organic matter, although they tend to be expensive (Oller et al. 2011). Nevertheless, biological treatments have been considered environmentally friendly and less expensive, since pollutants can be converted in products with null toxicity for the environment.
Nitrification and denitrification are well-documented technologies to remove organic matter and nutrients from industrial and domestic wastewaters (Zhu et al. 2008). In addition, desulfurization is also possible under nitrifying or denitrifying conditions (Beristain-Cardoso et al. 2009; Sekinet et al. 2020). Several biotechnologies have been developed to treat wastewater with low concentration of organic matter, such as ANAMMOX, SHARON, CANON, and OLAND (Zhu et al. 2008). However, when the wastewater has low C/N ratio an external donor is required, increasing the operating costs (Tam et al. 1992). Conversely, when wastewater has high C/N ratio (above 5.4/1 as COD/NO3−-N), the nitrification/denitrification has been a feasible technology to remove simultaneously organic and nitrogen compounds since an external carbon source is not required (Tam et al. 1992).
Conventional systems have been used to remove organic matter and nutrients (i.e., phosphorus and nitrogen); however, they require more space and higher building costs. In the last decade, hybrid bioreactors have gained attention since they can couple distinct biological processes in the same tank, reducing the land areas required for building. A hybrid bioreactor can be defined as a multi-modular system that allows coupling biological processes in the same reaction tank (Velasco-Garduño et al. 2018). In the following lines, a brief summary presents the promising results of using hybrid bioreactors. Long et al. (2009) evaluated an anaerobic–aerobic hybrid bioreactor with nitrogen removal efficiencies of 72%. In a hybrid moving bed biofilm reactor, pharmaceutical and personal care products were efficiently removed (Jiang et al. 2018). In 2003, Hibiya et al. (2003) reported promising results related to nitrogen removal in a membrane-aerated biofilm reactor, whereas Jianlong et al. (2008) showed high nitrogen and organic matter removal in a sequential hybrid biological reactor. Bhuvanesh et al. (2013) reported an efficient denitrifying hybrid reactor with immobilized granules for nitrate removal. Similarly, in a high-rate single airlift bioreactor, Mirghorayshi et al. (2013) observed the coupling of anammox and nitrification/denitrification for nitrogen and carbon removal. The literature above mentioned clearly shows the potential of hybrid reactors for ammonium and carbon removal, although most authors have only evaluated synthetic wastewater rather than industrial wastewater, and sulfide has not yet been studied. Also, it should be noted that until recently, the use of an up-flow vertical hybrid reactor for treating complex industrial wastewater polluted with COD, ammonium and sulfide is scarce in the literature.
Therefore, the aim of this study was to evaluate the performance of a new up-flow vertical hybrid reactor to treat synthetic and complex industrial wastewater polluted with COD, ammonium, and sulfide. In addition, to evaluate changes in the structure and the microbial diversity, sequencing of 16S rRNA gene amplicons with Illumina Miseq were also performed.
Materials and methods
Batch cultures
Batch cultures were carried out to evaluate the nitrification, since this biological step is crucial to couple nitrification and denitrification. Batch cultures were implemented in glass bottles of 500 ml with a working volume of 400 ml. The chemical composition of synthetic wastewater (SW) in g/L was as follows: NH4Cl-N (0.075); KH2PO4 (1.0); K2HPO4 (1); NaHCO3 (1); MgCl (0.01); CaCl2 (0.01), and 0.2 ml/L of enzymatic cofactors (Velasco-Garduño et al. 2018). Batch experiments were inoculated with 3 g VSS/L of activated sludge taken from an Industrial Wastewater Treatment Plant (IWTP) located in Mexico. Experiments were executed by duplicate, and pH was controlled at 7.03 ± 0.2 due to the bicarbonate and phosphate buffers in the synthetic medium. The dissolved oxygen level was controlled at 3.5 ± 0.3 mg/L using aquarium air stones. Batch cultures were carried out using synthetic wastewater (as control test) and complex industrial wastewater. The industrial wastewater was a mixture of two industrial effluents, food processing and textile industries. The chemical composition of the wastewater was as follows: 3700 ± 500 mg COD/L; 240 ± 50 mg NT/L, and 1430 ± 350 mg BOD5/L. The industrial wastewater had also a low concentration of metals (not quantified). The biodegradability index (BOD5/COD) of this kind of industrial wastewater was 0.38, and this value allowed to use a biological process. BOD5/COD between 0.3 and 0.6 is suitable for biological treatment, but seeding is required (Abdalla and Hammam 2014). Other authors consider that wastewater is easily biodegradable between 0.4 and 0.8 (Metcalf and Eddy 1985; Al-Momani et al. 2002). On the other hand, the integrated Gompertz model (Origin 8.0, OriginLab, Inc. ®) was used to estimate the kinetic parameters and to compute the ammonium consumption specific rate according to following equation:
| 1 |
where A (mg/L) is the maximum ammonium consumed, K (h−1) is the kinetic constant for the consumption rate, and B ( g VSS/L) is the initial biomass spiked to the batch cultures.
Experimental set-up
A cylindrical bioreactor built in acrylic, with a working volume of 4.3 L, was used for the study. The vertical hybrid reactor had a total height of 0.9 m and 9.5 cm of internal diameter. The bioreactor was divided into two modules by an acrylic mesh. The first module was the oxic section (0.4 m height) containing suspended biomass and 20% of kaldnes (polyethylene media) as biofilm support. The second module was the anoxic section (0.5 m height) packed with tezontle rock with an average diameter of 2 cm (Fig. 1). The raw industrial wastewater was stored under refrigeration at 2 ºC before and during its use to avoid chemical reactions. The air was supplied to the hybrid reactor using an aquarium pump of 2 L/min to maintain DO in oxic zone of 3.5 mg/L. The bioreactor was operated at a hydraulic retention time (HRT) of 1.6 days for a period of 180 days. The results will be presented in four stages (SI—SIV). The source of wastewater and the initial pollutant concentrations in each stage are presented in Table 1. The efficiencies (E%) of ammonium, COD, BOD5 and sulfide removal, and the production yields (Y = dimensionless value) were calculated as follows:
| 2 |
| 3 |
| 4 |
Fig. 1.
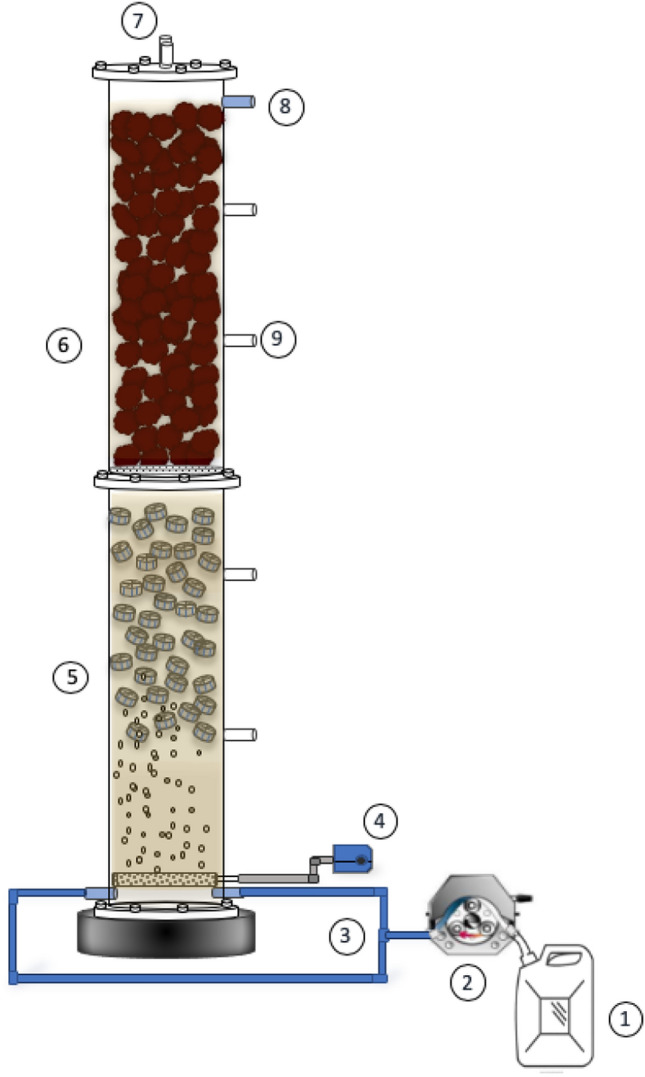
Schematic of the up-flow vertical hybrid reactor. (1) Influent container, (2) peristaltic pump, (4) air pump, (5) oxic module, (6) anoxic module, (7) gas output, (8) effluent, and (9) sampling ports
Table 1.
Wastewater source, pollutant and its initial concentration in each phase
| Wastewater source | COD influent (mg/L) | NH4+-N influent (mg/L) | HS− Influent (mg/L) | |
|---|---|---|---|---|
| Stage I | Synthetic | 252 ± 48 | 38 ± 5.6 | – |
| Stage II | Synthetic | 392 ± 66 | 156 ± 8.7 | – |
| Stage III | Synthetic | 418 ± 59 | 150 ± 12 | 48 ± 5 |
| Stage IV | Industrial | 443 ± 25 | 74 ± 3.0 | 24 ± 3 |
Bacterial community analysis
In each steady state, sludge samples (suspended sludge and biofilm) were taken from both modules and mixed for DNA extraction. Biomass attached to the support media was extracted using 0.1 M of EDTA. The methodology used for DNA extraction, analysis of PCR amplification products (5–20 ng of sample), amplicon multiplexing, and sequencing is detailed in Aguirre-Garrido et al. (2016). Bioinformatic analysis of the 16S rRNA sequences were performed using the MOTHUR software (Schloss et al. 2009; Kozich et al. 2013). After demultiplexing, with lengths of 400–490 bp, chimeric reads were first identified to be excluded using the chimera-vsearch program (Rognes et al. 2016). The composition of microbial communities was then determined with the Naïve Bayesian classifier provided by the RDP (Wang et al. 2007).
Analytical methods
Ammonium and oxygen were analyzed using the selective electrodes Hannah HI-4101 and Hanna HI-98186, respectively. Nitrate and nitrite were analyzed by HPLC (PerkinElmer series 200) following the methodology reported by Velasco-Garduño et al. (2018). N2 and N2O were detected by gas chromatography with a thermal conductivity detector (GOW-MAC Series 580). Liquid samples were filtered with a membrane (0.45 µm) to measure chemical oxygen demand by the closed reflux method. BOD5 was measured in a BODTrak™ II (HACH). Finally, sulfide was measured by the iodometric method, and volatile suspended solids (VSS) were determined according to the standard methodology (APHA 2005).
Results and discussion
Batch cultures
Initially, batch cultures were carried out to evaluate the nitrifying behavior, since this biological step is crucial to couple nitrification and denitrification. The time course of the nitrifying profile in presence of synthetic water (SW) and industrial wastewater (IW) is shown in the Fig. 2. Nitrifying culture, evaluated with SW, displayed an ammonium removal efficiency (ENH4+) of 100% within 25 h. The specific ammonium consumption rate (qNH4+) was of 2.89 ± 0.3 mg NH4+-N/g VSS-h. Nitrate was the end product, with a production yield (YNO3-) of 0.93 ± 0.03 mg NO3−-N/NH4+-N. There were also significant differences when IW was applied (Fig. 2); for example, activated sludge removed ammonium in 82.6 ± 0.2%. The qNH4+ diminished significantly compared to the nitrifying control, with a result of 1.3 ± 0.2 mg NH4+-N/g VSS-h. Nitritation or partial nitrification was induced due to nitrite was the end product instead of nitrate, with a production yield (YNO2-) of 0.95 ± 0.02 mg NO2−-N/NH4+-N. Other studies have found that nitritation is linked to dissolved oxygen limitation (0.1–1.5 mg/L) or the presence of strong inhibitors (Zhu et al. 2008; Kouba et al. 2017; Vela et al. 2018). In the present study, there was no oxygen limitation, therefore sulfur or dye compounds contained in the industrial wastewater might be induced the nitritation process (NH4+ NO2−). This gave clear evidence that complex industrial wastewater influenced strongly the nitrifying behavior of the activated sludge.
Fig. 2.
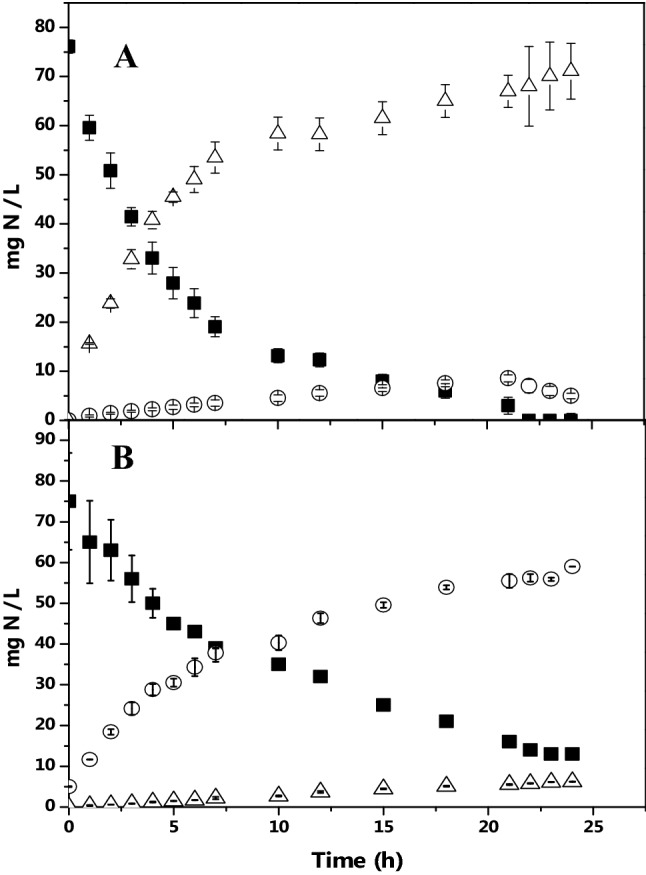
Nitrifying batch cultures. a Synthetic wastewater, b industrial wastewater; (■) NH4+-N, (Δ) NO3−-N, (○) NO2−-N
Reactor performance
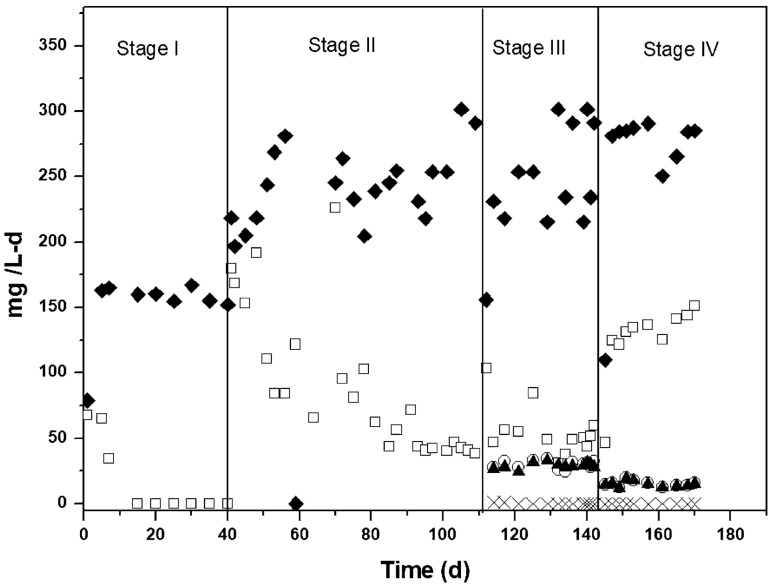
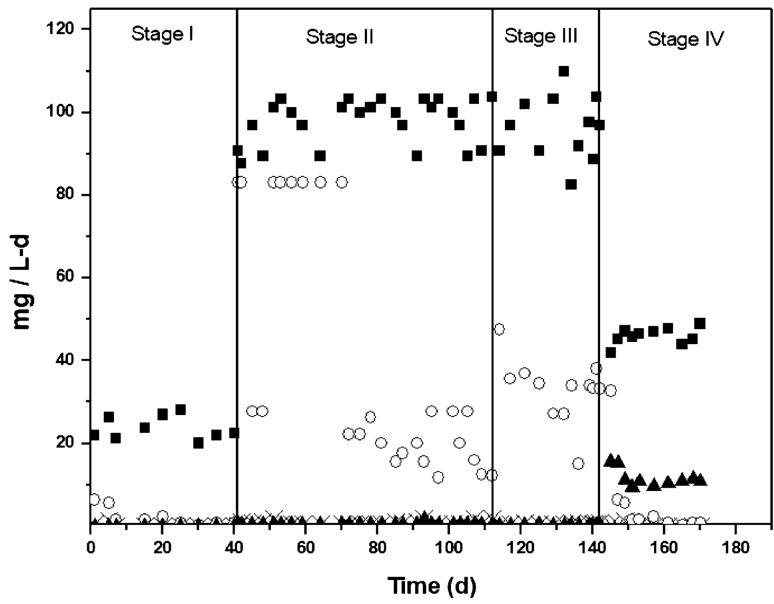
In stage-I and stage-II, the hybrid reactor was fed with synthetic wastewater containing COD-glucose and ammonium. In stage-III, the bioreactor was fed with COD-glucose and ammonium, plus sulfide. Finally, in the stage-IV, the bioreactor was fed with complex industrial wastewater containing COD, ammonium and sulfide. Figures 3 and 4 show the global profiles of COD, ammonium and sulfide. In the stage I, the hybrid reactor was fed at loading rates of 158 ± 6.5 mg COD/L-d and 23.8 ± 3.5 mg NH4+-N/L-d. The steady state was reached in 20 days, where COD and ammonium removals were 99.9 and 100%, respectively (Table 2). Nitrate residual was as low as 1.7 mg NO3−-N/L, with YNO3- of 0.04 ± 0.01, whereas nitrite was not detected in the effluent, suggesting that nitrification and denitrification played important roles on nitrogen biotransformation. Although N2 was detected, it was not easy to track and quantify because the reactor was continuously aerated.
Fig. 3.
COD and sulfur compounds profiles in the continuous hybrid bioreactor; (♦) CODinfluent, (□) CODeffluent, (○) HS−-Sinfluent, (×) HS−-Seffluent, (▲) SO42−-S
Fig. 4.
Nitrogen compounds profile in the continuous hybrid bioreactor; (■) NH4+influent, ( ×) NH4+effluent, (○) NO3−, (▲) NO2−
Table 2.
Production yields and removal efficiencies from the up-flow vertical hybrid reactor operated in continuous mode
| COD removal efficiency (%) | NH4+ removal efficiency (%) | HS− removal efficiency (%) | NO3-N yield | NO2-N yield | SO42-S yield | |
|---|---|---|---|---|---|---|
| Stage I | 99.9 ± 3.1 | 100.00 | 0.04 ± 0.01 | |||
| Stage II | 82.7 ± 2.8 | 99.2 ± 0.4 | 0.20 ± 0.06 | 0.005 ± 0.004 | ||
| Stage III | 81.0 ± 3.5 | 99.5 ± 0.2 | 99.7 ± 0.2 | 0.34 ± 0.09 | 0.003 ± 0.001 | 1.0 ± 0.11 |
| Stage IV | 51 ± 2.8 | 100.00 | 99.9 ± 0.03 | 0.02 ± 0.01 | 0.23 ± 0.020 | 0.98 ± 0.01 |
In the stage-II, loading rates increased to 256 ± 28 mg COD/L-d and 97 ± 5 mg NH4+-N/L-d (Fig. 4). In the steady state, COD degradation diminished from 99.9 to 82.7%. Ammonium removal did not significantly change (99.2 ± 0.4%). The YNO3- and YNO2- were 0.20 ± 0.06 and 0.005 ± 0.004, respectively. These results suggest that the remaining 80% of ammoniacal nitrogen consumed was converted mainly to biomass and N2.
In the stage-III, the hybrid reactor was fed with synthetic water containing sulfide at a loading rate of 30 ± 3 mg HS−-S/L-d (Fig. 3), whereas COD and ammonium were fed at loading rates of 261 ± 37 mg/L-d and 93.5 ± 7.5 mg N/L-d, respectively. Sulfide feeding did not affect both, ammonium and COD removal, since they remained at 99.5 ± 0.2% and 81 ± 3.5%, respectively. The nitrate yield increased from 0.20 ± 0.06 in the previous stage to 0.34 ± 0.09. The sulfide removal was of 99.7 ± 0.2% (Table 2) and it was completely oxidized to sulfate, with a production yield (YSO4-) of 1.0 ± 0.11 mg SO42−-S/mg HS−-S. Sulfide is widely known to inhibit the nitrification process; for example, Vela et al. (2018) showed that sulfide is a stronger inhibitor of nitrite oxidizing bacteria at concentrations from 0.22 to 13 mg/L. In the present work, previous biomass stabilization with ammonium and COD might have contributed to a greater sulfide tolerance. In this sense, Jiang et al. (2018) observed a high sulfide removal in a bioreactor acclimated previously with ammonium; also, Beristain-Cardoso et al. (2011) observed the same behavior in a continuous stirred-tank reactor, where high sulfide removal was achieved in a nitrifying sludge previously acclimated with ammonium and p-cresol. In the literature it has been reported that sulfide may be removed in both, chemical and biochemical reactions. For example, Celis-Garcia et al. (2008) observed that sulfide was oxidized mostly by biological processes, whereas Beristain-Cardoso et al. (2011) showed that sulfide was oxidized 12-fold-faster via biological reactions under nitrifying conditions, rather than by a chemical reaction. Sekine et al. (2020) reported the sulfide oxidation under nitrifying conditions in 8 h and indicated that this was possible because the nitrifying sludge normally has sulfur-oxidizing bacteria. González-Sánchez and Revah (2007) reported that sulfide oxidation via a chemical reaction can attain high rates only at pH above 9. In the present study, the pH in the effluent was around 8.0, suggesting that sulfide was removed mainly via biological processes.
In stage-IV, the complex industrial wastewater was fed into the hybrid reactor, but it was diluted with tap water to be compared in terms of COD with the previous stage. The bioreactor was fed with COD, ammonium and sulfide at loading rates of 277 ± 16 mg/L-d, 46.5 ± 2.0 mg/L-d and 15 ± 2.0 mg/L-d, respectively. In the steady state, the COD removal efficiency attained was of 51 ± 2.8%. The low COD removal might be attributed to dye compounds contained in the industrial wastewater, which are compounds of difficult biodegradation.
Even though the system was fed with complex industrial wastewater, sulfide removal was high (99.9 ± 0.03%). Sulfide was oxidized to sulfate with a yield of YSO4- of 0.98 ± 0.01. This yield value indicates that the complex industrial wastewater was completely desulfurized. Ammonium removal efficiency did not change significantly compared to the previous stage, since it remained at the 100%. YNO3- and YNO2- were 0.02 ± 0.01 and 0.23 ± 0.020, respectively, indicating that 23% of ammonium nitrogen consumed was recovered mainly as nitrite. The nitritation or partial nitrification in the continuous hybrid bioreactor was initially predicted in the section of batch cultures, since nitritation took place owing to the feeding of industrial wastewater. It is well known that phenol and dye pollutants inhibit strongly the nitrification with nitrite accumulation (He and Bishop 1994; Silva et al. 2011; Velasco-Garduño et al. 2018). For example, Hockenbury et al. (1977) observed that 52 industrial organic compounds produced a nitrite accumulation under nitrifying conditions, whereas Silva et al. (2011) observed that nitrification was inhibited in the presence of phenolic compounds such as 2-chlorophenol, phenol, p-cresol and p-hydroxybenzaldehyde. Similarly, Li and Bishop (2002) reported a nitrifying inhibition in presence of acid orange 7, and He and Bishop (1994) showed that acid orange 7 inhibited the whole nitrification process, being the nitrite oxidation the most affected step.
On the other hand, N2O was detected along with molecular nitrogen. Nitrous oxide can be produced either by nitritation (Rodriguez-Caballero et al. 2013) or denitrification. In the case of nitritation, the mechanisms involved for N2O production are not completely understood. In denitrification however, there are several factors involved on nitrous oxide reductase enzyme inhibition (e.g., oxygen limitation, sulfide, C/N ratio, azide, cyanide). Further studies should be addressed to determine the percentage of N2O produced under these experimental conditions, since this intermediary is considered as a greenhouse gas with a 300-fold stronger effect than CO2. Therefore, careful attention needs to be paid when evaluating the applicability of this biotechnology from an environmental perspective.
Bacterial community composition
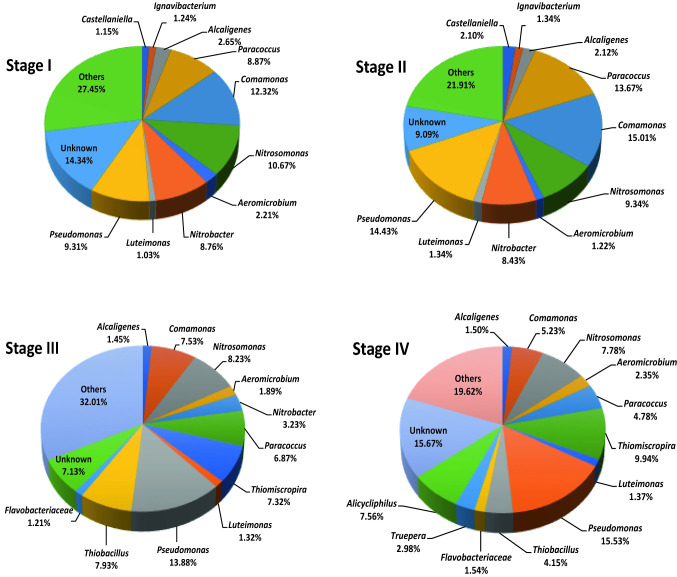
The tool Illumina MiSeq Sequencing showed that the microbial community structure and diversity were modified due to the chemical composition of the wastewater. Figure 5 shows the relative abundance of bacteria, at the genus level, in each steady state. In the stage-I, Paracoccus (8.87%), Comamonas (12.32%), Nitrosomonas (10.67%), Nitrobacter (8.76%), and Pseudomonas (9.31%) were the predominant genera in the microbial sludge. Nitrosomonas and Nitrobacter are nitrifying bacteria well known to oxidize ammonium to nitrite and nitrite up to nitrate, respectively. Comamonas and Paracoccus are bacteria well identified in activated sludge systems (Shchegolkova et al. 2016); for example, Comamonas nitrativorans is a denitrifier isolated from a denitrifying reactor (Etchebehere et al. 2001). Paracoccus denitrificans is a denitrifying bacterium with facultative metabolism, since it is able to use either oxygen or nitrite as electron acceptor for respiration (Uemoto et al. 1996). Pseudomonas are heterotrophic bacteria involved on organic matter removal under aerobic conditions, although some species such as P. stutzeri and P. aeruginosa may act as denitrifiers (Khanichaidecha et al. 2019).
Fig. 5.
Changes in the microbial diversity of bacteria at genus level in the operating hybrid reactor
In the stage-II, the abundance of Paracoccus (13.67%), Comamonas (15.01%), and Pseudomonas (14.43%) increased. This boost might be attributed to the effect of increasing loadings rates. The relative abundance of Nitrosomonas and Nitrobacter did not change significantly. In this case, nitrifiers and heterotrophs compete for the same oxidizing source (i.e., oxygen) but heterotrophic growth is kinetically more favorable than nitrification, although both biological processes may coexist (Ma et al. 2013). The microbial results evidence that nitrifying and denitrifying bacteria in the hybrid reactor were involved in nitrogen removal.
In the stage-III, the sulfide introduced into the hybrid reactor changed the microbial community structure with Comamonas, Paracoccus and Nitrobacter diminishing in abundance, since sulfide is known to be a strong inhibitor of the nitrite oxidation step (Vela et al. 2018). In addition, new genera such as Thiobacillus (7.93%) and Thiomiscropira (7.32%) proliferated in this stage. Thiomiscropira is a chemolithotrophic sulfur oxidizer identified in aerobic biofilters (Cytryn et al. 2005). In the case of Thiobacillus, T. denitrificans is a sulfide-oxidizing with the metabolic capability to use oxygen or nitrate as oxidizing source (Pokorna et al. 2015). Although autotrophic denitrifying bacteria are chemolithotrophics, P. denitrificans is also able to adapt to heterotrophic and mixotrophic growth (Pokorna et al. 2015). Here, this result suggests that sulfide might have been removed via two biological pathways; aerobic sulfide oxidation and lithotrophic denitrification.
In the last stage, Pseudomonas increased in abundance (~ 15.5%) which may be due to the presence of dyes in the industrial wastewater, since P. aeruginosa and P. putida have been reported to remove them (Bayoumi et al. 2014). Camomonas and Paracoccus diminished in abundance compared to the previous stage. This decline might be attributed to the presence of recalcitrant compounds contained in the industrial wastewater. On the other hand, Thiobacillus and Thiomiscropira remained in the microbial structure, although Thiobacillus diminished in abundance suggesting that this genus was more sensible to the presence of organic inhibitors. The genus Nitrobacter was no longer detected, which could justify the nitrite presence in the effluent. The industrial wastewater promoted the proliferation of other genera such as Truepera (2.98%) and Alicycliphilus (7.56%) which have also been identified in activated sludge systems (Sánchez et al. 2011; Morohoshi et al. 2016). For example, Alicycliphilus are able to use oxygen, nitrate or chlorate as oxidizing sources to biodegrade refractory organic compounds under oxic or anoxic conditions (Solís-González et al. 2019).
Conclusion
The new up-flow vertical hybrid bioreactor showed the potential to remove simultaneously organic matter, ammonium and sulfide by coupling nitrification/denitrification and desulfurization from synthetic and complex industrial wastewater. The nitrifying batch cultures, as well as the microbiota, suggested that ammonium was removed via nitritation/denitrification when the system was operated with industrial wastewater. Sulfide fed into the bioreactor and the chemical composition of the wastewater shifted the microbial community structure. Thiobacillus and Thiomiscropira were the main genera identified as sulfur oxidizers in the hybrid reactor, highlighting their potential as sulfur bioindicators.
Acknowledgements
The second author acknowledges the support of CONACyT-Mexico through a scholarship to attend the doctorate of Oscar Velasco. Part of the project was supported by PRODEP-SEP-UAM-L-CA-3.
Author contributions
Investigation: CS, GB, VG. Analysis of molecular biology: GB, AG. Supervision: BC. Review and editing: BC, RG.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Abdalla KZ, Hammam G. Correlation between biochemical oxygen demand and chemical oxygen demand for various wastewater treatment plants in Egypt to obtain the biodegradability indices. Int J Sci: Basic Appl Res. 2014;13:42–48. [Google Scholar]
- Aguirre-Garrido JF, Ramírez-Saad HC, Toro N, Martínez-Abarca F. Bacterial diversity in the soda saline Crater Lake from Isabel Island, Mexico. Microb Ecol. 2016;71:68–77. doi: 10.1007/s00248-015-0676-6. [DOI] [PubMed] [Google Scholar]
- Al-Momani F, Touraud E, Degorce Dumas JR, Roussy J, Thomas O. Biodegradability enhancement of textile dyes and textile wastewater by UV photolysis. J Photoch Photobio A. 2002;153:191–197. doi: 10.1016/S1010-6030(02)00298-8. [DOI] [Google Scholar]
- APHA Awwa WEF . Standard methods for the examination of water and wastewater. Alexandria: American Public Health Association; 2005. [Google Scholar]
- Bayoumi MN, Al-Wasify RS, Hamed SR. Bioremediation of textile wastewater dyes using local bacterial isolates. Int J Curr Microbiol App Sci. 2014;3:962–970. [Google Scholar]
- Beristain-Cardoso R, Texier AC, Razo-Flores E, Méndez-Pampín R, Gómez J. Biotransformation of aromatic compounds from wastewaters containing N and/or S, by nitrification/denitrification: a review. Rev Environ Sci Bio/Technol. 2009;8:325–342. doi: 10.1007/s11157-009-9172-0. [DOI] [Google Scholar]
- Beristain-Cardoso R, Pérez-González DN, González-Blanco G, Gómez J. Simultaneous oxidation of ammonium, p-cresol and sulfide using a nitrifying sludge in a multipurpose bioreactor: a novel alternative. Bioresour Technol. 2011;102:3623–3625. doi: 10.1016/j.biortech.2010.10.127. [DOI] [PubMed] [Google Scholar]
- Bhuvanesh S, Maneesh N, Sreekrishnan TR. Start-up and performance of a hybrid anoxic reactor for biological denitrification. Bioresour Technol. 2013;129:78–84. doi: 10.1016/j.biortech.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Celis-García LB, González-Blanco G, Meraz M. Removal of sulfur inorganic compounds by a biofilm of sulfate reducing and sulfide oxidizing bacteria in a down-flow fluidized bed reactor. J Chem Technol Biotechnol. 2008;83:260–268. doi: 10.1002/jctb.1802. [DOI] [Google Scholar]
- Cytryn E, van Rijn J, Schramm A, Gieseke A, de Beer D, Minz D. Identification of bacteria potentially responsible for oxic and anoxic sulfide oxidation in biofilters of a recirculating mariculture system. App Environ Microbiol. 2005;71:6134–6141. doi: 10.1128/AEM.71.10.6134-6141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchebehere C, Errazquin MI, Dabert P, Moletta R, Muxí L. Comamonas nitrativorans sp. nov., a novel denitrifier isolated from a denitrifying reactor treating landfill leachate. Int J Syst Evol Microbiol. 2001;51:977–983. doi: 10.1099/00207713-51-3-977. [DOI] [PubMed] [Google Scholar]
- González-Sánchez A, Revah S. The effect of chemical oxidation on the biological sulfide oxidation by an alkaliphilic sulfoxidizing bacterial consortium. Enzyme Microbiol Technol. 2007;40:292–298. doi: 10.1016/j.enzmictec.2006.04.017. [DOI] [Google Scholar]
- He Y, Bishop PL. Effect of acid orange 7 on nitrification process. J Environ Eng. 1994;120:108–121. doi: 10.1061/(ASCE)0733-9372(1994)120:1(108). [DOI] [Google Scholar]
- Hibiya K, Terada A, Tsuneda S, Hirata A. Simultaneous nitrification and denitrification by controlling vertical and horizontal microenvironment in a membrane-aerated biofilm reactor. J Biotechnol. 2003;100:23–32. doi: 10.1016/S0168-1656(02)00227-4. [DOI] [PubMed] [Google Scholar]
- Hockenbury MR, Leslie Grady CP (1977) Inhibition of nitrification-effects of selected organic compounds. J Water Pollut Control Fed 768–777. www.jstor.org/stable/25039349
- Hou N, Xia Y, Wang X, Liu H, Liu H, Xun L. H2S biotreatment with sulfide-oxidizing heterotrophic bacteria. Biodegradation. 2018;29:511–524. doi: 10.1007/s10532-018-9849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Ngo HH, Nghiem LD, Hai FI, Price WE, Zhang J, Liang S, Deng L, Guo W. Effect of hydraulic retention time on the performance of a hybrid moving bed biofilm reactor-membrane bioreactor system for micropollutants removal from municipal wastewater. Bioresour Technol. 2018;247:1228–1232. doi: 10.1016/j.biortech.2017.09.114. [DOI] [PubMed] [Google Scholar]
- Jianlong W, Yongzhen P, Shuying W, Yongqing GAO. Nitrogen removal by simultaneous nitrification and denitrification via nitrite in a sequence hybrid biological reactor. Chin J Chem Engin. 2008;16:778–784. doi: 10.1016/S1004-9541(08)60155-X. [DOI] [Google Scholar]
- Khanichaidecha W, Nakaruk A, Ratananikom K, Eamrat R, Kazama F. Heterotrophic nitrification and aerobic denitrification using pure-culture bacteria for wastewater treatment. J Water Reuse Desalin. 2019;9:10–17. doi: 10.2166/wrd.2018.064. [DOI] [Google Scholar]
- Kouba V, Proksova E, Wiesinger H, Vejmelkova D, Bartacek J. Good servant, bad master: sulfide influence on partial nitritation of sewage. Water Sci Technol. 2017;76:3258–3268. doi: 10.2166/wst.2017.490. [DOI] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bishop PL. In situ identification of azo dye inhibition effects on nitrifying biofilms using microelectrodes. Water Sci Technol. 2002;46:207–214. doi: 10.2166/wst.2002.0479. [DOI] [PubMed] [Google Scholar]
- Long Y, Hu LF, Shen DS. Nitrogen transformation in the hybrid bioreactor landfill. Bioresour Technol. 2009;100:2527–2533. doi: 10.1016/j.biortech.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Ma J, Wang Z, Zhu C, Liu S, Wang Q, Wu Z. Analysis of nitrification efficiency and microbial community in a membrane bioreactor fed with low COD/N-ratio wastewater. PLoS ONE. 2013;8:e63059. doi: 10.1371/journal.pone.0063059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf and Eddy . Wastewater engineering: treatment, disposal and reuse. 3. New York: McGraw-Hill Inc.; 1985. [Google Scholar]
- Mirghorayshi M, Zinatizadeh AA, Van Loosdrecht M. Evaluating the process performance and potential of a high-rate single airlift bioreactor for simultaneous carbon and nitrogen removal through coupling different pathways from a nitrogen-rich wastewater. Bioresour Technol. 2013;260:44–52. doi: 10.1016/j.biortech.2018.03.048. [DOI] [PubMed] [Google Scholar]
- Morohoshi T, Okutsu N, Xie X, Ikeda T. Identification of quorum-sensing signal molecules and a biosynthetic Gene in Alicycliphilus sp. isolated from activated sludge. Sensors. 2016;16:1218. doi: 10.3390/s16081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller I, Malato S, Sánchez-Pérez J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ. 2011;409:4141–4166. doi: 10.1016/j.scitotenv.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Pokorna D, Zabranska J. Sulfur-oxidizing bacteria in environmental technology. Biotechnol Adv. 2015;33:1246–1259. doi: 10.1016/j.biotechadv.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Caballero A, Ribera A, Balcázar JL, Pijuan M. Nitritation versus full nitrification of ammonium-rich wastewater: comparison in terms of nitrous and nitric oxides emissions. Bioresour Technol. 2013;139:195–202. doi: 10.1016/j.biortech.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016 doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez O, Garrido L, Forn I, Massana R, Maldonado MI, Mas J. Molecular characterization of activated sludge from a seawater-processing wastewater treatment plant. Microb Biotechnol. 2011;4:628–642. doi: 10.1111/j.1751-7915.2011.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Enviro Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine M, Akizuki S, Kishi M, Kurosawa N, Toda T. Simultaneous biological nitrification and desulfurization treatment of ammonium and sulfide-rich wastewater: effectiveness of a sequential batch operation. Chemosphere. 2020;244:125381. doi: 10.1016/j.chemosphere.2019.125381. [DOI] [PubMed] [Google Scholar]
- Shchegolkova NM, Krasnov GS, Belova AA, Dmitriev AA, Kharitonov SL, Klimina KM, Melnikovs NV, Kudryavtseva AV. Microbial community structure of activated sludge in treatment plants with different wastewater compositions. Front Microbiol. 2016;7:90. doi: 10.3389/fmicb.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CD, Gómez J, Beristain-Cardoso R. Simultaneous removal of 2-chlorophenol, phenol, p-cresol and p-hydroxybenzaldehyde under nitrifying conditions: kinetic study. Bioresour Technol. 2011;102:6464–6468. doi: 10.1016/j.biortech.2011.03.105. [DOI] [PubMed] [Google Scholar]
- Solís-González CJ, Loza-Tavera H. Alicycliphilus: current knowledge and potential for bioremediation of xenobiotics. J Appl Microbiol. 2019;126:1643–1656. doi: 10.1111/jam.14207. [DOI] [PubMed] [Google Scholar]
- Tam NFY, Wong YS, Leung G. Effect of exogenous carbon sources on removal of inorganic nutrients by the nitrification denitrification process. Water Res. 1992;26:1229–1236. doi: 10.1016/0043-1354(92)90183-5. [DOI] [Google Scholar]
- Uemoto H, Saiki H (1996) Nitrogen removal by tubular gel containing Nitrosomonas europaea and Paracoccus denitrificans. Appl Environ Microbiol 62:4224–4228. https://www.ncbi.nlm.nih.gov/pubmed/8900015 [DOI] [PMC free article] [PubMed]
- Veeresh GS, Kumar P, Mehrotra I. Treatment of phenol and cresols in upflow anaerobic sludge blanket (UASB) process: a review. Water Res. 2005;39:154–170. doi: 10.1016/j.watres.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Vela JD, Dick GJ, Love NG. Sulfide inhibition of nitrite oxidation in activated sludge depends on microbial community composition. Water Res. 2018;138:241–249. doi: 10.1016/j.watres.2018.03.047. [DOI] [PubMed] [Google Scholar]
- Velasco-Garduño O, Mendoza-Reséndiz A, Fajardo-Ortiz C, Beristain-Cardoso R. Simultaneous ammonia and organic matter removal from industrial wastewater in a continuous novel hybrid carrousel bioreactor. Int J Environ Sci Technol. 2018;16:3429–3436. doi: 10.1007/s13762-018-2017-z. [DOI] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Peng Y, Li B, Guo J, Yang Q, Wang S (2008) Biological removal of nitrogen from wastewater. In: Reviews of Environmental contamination and toxicology. Springer, New York, pp 159–195. 10.1007/978-0-387-71724-1_5 [DOI] [PubMed]